Abstract
Compromised lung function is a common feature of COPD patients, but certain factors increase the rate of lung function decline in COPD patients. The objective of the current study was to investigate the effect of different clinically important factors responsible for rapid deterioration of lung function quantified as ≥ 60 ml decline in FEV1 over a period of one year. COPD patients recruited from the chest clinic of Penang hospital were followed-up for one year from August 2018 to August 2019. Rapid deterioration of lung function was defined as greater than 60 ml/year decline in force expiratory volume in one second. Among 367 included patients 73.84% were male, with mean age 65.26 (9.6) years and % predicted FEV1 51.07 (11.84). 30.27% patients showed mean decline of ≥ 60 ml in FEV1. The regression analysis showed that current smoking relative risk (RR) = 2.38 (1.78–3.07), p < 0.001); GOLD Stage III& IV RR = 1.43 (1.27–1.97), p < 0.001); mMRC score 3 to 4 RR = 2.03 (1.74–2.70), p < 0.01); SGRQ-C score ≥ 10 points difference RR = 2.01 (1.58–2.73), p < 0.01); SGRQ-C symptoms Score ≥ 10 points difference RR = 1.48 (1.23–2.29), p < 0.001); 6MWT < 350 m RR = 2.29 (1.87–3.34), p < 0.01); ≥ 3 exacerbation in study year RR = 2.28 (1.58–2.42, p < 0.001); 8 or more hospital admission days (RR = 3.62 (2.66–4.20), p < 0.001); Charlson comorbidity index ≥ 3 RR = 3.18 (2.23–3.76), p < 0.01) and emphysema RR = 1.31 (1.15–1.79), p < 0.01) were significant risk factors for the rapid deterioration of lung function (FEV1 decline ≥ 60 ml). Among different factors CCI score ≥ 3, abrupt decline in health status, exacerbation frequency ≥ 3, hospital admission days ≥ 8 and emphysema were reported as risk factors for rapid deterioration of lung function.
Subject terms: Diseases, Respiratory tract diseases, Risk factors
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with substantial economic burden and is among the leading causes of morbidity and mortality worldwide1. It is affecting more than 300 million word’s population and is responsible for 3 million deaths every year worldwide2. Spirometry test quantified as forced expiratory volume in 1 second (FEV1) is a GOLD (Global initiative for chronic obstructive lung disease) recommended COPD diagnostic test and is one of the most reliable and reproducible measurements of airflow obstruction3. Current diagnosis system relies on it as an indicator of disease progression, categorize COPD patients on the basis of disease severity, delineate risk factors, and lung function impairment under the influence of COPD3. The European Medicines Agency recommend to use the change in FEV1 value during baseline and follow-up as an indicator of efficacy of therapeutic interventions in COPD clinical trials4.
Compromised lung function is a common feature of COPD patients, but certain factors increase the rate of lung function decline in COPD patients. Excessive decline in lung function is associated with increased exacerbation, premature mortality and compromised quality of life in COPD patients5. Research shows that pharmacological therapies and interventions show more efficacy in patients with higher FEV1 than in patients with lower FEV16. Slowing down the progression of disease is an achievable goal in management of COPD3. Initially smoking was considered as primary cause for progression of COPD and quitting smoking was considered as most important preventive measure to slow down progression of disease7. Research shows smoking is not the only risk factor and quitting smoking is not the only preventive measure to reduce progression of disease8. Exposure to biomass fuel burning, pollution, atopy, alpha-1 antitrypsin deficiency, antioxidant deficiency, respiratory-tract infections, airway inflammation, asthma, emphysema, chronic bronchitis, and poor socioeconomic status are also potential risk factors for initiation and progression of COPD6,9,10.
Identification of factors responsible for rapid deterioration of lung function in COPD patients, has important public health implications. The ability to accurately identify patients at increased risk of deterioration in lung function and rapid progression of disease would help to inhibit lung function deterioration and limit disease in acute phase by taking in time measures. Knowledge about factors responsible for rapid progression of disease can also play an important role in disease management decisions and initiation or withdrawal of a therapy. This system of prognosis of disease proved its effectiveness in management of community-acquired pneumonia, where implementation of CURB-65 tool in clinical practice resulted in improved patient care11. Similar prognostic method in COPD to identify the risk factors responsible for rapid deterioration of lung function, can help to direct resources to the ones in need, reduce progression of disease, reduce exacerbation, improve patient care and reduced burden on healthcare system12.
However, the evidence of such factors on rapid deterioration of lung function is limited due to unavailability of sufficient number of studies with repeated lung function measurements during specific time period. Previous studies have examined the risk factors responsible for decline in FEV1 value in COPD patients, but they were performed in specific populations, such as patients suffering from severe COPD or frequent exacerbations8,13,14. Few studies assessed the impact of specific factors on FEV1 decline, such as specific therapies15, emphysema or chronic bronchitis16, exercise capacity17, Occupational exposures18, biomass fuel19, and clinical variables12. There is a lack of consistency in identified factors due to difference in study design, inclusion criteria, study duration and parameters investigated. Given the discrepancies between and limitations of the studies conducted to date, we investigated the effect of different clinically important factors responsible for rapid deterioration of lung function quantified as ≥ 60 ml decline in FEV1 over a period of one year in a single study. Addition of different potential factors in a multivariate analysis model gives true impact of a factor in presence of different factors.
Methodology
Study design and participants
This was a longitudinal prospective cohort. Sample size was calculated using Daniel formula on the basis of prevalence of disease20. The calculated sample size including 20% dropout was 367 COPD patients. Patients were recruited at the at chest clinic of Penang hospital. Patients were included if (1) they have confirmed diagnosis of COPD according to GOLD guidelines (FEV1/FVC ratio < 70%), (2) age ≥ 40 years, (3) no exacerbation in last 4 months, (5) enrolled in the chest clinic and having medical history of at least one year. Patients were excluded from the study if (1) they undergone through lung surgery or lung transplantation, (2) participating in any pulmonary rehabilitation program (PR), (3) inability to complete pulmonary function test and (4) inability to compete the questionnaire. Severity of COPD was defined based on GOLD 2018 classification. Ethical approval for this study was obtained from the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia (Registration number: NMRR-18-1482-42075). This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Clinical data and measured outcome
Data was collected from all included patients using a self-administered standardized questionnaire. The information obtained included social and demographic data, respiratory symptoms, smoking history, workplace history, previous asthma history, years with COPD, exacerbation history, risk factors, preventive care (influenza and pneumonia vaccination), comorbidities with Charlson Comorbidity Index (CCI), exercise capacity by six-minute walk distance test (6MWT), modified medical research council dyspnoea scale (mMRC), COPD assessment test (CAT), St. George’s Respiratory COPD specific Questionnaire (SGRQ-C) scale and pharmacological treatment during management phase and exacerbation phase.
