Abstract
Tumor necrosis factor (TNF) inhibitors have improved a lot the treatment of numerous diseases, with the well-known example of rheumatoid arthritis (RA). In the early 2000s, postmarketing data quickly revealed an alarming number of severe tuberculosis (TB) under such treatment. These findings were consistent with previous results in mice where TNF is essential for lymph node formation and granuloma organization. The effects of TNF inhibition on RA synovium structure are very similar to those on granuloma, with changes in cellular interactions, cytokine, and chemokine production. In addition to the role of TNF in granuloma, the interleukin (IL)-12/interferon (IFN)-γ pathway is required for an efficient host defense against TB. Primary and secondary immunodeficiencies affecting this pathway lead to severe bacillus Calmette-Guérin (BCG) reaction or full TB. Any chronic inflammation as in RA induces a systemic Th1 defect that predisposes to TB through specific downregulation of the IL-12Rß2 chain. When TNF inhibitors are initiated, this transiently increases this risk of TB, through effects on cellular interactions in a latent TB granuloma. At a later stage, when a better control disease activity is obtained, the risk of TB is reduced but not abrogated. Given the clear benefit from TNF inhibition, latent TB infection screening at baseline is essential for an optimal safety.
Keywords: tuberculosis, TNF inhibition, rheumatoid arthritis, cytokines
Subject terms: Antimicrobial responses, Predictive markers
Introduction
Tuberculosis (TB) is still the leading cause of death worldwide from an infectious disease among adults. One of the current challenges is the emergence of drug resistance, which is a major threat to control TB spreading.1 The clinical spectrum ranges from latent TB infection (LTBI) to active disease defining three categorical states.2,3 LTBI affects approximately one-third of the world population and is defined by persistent bacterial viability, but with adequate immune control and no clinical symptoms.4 The risk of progression to active TB is determined by the combination of bacterial factors with host and environmental factors. Immune response involved in disease control is impaired in different situations as immune deficiencies, chronic inflammation, malnutrition, and other comorbidities.
Among the key immune pathways required to control TB infection, the T-helper (Th) 1 response is essential, especially through the secretion of interferon (IFN)-γ. In addition, tumor necrosis factor (TNF)-α5–7 has a key role in granuloma formation, which is critical to control bacterial spreading throughout the body. This role of TNFα was demonstrated in mice in the 90s and then confirmed in observational studies with TB reactivation under TNFα inhibitors (TNFi).8–11
The importance of TNFα in chronic inflammation was first demonstrated in human and mouse models of rheumatoid arthritis (RA).12–15 This led to use TNFi to control disease activity.13,16 Through chronic inflammation alone, RA patients are more likely to develop TB when they are not adequately treated because of the defect in Th1 response due to chronic inflammation.17 In RA taken here as an example as well as in all inflammatory conditions, initiation of a TNFi increases the risk of TB reactivation through the combination of the cell-mediated immune defect linked to inflammation and the effect of TNFi on TB-related granuloma structure.18 Understanding of such increased risk in patients with inflammation-induced secondary immunodeficiency has shown the critical role of IFNγ in TB defense, as in children with a primary immunodeficiency of this pathway, who developed severe TB.19 These findings have led to the development of recommendations before initiating TNFi in inflammatory diseases.20
This review will first describe the association between TB and TNFi. The following part will discuss the involvement of TNFα in granuloma formation and the crucial role of the IL-12/IFNγ pathway in defense against TB. Finally, primary and secondary immunodeficiencies will illustrate these concepts in vivo.
TB and TNF inhibitors
First cases of TB reactivation before prevention
The first two TNFi on the market were infliximab, a monoclonal antibody targeting directly TNFα and etanercept, a fusion protein that acts as a “decoy receptor” for TNFα. They differ by their modes of administration and their pharmacokinetics.21,22
During initial clinical trials that evaluated the effect of infliximab in RA, only one case of TB was reported.23 The first alert came after the use of infliximab in Crohn’s disease and in RA. The drug was approved in 1998 and 70 cases of TB were reported up to 2001. Among them, 12 patients died. The median duration from the initiation of the treatment until TB development was 12 weeks. Nine cases of TB were reported in patients treated with etanercept.8 In 2002, postmarketing data showed a prevalence of 1/1000 cases of TB among patients treated with infliximab and 0.15/1000 for etanercept.24 These results and others had led to implement strategies to track LTBI before the initiation of anti-TNFα.25 Apart from infliximab and etanercept, other monoclonal antibodies targeting TNFα were developed (certolizumab pegol, adalimumab, and golimumab).26
Cases of TB reactivation with prevention in place
TB prevention includes LTBI screening at baseline with two methods: tuberculin skin test (TST) and IFNγ release assay (IGRA). The classical TST gives a full immune response to Mycobacterium antigens but its interpretation is difficult, particularly in individuals previously exposed to bacillus Calmette-Guérin (BCG).4 The IGRA test can distinguish between BCG-induced and M. tuberculosis-induced positive TST responses and is thus more specific.2 IGRA is an in vitro blood test of cell-mediated immune response measuring T-cell release of IFNγ after stimulation by M. tuberculosis antigens. Two tests are currently approved with T-SPOT.TB assay and the QuantiFERON-TB assay. Chest radiographs, CT scans, and review of clinical data are also performed.4,27 If LTBI is identified, different regimens are used to treat latent TB with either 3 months of bitherapy (isoniazid and rifampicin), 4 months of rifampicin or 6–12 months of isoniazid alone. In cases of active pan-susceptible TB at screening, four drugs are given for 2 months (isoniazid, rifampicin, pyrazinamide, and ethambutol) followed by 4 months with isoniazid and rifampicin. TNFi can be started after at least 3 weeks of treatment.3,28
