Abstract
Objective
The survival benefit of using mechanical circulatory support (MCS) in patients with acute myocardial infarction (AMI) is still controversial. It is necessary to explore the impact on clinical outcomes of MCS in patients with AMI undergoing stenting.
Design
Systematic review and meta-analysis.
Data sources
Embase, Cochrane Library, Medline, PubMed, Web of Science, ClinicalTrials.gov and Clinicaltrialsregister.eu databases were searched from database inception to February 2021.
Eligibility criteria
Randomised clinical trials (RCTs) on MCS use in patients with AMI undergoing stent implantation were included.
Data extraction and synthesis
Data were extracted and summarised independently by two reviewers. Risk ratios (RRs) and 95% CIs were calculated for clinical outcomes according to random-effects model.
Results
Twelve studies of 1497 patients with AMI were included, nine studies including 1382 patients compared MCS with non-MCS, and three studies including 115 patients compared percutaneous ventricular assist devices (pVADs) versus intra-aortic balloon pump (IABP). Compared with non-MCS, MCS was not associated with short-term (within 30 days) (RR=0.90; 95% CI 0.57 to 1.41; I2=46.8%) and long-term (at least 6 months) (RR=0.82; 95% CI 0.57 to 1.17; I2=37.6%) mortality reductions. In the subset of patients without cardiogenic shock (CS) compared with non-MCS, the patients with IABP treatment significantly had decreased long-term mortality (RR=0.49; 95% CI 0.27 to 0.90; I2=0), but without the short-term mortality reductions (RR=0.51; 95% CI 0.22 to 1.19; I2=17.9%). While in the patients with CS, the patients with MCS did not benefit from the short-term (RR=1.09; 95% CI 0.67 to 1.79; I2=46.6%) or long-term (RR=1.00; 95% CI 0.75 to 1.33; I2=22.1%) survival. Moreover, the application of pVADs increased risk of bleeding (RR=1.86; 95% CI 1.15 to 3.00; I2=15.3%) compared with IABP treatment (RR=1.86; 95% CI 1.15 to 3.00; I2=15.3%).
Conclusions
In all patients with AMI undergoing stent implantation, the MCS use does not reduce all-cause mortality. Patients without CS can benefit from MCS regarding long-term survival, while patients with CS seem not.
Keywords: acute myocardial infarction (AMI), percutaneous coronary intervention (PCI), stent implantation, mechanical circulatory support (MCS), intra-aortic balloon pump (IABP), percutaneous ventricular assist devices (pVADs)
Strengths and limitations of this study.
This meta-analysis is focusing on the effect of mechanical circulatory support (MCS) in patients with acute myocardial infarction (AMI) undergoing stenting.
This meta-analysis is the first to identity that MCS benefits patients with AMI without cardiogenic shock undergoing stenting intervention.
In limited number of randomised clinical trials comparing the effect of percutaneous ventricular assist devices (pVADs) with intra-aortic balloon pump, and different types of pVADs, the results should be interpreted with caution.
Introduction
Acute myocardial infarction (AMI) is the most common cause of hospitalisation in elderly patients.1 2 Emerging mechanical circulatory support (MCS), including intra-aortic balloon pump (IABP), percutaneous ventricular assist devices (pVADs), and extracorporeal life support are important haemodynamic supportive strategies for maintaining haemodynamic stability and organ perfusion in the acute phase of myocardial infarction (MI). However, the survival benefit of using MCS in patients with AMI is still controversial.
Although previous guidelines from various committees have recommended the necessity of using MCS in patients with severe cardiac ischaemia,3 4 accumulating evidence from randomised clinical trials (RCTs) and meta-analysis has shown that neither MCS devices have significantly improved the survival in patients with AMI with cardiogenic shock (CS).5–9 Therefore, in patients with AMI with CS, IABP and pVADs were recommended to class IIa and class IIb in US guidelines, respectively, and IABP to class IIaC–IIIA in European guidelines.10 11 Recent meta-analyses have focused on the use of MCS in patients with CS, but no benefits for survival were found.12–14 Even if the conclusions from MCS studies were something of a disappointment, these data are still worth to be further explored in detail.
As there are rapid advances in percutaneous coronary intervention (PCI) technology, stenting is better for reperfusion after AMI than balloon angioplasty.15 However, it is noteworthy that the patients with both stenting and balloon angioplasty were included in the studies on MCS.16 17 As the revascularisation strategy may be so heterogeneous, choosing the strategy must take into account the evidence. Therefore, in this study, we conduct a systematic review and meta-analysis to highlight the effect of MCS in patients with AMI undergoing stent implantation and further exploration on the effect of MCS performed in patients with or without CS.
Methods
The current meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (for the PRISMA checklist, see online supplemental file 1).18
bmjopen-2020-044072supp001.pdf (48KB, pdf)
Search strategy
We conducted a systematic search of the literature in February 2021 without restrictions on region, publication type or language. Embase, Cochrane Library, Medline, PubMed and Web of Science databases were included. ClinicalTrials.gov and Clinicaltrialsregister.eu were searched as well. The Medical Subject Headings search terms included the following: myocardial infarction, acute coronary syndrome, heart-assist devices, intra-aortic balloon pump and mechanical circulatory support. We excluded non-human studies. Details of the search strategy were presented in online supplemental file 2.
bmjopen-2020-044072supp002.pdf (113.2KB, pdf)
Study selection
In the current meta-analysis, only RCTs in which stent implantation was the dominant form of revascularisation and MCS was used during the perioperative period in patients with AMI were included. We excluded non-randomised trials, cohort studies, cross-sectional studies, ongoing trials, trials terminated early, and other publication types, including review, case reports or case series, editorials, letters and meeting abstracts. Studies in which patients underwent coronary artery bypass grafting (CABG), balloon angioplasty or systemic thrombolysis as the main revascularisation method were also excluded.
