Abstract
Liver damage upon exposure to ionizing radiation (IR), whether accidental or therapeutic, can contribute to liver dysfunction. Currently, radiotherapy (RT) is used for various cancers including hepatocellular carcinoma (HCC); however, the treatment dose is limited by radiation-induced liver disease (RILD) with a high mortality rate. Furthermore, the precise molecular mechanisms of RILD remain poorly understood. Here, we investigated RILD pathogenesis using various knockout mouse strains subjected to whole-liver irradiation. We found that hepatocytes released a large quantity of double-stranded DNA (dsDNA) after irradiation. The cGAS-STING pathway in non-parenchymal cells (NPCs) was promptly activated by this dsDNA, causing interferon (IFN)-I production and release and concomitant hepatocyte damage. Genetic and pharmacological ablation of the IFN-I signaling pathway protected against RILD. Moreover, clinically irradiated human peri-HCC liver tissues exhibited substantially higher STING and IFNβ expression than non-irradiated tissues. Increased serum IFNβ concentrations post-radiation were associated with RILD development in patients. These results delineate cGAS-STING induced type 1 interferon release in NPCs as a key mediator of IR-induced liver damage and described a mechanism of innate-immunity-driven pathology, linking cGAS-STING activation with amplification of initial radiation-induced liver injury.
Keywords: radiation-induced liver disease, dsDNA, cGAS-STING, type 1 interferon
Subject terms: Cell death and immune response, Pattern recognition receptors
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer death worldwide, and it is particularly common in developing countries.1 Hepatectomy is currently considered the most effective therapy for HCC,2,3 but the majority of patients diagnosed with HCC are not eligible for radical curative surgery. Radiotherapy (RT) is now listed in the National Comprehensive Cancer Network Guidelines (NCCN, Version 2.2019) as a locoregional treatment option for inoperable HCC.
The tolerance of healthy tissue to RT limits the dose of radiation that can be delivered during the treatment of malignancies. Despite continuing technical developments in RT, both the acute and delayed side effects of radiation in healthy tissue remain significant obstacles to cancer treatment. The liver is a highly radiosensitive organ, with a high risk of radiation-induced liver disease (RILD). This radiosensitivity has become a major limitation of RT in the treatment of HCC or other abdomino-pelvic tumors, and RILD is associated with a high mortality rate.4 When RT is combined with chemotherapy or immunotherapy, the risk of RILD is even higher.5,6 In contrast to the in vivo liver, isolated primary hepatocytes are radioresistant in vitro. Our group and others have demonstrated that cell-cell interactions between hepatocytes and liver non-parenchymal cells (NPCs) play an important role in RILD development.7–9 Nevertheless, the molecular mechanisms of these interactions remain largely elusive, despite decades of research.
Immune system recognition of DNA as a pathogen-associated molecular pattern provides a versatile mechanism to detect a large variety of microbial pathogens containing DNA. However, DNA is also a danger-associated molecular pattern (DAMP) when self-DNA, which is normally confined to the nucleus and mitochondria in eukaryotic cells, is inadvertently present in the cytosol.10 Cytosolic DNA in immune cells can trigger strong innate immune responses, including the production of type 1 IFNs and other inflammatory cytokines. A major sensor for cytosolic DNA is cGMP-AMP synthase (cGAS), which binds to double-stranded DNA (dsDNA), irrespective of the DNA sequence.11,12 DNA binding produces a conformational change in cGAS, leading to cGAS activation.5–9 Activated cGAS catalyzes the conversion of GTP and ATP to 2’3’-cGAMP, which functions as a second messenger that binds to and activates the adapter protein STING (stimulator of IFN genes), an endoplasmic reticulum membrane protein.12,13 STING, in turn, activates protein kinases IκB kinase (IKK) and TANK-binding kinase 1 (TBK1), leading to activation of transcription factor IFN regulatory factor 3 (IRF3), which enters the nucleus and induces a number of immune and inflammatory gene products, including type 1 IFNs. Genetic and biochemical studies have demonstrated that cGAS is an innate immune sensor that detects a wide spectrum of pathogens, including DNA-containing viruses like Herpesviruses and bacteria such as Mycobacterium tuberculosis.10 As a general sensor of cytosolic DNA, cGAS-STING activation has also been shown in several mouse models to produce autoimmune organ damage when self-DNA accumulates in the cytoplasm.14,15
Another potential source of self-DNA that can activate cGAS is tumor cell DNA. Extracellular tumor dsDNA induced by irradiation can be ingested by phagocytes, such as dendritic cells, and subsequently activate the cGAS-STING pathway.16 Indeed, recent studies suggest that STING-deficient mice are less responsive to irradiation and immunotherapy.17 However, self-DNA trafficking may occur in both physiological and pathological situations. Upon DNA sensing, several innate immune DNA-sensing pathways trigger an antimicrobial type 1 IFN response, which initially enhances host defenses but can also produce damage if it is activated by self-DNA.
The liver contains one of the main populations of polyploid cells within the body, and DNA constitutes a substantial part of the mass of mature hepatocytes in both mice and humans.18 Breakage of dsDNA induced by ionizing radiation is a key mechanism of targeted cell death following RT,19 which leads to release of dsDNA into the cytoplasm or extracellular space. It is, therefore, reasonable to hypothesize that massive hepatocyte apoptosis following RT will generate large amounts of ectopic dsDNA, which can induce activation of the innate immune system via the cGAS-STING pathway.
On the basis of these considerations, there is a critical need to elucidate the exact role of cytosolic dsDNA and subsequent activation of the cGAS-STING pathway in the development and progression of RILD. We uncovered a deleterious role of cGAS-STING activation in RILD, which is likely mediated through the release of significant amounts of type 1 IFNs from NPCs. Furthermore, our data identify cGAS-STING-mediated type 1 IFN production in NPCs as a key mediator of IR-induced liver damage, exposing innate immunity as a driver of liver pathology. Genetic or pharmacological ablation of the IFN-I signaling pathway protected against RILD. These results provide insights into the molecular pathogenesis of RILD and radiation-associated tissue damage. Elucidation of this process may guide future pharmacological interventions to control RILD by targeting STING or reducing sensing of type 1 IFNs.