Despite such prevention, cases of TB are described under TNFi. For instance, a Korean study showed that in a baseline negative LTBI group before TNFi therapy, 6/447 (1.34%) patients developed active TB during the study period. Such TB incidence is rather similar in a Spanish cohort of inflammatory bowel disease (IBD) patients despite LTBI screening at baseline.29,30 The incidence of TB also increases after LTBI treatment. Despite LTBI treatment, 2.7% (2/74) of patients are diagnosed with TB in a Brazilian study.31 Moreover, the occurrence of active TB appears to be biphasic with either the development of active disease within 3/6 months after the initiation of TNFi or later after 20 months of treatment.30,32,33 The biphasic occurrence of active TB relies on different rationales. Early development of M. tuberculosis infection after initiation of TNFi can be attributed to a false-negative screening test, caused by immunodepression induced by RA or IBD. Conversely, a development of active TB after several months of anti-TNFα treatment seems more consistent with a primary TB.29,30,32,33
Registry observations reported the number of TB under TNFi, especially in RA. In the British Society for Rheumatology Biologics Register, 40 cases of active TB were reported among 10,712 RA patients receiving TNFi. Overall, 38% of TB were pulmonary, 62% were extrapulmonary, 28% were disseminated, and 10 patients died within 12 months of diagnosis.34 A meta-analysis on 19 randomized clinical trials in various diseases (RA, psoriatic arthritis, or ankylosing spondylitis) showed that the occurrence of TB is 0.6% in the treatment groups (5339 patients; 32 events) while no event was reported in the control groups (2981 patients). Exposure to TNFi is associated with a threefold increase in TB risk.18 A recent meta-analysis performed with 52 observational studies (98,483 patients with rheumatic diseases) showed an overall TB incidence of 9.62 cases per 1000 exposed patients, without any difference between diagnoses. Conversely, there is a statistically significant difference in TB development regarding continents with an increased risk in South America and Asia compared with North America and Europe.35 Among the TNF blockers, the rate of TB is almost fourfold higher in patients treated with infliximab or adalimumab compared with etanercept.34 Variation in pharmacokinetics and mechanism of action between these TNFi may account for this difference.21,22
TNFα and granuloma formation
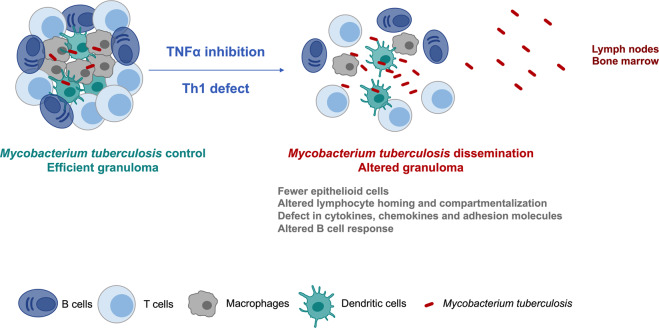
The development of TB under TNFi suggests that this cytokine plays a role in granuloma formation (Fig. 1). Granuloma consists of aggregates of macrophages, B and T lymphocytes, which are organized to control a pathogen or a foreign body that cannot be eliminated. In vivo studies corroborate that TNFα plays a role both in granuloma formation and maintenance.36
Fig. 1.
Consequences of tumor necrosis factor (TNF)-α inhibition and Th1 defect in granuloma formation. A granuloma able to control Mycobacterium tuberculosis infection corresponds to a cell organization infiltrated with T and B cells, macrophages, and dendritic cells. TNFα is required for granuloma formation, organization, and maintenance. Th1 deficit or TNF inhibition allow systemic dissemination of Mycobacterium tuberculosis (e.g., to lymph nodes and bone marrow) because of an altered granuloma formation. This impairment is due to fewer epithelioid cells and macrophages and a delayed cellular recruitment due to a defect in cytokines, chemokines, and adhesion molecules. There are also an altered B cell response (germinal center formation and humoral response) and defects in lymphocyte homing and compartmentalization
Mouse studies in TNF family deficient mice with lymph node/granuloma formation defects
TNFα is required for the formation of B cell follicles, follicular dendritic cell (DC) networks and germinal centers (GC) and for the maturation of the immune response.37 Through the induction of adhesion molecules, TNFα regulates lymphocyte homing and compartmentalization in lymphoid tissues. TNFα plays a role in lymphoid organ development and organization38–40 and binds to two different receptors: TNR-receptor (TNF-R) I and TNF-RII.41 Lymphotoxin (LT) α−/− (LT is also called TNF-ß) mice have a complete absence of lymph nodes and Peyer’s patches link to defective lymphoid tissue organogenesis.42 Other molecules are involved in lymph node development as adhesion molecules and chemokines.43 Mice deficient in TNFR-I or in TNFα fail to generate GCs after immunization with T-cell-dependent antigens. Similar results apply for LTα−/− and TNFRI−/− mice.37,44
If TNF ligands are required for lymphoid organ development and organization, it is logical that they play a role in granuloma formation. Granuloma are host-protective structures in response to persistent stimuli, which can be either inanimate with foreign-body or living with TB.45 The first evidence came from experiments on mice infected with BCG. Granuloma formation in the liver of these mice coincides with local TNF synthesis. The injection of anti-TNF antibody interferes with granuloma development and mycobacterial elimination.9 In TNF-RI KO mice, the number and the size of BCG-induced granulomas are decreased compared to controls. Granulomas in TNF-RI KO mice also contain fewer epithelioid cells.46 A defect in granuloma formation exists in Tnf−/− mice, which are highly susceptible to M. tuberculosis aerosol infection and all animals succumb with widespread TB dissemination.47 Tnf−/− mice infected with M. tuberculosis have a delayed cellular recruitment and chemokine production compared to WT mice. Granulomas in these mice lack epithelioid macrophages and the lymphocyte cuff.48 Therefore, experiments either with Tnf−/− mice or with anti-TNF antibody clearly show that TNFα is required for granuloma and lymph node organization (Fig. 1). Finally, in vitro granuloma models show that adalimumab specifically induces M. tuberculosis resuscitation in a TGF-ß1-dependent manner while etanercept potentiates it through the neutralization of TNFα and LT-α.49
