Abstract
Recent studies have demonstrated a central role for plasma cells in the development of autoimmune diseases, such as systemic lupus erythematosus (SLE). Currently, both the phenotypic features and functional regulation of autoreactive plasma cells during SLE pathogenesis remain largely unclear. In this study, we first found that a major subset of IL-17 receptor-expressing plasma cells potently produced anti-dsDNA IgG upon IL-17A (IL-17) stimulation in SLE patients and lupus mice. Using a humanized lupus mouse model, we showed that the transfer of Th17 cell-depleted PBMCs from lupus patients resulted in a significantly reduced plasma cell response and attenuated renal damage in recipient mice compared to the transfer of total SLE PBMCs. Moreover, long-term BrdU incorporation in lupus mice detected highly enriched long-lived BrdU+ subsets among IL-17 receptor-expressing plasma cells. Lupus mice deficient in IL-17 or IL-17 receptor C (IL-17RC) exhibited a diminished plasma cell response and reduced autoantibody production with attenuated renal damage, while the adoptive transfer of Th17 cells triggered the plasma cell response and renal damage in IL-17-deficient lupus mice. In reconstituted chimeric mice, IL-17RC deficiency resulted in severely impaired plasma cell generation but showed no obvious effect on germinal center B cells. Further mechanistic studies revealed that IL-17 significantly promoted plasma cell survival via p38-mediated Bcl-xL transcript stabilization. Together, our findings identified a novel function of IL-17 in enhancing plasma cell survival for autoantibody production in lupus pathogenesis, which may provide new therapeutic strategies for the treatment of SLE.
Keywords: Systemic lupus erythematosus (SLE), Plasma cell (PC), Autoantibody, Interleukin-17A (IL-17)
Subject terms: Autoimmunity, Plasma cells, Interleukins
Introduction
Recent studies have suggested a central role for autoreactive B cells in the development of autoimmune diseases, such as systemic lupus erythematosus (SLE),1,2 in which plasma cells secrete autoantibodies and contribute to autoimmune inflammation and tissue injury.3 Expanded autoreactive plasma cells with autoantibody production have been shown to trigger immune complex-deposited glomerulonephritis in SLE.4 In particular, long-lived plasma cells are nondividing cells with persistent antibody production and show resistance to current therapies for SLE in patients, including conventional immunosuppressive drugs and B-cell depletion.5,6 Currently, both the phenotypic features and functional regulation of plasma cells during autoimmune pathogenesis remain largely unclear. We and others recently reported a subset of autoreactive plasma cells with longevity signatures in SLE development and aging.7,8 Further studies revealed distinct transcriptional profiles of terminally differentiated signatures of mouse B cells with expression of certain cytokine receptors in plasma cells, including Il17ra and Il10rb.9 However, it remains largely unclear how proinflammatory cytokines contribute to the sustained plasma cell response and autoantibody production in SLE. As a key effector cytokine in autoimmune pathogenesis, IL-17A (IL-17) has been extensively investigated due to its systemic effects on various types of target cells in inflammatory diseases.10 Although it has been shown that IL-17 promotes germinal center (GC) B-cell differentiation and antibody class switching in lupus-prone BXD2 mice,11 comparable frequencies of GC B cells are observed in IL-17-deficient lupus-prone Fcgr2b−/− mice.12 To date, a direct mechanistic link between IL-17 and the plasma cell response during SLE development has not been established.
Extensive studies have demonstrated that T-cell overactivation and proinflammatory cytokine production promote persistent autoimmune inflammation and target organ damage in SLE.13,14 Many reports, including our recent study, have revealed pathogenic functions for IL-17-producing T helper (Th17) cells in autoimmune diseases, such as rheumatoid arthritis, psoriasis, multiple sclerosis, and Sjögren’s syndrome.15–17 Notably, blockade of IL-17 produces clinical improvement in psoriasis.18 Several reports have also indicated the crucial involvement of Th17 cells and IL-17 in the development of SLE.11,19,20 IL-17-deficient mice exhibit a diminished autoantibody response and reduced lupus progression, which are associated with reduced CD3+CD4−CD8− double-negative T-cell numbers and expanded regulatory T cells.12,19,21 Moreover, Th17 cells have been shown to trigger nephritis development during the pathogenesis of SLE.11,19,20 However, it remains unclear whether Th17 cells can modulate plasma cell function via IL-17 production during lupus development.
In this study, we first identified a major subset of plasma cells expressing IL-17 receptor A and C (RA/RC) with potent anti-dsDNA IgG production in SLE patients, which correlated closely with an increased Th17 frequency and enhanced disease activity. Using a humanized lupus mouse model, we revealed that Th17 cells from SLE patients promoted a plasma cell response with increased autoantibody production and nephritis development in vivo. Importantly, IL-17 signaling deficiency induced with IL-17 or IL-17 receptor C (IL-17RC) knockout led to a diminished plasma cell response and attenuated renal damage in lupus mice. In both SLE patients and lupus mice, IL-17RA/RC-expressing plasma cells were substantially expanded with increased anti-dsDNA autoantibody secretion upon IL-17 stimulation. Mechanistically, IL-17 directly promoted lupus plasma cell survival via p38-mediated Bcl-xL transcript stabilization. Together, our findings identify a novel function of IL-17 in promoting the plasma cell response for autoantibody production in the pathogenesis of SLE.
Materials and methods
Human samples
Fifty-two patients diagnosed with SLE fulfilling the 2012 American College of Rheumatology classification criteria were recruited from Shenzhen People’s Hospital.22 All experiments with human samples were approved by the Ethics Committee of the Second Affiliated Hospital of Jinan University (reference number: LL-KT-2019066). The demographic information of the SLE patients and healthy controls is shown in Supplementary Tables S1 and S2.
Mice and treatments
Female C57BL/6J (C57), NOD-SCID IL2Rγnull (NSG), B6.SJL-Ptprca Pepcb/BoyJ, Il17rc−/− (IL-17RC KO, C57 background), MRL/MPJ, and MRL/MpJ-Faslpr/J (MRL/Lpr) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Female Il17a−/− (IL-17 KO, C57 background) mice were kindly provided by Dr. Yoichiro Iwakura (University of Tokyo). All mice were maintained at the Laboratory Animal Unit of the University of Hong Kong. For immunization-induced lupus development, 6-week-old female C57 mice were s.c. immunized with chromatin components extracted from apoptotic cells, as we previously described.7 Mice immunized with PBS emulsified with adjuvants were used as controls. Female WT mice were fed 1 mg/mL bromodeoxyuridine (BrdU; Sigma-Aldrich, MO, USA) and 1% glucose in the drinking water during the first week and sacrificed at 15 weeks post immunization. All animal experiments were approved by the Committee on the Use of Live Animals in Teaching and Research (CULATR) at the University of Hong Kong.
Th17 cell isolation
IL-17A-secreting Th17 cells were isolated using a double-antibody-sandwich method.17 Briefly, SLE PBMCs or mouse Th17 cells generated from naive CD4+ T cells cultured under Th17-polarizing conditions were stimulated with PMA (20 ng/ml) and ionomycin (200 μg/ml) for 3 h. IL-17A capture reagents were prepared by mixing a biotinylated anti-mouse IL-17A antibody (TC11-8H4, BioLegend), a biotinylated anti-mouse CD4 antibody (GK1.5, BioLegend), and NeutrAvidin (Thermo Fisher Scientific) at a 3:1:3 ratio for mice or by mixing a biotinylated anti-human IL-17A antibody (Poly5189, BioLegend), a biotinylated anti-human CD4 antibody (A161A1, BioLegend), and NeutrAvidin (Thermo Fisher Scientific) at a 3:1:3 ratio for humans. The IL-17A capture reagents were added to cultured cells for a 3-h incubation at 37 °C. Then, the cells were washed and labeled with the secondary antibodies PE-conjugated anti-mouse IL-17A (TC11-18H10.1, BioLegend) and FITC-conjugated anti-mouse CD4 (GK1.5, BioLegend). Viable FITC+PE+ mouse cells were sorted and collected as Th17 cells with >99% purity using a BD FACSAria SORP (Becton Dickinson). For Th17 cell depletion in human PBMCs, viable FITC+PE+ cells were excluded with >99% purity using a BD FACSAria SORP (Becton Dickinson).