No instructions regarding treatment were provided to the healthcare professionals and treatment was decided solely by patient’s physician. Exacerbation was defined as worsening of COPD symptoms requiring emergency department visit or hospital admission. The frequency of exacerbation was calculated during follow-up period. Patients were categorized as suffering from 1, 2, 3 or more than 3 exacerbations during study period. Severity of exacerbation was defined as hospital admission for more than 7 days or as judged by the physician. Data on comorbidities were collected from patient’s medical files. CCI was used to measure the impact of comorbidities. CCI is a standardized scale with 15 chronic diseases, graded according to the severity of disease21.
Patients recruited into the study were then followed up for one year from August 2018 to August 2019. The procedures were repeated every 4th month during the follow-up. The yearly visits data is presented here. Data recorded at the 4th, and 8th month visits have not been analyzed in this study as data on some patient characteristics were not available at these time points.
Spirometry was performed according to American thoracic society guidelines22. Spirometry was performed 45 min after bronchodilation with 400 μg salbutamol via a spacer. The mean difference in FEV1 was reported as difference in baseline visit and yearly visit. In COPD patients different thresholds of ≥ 40 mL/year and ≥ 60 ml/year8,19, have been used in literature to define rapid decline in FEV1. For the purpose of this study, decline in FEV1 ≥ 60 ml/year was considered as rapid decline because it is significant and clinically important according to ATS/ERS recommendations23. Severity of COPD was categorised according to spirometry results, in accordance with GOLD 2018 guidelines3. Grade I COPD with FEV1 ≥ 80% predicted, grade II with FEV1 50% to 80% predicted, grade III with FEV1 30% to 50% predicted, and grade IV with FEV1 < 30% predicted.
Sputum analysis was done at the pathology lab of Penang hospital to assess the presence of inflammation. A sputum eosinophil percentage ≥ 2.5% was used to define eosinophilic inflammation24. Chronic bronchitis was defined by the presence of chronic cough and sputum production for three consecutive months in two consecutive years. Computed tomography scan (CT scan) were done at the imaging unit of Penang hospital to distinguish emphysematous regions from non-emphysematous gas trapping regions. The reports were evaluated by the radiologist to confirm the presence or absence of emphysema. The presence of emphysema on CT scan was defined as well demarcated areas of decreased attenuation, as compared with contiguous normal lung tissue. The whole lung was divided into six zones (left and right zones in the upper, middle and lower lung fields). Low attenuation areas (LAA) in each image section were scored on a scale of 0 to 4, where 0 = no LAA, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100% LAA. Grades for the images of each zone were added to yield the total LAA score.
For 6MWT, patients were requested to walk as far as possible at their own pace in 6 min in long hospital corridor (48.76 m long) adjacent to the chest clinic. The distance covered was recorded in meters. Patients were allowed to stop and rest during the test, but were instructed to resume walking as soon as they felt able to do so. The test was supervised by a well-trained researcher according to the ATS guidelines25.
Health status measures
Malaysian version of SGRQ-C and mMRC dyspnea scale were used to collect data about health status26,27. SGRQ-C is a self-administered, disease specific, COPD questionnaire comprising symptoms, activity and impact subscale. Each subscale score and total score range from 0 to 100, with 100 shows the worst quality of life. For SGRQ-C a threshold of 10 points increase from baseline is considered as clinically significant deterioration in health status28. Therefore, to assess the relationship of SGRQ-C scores and rate of lung function decline, the difference in SGRQ-C scores between follow-up and baseline were categorized as ≤ 10 points difference and > 10 points difference.
CAT is an FDA approved health status questionnaire used for assessment of COPD patients. CAT is easy to understand and consists of 8 items related to symptoms and activities. Each item has scores 0 to 5 from best to worst with a maximum total score of 4029.
MMRC dyspnoea scale is a 5-item WHO recommended scale to assess the degree of breathlessness in COPD patients26. It is widely used in clinical practice and used to categorise COPD patients based on symptoms burden as recommended in 2019 GOLD guidelines3.
Analysis
Frequencies and percentages were reported for categorical variables and the mean and standard deviation for continuous variables. Normality of the data was checked using Kolmogorov–Smirnov test and Shapiro–Wilk test. Characteristics of patients in different groups stratified according to FEV1 decline were compared using independent-samples t-test for continuous variables and Chi-square test for categorical variables. Univariate and multivariate Cox regression with robust variance were performed to estimate relative risk (RR) for different factors i.e. body mass index (BMI), smoking status (ex or current smoker), age, disease severity, degree of dyspnoea exercise capacity, difference in health status over a period of one year (SGRQ-C total, symptoms, activity and impact scores), exacerbation frequency (0, 1, 2, 3, or > 3), hospital admission days, CCI scores, inflammation, LABA (long-acting beta agonist) use, LAMA (long acting muscarinic antagonist) use, vaccination, and occupational hazards on decline in FEV1 > 60 ml/year after one year of follow up. First a univariate analysis was performed to assess important factors30. Factors having substantial impact (p values < 0.20) were added in multivariate model. The same analysis was again performed on a subset of 109 patients showing ≥ 60 ml decline in FEV1 over a period of one year to assess the impact of above-mentioned factors on rapid decline of FEV1. Because the amount of missing data was only 2.3%, a complete case analysis was performed30. A p value of < 0.01 was considered to be statistically significant. An adjustment for multiple testing was made with a Bonferroni correction when applicable. All analysis were performed using STATA software (https://www.stata.com/; StataCorp. 2009. Stata 11 Base Reference Manual. College Station, TX: Stata Press).
Results
A total of 367 patients were enrolled in the study during baseline. Most of the patients were male 271 (73.84%), with mean age 65.26 (9.6) years and % predicted FEV1 51.07 (11.84). During baseline 175 (47.68%) patients were suffering from moderate COPD (GOLD Stage I&II) and 192 (52.32%) patients were suffering from severe COPD (GOLD Stage III& IV). Mean CCI score was reported as 2.03 (1.44). When stratified according to decline in FEV1 significant difference was observed in BMI, current smoking, severity of disease, mMRC score, CAT Score, SGRQ–C scores, distance covered in 6MWT and CCI among the patients with ≤ 60 ml decline in FEV1 and ≥ 60 ml decline in FEV1. Baseline demographic and clinical data of COPD patients stratified according to decline in FEV1 are presented in Table 1.
Table 1.