Effects of TNFα inhibition on synovium structure
Studies of sections of RA synovium show that around 40% have GC-like structures, 30% infiltrates of cells without organization, and 30% a low density of cells. These GCs-like structures are also called ectopic lymphoid structures or tertiary lymphoid organs and are dependent on LT expression.50–52 In inflamed synovial tissue, activated macrophages are the primary source of TNFα. The number of macrophages and TNFα expression correlate with local disease activity and cytokine expression.53 When comparing biopsies from patients before and 4 weeks after a TNFi, there is a decrease in cellularity and expression of cytokines and adhesion molecules. When the biopsy is performed 48 h after the initiation of treatment, the decreased cell infiltration already exists and suggests that the clinical response is associated with an early inhibition of cell migration.54–56 Administration of infliximab or etanercept also increases concentrations of apoptotic monocytes/macrophages in RA synovium.55 TNFα blockade decreases synovial lymphocyte aggregates and impedes the induction of primary T-cell-dependent humoral responses by interference with GC responses.57–59 The response to anti-TNFα is correlated with the presence of these aggregates.57 Therefore, TNFα inhibition has pleiotropic effect on synovium structure acting both on innate and adaptive immune cells.
Similarities between TB-associated granulomas and GC in synovium and lymph nodes
There are similarities between TB granulomas and RA synovium structure. As mentioned above, TNFα is required for granuloma and GC formation, both in synovium and in lymph nodes. Ectopic lymphoid structures found in RA synovial tissue disappear after TNFi.57 Mechanisms underlying these changes could be mediated by the expression of adhesion molecules and cytokines or chemokines.54 Cellular interactions are also modified through the effect of TNFα on lymph node and synovium organization and compartmentalization.38–40 Study on paired synovium and lymph nodes from RA patients allowed to identify similarities and differences between these two structures. They share the same T-cell-B-cell organization. However, the number of follicular DC is higher in lymph nodes compared with paired synovium. Chemokine (C-C motif) ligand (CCL)19 and CCL21, and their receptor CCR7, are more expressed in paired lymph nodes and are associated with an accumulation of mature DC subset compared with RA synovium with a defect of DC maturation. Conversely, CCL20 the chemokine for immature DC, is expressed in synovium but not in paired lymph nodes. These results show that RA synovium lacks some characteristics of lymphoid organ.60
While TNFi increase the risk of TB among treated patients, results from primary and secondary immunodeficiencies show that IL-12/IFNγ pathway is required in efficient host defense against M. tuberculosis.
IL-12 and IFNγ pathway
Structure of IL-12 and its receptors versus IL-23
IL-12 is a heterodimer composed of IL-12p40 and IL-12p35 subunits (Fig. 2). The IL-12 receptor is made of the common IL-12Rß1 and specific IL-12Rß2 chains, and it activates JAK and STAT signaling molecules, especially STAT4.61,62 IL-12 is mainly produced by activated inflammatory cells as monocytes, macrophages, neutrophils, and DC.62
Fig. 2.
Similarities and differences between interleukin (IL)-12 and IL-23 and role in interferon (IFN)-γ production. IL-12 and IL-23 are heterodimer cytokines and share the p40 subunit which is associated with p35 to form IL-12, and with p19 to form IL-23. Their receptors also share the IL-12-receptor (IL-12R)-ß1 chain. IL-12Rß2, which is specific to IL-12R, is upregulated by IL-18. Both IL-12 and IL-23 lead to JAK-STAT activation but with different effects. IL-23 through the activation of STAT3, allows Th17 stabilization and the production of IL-17A, IL-17F, and IL-22. IL-12, mainly through the activation of STAT4, allows Th1 development and IFNγ production. Its effect is enhanced by IL-18 and there is a positive feedback loop of IFNγ on IL-12 production
Conversely, IL-23 shares with IL-12 the IL-12p40 subunit, linked to IL-23p19. IL-23 is a member of the IL-12 family and activates JAK and STAT signaling but preferentially STAT3. The receptor for IL-23 is a heterodimer composed of the common IL-12Rß1 and the specific IL-23R chains.63,64
Both cytokines are produced in response to signals associated with host defense and wound healing.65
Critical role in IFNγ production in defense against TB
IL-12 promotes Th1 development and IFNγ production while IL-23 is required for Th17 stabilization and production of IL-17A, IL-17F, and IL-22.65 Activation of T cells induces IL-12R transcription and expression of the ß2-chain, whose upregulation is enhanced by IL-12 itself, IFNγ and TNFα. Among T cells, IL-12Rß2 is confined to the Th1 lineage and its expression correlates with responsiveness to IL-12.66,67 Moreover, IL-12 is required for optimal Th1-cell immune response to intracellular bacteria.62 IL-12-induced IFNγ production requires the presence of low levels of TNFα and IL-1. There is a positive feedback loop that relies on the ability of IFNγ to enhance IL-12 production during inflammatory and Th1 responses.62 IFNγ production by Th1 lymphocytes and other cells is also induced by the synergistic action of IL-12 and IL-18 that are produced by DC and phagocytes.17
