Abstract
The use of natural killer (NK) cells is a promising and safe immunotherapeutic approach in the field of cancer immunotherapy. However, combination treatments are required to enhance the effector functions and therapeutic efficacy of NK cells. In this study, we investigated the potential of daratumumab (Dara), bortezomib, and dexamethasone (Dvd) to augment the antitumor effects of NK cells in a multiple myeloma (MM) xenograft mouse model. NK cells were expanded and activated using the K562-OX40 ligand and membrane-bound IL-18 and IL-21 in the presence of IL-2 and IL-15 from peripheral blood mononuclear cells from MM patients. A human MM xenograft model was established using human RPMI8226-RFP-FLuc cells in NOD/SCID IL-2Rγnull (NSG) mice. Tumor-bearing mice were divided into six treatment groups: no treatment, expanded NK cells (eNKs), Dara, Dara + eNKs, Dvd, and Dvd + eNKs. Dvd treatment strongly enhanced the cytotoxicity of eNKs by upregulating expression of NK cell activation ligands, downregulating expression of NK cell inhibitory ligands, and promoting antibody-dependent cellular cytotoxicity. The combination of eNKs with Dvd significantly prolonged mouse survival and reduced the tumor burden and serum M-protein level. Furthermore, Dvd pretreatment significantly increased eNK persistence and homing to MM sites. Our findings suggest that Dvd treatment potentiates the antimyeloma effects of NK cells expanded and activated ex vivo by modulating immune responses in MM-bearing mice.
Keywords: Multiple myeloma, Natural killer cell, Chemotherapy
Subject terms: Translational immunology, Myeloma
Introduction
Multiple myeloma (MM) is an incurable hematological malignancy in the majority of patients and is characterized by the abnormal proliferation of clonal malignant plasma cells.1,2 Although several novel agents (e.g., proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies) have been developed in recent decades to improve clinical outcomes in MM patients, most MM patients ultimately relapse and require additional therapy. More recently, treatment with chimeric antigen receptor (CAR) T cells has shown remarkable antitumor activity in MM and other hematological malignancies; however, CAR T-cell therapy may cause several undesirable and potentially lethal effects, such as severe cytokine release syndrome and neurotoxicity. In addition, CAR T-cell therapy significantly increases treatment costs while providing a limited progression-free survival (PFS) benefit.3,4
Natural killer (NK) cells are large granular innate immune cells that can eliminate tumor cells without prior sensitization.5 In MM patients, NK cell activity can be suppressed by several immunosuppressive factors in the bone marrow (BM) niche.6–8 Therefore, ex vivo activation and expansion of NK cells from MM patients are challenging.9 We recently established a novel protocol for ex vivo expansion and activation of NK cells from MM patients using K562-OX40 ligand and membrane-bound (mb) IL-18 and IL-21 (K562-OX40L-mbIL-18/-21) cells, which were generated by transducing K562-OX40L cells with a lentiviral vector encoding mbIL-18 and mbIL-21 in the presence of IL-2 and IL-15 (manuscript submitted for publication). MHC-I molecules expressed on MM cells inhibit NK cell activation to avoid NK cell-mediated tumor lysis. Interestingly, bortezomib, a proteasome inhibitor, downregulates the expression of the NK inhibitory ligands HLA-A, HLA-B, HLA-C, and HLA-E on the MM cell surface, augmenting NK cell-mediated killing of MM cells.10,11 Daratumumab (Dara), a CD38-targeting monoclonal antibody, mediates MM cell elimination via antibody-dependent cell-mediated cytotoxicity (ADCC), complement activation, and direct killing of MM cells.12–15 Therefore, a combinatorial approach using NK cells, bortezomib, and Dara may provide superior antitumor activity in MM. We hypothesize that pretreatment with Dara, bortezomib, and dexamethasone (Dvd) regimens renders MM cells more susceptible to NK cell-mediated lysis by suppressing expression of NK cell inhibitory ligands and inducing expression of NK cell-activating ligands on the surface of MM cells.
In this study, we evaluated the ability of the Dvd regimen to improve the therapeutic efficacy of adoptively transferred NK cells in an MM xenograft model. The combination of NK cells and Dvd significantly prolonged disease-free survival and overall survival in MM mice and reduced the serum M-protein level. The ability of Dvd to augment the antimyeloma effects of expanded NK cells (eNKs) is ascribed to the ability of the Dvd regimen to attract tumor-infiltrating NK cells to myeloma sites by upregulating various stress ligands. Thus, treatment with the Dvd regimen can enhance the clinical efficacy of NK cell adoptive transfer in patients with MM.
Materials and methods
Ethics declaration
All protocols for collecting samples from MM patients were approved by the institutional review board of Chonnam National University Hospital; informed consent was obtained before sample collection. All animal experiments were performed in accordance with protocols approved by the Chonnam National University Animal Use and Care Committee.
Cell lines, cytokines, and drugs
K562 (human myelogenous leukemia cell line), U266 and RPMI8226 (human MM cell lines), and Raji (human Burkitt’s lymphoma cell line) cells were purchased from the American Type Culture Center (ATCC, Manassas, VA, USA). Genetically modified K562-OX40L-mbIL-18/-IL-21 cells were used to expand and activate NK cells. All cell lines were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, US) and 1% penicillin/streptomycin at 37 °C in a humidified 5% CO2 incubator. NK cells were cultured in the presence of recombinant human interleukin IL-2 and IL-15 (PeproTech, Rocky Hill, NJ, USA).
Surface and intracellular staining for flow cytometry
The purity of eNKs and the expression of surface receptors were analyzed by flow cytometry after staining with fluorochrome-conjugated monoclonal antibodies (CD3, CD56, CD16, CD69, CD94, NKp30, NKp44, NKp46, NKG2D, NKG2A, CD158a, and CD158b). Briefly, eNKs (2 × 105 cells) were stained with appropriate antibodies for 15–20 min and then analyzed on a BD FACSCalibur. Tumor cells pretreated with Dara (10 μg/mL; Janssen Pharmaceuticals, Johnson & Johnson, New Jersey, USA), bortezomib (10 nM; Janssen Pharmaceuticals), and dexamethasone (50 nM; Daewon Pharmaceuticals, Seoul, Korea) for 24 h were stained with PE-conjugated antibodies specific for HLA-ABC, HLA-E, HLA-DR, MICA, MICB, ULBP1, ULBP2, and Fas. The intracellular levels of IFN-γ, granzyme-B, perforin, TRAIL, and FasL in Dvd-pretreated eNKs were measured using a BD Cytofix/Cytoperm™ kit (BD Biosciences, USA). Flow cytometry data were analyzed using FlowJo.
