Abstract
Nutritional diarrhea and subsequent performance degradation in weaned piglets are major challenges for the pig industry. Bile acids (BA) can be added to the diet as emulsifiers. This experiment was conducted to investigate the effects of chenodeoxycholic acid (CDCA), a major primary BA, on growth performance, serum metabolic profiles and gut health in weaned piglets. A total of 72 healthy weaned piglets were randomly assigned to the control (CON) and the CDCA groups, which were feed a basal diet and the basal diet supplemented with 200 mg/kg CDCA for 30 d, respectively. Our results demonstrated that CDCA significantly increased final BW and average daily gain (ADG), decreased feed-to-gain (F:G) ratio and tended to reduce diarrhea incidence. In addition, CDCA increased the villus height-to-crypt depth (V:C) ratio, elevated goblet cell numbers and the expression of tight junction proteins, suggesting the enhancement of intestinal barrier function. As an emulsifier, CDCA increased jejunal lipase activity and the mRNA expression of pancreatic lipases. CDCA supplementation also altered the serum metabolic profiles, including increasing the levels of indole 3-acetic acid, N′-formylkynurenine and theobromine that were beneficial for gut health. Moreover, the relative abundance of 2 beneficial gut bacteria, Prevotella9 and PrevotellaceaeTCG-001, were increased, whereas the relative abundance of a harmful bacteria, Dorea, was decreased in the gut of weaned piglets supplemented with CDCA. Importantly, the altered serum metabolic profiles showed a strong correlation with the changed gut bacteria. In conclusion, CDCA improved the growth performance of weaned piglets by improving intestinal morphology and barrier function, and enhancing lipid digestion, accompanied by alterations of serum metabolic profiles, and changes in relative abundance of certain gut bacteria.
Keywords: Chenodeoxycholic acid, Weaned piglet, Growth performance, Gut health, Serum metabolite, Gut microbiota
1. Introduction
In modern feeding conditions, weaning is an important time for pig feeding. Weaning stress, caused by sudden changes in psychology, diet and housing environment, often leads to villus atrophy, crypt hyperplasia, reduced gastrointestinal volume and decreased appetite (Lalles et al., 2007; Gresse et al., 2017). Furthermore, the gastrointestinal tract of postweaning piglets is still immature, the secretion of bile salts and endogenous digestive enzymes, such as lipase, are not enough to digest and absorb the fat in the feed, leading to early weaning syndrome, usually manifested by diarrhea, slow growth and reduced immunity (Gresse et al., 2017; Campbell et al., 2013; Mclamb et al., 2013). Therefore, it is of great importance to improve the digestibility and intestinal barrier function of weaned piglets.
BA derive from cholesterol in liver and are amphiphilic molecules consisting of hydrophilic and hydrophobic groups. This amphoteric molecular structure makes BA have strong surface activity and become natural emulsifiers which can effectively emulsify lipids into lipid droplets or chylomicron. Emulsification of lipids can increase the contact area between lipase and lipids, thereby facilitating the digestion and absorption of dietary lipids and liposoluble vitamins in the small intestine (Monte et al., 2009; Holm et al., 2013). Moreover, a previous study has reported that supplementation of diets with 60 and 80 mg/kg of BA extracted from pig bile (70.67% hyodeoxycholic acid [HDCA], 19.61% chenodeoxycholic acid [CDCA] and 8.00% hyocholic acid) can effectively enhance the intestinal lipase activity and lipoprotein lipase in broilers (Lai et al., 2018). Besides promoting lipid digestion, mounting evidence has revealed that BA act as signaling molecules in vivo to regulate the body metabolism, including BA synthesis, lipid metabolism, energy metabolism, liver insulin resistance and glucose homeostasis (Martinot et al., 2017; Molinaro et al., 2018). BA can be classified into primary and secondary BA. Primary BA are synthesized from cholesterol in the liver and are biotransformed by gut microbiota into secondary BA (Winston and Theriot, 2019; Jia et al., 2018). Therefore, gut microbiota plays important roles in BA metabolism. CDCA is a main natural primary BA in human and animal bile (Joyce and Gahan, 2016). In recent years, CDCA has been widely used to treat cholestasis and biliary tract diseases, such as gallstones, cholangitis, primary biliary cirrhosis and other cholestatic disorders (Li et al., 2016). Additionally, emerging evidence suggests that CDCA, as an endogenous farnesoid X receptor (FXR) agonist, can up-regulate BA transporter expression to protect hepatocytes and ameliorate insulin resistance by modulation of adipokines and inflammatory regulators (Staudinger et al., 2013; Shihabudeen et al., 2015). In addition, our previous results also showed that CDCA could alleviate lipopolysaccharide (LPS)-induced impairment of intestinal epithelial barrier function in both IPEC-J2 cells and mouse models, indicating the beneficial effects on gut barrier function (Song et al., 2019).
Although there are some studies on the effects of exogenous BA (e.g. desiccated pig bile, taurocholic acid [TCA] and sodium cholate) on poultry and aquatic animals (Ge et al., 2019; Peng et al., 2019; Maisonnier et al., 2003), studies on exogenous BA supplementation in the feed of weaned piglets are still rare. Therefore, in the present study, we hypothesized that exogenous CDCA supplementation might improve intestinal barrier function and lipid digestion, thereby improving growth performance. In addition, as a bile acid (BA), CDCA supplementation may cause changes in BA metabolic profiles in weaned piglets, which are closely related to the gut microbiota. Therefore, we tested the BA metabolic profiles and gut microbiota composition in weaned piglets. Moreover, we also measured the serum metabolic profiles to analyze the effect of CDCA supplementation on the whole-body metabolism of weaned piglets, and performed a correlation analysis on the serum metabolic profiles and gut microbiota.
2. Materials and methods
2.1. Animals management and dietary treatment
The animal protocol was approved by the permission number of SYXK (Guangdong) 2014-0136, and the animal care protocol for this experiment followed the guidelines of The Animal Ethics Committee of South China Agricultural University.
A total of 72 healthy 28-d-old weaned piglets (weaned at 21 d of age, Duroc × Landrace × Yorkshire) with an average initial BW of 7.02 ± 0.08 kg (mean ± SEM) were raised on a local commercial farm (Jixiang Animal Husbandry Co., Ltd., Yangjiang, Guangdong Province). The piglets were randomly allotted to 2 groups according to the gender and BW. Each group had 4 pens (replications), with each pen containing 9 piglets (male:female = 4:5). The CON group was fed a basal diet (Table 1) and the CDCA group was fed the basal diet supplemented with 200 mg/kg CDCA (Hangzhou Baoji Biotechnology Co. Ltd., Hangzhou, China). In addition, the basal diet contained 75 mg/kg of chlortetracycline and 50 mg/kg of quinocetone. The experimental period was 30 d. Feed and water were offered ad libitum throughout the period.
Table 1.