Baseline demographic and clinical data of COPD patients included in the study.
| Variables | Overall COPD patients (367) |
≤ 60 ml decline in FEV1 (253) |
≥ 60 ml decline in FEV1 (114) |
p value a |
|---|---|---|---|---|
| No. of patients | 367 (100%) | 253 (68.93%) | 114 (31.06%) | – |
| Age | 65.26 (9.6) | 64.38 (9.5) | 67.21 (9.5) | 0.37 |
| Male | 271 (73.84%) | 189 (73.25%) | 82 (71.92%) | 0.65 |
| BMI | 24.23 (4.27) | 24.85 (4.21) | 22.04 (3.89) | < 0.01 |
| Years with COPD | 7.2 (6.1) | 6.3 (5.6) | 9.3 (6.4) | 0.31 |
| Smoking status | ||||
| Current smokers | 68 (18.89%) | 29 (11.46%) | 39 (34.21%) | < 0.001 |
| Ex-smokers | 301 (82.01%) | 209 (82.60%) | 92 (80.70%) | 0.26 |
| Post-bronchodilator spirometry | ||||
| FEV1% | 51.07 (11.84) | 56.67 (11.54) | 43.41 (11.19) | < 0.01 |
| FEV1 (L) | 1.38 (0.54) | 1.56 (0.51) | 1.14 (0.60) | < 0.01 |
| GOLD Stage I & II | 175 (47.68%) | 119 (47.03%) | 56 (49.12%) | 0.29 |
| GOLD Stage III & IV | 192 (52.32%) | 134 (52.97%) | 58 (50.88%) | < 0.001 |
| Medication | ||||
| LABA | 236 (64.31%) | 183 (72.33%) | 53(46.49%) | < 0.01 |
| LAMA | 191 (52.04%) | 130 (68.06%) | 61 (53.51%) | 0.12 |
| Health status measures | ||||
| mMRC dyspnea | 2.76 (0.8) | 2.37 (0.8) | 3.30 (1.10) | < 0.001 |
| CAT Score | 20.05 (7.62) | 17.43 (6.75) | 26.68 (7.12) | < 0.01 |
| SGRQ-C Total | 46.48 (28.61) | 40.66 (35.48) | 59.30 (36.65) | < 0.001 |
| SGRQ-C Symptom Score | 51.46 (29.48) | 46.23 (25.95) | 58.70 (29.33) | < 0.001 |
| SGRQ-C Activity Score | 45.91 (28.17) | 44.42 (26.5) | 51.4 (29.53) | < 0.01 |
| SGRQ-C Impact Score | 40.47 (32.78) | 38.87 (29.72 | 48.07 (27.24) | 0.07 |
| 6MWT in meters | 429.8 (84.32) | 484.2 (73.45) | 339.4 (87.14) | < 0.001 |
| Comorbidities | ||||
| CCI | 2.03 (1.44) | 1.47 (0.92) | 2.9 (1.34) | < 0.01 |
Data are presented as n (%) and mean (SD) unless otherwise stated.
BMI, body mass index; COPD, Chronic Obstructive Pulmonary Disease; CAT, COPD assessment test; CCI, Charlson comorbidity Index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV1%, percentage predicted FEV1; GOLD, global initiative for Chronic Obstructive Lung Disease; GOLD Stage I (FEV1 ≥ 80% predicted); GOLD stage II (50% ≤ FEV1 < 80% predicted); GOLD Stage III (30% ≤ FEV1 < 50% predicted); GOLD Stage IV (FEV1 < 30% predicted); LABA, long-acting beta agonist; LAMA, long acting muscarinic antagonist; 6MWT, six minute walking distance travelled; mMRC, modified medical research council dyspnoea scale, SGRQ-C, St George’s Respiratory COPD specific Questionnaire.
aThe difference was assessed among groups stratified according to decline in FEV1.
Longitudinal assessment
Three hundred and sixty patients completed the follow-up. Seven patients lost the follow-up due to different reasons (Fig. 1).
Figure 1.
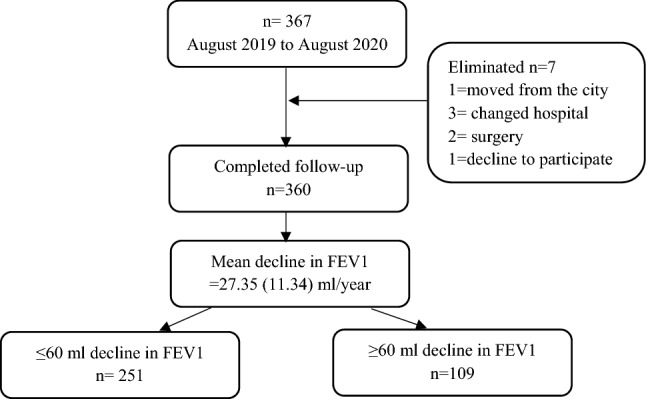
Consort diagram of the study population.
During the study period mean decline in FEV1 was observed as 27.35 (11.34) ml. Among the included patients 251 (69.72%) patients showed mean decline of ≤ 60 ml, while 109(30.27%) patients showed mean decline of ≥ 60 ml in FEV1. Smoking cessation was observed in 17(4.72%) patients. 31(8.3%) patients moved from moderate COPD to severe COPD at the end of the study year. Emphysema and chronic bronchitis were present in 198 (55%) and 220 (61.11%) patients. During the follow-up 130 (36.11%) patients didn’t suffer to an exacerbation, 148 (41.11%) patients suffered ≤ 2 exacerbation and 82 (22.77%) patients suffered ≥ 3 exacerbation (Figs. 2 and 3).
Figure 2.
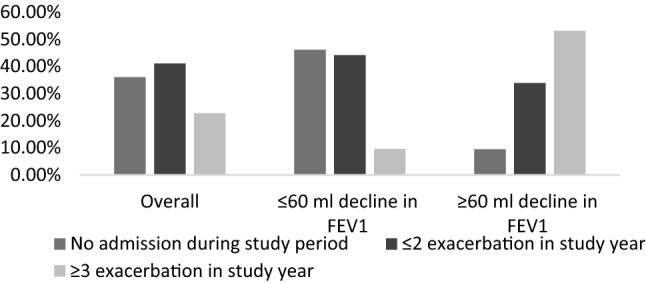
Frequency of exacerbation stratified according to FEV1 decline.
Figure 3.
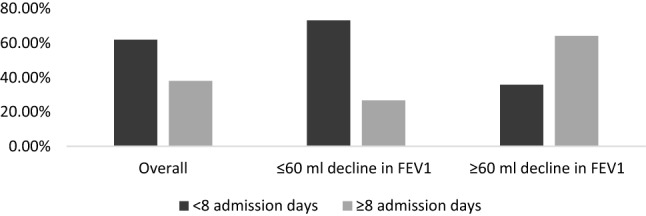
Hospital admission days stratified according to FEV1 decline.
Demographic and clinical characteristics of COPD patients stratified according to decline in FEV1 after one year of follow-up are presented in Table 2.
Table 2.
