Abstract
Idiopathic megacolon (IMC) and idiopathic megarectum (IMR) describe an abnormality of the colon or rectum, characterised by a permanent dilatation of the bowel diameter in the absence of an identifiable cause. We present a 23-year-old woman with chronic constipation and excessive straining during defecation who presented at the emergency department in partial gut obstruction with a palpable fecaloma. Manual faecal disimpaction and a sigmoid loop colostomy was initially done. A full thickness rectal biopsy was positive for ganglion cells. Further workup led to the diagnosis of chronic IMC and IMR. The patient underwent laparoscopic modified Duhamel procedure, with an uneventful postoperative course.
Keywords: gastrointestinal surgery, general surgery
Background
Adults who present with chronic constipation and bowel dilatation in the absence of a mechanical obstruction pose a diagnostic and therapeutic dilemma. Idiopathic megacolon (IMC) and idiopathic megarectum (IMR) are terms used to define a subgroup of patients with intractable constipation and persistent dilatation of the bowel in the absence of an organic cause.1 2 Although the exact incidence of IMC/IMR is unknown, it is regarded to be a rare condition. An electronic search we performed of the MEDLINE (PubMed Central) database using the search terms “idiopathic megacolon” and “idiopathic megarectum” yielded 26 case reports in adults in a 39-year period (submitted from 1979 to 2018). It affects males and females equally,1 and is characterised by recurrent faecal impaction that may begin during childhood or adult life.2 The dilatation of the affected segment is often grossly evident on examination, or plain radiography of the abdomen, or during laparotomy. It is characterised by intractable chronic constipation that responds poorly to pharmacological and non-surgical interventions hence, posing a significant disease burden, if left untreated. Due to the obscure nature of the disease, there is no recognised consensus on the management of IMC and IMR.
Case presentation
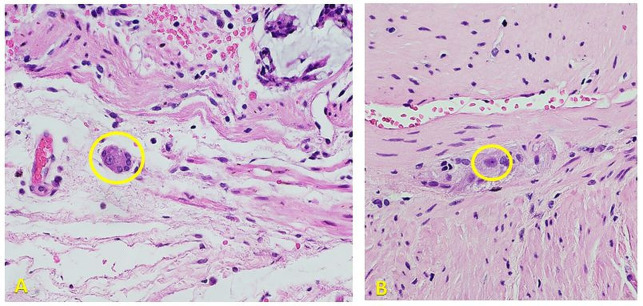
A 23-year-old woman reporting of a 1-year history of chronic constipation, with excessive straining during defecation and a feeling of incomplete evacuation, was referred to us. She reported an average of two to three bowel movements per week. Her symptoms were partially relieved with the use of laxatives and enemas. Birth and maternal history were unremarkable, as the patient reported regular bowel movements prior to onset of symptoms. The patient presented at the emergency department in partial gut obstruction, with a palpable left hemiabdominal mass. The palpable lesion was later assessed to be a fecaloma. The primary working impression was adult Hirschsprung’s disease (HD). A manual disimpaction transanally was attempted. When this failed, the abdomen was entered to access the sigmoid and break the fecaloma. A colotomy to further assist breaking the fecaloma, and successfully evacuate it was eventually done; and a sigmoid loop colostomy was brought out. A full thickness rectal biopsy was then done. Because the rectal biopsy was positive for ganglion cells (figure 1) further diagnostic workup was done, focusing on allied disorders of HD.3
Figure 1.
H&E stain, high power objective (×400). Photomicrographs show representative sections of the submitted rectal biopsy specimens showing the presence of ganglion cells in the submucosal plexus (A) and myenteric plexus (B). Philippine General Hospital, 2019.