Composition and nutrient levels of the basal diet (%).
| Ingredient | Content | Nutrient level | Content |
|---|---|---|---|
| Corn | 61.50 | Digestible energy, MJ/kg | 14.56 |
| Dehulled soybean meal | 20.50 | Crude protein | 18.50 |
| Expanded soybean meal | 5.00 | Crude fiber | 1.90 |
| 50% oil powder | 3.00 | Ca | 0.73 |
| Fish meal | 1.50 | Available P | 0.52 |
| Glucose | 2.50 | Lys | 1.3 |
| Lactose | 3.00 | Cys | 0.78 |
| Vitamin-mineral premix1 | 1.00 | Thr | 0.84 |
| Calcium hydrogen phosphate | 1.00 | Trp | 0.25 |
| Stone powder | 1.00 | ||
| Total | 100.00 |
Premix provided per kilogram of diet: vitamin A, 12,000 IU; vitamin D3, 2,400 IU; vitamin E, 60 IU; vitamin K3, 2.0 mg; vitamin B1, 2.0 mg; vitamin B6, 12.0 mg; vitamin B2, 10 mg; vitamin B12, 0.03 mg; niacin, 40 mg; D-pantothenic acid, 20.0 mg; biotin, 0.30 mg; folic acid, 2.1 mg; choline chloride, 500.0 mg; Cu, 25.0 mg; Fe, 150.0 mg; Mn, 30.0 mg; Zn, 150.0 mg; I, 0.5 mg; Se, 0.5 mg; Co, 0.3 mg and 4.0 mg of ethoxyquin.
2.2. Growth performance index, diarrhea incidence analysis and sample collection
At the beginning and end of the trial, all weaned piglets were weighed individually. Feed was replenished throughout the day and the feed intakes of piglets in each pen were recorded daily during the experiment. Average daily feed intake (ADFI), BW, ADG and F:G ratio were calculated for the piglets in each pen. Additionally, piglets were monitored for signs of diarrhea twice a day at 08:00 and 18:00 throughout the experimental period. The severity of diarrhea was assessed by fecal consistency: 0 solid, 1 semi-solid, 2 semi-liquid, 3 liquid. Diarrhea was defined as excretion of feces at level 2 or 3 for 2 continuous days (Feng et al., 2018). Diarrhea incidence was calculated according to the formula:
| Diarrhea incidence (%) = [Total number of pigs with diarrhea in each pen × Diarrhea days/(9 piglets × 30 d)] × 100 |
At the end of the trial (d 30), 3 female and 3 male piglets per group with similar BW to average BW (at least one piglet per pen) were selected for sample collection. The piglets were euthanized after 12 h fasting and blood was collected via jugular vein puncture before euthanasia. Next, the blood was incubated at room temperature for 30 min and centrifuged at 1,000 × g for 15 min. The obtained serum was stored at −20 °C until further analysis. Segments of the middle jejunum and distal ileum were collected, rinsed with 0.9% sodium chloride solution, and then divided into 2 sections. One section was fixed in 4% formaldehyde for subsequent morphological analysis and the other section was stored at −80 °C for further analysis. The pancreas were also collected and stored at −80 °C for further mRNA analysis. Moreover, 6 fecal samples in each group were collected from the rectum near the anus of the just slaughtered piglets and stored at −80 °C for 16S rRNA gene sequences.
2.3. Intestinal morphology and histology analysis
The fixed intestinal segments were dehydrated and then embedded in paraffin. Cross sections of a thickness of 5 μm were sectioned and stained with haematoxylin and eosin (H&E) as previously described (Wang et al., 2017). Images were obtained using a light microscopy. Five microscopic fields per sample were selected to measure villus height and crypt depth using an image analyzer (Image Pro Plus 6.0, Media Cybernetics Bethesda, MD, USA), and villus height-to-crypt depth (V:C) ratio was calculated. The identification of goblet cells in the jejunum and ileum was performed by using periodic acid-schiff (PAS) stain as previously described (McClemens et al., 2013).
2.4. Measurement of serum diamine oxidase (DAO)
Serum DAO was detected by using ELISA kits specific for pigs (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
2.5. Western blot (WB) analysis
Expression of tight junction proteins of jejunum was determined by WB analysis, which was conducted as previously described (Meng et al., 2017). Primary antibodies used include anti-zonula occludens 1 (ZO-1) (1:2,000, Santa Cruz, USA), anti-occludin (OCC) (1:500, Bioss, China), anti-claudin-1 (1:500, Bioss, China) and anti-tubulin antibodies (1:5,000, Bioss, China). Finally, chemiluminescence detection was performed with western ECL reagent on a FluorChem M Fluorescent Imaging System (ProteinSimple, Santa Clara, CA, USA). Densitometry analysis was perform using Image J software (NIH, Bethesda, MD, USA).
2.6. Real-time quantitative PCR analysis
RNA extraction and real-time PCR analyses were conducted as previously described (Meng et al., 2017). Briefly, total RNA was isolated from the pancreas by using an RNA extraction kit (Guangzhou Magen Biotechnology Co., Ltd., Guangdong, China). Reverse transcription was carried out with 2 μg RNA using M-MLV reverse transcriptase (Promega, Madison, WI, USA) to synthesize the cDNA. The pancreatic expression of lipase genes was determined by SYBR green real-time PCR using an Mx3005p instrument (Stratagene, La Jolla, CA, USA). The primer sequences are shown in Table 2. Beta-actin was used as an internal standard for normalization.
Table 2.
Gene primer sequence.
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| BSSL | CTCTCCTGGGCGAAAACGATTG | GTTGGCGTACAGGTTGACCG |
| PTL | CAGACTTCGCTTCCTAGTACTGCT | CAGACTTCGCTTCCTAGTACTGCT |
| PLA2 | TGCGAGACACACGACAACTG | ATTGTTTTTGCTGTTGCAGGTGAT |
| PLRP1 | TGCTTCCCGTGTCCAGATCAA | CCCATATCTCCAGCGAGCGAAA |
| β-actin | CCACGAAACTACCTTCAACTC | TGATCTCCTTCTGCATCCTGT |
BSSL = bile salt-stimulated lipase; PTL = pancreatic triglyceride lipase; PLA2 = pancreatic phospholipase A2; PLRP1 = pancreatic lipase-related protein 1.
2.7. Fecal BA measurement
Fecal BA quantification was conducted in the Shenzhen Health Time Gene Institute using an ultraperformance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). In brief, about 100 mg feces were ultrasonically extracted with 1 mL methanol. The obtained methanol extracts were centrifugated, filtered, and then quantified by the UPLC-MS/MS system as previously described (Fang et al., 2018). Individual BA were identified by comparing the retention time with the corresponding reference standard substance.
2.8. Serum metabolic profiles and serum BA levels
Serum metabolic profiles were analyzed by untargeted metabolomic profiling using LC-MS/MS in Wuhan Metware Biological Technology Co., Ltd. In our present study, 537 metabolites were identified in serum, including 11 individual BA. The fold-change of individual BA in serum after CDCA supplementation were calculated according to the raw peak intensity. Serum sample preparation and LC-MS/MS analysis conditions were conducted according to a previously described method (Sun et al., 2019). The identified metabolites were functionally annotated using the database of Kyoto Encyclopedia of Genes and Genomes (KEGG), and then the annotated metabolites were mapped to the KEGG pathway database.