Results
cGAS-STING deficiency attenuated radiation-induced hepatic injury in mice
The cGAS-STING–mediated cytosolic dsDNA sensing cascade is a major mechanism responsible for innate immune activation mediating liver injury and autoimmune diseases.14,20,21 To determine whether the cGAS-STING pathway mediates radiation-induced liver injury, WT, CGAS-deficient (Mb21d1−/−), and STING-deficient mice (Tmem173gt) underwent 30-Gy whole-liver irradiation, and liver tissues were collected at two time points to evaluate RILD in the acute stages. Our group and others have demonstrated that whole-liver irradiation leads to steatosis and apoptotic death within the first 48 h after irradiation and veno-occlusive inflammation cell infiltration within 4 months.7,22 As depicted in Fig. 1a–c, H&E staining showed IR-induced steatosis and inflammatory cell infiltration at two acute time points in the livers of irradiated mice, which were attenuated by deficiency of both cGAS and STING. At 48 h after irradiation, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assays demonstrated that apoptotic cells increased to 10.09 ± 4.61% per section of irradiated livers in WT mice compared to 0.89 ± 0.76% of sham-IR liver at the same time (Fig. 1a, d). However, the degree of apoptotic death was significantly ameliorated in Mb21d1−/− and Tmem173gt mice (Fig. 1d). Moreover, cGAS and STING deficiency was associated with lower serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) at 12, 24, and 48 h after irradiation, compared to irradiated WT mice. In the absence of radiation, both cGAS and STING deficiency had no significant effect on liver pathology, apoptotic death (Fig. 1b–d), or serum ALT or AST levels (data not shown), suggesting a strong link between cGAS-STING pathways and RILD pathogenesis.
Fig. 1.
cGAS-STING deficiency attenuated radiation-induced hepatic steatosis, and inflammation, in mice. WT, cGAS-, or STING-deficient mice underwent 30-cGy whole-liver irradiation to induce RILD. a Paraffin-embedded liver sections were stained with H&E or TUNEL. b Hepatic steatosis scores. c Histology score of infiltrated inflammatory cells. d Quantification of TUNEL+ cells based on the number of positive cells in each high-powered field. Serum ALT (e) and AST (f) levels were measured at multiple time points after irradiation. n = 10 per group. Values are mean ± SD. *P < 0.05, **P < 0.001. Statistical significance was determined using one-way ANOVA followed by Tukey-Kramer multiple comparisons test
Radiation causes dsDNA accumulation within liver sinusoids
The main stimulus for cGAS-STING is self- or virus-derived dsDNA. To elucidate whether release of dsDNA is caused by radiation, we first established a mouse RILD model using 30-cGy whole-liver irradiation. Using intravital two-photon confocal microscopy, we found that extracellular DNA released after irradiation accumulated in islets within hepatic sinusoids as large areas of DNA deposition (Fig. 2a). Notably, the majority of extracellular DNA lined the sinusoidal lumen (arrows), and extra-nuclear genetic material was stained by Sytox Green (a DNA-staining probe; Fig. 2a middle), indicating that loss of liver cell membranes had begun within 24 h after irradiation. Furthermore, more dsDNA accumulation was also detected within liver tissue interstitial fluid (TIF) in irradiated mice compared to sham-irradiated mice (Fig. 2b). These results demonstrated that irradiation caused marked dsDNA release and accumulation in the liver extracellular microenvironment. Because hepatocytes have substantial DNA content, we hypothesized that massive hepatocyte apoptosis following RT may release ectopic dsDNA into the interstitial fluid. To confirm this hypothesis, hepatocytes and liver NPCs were isolated 24 h after irradiation and cultured in vitro. The cell supernatant was collected to quantify dsDNA content after 24 h of culture (Fig. 2C). As shown in Fig. 2d, dsDNA concentration substantially increased from 205.60 ± 25.52 ng/mL to 1632.41 ± 145.18 ng/mL in the hepatocyte supernatant. However, only slight changes were observed in the NPC supernatant, suggesting that hepatocytes were the main source of extracellular dsDNA following irradiation.
Fig. 2.
In vivo multiphoton microscopy confirmation of DNA deposition in the liver microvasculature during radiation-induced injury. Mice underwent IR and after 24 h, were prepared for multiphoton microscopy. Arrows indicate extensive extracellular DNA deposition within the vessels. a Liver intravital multiphoton microscopy from an IR mouse and a sham-IR mouse (10× or 40× magnification) showing areas of DNA accumulation. Green: Sytox Green; red: phycoerythrin-conjugated anti-CD31. b dsDNA concentration in hepatic TIF. c Schematic representation depicting isolation of hepatocytes and NPCs in mice treated with 30-cGy whole-liver irradiation. d Both hepatocytes and NPCs were cultured in vitro for 24 h, and the supernatants were then harvested to measure dsDNA. Comparison of DNA sensors cGAS (e), STING (f), and TLR9 (g) gene and protein expression levels by real-time PCR and western blot analysis in different cell populations. Mean ± SD; n = 5. *P < 0.05 and ***P < 0.001 compared with hepatocytes
We next investigated which population of liver cells responded to extracellular dsDNA. Hepatocytes and liver NPCs were isolated after 24 h culture in vitro to determine expression levels of different DNA sensors including cGAS, STING, and TLR9. We found that mRNA and protein expression of all three DNA sensors was mainly in liver NPCs rather than hepatocytes (Fig. 2e–g). To determine DNA-sensing mRNA expression at the organ-level, we further analyzed liver tissues at 12, 24, and 48 h upon 30-Gy whole-liver irradiation. We observed a very similar trend for in vitro data from isolated primary NPCs as shown in Supplementary Fig. 1. These results are consistent with previous observations that liver NPCs are the main DNA sensors,23 whereas primary human hepatocytes lack detectable expression of STING.24
The CGAS-STING-type 1 IFN axis in NPCs mediates radiation-induced liver injury
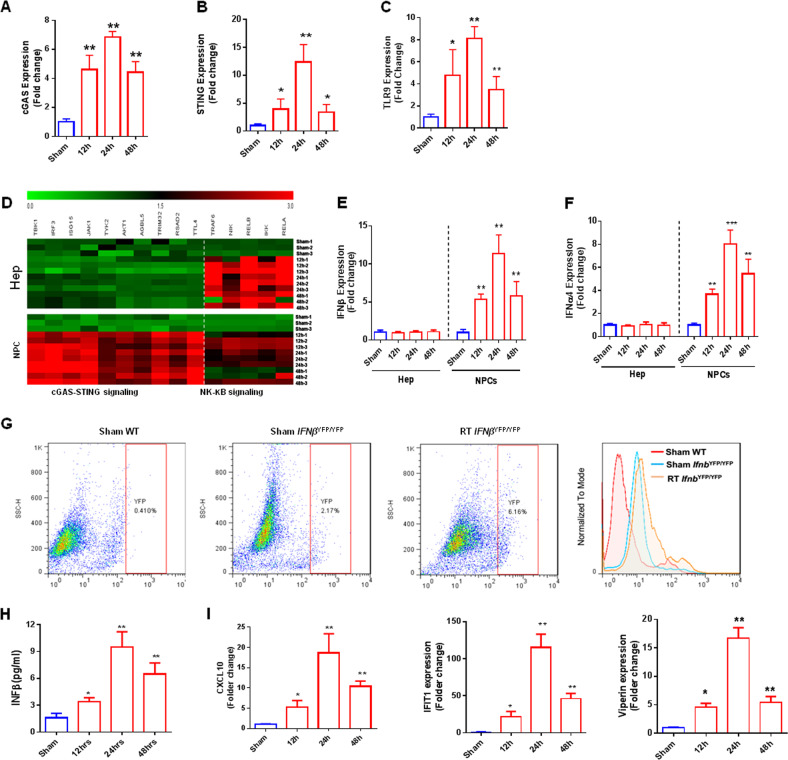
After determining the homeostatic expression of DNA-sensing pathways in liver NPCs, we next investigated how different liver cell populations react to extracellular DNA released by irradiation. For this, we performed whole-liver irradiation and isolated hepatocytes and liver NPCs (cell viability >90%) at different time points for gene expression analyses. We found a slight increase in cGAS expression by hepatocytes at 24 h after irradiation, but no significant changes in STING and TLR9 expression at any time point in these cells (data not shown). In contrast, liver NPCs consistently upregulated DNA-sensing pathways following irradiation, with cGAS, STING, and TLR9 reaching peak expression levels at 24 h after irradiation (Fig. 3a–c).
