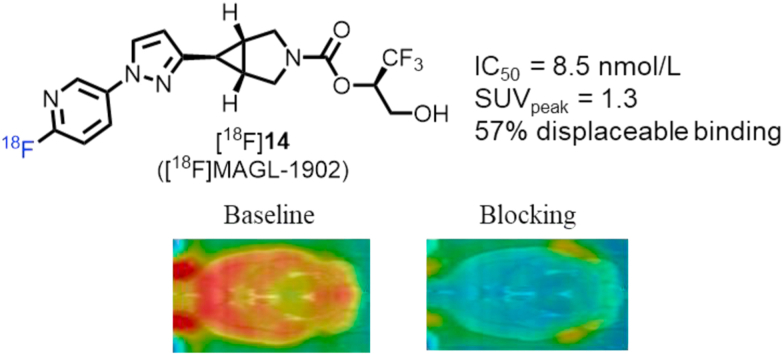
Abstract
As a serine hydrolase, monoacylglycerol lipase (MAGL) is principally responsible for the metabolism of 2-arachidonoylglycerol (2-AG) in the central nervous system (CNS), leading to the formation of arachidonic acid (AA). Dysfunction of MAGL has been associated with multiple CNS disorders and symptoms, including neuroinflammation, cognitive impairment, epileptogenesis, nociception and neurodegenerative diseases. Inhibition of MAGL provides a promising therapeutic direction for the treatment of these conditions, and a MAGL positron emission tomography (PET) probe would greatly facilitate preclinical and clinical development of MAGL inhibitors. Herein, we design and synthesize a small library of fluoropyridyl-containing MAGL inhibitor candidates. Pharmacological evaluation of these candidates by activity-based protein profiling identified 14 as a lead compound, which was then radiolabeled with fluorine-18 via a facile SNAr reaction to form 2-[18F]fluoropyridine scaffold. Good blood–brain barrier permeability and high in vivo specific binding was demonstrated for radioligand [18F]14 (also named as [18F]MAGL-1902). This work may serve as a roadmap for clinical translation and further design of potent 18F-labeled MAGL PET tracers.
KEY WORDS: Monoacylglycerol lipase (MAGL), Central nervous system (CNS), 2-Arachidonylglycerol (2-AG), Arachidonic acid (AA), Positron emission tomography (PET), Fluorine-18
Graphical abstract
A highly potent irreversible MAGL PET tracer [18F]14 was described, which exhibited favorable in vitro and in vivo characteristics, including excellent affinity, high brain uptake, and good binding specificity.
1. Introduction
As a serine hydrolase, monoacylglycerol lipase (MAGL) exerts a vital role in the endocannabinoid and eicosanoid signalling systems1, 2, 3, 4, 5. MAGL is widespreadly distributed in the body with particularly high expression in the brain. In the central nervous system (CNS), MAGL catalyses the metabolism of the endocannabinoid 2-arachidonylglycerol (2-AG) to arachidonic acid (AA), approximately constituting 50% AA production, which not only serves as a proinflammatory eicosanoid precursor, but also constitutes inflammatory signals6, 7, 8, 9, 10. In this case, simultaneous regulation of both endocannabinoid and eicosanoid system constitutes the dual-function of MAGL in the CNS. Recent studies have indicated that dysfunction of MAGL is associated with multiple disorders such as neuroinflammation, cognitive impairment, epileptogenesis, nociception, neurodegenerative diseases, and cancer pathogenesis11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23. Inhibition of MAGL not only reduces the production of pro-inflammatory eicosanoids, but also increases 2-AG signaling, thereby providing a promising therapeutic direction for the treatment of the disorders mentioned above. As such, the development of MAGL inhibitors with high affinity and selectivity has caught considerable interest in the field of medicinal chemistry and drug discovery24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35.