Investigations
Contrast enema
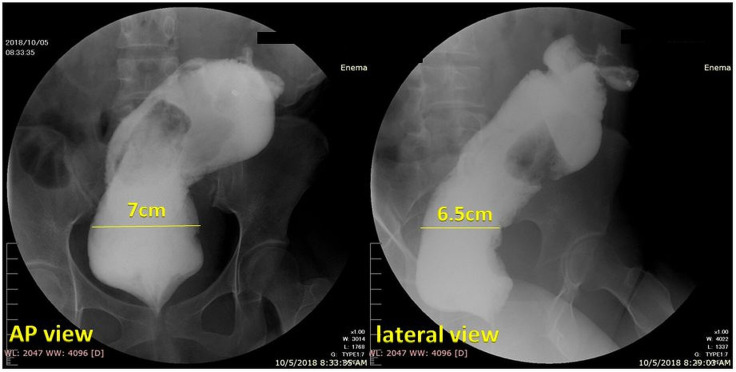
A water-soluble contrast enema showed a markedly dilated sigmoid colon and rectum (figure 2). The rectosigmoid index was 0.4 (N>1) and the rectal diameter at the pelvic brim was 7 cm in the AP view and 6.5 cm in the lateral view. There were persistent filling defects seen within the markedly dilated colonic segment representing retained faecal material. Although there is no consensus on the definition, most researchers agree that marked bowel dilatation is defined as cecal dilatation greater than 12 cm, and a dimension greater than 6.5 cm for the rectum and sigmoid colon when measured at the pelvic brim.4 Hence, we establish that the patient has both a megacolon and megarectum.
Figure 2.
Water-soluble contrast enema (anteroposterior (AP) view and lateral view). Opacified portions of the rectum and sigmoid colon were markedly dilated. The rectosigmoid index was 0.4 (N>1) and the rectal diameter at the pelvic brim was 7 cm in the AP view and 6.5 cm in the lateral view. There were persistent filling defects seen within the markedly dilated colonic segment representing retained faecal material. Philippine General Hospital, 2019.
Anal manometry
Anorectal manometry was performed with a closed water-filled 3 mm microballoon (table 1).
Table 1.
Anal manometry findings in a 23-year-old woman with chronic constipation
| Parameter | Results | Reference values |
| Resting pressure | 37 mm Hg | (N: 59–74) |
| Squeeze (pressure increase) | 67 mm Hg | (N: 65–78) |
| Rectal sensation thresholds | Elevated | |
| Rectoanal inhibitory reflex | (+) 34% relaxation | Positive if with at least 30% relaxation |
Philippine General Hospital, 2019.
Anal manometry is useful specifically for cases of IMR. The findings in the case presented (table 1) are compatible with findings summarised by Gattuso and Kamm5 among patients with IMR: (1) the presence of a rectoanal inhibitory reflex (RAIR), indicating an intact intramural enteric innervation (in contrast to patients with HD in whom RAIR is absent) and (2) low resting anal canal pressure which is an indicator predominantly of impaired internal anal sphincter function.6 7 Several mechanisms are postulated to cause this, including damage caused by manual disimpaction8 or an intrinsic abnormality of the sphincter itself (possibly shared with a smooth muscle abnormality of the rectum).5 The external anal sphincter is functionally intact, as reflected in the normal voluntary contraction or squeeze pressure. Several studies have also made the diagnosis of IMR on the basis of an elevated maximum tolerable volume (MTV) to latex balloon distension during anorectal physiological investigation9 10 with the assumption that this reflects rectal capacity. However, axial expansion of rectal balloons into the sigmoid colon may overestimate rectal volume thresholds.11 Hence the utility of measuring MTV is better suited as a screening tool for patients with constipation, since normal sensory threshold volumes would exclude the presence of megarectum.12
Differential diagnosis
In clinching the diagnosis, we looked to disease entities that presented with the same chief complaint of chronic constipation and markedly dilated colon and rectum. The term ‘allied disorders of HD’, as defined by Muto et al3 in the Japanese clinical practice guidelines for allied disorders of Hirschsprung’s disease (2018), refers to a disease group that is characterised by signs and symptoms similar to those of Hirschsprung’s disease, such as bowel obstruction, intestinal dilatation and chronic constipation, despite the presence of ganglionic cells in the rectum. These disorders are further classified into two: (1) diseases with intestinal ganglion cell abnormality (ie, intestinal neuronal dysplasia, intestinal hypoganglionosis and immaturity of ganglia) and (2) diseases without ganglion cell abnormality (ie, chronic idiopathic intestinal pseudo-obstruction, megacystis microcolon hypoperistalsis syndrome, internal anal sphincter achalasia). These disorders were then ruled out mainly from the histopathological review showing no quantitative and morphological abnormality in the ganglion cells (figure 3), and by ruling out all other secondary aetiologies. Hence, as a diagnosis of exclusion, we committed to managing the patient as a case of IMC and IMR.