Demographic and clinical data of COPD patients included in the study after one year follow-up.
| Variables | Overall | ≤ 60 ml decline in FEV1 (251) | ≥ 60 ml decline in FEV1 (109) | p value a |
|---|---|---|---|---|
| No. of patients | 360 (100%) | 251 (69.72%) | 109 (30.27%) | – |
| BMI | 23.97 (4.19) | 24.53 (5.2) | 21.62 (4.26) | < 0.01 |
| Current smokers | 51 (14.16%) | 24 (9.56%) | 27 (28.44%) | < 0.001 |
| Post-bronchodilator spirometry | ||||
| FEV1% | 51.03 (10.21) | 54.7 (10.43) | 37.36 (10.75) | < 0.01 |
| FEV1 (L) | 1.35 (0.66) | 1.51 (0.32) | 1.06 (0.42) | < 0.01 |
| GOLD Stage I & II | 142 (39.44%) | 111 (44.22%) | 31 (28.44%) | 0.21 |
| GOLD Stage III & IV | 218 (60.56%) | 140 (55.77%) | 78 (71.55%) | < 0.001 |
| Health status measures | ||||
| mMRC dyspnea | 2.92 (0.8) | 2.42 | 3.87 (0.9) | < 0.001 |
| CAT Score | 22.71 (6.88) | 18.46 (7.62) | 29.96 (7.37) | < 0.01 |
| SGRQ-C Total | 52.23 (26.44) | 44.26 (25.54) | 68.20 (26.12) | < 0.001 |
| SGRQ-C Symptom Score | 53.4 (28.32) | 51.35 (23.42) | 67.45 (30.28) | < 0.001 |
| SGRQ-C Activity Score | 48.69 (26.43) | 45.97 (26.5 | 59.41 (26.42) | < 0.01 |
| SGRQ-C Impact Score | 42.37 (30.82) | 39.36 (28.34) | 57.39 (27.21) | < 0.01 |
| 6MWT in meters | 418.5 (80.28) | 481.8 (76.42) | 323.3 (78.40) | < 0.001 |
| 6MWT > 350 m | 221 (61.39%) | 193 (76.89%) | 28 (25.69%) | |
| 6MWT < 350 m | 139 (38.61%) | 58 (23.10%) | 81 (74.31%) | |
| Medication | ||||
| LABA | 242 (67.22%) | 189 (75.30%) | 53(48.62%) | < 0.01 |
| LAMA | 186 (52.04%) | 131 (52.19%) | 55 (50.46%) | 0.17 |
| Comorbidity Index | ||||
| CCI | 2.12 (1.44) | 1.49 (0.92) | 3.1 (1.34) | < 0.001 |
| CCI < 3 | 255 (69.48%) | 219 (86.56%) | 36 (31.58%) | |
| CCI ≥ 3 | 112 (30.51%) | 34 (13.44%) | 78 (68.42%) | |
| Emphysema | 198 (55%) | 125 (49.80) | 73 (66.97%) | < 0.01 |
| Chronic bronchitis | 220 (61.11%) | 133 (52.99%) | 87 (79.82%) | < 0.01 |
| Exacerbation | ||||
| Exacerbation per year | 1.20 (1.4) | 0.91 (1.1) | 2.6 (1.7) | < 0.01 |
| No admission during study period | 130 (36.11%) | 116 (46.21%) | 14 (9.47%) | 0.19 |
| ≤ 2 exacerbation in study year | 148 (41.11%) | 111 (44.22%) | 37 (33.95% | < 0.001 |
| ≥ 3 exacerbation in study year | 82 (22.77%) | 24 (9.56%) | 58 (53.21%) | < 0.001 |
| Hospital stay days | 4.9 (4.8) | 4.5 (4.2) | 7.2 (5.3) | < 0.001 |
| < 8 admission days | 223 (61.94%) | 184 (73.30%) | 39 (35.78%) | |
| ≥ 8 admission days | 137 (38.06%) | 67 (26.69%) | 70 (64.22%) | |
| Eosinophilic inflammation | ||||
| Eosinophil count % | 1.7% (1–2.8) | 1.7% (1.1–2.8) | 1.8% (1.1–2.1) | 0.12 |
| Eosinophil count ≥ 3% | 141 (31.67%) | 62 (24.70%) | 52 (47.60%) | |
| Vaccination | 68 (18.89%) | 57 (22.71%) | 11 (10.09%) | < 0.01 |
| Occupational hazards | 48 (13.33) | 31 (12.35%) | 17 (15.60%) | < 0.01 |
Data are presented as n (%) and mean (SD) unless otherwise stated.
aThe difference was assessed among groups stratified according to decline in FEV1.
BMI, body mass index; COPD, Chronic Obstructive Pulmonary Disease; CAT, COPD assessment test; CCI, Charlson comorbidity Index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV1%, percentage predicted FEV1; GOLD, global initiative for Chronic Obstructive Lung Disease; GOLD Stage I (FEV1 ≥ 80% predicted); GOLD stage II (50% ≤ FEV1 < 80% predicted); GOLD Stage III (30% ≤ FEV1 < 50% predicted); GOLD Stage IV (FEV1 < 30% predicted); LABA, long-acting beta agonist; LAMA, long acting muscarinic antagonist; 6MWT, six minute walking distance travelled; mMRC, modified medical research council dyspnoea scale, SGRQ-C, St George’s Respiratory COPD specific Questionnaire.
After one year of follow-up statistically significant difference was observed in mMRC score (p < 0.01), CAT Score (p < 0.001), SGRQ-C total Score (p < 0.001) and SGRQ-C symptom score (p < 0.01) as compared to their baseline values. Mean change in health status scores after one year from baseline is represented in Table 3.
Table 3.
Change in health status measures scores from baseline to one year follow-up.
| Health status measures | Baseline values | One year follow-up values | Mean difference | p value |
|---|---|---|---|---|
| mMRC | 2.76 (0.8) | 2.92 (0.8) | 0.16 (0.04) | < 0.01 |
| CAT Score | 20.05 (7.62) | 22.71 (6.88) | 2.66 (1.2) | < 0.001 |
| SGRQ-C Total | 46.48 (28.61) | 52.83 (26.44) | 6.35 (4.53) | < 0.001 |
| SGRQ-C Symptom Score | 50.46 (29.48) | 55.4 (28.32) | 4.94 (3.61) | < 0.01 |
| SGRQ-C Activity Score | 44.91 (28.17) | 50.69 (26.43) | 5.78 (3.50) | 0.03 |
| SGRQ-C Impact Score | 39.11 (32.78) | 43.77 (30.82) | 4.66 (7.61) | 0.19 |
| 6MWT in meters | 429.8 (84.32) | 418.5 (80.28) | − 11.3 | < 0.01 |
Data are presented as mean (SD) unless otherwise stated.
COPD, Chronic Obstructive Pulmonary Disease; mMRC, modified medical research council dyspnoea scale; CAT, COPD assessment test; SGRQ-C, St George’s Respiratory COPD specific Questionnaire.
Risk factors influencing ≤ 60 ml decline in FEV1 in COPD patients
After one year of follow-up 251 patients showed ≤ 60 ml decline in FEV1. Table 4 shows the risk associated with different factors for ≤ 60 ml decline in FEV1. The multivariate regression analysis showed that age RR = 1.12 (0.79–1.34), p < 0.01; current Smokers RR = 3.27 (2.56–3.34), p < 0.001; GOLD Stage III& IV RR = 3.48 (2.72–4.36), p < 0.001; mMRC score 2 to 3 RR = 2.17 (1.64–2.88), p < 0.01; mMRC score 3 to 4 RR = 2.54 (2.13–3.20), p < 0.001; SGRQ-C score ≥ 10 points difference RR = 3.27 (2.78–3.91), p < 0.001; SGRQ-C symptoms Score ≥ 10 points difference RR 2.32 (1.93–3.18), p < 0.001; SGRQ-C activity Score ≥ 10 points difference RR = 1.74 (1.79–2.80), p < 0.001; 6MWT < 350 m RR = 2.69 (2.19–3.84), p < 0.001; ≤ 2 exacerbation in study year RR = 1.54 (1.15–1.84), p < 0.01; ≥ 3 exacerbation in study year RR = 3.49 (2.85–3.73), p < 0.001; 8 or more hospital admission days RR = 3.91 (3.36–4.30), p < 0.001; CCI score ≤ 3 RR = 1.49 (1.14–1.72), p < 0.01; CCI ≥ 3 RR = 3.46 (2.73–4.13), p < 0.001; emphysema RR = 1.24 (1.03–1.52), p < 0.01; chronic bronchitis RR = 1.12 (0.86–1.35), p < 0.01; eosinophil count ≥ 3% RR = 1.78 (0.82–2.12), p < 0.001; and occupational Hazards RR = 1.43 (1.14–1.88), p < 0.01 were significant risk factors for normal deterioration of lung function (FEV1 decline ≤ 60 ml) in COPD patients.
