Abstract
Background
Identifying the immune cells involved in coronavirus disease 2019 (COVID-19) disease progression and the predictors of poor outcomes is important to manage patients adequately.
Methods
This prospective observational cohort study enrolled 48 patients with COVID-19 hospitalized in a tertiary hospital in Oman and 53 non-hospitalized patients with confirmed mild COVID-19.
Results
Hospitalized patients were older (58 years vs 36 years, P < 0.001) and had more comorbid conditions such as diabetes (65% vs 21% P < 0.001). Hospitalized patients had significantly higher inflammatory markers (P < 0.001): C-reactive protein (114 vs 4 mg/l), interleukin 6 (IL-6) (33 vs 3.71 pg/ml), lactate dehydrogenase (417 vs 214 U/l), ferritin (760 vs 196 ng/ml), fibrinogen (6 vs 3 g/l), D-dimer (1.0 vs 0.3 μg/ml), disseminated intravascular coagulopathy score (2 vs 0), and neutrophil/lymphocyte ratio (4 vs 1.1) (P < 0.001). On multivariate regression analysis, statistically significant independent early predictors of intensive care unit admission or death were higher levels of IL-6 (odds ratio 1.03, P = 0.03), frequency of large inflammatory monocytes (CD14+CD16+) (odds ratio 1.117, P = 0.010), and frequency of circulating naïve CD4+ T cells (CD27+CD28+CD45RA+CCR7+) (odds ratio 0.476, P = 0.03).
Conclusion
IL-6, the frequency of large inflammatory monocytes, and the frequency of circulating naïve CD4 T cells can be used as independent immunological predictors of poor outcomes in COVID-19 patients to prioritize critical care and resources.
KEYWORDS: COVID-19, Lymphocyte subsets, Inflammatory markers, Immunological predictors, Mortality predictors
1. Introduction
A cluster of atypical viral pneumonia cases was identified in Wuhan, China, in December 2019. A novel coronavirus was identified as the cause, later named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Lai et al., 2020; Yu et al., 2020). The World Health Organization (WHO) declared it a pandemic on March 11, 2020, and by March 20, 2021, the total number of confirmed cases had exceeded 121 million worldwide, with over 2.6 million deaths (Shi et al., 2020). Some people infected with SARS-CoV-2 develop severe coronavirus disease 2019 (COVID-19) while others remain asymptomatic or have a milder illness course (Shi et al., 2020). Identifying predictors of poor outcomes is increasingly gaining importance to help to prioritize resources for high-risk patients and minimize death. Older age and certain comorbid conditions like chronic renal, lung, and heart diseases are established predictors of a worse prognosis in COVID-19 patients. In addition, hypoxemia, diarrhea, and high inflammatory markers like C- reactive protein (CRP) and interleukin 6 (IL-6) on admission are also predictors of a worse prognosis (Bhargava et al., 2020; Aziz et al., 2020; Shi et al., 2020; Prompetchara et al., 2020).
In these patients, immune cells, namely lymphocytes, have been heavily implicated in controlling disease progression and clinical outcomes. Some studies have demonstrated that higher leukocyte counts, specifically neutrophils (Shi et al., 2020; Chen et al., 2010; Prompetchara et al., 2020), and T cell lymphopenia (CD3+, CD8+ (Du et al., 2020; Urra et al., 2020), and CD4 (Song et al., 2020)) are associated with increased mortality in patients admitted with COVID-19 pneumonia. Moreover, it has been shown that older patients have lower counts and frequencies of naïve (CD45RA+CCR7+CD27+CD28+) CD4+ T cells contributing to the poor response of T cells (Koch et al., 2008). These cells are required for the effective handling of new infections or vaccines (Pawelec 2018; Li et al., 2011). It has also been shown that hospitalized COVID-19 patients have reduced (CD45RA+CCR7+CD27+CD28+) CD4+ naïve subsets of T cells compared to healthy uninfected controls (De Biasi et al., 2020). Furthermore, hospitalized patients with severe manifestations have a lower frequency of exhausted non-cytotoxic T cells (PD-1+CD57−CD8+) (Kusnadi et al., 2021).
Monocytes are other immune cells that are also vital for a normal and dysregulated immune response. Monocytopenia was found to be a predictor of worse outcomes in patients with severe community infections and sepsis (Aalto et al., 2007). Moreover, there is a reduction in the classic monocytes (CD14+CD16−) in severe SARS-CoV-2 infection and an increase in the inflammatory subsets (CD14+CD16+) (Payen et al., 2020). Monocytes were also recently divided based on size into small and large subsets, coupled with a level of CD14 and CD16 expression into different subsets with different functional abilities (Merah-Mourah et al., 2020).
The aim of this study was to identify changes in immune variables, namely naïve CD4+ and CD8+ (CD45RA+CCR7+CD27+CD28+), exhausted CD8+ (PD-1+CD57−) T cells, large and small inflammatory (CD14+CD16+) monocytes, and IL-6 levels early in the course of COVID-19 infection as immunological predictors of poor outcomes, including intensive care unit (ICU) admission and death.
2. Methods
2.1. Study design and patients
This was a prospective observational cohort study conducted from July 20, 2020 to August 27, 2020. A total of 101 COVID-19 cases confirmed by SARS-CoV-2 real-time PCR (RT-PCR) from nasopharyngeal swabs were enrolled: 53 non-hospitalized cases and 48 hospitalized cases. Inclusion criteria were patient age ≥13 years, both sexes, confirmed mild SARS-CoV-2 infection in the non-hospitalized group, and confirmed moderate infection in patients hospitalized at the Royal Hospital. Hospitalized patients were recruited within 48 hours of admission. Patients admitted directly to the ICU at the time of enrolment were excluded from the study. Mild cases were those that did not require admission to the hospital due to COVID-19-related illness or oxygen therapy. In contrast, moderate cases were identified as patients with hypoxemia ≤94% requiring oxygen support or those with one or more COVID-19-related organ involvement.
A dedicated clinical team was assigned to collect data and blood samples from the inpatients. Outpatients were approached through a daily list of confirmed COVID-19 patients provided by the Center of Operation Management for COVID-19 at the Ministry of Health, Oman. Telephone calls were conducted to obtain patient consent to participate in the study after an explanation of the research idea. Patients in the community were visited by two designated researchers the next day. One nominated researcher maintained communication to ensure the adherence of participants. Patient demographics and clinical characteristics were obtained from non-hospitalized patients directly or for hospitalized patients through the electronic hospital records using a unified data collection form. Informed consent was obtained from all enrolled patients. The study was approved by the Central Research Committee at the Ministry of Health in Oman (MoH/CSR/20/23605).
2.2. Measurement of inflammatory markers and lymphocyte subsets
Blood was collected at a median 6 days (interquartile range (IQR) 2–8 days) from the onset of symptoms for the hospitalized patients and 7.5 days (IQR 6.75–8.25 days) for the non-hospitalized patients. . Blood was sent for complete blood count, renal function tests, liver function tests, lactate dehydrogenase (LDH), ferritin, D-dimer, coagulation profile, IL-6, and lymphocyte subset analysis. The IL-6 concentration was measured on serum samples using the fully automated Elecsys IL-6 immunoassay (electrochemiluminescence immunoassay) on a Cobas e601 immunoassay analyzer (Roche Diagnostics, Switzerland) following the manufacturer's protocol. The IL-6 cut-off used was 7.0 pg/ml.
