Abstract
Randomised controlled trials have shown a neutral or even unfavourable risk–benefit balance of aspirin for primary prevention of cardiovascular events. Using Danish nationwide registries, we investigated aspirin use and associated risks during the past two decades (1998–2018). We linked individual patient data on repeated aspirin redemptions with registered hospital ICD-10 diagnoses of atherosclerotic cardiovascular disease and bleedings. The prevalence of aspirin use among 1.1 million Danish adults fluctuated over the 20-year study period peaking in 2008 with 8.5% (5.4% primary prevention) and dropping to 5.1% (3.1% primary prevention) in 2018. Aspirin use showed strong age dependency, and 21% of individuals > 80 years were treated with aspirin for primary prevention in 2018. Medication adding to bleeding risk was used concurrently by 21% of all aspirin users in 2018. The incidence of major bleedings were similar with primary and secondary prevention aspirin use and highest in elderly (2 per 100 patient years among individuals > 80 years in 2018). In conclusion, low-dose aspirin use for primary prevention of cardiovascular events remains prevalent. The widespread use of aspirin, especially among older adults, and substantial concomitant use of medications adding to bleeding risk warrant increased focus on discontinuation of inappropriate aspirin use.
Subject terms: Epidemiology, Cardiology
Introduction
Aspirin is a well-established and inexpensive antiplatelet drug used to prevent cardiovascular events in patients with atherosclerotic cardiovascular disease (ASCVD)1. While a favourable risk–benefit balance is well-established for low-dose (75–150 mg) aspirin use in patients with ASCVD2, the net clinical benefit of primary aspirin treatment in patients without established ASCVD is less clear3–5. Thirty years ago, the first clinical trials reported a potential cardiovascular protective effect of relatively high-dose (500 mg) aspirin in individuals without ASCVD6,7. Since then, several randomized clinical trials have investigated the risk–benefit profile of primary prevention of cardiovascular events with low-to moderate dose (75–325 mg) aspirin in patients without established ASCVD3,4. Based on accruing evidence throughout the 2000s, international guidelines recommended primary prevention aspirin in adults with conventional risk factors for ASCVD. Around 2010 guideline directions for the primary prevention use of aspirin increasingly had diverged8, and several therapeutic antithrombotic alternatives to aspirin for ASCVD and other conditions with heightened thrombotic risk had emerged9,10. In 2016 the European Society of Cardiology unilaterally decided to recommend against aspirin in patients without overt ASCVD11. In 2018, clinical trials investigated the effect of primary prevention aspirin in healthy elderly (ASPREE)12–14, patients with diabetes (ASCEND)15, and patients with multiple risk factors for CV disease excluding patients with diabetes (ARRIVE)16. The trial data consistently supported that primary prevention with aspirin in general should be avoided as small absolute ASCVD benefits were exceeded or balanced by bleeding hazards. Moreover, the largest trial did not support any preventive effect of aspirin on cancer incidences in older adults17. In 2019 the American Heart Association and American College of Cardiology (AHA/ACC) adopted a recommendation against the routine use of aspirin for primary cardiovascular prevention, particularly in individuals older than 70 years or patients with increased risk of bleeding18.
The historical uncertainty regarding primary prevention aspirin use may have led to what in retrospect could be perceived as overtreatment. To clarify the extent of such potentially inappropriate aspirin use—before and now—we performed a nationwide drug utilization study exploring aspirin use for primary prevention during the past two decades (1998–2018).
Methods
Data sources
We linked data from Danish registries using the Civil Personal Register number, a unique identifier assigned to all Danish residents since 196819. From the Danish Prescription Registry20, we obtained data on all prescription drugs (ATC-code, quantity, and date of dispensing). From the Civil Registration System21, we obtained data on death and migration to and from Denmark. From the Danish National Patient Register22, we obtained data on all diagnoses according to ICD-10 (International Classification of Diseases, 10th revision). We defined ASCVD as those with a diagnosis of myocardial infarction, stable or unstable angina, coronary revascularization, peripheral artery disease, ischaemic stroke, or transient ischaemic attack (see supplementary Table 1 for ICD codes).