Generation of humanized lupus mice
Female NSG mice were adoptively transferred with 1 × 108 PBMCs or Th17-depleted PBMCs from active SLE patients. For transfer, PBMCs from one SLE patient were divided into two samples with one depleted of Th17 cells, as described previously.23 The human plasma cell response and human IgG renal deposition were assessed 20 days after cell transfer in the humanized lupus mice.
Adoptive cell transfer
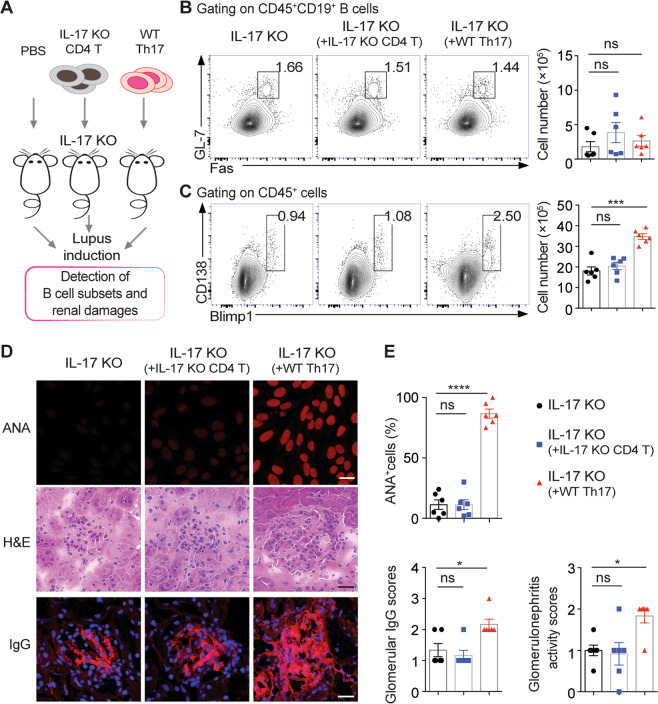
For Th17 polarization, naive CD4+ T cells were isolated from the spleen of naive C57 mice with a naive CD4+ T-cell isolation kit (Miltenyi Biotec, CA, USA) and stimulated with TGF-β1, IL-6, IL-23, and anti-CD3/CD28 monoclonal antibody beads (Gibco, Thermo Fisher Scientific, USA) for 72 h. Th17 cells were purified as we previously described.17 IL-17 KO mice were adoptively transferred with 1 × 106 Th17 or IL-17 KO CD4+ T cells and immunized with chromatin to induce lupus. None of the transferred IL-17 KO mice with lupus induction were used as controls. B-cell responses, serum autoantibody levels, and renal damage in the IL-17 KO recipients with lupus induction for 6 weeks were assessed.
Generation of mixed bone marrow chimeras
Mixed bone marrow chimeric mice were generated as described previously.24 In brief, recipient BoyJ mice received 800 cGy of X-ray irradiation. One day later, the lethally irradiated recipients were reconstituted with a mixed inoculum of 60% IL-17RC KO bone marrow cells and 40% bone marrow cells from BoyJ mice. Lupus induction was performed in chimeric mice after 12 weeks, which allowed full reconstitution of the lymphoid system. Plasma cell subsets were analyzed within WT (BoyJ, CD45.1+) and IL-17RC KO (CD45.2+) counterparts by flow cytometry after 6 weeks of lupus induction.
Plasma cell culture
Human LineagenegCD27hiCD38hi plasma cells and IL-17RA/RC+ and IL-17RA/RC− plasma cell subsets were sorted with a FACSAria SORP cytometer (BD Biosciences) from the PBMCs of active SLE patients. Murine CD138+ plasma cells were isolated using anti-CD138 MicroBeads (Miltenyi Biotec). CD19−CD138+ plasma cells and IL-17RA/RC+ and IL-17RA/RC− plasma cell subsets were then sorted with a FACSAria SORP cytometer (BD Biosciences).
Plasma cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 50 μM β-ME, and antibiotics. IL-17A (20 ng/mL), neutralizing anti-IL-17RA antibodies (10 ng/mL), and the p38 inhibitor SB203580 (5 μM) were added to cultured plasma cells as indicated.
Flow-cytometric analysis
For multicolor flow-cytometric analysis, single-cell suspensions were prepared from the peripheral blood, spleen, kidneys, and bone marrow (BM) of control and lupus mice. Murine kidneys were digested after perfusion, and renal-infiltrated leukocytes were enriched with Percoll (Sigma-Aldrich, MO, USA), as described previously.25 The Zombie Aqua fixable viability dye (BioLegend, CA, USA) was added to single-cell suspensions to exclude dead cells. Nonspecific Fc-gamma receptor (FcƔR)-mediated binding of antibodies in the cell suspensions was blocked with an Fc receptor-binding inhibitor (BioLegend).
For surface marker detection, cells were labeled with fluorescent dye-conjugated antibodies as described in Supplementary Table S2. For analysis of intracellular cytokine production, cells were stimulated with PMA (20 ng/ml), ionomycin (1 μg/ml), and monensin (5 μg/ml) for at least 4 h and stained with combinations of fluorescent dye-conjugated anti-CD45 and anti-CD4 antibodies. Then, the cells were washed, fixed, permeabilized, and stained with fluorescent dye-conjugated anti-IL-17 antibodies (Supplementary Table S3). The incorporation of BrdU was detected using a BrdU flow kit (BD Biosciences). The phosphorylation of p65, p38, and JAK1 in plasma cells was detected using the BD Phosflow™ Kit (BD Biosciences) according to the manufacturer’s instructions. All flow-cytometric data were acquired using a BD LSR Fortessa cytometer (BD Biosciences) and analyzed with FlowJo software (BD Biosciences).
Confocal fluorescence microscopy
Anti-nuclear autoantibodies (ANAs) were detected by indirect immunofluorescence staining of HEp-2 cells (Bio-Red, CA, USA) with serum samples. For each slide analyzed with ANA staining, both IgG and nuclear staining results were counted using MetaMorph software (MetaMorph, TN, USA). The percentages of ANA+ cells were calculated as (ANA+ staining counts/total nuclei counts per field) × 100 with five independent fields per slide assessed.
For the detection of immune complex deposition, frozen kidney sections from lupus mice were stained with fluorescent dye-conjugated IgM and IgG antibodies. Hoechst 33258 (Sigma-Aldrich) was used to visualize cell nuclei. Fluorescence images were captured with a ×40 oil immersion objective by an LSM780 confocal microscope (Carl Zeiss, Jena, Germany).
ELISA and ELISPOT
The dsDNA-specific IgG levels in serum were measured using a human anti-dsDNA ELISA kit (MyBioSource, CA, USA). dsDNA-specific IgG secretion by plasma cells was detected with an ELISPOT assay, as we described previously.7
Quantitative real-time PCR (qPCR)
RNA extraction from sorting-purified plasma cells was performed with the RNeasy Mini Kit (Qiagen, Hilden, Germany) and reverse-transcribed with TB Green Premix Ex Taq II (TakaRa, Dalian, China). To detect mRNA stability, plasma cells were then incubated with actinomycin D (ActD, 5 µg/mL) to block mRNA synthesis. RNA extraction was performed at 0, 30, 60, and 120 min after ActD treatment. qPCR analysis was performed using PrimeScript RT Master Mix (TakaRa) according to the manufacturer’s instructions. The PCR primers used were as follows: Bcl-xL (forward: 5′-CTGGGACACTTTTGTGGATCTCT-3′; reverse: 5′-GAAGCGCTCCTGGCCTT-3′) and 18S (forward: 5′-AACCCGTTGAACCCCATT-3′; reverse: 5′-CCATCCAATCGGTAGTAGCG-3′). RT-PCR analysis was performed using the ABI Prism 7900HT Real-time PCR System (Applied Biosystems, CA, USA). The relative expression levels of the Bcl-xL transcript were normalized to those of the internal standard (18S) and calculated via the 2−∆∆Ct method.