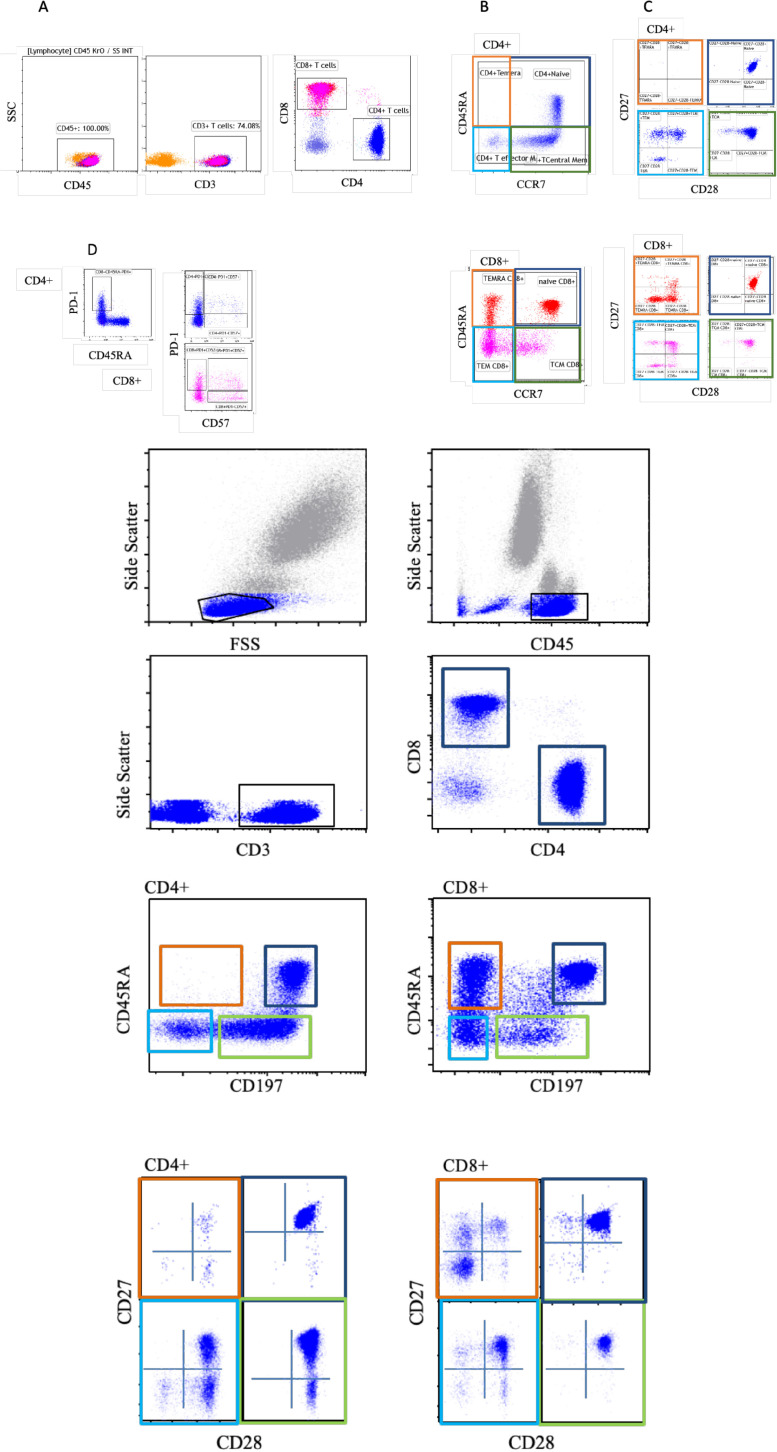
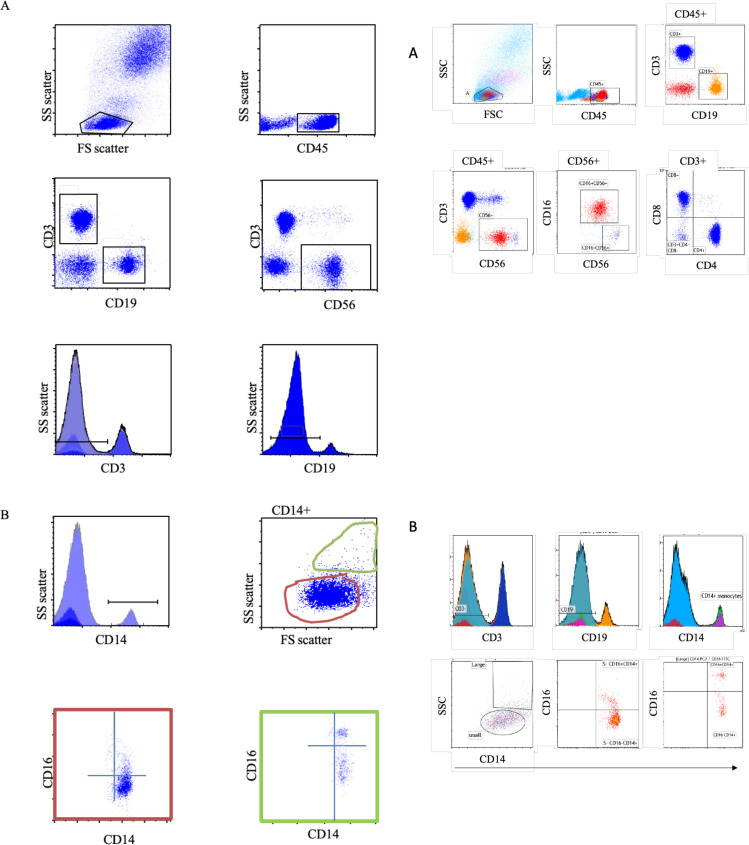
The assessment of the different basic lymphocyte and detailed T cell subsets was performed using flow cytometry. The DURAClone IM T cell subsets tube (Beckman Coulter) that includes CD45RA (clone, 2H4), CD197 (CCR7) (clone, G043H7), CD28 (clone, CD28.2), CD279 (PD-1) (clone, PD-1.3.5), CD27 (clone, 1A4.CD27), CD4 (clone, 13B8.2), CD8 (clone, B9.11), CD3 (clone, UCHT-1), CD57 (clone, NC1), and CD45 (clone, J33) was used to assess the different T cell subsets. The DURAClone IM phenotyping basic tube (Beckman Coulter) that includes CD16 (3G8), CD56 (N901), CD19 (J3_119), CD14 (RMO52), CD4 (13B8.2), CD8 (B9.11), CD3 (UCHT-1), and CD45 (J33) was used for basic lymphocyte staining. Gating strategies are presented in Figure 1, Figure 2 . A total of 100 000 events were collected.
Figure 1.
Distribution of CD4 and CD8 T cell subsets in the blood. (A) Representative flow cytometry analysis of the gating strategy, leukocytes (CD45+), T cell (CD3+), and CD4 and CD8 T cells. (B) Based on CD45RA and CCR7 expression, CD4 and CD8 have four main subsets: naïve (CD45RA+CCR7+), central memory (TCM, CD45RA−CCR7+), effector memory (TEM, CD45RA−CCR7−), and revertant effector memory TEMRA (CD45RA+CCR7−); CD4+ subset in the upper panel and CD8+ subsets in the lower panel. (C) The four main subsets, naïve (dark blue), TCM (green), TEM (light blue), and TEMRA (orange), are further divided into a different subset based on surface expression of CD27 and CD28. (D) CD4 is separated into T follicular helper cells (PD-1+CD45RA−). CD4 and CD8 are divided into cytotoxic (PD-1+CD57+) and senescence cells (PD-1−CD57+); CD4+ subset in the left panel and CD8+ subsets in the right panel.
Figure 2.
Distribution of lymphocytes and monocytes. (A) Representative flow cytometry analysis of the gating strategy, leukocytes (CD45+), T cell (CD3+), B cell (CD19), NK cell (CD56), and CD4 and CD8 T cells. (B) Gating on CD3− followed by gating on CD19− and then CD14+ (upper two rows). Based on the size of CD14+ monocytes (small (orange) and large (green)), followed by expression of CD14 and CD16 (lower row).