Ex vivo NK cell activation and expansion
NK cells were expanded using our recently established K562-OX40L-mbIL-18/-21 feeder cells. Briefly, peripheral blood mononuclear cells isolated from MM patients were cocultured with gamma-irradiated (100 Gy) K562-OX40L-mbIL-18/-21 feeder cells in RPMI-1640 medium containing 10% FBS, 1% penicillin/streptomycin, and 4 mM L-glutamine. The cells were exposed to 10 U/mL recombinant IL-2 until day 7. Subsequently, the IL-2 concentration was increased to 100 U/mL, and 5 ng/mL recombinant IL-15 was added to the cell culture medium. The cell culture medium containing cytokines was refreshed every 2–3 days. On day 14, eNKs with a purity of >90% were used for in vitro and in vivo experiments (Supplementary Fig. 1).
ADCC and cytotoxicity assay
Dara-mediated ADCC and cytotoxicity were measured using a flow cytometry-based cytotoxicity assay. Briefly, tumor cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE, Life Technologies, USA) for 10 min. Subsequently, tumor cells were coated with 10 μg/mL Dara for 45 min and cocultured with ex vivo eNKs at various effector-to-target (E:T) ratios. To estimate cytotoxicity, target cells were pretreated with Dara (10 μg/mL), bortezomib (10 nM), and dexamethasone (50 nM) for 24 h and then cocultured with eNKs at various ratios at 37 °C for 4 h in a 5% CO2 incubator. After incubation, 1 μL of propidium iodide (Life Technologies, USA) was added, and the cells were analyzed on a BD FACSCalibur. The percentage of dead cells among the CFSE-positive cells was calculated by deducting the percentage of cells that spontaneously died.
Degranulation assay
To assess the effects of Dvd pretreatment of tumor cells on the ability of NK cells to degranulate, we cocultured Dvd-pretreated tumor cells (K562, U266, RPMI8226, and Raji) with eNKs (E:T of 1:1) in the presence of a PE-conjugated anti-CD107a antibody in 96-well U-bottom plates for 1 h. Brefeldin A and monensin (BD Biosciences) were added, and the cells were incubated for an additional 3 h at 37 °C in a 5% CO2 incubator.16 After incubation, the cells were stained for CD3 and CD56 for 15 min. The stained cells were analyzed on a BD FACSCalibur, and flow cytometry data were analyzed using FlowJo.
MM xenograft model
NOD/SCID IL-2Rγnull (NSG) mice were purchased from the Jackson Laboratory (Bar Harbor, MA, USA). To establish an MM xenograft model, we intravenously injected human RPMI8226-RFP-FLuc cells (5 × 106 per mouse) into 9–12-week-old male and female mice. Tumor growth was monitored by bioluminescence imaging using the Night Owl System (Berthold Technologies, Bad Wildbad, Germany).
Combination treatment of eNKs with Dvd in the MM xenograft model
On day 0, mice were intravenously injected with 5 × 106 RPMI8226-RFP-FLuc cells. Ten days after tumor inoculation, the mice (n = 23 per group) were divided into six treatment groups: no treatment (PBS control), Dara, eNKs, Dara + eNKs, Dvd, and Dvd + eNKs. The mice were treated with Dara (8 mg/kg/day) by intraperitoneal injection, bortezomib (0.5 mg/kg/day) by subcutaneous injection, and dexamethasone (0.6 mg/kg/day) by intravenous injection on days 10, 17, 24, and 31. Freshly harvested eNKs (2 × 107 cells/mouse) were infused on days 11, 18, 25, and 32. Tumor growth was monitored weekly by bioluminescence imaging in both the dorsal and ventral views; 10 min before imaging, the mice were intraperitoneally injected with D-Luciferin (150 mg/kg/mouse, Perkin Elmer, USA). The Night Owl System (Berthold Technologies) was used for imaging.17 Serum M-protein levels were assessed by measuring the level of the human lambda free chain (Bethyl Laboratories, USA).18 In vivo persistence and tumor infiltration of eNKs and myeloma clearance at various MM sites were assessed by flow cytometry.
Quantification of cytokine release in MM-bearing mice
We evaluated the levels of human immune effector and regulatory cytokines in the serum of mice using the BD OptEIA™ enzyme-linked immunosorbent (ELISA) assay (IFN-γ, TNF-α, and IL-10) and human granzyme-B and perforin ELISA development kit (Mabtech AB, Sweden).
Immunohistochemistry
To further evaluate the in vivo homing of eNKs to MM sites, we collected femur and tibia samples from mice. Femur and tibia samples were fixed in 10% formalin and embedded in paraffin blocks for immunohistochemistry. The paraffin sections were stained with hematoxylin and eosin and antibodies specific for NK activation and MM markers (CD56, CD16, NKG2D, CD138 and CD38). The stained sections were evaluated by an experienced pathologist.
Statistical analysis
Data were analyzed using GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA). Statistical significance was determined using Student’s t tests or one-way ANOVA. P values < 0.05 were considered significant. Data are expressed as the mean ± standard deviation or standard error of the mean.
