Abstract
Difficulty swallowing has been reported following whiplash injury; however, the reasons remain poorly understood. A possible factor may be the observed changes in pharyngeal volume. The current exploratory study was designed to examine the prevalence of self-reported dysphagia after whiplash and the relationship with recovery status and change in pharyngeal volume. Data were available from a longitudinal study of adults with whiplash. Data included magnetic resonance imaging (MRI) of the cervical spine, the Dysphagia Handicap Index (DHI), and Neck Disability Index (NDI) collected over four timepoints (< 1 week, 2 weeks, 3 months, and 12 months post-injury). Initial cross-sectional analysis examined 60 patients with DHI data from at least one timepoint. A second, longitudinal analysis was conducted on 31 participants with MRI, NDI, and DHI data at both early (< 1–2 weeks) and late (3–12 months) timepoints. The pharynx was contoured on axial T2-weighted MRI slices using OsiriX image processing software and pharyngeal volume (mm3) was quantified. In the 60-patient cohort, prevalence of self-reported dysphagia (DHI ≥ 3) was observed in 50% of participants at least once in 12 months (M = 4.9, SD 8.16, range 0–40). In the longitudinal cohort (n = 31), mean total DHI significantly (p = 0.006) increased between early and late stages. There was no relationship (p = 1.0) between dysphagia and recovery status, per the NDI% score. Pharyngeal volume remained stable and there was no relationship between dysphagia and pharyngeal volume change (p = 1.0). This exploratory study supports the need for further work to understand the nature of dysphagia, extent of functional compromise, and the underlying pathophysiology post-whiplash.
Keywords: Dysphagia, Deglutition, Deglutition disorders, Swallowing, Whiplash, Pharyngeal volume, Whiplash-associated disorders
Introduction
Swallowing problems after whiplash have been described [1–8], with prevalence rates ranging from 7 [6] to 34% [1]. However, in the majority of studies to date [1, 4, 6, 8, 9], swallowing deficits are mentioned only as a secondary finding in broader investigations into post-whiplash symptomatology, without the measurement of swallowing biomechanics or use of validated measurement tools. A range of swallow-related deficits have been described, including reduced chewing endurance [2, 3, 10, 11], specific food texture avoidance [2], pain related to swallowing [2, 5, 12], and social impacts related to “problems eating” [2]. While observations and reports of “swallowing difficulty” [5, 13] or “dysphagia” [7, 8] have been limited to single case studies [5, 7, 13], there appears to be an emerging body of evidence to suggest swallowing problems are an under-recognized consequence of a whiplash.
Changes in pharyngeal volume as demonstrated on magnetic resonance imaging (MRI) have been demonstrated in those with poor recovery after whiplash in comparison to healthy controls [14] and those who nominate recovery [15]. Specifically, significant temporal reductions in the cross-sectional area (CSA) and the shape ratio (SR) of the pharyngeal lumen corresponding to the C2 vertebrae were reported to characterize those who continued to complain of neck disability 6 months post-whiplash [15]. It was posited that narrowing of the pharyngeal region may be the consequence of a peri-traumatic compressive muscular response occurring in all those exposed to and injured from a whiplash, but only persisting in those with more severe pain and disability [15].
The relationship between pharyngeal volume and dysphagia has been a recent topic of interest in dysphagia research [16–19]. Increased pharyngeal volume measured by acoustic pharyngometry (AP) was associated with increased vallecular residue and reduced pharyngeal constriction in a group of 44 healthy seniors [18], demonstrating a link between volumetric measures, swallowing biomechanics, and swallow function. The feasibility of AP in dysphagia measurement was established in Molfenter and colleagues’ earlier study, where the only predictor of swallowing impairment was pharyngeal atrophy measured by AP [19]. Further work [16] has also demonstrated age-related volumetric changes in the pharynx, calculated from three axial slices corresponding to C2 and C3 spinal vertebrae using pixel-based measures. They proposed loss of muscle bulk as a plausible explanation for increased pharyngeal volume. Increased pharyngeal width in older healthy individuals (65–80 years old) compared with younger controls (18–57 years) was observed from videofluroscopic swallow study data, suggestive of age-related tissue changes including reduced pharyngeal muscle mass and contraction [20]. Finally, a recent study using computed tomography to investigate the volumetric mechanisms during swallowing demonstrated a reduction in pharyngo-laryngeal volume during maximal pharyngeal contraction and an increase in volume with tongue loading [17].
In whiplash, changes in pharyngeal volume and thus pharyngeal lumen width in those with poor recovery [15, 21] may be a contributing factor to a post-whiplash dysphagia. Although the current evidence regarding dysphagia after whiplash is minimal, there are emerging patterns to suggest dysphagia is experienced by a proportion of patients. Unlike the case of sarcopenia, however, decreases in pharyngeal volume have been found after whiplash. In this context, whether muscle tension can be attributed to this narrowing is unknown but has been proposed [15] and given our increased understanding of muscle tension dysphagia [22, 23] may be relevant to explain any dysphagia resulting from whiplash, particularly in the common context of persistent pain, psychological distress, and neck disability.
As there has been, to our knowledge, no systematic research into dysphagia post-whiplash, the current study was considered exploratory and designed in two parts. Part 1 aimed to investigate the percent prevalence of self-reported dysphagia post-whiplash. In Part 2, the aim was to explore changes in self-reported dysphagia over time and the relationship between dysphagia and both recovery status and changes in pharyngeal volume on MRI. It was hypothesized that self-reported dysphagia would worsen over time. Given previous findings of reductions in pharyngeal volume in those with persistent neck pain and disability [15], it was also hypothesized that dysphagia would be worse in those who were not recovered and those demonstrating a reduction in pharyngeal volume.
Method
Participants
Secondary analysis was carried out on participants from a longitudinal cohort study (ClinicalTrials.gov Identifier: NCT02157038), investigating the neuromuscular mechanisms underlying poor recovery following whiplash injury after motor vehicle collision (MVC). Ethical approval for the current secondary analysis study was obtained through Northwestern University, Feinberg School of Medicine. The parent study included 97 acutely injured adults, recruited at time of presentation to an urban emergency medicine department with Level 1 trauma designation, following MVC. Participants in the parent study included all individuals presenting with whiplash injury from an MVC without radiologic confirmation of cervical spine fracture, need for surgical intervention, and without known acquired or traumatic neurologic injury. Participants in the parent study were asked to attend for MRI of the cervical spine and completion of a series of questionnaires capturing; (1) neck-related disability using the Neck Disability Index (NDI) [24], (2) psychological distress using the (i) Tampa scale of Kinesiophobia (TSK) [25, 26], (ii) Vanderbilt Pain Management Inventory (PMI) [27, 28], (iii) Centre of Epidemiological Studies Depression scale (CES-D 10) [29], and (iv) Impact of Events Scale (IES) [30]), and (3) self-reported dysphagia using the Dysphagia Handicap Index (DHI) [31]. The plan was for each participant to be reviewed at four points within the first 12 months of injury: < 1 week, 2 weeks, and at 3 months and 12 months, with MRI and questionnaires completed at all timepoints.
This study was based on results from the MRIs, the DHI, and NDI. For Part 1 of the current study, all participants from the parent study who completed a DHI [31] questionnaire at least once within a 12-month follow-up period (n = 60) were included. For Part two, a subset (n = 31) of the 60 participants who underwent MRI and completed the NDI [24] and DHI at both an early (< 1–2 weeks post-injury) and a late (3–12 months post-injury) assessment timepoint were included for longitudinal analysis.
Outcome Measures
Questionnaires
The DHI is a 25-item self-reported questionnaire, rated as a 3-point Likert scale investigating self-perceived dysphagia across 3 specific domains; (i) physical, (ii) functional, and (iii) emotional. Individual items responses of never, sometimes, or always are given a 0, 1, or 2 categorical value and converted to raw item scores of 0, 2, and 4. Items relating to physical discomfort associated with dysphagia are summed to obtain an individual physical domain score (maximum score/36). A functional domain score is calculated from the sum of items relating to the self-perceived impact of dysphagia on daily activities (maximum score/36). Items related to an individual’s emotional response to dysphagia are summed to calculate an emotional domain score (maximum score/28). Domain scores are summed to obtain a total DHI score (maximum score/100), where higher scores demonstrate more severe handicap. The cut-off score representative of a normal control group during the validation process has been previously established as < 3 [31] and this was used in the current study to denote the presence (≥ 3) or absence (< 3) of self-reported dysphagia.