Population and study drugs
We included all adults (> 18 years) redeeming a prescription for low-dose aspirin in strengths ≤ 150 mg (ATC codes B01AC06 and N02BA01). In Denmark, higher doses of aspirin are not used for prevention of CV events. For several analyses, we had to estimate whether an individual was a ‘current user’ of aspirin at a given date. We considered an individual as a ‘current user’ if they had recently filled a prescription for aspirin with enough tablets to cover that day. The duration of each prescription was estimated as the number of tablets dispensed, that is assuming a consumption of one tablet per day, while adding 20% to the duration to account for noncompliance and irregular prescription refills.
Analyses
First, we calculated the annual rate of new users per 1000 adults in the population (as an estimate of incidence) and proportion of total users (point prevalence) using the total Danish adult population on 1 January of the relevant year as the denominator. New users were defined as individuals without low-dose aspirin prescription use in a preceding 5-year period or since the establishment of the Danish National Prescription Database in 1995. Aspirin use was divided into primary and secondary prevention based on cardiovascular diagnoses at the time of redeeming the prescription. Further, the sex and age-specific (using one-year intervals) prevalence proportion was reported each 10 years (1998, 2008 and 2018). Second, we described treatment duration, using the ‘proportion of patients covered’ method23. In brief, we followed all individuals from the date of their first aspirin prescription fill. Over time, we estimated the proportion of all subjects still alive after X days and still using aspirin at that day. As such, an individual could be regarded as ‘dropped out of treatment’ at one point in time and later be reclassified as ‘current user’ upon redeeming a new prescription. Subgroup analyses were carried out according to age, sex, and calendar year of first prescription. Third, we identified the proportion of individuals who, after their first aspirin prescription, filled at least one prescription for aspirin each year for 5 consecutive years (defined as years from first prescription). Fourth, in order to explore potential bleeding risk in relation to aspirin use, we estimated the prevalence of concomitant use (≥ 1 prescription) of co-medication known to affect the risk of gastrointestinal bleeding among users of aspirin (increase risk of bleeding: Non-steroidal anti-inflammatory drugs (NSAID), serotonin reuptake inhibitors (SSRI), other antiplatelet drugs, oral anticoagulants, and systemic glucocorticoids; lower risk of bleeding: Proton pump inhibitors (PPI)) standardised to the age-distribution of 2018. Only prescriptions redeemed by individuals currently using aspirin or during the 30 days leading up to aspirin use were included. As a sensitivity analysis, we also analysed this using a 90-day look back period (which did not change the estimates substantially). Fifth, we estimated age-standardised annual bleeding rate by including established hospital diagnoses of bleeding (see Supplementary Table 1). Data was analysed using Stata Version 16.1 (StataCorp, College Station, TX, USA).
Ethics
According to Danish law, anonymized studies based solely on registry data do not require approval from an ethics review board. In terms of data protection, the study was registered at the University of Southern Denmark’s inventory (record no. 10.446).
Results
We identified 1.1 million adult aspirin users during the 21-year study period, during which the adult Danish population increased from 4.19 to 4.62 million individuals. Among users, 161,022 (14%) individuals redeemed only one prescription, whereas 147,659 (13%) redeemed two to four, and 818,484 (73%) individuals redeemed ≥ five prescriptions.
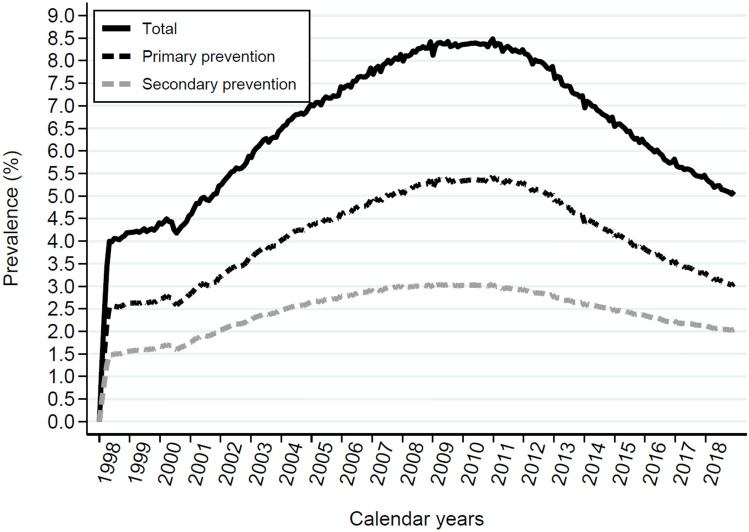
The prevalence of aspirin use followed a unimodal curve over the study period (Fig. 1). The prevalence of aspirin users was 4.2% in 1998, 8.5% in 2008, and 5.1% in 2018. Primary prevention use amounted to 2.6% in 1998, 5.4% in 2008, and 3.1% in 2018, accounting for 62%, 64, and 61% of all prescribed low-dose aspirin the respective years.
