Abstract
The objective of this study was to investigate the effects of natural capsicum extract (NCE, containing 2% natural capsaicin, the rest is carrier) replacing chlortetracycline (CTC) on performance, digestive enzyme activities, antioxidant capacity, inflammatory cytokines, and gut health in weaned pigs. A total of 108 weaned pigs (Duroc × [Landrace × Yorkshire], initial body weight = 8.68 ± 1.34 kg; weaned on d 28) were randomly allotted into 3 treatments with 6 replicate pens per treatment (3 barrows and 3 gilts per pen). The treatments include a corn-soybean meal basal diet as a control group (CON), a CTC group (basal diet + CTC at 75 mg/kg), and a NCE group (basal diet + NEC at 80 mg/kg). Compared with CON and CTC, NCE had increased (P < 0.05) average daily gain in phase 2 (d 15 to 28) and overall (d 1 to 28), and higher (P < 0.05) apparent total tract digestibility of gross energy, dry matter, crude protein, and organic matter in phase 1 (d 1 to 14). These pigs also had increased (P < 0.05) pancrelipase activity in pancreas, α-amylase, lipase and protease activities in the jejunal mucosa, and lipase activity in the ileal mucosa on d 28. Moreover, NCE had increased (P < 0.05) the contents of growth hormone, β-endorphin, 5-hydroxytryptamine, total antioxidant capacity, total superoxide dismutase, catalase, and IL-10, as well as decreased (P < 0.05) contents of malondialdehyde, tumor nuclear factor-α, interferon-γ, and interleukin-6 in serum on d 28 compared with CON and CTC. NCE showed higher (P < 0.05) propionic acid, butyric acid and total volatile fatty acids (VFA) contents, and increased (P < 0.05) relative abundance of Faecalibacterium in colon, as well as higher (P < 0.05) propionic acid and total volatile fatty acids in cecum on d 28 compared with CON. In conclusion, NCE replacing CTC could enhance performance via improving digestive enzyme activities, antioxidant capacity, anti-inflammatory function, gut VFA composition and microbiota community in weaned pigs, and it could be used as a potential target for the development of feed additives.
Keywords: Antioxidant capacity, Chlortetracycline, Natural capsicum extract, Performance, Weaned pig
1. Introduction
After weaning, pigs are easily exposed to weaning stress due to their undeveloped immune and digestive systems and the sudden changes on environment and diet, which may result in a reduction of feed intake and nutrient absorption, as well as an increase of gastrointestinal disorder, diarrhea rate and mortality rate (Yin et al., 2014). Antibiotics, especially chlortetracycline (CTC), can be used to alleviate weaning stress and enhance performance via modulating the intestinal microbiota community in weaned pigs (Kiarie et al., 2018). However, the abuse of antibiotics in feed can increase the risk of bacterial cross-resistance and residue of antibiotics in animal products, which might be harmful for the human health (Cromwell, 2002). Previous studies in our laboratory had proved that organic acids (Long et al., 2018a), essential oil (Zeng et al., 2015), and Forsythia suspensa extract (Long et al., 2019) could be used as substitutes of antibiotics in weaned pigs. Among all these substitutes, natural plant extracts could not only inhibit the growth of harmful bacteria without producing drug resistance, but could also regulate and improve immune function of animals (Huang et al., 2012). Therefore, more and more natural plant extracts replacing antibiotics have been used to enhance performance and gut health in weaned pigs (Kong et al., 2007; Liu et al., 2011).
Natural capsicum extract (NCE), a kind of natural plant extract from Chili pepper (Capsicum annum), could be a potential feed additive on alleviating diarrhea and increasing performance in weaned pigs challenged with Escherichia coli (Liu et al., 2012, 2013). Moreover, NCE could also be beneficial for enhancing intestinal mucosa health and stimulating immune responses in weaned pigs (Liu et al., 2014). The main bioactive ingredient of NCE is capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide), which is a kind of vanillamide alkaloids and the main source for the spicy taste of red pepper. Previous studies have demonstrated that the capsaicin has anti-inflammatory (Spiller et al., 2008), antioxidant (Bogusz et al., 2018), and anti-tumor (Chapa-Oliver and Mejía-Teniente, 2016) functions, which had potential effects on performance and behavior in animals (Nmaju et al., 2018). Furthermore, the capsaicin from plant extracts also had positive effects on inhibiting the growth of harmful bacteria, and increasing the feed intake, secretion of digestive enzymes, and digestibility of crude protein (CP) in animals (Manzanilla et al., 2004; Srinivasan, 2016).
Although NCE has been used in animal production to a certain extent, researches on the efficiency of NCE as substitute of CTC in piglet diets are still relatively insufficient. Moreover, the mechanism of capsaicin in NCE on improving performance and intestinal health in weaned pigs still remains to be estimated. Based on previous studies, we hypothesized that NCE could replace CTC and enhance performance in weaned pigs via improving digestive enzyme activities, antioxidant capacity, inflammatory cytokines, gut volatile fatty acids (VFA) and microbiota community. Therefore, the objective of this study was to verify this hypothesis and provide a theoretical basis for the efficient use of NCE on alleviating weaning stress in weaned pigs.
2. Materials and methods
The animal procedures in this study were agreed by the Institutional Animal Care and Use Committee of China Agricultural University (Beijing, China).
2.1. Natural capsicum extract and chlortetracycline
The NCE (in powder form) was provided by Guangzhou Leader Bio-Technology Co., Ltd. (Guangzhou, China), and the active ingredient is natural capsaicin (2%), and the rest is carrier, which mainly contains fatty acids (stearic acid). The CTC was supplied by Beijing Tonglixike Agricultural and Technology Co. Ltd. (Beijing, China).
2.2. Experimental animals, design, and management
A total of 108 weaned pigs (Duroc × [Landrace × Yorkshire]; initial body weight = 8.68 ± 1.34 kg; weaned on d 28) were randomly allotted into 3 treatments with 6 replicate pens per treatment (3 barrows and 3 gilts per pen). The breed of barrow as the father of weaned pigs was Duroc, and the breed of crossed sows as the mother of weaned pigs were Landrace × Yorkshire. These 108 weaned pigs were selected from all the litters of 22 sows (about 220 pigs). The treatments include a corn-soybean meal basal diet as a control group (CON), a CTC group (basal diet + CTC at 75 mg/kg), and a NCE group (basal diet + NCE at 80 mg/kg). There are 2 phases in this experiment: phases 1 (d 1 to 14) and phase 2 (d 15 to 28). Chromic oxide was used as an indigestible marker. The nutrient level in basal diet met the requirements recommended by NRC (2012) (Table 1).
Table 1.
Ingredients and nutrient composition in basal diets (%, as-fed basis).
| Item | Phase 1, d 1 to 14 | Phase 2, d 15 to 28 |
|---|---|---|
| Ingredients | ||
| Soybean meal | 18.00 | 20.00 |
| Maize | 55.64 | 56.00 |
| Spray dried plasma protein | 4.00 | 0.00 |
| Extruded full-fat soybean | 12.00 | 10.00 |
| Whey powder | 2.00 | 5.50 |
| Fish meal | 2.00 | 4.00 |
| Soybean oil | 2.80 | 1.73 |
| Limestone | 1.12 | 0.80 |
| Dicalcium phosphate | 0.90 | 0.52 |
| Salt | 0.30 | 0.30 |
| Methionine | 0.10 | 0.06 |
| Lysine | 0.22 | 0.26 |
| Tryptophan | 0.00 | 0.01 |
| Threonine | 0.01 | 0.07 |
| Chromic oxide | 0.25 | 0.25 |
| Zinc oxide | 0.16 | 0.00 |
| Non-antibiotic premix1 | 0.50 | 0.50 |
| Nutrient levels2 | ||
| Gross energy, kcal/kg | 4,018 | 3,997 |
| Digestible energy, kcal/kg | 3,542 | 3,490 |
| Crude protein | 21.50 | 19.80 |
| Calcium | 0.80 | 0.71 |
| Gross phosphorus | 0.60 | 0.53 |
| Digestible phosphorus | 0.40 | 0.33 |
| Lysine | 1.55 | 1.43 |
| Methionine | 0.46 | 0.43 |
| Threonine | 0.91 | 0.89 |
| Tryptophan | 0.26 | 0.25 |
Non-antibiotic premix for per kilogram diet included: vitamin A, 12,000 IU; vitamin D3, 2,500 IU; vitamin E, 30 IU; vitamin K3, 30 mg; vitamin B12, 12 μg; riboflavin, 4 mg; pantothenic acid, 15 mg; nicotinic acid, 40 mg; choline chloride, 400 mg; folic acid, 0.7 mg; vitamin B1, 1.5 mg; vitamin B6, 3 mg; biotin, 0.1 mg; manganese, 40 mg; iron, 90 mg; zinc, 100 mg; copper, 8.8 mg; iodine, 0.35 mg; selenium, 0.3 mg.
