Abstract
Nature has endowed gaseous molecules such as O2, CO2, CO, NO, H2S, and N2 with critical and diverse roles in sustaining life, from supplying energy needed to power life and building blocks for life's physical structure to mediating and coordinating cellular functions. In this article, we give a brief introduction of the complex functions of the various gaseous molecules in life and then focus on carbon monoxide as a specific example of an endogenously produced signaling molecule to highlight the importance of this class of molecules. The past twenty years have seen much progress in understanding CO's mechanism(s) of action and pharmacological effects as well as in developing delivery methods for easy administration. One remarkable trait of CO is its pleiotropic effects that have few parallels, except perhaps its sister gaseous signaling molecules such as nitric oxide and hydrogen sulfide. This review will delve into the sophistication of CO-mediated signaling as well as its validated pharmacological functions and possible therapeutic applications.
KEY WORDS: Carbon monoxide, Gasotransmitter, Gaseous signaling molecule, CO releasing molecule, Organic CO prodrug, Homeostasis, Pleiotropic effect, Yin and Yang
Graphical abstract
The pleiotropic effect of carbon monoxide is reviewed and discussed in the context of maintaining the homeostasis of life's processes and its therapeutic potential.
1. Introduction
There are many ways of looking at life on earth, depending on the specific context. However, at a fifty-thousand-foot view, life needs three key components to sustain: an appropriately organized physical structure, well-coordinated signaling mechanisms to mediate the functions of the physical structure, and energy to power life. Missing any of the three components, life would cease to exist. In modern biology, the discussions of physiological and pathological processes of life commonly (and rightfully) involve the examination of DNA, RNA, proteins, peptides, saccharides, lipids, metal ions, essential nutrients such as amino acids and carbohydrates, and signaling molecules including hormones, neurotransmitters, cytokines, chemokines, metabolites, and other small molecules. Often overlooked is a group of gaseous molecules that play critical roles in sustaining life. With this article, we hope to look at some of the life's processes from a “gas”-centric perspective. As such, we hope to highlight the diverse roles that nature has endowed upon a relatively small number of gaseous molecules by starting with a brief description of how they help to sustain life on a global level, then transitioning to their endogenous signaling roles in mammals, and finally zeroing in on one example, carbon monoxide, to unveil the amazing roles that this small molecule plays in health and disease and its promise as a potential therapeutic agent.
If one looks at life on earth, a central issue to consider is how to capture and use energy to power life. Long before atmospheric oxygen was available for aerobic respiration, Earth's atmosphere was abundant with gases such as methane, nitric oxide, hydrogen sulfide, and carbon monoxide, which early life probably harnessed for energy in redox mechanisms1. In Earth's modern ecosystem, solar energy is the dominant form of energy sustaining life through photosynthesis. In this context, it is amazing to see how two gaseous molecules serve as the key conduits of energy flow among species that rely on solar energy: oxygen and carbon dioxide. While phototrophs capture and store solar energy by transforming carbon dioxide into organic compounds and splitting water to emit oxygen, heterotrophs, such as human, consume such organic compounds as an energy source through “burning” or oxidation by oxygen with carbon dioxide and water as the ultimate end products of the organic component. Consequently, the reciprocal redox cycling of these two small gaseous molecules forms the chemical basis for powering life on earth that relies on solar energy. The story of nitrogen is just as intriguing; microorganism's ability to fix N2 in organic molecules is another pillar that supports life on earth. Further, the products as a result of photosynthesis are also essential building blocks for the physical structure of life both in plants and in animals.
At an individual organism level, the ability for humans to use oxygen for oxidation of organic molecules in a controlled fashion through an intricate web of enzymes is what allows us to capture the energy stored in these organic compounds and to survive on this earth. Even the byproduct, carbon dioxide, is not simply a waste or a bystander2; it helps to regulate pH, stimulate breathing, and influence the hemoglobin's affinity for oxygen (the Bohr effect). While a small portion of carbon dioxide is transported as carbaminohemoglobin, carbonic anhydrase is the enzyme responsible for converting carbon dioxide into (bi)carbonate reversibly and is an important target for drug design for a variety of pathological conditions including cancer, neuropathic pain, fluid retention, epilepsy, and glaucoma, among others3.
Beyond carbon dioxide and oxygen, three other gaseous molecules are recognized as endogenous signaling molecules in human: nitric oxide (NO)4, hydrogen sulfide (H2S)5,6, and carbon monoxide (CO)7,8. These three small signaling molecules are often referred to as “gasotransmitters”9,10, with some dissention11. They have similar and overlapping roles at times. Research on NO led to the 1998 Nobel prize in Physiology and Medicine being awarded to Robert F. Furchgott, Louis J. Ignarro and Ferid Murad for their seminal contributions. It is also important to note one thing. Though these three molecules are gaseous in the pure form under normal conditions, they largely exist in the body in the dissolved form, allowing for the possible interconversion of these two forms depending on location and conditions. These gaseous molecules also share three important traits: rapid diffusion, easy permeation through various barriers, and rapid excretion without the need for metabolism (especially CO) through exhalation. Among these three gaseous signaling molecules, NO has the shortest half-life, and thus is most suited for local signaling unless meta-stable precursors such as S-nitrosothiols are involved12,13. HNO, the one-electron reduced and protonated congener of NO, exhibits some biological activities similar to that of NO14. Hydrogen sulfide is much more stable, but still undergoes rapid conversions to a variety of species through ionization, redox reactions, sulfur exchange, or chelation to metal ions15,16. CO is relatively metabolic inert except for its high affinity for certain transition metals such as nickel17 and iron18 and undergoes minimal chemical transformation in mammalian cells under physiological conditions19, and thus is suitable for both local and global signaling over time as the biological half-life in humans is in the range of hours20, 21, 22, 23. Recent years have seen sulfur dioxide (SO2) emerging as a possible signaling molecule24,25. It is endogenously produced and has well-recognized biological functions. Another interesting gaseous molecule is molecular hydrogen (H2)26, which has been extensively studied for its possible pharmacological effects27. To the best of our knowledge, there is no human enzyme that produces H2. Therefore, it would not be considered as an endogenous signaling molecule in the traditional sense. However, bacteria in the gut is known to produce H228. Then, could H2-mediated signaling be part of the bacteria-host symbiotic relationship? Furthermore, some pathogens also use H2 as fuel, such as Helicobacter pylori. In terms of therapeutic application, it is very interesting to note that hydrogen-rich water is already commercially available in some parts of the world including China and Japan26,29. We defer to several excellent recent reviews for in-depth discussions of this subject26,27,29. There are also other gaseous molecules in the body including CH4 (gut bacteria)28,30, NH331,32, and N233 that play various roles in normal human physiology.
Available data on these gaseous species offers a complex and sophisticated picture of how a human body uses gaseous molecules for sustaining and controlling normal physiology. They also offer opportunities for developing therapeutic agents. Covering all these gaseous molecules would require more than a book. In this article, we start with one endogenous gaseous signaling molecules: carbon monoxide (CO)! Other stories will follow at a later time.
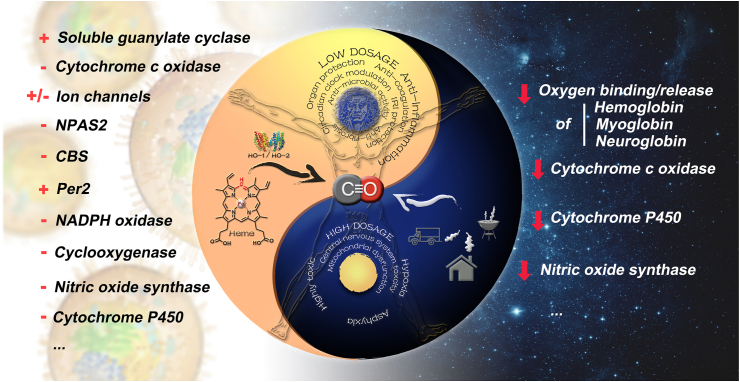
Below we discuss the molecular, physiological, and pharmacological traits of CO. We will also briefly discuss some of CO's known molecular mechanisms of actions. As is true with everything is life, CO has two sides: it possesses very promising therapeutic utilities at low doses and is poisonous at high doses. Further, it is very important to point out that CO has a high enough safety margin for such applications. Fig. 1 summarizes the Yin and Yang sides of CO, the details of which are described in subsequent sections.
Figure 1.
The Yin and Yang of CO. The left side (Yang) showcases the beneficial effects of CO and the top circle shows some of CO's therapeutic indications, either therapeutically delivered or endogenously produced through heme degradation by heme oxygenase (HO). The left side lists some of known CO's pharmacological targets (+positive modulation; –negative modulation). The right side (Yin) highlights the toxic consequences of high-level exposure to CO-containing products from various sources. The higher binding affinity of CO over O2 to respiratory hemeproteins such as hemoglobin, myoglobin, and neuroglobin compromises their ability to transport and store oxygen, leading to hypoxia and asphyxia. Persistent mitochondria dysfunction caused by cytochrome inhibition and disruption of NO signaling pathway by nitric oxide synthase inhibition also contributes to CO's toxicity in the central nervous system and cardiac system ( persistent inhibition).
persistent inhibition).
2. CO is endogenously produced, can diffuse throughout the body, and is essential for life
In mammals, CO is produced mostly by heme degradation by an enzyme called heme oxygenase (HO)34, which has two enzymatically active isoforms (HO-1 and HO-2) and is present in all cells. Daily production of CO is on the scale of about 400 μmol per day35, largely as part of red blood cell turnover35,36. However, there are other secondary sources including cytochrome P450 reductase37, human acireductone dioxygenase38, tyrosinase39, lipid peroxidation40, and numerous minor sources36,41. Further, microbiome sources of CO have not been extensively studied and could turn out to be very important in health and disease42,43. Regardless of the source, CO mostly exists in the form of carboxyhemoglobin (COHb) because of its 240-fold higher affinity to hemoglobin than oxygen and essentially reaches to all parts of the body through blood circulation. CO is known to be highly permeable and diffusible through both endothelium and skin epithelium44,45. There is strong evidence that orally administered CO donors allow delivery of CO into the systemic circulation46,47. Therefore, some common barriers for drug delivery, such as the intestinal barrier and blood–brain barrier are not considered a major issue for CO, which is capable of diffusing in and out of the blood circulation. However, because of CO's affinity for hemoglobin, at any given time the proportion that diffuses into tissues is thought to be less than 20%48.
CO is considered essential for life with multiple targets49, 50, 51, 52; and the physiological roles of the CO–HO axis have been extensively studied over the past twenty years53, 54, 55. HO-1 is inducible in response to various stress signals and is cytoprotective and anti-inflammatory. During stress, increased production of CO is on the order of 4000 μmol per day56, which can be measured in exhaled breath57, 58, 59. Lack of HO-1 results in a pro-inflammatory phenotype. Though inflammation promotes malignant growth and contributes to cancer progression60, 61, 62, 63, 64, 65, the effect of HO-1 on cancer is more complex than inflammation alone66. Further, HO-1 knockout animals were reported to die within a year with indications of inflammation in multiple organs, which is consistent with the known anti-inflammatory role of CO67.
It is clear that CO is essential in sustaining life, has multiple targets and thus pleiotropic effects (see below), has high diffusivity, and is very stable. Therefore, CO's effect can be both local and global as well as long-lasting. Further, CO has overlapping targets and functions with NO and H2S. As a result, the study of CO's physiological and pharmacological functions needs to take a holistic approach by combining molecular-level details with global-level network interactions and feedback controls.
