Inflammation is critical for the induction of immune responses to pathogens and other foreign substances. However, excessive local and systemic inflammation can have dire consequences beyond the original site of inflammation. A link between peripheral inflammation and neuropathology has been suggested for many diseases, including influenza infection, liver cirrhosis, and irritable bowel syndrome.1–3 Arguably, the vast majority of inflammatory diseases have been associated with deleterious neurological outcomes. Likewise, immunotherapies, such as CAR T-cell treatment, are known for their high risk of cytokine release syndrome and associated neurotoxicity. However, the mechanism behind this phenomenon of inflammation-induced neuropathology remains largely misunderstood. Preclinical evidence from animal models that accurately depict this phenomenon is limited; these models will be essential to uncover the role of peripheral inflammation in neurological complications of disease. Beginning to fill this gap in understanding, Zhou et al. reported critical evidence linking lymphocytes from the inflamed gut to neurological sequelae in infants with necrotizing enterocolitis (NEC).4 These results pave the way to understanding how peripheral inflammation can adversely enhance immune activation and result in downstream neurological disease.
NEC is a devastating gastrointestinal disease in premature infants that involves sudden necrosis of the intestines and results in death in >30% of cases. Gut inflammation, bacterial dysbiosis, and TLR4 activation in the intestinal epithelium have been demonstrated to play a role in the mechanism of severe NEC.5 Unfortunately, ~45% of survivors of NEC experience long-term consequences, including severe brain injury resulting in hearing, visual, psychomotor, and cognitive impairments.6 However, the connection between NEC and brain injury has remained elusive. Zhou et al. were the first to define the mechanism through which inflammation and necrosis of the gut lead to off-target neuropathology facilitated by CD4+ T cells in newborns with NEC. Critically, these results may not be limited to neonatal disease, but may be broadly applicable to neuroinflammatory disorders of varying origins.
The characteristics of brain damage in infants with NEC include increased CD4+ T cells and activated microglia compared to infants without NEC.4 Likewise, in Zhou et al.’s mouse model of NEC, the brain shows increased abundance of CD4+ and CD8+ T cells. Brain injury in this model is associated with increased microglial activation, expression of the proinflammatory cytokine TNF-α and the neuroinflammatory marker Lcn2, and demyelination, as shown by decreased myelin basic protein levels.4,5 Further analysis suggests that the CD4+ T-cell populations mainly consist of T-helper 17 (Th17) and Th1 cells. These T cells have been implicated in autoimmunity and neurodegenerative disorders, such as multiple sclerosis,7 while other T-helper cell phenotypes did not differ from those in control brains. Depleting CD4+ T cells in NEC mice subsequently prevented damage to the CNS without altering inflammation in the ileum, suggesting that this mechanism differs from the cause of intestinal pathology in NEC mice. Similarly, injection of CD4+ T cells isolated from the brains of NEC mice into the brains of Rag1−/− mice, which lack functional T and B cells, was sufficient to induce inflammatory cytokine production, microglial activation, and loss of myelin. In addition, control CD4+ T cells did not induce brain damage, suggesting that this phenomenon is mediated by CD4+ T cells, but these T cells require a previous inflammatory trigger to induce brain damage.
CD4+ T cells perform several functions, such as facilitating the activation of macrophages, T cells, and B cells and producing cytokines. To investigate the mechanism of CD4+ T-cell-mediated brain damage, Zhou et al. established a novel in vitro brain organoid model and added CD4+ T cells isolated from control and NEC mouse brains. While control brain T cells did not have any effect, the addition of CD4+ T cells from NEC brains significantly increased inflammatory cytokine expression as well as a loss of myelin. Using a Transwell system that separates the brain organoids from NEC brain-derived T cells, they found that T cells exert an indirect effect that does not require cell–cell contact to induce inflammation and demyelination. Since CD4+ T cells infiltrating NEC mouse brains exhibit increased IFN-γ production, the authors investigated the role of IFN-γ in mediating this response. Consistent with their hypothesis, the addition of recombinant IFN-γ (rIFN-γ) to mouse brain organoid cultures increased inflammation and decreased myelination. These results were consistent with those obtained in human brain organoids, where rIFN-γ treatment induced inflammation and demyelination. Further in vivo investigation showed that injection of rIFN-γ into the brains of naïve mice induced signs of brain injury and inflammation, as observed in NEC mice. Ex vivo knockdown of IFN-γ in CD4+ T cells isolated from NEC brains prevented brain injury when transferred to Rag1−/− mice, and injection of anti-IFN-γ neutralizing antibody in the brains of NEC mice prevented inflammation and brain damage. Collectively, these results demonstrated that CD4+ T cells act as critical mediators of off-target inflammation and damage in the CNS of NEC patients via IFN-γ release (Fig. 1A). However, one question remained: where do these off-target T cells come from?