The nutrient levels were analyzed values except for the digestible energy and digestible phosphorus.
The trial was conducted at the Feng Ning Swine Research Unit of China Agricultural University (Chengde, Hebei). In the nursery room, the temperature was kept at 24 ± 2 °C, and the relative humidity was maintained at 60% to 70%. Pigs were housed in 1.5 m × 1.5 m experimental pens with plastic slatted floors, duckbill drinkers, and adjustable feeders. Pigs were fed ad libitum in mash form and had free access to water. The diarrhea score was recorded daily and the diarrhea rate was calculated following the method recommended by Pan et al. (2016). On d 1, 14 and 28, pigs and feed were weighed to calculated the average daily gain (ADG), average daily feed intake (ADFI), and gain-to-feed ratio (G:F) respectively.
2.3. Sampling and chemical analysis
The fresh fecal samples (about 1 kg) were collected from each pen during 3 d in phase 1 (from d 12 to 14) and 2 (d 26 to 28) and were dried in a 65 °C oven for 72 h. The feed and dried fecal samples were ground and then passed through a 1-mm sieve for the measurement of dry matter (DM), CP, gross energy (GE), ash, organic matter (DM-ash), and chromium (Cr) following the methods of AOAC (2012). An automatic isoperibolic oxygen bomb calorimeter (Parr 1281, Automatic Energy Analyzer; Moline, IL) was used to measure GE. An atomic absorption spectrophotometer (Z-5000; Hitachi, Tokyo, Japan) was used to measure the content of Gr. The apparent total tract digestibility (ATTD) of nutrients was calculated following the formula by Long et al. (2018a) as follows:
| ATTDnutrient = 1- (Nutrientfeces × Crdiet)/(Nutrientdiet × Crfeces) |
The blood sample (8 mL) was collected from one pig weighing near the average body weight in each pen via the jugular vein into vacutainer (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ) on the morning of d 14 and 28. The blood samples were centrifuged at 3,000×g for 15 min at 4 °C to get the serum and kept at −20 °C until analysis. The immunoglobulins (IgG, IgM and IgA) and tumor necrosis factor alpha (TNF-α) in serum were measured by an ELISA kit (IgG, IgM, IgA and TNF-α quantitation kit; Bethyl Laboratories, Inc., TX, USA). The serum total cholesterol, total triglyceride, low density lipoprotein cholesterol (LDLC), high density lipoprotein cholesterol (HDLC) and urea contents were measured in an automatic biochemical analyzer (RA-1000, Bayer Corp., Tarrytown, NY, USA) following the instructions of the corresponding reagent kits (Zhongsheng Biochemical Co., Ltd., Beijing, China) (Long et al., 2018b). The serum growth hormone, 5-hydroxytryptamine (5-HT) and β-endorphin (β-EP) contents measured by using a radioimmunoassay (Sn-96513, Shanghai, China). The serum total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), catalase (CAT), total antioxidant capacity (T-AOC), malondialdehyde (MDA), Interferon-γ (IFN-γ), interleukin-6 (IL-6), and IL-10 levels were measured by a spectrophotometer (LengGuang SFZ1606017568, Shanghai, China) according to the instructions of the corresponding reagent kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China).
On d 28, 18 barrows (closest to the average body weight in each pen, n = 6) were selected and injected with pentobarbital sodium for complete anesthesia, then slaughtered for the collection of the pancreas, jejunum and ileum (about 15 cm) as 1/3 distal part. The pancreas and the jejunal and ileal mucosa were carefully scraped using a sterile glass slide, and the digesta samples in the middle part of cecum and colon were also collected. All these samples were placed in 2-mL cryotubes, frozen in liquid nitrogen immediately, and then stored at −80 °C until analysis. The corresponding kits (Nanjing Jiancheng Institute of Bioengineering, China) and UV-VIS spectrophotometer (UV1100, MAPADA, Shanghai, China) were used to determine protease, lipase and α-amylase activities in the jejunum and ileum mucosa, and the α-amylase, pancrelipase, trypsin and chymotrypsin activities in pancreas, the operation steps were carried out according to the kit instructions (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China).
These pigs were also used for the collection of about 5-cm fragment histological samples in duodenum, jejunum, and ileum as 1/3 distal part. These histological samples were immediately fixed in 50-mL tubes filled in with 10% neutral buffered formalin for about 48 h, and then washed, excised, dehydrated, and embedded in the paraffin wax. About 5 transverse sections of these histological tissues were sliced, installed on glass slides and dyed with eosin and hematoxylin. A calibrated 10-fold eyepiece graticule was used to measure about 15 orientated villi and their adjoining crypts randomly on each slice for the calculation of the average villus height, crypt depth and their ratio (villus height/crypt depth).
The digesta samples in cecum and colon were used for the measurement of VFA contents. Samples about 1.5 g of fresh digesta were taken into centrifuge tubes, mixed it with 1.5 mL sterile water, and centrifuged at 15,000 × g for 15 min at 4 °C. A gas chromatograph sample bottle was used to transfer the supernatant, mixed it with 200 μL of meta-phosphoric acid, placed in ice for 30 min, and then centrifuged at 15,000 × g for 15 min at 4 °C. The VFA contents in digesta samples were measured by a Hewlett Packard 5890 gas chromatograph (HP, Pennsylvania, USA) following the procedure mentioned by Long et al. (2018a) and Li et al. (2019).
The digesta samples in colon were also used for the measurement of microbiota community. The DNA kit (Omega bio-tek, orcross, USA) was used to extract bacterial genomic DNA from colon digesta samples. The 16S rRNA of the colon digesta samples was amplified and targeted to the V3–V4 region by using a pair of primers (338F ACTCCTACGGGAGGCAGCAG and 806R GGACTACHVGGGTWTCTAAT). PCR was performed by using the KAPA HiFiHotstart PCR kit with a high-fidelity enzyme. Then, the PCR products were extracted from 2% agarose gel, and purified by using the AxyPrep DNA gel extraction kit (Axygen Biosciences, California, USA) and QuantiFluor-st (Promega, USA). The purified PCR products were combined and paired terminal sequencing (2 × 250 bp) was performed on Illumina MiSeq platform according to the standard protocol. Using QIIME (version 1.17) to demultiplex and quality filter the original fastq files. According to the overlapping order, only the sequences with overlapping exceeding 10 bp were assembled. Using UPARSE to categorize the operational classification unit (OTU) with 97% similarity, and using UCHIME to identify and remove chimeric sequences. The RDP classifier at 80% confidence level to RDP OTU database (https://rdp.cme.msu.edu/) was used base on the analysis of the classification (Cole et al., 2014). The QIIME was used to analyze the α-diversity (Mahnert et al., 2015). According to the studies of Segata et al. (2011) and Ba et al. (2016), the linear discriminant analysis (LDA) effect size (LEfSe) tool was used to analyze LEfSe.
2.4. Statistical analysis
The mixed procedure of SAS (2008) (version 9.2) was used for statistical analysis. The individual pen was used as statistical unit for performance and diarrhea rate, and the individual pig was used as statistical unit for other data. The difference of diarrhea score and rate were analyzed by chi-square contingency test, and the statistical differences of other data except for the microbiota were analyzed via the Student-Neuman-Keul's Multiple Range Tests. Differences of microbiota abundance in colon digesta were analyzed by LEfSe using Kruskal–Wallis rank sum test. The LDA scores (threshold: ≥2) were used to express the effect size. A significant difference was defined as P ≤ 0.05, and a trend of difference was defined as 0.05 < P ≤ 0.10.
3. Results
3.1. Performance and diarrhea rate
As shown in Table 2, compared with CON and CTC, pigs fed NCE had increased (P < 0.05) average daily gain in phase 2 and overall (d 1 to 28). There was a tendency for an increase of G:F in pigs fed NCE compared with CON (P = 0.10) in overall. However, there were no differences of diarrhea rate and diarrhea score among the treatments.
Table 2.