2.9. DNA extraction and 16S rRNA microbiome analysis
The Illumina MiSeq sequencing protocol was based on a previously published method (Zheng et al., 2016). Briefly, the total DNA of fecal samples was extracted using an E.Z.N.A. stool DNA Kit (Omega) following the manufacturer instructions. The V3–V4 regions of the bacteria 16S rRNA gene were amplified by PCR using primers 341F (5′-CTCCTACGGGAGGCAGCAGT-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The purified products were mixed together and then sequenced using the Illumina Hiseq 2500 platform. Qiime software package was used to process the raw sequencing data. The resulting 16S rRNA gene sequences were compared for relative abundance of bacterial taxa. Operational taxonomic units (OTU) clustering was conducted by Uparse software and delimited at the cutoff of 97% using USEARCH v.10. The alpha and beta diversity analysis were performed using Qiime v1.8.0. The alpha diversity was analyzed by Shannon index and Chao1 index. The beta diversity was analyzed using principal component analysis (PCoA) and principal coordinate analysis on unweighted UniFrac distance matrices. Metastat analysis was used for comparative analysis of bacterial species differences between 2 groups. Finally, PICRUSt function was predicted based on the obtained data, and differential functions and metabolic pathways between 2 groups were obtained. Moreover, the Spearman correlation between serum differential metabolites and intestinal differential OTU was analyzed.
2.10. Statistical analysis
All data were presented as mean values ± the standard error of the mean (SEM). Statistical analyses of the data were tested by two-tailed Student's t-test using Sigmaplot 14.0 (Systat Software, Inc., San Jose, CA). Differences were considered statistiacally significant at P < 0.05. The serum differential metabolites between the CON and CDCA groups were determined by Variable Importance in Projection (VIP) ≥1, and fold change ≥ 2 or fold change ≤ 0.5. Correlation analysis between serum differential metabolites and intestinal differential OTU was investigated by Spearman correlation analysis.
3. Results
3.1. Dietary supplementation of CDCA improved the growth performance of weaned piglets
As shown in Table 3, after 30 d of feeding, compared with the CON group, CDCA supplementation significantly increased the final BW and ADG of weaned piglets (P < 0.05 and P < 0.01, respectively), with no significant effect on ADFI (P > 0.05). As a result, F:G ratio of the CDCA group was significantly lower than that of the CON group (P < 0.05). In addition, CDCA supplementation had a tendency to reduce the diarrhea incidence of weaned piglets (P = 0.082).
Table 3.
Growth performance and diarrhea incidence of weaned piglets1.
| Item | CON | CDCA | P-value |
|---|---|---|---|
| Initial BW (n = 36), kg | 7.00 ± 0.12 | 7.05 ± 0.12 | 0.868 |
| Final BW (n = 36), kg | 18.61 ± 0.47 | 20.25 ± 0.49 | 0.018 |
| ADG (n = 36), g | 386.38 ± 13.14 | 439.41 ± 13.94 | 0.007 |
| ADFI (n = 4), g | 617.54 ± 39.05 | 685.70 ± 34.94 | 0.241 |
| F:G (n = 4) | 1.53 ± 0.02 | 1.48 ± 0.03 | 0.025 |
| Diarrhea incidence (n = 4), % | 13.29 ± 1.03 | 10.32 ± 0.99 | 0.082 |
CDCA = chenodeoxycholic acid; ADG = average daily gain; ADFI = average daily feed intake; F:G = feed-to-gain ratio.
Data are presented as the means ± SEM. The P-values were determined using two-tailed Student's t-test.
3.2. Dietary supplementation of CDCA enhanced intestinal barrier function of weaned piglets
As shown in Fig. 1A and B, compared with the CON group, CDCA supplementation improved the intestinal morphology, with an increase of V:C ratio of the jejunum and ileum (P < 0.05). In addition, PAS staining results showed that piglets supplemented with CDCA had more PAS-positive stained cells in the jejunum and ileum (P < 0.05, Fig. 1C and D). Furthermore, CDCA treatment significantly increased the expression of tight junction proteins, such as ZO-1, OCC and claudin-1 in jejunum (P < 0.05, Fig. 2A and B), thereby reducing intestinal permeability, manifested as the decreased serum DAO level (P < 0.05, Fig. 2C). The expression of tight junction proteins in the ileum was similar to that of the jejunum (Data not shown). These results suggested that CDCA supplementation enhanced intestinal barrier function of weaned piglets.
Fig. 1.
Effects of dietary chenodeoxycholic acid (CDCA) supplementation on the intestinal morphology and mucosa mucopolysaccharide of weaned piglets (n = 6). (A) The intestinal morphology of the jejunum and ileum (scale bar, 1,000 μm). (B) The villus height-to-crypt depth ratio in the jejunum and ileum. (C) The intestinal mucosa mucopolysaccharide of jejunum and ileum. Samples were stained with periodic acid-schiff (PAS) (scale bar, 200 μm). (D) Statistical diagram of the number of PAS positive cells in each villus. Samples were stained with H&E. ∗P < 0.05, versus the CON group.
Fig. 2.
Effects of dietary chenodeoxycholic acid (CDCA) supplementation on intestinal barrier function in weaned piglets. (A) Western blot analysis of zonula occludens 1 (ZO-1), occludin (OCC) and claudin-1 in jejunum of weaned piglets. Tubulin was used as the loading control. (B) Mean ± SEM of immunoblotting bands of ZO-1, OCC and Claudin-1 (n = 4). (C) Serum diamine oxidase (DAO) level (n = 6). ∗P < 0.05 versus the CON group.
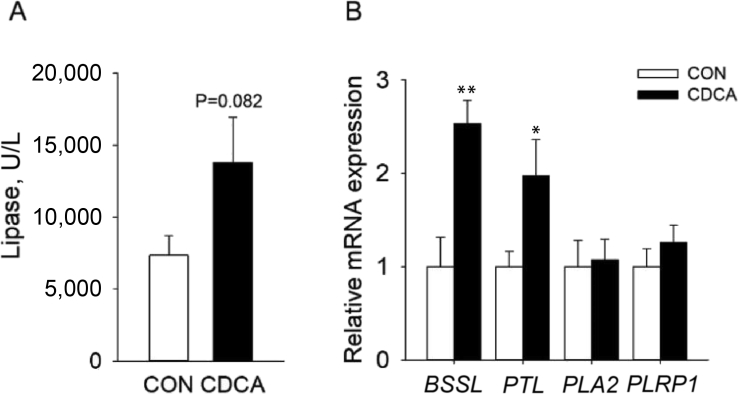
3.3. Effects of dietary CDCA supplementation on lipase activity and mRNA expression of pancreatic lipases in weaned piglets
As shown in Fig. 3A, dietary CDCA supplementation showed a tendency to increase jejunal lipase activity (P = 0.082). In addition, CDCA supplementation significantly increased the mRNA expression levels of bile salt-stimulated lipase (BSSL) and pancreatic triglyceride lipase (PTL) in the pancreas (P < 0.01 and P < 0.05, respectively), with no significant effect on the mRNA expression levels of pancreatic phospholipase A2 (PLA2) and pancreatic lipase-related protein 1 (PLRP1) (P > 0.05, Fig. 3B). These findings implied that CDCA might stimulate lipid digestion by upregulating pancreatic expression of lipase and intestinal activity of lipase.
