Conventional dendritic cells (cDCs) are categorized into two main subsets, cDC1 and cDC2, distinguished by their differential expression of cell surface markers, and the development of these two cDC subsets is dependent on interferon regulatory factor 8 (IRF8) and IRF4, respectively.1 Kim et al.2 recently reported that cDC1 can be distinguished from cDC2 based on an AP1–IRF composite element (AICE)-dependent gene program. IRF8 at a high concentration engages AICEs at enhancer regions to direct a program toward establishing the cDC1 identity. These findings provide a molecular basis to explain the distinct transcriptional signatures of cDC1s and cDC2s.
Basic leucine zipper transcription factor ATF-like 3 (BATF3) and IRF8 are the main transcription factors essential for the generation of cDC1s.1,3 Using chromatin immunoprecipitation sequencing, Kim et al. found that the cDC1-specific transcriptional program relied on the selective engagement of AICE-dependent enhancers, which can be activated in the presence of a high amount of IRF8.2 AICE, along with the Ets–IRF composite element (EICE) and IFN-stimulated response element, is a DNA motif recognized and bound by IRF8 and IRF4 to initiate transcription after binding to the BATF3–JUN dimer.4 The authors found that AICEs in the cDC genome were only enriched in peaks specific to IRF8, whereas EICEs were enriched in IRF8- and IRF4-binding peaks. The IRF8-binding peaks associated with cDC1-specific genes were enriched in both AICEs and EICEs, suggesting that the cDC1-specific transcriptional program relies on high IRF8 levels and engagement of AICE-dependent enhancers. Specifically, two potential AICEs and two potential EICEs were found at the IRF8-binding sites in the enhancer region of the cDC1-specific gene Snx22 in mice.2 These results demonstrate that the expression of cDC1-specific genes requires the activation of EICE- and AICE-dependent enhancers (Fig. 1).
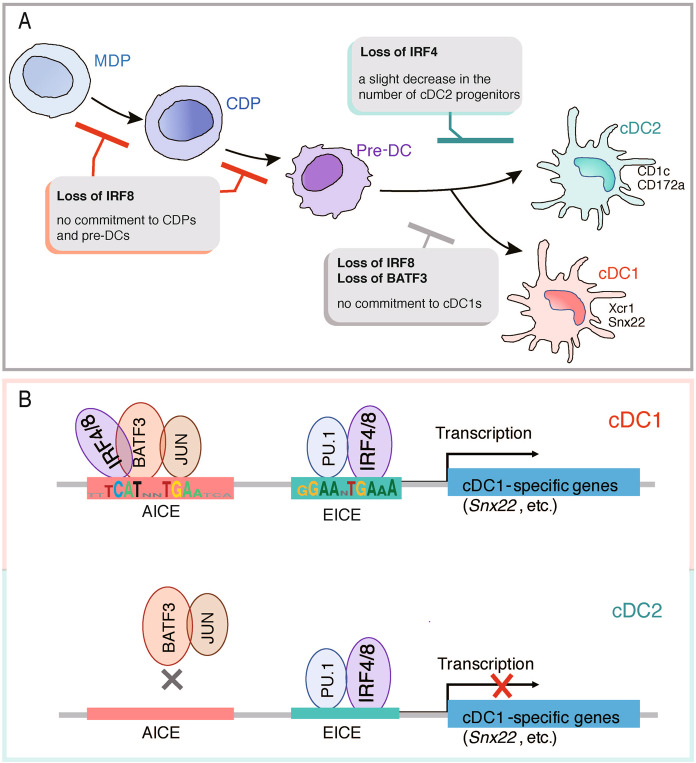
Fig. 1.
Different transcriptional regulation between cDC1s and cDC2s based on the AP1–IRF composite element-dependent program. A In the commitment of bone marrow progenitor cells toward conventional dendritic cells (cDCs), loss of interferon regulatory factor 8 (IRF8) is linked to defects in the generation of common DC progenitors (CDPs) and the specification of CDPs toward the cDC1 lineage; IRF8 and basic leucine zipper transcription factor ATF-like 3 (BATF3) are necessary for the development and function of cDC1s. B The expression of cDC1-specific genes relies on the AP1–IRF composite element (AICE)-dependent program. In cDC1s, BATF3–JUN heterodimers form heterocomplexes with IRF4 or IRF8 on AICEs to initiate transcription, which requires high IRF concentrations. In cDC2s, the contribution to gene transcription from AICEs is minimal, and there is no BATF3 binding at AICEs for cDC1-specific genes. MDP, macrophage DC progenitor; EICE, Ets–IRF composite element
Given the dependency of IRF8 and IRF4 on the development and function of cDC subsets, Kim et al. compared their individual capacities to promote cDC1 development. After retroviral-mediated expression in mouse Irf4–/–Irf8–/– bone marrow progenitors, neither Irf4 nor Irf8 restored the proportion of the cDC1 population to that found in wild-type mice, although Irf8 was slightly more efficient at recovering than Irf4.2 These results provide an energetic rationale for the cDC1-specific requirement for BATF3. Indeed, in the context of BATF3 expression, high amounts of IRF4 and IRF8 can restore the development and cross-presentation function of cDC1s. Next, studies confirmed that IRF4 and IRF8 induced similar transcriptional programs when expressed in similar amounts in cDCs; most of the transcriptional differences observed between cDC1s and cDC2s were explainable by the amount, but not the type, of the IRF protein being expressed. For instance, the expression of cDC1-specific gene Itgae, encoding CD103, was restored in the presence of high IRF4 or IRF8 levels but not low IRF conditions.2 Altogether, these results demonstrate that IRF4 and IRF8 have an equivalent cDC transcriptional impact, showing that high IRF levels are required for the initiation of an AICE-dependent transcriptional program to promote the development and function of cDC1s.