In the parent study, all participants also completed the NDI questionnaire at each of the four timepoints. The NDI is commonly used in wider neck-disorders research as a marker of neck-related interference in daily life and long-term recovery [32–35]. Based on clinical practice guidelines [36], a mean total NDI score of ≥ 30/100 is used to classify poor recovery. A score > 8 and < 30/100 typically identifies those with mild neck -related symptoms and persistent non-recovery. An overall score ≤ 8/100 is used to classify full recovery. In the longitudinal analysis of the current study, a raw NDI score at 12 months was dichotomized into either a non-recovered (> 8) or fully recovered (≤ 8) classification, to investigate whether dysphagia was more likely to be self-reported in those with non-recovery.
Magnetic Resonance Imaging (MRI) of the Cervical Spine
All participants in the parent study underwent serial T2-weighted MRI of the cervical spine at each of the four timepoints post-injury. Imaging was performed on a Siemens 3.0 T Prisma Syngo MR D13D magnetic resonance scanner, equipped with a 64-channel head/neck coil (Erlangen, Germany). A localizer scan was acquired followed by 3D T2-weighted sagittal isotropic imaging representing all regions of pharyngeal anatomy. Imaging parameters for the sagittal T2 included repetition time (TR) = 1500 ms, echo time (TE) = 135.0 ms, flip angle = 140 degrees, bandwidth = 625 Hz/Px and, an imaging matrix of 320 × 320. The field of view was 250 × 250 mm. The number of slices was 64 per slab, with a slice thickness of 0.80 mm. The slice oversampling was 12.5% with a turbo factor of 88 and voxel size of 0.4 × 0.4 × 0.8 mm. The acquisition time was 4:08 min.
For the current study, pharyngeal volume was measured for each of the participants eligible to be included in the Part 2, longitudinal analysis. Pharyngeal volume was measured by a contour tracing tool with OsiriX image processing software [37]. Using a corresponding sagittal image as a reference point, the pharynx was manually segmented on all axial slices (Fig. 1). To control for consistency, the most superior aspect of the pharyngeal region of interest (ROI) was marked by the apicodental junction or neck of the dens of the C2 vertebrae. Due to individual differences in anatomy and imaging, the most inferior aspect of the ROI was identified during the contouring process as the slice where the pharynx could no longer be visualized. The final axial slice consistently corresponded to the inferior aspect of a uni- or bilateral pyriform sinus/es. The first axial slice after observing this inferior aspect of the pyriform sinuses was considered the subglottic airway and was not included in the volume measure. To ensure the pyriform sinuses were included in the ROI to represent the hypopharynx, the supraglottic airway was included in the volume measure. This was due to the inability of the contouring tool to trace around separate regions within the same axial slice. After contouring, each slice was summed to calculate a total volume (mm3) of the contoured pharynx, calculated for each participant at each time point (Fig. 2).
Fig. 1.
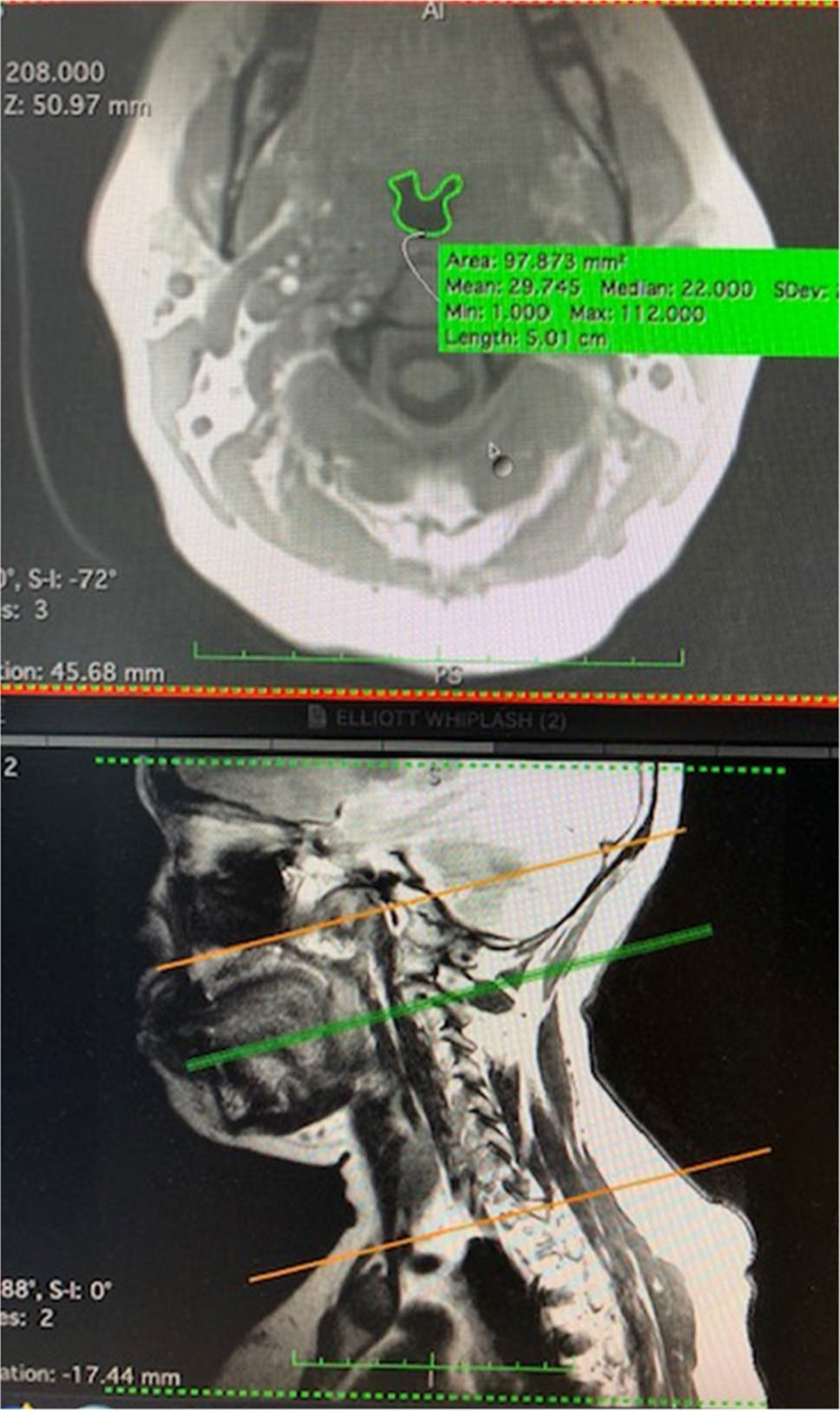
Pharyngeal contouring on MRI. This figure illustrates an axial slice of the C-spine (top image), where the green trace delineates the pharyngeal lumen. This axial slice represents C2 of the cervical spine, corresponding to the green line on the sagittal image (bottom image). The orange lines marks the region of interest from which axial slices were taken to represent the pharynx
Fig. 2.