Histological assessment
Paraffin-embedded kidney sections were stained with H&E according to standard procedures. Semiquantitative data for pathological changes, including glomerulonephritis activity scores and glomerular IgG scores, were assessed in a blinded fashion, and 15 cortical glomeruli from each mouse were evaluated as described previously.26 In brief, glomerulonephritis activity was scored on a scale of 0 to 3 by the extent of glomerular cell proliferation and leukocyte exudation as follows: no proliferation (0), <25% (1), 25–50% (2), and >50% (3) of glomeruli. Glomerular IgG deposition was graded by assessing the intensity of IgG staining as follows: no staining (0), <25% (1, weak), 25–50% (2, moderate), or >50% (3, strong) of glomeruli.27
Statistics
Results are presented as the mean ± standard error of the mean (SEM). Flow-cytometric data analysis was performed using FlowJo (BD, USA). Statistical analysis was performed using GraphPad Prism (GraphPad Software, CA, USA). Comparisons between two groups were performed by a two-tailed, unpaired Student’s t test, and the nonparametric Mann–Whitney U test. Comparisons among more than two groups were performed using one-way ANOVA followed by the Newman–Keuls test as indicated. Correlations were assessed with Pearson’s correlation coefficient. P < 0.05 was considered statistically significant.
Results
IL-17RA/RC-expressing plasma cells are expanded with potent autoantibody production in SLE patients
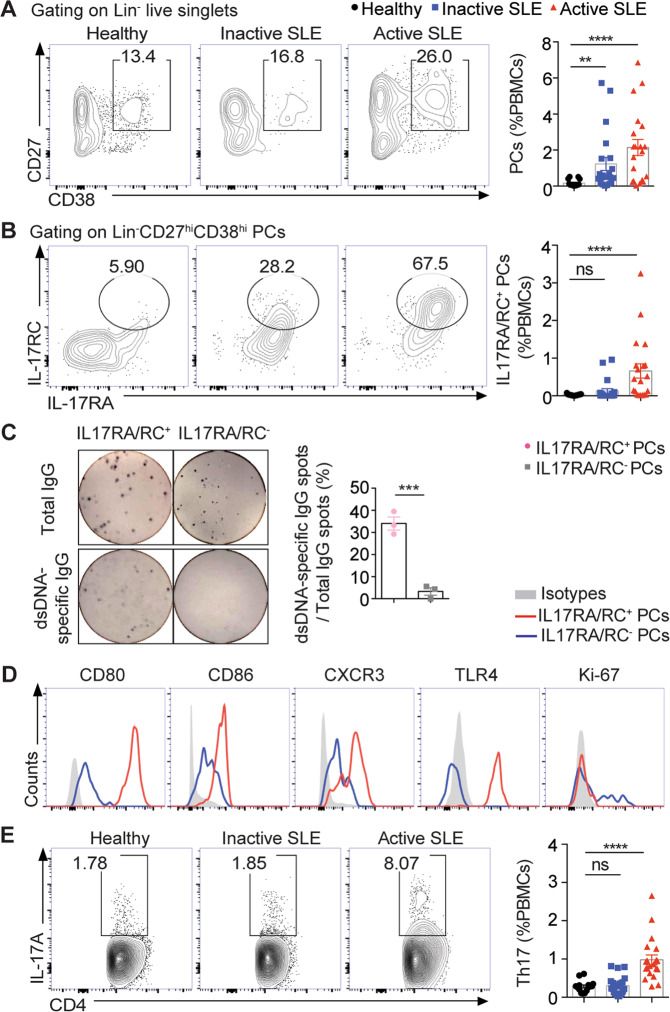
To examine the kinetic changes of plasma cells during lupus progression, we analyzed PBMC samples from a cohort of healthy controls, inactive SLE patients (SLEDAI < 6), and active SLE patients (SLEDAI > = 6) by flow cytometry (see Supplementary Table S1 for patient characteristics). As shown in Fig. 1a, the frequencies of circulating plasma cells were markedly increased in the active SLE patients, in which a major subset of plasma cells was found to express IL-17 receptors A and C (RA/RC), with more than 20-fold increases in frequencies when compared with the same subsets in healthy controls (Fig. 1b and Supplementary Fig. S1). To determine the autoantibody-producing capacity of lupus plasma cells, both IL-17RA/RC+ and IL-17RA/RC− plasma cell subsets in the PBMCs of the active SLE patients were sorting-purified for enzyme-linked immunospot (ELISPOT) analysis (Supplementary Fig. S2). IL-17RA/RC+ plasma cells were found to contain approximately fivefold higher frequencies of anti-dsDNA IgG-secreting cells than IL-17RA/RC- counterparts in the active lupus patients (Fig. 1c). Phenotypic analysis revealed that IL-17RA/RC+ plasma cells exhibited significantly higher levels of surface CD80, CD86, TLR4, and CXCR3 expression than their IL-17RA/RC− counterparts in lupus patients (Fig. 1d). Since we previously reported the accumulation of autoreactive TLR4+CXCR4+ plasma cells in SLE,7 here, we further examined the IL-17R expression profile in the TLR4+CXCR4+ plasma cell population. As shown in Supplementary Fig. S3, ~80% of the TLR4+CXCR4+ plasma cells in SLE PBMCs expressed IL-17RA.
Fig. 1.
Expanded IL-17RA/RC+ plasma cells in systemic lupus erythematosus (SLE) patients. a, b Representative flow-cytometric profiles and plots show lineage−CD27hiCD38hi plasma cells (PCs) and IL-17RA/RC+ PCs in the PBMCs of healthy donors (n = 15), inactive SLE patients (SLEDAI < 6, n = 21), and active SLE patients (SLEDAI > = 6, n = 20). c Representative ELISPOT detection results and a data plot show the total IgG spots (upper panel) and dsDNA-specific IgG-secreting cells (lower panel) in IL-17RA/RC+ PCs and IL-17RA/RC− PCs purified from the PBMCs of active SLE patients by sorting (n = 3). d Representative flow-cytometric histograms show the expression profiles of IL-17RA/RC+ PCs and IL-17RA/RC− counterparts from the PBMCs of active SLE patients (n = 5). e Representative flow-cytometric profiles and a plot show the frequencies of Th17 cells in the PBMCs of healthy donors (n = 15), inactive SLE patients (n = 21), and active SLE patients (n = 20). Data are shown as the mean ± SEM; one-way ANOVA in a, b, and e; unpaired, two-tailed Student’s t test in c; ns no significance; **P < 0.01; ***P < 0.001; ****P < 0.0001
Moreover, IL-17RA/RC+ plasma cells were also negative for Ki67 staining, indicating the nondividing property of IL-17RA/RC+ plasma cells in SLE patients (Fig. 1d). Notably, treatment with recombinant IL-17 markedly increased the frequencies of anti-dsDNA IgG-producing cells among plasma cells from the active SLE patients, while treatment with an anti-IL-17R neutralizing antibody significantly dampened autoantibody secretion by lupus plasma cells (Supplementary Fig. S4), indicating a critical role for IL-17 in promoting autoantibody production by plasma cells in SLE. We also detected significantly increased frequencies of Th17 cells in the active SLE patients compared with the healthy subjects and inactive SLE patients (Fig. 1e).