Figure 3.
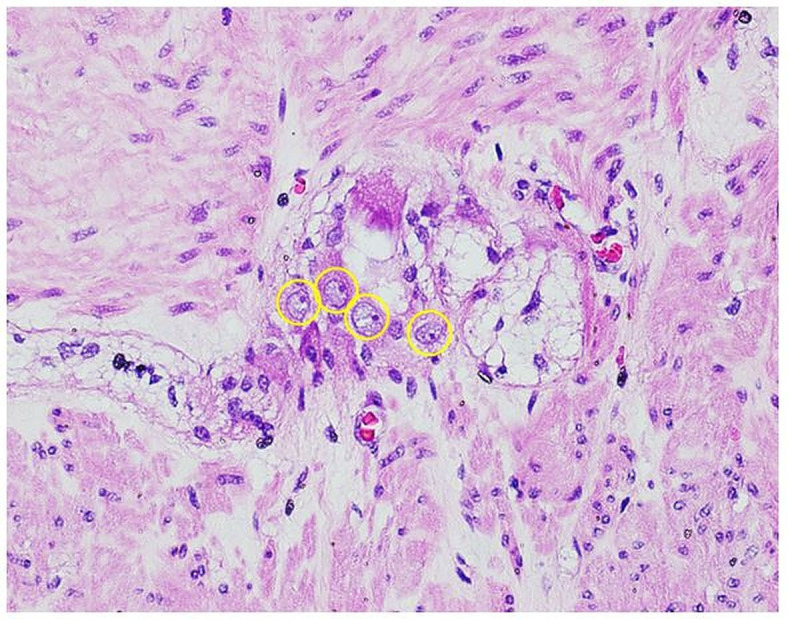
H&E stain, high power objective (×400). Photomicrograph shows a representative ganglion in the submitted resected bowel segment. Cells with a large vesicular nucleus, prominent nucleolus, and basophilic cytoplasm, which are characteristic of morphologically mature ganglion cells, are seen present and regularly distributed throughout the sampled length of the submitted bowel segment. Philippine General Hospital, 2019.
Treatment
Patients with IMC and IMR are initially managed conservatively, usually with the use of oral laxatives or enemas. However, medical treatment may fail to relieve symptoms in up to 70% of patients.13 14 The role of behavioural retraining and biofeedback have been explored,15 but as of this writing no long-term studies have been conducted evaluating the efficacy of these in patients with IMC/IMR. When conservative therapy is ineffective, surgical management may become necessary. Development of complications (eg, faecal impaction, recurrent sigmoid volvulus) is another indication to intervene surgically. Because the exact pathogenesis is yet to be elucidated, there is a variety of surgical options available and choosing the appropriate procedure may not be very straightforward. Surgical options that have been employed include: subtotal colectomy, rectal resection procedures including proctectomy, Duhamel procedure and pull through procedures and pelvic floor procedures. For the case presented here, the bowel dilatation was mainly at the upper rectum and distal sigmoid, hence a resection of the distal sigmoid and rectum was deemed sufficient. This was done through a laparoscopic modified Duhamel procedure.
The original operation described by Duhamel16 involved exclusion of the rectum in the resection and performing an end to side anastomosis between the colon and native rectum at the skin level. We adapted the modification described by Martin and Altemeier,17 performing a side-to-side anastomosis between the native rectum and the colon and performing the end to side anastomosis above the level of the internal anal sphincter. Advantages of this modified Duhamel procedure include (1) maintaining a faecal reservoir as native rectum is retained, (2) avoiding incontinence from operative injury as anastomosis lies above the sphincter complexes and (3) preserving urinary and sexual function as hypogastric nerves are not involved in dissection.