Fig. 3.
Evaluation of DNA sensor gene expression in different hepatic populations after radiation-induced injury. Gene expression of various DNA sensors in liver NPCs and hepatocytes after hepatic irradiation: a cGAS, b STING, and c TLR9. d Heatmap of cGAS-SING-related genes (TBK1, IRF3, ISG15, JAK1, TYK2, AKT1, AGBL5, TRIM32, RSAD2, TTL4) and NF-κB-related genes (TRAF6, NIK, RELB, IKK, RELA) at different time points after radiation-induced hepatic injury. Green = decreased expression; black = no variation; red = increased expression. The graphs represent the mean expressions of selected genes relative to sham-IR hepatocytes (▵▵CT). Gene expression of different cytokines in hepatocytes and NPCs after hepatic irradiation: e IFNβ and f IFNa4. g Flow cytometry for evaluating type 1 IFN production by NPCs in IFNβYFP/YFP mice. h Levels of type 1 IFNs in hepatic TIF. i Evaluation of type 1 IFN-regulated gene expression in hepatocytes after induction of hepatic radiation injury. Mean ± SD; n = 5. *P ≤ 0.05 and ***P ≤ 0.001 compared with control hepatocytes
cGAS-STING activation affects two downstream pathways, increasing type 1 IFNs through IRF3 and pro-inflammatory responses through nuclear factor (NF)-κB.25 Therefore, we next evaluated the expression of cGAS-STING-related genes (TBK1, IRF3, ISG15, JAK1, TYK2, AKT1, AGBL5, TRIM32, RSAD2, TTL4) and NF-κB-related genes (TRAF6, NIK, RELB, IKK, RELA) in hepatocytes and NPCs. As depicted in Fig. 3d, expression of cGAS-STING-related genes, which encode proteins that contribute to IFN secretion, was significantly upregulated in NPCs but not in hepatocytes after irradiation. In contrast, genes related to NF-κB signaling were significantly increased in both NPCs and hepatocytes after irradiation. Therefore, because of the higher levels of DNA sensors in liver NPCs, we speculated that type 1 IFNs were mainly produced in NPCs. Next, we assessed IFNα and IFNβ mRNA levels in NPCs and hepatocytes. No change was detected in hepatocytes after irradiation, whereas expression of both IFNα and IFNβ significantly increased in NPCs, peaking at 24 h after irradiation (Fig. 3e, f). To further address this issue, we used a mouse strain in which type 1 IFN production is under the control of yellow fluorescent protein (IFNβYFP/YFP mice). We found that 2.17% of NPCs from IFNβYFP/YFP mice constitutively expressed type 1 IFNs (as observed in mice receiving sham RT), and that the percentage of NPCs expressing IFNβ increased almost 3-fold after whole-liver irradiation (6.16%) (Fig. 3g). Accordingly, type 1 IFNs increased in liver TIF and peaked at 24 h after irradiation as shown in Fig. 3h. Therefore, irradiation led to enhanced expression of DNA-sensing and inflammatory pathways in different hepatic populations, with a significantly more pronounced effect in liver NPCs.
Type 1 IFN can reportedly directly enhance hepatocyte injury.23,26 We next tested the hypothesis that type 1 IFNs released by NPCs are sensed by hepatocytes during RILD. Indeed, we found that various genes involved in type 1 IFN-mediated cell activation, including CXCL10, IFN-induced protein with tetratricopeptide repeats 1 (IFIT1), and viperin, were significantly upregulated in isolated hepatocytes after irradiation (Fig. 3j). Interestingly, all of these genes were expressed at 12 h and were markedly increased at 24 h after irradiation, concomitant with the peak in extracellular DNA release, activation of liver NPCs, and increased expression liver enzymes (serum AST and ALT). Thus, we speculated that DNA triggers type 1 IFN production from NPCs, which subsequently acts on hepatocytes during RILD.
Removing extracellular DNA abrogates type 1 IFN production and reduces radiation-induced liver injury
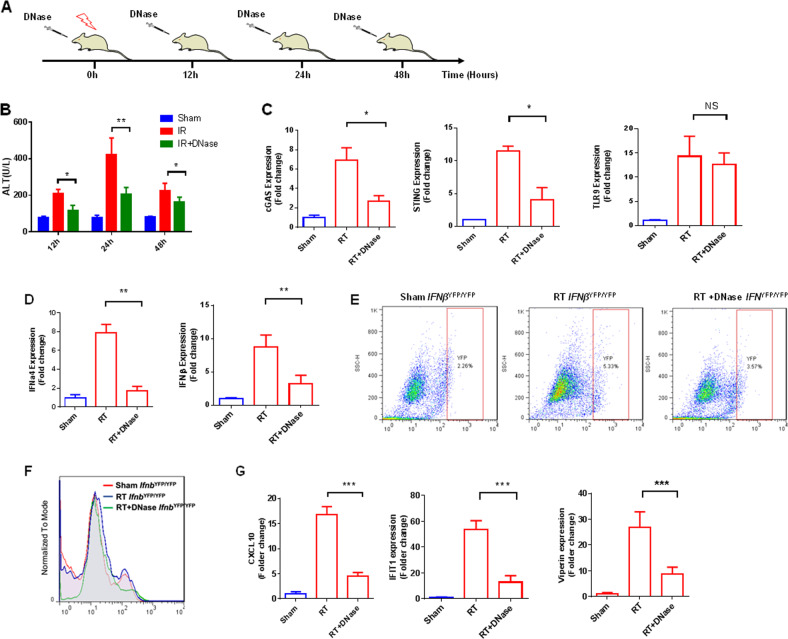
To confirm that extracellular DNA released during cell death triggers liver NPC activation and hepatocyte injury, we treated mice with a commercially available DNase at different time points after irradiation, as shown in Fig. 4a. Firstly, to determine whether DNase was able to remove extracellular DNA in the liver microenvironment after irradiation, we collected liver TIF at multiple time points after irradiation and measured the DNA concentration. As expected, DNase injection dramatically lowered TIF DNA levels after irradiation (Supplementary Fig. 2). Next, we examined the effects of DNase treatment on radiation-induced liver injury. When treated with systemic DNase, plasma levels of ALT after irradiation were significantly reduced (Fig. 4b). Furthermore, dsDNA removal with DNase abolished expression of both cGAS and STING in liver NPCs of irradiated mice, although TLR9 expression was unaffected (Fig. 4c). Moreover, expression of genes involved in the production of type 1 IFNs (IFNa4 and IFNβ) was significantly reduced in NPCs, as shown in Fig. 4d. Furthermore, in vivo IFNβYFP/YFP mice model, DNase treatment substantially limited the post-radiation increase in the percentage of liver NPCs expressing IFNβ (Fig. 4e, f). Strikingly, the decrease in extracellular DNA and type 1 IFN release after DNase treatment were accompanied by a significant reduction in expression levels of type 1 IFN-regulated genes in isolated primary hepatocytes (Fig. 4g), suggesting a strong link between radiation-induced extracellular DNA release and the cGAS-STING-IFN axis in regulating RILD.
