Abstract
Introduction
Diagnostic confirmation of SARS-CoV-2 by self-collection of specimens is a reliable method compared with healthcare worker collected samples. Citizens’ preferences for collection methods are unknown, but at-home collection could have several advantages.
Methods
This study investigated the preference for guided at-home self-collection versus at-hospital specimen collection by healthcare workers.
Results
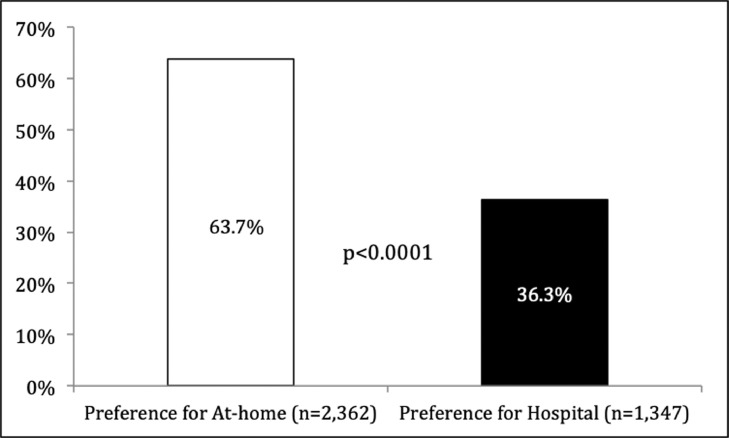
Among the 3709 participants, at-home swab collection was the preferred setting for 2362 (63.7%) compared with 1347 (36.3%) reporting a preference for an at-hospital swabbing procedure.
Conclusion
A high preference for guided at-home self-collection of oropharyngeal/nasal SARS-CoV-2 specimens exists and could be a future norm beyond COVID-19.
Keywords: COVID-19, Diagnostics, Self-collection, Oropharyngeal/nasal swab, SARS-CoV-2, Patient-reported
Introduction
Since its emergence, SARS-CoV-2 causing COVID-19 has infected more than 179 million persons (World Health Organization, 2021). The gold standard for diagnostic confirmation of SARS-CoV-2 is reverse transcriptase-polymerase chain reaction (RT-PCR) using nasopharyngeal specimens collected by a swab. We (Therchilsen et al., 2020) and others (Tu et al., 2020) have found that self-collection of SARS-CoV-2 specimens is a reliable method compared with healthcare worker (HCW) collected samples with acceptable agreement of Cohens kappa 0.82 and without any significant difference in diagnostic sensitivity (84.2% and 89.5%, respectively, P = 0.81). Self-collection has also been found to be acceptable for citizens (Valentine-Graves et al., 2020). Reliability and acceptability have been established, but citizens’ preferences towards collection methods are unknown. In order to succeed in impeding SARS-CoV-2 transmission, the inclusion of citizens’ preferences is essential as previous research has shown that anticipated use of at-home self-tests for other infectious diseases can be misguiding due to behaviour that can be uncovered by assessing citizens or patients thoughts (Colfax et al., 2002). COVID-19 has placed stress on hospital bed capacity, equipment, and healthcare personnel, making healthcare resource allocation a higher priority than usual (Emanuel et al., 2020). In the Danish population of 5.8 million citizens, health authorities report that more than 35 million PCR tests for SARS-CoV-2 have been conducted at testing facilities, and a PCR test has been carried out on 84% of the population (Sundhedsstyrelsen, 2021). There are 662 test centres (PCR and antigen) in Denmark, resulting in massive employment (Danish-Regions, 2021). This pressure on the healthcare system could be accommodated by moving diagnostics from at-hospital to at-home. It has previously been shown that a hierarchy of willingness to test for SARS-CoV-2 is ordered by the degree of contact required and that at-home specimen collection options could result in approximately one-third more symptomatic persons being tested without any difference in willingness to test across sociodemographic factors (Siegler et al., 2020). At-home testing is not a new diagnostic approach, it has been used for diagnostics and management of various conditions for years, and the US Food and Drug Administration (FDA) has recently authorized the first at-home test for COVID-19 in their commitment to expanding access to testing (FDA, 2020). Our study investigates citizens’ preferences for a SARS-CoV-2 specimen collection setting of either at-hospital or at-home.