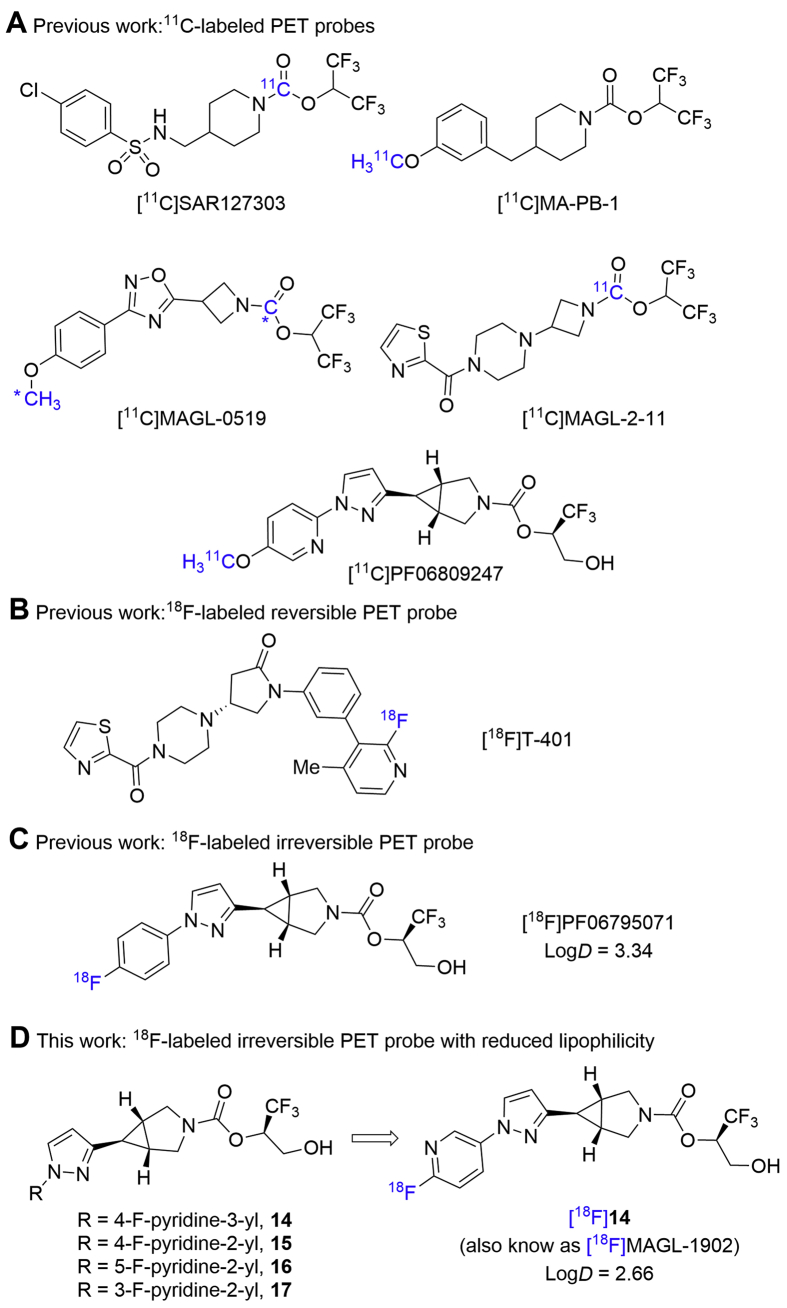
Complementary to routine clinical diagnostic application, positron emission tomography (PET) is a well-characterized noninvasive nuclear imaging tool, which has emerged to be invaluable for target engagement and phase 0 studies in the discovery of CNS drugs36, 37, 38. Our interest focuses on the discovery of highly MAGL-specific (PET) tracers to enable preclinical and clinical drug development. A MAGL PET tracer would not only allow a deep understanding of biology in vivo such as target expression/distribution and relationship with multiple diseases, but also enable a facile clinical translation of MAGL inhibitors. So far, our group39, 40, 41, 42 and others43, 44, 45, 46 have reported several first-in-class 11C-labeled MAGL PET probes with favorable brain permeability and target specificity, such as [11C]SAR127303, [11C]MA-PB-1, [11C]MAGL-0519, [11C]MAGL-2-11 and [11C]PF-06809247. However, the short half-life of carbon-11 (t1/2 = 20.4 min) requires on site tracer production, and their utilization is restricted to imaging facilities equipped with a cyclotron. On the other hand, the relatively longer half-life of fluorine-18 (t1/2 = 109.7 min) allows for multistep synthesis, extended acquisition time, and transportation over a long distance, thus enabling its off-site use, and has demonstrated great commercialization value. Furthermore, relatively-slow radioactive decay and short positron range of fluorine-18 favorably improves the resolution and counting statistics of PET images. So far, the discovery of 18F-labeled MAGL PET probes is still in its infancy. Only recently, during the preparation of this manuscript, [18F]T-40147 was developed as an 18F-labeled reversible MAGL PET tracer by Koike and co-workers, whereas [18F]PF0679507148 was disclosed by our group as an 18F-labeled irreversible MAGL PET probe. By taking advantage of the unique azabicyclo[3.1.0]hexane scaffold, herein we designed and synthesized a focused library of fluoropyridyl-containing MAGL inhibitor candidates (14–17, Fig. 1). Our hypothesis was that, the incorporation of a fluoropyridyl moiety in 14–17 (particularly for 2-fluoropyridyl) instead of the phenyl group in [18F]PF06795071 may facilitate SNAr labeling with fluorine-18 and decrease the lipophilicity of candidate ligands, thereafter further improving target binding specificity. In this work, our preliminary pharmacological screening and molecular docking studies of these candidates identified 14 as a lead compound. Radiolabeling of 14 with fluorine-18 was achieved via a facile SNAr reaction. MAGL PET ligand [18F]14 (also named as [18F]MAGL-1902) exhibited excellent brain permeability, high in vivo specific binding, and heterogeneous radioactivity accumulation in various brain regions, which was consistent with MAGL expression profile in the brain. This work may serve as a roadmap for PET imaging translation in higher species and guideline for further design of potent 18F-labeled MAGL PET tracers.