Effects of natural capsicum extract on performance and diarrhea rate in weaned pigs.
| Item | CON | CTC | NCE | SEM | P-value |
|---|---|---|---|---|---|
| Initial body weight, kg | 8.68 | 8.68 | 8.68 | 0.01 | 0.85 |
| d 14 body weight, kg | 14.33 | 14.54 | 14.99 | 0.25 | 0.22 |
| d 28 body weight, kg | 20.67 | 21.14 | 22.50 | 0.26 | <0.01 |
| d 1 to 14 | |||||
| ADG, g | 404 | 418 | 451 | 17.89 | 0.33 |
| ADFI, g | 748 | 783 | 744 | 40.85 | 0.51 |
| G:F, g/g | 0.55 | 0.54 | 0.61 | 0.03 | 0.40 |
| Diarrhea score | 3.60 | 3.10 | 3.40 | 0.15 | 0.12 |
| Diarrhea rate, % | 2.86 | 0.71 | 2.38 | 0.82 | 0.84 |
| d 15 to 28 | |||||
| ADG, g | 453b | 472b | 536a | 12.23 | 0.01 |
| ADFI, g | 903 | 956 | 1028 | 38.18 | 0.13 |
| G:F, g/g | 0.50 | 0.49 | 0.52 | 0.02 | 0.27 |
| Diarrhea score | 3.50 | 3.40 | 3.60 | 0.14 | 0.60 |
| Diarrhea rate, % | 4.86 | 4.28 | 6.43 | 1.47 | 0.59 |
| d 1 to 28 | |||||
| ADG, g | 428b | 445b | 493a | 9.36 | 0.02 |
| ADFI, g | 825 | 870 | 886 | 32.46 | 0.16 |
| G:F, g/g | 0.52 | 0.51 | 0.56 | 0.01 | 0.10 |
| Diarrhea score | 3.55 | 3.25 | 3.50 | 0.10 | 0.12 |
| Diarrhea rate, % | 3.77 | 2.50 | 4.40 | 0.85 | 0.33 |
CON = control; CTC = chlortetracycline; NCE = natural capsicum extract; SEM = standard error of the mean; ADG = average daily gain; ADFI = average daily feed intake; G:F = gain-to-feed ratio.
a, b Within a row, different superscripts mean significant difference (P < 0.05).
3.2. The apparent total tract digestibility of nutrients
Pigs fed NCE had higher (P < 0.05) ATTD of CP, DM, OM and GE compared with CON and CTC in phase 1. The ATTD of CP in pigs fed NCE tended to be increased (P = 0.08) compared with CON in phase 2 (Table 3).
Table 3.
Effects of NCE on apparent total tract digestibility of nutrients in weaned pigs.
| Item | CON | CTC | NCE | SEM | P-value |
|---|---|---|---|---|---|
| d 14 | |||||
| DM | 0.82b | 0.83b | 0.89a | 0.01 | <0.01 |
| OM | 0.85b | 0.86b | 0.91a | 0.01 | <0.01 |
| CP | 0.75b | 0.77b | 0.85a | 0.01 | <0.01 |
| GE | 0.81b | 0.84b | 0.89a | 0.01 | <0.01 |
| d 28 | |||||
| DM | 0.77 | 0.75 | 0.78 | 0.01 | 0.21 |
| OM | 0.81 | 0.79 | 0.81 | 0.01 | 0.22 |
| CP | 0.66 | 0.63 | 0.69 | 0.02 | 0.08 |
| GE | 0.77 | 0.75 | 0.79 | 0.01 | 0.13 |
ATTD = apparent total tract digestibility; NCE = natural capsicum extract; CON = control; CTC = chlortetracycline; SEM = standard error of the mean; DM = dry matter; OM = organic matter; CP = crude protein; GE = gross energy.
a, b Within a row, different superscripts mean significant difference (P < 0.05).
3.3. Digestive enzyme activity
According to Table 4, compared with the CON and CTC, pigs fed NCE had increased (P < 0.05) pancrelipase activity, as well as α-amylase, lipase and protease activities in the jejunal mucosa on d 28. In the ileal mucosa, pigs fed NCE also showed increased (P < 0.05) lipase activity on d 28 compared with CON and CTC, these pigs also had increased (P < 0.05) entero–proteinase activity on d 28 compared with CON.
Table 4.
Effects of natural capsicum extract on pancreatic digestive enzyme activities in weaned pigs (U/mg).
| Item | CON | CTC | NCE | SEM | P-value |
|---|---|---|---|---|---|
| Pancreas | |||||
| α-amylase | 20.71 | 16.13 | 15.93 | 2.12 | 0.20 |
| Trypsin | 43.97 | 41.75 | 47.16 | 4.04 | 0.57 |
| Chymotrypsin | 4.45 | 4.60 | 6.12 | 0.62 | 0.15 |
| Pancrelipase | 151.72b | 180.51b | 250.79a | 12.34 | <0.01 |
| Jejunal mucosa | |||||
| α-amylase | 66.24b | 88.14b | 337.25a | 25.90 | <0.01 |
| Trypsin | 13.01b | 16.09b | 16.89a | 0.76 | 0.02 |
| Chymotrypsin | 5.56b | 6.59b | 7.19a | 0.22 | <0.01 |
| Ileal mucosa | |||||
| α-amylase | 43.18 | 38.66 | 34.65 | 6.10 | 0.64 |
| Lipase | 13.82b | 17.59a | 18.50a | 0.86 | 0.02 |
| Entero-proteinase | 5.95b | 6.62ab | 7.32a | 0.28 | 0.04 |
NCE = natural capsicum extract; CON = control; CTC = chlortetracycline; SEM = standard error of the mean.
a, b Within a row, different superscripts mean significant difference (P < 0.05).
3.4. Serum hormone and metabolites
As shown in Table 5, pigs fed NCE had higher (P < 0.05) concentrations of β-EP, growth hormone and 5-HT in serum on d 28 compared with CON. Pigs fed CTC had higher (P < 0.05) concentrations of β-EP and 5-HT in serum on d 28 compared with CON. There is no significant difference on total cholesterol, urea, total triglyceride, LDLC and HDLC concentrations on d 28 among treatments (P > 0.05).
Table 5.
Effects of natural capsicum extract on serum hormone and metabolites in weaned pigs.
| Item | CON | CTC | NCE | SEM | P-value |
|---|---|---|---|---|---|
| β-Endorphin, pg/mL | 109b | 177a | 191a | 5.19 | <0.01 |
| Growth hormone, ng/mL | 3.62b | 4.05b | 7.29a | 0.34 | <0.01 |
| Total cholesterol, mmol/L | 1.42 | 1.4 | 1.57 | 0.13 | 0.62 |
| Total triglyceride, mmol/L | 1.86 | 2.00 | 1.99 | 0.09 | 0.46 |
| Low density lipoprotein cholesterol, mmol/L | 0.73 | 0.65 | 0.75 | 0.07 | 0.55 |
| High density lipoprotein cholesterol, mmol/L | 0.46 | 0.47 | 0.52 | 0.04 | 0.57 |
| Urea, mmol/L | 2.49 | 2.88 | 2.46 | 0.40 | 0.50 |
| 5-hydroxytryptamine, ng/mL | 97.40c | 176.40b | 226.40a | 54.47 | <0.01 |
CON = control; CTC = chlortetracycline; NCE = natural capsicum extract; SEM = standard error of the mean.
a–c Within a row, different superscripts mean significant difference (P < 0.05).
3.5. Antioxidant status and inflammatory cytokines
As shown in Table 6, pigs fed NCE had higher (P < 0.05) T-SOD, CAT and T-AOC contents, as well as lower (P < 0.05) MDA level in serum on d 28 compared with CON. Pigs fed CTC and NCE had higher (P < 0.05) IL-10 content, and lower (P < 0.05) TNF-α, IFN-γ and IL-6 contents on d 28 compared with CON.
Table 6.
Effects of natural capsicum extract on serum antioxidant status and immune function in weaned pigs.
| Item | CON | CTC | NCE | SEM | P-value |
|---|---|---|---|---|---|
| Antioxidant status | |||||
| Total antioxidant capacity, U/mL | 9.69b | 10.45b | 14.80a | 0.34 | <0.01 |
| Total superoxide dismutase, U/mL | 56.65c | 64.65b | 80.59a | 0.84 | <0.01 |
| Catalase, U/mL | 34.89b | 37.23b | 63.70a | 3.01 | 0.02 |
| Glutathione peroxidase, U/mL | 830.49 | 799.68 | 799.44 | 13.91 | 0.25 |
| Malondialdehyde, nmol/mL | 6.74a | 5.71ab | 4.34c | 0.36 | 0.02 |
| Immune function | |||||
| Immunoglobulin A, g/L | 1.05 | 0.93 | 0.95 | 0.05 | 0.19 |
| Immunoglobulin G, g/L | 21.34 | 21.01 | 21.05 | 0.28 | 0.68 |
| Immunoglobulin M, g/L | 2.37 | 2.368 | 2.34 | 0.02 | 0.70 |
| Tumor nuclear factor-α, pg/mL | 93.93a | 72.87b | 35.03c | 3.29 | <0.01 |
| Interferon-γ, pg/mL | 71.70a | 64.39b | 23.74c | 2.42 | <0.01 |
| Interleukin-6, pg/mL | 182.79a | 169.82b | 82.87c | 7.11 | <0.01 |
| Interleukin-10, pg/mL | 12.06c | 13.53b | 18.94a | 0.80 | <0.01 |
CON = control; CTC = chlortetracycline; NCE = natural capsicum extract; SEM = standard error of the mean.
a-c Within a row, different superscripts mean significant difference (P < 0.05).