SLE Th17 cells promote the plasma cell response and renal IgG deposition in humanized lupus mice
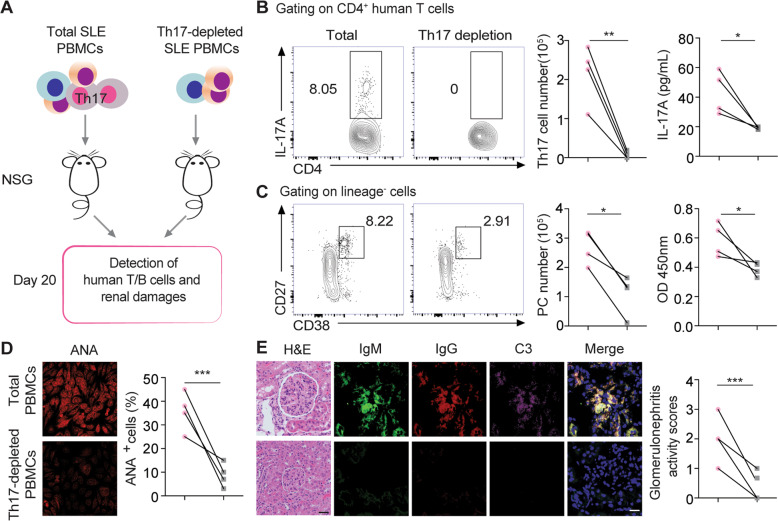
To determine the role of Th17 cells in driving the plasma cell response during lupus development, total PBMC and Th17 cell-depleted PBMC samples from active SLE patients were adoptively transferred into T/B/NK cell-deficient NSG mice (Fig. 2a). On day 20 post SLE PBMC transfer, both human Th17 cells and lineage (Lin)−CD27hiCD38hi plasma cells were readily detected in recipient mice, as were significantly elevated serum levels of human IL-17 and anti-dsDNA IgG antibodies. In mice transferred with Th17 cell-depleted SLE PBMCs, the number of human Lin−CD27hiCD38hi plasma cells showed a marked threefold reduction in the spleen compared with that in recipient mice transferred with total SLE PBMCs (Fig. 2b, c).
Fig. 2.
Systemic lupus erythematosus (SLE) Th17 cells promote the plasma cell response in humanized lupus mice. a The schematic diagram shows humanized NSG mice reconstituted with total PBMCs or Th17 cell-depleted SLE PBMCs from SLE patients (n = 4 per group). b, c Representative flow-cytometric profiles and data plots show splenic human Th17 cells (upper panel), serum human IL-17A levels (upper panel), plasma cells (lower panel), and anti-dsDNA IgG levels (lower panel) in NSG-recipient mice on day 20 post reconstitution with PBMCs or Th17 cell-depleted PBMCs from active SLE patients (n = 4 per group). Representative images and plots show the ANA staining (d) of human IgG in serum samples (1:5 dilution), H&E staining, and human IgG (red), IgM (green), and C3 (violet) staining in kidney sections (e) from NSG recipients on day 20 post PBMC transfer (n = 4 per group). Original magnification: ×40; scale bars, 20 μm. Data are represented as the mean ± SEM; unpaired, two-tailed Student’s t test; *P < 0.05; **P < 0.01; ***P < 0.001
In NSG recipients, transfer of total SLE PBMCs resulted in significantly elevated serum levels of anti-dsDNA IgG and anti-nuclear autoantibodies (ANAs) with pronounced glomerular immune complex (IgG, IgM, and C3) deposition in the kidneys (Fig. 2c–e). In contrast, serum ANA levels were barely detectable with no obvious renal IgG deposition in NSG recipients transferred with Th17 cell-depleted SLE PBMCs (Fig. 2c–e), indicating a pivotal role for Th17 cells in driving the plasma cell response, autoantibody production, and renal pathology during lupus development.
IL-17RA/RC-expressing plasma cells with longevity signatures are expanded in lupus mice
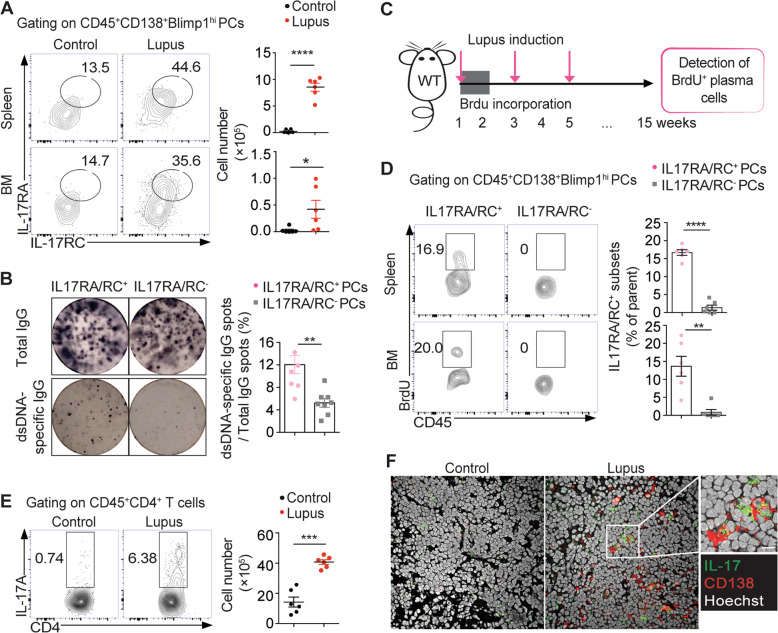
Consistent with the findings from SLE patients, markedly increased IL17RA/RC+ plasma cell levels were observed in the spleen and bone marrow of lupus mice (Fig. 3a). Similarly, IL-17RA/RC+ plasma cells were found to contain significantly higher frequencies of anti-dsDNA IgG-secreting cells than IL-17RA/RC− plasma cells in lupus mice (Fig. 3b). To examine the lifespan of IL-17RA/RC− plasma cells in lupus mice, we analyzed long-lived plasma cells in mice at 15 weeks after BrdU incorporation (Fig. 3c). Interestingly, we observed that long-lived BrdU+ plasma cells accumulated within the IL-17RA/RC+ plasma cell subset in the spleen and bone marrow of lupus mice. Approximately 18% of IL-17RA/RC+ plasma cells were positive for BrdU incorporation (Fig. 3d). Similarly, expanded IL-17RA/RC+ plasma cells were also observed in MRL/Lpr mice, a model of spontaneous lupus development (Supplementary Fig. S5).
Fig. 3.
Expanded IL-17RA/RC+ plasma cells harbor long-lived signatures in lupus mice. a Representative flow-cytometric profiles and plots show IL-17RA/RC+CD138+Blimp1hi PCs in spleens and bone marrow from adjuvant-treated control and lupus mice at 15 weeks post immunization (n = 6 per group). b Representative ELISPOT detection results and a data plot show the total IgG spots (upper panel) and dsDNA-specific IgG-secreting cells (lower panel) in sorting-purified IL-17RA/RC+ PCs and IL-17RA/RC− PCs from the spleen of lupus mice (n = 6). c The schematic diagram shows long-term BrdU incorporation in WT lupus mice (n = 6). d Representative flow-cytometric profiles and a plot show BrdU+ subsets within the IL-17RA/RC+ and IL-17RA/RC− PC subsets in the spleen and bone marrow of long-term BrdU-incorporated lupus mice at 15 weeks post immunization (n = 6). e Representative flow-cytometric profiles and a plot show Th17 cells in spleens from lupus and control mice (n = 6 per group). f The representative confocal image shows IL-17-producing cells and CD138+ PCs in the spleen of control and lupus mice. Original magnification: ×40; scale bars, 10 μm. Data are represented as the mean ± SEM; unpaired, two-tailed Student’s t test; **P < 0.01; ***P < 0.001; ****P < 0.0001
In addition, we also detected significantly increased Th17 cell numbers in the spleen of lupus mice (Fig. 3e). Morphological examination revealed the adjacent locations of IL-17-producing cells and CD138+ plasma cells in the spleen of lupus mice compared with that of control mice by confocal microscopy (Fig. 3f), indicating a role for IL-17 signaling in modulating the plasma cell response in lupus.