Table 4.
Multiple logistic regression models to assess the risk factors associated with ≤ 60 ml decline in FEV1 in COPD patients.
| Risk factors | ≤ 60 ml decline in FEV1 (251) | ||
|---|---|---|---|
| RR | 95% CI | Sig | |
| BMI | 1.21 | 0.85–1.31 | 0.11 |
| Age | 1.12 | 0.79–1.34 | 0.01 |
| Gender | 0.73 | 0.29–1.04 | 0.08 |
| Smoking status | |||
| Current smokers | 3.27 | 2.56–3.34 | < 0.001 |
| Disease severity | |||
| GOLD Stage I & II | 0.74 | 0.47–1.13 | 0.21 |
| GOLD Stage III & IV | 3.48 | 2.72–4.36 | < 0.001 |
| mMRC Dyspnea | |||
| mMRC score 2 to 3 | 2.17 | 1.64–2.88 | < 0.01 |
| mMRC score 3 to 4 | 2.54 | 2.13–3.20 | < 0.001 |
| Difference in health status measure Scores over a period of one year | |||
| SGRQ-C score ≥ 10 points difference | 3.27 | 2.78–3.91 | < 0.001 |
| SGRQ-C symptoms Score ≥ 10 points difference | 2.32 | 1.93–3.18 | < 0.001 |
| SGRQ-C activity Score ≥ 10 points difference | 1.74 | 1.79–2.80 | < 0.001 |
| Exercise capacity | |||
| 6MWT > 350 m | 1.13 | 0.81–1.29 | 0.07 |
| 6MWT < 350 m | 2.69 | 2.19–3.84 | < 0.001 |
| Exacerbation | |||
| ≤ 2 exacerbation in study year | 1.54 | 1.15–1.84 | 0.01 |
| ≥ 3 exacerbation in study year | 3.49 | 2.85–3.73 | < 0.001 |
| Hospital admission days | |||
| 1 to 7 | 1.31 | 0.91–1.49 | 0.05 |
| 8 or more | 3.91 | 3.36–4.30 | < 0.001 |
| Comorbidities | |||
| CCI ≤ 3 | 1.49 | 1.14–1.72 | < 0.01 |
| CCI ≥ 3 | 3.46 | 2.73–4.13 | < 0.001 |
| Emphysema | 1.24 | 1.03–1.52 | < 0.01 |
| Chronic Bronchitis | 1.12 | 0.86–1.35 | < 0.01 |
| Inflammation | |||
| Eosinophil count ≥ 3% | 1.78 | 0.82–2.12 | < 0.001 |
| Vaccination | 0.53 | 0.27–0.69 | 0.42 |
| Medication | |||
| LABA | 0.72 | (0.64–0.88) | < 0.01 |
| LAMA | 0.79 | (0.62–0.92) | < 0.01 |
| Occupational Hazards | 1.43 | 1.14–1.88 | < 0.01 |
BMI, body mass index; CI, confidence interval; COPD, Chronic Obstructive Pulmonary Disease; CCI, Charlson comorbidity Index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV1%, percentage predicted FEV1; GOLD, global initiative for Chronic Obstructive Lung Disease; GOLD I (FEV1% > 80); GOLD II (FEV1% 50–79); GOLD III (FEV1% 30–49); GOLD IV (FEV1% < 30); mMRC, modified medical research council dyspnoea scale, SGRQ-C, St George’s Respiratory COPD specific Questionnaire; LABA, long-acting beta agonist; LAMA, long acting muscarinic antagonist; RR, Relative Risk; spirometry was post bronchodilator; Vaccination, Pneumonia and influenza vaccination; 6MWT, six minute walking distance test.
Risk factors influencing the rapid lung function deterioration (≥ 60 ml decline in FEV1) in COPD patients
After one year follow-up 109 patients showed rapid deterioration in lung function (≥ 60 ml decline in FEV1). Table 5 shows the risk associated with different factors for ≥ 60 ml decline in FEV1. The regression analysis showed that current smoking RR = 2.38 (1.78–3.07), p < 0.001; GOLD Stage III& IV RR = 1.43 (1.27–1.97), p < 0.001; mMRC score 3 to 4 RR = 2.03 (1.74–2.70), p < 0.01; SGRQ-C score ≥ 10 points difference RR = 2.01 (1.58–2.73), p < 0.01; SGRQ-C symptoms Score ≥ 10 points difference RR = 1.48 (1.23–2.29), p < 0.001; 6MWT < 350 m RR = 2.29 (1.87–3.34), p < 0.01; ≥ 3 exacerbation in study year RR = 2.28 (1.58–2.42, p < 0.001; 8 or more hospital admission days RR = 3.62 (2.66–4.20), p < 0.001; CCI ≥ 3 RR = 3.18 (2.23–3.76), p < 0.01 and emphysema RR = 1.31 (1.15–1.79), p < 0.01 were significant risk factors for the rapid deterioration of lung function (FEV1 decline ≥ 60 ml).
Table 5.
Multiple logistic regression models to assess the risk factors associated with ≥ 60 ml decline in FEV1 in COPD patients.