Endpoints
The primary outcome was all-cause mortality, including short-term mortality and long-term mortality. Short-term mortality was defined as mortality occurring within 30 days. Long-term mortality occurred for at least 6 months of follow-up. The secondary endpoints included reinfarction, repeat revascularisation, stroke-TIA (transient ischaemic attack), bleeding (moderate to major bleeding, blood transfusion or surgery to control the bleeding), arrhythmias and vascular complications (peripheral ischaemic vascular complications, major dissection, pseudoaneurysm or arteriovenous fistula) occurring within 30 days. The endpoint definitions as applied in each study were presented in online supplemental file 3.
bmjopen-2020-044072supp003.pdf (74.9KB, pdf)
Data extraction and quality assessment
Data from the included studies were extracted and summarised independently by two reviewers (YS and YW). All extracted data from the included studies were independently collected in duplicate using a standardised data extraction form, which contained authors or trials’ name, year of publication, study design (interventions, sample size, percentage of stenting and bare-metal stent, PCI success, timing of MCS, time point of reported mortality, follow-up), baseline characteristics of study population (sex, age, AMI, CS, infarct-related artery, hypertension, diabetes mellitus, previous stroke/TIA and MI, prior PCI and CABG), the endpoint definitions, the centre and the time of the study enrolment. Any disagreements were resolved by a third reviewer (XS). The quality of the included studies was assessed using the Cochrane Collaboration tool,19 which included selection bias, performance bias, detection bias, attrition bias, reporting bias and other possible biases. Each source of bias was classified as low, unclear and high risk.
Statistical analysis
We applied risk ratios (RRs) and 95% CIs to summarise the statistics. Statistical heterogeneity was assessed using Cochran’s Q test and quantified using the I2 statistic; p<0.10. Heterogeneity was categorised by I2 as follows20: insignificant heterogeneity, I2 <25%; low heterogeneity, 25% ≤I2 <50%; moderate heterogeneity, 50% ≤I2 <75%; high heterogeneity, I2 ≥75%. Considering the clinical differences among the included studies, a random-effects model was applied for statistical analyses. Sensitivity analyses were performed for primary outcomes with the following: (1) inconsistent length of follow-up, (2) different types of MCS use, (3) exclusion of research with a high risk of bias and (4) the one-study-out method. Funnel plots, Begg and Mazumdar rank correlation, and Egger’s regression intercept were used to evaluate publication bias.20 If publication bias existed, the trim-and-fill method was performed for estimating the number of missing studies and RR adjustment.21 Statistical analyses were performed using STATA/SE V.12.0 (Stata Corp, College Station, Texas, USA). P<0.05 was considered statistically significant.
Results
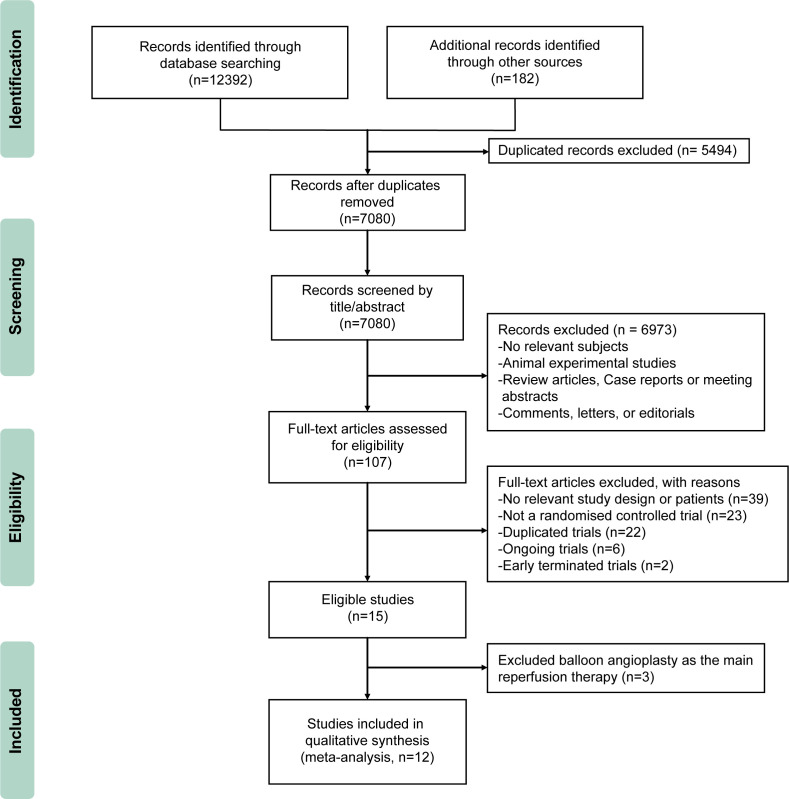
There are 12 574 citations identified through the search criteria. The full texts of 107 potentially eligible articles were scrutinised in detail. Among these, 15 RCTs used PCI as the main revascularisation therapy, 3 RCTs on balloon angioplasty use were excluded22–24 and 12 RCTs finally met the inclusion criteria.8 9 25–35 The study selection process is outlined in figure 1.
Figure 1.
Flow diagram of study selection. Randomised clinical trials were included.