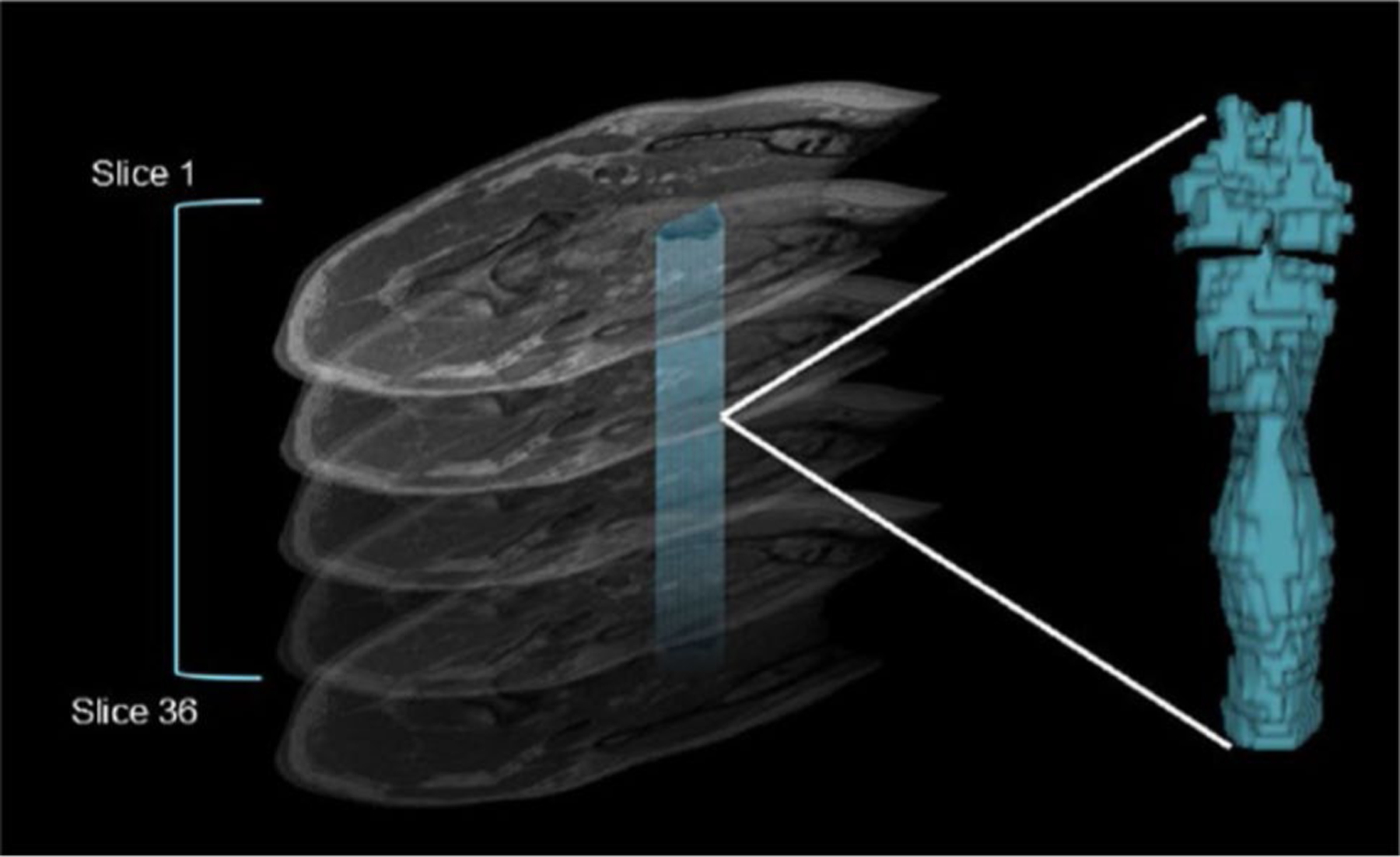
Calculation of pharyngeal volume from an MRI series of the cervical spine. The figure illustrates stacking of all relevant axial slices from the cervical spine MRI series. Individual volume measures for each slice were calculated by manually tracing around the pharyngeal lumen seen on each axial image. Pixel-based measures were used to calculate an individual volume for each. Individual volumes were summed to calculate a total pharyngeal volume for each MRI series. A 3D model can be generated to represent pharyngeal volume, pictorially
Analysis
Part 1—Prevalence of Dysphagia
For Part 1 of the current study, DHI domain and total scores for each participant were analyzed descriptively. First, raw total DHI scores across all timepoints were examined to identify the presence or absence of self-reported dysphagia based on a DHI cut-off score of ≥ 3 being outside normal limits [24]. Individual DHI scores were dichotomized to represent dysphagia (DHI ≥ 3) or no dysphagia (DHI < 3) and any participant with a total DHI score ≥ 3 at any timepoint was classified as dysphagia and included in the prevalence count. Secondly, mean physical, functional , and emotional domain and mean total DHI scores were calculated over all available timepoints for each of the 60 participants. Individual mean domain scores were calculated as a percent of the total maximum domain score. Thirdly, individual item responses ≥ 3 within each domain were identified to represent dysphagia and presented as a percentage of the total number of domain items. Finally, mean domain scores and a mean total DHI score were calculated for the whole cohort. A Kolmogorov–Smirnov test revealed non-normal data distribution for these variables. A Kruskal–Wallis test was used to test the difference between physical, functional, and emotional domain percent scores and the Mann–Whitney U was used for post hoc analysis.
Part 2—Change in Dysphagia and Recovery
For Part 2, descriptive analysis of DHI scores at early and late timepoints was performed, to investigate dysphagia change over 12 months from whiplash. Mean DHI scores were computed for each participant at early (< 1–2 weeks) and late (3–12 months) stages of recovery. Binary classification of dysphagia (yes or no [24]) was conducted based on mean early and late mean scores. Based on this classification, the percentage of participants with and without self-reported dysphagia was calculated. A Wilcoxon signed rank test was performed to test the difference between early and late mean DHI. A McNemar test was used to compare dysphagia status (present/absent) at early and late timepoints.
Descriptive analysis of NDI scores was performed for all those who had completed both an early and late NDI questionnaire. A Kolmogorov–Smirnov test revealed normal data distribution. Mean NDI scores were calculated for individuals at both early and late timepoints and dichotomized to represent either a recovered or non-recovered classification [36] based on a pre-determined cut-off of 8/100 (> 8; non-recovered). Based on this classification, the percentage of participants with recovery and non-recovery was calculated. A Wilcoxon signed rank test was used to investigate the difference between early and late mean NDI. A McNemar test was performed to compare recovery status (recovered/non-recovered) at early vs. late timepoints.
The Relationship Between Dysphagia and Recovery Status
Based on the established chronicity of symptoms [36], a 12-month NDI was considered most suitable for determining overall self-reported recovery status and evaluating the relationship between recovery and dysphagia. From the longitudinal cohort, those who had completed both a DHI and NDI at the 12-month timepoint were included in this analysis. Raw 12-month NDI scores for those were dichotomized to represent recovery or non-recovery. Corresponding raw DHI scores at the 12-month timepoint were also dichotomized to represent dysphagia and no dysphagia. Because of small expected frequencies in some cells, a Fisher exact test was used to investigate the proportion of those with self-reported dysphagia and non-recovery at 12 months.
Pharyngeal Volume and Relationship Between Volume and Dysphagia
From the MRI data, total pharyngeal volume (mm3) for each MRI series was calculated and a Kolmogorov–Smirnov test revealed normal data distribution. Volume scores at timepoints 1 and 2 (early) and 3 and 4 (late) were summed and averaged for each participant, to obtain an early and late mean volume. A paired sample t-test was used to investigate the difference between mean volumes. For each participant, the difference in volume between early vs. late timepoints was calculated by subtracting mean volume from the early phase from the mean volume of the late phase. Volume % change scores for each participant were dichotomized to represent either an increase or decrease in volume. Because of small expected frequencies in some cells, a Fisher exact test was used to compare dysphagia status (present/absent) with pharyngeal volume change (increased/decreased).
Results
Participants
For Part 1 of this study, a total of 60 of the 97 participants from the parent study completed both an NDI and a DHI questionnaire at one or more of the 4 timepoints and were thus included. The remaining 37 participants did not complete an NDI and/or DHI at these timepoints and were thus excluded. A total of 31 participants completed an NDI, DHI, and had a corresponding MRI at both an early and a late timepoint and were thus included in the longitudinal analysis (part 2). Demographics of the total n = 60 cohort and the longitudinal n = 31 cohort is detailed in Table 1. Statistical comparisons revealed that key demographics across the 2 group did not differ significantly.