3. CO is known to be important in balancing human immunity and has anti-inflammatory, cytoprotective, and organ-protective effects
One of the most widely studied properties of CO is its anti-inflammatory, cytoprotective and organ-protective effects, with a large number of publications supporting this view7,8. CO has been shown to be protective of organs such as kidney68, 69, 70, 71, heart72,73, liver74,75, brain76,77, and gastrointestinal (GI) system78, 79, 80, 81, 82, 83, 84, among others7,8 after various injurious events such as ischemia–reperfusion injury, trauma, and chemically induced injury. Along a similar line, CO seems to show beneficial effects in treating autoimmune-related conditions85, 86, 87. In addition, CO has been examined in animal models of sickle cell disease46, preeclampsia88,89, and gastroparesis90 and shown to have beneficial effects. The pleiotropic health effects of CO suggest its network-like pharmacological functions and speak to its unique traits and importance in maintaining overall human health. All these suggest the need to take a holistic approach to understanding CO's true physiological and pharmacological functions.
4. CO has been reported to help the body in fending off bacterial infection and in sensitizing cancer toward chemotherapy
One of the remarkable traits of CO is its ability to suppress pathologically relevant inflammation while still strengthening the body's ability to fend off infection and other pathological changes. This is very different from other anti-inflammation therapies such as corticosteroids and anti-tumor necrosis factor (TNF)-α agents. For example, CO has been shown to be beneficial in treating bacterial sepsis, which requires the ability to suppress inflammation while still allowing the body to fight off the infection44,91,92. In a recent study, Otterbein and colleagues93 have shown that macrophages can sense CO and kill bacteria through activation of inflammasome. Similarly, CO blocks the proinflammatory cytokine TNF, while enhancing expression of the anti-inflammatory cytokine IL-10 in macrophages and in tissue94,95. CO likewise has a direct manner of action against pathogens by inhibiting bacterial oxidases and essential hemoproteins96. In the area of cancer, CO has been shown to sensitize cancer cells toward chemotherapy64,97 through an anti-Warburg effect, and to help overcome cisplatin resistance98,99, while simultaneously offer protection of organs such as the heart, kidney, and liver that are prone to chemically-induced injuries72,100, 101, 102, 103, 104, 105. Very few, if any, known therapies can offer such seemingly directional controls in achieving therapeutic effects. One can also say that CO helps to restore a balance in eradicating pathological changes.
5. CO's remote-conditioning property reflects its health effect in a holistic fashion
Pre-conditioning106 and remote ischemic conditioning (RIC)107,108 refer to the protective effects of relatively minor ischemic events either locally beforehand or remotely against major damages resulting from ischemia-related events109,110. Despite success in some studies and in pre-clinical models111, 112, 113, 114, 115, 116, not all studies observed the same benefit of such conditioning117, 118, 119, 120. Thus, a deeper understanding of the mechanism of action might offer a chance to elucidate critical factors that contribute to success in such conditioning and may lead to approaches for pharmacological conditioning121. Mechanistic studies in animal models have led to suggestions of involvement of NO122, hypoxia-inducible factor 1α (HIF-1α)123,124, the PTEN/AKT signaling pathway124, exosomal miR-21125, NF-κB126, 127, 128, and IκBα129 in pre-conditioning or RIC. Understandably, many such molecular targets are inter-related. No matter what the mechanism is, RIC will likely involve the diffusion of a “signal” from a remote site to the location where ischemic damage happens. CO is such a molecule. HO-1 has been suggested as playing a key role in anesthetic-induced conditioning130 and CO has been suggested as a mediator in the conditioning process131,132. In an animal model study, it was shown that inhalation of CO was able to recapitulate the HO-1 induced protective conditioning in a liver ischemic reperfusion injury model133. As discussed in Section 2, there have been many other reports of CO's role in organ protection after ischemic reperfusion injury134, 135, 136. Thus, HO-1 activation and CO production as a result of minor and repeated ischemic events at a remote site could lead to the protection of major ischemic injuries through distance. Again, the chemically inert nature, diffusivity, and long half-life of CO play an important role in allowing CO to be a signaling molecule through distance and over time.
6. There is a large amount of data allowing for some understanding of CO's molecular mechanism(s) of actions
The examination of CO's pleiotropic pharmacological effects is supported by extensive and rigorous mechanistic studies, which are very critical to the advancement of this field. Some of the identified molecular targets of CO include the soluble form of guanylyl cyclase, mitochondrial oxidases, nitric oxide synthases, prolyl hydroxylase, neuronal PAS domain protein 2 (NPAS2), the CLOCK transcription factor, Per-2, mitogen-activated protein (MAP) kinases, catalase, PPARγ, HIF-1α, Nrf2, ion channels, and cystathionine β-synthase (CBS), among others71,95,101,137, 138, 139, 140, 141. A detailed discussion of this aspect would require a separate paper or a book. However, the references provided hopefully would allow readers to delve into the details, should they be interested. The multi-target nature of CO is very remarkable. Understanding the intricate interplay among all the molecular targets in a holistic fashion will be very valuable to achieve a deeper understanding of the “network” effect of CO, especially considering that CO has overlapping targets and functions with NO and H2S. Fortunately, this area has attracted a great deal of attention and research activities9,10.
7. CO has healing powers, is safe at therapeutic levels, and yet is harmful or lethal at high levels
Although CO plays an essential role in human physiology in managing inflammation as one of its functions and is being developed for therapeutic applications, there is no doubt that CO is toxic or lethal at high levels. Although the highest recorded non-fatal COHb was 73%142, each year many people die of CO poisoning143. Therefore, it is very important to understand the normal physiological concentration range and safety margin of CO.
In non-smokers, COHb levels averaged about 2% in one study, while smokers averaged 8%144. Hookah smoking has been the subject of clinical trials based on the link between CO inhalation and its vasodilatory mechanism of action (NCT03067701). Interestingly, despite the overall harmful effect of cigarette smoking, some studies found an inverse correlation between smoking and incidents of ulcerative colitis145, 146, 147 while others offer a different view148, 149, 150. Animal model experiments have also duplicated the inverse relationship between smoking and ulcerative colitis151,152. Further research points to CO's beneficial effects in colitis as a likely molecular link and suggests that the COHb level achieved through smoking is high enough for therapeutic values, though one has to be mindful of all the other harmful substance inhaled during smoking79, 80, 81, 82,84,152, 153, 154. If one considers the fact that Hb level in a healthy person is about 7.5 mmol/L, the CO concentration in the form of COHb in the blood can range from 150 (non-smokers) to 600 μmol/L (smokers), which is within human safety tolerance. These concentrations are also comparable to or higher than the peak plasma concentrations of approved drugs such as Tylenol (>100 μmol/L), naproxen (>300 μmol/L), Zantac (1.5 μmol/L), doxorubicin (1–2 μmol/L)155, imatinib (3.5 μmol/L), and 5-fluorouracil (>300 μmol/L). As a matter of fact, CO has been studied for safety in several human clinical trials. As a result, the U.S. Food and Drug Administration has approved clinical trials with COHb level being as high as 14% as an upper threshold105. All indications are that CO can be developed as a safe therapeutic agent with safety margins being higher or comparable to even nutrients such as glucose and potassium as well as therapeutics such as insulin, heparin, digitalis, warfarin, and others7,133.
Herein lies an important message that is not always as widely understood in the general public as one would have hoped. As an important historical figure, Paracelsus (a Swiss physician, alchemist, and philosopher, 1493–1541) would say, it is the dose that makes a poison or a remedy156. CO's therapeutic effect at low doses does not diminish its health risk or lethality at high levels, and vice versa. Further, simply because a drug is useful in treating a specific pathological condition does not mean that it is good for a healthy person to take as a preventative measure. This is a message that I (Wang) always tell students: just because taxol is a good drug to treat certain forms of cancer, it does not mean that a healthy person should take taxol to prevent the same cancer because all drugs are inherently toxic if abused. The same message is true for CO as well as medicinal plants, herbal supplements, and natural compounds. Prophylactic usage is very different from treatment and should only be based on proven science. Just because CO has the potential to be used as a therapeutic agent for treating certain conditions such as inflammation, it does not mean that a healthy person can “self-medicate” in any form or fashion. All therapeutic agents should be used only based on clinically proven science and under the supervision of appropriately trained medical professionals. Otherwise, there could be harmful or lethal consequences.
8. Much progress has been made in developing CO-based therapeutics, but challenges remain
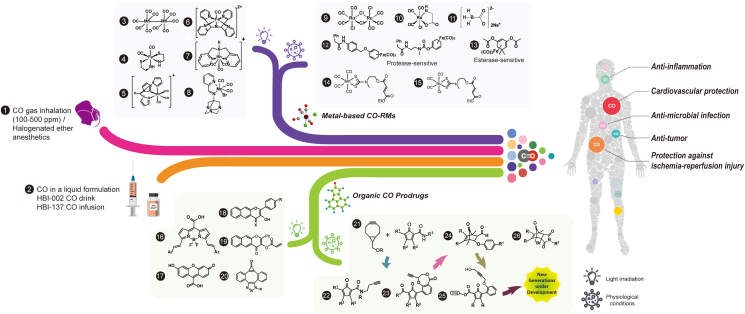
Because of the demonstrated pharmacological activity of CO, there is much interest in developing CO-based therapeutics for various indications, as summarized in a few recent reviews7,8,157, 158, 159, 160. Thus, we shall only provide a brief synopsis without duplicating what is in these reviews. In addition to efficacy, there are two key factors to consider in developing CO-based therapeutics: toxicity and delivery. For toxicity, Sections 1, 6 specifically address this issue. Three recent reviews address the safety issue in detail and show that for pharmaceutical development, CO has a sufficient safety margin, which is probably higher than or comparable to many approved therapeutics105,160,161. For delivery, using CO gas as the delivery form actually poses several issues. First, CO is lethal at high doses. A practical problem in using CO in a tank is that it is odorless, colorless, and tasteless, making it hard to detect if leakage happens. Even with the availability of CO detectors, CO leakage still poses a safety issue for healthcare workers and family members in the same household. Further, delivering a precise dose of gaseous CO through inhalation is a challenge by itself and in terms of patient compliance. As a result, there have been strong interests in developing alternative CO delivery methods including oral formulations and metal-immobilized carbonyls as CO-releasing molecules (CORMs)159. For those who are interested in more details, there are two earlier reviews and three recent reviews that cover the subject comprehensively8,158, 159, 160,162. Recent years have seen a growing interest in developing metal-free CO prodrugs belonging to different structural scaffolds7,158,161, 162, 163, 164, 165, 166, 167, 168, 169. These include prodrugs with tunable release rates (2 min to days)153,162,165,170, targeted delivery75,171,172, triggered release (pH-173, 174, 175, reactive oxygen species (ROS)-176, thiol-177, esterase-sensitive174,178, photo-sensitive164,167, 168, 169,172,179 as well as dual-triggered166,174,177 prodrugs), the ability to deliver two payloads in one prodrug180, and the ability to target the mitochondria75,158,171. These prodrugs have shown anti-inflammatory162 and antibacterial (H. pylori)180 effects in vitro and have been studied in animal models of colitis153, gastric injuries181, liver injuries75,174, kidney injuries71, and systemic inflammation174 with very pronounced efficacy.