Briefly, 100 μl of blood was added to the tube containing the desired cocktail of antibodies and incubated for 20 min at room temperature. Next, 100 μl of lysing solution OptiLyse B or VersaLyse was added according to the manufacturer's recommendation. This was followed by a wash step. The acquisition of samples was done using Navios flow cytometry (Beckman Coulter) and the analysis was performed using Kaluza version 2.1 (Beckman Coulter).
2.3. Statistical analysis
Results were expressed as the median and interquartile range (IQR) and the frequency and percentage (%) for continuous and categorical variables, respectively. The assessment of differences between inpatients and outpatients was performed using the Chi-square test or Fisher's exact test for categorical variables, and the Mann–Whitney U-test for continuous variables.
Selected variables from the demographic characteristics, clinical presentation, inflammatory markers, and all significant immune subsets were subjected to univariable logistic regression analysis with the composite outcome of ICU admission and mortality in the first 30 days. Variables significant in the univariable analysis (P < 0.05) were assessed using multivariable logistic regression analysis to determine the independent predictors of the composite outcome.
An alpha threshold of 0.05 was used for statistical significance. The statistical analysis was performed using RStudio (RStudio Team (2016), RStudio (version 1.1.456): Integrated Development for R; RStudio, Inc., Boston, MA, USA).
3. Results
3.1. Demographic data, clinical characteristics, and main laboratory findings
During the period between July 2020 and August 2020, a total of 53 non-hospitalized patients with mild SARS-CoV-2 infection and 48 hospitalized patients with moderate SARS-CoV-2 infection were recruited. Demographic and clinical characteristics, and laboratory investigations, including immunological and inflammatory biomarkers, were compared between the two groups.
Hospitalized patients were found to be older: 58 years vs 36 years (P < 0.001). However, there was no difference in sex distribution (male: 54% of hospitalized vs 43% of non-hospitalized, P = 0.32). Comorbid conditions were more frequent in the admitted group, such as diabetes (65% vs 21%, P < 0.001). While shortness of breath was more frequent in hospitalized patients (80% vs 17%, P < 0.001), fatigue (56.6% vs 20.83%, P < 0.001) and diarrhea (34.0% vs 14.58%, P = 0.037) were reported more in the non-hospitalized group (Table 1 , Supplementary Material Appendix 1).
Table 1.
Comorbid conditions and symptoms of hospitalized (n = 48) and non- hospitalized (n = 53) patients with COVID-19. All variables are reported as the number and percentage (%). The P-values were calculated using Fisher's exact test, with 2 × 2 contingency tables
| Status |
P-value | ||
|---|---|---|---|
| Hospitalizedn (%) | Non-hospitalizedn (%) | ||
| Comorbid conditions | |||
| Pregnancy | 0 (0) | 2 (4) | 0.3190 |
| Obesity | 6 (13) | 14 (26) | 0.0000 |
| Cancer | 0 (0) | 1 (2) | 1.0000 |
| Diabetes | 31 (65) | 11 (21) | 0.0000 |
| HTN | 32 (67) | 4 (8) | 0.0000 |
| HIV/immune deficiency | 2 (4) | 0 (0) | 0.2234 |
| Heart disease | 10 (21) | 3 (6) | 0.0353 |
| Asthma | 3 (6) | 3 (6) | 1.0000 |
| Chronic lung disease | 6 (13) | 0 (0) | 0.0097 |
| Chronic liver disease | 0 (0) | 2 (4) | 0.4962 |
| Chronic hematological disease | 3 (6) | 1 (2) | 0.3439 |
| Chronic renal disease | 25 (52) | 1 (2) | 0.0000 |
| Chronic neurological disease | 1 (2) | 0 (0) | 0.4752 |
| Organ or bone marrow recipient | 3 (6) | 0 (0) | 0.1038 |
| On ACEI/ARB | 2 (4) | 2 (4) | 0.6664 |
| On oral hypoglycemic agents/ insulin | 19 (40) | 3 (6) | 0.0001 |
| On active chemotherapy | 1 (2) | 0 (0) | 0.4752 |
| On long-term steroids | 4 (8) | 1 (2) | 0.1880 |
| On biologics | 1 (2) | 0 (0) | 0.4752 |
| Symptoms | |||
| Shortness of breath | 38 (79) | 9 (17) | 0.0000 |
| Fever | 30.(62.5%) | 41 (77.4) | 0.1286 |
| Cough | 26.00 (54.17) | 27 (50.9) | 0.8425 |
| Fatigue | 10.00 (20.83) | 30 (56.6) | 0.0003 |
| Diarrhea | 7.00 (14.58) | 18 (34) | 0.0368 |
| Nausea/vomiting | 6.00 (12.5) | 16 (30.2) | 0.0520 |
| Loss of appetite | 6.00 (12.5) | 4 (7.5) | 0.5117 |
| Muscle/joint pain | 5.00 (10.42) | 14 (26.4) | 0.0454 |
| Headache | 4.00 (8.33) | 11 (20.8) | 0.0977 |
| Sore throat | 3.00 (6.25) | 26 (49.1) | 0.0000 |
| Chills | 3.00 (6.25) | 3 (5.7) | 1.0000 |
| Loss of smell | 2.00 (4.17) | 25 (47.2) | 0.0000 |
| Neurological deficits or seizure | 2.00 (4.17) | 0 (0) | 0.2234 |
| Runny nose | 1.00 (2.8) | 23 (43.4) | 0.0000 |
| Rash | 0.00 (0) | 0 (0) | NA |
| Epistaxis | 0.00 | 0 | NA |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; HTN, hypertension; NA, not applicable.
The hospitalized group had higher inflammatory markers: CRP (114 vs 4 mg/l, P < 0.001), LDH (417 vs 214 U/l, P < 0.001), ferritin (760 vs 196 ng/ml, P < 0.001), fibrinogen (6 vs 3 g/l, P < 0.001), D-dimer (1.0 vs 0.3 μg/ml, P < 0.001), disseminated intravascular coagulopathy (DIC) score (2.0 vs 0.0, P < 0.001), and higher neutrophil/lymphocyte ratio (4 vs 1.1, P < 0.001) (Table 2 , Supplementary Material Appendix 1).
Table 2.