IL-17 signaling deficiency severely impairs the plasma cell response with attenuated renal damage in lupus mice
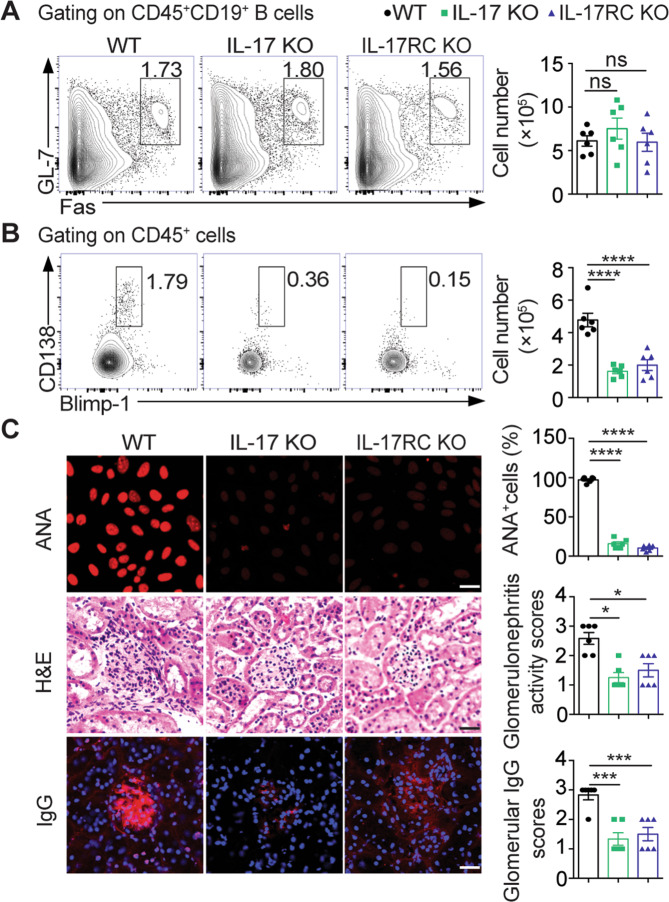
To determine the role of IL-17 signaling in the plasma cell response in lupus, we immunized both IL-17-deficient and IL-17RC-deficient mice for lupus induction. Interestingly, we found comparable numbers of germinal center (GC) B cells but dramatically reduced numbers of CD138+Blimp1hi plasma cells in both IL-17 KO and IL-17RC KO mice compared with wild-type (WT) lupus mice (Fig. 4a, b). Upon lupus induction, both IL-17 KO and IL-17RC KO mice showed profoundly decreased serum ANA levels and ameliorated glomerular damage (Fig. 4c), indicating an indispensable role for IL-17 signaling in maintaining the plasma cell response and renal damage during lupus development.
Fig. 4.
IL-17 signaling deficiency impairs the plasma cell response and attenuates renal damage in lupus mice. a, b Representative flow-cytometric profiles and plots show GC B cells and PCs in the spleen of wild-type (WT), IL-17 KO, and IL-17RC KO mice with lupus induction for 15 weeks (n = 6 per group). c Representative images and data plots show ANA staining in serum samples (1:100 dilution) and H&E and IgG (red) staining in kidney sections from WT, IL-17 KO, and IL-17RC KO mice with lupus induction for 15 weeks (n = 6 per group). Original magnification: ×40; scale bars, 20 μm. Data are represented as the mean ± SEM; one-way ANOVA; ns no significance; ***P < 0.001; ****P < 0.0001
Th17 cells promote the plasma cell response and lupus progression via IL-17 secretion
To further determine the effector function of Th17 cells in lupus progression, we first expanded Th17 cells by culturing naive CD4+ T cells from WT mice to induce Th17 cell differentiation under polarizing conditions.28 Then, purified WT Th17 cells or IL-17 KO CD4+ T cells were adoptively transferred into IL-17 KO-recipient mice prior to lupus induction (Fig. 5a and Supplementary Fig. S6).17 Both recipient groups treated with WT Th17 or IL-17 KO T-cell transfer exhibited similar splenic GC B-cell population sizes (Fig. 5b). Notably, WT Th17 cell transfer led to significantly expanded CD138+Blimp1hi plasma cells in the spleen of IL-17 KO recipients, accompanied by elevated levels of serum anti-dsDNA IgG and ANAs and increased glomerular immune complex deposition in the kidneys (Fig. 5c–e). However, the transfer of IL-17 KO CD4+ T cells did not result in any obvious changes in the plasma cell response or renal pathology in IL-17 KO-recipient mice (Fig. 5c–e), suggesting that Th17 cells promote the plasma cell response and autoantibody production via IL-17 production in lupus mice.
Fig. 5.
Th17 cells promote plasma cell expansion via IL-17 secretion in lupus mice. a The schematic diagram shows the protocol for the adoptive transfer of WT Th17 or IL-17 KO CD4+ T cells into IL-17 KO recipients with lupus induction for 6 weeks (n = 6 per group). b, c Representative flow-cytometric profiles and data plots show GC B cells and PCs in the spleen of IL-17 KO recipients transferred with WT Th17 or IL-17 KO CD4+ T cells for 6 weeks (n = 6 per group). Representative images (d) and plots (e) show ANA staining in serum samples (1:100 dilution), H&E staining, and IgG staining (red) in kidney sections from IL-17 KO recipients transferred with WT Th17 or IL-17 KO CD4+ T cells for 6 weeks (n = 6 per group). Original magnification: ×40; scale bars, 20 μm. Data are represented as the mean ± SEM; one-way ANOVA; ns no significance; *P < 0.05; ***P < 0.001; ****P < 0.0001
Intrinsic IL-17 signaling sustains the plasma cell response for autoantibody production in lupus mice
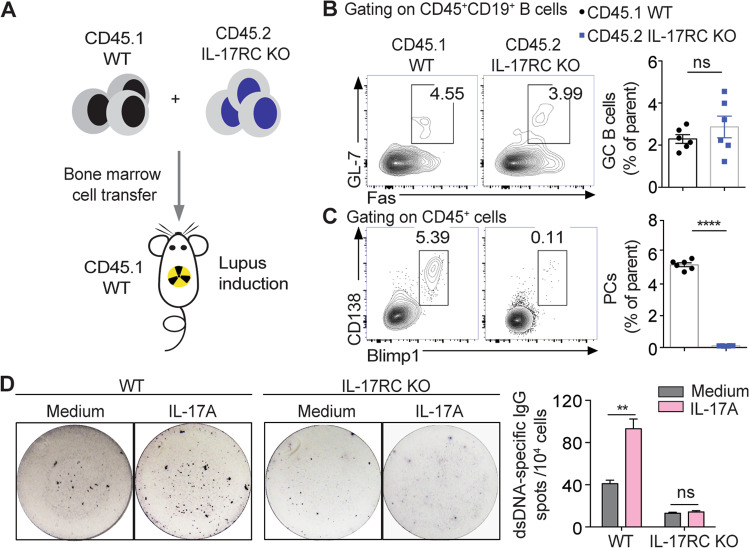
To elucidate whether B-cell-intrinsic IL-17 signaling is involved in regulating the plasma cell response in vivo, we generated chimeric mice reconstituted with mixed bone marrow cell suspensions from CD45.1+ BoyJ mice and CD45.2+ IL-17RC KO mice for lupus induction (Fig. 6a). The flow-cytometric analysis showed comparable frequencies of splenic GC B cells, but significantly reduced frequencies of CD138+Blimp1hi plasma cells in the CD45.2+ IL-17RC KO population compared with the CD45.1+ WT population (Fig. 6b, c).
Fig. 6.
Intrinsic IL-17 signaling is required for plasma cell expansion in lupus mice. a The schematic diagram shows the generation of chimeric mice (lethally irradiated BoyJ recipients) reconstituted with CD45.1+ BoyJ and CD45.2+ IL-17RC KO bone marrow cells with lupus induction for 6 weeks (n = 6). b, c Representative flow-cytometric profiles and data plots show GC B cells and PCs within the CD45.1+ BoyJ and CD45.2+ IL-17RC KO subpopulations in spleens from chimeric recipients with lupus induction (n = 6). d Representative ELISPOT detections and a data plot show the dsDNA-specific IgG-secreting cells in sorting-purified plasma cells from WT and IL-17RC KO lupus mice with or without IL-17A treatment for 72 h. Data were obtained from three independent experiments in d and are shown as the mean ± SEM; unpaired, two-tailed Student’s t test in b and c; one-way ANOVA in d; ns no significance; **P < 0.01; ****P < 0.0001
To further confirm the effector function of IL-17 signaling in lupus plasma cells, we cultured splenic CD19−CD138+ plasma cells from WT and IL-17RC KO lupus mice with or without recombinant IL-17 treatment. As shown in Fig. 6d, IL-17 treatment significantly increased the frequencies of anti-dsDNA IgG-secreting cells among the WT lupus plasma cells, but showed no obvious effects on the IL-17RC KO lupus plasma cells.