Fig. 3.
Effects of dietary chenodeoxycholic acid (CDCA) supplementation on lipase activity and pancreatic lipase mRNA expression in weaned piglets (n = 6). (A) The lipase activity in jejunal contents. (B) The mRNA expression of pancreatic lipases such as bile salt-stimulated lipase (BSSL), pancreatic triglyceride lipase (PTL), pancreatic phospholipase A2 (PLA2) and pancreatic lipase-related protein 1 (PLRP1) in pancreas. ∗P < 0.05 and ∗∗P < 0.01 versus the CON group.
3.4. Effects of dietary CDCA supplementation on the BA metabolic profiles in feces and serum of weaned piglets
The BA metabolic profiles in feces and serum of weaned piglets were measured. As shown in Fig. 4A, lithocholic acid (LCA) and HDCA were the major BA whereas CDCA, glycochenodeoxycholic acid (GCDCA), deoxycholic acid (DCA), ursodeoxycholic acid (UDCA), glycolithocholic acid (GLCA), and TCA were the minor ones in feces. CDCA supplementation significantly increased the fecal HDCA, total BA and secondary BA (P < 0.05, Fig. 4A). In contrast, CDCA supplementation had no significant effect on serum BA metabolic profiles (P > 0.05, Fig. 4B).
Fig. 4.
Effects of dietary chenodeoxycholic acid (CDCA) supplementation on the bile acid (BA) metabolic profiles in feces and serum of weaned piglets (n = 6). (A) The BA metabolic profile in feces. (B) The BA metabolic profile in serum. GCDCA = glycochenodeoxycholic acid; TCA = taurocholic acid; LCA = lithocholic acid; DCA = deoxycholic acid; HDCA = hyodeoxycholic acid; UDCA = ursodeoxycholic acid; GLCA = glycolithocholic acid; GUDCA = glycoursodeoxycholic acid; GDCA = glycodeoxycholic acid; GCA = glycocholic acid; TCDCA = taurochenodeoxycholic acid; TDCA = taurodeoxycholic acid; TUDCA = tauroursodeoxycholic acid. ∗P < 0.05 versus the CON group.
3.5. Dietary supplementation of CDCA changed the serum metabolic profiles of weaned piglets
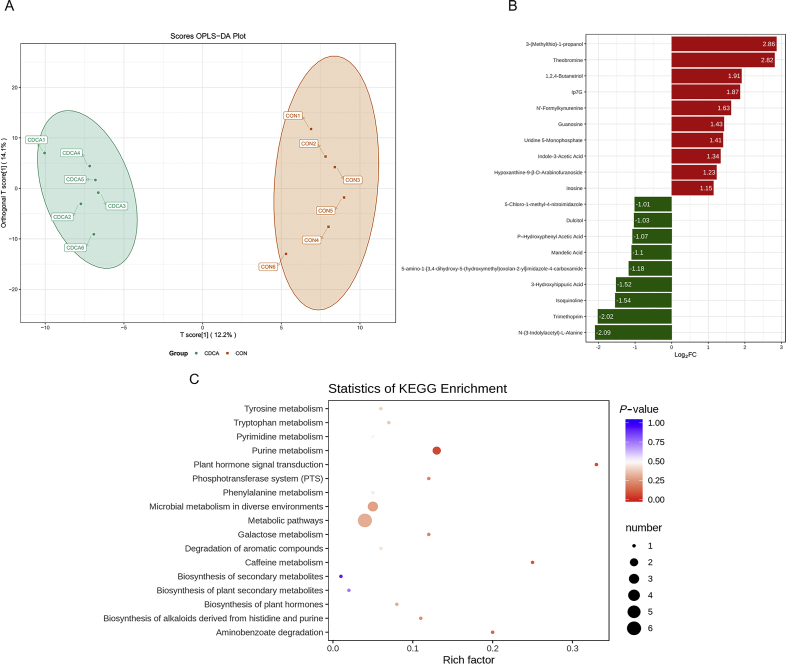
In this study, we used an untargeted UPLC-MS/MS approach for comprehensive serum metabolic profiles. A total of 537 metabolites were detected in serum samples, including amino acids, organic acids, indole and its derivatives, BA, free fatty acids, lipids, nucleotide, benzene and substituted derivatives, hormones and vitamins. The orthogonal partial least squares-discriminant analysis (OPLS-DA) scores plot is shown in Fig. 5A. There was a clear separation between the CON and the CDCA groups in the plot regarding serum samples, which indicated that the serum metabolic profiles of the CDCA group were distinct from that of the CON group. The 19 serum metabolites with the largest change are shown in Fig. 5B. Among them, the up-regulated serum metabolites included 3-(methylthio)-1-propanol, theobromine, 1,2,4-butanetrio, IP7G, N′-formylkynurenine, guanosine, uridine 5-monophosphate, indole-3-acetic acid, hypoxanthine-9-β-D-arabinofuranoside and inosine, and the down-regulated serum metabolites included 5-chloro-1-methyl-4-nitroimidazole, dulcitol, p–hydroxyphenyl acetic acid, mandelic acid, 5-amino-1-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]imidazole-4-carboxamide, 3-hydroxyhippuric acid, isoquinoline, trimethoprim and N-(3-indolylacetyl)-L-alanine.
Fig. 5.
Effects of dietary chenodeoxycholic acid (CDCA) supplementation on serum metabolic profiles of weaned piglets (n = 6). (A) Orthogonal partial least squares-discriminant analysis (OPLS-DA) scores plot for the CON and CDCA groups for serum metabolites. (B) The top 10 up-regulated (red-marked) and top 9 down-regulated (green-marked) serum differential metabolites. (C) Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment of serum differential metabolites. The color of the point was P-value, and the redder, the more significant enrichment. The size of the spot represented the number of different metabolites enriched.
By using KEGG pathway analysis, we attempted to identify the effects of CDCA on the metabolic pathways in weaned piglets. As shown in Figs. 5C and 17 metabolic pathways altered in response to CDCA supplementation, and purine metabolism, tryptophan metabolism and microbial metabolism in diverse environments were the predominantly involved metabolic pathways.