Laboratory findings of the hospitalized (n = 48) and non-hospitalized (n = 53) patients with COVID-19. All variables are reported as the median (interquartile range). The P-value was calculated by non-parametric Mann–Whitney U-test
| Laboratory tests | HospitalizedMedian (IQR) | Non-hospitalizedMedian (IQR) | P-value |
|---|---|---|---|
| Hemoglobin (g/dl) | 12.15 (10.35–13.38) | 13.4 (12.30–14.50) | 0.0006 |
| White blood cell count (× 109/l) | 6.55 (5.07–8.48) | 3.9 (3.40–5.05) | 0.0000 |
| Platelet count (× 109/l) | 180 (152.2–245.2) | 208.0 (179.0–229.5) | 0.3235 |
| Neutrophil count (× 109/l) | 4.9 (2.95–6.47) | 1.6 (1.20–2.70) | 0.0000 |
| lymphocyte count (× 109/l) | 1.05 (0.70–1.60) | 1.7 (1.30–2.05) | 0.0003 |
| Neutrophil/lymphocyte ratio | 3.775 (2.07–6.12) | 1.05 (0.60–1.77) | 0.0000 |
| CRP (mg/l) | 114 (53.8–176.4) | 4 (4–23.2) | 0.0000 |
| LDH (U/l) | 416.5 (321.5–528.0) | 214 (187.0–246.0) | 0.0000 |
| Ferritin (ng/ml) | 760 (312–1645) | 196 (54–346) | 0.0000 |
| Urea (mmol/l) | 10.3 (4.15–40.30) | 3.9 (3.20–4.60) | 0.0000 |
| Creatinine (mmol/l) | 151 (69.5–484.0) | 66.0 (53.0–75.0) | 0.0000 |
| eGFR (ml/min/1.73m2) | 43 (9.00–90.00) | 90.00 (90.00–90.00) | 0.0000 |
| ALT (U/l) | 30.5 (21.0–63.0) | 27.00 (18.75–47.25) | 0.4888 |
| AST (U/l) | 39.0 (27.5–100.5) | 27.00 (21.50–35.00) | 0.0283 |
| ALP (U/l) | 79 (62.5–132.0) | 74.00 (59.50–90.50) | 0.1360 |
| GGT (U/l) | 47.5 (34.25–195.50) | 36.00 (21.50–49.00) | 0.0644 |
| Bilirubin (μmol/l) | 7.00 (5.00–10.75) | 8.00 (5.75–10.00) | 0.3394 |
| Albumin (g/l) | 38 (35.00–40.00) | 44.50 (42.00–46.00) | 0.0000 |
| PT (second) | 11.1 (10.90–12.18) | 10.5 (10.30–10.88) | 0.0000 |
| aPTT (second) | 35.15 (32.23–38.00) | 28.65 (26.80–31.48) | 0.0000 |
| Fibrinogen (g/l) | 5.585 (3.75–6.71) | 3.340 (2.80–4.24) | 0.0000 |
| D-dimer (mg/l FEU) | 1.07 (0.78–4.13) | 0.31 (0.232–0.597) | 0.0000 |
| DIC score | 2 (0–3) | 0 (0–0) | 0.0000 |
ALP, alkaline phosphatase; ALT, serum alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, serum aspartate aminotransferase; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; eGFR, estimated glomerular filtration rate; FEU, fibrinogen-equivalent units; GGT, gamma-glutamyl transferase; IQR, interquartile range; LDH, lactate dehydrogenase; PT, prothrombin time.
3.2. Immunological features
3.2.1. CD3 lymphopenia in the hospitalized group
Despite having higher total white blood cell and neutrophil counts (Table 2), hospitalized patients had a lower lymphocyte count and a lower percentage of the CD3+ T cell subset (P = 0.001). At the same time, the ratio of CD4+ T cells and CD8+ T cells was normal (Table 3 ), and there was no difference between the two groups when comparing the percentage of CD19+ B cells and CD16+56+ natural killer (NK) cells (P = 0.29 and P = 0.42, respectively) (Table 3, Supplementary Material Appendix 1).
Table 3.
Immunological findings of the hospitalized (n = 48) and non-hospitalized (n = 53) patients with COVID-19. All variables are reported as the median (interquartile range). The P-value was calculated by non-parametric Mann–Whitney U-test
| Immune markers | HospitalizedMedian (IQR) | Non-hospitalizedMedian (IQR) | P-value |
|---|---|---|---|
| IL-6 | 33 (8.36–86.28) | 3.71 (1.58–12.58) | 0.0000 |
| CD3+ | 62.03 (53.18–71.11) | 70.85 (64.30–73.63) | 0.0100 |
| CD4+ | 56.99 (50.34–67.47) | 61.47 (52.46–66.14) | 0.3166 |
| CD8+ | 34.4 (25.12–42.83) | 31.88 (26.09–38.28) | 0.3166 |
| CD19+ | 14.22 (7.27–21.54) | 11.23 (8.01–13.69) | 0.2922 |
| CD56+ | 18.27 (9.90–26.58) | 15.33 (11.17–20.67) | 0.2986 |
| TEM CD4+ | 13.3 (6.82–22.42) | 10.33 (8.21–14.27) | 0.1432 |
| Naïve CD4+ | 25.4 (14.99–36.88) | 32.75 (23.51–39.50) | 0.0343 |
| TCM CD4+ | 50.91 (43.31–42.83) | 53.02 (46.72–58.33) | 0.7299 |
| TEMRA CD4+ | 0.37 (0.05–1.51) | 0.17 (0.05–0.80) | 0.3368 |
| CD27−CD28−Naïve CD4+ | 0.03 (0.000.20) | 0.02 (0.00–0.05) | 0.4492 |
| CD27−CD28+Naïve CD4+ | 0.81 (0.19–1.72) | 0.34 (0.18–0.65) | 0.0548 |
| CD27+CD28−Naïve CD4+ | 0.19 (0.06–0.45) | 0.11 (0.04–0.23) | 0.0397 |
| CD27+CD28+Naïve CD4+ | 99 (97.15–99.67) | 99.48 (98.90–99.71) | 0.0213 |
| CD27−CD28−TCM CD4+ | 0.54 (0.11–1.58) | 0.29 (0.05–1.07) | 0.4597 |
| CD27−CD28+TCM CD4+ | 5.68 (4.88–7.63) | 6.57 (4.22–7.97) | 0.4769 |
| CD27+CD28−TCM CD4+ | 0.15 (0.05–0.40) | 0.09 (0.04–0.19) | 0.1800 |
| CD27+CD28+TCM CD4+ | 93.24 (89.94–94.59) | 92.55 (91.38–94.62) | 0.9257 |
| CD27−CD28−TEM CD4+ | 22.3 (4.98–42.01) | 8.46 (2.44–29.36) | 0.0698 |
| CD27−CD28+TEM CD4+ | 27.34 (20.20–35.31) | 31.99 (22.05–37.79) | 0.1589 |
| CD27+CD28−TEM CD4+ | 0.79 (0.28–1.74) | 0.76 (0.35–1.42) | 0.8819 |
| CD27+CD28+TEM CD4+ | 47.37 (25.23–58.87) | 52.47 (39.59–61.43) | 0.0737 |
| CD27−CD28−TEMRA CD4+ | 51.92 (0.00–79.48) | 50.00 (11.99–77.08) | 0.8673 |
| CD27−CD28+ TEMRA CD4+ | 12 (2.35–23.09) | 18.00 (6.07–27.75) | 0.3042 |
| CD27+CD28− TEMRA CD4+ | 3.685 (0.00–9.55) | 3.23 (0.00–6.02) | 0.3909 |
| CD27+CD28+ TEMRA CD4+ | 10.265 (1.80–39.20) | 19.23 (3.53–51.09) | 0.2330 |
| PD-1−CD57+CD4+ | 1.46 (0.38–3.59) | 0.89 (0.50–3.64) | 0.9285 |
| PD-1+CD57−CD4+ | 17.59 (14.16–23.48) | 13.63 (10.24–18.20) | 0.0007 |
| PD-1+CD57+CD4+ | 5.48 (1.66–12.88) | 2.97 (1.56–5.98) | 0.0396 |
| CD45RA−PD-1+CD4+ | 13.48 (9.29–20.30) | 12.57 (8.19–17.09) | 0.4413 |
| Naïve CD8+ | 14.31 (4.48–36.24) | 27.82 (14.84–37.89) | 0.0099 |
| TCM CD8+ | 8.64 (4.71–13.83) | 10.5 (7.58–16.86) | 0.0523 |