Figure 1.
Panel (A) shows Aspirin use expressed as total point prevalence among the adult population in Denmark within a 30-day time period (black line) and separated into primary prevention (black dotted line) and secondary prevention (grey dotted line). Panel shows the distribution of aspiring use in.
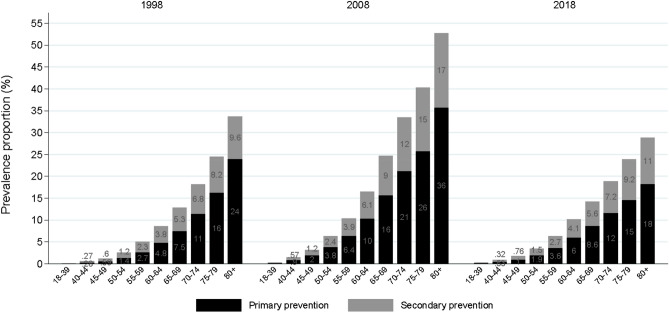
Figure 2 depicts the age continuum for prevalence of aspirin use for selected years in the study period. There was a general, steadily increasing use of aspirin for both primary and secondary with increasing age. The prevalence of aspirin use for primary prevention among those aged at least 80 years was approximately 27% in 1998, 40% in 2008, and 20% in 2018.
Figure 2.
Prevalence of aspirin use stratified by age groups and primary vs. secondary prevention in 1998, 2008 and 2018. The age continuum for aspirin users divided into primary prevention (black bars) and secondary prevention (grey bars) use at selected years in the study period.
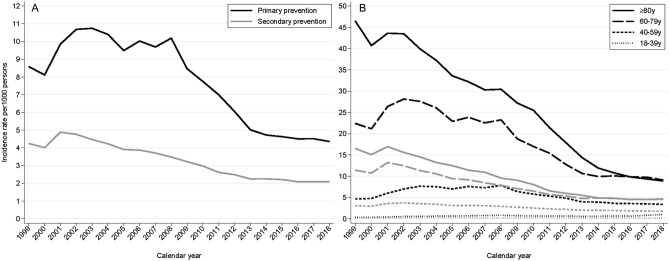
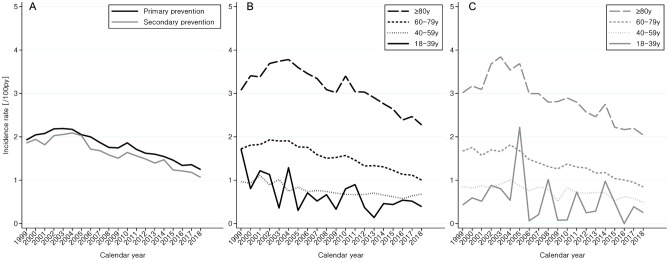
The incidence rate was independent of whether aspirin was used for primary or secondary prevention (Fig. 3A). While the proportion of incident users who used aspirin for secondary prevention was relatively stable over time, the incidence rate for primary prevention declined after 2008 from 11 to 4 incident users per 1000 person-years (Fig. 3A). The incidence rate was age-dependent and highest in patients older than 80 years to 2015 and thereafter was equivalent to people aged 60–80 years (Fig. 3B).
Figure 3.
Incidence rate of new aspirin users stratified by primary and secondary prevention and age groups. The incidence of new users redeeming their first of at least two prescriptions divided into primary (black lines) and secondary prevention (grey lines) for the entire adult population (Panel A), and age intervals for primary (black lines) and secondary prevention (grey lines) (Panel B).