Fig. 271,75,153,161,164, 165, 166, 167, 168,170,173,175, 176, 177,180,104,169,182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200 summarizes the conceptual development of CO delivery forms; and we hope that with this many CO delivery options available, some will eventually be developed into therapeutic agents. Even with all the remarkable progress, it is important to note that challenges remain in areas such as (1) understanding the interplay of dosage, duration of exposure, and efficacy, (2) deciding key parameters to measure in studying CO's pharmacokinetics47, (3) tissue-specific delivery when needed, (4) ways to minimize systemic CO exposure when needed, (5) methods for real-time detection of free and bound CO, and (6) a deeper understanding of the consequence of the “network”-like pleiotropic effects of CO at the molecular level. Recently, it has been reported that many of the previously described biological effects of metal-based CORMs, including antimicrobial201, 202, 203, 204, 205, anticoagulation206, 207, 208, and ion channel regulation209 are actually CO independent. Instead, much of these activities can be attributed to the generation of reactive oxygen species203, 204, 205, and reactivity toward thiol201, sulfite210, proteins and enzymes201,208,209,211, 212, 213, 214, 215, 216, 217, and electron-deficient organic functional groups such as an arylnitro group218 by certain metal-based CORMs in as well as their general solution chemistry including water-shift reactions203,219. All these issues indeed suggest that some of these metal-based CORMs may be more than CO donors per se. Such results further suggest the need to use rigorous controls and assessment of the general molecular reactivity of CO donors and their side products after CO release, whether they are metal-based CORMs or organic prodrugs. Therefore, much more work is needed.
Figure 2.
Summary of CO delivery approaches. Refs.: (1)161,182, 183, 184, 185, (2)186, (3)187, (4)188, (5)189, (6)190, (7)191, (8)192, (9)187, (10)104, (11)193, (12)194, (13)195, (14) and (15)196, (16)168, (17)169, (18)164, 165, 166,177, (19)167, (20)197, (21)75,198, (22)71,153,170,199, (23)200, (24)173,176, (25)180, (26)175.
In discussing CO-based therapeutics, it is critical to also analyze one issue: pharmacokinetics. As discussed earlier, CO's biological half-life (t1/2) in humans is commonly cited as about 300 min under atmospheric conditions20. However, such studies were mostly in the context of CO poisoning, presumably through inhalation. One may also need to consider the likelihood that the t1/2 may vary depending on the duration of CO exposure and alveolar ventilation220, the form of CO delivery47,221,222, and the level of CO in the blood21, 22, 23,223, 224, 225, 226, 227, 228. For example, in a study of human waterpipe usage, COHb level (averaging 6.2% COHb) returned to the baseline level within 8 h, implying a t1/2 of much shorter than 300 min229. In any case, CO administration in the form of short inhalation of 1000 ppm (once every min for 10 min) at 40 min interval was sufficient to give a sustain level of COHb230. In most animal model studies, once daily administration of CO is commonly used for yielding pharmacological efficacy. However, the biological half-life of CO as represented by COHb levels seems to vary considerably among porcine, dog, rabbit, rodent, and human231, 232, 233, 234. For example, in mice when administered in the form of CO prodrugs, the t1/2 of COHb is normally about 1–2 h depending on the route of administration47,75. In porcine after bolus administration of CO-saturated blood, the t1/2 of COHb is also about 1 h235. There have been other pharmacokinetic studies of COHb profiles using different donors and under different conditions8,229. Such results mean that much more work is needed in examining the relationship between pharmacokinetics and pharmacodynamics, which may vary depending on the specific pharmacological indications.
9. Concluding remarks
With all the discussions above, it is clear that CO is essential to human health, has a wide range of pharmacological effects, and has therapeutic potential. The inter-twined pleiotropic effects of CO mean that we will need to take an integrated and holistic approach to understanding CO's functional roles, especially in the context of its overlapping functions with NO and H2S. Beyond CO, it is important to note the network-like roles of the various gaseous molecules in sustaining life. This is an area that will require elevated levels of activities to fully tap into the therapeutic potential of these gaseous molecules.
As an afterthought, it is important to note that the conception of the roles of gaseous molecules in life's processes did not start with the commencement of modern molecular science. Quite to the contrary, the central roles that gaseous entities play in life had been recognized long time ago by human ancestors throughout the ancient civilizations, even though at that time, understanding at the molecular level was in the far distant future. For example, more than five thousand years ago in the ancient India, breath and gas (prana, “प्राण” in Hindu) were acknowledged as a vital element for life in the oldest philosophical medicine system known as “Ayurveda”236. Further, the book On Medicine, published around 100 AD, describes how Hippocrates (460–370 BC), the Greek physician often considered as the father of medicine, conjures the role of breaths and gases (pneuma, “πνεῦμα” in Greek) in health and disease. In a similar period of time, the concept of “Qi” (or Chi, “氣” in traditional Chinese) also emerged in Chinese medical records such as the “The Yellow Emperor's Classic of Medicine” (circa 2nd century BCE). However, all these ancient concepts seem to have a broader meaning than its literal translation of the physical concept of “air” or “gas” and to embody philosophical concepts as well, reflecting the recognition of the vital nature of gaseous entities in life. Finally, it is also interesting to note that one of the earliest medicines that continues its use in the modern days is laughing gas, or nitrous oxide, which was first introduced in the 1770's237.
It is our hope that this article will serve as a teaser to nature's marvels embedded in gaseous molecules and will help to stimulate additional interests in the same. As a result, we look forward to seeing a boom in research activities in this area so that we can collectively take a quantum leap in moving this field forward. We also hope that readers will lend their collective wisdom to the exploration of the holistic roles of gaseous signaling molecules in maintaining human health.
Acknowledgments
We acknowledge the general financial support of the Georgia Research Alliance through an Eminent Scholar endowment and internal financial sources at Georgia State University, USA. CPH's work was supported by The German Research Foundation (Deutsche Forschungsgemeinschaft—DFG), Germany, grant number: DFG #374031971 CRC/TR 240, Projekt B03. We would also like to express our gratitude to many friends and colleagues, who shall remain anonymous, for their thoughtful personal counsel, insightful advice, and constructive suggestions during the preparation of this manuscript.
Author contributions
Xiaoxiao Yang and Binghe Wang drafted this manuscript. Christopher P. Hopper, Wen Lu, and Bowen Ke gave their intellectual input and revised the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Olson K.R., Donald J.A., Dombkowski R.A., Perry S.F. Evolutionary and comparative aspects of nitric oxide, carbon monoxide and hydrogen sulfide. Respir Physiol Neurobiol. 2012;184:117–129. doi: 10.1016/j.resp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Tresguerres M., Buck J., Levin L.R. Physiological carbon dioxide, bicarbonate, and pH sensing. Pflugers Arch. 2010;460:953–964. doi: 10.1007/s00424-010-0865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Supuran C.T. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat. 2018;28:709–712. doi: 10.1080/13543776.2018.1523897. [DOI] [PubMed] [Google Scholar]
- 4.Ignarro L.J. Nitric oxide is not just blowing in the wind. Br J Pharmacol. 2019;176:131–134. doi: 10.1111/bph.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura H. Hydrogen sulfide and polysulfide signaling. Antioxid Redox Signal. 2017;27:619–621. doi: 10.1089/ars.2017.7076. [DOI] [PubMed] [Google Scholar]
- 6.Paul B.D., Snyder S.H. Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem Pharmacol. 2018;149:101–109. doi: 10.1016/j.bcp.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji X., Damera K., Zheng Y., Yu B., Otterbein L.E., Wang B. Toward carbon monoxide-based therapeutics: critical drug delivery and developability issues. J Pharm Sci. 2016;105:406–416. doi: 10.1016/j.xphs.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motterlini R., Otterbein L.E. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 9.Papapetropoulos A., Foresti R., Ferdinandy P. Pharmacology of the 'gasotransmitters' NO, CO and H2S: translational opportunities. Br J Pharmacol. 2015;172:1395–1396. doi: 10.1111/bph.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagone P., Mazzon E., Bramanti P., Bendtzen K., Nicoletti F. Gasotransmitters and the immune system: mode of action and novel therapeutic targets. Eur J Pharmacol. 2018;834:92–102. doi: 10.1016/j.ejphar.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Wareham L.K., Southam H.M., Poole R.K. Do nitric oxide, carbon monoxide and hydrogen sulfide really qualify as 'gasotransmitters' in bacteria?. Biochem Soc Trans. 2018;46:1107–1118. doi: 10.1042/BST20170311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y.L., Houk K.N. Thionitroxides, RSNHO∗: the structure of the SNO moiety in "S-nitrosohemoglobin", a possible NO reservoir and transporter. J Am Chem Soc. 2006;128:1422–1423. doi: 10.1021/ja057097f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynia-Smith S.L., Smith B.C. Nitrosothiol formation and S-nitrosation signaling through nitric oxide synthases. Nitric Oxide. 2017;63:52–60. doi: 10.1016/j.niox.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Fukuto J.M. A recent history of nitroxyl chemistry, pharmacology and therapeutic potential. Br J Pharmacol. 2019;176:135–146. doi: 10.1111/bph.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabil O., Vitvitsky V., Banerjee R. Sulfur as a signaling nutrient through hydrogen sulfide. Annu Rev Nutr. 2014;34:171–205. doi: 10.1146/annurev-nutr-071813-105654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y., Yu B., De La Cruz L.K., Roy Choudhury M., Anifowose A., Wang B. Toward hydrogen sulfide based therapeutics: critical drug delivery and developability issues. Med Res Rev. 2018;38:57–100. doi: 10.1002/med.21433. [DOI] [PubMed] [Google Scholar]