3.6. Effects of dietary supplementation of CDCA on gut microbiota of weaned piglets
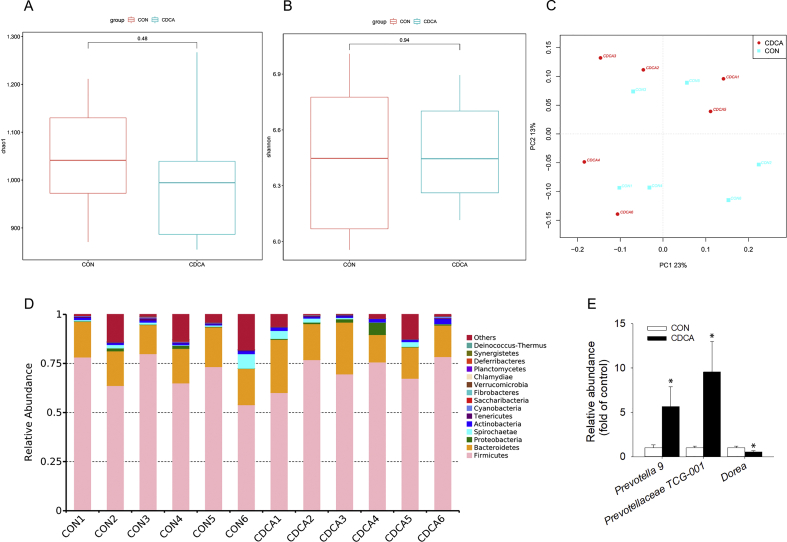
The fecal microbiota composition was evaluated by 16S rRNA gene sequencing to investigate the effects of CDCA supplementation on the gut bacteria of weaned piglets. Shannon index and Chao1 value were used as parameters of the alpha diversity of gut microbiota in this study. As shown in Fig. 6A and B, Shannon index and Chao1 value suggested that the CDCA supplementation had no significant differences in microbiota alpha diversity. Moreover, PCoA result showed that there was not a distinct clustering pattern between the CON and CDCA groups, which meant no significant differences in microbiota beta diversity (Fig. 6C). These results indicated that CDCA supplementation had no significant effect on the diversity of gut microbiota. Fig. 6D shows that the relative abundance of bacteria at the phylum level and the main phylum were Firmicutes and Bacteroidetes. Although CDCA treatment did not change the relative abundance of gut microbiota at the phylum level, it increased the relative abundance of Prevotella 9 and Prevotellaceae TCG-001, and decreased the relative abundance of Dorea at the genus level (P < 0.05, Fig. 6E).
Fig. 6.
Effects of dietary chenodeoxycholic acid (CDCA) supplementation on gut microbiota of weaned piglets (n = 6). (A) Chao1 value (A). (B) Shannon index of gut microbiota, (C) Principal component analysis (PCoA) scores plot for gut microbiota of the CON and CDCA groups. (D) Bar graphs represent relative abundance of gut bacteria at the phylum level. (E) The relative abundance of gut bacteria with significant differences in the genus level after CDCA supplementation. ∗P < 0.05 versus the CON group.
3.7. Correlations between serum metabolic profiles and gut bacteria
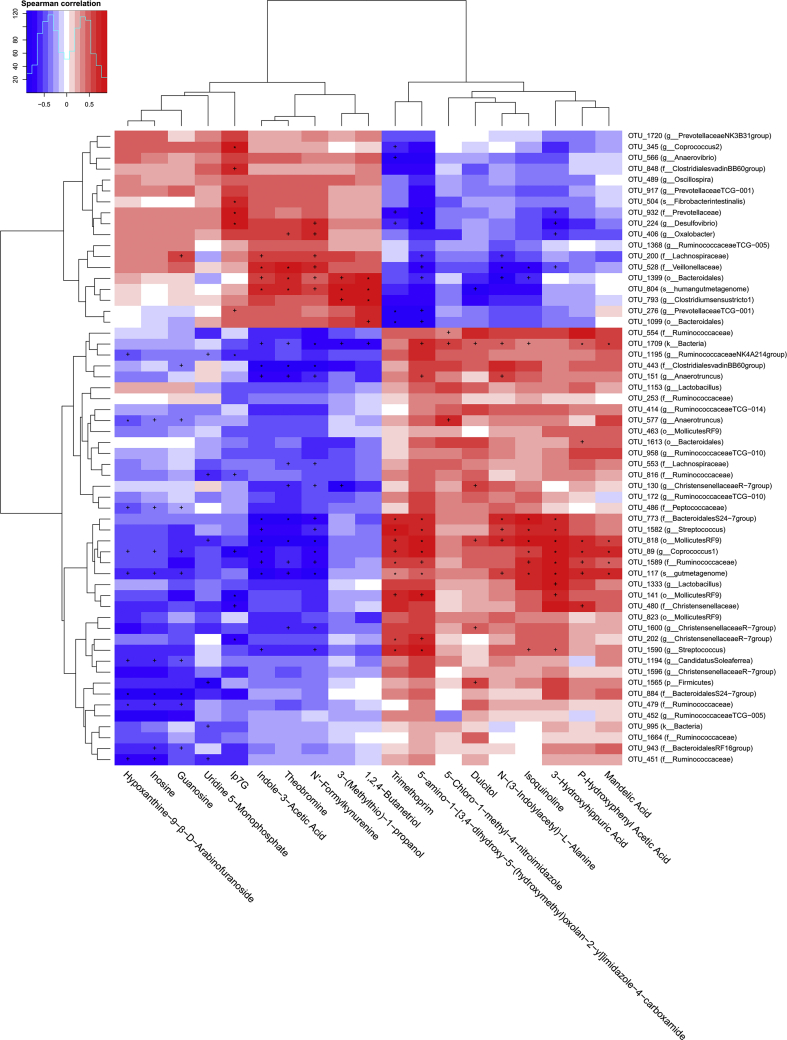
To explore the relationship between serum metabolic profiles and gut microbiota, Spearman correlation analysis was conducted between the serum differential metabolites and the 59 differential OTU. Red represented positive correlation and blue represented negative correlation. As shown in Fig. 7, the color of the up-regulated metabolites was just the contrary to that of the down-regulated serum metabolites, which meant the correlation between differential OTU and up-regulated metabolites was opposite to the correlation between the differential OTU and down-regulated serum metabolites. Moreover, we also found that each serum differential metabolite was significantly correlated with multiple differential OTU. These results indicated that these differential OTU were closely associated with, and might contribute to, the altered serum metabolic profiles in response to CDCA supplementation.
Fig. 7.
Correlations between serum differential metabolites and differential operational taxonomic units (OTU) of weaned piglets (n = 6). Red and blue cells represent positive and negative correlations, respectively. The significant correlations are indicated by “+” and “∗” (+P < 0.05 and ∗P < 0.01).
4. Discussion
In the present study, the results demonstrated that CDCA supplementation increased final BW and ADG, and reduced F:G ratio and diarrhea incidence of weaned piglets, indicating that CDCA could improve the growth performance of piglets. Consistent with our results, broilers fed diets supplemented with desiccated pig bile had higher final BW and ADG, and lower F:G ratio compared with the broilers fed diets without BA (Ge et al., 2019). However, a study conducted in mice showed that CDCA could attenuate high-fat diet-induced obesity and improve glucose tolerance via Takeda G-coupled protein receptor 5 (TGR5) (Chen et al., 2017). On the other hand, it has been shown that dietary supplementation of CDCA (60 mg/kg feed) for 11 d had no effect on ADG, ADFI and F:G ratio for weaned piglets (Meer et al., 2012). The differences between these previous results and our results may be due to the differences in experimental animals, the diet fed to animals, the different doses used and the duration of administration.