Methods
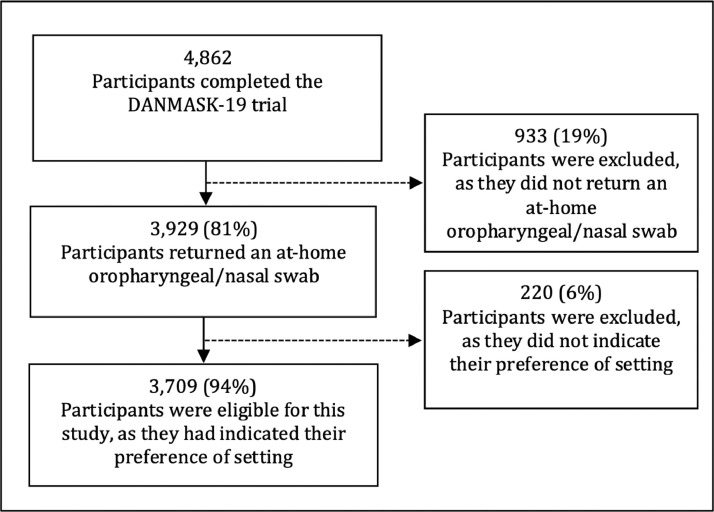
This is a substudy of the randomized controlled trial entitled Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers (DANMASK-19) (Bundgaard et al., 2020) (ClinicalTrials.gov: NCT04337541). The target population was Danish citizens above 18 years who spend more than 3 hours per day outside the home. In addition to the criteria from the DANMASK-19 trial, inclusion criteria for this study were that participants had conducted an at-home oropharyngeal/nasal swab and completed the survey. Nationwide recruitment of participants involved media advertisements and contacting public organizations and private companies. Participants were enrolled during April and May 2020. In this period, Danish authorities had not yet begun recommending mask use in the community outside of hospitals (YouGov, 2021). However, recommended public health measures included quarantining persons with SARS-CoV-2 infection, social distancing, frequent hand hygiene and cleaning, limiting visitors to hospitals and nursing homes, and limiting the number of persons seen (Sundhedsstyrelsen, 2020, n.d.). Approximately half of the participants in the DANMASK-19 trial were capital region residents, and a small majority of participants were female. The occupations of participants were limited to non-mask-wearing jobs and were distributed across various sectors. In order to reduce confounding factors related to recruitment, participation was free of charge, and we used nationwide advertisement through multiple channels. Participants received surveys by e-mail and reminders by phone (text messages). All participants conducted 1 or 2 at-home swabs according to the trial description before reporting their preferred swab procedure setting. Participants who had performed at least 1 at-home swab procedure and responded to a preferred setting for future swab procedures were eligible for the present substudy. Participants received materials and instructions at home and free of charge. In order to perform the self-collected swab procedure at home, a detailed 2-minute video guidance of an oropharyngeal/nasal swab (Therchilsen et al., 2020) procedure carried out by an HCW and a written step-by-step manual were provided (Bundgaard et al., 2020). Participants returned the test material by a prepaid express courier. At-hospital SARS-CoV-2 testing by swab was free of charge during the study period for all Danes. Surveys were sent by e-mail to assess participants’ preference for SARS-CoV-2 swab setting for future specimen collections. Participants answered the surveys through Research Electronic Data Capture (REDCap) software (Harris et al., 2009) and the study question was “In the future, what would you prefer if you had to be swab tested?” with 3 answering categories a) self-swab at home – and I think it was easy, b) self-swab at home – but I think it was difficult or c) swab at the hospital by a health care professional. The surveys can be found on Annals.org in the supplement to the DANMASK-19 trial. Baseline characteristics are presented as frequency (percentages) for categorical variables and mean (standard deviation) for continuous variables. Differences according to preferences were assessed by χ2-test. The sample size of the DANMASK-19 trial was determined to provide adequate power for assessment of the combined composite primary outcome, with an estimated SARS-CoV-2 infection of at least 2% and assuming that wearing a face mask halves the risk of infection. A total of 4636 participants would provide the trial with 80% power at a significance level of 5% (2-sided a level), which was reached in the DANMASK-19 trial. In this substudy, 3709 of the 4862 participants were eligible (Figure 1 ).
Figure 1.
Flowchart of participants
Results
A total of 3929 participants conducted an at-home oropharyngeal/nasal swab, and 3709 (94.4%) were eligible for the study (mean age [SD] 48.1 [14] years; 2408 (64.9%) females). An at-home swab procedure for SARS-CoV-2 testing was the preferred setting for 2362 (63.7%) participants compared with 1347 (36.3%) reporting a preference for an at-hospital swab procedure by an HCW (P<0.0001) (Figure 2 ). Among the citizens who preferred at-home testing, a total of 1651 (69.9%) reported that the swabbing procedure at home was easy when guided by a video, whereas 711 (30.1%) found the test difficult but still preferred to perform the test at-home (P<0.0001).
Figure 2.
Preferences for setting of specimen collection
Discussion
This study found a high preference for at-home oropharyngeal/nasal swab collection among participants who carried out an at-home collection during the DANMASK-19 trial. These findings support the recently released FDA statement on at-home testing, and the preference for at-home testing may potentially be even higher for potentially be even higher for diagnostic tests that do not require laboratory analysis. Home collection does, however, raise several important issues. These issues include whether lay people can properly perform the swabbing procedure and if specimen transportation from home to the laboratory is safe, fast enough and under proper conditions (e.g., temperature)—both for sample integrity and keeping time from specimen collection to result short. Previous studies have validated and found that self-collected oropharyngeal/nasal swab for SARS-CoV-2 testing is reliable compared with HCW-collected samples (Therchilsen et al., 2020; Tu et al., 2020). Where sample integrity to some degree can be addressed using a robust DNA/RNA preservation buffer, the logistics for delivery of swab materials to and from participants necessitates local arrangements. The high stated preference supports that self-collection—especially at-home—of SARS-CoV-2 specimens could offer one solution for reducing healthcare personnel resources for testing and personal protective equipment usage while avoiding infectious individuals transmitting the virus in the community or to hospital personnel when leaving their home for an at-hospital test. Self-testing could become a future norm beyond COVID-19, similar to HIV or pregnancy self-tests (Boum et al., 2021).