Results
Dvd pretreatment downregulates expression of NK cell inhibitory ligands and upregulates expression of NK cell-activating ligands on tumor cells in vitro
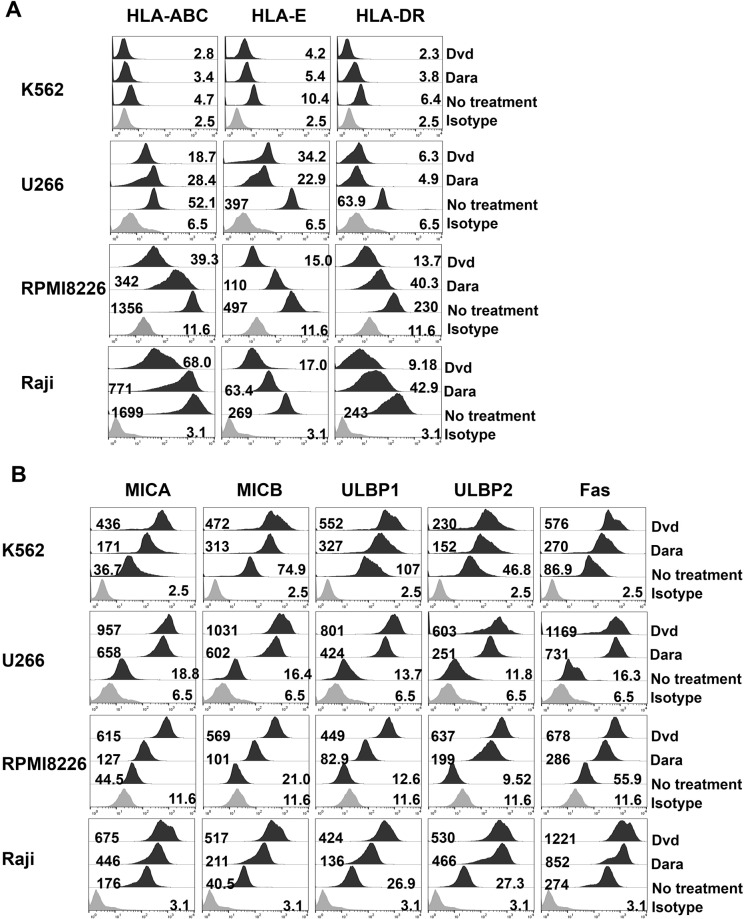
To explore the effects of Dvd on the NK cell-mediated killing of cancer cells, we analyzed the expression of inhibitory and activating ligands on the surface of U266, RPMI8226, and Raji cells treated with Dvd for 24 h. Dvd treatment significantly downregulated the expression of HLA-A, HLA-B, HLA-C, HLA-E, and HLA-DR and upregulated that of NKG2D ligands (MICA, MICB, ULBP1, and ULBP2) and the Fas receptor in tumor cells (all p < 0.0001, Fig. 1 and Supplementary Fig. 2). These data suggest that the Dvd regimen may sensitize tumor cells to NK cell-mediated killing via death receptor signaling.
Fig. 1.
Dvd treatment downregulates the expression of NK inhibitory molecules and upregulates the expression of NK-activating ligands on tumor cells. (A and B) Flow cytometry histograms showing the surface expression of HLA-A, HLA-B, HLA-C, HLA-E, HLA-DR, MICA, MICB, ULBP1, ULBP2, and Fas on K562, U266, RPMI8226, and Raji cells after treatment with Dara or Dvd. Dvd-pretreated myeloma cells exhibited significantly lower levels of NK inhibitory molecules (HLA-A, HLA-B, HLA-C, HLA-E, and HLA-DR) and higher levels of NKG2D ligands (MICA, MICB, ULBP1, and ULBP2) and the Fas receptor than the other groups (see also Supplementary Fig. 2)
eNKs exert potent antitumor effects on Dvd-pretreated tumor cells in vitro
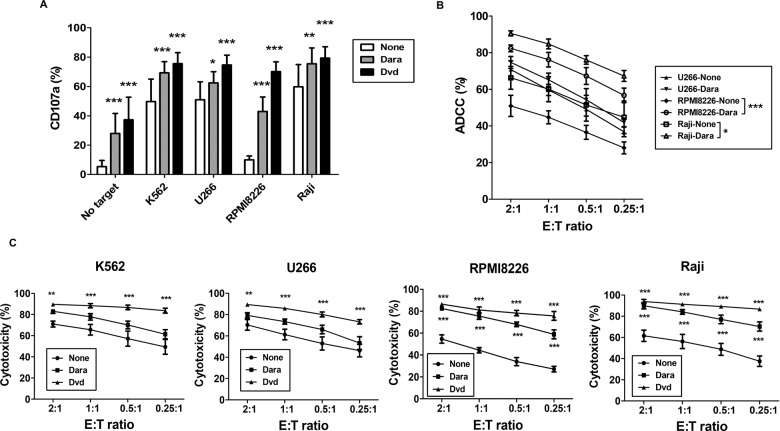
Dvd has various effects on immune cells and tumor cells. Here, we investigated the effect of Dvd on eNK function by assessing the expression of IFN-γ, granzyme-B, perforin, TRAIL, FasL, and NK2GA in eNKs (Fig. 2A). Dvd treatment substantially increased IFN-γ and perforin expression and decreased NKG2A expression in eNKs but had no effect on the expression of granzyme-B, TRAIL, or FasL (Fig. 2B). We also evaluated the effect of Dvd pretreatment of tumor cells on the cytotoxic ability of eNKs. Dvd pretreatment of the tumor cells significantly induced CD107a expression in eNKs (Fig. 3A). In addition, Dara treatment significantly improved the killing of CD38 high-expressing RPMI8226 and Raji cells by eNKs at all E:T ratios assessed (Fig. 3B). In addition, NK-resistant target cells pretreated with Dvd showed superior antitumor effects at all E:T ratios (Fig. 3C). These data suggest that Dvd pretreatment of tumor cells augments eNK-mediated cytotoxicity and is a promising strategy to enhance the antimyeloma activity of eNKs.
Fig. 2.