Operative technique
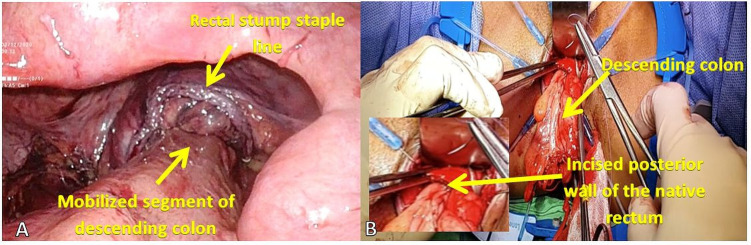
The procedure was performed with the patient in dorsal lithotomy position under general anaesthesia. A 10 mm optical port was placed by open technique through a supraumbilical incision. Three additional 5 mm ports were placed under laparoscopic guidance at the right and left flank and left lower quadrant, and another 10 mm port was inserted at the right lower quadrant to accommodate the endoscopic stapler. Takedown of the previous stoma (sigmoid loop colostomy) was done and the previous stoma site was temporarily closed to be able to maintain pneumoperitoneum. A medial-to-lateral mobilisation of the descending colon and sigmoid was done with high ligation of the inferior mesenteric artery and vein, and full splenic flexure mobilisation. The retrorectal space was dissected until the pelvic floor was visualised. Under laparoscopic guidance, the previous sigmoid colostomy was taken down and the colon was divided proximally at the nondilated descending colon. Distally, the rectum was divided using an endoscopic stapler at the level of the peritoneal reflection and the resected bowel segment was delivered through the previous colostomy site. The perineal portion of the surgery was started with gentle anal dilatation and a curvilinear incision was made along the posterior rectal wall 1 cm above the dentate line (figure 4). The colon was pulled down through posterior rectal incision, and anastomosed to the upper and lower lips of posterior rectal wall incision. A side-to-side anastomosis between the native rectum and the descending colon was done using sequential firing of 75 mm linear cutter gastrointestinal stapler (NTLC75 Ethicon). A protecting loop ileostomy was then fashioned.
Figure 4.
(A) Laparoscopic view of the normal colon mobilised to create a retrorectal anastomosis. (B) Perineal view of the pulled through segment of colon for full thickness coloanal handsewn anastomosis. Philippine General Hospital, 2019.
Outcome and follow-up
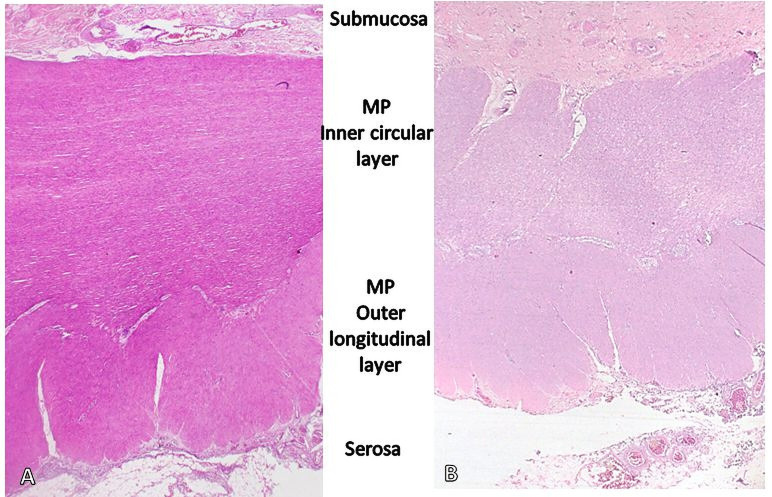
The patient was discharged after the fifth postoperative day with no complications. Final histopathological review of the resected sigmoid and rectum showed muscular hypertrophy (figure 5) and no identifiable quantitative or morphological abnormality in the ganglion cells (figure 3). The patient was followed up at 30 and 60 days after surgery with noted intact anastomosis and good sphincter tone.
Figure 5.
H&E stain, lower magnification (×20). Photomicrograph shows muscular hypertrophy in the proximal margin of the resected bowel segment. The muscularis propria (MP) of the proximal resection margin (A) and distal resection margin (B) are seen, side by side, using the same magnification. More muscle bulk is seen in the proximal segment which corresponds to the grossly dilated sigmoid and upper rectum compared with the distal segment of the resected bowel which corresponds to the transition to the grossly non-dilated part of the rectum. Philippine General Hospital, 2019.