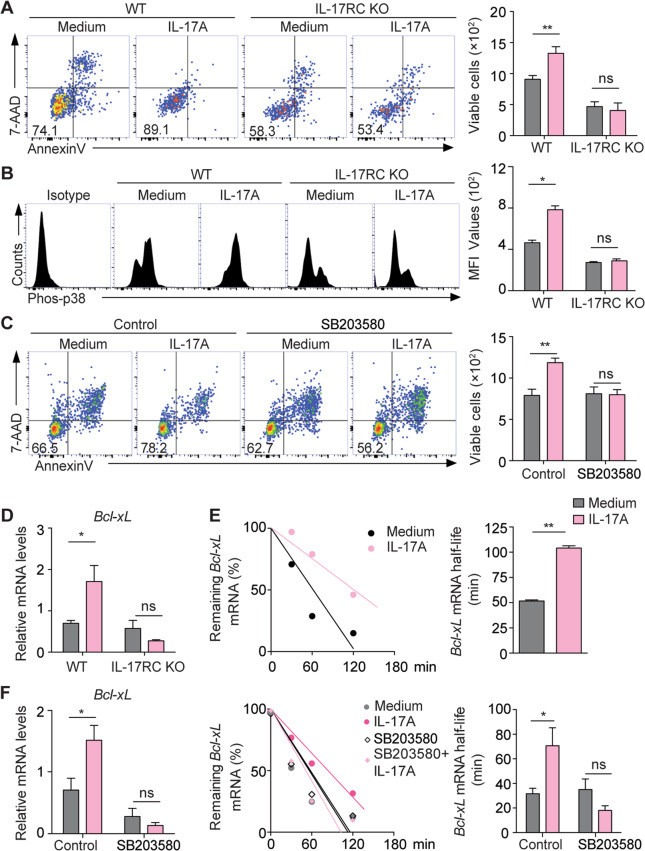
IL-17 promotes plasma cell survival via p38-mediated Bcl-xL transcript stabilization
To determine the molecular mechanisms underlying IL-17 effector function in lupus plasma cells, we cultured splenic CD19−CD138+ plasma cells from WT and IL-17RC KO lupus mice with or without recombinant IL-17. IL-17 treatment markedly increased the survival of the plasma cells from WT lupus mice, but showed no protective effects on the viability of the IL-17RC KO lupus plasma cells (Fig. 7a). Furthermore, flow-cytometric analysis detected markedly increased levels of phosphorylated (Phos)-p38 but comparable levels of Phos-p65 and Phos-JAK1 in WT lupus plasma cells upon IL-17 stimulation (Fig. 7b and Supplementary Fig. S7). Notably, the preincubation of lupus plasma cells with the p38 inhibitor SB203580 almost completely abrogated the IL-17-mediated protective effects on cell survival in culture (Fig. 7c).
Fig. 7.
IL-17 promotes plasma cell survival via p38-mediated Bcl-xL mRNA stabilization. a, b Representative flow-cytometric profiles and plots show viable cells (7-AAD−Annexin V−) and the levels of phosphorylated (Phos)-p38 in PCs from WT and IL-17RC KO lupus mice with or without IL-17A treatment for 72 h. c Representative flow-cytometric profiles and a plot show the number of viable cells in IL-17A-treated PCs from WT lupus mice in the presence of SB203580 or an equal volume of DSMO. d The plot shows the mRNA levels of Bcl-xL in PCs from WT and IL-17RC KO lupus mice with or without IL-17A treatment for 6 h. e Representative plots show the stability of Bcl-xL mRNA in WT plasma cells with or without IL-17 treatment for 72 h. f Representative plots show the mRNA levels (left panel) and stability of Bcl-xL (middle and right panels) in IL-17A-treated plasma cells from WT lupus mice in the presence of SB203580 or an equal volume of DSMO. Data were obtained from three independent experiments and are shown as the mean ± SEM; one-way ANOVA in a–d and f; unpaired, two-tailed Student’s t test in e; ns no significance; *P < 0.05; **P < 0.01
IL-17 has been shown to enhance the expression of multiple genes via posttranscriptional regulation of mRNA stability.29 To this end, we detected an approximately twofold increase in Bcl-xL transcript levels in IL-17-treated WT plasma cells, but not in IL-17RC KO plasma cells from lupus mice by quantitative PCR analysis (Fig. 7d). Moreover, we treated WT lupus plasma cells with actinomycin D to block mRNA synthesis and examined the mRNA half-life of Bcl-xL using qPCR analysis. Indeed, IL-17A treatment significantly prolonged the mRNA half-life of Bcl-xL in plasma cells compared with no treatment (Fig. 7e). Remarkably, inhibition of p38 activation with SB203580 completely abrogated IL-17A-induced Bcl-xL transcript upregulation and mRNA stabilization (Fig. 7f). Together, these results demonstrate that IL-17 promotes the survival of plasma cells via p38-mediated Bcl-xL mRNA stabilization.
Discussion
Autoimmune inflammation with sustained autoantibody production has long been implicated in the development of SLE, but the direct functional link between IL-17 and the plasma cell response remains unclear. In this study, we first identified a major subset of IL-17RA/RC+ plasma cells with potent anti-dsDNA antibody production upon IL-17 stimulation in active SLE patients, which showed positive correlations with increased circulating Th17 cell levels, serum autoantibody levels, and disease activity in both SLE patients and lupus mice. In particular, long-lived plasma cells accumulated within the IL-17RA/RC+ plasma cell subset. Lupus mice deficient in IL-17 or IL-17RC exhibited a diminished plasma cell response and attenuated renal damage upon lupus induction. Further mechanistic studies demonstrated that IL-17 directly promoted plasma cell survival via p38-mediated Bcl-xL transcript stabilization. Together, our findings reveal a novel function of IL-17 in driving prolonged autoantibody production by plasma cells during the pathogenesis of SLE.
Autoreactive plasma cells are known to continuously produce autoantibodies in inflamed tissue and organs during SLE pathogenesis, but the microenvironmental factors involved in maintaining these pathogenic plasma cells remain poorly characterized.3,7,30 As a therapeutic challenge, autoreactive long-lived plasma cells are resistant to conventional immunosuppressive drugs and B-cell depletion therapy.31 We recently identified a novel subset of autoreactive long-lived plasma cells with surface CXCR4 and TLR4 expression in SLE patients.7 Here, we further show that most TLR4+CXCR4+ plasma cells in SLE PBMCs express IL-17R. Since recent evidence indicates crosstalk between TLR4 and IL-17 signaling, the molecular mechanisms by which IL-17 regulates TLR4 or CXCR4 expression in plasma cells need further investigation.32
Using long-term BrdU incorporation in lupus mice, we demonstrated that long-lived plasma cells were enriched within the IL-17R-expressing plasma cell subset. It has been reported that several transcription factors, such as Blimp1, ZBTB20, XBP1, and ATG5, are required for the survival of plasma cells.33–37 Thus, further studies are needed to investigate the transcriptional network of IL-17 signaling involved in maintaining the survival of autoreactive plasma cells in autoimmune diseases.
As a key effector T-cell subset, Th17 cells play a pivotal role in autoimmune inflammation.38 To define the indispensable function of Th17 cells in lupus development, we established a humanized lupus model by transferring PBMCs from active SLE patients into NSG mice that lack mature T, B, and NK cells, in which the hallmarks of lupus, including increased serum levels of human anti-dsDNA autoantibodies and glomerular IgG deposition in the kidneys, are observed at 20 days post transfer. Notably, the transfer of Th17 cell-depleted SLE PBMCs significantly dampened the plasma cell response and diminished glomerular immune complex deposition in the kidneys of NSG recipients. Recent clinical studies have reported elevated concentrations of serum IL-17 in patients with lupus flares.39 Consistently, we found that increased Th17 cell frequencies correlated closely with disease activity and autoantibody responses in SLE patients.