Fig. 4.
DNase treatment after hepatic irradiation. a Schematic representation of irradiation and DNase (1000 U/L) administration over time. b Serum ALT levels to assess hepatic injury at 12, 24, and 48 h after DNase treatment. c Comparison of gene expression of various sensors in hepatic NPCs between DNase-treated and DNase-untreated groups. Cells were collected 24 after IR, and relative expression was determined using control NPCs as reference. d Comparison of expression of genes involved in the production of type 1 IFNs in DNase-treated and untreated NPCs. e, f Flow cytometry to evaluate type 1 IFN production in DNase-treated and DNase-untreated NPCs. Cells were collected from IFNYFP/YFP mice 24 h after 30-Gy whole-liver irradiation. g Evaluation of type 1 IFN-regulated gene expression in DNase-treated and DNase-untreated hepatocytes. Mean ± SD; n = 5. *P < 0.05, **P < 0.01 and ***P < 0.001
Blockade of type 1 IFN signaling protects mice from radiation-induced liver injury without affecting tumor growth
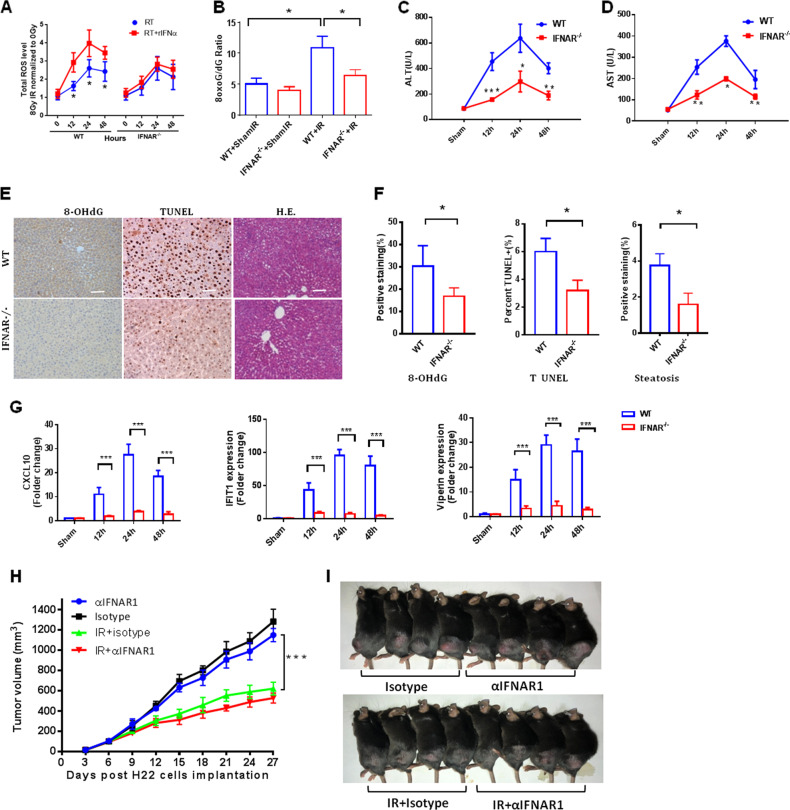
Type 1 IFN could directly mediate hepatocyte injury.23,26 To gain further insight regarding type 1 IFN regulation of liver injury, we examined the direct effects of altering type 1 IFN signaling on hepatocyte injury. Radiation induces the formation of reactive oxygen species (ROS) that play a major role in physiologic and pathologic events, causing cell death and perpetuating damage. Superoxide dismutases (SODs) are essential to convert superoxide to hydrogen peroxide that is subsequently removed by catalase.27 Type 1 IFN led to a downregulation of SODs,26 which potentially will exaggerate increased steady-state levels of ROS after IR. To determine whether type 1 IFN enhanced IR-induced oxidative stress, we firstly irradiated primary WT mouse hepatocytes in vitro with or without recombinant IFN-α (rIFN-α). As expected, we observed significantly higher ROS levels at all time points after IR with the presence of rIFN-α (Fig. 5a). When type 1 IFN signaling was blocked using primary hepatocytes isolated from IFNα and IFNβ receptor-deleted mice (IFNAR−/−), the exaggerated accumulation of ROS was prevented, indicating that type 1 IFN is sufficient to maximize IR-induced ROS accumulation (Fig. 5a).
Fig. 5.