Figure 1.
Representative PET probes for brain MAGL imaging.
2. Results and discussion
2.1. Chemical synthesis
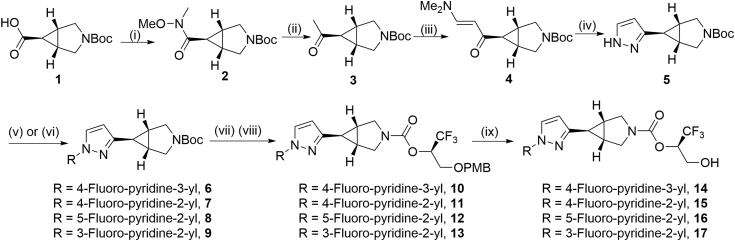
To synthesize irreversible MAGL inhibitors 14–17 containing 3-azabicyclo[3.1.0]hexane core unit, we took advantage of a general strategy shown in Scheme 1 with tert-butyloxycarbonyl (Boc)-protected 3-azabicyclo[3.1.0]hexane-6-carboxylic acid 1 as the starting material. Briefly, the coupling of 1 with N,O-dimethylhydroxylamine hydrogen chloride (NHMeOMe·HCl) occurred smoothly to deliver the Weinreb amide 2. Subsequent Grignard addition converted 2 to the corresponding ketone 3 in 99% yield over two steps. Ketone 3 was then transformed into pyrazole 5 via enamine formation and cyclization with hydrazine in 97% total yield. Copper-mediated cross-coupling reaction of pyridyl boronic acid or direct nucleophilic SNAr substitution of 2-fluoropyridine derivatives with 5 provided compounds 6–9 in 8%−52% yield. TFA-triggered removal of the Boc group in 6–9 followed by coupling with an activated carbonate in situ generated from (R)-1,1,1-trifluoro-3-(4-methoxybenzyloxy)propan-2-ol readily proceeded to provide carbamates 10–13 in moderate yields (26%–41%). Ultimately, the desired MAGL inhibitors 14–17 were obtained in a highly efficient manner by deprotection of p-methoxybenzyl (PMB) group.
Scheme 1.
Synthesis of irreversible MAGL inhibitors 14–17. Conditions: (i) NHMeOMe·HCl, EDC·HCl, DIPEA, HOBT, CH2Cl2, rt, 2 h; (ii) MeMgBr, THF, rt, 1 h; 99% yield over 2 steps; (iii) DMF−DMA, DMF, 110 °C, 16 h; (iv) NH2NH2, EtOH, 80 °C, 16 h; 97% yield over 2 steps; (v) (6-fluoropyridin-3-yl)boronic acid, pyridine, Cu(OAc)2, 4 Å molecular sieves, CH2Cl2, 30 °C, 30 h; 8% yield for 6; (vi) 2,4-difluoropyridine (for 7), 2,5-difluoropyridine (for 8) or 2,3-difluoropyridine (for 9), Cs2CO3, DMF, 120 °C, 24 h; 51% yield for 7; 48% yield for 8; 52% yield for 9; (vii) TFA, rt, 1 h; (viii) Et3N, 1,1ʹ-[carbonylbis(oxy)]dipyrrolidine-2,5-dione, (R)-1,1,1-trifluoro-3-(4-methoxybenzyloxy)propan-2-ol, CH2Cl2, 30 °C, 30 h; 26% yield for 10; 41% yield for 11;31% yield for 12; 33% yield for 13; (ix) TFA, CH2Cl2, rt, 4 h; 96% yield for 14; 67% yield for 15; 74% yield for 16; 92% yield for 17; NHMeOMe·HCl=N,O-dimethylhydroxylamine hydrogen chloride; EDC∙HCl N-(3-dimethylaminopropyl)-Nʹ-ethylcarbodiimide hydrochloride; DIPEA=N,N-diisopropylethylamine; HOBT = 1-hydroxybenzotriazole hydrate; Et3N = triethylamine; DMF = N,N-dimethylformamide; TFA = trifluoroacetic acid; PMB = p-methoxybenzyl.