IL-18 is a member of the IL-1family. IL-18R is made of the IL-18Rα and IL-18Rß chains. IL-12 increases the expression of IL-18Rß, that is essential for IL-18 signal transduction.68 IL-18 acts as an immunoregulatory cytokine inducing IFNγ production from natural killer cells and IL-17 from T cells which promotes autoimmune responses. However, IL-18 alone is ineffective to induce IFNγ production from Th1 cells, which requires the presence of IL-12. IL-18 upregulates the expression of IL-12Rß2. Therefore, both cytokines and IL-12Rß2 are required for the efficient production of IFNγ.69,70 Conversely, IL-18 binding protein (IL-18BP) is the natural inhibitor of IL-18 function that downregulates Th1 responses and the induction of IFNγ. IFNγ increases gene expression and synthesis of IL-18BP.71 As opposed to IL-18, IL-17 downregulates IL-12Rß2 chain and decreases IFNγ production (Fig. 2).72 Therefore, the IL-12Rß2 chain plays a crucial role in the regulation of IFNγ production.62,73,74
T cells also increase IL-12 production through the production of cytokines mentioned above but also across direct cell-cell interactions, notably through ligands of the TNF family.75 As Th1 response plays a role in host defense against TB, a defect in IL-12 or in IFNγ pathways impairs anti-mycobacterial immunity.76–78 As a consequence, TST and IGRA have reduced sensitivity in immunocompromised patients.79 IGRA performance depends on intact cellular Th1 responses. A defect in such response can lead to false-negative results.80 Among the IGRA tests, QuantiFERON-TB and T-SPOT.TB assays are based on different methods. T-SPOT.TB assay requires a lymphocyte adjustment, which decreases the risk of false-negative results in patients with reduced lymphocyte count compared with QuantiFERON-TB test.27,81
Primary and secondary immunodeficiencies of the IL-12 and IFNγ pathway
Mendelian susceptibility to mycobacterial disease (MSMD)
IL-12 and IFNγ pathways are critical in defense against mycobacteria. The genetic theory of TB control was supported by genetic epidemiologic studies, mainly based on familial and twin observations of TB.82 Severe TB and even deaths after BCG vaccination support this hypothesis, as the variability in response to primary infection with M. tuberculosis.83
BCG vaccination is harmless for most children although it can lead to a benign regional adenitis.84 In rare cases, disseminated infection may occur. Two types of idiopathic disseminated BCG infections are distinguished based on clinical outcome and type of granuloma. Type I granulomas that resemble tuberculoid ones are associated with survival whereas type II granulomas are diffuse and lepromatous-like and 16/17 children died.85 Patients with these types of granulomas can develop after vaccination many symptoms including fever, cachexia, hepatosplenomegaly, lymph node enlargement, diffuse pneumonitis, osteolytic lesions, granulomatous dermatitis, and bone marrow failure.86,87 Some of these severe reactions are linked to inborn errors of IFNγ immunity, that are included in MSMD. Eleven gene defects can induce MSMD: nine are inherited autosomally (IFNγR1, IFNγR2, STAT1, IL-12p40 (or IL-12B), IL-12Rß1, IRF8, ISG15, SPPL2A, and TYK2) and two are X-linked (IKBKG (NEMO), CYBB).88 Patients with MSMD are predisposed to clinical disease caused by weakly virulent mycobacteria (BCG vaccines and non-tuberculous environmental mycobacteria) but also to M. tuberculosis.19. Severe TB can lead to death from miliary TB, meningitis, and osteitis.82
The first identified MSMD was the inherited deficiency in IFNγR1, which is linked to virulent mycobacterial infection with disseminated disease. In turn, IFNγ fails to upregulate the production of TNF by macrophages and induces a defect in antigen processing and presentation.86,89,90 Defects in IFNγR2 are also described, but are less frequent.91 MSMD includes defects in IL-12/23p40 and IL-12Rß1.76–78 The most common MSMD is the autosomal recessive deficiency in IL-12Rß1 in which patient leukocytes are not sensitive to IL-12 or IL-23 and produce low levels of IFNγ.65,92 The clinical phenotype is very heterogeneous ranging from asymptomatic cases to early death in infancy. Mycobacterial infections (e.g., BCG, M. avium) are the most frequent infections but severe TB can occur.19 Autosomal recessive deficiency in IL-12p40 results in a similar phenotype of IL-12Rß1 deficit.93,94 Among 41 vaccinated patients, 40 developed BCG disease. However, adverse reactions to BCG vaccination were more frequent in IL-12p40 deficient patients than in IL-12Rß1 ones. Ten patients among 14 died from disseminated BCG infection.94
Secondary defects of the IL-12 and IFNγ pathway during chronic inflammation
During any chronic inflammation, there is a defect in Th1 immune response in peripheral circulation. Focusing on RA as an example, RA-PBMCs show a lower response to IL-12 and IL-18 to produce IFNγ compared with PBMCs from healthy controls. RA patients with active disease have a more important decrease of cell-mediated immunity compared with less active patients in blood. Conversely, Th1 response is overexpressed in inflamed joints.17,95 Similar results apply for Crohn’s disease and multiple sclerosis.96,97 This systemic defect can explain the higher prevalence of TB among RA patients, independently of the use of immunosuppressive treatments, compared to the general population.98,99 Such Th1 defect is sensitive to TNFi, as responders but not non-responders, upregulate blood expression of IFNγ and restore to normal levels production of IFNγ by PBMC in response to IL-12 and IL-18.100 TNFi decrease the expression of adhesion molecules and may thus suppress the migration of Th1 cells from peripheral blood to synovium.54,100 Moreover, IL-12 hypo-responsiveness is associated with a selective downregulation of IL-12Rß2 expression in RA-PBMC. Addition of IL-17 to cultures further increases the systemic Th1 defect.72,74 Other explanations of susceptibility to M. Tuberculosis during chronic inflammation come from diabetes mellitus (DM). Similarly, DM patients respond with less IL-1ß, IL-12, and IL-18 and decreased secretion of IFNγ upon stimulation. The frequency of Th1 cells is also decreased in DM patients with a lower Th1/Th2 ratio that can contribute to TB susceptibility. DC and neutrophil functions are also impaired. Natural killer cells which are another source of IFNγ, supposed to enhance macrophage microbicidal activity against M.Tuberculosis, are altered.101 Overall, chronic inflammation, through innate and adaptive immune dysfunction, induces a defect of IL-12 and IFNγ pathways.