Blockade of type 1 IFN signaling protects mice from radiation-induced liver injury without affecting tumor growth. Measurement of intracellular total ROS (a) in irradiated primary WT mouse hepatocytes and IFNAR−/− cells with or without rIFN-α (n = 7) at 0, 12, 24, and 48 h postirradiation (30 Gy). b 8-oxo-G/deoxyguanosine ratios in IFNAR−/− versus WT mice 24 h after whole-liver irradiation (30 Gy IR). Serum c ALT and d AST levels in IFNAR−/− and WT mice at 12, 24, and 48 h after IR. e 8-hydroxy-2′-deoxyguanosine (8-OHdG) and TUNEL staining in the liver was determined by immunohistochemistry. Steatosis was analyzed in H&E staining. Scale bar: 100 μm. f Quantification of TUNEL-positive cells in each high-power field and positive 8-OHdG signal in a unit area, and steatosis score at the indicated region. g Expression of CXCL10, IFIT1, and viperin in IFNAR−/− mouse hepatocytes versus WT cells at 12, 24, and 48 h after irradiation. h H22 tumor size (mean ± SD, n = 7 mice/group) irradiated with 8 Gy for 3 consecutive days in mice that received intraperitoneal injection with 250 mg of a-IFNAR1 antibody (Clone MAR1-5A3, BioXCell) or matched isotype control (clone MOPC21, BioXCell) 1 day prior to whole-liver irradiation (8 Gy x 3) followed by injections every other day. Significant differences were determined using two-way ANOVA. i Representative mice with subcutaneous H22 tumors at termination, 27 days after tumor inoculation. Mean ± SD; n = 5. *P < 0.05, **P < 0.01, and ***P < 0.001
To further investigate the causative role of IFN-I in RT-induced oxidative tissue damage, we subjected IFNAR−/− mice to whole-liver irradiation. In agreement with in vitro experiments, IFNAR−/− mice exhibited a reduced 8-oxo-guanine/deoxyguanosine (8-oxo-G/dG) ratio, which is a readout for oxidative DNA damage (Fig. 5b). Serum ALT and AST levels in IFNAR−/− mice were significantly lower than those of WT mice throughout the observation period after irradiation, including at the earliest time point (12 h) (Fig. 5c, d). Histological examination of liver biopsy specimens showed that IFNα and IFNβ receptor deletion significantly reduced radiation-induced oxidative DNA damage measured by 8-OhdG staining, apoptosis, and extent of steatosis compared with WT mice at 48 h after IR (Fig. 5e, f). To elucidate the clinical relevance of these findings, we investigated whether pharmacological blockade of the IFN-I signaling pathway had a beneficial effect on IR-induced oxidative tissue damage. In line with these gene knockout models, antibody blockade of IFNAR1 abrogated oxidative tissue damage in mice upon IR (Supplementary Figure 3A) and prevented the elevation of serum ALT (Supplementary Figure 3B and 3C). Thus, type 1 IFN signaling appears to be crucial for the initial phase of radiation-induced liver injury. Importantly, type 1 IFN-induced genes such as CXCL10, IFIT1, and viperin were also dramatically reduced in hepatocytes from IFNAR−/− mice after whole-liver irradiation (Fig. 5g).
To investigate whether blockade of type 1 IFN affected the therapeutic ratio of RT, we injected murine HCC H22 cell lines subcutaneously and treated animals with 8 Gy for 3 consecutive days and concurrently administered the IFNAR1-specific antibody when the tumor volume reached ~100 mm3. As shown in Fig. 5h, i, RT alone substantially improved tumor control, but blockade of type 1 IFN signaling using IFNAR1-specific antibody alone or with concurrent RT did not affect tumor growth.
STING and IFNβ expression is increased in irradiated peritumoral normal liver tissues of HCC patients who received RT
To further address the relevance of cGAS-STING to human RILD, we examined downstream STING and IFNβ expression in peritumoral normal liver tissue sections from human subjects who received pre-operative radiotherapy (POR). Compared with peritumoral sections of patients without POR, sections of patients with POR exhibited increased inflammatory infiltration, which is consistent with our findings in mice (data not shown). When expression of STING and IFNβ was examined, the intensity of staining in peritumoral normal liver tissue of patients with POR was much stronger than that in subjects without POR (Fig. 6a–c). Furthermore, STING-positive cells were mainly NPCs that lined the hepatic sinusoidal space with small nuclei as shown in Fig. 6a (red arrows). Similar to our results in mice, human liver sections did not contain STING-positive hepatocytes as demonstrated in Fig. 6a. RNA from paraffin-embedded tissues was used to analyze gene expression of IFNβ and the downstream signaling molecules CXCL10 and IFIT1. As shown in Fig. 6d, expression of all of these genes was substantially increased in POR+ patients compared with POR- subjects.
Fig. 6.
STING expression is increased in irradiated peritumoral normal liver tissues of humans receiving hepatic radiation. a POR or matched control peritumoral normal liver sections (collected at 4 weeks after RT completion) were stained with H&E or for STING or IFNβ IHC. Quantification of b STING or c IFNβ expression in irradiated or non-irradiated peritumoral normal liver tissues (n = 20). d Evaluation of type 1 IFNs and regulated gene expression in irradiated or non-irradiated peritumoral normal liver tissues (n = 20). e IFNβ serum levels pre-irradiation (RT) (blue) and 7 days after irradiation completion (red) in RILD, which were borderline compared to control groups. Mean ± SD; n = 5. *P < 0.05 and ***P < 0.001. f Proposed schematic diagram of cGAS-STING-mediated type 1 IFN release contributes to RILD progression. Radiation-induced liver cell death (mainly hepatocytes, Hep) leads to massive DNA release and deposition within the hepatic tissue interstitial fluid (TIF). Non-parenchymal cells (NPCs) engulf the dsDNA, which leads to cGAS-STING activation and elicits the expression of type 1 IFN. Type 1 IFNs acts on primarily hepatocytes by increasing oxidative stress, which potentially deteriorates RILD. Controlling the innate immune response during RILD may reduce IR-triggered liver injury, thereby suggesting a potently useful strategy for patients undergoing hepatic irradiation
We also retrospectively analyzed serum samples from three groups of patients with HCC: RILD (n = 19), HCC patients fulfilling the diagnostic criteria for RILD;22 borderline (n = 19), a matched group of HCC patients whose liver enzyme levels exceeded normal limits but who did meet RILD criteria; and control (n = 19, another matched group of HCC patients whose liver function remained normal after RT (Supplementary Table 2). We found that radiation-induced increases in IFNβ correlated with RILD development. Compared to baseline, serum IFNβ increased significantly in the RILD group after RT, peaking shortly after the completion of therapy (on day 7). Serum IFNβ also increased significantly, albeit to a lesser extent, after RT in the borderline group, whereas no significant increase was observed in the control group (Fig. 6e). Together, these results strongly suggest that STING and type 1 IFNs have a deleterious role in the pathogenesis of RILD in both mice and humans.
Discussion
Radiation toxicity remains a challenging obstacle to the therapeutic efficiency of liver RT, but the precise molecular mechanisms of RILD remain poorly understood. CGAS-STING is a powerful innate immunity pathway. In the present study, we used various knockout mouse models to confirm that cGAS-STING signaling plays a deleterious role in IR-induced liver injury. We revealed a novel pathway involving direct toxic injury, DNA release from dead hepatocytes, and activation of a cascade through cGAS-STING–mediated sensing of liver self-DNA. This cascade culminates in further type 1 IFN production and liver injury (Fig. 6f). These data have a substantial impact on our understanding of why patients with RILD develop worsening hepatitis, even when the initial ionizing radiation stimulus has been removed. Clinically, higher serum IFNβ changes post-IR were found in RILD patients comparing to those without RILD. Moreover, pharmacological ablation of the IFN-I signaling pathway protected against IR-induced liver injury without affecting tumor growth in the context of RT. Accordingly, our results shed light on potential adjunct therapeutic strategies directed at dampening the direct toxic effects of RT and the indirect effects of radiation triggered by activation of the hepatic immune system.