2.2. Molecular docking studies
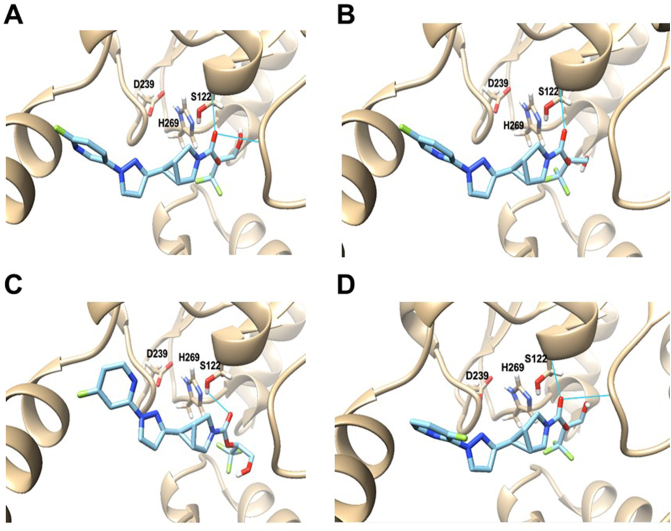
To investigate possible molecular interaction of candidate inhibitors 14–17 with MAGL, a “pre-covalent” MAGL–inhibitor complex was constructed by use of molecular docking study. The published MAGL crystal structure (PDB ID: 3PE6)42,49 was used as the model, and compounds 14–17 were then docked into this model by Autodock Vina in a pre-covalent state. As depicted in Fig. 2, all these candidate inhibitors exhibited good interaction with the binding pocket of MAGL and their leaving group oriented towards the Ser122-His269-Asp239 catalytic triad of MAGL, which is a crucial functional unit for the catabolism of 2-AG. In these binding poses, the carbonyl oxygen of candidates 14–17 resided close to the residues of Ser122, which possibly formed H-bonding interactions. These results suggested great promise of compounds 14–17 to decrease the likelihood of 2-AG to approach the MAGL binding site, thereby being good MAGL inhibitors.
Figure 2.
Molecular docking structures of compounds 14 (A), 15 (B) 16 (C) and 17 (D) onto MAGL (pre-covalent docking state). H-bonding interactions between compounds 14–17 and MAGL residues were labeled by blue lines. PDB ID: 3PE6.
2.3. Pharmacology
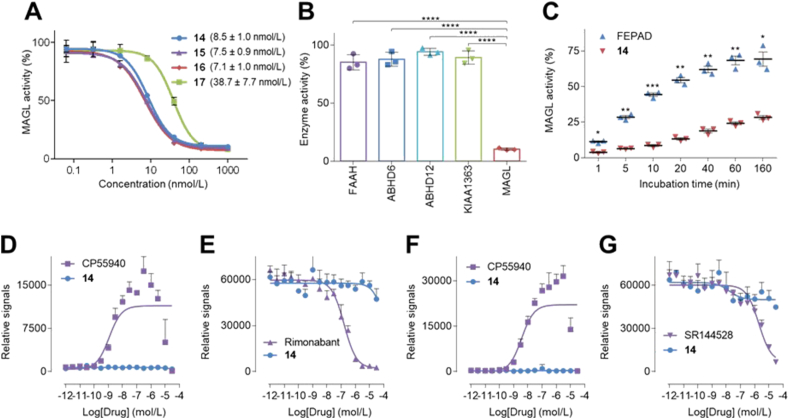
To probe the potency and selectivity, compounds 14–17 were evaluated in vitro in mouse brain lysates by activity-based protein profiling (ABPP) with the serine hydrolase directed probe FP-rhodamine50. As shown in Fig. 3A, compounds 14–16 demonstrated excellent inhibitory activity towards MAGL with single-digit nanomolar IC50 values (8.5 nmol/L for 14, 7.5 nmol/L for 15 and 7.1 nmol/L for 16). Considering the unique 2-fluoropyridine scaffold in 14, which enables facile synthesis of the precursor and radiolabeling with fluorine-18, we prioritized this probe for further pharmacological evaluation. As such, the selectivity of compound 14 for inhibition of MAGL over other serine hydrolases, e.g., FAAH, ABHD6, ABHD12 and KIAA1363, was determined by ABPP in mouse brain lysates. In these experiments an excellent selectivity profile was demonstrated with no significant inhibition of serine hydrolase activity of these off-targets (Fig. 3B). To evaluate the reversibility of inhibition, a time-dependent ABPP study was carried out with compound 14 and a known reversible MAGL inhibitor, FEPAD50, which served as a positive control (Fig. 3C). In this assay, MAGL activity recovered rapidly over time in FEPAD-treated samples, whereas MAGL activity only slowly increased in compound 14-treated samples, indicating irreversible binding. Furthermore, no direct agonism or antagonism was found for compound 14 with the cannabinoid receptors CB1 and CB2 (Fig. 3D−G). An off-target pharmacological screening in major CNS targets, including common GPCRs, enzymes, ion channels and transporters was further carried out for compound 14 at a testing concentration of 10 μmol/L. As illustrated in Supporting Information Fig. S2A, only norepinephrine transporter (NET) was identified with greater than 50% target activity at 10 μmol/L of compound 14, and a follow-up NET binding assay using [3H]nisoxetine showed the Ki value of 14 to be 4.08 μmol/L (Fig. S2B), indicating more than 400-fold selectivity towards MAGL among other CNS targets tested.