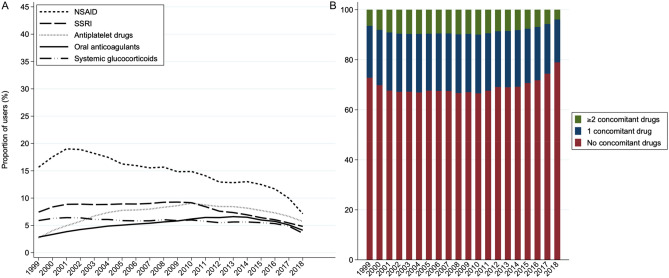
The proportion of aspirin users redeeming a concomitant prescription of at least one drug known to affect the risk of gastrointestinal bleeding differed slightly between primary and secondary aspirin users (see Fig. 4 and Supplementary Fig A). Among primary prevention aspirin users, the concomitant use of a drug class known to increase bleeding (NSAID, SSRIs, other platelet inhibitors, systemic glucocorticoids and oral anticoagulants) was highest in 2002–2010 with approximately 33%, and in 2018 it had dropped to its lowest prevalence of 21% (Fig. 4). The largest change among primary prevention aspirin users was for NSAID, which had a prevalence of 19% in 2001–2002 that had dropped to 7.1% in 2018 (Fig. 4A). Use of SSRIs, other platelet inhibitors, systemic glucocorticoids and oral anticoagulants fluctuated slightly over the study period, and in 2018 among primary prevention aspirin the proportions of concomitant users for these drug classes were 4.8%, 5.7%, 3.6% and 4.1%, respectively. Use of PPI in primary prevention aspirin users almost doubled over the study period from 8.6% in 1999 to 16% in 2018.
Figure 4.
Primary prevention use of aspirin and concomitant use of drugs known to increase the risk of gastrointestinal bleeding. The prevalence of concomitant use of drugs known to affect the risk of gastrointestinal bleeding (Panel A) divided into non-steroidal anti-inflammatory drugs (NSAID), serotonin reuptake inhibitors (SSRI), other antiplatelet drugs, oral anticoagulants, systemic glucocorticoids and proton pump inhibitors (PPI) among primary prevention aspirin users. Panel (B) shows the percentage of aspirin users treated simultaneously with either none, one or more than one drug, respectively, known to increase the risk of bleeding.
The incidence of major bleedings in aspirin users was similar with primary and secondary prevention use and has been declining since 2002 (Fig. 5). The bleeding incidence was higher with increasing age, and in 2018 it amounted to 2.2 per 100 patient years among primary prevention aspirin users aged 80 years or more.
Figure 5.
Incident bleeding events in aspirin users. The incidence of major bleedings with primary prevention aspirin use (black lines) or secondary prevention aspirin use (grey lines) for the entire population (Panel A) of, or according to age intervals as either primary prevention (Panel B) use or secondary prevention (panel C) and age-standardised to 2018.
Persistence to treatment estimated by proportion of patients covered (Supplementary Fig B) was generally lower with aspirin used for primary prevention than for secondary prevention. Patients who initiated aspirin therapy between 2002–2005 had a higher treatment persistence than patients who initiated aspirin in 2006–2012 or from 2013–2018 (Supplementary Fig B). In general, patients older than 60 years had a high treatment persistence, with almost 60% of both primary and secondary prevention aspirin users redeeming prescription for at least 5 years (Supplementary Fig B).
Discussion
In 2018, low dose-aspirin was still more frequently prescribed for primary than secondary prevention. A total of 3.6% of the entire adult population used aspirin without a registered diagnosis of ASCVD, whereas more than 20% of Danish citizens aged 80 years or older were treated with aspirin for primary prevention, and this group also had a substantial annual hospital-diagnosed bleeding risk of 2.2%.