3.6. The VFA in cecum and colon
As shown in Table 7, pigs fed NCE had higher (P < 0.05) propionic acid, butyric acid and total VFA contents in colon on d 28 compared with CON and CTC. Pigs fed NCE had increased (P < 0.05) propionic acid and total VFA contents in cecum on d 28 compared with CON and CTC. Pigs supplemented with CTC had higher (P < 0.05) acetic acid content in cecum on d 28 compared with CON and NCE.
Table 7.
Effects of natural capsicum extract on volatile fatty acid contents in large intestine in weaned pigs (mg/g).
| Item | CON | CTC | NCE | SEM | P-value |
|---|---|---|---|---|---|
| Colon | |||||
| Acetic acid | 3.52 | 3.89 | 3.77 | 0.18 | 0.55 |
| Propionic acid | 2.25b | 2.27b | 2.81a | 0.11 | 0.03 |
| Butyric acid | 1.05b | 1.20b | 1.76a | 0.09 | <0.01 |
| Total volatile fatty acid | 7.11b | 7.62b | 8.79a | 0.24 | 0.02 |
| Cecum | |||||
| Acetic acid | 4.02b | 4.55a | 4.23b | 0.08 | 0.01 |
| Propionic acid | 2.69b | 2.72b | 7.31a | 0.17 | <0.01 |
| Butyric acid | 1.31 | 1.11 | 1.60 | 0.26 | 0.56 |
| Total volatile fatty acid | 8.26b | 8.54b | 13.40a | 0.35 | <0.01 |
CON = control; CTC = chlortetracycline; NCE = natural capsicum extract; SEM = standard error of the mean.
a-b Within a row, different superscripts mean significant difference (P < 0.05).
3.7. Gut morphology
As shown in Table 8, pigs supplemented with NCE tended to have higher (P = 0.08) villus height in ileum on d 28 compared with CON.
Table 8.
Effects of natural capsicum extract on intestinal morphology in weaned pigs.
| Item | CON | CTC | NCE | SEM | P-value |
|---|---|---|---|---|---|
| Duodenum | |||||
| Villus height, μm | 406.24 | 428.08 | 490.72 | 28.95 | 0.47 |
| Crypt depth, μm | 490.66 | 571.28 | 509.70 | 70.69 | 0.97 |
| Villus height:Crypt depth | 0.86 | 0.76 | 0.97 | 0.17 | 0.90 |
| Jejunum | |||||
| Villus height, μm | 546.01 | 471.09 | 485.28 | 81.06 | 0.83 |
| Crypt depth, μm | 437.63 | 370.54 | 343.55 | 28.35 | 0.39 |
| Villus height:Crypt depth | 1.39 | 1.30 | 1.42 | 0.14 | 0.33 |
| Ileum | |||||
| Villus height, μm | 338.61b | 374.78ab | 464.24a | 65.19 | 0.08 |
| Crypt depth, μm | 278.25 | 349.48 | 293.23 | 50.33 | 0.13 |
| Villus height:Crypt depth | 1.31 | 1.16 | 1.60 | 0.37 | 0.20 |
CON = control; CTC = chlortetracycline; NCE = natural capsicum extract; SEM = standard error of the mean.
a, b Within a row, different superscripts mean significant difference (P < 0.05).
3.8. Gut microbiota community in colon
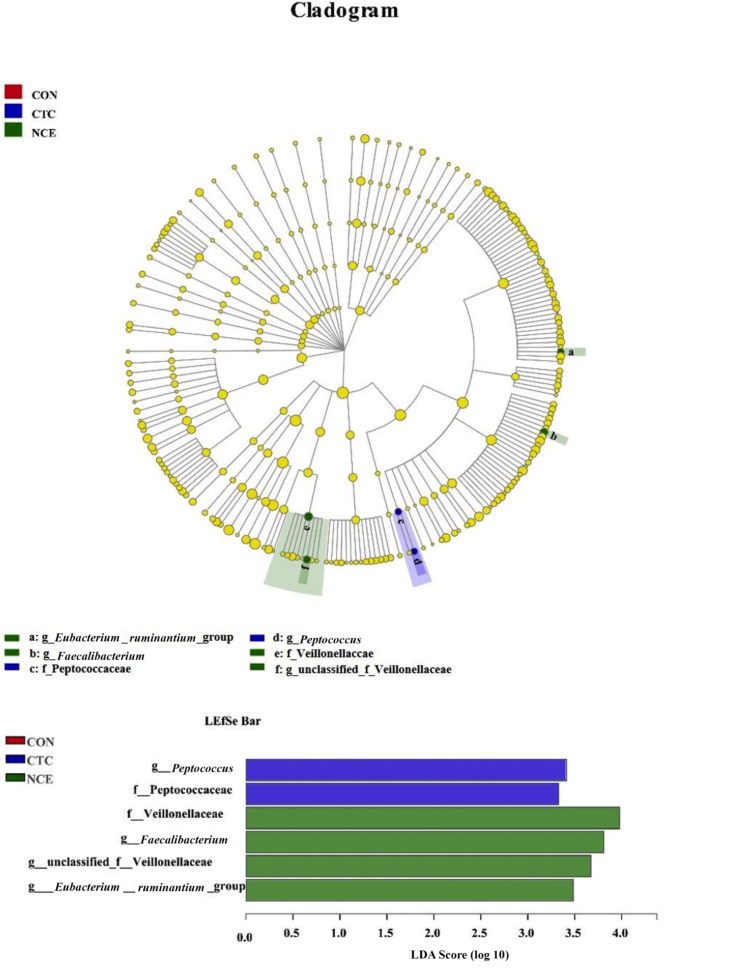
As shown in Table 9, there were no significant differences of α-diversity among treatments. As shown in Table 10 and Fig. 1, pigs fed NCE showed a numerical increase of relative abundance of Firmicutes (3.71% vs. 8.13%), and a numerical reduction of Bacteroidetes (91.38% vs. 88.91%) in phylum level in colon digesta on d 28 compared with CON. These pigs also had a numerical increase of relative abundance of Lactobacillus (38.37% vs. 23.62%), and a numerical reduction of the relative abundance of Streptococcus (6.93% vs. 20.57%) in genus level in colon digesta on d 28 compared with CON. Moreover, pigs fed NCE had increased (P < 0.05) relative abundance of Faecalibacterium, unclassified_f__Veillonellaceae, and [Eubacterium]_ruminantium_group, as well as tended to show increased (P = 0.09) relative abundance of Lachnospira in genus level in colon digesta on d 28 compared with CON. Pigs fed CTC showed increased (P < 0.05) relative abundance of Peptococcus in genus level in colon digesta on d 28 compared with CON.
Table 9.
Effects of natural capsicum extract on α-diversity in the OTU level on intestinal microbiota in colon digesta of weaned pigs.
| Item | CON | CTC | NCE | SEM | P-value |
|---|---|---|---|---|---|
| Sobs | 469.50 | 454.50 | 449.50 | 30.93 | 0.89 |
| Shannon | 3.64 | 3.96 | 3.94 | 0.20 | 0.49 |
| Simpson | 0.09 | 0.05 | 0.06 | 0.02 | 0.25 |
| Ace | 525.92 | 510.71 | 513.79 | 32.31 | 0.94 |
| Chao | 538.19 | 517.23 | 519.85 | 32.70 | 0.89 |
OTU = operational taxonomic unit; CON = control; CTC = chlortetracycline; NCE = natural capsicum extract; SEM = standard error of the mean.
Table 10.