We used intravital microscopy to demonstrate massive deposition of extracellular DNA within the liver after irradiation. Primary hepatocytes isolated from irradiated liver released much higher quantities of DNA into the surrounding media than primary NPCs, suggesting that hepatocytes were the major source of the accumulated DNA. It is worth mentioning that some extracellular DNA released by necrosis would be washed from the vessels by blood flow, and some DNA may be degraded by serum DNase I. Therefore, the actual amount of DNA released from irradiated tissue may be even higher than that detected using our methodology. However, blood flow within the liver is relatively slow compared with other organs, which may account for the substantial amount of DNA retention within the hepatic microcirculation.
The cGAS-STING pathway is essential for host protection against DNA pathogens and acts through various mechanisms. However, overstimulation of this innate immune signaling pathway, speculatively by chronic infection or inappropriately digested apoptotic DNA, will lead to severe inflammation, augmented autoimmune multiple organ injury, and abnormal production of cytokines, including type 1 IFNs.21,28 In the pathogenesis of RILD, inflammation is a key to converting primary DNA damage into veno-occlusive disease, which is characterized by inflammatory damage and delayed liver fibrosis.22,29 In the present study, we confirmed a deleterious role of cGAS-STING in early radiation-induced apoptosis and steatohepatitis. As supporting evidence, serum ALT and AST after irradiation were significantly lower in cGAS-/- or STING-/- mice than in WT mice.
Accumulating evidence has demonstrated the essential role of innate immunity in the development of hepatic steatosis, but the exact mechanisms remain unclear.7,30,31 NPCs have drawn particular attention as mediators of innate immune responses because pro-inflammatory activation is strongly associated with hepatic steatosis and inflammation. Iracheta-Vellve et al. suggested that early proapoptotic activation of IRF3 by CCl4 was hepatocyte specific and mediated by STING.32 The same group also demonstrated that STING and IRF3 were key determinants of alcoholic liver disease via hepatocyte apoptosis.20 Qiao et al. reported that knockdown of STING in a normal human fetal liver cell line attenuated free fatty acid–induced apoptosis.33 Lipotoxicity-induced hepatic protein inclusions were also observed after STING-TBK1 activation in a human HCC line.34 However, STING protein was not expressed in hepatocytes of adult humans or mice.23 In our current work, western blot analysis demonstrated that hepatocytes in livers of adult mice did not express STING protein. When investigating the role of STING in non-alcoholic steatohepatitis, NPCs (particularly Kupffer cells) are of critical importance. Kupffer cells are an integral part of the innate immune system, acting as scavengers and phagocytes in the liver.35 NPCs are the most important population responding to hepatocyte injury, leading to the production of chemokines, recruitment of monocytes, and induction of pro-inflammatory cytokines,36 and we previously demonstrated that early depletion of liver Kupffer cells from NPCs prevented the development of RILD in an animal model.7
Although our knowledge of RILD pathogenesis has improved in recent years, the molecular pathogenic mechanisms remain unclear. Hepatocytes are known to be radioresistant in vitro, but they are radiosensitive in vivo.37 Increasing evidence has attributed this discrepancy to the liver microenvironment composed of NPCs and their released cytokines.38 In the current work, cGAS or STING deficiency attenuated dsDNA-induced induction of type 1 IFNs and downstream gene expression of CXCL10 in hepatocytes, alleviating radiation-induced liver injury. Araujo et al. demonstrated the crucial role of type 1 IFN signaling in drug-induced hepatic injury.23 An increase in type 1 IFN-producing Kupffer cells was crucial for the development of non-alcoholic steatohepatitis, acting by the promotion of blood monocyte infiltration through the production of two chemokines: monocyte chemoattractant protein 1 (MCP-1) and IFN-inducible protein 10 (IP-10).36
The exact mechanisms whereby type 1 IFNs enhance hepatocyte injury remain elusive. Recent reports indicated that type 1 IFNs led to downregulation of superoxide dismutase 1 (Sod1; an enzyme that catalyzes the removal of free hepatic superoxide radicals) and produces oxidative liver damage in superoxide-deficient and WT mice. Genetic and pharmacological ablation of the type 1 IFN signaling pathway protected against virus-induced liver damage. These findings delineated type 1 IFN-mediated oxidative stress as a key mediator of virus-induced liver damage and identified a mechanism of innate-immunity-driven pathology, linking type 1 IFN signaling with antioxidant host defenses and infection-associated tissue damage. Furthermore, a major adverse effect of type 1 IFN administration in patients involves the downregulation of Sod1, promoting hepatocyte necrosis and morbidity.23,26 It is well established that liver injury after irradiation arises mainly from two mechanisms: direct DNA damage from ionizing radiation and indirect injury from oxidative stress and ROS production, leading to hepatocellular apoptosis, acute inflammatory responses, and delayed fibrosis in irradiated regions. Importantly, we found that patients with RILD had dramatic increases in IFNβ, which were not observed in patients without RILD, which again supported a correlation between type 1 IFNs and radiation-induced liver injury. Therefore, we suggest that type 1 IFNs may be extremely hepatotoxic, acting primarily by increasing oxidative stress and ROS, which lead to indirect cell injury.
Emerging data indicate that IFN signaling has paradoxical effects on tumor control.39,40 IFN signaling can enhance the generation of an antitumor response, with DCs and T cells as well-recognized targets.41 Suppressive effects are also recognized, including induction of the immune checkpoint mediator PD-L1.42,43 Radiation may amplify these effects through the induction of type 1 IFN. Benci et al. showed that prolonged IFN signaling renders cancer cells more resistant to immune checkpoint blockade with or without RT.39 There is a growing body of evidence indicating that activation of type 1 IFN signaling in tumors correlates with worse outcomes in patients and with their resistance to therapies, including RT.44–47 Chen et al. reported that type 1 IFN can protect cancer cells from T cell-mediated cytotoxicity after radiation, and type 1 IFN signaling in cancer cells reduces benefits from the antitumor immune response after radiation, adding another layer of complexity.48 In our murine H22 tumor model, blockade of type 1 IFN signaling using an IFNAR1-specific antibody alone or with RT did not affect tumor growth. This further suggests type 1 IFN signaling could potentially be a target for interventions designed to preserve normal liver function without affecting the tumor response to RT.
In summary, we identified a novel cascade in which irradiation-induced cell death leads to massive DNA deposition within the liver, followed by cGAS-STING activation that causes IFN-I production and release and concomitant hepatocyte damage. Type 1 IFN-mediated effects, governed mainly by liver NPCs, play a major role in RILD. This was also supported by clinical data showing that increased serum IFNβ concentrations post-radiation were associated with RILD development in patients. Genetic and pharmacological ablation of the IFN-I signaling pathway protected against RILD. These results demonstrate that cGAS-STING induced IFN-I release in NPCs as a key mediator of IR-induced liver damage. They also reveal a mechanism of innate-immunity-driven pathology that links cGAS-STING activation with amplification of initial radiation-induced liver injury. Notably, ablation of the IFN-I signaling pathway did not affect tumor growth in the context of RT. Collectively these results provide strong rationale for targeting STING or inhibiting type 1 IFN signaling as a therapeutic or preventive approach for RILD management.