This study has several strengths and limitations. Although COVID-19 has drawn considerable attention from most people, the DANMASK-19 trial may have recruited people with a very high interest in testing for COVID-19 or people concerned about their own SARS-CoV-2 infection status. Furthermore, we had a slight overrepresentation of females. Participants were not aware of the reliability of at-home specimen collection, which might have influenced their response. The study utilised free delivery of guidance material, test material and a free helpline. Participants represented broad societal groups, a broad age span above 18 years of age, and several occupational areas across the whole country. These aspects should be taken into account when considering the generalizability of the study.
Conclusions
The preferred setting for testing for SARS-CoV-2 among citizens is guided at-home self-collection. Since at-home collection has previously been found to be a reliable test method compared with testing in the healthcare system, healthcare authorities should consider adopting at-home collection options for the current and future pandemics.
Acknowledgments
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Source
The Salling Foundations supported the study with an unrestricted grant with no influence on study design, conduct, or reporting (grant number: NA).
Ethical Approval
The protocol of the DANMASK-19 trial was presented to the independent regional scientific ethics committee of the Capital Region of Denmark and the study did not require ethics approval (H-20023709) in accordance with Danish legislation. Further details of this can be found in the supplement to the DANMASK-19 trial.
References
- Boum Y, Eyangoh S, Okomo M-C. Beyond COVID-19 - will self-sampling and testing become the norm? Lancet Infect Dis. 2021;21:S1473–S3099. doi: 10.1016/S1473-3099(21)00197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard H, Bundgaard JS, Raaschou-Pedersen DET, von Buchwald C, Todsen T, Norsk JB, et al. Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers. Ann Intern Med. 2020;174:335–343. doi: 10.7326/M20-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colfax GN, Lehman JS, Bindman AB, Vittinghoff E, Vranizan K, Fleming PL, et al. What happened to home HIV test collection kits? Intent to use kits, actual use, and barriers to use among persons at risk for HIV infection. AIDS Care. 2002;14:675–682. doi: 10.1080/0954012021000005533a. [DOI] [PubMed] [Google Scholar]
- Danish-Regions . 2021. Danish Regions - testing overview.https://www.regioner.dk/sundhed/coronaviruscovid-19/overblik-over-tests accessed June 27, 2021. [Google Scholar]
- Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, et al. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- FDA . 2020. Coronavirus (COVID-19) Update: FDA Authorizes First COVID-19 Test for Self-Testing at Home.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-covid-19-test-self-testing-home accessed May 25, 2021. [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler AJ, Hall E, Luisi N, Zlotorzynska M, Wilde G, Sanchez T, et al. 2020. Willingness to seek laboratory testing for SARS-CoV-2 with home, drive-through, and clinic-based specimen collection locations. MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundhedsstyrelsen . 2021. Danish Health Authorities - Corona figure reportings.https://www.sst.dk/da/corona/covid-19-og-ny-coronavirus/coronatal accessed June 27, 2021. [Google Scholar]
- Sundhedsstyrelsen . 2020. Danish Health Authority: COVID-19 guidelines - Prevention against spread of infection.https://www.sst.dk/da/Udgivelser/2020/COVID-19-Forebyggelse-af-smittespredning accessed June 27, 2021. [Google Scholar]
- Therchilsen JH, von Buchwald C, Koch A, Nielsen SD, Rasmussen DB, Thudium RF, et al. Self-Collected versus Healthcare Worker-Collected Swabs in the Diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2. Diagnostics. 2020;10:1–10. doi: 10.3390/diagnostics10090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y-P, Jennings R, Hart B, Cangelosi GA, Wood RC, Wehber K, et al. Swabs Collected by Patients or Health Care Workers for SARS-CoV-2 Testing. N Engl J Med. 2020;383:494–496. doi: 10.1056/NEJMc2016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine-Graves M, Hall E, Guest JL, Adam E, Valencia R, Shinn K, et al. At-home self-collection of saliva, oropharyngeal swabs and dried blood spots for SARS-CoV-2 diagnosis and serology: Post-collection acceptability of specimen collection process and patient confidence in specimens. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2021. WHO Coronavirus (COVID-19) Dashboard.https://covid19.who.int accessed May 25, 2021. [Google Scholar]
- YouGov . 2021. Personal measures taken to avoid COVID-19 n.d.https://yougov.co.uk/topics/international/articles-reports/2020/03/17/personal-measures-taken-avoid-covid-19 accessed June 27. [Google Scholar]