Table 1.
Participant demographics for Part 1 and 2 of analysis
| Demographics | Part 1 Cohort n = 60 | Part 2 Cohort n = 31 | P |
|---|---|---|---|
| N (%) | N (%) | ||
| At least one DHI^ | 60 (100) | 31 (100) | n/a |
| Mean age (years; days) | 35.2; SD 10.71 | 33.04; SD 9.76 | 0.94* |
| Gender n male (%) | 49 (82) | 7 (23) | 0.68** |
| Race | |||
| African American | 17 | 12 | 0.14** |
| Asian | 5 | 3 | 0.45** |
| Caucasian | 28 | 12 | 0.09** |
| Hispanic | 8 | 3 | 0.38** |
| Other | 2 | 1 | *** |
| Early review | 31 (52) | 31 (100) | n/a |
| Late review | 59 (98) | 31 (100) | n/a |
| Early and late review | 31 (52) | 31 (100) | n/a |
| Mean days from MVC+ to timepoint 1 | 5.27; SD 1.47 | 5.48; SD 1.34 | 0.32* |
| Mean days from MVC to timepoint 2 | 15.77; SD 6.30 | 14.87; SD 5.11 | 0.39* |
| Mean days from MVC to timepoint 3 | 107.78; SD 15.42 | 109.68; SD 16.48 | 0.12* |
| Mean days from MVC to timepoint 4 | 318.31; SD 86.36 | 386.54; SD 37.56 | 0.87* |
Dysphagia Handicap Index [26]
Paired t-test
McNemar
not calculated (p < 1)
Motor vehicle collision
Part 1—Prevalence of Self-Reported Dysphagia and Relationship with Recovery Status
Dysphagia Prevalence
In 60 participants post-whiplash, mean total DHI was 4.92 (median 2, SD 8.16, range 0–40). A total of 30 (50%) had total scores which fell outside the normal range of self-reported dysphagia (≥ 3 on the DHI) [31] at some points within 12 months of injury. Analysis by DHI domains revealed that most participant complaints fell within the physical domain (mean 3.47, SD 4.52, range 0–20) compared with the functional domain (mean 0.79, SD 2.54, range 0–16) and emotional domain (mean 0.66, SD 1.76, range 0–7), where mean scores fell below the cut-off. A Kruskal–Wallis test demonstrated a significant (p < 0.001) difference between percent scores for each domain. Post hoc analysis via the Mann–Whitney U test showed the physical domain attracted more complaints than either of the other two domains (p < 0.001), which did not differ from each other (p = 0.598).
Individual DHI items that were reported by any participant are listed in Table 2. Sorting by item revealed the top 4 complaints raised by the cohort included dry mouth (58%), needing to wash food down with liquid (48%), coughing with liquid (32%), and coughing with solid food (20%). From 25 items, two items were never reported and were the need to find alternative means to eat/drink (e.g., feeding tube) and a feeling of “anger” as a result of a swallowing problem.
Table 2.
Self-reported dysphagia across DHI domains (N = 60)
| Item responses | DHI Domain | n | Outside normal range %* |
|---|---|---|---|
| My mouth is dry | P | 32 | 53 |
| I need to drink fluids to wash food down | P | 29 | 48 |
| I cough when I drink liquid | P | 19 | 32 |
| I cough when I eat solid food | P | 12 | 20 |
| It takes me longer to eat a meal than it used to | E | 9 | 15 |
| I choke when I take my medication | P | 9 | 15 |
| I have to swallow again before food will go down | P | 7 | 12 |
| I feel a strangling sensation when I swallow | P | 7 | 12 |
| I eat smaller meals more often due to my swallowing problem | F | 6 | 10 |
| I don’t enjoy eating as much as I used to | E | 6 | 10 |
| I’m afraid that I’ll choke and stop breathing because of my swallowing problem | E | 6 | 10 |
| I cough up food after I swallow | P | 6 | 10 |
| I have changed the way I swallow to make it easier to eat | F | 5 | 8 |
| I’m embarrassed to eat in public | E | 5 | 8 |
| I am nervous because of my swallowing problem | E | 3 | 5 |
| I eat less because of my swallowing problem | F | 2 | 3 |
| I feel handicapped because of my swallowing problem | E | 2 | 3 |
| I’ve changed my diet due to my swallowing problem | F | 2 | 3 |
| I’ve lost weight because of my swallowing problem | P | 1 | 2 |
| I avoid some foods because of my swallowing problem | F | 1 | 2 |
| I feel depressed because I can’t eat what I want | E | 1 | 2 |
| I don’t socialize as much due to my swallowing problem | F | 1 | 2 |
| I avoid eating because of my swallowing problem | F | 1 | 2 |
P physical, F functional, E emotional (DHI domains)
Percentage of domain responses outside normal range cut = off≥ 3 [26]
Part 2—Dysphagia Change and the Relationship with Recovery Status and Pharyngeal Dimensions
Change in Dysphagia
Direct comparison between early and late mean total DHI was carried out for n = 31 participants and is illustrated in Fig. 3. Mean early DHI was 1.29 (median 0, SD 2.60, range 0–10) and mean late DHI was 2.39 (median 1, SD 2.83, range 0–9). Although mean scores at both timepoints were below the cut-off, 6 participants (19.4%) at the early timepoint had mean DHI scores ≥ 3, indicating the presence of dysphagia. At the late-stage review, 11 participants (35.5%) had mean DHI scores ≥ 3 (Fig. 4). A Wilcoxon signed rank test demonstrated a significant difference between early and late mean DHI (p = 0.006). A McNemar test demonstrated a trend (p = 0.063) towards a greater proportion of self-reported dysphagia in the late vs. early stage of recovery.
Fig. 3.
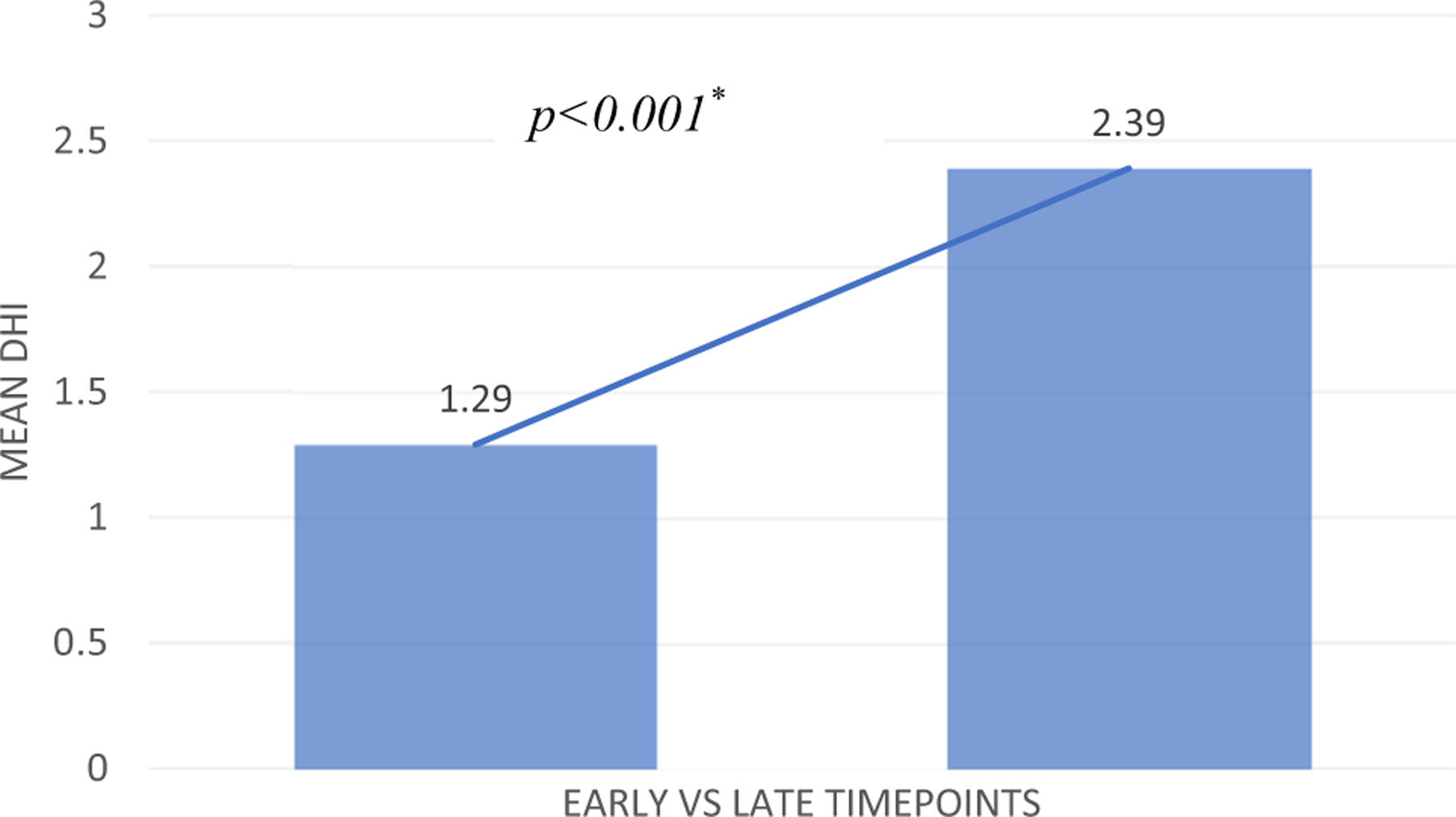
Early vs. late self-reported dysphagia in longitudinal cohort. Increase in mean Dysphagia Handicap Index (DHI) scores between early (< 1–2 weeks) to late (3–12 months) post-whiplash (n = 31)
Fig. 4.