Although previous studies have revealed the cellular sources of IL-17, including Th17 cells, γδ T cells, neutrophils, and B cells, in humans and mice,40–42 we found Th17 cells to be the major producer of IL-17 in the PBMCs of SLE patients (data not shown). In addition to IL-17, Th17 cells also produce several other cytokines, such as IL-17F, IL-21, IL-22, and GM-CSF, to prime proinflammatory responses.43 Here, we showed that IL-17 deficiency profoundly impaired plasma cell generation and the autoantibody response, resulting in attenuated renal pathology in lupus mice. Moreover, the transfer of IL-17-producing Th17 cells significantly enhanced plasma cell expansion and renal IgG deposition, but the transfer of IL-17 KO CD4 T cells could not restore plasma cell generation and the autoantibody response in IL-17-deficient recipients. Thus, these results indicate that Th17 cells exert their function in triggering the plasma cell response and lupus pathogenesis via IL-17 secretion.
Previous reports have shown IL-17 has multiple functions in germinal center responses. IL-17 promotes the generation of follicular T helper (Tfh) cells and upregulates the expression of G-protein signaling (RGS)13 and RGS16 in GC B cells, which both lead to an enhanced germinal center response in lupus-prone BXD2 mice.11,44–46 Interestingly, IL-17 deficiency does not affect the frequencies of GC B cells in lupus-prone Fcγr2b−/− mice.12 Although extensive studies have suggested the importance of Tfh cells in promoting the humoral immune response, we recently identified a crucial function for IL-17 in promoting the differentiation of innate B-1a cells and their natural secretion of IgM.47,48 IL-17-mediated NF-κB activation and Prdm1 transcript induction in B-1a cells maintain natural antibody production in response to influenza infection.47 In this study, we identified a major plasma cell subset expressing high levels of the IL-17 receptor complex (IL-17RA and IL-17RC) in both SLE patients and lupus mice. Importantly, we showed that IL-17RC deficiency within B-cell compartments did not result in any obvious changes in GC B cells but significantly reduced plasma cell generation in mixed BM-reconstituted chimeric mice, indicating an essential role for intrinsic IL-17 signaling in maintaining the plasma cell response during lupus development. Recent studies have shown that enhanced Th17 responses are involved in multiple autoimmune disorders,10–13 which supports the conclusion that Th17 cells may exert multiple effects on B-cell subsets in autoimmune pathogenesis.
Consistent with early findings, we observed significantly decreased frequencies and numbers of GC B cells and plasma cells in IL-17 KO and IL-17RC KO mice on day 10 post immunization for lupus induction during the disease onset stage (data not shown).46 Since the long-term autoantibody response is critically involved in target organ damage during lupus development, our current findings reveal the novel function of IL-17 signaling involved in modulating the prolonged response of autoreactive plasma cells during the chronic stage of lupus development when the GC response is possibly contracted.46
IL-17 binds to the heterodimeric receptor complex composed of the IL-17RA and IL-17RC subunits to exert its cellular function.49,50 Although recent studies have suggested that ligation of IL-17RC by the IL-17F homodimeric complex may drive signal cascades independent of IL-17RA,51 the coexpression of IL-17RA and IL-17RC is essential for optimal cascade activation in the IL-17 signaling pathway.52,53 Moreover, different expression patterns of IL-17RA and IL-17RC have been observed in various types of cells.51,54,55 Here, we detected coexpression of the IL-17RA and IL-17RC subunits in a subset of long-lived plasma cells. Notably, IL-17RA/RC-expressing plasma cells were markedly expanded with potent production of autoantibodies in both SLE patients and lupus mice, indicating a previously unrecognized function of IL-17 in regulating the long-lived plasma cell response in SLE. In addition, we showed that deficiency in IL-17RC largely dampened IL-17-promoted long-lived plasma cell expansion in lupus mice, which further highlights the importance of intrinsic IL-17 signaling in regulating plasma cell function. Since IL-17-F also binds to the IL-17RA/RC complex, a potential role for IL-17F could not be excluded in the current study. Interestingly, it has been reported that compared with those from healthy donors, activated T cells from SLE patients secrete increased amounts of IL-17A but fail to produce IL-17F, indicating a dysregulated balance between IL-17A and IL-17F in SLE.56 Moreover, recent evidence has indicated that IL-17 receptor D (IL-17RD) may directly bind to IL-17 and constitute an alternative signaling pathway for IL-17 by forming a heterodimeric receptor complex with IL-17RA, as revealed by findings indicating that deficiency in IL-17RD abrogates psoriasis-like skin inflammation development.57 Thus, the potential role of IL-17RD in modulating the long-lived plasma cell response during lupus development remains to be established. Although our current findings suggest a pivotal role for IL-17R-expressing plasma cells in the development of lupus nephritis, it is possible that SLE with nervous system involvement or vessel disease may manifest different immunological profiles with diverse effector mechanisms.
In regard to the molecular mechanism involving IL-17 in modulating the autoreactive plasma cell response in SLE, we found that IL-17 promotes the survival of lupus plasma cells via p38 activation-mediated Bcl-xL mRNA stabilization in culture. Although previous studies have indicated pivotal roles for the PI3K/NF-κB/p65 pathway and JAK/STAT pathway in target cells upon IL-17 stimulation,58,59 our findings that IL-17 signaling-mediated p38 activation enhances Bcl-xL mRNA stabilization and plasma cell survival provide new insights into the crucial function of IL-17 in driving plasma cell expansion with prolonged autoantibody production during lupus development, which further supports the conclusion that IL-17 has diverse functions in autoimmune inflammation.29 Further studies on the roles of tissue stromal cells and their derived proinflammatory factors within target organs in promoting plasma cell differentiation and antibody production may contribute to the development of effective therapies for SLE.60
In conclusion, our study demonstrates for the first time that autoreactive plasma cells are the major target cell population of Th17 cell-derived IL-17 during lupus pathogenesis. Mechanistically, IL-17 promotes plasma cell survival via activation of p38-mediated Bcl-xL mRNA stabilization. Thus, further validation of these findings may facilitate the development of novel strategies to target IL-17 and plasma cells for effective treatment of SLE.
Supplementary information
Acknowledgements
This study was funded by grants from the National Natural Science Foundation of China (Nos. 81771761, 91842304, and 81901635), Chongqing International Institute for Immunology (2020YJC10), and Sanming Project of Medicine in Shenzhen (SZSM201512019). We thank Mr. Otis Ko for the technical support and service of the Medical Faculty Core Facility and Laboratory Animal Unit at The University of Hong Kong. We are grateful to Dr. Yoichiro Iwakura (University of Tokyo) for providing Il17a−/− mice.
Author contributions
K.M.: experimental design and paper writing; W.D., F.X., E. H., Y.T., C.D., L.L. M.H., Y.C., and S.Y.: mouse experiments and paper preparation; N.P., J.L., D.H., Q.H., X.H., X.C., Q.J., and D.L.: clinical data acquisition and analysis; and L.L.: experimental design, paper preparation, and funding acquisition.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-020-00540-4) contains supplementary material.