| TEM CD8+ | 30.23 (18.20–40.65) | 29.26 (22.32–37.58) | 0.9394 |
| TEMRA CD8+ | 31.85 (16.54–45.24) | 23.46 (15.30–31.33) | 0.0731 |
| CD27−CD28−Naïve CD8+ | 00 (00–0.85) | 0.07 (0.00–0.33) | 0.3339 |
| CD27−CD28+Naïve CD8+ | 0.270 (0.06–1.020) | 0.19 (0.09–0.41) | 0.3816 |
| CD27+CD28−Naïve CD8+ | 3.92 (2.44–7.05) | 2.67 (1.22–5.79) | 0.1244 |
| CD27+CD28+Naïve CD8+ | 94.93 (87.39–97.33) | 96.2 (90.48–98.47) | 0.0913 |
| CD27−CD28−TCM CD8+ | 1.67 (0.72–5.57) | 0.74 (0.40–2.14) | 0.0262 |
| CD27−CD28+TCM CD8+ | 2.06 (1.14–4.32) | 1.21 (0.82–2.56) | 0.0486 |
| CD27+CD28−TCM CD8+ | 5.63 (3.45–8.76) | 5.33 (3.29–7.57) | 0.7533 |
| CD27+CD28+TCM CD8+ | 88.88 (83.05–93.55) | 91.69 (87.16–94.55) | 0.0688 |
| CD27−CD28−TEM CD8+ | 47.25 (24.14–57.68) | 25.76 (15.45–40.84) | 0.0017 |
| CD27−CD28+TEM CD8+ | 6.41 (3.63–10.93) | 6.26 (4.81–9.65) | 0.6761 |
| CD27+CD28−TEM CD8+ | 12.95 (7.86–20.89) | 15.99 (12.64–20.75) | 0.1252 |
| CD27+CD28+TEM CD8+ | 28.71 (21.38–45.66) | 43.95 (32.92–59.75) | 0.0007 |
| CD27−CD28−TEMRA CD8+ | 76.65 (57.97–87.09) | 71.34 (61.51–79.33) | 0.1413 |
| CD27−CD28+ TEMRA CD8+ | 1.31 (0.55–3.25) | 0.91 (0.41–1.65) | 0.1460 |
| CD27+CD28− TEMRA CD8+ | 16.33 (10.29–27.73) | 21.07 (15.00–28.39) | 0.0286 |
| CD27+CD28+ TEMRA CD8+ | 4.13 (1.59–9.58) | 5.10 (3.10–10.24) | 0.1906 |
| PD-1−CD57+CD8+ | 14.91 (9.49–26.96) | 13.10 (6.22–23.24) | 0.1499 |
| PD-1+CD57−CD8+ | 24.34 (20.34–33.40) | 29.70 (23.13–34.56) | 0.1010 |
| PD-1+CD57+CD8+ | 16.86 (11.35–27.96) | 11.98 (8.96–15.41) | 0.0078 |
| CD45RA−PD-1+CD8+ | 21.48 (13.53–33.84) | 13.88 (11.78–19.79) | 0.0023 |
| CD3+CD4−CD8− | 4.08 (3.00–6.19) | 4.55 (3.69–6.44) | 0.2362 |
| CD16−CD56+ | 3.01 (2.28–4.37) | 3.07 (2.28–5.82) | 0.5297 |
| CD16+CD56+ | 95.3 (92.88–96.60) | 95.06 (92.31–96.03) | 0.4171 |
| CD14+ | 3.91 (2.19–6.31) | 7.43 (6.09–10.10) | 0.0000 |
| LCD14+ | 0.59 (0.30–1.02) | 0.64 (0.44–1.03) | 0.2038 |
| LCD16−CD14+ | 72.7 (59.38–87.69) | 84.71 (80.46–88.61) | 0.0008 |
| LCD16+CD14+ | 27.3 (12.31–40.62) | 15.29 (11.39–9.54) | 0.0009 |
| SCD14+ | 2.46 (1.21–4.10) | 4.58 (3.34–5.53) | 0.0000 |
| sCD16−CD14+ | 81.52 (71.72–88.07) | 83.16 (73.99–89.12) | 0.9422 |
| sCD16+CD14+ | 18.48 (11.93–27.70) | 16.84 (10.88–26.01) | 0.9257 |
IL-6, interleukin 6; IQR, interquartile range.
3.2.2. Reduced naïve and increased effector and increased cytotoxic and exhausted CD4+ T cells in the hospitalized group
The assessment of the CD4+ T cell maturation and differentiation stages revealed a significantly higher increase in the total naïve (CD45RA+CCR7+) CD4+ T cells in the non-hospitalized group, with a median of 32.75% (IQR 23.51–39.50%) compared to 5.40% (IQR 14.99–36.88%) ) in the hospitalized group (P = 0.034). This increase was mirrored by the increase in the naïve (CD27+CD28+CD45RA+CCR7+) CD4+ T cells subsets, with a median of 99.48% (IQR 98.90–99.71%) vs 99.00% (IQR 97.15–99.67%) in the non-hospitalized group and admitted group, respectively (P = 0.021) (Table 3). On the other hand, there was an expansion of effector CD4+ T cells in hospitalized patients compared to non-hospitalized patients. These patients had a higher frequency of exhausted (PD-1+CD57−) CD4+ T cells with a median of 17.59% (IQR 14.16–23.48%) compared to 13.63% (IQR 10.24–18.20%) (P = 0.001) and exhausted cytotoxic (PD-1+CD57+CD4+) CD4+ T cells with a median of 5.48% (IQR 1.66–12.89%) compared to 2.97% (IQR 1.56–5.98%) in the non-hospitalized patients (P = 0.039) (Table 3, Supplementary Material Appendix 1).
3.2.3. Reduced naïve and increased cytotoxic effector and exhausted CD8+ T cells in the hospitalized group
Similar to total CD4+ T cells, there was no statistically significant difference in total CD8+ T cells (P = 0.317). However, there was an increase in the naïve CD8+ T cells seen in the non-hospitalized patients, with a median of 27.82% (IQR 14.84–37.89%) compared to14.31% (IQR 4.485–36.24% ) in the hospitalized group (P = 0.010). In addition, the non-hospitalized group had a higher frequency of cells with lower cytotoxic characteristics. Examples include effector memory (TEM) CD27+CD28+CD8+ T cells (43.95% (IQR 32.92–59.75%) vs 28.71% (IQR 21.38–45.66%), P = 0.01) and revertant effector memory (TEMRA) CD27+CD28−CD8+ T cells (21.07% (IQR 15.00–28.39%) vs 16.33% (IQR 10.29–27.73%), P = 0.029), in the non-hospitalized vs hospitalized group (Table 3, Supplementary Material Appendix 1).
In contrast, the hospitalized group had a high percentage of cytotoxic TEM CD27−CD28−CD8+ T cells, with a median 47.25% (IQR 24.14–57.68%) compared to 25.76% (IQR 15.45–40.84%) in the non-hospitalized group (P = 0.002). Moreover, the percentage of cytotoxic exhausted (CD57+PD-1+) CD8+ T cells was higher in the hospitalized group, with a median of 16.86% (IQR 11.35–27.96%) vs 11.98% (IQR 8.96–15.41%) (P = 0.008) (Table 3, Supplementary Material Appendix 1). The hospitalized group had a higher frequency of cells with cytotoxic characteristics.
3.2.4. Large inflammatory (CD14+CD16+) monocytes in the hospitalized group
Hospitalized patients exhibited a lower percentage of CD14+ monocytes than non-hospitalized patients, with a median of 3.91% (2.20–6.31%) vs 7.43% (IQR 6.09–10.10%), respectively (P < 0.001). As a majority of the monocytes were of small size, the hospitalized group had a lower median of 2.46% (IQR 1.21–4.10%) CD14+ small monocytes compared to the non-hospitalized group with a median of 4.58% (IQR 3.34–5.53%) (P < 0.001) (Table 3).