Characteristics of the studies
The centre and the time of enrolment in each study had shown that no duplicate of the sample population of included studies was found (see online supplemental file 4). The detailed designs of the included studies are listed in table 1. Among the 12 RCTs, 1497 patients with AMI were included, 94.5% of patients (1391 of 1471) underwent stent implantation, 86.9% of patients (959 of 1104) had successful PCI. The follow-up period ranged from in-hospital only to 6 years. Nine studies including 1382 patients with AMI compared MCS with non-MCS (693 in the MCS group and 689 in the non-MCS group).8 9 25 28–31 33–35 Three studies including 115 patients with AMI compared pVADs versus IABP (58 in the pVADs group and 57 in the IABP group).26 27 32 Eight RCTs included patients with CS, among which five studies that included 806 patients compared MCS with non-MCS (414 in the MCS group and 392 in the non-MCS group),8 9 29 31 33 35 the remaining three studies compared pVADs versus IABP. Four RCTs included patients without CS, and the type of MCS used was IABP, which included 576 patients, compared MCS with non-MCS (279 in the IABP group and 297 in the non-IABP group).25 28 30 34 The summarised patients’ characteristics of included studies are shown in table 2. The mean age of the population was 63.8 years, and 73.3% were men, 77.1% (1046 of 1357) had left coronary artery-related infarction, 52.9% (726 of 1373) had hypertension, 27.7% (381 of 1373) had diabetes mellitus, 4.3% (49 of 1172) had a previous diagnosis of stroke/TIA, 14.3% (193 of 1347) had a previous diagnosis of MI, 17.6% (208 of 1185) underwent PCI previously and 5.0% (37 of 742) underwent CABG previously.
Table 1.
Study design of the included studies
| Study | Year | MCS group/non-MCS group | No of Patients | Stenting, % | BMS, % | PCI success, % | Timing of MCS | Time point of reported mortality | Follow-up |
| SEMPER FI25 | 2020 | IABP+stenting/stenting | 50/50 | 100 | NA | 90/96 | After stenting | 30 days, 6 months | 6 months |
| Zhou et al29 | 2017 | IABP+stenting/stenting | 42/42 | 100 | NA | NA | Before stenting | In-hospital, 12 months | 12 months |
| IABP-SHOCK II8 9 | 2012, 2013 | IABP+stenting/stenting | 301/299 | 89.8 | 50.2/51.5 | 82.2/81.5 | Before or after stenting | 30 days, 6 months | 6 years |
| CRISP AMI28 | 2011 | IABP+stenting/stenting | 161/176 | 96.9 | 53.7/53.1 | 92.9/95.3 | Before stenting | 30 days, 6 months | 6 months |
| Gu et al30 | 2011 | IABP+stenting/stenting | 51/55 | 100 | NA | NA | Before stenting | 30 days, 6 months | 6 months |
| Prondzinsky et al31 | 2010 | IABP+stenting/stenting | 19/21 | 85 | NA | NA | After stenting | In-hospital | In-hospital |
| Arias et al33 | 2005 | IABP+stenting/stenting | 31/9 | 100 | NA | NA | Before stenting | In-hospital | In-hospital |
| Vijayalakshmi et al34 | 2007 | IABP+stenting/stenting | 17/16 | 100 | NA | NA | After stenting | In-hospital, 30 days | 30 days |
| IMPRESS26 | 2017 | pVADs+stenting/IABP+stenting | 24/24 | 97.9 | 4/8 | NA | After stenting | 30 days, 6 months | 6 months |
| Seyfarth et al32 | 2008 | pVADs+stenting/IABP+stenting | 13/13 | NA | NA | 100/92 | After stenting | 30 days | 30 days |
| Thiele et al27 | 2005 | pVADs+stenting/IABP+stenting | 21/20 | 95.1 | NA | 80/74 | Before or after stenting | 30 days | 30 days |
| Lackermair et al35 | 2020 | ECLS+stenting/stenting | 21/21 | 100 | NA | NA | After stenting | 30 days, 12 months | 12 months |
BMS, bare-metal stent; ECLS, extracorporeal life support; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; NA, not available; PCI, percutaneous coronary intervention, including stenting and balloon angioplasty; pVADs, percutaneous ventricular assist devices.
Table 2.
Baseline clinical characteristics of the included studies
| Study | Male, % | Mean age, year | AMI, % | CS, % | Infarct-related artery (LM/LAD/LCX/RCA), % | Hypertension, % | Diabetes mellitus, % | Previous stroke/TIA, % | Prior MI, % | Prior PCI, % | Prior CABG, % |
| SEMPER FI25 | 74/78 | 64/63 | 100 | 0 | 1.0/50.0/11.0/33.0 | 24/32 | 8/18 | 2/2 |
6/10 | 8/12 | 2/8 |
| Zhou et al29 | 67/62 | 57.2/56.7 | 100 | 100 | 0/34.5/38.1/27.4 | NA | NA | NA | NA | NA | NA |
| IABP-SHOCK II8 9 | 70.6/67.1 | 70/69 | 100 | 100 | 9/42.2/18.7/25.3 | 72/66.6 | 35.4/30.1 | 8/6.7 | 23.7/20.4 | 21.1/17.4 | 6.7/4.0 |
| CRISP AMI28 | 82/81.8 | 56.1/57.7 | 100 | 0 | 0/97.3/0/0.3 | 24.2/34.1 | 16.8/20.5 | 0/0.6 | 0/0 | 1.9/1.1 | NA |
| Gu et al30 | 56.9/65.5 | 67.4/66.6 | 100 | 0 | 3.8/47.2/11.3/37.7 | 68.6/60 | 35.5/34.5 | NA | 3.9/5.5 | 68.6/70.9 | NA |
| Prondzinsky et al31 | 74/81 | 62.1/66.1 | 100 | 100 | NA | 42.1/47.6 | 52.6/47.6 | NA | 21.1/23.8 | NA | NA |
| Arias et al33 | NA | NA | 100 | 100 | 0/60.0/22.5/8.3 | NA | NA | NA | NA | NA | NA |
| Vijayalakshmi et al34 | 82/88 | 40/45 | 100 | 0 | NA | 25/38 | 18/25 | NA | 12/25 | NA | NA |
| IMPRESS26 | 75/83 | 58/59 | 100 | 100 | 6.3/64.6/18.8/10.4 | 20/29 | 9/13 | 0/4 | 5/4 | NA | NA |
| Seyfarth et al32 | 62/85 | 65/67 | 100 | 100 | NA | 54/69 | 39/23 | NA | NA | NA | NA |
| Thiele et al27 | 76/75 | 63/65 | 100 | 100 | NA | 76/75 | 52/55 | NA | 62/45 | NA | NA |
| Lackermair et al35 | 76.2/95.2 | 62/70 | 100 | 100 | 9.5/54.8/16.7/19.0 | 55/75 | 15/30 | 0/10 | 15/30 | 20/20 | 0/0 |
AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; CS, cardiogenic shock; LAD, left anterior descending; LCX, left circumflex; LM, left main; MI, myocardial infarction; NA, not available; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIA, transient ischaemic attack.