Effects of natural capsicum extract on the relative abundance of intestinal microbiota in colon digesta of weaned pigs (%).
| Item | CON | CTC | NCE | SEM | P-value |
|---|---|---|---|---|---|
| Phylum level | |||||
| Firmicutes | 88.91 | 89.35 | 91.38 | 0.05 | 0.84 |
| Bacteroidetes | 8.13 | 6.84 | 3.71 | 0.04 | 0.58 |
| Actinobacteria | 0.97 | 1.54 | 1.04 | 0.01 | 0.23 |
| Proteobacteria | 0.27 | 1.22 | 2.75 | 0.02 | 0.67 |
| Tenericutes | 1.09 | 0.53 | 0.67 | 0.01 | 0.50 |
| Spirochaetae | 0.33 | 0.22 | 0.24 | 0.01 | 0.78 |
| Cyanobacteria | 0.10 | 0.17 | 0.13 | 0.01 | 0.44 |
| Genus level | |||||
| Lactobacillus | 23.62 | 37.99 | 38.37 | 0.15 | 0.43 |
| Streptococcus | 20.57 | 10.18 | 6.93 | 0.09 | 0.49 |
| Blautia | 3.17 | 5.55 | 6.12 | 0.02 | 0.17 |
| Ruminococcaceae_UCG-014 | 2.12 | 2.89 | 3.24 | 0.02 | 0.50 |
| Lachnospira | 0.22 | 0.90 | 1.60 | 0.01 | 0.09 |
| Faecalibacterium | 0.23b | 0.83b | 1.23a | 0.01 | 0.05 |
| Clostridium_sensu_stricto_1 | 1.98 | 3.01 | 0.86 | 0.02 | 0.13 |
| unclassified_f__Veillonellaceae | 0.03b | 0.31b | 0.83a | 0.01 | 0.01 |
| Ruminococcaceae_UCG-008 | 0.52 | 1.64 | 0.69 | 0.01 | 0.08 |
| Peptococcus | 0.12b | 0.40a | 0.33b | 0.01 | 0.05 |
| [Eubacterium]_ruminantium_group | 0.00 | 0.03 | 0.33 | 0.01 | 0.05 |
CON = control; CTC = chlortetracycline; NCE = natural capsicum extract; SEM = standard error of the mean.
a, b Within a row, different superscripts mean significant difference (P < 0.05).
Fig. 1.
Effects of natural capsicum extract (NCE) on the microbiota communities in colon digesta of weaned pigs. Key phylotypes of microbiota communities in colon digesta of weaned pigs fed basal diet as control (CON, noted in red), basal diet with chlortetracycline (CTC, noted in blue), and basal diet with NCE (noted in green) were shown using LEfSe analysis via the Kruskal–Wallis rank sum test to measure the significant difference. LDA scores (threshold: ≥2) was used to determine the effect size. LDA = linear discriminant analysis. The CON had no significant different microbiota community compared with CTC and NCE, so CON was not shown in the figure.
4. Discussion
After weaning, pigs are commonly faced with weaning stress from sudden changes on environment and diet, which may lead to severe diarrhea and poor performance. Previous studies have demonstrated that plant extracts could be used to alleviate negative impact from weaning stress and improve performance in weaned pigs via improving nutrient digestibility, anti-inflammatory function and antioxidant status (Kong et al., 2007; Huang et al., 2012; Liu et al., 2011; Long et al., 2019). Fan et al. (2017) has reported that dietary supplemented with chili meal (containing capsaicin) together with soybean oil could enhance the ADG in growing pigs, but its effect on weaned pigs and possible mechanism remained to be studied. In this study, the NCE, in which the main active compound was 2% capsaicin, could also increase ADG and tended to enhance G:F compared with CON and CTC, indicating NCE could potentially replace CTC on alleviating weaning stress and improve performance in weaned pigs. The possible mechanism of this finding might be that the capsaicin in plant extracts could promote gastric motility, prolong the residence time of feed in the stomach, improve the digestibility of protein, and thus enhance growth performance in weaned pigs (Manzanilla et al., 2004, 2009). Moreover, the capsaicin in NCE could also help increase intestinal permeability by binding to vanilloid receptor-1 like proteins (Isoda et al., 2001) and improve the digestibility of amino acids (Jamroz et al., 2003), which benefited the absorption and utilization of nutrients in weaned pigs. Furthermore, the capsaicin in NCE could also improve the integrity and tight junction of small intestine, which might help improve gut immunity and health status in pigs after weaning (Liu et al., 2014). Besides, Jamroz et al. (2005) reported that plant extracts (containing 2% capsaicin) could increase the growth of Lactobacillus and reduce the number of E. coli in large intestine, which might be beneficial for alleviating weaning stress and improving performance.
In order to better understand the effect of NCE on modulating nutrient utilization, we measured the ATTD of nutrients, and we also found NCE enhanced the ATTD of DM, CP, GE, and OM in phase 1 and tended to increase the ATTD of CP in phase 2, which was beneficial for improving ADG and G:F in weaned pigs in the present study. This finding indicated the utilization of energy and protein were improved by NCE in weaned pigs. One of the possible reasons for this finding might be that the capsaicin in NCE could effectively increase the activity of some digestive enzymes, including lipase, amylase, trypsin and chymotrypsin in pancreas (Platel and Srinivasan, 1996, 2000). In the current study, we found the ADG was increased mainly in phase 2 and overall by NCE, and the ATTD of nutrients was increased mainly in phase 1 by NCE. Since the weaning stress impaired the gut epithelium functional and structural integrities in pigs (Ji et al., 2019), the improvement of intestinal development and health was of vital importance for weaned pigs (Xiong et al., 2019). We suspected that the increased ATTD of nutrients in phase 1 might be used for the maturation of organs, especially the development of intestine, alleviating the negative impacts of weaning stress, and more nutrients could be available and used for growth performance in phase 2. For example, some amino acids could be used as functional nutrients in improving intestinal morphology and functions after weaning for pigs (Mou et al., 2019). Moreover, the ATTD of CP was tended to be increased by the NCE in phase 2, which could also help explain the improved performance in phase 2 and overall of weaned pigs in the current study.
In order to fully explain the possible mechanism of the enhanced ATTD of nutrients, we measured the digestive enzyme activities in the pancreas and small intestinal mucosa and we found that the supplementation of NCE could significantly increase the activities of pancreatic lipase activity, jejunal mucosa α-amylase, lipase and protease activities, and ileal mucosa protease and lipase activities in weaned pigs on d 28, which was beneficial for improving growth performance in phase 2 in this study. These endogenous α-amylase, lipase and protease were important for the decomposition, digestion and absorption of crude protein, lipid and carbohydrates from large molecules to amino acids, triglycerides and glucose in weaned pigs. Therefore, the increase level of digestive enzyme activities by capsaicin could be the reasons for the improvement in nutrient digestibility and performance of weaned pigs. Kalpana and Srinivasan (1996) has also reported that capsaicin could improve the activities of intestinal mucosal digestive enzymes (lipase and amylase) in mice, which revealed that capsaicin could modulate the digestive enzymes activities. The current finding might also be due to that capsaicin could increase the content of cholecystokinin in plasma, which could promote the growth of the pancreas and improve the symptoms for insufficient secretion of pancreatic amylase (Otsuki and Williams, 1983; Yamamoto et al., 2003). Moreover, the capsaicin could also enhance the development and immune function of ileum, which might be beneficial for the enhanced enzymes activities of protease and lipase in ileum (Liu et al., 2014).
For weaned pigs, the villus height might be reduced due to the cell apoptosis caused by changes of feed composition after weaning (Van der Peet-Schwering et al., 2007), which could reduce the nutrition absorption capacity. The current study found that NCE tended to increase villus height in ileum, which might be the reason that the capsaicin in NCE could increase the development of villus cells, and thus improve nutrient absorption and digestibility in weaned pigs (Huang et al., 2012; Jarupan et al., 2018).
After weaning, the oxidative stress caused by weaning stress might damage DNA, bio-membrane lipids, and proteins in tissues (Zhang et al., 2011). The antioxidant enzymes, such as T-SOD and CAT, could help alleviate the impact of oxidative stress, and MDA is a products of lipid peroxidation. Frankič et al. (2010) has reported that the carvacrol, cinnamaldehyde and capsaicin in plant extract could effectively protect lymphocytes against oxidative DNA damage, suggesting in their potentially beneficial effects on the improving immune system and antioxidant status in weaned pigs. Thiamhirunsopit et al. (2014) also reported that dietary supplementation with chili meal (containing 0.43 g/kg capsaicin) could reduce the serum MDA level. In the present study, pigs supplemented with NCE had higher concentrations of T-AOC, T-SOD and CAT, and lower level of MDA in serum, which indicated NCE could increase antioxidant capacities in pigs and alleviate the oxidative stress in weaned pigs. The reason for this finding might be the phenolic structure of capsaicin in NCE could be a trap for free radicals, which could scavenge hydrogen peroxide free radicals, prevent lipid peroxidation and protein oxidation, and therefore play a role to alleviate the oxidative stress and improve ADG in weaned pigs (Bogusz et al., 2018; Wang et al., 2019). Moreover, the possible mechanism for the increased antioxidant enzymes activities was that the capsaicin in NCE could also increase the activity of SOD and promote the expression of GPx 1 gene (one of the antioxidant genes) in the liver, which could reduce the content of hydrogen peroxide and other oxides in this study (Kim et al., 2008; KÜRÜM et al., 2015). While Kogure et al. (2002) also pointed out that the major antioxidant site of capsaicin was the C7-benzyl carbon.