Although there was no difference in the total large CD14+ monocytes, large inflammatory (CD14+CD16+) monocytes were seen at a higher percentage in the hospitalized group, with a median of 27.3% (IQR 12.31–40.62%) compared to the non-hospitalized group with 15.29% (IQR 11.39–9.54%) (P < 0.001) (Table 3, Supplementary Material Appendix 1).
3.2.5. Higher IL-6 levels in the hospitalized group
In line with previous findings, IL-6 was higher in the hospitalized disease group, with a median of 33 pg/ml (IQR 8.36–86.28 pg/ml) compared to the non-hospitalized group with 3.71 pg/ml (IQR 1.58–12.58 pg/ml) (P < 0.001) (Table 3, Supplementary Material Appendix 1).
3.2.6. ICU admission and death
Ten of the 48 hospitalized patients (21%) required ICU admission. A total of seven patients died: five were among those who were moved to the ICU, one died while in the ward, and one of the non-hospitalized patients died before 30 days of illness. Therefore, the total composite endpoint was 12 events (2%).
3.2.7. Factors associated with ICU admission or death
Univariable regression analysis was used to examine potential parameters predictive of ICU admission or death within 30 days (Table 4 , Supplementary Material Appendix 1). Significant univariable factors were then subjected to multivariable regression analysis. It was found that the increase in IL-6 level (pg/ml) increased the composite endpoint odds by 1.03 (P = 0.03). Similarly, an increase in the percentage of the large inflammatory monocytes (CD16+CD14+) subset was found to be associated with an increase in the composite endpoint odds by 1.117 (P = 0.01). On the other hand, an increase in the frequency of the naïve CD4+ (CD45RA+CCR7+CD27+CD28+) decreased the odds of the composite endpoint by 0.476 (P = 0.03) (Table 4, Supplementary Material Appendix 1).
Table 4.
Univariable and multivariable analysis of the selected parameters
| Variable | Univariable analysis | Multivariable analysis |
|
|---|---|---|---|
| P-value | OR | P-value | |
| Age | 0.022 | 0.924 | 0.089 |
| Sex (% male) | 0.471 | ||
| BMI | 0.516 | ||
| Admission status | 0.010 | 1.208 | 0.921 |
| Diabetes | 0.020 | 0.268 | 0.340 |
| HTN | 0.024 | ||
| HIV/immune deficiency | 0.152 | ||
| Heart disease | 0.621 | ||
| Asthma | 0.711 | ||
| Chronic lung disease | 0.711 | ||
| Chronic renal disease | 0.524 | ||
| White blood cell count (× 109/l) | 0.211 | ||
| Neutrophil count (× 109/l) | 0.104 | ||
| lymphocyte count (× 109/l) | 0.053 | ||
| Neutrophil/lymphocyte ratio | 0.181 | ||
| CRP (mg/l) | 0.044 | ||
| LDH (U/l) | 0.014 | ||
| Ferritin (ng/ml) | 0.003 | ||
| Fibrinogen | 0.914 | ||
| D-dimer | 0.039 | ||
| IL-6 | 0.001 | 1.030 | 0.032 |
| CD3+ | 0.143 | ||
| CD4+ | 0.303 | ||
| CD8+ | 0.556 | ||
| TEM CD4+ | 0.869 | ||
| Naïve CD4+ | 0.281 | ||
| TCM CD4+ | 0.101 | ||
| TEMRA CD4+ | 0.211 | ||
| CD27+CD28+Naïve CD4+ | 0.005 | 0.476 | 0.030 |
| CD27+CD28+TCMD4+ | 0.460 | ||
| CD27−CD28+TEM CD4+ | 0.862 | ||
| CD27+CD28+TEM CD4+ | 0.334 | ||
| Naïve CD8+ | 0.593 | ||
| TCM CD8+ | 0.100 | ||
| TEM CD8+ | 0.377 | ||
| TEMRA CD8+ | 0.489 | ||
| CD27+CD28+Naïve CD8+ | 0.212 | ||
| CD27+CD28+TCM CD8+ | 0.168 | ||
| CD27−CD28−TEM CD8+ | 0.913 | ||
| CD27−CD28+TEM CD8+ | 0.303 | ||
| CD27+CD28−TEM CD8+ | 0.133 | ||
| CD27+CD28+TEM CD8+ | 0.524 | ||
| CD27−CD28−TEMRA CD8+ | 0.328 | ||
| CD27−CD28+TEMRA CD8+ | 0.488 | ||
| CD27+CD28−TEMRA CD8+ | 0.476 | ||
| CD27+CD28+TEMRA CD8+ | 0.345 | ||
| PD-1−CD57+ CD4+ | 0.368 | ||
| PD-1+CD57−CD4+ | 0.572 | ||
| PD-1+CD57+ CD4+ | 0.748 | ||
| CD45RA−PD-1+ CD4+ | 0.035 | 1.139 | 0.055 |
| PD-1−CD57+ CD8+ | 0.332 | ||
| PD-1+CD57− CD8+ | 0.531 | ||
| PD-1+CD57+ CD8+ | 0.076 | 0.893 | 0.110 |
| CD14 | 0.022 | 0.862 | 0.437 |
| Large CD14 | 0.614 | ||
| Large CD16nCD14p | 0.000 | ||
| Large CD16pCD14p | 0.000 | 1.117 | 0.010 |
| Small CD14 | 0.021 | ||
| Small CD16nCD14p | 0.521 | ||
| Small CD16pCD14p | 0.631 | ||
BMI, body mass index; CRP, C-reactive protein; HTN, hypertension; IL-6, interleukin 6; LDH, lactate dehydrogenase; OR, odds ratio.
4. Discussion
Earlier studies have shown that in hospitalized patients, inflammatory biomarkers such as CRP, ferritin, LDH, D-dimer, and IL-6 can be used to predict clinical outcomes in COVID-19 patients (Bhargava et al., 2020; Aziz et al., 2020; Shi et al., 2020; Prompetchara et al., 2020). The immune system plays a significant role in the clinical manifestations and progression of the disease, including the inflammatory markers mentioned above. Therefore, the focus on immunological predictors that can be used early in the disease course to enable the relocation of resources towards those at risk of getting the severe disease should be prioritized. In this study, it was observed that, in addition to elevated levels of IL-6, the higher percentage of large inflammatory CD14+CD16+ monocytes and lower percentage of naïve CD27+CD28+CD4+ T cells were independent early immunological prognostic predictors of worse outcomes in patients with COVID-19.
Similar to other existing data, admitted patients in the present study were found to be older and more frequently diabetic and hypertensive compared to those who did not require admission. Moreover, underlying chronic heart, lung, and kidney diseases were noted more often in admitted patients than non-admitted patients, which is in agreement with previous studies (Wang et al., 2020; Yang et al., 2020; Ruan et al., 2020; Wu and McGoogan 2020). In addition, the current literature has ample information suggesting that high inflammatory markers can be used as a predictor of worse outcomes in admitted patients with SARS-CoV-2 infection. These include the white blood cell count, absolute lymphocyte count, LDH, CRP, procalcitonin, D-dimer, ferritin, and erythrocyte sedimentation rate (Barrett et al., 2020; Wang et al., 2020). This was also confirmed in the present study, in which admitted patients had higher inflammatory markers including CRP, LDH, ferritin, fibrinogen, D-dimer, DIC score, and higher neutrophil/lymphocyte ratio.