Strength and limitations
A major strength of our study is the nationwide capture of prescription redemptions and relevant diagnosis from national registers. However, a limitation of these data is that we were unable to include data related to non-prescription aspirin use (i.e. related to over the counter (OTC) sale) of aspirin. In Denmark, non-prescription OTC-sale is restricted to pharmacies and amounted to about 10% of the total low-dose aspirin sale in 2006–2018 (available from medstat.dk24). However, as prescription sale of aspirin is reimbursed in Denmark, it is plausible that OTC sale mostly was for people without a prescription (i.e. in most cases not based on an established ASCVD diagnosis). Therefore, the fact that we did not have access to individual non-prescription sale of aspirin most likely leads to an underestimation of the primary prevention use of aspirin in the Danish population. Another issue is misclassification of primary vs. secondary prevention, since patients only treated in primary care may not have a central registration of diagnoses for ASCVD and/or bleeding, which potentially could lead to some underestimation of ASCVD and bleeding diagnoses in our dataset. However, as manifestations of ASCVD are generally symptomatic and treated in specialized secondary care facilities, where the diagnoses are also established, substantial misclassification for ASCVD is not likely. The high accuracy of cardiovascular ICD diagnoses in Denmark support this notion25. In contrast, we have likely underestimated harms in terms of minor bleeding events, as only severe bleeding events necessitate in-hospital management.
Previous literature
There are few other contemporary studies investigating population-based aspirin use. In a recent cohort from the 2017 US National Health Interview Survey (NHIS) (n = 14,328), self-reported aspirin use ranged from 7% among individuals aged 40–50 years to 46% among individuals aged 80 years or more26. In contrast to our registry data, the NHIS defined primary prevention aspirin use as no self-reported angina, coronary heart disease, myocardial infarction, or stroke. The 2017 US prevalence numbers are much higher than the corresponding numbers in our cohort, where less than 2% of the individuals aged 40–50 years and 20% of individuals aged 80 years or more were taking aspirin in 2017. This difference may in part be due to unregistered OTC use in Denmark in particular the early periods of our study periods, but even more likely due to differences in prescription patterns. Thus, as previously mentioned opinions on the risk–benefit balance of aspirin differed between Europe and the US during the 2010s. In Denmark, the prevalence of aspirin use for primary prevention among citizens aged 80 years peaked at 36% in 2010, and was reduced to almost half of that by 2018. The lower prevalence of aspirin use in our data compared to NHIS data may also reflect differences between registry data and self-reported diagnoses and aspirin use, notably there was final response rate of only 53% in the 2017 NHIS26. Our registry-based estimates relying on prescription data and physician-diagnosed ASCVD based on ICD codes include the whole population are probably more accurate and conservative, which result in lower prevalence estimates.
Our findings and the US NHIS data support that overtreatment with aspirin occurred during the past decades and, based on 2018 data, still occurs. A recent meta-analysis including populations with and without diabetes concludes that the benefit of aspirin for primary prevention in terms of preventing major cardiovascular events is a relative risk reduction of 11% (95% CI 6–16%), which has to be compared to a 43% relative risk increase of major bleeding events (95% CI 30–56%)27.
Collectively, the large extent of primary prevention aspirin use in light of the updated evidence suggest that a substantial amount of the treatment is potentially inappropriate and could be discontinued, preferably based on shared decision making between prescriber and patient28. However, the decision on whether to initiate, continue or discontinue aspirin for primary prevention in and by individual patients can be challenging. As corroborated by our data, increasing age generally leads to increased bleeding risk. Thus, the current US guidelines discourage routine aspirin use in patients over 70 years, but offer little help in deciding which older patients (if any) without established ASCVD, who should be treated with aspirin. Patients with type 2 diabetes is another large and challenging patient group. European and U.S. guidelines has historically offered divergent interpretations of the available clinical trial evidence in these patients29. The recent ASCEND trial15 with a mean follow-up of 7.4 years showed that in patients with type 2 diabetes, an absolute 1.1%-point reduction of serious vascular events (8.5% with aspirin treatment vs 9.6% with placebo) was balanced by a 0.9%-point excess of major bleeding events (4.1% with aspirin treatment vs 3.2% with placebo). Notably, the most favourable risk–benefit balance was observed in individuals with the lowest vascular risk score (baseline 5-year risk of serious vascular events < 5%). In contrast, among the subgroup with the highest risk of ASCVD (baseline 5-year risk of serious vascular events ≥ 10%), the avoided number of deaths from cardiovascular causes was numerically lower than the increase in fatal bleeding events15. The most recent European and US guidelines on the use of antiplatelet agents for patients with type 2 diabetes recommends that primary prevention use of low dose aspirin may be considered ‘in patients at high/very high cardiovascular risk, in the absence of clear contraindications’ or ‘in those, who are at increased cardiovascular risk, after a discussion with the patient on the benefits versus increased risk of bleeding’30,31. Thus, there seems to be a great need for clear, supportive guidelines and easy understandable information to help clinicians and patients balancing the risks and benefits of aspirin for primary prevention based on individual patient characteristics.