Understanding the risk of latent TB reactivation with TNFi
Chronic inflammation as in RA induces a systemic Th1 immune defect concomitantly with an increased migration of Th1 cells from peripheral blood to inflamed synovium in active RA. IL-17 known to be involved in RA pathogenesis, has an inhibitory effect on IFNγ production through a specific inhibition of the IL-12Rß2 chain. These elements contribute to the systemic Th1 defect and explain the increased incidence of TB among RA patients (Fig. 3A).17,74,95,98,99,102 The initiation of TNFi leads to transient increase of the risk of TB reactivation because it induces changes in cellular interactions both in synovium and lymph nodes, but also in pre-existing TB granuloma (Fig. 3B).18 At a later stage, control of disease activity in responders reduces this Th1 defect thus reducing the risk of TB reactivation, however the effect on TB granuloma formation persists, possibly leading to de novo TB (Fig. 3C).102
Fig. 3.
Risk of Mycobacterium tuberculosis according to disease stages. A Chronic inflammatory diseases are characterized by a systemic Th1 defect with a lower production of interferon (IFN)-γ in response to interleukin (IL)-12 + IL-18 through the downregulation of the IL-12-receptor (IL-12R)-ß2 chain by IL-17. Rheumatoid synovium is characterized by lymphoid aggregates where Th1 cells play a key role. When there is latent tuberculosis (TB) infection, the risk of TB is even more increased in the context of chronic inflammation. B There is an increased risk of TB at treatment initiation. Starting anti-tumor necrosis factor (TNF)-α reduces synovium aggregates and altered granuloma formation leading to TB dissemination. Indeed, there is a strong and temporary deficit of IFNγ production. This deficiency is added to the systemic one and requires the prevention of TB reactivation. C Finally, when the disease is controlled, Th1 cells recover their ability to produce IFNγ and the risk of TB is lowered. Concurrently, synovium aggregates are reduced
Effect of IL-17 blockers on the risk of TB
IL-17 is well-known to play a role in the host defense against extra-cellular bacterial infections and fungi. However, the deficiency or the blockade of the IL-17 pathway has been associated with increased dissemination of bacterial infections and its role in M. tuberculosis defense has been suggested.103–105 The use of IL-17 inhibitors in psoriasis and psoriatic arthritis patients with treated LTBI show no cases of TB reactivation under treatment. Postmarketing surveillance data reveal the occurrence of five cases of de novo active TB among 7355 patients treated with secukinumab, an anti-IL-17A monoclonal antibody. Moreover, even when patients have untreated LTBI, no cases of TB reactivation are described after 52 weeks of follow-up.106,107 Even less studied in RA, IL-17 pathway inhibitors slightly increase the infectious risk but no mycobacterial infections have been described.108–110
Therefore, the use of IL-17 inhibitors appears rather safe regarding the risk of M. tuberculosis reactivation or de novo infection so far.111
Conclusion
The use of TNFi has been a major progress in the treatment of chronic inflammatory diseases starting with RA. The main adverse event was the reactivation of TB, specifically in patients with severe forms of RA. The understanding of such phenomenon relies on mouse models deficient for TNF signaling which showed defects in granuloma formation and maintenance. Inhibition of TNF adds this inhibitory effect on granuloma to the cell-mediated systemic immune defect associated with chronic inflammation. The resulting decrease of IFNγ production results from a specific inhibition of the IL-12Rß2 chain that is required for IL-12 signaling. This defect is further increased in the presence of IL-17 produced during inflammation. The importance of IL-12/IFNγ pathway is well illustrated by the severity of any mycobacterial infection in primary immunodeficiencies of this pathway.
Control of inflammation with TNFi reduces but does not abolish the secondary defect of this pathway. Given the benefit-risk balance of TNFi in chronic inflammatory diseases, consideration of TB screening before the initiation of treatment is an absolute necessity to use these biologics safely.
Acknowledgements
M.R. is supported by the Ecole de l’Inserm Liliane Bettencourt. P.M. is a senior member of the Institut Universitaire de France. His laboratory is supported in part by the IHU OPERA. This review is based on a talk given during the 17th International Congress of Immunology that took place on 19–23 October 2019 in Beijing, China.
Author contributions
M.R.: writing and figures. P.M.: concept and proof reading.
Competing interests
The authors declare no competing interests.