bmjopen-2020-044072supp004.pdf (63.6KB, pdf)
Outcomes of synthesis analyses
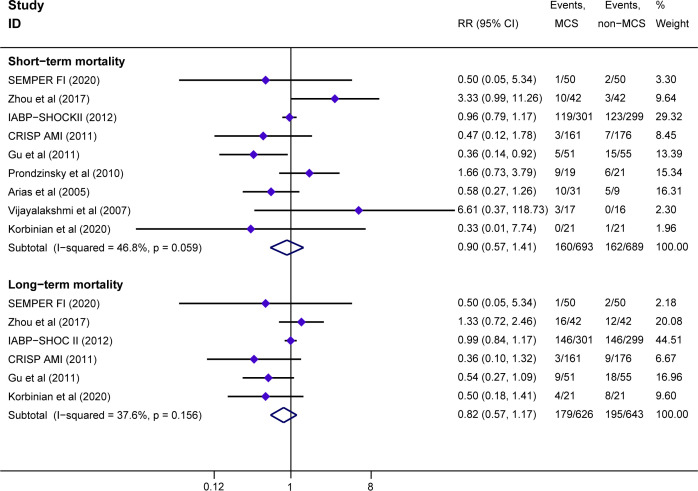
Of the included nine RCTs comparing the survival benefit of MCS with non-MCS, all studies reported short-term mortality, and six studies reported long-term mortality. MCS was not associated with a short-term mortality reduction compared with non-MCS (MCS=23.1%, 160 of 693; non-MCS=23.5%, 162 of 689; RR=0.90; 95% CI 0.57 to 1.41; p=0.640), with low heterogeneity among studies (I2=46.8%; p=0.059) (figure 2). Similarly, there was no significant difference between the MCS group and the non-MCS group in the long-term mortality (MCS=28.6%, 176 of 626; non-MCS=30.3%, 195 of 643; RR=0.82; 95% CI 0.57 to 1.17; p=0.276), with low heterogeneity among studies (I2=37.6%; p=0.156) (figure 2). The results of prespecified sensitivity analyses were not significantly different from those of the primary analysis (see online supplemental file 5).
Figure 2.
Forest plots for the all-cause mortality. Risk ratio (RR) of all-cause mortality of patients with AMI treated with MCS compared with non-MCS in short term or long term. Non-MCS, no plan of MCS use during perioperative period; short-term mortality, occurring within 30 days; long-term mortality, occurring at least 6 months of follow-up. AMI, acute myocardial infarction; MCS, mechanical circulatory support.
bmjopen-2020-044072supp005.pdf (46.6KB, pdf)
For the secondary endpoints, MCS had no effect on the incidence of reinfarction (MCS=2.6%, 14 of 530; non-MCS=1.6%, 9 of 546; RR=1.51; 95% CI 0.64 to 3.59; p=0.346; I2=0, p=0.498), repeat revascularisation (MCS=7.9%, 7 of 89; non-MCS=4.3%, 4 of 92; RR=1.60; 95% CI 0.49 to 5.24; p=0.435; I2=0, p=0.668), stroke-TIA (MCS=1.1%, 5 of 462; non-MCS=1.3%, 6 of 475; RR=1.51; 95% CI 0.64 to 3.59; p=0.988; I2=54.9%, p=0.137), bleeding (MCS=13.9%, 78 of 563; non-MCS=11.7%, 68 of 580; RR=1.58; 95% CI 0.76 to 3.26; p=0.218; I2=37.6%, p=0.186), arrhythmias (MCS=5.1%, 3 of 59; non-MCS=1.7%, 1 of 58; RR=2.21; 95% CI 0.34 to 14.24; p=0.404; I2=0, p=0.815), and vascular complications (MCS=4.9%, 25 of 511; non-MCS=3.0%, 16 of 530; RR=1.60; 95% CI 0.86 to 2.96; p=0.134; I2=0, p=0.676) (see online supplemental file 6).
bmjopen-2020-044072supp006.pdf (232.3KB, pdf)
Head-to-head comparisons between different types of MCS were also performed. There were three RCTs comparing pVADs versus IABP. The pooled analysis revealed that pVADs were not associated with a decrease in short-term mortality compared with IABP (pVADs=44.8%, 26 of 58; IABP=47.4%, 27 of 57; RR=0.95; 95% CI 0.64 to 1.41; p=0.786), with insignificant heterogeneity among studies (I2=0; p=0.986) (see online supplemental file 7). Long-term mortality was not analysed because only one study was reported. For the secondary outcome analysis, only bleeding and vascular complications met the quantitative requirements for the analysis. Compared with IABP, pVADs increased the rate of bleeding (pVADs=66.7%, 30 of 45; IABP=36.4%, 16 of 44; RR=1.86; 95% CI 1.15 to 3.00; p=0.011; I2=15.3%, p=0.227) and trended towards increased vascular complications (pVADs=15.5%, 9 of 58; IABP=0, 0 of 57; RR=5.48; 95% CI 0.96 to 31.11; p=0.055; I2=0, p=0.671) (see online supplemental file 7). The results of the prespecified sensitivity analyses were not significantly different from those of the primary analysis (see online supplemental file 8).