The immune CD4+ and CD8+ T cells can be divided into four main subsets based on the surface expression of CCR7 and CD45RA. They reflect different maturation and T cell differentiation stages that are functionally distinct. The subsets include naïve CD4+ T cell subsets (CCR7+CD45RA+), central memory (TCM) CD4+ T cells (CCR7+CD45RA−), effector memory CD4+ T cells (TEM), and RA+ revertant effector memory cells (CCR−CD45RA+) (TEMRA) (Okada et al., 2008; Romero et al., 2007). The main four subsets of both CD4+ and CD8+ can be further divided into different functional subsets based on the expression of CD27 and CD28 with different cytokine expression (Amyes et al., 2005; Romero et al., 2007; Koch et al., 2008). TEM that are CD27−CD28− are mainly interferon-gamma (IFN-γ) producers (Th1), while CD27−CD28+ TEM are IL-2 (Th0), IFN-γ (Th1), and IL-4 (Th2) producers (Okada et al., 2008)]. TEM and TEMRA T cells are good cytokine producers, including IL-2, IFN-γ, and tumor necrosis factor alpha (TNF-α). Moreover, CD27−CD28− T cells have high effector capability similar to the terminal effector T cells TEMRA subset ] (Romero et al., 2007; Koch et al., 2008). Similarly, the combination of PD-1 and CD57 can identify cells with exhausted and or cytotoxic phenotype (Kraaijeveld et al., 2018; Alshekaili et al., 2018).
On the other hand, the naïve T cell is mainly CD27+CD28+ and is a good producer of IL-2, which is required for activation and proliferation (Okada et al., 2008; Koch et al., 2008). CD27+CD28+ naïve T cells are crucial in response to a new virus or vaccine. Those with a reduced frequency of naïve T cells, such as the elderly, are at risk of getting a significant disease when compared to those with plenty of naïve T cells that can respond better to such new viruses (Cunha et al., 2020). This is one of the important explanations for the increased mortality in the elderly after infection with SARS-CoV-2. The present study findings are in line with this: those patients with a low percentage of naïve CD4+ T cells are at a higher risk of mortality.
De Biasi et al. compared the immune system in hospitalized patients with mild to moderate disease (n = 39) to that in a healthy uninfected group (n = 25). They showed a low count of total CD4+ and CD8+ T cells and their naïve and TCM subsets in the patient group. Moreover, these patients had a higher frequency of cells with the senescent/exhausted phenotype (CD57+PD-1+) (De Biasi et al., 2020). Similarly, we found a reduced percentage of naïve CD4+ and CD8+ T cells and increased exhausted CD4+ and CD8+ T cells in the hospitalized group early in the disease course. In addition, these cells were more cytotoxic compared to the mild non-hospitalized patients in agreement with findings with the other published data (Kusnadi et al., 2021).
Monocytes are the key immune cells and are good producers of inflammatory cytokines like IL-6 (Giavridis et al., 2018; Norelli et al., 2018). They acquire a larger size upon activation and in viral infections (Polilli et al., 2020), including in patients with severe COVID-19 (Lippi and Plebani 2020). Moreover, monocytes can be divided according to the differentiation stage using a combination of CD markers, into immature (CD14+CD16−), differentiated and inflammatory type (CD14+CD16+), and non-classical (CD14−CD16+) (Sánchez-Cerrillo et al., 2020). Examining monocytes in this way has revealed a higher percentage of large inflammatory monocytes CD14+CD16+ in hospitalized patients, in line with previous suggestions that patients with severe manifestation have bigger sized monocytes (Khartabil et al., 2020; Lippi and Plebani 2020; Polilli et al., 2020).
In conclusion, the current study identified elevated levels of IL-6, a higher percentage of CD14+CD16+ inflammatory large monocytes, and a lower percentage of circulating naïve (CD27+CD28+CD45RA+CCR7+) CD4+ T cells early in the disease course as independent early predictors of ICU admission or death in patients with SARS-CoV-2 infection. Such predictors could be used for the early identification of patients who might deteriorate and thus need early aggressive interventions. Larger studies are required to validate the current findings, aiming towards better early clinical management.
Declaration of Competing Interest
The authors declare that they have no financial interests or personal relationships that might influence the work presented in this paper.
Acknowledgments
Author contributions
Asma Al Balushi: Conceptualized and designed the study, wrote the research proposal, data collection for inpatients and field visits, communicated with patients, drafted and reviewed the manuscript, and overall workflow and integrity supervision. Jalila AlShekaili: Reviewed the study design, reviewed the research proposal, supervised receiving blood samples at the immunology laboratory, supervised the flow cytometry work and IL-6 assessment run and analysis, and interpreted the data, wrote up the first few drafts and approved the final draft of the manuscript. Mahmood Al Kindi: Reviewed the study design, reviewed the research proposal, supervised receiving blood samples at the immunology laboratory, supervised the flow cytometry work and IL-6 assessment run and analysis, analyzed the data, wrote up the first few drafts, and approved the final draft of the manuscript. Zainab Ansari: Field visits, data and sample collection from outpatients and inpatients, and data entry. Murtadha Al-Khabori: Data analysis, writing up results, and manuscript review. Faryal Khamis: Manuscript review and facilitating research work for inpatients. Zaiyana Ambusaidi: Data and sample collection from inpatients and data entry. Afra Al Balushi: Data and sample collection from inpatients and data entry. Aisha Al Huraizi: Data and sample collection from inpatients and data entry. Sumaiya Al Sulaimi: Data and sample collection from inpatients and data entry. Fatma Al Fahdi: Data and sample collection from inpatients and data entry. Iman Al Balushi: Data collection and entry for outpatients and second review of all data. Nenad Pandak: Manuscript review and facilitating research work for inpatients. Tom Fletcher: Overall supervision of the study and manuscript review. Iman Nasr: Proposal review, registered the study, communication with laboratories and companies, manuscript review, and overall workflow and integrity supervision.
Acknowledgements
We thank the Ministry of Higher Education, Research and Innovation (MoHERI) for their great contribution in funding this research. We also thank the Tropical and Infectious Diseases Unit at the Royal Liverpool University Hospital for their support and help in facilitating this research and overall supervision of the primary author as part of a fellowship training program. We thank Dr Lamya Al Balushi from the Directorate General of Health Services, Ministry of Health, Oman for her help in facilitating the fieldwork.
Funding
The study was supported by the Ministry of Higher Education, Research and Innovation (MoHERI) in Oman (TRC/CRP/MOH/COVID-19/20/26).
Ethical approval
Ethical approval was obtained through the Central Research Committee at the Ministry of Health in Oman (MoH/CSR/20/23605).
Consent
Informed consent was obtained from all enrolled patients.