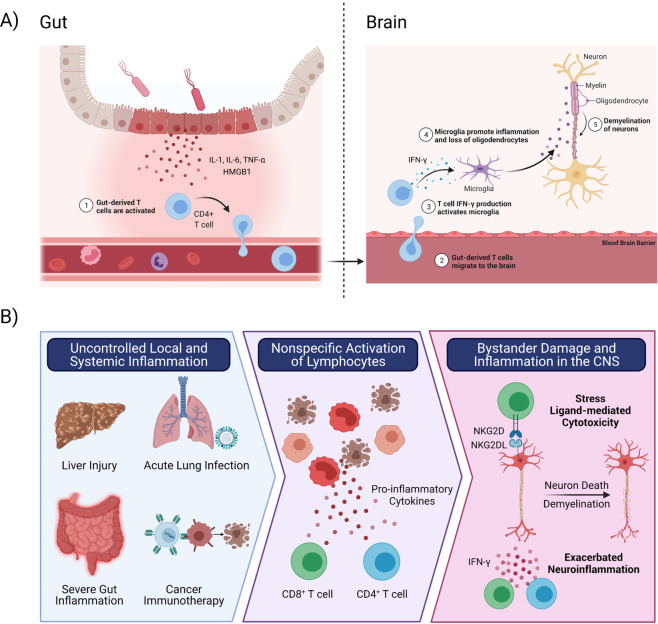
Fig. 1. Mechanisms of hyperinflammation-induced T cell activation and innate-like cytotoxicity.
A NEC is associated with severe inflammation of the gut and subsequent neurological impairment. Gut-derived CD4+ T cells are activated in the gut and recruited to the brain, where they produce IFN-γ. CD4+ T cell derived IFN-γ is required in brain organoid and NEC mouse models to induce microglial activation and neuroinflammation. This results in a loss of oligodendrocytes and myelin, causing severe neurological impairments in NEC patients. B) Excessive systemic inflammation (such as during CAR-T cell therapy and Ebola virus disease) and local inflammation in the liver (e.g. steatohepatitis and acute liver failure), lung (including Influenza and Respiratory Syncytial Virus infections), and the gut (Inflammatory Bowel Disease and NEC) has been associated with neurological disease. Zhou et al.’s work supports recent evidence that inflammation induces bystander activation of CD4+ and CD8+ T cells. These T cells traffic to the CNS where T cell derived IFN-γ promotes neuroinflammation and microglia-mediated demyelination. In neuroinflammatory conditions, expression of stress ligands on CNS resident cells can facilitate bystander CD8+ T cell cytotoxicity and demyelination. Created with BioRender.com
To investigate whether NEC mouse T cells might be derived from the inflamed gut of NEC mice, Zhou et al. isolated CD4+ T cells from the intestines and found that addition to mouse brain organoids and injection into the brains of naïve mice increased inflammation, microglial activation, and demyelination. Similarly, they found that NEC mouse gut-derived CD4+ T cells injected into the peritoneum of Rag1−/− mice were capable of migrating to the brain, suggesting that these cells could feasibly be recruited from the gut to induce brain injury. Sequencing the TCRs of brain- and gut-derived CD4+ T cells demonstrated a 25% overlap in NEC mice, compared to 2% in control mice. Thus, Zhou et al. demonstrated that gut-derived CD4+ T cells are responsible for brain injury in NEC patients (Fig. 1A).
Although Zhou et al. clearly identified a gut origin of these pathogenic CD4+ T cells, it remains unclear how these cells are activated and recruited to the CNS. Severe inflammation is known to play a key role in the development of NEC, with prominent monocyte-derived production of IL-6, IL-8, and TNF-α that promotes the expansion of pathogenic Th17 cells.8,9 The authors also previously identified proinflammatory HMGB1 in NEC mouse intestines, which has been suggested to induce Th17 cell IFN-γ production.5 Thus, inflammation in the gut of NEC patients appears to be a critical driver of T-cell activation and IFN-γ production.