References
- 1.Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393:1642–1656. doi: 10.1016/S0140-6736(19)30308-3. [DOI] [PubMed] [Google Scholar]
- 2.Pai M, et al. Tuberculosis. Nat. Rev. Dis. Prim. 2016;2:16076. doi: 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 3.Drain PK, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin. Microbiol. Rev. 2018;31:e00021–18. doi: 10.1128/CMR.00021-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med. 2015;372:2127–2135. doi: 10.1056/NEJMra1405427. [DOI] [PubMed] [Google Scholar]
- 5.Manca C, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc. Natl Acad. Sci. USA. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann SH. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann. Rheum. Dis. 2002;61:ii54–ii58. doi: 10.1136/ard.61.suppl_2.ii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goletti D, et al. Preventive therapy for tuberculosis in rheumatological patients undergoing therapy with biological drugs. Expert. Rev. Anti. Infect. Ther. 2018;16:501–512. doi: 10.1080/14787210.2018.1483238. [DOI] [PubMed] [Google Scholar]
- 8.Keane J, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 9.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 10.Miller EA, Ernst JD. Illuminating the black box of TNF action in tuberculous granulomas. Immunity. 2008;29:175–177. doi: 10.1016/j.immuni.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Flynn JL, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 12.Akdis M, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016;138:984–1010. doi: 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin. Immunopathol. 2017;39:365–383. doi: 10.1007/s00281-017-0619-z. [DOI] [PubMed] [Google Scholar]
- 14.Alexopoulou L, Pasparakis M, Kollias G. A murine transmembrane tumor necrosis factor (TNF) transgene induces arthritis by cooperative p55/p75 TNF receptor signaling. Eur. J. Immunol. 1997;27:2588–2592. doi: 10.1002/eji.1830271018. [DOI] [PubMed] [Google Scholar]
- 15.Brennan FM, Chantry D, Jackson A, Maini R, Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;2:244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 16.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawashima M, Miossec P. Decreased response to IL-12 and IL-18 of peripheral blood cells in rheumatoid arthritis. Arthritis Res. Ther. 2004;6:R39–R45. doi: 10.1186/ar1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minozzi S, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin. Drug Saf. 2016;15:11–34. doi: 10.1080/14740338.2016.1240783. [DOI] [PubMed] [Google Scholar]
- 19.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin. Immunol. 2014;26:454–470. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmon D, GTI and AFSSAPS. Groupe Tuberculose et infliximab. Agence Française de Sécurité Sanitaire de Produits de Santé Recommendations about the prevention and management of tuberculosis in patients taking infliximab. Jt. Bone Spine. 2002;69:170–172. doi: 10.1016/s1297-319x(02)00387-1. [DOI] [PubMed] [Google Scholar]
- 21.Furst DE, Wallis R, Broder M, Beenhouwer DO. Tumor necrosis factor antagonists: different kinetics and/or mechanisms of action may explain differences in the risk for developing granulomatous infection. Semin. Arthritis Rheum. 2006;36:159–167. doi: 10.1016/j.semarthrit.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Botsios C. Safety of tumour necrosis factor and interleukin-1 blocking agents in rheumatic diseases. Autoimmun. Rev. 2005;4:162–170. doi: 10.1016/j.autrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Maini R, et al. et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 24.Furst DE, Cush J, Kaufmann S, Siegel J, Kurth R. Preliminary guidelines for diagnosing and treating tuberculosis in patients with rheumatoid arthritis in immunosuppressive trials or being treated with biological agents. Ann. Rheum. Dis. 2002;61:ii62–ii63. doi: 10.1136/ard.61.suppl_2.ii62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Reino JJ, et al. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–2127. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 26.Murdaca G, et al. Infection risk associated with anti-TNF-alpha agents: a review. Expert Opin. Drug Saf. 2015;14:571–582. doi: 10.1517/14740338.2015.1009036. [DOI] [PubMed] [Google Scholar]
- 27.Redelman-Sidi G, Sepkowitz KA. IFN-gamma release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am. J. Respir. Crit. Care Med. 2013;188:422–431. doi: 10.1164/rccm.201209-1621CI. [DOI] [PubMed] [Google Scholar]
- 28.Cantini F, et al. Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun. Rev. 2015;14:503–509. doi: 10.1016/j.autrev.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Jauregui-Amezaga A, et al. Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J. Crohns Colitis. 2013;7:208–212. doi: 10.1016/j.crohns.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Lee EH, et al. Active tuberculosis incidence and characteristics in patients treated with tumor necrosis factor antagonists according to latent tuberculosis Infection. Sci. Rep. 2017;7:6473. doi: 10.1038/s41598-017-06899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sartori NS, Picon P, Papke A, Neyeloff JL, da Silva Chakr RM. A population-based study of tuberculosis incidence among rheumatic disease patients under anti-TNF treatment. PLoS One. 2019;14:e0224963. doi: 10.1371/journal.pone.0224963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen DY, et al. Biphasic emergence of active tuberculosis in rheumatoid arthritis patients receiving TNFalpha inhibitors: the utility of IFNgamma assay. Ann. Rheum. Dis. 2012;71:231–237. doi: 10.1136/annrheumdis-2011-200489. [DOI] [PubMed] [Google Scholar]
- 33.Soare A, et al. Risk of active tuberculosis in patients with inflammatory arthritis receiving TNF inhibitors: a look beyond the baseline tuberculosis screening protocol. Clin. Rheumatol. 2018;37:2391–2397. doi: 10.1007/s10067-017-3916-y. [DOI] [PubMed] [Google Scholar]