Nevertheless, the transcriptional divergence between IRF4 and IRF8 in DCs was also identified in this work: some genes in the cDCs were preferentially regulated by IRF4 or IRF8. A minority of cDC1-specific genes were activated selectively by IRF8 at a high level but did not respond to high levels of IRF4, such as Xcr1, which encodes a cDC1-specific chemokine receptor. Kim et al.2 generated a series of chimeric IRF8 and IRF4 proteins with different combinations of N-terminal DNA-binding domains, linker regions, and carboxy-terminal IRF-associated domains. XCR1 was expressed only in response to chimeric proteins containing the IRF8 DNA-binding domain but not the IRF4 DNA-binding domain, implying that cis-acting elements controlling the expression of specific genes can differentiate IRF4 from IRF8.
cDCs arise from a cascade of bone marrow DC-committed progenitor cells, including common DC progenitors (CDPs) and pre-DCs; CDPs differentiate into pre-DCs, which give rise mainly to the cDC1 and cDC2 lineages.1 IRF8 deficiency is linked to defects in the generation of CDPs and their specification toward the cDC1 lineage;4,5 however, this deficiency did not impair the development of macrophage DC progenitors,2 which differentiate into CDPs. To determine the developmental stage at which IRF4 and IRF8 exert their influence, Kim et al.2 examined cDC bone marrow progenitors in wild-type, Irf4–/–, Irf8–/–, and Irf4–/–Irf8–/– mice. The combined lack of Irf4 and Irf8 eliminated all cDC progenitors (CDPs and pre-DCs); however, the Irf4–/– mice developed a progenitor population similar to that of the wild type mice, except the former showed a slight decrease in the number of cDC2 progenitors. These findings indicate that IRF8 acts earlier than IRF4 in cDC progenitor development (Fig. 1).
cDC1s play essential roles in activating antitumor immunity, and the abundance of cDC1s within tumors correlates with improved patient outcomes.6 Thus, by revealing the molecular basis underlying the distinct transcriptional signatures of cDC1s and cDC2s, Kim et al. provided new directions for developing immunomodulatory agents and improving DC-based therapeutic strategies. The differentiation and identity of functional DC subsets have constituted a research hotspot in the field of acquired immunity; recently, DCs have also been the subject of transcriptomic, epigenomic, and metabolomic analyses. Further work in this direction should focus on metabolic reprogramming, epigenetic modification, and transcriptional regulation in relation to DC maturation, localization, and functional specialization.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China [grant number 81870152] and the Scientific and Technological Developing Plan of Jilin Province [grant number 20200201588JC].
Competing interests
The authors declare no competing interests.
References
- 1.Schlitzer A, et al. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat. Immunol. 2015;16:718–728. doi: 10.1038/ni.3200. [DOI] [PubMed] [Google Scholar]
- 2.Kim S, et al. High amount of transcription factor IRF8 engages AP1-IRF composite elements in enhancers to direct type 1 conventional dendritic cell identity. Immunity. 2020;53:759–774 e7598. doi: 10.1016/j.immuni.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durai V, et al. Cryptic activation of an Irf8 enhancer governs cDC1 fate specification. Nat. Immunol. 2019;20:1161–1173. doi: 10.1038/s41590-019-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grajales-Reyes GE, et al. Batf3 maintains autoactivation of Irf8 for commitment of a CD8alpha(+) conventional DC clonogenic progenitor. Nat. Immunol. 2015;16:708–717. doi: 10.1038/ni.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sichien D, et al. IRF8 transcription factor controls survival and function of terminally differentiated conventional and plasmacytoid dendritic cells, respectively. Immunity. 2016;45:626–640. doi: 10.1016/j.immuni.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, et al. Vaccine efficacy against primary and metastatic cancer with in vitro-generated CD103(+) conventional dendritic cells. J. Immunother. Cancer. 2020;8:4. doi: 10.1136/jitc-2019-000474. [DOI] [PMC free article] [PubMed] [Google Scholar]