Figure 3.
Pharmacological evaluation of compounds 14–17. (A) Concentration–response curves of candidates 14–17 for inhibiting mouse brain MAGL activity; (B) Selectivity of 14 at a concentration of 0.2 μmol/L among MAGL and other serine hydrolases, e.g., FAAH, ABHD6, ABHD12 and KIAA1363; (C) Time-dependent ABPP activity of 14 at a concentration of 1 μmol/L with the reversible inhibitor FEPAD (1 μmol/L) as control. (D) CB1 agonist assay with CP55940 as control; (E) CB1 antagonist assay with rimonabant as control; (F) CB2 agonist assay with CP55940 as control; (G) CB2 antagonist assay with SR144528 as control. All data are indicated as mean ± SD, n = 3. A student's two-tailed t-test was carried out for statistical analysis and asterisks referred to the statistical significance: ∗P < 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001, and ∗∗∗∗P ≤ 0.0001.
2.4. Radiochemistry
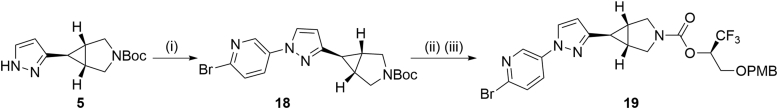
With promising pharmacology results, we commenced with the labeling of compound 14 with fluorine-18. The synthesis of bromopyridine precursor 19 was obtained as per the general strategy depicted in Scheme 2. Beginning with pyrazole derivative 5, 2-bromopyridyl moiety was successfully incorporated via a copper-promoted cross-coupling reaction with (6-bromopyridin-3-yl)boronic acid. Deprotection of the Boc group was achieved with TFA, and the corresponding amine intermediate readily underwent alkoxyl-carbonylation reaction with (R)-1,1,1-trifluoro-3-(4-methoxybenzyloxy)propan-2-ol and 1,1ʹ-[carbonylbis(oxy)]dipyrrolidine-2,5-dione, thus providing precursor 19 in 54% yield over 2 steps.
Scheme 2.
Synthesis of precursor 19 and its radiolabeling en route to MAGL PET tracer 20 ([18F]14). Conditions: (i) (6-bromopyridin-3-yl)boronic acid, Cu(OAc)2, pyridine, 4 Å molecular sieves, CH2Cl2, 30 °C, 30 h; 9% yield; (ii) TFA, rt, 1 h; (iii) Et3N, 1,1ʹ-[carbonylbis(oxy)]dipyrrolidine-2,5-dione, (R)-1,1,1-trifluoro-3-(4-methoxybenzyloxy)pro-pan-2-ol, CH2Cl2, 30 °C, 30 h; 54% yield over two steps. TFA = trifluoroacetic acid; PMB = p-methoxybenzyl.