Intestinal tight junction protein plays an important role in maintaining the stability of intestinal epithelial barrier and permeability (Capaldo et al., 2017). A previous study has reported that CDCA supplementation reduced intestinal permeability of weaned piglets (Meer et al., 2012). Furthermore, our previous study also revealed that CDCA could attenuate LPS-induced decrease in expression of tight junction proteins and increase in intestinal permeability of mice (Song et al., 2019). Consistent with these results, our present research suggested that CDCA supplementation could increase the V:C ratio, increase the number of goblet cells which secrete mucopolysaccharides to form mucus layers critical for maintaining the integrity of the intestinal epithelium barrier, promote expression of tight junction proteins and reduce intestinal permeability. Taken together, these results provided strong evidence that CDCA supplementation could enhance intestinal barrier function, thus improving the growth performance of weaned piglets.
As an emulsifier, dietary supplementation of CDCA might affect lipid metabolism. Previous research has reported that supplementation of diets with BA extracted from pig bile can effectively enhance the intestinal lipase activity and lipoprotein lipase in broilers (Lai et al., 2018). Similarly, our results showed that CDCA supplementation tended to increase jejunal lipase activity and significantly increased the mRNA expression of BSSL and PTL in the pancreas. BSSL is the main lipid digestion enzyme of humans and animals in the neonatal stage (Andersson et al., 2011). Additionally, cholesterol is a BSSL specific substrate, and BSSL is the major pancreatic lipase for the digestion of cholesterol in both neonatal and adult stages (Li et al., 2007). PTL is the primary enzyme that hydrolyzes triglycerides in emulsified lipid particles after weaning and in adulthood. After weaning, PTL gene begins to be expressed at a low level, and then gradually increases to the adult level (Li et al., 2007; Hui and Howles, 2005). In our research, the increased mRNA expression of BSSL and PTL in the pancreas indicated the increased secretion of related pancreatic lipase in the digestive tract of weaned piglets, which was conducive to the digestion and absorption of cholesterol and triglyceride in the digestive tract, thereby improving the growth performance of weaned piglets.
As a BA, CDCA supplementation might affect the BA metabolic profiles, so we investigated the composition of BA in feces and serum. Our results indicated that CDCA supplementation had no effect on the individual BA levels in serum, but markedly increased the level of HDCA in feces, which in turn led to the increase of total BA and secondary BA levels. HDCA is a major secondary BA of pigs (Sehayek et al., 2001; Watanabe and Fujita, 2014). When CDCA reaches the distal small intestine, it can be converted to HDCA by gut microbiota (Jia et al., 2018). In the present study, the unchanged circulatory BA levels in serum suggested that dietary CDCA supplementation at the dose of 200 mg/kg feed did not affect intestinal BA transport and reabsorption in weaned piglets. Therefore, the CDCA supplemented in the diet might be converted into HDCA at the distal small intestine, and the excess HDCA was excreted in the feces, resulting in the increase of HDCA, total BA and secondary BA levels in the feces.
Growing evidence suggests that BA are intensively related to gut microbiota, and they interact with each other (Jia et al., 2018; Joyce and Gahan, 2016; Wahlstrom et al., 2016). Our results showed that CDCA supplementation had no effect on the alpha and beta diversity of gut microbiota, and the relative abundance of gut microbiota at the phylum level. However, at the genus level, CDCA supplementation increased the relative abundance of Prevotella 9 and Prevotellaceae TCG-001, and decreased the relative abundance of Dorea. Both Prevotella 9 and Prevotellaceae TCG-001 belong to the Prevotellaceae family. Prevotellaceae has been demonstrated to be associated with obesity, and the numbers of Prevotellaceae are highly enriched in obese individuals (Zhang et al., 2009). In addition, Prevotella has been shown to improve glucose metabolism in humans and mice, and is often considered to be a beneficial bacteria for health (Kovatcheva-Datchary et al., 2015). However, Dorea has been reported to be highly elevated in patients with irritable bowel syndrome (IBS) and positively correlated to IBS, so it is generally considered to be harmful for gut health (Rajilic-Stojanovic et al., 2011). However, whether the changes in the relative abundance of Prevotella 9, Prevotellaceae TCG-001 and Dorea in the gut in response to CDCA supplementation are related to the improvement of growth performance in weaned piglets requires further in-depth analysis.
An untargeted metabolomics was conducted to investigate the effect of CDCA supplementation on serum metabolic profiles. According to our OPLS-DA scores plot results, the serum metabolic profiles of the CDCA group were significantly different from that of the CON group and 19 serum differential metabolites were identified. Among them, we noticed 2 of the microbiota-derived tryptophan metabolites, indole-3-acetic acid and N′-formylkynurenine, were significantly increased after CDCA supplementation. KEGG pathway analysis also showed that CDCA could affect tryptophan metabolism. It has been suggested that tryptophan metabolites produced by gut bacteria, especially indole derivatives, played a critical role in the integrity of the intestinal barrier and mucosal protection from inflammation, which are beneficial for gut health (Postler and Ghosh, 2017; Marsland, 2016; Krishnan et al., 2018). In addition, a previous study has shown that a diet including 0.25% theobromine could inhibit the growth of harmful bacteria in rats, including Escherichia coli, Streptococcus spp., and Clostridium histolyticum-C (Martín-Peláez et al., 2017). In our study, the increased serum theobromine level, which was closely related to changes in gut microbiota, also meant the elevated level of theobromine in the intestine, so it might inhibit the growth of some harmful bacteria in the gut. Spearman correlation analysis between the serum differential metabolites showed that the elevated serum levels of indole-3-acetic acid and N′-formylkynurenine were negatively correlated with OTU_1590 (g_Streptococcus) and OTU_1582 (g_Streptococcus), and positively correlated with OTU_200 (f_Lachnospiraceae) and OTU_1399 (o-Bacteroidales). Streptococcus is a common harmful bacteria, whereas Lachnospiraceae and Bacteroidales are important short-chain fatty acids producing bacteria in the intestine of weaned piglets (Tao et al., 2016; Xu et al., 2018). Short-chain fatty acids are the main source of energy for intestinal mucosal epithelium, and they maintain intestinal homeostasis in many different aspects, such as inhibiting the growth of pathogenic bacteria, promoting the secretion of colonic mucus, and enhancing the function of the mucus barrier by lowering the pH value in the intestinal tract (Barcelo et al., 2000; Koh et al., 2016). These results indicated that the elevated serum levels of indole-3-acetic acid, N′-formylkynurenine and theobromine and the resulting changes in certain gut bacteria might be related to CDCA-improved growth performance of weaned piglets.
A correlation analysis was conducted between serum differential metabolites and intestinal differential OTU to further study the relationship between serum metabolic profiles and the gut microbiota. Interestingly, we found that the correlation between the differential metabolites that increased in serum and the differential OTU was just the opposite to the correlation between the differential metabolites that decreased in serum and the differential OTU. Moreover, each serum differential metabolite was significantly correlated with multiple serum differential OTU, which indicated that the changes in certain gut bacteria was contributing to the alterations of serum metabolic profiles.