| Risk factors | ≥ 60 ml decline in FEV1 (109) | ||
|---|---|---|---|
| RR | 95% CI | Sig | |
| BMI | 1.15 | 0.73–1.35 | 0.17 |
| Age | 0.74 | 0.35–1.01 | 0.36 |
| Gender | 0.67 | 0.37–0.97 | 0.14 |
| Smoking status | |||
| Current smokers | 2.38 | 1.78–3.07 | < 0.001 |
| Disease severity | |||
| GOLD Stage I & II | 0.87 | 0.61–1.25 | 0.42 |
| GOLD Stage III & IV | 1.43 | 1.27–1.97 | < 0.001 |
| mMRC dyspnea | |||
| mMRC score 2 to 3 | 1.36 | 1.17–1.82 | 0.22 |
| mMRC score 3 to 4 | 2.03 | 1.74–2.70 | < 0.01 |
| Difference in health status measure Scores over a period of one year | |||
| SGRQ-C score ≥ 10 points difference | 2.01 | 1.58–2.73 | < 0.01 |
| SGRQ-C symptoms Score ≥ 10 points difference | 1.48 | 1.23–2.29 | < 0.001 |
| SGRQ-C activity Score ≥ 10 points difference | 1.15 | 1.01–1.48 | 0.22 |
| Exercise capacity | |||
| 6MWT > 350 m | 0.81 | 0.21–0.97 | 0.53 |
| 6MWT < 350 m | 2.29 | 1.87–3.34 | < 0.01 |
| Exacerbation | |||
| ≤ 2 exacerbation in study year | 1.07 | 0.61–1.44 | 0.18 |
| ≥ 3 exacerbation in study year | 2.28 | 1.58–2.42 | < 0.001 |
| Hospital admission days | |||
| 1 to 7 | 1.04 | 0.53–1.32 | 0.23 |
| 8 or more | 3.62 | 2.66–4.20 | < 0.001 |
| Comorbidities | |||
| CCI ≤ 3 | 1.12 | 0.46–1.42 | 0.30 |
| CCI ≥ 3 | 3.18 | 2.23–3.76 | < 0.01 |
| Emphysema | 1.31 | 1.15–1.79 | < 0.01 |
| Chronic Bronchitis | 1.18 | 0.77–1.45 | 0.05 |
| Inflammation | |||
| Eosinophil count ≥ 3% | 1.39 | 0.82–2.12 | < 0.001 |
| Vaccination | 0.67 | 0.29–0.86 | 0.32 |
| Medication | |||
| LABA | 0.75 | (0.64–0.86) | 0.03 |
| LAMA | 0.82 | (0.72–1.09) | 0.15 |
| Occupational hazards | 1.21 | 0.73–1.47 | 0.10 |
BMI, body mass index; CI, confidence interval; COPD, Chronic Obstructive Pulmonary Disease; CCI, Charlson comorbidity Index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV1%, percentage predicted FEV1; GOLD, global initiative for Chronic Obstructive Lung Disease; GOLD I (FEV1% > 80); GOLD II (FEV1% 50–79); GOLD III (FEV1% 30–49); GOLD IV (FEV1% < 30); mMRC, modified medical research council dyspnoea scale, SGRQ-C, St George’s Respiratory COPD specific Questionnaire; LABA, long-acting beta agonist; LAMA, long acting muscarinic antagonist; RR, Relative Risk; spirometry was post bronchodilator; Vaccination, Pneumonia and influenza vaccination; 6MWT, six minute walking distance test.
Discussion
In COPD management multidimensional assessment is an established strategy for better assessment of patients. But it is still confusing which factors are best predictor for rapid deterioration of lung function. This study reports impact of different factors on rapid deterioration of lung function in COPD patients. current smoking, disease severity, mMRC score 3 to 4, SGRQ-C score ≥ 10 points difference, SGRQ-C symptom score ≥ 10 points difference, 6MWT < 350 m, ≥ 3 exacerbation per year, 8 or more hospital admission days, CCI ≥ 3 and emphysema were significantly associated with decline in FEV1 ≥ 60 ml/year. During the study year mean annual decline in FEV1 was observed as 27.35 (11.34) ml/year, whereas, 30.27% patients showed more than 60 ml/year decline in FEV1. This is quite close to a previously reported study in which they reported that one third of the patients showed a rapid decline of 78 ml/ year in FEV1 value31. Another study reported mean decline of 66 ml/year in FEV1 in patients with rapidly declining FEV1 as compared to mean decline of 28 ml/year in rest of the patients8.
Health status of COPD patients was assessed using SGRQ-C questionnaire. The rate of progression in lung function decline increased with increase in the difference between baseline and follow-up scores. Patients who reported greater difference in baseline and follow-up SGRQ-C scores showed rapid deterioration of lung function. The rate was significant when patients showed ≥ 10 points difference in total and symptom score over a period of one year. Our results are in line with the study of Alma et al. who reported that, a difference of 10 points in SGRQ-C score was associated with significant deterioration in health status of COPD patients28. Our results strengthen the fact that deterioration in health status is associated with rapid decline in lung function.
Severity of Dyspnea was assessed using mMRC dyspnea scale. MMRC dyspnea scale is a well-established tool to assess the severity of dyspnea. Higher score indicates higher level of breathlessness26. Higher dyspnea score was significantly related to rapid deterioration of lung function. In fact, it was one of the strongest risk factors for FEV1 decline. Dyspnoea is associated with physiological, cognitive, psychological and emotional responses. These responses may stimulate the rapid deterioration of lung function. In a large prospective study dysponea was reported to be a better predictor of mortality than the BODE index32. Thus, controlling dyspnea can slow down the progression of COPD. Different therapeutic interventions such as desensitization, relaxation and breathing retraining, monitored activity training and self-management can help to minimize the effects of dysponea and limit the rate of lung function decline33. Severity of disease showed significant impact on the progression of disease. People in GOLD grade I and II didn’t showed significant decline in lung function. Patients with severe airway obstruction were prone to rapid disease progression. This may be due to the fact that in severe airway obstruction, lungs have reduced respiratory reserves and decompensate in response to small triggers11. GOLD III and GOLD IV patients experience severe level of dyspnoea. Dyspnoea, causes forceful berating and utilization of energy to breath. This causes load on lungs and results in rapid destruction of airways32.
Emphysema and chronic bronchitis are important clinical manifestations of the COPD and are present in majority of the COPD patients. In patients with COPD emphysema was associated with rapid deterioration of lung function. Previous research reported that emphysema was associated with rapid deterioration of lung function in severe COPD patients16. Another research showed that presence of emphysema in COPD patients was associated with higher decline in FEV1 irrespective of the severity of disease34. Thus, assessment of emphysema should be included in routine clinical practice to identify patients at increased risk of lung function decline.
Exacerbation causes deterioration of COPD symptoms increase the risk of mortality and is associated with substantial economic burden on healthcare system35–37. Thus, prevention of exacerbation is a key goal in the management of COPD6. Impact of exacerbation frequency and hospital admission days was observed on rapid deterioration of lung function. For exacerbation frequency a significant difference was observed on rapid deterioration of lung function in patients suffering from 3 or more exacerbations during study period. Our findings are in line with the current literature. Previous studies demonstrated that frequent exacerbations cause rapid deterioration of lung function and exercise capacity38. Severe exacerbation requires long hospital stay. COPD patients who spent more than 8 days admitted in hospital showed rapid deterioration of lung function. This in in line with the previous research, which reported that each severe exacerbation event was associated with 87 ml/yr decline in FEV1 in COPD patients39.
Impact of comorbidities on rapid progression of disease was observed for CCI score. CCI score ≥ 3 was significantly associated with rapid deterioration of lung function. COPD is caused by inflammation of lungs. The drainage of inflammatory mediators from respiratory system into systemic circulation may trigger or worsen the effect of different comorbid conditions, such as peripheral vascular disease, hypertension, osteoporosis, diabetes and depression, which in turn results in worsening of COPD40,41. In a previous research coronary heart disease and peripheral vascular disease in addition to depression and anxiety were reported to have substantial effect on health status of COPD patients42. This shows a substantial relation among comorbidities and health status of COPD patients. Thus management of comorbid conditions can help to limit the disease in acute stage, reduce mortality and improve health status.