Effect of Dvd treatment on effector molecules and inhibitory receptors in eNKs. A Representative flow cytometry plots showing the expression of IFN-γ, granzyme-B, perforin, TRAIL, FasL, and NKG2A in eNKs. eNKs were harvested on day 14 and treated with Dvd or Dara for 12 h. B Significantly increased expression of IFN-γ and perforin and decreased expression of NKG2A was observed with eNKs administered in combination with Dvd compared with all other treatments (mean ± standard deviation (SD); n = 5). *p < 0.05, **p < 0.001, and ***p < 0.0001
Fig. 3.
eNKs exert a strong cytotoxic effect on Dvd-pretreated tumor cells. A Percentage of CD107a-expressing eNKs (CD3−CD56+) after coculture with Dvd-pretreated K562, U266, RPMI8226, or Raji cells (E:T ratio of 1:1) for 4 h. Flow cytometry revealed a higher level of CD107a in eNKs cocultured with Dvd-treated tumor cells. B The ADCC effect of Dara on K562, U266, RPMI8226, and Raji cells determined by a standard flow cytometry-based cytotoxicity assay. Dara significantly induced ADCC in RPMI8226 and Raji cells at all E:T ratios. C eNK-mediated cytotoxicity in Dvd-pretreated tumor cells (K562, U266, RPMI8226, and Raji) measured by a standard flow cytometry-based cytotoxicity assay. eNKs showed potent antitumor activity against Dvd-pretreated tumor cells at all E:T ratios. *p < 0.05, **p < 0.001, and ***p < 0.0001
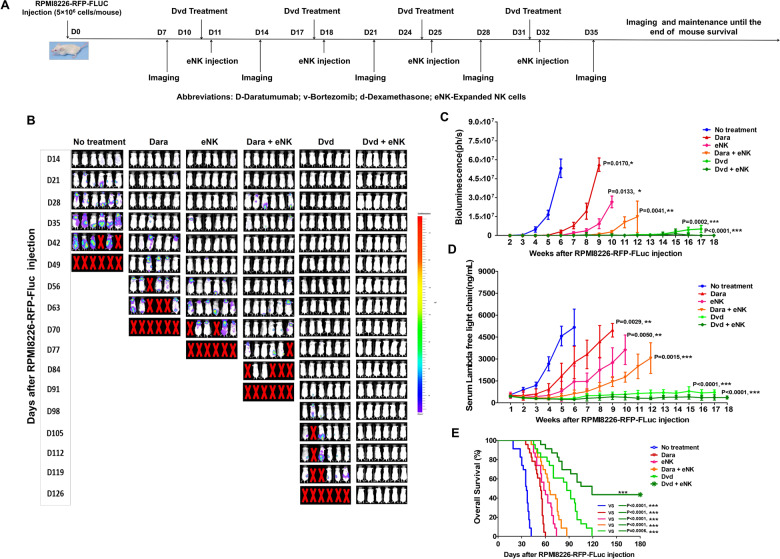
Dvd pretreatment enhances the antimyeloma activity of eNKs and prolongs survival in the RPMI8226-RFP-FLuc xenograft model
The combination of either Dara or bortezomib with adoptive NK cell transfer has been shown to extend mouse survival by enhancing NK cell activation in a human MM xenograft model.11,19,20 Using RPMI8226-RFP-FLuc cells, we established a human MM mouse model that accurately mimics human MM. All untreated tumor-bearing mice showed rapid tumor growth that led to death within 7 weeks. Notably, Dara, eNK, Dara + eNK, and Dvd treatments significantly inhibited tumor growth (Fig. 4A, B; Supplementary Fig. 3A). Among all treatment groups, the combination of Dvd + eNKs provided the strongest inhibition of tumor growth; 9 mice in the Dvd + eNK group exhibited no bioluminescence (Fig. 4B and C), and none of the mice had visible tumors or detectable serum M-protein (Fig. 4C and D, Supplementary Fig. 3B, Table 1). Plasmacytomas could be detected in all treatment groups, except the Dvd + eNK group (Table 1). Furthermore, mice treated with Dvd + eNKs exhibited the longest survival (Fig. 4E; ***, p < 0.001), and no significant body weight loss was observed in mice treated with Dvd or Dvd + eNKs (Supplementary Fig. 3C). These results indicate that Dvd treatment combined with eNKs may induce long-term systemic antimyeloma effects.
Fig. 4.
eNKs combined with Dvd exert potent antimyeloma effects in the RPMI8226-RFP-FLuc xenograft model. A Schematic summarizing the treatment of RPMI8226-RFP-FLuc-bearing mice with eNKs combined with Dvd. NSG mice were intravenously injected with 5 × 106 RPMI8226-RFP-FLuc cells, and tumor growth was monitored weekly by bioluminescence imaging. B Representative bioluminescence imaging of six mice from each group (dorsal view). C Graph showing the bioluminescence intensity in each group. Treatment with Dvd + eNKs provided the strongest antitumor effect. D Serum M-protein levels determined by quantifying the level of the human lambda free light chain in the peripheral blood. Mice treated with Dvd + eNKs had the lowest serum M-protein levels. E Kaplan–Meier survival curves showing mouse survival in each group (n = 23 mice/group). Statistical significance was determined using the log-rank test. Mice treated with Dvd + eNKs exhibited the longest survival. *p < 0.05, **p < 0.01, and ***p < 0.001
Table 1.
The pathophysiological findings in RPMI8226-RFP-FLuc myeloma-bearing mice
| Treatment group | Number of mice with paralysis (Total = 23 mice) |
RPMI8226-RFP-FLuc BLI signal | Serum M-protein level (human λ light chain) |
Number of mice with plasmacytoma (Total = 23 mice) |
|
|---|---|---|---|---|---|
| Skeletal regions | Nonskeletal regions | ||||
| No treatment | 23 | Very high | Low | Very high | 17 |
| eNKs | 17 | High | Low | High | 7 |
| Dara | 18 | Very high | Low | Very high | 14 |
| Dara + eNKs | 16 | Medium | Low | Medium | 7 |
| Dvd | 12 | Low | BDL | Low | 5 |
| Dvd + eNKs | 9 | Very low | BDL | Very low | No |
BLI bioluminescence imaging, eNKs expanded natural killer cells, Dara daratumumab, Dvd daratumumab, bortezomib, and dexamethasone, BDL below the detection level
Dvd pretreatment enhances the function, persistence, and homing of eNKs in vivo
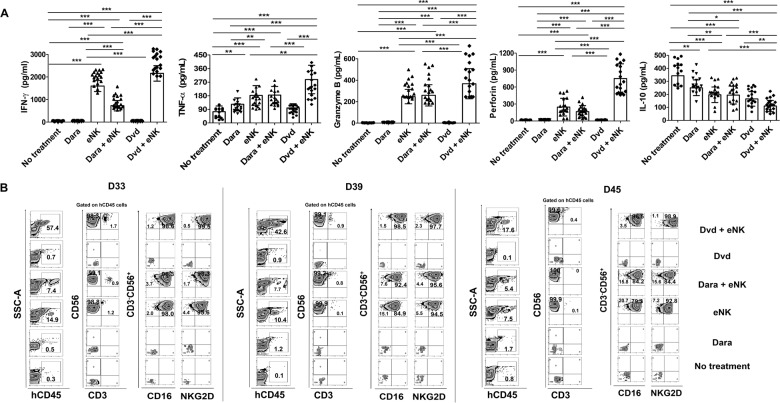
To elucidate the immunological mechanisms underlying the enhanced antimyeloma effects of Dvd pretreatment combined with eNKs, we evaluated the effect of combination therapy on the tumor microenvironment.21 The day after the last eNK infusion, we collected serum from mice and assessed the levels of human IFN-γ, granzyme-B, perforin, TNF-α, and IL-10 (Fig. 5A). The levels of human IFN-γ, granzyme-B, perforin, and TNF-α were highest in the mice treated with Dvd + eNKs. In addition, Dvd + eNK-treated mice had the lowest levels of the immunosuppressive cytokine IL-10. These data suggest that the combination of Dvd + eNKs exerts potent antimyeloma effects by inhibiting the production of immunosuppressive cytokines in tumor cells. Next, we investigated the persistence of eNKs in the circulation. Although circulating eNKs were detected in all mice infused with eNKs, the percentage of circulating eNKs decreased gradually over time. Importantly, Dvd + eNK-treated mice displayed a significantly higher percentage of eNKs in the circulation than mice treated with either eNKs alone or Dara + eNKs (Fig. 5B, Supplementary Fig. 4).