Discussion
IMC and IMR are terms used to define a subgroup of patients with intractable constipation and persistent dilatation of the bowel in the absence of an organic cause.1 2 Although the exact incidence of IMC/IMR is unknown, it is a rare condition. An electronic search of the MEDLINE (PubMed Central) database using the search terms “idiopathic megacolon” and “idiopathic megarectum” yielded 26 case reports in adults in a 39-year period (submitted from 1979 to 2018). It affects males and females equally,1 and is characterised by recurrent faecal impaction that may begin during childhood or adult life.2 The dilatation of the affected segment is often grossly evident on examination, or plain radiography of the abdomen, or during laparotomy.
In a histopathological review of 63 IMC resections between 1997 and June 2004 by Meier-Ruge et al,9 IMC/IMR was characterised by a total atrophy of the collagenous tendinous connective tissue membrane of the myenteric plexus and the tendinous collagen fibre net of the muscularis propria. The myenteric plexus was noted to be normal, supporting the hypothesis that a primary metabolic defect of muscularis propria may be the underlying cause of IMC/IMR.18
Because the pathogenesis is unclear, a variety of surgical treatments have been employed. In a systematic review on the surgical options for IMC and IMR by Gladman et al,2 subtotal colectomy was successful in 71.1% but was associated with significant morbidity related to bowel obstruction. Rectal resection procedures, including proctectomy, Duhamel procedure and pull-through procedures, achieved a successful outcome in 71%–87% of patients but were also associated with significant mortality (3%–25%) and morbidity (6%–29%), mainly from postoperative pelvic sepsis. Vertical reduction rectoplasty offered promising short-term success (83%).2 Pelvic-floor procedures such as internal sphincterotomy and puborectalis division were associated with poor outcomes,2 presumably due to the persistence of the bowel dilatation. Haddad19 and Stabile et al20 examined the outcome of modified Duhamel procedure for IMC and IMR in a total of 70 patients. A successful outcome was reported in 59 patients (84%) with restoration of normal bowel function. However, significant complications were noted including pelvic abscesses and anastomotic strictures each occurring in up to 15% of cases.20
Despite conflicting data among the surgical options, the cornerstone of management is resection of the pathological dilated bowel segment, yielding the most consistent alleviation of chronic constipation, and restoration of bowel continuity. This may be done with or without a protecting stoma. Here, the bowel dilatation was mainly at the upper rectum and distal sigmoid, hence a resection of the distal sigmoid and rectum was deemed sufficient.
Learning points.
Chronic constipation in adults is often a diagnostic and therapeutic dilemma hence a thorough history and physical examination with appropriate diagnostic modalities are important in clinching the diagnosis and applying the appropriate management.
Idiopathic megacolon and megarectum (IMC/IMR) are rare disease entities that cause significant disease burden should they remain unrecognised and untreated.
Here, we presented a case of IMC and IMR in an adult where relief of symptoms was achieved through faecal disimpaction and colonic diversion, followed by definitive surgery with an uneventful postoperative course.
Laparoscopic modified Duhamel procedure can be a surgical option for adult patients with IMC and IMR.
Acknowledgments
Deepest gratitude to Dr Vannah Lee for reviewing and annotating the photomicrographs and imparting her expertise on the case presented.