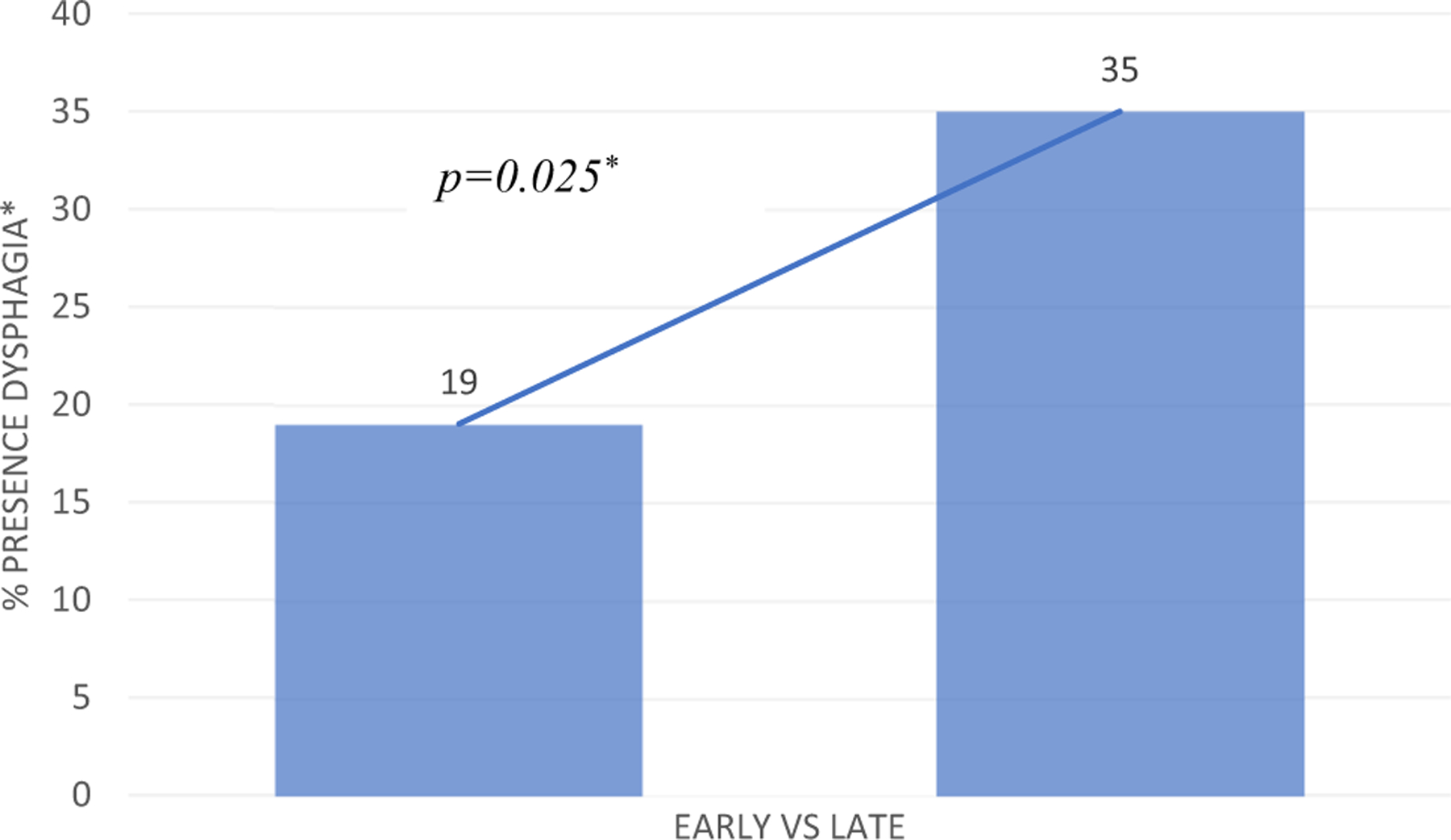
Presence vs absence of dysphagia in longitudinal cohort. Presence (%) of self-reported dysphagia from early to late timepoints, based on a cut-off mean DHI over ≥ 3 [26]. *Wilcoxon signed rank test
Change in Recovery Status
Direct comparison between early and late mean total NDI was carried out for n = 31 participants. Analysis of total NDI scores revealed a significant decrease (Wilcoxon p < 0.001) in mean NDI between early (mean 33.47, median 32, SD 15.69, range 0–79) and late (mean 17.82, median 14, SD 14.64, range 0–52) review. Based on NDI scores, at the early stage, 30 were non-recovered (NDI > 8) and 1 was recovered (NDI < 8). At the late stage, 21 were non-recovered and 10 were recovered. A McNemar test demonstrated a significant (p = 0.004) difference in those with non-recovery at the early (96.8%) vs. late (67.7%) timepoints.
Relationship Between Dysphagia and Recovery Status
At the 12-month review, a total of 24 participants had completed an NDI and DHI. For this cohort at 12 months, the mean NDI was 18.25 (median 13, SD 15.36, range 0–50), with 17 (65%) classified as non-recovered. The mean DHI at 12 months was 1.75 (median 0, SD 3.55, range 0–16), with n = 6 (25%) falling outside the normal range. Out of those non-recovered participants (n = 17), five self-reported dysphagia. Of the seven individuals who had recovered by 12 months, one reported dysphagia and six did not. There was no evidence of a relationship between recovery as determined by the NDI and dysphagia status (Fisher exact p = 1).
Pharyngeal Volume
For the 31 participants, the mean number of axial slices used to calculate pharyngeal volume for each series was 17.38 (SD 3.14; Range 11–24). Mean early pharyngeal volume was 16.02 mm3 (SD 3.73, range 7.19–23 mm3) and mean late volume was 16.66 mm3 (SD 4.1, range 6.38–24 mm3). A paired t-test revealed no statistically significant difference (p = 0.304) between early and late timepoints. Mean volume change score between early and late timepoints was 0.65 mm3 (SD 3.73, range − 5.12 to 10.01 mm3). A total of 16 (52%) participants had an increase in pharyngeal volume and 15 (48%) showed a decrease in volume over time.