- 17.Maroney M.J., Ciurli S. Nonredox nickel enzymes. Chem Rev. 2014;114:4206–4228. doi: 10.1021/cr4004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collman J.P., Brauman J.I., Doxsee K.M. Carbon monoxide binding to iron porphyrins. Proc Natl Acad Sci U S A. 1979;76:6035–6039. doi: 10.1073/pnas.76.12.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uffen R.L. Metabolism of carbon monoxide. Enzyme Microb Technol. 1981;3:197–206. [Google Scholar]
- 20.Buboltz J.B., Robins M. StatPearls Publishing; Treasure Island, Florida: 2020. Hyperbaric treatment of carbon monoxide toxicity. [PubMed] [Google Scholar]
- 21.Weaver L.K., Howe S., Hopkins R., Chan K.J. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;117:801–808. doi: 10.1378/chest.117.3.801. [DOI] [PubMed] [Google Scholar]
- 22.Shimazu T., Ikeuchi H., Sugimoto H., Goodwin C.W., Mason A.D., Jr., Pruitt B.A., Jr. Half-life of blood carboxyhemoglobin after short-term and long-term exposure to carbon monoxide. J Trauma. 2000;49:126–131. doi: 10.1097/00005373-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Shimazu T. Half-life of blood carboxyhemoglobin. Chest. 2001;119:661–662. doi: 10.1378/chest.119.2.661. [DOI] [PubMed] [Google Scholar]
- 24.Wang X.B., Cui H., Liu X., Du J.B. Sulfur dioxide: foe or friend for life?. Histol Histopathol. 2017;32:1231–1238. doi: 10.14670/HH-11-904. [DOI] [PubMed] [Google Scholar]
- 25.Wang W., Ji X., Du Z., Wang B. Sulfur dioxide prodrugs: triggered release of SO2via a click reaction. Chem Commun. 2017;53:1370–1373. doi: 10.1039/c6cc08844a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iida A., Nosaka N., Yumoto T., Knaup E., Naito H., Nishiyama C. The clinical application of hydrogen as a medical treatment. Acta Med Okayama. 2016;70:331–337. doi: 10.18926/AMO/54590. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J.Y., Liu C., Zhou L., Qu K., Wang R., Tai M.H. A review of hydrogen as a new medical therapy. Hepatogastroenterology. 2012;59:1026–1032. doi: 10.5754/hge11883. [DOI] [PubMed] [Google Scholar]
- 28.Naito Y., Uchiyama K., Takagi T. Redox-related gaseous mediators in the gastrointestinal tract. J Clin Biochem Nutr. 2018;63:1–4. doi: 10.3164/jcbn.18-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikami T., Tano K., Lee H., Lee H., Park J., Ohta F. Drinking hydrogen water enhances endurance and relieves psychometric fatigue: a randomized, double-blind, placebo-controlled study. Can J Physiol Pharmacol. 2019;97:857–862. doi: 10.1139/cjpp-2019-0059. [DOI] [PubMed] [Google Scholar]
- 30.Jia Y., Li Z., Liu C., Zhang J. Methane medicine: a rising star gas with powerful anti-inflammation, antioxidant, and antiapoptosis properties. Oxid Med Cell Longev. 2018 doi: 10.1155/2018/1912746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adeva M.M., Souto G., Blanco N., Donapetry C. Ammonium metabolism in humans. Metabolism. 2012;61:1495–1511. doi: 10.1016/j.metabol.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Tomasova L., Konopelski P., Ufnal M. Gut bacteria and hydrogen sulfide: the new old players in circulatory system homeostasis. Molecules. 2016;21:1558–1575. doi: 10.3390/molecules21111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farhi L.E. Atmospheric nitrogen and its role in modern medicine. JAMA. 1964:984–993. doi: 10.1001/jama.1964.03060370040009. [DOI] [PubMed] [Google Scholar]
- 34.Wu L., Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 35.Wang R. CRC Press; Boca Raton, Florida: 2001. Carbon monoxide and cardiovascular functions. [Google Scholar]
- 36.Vreman H.J., Wong R.J., Stevenson D.K. Sources, sinks, and measurements of carbon monoxide. In: Wang R., editor. Carbon monoxide and cardiovascular functions. CRC Press; Boca Raton, Florida: 2001. pp. 273–307. [Google Scholar]
- 37.Vukomanovic D., Rahman M., Jia Z., Nakatsu K. Drug-enhanced carbon monoxide production from heme by cytochrome P450 reductase. Med Gas Res. 2017;7:37–44. doi: 10.4103/2045-9912.202908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshpande A.R., Pochapsky T.C., Petsko G.A., Ringe D. Dual chemistry catalyzed by human acireductone dioxygenase. Protein Eng Des Sel. 2017;30:109–206. doi: 10.1093/protein/gzw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyahara S., Takahashi H. Biological CO evolution. J Biochem. 1971;69:231–233. doi: 10.1093/oxfordjournals.jbchem.a129450. [DOI] [PubMed] [Google Scholar]
- 40.Wolff D.G., Bidlack W.R. The formation of carbon monoxide during peroxidation of microsomal lipids. Biochem Biophys Res Commun. 1976;73:850–857. doi: 10.1016/0006-291x(76)90199-6. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers P.A., Vreman H.J., Dennery P.A., Stevenson D.K. Sources of carbon monoxide (CO) in biological systems and applications of CO detection technologies. Semin Perinatol. 1994;18:2–10. [PubMed] [Google Scholar]
- 42.Maharshak N., Ryu H.S., Fan T.J., Onyiah J.C., Schulz S., Otterbein S.L. Escherichia coli heme oxygenase modulates host innate immune responses. Microbiol Immunol. 2015;59:452–455. doi: 10.1111/1348-0421.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hino S., Tauchi H. Production of carbon monoxide from aromatic amino acids by Morganella morganii. Arch Microbiol. 1987;148:167–171. [Google Scholar]
- 44.Nakahira K., Choi A.M. Carbon monoxide in the treatment of sepsis. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1387–L1393. doi: 10.1152/ajplung.00311.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakurai Y. The study on the skin permeability of carbon monoxide and measurement of carboxy-hemoglobin in blood. Jpn J Leg Med. 1963;17:314–326. [PubMed] [Google Scholar]
- 46.Belcher J.D., Gomperts E., Nguyen J., Chen C., Abdulla F., Kiser Z.M. Oral carbon monoxide therapy in murine sickle cell disease: beneficial effects on vaso-occlusion, inflammation and anemia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M., Yang X., Pan Z., Wang Y., De La Cruz L.K., Wang B. Towards “CO in a pill”: pharmacokinetic studies of carbon monoxide prodrugs in mice. J Control Release. 2020;327:174–175. doi: 10.1016/j.jconrel.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawano M. Demonstration and quantification of the redistribution and oxidation of carbon monoxide in the human body by tracer analysis. Med Gas Res. 2016;6:59–63. doi: 10.4103/2045-9912.184598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahan V.L. Cardiac function dependence on carbon monoxide. Med Gas Res. 2020;10:37–46. doi: 10.4103/2045-9912.279982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suliman H.B., Zobi F., Piantadosi C.A. Heme oxygenase-1/carbon monoxide system and embryonic stem cell differentiation and maturation into cardiomyocytes. Antioxid Redox Signal. 2016;24:345–360. doi: 10.1089/ars.2015.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nemecek D., Dvorakova M., Sedmikova M. Heme oxygenase/carbon monoxide in the female reproductive system: an overlooked signalling pathway. Int J Biochem Mol Biol. 2017;8:1–12. [PMC free article] [PubMed] [Google Scholar]
- 52.McLean M., Bowman M., Clifton V., Smith R., Grossman A.B. Expression of the heme oxygenase-carbon monoxide signalling system in human placenta. J Clin Endocrinol Metab. 2000;85:2345–2349. doi: 10.1210/jcem.85.6.6705. [DOI] [PubMed] [Google Scholar]
- 53.Ayer A., Zarjou A., Agarwal A., Stocker R. Heme oxygenases in cardiovascular health and disease. Physiol Rev. 2016;96:1449–1508. doi: 10.1152/physrev.00003.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryter S.W. Heme oxygenase-1/carbon monoxide as modulators of autophagy and inflammation. Arch Biochem Biophys. 2019;678:108186. doi: 10.1016/j.abb.2019.108186. [DOI] [PubMed] [Google Scholar]
- 55.Waza A.A., Hamid Z., Ali S., Bhat S.A., Bhat M.A. A review on heme oxygenase-1 induction: is it a necessary evil. Inflamm Res. 2018;67:579–588. doi: 10.1007/s00011-018-1151-x. [DOI] [PubMed] [Google Scholar]
- 56.Vreman H., Wong R., Stevenson D. Carbon monoxide in breath, blood, and other tissues. In: Penney D.G., editor. Carbon monoxide toxicity. Taylor Francis; 2000. pp. 19–60. [Google Scholar]
- 57.Nath K.A., Balla G., Vercellotti G.M., Balla J., Jacob H.S., Levitt M.D. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang L.L., Ma Y.J., Zhang H.D. Carbon monoxide breath test assessment of mild hemolysis in Gilbert's syndrome. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H.D., Ma Y.J., Liu Q.F., Ye T.Z., Meng F.Y., Zhou Y.W. Human erythrocyte lifespan measured by Levitt's CO breath test with newly developed automatic instrument. J Breath Res. 2018;12 doi: 10.1088/1752-7163/aaacf1. [DOI] [PubMed] [Google Scholar]
- 60.Barbagallo I., Marrazzo G., Frigiola A., Zappala A., Li V.G. Role of carbon monoxide in vascular diseases. Curr Pharm Biotechnol. 2012;13:787–796. doi: 10.2174/138920112800399086. [DOI] [PubMed] [Google Scholar]
- 61.Simon T., Anegon I., Blancou P. Heme oxygenase and carbon monoxide as an immunotherapeutic approach in transplantation and cancer. Immunotherapy. 2011;3:15–18. doi: 10.2217/imt.11.43. [DOI] [PubMed] [Google Scholar]
- 62.Ryter S.W., Choi A.M. Heme oxygenase-1/carbon monoxide: novel therapeutic strategies in critical care medicine. Curr Drug Targets. 2010;11:1485–1494. doi: 10.2174/1389450111009011485. [DOI] [PubMed] [Google Scholar]
- 63.Ryter S.W., Choi A.M. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol. 2009;41:251–260. doi: 10.1165/rcmb.2009-0170TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wegiel B., Hanto D.W., Otterbein L.E. The social network of carbon monoxide in medicine. Trends Mol Med. 2013;19:3–11. doi: 10.1016/j.molmed.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wegiel B., Nemeth Z., Correa-Costa M., Bulmer A.C., Otterbein L.E. Heme oxygenase-1: a metabolic nike. Antioxid Redox Signal. 2014;20:1709–1722. doi: 10.1089/ars.2013.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiang S.K., Chen S.E., Chang L.C. A Dual role of heme oxygenase-1 in cancer cells. Int J Mol Sci. 2019;20:39–56. doi: 10.3390/ijms20010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poss K.D., Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uddin M.J., Pak E.S., Ha H. Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress. Korean J Physiol Pharmacol. 2018;22:567–575. doi: 10.4196/kjpp.2018.22.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng Y., Rong J. Therapeutic potential of heme oxygenase-1/carbon monoxide system against ischemia–reperfusion injury. Curr Pharm Des. 2017;23:3884–3898. doi: 10.2174/1381612823666170413122439. [DOI] [PubMed] [Google Scholar]