Since piglets are usually weaned early, their gastrointestinal digestive systems are not yet mature. In addition, weaning stress usually causes damage to intestinal morphology, reduction of digestive and absorption capacity, and destruction of the intestinal barrier, which leads to the invasion of pathogenic and harmful micro-organisms, gut microbiota dysbiosis and the production of a large amount of toxic and harmful substances such as LPS. The results of this study showed that CDCA had positive effects on growth performance, intestinal morphology and intestinal epithelial barrier function, serum metabolome and certain gut bacteria. The potential underlying mechanism may be as follows. First, CDCA is a type of pig BA, and the supplementation of CDCA can make up for the deficiency of BA secretion in the intestinal tract of weaned piglets, so as to promote intestinal lipid digestion and improve the growth performance of piglets. Second, BA including CDCA can be secreted into the intestine as an antibacterial substance to directly affect the gut microbiota, or indirectly affect the gut microbiota as an inducer of genes encoding antibacterial peptides and lectins through the BA receptors (Kurdi et al., 2006; D'Aldebert et al., 2009). Then, the serum metabolites that were closely related to the intestinal gut microbiota were changed, leading to alternations of the serum metabolome. Third, it has been shown that CDCA can also act as a signaling molecule to alleviate the impairment of intestinal epithelial barrier function induced by LPS through the FXR-myosinlightchainkinase (MLCK) pathway, including increasing the expression of tight junction proteins and reducing intestinal permeability (Song et al., 2019). Therefore, we speculated that the improvement of intestinal barrier function and growth performance in weaned piglets might also be related to the role of CDCA as a signaling molecule.
5. Conclusions
In summary, our findings demonstrated that CDCA supplementation enhanced the growth performance of weaned piglets, and this beneficial effect could partly be attributed to the following: (1) CDCA supplementation improved the intestinal health, which was manifested in the improvement of intestinal morphology and intestinal barrier function; (2) CDCA supplementation enhanced the expression and activity of lipases; (3) CDCA supplementation increased the relative abundance of beneficial gut bacteria, Prevotella 9 and Prevotellaceae TCG-001, and decreased that of harmful bacteria, Dorea; and (4) CDCA supplementation also altered the serum metabolic profiles, including increasing indole3-acetic acid, N′-formylkynurenine and theobromine that were beneficial for gut health. These findings provided an experimental and theoretical basis for the application of CDCA in the production of piglets.
Author contributions
Min Song: Methodology, Investigation, Data curation, Writing-Original draft preparation. Fenglin Zhang, Lin Chen, Qiang Yang, Han Su, Xiaohua Yang, Haiwen He, Mingfa Ling, Jisong Zheng, Chen Duan, and Xumin Lai: Investigation, Validation. Mushui Pan, Xiaotong Zhu, Lina Wang, Ping Gao and Gang Shu: Writing - Reviewing and Editing. Qingyan Jiang: Conceptualization, Funding acquisition. Songbo Wang: Conceptualization, Writing - Reviewing and Editing, Funding acquisition.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31672508, 31972636, 31790411), the National Key Research and Development Program of China (2017YFD0500501), South China Agricultural University Doctoral Innovative Talent Cultivation Program (CX2019N006), Guangdong Key areas Research and Development Project (2019B020218001) and Guangdong Provincial Promotion Project on Preservation and Utilization of Local Breed of Livestock and Poultry.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Qingyan Jiang, Email: qyjiang@scau.edu.cn.
Songbo Wang, Email: songbowang@scau.edu.cn.
References
- Andersson E.L., Hernell O., Blackberg L., Falt H., Lindquist S. BSSL and PLRP2: key enzymes for lipid digestion in the newborn examined using the Caco-2 cell line. J Lipid Res. 2011;52:1949–1956. doi: 10.1194/jlr.M015685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo A., Claustre J., Moro F., Chayvialle J.A., Cuber J.C., Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.M., Crenshaw J.D., Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. 2013;4:45–48. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo C.T., Powell D.N., Kalman D. Layered defense: how mucus and tight junctions seal the intestinal barrier. J Mol Med (Berl) 2017;95:927–934. doi: 10.1007/s00109-017-1557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Yan L., Guo Z., Chen Y., Li M., Huang C. Chenodeoxycholic acid attenuates high-fat diet-induced obesity and hyperglycemia via the G protein-coupled bile acid receptor 1 and proliferator-activated receptor gamma pathway. Exp Ther Med. 2017;14:5305–5312. doi: 10.3892/etm.2017.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aldebert E., Mve M.J.B., Mergey M., Wendum D., Coilly A., Fouassier L. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136:1435–1443. doi: 10.1053/j.gastro.2008.12.040. [DOI] [PubMed] [Google Scholar]
- Fang W., Zhang L., Meng Q., Wu W., Lee Y.K., Xie J. Effects of dietary pectin on the profile and transport of intestinal bile acids in young pigs. J Anim Sci. 2018;96:4743–4754. doi: 10.1093/jas/sky327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Wu Y., Chen G., Fu S., Li B., Huang B. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a gpr109a-dependent manner. Cell Physiol Biochem. 2018;47:1617–1629. doi: 10.1159/000490981. [DOI] [PubMed] [Google Scholar]
- Ge X.K., Wang A.A., Ying Z.X., Zhang L.G., Su W.P., Cheng K. Effects of diets with different energy and bile acids levels on growth performance and lipid metabolism in broilers. Poultry Sci. 2019;98:887–895. doi: 10.3382/ps/pey434. [DOI] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Holm R., Mullertz A., Mu H. Bile salts and their importance for drug absorption. Int J Pharm. 2013;453:44–55. doi: 10.1016/j.ijpharm.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Hui D.Y., Howles P.N. Molecular mechanisms of cholesterol absorption and transport in the intestine. Semin Cell Dev Biol. 2005;16:183–192. doi: 10.1016/j.semcdb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Jia W., Xie G., Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce S.A., Gahan C.G.M. Bile acid modifications at the microbe-host interface: potential for nutraceutical and pharmaceutical interventions in host health. Annu Rev Food Sci Technol. 2016;7:313–333. doi: 10.1146/annurev-food-041715-033159. [DOI] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Kovatcheva-Datchary P., Nilsson A., Akrami R., Lee Y.S., De Vadder F., Arora T. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metabol. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Krishnan S., Ding Y., Saedi N., Choi M., Sridharan G.V., Sherr D.H. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23:1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdi P., Kawanishi K., Mizutani K., Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979–1986. doi: 10.1128/JB.188.5.1979-1986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai W., Huang W., Dong B., Cao A., Zhang W., Li J. Effects of dietary supplemental bile acids on performance, carcass characteristics, serum lipid metabolites and intestinal enzyme activities of broiler chickens. Poultry Sci. 2018;97:196–202. doi: 10.3382/ps/pex288. [DOI] [PubMed] [Google Scholar]