Relationship Between Pharyngeal Volume and Self-Reported Dysphagia
Of those with an increase in pharyngeal volume (n = 16), four self-reported dysphagia and twelve did not. In those with a reduction in pharyngeal volume (n = 15), four also reported dysphagia and eleven did not. A Fisher exact test demonstrated no statistical difference (p = 1.0) in the expected proportion of patients with an increase or decrease in pharyngeal volume and those with and without self-reported dysphagia.
Discussion
In a cohort of 60 individuals with whiplash injury after MVC, half self-reported dysphagia at some point within 12 months of injury. Prevalence of dysphagia in this study is higher than previous reports of between 7 and 34% [1, 4, 6, 8, 9]. The higher prevalence in our study could possibly be explained by using a validated self-reported dysphagia tool, whereby a range of swallow-related complaints were measured, potentially demonstrating more sensitivity to difficulties experienced after whiplash and suggesting more depth of measurement compared with previous studies. To our knowledge, no prior study has used validated swallow measures and unlike this study, many did not explicitly set out to investigate post-whiplash swallowing in isolation [1, 6, 8, 9]. The high prevalence of post-whiplash dysphagia demonstrated in this preliminary study as determined by an established, validated tool highlights this as a previously under-reported consequence following whiplash, warranting the need for further investigation.
Analysis by DHI domain revealed swallow-related symptoms that fell within the physical domain were significantly more prevalent compared to functional and social complaints. While the differences between domains were statistically significant, further research is needed to determine whether these differences are clinically relevant. Importantly, while the mean total DHI score of the n = 60 cohort was outside the normal range and indicated the presence of dysphagia, the mean scores of each individual domain were low and indicated an absence of dysphagia in most individuals. This may be explained by the small numbers represented in each group and further analysis with larger participant numbers is recommended to more confidently ascertain whether a meaningful difference exists. However, the statistical difference is interesting. Future research using instrumental measurement is necessary to determine the exact nature of these physical complaints and whether they exist in the absence of significant social and/or functional implications. If so, this may be related to the limited focus on dysphagia from those health professionals typically managing post-whiplash sequelae or could be explained by the patient experience of more specific neck-related disability, whereby swallowing concerns are not as apparent. If this is the case, this is unsurprising given the lack of whiplash-related cases in typical speech pathology caseloads. Regardless, these are interesting preliminary findings which warrant further consideration.
The most common swallowing-related issues being self-reported in our cohort following whiplash included coughing on solids and liquids, washing food down with liquids and a dry mouth, closely followed by a longer time to complete meals, and needing smaller meals. Some of these complaints may be likened to the symptom profile described by Gronqvist et al. [2] in a study of 50 people following whiplash. In comparison to normal controls, individuals post-whiplash reported difficulty eating “tough” and large pieces of food compared to normal controls. Several other studies [3, 10, 12, 38] reported fatigue, pain, and jaw dysfunction impacted chewing endurance after whiplash, contributing to mealtime fatigue. Although impossible to make direct comparisons, it is viable that these problems could feasibly explain the complaints described by our cohort, specifically those related to difficulties with solid textures. However, more research needs to be carried out to determine whether or not pain, fatigue, and/or jaw dysfunction are underlying problems which may explain dysphagia symptoms specifically associated with whiplash. The frequent complaints of coughing on solids and liquids are interesting; however, our study cannot confirm the nature of these cough symptoms, specifically whether they are related to any potential aspiration. However, the fact that these complaints were the most prevalent further establishes the need for instrumental assessment to determine whether these complaints of cough are associated with aspiration or swallow inefficiency and any impairment in swallow biomechanics. Finally, the scope of our study did not allow us to surmise any potential physiologic explanation for dry mouth and whether this was associated with problems of swallowing. Future investigations should consider whether dry mouth is in any way connected with altered chewing or swallowing behaviors after whiplash. Given the high incidence of persistent pain [39], psychological distress [40], and opioid use [41] observed in individuals following whiplash, future studies should consider their impact on not only dry mouth but swallowing in general. Overall, the current data highlight that our understanding of the presenting symptoms of dysphagia post-whiplash is only just emerging. Future work needs to include tools which encompass a diverse and wide range of symptom descriptors, to ensure all characteristics of swallow-related changes being experienced by this population can be fully elucidated and effectively managed.
The longitudinal analysis of 31 participants demonstrated a rise in self-reported dysphagia over time. Swallow change after initial whiplash injury has not been previously investigated and these findings provide an interesting insight into whiplash-related swallow complaints. The suggestion that dysphagia may be a late-appearing symptom may guide health professionals to consider these complaints in the later stages of recovery in order to more effectively assess and manage those with persistent symptomatology. However, prior to this, research into the nature of swallow change after whiplash needs to incorporate instrumental assessment of swallow biomechanics to fully understand the extent of these patient complaints and the nature of change over time. Whether a deterioration in swallowing aligns with other persistent symptoms post-whiplash could be a worthwhile avenue for exploration. It must be acknowledged that although our findings suggest a significant worsening of self-reported dysphagia over time, it is possible that repeatedly measuring dysphagia contributed to an increase in focused attention and thus awareness of symptoms, allowing participants with possible early dysphagia to better report what may have existed immediately after whiplash. Future investigations should take this into consideration and aim to replicate findings of swallow change in larger cohorts with varying levels of whiplash-related disability and across a wider age-range of males and females.
Although poorly understood [42, 43], the maintenance of widespread pain and disability post-whiplash is well known and symptoms of pain and disability in the neck and head is common [44]. High levels of trauma-related distress are also a common presentation in those classified with poor recovery [45–50]. Although a significant number of participants in the longitudinal cohort transitioned out of a poor recovery status as determined by the NDI, the majority still reported overall non-recovery (67.7%) at late-stage review, consistent with what is commonly observed in this population [51]. Despite this, there was no relationship between recovery status as determined by NDI % scores and the presence vs. absence of dysphagia as determined by DHI cut-off scores. This was surprising and contrary to our hypothesis that dysphagia would be higher in those classified as non-recovered. The limitations of using the NDI in isolation to predict recovery status is worth considering and suggests the need for multidimensional assessment to investigate whether adverse neuropsychiatric distress and widespread pain and disability add to the profile of a post-whiplash dysphagia.
The current study attempted to explore one potential mechanism to explain self-reported dysphagia. Given previous findings demonstrating reduced pharyngeal volume over time in those with persistent disability following whiplash [14, 15], we were interested in whether dysphagia would be more prevalent in those with reduced pharyngeal volume. We wondered whether reductions in pharyngeal lumen could be explained by muscle tensioning in the context of persistent pain, neck disability, and potentially other musculoskeletal problems arising following whiplash. However, contrary to our hypothesis, the current data did not establish a relationship between pharyngeal volume and self-reported dysphagia after whiplash. The current preliminary data demonstrated neither an increase nor a decrease in pharyngeal dimensions over time and although self-reported dysphagia worsened over time, there was no influence from pharyngeal dimension change on self-reported dysphagia. Given the absence of traumatic injury following whiplash in this cohort and the pathophysiology underpinning muscle tension dysphagia [22], increased muscle tension is still a considered a plausible reason to explain a whiplash-associated dysphagia; however, it is recommended that future studies consider other methodologies to explore muscle tensioning. While pharyngeal volume is still of interest and may be worthwhile to investigate further in larger cohorts, it may not be the most effective way to measure muscle tension. A consideration of post-whiplash voice quality and function and laryngoscopic examination may prove to be more informative. Future studies could also consider additional investigation into laryngeal hyperresponsiveness and whether this is a potential factor to explain these swallowing complaints.
While this study has shown self-reported dysphagia appears to exist following whiplash, further research into the cause for these reports is needed, to start to build a strong theoretical basis to guide management. What is particularly important to establish is whether patient-reported outcomes related to swallowing are in concordance with swallow biomechanics. This may guide investigations into whether these problems are of motor or sensory origin, ultimately increasing our understanding of underlying physiology.