- 70.Abe T., Yazawa K., Fujino M., Imamura R., Hatayama N., Kakuta Y. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab Invest. 2017;97:468–477. doi: 10.1038/labinvest.2016.157. [DOI] [PubMed] [Google Scholar]
- 71.Correa-Costa M., Gallo D., Csizmadia E., Gomperts E., Lieberum J.L., Hauser C.J. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc Natl Acad Sci U S A. 2018;115:E2302–E2310. doi: 10.1073/pnas.1716747115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suliman H.B., Carraway M.S., Ali A.S., Reynolds C.M., Welty-Wolf K.E., Piantadosi C.A. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim H.H., Choi S. Therapeutic aspects of carbon monoxide in cardiovascular disease. Int J Mol Sci. 2019;19:2381–2392. doi: 10.3390/ijms19082381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun J., Guo E., Yang J., Yang Y., Liu S., Hu J. Carbon monoxide ameliorates hepatic ischemia/reperfusion injury via sirtuin 1-mediated deacetylation of high-mobility group box 1 in rats. Liver Transpl. 2017;23:510–526. doi: 10.1002/lt.24733. [DOI] [PubMed] [Google Scholar]
- 75.Zheng Y., Ji X., Yu B., Ji K., Gallo D., Csizmadia E. Enrichment-triggered prodrug activation demonstrated through mitochondria-targeted delivery of doxorubicin and carbon monoxide. Nat Chem. 2018;10:787–794. doi: 10.1038/s41557-018-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Che X., Fang Y., Si X., Wang J., Hu X., Reis C. The role of gaseous molecules in traumatic brain injury: an updated review. Front Neurosci. 2018;12:392–400. doi: 10.3389/fnins.2018.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi Y.K., Maki T., Mandeville E.T., Koh S.H., Hayakawa K., Arai K. Dual effects of carbon monoxide on pericytes and neurogenesis in traumatic brain injury. Nat Med. 2016;22:1335–1341. doi: 10.1038/nm.4188. [DOI] [PubMed] [Google Scholar]
- 78.Wang P., Yao L., Zhou L.L., Liu Y.S., Chen M.D., Wu H.D. Carbon monoxide improves neurologic outcomes by mitochondrial biogenesis after global cerebral ischemia induced by cardiac arrest in rats. Int J Biol Sci. 2016;12:1000–1009. doi: 10.7150/ijbs.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hegazi R.A.F., Rao K.N., Mayle A., Sepulveda A.R., Otterbein L.E., Plev S.E. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med. 2005;202:1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheikh S.Z., Hegazi R.A., Kobayashi T., Onyiah J.C., Russo S.M., Matsuoka K.M. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol. 2011;186:5506–5513. doi: 10.4049/jimmunol.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steiger C., Uchiyama K., Takagi T., Mizushima K., Higashimura Y., Gutmann M. Prevention of colitis by controlled oral drug delivery of carbon monoxide. J Control Release. 2016;239:128–136. doi: 10.1016/j.jconrel.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 82.Takagi T., Naito Y., Mizushima K., Akagiri S., Suzuki, Hirata I. Inhalation of carbon monoxide ameliorates TNBS-induced colitis in mice through the inhibition of TNF-α expression. Digest Dis Sci. 2010;55:2797–2804. doi: 10.1007/s10620-009-1112-x. [DOI] [PubMed] [Google Scholar]
- 83.Takagi T., Naito Y., Uchiyama K., Suzuki T., Hirata I., Mizushima K. Carbon monoxide liberated from carbon monoxide-releasing molecule exerts an anti-inflammatory effect on dextran sulfate sodium-induced colitis in mice. Digest Dis Sci. 2011;56:1663–1671. doi: 10.1007/s10620-010-1484-y. [DOI] [PubMed] [Google Scholar]
- 84.Uddin M.J., Jeong S.O., Zheng M., Chen Y., Cho G.J., Chung H.T. Carbon monoxide attenuates dextran sulfate sodium-induced colitis via inhibition of GSK-3beta signaling. Oxid Med Cell Longev. 2013;2013:210563. doi: 10.1155/2013/210563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nikolic I., Saksida T., Mangano K., Vujicic M., Stojanovic I., Nicoletti F. Pharmacological application of carbon monoxide ameliorates islet-directed autoimmunity in mice via anti-inflammatory and anti-apoptotic effects. Diabetologia. 2014;57:980–990. doi: 10.1007/s00125-014-3170-7. [DOI] [PubMed] [Google Scholar]
- 86.Nikolic I., Vujicic M., Stojanovic I., Stosic-Grujicic S., Saksida T. Carbon monoxide-releasing molecule-A1 inhibits Th1/Th17 and stimulates Th2 differentiation in vitro. Scand J Immunol. 2014;80:95–100. doi: 10.1111/sji.12189. [DOI] [PubMed] [Google Scholar]
- 87.Nikolic I., Saksida T., Vujicic M., Stojanovic I., Stosic-Grujicic S. Anti-diabetic actions of carbon monoxide-releasing molecule (CORM)-A1: immunomodulation and regeneration of islet beta cells. Immunol Lett. 2015;165:39–46. doi: 10.1016/j.imlet.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Venditti C.C., Casselman R., Young I., Karumanchi S.A., Smith G.N. Carbon monoxide prevents hypertension and proteinuria in an adenovirus sFlt-1 preeclampsia-like mouse model. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Venditti C.C., Casselman R., Murphy M.S., Adamson S.L., Sled J.G., Smith G.N. Chronic carbon monoxide inhalation during pregnancy augments uterine artery blood flow and uteroplacental vascular growth in mice. Am J Physiol Regul Integr Comp Physiol. 2013;305:R939–R948. doi: 10.1152/ajpregu.00204.2013. [DOI] [PubMed] [Google Scholar]
- 90.Kashyap P.C., Choi K.M., Dutta N., Linden D.R., Szurszewski J.H., Gibbons S.J. Carbon monoxide reverses diabetic gastroparesis in NOD mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G1013–G1019. doi: 10.1152/ajpgi.00069.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsoyi K., Hall S.R., Dalli J., Colas R.A., Ghanta S., Ith B. Carbon monoxide improves efficacy of mesenchymal stromal cells during sepsis by production of specialized proresolving lipid mediators. Crit Care Med. 2016;44:e1236–e1245. doi: 10.1097/CCM.0000000000001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang P., Huang J., Li Y., Chang R., Wu H., Lin J. Exogenous carbon monoxide decreases sepsis-induced acute kidney injury and inhibits NLRP3 inflammasome activation in rats. Int J Mol Sci. 2015;16:20595–20608. doi: 10.3390/ijms160920595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wegiel B., Larsen R., Gallo D., Chin B.Y., Harris C., Mannam P. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest. 2014;124:4926–4940. doi: 10.1172/JCI72853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uddin M.J., Li C.S., Joe Y., Chen Y., Zhang Q., Ryter S.W. Carbon monoxide inhibits tenascin-C mediated inflammation via IL-10 expression in a septic mouse model. Mediators Inflamm. 2015;2015:613249. doi: 10.1155/2015/613249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Otterbein L.E., Bach F.H., Alam J., Soares M., Tao Lu H., Wysk M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 96.Davidge K.S., Motterlini R., Mann B.E., Wilson J.L., Poole R.K. Carbon monoxide in biology and microbiology: surprising roles for the "Detroit perfume". Adv Microb Physiol. 2009;56:85–167. doi: 10.1016/S0065-2911(09)05603-3. [DOI] [PubMed] [Google Scholar]
- 97.Wegiel B., Gallo D., Csizmadia E., Harris C., Belcher J., Vercellotti G.M. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013;73:7009–7021. doi: 10.1158/0008-5472.CAN-13-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawahara B., Ramadoss S., Chaudhuri G., Janzen C., Sen S., Mascharak P.K. Carbon monoxide sensitizes cisplatin-resistant ovarian cancer cell lines toward cisplatin via attenuation of levels of glutathione and nuclear metallothionein. J Inorg Biochem. 2019;191:29–39. doi: 10.1016/j.jinorgbio.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 99.Kawahara B., Sen S., Mascharak P.K. Reaction of carbon monoxide with cystathionine beta-synthase: implications on drug efficacies in cancer chemotherapy. Future Med Chem. 2020;12:325–337. doi: 10.4155/fmc-2019-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoon Y.E., Lee K.S., Lee Y.J., Lee H.H., Han W.K. Renoprotective effects of carbon monoxide-releasing molecule 3 in ischemia–reperfusion injury and cisplatin-induced toxicity. Transplant Proc. 2017;49:1175–1182. doi: 10.1016/j.transproceed.2017.03.067. [DOI] [PubMed] [Google Scholar]
- 101.Tayem Y., Johnson T.R., Mann B.E., Green C.J., Motterlini R. Protection against cisplatin-induced nephrotoxicity by a carbon monoxide-releasing molecule. Am J Physiol Renal Physiol. 2006;290:F789–F794. doi: 10.1152/ajprenal.00363.2005. [DOI] [PubMed] [Google Scholar]
- 102.Musameh M.D., Green C.J., Mann B.E., Motterlini R., Fuller B.J. CO liberated from a carbon monoxide-releasing molecule exerts a positive inotropic effect in doxorubicin-induced cardiomyopathy. J Cardiovasc Pharmacol. 2010;55:168–175. doi: 10.1097/FJC.0b013e3181ca4bbc. [DOI] [PubMed] [Google Scholar]
- 103.Soni H., Pandya G., Patel P., Acharya A., Jain M., Mehta A.A. Beneficial effects of carbon monoxide-releasing molecule-2 (CORM-2) on acute doxorubicin cardiotoxicity in mice: role of oxidative stress and apoptosis. Toxicol Appl Pharmacol. 2011;253:70–80. doi: 10.1016/j.taap.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 104.Clark J.E., Naughton P., Shurey S., Green C.J., Johnson T.R., Mann B.E. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2–8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 105.Yang X., de Caestecker M., Otterbein L.E., Wang B. Carbon monoxide: an emerging therapy for acute kidney injury. Med Res Rev. 2020;40:1147–1177. doi: 10.1002/med.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murry C.E., Jennings R.B., Reimer K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 107.Hess D.C., Blauenfeldt R.A., Andersen G., Hougaard K.D., Hoda M.N., Ding Y. Remote ischaemic conditioning—a new paradigm of self-protection in the brain. Nat Rev Neurol. 2015;11:698–710. doi: 10.1038/nrneurol.2015.223. [DOI] [PubMed] [Google Scholar]
- 108.Zhao W., Li S., Ren C., Meng R., Jin K., Ji X. Remote ischemic conditioning for stroke: clinical data, challenges, and future directions. Ann Clin Transl Neurol. 2019;6:186–196. doi: 10.1002/acn3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Donatelli F., Pietropaoli L. Remote ischemic preconditioning: the hunt is still open. Minerva Anestesiol. 2018;84:1243–1245. doi: 10.23736/S0375-9393.18.13219-6. [DOI] [PubMed] [Google Scholar]
- 110.Donato M., Evelson P., Gelpi R.J. Protecting the heart from ischemia/reperfusion injury: an update on remote ischemic preconditioning and postconditioning. Curr Opin Cardiol. 2017;32:784–790. doi: 10.1097/HCO.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 111.Zhou H., Yang L., Wang G., Zhang C., Fang Z., Lei G. Remote ischemic preconditioning prevents postoperative acute kidney injury after open total aortic arch replacement: a double-blind, randomized, sham-controlled trial. Anesth Analg. 2019;129:287–293. doi: 10.1213/ANE.0000000000004127. [DOI] [PubMed] [Google Scholar]