- 34.Dixon WG, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Ann. Rheum. Dis. 2010;69:522–528. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sartori NS, de Andrade NPB, da Silva Chakr RM. Incidence of tuberculosis in patients receiving anti-TNF therapy for rheumatic diseases: a systematic review. Clin. Rheumatol. 2020;39:1439–1447. doi: 10.1007/s10067-019-04866-x. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 37.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Gommerman JL, Browning JL. Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Nat. Rev. Immunol. 2003;3:642–655. doi: 10.1038/nri1151. [DOI] [PubMed] [Google Scholar]
- 40.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 41.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug. Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto M, Fu YX, Molina H, Chaplin DD. Lymphotoxin-alpha-deficient and TNF receptor-I-deficient mice define developmental and functional characteristics of germinal centers. Immunol. Rev. 1997;156:137–144. doi: 10.1111/j.1600-065x.1997.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 43.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto M, et al. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 45.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012;12:352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 46.Senaldi G, et al. Corynebacterium parvum- and Mycobacterium bovis bacillus Calmette-Guerin-induced granuloma formation is inhibited in TNF receptor I (TNF-RI) knockout mice and by treatment with soluble TNF-RI. J. Immunol. 1996;157:5022–5026. [PubMed] [Google Scholar]
- 47.Bean AG, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 48.Roach DR, et al. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 49.Arbués A, et al. TNF-α antagonists differentially induce TGF-β1-dependent resuscitation of dormant-like Mycobacterium tuberculosis. PLoS Pathog. 2020;16:e1008312. doi: 10.1371/journal.ppat.1008312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corsiero E, et al. Role of lymphoid chemokines in the development of functional ectopic lymphoid structures in rheumatic autoimmune diseases. Immunol. Lett. 2012;145:62–67. doi: 10.1016/j.imlet.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann. N. Y Acad. Sci. 2003;987:140–149. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 52.Braun A, Takemura S, Vallejo AN, Goronzy JJ, Weyand CM. Lymphotoxin beta-mediated stimulation of synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2004;50:2140–2150. doi: 10.1002/art.20356. [DOI] [PubMed] [Google Scholar]
- 53.Tak PP, et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–225. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- 54.Tak PP, et al. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor alpha monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1077–1081. doi: 10.1002/art.1780390702. [DOI] [PubMed] [Google Scholar]
- 55.Catrina, A. I. et al. Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended report. Arthritis Rheum.52, 61–72 (2005). [DOI] [PubMed]
- 56.Smeets TJ, Kraan MC, van Loon ME, Tak PP. Tumor necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum. 2003;48:2155–2162. doi: 10.1002/art.11098. [DOI] [PubMed] [Google Scholar]
- 57.Klaasen R, et al. The relationship between synovial lymphocyte aggregates and the clinical response to infliximab in rheumatoid arthritis: a prospective study. Arthritis Rheum. 2009;60:3217–3224. doi: 10.1002/art.24913. [DOI] [PubMed] [Google Scholar]
- 58.Cantaert T, et al. B lymphocyte autoimmunity in rheumatoid synovitis is independent of ectopic lymphoid neogenesis. J. Immunol. 2008;181:785–794. doi: 10.4049/jimmunol.181.1.785. [DOI] [PubMed] [Google Scholar]
- 59.Salinas GF, et al. Anti-TNF treatment blocks the induction of T cell-dependent humoral responses. Ann. Rheum. Dis. 2013;72:1037–1043. doi: 10.1136/annrheumdis-2011-201270. [DOI] [PubMed] [Google Scholar]
- 60.Page G, Miossec P. Paired synovium and lymph nodes from rheumatoid arthritis patients differ in dendritic cell and chemokine expression. J. Pathol. 2004;204:28–38. doi: 10.1002/path.1607. [DOI] [PubMed] [Google Scholar]
- 61.Watford WT, et al. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 62.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 63.Parham C, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 64.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 65.Teng MW, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 66.Rogge L, et al. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boraschi D, Dinarello CA. IL-18 in autoimmunity: review. Eur. Cytokine Netw. 2006;17:224–252. [PubMed] [Google Scholar]
- 69.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv. Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 70.Nakahira M, et al. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J. Immunol. 2002;168:1146–1153. doi: 10.4049/jimmunol.168.3.1146. [DOI] [PubMed] [Google Scholar]
- 71.Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toh ML, et al. IL-17 inhibits human Th1 differentiation through IL-12R beta 2 downregulation. Cytokine. 2009;48:226–230. doi: 10.1016/j.cyto.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 73.Cruz A, et al. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 74.Toh ML, Kawashima M, Hot A, Miossec P, Miossec P. Role of IL-17 in the Th1 systemic defects in rheumatoid arthritis through selective IL-12Rbeta2 inhibition. Ann. Rheum. Dis. 2010;69:1562–1567. doi: 10.1136/ard.2009.111757. [DOI] [PubMed] [Google Scholar]
- 75.Schulz O, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 76.Altare F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 77.Altare F, et al. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J. Clin. Investig. 1998;102:2035–2040. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Jong R, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 79.Pai M, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin. Microbiol. Rev. 2014;27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang B, et al. Interferon-gamma release assay in the diagnosis of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. Clin. Rheumatol. 2011;30:1535–1541. doi: 10.1007/s10067-011-1771-9. [DOI] [PubMed] [Google Scholar]