bmjopen-2020-044072supp007.pdf (130.6KB, pdf)
bmjopen-2020-044072supp008.pdf (65.6KB, pdf)
Outcomes of subgroup analyses
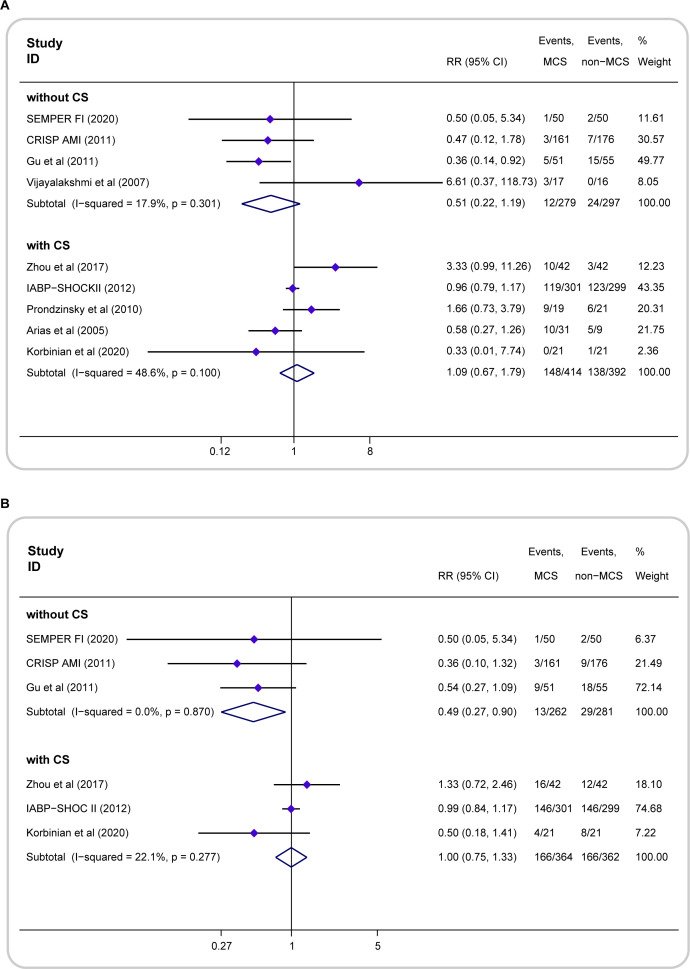
In the subset of patients without CS, compared with the treatment without MCS, the MCS treatment did not change the short-term mortality (MCS=4.3%, 12 of 279; non-MCS=8.1%, 24 of 297; RR=0.51; 95% CI 0.22 to 1.19; p=0.121; I2=17.9%, p=0.301) (figure 3A), but significantly decreased the long-term mortality (MCS=5.0%, 13 of 262; non-MCS=10.3%, 29 of 281; RR=0.49; 95% CI 0.27 to 0.90; p=0.020; I2=0, p=0.870) (figure 3B). In patients with CS, MCS did not reduce short-term mortality (MCS=35.7%, 148 of 414; non-MCS=35.2%, 138 of 392; RR=1.09; 95% CI 0.67 to 1.79; p=0.726; I2=46.6%, p=0.100) (figure 3A) or long-term mortality (MCS=45.6%, 166 of 364; non-MCS=45.9%, 166 of 362; RR=1.00; 95% CI 0.75 to 1.33; p=0.984; I2=22.1%, p=0.227) (figure 3B). Due to the limited number of studies, subgroup analyses of the secondary outcomes were not performed.
Figure 3.
Forest plots showing the all-cause mortality for the subgroup analyses. (A) Risk ratio (RR) of short-term mortality of patients with AMI with or without CS treated with MCS compared with non-MCS. (B) RR of long-term mortality of patients with AMI with or without CS treated with MCS compared with non-MCS. AMI, acute myocardial infarction; CS, cardiogenic shock; MCS, mechanical circulatory support.
All patients had CS in studies comparing pVADs versus IABP, and the results have been described in the synthesis analyses.
Study quality and publication bias
The results of the literature quality assessment using the Cochrane Collaboration tool are shown in online supplemental file 9. Three trials had a high risk of bias.29 33 35 There was no evidence of publication bias for included studies except the Egger’s test for short-term mortality of studies comparing pVADs use versus IABP use (p<0.05) (online supplemental file 10 and 11). Then the trim-and-fill method was performed for the estimation of the number of missing studies (pVADs vs IABP) that might exist. Result suggested two theoretically missing studies with an adjusted RR of short-term mortality (RR=0.92; 95% CI 0.67 to 1.26; p=0.59).
bmjopen-2020-044072supp009.pdf (52KB, pdf)
bmjopen-2020-044072supp010.pdf (107.1KB, pdf)
Discussion
Summary of the evidence
It is the first study that evaluates the efficacy and safety of MCS in patients with AMI undergoing stent implantation. Compared with non-MCS, MCS does not reduce mortality in patients with AMI undergoing stent implantation and other adverse events (reinfarction, repeat revascularisation, stroke-TIA, bleeding, arrhythmias and vascular complications) in patients with AMI undergoing stent implantation. Surprisingly, the results show that MCS reduced long-term mortality by half in patients without CS compared with non-MCS (5.0% vs 10.3%). This benefit does not exist in patients with CS. The strategy of using pVADs did not outperform IABP regarding all-cause mortality in patients with CS, but increased rate of bleeding compared with IABP.