- 112.Zarbock A., Schmidt C., Van Aken H., Wempe C., Martens S., Zahn P.K. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. J Am Med Assoc. 2015;313:2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 113.Zarbock A., Kellum J.A., Van Aken H., Schmidt C., Kullmar M., Rosenberger P. Long-term effects of remote ischemic preconditioning on kidney function in high-risk cardiac surgery patients: follow-up results from the RenalRIP trial. Anesthesiology. 2017;126:787–798. doi: 10.1097/ALN.0000000000001598. [DOI] [PubMed] [Google Scholar]
- 114.Zarbock A., Kellum J.A. Remote ischemic preconditioning and protection of the kidney—a novel therapeutic option. Crit Care Med. 2016;44:607–616. doi: 10.1097/CCM.0000000000001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sprick J.D., Mallet R.T., Przyklenk K., Rickards C.A. Ischaemic and hypoxic conditioning: potential for protection of vital organs. Exp Physiol. 2019;104:278–294. doi: 10.1113/EP087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ren X., Roessler A.E., Lynch TLt, Haar L., Mallick F., Lui Y. Cardioprotection via the skin: nociceptor-induced conditioning against cardiac MI in the NIC of time. Am J Physiol Heart Circ Physiol. 2019;316:H543–H553. doi: 10.1152/ajpheart.00094.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benstoem C., Stoppe C., Liakopoulos O.J., Ney J., Hasenclever D., Meybohm P. Remote ischaemic preconditioning for coronary artery bypass grafting (with or without valve surgery) Cochrane Database Syst Rev. 2017;5 doi: 10.1002/14651858.CD011719.pub3. Cd011719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao W., Zhang J., Sadowsky M.G., Meng R., Ding Y., Ji X. Remote ischaemic conditioning for preventing and treating ischaemic stroke. Cochrane Database Syst Rev. 2018;7 doi: 10.1002/14651858.CD012503.pub2. Cd012503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krag A.E., Hvas A.M., Hvas C.L., Kiil B.J. Remote ischemic preconditioning in microsurgical head and neck reconstruction: a randomized controlled trial. Plast Reconstr Surg Glob Open. 2020;8 doi: 10.1097/GOX.0000000000002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Freitas S., Hicks C.W., Mouton R., Garcia S., Healy D., Connolly C. Effects of ischemic preconditioning on abdominal aortic aneurysm repair: a systematic review and meta-analysis. J Surg Res. 2019;235:340–349. doi: 10.1016/j.jss.2018.09.049. [DOI] [PubMed] [Google Scholar]
- 121.Liu Z., Gong R. Remote ischemic preconditioning for kidney protection: GSK3beta-centric insights into the mechanism of action. Am J Kidney Dis. 2015;66:846–856. doi: 10.1053/j.ajkd.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jung H., Choi E.K., Baek S.I., Cho C., Jin Y., Kwak K.H. The effect of nitric oxide on remote ischemic preconditioning in renal ischemia reperfusion injury in rats. Dose Response. 2019;17 doi: 10.1177/1559325819853651. 1559325819853651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Du X., Yang J., Liu C., Wang S., Zhang C., Zhao H. Hypoxia-inducible factor 1alpha and 2alpha have beneficial effects in remote ischemic preconditioning against stroke by modulating inflammatory responses in aged rats. Front Aging Neurosci. 2020;12:54. doi: 10.3389/fnagi.2020.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang W., Chen C., Jing R., Liu T., Liu B. Remote ischemic preconditioning protects cisplatin-induced acute kidney injury through the PTEN/AKT signaling pathway. Oxid Med Cell Longev. 2019;2019:7629396. doi: 10.1155/2019/7629396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pan T., Jia P., Chen N., Fang Y., Liang Y., Guo M. Delayed remote ischemic preconditioning confers renoprotection against septic acute kidney injury via exosomal miR-21. Theranostics. 2019;9:405–423. doi: 10.7150/thno.29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shin H.J., Won N.H., Lee H.W. Remote ischemic preconditioning prevents lipopolysaccharide-induced liver injury through inhibition of NF-kappaB activation in mice. J Anesth. 2014;28:898–905. doi: 10.1007/s00540-014-1850-6. [DOI] [PubMed] [Google Scholar]
- 127.Koh W.U., Kim J., Lee J., Song G.W., Hwang G.S., Tak E. Remote ischemic preconditioning and diazoxide protect from hepatic ischemic reperfusion injury by inhibiting HMGB1-induced TLR4/MyD88/NF-kappaB signaling. Int J Mol Sci. 2019;20:5899–5912. doi: 10.3390/ijms20235899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liang W., Lin C., Yuan L., Chen L., Guo P., Li P. Preactivation of Notch1 in remote ischemic preconditioning reduces cerebral ischemia–reperfusion injury through crosstalk with the NF-kappaB pathway. J Neuroinflammation. 2019;16:181–196. doi: 10.1186/s12974-019-1570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim Y.H., Kim Y.S., Kim B.H., Lee K.S., Park H.S., Lim C.H. Remote ischemic preconditioning ameliorates indirect acute lung injury by modulating phosphorylation of IkappaBalpha in mice. J Int Med Res. 2019;47:936–950. doi: 10.1177/0300060518818300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bauer I., Raupach A. The role of heme oxygenase-1 in remote ischemic and anesthetic organ conditioning. Antioxidants (Basel) 2019;8:E403. doi: 10.3390/antiox8090403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Andreadou I., Iliodromitis E.K., Rassaf T., Schulz R., Papapetropoulos A., Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol. 2015;172:1587–1606. doi: 10.1111/bph.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bilban M., Haschemi A., Wegiel B., Chin B.Y., Wagner O., Otterbein L.E. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med. 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- 133.Motterlini R., Mann B.E., Foresti R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Investig Drugs. 2005;14:1305–1318. doi: 10.1517/13543784.14.11.1305. [DOI] [PubMed] [Google Scholar]
- 134.Mahan V.L., Zurakowski D., Otterbein L.E., Pigula F.A. Inhaled carbon monoxide provides cerebral cytoprotection in pigs. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Loop T., Schlensak C., Goebel U. Cytoprotection by inhaled carbon monoxide before cardiopulmonary bypass in preclinical models. Curr Pharm Biotechnol. 2012;13:797–802. doi: 10.2174/138920112800399130. [DOI] [PubMed] [Google Scholar]
- 136.Ozaki K.S., Kimura S., Murase N. Use of carbon monoxide in minimizing ischemia/reperfusion injury in transplantation. Transplant Rev. 2012;26:125–139. doi: 10.1016/j.trre.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Motterlini R., Foresti R. Biological signaling by carbon monoxide and carbon monoxide-releasing molecules. Am J Physiol Cell Physiol. 2017;312:C302–C313. doi: 10.1152/ajpcell.00360.2016. [DOI] [PubMed] [Google Scholar]
- 138.Haschemi A., Chin B.Y., Jeitler M., Esterbauer H., Wagner O., Bilban M. Carbon monoxide induced PPARgamma SUMOylation and UCP2 block inflammatory gene expression in macrophages. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bilban M., Bach F.H., Otterbein S.L., Ifedigbo E., d'Avila J.C., Esterbauer H. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 140.Zuckerbraun B.S., Chin B.Y., Bilban M., d'Avila J.C., Rao J., Billiar T.R. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 141.Dioum E.M., Rutter J., Tuckerman J.R., Gonzalez G., Gilles-Gonzalez M.A., McKnight S.L. NPAS2: a gas-responsive transcription factor. Science. 2002;298:2385–2387. doi: 10.1126/science.1078456. [DOI] [PubMed] [Google Scholar]
- 142.Hampson N.B. Carboxyhemoglobin: a primer for clinicians. Undersea Hyperb Med. 2018;45:165–171. [PubMed] [Google Scholar]
- 143.Wu P.E., Juurlink D.N. Carbon monoxide poisoning. Can Med Assoc J. 2014;186:611. doi: 10.1503/cmaj.130972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boehm R.E., Arbo B.D., Leal D., Hansen A.W., Pulcinelli R.R., Thiesen F.V. Smoking fewer than 20 cigarettes per day and remaining abstinent for more than 12 hours reduces carboxyhemoglobin levels in packed red blood cells for transfusion. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kridin K., Zamir H., Cohen A.D. Cigarette smoking associates inversely with a cluster of two autoimmune diseases: ulcerative colitis and pemphigus. Immunol Res. 2018;66:555–556. doi: 10.1007/s12026-018-9021-8. [DOI] [PubMed] [Google Scholar]
- 146.Begon J., Juillerat P., Cornuz J., Clair C. Smoking and digestive tract: a complex relationship. Part 1: inflammatory bowel disease and cigarette smoking. Rev Med Suisse. 2015;11:1282–1287. [PubMed] [Google Scholar]
- 147.Lakatos P.L., Szamosi T., Lakatos L. Smoking in inflammatory bowel diseases: good, bad or ugly?. World J Gastroenterol. 2007;13:6134–6139. doi: 10.3748/wjg.v13.i46.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jaruvongvanich V., Poonsombudlert K., Ungprasert P. Smoking and risk of microscopic colitis: a systematic review and meta-analysis. Inflamm Bowel Dis. 2019;25:672–678. doi: 10.1093/ibd/izy296. [DOI] [PubMed] [Google Scholar]
- 149.Burke K.E., Ananthakrishnan A.N., Lochhead P., Olen O., Ludvigsson J.F., Richter J.M. Smoking is associated with an increased risk of microscopic colitis: results from two large prospective cohort studies of US women. J Crohns Colitis. 2018;12:559–567. doi: 10.1093/ecco-jcc/jjy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Adams S.H., Park M.J., Schaub J.P., Brindis C.D., Irwin C.E. Medical vulnerability of young adults to severe COVID-19 illness—data from the national health interview survey. J Adolesc Health. 2020;67:362–368. doi: 10.1016/j.jadohealth.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Daniluk J., Daniluk U., Reszec J., Rusak M., Dabrowska M., Dabrowski A. Protective effect of cigarette smoke on the course of dextran sulfate sodium-induced colitis is accompanied by lymphocyte subpopulation changes in the blood and colon. Int J Colorectal Dis. 2017;32:1551–1559. doi: 10.1007/s00384-017-2882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lo Sasso G., Phillips B.W., Sewer A., Battey J.N.D., Kondylis A., Talikka M. The reduction of DSS-induced colitis severity in mice exposed to cigarette smoke is linked to immune modulation and microbial shifts. Sci Rep. 2020;10:3829. doi: 10.1038/s41598-020-60175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ji X., Zhou C., Ji K., Aghoghovbia R., Pan Z., Chittavong V., Ke B., Wang B. Click and release: a chemical strategy toward developing gasotransmitter prodrugs by using an intramolecular Diels–Alder reaction. Angew Chem Int Ed Engl. 2016;55:15846–15851. doi: 10.1002/anie.201608732. [DOI] [PubMed] [Google Scholar]