Limitations
There are several limitations to the study. Although there were no direct methods to control for other factors within the 12-month follow-up period which may have influenced swallowing or recovery status, exclusion criteria were ensured throughout the follow period to exclude any participant with any history or new-onset traumatic or acquired neurologic disease or new traumatic injury. Also, previous research [42, 52] has detailed the post-whiplash clinical presentation is not influenced by ongoing treatment. In the case of persistent symptoms at 3 months post-injury, for example, it is well established that function is not likely to change for the better in the long term. There was also no baseline dysphagia screen to exclude pre-existing dysphagia. This is a limitation of the study and can be explained by the swallow-related outcomes being added later in the study protocol due to clinical observations of reported swallow changes from participants. Importantly, however, as we excluded previous traumatic and acquired injury and neurological conditions, a dysphagia potentially arising from these conditions was controlled for. Future research should include more rigorous methods to control for confounding factors.
Prior research has not developed validated tools to detect dysphagia following non-catastrophic trauma involving the head/neck. Self-reported dysphagia was examined in the current study using the DHI. The DHI was developed predominately for those with neurological conditions and head and neck cancer [31] and as such, it may not include all relevant symptoms experienced by a population of patients following non-catastrophic trauma involving the head/neck. More information regarding symptoms is needed to determine the best patient-reported tool/s to detect the specific issues experienced post-whiplash. Furthermore, the final 7-point rating scale of overall dysphagia severity was not part of data collection in this cohort. As a result, we were unable to ascertain overall self-perceived dysphagia severity for participants, which may have added to the breadth of information.
This study was also exploratory and designed to determine if there was enough evidence to suggest avenues for further investigation into swallowing problems after whiplash. It used secondary analysis of data taken as part of a larger parent study, where dysphagia was not part of the original aims. The current study did not include any instrumental measures of dysphagia. The main aim of the parent study was to investigate neuromuscular changes after whiplash. As such, information relating to post-whiplash swallowing biomechanics and functional consequences was not an aim of the study and thus remains unknown. Until that work is conducted, there is insufficient evidence to create the profile for post-whiplash dysphagia and insufficient data on which to diagnose, propose cause, or offer any intervention to target specific impairments. Although this weakened the conclusions, it does provide supporting evidence that dysphagia post-whiplash is an issue for half of patients—and, as such, is deserved of further systematic investigation. Current work is in progress, including the analysis of videofluroscopic swallow studies of individuals previously exposed to whiplash.
Conclusion
Half of those exposed to and injured from whiplash, self-reported dysphagia at some point within 12 months of injury, with significant worsening observed over time. Despite this and overall high rates of non-recovery, there was no relationship demonstrated between recovery status and self-reported dysphagia at 12 months. Pharyngeal lumen size in this cohort remained stable and did not influence swallow-related outcomes. Dysphagia is under-recognized following whiplash; however, more work needs to be carried out to explore both swallow biomechanics and physiological underpinnings.
Funding
The project described was supported by the National Institutes of Health (NIH) through Grant Number R01HD079076: Eunice Kennedy Shriver National Institute of Child Health & Human Development; National Center for Medical Rehabilitation Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Data Availability Data were taken at the Department of Physical Therapy and Human Movement Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
References
- 1.Carroll LJ, Ferrari R, Cassidy JD. Reduced or painful jaw movement after collision-related injuries—a population-based study. J Am Dent Assoc. 2007;138(1):86–93. 10.14219/jada.archive.2007.0026. [DOI] [PubMed] [Google Scholar]
- 2.Gronqvist J, Haggman-Henrikson B, Eriksson PO. Impaired jaw function and eating difficulties in whiplash-associated disorders. Swed Dent J. 2008;32(4):171–7. [PubMed] [Google Scholar]
- 3.Häggman-Henrikson B, Österlund C, Eriksson P, Häggman-Henrikson B, Osterlund C, Eriksson PO. Endurance during chewing in whiplash-associated disorders and TMD. J Dent Res. 2004;83(12):946–50. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari R, Russell AS, Carroll LJ, Cassidy JD. A re-examination of the whiplash associated disorders (WAD) as a systemic illness. Ann Rheum Dis. 2005;64(9):1337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordoni B, Marelli F, Morabito B. The tongue after whiplash: case report and osteopathic treatment. Int Med Case Rep J. 2016;9:179–82. 10.2147/IMCRJ.S111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturzenegger M, Radanov BP, Di Stefano G. The effect of accident mechanisms and initial findings on the long-term course of whiplash injury. J Neurol. 1995;242(7):443–9. [DOI] [PubMed] [Google Scholar]
- 7.Hildingsson C, Hietala SO, Toolanen G. Scintigraphic findings in acute whiplash injury of the cervical spine. Injury. 1989;20(5):265–6. 10.1016/0020-1383(89)90164-2. [DOI] [PubMed] [Google Scholar]
- 8.Gargan MF, Bannister GC. The rate of recovery following whiplash injury. Eur Spine J. 1994;3(3):162–4. [DOI] [PubMed] [Google Scholar]
- 9.Pennie B, Agambar L. Patterns of injury and recovery in whiplash. Injury. 1991;22(1):57–9. [DOI] [PubMed] [Google Scholar]
- 10.Kalezic N, Noborisaka Y, Nakata M, Crenshaw AG, Karlsson S, Lyskov E, Eriksson PO. Cardiovascular and muscle activity during chewing in whiplash-associated disorders (WAD). Arch Oral Biol. 2010;55(6):447–53. 10.1016/j.archoralbio.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Severinsson Y, Bunketorp O, Wenneberg B. Jaw symptoms and signs and the connection to cranial cervical symptoms and posttraumatic stress during the first year after a whiplash trauma. Disabil Rehabil. 2010;32(24):1987–98. 10.3109/09638281003797323. [DOI] [PubMed] [Google Scholar]
- 12.Lampa E, Wanman A, Nordh E, Haggman-Henrikson B. Effects on jaw function shortly after whiplash trauma. J Oral Rehabil. 2017;44(12):941–7. 10.1111/joor.12571. [DOI] [PubMed] [Google Scholar]
- 13.Anagnostara A, Athanassopoulou A, Kailidou E, Markatos A, Eystathidis A, Papageorgiou S. Traumatic retropharyngeal hematoma and prevertebral edema induced by whiplash injury. Emerg Radiol. 2005;11(3):145–9. [DOI] [PubMed] [Google Scholar]
- 14.Elliott J, Cannata E, Christensen E, Demaris J, Kummrow J, Manning E, Nielsen E, Romero T, Barnes C, Jull G. MRI analysis of the size and shape of the oropharynx in chronic whiplash. Otolaryngol—Head Neck Surg. 2008;138(6):747–51. 10.1016/j.otohns.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Elliott JM, Pedler AR, Theodoros D, Jull GA. Magnetic resonance imaging changes in the size and shape of the oropharynx following acute whiplash injury. J Orthop Sports Phys Ther. 2012;42(11):912–8. 10.2519/jospt.2012.4280. [DOI] [PubMed] [Google Scholar]
- 16.Molfenter SM, Amin MR, Branski RC, Brumm JD, Hagiwara M, Roof SA, Lazarus CL. Age-related changes in pharyngeal lumen size: a retrospective MRI analysis. Dysphagia. 2015;30(3):321–7. 10.1007/s00455-015-9602-9. [DOI] [PubMed] [Google Scholar]
- 17.Iida T, Kagaya H, Inamoto Y, Shibata S, Saitoh E, Kanamori D, Hashimoto S, Katada K, Tohara H, Ueda K. Measurement of pharyngo-laryngeal volume during swallowing using 320-row area detector computed tomography. Dysphagia. 2017;32(6):749–58. 10.1007/s00455-017-9818-y. [DOI] [PubMed] [Google Scholar]