Fig. 5.
Dvd pretreatment improves in vivo effector function and the persistence of eNKs in RPMI8226-RFP-FLuc-bearing mice. A In vivo effector function of circulating eNKs, determined based on the levels of various immune effectors and immunosuppressive cytokines the day after the last eNK infusion. Mice treated with Dvd + eNKs had the highest levels of IFN-γ, granzyme-B, perforin, and TNF-α and the lowest level of IL-10. B Representative flow cytometry plots showing the in vivo persistence of circulating eNKs (CD3−CD56+CD16+ cells and CD3−CD56+NKG2D+ cells) in mouse peripheral blood at various time points (D33, D39, and D45). *p < 0.01, **p < 0.001, ***p < 0.0001
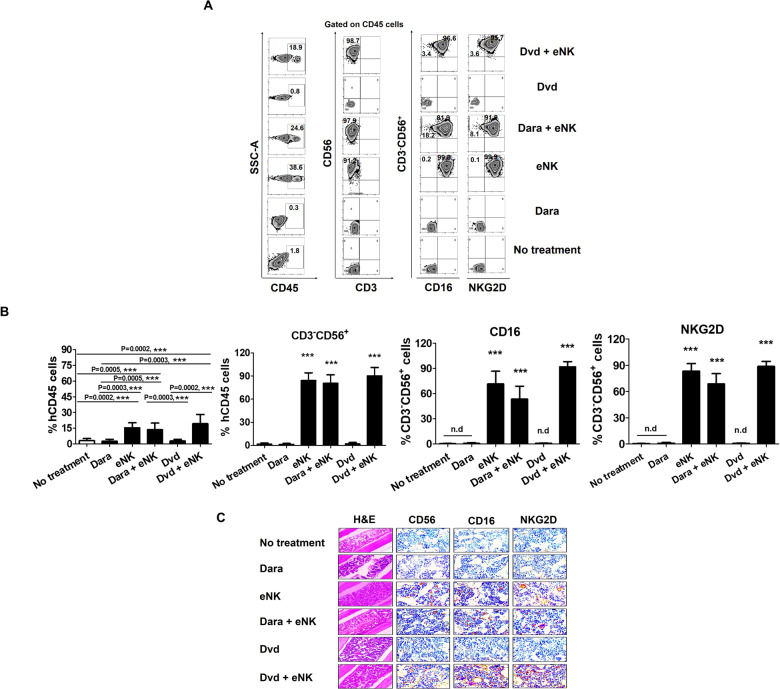
Next, we investigated the homing and tumor-targeting ability of our novel eNKs in vivo in an RPMI8226-RFP-FLuc human MM xenograft model. Tissue samples (BM, brain, heart, kidneys, liver, lungs, and spleen) were collected to evaluate the distribution of eNKs. Mice treated with Dvd + eNKs showed the highest levels of eNKs in the BM (Fig. 6A) and other tissues (kidneys, liver, lungs, and spleen; Supplementary Fig. 5) among all treatment groups. We also evaluated the functional stability of eNKs by assessing NK cell purity and the expression of activation markers (CD16 and NKG2D) in the BM (Fig. 6B) and other tissues (Supplementary Fig. 5). eNKs were highly stable even under in vivo conditions, remaining in a state unaffected by factors in the tumor microenvironment for a long time.
Fig. 6.
Dvd treatment enhances eNK homing in vivo in RPMI8226-RFP-FLuc-bearing mice. A In vivo homing of eNKs in RPMI8226-RFP-FLuc-bearing mice (n = 9 mice per group) evaluated by flow cytometry. Mice were sacrificed at the experimental endpoint, and BM samples were analyzed by flow cytometry for human NK cell (CD3−CD56+) and activation receptor (CD16 and NKG2D) levels. B Quantification (mean ± SD) of in vivo eNK homing in the BM based on flow cytometry data. eNKs from mice treated with Dvd + eNKs showed the strongest in vivo homing ability. C Immunohistochemical staining for human CD56, CD16, and NKG2D in femur and tibia sections (no treatment, day 36; Dara, day 60; eNKs, day 63; Dara + eNKs, day 75; Dvd and Dvd + eNKs, day 91). The BM of mice treated with Dvd + eNKs had the highest staining intensity for NK cell markers (magnification: ×10 for hematoxylin and eosin; ×600 for CD56, CD16, and NKG2D immunostaining. *p < 0.01, **p < 0.001, ***p < 0.0001
We also examined the tumor-targeting ability of eNKs and the expression of NK cell activation markers in the RPMI8226-RFP-FLuc MM mouse model. Formalin-fixed paraffin-embedded femur and tibia sections were immunohistochemically stained for the NK cell markers CD56, CD16, and NKG2D. Interestingly, we detected eNKs in the BM of all mice infused with eNKs, and Dvd + eNK-treated mice showed the highest expression of NK cell markers among the treatment groups (Fig. 6C). The in vivo persistence and biodistribution of eNKs were strongly correlated with their antimyeloma effect. eNKs alone or in combination with Dara suppressed tumor growth until the last eNK infusion. Dara + eNK-treated mice exhibited a slight reduction in the percentage of eNKs, possibly due to the cytotoxic effect of Dara on eNKs. Although Dara effectively induces NK cell function, Dara could not protect NK cells with high CD38 expression from daratumumab-mediated NK fratricide, resulting in ADCC, which affects the persistence of circulating eNKs. Interestingly, all of these obstacles were overcome by Dvd, and the combination of Dvd + eNKs prolonged the PFS of tumor-bearing mice. These findings suggest that the combination of Dara, bortezomib, and dexamethasone increases the infiltration of eNKs into MM sites, and Dvd pretreatment enhances the ability of eNKs to eliminate tumor cells by suppressing the production of immunosuppressive factors in the MM microenvironment.