Materials and methods
Animals and in vivo multiphoton imaging
C57BL mice aged 6–8 weeks were used in experiments. IFNAR−/− mice were purchased from NanJing KeyGen Biotech (Nanjing, China). cGAS−/− (Mb21d1−/−) and IFNβ1-eYFP mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). STING-deficient mice (STING−/−, Tmem173gt) were kindly provided by Dr. Liufu Deng’s Lab at Shanghai Jiaotong University. The mice were maintained under controlled conditions (24 ± 2 °C, 40–70% relative humidity, 12-h light/12-h dark cycle) and given a normal laboratory diet and water ad libitum. All mice received humane care in compliance with institutional animal care guidelines, and protocols were approved by the local institutional committee. All protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of Zhongshan Hospital Fudan University. All efforts were made to minimize the number of mice used and their suffering.
Multiphoton confocal intravital imaging was performed as previously described.49 Briefly, mice were anesthetized, and a midline laparoscopy was performed to expose the liver for imaging. Image experiments were performed using a multiphoton laser-scanning microscope (FV1200MPE; Olympus, Tokyo, Japan). Before surgery, the mice were injected with 1 µL Sytox Green, (Invitrogen, Carlsbad, CA, USA) and phycoerythrin (PE)-conjugated anti-CD31 (4 µg; eBiosciences, San Diego, CA, USA). Mice injected with PE-rat anti-mouse IgG served as positive controls. Multiphoton z-stack images were obtained from 200 μm below the surface of the cortex to a depth of 500 μm.
Isolation of mouse primary hepatocytes and liver NPCs
Primary hepatocyte purification was performed as described previously.17,18 Briefly, mice were anesthetized and underwent liver perfusion with collagenase (C213; Sigma-Aldrich, St. Louis, MO, USA) through the portal vein at 12, 24, and 48 h post-radiation. After perfusion, the liver was transferred to a sterile glass container and dissociated with forceps while remaining in Williams’ Emedium. Cell suspensions were filtered through a 40-µm sterile nylon mesh, transferred to a 50-mL tube, and then centrifuged twice at 60g for 3 min at 4 °C. Following centrifugation, cell viability was determined by staining with trypan blue dye.
Liver NPC isolation was performed as described previously.17,18 In brief, the liver of each mouse was removed at 24, 48, and 72 h after radiation, minced into small pieces, and digested using a solution of RPMI medium supplemented with 10% fetal bovine serum and collagenase VIII (1 mg/mL; Sigma-Aldrich). After incubation under agitation for 1 h at 37 °C, the liver homogenate was filtered through a 70-µm cell strainer to remove undigested tissue. The filtrate was subsequently transferred to a 50-mL tube and differentially centrifuged (1st: 300g for 5 min at 4 °C; 2nd and 3rd: 60g for 3 min at 4 °C; and 4th: 300g for 5 min at 4 °C). The pellet was reconstituted for further analysis. For flow cytometry, 5 × 105 cells were used, and each sample was read in an BD FACSCanto II cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). FlowJo and AccuriTM C6 software (FlowJo, LLC, Ashland, OR, USA) were used to analyze the results.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from NPCs and hepatocytes using the TRIzol kit (9109; Takara, Kyoto, Japan), according to the manufacturer’s instructions. Total RNA was quantified using NanoDrop One (Thermo Fisher Scientific, Waltham, MA, USA), and 1 µg RNA was used to perform reverse transcription using the Bestar qPCR RT kit (#2220; DBI Bioscience, Ludwigshafen, Germany). Gene expression analyses were performed using SYBR Green PCR master mix (Roche, Basel, Switzerland) in a LightCycler 480 real-time PCR system (Roche) using a 20-μL reaction system. The PCR settings were 95 °C for 2 min, followed by 40 cycles of 94 °C for 20 s, 58 °C for 20 s, and 72 °C for 20 s. Data from more than three biological replicates were subjected to final quantitation and statistical analysis using the 2−▵▵Ct method normalized with respect to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The sequences of primers used for qRT-PCR analysis are listed in Supplementary Table 1. Heatmaps were created with Mev software (http://mev.tm4.org/) using the relative expression of genes in different groups.
Immunoblotting
Whole-tissue protein extracts were assessed by discontinuous SDS-PAGE and standard western blot analysis using anti-cGAS (ab179785; Abcam, Cambridge, UK), anti-STING (Cat no.13647, Cell Signaling Technology, Danvers, MA USA) and anti-TLR9 antibodies (ab52967, Abcam). Protein expression levels were normalized to GAPDH (ab181602, Abcam) levels.
Statistics
Experimental data were analyzed using Student’s t-test and one-way analysis of variance (ANOVA) with Tukey-Kramer’s post-hoc tests. All statistical testing was performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). The significance level was set at P < 0.05. Data are presented as mean ± SD unless otherwise indicated. Data shown are representative of at least three independent experiments.
Study approval
We retrospectively constructed a patient database using data collected from 2005–2019 in our institute. Data were obtained from patients who received the standard of care treatment. Peritumoral normal liver tissues were collected from 20 patients with HCC who underwent surgical resection post-RT from July 2005 to October 2013 at the Zhongshan Hospital Fudan University (Shanghai, People’s Republic of China) as previously prescribed.50 Serum samples were obtained from the patient’s specimen bank of Fudan Liver Cancer Institute (Shanghai, People’s Republic of China). Patients were informed that their clinical data and tissue samples could be used for anonymized scientific analyses, for which they gave written consent. Ethical approval for the study protocol was obtained from the research ethics committee of Zhongshan Hospital Fudan University (approval number B2017-062R).
Supplementary information
Acknowledgements
This work was supported by the National Nature Science Foundation of China (No. 81773220 and U1505229).
Author contributions
Study concept and design: S.D. and Z.Z.; acquisition of data: G.C. and S.D.; analysis and interpretation of data: S.D. and G.C.; acquisition of patient specimens: P.Y., Y.C., Y.H. and S.D.; drafting of the manuscript: S.D. and Z.Z.; critical revision of the manuscript: J.Z., J.F. and Z.Z.; obtained funding: administrative, Z.Z.; technical or other material support: S.D. and G.C.; study supervision: Z.Z.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-020-0395-x) contains supplementary material.