Efficacy of MCS in patients without CS
In the analysis of MCS in patients without CS, only IABP-related studies were found in included RCTs. Regarding the work mechanism of IABP, deflation of the pump reduces ventricular workload and helps the ventricle push blood into the aorta during systole, and the inflation of the pump improves coronary blood flow during diastole.36 The evidence from clinical trials also shows the beneficial effect of IABP on haemodynamic situation, as these pumps can significantly improve myocardial coronary blood flow and myocardial ischaemia.37 38 IABP might have a greater effect on patients with critically reduced coronary artery perfusion and temporarily impaired left ventricular function.39 The haemodynamic situation of patients without CS may recover more quickly with IABP support, avoiding more serious clinical consequences, such as progression to CS, severe left heart failure or even death. In the last decades, the application of PCI technology significantly reduces mortality in patients with ST-elevation MI.11 40 In particular, stenting reduces acute risk of major complications and restenosis.15 One of our findings shows that IABP can give long-term mortality reduction in patients with AMI without CS. This suggests that the use of IABP in patients with AMI without CS with contemporary recommended reperfusion strategy (stent implantation) is reasonable. Some previous meta-analyses showed similar conclusions, but there was no detailed distinction between the revascularisation strategies of thrombolytic therapy or PCI (balloon angioplasty and stent implantation).16 17 41 This result from the meta-analysis generates a promising hypothesis that IABP reduced long-term mortality in patients with AMI without CS receiving stenting treatment. Studies with randomised control and decent sample size are warranted to confirm this finding.
Efficacy of MCS in patients with CS
Previous guidelines have recommended IABP as the complementary strategy to maintaining haemodynamic stability in patients with combined AMI and CS.3 4 42 However, accumulating evidence from large-scale RCTs, including IABP-SHOCK II and PAMI-II, as well as registry data, did not show that the use of IABP brought benefit to the mortality reduction in patients with AMI with CS.8 9 22 43 44 The findings from our study also showed that MCS was not associated with an all-cause mortality reduction in patients with CS undergoing stent implantation. Patients with AMI and CS are considered a high-risk group with more impaired cardiac function and haemodynamic instability and are more likely to develop circulatory failure during PCI.45 The IABP SHOCK trial showed that IABP treatment does not significantly improve the haemodynamic profile in patients with combined AMI and CS.46 Even though patients with AMI with CS had combined use of currently advanced reperfusion strategy and MCS, mortality was not reduced in our meta-analysis, which is consistent with the results from previous meta-analyses focusing on the impact of IABP on patients with CS.14 47–49 Therefore, IABP should be used with caution when patients have a combination of AMI and CS.
Several clinical trials exploring the efficacy of using different types of MCS in patients with CS, including extracorporeal membrane oxygenation, pVADs or the combination of two types of MCS are ongoing (NCT04184635, NCT03637205, NCT01633502, NCT03813134, NCT02301819, NCT03729765, NCT03431467, NCT03947619). We look forward to seeing those studies provide new insights into better MCS strategies in improving survival in patients with AMI and CS. Moreover, we compared the efficacy of survival between pVADs and IABP in patients with combined AMI and CS. The results showed that pVADs did not reduce the all-cause mortality, but significantly increased the rate of bleeding. These results are consistent with previous studies which focus on the impact of pVADs in patients with CS.6 13
Clinical implications
In patients with AMI without CS who received stent implantation, the application of IABP may improve the long-term survival. However, in patients with CS, neither IABP nor pVADs improve the survival outcome. Moreover, pVADs may bring higher risk of bleeding in patients with AMI with CS. This result generates an interesting hypothesis that IABP reduced long-term mortality in patients with AMI without CS receiving stenting treatment. RCT studies with a large number of patients are warranted to confirm this finding.
Limitations
There are several limitations in the current meta-analysis. First, the number of included RCTs and participants was limited. Second, due to few reports of secondary endpoints, subsequent subgroup analyses could not be performed. Third, there was high heterogeneity in the pooled CS group, which may be explained by inconsistent timing of initiation, higher incidence of interactions and inconsistent time points for reporting mortality. Fourth, there were a very limited number of RCTs comparing the efficacy between pVADs with IABP, and the pVADs have large heterogeneity.
Conclusions
Overall, the use of MCS does not reduce mortality in patients with AMI undergoing stent implantation. However, IABP demonstrated a promising effect in reducing the long-term mortality in patients with AMI without CS. However, in patients with CS, MCS is not associated with a mortality reduction and pVADs further increase risk of bleeding.
bmjopen-2020-044072supp011.pdf (36KB, pdf)
Supplementary Material
Footnotes
Authors contributions: JC contributed to the conception and revision of the review. YS and YW performed systematic literature search, study selection data extraction and data analyses. YS contributed to writing and revision of the manuscript. XS contributed to reviewing the data and manuscript. YT, MJ, YB, SL, WJ, YL and HY checked the manuscript.
Funding: This work was supported in part by the National Natural Science Foundation of China (grant nos. 81870171, 81800393, 81770403, 81974054), the Hunan Distinguished Young Scholars (grant nos. 2017RS3015, 2020JJ2057), the National Key Research and Development Projects (grant nos. 2018YFC1311300, SQ2019YFF020012, YS2019YFF020012) and the Hunan Youth Talent Project (grant no. 2019RS2014).