- 154.Takagi T., Naito Y., Uchiyama K., Okuda T., Suzuki T., Tsuboi H. Colonic insufflation with carbon monoxide gas inhibits the development of intestinal inflammation in rats. Med Gas Res. 2012;2:23. doi: 10.1186/2045-9912-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barpe D.R., Rosa D.D., Froehlich P.E. Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur J Pharm Sci. 2010;41:458–463. doi: 10.1016/j.ejps.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 156.Grandjean P. Paracelsus revisited: the dose concept in a complex world. Basic Clin Pharmacol Toxicol. 2016;119:126–132. doi: 10.1111/bcpt.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Christopher P.H., Lorenz M., Christoph S., Leo E.O. Where is the clinical breakthrough of heme oxygenase-1/carbon monoxide therapeutics?. Curr Pharm Des. 2018;24:2264–2282. doi: 10.2174/1381612824666180723161811. [DOI] [PubMed] [Google Scholar]
- 158.Soboleva T., Simons C.R., Arcidiacono A., Benninghoff A.D., Berreau L.M. Extracellular vs intracellular delivery of CO: does it matter for a stable, diffusible gasotransmitter?. J Med Chem. 2019;62:9990–9995. doi: 10.1021/acs.jmedchem.9b01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mann B.E. CO-releasing molecules: a personal view. Organometallics. 2012;31:5728–5735. [Google Scholar]
- 160.Yang X.X., Ke B.W., Lu W., Wang B.H. CO as a therapeutic agent: discovery and delivery forms. Chin J Nat Med. 2020;18:284–295. doi: 10.1016/S1875-5364(20)30036-4. [DOI] [PubMed] [Google Scholar]
- 161.Hopper C.P., Wollborn J. Delivery of carbon monoxide via halogenated ether anesthetics. Nitric Oxide. 2019;89:93–95. doi: 10.1016/j.niox.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 162.Ji X., Wang B. Strategies toward organic carbon monoxide prodrugs. Acc Chem Res. 2018;51:1377–1385. doi: 10.1021/acs.accounts.8b00019. [DOI] [PubMed] [Google Scholar]
- 163.Ji X., Pan Z., Yu B., De La Cruz L.K., Zheng Y., Ke B. Click and release: bioorthogonal approaches to "on-demand" activation of prodrugs. Chem Soc Rev. 2019;48:1077–1094. doi: 10.1039/c8cs00395e. [DOI] [PubMed] [Google Scholar]
- 164.Soboleva T., Berreau L.M. 3-Hydroxyflavones and 3-hydroxy-4-oxoquinolines as carbon monoxide-releasing molecules. Molecules. 2019;24:1252. doi: 10.3390/molecules24071252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Anderson S.N., Richards J.M., Esquer H.J., Benninghoff A.D., Arif A.M., Berreau L.M. A structurally-tunable 3-hydroxyflavone motif for visible light-induced carbon monoxide-releasing molecules (CORMs) ChemistryOpen. 2015;4:590–594. doi: 10.1002/open.201500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Popova M., Soboleva T., Benninghoff A.D., Berreau L.M. CO sense and release flavonols: progress toward the development of an analyte replacement PhotoCORM for use in living cells. ACS Omega. 2020;5:10021–10033. doi: 10.1021/acsomega.0c00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peng P., Wang C., Shi Z., Johns V.K., Ma L., Oyer J. Visible-light activatable organic CO-releasing molecules (PhotoCORMs) that simultaneously generate fluorophores. Org Biomol Chem. 2013;11:6671–6674. doi: 10.1039/c3ob41385c. [DOI] [PubMed] [Google Scholar]
- 168.Palao E., Slanina T., Muchova L., Solomek T., Vitek L., Klan P. Transition-metal-free CO-releasing BODIPY derivatives activatable by visible to NIR light as promising bioactive molecules. J Am Chem Soc. 2016;138:126–133. doi: 10.1021/jacs.5b10800. [DOI] [PubMed] [Google Scholar]
- 169.Antony L.A., Slanina T., Sebej P., Solomek T., Klan P. Fluorescein analogue xanthene-9-carboxylic acid: a transition-metal-free CO releasing molecule activated by green light. Org Lett. 2013;15:4552–4555. doi: 10.1021/ol4021089. [DOI] [PubMed] [Google Scholar]
- 170.Pan Z., Ji X., Chittavong V., Li W., Ji K., Zhu M. Organic CO-prodrugs: structure co-release rate relationship studies. Chem Eur J. 2017;23:9838–9845. doi: 10.1002/chem.201700936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Soboleva T., Esquer H.J., Anderson S.N., Berreau L.M., Benninghoff A.D. Mitochondrial-localized versus cytosolic intracellular CO-releasing organic PhotoCORMs: evaluation of co effects using bioenergetics. ACS Chem Biol. 2018;13:2220–2228. doi: 10.1021/acschembio.8b00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Popova M., Soboleva T., Ayad S., Benninghoff A.D., Berreau L.M. Visible-light-activated quinolone carbon-monoxide-releasing molecule: prodrug and albumin-assisted delivery enables anticancer and potent anti-inflammatory effects. J Am Chem Soc. 2018;140:9721–9729. doi: 10.1021/jacs.8b06011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ji X., de La Cruz L.K., Pan Z., Chittavong V., Wang B. pH-sensitive metal-free carbon monoxide prodrugs with tunable and predictable release rates. Chem Commun. 2017;53:9628–9631. doi: 10.1039/c7cc04866a. [DOI] [PubMed] [Google Scholar]
- 174.Ji X., Pan Z., Li C., Kang T., De La Cruz L.K.C., Yang L. Esterase-sensitive and pH-controlled carbon monoxide prodrugs for treating systemic inflammation. J Med Chem. 2019;62:3163–3168. doi: 10.1021/acs.jmedchem.9b00073. [DOI] [PubMed] [Google Scholar]
- 175.Kueh J.T.B., Stanley N.J., Hewitt R.J., Woods L.M., Larsen L., Harrison J.C. Norborn-2-en-7-ones as physiologically-triggered carbon monoxide-releasing prodrugs. Chem Sci. 2017;8:5454–5459. doi: 10.1039/c7sc01647f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pan Z., Zhang J., Ji K., Chittavong V., Ji X., Wang B. Organic CO Prodrugs activated by endogenous ROS. Org Lett. 2018;20:8–11. doi: 10.1021/acs.orglett.7b02775. [DOI] [PubMed] [Google Scholar]
- 177.Soboleva T., Esquer H.J., Benninghoff A.D., Berreau L.M. Sense and release: a thiol-responsive flavonol-based photonically driven carbon monoxide-releasing molecule that operates via a multiple-input AND logic gate. J Am Chem Soc. 2017;139:9435–9438. doi: 10.1021/jacs.7b04077. [DOI] [PubMed] [Google Scholar]
- 178.Ji X., Ji K., Chittavong V., Yu B., Pan Z., Wang B. An esterase-activated click and release approach to metal-free CO-prodrugs. Chem Commun. 2017;53:8296–8299. doi: 10.1039/c7cc03832a. [DOI] [PubMed] [Google Scholar]
- 179.Poloukhtine A., Popik V.V. Mechanism of the cyclopropenone decarbonylation reaction. A density functional theory and transient spectroscopy study. J Phys Chem A. 2006;110:1749–1757. doi: 10.1021/jp0563641. [DOI] [PubMed] [Google Scholar]
- 180.De La Cruz K., Benoit S.L., Pan Z., Maier R., Ji X., Wang B. Click, release, and fluoresce: a chemical strategy for a cascade prodrug system for codelivery of carbon monoxide, a drug payload, and a fluorescent reporter. Org Lett. 2018;20:897–900. doi: 10.1021/acs.orglett.7b03348. [DOI] [PubMed] [Google Scholar]
- 181.Bakalarz D., Surmiak M., Yang X., Wójcik D., Korbut E., Śliwowski Z. Organic carbon monoxide prodrug, BW-CO-111 in protection against chemically induced gastric mucosal damage. Acta Pharm Sin B. 2021;11:456–475. doi: 10.1016/j.apsb.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.CO gas 100–500 ppm, Clinical trials: NCT00122694, NCT02425579, NCT01214187, NCT01523548. Available from https://www.clinicaltrials.gov.
- 183.Bathoorn E., Slebos D.J., Postma D.S., Koeter G.H., van Oosterhout A.J., van der Toorn M. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J. 2007;30:1131–1137. doi: 10.1183/09031936.00163206. [DOI] [PubMed] [Google Scholar]
- 184.Mitchell L.A., Channell M.M., Royer C.M., Ryter S.W., Choi A.M., McDonald J.D. Evaluation of inhaled carbon monoxide as an anti-inflammatory therapy in a nonhuman primate model of lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L891–L897. doi: 10.1152/ajplung.00366.2009. [DOI] [PubMed] [Google Scholar]
- 185.Mayr F.B., Spiel A., Leitner J., Marsik C., Germann P., Ullrich R. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2005;171:354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 186.HBI-002 CO drink; HBI-137 CO infusion; NCT03926819; Developed by Hillhurst Biopharmaceuticals. Available from clinicaltrials.gov and www.hillhurstbio.com.
- 187.Motterlini R., Clark J.E., Foresti R., Sarathchandra P., Mann B.E., Green C.J. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 188.Kretschmer R., Gessner G., Gorls H., Heinemann S.H., Westerhausen M. Dicarbonyl-bis(cysteamine)iron(II): a light induced carbon monoxide releasing molecule based on iron (CORM-S1) J Inorg Biochem. 2011;105:6–9. doi: 10.1016/j.jinorgbio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 189.Niesel J., Pinto A., Peindy N'Dongo H.W., Merz K., Ott I., Gust R., Schatzschneider U. Photoinduced CO release, cellular uptake and cytotoxicity of a tris(pyrazolyl)methane (TPM) manganese tricarbonyl complex. Chem Commun (Camb) 2008;15:1798–1800. doi: 10.1039/b719075a. [DOI] [PubMed] [Google Scholar]
- 190.Jackson C.S., Schmitt S., Dou Q.P., Kodanko J.J. Synthesis, characterization, and reactivity of the stable iron carbonyl complex [Fe(CO)(N4Py)](ClO4)2: photoactivated carbon monoxide release, growth inhibitory activity, and peptide ligation. Inorg Chem. 2011;50:5336–5338. doi: 10.1021/ic200676s. [DOI] [PubMed] [Google Scholar]
- 191.Brückmann N.E., Wahl M., Reiß G.J., Kohns M., Wätjen W., Kunz P.C. Polymer conjugates of photoinducible co-releasing molecules. Eur J Inorg Chem. 2011;2011:4571–4577. [Google Scholar]
- 192.Jimenez J., Chakraborty I., Carrington S.J., Mascharak P.K. Light-triggered CO delivery by a water-soluble and biocompatible manganese photoCORM. Dalton Trans. 2016;45:13204–13213. doi: 10.1039/c6dt01358a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Motterlini R., Sawle P., Hammad J., Bains S., Alberto R., Foresti R. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005;19:284–286. doi: 10.1096/fj.04-2169fje. [DOI] [PubMed] [Google Scholar]
- 194.Sitnikov N.S., Li Y., Zhang D., Yard B., Schmalz H.G. Design, synthesis, and functional evaluation of CO-releasing molecules triggered by Penicillin G amidase as a model protease. Angew Chem Int Ed Engl. 2015;54:12314–12318. doi: 10.1002/anie.201502445. [DOI] [PubMed] [Google Scholar]
- 195.Romanski S., Kraus B., Schatzschneider U., Neudorfl J.M., Amslinger S., Schmalz H.G. Acyloxybutadiene iron tricarbonyl complexes as enzyme-triggered CO-releasing molecules (ET-CORMs) Angew Chem Int Ed Engl. 2011;50:2392–2396. doi: 10.1002/anie.201006598. [DOI] [PubMed] [Google Scholar]