eNKs combined with Dvd enhance MM clearance in the RPMI8226-RFP-FLuc xenograft model
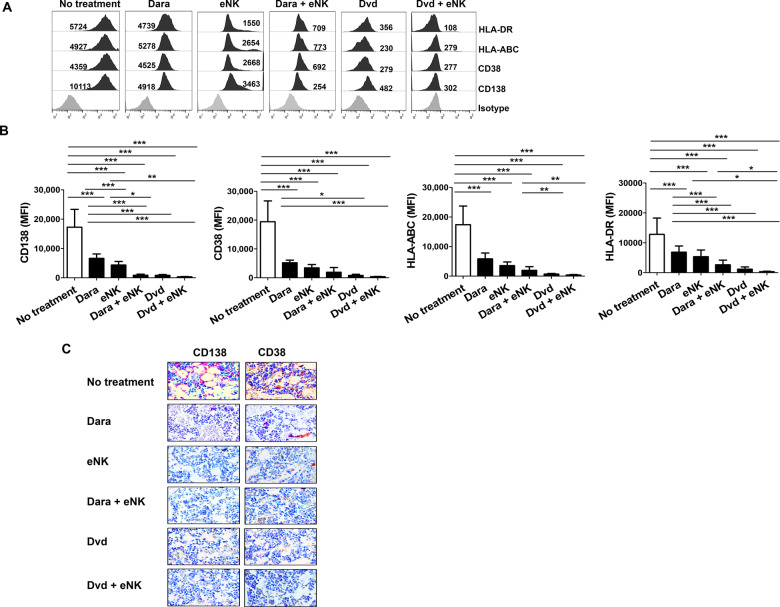
The combination of Dvd + eNKs showed a remarkable antimyeloma effect in the MM mouse model, as assessed by bioluminescence imaging and serum M-protein measurement.22 We next evaluated the presence of residual RPMI8226-RFP-FLuc cells in different mouse organs. Mice were sacrificed at the experimental endpoint, and single-cell suspensions were generated from the BM, brain, heart, kidneys, liver, lungs, and spleen. The expression levels of MM markers (CD138 and CD38) and NK inhibitory molecules (HLA-A, HLA-B, HLA-C, and HLA-DR) were evaluated by flow cytometry. Dvd-treated mice with or without eNKs showed the lowest levels of CD138, CD38, HLA-ABC, and HLA-DR in all tissues (BM: Fig. 7A and B; other tissues: Supplementary Fig. 6). To further confirm MM clearance in vivo, we stained femur and tibia tissues for the MM markers CD138 and CD38. Interestingly, Dvd + eNK-treated mice had the lowest levels of MM markers in the BM (Fig. 7C), suggesting that the combination of eNKs with Dvd showed a superior antimyeloma effect to other treatments in this model.
Fig. 7.
Dvd combined with eNKs enhances MM clearance in the RPMI8226-RFP-FLuc xenograft model. A Residual RPMI8226-RFP-FLuc cells in the BM evaluated by flow cytometry. Representative histograms showing the expression (MFI value) of CD138, CD38, HLA-A, HLA-B, HLA-C, and HLA-DR. B Quantification (mean ± SD) of residual myeloma cells in the BM. Mice treated with Dvd alone or with eNKs exhibited lower levels of CD138, CD38, HLA-A, HLA-B, HLA-C, and HLA-DR in the BM than the other groups. C Immunohistochemical staining of human CD138 and CD38 in femur and tibia sections (no treatment, day 36; Dara, day 60; eNKs, day 63; Dara + eNKs, day 75; Dvd and Dvd + eNKs, day 91) (magnification, ×600). Mice treated with Dvd alone or with eNKs showed a drastic reduction in CD138 and CD38 expression in the BM compared with that of the other groups. *p < 0.01, **p < 0.001, ***p < 0.0001
Discussion
In contrast to cytotoxic T cells, NK cells can kill cancer cells without prior sensitization and do not cause graft-versus-host disease or cytokine release syndrome; hence, eNKs have many advantages over CAR T cells in cancer treatment.23,24 However, the in vivo function of NK cells can be attenuated by immunosuppressive factors in the tumor microenvironment, calling for the development of combination treatments to combat immunosuppression.6,25 In this study, we developed a combination approach to enhance the effector function of adoptively transferred eNKs. The combination of Dvd + eNKs showed superior antimyeloma effects, prolonging disease-free survival and overall survival in the RPMI8226-RFP-FLuc MM xenograft model. Moreover, Dvd treatment augmented the antimyeloma effects of eNKs in vivo by enhancing eNK persistence, homing, and infiltration to myeloma sites.