In the current study, the serum β-EP content of NCE-fed pigs was significantly increased, which might be related to the increase of proopiomelanocortin (POMC) by capsaicin in NCE. Lee et al. (2012) demonstrated that intraperitoneal injection of capsaicin solution could significantly increase the mRNA expression of POMC, and the POMC was an important precursor of opioid peptide β-EP. In the current study, the increased content of β-EP could reduce the activity of energy expenditure, and thus improved performance in pigs, which might be due to that β-EP had positive effects on regulating blood glucose metabolism and analgesics (Dalayeun et al., 1993). As a monoamine neurotransmitter, 5-HT is directly involved in regulating mental and emotional changes. It is generally believed that abnormal brain content is the main cause of depression (Yu et al., 2011). Therefore, the increased serum 5-HT content in pigs fed NCE indicated that capsaicin in NCE might have a positive effect on regulating the mood of weaned pigs, which might help pigs to minimize the adverse effects from inflammation and oxidative stress response after weaning, and thus improve their performance. Moreover, the higher level of growth hormone in serum of pigs fed NCE could also be beneficial for the improved growth performance.
In this study, we found pigs fed NCE had increased IL-10 content (one of anti-inflammatory cytokines), and lower TNF-α, IFN-γ and IL-6 contents (pro-inflammatory cytokines) in serum, which indicated the anti-inflammatory function was improved by NCE. Manjunatha and Srinivasan (2006) also reported that dietary curcumin and capsaicin supplementation could alleviate carrageenan-induced inflammation. The current finding might be due to the ability of capsaicin in NCE on decreasing the secretion of TNF-α and IL-1β (Liu et al., 2012). Moreover, the capsaicin in NCE could potentially increase immune response via decreasing pro-inflammatory cytokines, including TNF-α and IL-1β (Spiller et al., 2008). Previous study has demonstrated that capsaicin in NCE could alleviate inflammatory response via reducing the gene expression of TNF-α, IL-1β, and IL-6 (Mendivil et al., 2019). Moreover, the capsaicin in NCE could also modulate the release of some inflammatory cytokines (such as IL-10, TNF-α, IL-1β, IL-6, IL-12) through the down-regulation of NF-ĸB pathway (Zhou et al., 2014).
The VFA are the main products of microorganisms-fermented dietary fiber in large intestine, which can be quickly absorbed by the intestinal cells and may has a role as probiotic for animals. In the liver, propionic acid can be converted into glucose through the gluconeogenesis pathway. In the colon, butyric acid can not only supply part of the energy for the metabolic activities of colon cells, but also stimulate the proliferation of epithelial cells to promote the growth and development of the large intestine (Rerat et al., 1987; Bugaut and Bentejac, 1993; Guilloteau et al., 2010). In addition, VFA can also form an acidic environment in large intestine and inhibit some harmful bacteria. Yang et al. (2019) reported the essential oils from plant herbs together with organic acids could modulate the concentration of VFA composition and microflora community, and therefore increase performance in weaned pigs. In the current study, NCE could also increase the concentrations of propionic acid, butyric acid and total volatile fatty acids in large intestine, which had a positive effect on intestinal health and microbiota composition in weaned pigs. One of the reasons for this finding might be the beneficial effects of capsaicin on increasing the butyrate level in large intestine and enhancing the immune response via decreasing serum TNF-α, IL-1β, and IL-6 levels (Song et al., 2017). Moreover, this finding might also be due to that capsaicin in NCE could effectively increase the relative abundance of butyric acid-producing or VFA-producing bacteria (Kang et al., 2016).
In order to figure out the possible mechanism of NCE on increasing VFA contents in large intestine, we measured the microbiota community via 16S rRNA sequencing technology. We found pigs fed NCE showed a numerical increase of relative abundance of Firmicutes (3.71% vs. 8.13%), and a numerical reduction of Bacteroidetes (91.38% vs. 88.91%) in phylum level in colon digesta compared with CON, which might be related to the capsaicin in NCE could increase the Firmicutes/Bacteroidetes ratio in phylum level and decreased the relative abundance of Bacteroides in genus level (Song et al., 2017). In this study, pigs fed NCE also had a numerical increase of relative abundance of Lactobacillus (38.37% vs. 23.62%) in genus level in colon digesta compared with CON, which was beneficial for improving intestinal health. Manzanilla et al. (2009) found that dietary supplementation with plant extracts (containing 2% capsaicin) could increase the abundance of Lactobacillus, inhibit the growth of some harmful bacteria (E. coli or Salmonella), and thus improve the intestinal health of piglets (Tsuchiya et al., 2011), which might be a possible explanation for the current finding. The essential oil in red pepper could also increase the content of gut Lactobacillus (Cairo et al., 2018). In this study, we found pigs fed NCE had increased relative abundance of Faecalibacterium in genus level in colon digesta compared with CON. Faecalibacterium is one of the butyric acid-producing bacteria, which could be increased in large intestine by dietary supplementation of capsaicin from NCE (Kang et al., 2016; Wang et al., 2020). These findings could support that capsaicin in NCE might have a role on improving the intestinal health in weaned pigs via modulating the gut microbiota composition. Furthermore, pigs fed NCE also had increased relative abundance of [Eubacterium]_ruminantium_group (belonging to Ruminococcaceae family) and tended to show increased relative abundance of Lachnospira (belonging to Lachnospiraceae family) in genus level in colon digesta compared with CON, which might also be due to the beneficial effect of capsaicin. Kang et al. (2017) reported capsaicin could increase the relative abundance of butyrate-producing or VFA-producing bacteria, especially Ruminococcaceae and Lachnospiraceae, and thus enhance the contents of butyric acid and VFA in large intestine of pigs in the current study. Although the current study found some novel ideas about the effects of NCE on microbiota community, its mechanism still remained to be further investigation in swine.
5. Conclusion
In conclusion, dietary NCE supplementation replacing CTC could enhance performance via improving digestive enzyme activities, antioxidant capacity, nutrient digestibility, anti-inflammatory function, gut morphology in weaned pigs. Moreover, natural capsicum extract could also improve gut health via modulating gut VFA and microbiota composition in weaned pigs, and thus it can be used as a potential target for the development of feed additives.
Author contributions
Shenfei Long: Conceptualization, Writing-Original Draft; Sujie Liu: Methodology, Investigation, Validation; Jian Wang: Data Curation, Software; Shad Uddin Mahfuz: Visualization; Xiangshu Piao: Supervision.