- Lalles J.P., Bosi P., Smidt H., Stokes C.R. Nutritional management of gut health in pigs around weaning. Proc Nutr Soc. 2007;66:260–268. doi: 10.1017/S0029665107005484. [DOI] [PubMed] [Google Scholar]
- Li X., Lindquist S., Lowe M., Noppa L., Hernell O. Bile salt-stimulated lipase and pancreatic lipase-related protein 2 are the dominating lipases in neonatal fat digestion in mice and rats. Pediatr Res. 2007;62:537–541. doi: 10.1203/PDR.0b013e3181559e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yuan Z., Liu R., Hassan H.M., Yang H., Sun R. UDCA and CDCA alleviate 17 alpha-ethinylestradiol-induced cholestasis through PKA-AMPK pathways in rats. Toxicol Appl Pharmacol. 2016;311:12–25. doi: 10.1016/j.taap.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Maisonnier S., Gomez J., Bree A., Berri C., Baeza E., Carre B. Effects of microflora status, dietary bile salts and guar gum on lipid digestibility, intestinal bile salts, and histomorphology in broiler chickens. Poultry Sci. 2003;82:805–814. doi: 10.1093/ps/82.5.805. [DOI] [PubMed] [Google Scholar]
- Marsland B.J. Regulating inflammation with microbial metabolites. Nat Med. 2016;22:581–583. doi: 10.1038/nm.4117. [DOI] [PubMed] [Google Scholar]
- Martín-Peláez S., Camps-Bossacoma M., Massot-Cladera M., Rigo-Adrover M., Franch À., Pérez-Cano F.J. Effect of cocoa's theobromine on intestinal microbiota of rats. Mol Nutr Food Res. 2017;61:1700238. doi: 10.1002/mnfr.201700238. [DOI] [PubMed] [Google Scholar]
- Martinot E., Sedes L., Baptissart M., Lobaccaro J.M., Caira F., Beaudoin C. Bile acids and their receptors. Mol Aspect Med. 2017;56:2–9. doi: 10.1016/j.mam.2017.01.006. [DOI] [PubMed] [Google Scholar]
- McClemens J., Kim J.J., Wang H., Mao Y.K., Collins M., Kunze W. Lactobacillus rhamnosus ingestion promotes innate host defense in an enteric parasitic infection. Clin Vaccine Immunol. 2013;20:818–826. doi: 10.1128/CVI.00047-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclamb B.L., Ga J., Oe L., Chad S., Ma J., Colette K.-L. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PloS One. 2013;8 doi: 10.1371/journal.pone.0059838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer Yvd, Gerrits W.J.J., Bosch Mvd, Holst J.J., Moreto M., Buurman W.A. Chenodeoxycholic acid reduces intestinal permeability in newly weaned piglets. J Anim Sci. 2012;90:302–304. doi: 10.2527/jas.50998. [DOI] [PubMed] [Google Scholar]
- Meng Y., Zhang J., Zhang F., Ai W., Zhu X., Shu G. Lauric acid stimulates mammary gland development of pubertal mice through activation of GPR84 and PI3K/akt signaling pathway. J Agric Food Chem. 2017;65:95–103. doi: 10.1021/acs.jafc.6b04878. [DOI] [PubMed] [Google Scholar]
- Molinaro A., Wahlstrom A., Marschall H.U. Role of bile acids in metabolic control. Trends Endocrinol Metabol. 2018;29:31–41. doi: 10.1016/j.tem.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Monte M.J., Marin J.J., Antelo A., Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15:804–816. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X.R., Feng L., Jiang W.D., Wu P., Liu Y., Jiang J. Supplementation exogenous bile acid improved growth and intestinal immune function associated with NF-kappaB and TOR signalling pathways in on-growing grass carp (Ctenopharyngodon idella): enhancement the effect of protein-sparing by dietary lipid. Fish Shellfish Immunol. 2019;92:552–569. doi: 10.1016/j.fsi.2019.06.047. [DOI] [PubMed] [Google Scholar]
- Postler T.S., Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metabol. 2017;26:110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajilic-Stojanovic M., Biagi E., Heilig H.G.H.J., Kajander K., Kekkonen R.A., Tims S. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Sehayek E., Ono J.G., Duncan E.M., Batta A.K., Salen G., Shefer S. Hyodeoxycholic acid efficiently suppresses atherosclerosis formation and plasma cholesterol levels in mice. J Lipid Res. 2001;42:1250–1256. [PubMed] [Google Scholar]
- Shihabudeen M.S., Roy D., James J., Thirumurugan K. Chenodeoxycholic acid, an endogenous FXR ligand alters adipokines and reverses insulin resistance. Mol Cell Endocrinol. 2015;414:19–28. doi: 10.1016/j.mce.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Song M., Ye J., Zhang F., Su H., Yang X., He H. Chenodeoxycholic acid (CDCA) protects against the lipopolysaccharide-induced impairment of the intestinal epithelial barrier function via the FXR-MLCK pathway. J Agric Food Chem. 2019;67:8868–8874. doi: 10.1021/acs.jafc.9b03173. [DOI] [PubMed] [Google Scholar]
- Staudinger J.L., Woody S., Sun M., Cui W. Nuclear-receptor-mediated regulation of drug- and bile-acid-transporter proteins in gut and liver. Drug Metab Rev. 2013;45:48–59. doi: 10.3109/03602532.2012.748793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Kang Q., Pan Y., Li N., Wang X., He Y. Serum metabolite profiling of familial adenomatous polyposis using ultra performance liquid chromatography and tandem mass spectrometry. Canc Biol Ther. 2019;20:1017–1028. doi: 10.1080/15384047.2019.1595277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J.H., Duan J.A., Jiang S., Guo J.M., Qian D.W. Simultaneous determination of six short-chain fatty acids in colonic contents of colitis mice after oral administration of polysaccharides from Chrysanthemum morifolium Ramat by gas chromatography with flame ionization detector. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1029–1030:88–94. doi: 10.1016/j.jchromb.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Wahlstrom A., Sayin S.I., Marschall H.U., Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metabol. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Wang S., Liang X., Yang Q., Fu X., Zhu M., Rodgers B.D. Resveratrol enhances brown adipocyte formation and function by activating AMP-activated protein kinase (AMPK) alpha 1 in mice fed high-fat diet. Mol Nutr Food Res. 2017;61:1600746. doi: 10.1002/mnfr.201600746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Fujita K. Dietary hyodeoxycholic acid exerts hypolipidemic effects by reducing farnesoid X receptor antagonist bile acids in mouse enterohepatic tissues. Lipids. 2014;49:963–973. doi: 10.1007/s11745-014-3947-y. [DOI] [PubMed] [Google Scholar]
- Winston J.A., Theriot C.M. Diversification of host bile acids by members of the gut microbiota. Gut Microb. 2019:1–14. doi: 10.1080/19490976.2019.1674124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.D., Cai L., Ni Y.S., Tian S.Y., Lu Y.Q., Wang L.N. Comparisons of effects on intestinal short-chain fatty acid concentration after exposure of two glycosidase inhibitors in mice. Biol Pharm Bull. 2018;41:1024–1033. doi: 10.1248/bpb.b17-00978. [DOI] [PubMed] [Google Scholar]
- Zhang H., DiBaise J.K., Zuccolo A., Kudrna D., Braidotti M., Yu Y. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci Unit States Am. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatr. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]