Bortezomib downregulates the expression of HLA-A, HLA-B, and HLA-C in MM cell lines.19,26 Most cancer cells express high levels of inhibitory molecules, including HLA-ABC and HLA-E, which enable them to escape NK-mediated lysis. In this case, although eNKs are highly cytotoxic in nature, their function is inhibited by these inhibitory molecules.10,11,27 Moreover, several studies have reported that bortezomib sensitizes tumor cells to NK cell-mediated lysis by inducing the expression of stress ligands. Recently, HLA-E and HLA-DR molecules have been shown to strongly inhibit the function of NK cells by interacting with NKG2A28 and FCRL-6.29–31 Recent studies have shown that Dara exerts potent antitumor effects.32 However, Dara negatively affects NK cells expressing high levels of CD38, causing NK cell death in vitro.14,20,33,34 In addition, Dara enhances NK cell degranulation, ADCC, and tumor cell killing. In this study, we showed that Dvd pretreatment upregulated the expression of NKG2D ligands and downregulated the expression of NK inhibitory ligands, particularly MHC class I molecules. We also found that Dvd treatment suppressed the expression of HLA-DR in vitro, overcoming NK cell inhibition. This is the first study to report the effects of Dvd on the expression of MHC class II molecules. However, this study assessed the tumor sensitization effect of the Dvd combination in only human MM cell lines but not primary MM cells. Therefore, further investigations are needed to explore the effect of Dvd in primary MM cells.
The wide clinical implementation of NK cells is limited by their short lifespan, poor expansion, and short in vivo persistence without the administration of cytokines.35–37 We and others have shown that K562 cells expressing mb IL-21 and IL-15 enhanced NK cell expansion via their ability to increase telomere length.38–41 In addition, IL-18 was reported to augment NK cell expansion and activation.42 We recently developed a cost-effective NK expansion method using genetically engineered K562-OX40L-mbIL-18/-21 feeder cells; this method allows the production of large amounts of highly cytotoxic NK cells, overcoming the need for exogenous cytokines (manuscript submitted for publication).
Administration of high cytokine doses is required to maintain human NK cell persistence in mouse models.7,16,43,44 Imamura et al. reported that the expression of mb IL-15 promoted NK cell survival and expansion in vitro and in vivo in the absence of exogenous cytokines.45 In addition, a recent study showed that the expression of IL-15 and a CD19-targeting CAR in eNKs enhanced their long-term antitumor activity and persistence in a mouse model of lymphoma.46 In this study, although we did not administer exogenous cytokines or CAR NK cells producing IL-15, eNKs were maintained in the circulation for extended periods. Interestingly, Dvd + eNK-treated mice showed a significantly higher percentage of circulating eNKs than mice treated with eNKs alone or in combination with Dara. Furthermore, Dvd + eNK-treated mice had the longest PFS. Importantly, infusion of eNKs alone or in combination with Dvd pretreatment did not cause graft-versus-host disease or cytokine release syndrome in our in vivo model. Clinical trials using NK cells expanded by coculture with genetically modified K562 feeder cells in patients with various cancers have been performed by several groups.47,48 The limitation of this study is that this approach was not tested for clinical application using large-scale expansion of clinical-grade NK cells under GMP conditions with xeno- and serum component-free medium.
In conclusion, we investigated the ability of Dvd pretreatment to enhance the therapeutic efficacy of eNKs in vivo using a novel MM mouse model. Our findings indicate that the Dvd regimen profoundly enhances the antitumor effects of eNKs, suggesting that the combination of Dvd + eNKs is a promising immunotherapeutic approach for the treatment of MM.
Supplementary information
Acknowledgements
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2018R1A2B6006200, 2018R1A5A2024181, and 2020R1A2C2010098).
Author contributions
J.L.T., S.H.J., D.C., J.J.L. designed the study. J.L.T., M.C.V., T.H.C., M.T.T., and K.H.L. performed the experiments and interpreted the data. S.Y.A., M.K., G.Y.S., D.H.Y., J.S.A., and H.J.K. contributed intellectually to the study. J.L.T., S.Y.A., M.C.V., and T.H.C. wrote the paper. D.C. and J.J.L. supervised the study and wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Jaya Lakshmi Thangaraj, Seo-Yeon Ahn
Contributor Information
Duck Cho, Email: duck.cho@skku.edu.
Je-Jung Lee, Email: drjejung@chonnam.ac.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-021-00686-9.
References
- 1.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin. Proc. 2016;91:101–119. doi: 10.1016/j.mayocp.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Hujaily E. M., Oldham R. A., Hari P., Medin J. A. Development of Novel Immunotherapies for Multiple Myeloma. Int. J. Mol. Sci.17, 1506 10.3390/ijms17091506 (2016). [DOI] [PMC free article] [PubMed]
- 4.Berahovich R., et al. CAR-T Cells Based on Novel BCMA Monoclonal Antibody Block Multiple Myeloma Cell Growth. Cancers (Basel).10, 323 10.3390/cancers10090323 (2018). [DOI] [PMC free article] [PubMed]
- 5.Leung W. Infusions of allogeneic natural killer cells as cancer therapy. Clin. Cancer Res. 2014;20:3390–3400. doi: 10.1158/1078-0432.CCR-13-1766. [DOI] [PubMed] [Google Scholar]
- 6.Garg TK, et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica. 2012;97:1348–1356. doi: 10.3324/haematol.2011.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung IH, et al. In Vivo Study of Natural Killer (NK) Cell Cytotoxicity Against Cholangiocarcinoma in a Nude Mouse Model. Vivo. 2018;32:771–781.. doi: 10.21873/invivo.112307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittari G, et al. Restoring Natural Killer Cell Immunity against Multiple Myeloma in the Era of New Drugs. Front. Immunol. 2017;8:1444. doi: 10.3389/fimmu.2017.01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahaweni NM, Ehlers FAI, Bos GMJ, Wieten L. Tuning Natural Killer Cell Anti-multiple Myeloma Reactivity by Targeting Inhibitory Signaling via KIR and NKG2A. Front. Immunol. 2018;9:2848. doi: 10.3389/fimmu.2018.02848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–1317. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luna JI, et al. Bortezomib Augments Natural Killer Cell Targeting of Stem-Like Tumor Cells. Cancers (Basel) 2019;11:85. doi: 10.3390/cancers11010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Weers M, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. (Baltim., Md: 1950) 2011;186:1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 13.Plesner T, Krejcik J. Daratumumab for the Treatment of Multiple Myeloma. Front. Immunol. 