Conflicts of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgement
This research is supported by the Beijing Municipal Natural Science Foundation (6202019) and National Natural Science Foundation of China (31772612).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- AOAC . 19th ed. Off. Assoc. Anal. Chem.; Arlington, VA: 2012. Official methods of analysis. [Google Scholar]
- Ba Q., Li M., Chen P.Z., Huang C., Duan X.H., Lu L.J., Li J.Q., Chu R.A., Xie D., Song H.Y., Wu Y.N., Ying H., Jia X.D., Wang H. Sex-Dependent Effects of cadmium exposure in early life on gut microbiota and fat accumulation in mice. Environ Health Perspect. 2016;125:437–446. doi: 10.1289/EHP360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogusz S., Jr., Libardi S.H., Dias F.F., Coutinho J.P., Bochi V.C., Rodrigues D., Mt Melo A., Godoy H.T. Brazilian Capsicum peppers: capsaicinoid content and antioxidant activity. J Sci Food Agric. 2018;98:217–224. doi: 10.1002/jsfa.8459. [DOI] [PubMed] [Google Scholar]
- Bugaut M., Bentejac M. Biological Effects of short-chain fatty acids in nonruminant mammals. Annu Rev Nutr. 1993;13:217–241. doi: 10.1146/annurev.nu.13.070193.001245. [DOI] [PubMed] [Google Scholar]
- Cairo P.L., Gois F.D., Sbardella M., Silveira H., de Oliveira R.M., Allaman I.B., Cantarelli V.S., Costa L.B. Effects of dietary supplementation of red pepper (Schinus terebinthifolius Raddi) essential oil on performance, small intestinal morphology and microbial counts of weanling pigs. J Sci Food Agric. 2018;98:541–548. doi: 10.1002/jsfa.8494. [DOI] [PubMed] [Google Scholar]
- Chapa-Oliver A.M., Mejía-Teniente L. Capsaicin: from plants to a cancer-suppressing agent. Molecules. 2016;21:931. doi: 10.3390/molecules21080931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.R., Wang Q., Fish J.A., Chai B., McGarrell D.M., Sun Y., Brown C.T., Porras-Alfaro A., Kuske C.R., Tiedje J.M. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:633–642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell G.L. Why and how antibiotics are used in swine production. Anim Biotechnol. 2002;13:7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- Dalayeun J.F., Nores J.M., Bergal S. Physiology of β-endorphins. A close-up view and a review of the literature. Biomed Pharmacother. 1993;47:311–320. doi: 10.1016/0753-3322(93)90080-5. [DOI] [PubMed] [Google Scholar]
- Fan Y.F., Yang Y.Y., Yang P., Xia T., Ma Y.X. Available energy content, nutrients digestibility of chili meal and effects on performance of growing pigs. Anim Feed Sci Technol. 2017;229:97–105. doi: 10.1016/j.anifeedsci.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankič T., Levart A., Salobir J. The effect of vitamin E and plant extract mixture composed of carvacrol, cinnamaldehyde and capsaicin on oxidative stress induced by high PUFA load in young pigs. Animal. 2010;4:572–578. doi: 10.1017/S1751731109991339. [DOI] [PubMed] [Google Scholar]
- Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- Huang C.W., Lee T.T., Shih Y.C., Yu B. Effects of dietary supplementation of Chinese medicinal herbs on polymorphonuclear neutrophil immune activity and small intestinal morphology in weanling pigs. J Anim Physiol Anim Nutr. 2012;96:285–294. doi: 10.1111/j.1439-0396.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- Isoda H., Han J., Tominaga M., Maekawa T. Effects of capsaicin on human intestinal cell line caco-2. Cytotechnology. 2001;36:155–161. doi: 10.1023/A:1014053306343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamroz D., Orda J., Kamel C., Wiliczkiewicz A., Wertelecki T., Skorupińska J. The influence of phytogenic extracts on performance, nutrient digestibility, carcass characteristics, and gut microbial status in broiler chickens. J Anim Feed Sci. 2003;12:583–596. [Google Scholar]
- Jamroz D., Wiliczkiewicz A., Wertelecki T., Orda J., Skorupińska J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br Poultry Sci. 2005;46:485–493. doi: 10.1080/00071660500191056. [DOI] [PubMed] [Google Scholar]
- Jarupan T., Rakangthong C., Bunchasak C., Poeikhampha T., Kromkhun P. Effect of colistin and liquid methionine with capsaicin supplementation in diets on growth performance and intestinal morphology of nursery pigs. Int J Pharm Med Biol Sci. 2018;7:46–51. [Google Scholar]
- Ji F., Wang L., Yang H., Hu A., Yin Y. Review: the roles and functions of glutamine on intestinal health and performance of weaning pigs. Animal. 2019;13:2727–2735. doi: 10.1017/S1751731119001800. [DOI] [PubMed] [Google Scholar]
- Kalpana P., Srinivasan K. Influence of dietary spices or their active principles on digestive enzymes of small intestinal mucosa in rats. Int J Food Sci Nutr. 1996;47:55–59. doi: 10.3109/09637489609028561. [DOI] [PubMed] [Google Scholar]
- Kang C., Wang B., Kaliannan K., Wang X., Lang H., Hui S., Huang L., Zhang Y., Zhou M., Chen M., Mi M. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. mBio. 2017;8 doi: 10.1128/mBio.00470-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Zhang Y., Zhu X., Liu K., Wang X., Chen M., Wang J., Chen H., Hui S., Huang L., Zhang Q. Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J Clin Endocrinol Metabol. 2016;101:4681–4689. doi: 10.1210/jc.2016-2786. [DOI] [PubMed] [Google Scholar]
- Kim M.R., Lee K.N., Yon J.M., Lee S.R., Jin Y., Baek I.J., Lee B.J., Yun Y.W., Nam S.Y. Capsaicin prevents ethanol-induced teratogenicity in cultured mouse whole embryos. Reprod Toxicol. 2008;26:292–297. doi: 10.1016/j.reprotox.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Kogure K., Goto S., Nishimura M., Yasumoto M., Terada H. Mechanism of potent antiperoxidative effect of capsaicin. Biochim Biophys Acta Gen Subj. 2002;1573:84–92. doi: 10.1016/s0304-4165(02)00335-5. [DOI] [PubMed] [Google Scholar]
- Kiarie E., Voth C., Wey D., Zhu C., Vingerhoeds P., Borucki S., Squires E. Comparative efficacy of antibiotic growth promoter and benzoic acid on growth performance, nutrient utilization, and indices of gut health in nursery pigs fed corn–soybean meal diet. Can J Anim Sci. 2018;98:868–874. [Google Scholar]
- Kong X.F., Wu G.Y., Liao Y.P., Hou Z.P., Liu H.J., Yin F.G., Li T.J., Huang R.L., Zhang Y.M., Deng D., Kang P. Effects of Chinese herbal ultra-fine powder as a dietary additive on growth performance, serum metabolites and intestinal health in early-weaned piglets. Livest Sci. 2007;108:272–275. [Google Scholar]
- Kürüm A., Kocamiş H., Deprem T., Mu Ç.I.N.A.R. Determining gene expression profile of GPX 1 in the liver of diabetic rats treated with capsaicin by Real-Time PCR. Kafkas Üniversitesi Veteriner Fakültesi Dergisi. 2015;21:259–263. [Google Scholar]
- Lee J.S., Kim S.G., Kim H.K., Baek S.Y., Kim C.M. Acute effects of capsaicin on proopiomelanocortin mRNA levels in the arcuate nucleus of Sprague-Dawley rats. Psychiatr Invest. 2012;9:187–190. doi: 10.4306/pi.2012.9.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.W., Tong J.M., Zhou D.W. Utilization of Chinese herbal feed additives in animal production. Agric Sci China. 2011;10:1262–1272. [Google Scholar]
- Liu Y., Song M., Che T.M., Bravo D., Pettigrew J.E. Anti-inflammatory effects of several plant extracts on porcine alveolar macrophages in vitro. J Anim Sci. 2012;90:2774–2783. doi: 10.2527/jas.2011-4304. [DOI] [PubMed] [Google Scholar]
- Li M., Long S.F., Wang Q.Q., Zhang L.H., Hu J.X., Yang J., Cheng Z.B., Piao X.S. Mixed organic acids improve nutrients digestibility, volatile fatty acids composition and intestinal microbiota in growing-finishing pigs fed high-fiber diet. Asian-Australas J Anim Sci. 2019;32:856–864. doi: 10.5713/ajas.18.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T.M., Almeida J.A., Lee J.J., Bravo D., Maddox C.W., Pettigrew J.E. Dietary plant extracts alleviate diarrhea and alter immune responses of weaned pigs experimentally infected with a pathogenic Escherichia coli. J Anim Sci. 2013;91:5294–5306. doi: 10.2527/jas.2012-6194. [DOI] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T.M., Bravo D., Maddox C.W., Pettigrew J.E. Effects of capsicum oleoresin, garlic botanical, and turmeric oleoresin on gene expression profile of ileal mucosa in weaned pigs. J Anim Sci. 2014;92:3426–3440. doi: 10.2527/jas.2013-6496. [DOI] [PubMed] [Google Scholar]