Disclaimer: The sponsors or funders had no involvement in any part of this study. All authors confirm the independence of researchers from funding sources.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1–25. 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunwald E. The war against heart failure: the Lancet lecture. The Lancet 2015;385:812–24. 10.1016/S0140-6736(14)61889-4 [DOI] [PubMed] [Google Scholar]
- 3.Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the European Society of cardiology. Eur Heart J 2008;29:2909–45. 10.1093/eurheartj/ehn416 [DOI] [PubMed] [Google Scholar]
- 4.Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines. J Am Coll Cardiol 2009;54:2205–41. 10.1016/j.jacc.2009.10.015 [DOI] [PubMed] [Google Scholar]
- 5.Sjauw KD, Engström AE, Vis MM, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J 2009;30:459–68. 10.1093/eurheartj/ehn602 [DOI] [PubMed] [Google Scholar]
- 6.Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J 2009;30:2102–8. 10.1093/eurheartj/ehp292 [DOI] [PubMed] [Google Scholar]
- 7.Unverzagt S, Machemer MT, Solms A. Intra-Aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane database of syst rev 2011;7:Cd007398. 10.1002/14651858.CD007398.pub2/full [DOI] [PubMed] [Google Scholar]
- 8.Thiele H, Zeymer U, Neumann F-J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96. 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 9.Thiele H, Zeymer U, Neumann F-J, et al. Intra-Aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet 2013;382:1638–45. 10.1016/S0140-6736(13)61783-3 [DOI] [PubMed] [Google Scholar]
- 10.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines. Circulation 2013;127:e362–425. 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- 11., Windecker S, Kolh P, et al. , Authors/Task Force members . 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–619. 10.1093/eurheartj/ehu278 [DOI] [PubMed] [Google Scholar]
- 12.Pavasini R, Cirillo C, Campo G, et al. Extracorporeal circulatory support in acute coronary syndromes: a systematic review and meta-analysis. Crit Care Med 2017;45:e1173–83. 10.1097/CCM.0000000000002692 [DOI] [PubMed] [Google Scholar]
- 13.Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 2017;38:3523–31. 10.1093/eurheartj/ehx363 [DOI] [PubMed] [Google Scholar]
- 14.Unverzagt S, Buerke M, de Waha A, et al. Intra-Aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev 2015;75:Cd007398. 10.1002/14651858.CD007398.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SC, Dove JT, Jacobs AK, et al. ACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines)-executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. Circulation 2001;103:3019–41. 10.1161/01.cir.103.24.3019 [DOI] [PubMed] [Google Scholar]
- 16.Ye L, Zheng M, Chen Q, et al. Effects of intra-aortic balloon counterpulsation pump on mortality of acute myocardial infarction. PLoS One 2014;9:e108356. 10.1371/journal.pone.0108356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng X-Y, Wang Y, Chen Y, et al. The effectiveness of intra-aortic balloon pump for myocardial infarction in patients with or without cardiogenic shock: a meta-analysis and systematic review. BMC Cardiovasc Disord 2016;16:148. 10.1186/s12872-016-0323-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W–94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavasini R, Guralnik J, Brown JC, et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016;14:215. 10.1186/s12916-016-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone GW, Marsalese D, Brodie BR, et al. A prospective, randomized evaluation of prophylactic intraaortic balloon counterpulsation in high risk patients with acute myocardial infarction treated with primary angioplasty. second primary angioplasty in myocardial infarction (PAMI-II) trial Investigators. J Am Coll Cardiol 1997;29:1459–67. 10.1016/s0735-1097(97)00088-0 [DOI] [PubMed] [Google Scholar]
- 23.van 't Hof AW, Liem AL, de Boer MJ, et al. A randomized comparison of intra-aortic balloon pumping after primary coronary angioplasty in high risk patients with acute myocardial infarction. Eur Heart J 1999;20:659–65. 10.1053/euhj.1998.1348 [DOI] [PubMed] [Google Scholar]
- 24.Ohman EM, George BS, White CJ, et al. Use of aortic counterpulsation to improve sustained coronary artery patency during acute myocardial infarction. Results of a randomized trial. The randomized IABP Study Group. Circulation 1994;90:792–9. 10.1161/01.cir.90.2.792 [DOI] [PubMed] [Google Scholar]
- 25.van Nunen LX, van 't Veer M, Zimmermann FM, et al. Intra-Aortic balloon pump counterpulsation in extensive myocardial infarction with persistent ischemia: the semper FI pilot study. Catheter Cardiovasc Interv 2020;95:128–35. 10.1002/ccd.28289 [DOI] [PubMed] [Google Scholar]
- 26.Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol 2017;69:278–87. 10.1016/j.jacc.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 27.Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005;26:1276–83. 10.1093/eurheartj/ehi161 [DOI] [PubMed] [Google Scholar]
- 28.Patel MR, Smalling RW, Thiele H, et al. Intra-Aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA 2011;306:1329–37. 10.1001/jama.2011.1280 [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Yu K, Wang X-H, et al. Analysis on application timing of IABP in emergency PCI treatment of patients with combined acute myocardial infarction and cardiac shock. Eur Rev Med Pharmacol Sci 2017;21:2934–9. [PubMed] [Google Scholar]
- 30.Gu J, Hu W, Xiao H, et al. Prophylactic intra-aortic balloon pump reduces C-reactive protein levels and early mortality in high-risk patients undergoing percutaneous coronary intervention. Acta Cardiol 2011;66:499–504. 10.1080/AC.66.4.2126599 [DOI] [PubMed] [Google Scholar]