Footnotes
Twitter: @villanuevamaui
Contributors: MEPV: primary author and surgeon of the case, editor, followed up the patient. MPJL: consultant superviser of the case, editor. MASO: surgical assist for the case, editor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Autschbach F, Gassler N. Idiopathic megacolon. Eur J Gastroenterol Hepatol 2007;19:399–400. 10.1097/MEG.0b013e3280116cb8 [DOI] [PubMed] [Google Scholar]
- 2.Gladman MA, Scott SM, Lunniss PJ, et al. Systematic review of surgical options for idiopathic megarectum and megacolon. Ann Surg 2005;241:562–74. 10.1097/01.sla.0000157140.69695.d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muto M, Matsufuji H, Taguchi T, et al. Japanese clinical practice guidelines for allied disorders of Hirschsprung's disease, 2017. Pediatr Int 2018;60:400–10. 10.1111/ped.13559 [DOI] [PubMed] [Google Scholar]
- 4.Infante JM, Alonso MH, Gallardo BP. Megarrecto Y megacolon idiopático. Revista Española de Enfermedades Digestivas 2009;101. [Google Scholar]
- 5.Gattuso JM, Kamm MA. Clinical features of idiopathic megarectum and idiopathic megacolon. Gut 1997;41:93–9. 10.1136/gut.41.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frenckner B, Euler CV. Influence of pudendal block on the function of the anal sphincters. Gut 1975;16:482–9. 10.1136/gut.16.6.482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read NW, Sun WM. Anorectal manometry. In: Henry MM, Swash M, eds. The pelvic floor. 2 edn, 1992: 119–45. [Google Scholar]
- 8.Gattuso JM, Kamm MA, Halligan SM, et al. The anal sphincter in idiopathic megarectum. Diseases of the Colon Rectum 1996;39:435–9. 10.1007/BF02054060 [DOI] [PubMed] [Google Scholar]
- 9.Meier-Ruge WA, Müller-Lobeck H, Stoss F, et al. The pathogenesis of idiopathic megacolon. Eur J Gastroenterol Hepatol 2006;18:1209–15. 10.1097/01.meg.0000236883.13720.c2 [DOI] [PubMed] [Google Scholar]
- 10.Lane RH, Todd IP. Idiopathic megacolon: a review of 42 cases. Br J Surg 1977;64:305–10. 10.1002/bjs.1800640502 [DOI] [PubMed] [Google Scholar]
- 11.O Súilleabháin CB, Anderson JH, McKee RF, et al. Strategy for the surgical management of patients with idiopathic megarectum and megacolon. Br J Surg 2001;88:1392–6. 10.1046/j.0007-1323.2001.01871.x [DOI] [PubMed] [Google Scholar]
- 12.Mimura T, Nicholls T, Storrie JB, et al. Treatment of constipation in adults associated with idiopathic megarectum by behavioural retraining including biofeedback. Colorectal Dis 2002;4:477–82. 10.1046/j.1463-1318.2002.00372.x [DOI] [PubMed] [Google Scholar]
- 13.Verduron A, Devroede G, Bouchoucha M, et al. Megarectum. Dig Dis Sci 1988;33:1164–74. 10.1007/BF01535795 [DOI] [PubMed] [Google Scholar]
- 14.Siproudhis L, Le Gall R, Ropert A, et al. [Does manometric megarectum have a symptomatic role in patients complaining of dyschezia?]. Gastroenterol Clin Biol 1993;17:162–7. [PubMed] [Google Scholar]
- 15.Madoff RD, Orrom WJ, Rothenberger DA, et al. Rectal compliance: a critical reappraisal. Int J Colorectal Dis 1990;5:37–40. 10.1007/BF00496148 [DOI] [PubMed] [Google Scholar]
- 16.Duhamel B. A new operation for the treatment of Hirschsprung's disease. Arch Dis Child 1960;35:38–9. 10.1136/adc.35.179.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin LW, Altemeier WA. Clinical experience with a new operation (modified Duhamel procedure) for Hirschsprung's disease. Ann Surg 1962;156:678–81. 10.1097/00000658-196210000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladman MA, Knowles CH. Novel concepts in the diagnosis, pathophysiology and management of idiopathic megabowel. Colorectal Dis 2008;10:531–8. 10.1111/j.1463-1318.2007.01457.x [DOI] [PubMed] [Google Scholar]
- 19.Haddad J. Treatment of acquired megacolon by retrorectal lowering of the colon with a perineal colostomy: modified Duhamel operation. Dis Colon Rectum 1969;12:421–9. 10.1007/BF02617726 [DOI] [PubMed] [Google Scholar]
- 20.Stabile G, Kamm MA, Hawley PR, et al. Results of stoma formation for idiopathic megarectum and megacolon. Int J Colorectal Dis 1992;7:82–4. 10.1007/BF00341291 [DOI] [PubMed] [Google Scholar]