The 6MWT is a validated test to measure exercise capacity in COPD patients. Impaired exercise capacity is a well-established predictor of impaired lung function, exacerbation and mortality in COPD patients. Patients with mean walking distance < 350 were at increased risk of lung function deterioration. Exercise capacity decrease with the progression of disease. According to Celli reduced exercise capacity is the most reliable predictor of disease progression and should be considered in prognosis or worsening of COPD overtime43. Pinto-Plata et al. found that consistent decline in 6MWT was reliable predictor of COPD worsening over time and increased risk of mortality44. Thus, 6MWT can be used to assess the risk of rapid progression of disease over time.
In recent research inflammatory biomarkers are getting importance to categorize COPD patients and predict different outcomes like exacerbation and disease progression. Presence of high percentage of eosinophil cells in small airways was associated with increased risk of rapid decline in FEV1. Continuous cough, damage of airways or bacterial infection may increase the release of eosinophil from bronchial glands into the lumen9. Previous studies also reported that higher eosinophil count was associated with deterioration of lung function over time45. Our study strengthens this evidence.
This study may have few limitations. Patients reported outcomes may involve recall biasness, which can result in underreporting or over-reporting of an outcome. Biasness was reduced by cross matching the answers with patient medical files. Another limitation of the study was relatively small follow-up period of one year and sample sizes for subgroup analysis. Number of patients in rapid decline group were relatively small, which can affect the statistical power of the study to find risk factors for rapid deterioration of lung function. But despite few limitations, this study has several strengths. To ensure generalizability all eligible patients were included and no instructions regarding treatment were given to the healthcare professionals and treatment was decided solely by patient’s physician. Detailed characterization of the rapid decline group was performed. For comorbidities CCI score was used which gives better understanding of the impact of comorbidities according to severity and avoid underestimation of the impact of comorbidities. Most of the identified factors i.e. health status, symptoms, and occupational hazards were modifiable. Knowledge about these modifiable factors can ensure optimal monitoring of specific patients and identifying specific subgroups that may benefit from future novel therapies. Finding the risk factors to trigger disease progression can also help the health care practitioners and policy makers to limit disease in acute stage by taking in time measures. Moreover, during study period 32 (8.71%) patients showed no change in FEV1 value. This shows that lung function decline in COPD patients can be inhibited for longer period of time. Future investigations should focus on finding the factors which can be helpful in inhibiting the deterioration of lung function in COPD patients over a longer period of time. Emphasis should be placed on factors that can be easily adopted in clinical practice.
Conclusions
In COPD management multidimensional assessment is an established strategy for better assessment of patients. During the study period mean annual decline in FEV1 was observed as 27.35 ml, whereas, 30.27% patients showed ≥ 60 ml decline in FEV1 value. Among different factors increased symptoms load, CCI score ≥ 3, abrupt decline in health status, exacerbation frequency ≥ 3, hospital admission days ≥ 8, chronic bronchitis and emphysema were reported as risk factors for rapid deterioration of lung function. Most of the identified factors were modifiable and can be controlled through proper management. Thus assessment of above mentioned factors should be included in routine clinical practice to identify patients at increased risk of lung function decline.
Acknowledgements
We would like to thank the Director-General of Health, Malaysia for his permission to publish this study. We are also thankful to the patients and staff from the Chest Clinic of the Penang Hospital.
Author contributions
A.R., S.S., G.A., S.N.H., S.S., R.H., M.A. and M.F.R. made substantial contributions to conception and design of the study, and analysis and interpretation of the data. All authors drafted the work or revised it critically for important intellectual content. All authors reviewed, critiqued, and approved the final version submitted for publication.
Data availability
Data is available on request from corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ur Rehman A, Ahmad Hassali MA, Muhammad SA, Shah S, Abbas S, Hyder Ali IAB, et al. The economic burden of Chronic Obstructive Pulmonary Disease (COPD) in USA, Europe and Asia: Results from a systematic review of the literature. Expert Rev. Pharmacoecon. Outcomes Res. 2020;20:661–672. doi: 10.1080/14737167.2020.1678385. [DOI] [PubMed] [Google Scholar]
- 2.López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. doi: 10.1111/resp.12660. [DOI] [PubMed] [Google Scholar]
- 3.GOLD. Global Initiative for Chronic Obstructive Lung Disease. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.6-FINAL-08Nov2018-wms.pdf (2019).
- 4.European Medicines Agency. Guidelines on clinical investigation of medicinal products in the treatment of chronic obstructive pulmonary disease (COPD). European Medicines Agency www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/08/WC500130880. Accessed March 2020.
- 5.Young R, Hopkins R, Eaton T. Forced expiratory volume in one second: Not just a lung function test but a marker of premature death from all causes. Eur. Respir. J. 2007;30(4):616–622. doi: 10.1183/09031936.00021707. [DOI] [PubMed] [Google Scholar]
- 6.ur Rehman A, Hassali MAA, Abbas S, Ali IABH, Harun SN, Muneswarao J, et al. Pharmacological and non-pharmacological management of COPD; limitations and future prospects: a review of current literature. J. Public Health. 2020;28:357–366. doi: 10.1007/s10389-019-01021-3. [DOI] [Google Scholar]
- 7.Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: Definition, assessment, and prevention. The Lancet. 2015;385(9979):1778–1788. doi: 10.1016/S0140-6736(15)60647-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence PJ, Kolsum U, Gupta V, Donaldson G, Singh R, Barker B, et al. Characteristics and longitudinal progression of chronic obstructive pulmonary disease in GOLD B patients. BMC Pulm. Med. 2017;17(1):42. doi: 10.1186/s12890-017-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contoli M, Baraldo S, Conti V, Gnesini G, Marku B, Casolari P, et al. Airway inflammatory profile is correlated with symptoms in stable COPD: A longitudinal proof-of-concept cohort study. Respirology. 2020;25(1):80–88. doi: 10.1111/resp.13607. [DOI] [PubMed] [Google Scholar]
- 10.Borné Y, Ashraf W, Zaigham S, Frantz S. Socioeconomic circumstances and incidence of chronic obstructive pulmonary disease (COPD) in an urban population in Sweden. COPD J. Chronic Obstr. Pulm. Dis. 2019;16(1):51–57. doi: 10.1080/15412555.2019.1582618. [DOI] [PubMed] [Google Scholar]
- 11.Chalmers JD, Singanayagam A, Akram AR, Mandal P, Short PM, Choudhury G, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax. 2010;65(10):878–883. doi: 10.1136/thx.2009.133280. [DOI] [PubMed] [Google Scholar]