Conclusion
Low-dose aspirin use for primary prevention of ASCVD is prevalent and increases with age. In 2018, it was still more prevalent than secondary prevention use. The widespread use of aspirin, especially among older adults, and substantial concomitant use of medications adding to bleeding risk warrants increased focus on discontinuation of inappropriate aspirin use.
Supplementary Information
Abbreviations
- ASCVD
Atherosclerotic cardiovascular disease
- NHIS
US National health interview survey
- AHA/ACC
American Heart Association and American College of Cardiology
- ATC
Anatomical therapeutic chemical classification
- CI
Confidence interval
- ICD-10
International classification of diseases, 10th revision
- NSAID
Nonsteroidal antiinflammatory drugs
- OTC
Over the counter
Author contributions
M.B.C., E.J.S., M.S., A.P., and E.L.G. conceived and designed the study. M.T.E. analysed the data. M.C. and E.J.S. drafted the manuscript. M.B.C., E.J.S., M.S., M.T.E., A.P., and E.L.G. interpreted the data and revised the manuscript critically. All authors approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. M.B.C. and A.P. are the guarantors of this study.
Funding
There was no specific funding for this study.
Data availability
The data that support the findings of this study are available from Statistics Denmark and restrictions apply to the availability of these data, which were used under license for the current study and therefore are not publicly available. Data are however available from the authors upon reasonable request and with permission of Statistics Denmark.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work. Authors MC, EJS, and MTE have no conflicts of interest in relation to this manuscript. Author MS reports grant from Novo Nordisk Foundation, outside the submitted work (NNF19OC0054908). Author AP reports participation in research projects funded by Alcon, Almirall, Astellas, Astra-Zeneca, Boehringer-Ingelheim, Novo Nordisk, Servier and LEO Pharma, all with funds paid to the institution where he was employed (no personal fees) and with no relation to the work reported in this paper. Outside this manuscript, ELG has received speaker honoraria or consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, MSD, Lundbeck Pharma, MundiPharma, Portola Pharmaceuticals and Roche. HELG is an investigator in the SATELLITE, FLAVOUR and ETESIAN studies (AstraZeneca) and has received research grants from Boehringer Ingelheim.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-93179-8.
References
- 1.Patrono C, Morais J, Baigent C, Collet J-P, Fitzgerald D, Halvorsen S, et al. Antiplatelet agents for the treatment and prevention of coronary atherothrombosis. J. Am. Coll. Cardiol. 2017;70(14):1760–1776. doi: 10.1016/j.jacc.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Antithrombotic Trialists’ (ATT) Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: COLLABORATIVE meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raber I, McCarthy CP, Vaduganathan M, Bhatt DL, Wood DA, Cleland JGF, et al. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet. 2019;393(10186):2155–2167. doi: 10.1016/S0140-6736(19)30541-0. [DOI] [PubMed] [Google Scholar]
- 4.Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat. Rev. Cardiol. 2019;2:2. doi: 10.1038/s41569-019-0225-y. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen M, Grove EL, Hvas A-M. Primary prevention of cardiovascular events with aspirin: Toward more harm than benefit—A systematic review and meta-analysis. Semin. Thromb. Hemost. 2019;45(05):478–489. doi: 10.1055/s-0039-1687905. [DOI] [PubMed] [Google Scholar]
- 6.Peto R, Gray R, Collins R, Wheatley K, Hennekens C, Jamrozik K, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br. Med. J. (Clin. Res. Ed.). 1988;296(6618):313–316. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steering Committee of the Physicians’ Health Study Research Group Final report on the aspirin component of the ongoing physicians’ health study. N. Engl. J. Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 8.Matthys F, De Backer T, De Backer G, Stichele RV. Review of guidelines on primary prevention of cardiovascular disease with aspirin: How much evidence is needed to turn a tanker? Eur. J. Prev. Cardiol. 2014;21(3):354–365. doi: 10.1177/2047487312472077. [DOI] [PubMed] [Google Scholar]