Recently, the concept of inflammation-induced bystander damage caused by T cells has emerged as a novel mechanism of disease. Innate-like bystander cytotoxicity and cytokine production by antigen-unrelated CD4+ and CD8+ T cells has been implicated in the pathogenesis of diseases such as experimental autoimmune encephalitis (the mouse model of multiple sclerosis) and hepatitis A virus infection.7,10 The similar inflammatory profiles of these diseases and NEC patient guts suggest that this mechanism of inflammation-induced bystander T-cell-mediated brain damage may be applicable to many hyperinflammatory diseases (Fig. 1B). In addition, although Zhou et al. demonstrated that CD4+ T cells are sufficient to induce disease in this model, other bystander lymphocytes may also contribute. CD8+ T cells were also identified in the brains of NEC mice and can induce harmful innate-like cytotoxicity and cytokine production.10,11 CD8+ T cells have been demonstrated to perform antigen-independent, NKG2D- or NKp30-mediated killing of cells expressing stress ligands.10 Although these ligands are traditionally expressed on tumor cells or virus-infected cells, recent evidence suggests that hyperinflammation can facilitate stress ligand expression on noninfected cells in inflamed tissues, such as the liver, gut, and brain.10,12,13 Thus, both peripheral and CNS hyperinflammation may be required for stress ligand-induced brain damage by bystander CD8+ T cells (Fig. 1B). Proinflammatory cytokines, such as IL-12 and IL-18, are also capable of inducing CD8+ T-cell IFN-γ release without antigen stimulation.14 The phenomenon observed in NEC mouse brains closely resembles the bystander CD8+ T-cell IFN-γ production and subsequent demyelination observed in a model of mouse hepatitis virus infection, suggesting that this mechanism can be mediated by CD8+ or CD4+ T cells.11 Although the inflammatory profile of a disease may skew the cellular response toward either CD4+ or CD8+ T-cell-mediated damage, Zhou et al.’s results further support the theory that peripheral hyperinflammation can induce excessive activation of lymphocytes independent of antigen, with grave neurological consequences when this response is dysregulated (Fig. 1B).
Although inflammation is essential for coordinating immune responses to foreign antigens, its dysregulation in the intestines and brain of NEC patients can lead to devastating tissue damage. Zhou et al. demonstrated a fundamental connection between gut-derived CD4+ T cells and neuropathology in NEC patients. These results further indicate a potential antigen-independent mechanism of brain damage in NEC patients that involves CD4+ T-cell IFN-γ production. Considering the shared hallmarks of NEC-induced brain damage and many neuroinflammatory diseases, such as multiple sclerosis and hepatic encephalopathy, we suggest that this mechanism of inflammation-induced T-cell activation and subsequent neurological sequelae may be broadly applicable to other neurological diseases (Fig. 1B). Further investigation of this phenomenon will pave the way to understanding the complex role of peripheral inflammation in neuropathology as well as the development of effective immunoregulatory treatments.
Acknowledgements
Elizabeth Balint is a recipient of the Frederick Banting and Charles Best Canada Graduate Scholarship-Master’s from the Canadian Institutes of Health Research. Ali Ashkar is also a recipient of a CIHR Tier 1 Canada Research Chair.
References
- 1.Kawada JI, et al. J. Infect. Dis. 2003;188:690–698. doi: 10.1086/377101. [DOI] [PubMed] [Google Scholar]
- 2.Rubio T, et al. Sci. Rep. 2021;11:1–14. doi: 10.1038/s41598-020-80941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houser, M. C. npj Park. Dis. 3, 10.1038/s41531-016-0002-0 (2017).
- 4.Zhou Q, et al. Sci. Transl. Med. 2021;13:1–13. doi: 10.1126/scitranslmed.aay6621. [DOI] [Google Scholar]
- 5.Niño, D. F. et al. Sci. Transl. Med. 10, 10.1126/scitranslmed.aan0237 (2018).
- 6.Van Vliet EOG, De Kieviet JF, Oosterlaan J, Van Elburg RM. JAMA Pediatr. 2013;167:662–668. doi: 10.1001/jamapediatrics.2013.1199. [DOI] [PubMed] [Google Scholar]
- 7.Lee, H. G. et al. Nat. Commun. 10, 10.1038/s41467-019-08482-w (2019).
- 8.Pang Y, Du X, Xu X, Wang M, Li Z. Int. Immunopharmacol. 2018;59:354–360. doi: 10.1016/j.intimp.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Seo Ymi, Lin YK, Im SA, Sung IK, Youn YA. Cytokine. 2021;137:155343. doi: 10.1016/j.cyto.2020.155343. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, et al. Immunity. 2018;48:161–173.e5. doi: 10.1016/j.immuni.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Dandekar AA, Anghelina D, Perlman S. Am. J. Pathol. 2004;164:363–369. doi: 10.1016/S0002-9440(10)63126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Scaleia R, et al. Inflamm. Bowel Dis. 2012;18:1910–1922. doi: 10.1002/ibd.22899. [DOI] [PubMed] [Google Scholar]
- 13.Saikali P, et al. J. Neurosci. 2007;27:1220–1228. doi: 10.1523/JNEUROSCI.4402-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg RE, Cordes CJ, Forman J. Eur. J. Immunol. 2002;32:2807–2816. doi: 10.1002/1521-4141(2002010)32:10<2807::AID-IMMU2807>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]