- 12.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. New Engl. J. Med.. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 13.Casanova C, de Torres JP, Aguirre-Jaíme A, Pinto-Plata V, Marin JM, Cordoba E, et al. The progression of chronic obstructive pulmonary disease is heterogeneous: The experience of the BODE cohort. Am. J. Respir. Crit. Care Med. 2011;184(9):1015–1021. doi: 10.1164/rccm.201105-0831OC. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Villar A, Represas-Represas C, Mouronte-Roibás C, Ramos-Hernández C, Priegue-Carrera A, Fernández-García S, et al. Reliability and usefulness of spirometry performed during admission for COPD exacerbation. PLoS ONE. 2018;13(3):e0194983. doi: 10.1371/journal.pone.0194983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am. J. Respir. Crit. Care Med. 2008;178(4):332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2016;194(2):178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spruit MA, Watkins ML, Edwards LD, Vestbo J, Calverley PM, Pinto-Plata V, et al. Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir. Med. 2010;104(6):849–857. doi: 10.1016/j.rmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Alif SM, Dharmage S, Benke G, Dennekamp M, Burgess J, Perret JL, et al. Occupational exposure to solvents and lung function decline: A population based study. Thorax. 2019;74(7):650–658. doi: 10.1136/thoraxjnl-2018-212267. [DOI] [PubMed] [Google Scholar]
- 19.Ramírez-Venegas A, Sansores RH, Quintana-Carrillo RH, Velázquez-Uncal M, Hernandez-Zenteno RJ, Sánchez-Romero C, et al. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am. J. Respir. Crit. Care Med. 2014;190(9):996–1002. doi: 10.1164/rccm.201404-0720OC. [DOI] [PubMed] [Google Scholar]
- 20.Naing L, Winn T, Rusli B. Practical issues in calculating the sample size for prevalence studies. Arch. Orofac. Sci. 2006;1:9–14. [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Association MSotAL. American Thoracic Society Standardization of Spirometry, 1994 Update. 2012. [DOI] [PubMed]
- 23.Pellegrino R, Viegi G, Brusasco V, Crapo R, Burgos F, Casaburi R, et al. ATS/ERS Task Force: standardisation of lung function testing. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 24.George L, Brightling CE. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther. Adv. Chronic Dis. 2016;7(1):34–51. doi: 10.1177/2040622315609251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 26.Mahler, D. A. mMRC (Modified Medical Research Council) Dyspnea Scale. https://www.mdcalc.com/mmrc-modified-medical-research-council-dyspnea-scale#creator-insights. Accessed December 6 2019.
- 27.Rehman AU, Hassali MAA, Harun SN, Abbas S, Muneswarao J, Ali IABH, et al. Validation and clinical interpretation of the St George’s respiratory questionnaire for COPD (SGRQ-C) after adaptation to Malaysian language and culture, in patients with COPD. Health Qual. Life Outcomes. 2020;18:138. doi: 10.1186/s12955-020-01393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alma HJ, De Jong C, Jelusic D, Wittmann M, Schuler M, Sanderman R, et al. Thresholds for clinically important deterioration versus improvement in COPD health status: results from a randomised controlled trial in pulmonary rehabilitation and an observational study during routine clinical practice. BMJ Open. 2019;9(6):e025776. doi: 10.1136/bmjopen-2018-025776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones P, Brusselle G, Dal Negro R, Ferrer M, Kardos P, Levy M, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur. Respir. J. 2011;38(1):29–35. doi: 10.1183/09031936.00177210. [DOI] [PubMed] [Google Scholar]
- 30.Jones MP. Indicator and stratification methods for missing explanatory variables in multiple linear regression. J. Am. Stat. Assoc. 1996;91(433):222–230. doi: 10.1080/01621459.1996.10476680. [DOI] [Google Scholar]
- 31.Koskela J, Katajisto M, Kallio A, Kilpeläinen M, Lindqvist A, Laitinen T. Individual FEV1 trajectories can be identified from a COPD cohort. COPD J. Chronic Obstr. Pulm. Dis. 2016;13(4):425–430. doi: 10.3109/15412555.2015.1043423. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Feng J, Li Y, Jia W, Qian H. A new pulmonary rehabilitation maintenance strategy through home-visiting and phone contact in COPD. Patient Prefer. Adherence. 2018;12:97. doi: 10.2147/PPA.S150679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura M, Makita H, Nagai K, Konno S, Nasuhara Y, Hasegawa M, et al. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012;185(1):44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]
- 35.Shah S, Abbas G, Hanif M, Anees-Ur-Rehman ZM, Riaz N, Altaf A, Hassan SU, Saleem U, Shah A. Increased burden of disease and role of health economics: Asia-pacific region. Expert Rev. Pharmacoecon. Outcomes Res. 2019;19(5):517–528. doi: 10.1080/14737167.2019.1650643. [DOI] [PubMed] [Google Scholar]
- 36.Rehman A, Hassali MAA, Muhammad SA, Harun SN, Shah S, Abbas S. The economic burden of chronic obstructive pulmonary disease (COPD) in Europe: results from a systematic review of the literature. Eur. J. Health Econ. 2020;21(2):181–194. doi: 10.1007/s10198-019-01119-1. [DOI] [PubMed] [Google Scholar]
- 37.Rehman U, Hassali M, Muhammad S, Shakeel S, Chin O, Ali I, et al. Economic burden of chronic obstructive pulmonary disease patients in Malaysia: A longitudinal study. Pharmacoecon. Open. 2020;5:35–44. doi: 10.1007/s41669-020-00214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala J, Masa F, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: A 2 year follow up study. Thorax. 2004;59(5):387–395. doi: 10.1136/thx.2003.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dransfield MT, Kunisaki KM, Strand MJ, Anzueto A, Bhatt SP, Bowler RP, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017;195(3):324–330. doi: 10.1164/rccm.201605-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradford E, Jacobson S, Varasteh J, Comellas AP, Woodruff P, O’Neal W, et al. The value of blood cytokines and chemokines in assessing COPD. Respir. Res. 2017;18(1):180. doi: 10.1186/s12931-017-0662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter RE, Wilk JB, Larson MG, Vasan RS, Keaney JF, Jr, Lipinska I, et al. Systemic inflammation and COPD: the Framingham heart study. Chest. 2008;133(1):19–25. doi: 10.1378/chest.07-0058. [DOI] [PubMed] [Google Scholar]
- 42.Frei A, Muggensturm P, Putcha N, Siebeling L, Zoller M, Boyd CM, et al. Five comorbidities reflected the health status in patients with chronic obstructive pulmonary disease: the newly developed COMCOLD index. J. Clin. Epidemiol. 2014;67(8):904–911. doi: 10.1016/j.jclinepi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Celli BR. Predictors of mortality in COPD. Respir. Med. 2010;104(6):773–779. doi: 10.1016/j.rmed.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Pinto-Plata V, Cote C, Cabral H, Taylor J, Celli B. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur. Respir. J. 2004;23(1):28–33. doi: 10.1183/09031936.03.00034603. [DOI] [PubMed] [Google Scholar]
- 45.Fermont JM, Masconi KL, Jensen MT, Ferrari R, Di Lorenzo VA, Marott JM, et al. Biomarkers and clinical outcomes in COPD: a systematic review and meta-analysis. Thorax. 2019;74(5):439–446. doi: 10.1136/thoraxjnl-2018-211855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available on request from corresponding author.