References
- 1.Tsokos GC. Systemic lupus erythematosus. N. Engl. J. Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Malkiel S, Barlev AN, Atisha-Fregoso Y, Suurmond J, Diamond B. Plasma cell differentiation pathways in systemic lupus erythematosus. Front. Immunol. 2018;9:427. doi: 10.3389/fimmu.2018.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiepe F, et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat. Rev. Rheumatol. 2011;7:170–178. doi: 10.1038/nrrheum.2011.1. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Q, et al. Autoantibodies from long-lived ‘memory’ plasma cells of NZB/W mice drive immune complex nephritis. Ann. Rheum. Dis. 2013;72:2011–2017. doi: 10.1136/annrheumdis-2013-203455. [DOI] [PubMed] [Google Scholar]
- 5.Mahevas M, Michel M, Weill JC, Reynaud CA. Long-lived plasma cells in autoimmunity: lessons from B-cell depleting therapy. Front. Immunol. 2013;4:494. doi: 10.3389/fimmu.2013.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma K, et al. The expanding functional diversity of plasma cells in immunity and inflammation. Cell Mol. Immunol. 2020;17:421–422. doi: 10.1038/s41423-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma K, et al. TLR4(+)CXCR4(+) plasma cells drive nephritis development in systemic lupus erythematosus. Ann. Rheum. Dis. 2018;77:1498–1506. doi: 10.1136/annrheumdis-2018-213615. [DOI] [PubMed] [Google Scholar]
- 8.Pioli PD, Casero D, Montecino-Rodriguez E, Morrison SL, Dorshkind K. Plasma cells are obligate effectors of enhanced myelopoiesis in aging bone marrow. Immunity. 2019;51:351–366 e356. doi: 10.1016/j.immuni.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi W, et al. Transcriptional profiling of mouse B cell terminal differentiation defines a signature for antibody-secreting plasma cells. Nat. Immunol. 2015;16:663–673. doi: 10.1038/ni.3154. [DOI] [PubMed] [Google Scholar]
- 10.Beringer A, Miossec P. Systemic effects of IL-17 in inflammatory arthritis. Nat. Rev. Rheumatol. 2019;15:491–501. doi: 10.1038/s41584-019-0243-5. [DOI] [PubMed] [Google Scholar]
- 11.Hsu HC, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 12.Pisitkun P, et al. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity. 2012;37:1104–1115. doi: 10.1016/j.immuni.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah K, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res. Ther. 2010;12:R53. doi: 10.1186/ar2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salem D, Subang R, Kuwana M, Levine JS, Rauch J. T cells from induced and spontaneous models of SLE recognize a common T cell epitope on beta2-glycoprotein I. Cell Mol. Immunol. 2019;16:685–693. doi: 10.1038/s41423-018-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 16.Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc. Natl Acad. Sci. USA. 2008;105:14993–14998. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin X, et al. Th17 cells play a critical role in the development of experimental Sjogren’s syndrome. Ann. Rheum. Dis. 2015;74:1302–1310. doi: 10.1136/annrheumdis-2013-204584. [DOI] [PubMed] [Google Scholar]
- 18.Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann. Rheum. Dis. 2013;72:ii116–ii123. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- 19.Amarilyo G, Lourenco EV, Shi FD, La Cava A. IL-17 promotes murine lupus. J. Immunol. 2014;193:540–543. doi: 10.4049/jimmunol.1400931. [DOI] [PubMed] [Google Scholar]
- 20.Turner JE, et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J. Am. Soc. Nephrol. 2010;21:974–985. doi: 10.1681/ASN.2009070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YU, Lim H, Jung HE, Wetsel RA, Chung Y. Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells. PLoS One. 2015;10:e0120294. doi: 10.1371/journal.pone.0120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petri M, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streeck H, et al. Rapid ex vivo isolation and long-term culture of human Th17 cells. J. Immunol. Methods. 2008;333:115–125. doi: 10.1016/j.jim.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 25.Steinmetz OM, et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J. Immunol. 2009;183:4693–4704. doi: 10.4049/jimmunol.0802626. [DOI] [PubMed] [Google Scholar]
- 26.Pawar RD, et al. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J. Am. Soc. Nephrol. 2007;18:1721–1731. doi: 10.1681/ASN.2006101162. [DOI] [PubMed] [Google Scholar]
- 27.Austin HA, 3rd, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–695. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 28.Yang M, et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am. J. Pathol. 2012;180:2375–2385. doi: 10.1016/j.ajpath.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Hartupee J, Liu CN, Novotny M, Li XX, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 30.Starke C, et al. High frequency of autoantibody-secreting cells and long-lived plasma cells within inflamed kidneys of NZB/W F1 lupus mice. Eur. J. Immunol. 2011;41:2107–2112. doi: 10.1002/eji.201041315. [DOI] [PubMed] [Google Scholar]
- 31.DiLillo DJ, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J. Immunol. 2008;180:361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 32.Tang H, et al. TLR4 activation is required for IL-17-induced multiple tissue inflammation and wasting in mice. J. Immunol. 2010;185:2563–2569. doi: 10.4049/jimmunol.0903664. [DOI] [PubMed] [Google Scholar]
- 33.Reimold AM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 34.Hu CC, Dougan SK, McGehee AM, Love JC, Ploegh HL. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J. 2009;28:1624–1636. doi: 10.1038/emboj.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pengo N, et al. Plasma cells require autophagy for sustainable immunoglobulin production. Nat. Immunol. 2013;14:298–305. doi: 10.1038/ni.2524. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro-Shelef M, et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Bhattacharya D. Adjuvant-specific regulation of long-term antibody responses by ZBTB20. J. Exp. Med. 2014;211:841–856. doi: 10.1084/jem.20131821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, et al. Arginase-1-dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci. Transl. Med. 2016;8:331ra340. doi: 10.1126/scitranslmed.aae0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J. Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 41.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 42.Schlegel PM, Steiert I, Kotter I, Muller CA. B cells contribute to heterogeneity of IL-17 producing cells in rheumatoid arthritis and healthy controls. PLoS ONE. 2013;8:e82580. doi: 10.1371/journal.pone.0082580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirota K, et al. Autoimmune Th17 cells induced synovial stromal and innate lymphoid cell secretion of the cytokine GM-CSF to initiate and augment autoimmune arthritis. Immunity. 2018;48:1220–1232 e1225. doi: 10.1016/j.immuni.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie S, et al. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J. Immunol. 2010;184:2289–2296. doi: 10.4049/jimmunol.0903133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SY, et al. Inhibition of IL-17 ameliorates systemic lupus erythematosus in Roquin(san/san) mice through regulating the balance of TFH cells, GC B cells, Treg and Breg. Sci. Rep. 2019;9:5227. doi: 10.1038/s41598-019-41534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitsdoerffer M, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl Acad. Sci. USA. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, et al. IL-17A promotes pulmonary B-1a cell differentiation via induction of Blimp-1 expression during influenza virus infection. PLoS Pathog. 2016;12:e1005367. doi: 10.1371/journal.ppat.1005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin X, et al. IL-10-producing regulatory B cells restrain the T follicular helper cell response in primary Sjogren’s syndrome. Cell Mol. Immunol. 2019;16:921–931. doi: 10.1038/s41423-019-0227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ. Res. 2012;110:675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor PR, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat. Immunol. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goepfert A, Lehmann S, Blank J, Kolbinger F, Rondeau JM. Structural analysis reveals that the cytokine IL-17F forms a homodimeric complex with receptor IL-17RC to drive IL-17RA-independent signaling. Immunity. 2020;52:499–512 e495. doi: 10.1016/j.immuni.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Wright JF, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J. Immunol. 2008;181:2799–2805. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 53.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 55.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hedrich CM, Rauen T, Kis-Toth K, Kyttaris VC, Tsokos GC. cAMP-responsive element modulator alpha (CREMalpha) suppresses IL-17F protein expression in T lymphocytes from patients with systemic lupus erythematosus (SLE) J. Biol. Chem. 2012;287:4715–4725. doi: 10.1074/jbc.M111.323261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su Y, et al. Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci. Immunol. 2019;4:eaau9657. doi: 10.1126/sciimmunol.aau9657. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H, et al. IL-17 induces expression of vascular cell adhesion molecule through signalling pathway of NF-kappaB, but not Akt1 and TAK1 in vascular smooth muscle cells. Scand. J. Immunol. 2013;77:230–237. doi: 10.1111/sji.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Subramaniam SV, Cooper RS, Adunyah SE. Evidence for the involvement of JAK/STAT pathway in the signaling mechanism of interleukin-17. Biochem. Biophys. Res. Commun. 1999;262:14–19. doi: 10.1006/bbrc.1999.1156. [DOI] [PubMed] [Google Scholar]
- 60.Robert M, Miossec P. Interleukin-17 and lupus: enough to be a target? For which patients? Lupus. 2020;29:6–14. doi: 10.1177/0961203319891243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.