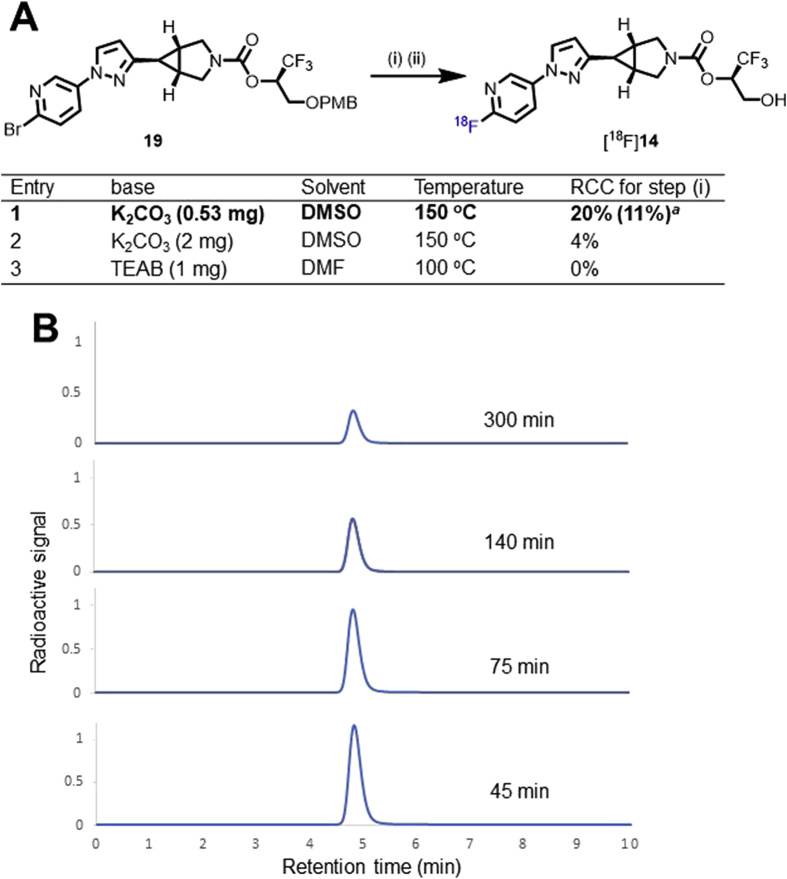
With the precursor 19 in hand, we performed its radiolabeling with fluorine-18 to synthesize MAGL PET tracer [18F]14. As illustrated in Scheme 3A, SNAr reaction of 19 with fluorine-18 was achieved by use of K2CO3/K222 (0.53 mg/9.4 mg) in DMSO at 150 °C for 10 min (entry 1). Increase of the amount of K2CO3 or use of other base such as tetraethylammonium bicarbonate (TEAB) failed to improve this reaction (entries 2 and 3). Complete deprotection of the PMB group was achieved by use of 6 mol/L HCl at 100 °C for 4 min, thus providing an access to radioligand [18F]14. This protocol features several merits such as reasonable radiochemical yield (11% RCY, decay-corrected), good molar activity (up to 210 GBq/μmol) and high radiochemical purity (>99%) at end of synthesis. Of note, no radiolysis was observed up to 5 h in ethanol-containing saline (5%), which implicated excellent formulation stability for radioligand [18F]14 (Scheme 3B). In addition, in the discovery of CNS PET tracers, favorable physicochemical property is crucial to increase the likelihood of the passive blood–brain barrier (BBB) permeability and decrease the risk of non-specific binding. In this case, the lipophilicity of ligand [18F]14 (logD = 2.66 ± 0.01, n = 3) was determined by the ‘shake flask method’, also well-characterized as liquid–liquid partition between n-octanol and PBS51, the value of which was aligned within the favorable space (1.0–3.5) of PET CNS tracers52, 53, 54.
Scheme 3.
(A) Radiosynthesis of MAGL PET tracer [18F]14. Conditions: (i) 18F−, K2CO3, K222, DMSO, 150 °C, 10 min; (ii) 6 mol/L HCl, 100 °C, 4 min. aThe value in parenthesis refers to an average radiochemical yield over two steps (decay-corrected). (B) Stability of tracer [18F]14 in saline containing 5% of EtOH. RCC = radiochemical conversion.