- Long S.F., Liu L., Liu S.J., Mahfuz S., Piao X.S. Effects of Forsythia suspense extract as an antibiotics substitute on growth performance, nutrient digestibility, serum antioxidant capacity, fecal Escherichia coli concentration and intestinal morphology of weaned piglets. Animals. 2019;9:729. doi: 10.3390/ani9100729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.F., Xu Y.T., Pan L., Wang Q.Q., Wang C.L., Wu J.Y., Wu Y.Y., Han Y.M., Piao X.S. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim Feed Sci Technol. 2018;235:23–32. [Google Scholar]
- Long S.F., Xu Y.T., Wang C.L., Li C.L., Liu D.W., Piao X.S. Effects of dietary supplementation with a combination of plant oils on performance, meat quality and fatty acid deposition of broilers. Asian-Australas J Anim Sci. 2018;31:1773–1780. doi: 10.5713/ajas.18.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnert A., Moissl-Eichinger C., Berg G. Microbiome interplay: plants alter microbial abundance and diversity within the built environment. Front Microbiol. 2015;6:887. doi: 10.3389/fmicb.2015.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunatha H., Srinivasan K. Protective effect of dietary curcumin and capsaicin on induced oxidation of low-density lipoprotein, iron-induced hepatotoxicity and carrageenan-induced inflammation in experimental rats. FEBS J. 2006;273:4528–4537. doi: 10.1111/j.1742-4658.2006.05458.x. [DOI] [PubMed] [Google Scholar]
- Manzanilla E.G., Pérez J.F., Martín M., Blandón J.C., Gasa J. Dietary protein modifies effect of plant extracts in the intestinal ecosystem of the pig at weaning. J Anim Sci. 2009;87:2029–2037. doi: 10.2527/jas.2008-1210. [DOI] [PubMed] [Google Scholar]
- Manzanilla E.G., Perez J.F., Martin M., Kamel C., Gasa J. Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs. J Anim Sci. 2004;82:3210–3218. doi: 10.2527/2004.82113210x. [DOI] [PubMed] [Google Scholar]
- Mendivil E.J., Sandoval-Rodriguez A., Meza-Ríos A., Zuñiga-Ramos L., Dominguez-Rosales A., Vazquez-Del Mercado M., Sanchez-Orozcoa L., Santos-Garcia A., Armendariz-Borunda J. Capsaicin induces a protective effect on gastric mucosa along with decreased expression of inflammatory molecules in a gastritis model. J Nutraceuticals, Funct Med Foods. 2019;59:345–351. [Google Scholar]
- Mou Q., Yang H.S., Yin Y.L., Huang P.F. Amino acids influencing intestinal development and health of the piglets. Animals. 2019;9:302. doi: 10.3390/ani9060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nmaju A.U., Joshua I.E., Okon U.E., Nwankwo A.A., Osim E.E. Long-term consumption of capsicum annum (chilli pepper) and capsaicin diets suppresses pain perception and improves social behaviour of CD-1 mice. Nutr Food Sci. 2018;48:911–921. [Google Scholar]
- Otsuki M., Williams J.A. Amylase secretion by isolated pancreatic acini after chronic cholecystokinin treatment in vivo. Am J Physiol Gastrointest Liver Physiol. 1983;244:683–688. doi: 10.1152/ajpgi.1983.244.6.G683. [DOI] [PubMed] [Google Scholar]
- Pan L., Ma X.K., Wang H.L., Xu X., Zeng Z.K., Tian Q.Y., Zhao P.F., Zhang S., Yang Z.Y., Piao X.S. Enzymatic feather meal as an alternative animal protein source in diets for nursery pigs. Anim Feed Sci Technol. 2016;212:112–121. [Google Scholar]
- Platel K., Srinivasan K. Influence of dietary spices or their active principles on digestive enzymes of small intestinal mucosa in rats. Int J Food Sci Nutr. 1996;47:55–59. doi: 10.3109/09637489609028561. [DOI] [PubMed] [Google Scholar]
- Platel K., Srinivasan K. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Food Nahrung. 2000;44:42–46. doi: 10.1002/(SICI)1521-3803(20000101)44:1<42::AID-FOOD42>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Rerat A., Fiszlewicz M., Giusi A., Vaugelade P. Influence of meal frequency on postprandial variations in the production and absorption of volatile fatty acids in the digestive tract of conscious pigs. J Anim Sci. 1987;64:448–456. doi: 10.2527/jas1987.642448x. [DOI] [PubMed] [Google Scholar]
- SAS . 2 Ed. SAS Inc.; Cary, NC, USA: 2008. SAS/STAT users guide: statistics. Version 9. [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller F., Alves M.K., Vieira S.M., Carvalho T.A., Leite C.E., Lunardelli A., Poloni J.A., Cunha Fernando Q., de Oliveira J.R. Anti-inflammatory effects of red pepper (Capsicum baccatum) on carrageenan-and antigen-induced inflammation. J Pharm Pharmacol. 2008;60:473–478. doi: 10.1211/jpp.60.4.0010. [DOI] [PubMed] [Google Scholar]
- Song J.X., Ren H., Gao Y.F., Lee C.Y., Li S.F., Zhang F., Li L., Chen H. Dietary capsaicin improves glucose homeostasis and alters the gut microbiota in obese diabetic ob/ob mice. Front Physiol. 2017;8:602. doi: 10.3389/fphys.2017.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K. Biological activities of red pepper (capsicum annuum) and its pungent principle capsaicin: a review. Crit Rev Food Sci Nutr. 2016;56:1488–1500. doi: 10.1080/10408398.2013.772090. [DOI] [PubMed] [Google Scholar]
- Thiamhirunsopit K., Phisalaphong C., Boonkird S., Kijparkorn S. Effect of chili meal (Capsicum frutescens LINN.) on growth performance, stress index, lipid peroxidation and ileal nutrient digestibility in broilers reared under high stocking density condition. Anim Feed Sci Technol. 2014;192:90–100. [Google Scholar]
- Tsuchiya K., Urabe M., Yamamoto R., Asada Y., Lee J.F. Effects of nω-nitro-l-arginine and capsaicin on neurogenic vasomotor responses in isolated mesenteric arteries of the monkey. J Pharm Pharmacol. 2011;46:155–157. doi: 10.1111/j.2042-7158.1994.tb03763.x. [DOI] [PubMed] [Google Scholar]
- Van der Peet-Schwering C.M., Jansman A.J., Smidt H., Yoon I. Effects of yeast culture on performance, gut integrity, and blood cell composition of weanling pigs. J Anim Sci. 2007;85:3099–3109. doi: 10.2527/jas.2007-0110. [DOI] [PubMed] [Google Scholar]
- Wang F., Huang X., Chen Y., Zhang D., Chen D., Chen L., Lin J. Study on the Effect of capsaicin on the intestinal flora through high-throughput sequencing. ACS Omega. 2020;5:1246–1253. doi: 10.1021/acsomega.9b03798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Yu L., Li F., Zhang G., Zhou W., Jiang X. Synthesis of amide derivatives containing capsaicin and their antioxidant and antibacterial activities. J Food Biochem. 2019;43:13061. doi: 10.1111/jfbc.13061. [DOI] [PubMed] [Google Scholar]
- Xiong X., Tan B., Song M., Ji P., Kim K., Yin Y.L., Liu Y.H. Nutritional intervention for the intestinal development and health of weaned pigs. Front Vet Sci. 2019;6:46. doi: 10.3389/fvets.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Otani M., Jia D., Fukumitsu K., Yoshikawa H., Akiyama T., Otsuki M. Differential mechanism and site of action of CCK on the pancreatic secretion and growth in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285:681–687. doi: 10.1152/ajpgi.00312.2002. [DOI] [PubMed] [Google Scholar]
- Yang C., Zhang L., Cao G., Feng J., Yue M., Xu Y., Dai B., Han Q., Guo X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J Anim Sci. 2019;97:133–143. doi: 10.1093/jas/sky426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Wu M.M., Xiao H., Ren W.K., Duan J.L., Yang G., Li T.J., Yin Y.L. Development of an antioxidant system after early weaning in piglets. J Anim Sci. 2014;92:612–619. doi: 10.2527/jas.2013-6986. [DOI] [PubMed] [Google Scholar]
- Yu H.L., Deng X.Q., Li Y.J., Li Y.C., Quan Z.S., Sun X.Y. N-palmitoylethanolamide, an endocannabinoid, exhibits antidepressant effects in the forced swim test and the tail suspension test in mice. Pharmacol Rep. 2011;63:834–839. doi: 10.1016/s1734-1140(11)70596-5. [DOI] [PubMed] [Google Scholar]
- Zeng Z.K., Xu X., Zhang Q., Li P., Zhao P.F., Li Q.Y., Liu J.D., Piao X.S. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim Sci J. 2015;86:279–285. doi: 10.1111/asj.12277. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Piao X.L., Piao X.S., Lu T., Wang D., Kim S.W. Preventive effect of Coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem Toxicol. 2011;49:61–69. doi: 10.1016/j.fct.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Guan X., Zhu W., Liu Z., Wang X., Yu H., Wang H. Capsaicin inhibits Porphyromonas gingivalis growth, biofilm formation, gingivomucosal inflammatory cytokine secretion, and in vitro osteoclastogenesis. Eur J Clin Microbiol. 2014;33:211–219. doi: 10.1007/s10096-013-1947-0. [DOI] [PubMed] [Google Scholar]