2018;9:1228. doi: 10.3389/fimmu.2018.01228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nijhof IS, et al. Daratumumab-mediated lysis of primary multiple myeloma cells is enhanced in combination with the human anti-KIR antibody IPH2102 and lenalidomide. Haematologica. 2015;100:263–268. doi: 10.3324/haematol.2014.117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmeister CC, Lonial S. How to Integrate Elotuzumab and Daratumumab Into Therapy for Multiple Myeloma. J. Clin. Oncol. 2016;34:4421–4430.. doi: 10.1200/JCO.2016.69.5908. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell. Stem Cell. 2018;23:181–192. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KW, et al. Combined NK Cell Therapy and Radiation Therapy Exhibit Long-Term Therapeutic and Antimetastatic Effects in a Human Triple Negative Breast Cancer Model. Int J. Radiat. Oncol. Biol. Phys. 2020;108:115–125.. doi: 10.1016/j.ijrobp.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki O, et al. Antimyeloma activity of NK012, a micelle-forming macromolecular prodrug of SN-38, in an orthotopic model. Int J. Cancer. 2014;134:218–223. doi: 10.1002/ijc.28333. [DOI] [PubMed] [Google Scholar]
- 19.Gras Navarro A., et al. Pretreatment of Glioblastoma with Bortezomib Potentiates Natural Killer Cell Cytotoxicity through TRAIL/DR5 Mediated Apoptosis and Prolongs Animal Survival. Cancers.11, 996 10.3390/cancers11070996 (2019). [DOI] [PMC free article] [PubMed]
- 20.Wang Y, et al. Fratricide of NK Cells in Daratumumab Therapy for Multiple Myeloma Overcome by Ex Vivo-Expanded Autologous NK Cells. Clin. Cancer Res. 2018;24:4006–4017.. doi: 10.1158/1078-0432.CCR-17-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang QM, et al. Enhanced Cancer Immunotherapy with Smad3-Silenced NK-92 Cells. Cancer Immunol. Res. 2018;6:965–977.. doi: 10.1158/2326-6066.CIR-17-0491. [DOI] [PubMed] [Google Scholar]
- 22.Chesi M, et al. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood. 2012;120:376–385. doi: 10.1182/blood-2012-02-412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezvani K, Rouce RH. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front. Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu E, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020;382:545–553.. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leivas A, et al. Novel treatment strategy with autologous activated and expanded natural killer cells plus anti-myeloma drugs for multiple myeloma. Oncoimmunology. 2016;5:e1250051. doi: 10.1080/2162402X.2016.1250051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanker A, et al. Bortezomib Improves Adoptive T-cell Therapy by Sensitizing Cancer Cells to FasL Cytotoxicity. Cancer Res. 2015;75:5260–5272. doi: 10.1158/0008-5472.CAN-15-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlsten M, et al. Bortezomib sensitizes multiple myeloma to NK cells via ER-stress-induced suppression of HLA-E and upregulation of DR5. Oncoimmunology. 2019;8:e1534664. doi: 10.1080/2162402X.2018.1534664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamiya T, Seow SV, Wong D, Robinson M, Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Investig. 2019;129:2094–2106.. doi: 10.1172/JCI123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niehrs A, Altfeld M. Regulation of NK-Cell Function by HLA Class II. Front. Cell Infect. Microbiol. 2020;10:55. doi: 10.3389/fcimb.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schreeder DM, et al. Cutting edge: FcR-like 6 is an MHC class II receptor. J. Immunol. (Baltim., Md: 1950) 2010;185:23–27. doi: 10.4049/jimmunol.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson DB, et al. Tumor-specific MHC-II expression drives a unique pattern of resistance to immunotherapy via LAG-3/FCRL6 engagement. JCI Insight. 2018;3:e120360. doi: 10.1172/jci.insight.120360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verkleij C. P. M., et al. Preclinical Rationale for Targeting the PD-1/PD-L1 Axis in Combination with a CD38 Antibody in Multiple Myeloma and Other CD38-Positive Malignancies. Cancers. 12, 3713 10.3390/cancers12123713 2020. [DOI] [PMC free article] [PubMed]
- 33.Ochoa MC, et al. Daratumumab in combination with urelumab to potentiate anti-myeloma activity in lymphocyte-deficient mice reconstituted with human NK cells. Oncoimmunology. 2019;8:1599636. doi: 10.1080/2162402X.2019.1599636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reina-Ortiz C, et al. Expanded NK cells from umbilical cord blood and adult peripheral blood combined with daratumumab are effective against tumor cells from multiple myeloma patients. Oncoimmunology. 2020;10:1853314. doi: 10.1080/2162402X.2020.1853314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granzin M, et al. Shaping of Natural Killer Cell Antitumor Activity by Ex Vivo Cultivation. Front. Immunol. 2017;8:458. doi: 10.3389/fimmu.2017.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121:258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujisaki H, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denman CJ, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seo H, et al. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat. Commun. 2017;8:15776. doi: 10.1038/ncomms15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim DP, et al. Effect of exposure to interleukin-21 at various time points on human natural killer cell culture. Cytotherapy. 2014;16:1419–1430. doi: 10.1016/j.jcyt.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senju H, et al. Effect of IL-18 on the Expansion and Phenotype of Human Natural Killer Cells: application to Cancer Immunotherapy. Int J. Biol. Sci. 2018;14:331–340.. doi: 10.7150/ijbs.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geller MA, et al. Intraperitoneal delivery of human natural killer cells for treatment of ovarian cancer in a mouse xenograft model. Cytotherapy. 2013;15:1297–1306. doi: 10.1016/j.jcyt.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oyer JL, et al. Natural killer cells stimulated with PM21 particles expand and biodistribute in vivo: clinical implications for cancer treatment. Cytotherapy. 2016;18:653–663. doi: 10.1016/j.jcyt.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Imamura M, et al. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood. 2014;124:1081–1088. doi: 10.1182/blood-2014-02-556837. [DOI] [PubMed] [Google Scholar]
- 46.Liu E, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32:520–531.. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciurea SO, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130:1857–1868.. doi: 10.1182/blood-2017-05-785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SC, et al. Phase I Trial of Expanded, Activated Autologous NK-cell Infusions with Trastuzumab in Patients with HER2-positive Cancers. Clin. Cancer Res. 2020;26:4494–4502.. doi: 10.1158/1078-0432.CCR-20-0768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.