- 196.Motterlini R., Nikam A., Manin S., Ollivier A., Wilson J.L., Djouadi S. HYCO-3, a dual CO-releaser/Nrf2 activator, reduces tissue inflammation in mice challenged with lipopolysaccharide. Redox Biology. 2019;20:334–348. doi: 10.1016/j.redox.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Feng W., Feng S., Feng G. CO release with ratiometric fluorescence changes: a promising visible-light-triggered metal-free CO-releasing molecule. Chem Commun. 2019;55:8987–8990. doi: 10.1039/c9cc04026a. [DOI] [PubMed] [Google Scholar]
- 198.Wang D., Viennois E., Ji K., Damera K., Draganov A. A click-and-release approach to CO prodrugs. Chem Commun. 2014;50:15890–15893. doi: 10.1039/c4cc07748b. [DOI] [PubMed] [Google Scholar]
- 199.Ji X., Ji K., Chittavong V., Aghoghovbia R.E., Zhu M., Wang B. Click and fluoresce: a bioorthogonally activated smart probe for wash-free fluorescent labeling of biomolecules. J Org Chem. 2017;82:1471–1476. doi: 10.1021/acs.joc.6b02654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ji X., Ji K., Chittavong V., Yu B., Pan Z., Wang B. An esterase-activated click and release approach to metal-free CO-prodrugs. Chem Commun. 2017;53:8296–8299. doi: 10.1039/c7cc03832a. [DOI] [PubMed] [Google Scholar]
- 201.Southam H.M., Smith T.W., Lyon R.L., Liao C., Trevitt C.R., Middlemiss L.A. A thiol-reactive Ru(II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO-releasing molecule-3. Redox Biol. 2018;18:114–123. doi: 10.1016/j.redox.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wareham L.K., McLean S., Begg R., Rana N., Ali S., Kendall J.J. The broad-spectrum antimicrobial potential of [Mn(CO)4(S2CNMe(CH2CO2H))], a water-soluble co-releasing molecule (CORM-401): intracellular accumulation, transcriptomic and statistical analyses, and membrane polarization. Antioxid Redox Signal. 2018;28:1286–1308. doi: 10.1089/ars.2017.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Seixas J.D., Chaves-Ferreira M., Montes-Grajales D., Goncalves A.M., Marques A.R., Saraiva L.M. An N-acetyl cysteine ruthenium tricarbonyl conjugate enables simultaneous release of CO and ablation of reactive oxygen species. Chemistry. 2015;21:14708–14712. doi: 10.1002/chem.201502474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tavares A.F., Teixeira M., Romão C.C., Seixas J.D., Nobre L.S., Saraiva L.M. Reactive oxygen species mediate bactericidal killing elicited by carbon monoxide-releasing molecules. J Biol Chem. 2011;286:26708–26717. doi: 10.1074/jbc.M111.255752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tavares A.F., Nobre L.S., Saraiva L.M. A role for reactive oxygen species in the antibacterial properties of carbon monoxide-releasing molecules. FEMS Microbiol Lett. 2012;336:1–10. doi: 10.1111/j.1574-6968.2012.02633.x. [DOI] [PubMed] [Google Scholar]
- 206.Nielsen V.G. Ruthenium, not carbon monoxide, inhibits the procoagulant activity of Atheris, Echis, and Pseudonaja venoms. Int J Mol Sci. 2020;21:2970. doi: 10.3390/ijms21082970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nielsen V.G., Wagner M.T., Frank N. Mechanisms responsible for the anticoagulant properties of neurotoxic dendroaspis venoms: a viscoelastic analysis. Int J Mol Sci. 2020;21:2082. doi: 10.3390/ijms21062082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nielsen V.G. The anticoagulant effect of Apis mellifera phospholipase A2 is inhibited by CORM-2 via a carbon monoxide-independent mechanism. J Thromb Thrombolysis. 2020;49:100–107. doi: 10.1007/s11239-019-01980-0. [DOI] [PubMed] [Google Scholar]
- 209.Gessner G., Sahoo N., Swain S.M., Hirth G., Schönherr R., Mede R. CO-independent modification of K+ channels by tricarbonyldichlororuthenium(II) dimer (CORM-2) Eur J Pharmacol. 2017;815:33–41. doi: 10.1016/j.ejphar.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.McLean S., Mann B.E., Poole R.K. Sulfite species enhance carbon monoxide release from CO-releasing molecules: implications for the deoxymyoglobin assay of activity. Anal Biochem. 2012;427:36–40. doi: 10.1016/j.ab.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 211.Santos-Silva T., Mukhopadhyay A., Seixas J.D., Bernardes G.J., Romao C.C., Romao M.J. Towards improved therapeutic CORMs: understanding the reactivity of CORM-3 with proteins. Curr Med Chem. 2011;18:3361–3366. doi: 10.2174/092986711796504583. [DOI] [PubMed] [Google Scholar]
- 212.Santos M.F., Seixas J.D., Coelho A.C., Mukhopadhyay A., Reis P.M., Romão M.J. New insights into the chemistry of fac-[Ru(CO)3]2+ fragments in biologically relevant conditions: the CO releasing activity of [Ru(CO)3Cl2(1,3-thiazole)], and the X-ray crystal structure of its adduct with lysozyme. J Inorg Biochem. 2012;117:285–291. doi: 10.1016/j.jinorgbio.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 213.Santos-Silva T., Mukhopadhyay A., Seixas J.D., Bernardes G.J., Romão C.C., Romão M.J. CORM-3 reactivity toward proteins: the crystal structure of a Ru(II) dicarbonyl-lysozyme complex. J Am Chem Soc. 2011;133:1192–1195. doi: 10.1021/ja108820s. [DOI] [PubMed] [Google Scholar]
- 214.Pontillo N., Ferraro G., Messori L., Tamasi G., Merlino A. Ru-based CO releasing molecules with azole ligands: interaction with proteins and the CO release mechanism disclosed by X-ray crystallography. Dalton Trans. 2017;46:9621–9629. doi: 10.1039/c7dt01991b. [DOI] [PubMed] [Google Scholar]
- 215.Seixas J.D., Santos M.F., Mukhopadhyay A., Coelho A.C., Reis P.M., Veiros L.F. A contribution to the rational design of Ru(CO)3Cl2L complexes for in vivo delivery of CO. Dalton Trans. 2015;44:5058–5075. doi: 10.1039/c4dt02966f. [DOI] [PubMed] [Google Scholar]
- 216.Aki T., Unuma K., Noritake K., Hirayama N., Funakoshi T., Uemura K. Formation of high molecular weight p62 by CORM-3. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Aki T., Unuma K., Noritake K., Kurahashi H., Funakoshi T., Uemura K. Interaction of carbon monoxide-releasing ruthenium carbonyl CORM-3 with plasma fibronectin. Toxicol Vitro. 2018;50:201–209. doi: 10.1016/j.tiv.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 218.Yuan Z., Yang X., De La Cruz L.K., Wang B. Nitro reduction-based fluorescent probes for carbon monoxide require reactivity involving a ruthenium carbonyl moiety. Chem Commun (Camb) 2020;56:2190–2193. doi: 10.1039/c9cc08296d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Johnson T.R., Mann B.E., Teasdale I.P., Adams H., Foresti R., Green C.J. Metal carbonyls as pharmaceuticals? [Ru(CO)3Cl(glycinate)], a CO-releasing molecule with an extensive aqueous solution chemistry. Dalton Trans. 2007;2007:1500–1508. doi: 10.1039/b613629j. [DOI] [PubMed] [Google Scholar]
- 220.Takeuchi A., Vesely A., Rucker J., Sommer L.Z., Tesler J., Lavine E. A simple "new" method to accelerate clearance of carbon monoxide. Am J Respir Crit Care Med. 2000;161:1816–1819. doi: 10.1164/ajrccm.161.6.9907038. [DOI] [PubMed] [Google Scholar]
- 221.Misra H., Lickliter J., Kazo F., Abuchowski A. PEGylated carboxyhemoglobin bovine (SANGUINATE): results of a phase I clinical trial. Artif Organs. 2014;38:702–707. doi: 10.1111/aor.12341. [DOI] [PubMed] [Google Scholar]
- 222.Shephard R.J. The influence of small doses of carbon monoxide upon heart rate. Respiration. 1972;29:516–521. doi: 10.1159/000192921. [DOI] [PubMed] [Google Scholar]
- 223.Plumb J.O.M., Kumar S., Otto J., Schmidt W., Richards T., Montgomery H.E. Replicating measurements of total hemoglobin mass (tHb-mass) within a single day: precision of measurement; feasibility and safety of using oxygen to expedite carbon monoxide clearance. Physiol Rep. 2018;6 doi: 10.14814/phy2.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Levin B.C., Paabo M., Gurman J.L., Harris S.E., Braun E. Toxicological interactions between carbon monoxide and carbon dioxide. Toxicology. 1987;47:135–164. doi: 10.1016/0300-483x(87)90165-x. [DOI] [PubMed] [Google Scholar]
- 225.Garvican L.A., Burge C.M., Cox A.J., Clark S.A., Martin D.T., Gore C.J. Carbon monoxide uptake kinetics of arterial, venous and capillary blood during CO rebreathing. Exp Physiol. 2010;95:1156–1166. doi: 10.1113/expphysiol.2010.054031. [DOI] [PubMed] [Google Scholar]
- 226.Bruce E.N., Bruce M.C. A multicompartment model of carboxyhemoglobin and carboxymyoglobin responses to inhalation of carbon monoxide. J Appl Physiol. 2003;95:1235–1247. doi: 10.1152/japplphysiol.00217.2003. [DOI] [PubMed] [Google Scholar]
- 227.Cronenberger C., Mould D.R., Roethig H.J., Sarkar M. Population pharmacokinetic analysis of carboxyhaemoglobin concentrations in adult cigarette smokers. Br J Clin Pharmacol. 2008;65:30–39. doi: 10.1111/j.1365-2125.2007.02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jay G.D., McKindley D.S. Alterations in pharmacokinetics of carboxyhemoglobin produced by oxygen under pressure. Undersea Hyperb Med. 1997;24:165–173. [PubMed] [Google Scholar]
- 229.Kharasch E.D., Powers K.M., Artru A.A. Comparison of Amsorb, sodalime, and Baralyme degradation of volatile anesthetics and formation of carbon monoxide and compound a in swine in vivo. Anesthesiology. 2002;96:173–182. doi: 10.1097/00000542-200201000-00031. [DOI] [PubMed] [Google Scholar]
- 230.Zevin S., Saunders S., Gourlay S.G., Jacob P., Benowitz N.L. Cardiovascular effects of carbon monoxide and cigarette smoking. J Am Coll Cardiol. 2001;38:1633–1638. doi: 10.1016/s0735-1097(01)01616-3. [DOI] [PubMed] [Google Scholar]
- 231.Shinomiya K., Orimoto C., Shinomiya T. Experimental exposure to carbon monoxide in rats (I)—relation between the degree of carboxyhemoglobin saturation and the amount of carbon monoxide in the organ tissues of rats. Nihon Hoigaku Zasshi. 1994;48:19–25. [PubMed] [Google Scholar]
- 232.Tyuma I., Ueda Y., Imaizumi K., Kosaka H. Prediction of the carbonmonoxyhemoglobin levels during and after carbon monoxide exposures in various animal species. Jpn J Physiol. 1981;31:131–143. doi: 10.2170/jjphysiol.31.131. [DOI] [PubMed] [Google Scholar]
- 233.Wu W.X. Factors influencing carboxyhemoglobin kinetics in inhalation lung injury. Chin J Intern Med. 1992;31:689–691. [PubMed] [Google Scholar]
- 234.Wazawa H., Yamamoto K., Yamamoto Y., Matsumoto H., Fukui Y. Elimination of CO from the body: an experimental study on the rabbit. Nihon Hoigaku Zasshi. 1996;50:258–262. [PubMed] [Google Scholar]
- 235.Aberg A.M., Hultin M., Abrahamsson P., Larsson J.E. Circulatory effects and kinetics following acute administration of carbon monoxide in a porcine model. Life Sci. 2004;75:1029–1039. doi: 10.1016/j.lfs.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 236.Rao A.V. Mind in ayurveda. Indian J Psychiatry. 2002;44:201–211. [PMC free article] [PubMed] [Google Scholar]
- 237.Sneader W. John Wiley and Sons; Hoboken, NJ: 2005. Drug discovery: a history; pp. 74–87. [Google Scholar]