References
- Aalto Hannele, Takala Annika, Kautiainen Hannu, Siitonen Sanna, Repo Heikki. Monocyte CD14 and soluble CD14 in predicting mortality of patients with severe community acquired infection'. Scandinavian Journal of Infectious Diseases. 2007;39:596–603. doi: 10.1080/00365540701199808. [DOI] [PubMed] [Google Scholar]
- Alshekaili Jalila, Chand Rochna, Lee Cindy Eunhee, Corley Susan, Kwong Kristy, Papa Ilenia, Fulcher David A., Randall Katrina L., Leiding Jennifer W., Ma Cindy S., Wilkins Marc R., Uzel Gulbu, Goodnow Chris C., Vinuesa Carola G., Tangye Stuart G., Cook Matthew C. STAT3 regulates cytotoxicity of human CD57+ CD4+ T cells in blood and lymphoid follicles', Scientific Reports. 2018;8:3529. doi: 10.1038/s41598-018-21389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyes E., McMichael A.J., Callan M.F. Human CD4+ T cells are predominantly distributed among six phenotypically and functionally distinct subsets'. J Immunol. 2005;175:5765–5773. doi: 10.4049/jimmunol.175.9.5765. [DOI] [PubMed] [Google Scholar]
- Aziz M., Haghbin H., Lee-Smith W., Goyal H., Nawras A., Adler D.G. Gastrointestinal predictors of severe COVID-19: systematic review and meta-analysis. Ann Gastroenterol. 2020;33:615–630. doi: 10.20524/aog.2020.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett Brendan, Pamphile Styve, Yang Fan, Naeem Farnia, Kim Jinsung, Annam Jayabhargav, Borczuk Rachel, Yellin Shira, Bass Carly, Fowler Sabrina, Mosheyev Maykl, Mayer Yael Jessica, Friedman Benjamin W. Inflammatory markers are poorly predictive of clinical outcomes among hospitalized patients with COVID-19. The American journal of emergency medicine. 2020 doi: 10.1016/j.ajem.2020.11.038. S0735-6757(20)31062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A., Fukushima E.A., Levine M., Zhao W., Tanveer F., Szpunar S.M., Saravolatz L. Predictors for Severe COVID-19 Infection'. Clin Infect Dis. 2020;71:1962–1968. doi: 10.1093/cid/ciaa674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84:1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha Lucas Leite, Perazzio Sandro Felix, Azzi Jamil, Cravedi Paolo, Riella Leonardo Vidal. Remodeling of the Immune Response With Aging: Immunosenescence and Its Potential Impact on COVID-19 Immune Response. Frontiers in Immunology. 2020:11. doi: 10.3389/fimmu.2020.01748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi Sara, Meschiari Marianna, Gibellini Lara, Bellinazzi Caterina, Borella Rebecca, Fidanza Lucia, Gozzi Licia, Iannone Anna, Tartaro Domenico Lo, Mattioli Marco, Paolini Annamaria, Menozzi Marianna, Milić Jovana, Franceschi Giacomo, Fantini Riccardo, Tonelli Roberto, Sita Marco, Sarti Mario, Trenti Tommaso, Brugioni Lucio, Cicchetti Luca, Facchinetti Fabio, Pietrangelo Antonello, Clini Enrico, Girardis Massimo, Guaraldi Giovanni, Mussini Cristina, Cossarizza Andrea. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nature Communications. 2020;11:3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M., Guo G.Y., Du J., Zheng C.L., Zhu Q., Hu M., Li X.Y., Peng P., Shi H.Z. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020:55. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavridis T., van der Stegen S.J.C., Eyquem J., Hamieh M., Piersigilli A., Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khartabil T.A., Russcher H., van der Ven A., de Rijke Y.B. A summary of the diagnostic and prognostic value of hemocytometry markers in COVID-19 patients. Crit Rev Clin Lab Sci. 2020;57:415–431. doi: 10.1080/10408363.2020.1774736. [DOI] [PubMed] [Google Scholar]
- Koch Sven, Larbi Anis, Derhovanessian Evelyna, Özcelik Dennis, Naumova Elissaveta, Pawelec Graham. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immunity & Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijeveld R., de Graav G.N., Dieterich M., Litjens N.H.R., Hesselink D.A., Baan C.C. Co-inhibitory profile and cytotoxicity of CD57(+) PD-1(-) T cells in end-stage renal disease patients. Clin Exp Immunol. 2018;191:363–372. doi: 10.1111/cei.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnadi Anthony, Ramírez-Suástegui Ciro, Fajardo Vicente, Chee Serena J, Meckiff Benjamin J, Simon Hayley, Pelosi Emanuela, Seumois Grégory, Ay Ferhat, Vijayanand Pandurangan, Ottensmeier Christian H. Severely ill COVID-19 patients display impaired exhaustion features in SARS-CoV-2-reactive CD8+ T cells. Science Immunology. 2021;6:eabe4782. doi: 10.1126/sciimmunol.abe4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Chih-Cheng, Liu Yen Hung, Wang Cheng-Yi, Wang Ya-Hui, Hsueh Shun-Chung, Yen Muh-Yen, Ko Wen-Chien, Hsueh Po-Ren. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. Journal of Microbiology, Immunology and Infection. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Manwani B., Leng S.X. Frailty, inflammation, and immunity. Aging Dis. 2011;2:466–473. [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin Chem Lab Med. 2020;58:1063–1069. doi: 10.1515/cclm-2020-0240. [DOI] [PubMed] [Google Scholar]
- Merah-Mourah F., Cohen S.O., Charron D., Mooney N., Haziot A. Identification of Novel Human Monocyte Subsets and Evidence for Phenotypic Groups Defined by Interindividual Variations of Expression of Adhesion Molecules. Scientific Reports. 2020;10:4397. doi: 10.1038/s41598-020-61022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M., Sanvito F., Ponzoni M., Doglioni C., Cristofori P., Traversari C., Bordignon C., Ciceri F., Ostuni R., Bonini C., Casucci M., Bondanza A. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- Okada R., Kondo T., Matsuki F., Takata H., Takiguchi M. Phenotypic classification of human CD4+ T cell subsets and their differentiation. Int Immunol. 2008;20:1189–1199. doi: 10.1093/intimm/dxn075. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Age and immunity: What is "immunosenescence"? Exp Gerontol. 2018;105:4–9. doi: 10.1016/j.exger.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Payen Didier, Cravat Maxime, Maadadi Hadil, Didelot Carole, Prosic Lydia, Dupuis Claire, Losser Marie-Reine, Bittencourt Marcelo De Carvalho. 'A Longitudinal Study of Immune Cells in Severe COVID-19 Patients. Frontiers in Immunology. 2020;11 doi: 10.3389/fimmu.2020.580250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polilli E., Sozio F., Frattari A., Persichitti L., Sensi M., Posata R., Di Gregorio M., Sciacca A., Flacco M.E., Manzoli L., Di Iorio G., Parruti G. Comparison of Monocyte Distribution Width (MDW) and Procalcitonin for early recognition of sepsis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- Romero Pedro, Zippelius Alfred, Kurth Isabel, Pittet Mikaël J., Touvrey Cédric, Iancu Emanuela M., Corthesy Patricia, Devevre Estelle, Speiser Daniel E., Rufer Nathalie. Four Functionally Distinct Populations of Human Effector-Memory CD8+T Lymphocytes. The Journal of Immunology. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Cerrillo, Ildefonso, Pedro Landete, Beatriz Aldave, Santiago Sánchez-Alonso, Ana Sánchez Azofra, Ana Marcos-Jiménez, Elena Ávalos, Ana Alcaraz-Serna, Ignacio de los Santos, Tamara Mateu-Albero, Laura Esparcia, Celia López-Sanz, Pedro Martínez-Fleta, Ligia Gabrie, Luciana del Campo Guerola, María José Calzada, Isidoro González-Álvaro, Arantzazu Alfranca, Francisco Sánchez-Madrid, Cecilia Muñoz-Calleja, Joan B Soriano, Julio Ancochea, and Enrique Martín-Gayo. 2020. 'Differential Redistribution of Activated Monocyte and Dendritic Cell Subsets to the Lung Associates with Severity of COVID-19′, medRxiv: 2020.05.13.20100925.
- Shi Yufang, Wang Ying, Shao Changshun, Huang Jianan, Gan Jianhe, Huang Xiaoping, Bucci Enrico, Piacentini Mauro, Ippolito Giuseppe, Melino Gerry. COVID-19 infection: the perspectives on immune responses. Cell Death & Differentiation. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Cong-Ying, Xu Jia, He Jian-Qin, Lu Yuan-Qiang. Immune dysfunction following COVID-19, especially in severe patients. Scientific Reports. 2020;10:15838. doi: 10.1038/s41598-020-72718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urra J.M., Cabrera C.M., Porras L., Ródenas I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lu X., Li Y., Chen H., Chen T., Su N., Huang F., Zhou J., Zhang B., Yan F., Wang J. Clinical Course and Outcomes of 344 Intensive Care Patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. Jama. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Du L., Ojcius D.M., Pan C., Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020;22:74–79. doi: 10.1016/j.micinf.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]