- 31.Prondzinsky R, Lemm H, Swyter M, et al. Intra-Aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP shock trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med 2010;38:152–60. 10.1097/CCM.0b013e3181b78671 [DOI] [PubMed] [Google Scholar]
- 32.Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52:1584–8. 10.1016/j.jacc.2008.05.065 [DOI] [PubMed] [Google Scholar]
- 33.Arias EA, González-Chon O, García-López SMC, et al. [Impact of the intra-aortic balloon pump in the mortality due to cardiogenic shock secondary to acute myocardial infarction]. Arch Cardiol Mex 2005;75:260–6. [PubMed] [Google Scholar]
- 34.Vijayalakshmi K, Kunadian B, Whittaker VJ, et al. Intra-Aortic counterpulsation does not improve coronary flow early after PCI in a high-risk group of patients: observations from a randomized trial to explore its mode of action. J Invasive Cardiol 2007;19:339–46. [PubMed] [Google Scholar]
- 35.Lackermair K, Brunner S, Orban M, et al. Outcome of patients treated with extracorporeal life support in cardiogenic shock complicating acute myocardial infarction: 1-year result from the ECLS-Shock study. Clin Res in Cardiol 2020;136. 10.1007/s00392-020-01778-8 [DOI] [PubMed] [Google Scholar]
- 36.Kantrowitz A. Experimental augmentation of coronary flow by retardation of the arterial pressure pulse. Surgery 1953;34:678–87. [PubMed] [Google Scholar]
- 37.Gold HK, Leinbach RC, Sanders CA, et al. Intraaortic balloon pumping for control of recurrent myocardial ischemia. Circulation 1973;47:1197–203. 10.1161/01.CIR.47.6.1197 [DOI] [PubMed] [Google Scholar]
- 38.Fuchs RM, Brin KP, Brinker JA, et al. Augmentation of regional coronary blood flow by intra-aortic balloon counterpulsation in patients with unstable angina. Circulation 1983;68:117–23. 10.1161/01.CIR.68.1.117 [DOI] [PubMed] [Google Scholar]
- 39.Grieshaber P, Niemann B, Roth P, et al. Prophylactic intra-aortic balloon counterpulsation in cardiac surgery: it is time for clear evidence. Crit Care 2014;18:662. 10.1186/s13054-014-0662-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campo G, Pavasini R, Morciano G, et al. Clinical benefit of drugs targeting mitochondrial function as an adjunct to reperfusion in ST-segment elevation myocardial infarction: a meta-analysis of randomized clinical trials. Int J Cardiol 2017;244:59–66. 10.1016/j.ijcard.2017.06.040 [DOI] [PubMed] [Google Scholar]
- 41.Chen S, Yin Y, Ling Z, et al. Short and long term effect of adjunctive intra-aortic balloon pump use for patients undergoing high risk reperfusion therapy: a meta-analysis of 10 international randomised trials. Heart 2014;100:303–10. 10.1136/heartjnl-2013-304198 [DOI] [PubMed] [Google Scholar]
- 42.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American heart association Task force on practice guidelines (writing Committee to revise the 2002 guidelines for the management of patients with unstable Angina/Non ST-elevation myocardial infarction): developed in collaboration with the American College of emergency physicians, the Society for cardiovascular angiography and interventions, and the Society of thoracic surgeons: endorsed by the American association of cardiovascular and pulmonary rehabilitation and the Society for academic emergency medicine. Circulation 2007;116:e148–304. 10.1161/CIRCULATIONAHA.107.181940 [DOI] [PubMed] [Google Scholar]
- 43.Zeymer U, Hochadel M, Hauptmann K-E, et al. Intra-Aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: results of the ALKK-PCI registry. Clin Res Cardiol 2013;102:223–7. 10.1007/s00392-012-0523-4 [DOI] [PubMed] [Google Scholar]
- 44.Anderson RD, Ohman EM, Holmes Jr DR. Use of intraaortic balloon counterpulsation in patients presenting with cardiogenic shock: observations from the GUSTO-I study. J Am Coll Cardiol 1997;30:708–15. 10.1016/s0735-1097(97)00227-1 [DOI] [PubMed] [Google Scholar]
- 45.Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294:448–54. 10.1001/jama.294.4.448 [DOI] [PubMed] [Google Scholar]
- 46.Prondzinsky R, Unverzagt S, Russ M, et al. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP shock trial. Shock 2012;37:378–84. 10.1097/SHK.0b013e31824a67af [DOI] [PubMed] [Google Scholar]
- 47.Ahmad Y, Sen S, Shun-Shin MJ, et al. Intra-Aortic balloon pump therapy for acute myocardial infarction: a meta-analysis. JAMA Intern Med 2015;175:931–9. 10.1001/jamainternmed.2015.0569 [DOI] [PubMed] [Google Scholar]
- 48.Shi W, Wang W, Wang K, et al. Percutaneous mechanical circulatory support devices in high-risk patients undergoing percutaneous coronary intervention: a meta-analysis of randomized trials. Medicine 2019;98:e17107. 10.1097/MD.0000000000017107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JM, Park J, Kang J, et al. The efficacy and safety of mechanical hemodynamic support in patients undergoing high-risk percutaneous coronary intervention with or without cardiogenic shock: Bayesian approach network meta-analysis of 13 randomized controlled trials. Int J Cardiol 2015;184:36–46. 10.1016/j.ijcard.2015.01.081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-044072supp001.pdf (48KB, pdf)
bmjopen-2020-044072supp002.pdf (113.2KB, pdf)
bmjopen-2020-044072supp003.pdf (74.9KB, pdf)
bmjopen-2020-044072supp004.pdf (63.6KB, pdf)
bmjopen-2020-044072supp005.pdf (46.6KB, pdf)
bmjopen-2020-044072supp006.pdf (232.3KB, pdf)
bmjopen-2020-044072supp007.pdf (130.6KB, pdf)
bmjopen-2020-044072supp008.pdf (65.6KB, pdf)
bmjopen-2020-044072supp009.pdf (52KB, pdf)
bmjopen-2020-044072supp010.pdf (107.1KB, pdf)
bmjopen-2020-044072supp011.pdf (36KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information.