References
- 1.Jemal A, et al. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90.. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Karaman B, Battal B, Sari S, Verim S. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J. Gastroenterol. 2014;20:18059–18060. doi: 10.3748/wjg.v20.i47.18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerum S, Jensen AD, Roeder F. Stereotactic body radiation therapy in patients with hepatocellular carcinoma: a mini-review. World J. Gastrointest. Oncol. 2019;11:367–376. doi: 10.4251/wjgo.v11.i5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koay EJ, Owen D, Das P. Radiation-induced liver disease and modern radiotherapy. Semin. Radiat. Oncol. 2018;28:321–331. doi: 10.1016/j.semradonc.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson LA, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int. J. Radiat. Oncol. Biol. Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, et al. Liver toxicity induced by combined external-beam irradiation and radioimmunoglobulin therapy. Radiat. Res. 1995;141:294–302. [PubMed] [Google Scholar]
- 7.Du SS, et al. Inactivation of kupffer cells by gadolinium chloride protects murine liver from radiation-induced apoptosis. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:1225–1234. doi: 10.1016/j.ijrobp.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 8.Christiansen H, et al. Irradiation leads to susceptibility of hepatocytes to TNF-alpha mediated apoptosis. Radiother. Oncol. 2004;72:291–296. doi: 10.1016/j.radonc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Dong Y, et al. Activation of the JNK-c-Jun pathway in response to irradiation facilitates Fas ligand secretion in hepatoma cells and increases hepatocyte injury. J. Exp. Clin. Cancer Res. 2016;35:114. doi: 10.1186/s13046-016-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016;17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 11.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao D, et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl. Acad. Sci. USA. 2015;112:E5699–E5705. doi: 10.1073/pnas.1516465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray EE, Treuting PM, Woodward JJ, Stetson DB. Cutting edge: cGAS is required for lethal autoimmune disease in the Trex1-deficient mouse model of aicardi-goutieres syndrome. J. Immunol. 2015;195:1939–1943. doi: 10.4049/jimmunol.1500969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng L, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demaria O, et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA. 2015;112:15408–15413. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang MJ, Chen F, Lau JTY, Hu YP. Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death Dis. 2017;8:e2805. doi: 10.1038/cddis.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrasek J, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl. Acad. Sci. USA. 2013;110:16544–16549. doi: 10.1073/pnas.1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc. Natl. Acad. Sci. USA. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence TS, et al. Hepatic toxicity resulting from cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 23.Araujo A. M., et al. Liver immune cells release type 1 interferon due to DNA sensing and amplify liver injury from acetaminophen overdose. Cells7, 1–16. 10.3390/cells7080088 (2018). [DOI] [PMC free article] [PubMed]
- 24.Thomsen MK, et al. Lack of immunological DNA sensing in hepatocytes facilitates hepatitis B virus infection. Hepatology. 2016;64:746–759. doi: 10.1002/hep.28685. [DOI] [PubMed] [Google Scholar]
- 25.Barber GN. STING: infection, inflammation and cancer. Nat. Rev. Immunol. 2015;15:760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharya A, et al. Superoxide dismutase 1 protects hepatocytes from type I interferon-driven oxidative damage. Immunity. 2015;43:974–986. doi: 10.1016/j.immuni.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mapuskar KA, Anderson CM, Spitz DR, Batinic-Haberle I, Allen BG. Utilizing superoxide dismutase mimetics to enhance radiation therapy response while protecting normal tissues. Semin. Radiat. Oncol. 2019;29:72–80. doi: 10.1016/j.semradonc.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata S. Apoptosis and autoimmune diseases. Ann. N. Y. Acad. Sci. 2010;1209:10–16. doi: 10.1111/j.1749-6632.2010.05749.x. [DOI] [PubMed] [Google Scholar]
- 29.Munoz-Schuffenegger P, Ng S, Dawson LA. Radiation-induced liver toxicity. Semin. Radiat. Oncol. 2017;27:350–357. doi: 10.1016/j.semradonc.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Deng ZB, et al. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology. 2009;50:1412–1420. doi: 10.1002/hep.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, et al. Myeloid cell-specific disruption of Period1 and Period2 exacerbates diet-induced inflammation and insulin resistance. J. Biol. Chem. 2014;289:16374–16388. doi: 10.1074/jbc.M113.539601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iracheta-Vellve A, et al. Endoplasmic reticulum stress-induced hepatocellular death pathways mediate liver injury and fibrosis via stimulator of interferon genes. J. Biol. Chem. 2016;291:26794–26805. doi: 10.1074/jbc.M116.736991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao JT, et al. Activation of the STING-IRF3 pathway promotes hepatocyte inflammation, apoptosis and induces metabolic disorders in nonalcoholic fatty liver disease. Metabolism. 2018;81:13–24. doi: 10.1016/j.metabol.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Cho CS, et al. Lipotoxicity induces hepatic protein inclusions through TANK binding kinase 1-mediated p62/sequestosome 1 phosphorylation. Hepatology. 2018;68:1331–1346. doi: 10.1002/hep.29742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J. Biol. Chem. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alati T, Van Cleeff M, Strom SC, Jirtle RL. Radiation sensitivity of adult human parenchymal hepatocytes. Radiat. Res. 1988;115:152–160. [PubMed] [Google Scholar]
- 38.Radoshevich L, Dussurget O. Cytosolic innate immune sensing and signaling upon infection. Front. Microbiol. 2016;7:313. doi: 10.3389/fmicb.2016.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benci JL, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167:1540–1554.e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katlinski KV, et al. Inactivation of interferon receptor promotes the establishment of immune privileged tumor microenvironment. Cancer Cell. 2017;31:194–207. doi: 10.1016/j.ccell.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo S-R, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spranger S, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taube JM, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weichselbaum RR, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc. Natl. Acad. Sci. USA. 2008;105:18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boelens MC, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159:499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erdal E, Haider S, Rehwinkel J, Harris AL, McHugh PJ. A prosurvival DNA damage-induced cytoplasmic interferon response is mediated by end resection factors and is limited by Trex1. Genes Dev. 2017;31:353–369. doi: 10.1101/gad.289769.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Post AEM, et al. Interferon-stimulated genes are involved in cross-resistance to radiotherapy in tamoxifen-resistant breast cancer. Clin. Cancer Res. 2018;24:3397–3408. doi: 10.1158/1078-0432.CCR-17-2551. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, et al. IFN protects cancer cells from CD8+ T cell-mediated cytotoxicity after radiation. J. Clin. Invest. 2019;129:4224–4238. doi: 10.1172/JCI127458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H, et al. ADAMTS13 controls vascular remodeling by modifying VWF reactivity during stroke recovery. Blood. 2017;130:11–22. doi: 10.1182/blood-2016-10-747089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu ZF, et al. Toll-like receptor 4 and its associated proteins as prognostic factors for HCC treated by post-radiotherapy surgery. Oncol. Lett. 2018;15:9599–9608. doi: 10.3892/ol.2018.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.