- 81.Meier NR, et al. Risk factors for indeterminate interferon-gamma release assay for the diagnosis of tuberculosis in children-a systematic review and meta-analysis. Front. Pediatr. 2019;7:208. doi: 10.3389/fped.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boisson-Dupuis S, et al. IL-12Rbeta1 deficiency in two of fifty children with severe tuberculosis from Iran, Morocco, and Turkey. PLoS One. 2011;6:e18524. doi: 10.1371/journal.pone.0018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boisson-Dupuis S, et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol. Rev. 2015;264:103–120. doi: 10.1111/imr.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lotte A, et al. BCG complications. Estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv. Tuberc. Res. 1984;21:107–193. [PubMed] [Google Scholar]
- 85.Emile JF, et al. Correlation of granuloma structure with clinical outcome defines two types of idiopathic disseminated BCG infection. J. Pathol. 1997;181:25–30. doi: 10.1002/(SICI)1096-9896(199701)181:1<25::AID-PATH747>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 86.Jouanguy E, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 87.Mackay A, et al. Fatal disseminated BCG infection in an 18-year-old boy. Lancet. 1980;2:1332–1334. doi: 10.1016/s0140-6736(80)92398-3. [DOI] [PubMed] [Google Scholar]
- 88.Rosain J, et al. Mendelian susceptibility to mycobacterial disease: 2014-2018 update. Immunol. Cell. Biol. 2019;97:360–367. doi: 10.1111/imcb.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Newport MJ, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 90.Altare F, et al. A causative relationship between mutant IFNgR1 alleles and impaired cellular response to IFNgamma in a compound heterozygous child. Am. J. Hum. Genet. 1998;62:723–726. doi: 10.1086/301750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kilic SS, et al. Severe disseminated mycobacterial infection in a boy with a novel mutation leading to IFN-gammaR2 deficiency. J. Infect. 2012;65:568–572. doi: 10.1016/j.jinf.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 92.Sarrafzadeh SA, et al. Molecular, immunological, and clinical features of 16 Iranian patients with mendelian susceptibility to mycobacterial disease. J. Clin. Immunol. 2019;39:287–297. doi: 10.1007/s10875-019-0593-4. [DOI] [PubMed] [Google Scholar]
- 93.de Beaucoudrey L, et al. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine. 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prando C, et al. Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds. Medicine. 2013;92:109–122. doi: 10.1097/MD.0b013e31828a01f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kawashima M, Miossec P. mRNA quantification of T-bet, GATA-3, IFN-gamma, and IL-4 shows a defective Th1 immune response in the peripheral blood from rheumatoid arthritis patients: link with disease activity. J. Clin. Immunol. 2005;25:209–214. doi: 10.1007/s10875-005-4092-4. [DOI] [PubMed] [Google Scholar]
- 96.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 97.Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74:5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yun JE, et al. The incidence and clinical characteristics of Mycobacterium tuberculosis infection among systemic lupus erythematosus and rheumatoid arthritis patients in Korea. Clin. Exp. Rheumatol. 2002;20:127–132. [PubMed] [Google Scholar]
- 99.Allali F, Rkain H, Faik A, El Hassani S, Hajjaj-Hassouni N. Prevalence and clinical characteristics of tuberculosis in rheumatoid arthritis patients. Clin. Rheumatol. 2005;24:656–657. doi: 10.1007/s10067-004-1037-x. [DOI] [PubMed] [Google Scholar]
- 100.Kawashima M, Miossec P. Effect of treatment of rheumatoid arthritis with infliximab on IFN gamma, IL4, T-bet, and GATA-3 expression: link with improvement of systemic inflammation and disease activity. Ann. Rheum. Dis. 2005;64:415–418. doi: 10.1136/ard.2004.022731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ayelign B, Negash M, Genetu M, Wondmagegn T, Shibabaw T. Immunological impacts of diabetes on the susceptibility of mycobacterium tuberculosis. J. Immunol. Res. 2019;2019:6196532. doi: 10.1155/2019/6196532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kosmaczewska A, et al. Patients with the most advanced rheumatoid arthritis remain with Th1 systemic defects after TNF inhibitors treatment despite clinical improvement. Rheumatol. Int. 2014;34:243–253. doi: 10.1007/s00296-013-2895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miossec P. Local and systemic effects of IL-17 in joint inflammation: a historical perspective from discovery to targeting. Cell. Mol. Immunol. 2021;18:1–6. doi: 10.1038/s41423-021-00644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gopal R, et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014;10:e1004099. doi: 10.1371/journal.ppat.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455–462. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nogueira M, Warren RB, Torres T. Risk of tuberculosis reactivation with interleukin (IL)-17 and IL-23 inhibitors in psoriasis - time for a paradigm change. J. Eur. Acad. Dermatol. Venereol. 2021;35:824–834. doi: 10.1111/jdv.16866. [DOI] [PubMed] [Google Scholar]
- 107.Fowler E, Ghamrawi RI, Ghiam N, Liao W, Wu JJ. Risk of tuberculosis reactivation during interleukin-17 inhibitor therapy for psoriasis: a systematic review. J. Eur. Acad. Dermatol. Venereol. 2020;34:1449–1456. doi: 10.1111/jdv.16254. [DOI] [PubMed] [Google Scholar]
- 108.Martin DA, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res. Ther. 2013;15:R164. doi: 10.1186/ar4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blanco FJ, et al. Secukinumab in active rheumatoid arthritis: a phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol. 2017;69:1144–1153. doi: 10.1002/art.40070. [DOI] [PubMed] [Google Scholar]
- 110.Genovese MC, et al. Safety and efficacy of open-label subcutaneous ixekizumab treatment for 48 weeks in a phase II study in biologic-naive and TNF-IR patients with rheumatoid arthritis. J. Rheumatol. 2016;43:289–297. doi: 10.3899/jrheum.140831. [DOI] [PubMed] [Google Scholar]
- 111.Elewski BE, et al. Association of secukinumab treatment with tuberculosis reactivation in patients with psoriasis, psoriatic arthritis, or ankylosing spondylitis. JAMA Dermatol. 2021;157:43–51. doi: 10.1001/jamadermatol.2020.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]