- 18.Molfenter SM, Lenell C, Lazarus CL. Volumetric changes to the pharynx in healthy aging: consequence for pharyngeal swallow mechanics and function. Dysphagia. 2019;34(1):129–37. 10.1007/s00455-018-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molfenter SM, Brates D, Herzberg E, Noorani M, Lazarus C. The swallowing profile of healthy aging adults: comparing noninvasive swallow tests to videofluoroscopic measures of safety and efficiency. J Speech Lang Hear Res. 2018;61(7):1603–12. 10.1044/2018_JSLHR-S-17-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard R, Kendall KA, McKenzie S. Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia. 2004;19(2):133–41. 10.1007/s00455-003-0508-6. [DOI] [PubMed] [Google Scholar]
- 21.Elliott J, Jull G, Noteboom JT, Galloway G. MRI study of the cross-sectional area for the cervical extensor musculature in patients with persistent whiplash associated disorders (WAD). Man Ther. 2008;13(3):258–65. 10.1016/j.math.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Kang CH, Hentz JG, Lott DG. Muscle tension dysphagia: symptomology and theoretical framework. Otolaryngol-Head Neck Surg. 2016;155(5):837–42. [DOI] [PubMed] [Google Scholar]
- 23.Kang CH, Zhang N, Lott DG. Muscle tension dysphagia: contributing factors and treatment efficacy. Ann Otol Rhinol Laryngol. 2020. 10.1177/0003489420966339. [DOI] [PubMed] [Google Scholar]
- 24.Vernon H The Neck Disability Index: state-of-the-art, 1991–2008. J Manip Physiol Ther. 2008;31(7):491–502. 10.1016/j.jmpt.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Crombez G, Vlaeyen JWS, Heuts PHTG, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80(1):329–39. 10.1016/S0304-3959(98)00229-2. [DOI] [PubMed] [Google Scholar]
- 26.Walton D, Elliott JM. A higher-order analysis supports use of the 11-item version of the tampa scale for kinesiophobia in people with neck pain. Phys Ther. 2013;93(1):60–8. 10.2522/ptj.20120255. [DOI] [PubMed] [Google Scholar]
- 27.Smith CA, Wallston KA, Dwyer KA. On babies and bathwater: disease impact and negative affectivity in the self-reports of persons with rheumatoid arthritis. Health Psychol. 1995;14(1):64. 10.1037/0278-6133.14.1.64. [DOI] [PubMed] [Google Scholar]
- 28.Brown GK, Nicassio PM. Development of a questionnaire for the assessment of active and passive coping strategies in chronic pain patients. Pain. 1987;31(1):53. [DOI] [PubMed] [Google Scholar]
- 29.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am J Prev Med. 1994;10(2):77–84. 10.1016/S0749-3797(18)30622-6. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–18. 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Silbergleit AK, Schultz L, Jacobson BH, Beardsley T, Johnson AF. The dysphagia handicap index: development and validation. Dysphagia. 2012;27(1):46–52. 10.1007/s00455-011-9336-2. [DOI] [PubMed] [Google Scholar]
- 32.Abbott R, Peolsson A, West J, Elliott JM, Aslund U, Karlsson A, Leinhard OD. The qualitative grading of muscle fat infiltration in whiplash using fat and water magnetic resonance imaging. Spine J. 2018;18(5):717–25. 10.1016/j.spinee.2017.08.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson A, Leinhard OD, Aslund U, West J, Romu T, Smedby O, Zsigmond P, Peolsson A. An investigation of fat infiltration of the multifidus muscle in patients with severe neck symptoms associated with chronic whiplash-associated disorder. J Orthop Sports Phys Ther. 2016;46(10):886–93. 10.2519/jospt.2016.6553. [DOI] [PubMed] [Google Scholar]
- 34.Elliott JM, Courtney DM, Rademaker A, Pinto D, Sterling MM, Parrish TB. The rapid and progressive degeneration of the cervical multifidus in whiplash: a MRI study of fatty infiltration. Spine. 2015;40(12):E694–700. 10.1097/BRS.0000000000000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pink J, Petrou S, Williamson E, Williams M, Lamb SE. Economic and health-related quality of life outcomes of whiplash associated disorders. Spine. 2016;41(17):1378–86. 10.1097/brs.0000000000001512. [DOI] [PubMed] [Google Scholar]
- 36.Brauer S Acute whiplash: guidelines for the management of acute whiplash-associated disorders. Aust J Physiother. 2008;54(2):147. [Google Scholar]
- 37.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17(3):205–16. 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haggman-Henrikson B, Zafar H, Eriksson PO. Disturbed jaw behavior in whiplash-associated disorders during rhythmic jaw movements. J Dent Res. 2002;81(11):747–51. [DOI] [PubMed] [Google Scholar]
- 39.Papageorgiou AC, Silman AJ, Macfarlane GJ. Chronic widespread pain in the population: a seven year follow up study. Ann Rheum Dis. 2002;61(12):1071. 10.1136/ard.61.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell L, Smith A, McGregor L, Sterling M. Psychological factors and the development of chronic whiplash-associated disorder(s): a systematic review. Clin J Pain. 2018;34(8):755. [DOI] [PubMed] [Google Scholar]
- 41.Berecki-Gisolf J, Hassani-Mahmooei B, Collie A, McClure R. Prescription opioid and benzodiazepine use after road traffic injury. Pain Med. 2016;17(2):304–13. 10.1111/pme.12890. [DOI] [PubMed] [Google Scholar]
- 42.Walton DM, Elliott JM. An integrated model of chronic whiplash-associated disorder. J Orthop Sports Phys Ther. 2017;47(7):462–71. 10.2519/jospt.2017.7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walton DM, Pretty J, MacDermid JC, Teasel RW. Risk factors for persistent problems following whiplash injury: results of a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2009;39(5):334–50. 10.2519/jospt.2009.2765. [DOI] [PubMed] [Google Scholar]
- 44.Côté P, Cassidy JD, Carroll L, Frank JW, Bombardier C. A systematic review of the prognosis of acute whiplash and a new conceptual framework to synthesize the literature. Spine. 2001;26(19):E445–58. [DOI] [PubMed] [Google Scholar]
- 45.Borsbo B, Peolsson M, Gerdle B. Catastrophizing, depression, and pain: correlation with and influence on quality of life and health— a study of chronic whiplash-associated disorders. J Rehabil Med. 2008;40(7):562–9. 10.2340/16501977-0207. [DOI] [PubMed] [Google Scholar]
- 46.Buitenhuis J, de Jong PJ, Jaspers JP, Groothoff JW. Relationship between posttraumatic stress disorder symptoms and the course of whiplash complaints. J Psychosom Res. 2006;61(5):681–9. 10.1016/j.jpsychores.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Coppieters I, Ickmans K, Cagnie B, Nijs J, De Pauw R, Noten S, Meeus M. Cognitive performance is related to central sensitization and health-related quality of life in patients with chronic whiplash-associated disorders and fibromyalgia. Pain Physician. 2015;18(3):E389–401. [PubMed] [Google Scholar]
- 48.Daenen L, Nijs J, Roussel N, Wouters K, Van Loo M, Cras P. Dysfunctional pain inhibition in patients with chronic whiplash-associated disorders: an experimental study. Clin Rheumatol. 2013;32(1):23–31. [DOI] [PubMed] [Google Scholar]
- 49.Davis CG. Mechanisms of chronic pain from whiplash injury. J Forensic Leg Med. 2013;20(2):74–85. 10.1016/j.jflm.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Elliott JM, Noteboom JT, Flynn TW, Sterling M. Characterization of acute and chronic whiplash-associated disorders. J Orthop Sports Phys Ther. 2009;39(5):312–23. 10.2519/jospt.2009.2826. [DOI] [PubMed] [Google Scholar]
- 51.Carroll LJ, Holm LW, Hogg-Johnson S, Côtè P, Cassidy JD, Haldeman S, Nordin M, Hurwitz EL, Carragee EJ, van der Velde G, Peloso PM, Guzman J. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the bone and joint decade 2000–2010 task force on neck pain and its associated disorders. J Manip Physiol Ther. 2009;32(2):S97–107. 10.1016/j.jmpt.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Kamper SJ, Rebbeck TJ, Maher CG, McAuley JH, Sterling M. Course and prognostic factors of whiplash: a systematic review and meta-analysis. Pain. 2008;138(3):617–29. 10.1016/j.pain.2008.02.019. [DOI] [PubMed] [Google Scholar]