2.5. Preliminary PET imaging and whole-body biodistribution studies of [18F]14
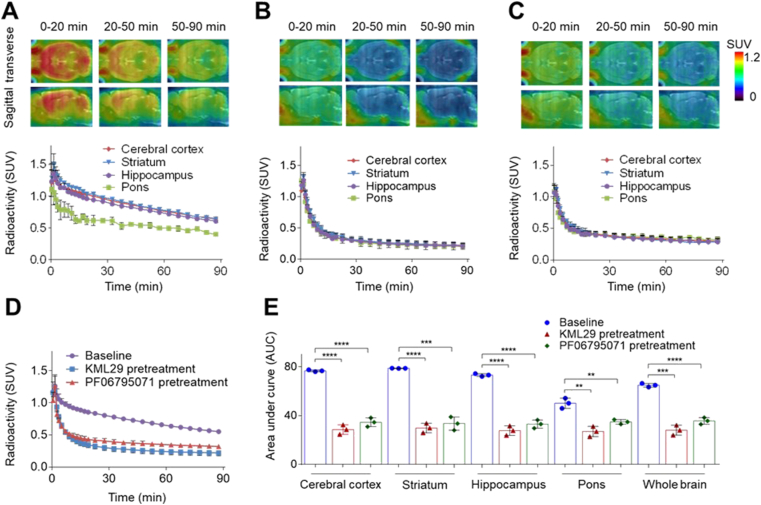
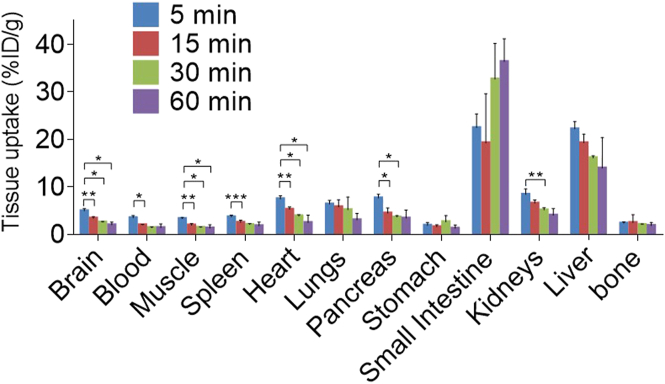
Radioligand [18F]14 was then advanced to PET imaging evaluation. Dynamic rat brain PET images were collected under baseline and blocking conditions for 90 min post intravenous administration of [18F]14 to Sprague–Dawley (SD) rats. Fig. 4 illustrated the co-registration of summed PET images (0–20, 20–50 and 50–90 min) with magnetic resonance imaging (MRI) images as well as the corresponding time–activity curves (TAC). The baseline scan demonstrated good BBB penetration ability for [18F]14 (Fig. 4A), and the maximum brain uptake was achieved at 1.5 min post tracer injection with the standard uptake value (SUV) of 1.3, as indicated by the whole-brain TAC (Fig. 4D). In addition, radioligand [18F]14 also implicated a heterogeneous distribution pattern, and high radioactivity were accumulated in the striatum, hippocampus and cerebral cortex, whereas pons exhibited low radioactivity accumulation. Of note, the slow elimination of [18F]14 from rat brain over time is possibly attributed to a slow hydrolysis of the inhibitor‒MAGL adduct, which was also observed in the ABPP studies. Following a 30 min pretreatment with KML29 under a dose of 0.3 mg/kg, a well-characterized MAGL inhibitor, the uptake of [18F]14 in various brain regions all reduced significantly, thereby leading to the abolishment of heterogeneous distribution pattern in baseline scans (Supporting Information Fig. S3). Of note, increasing the dose of KML29 to 3 mg/kg could further enhanced this radioactivity reduction in all brain regions with a robust attenuation of the whole-brain uptake by 57% as per the area under the curve (AUC), which suggested high in vivo binding specificity and a dose-dependent blocking of the binding of [18F]14 in rat brains (Fig. 4B, E and Fig. S3). To further assess the in vivo specificity of [18F]14, we then carried out another pretreatment experiment with PF06795071 (3 mg/kg), a potent MAGL inhibitor disclosed by Pfizer31. As expected, a robust blocking was seen in the rat brain images and the corresponding TAC (45% reduction of whole-brain uptake as per AUC, Fig. 4C and E). Motivated by these promising results, we then conducted whole-body biodistribution studies aiming to further examine in vivo uptake and washout of [18F]14 in peripheral organs of rodents. As illustrated in Fig. 5 and Supporting Information Table S1, CD-1 mice were used as objects and four time points (5, 15, 30 and 60 min) was selected post injection of [18F]14. Radioactivity was robustly accumulated in multiple peripheral organs of CD-1 mice, such as the heart, liver kidneys, lungs, small intestine, and pancreas with levels of higher than 5% ID/g (injected dose per gram of wet tissue) at 5 min post tracer injection. Following initial high uptake, the radioactivity of [18F]14 in the kidneys and liver slowly washed out, and high radioactivity level was observed in the small intestine at 60 min after injecting [18F]14, which implicated a possible hepatobiliary and urinary elimination pathway for [18F]14. To further showcase the pharmacokinetic properties of [18F]14, we performed whole-body PET imaging studies in mice. As showed in Supporting Information Fig. S4, initial high radioactivity level was observed in major peripheral organs such as liver, heart, lungs and kidneys, followed by steady wash-out, which is in line with the results from whole-body bio-distribution studies. At 60 min post tracer injection, the radioactivity level reached a relatively steady state in almost all the organs. The relative low uptake of [18F]14 in brown fat tissues (BAT) was probably caused by anesthesia and the radioactivity in BAT gradually accumulated over 5 min, and no significant elimination was observed, which complies with the irreversible binding profile. To probe the stability of [18F]14 in vivo, we performed a radiometabolic analysis in the brain and plasma homogenate of SD rats. With our previously developed method, we demonstrated that most of the radioactivity in rat brain was bound irreversibly to the brain tissues and the bound radioactivity fraction was determined to be 84% and 75% at 5 and 30 min post tracer injection (n = 3), respectively. In the meanwhile, we also investigated the metabolites of unbound radioactivity in rat brains and an average of 78% and 64% of parent radioactivity was observed at 5 and 30 min post tracer injection, respectively (n = 2). For radioactivity in plasma, the parent fraction was determined with an average of 70% and 46% of radioactivity at 5 and 30 min post tracer injection, respectively (n = 2). These results indicated favorable in vivo stability of [18F]14 in rats.