- 9.Fei Y, Lam CK, Cheung BMY. Efficacy and safety of newer P2Y12 inhibitors for acute coronary syndrome: A network meta-analysis. Sci. Rep. 2020;10(1):16794. doi: 10.1038/s41598-020-73871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danelich IM, Reed BN, Hollis IB, Cook AM, Rodgers JE. Clinical update on the management of atrial fibrillation. Pharmacotherapy. 2013;33(4):422–446. doi: 10.1002/phar.1217. [DOI] [PubMed] [Google Scholar]
- 11.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. Effect of aspirin on disability-free survival in the healthy elderly. N. Engl. J. Med. 2018;379(16):1499–1508. doi: 10.1056/NEJMoa1800722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N. Engl. J. Med. 2018;379(16):1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N. Engl. J. Med. 2018;379(16):1519–1528. doi: 10.1056/NEJMoa1803955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ASCEND Study Collaborative Group. Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N. Engl. J. Med. 2018;379(16):1529–1539. doi: 10.1056/NEJMoa1804988. [DOI] [PubMed] [Google Scholar]
- 16.Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): A randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036–1046. doi: 10.1016/S0140-6736(18)31924-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeil JJ, Gibbs P, Orchard SG, Lockery JE, Bernstein WB, Cao Y, et al. Effect of aspirin on cancer incidence and mortality in older adults. J. Natl. Cancer Inst. 2021;113(3):258–265. doi: 10.1093/jnci/djaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2019;2:2. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V, et al. The Danish health care system and epidemiological research: From health care contacts to database records. Clin. Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: The Danish national prescription registry. Int. J. Epidemiol. 2017;46(3):798. doi: 10.1093/ije/dyw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil registration system as a tool in epidemiology. Eur. J. Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish national patient registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015;449:2. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen L, Pratt N, Hansen MR, Hallas J, Pottegård A. Using the ‘proportion of patients covered’ and the Kaplan-Meier survival analysis to describe treatment persistence. Pharmacoepidemiol. Drug Saf. 2018;27(8):867–871. doi: 10.1002/pds.4582. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt M, Hallas J, Laursen M, Friis S. Data resource profile: Danish online drug use statistics (MEDSTAT) Int. J. Epidemiol. 2016;45(5):1401–1402g. doi: 10.1093/ije/dyw116. [DOI] [PubMed] [Google Scholar]
- 25.Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: A validation study. BMJ Open. 2016;6(11):e012832. doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: Results from the 2017 national health interview survey. Ann. Intern. Med. 2019;2:2. doi: 10.7326/M19-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: A systematic review and meta-analysis. JAMA. 2019;321(3):277–287. doi: 10.1001/jama.2018.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang KF, Shah SJ, Stafford RS. A practical approach to low-dose aspirin for primary prevention. JAMA. 2019;2:2. doi: 10.1001/jama.2019.8388. [DOI] [PubMed] [Google Scholar]
- 29.Nicolucci A, De Berardis G, Sacco M, Tognoni G. AHA/ADA vs. ESC/EASD recommendations on aspirin as a primary prevention strategy in people with diabetes: how the same data generate divergent conclusions. Eur. Heart J. 2007;28(16):1925–1927. doi: 10.1093/eurheartj/ehm248. [DOI] [PubMed] [Google Scholar]
- 30.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association 10. Cardiovascular disease and risk management: Standards of medical care in diabetes—2021. Dia Care. 2021;44(Supplement 1):S125–S150. doi: 10.2337/dc21-S010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Statistics Denmark and restrictions apply to the availability of these data, which were used under license for the current study and therefore are not publicly available. Data are however available from the authors upon reasonable request and with permission of Statistics Denmark.