Figure 4.
Summed PET images and representative time–activity curves (TACs) of [18F]14 in rat brains under (A) Baseline conditions; (B) Pretreatment conditions with KML29 (3 mg/kg); (C) Pretreatment conditions with PF06795071 (3 mg/kg). (D) Whole-brain TACs; (E) Area under curves. ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001, and ∗∗∗P ≤ 0.0001.
Figure 5.
Ex vivo whole-body biodistribution studies. The statistical significance was expressed with asterisks: ∗P < 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001.
3. Conclusions
We have successfully designed and synthesized a focused library of fluoropyridyl-containing MAGL inhibitor candidates on the basis of unique azabicyclo[3.1.0]hexane scaffold. The molecular interaction between these candidates and MAGL binding pocket was predicted by molecular docking studies. Pharmacological assessment by ABPP in mouse brain lysates identified 14 as a potent and selective lead compound. The radioligand [18F]14 (also called [18F]MAGL-1902) was achieved via a facile SNAr reaction on the 2-fluoropyridine scaffold with reasonable radiochemical yield, favorable molar activity and high radiochemical purity. Good BBB permeability, characteristic heterogeneous brain distribution and high in vivo binding specificity were demonstrated by PET studies. To further showcase the translation value of this [18F]14, comprehensive PET imaging studies in mouse models of MAGL deficiency and higher species with kinetic modeling is necessary in the future work. This work may provide a roadmap and guideline for further design of potent 18F-labeled MAGL PET tracers and translation into higher species, such as nonhuman primates and human subjects.
4. Experimental
4.1. General information
The experimental procedures used in this work were slightly modified from literatures41,48. All the chemicals used in the synthesis of MAGL inhibitors and the corresponding precursor were directly acquired from commercial vendors without any purification. Silica gel was used for the purification of synthetic compounds by column chromatography and 0.25 mm silica gel plates were used as indicator for TLC. To obtain the NMR spectra of synthetic compounds, a 300 MHz Bruker spectrometer was used. “ppm” was used to indicate the chemical shifts (δ) and “Hertz” was the unit of coupling constants. The abbreviations of multiplicities for peaks in HNMR and FNMR spectra were described as follows: s (singlet), d (doublet), dd (doublet of doublets), t (triplet), q (quartet), m (multiple), and br (broad signal). For the measurement of mass spectrometer, Agilent 6430 Triple Quad LC/MS was adopted with ESI as the ionization approach. No promiscuity was observed in the assay of PAINS (Pan Assay Interference Compounds) for all candidate compounds 14–17 with two in silico filters (http://zinc15.docking.org/patterns/home and http://www.swissadme.ch/index.php)55. High purity (≥95%) was also determined for lead compound 14 by a reverse-phase HPLC (Agilent 5 μm, Eclipse plus C18 column (100 mm × 4.6 mm). Unless noted otherwise, molar activity was determined at the end of synthesis. All animal studies were carried out following the ethical rules of Massachusetts General Hospital and National Institute of Radiological Sciences. CD-1 mice (female, 22–24 g, 7 weeks), SD rats (male, 210–230 g, 7 weeks) were feeded ad libitum with food and water under a condition of 12 h light/12 h dark cycle.
4.2. Radiosynthesis of [18F]14
[18F]F− was generated by the 18O(p,n)18F reaction performed in the cyclotron using 18 MeV protons and >98% enriched H218O (ROTEM Industries, Arava, Israel). An automated synthetic module was used in this work. The [18F]F− generated from the cyclotron was purified from H218O by use of an anion-exchange cartridge (Sep-Pak QMA Plus Light cartridge; Waters). The elution of [18F]F− from the cartridge was achieved with a solution of K2CO3 (0.53 mg) and Kryptofix 222 (9.4 mg) in water (250 μL) and acetonitrile (250 μL). The eluted [18F]F− solution was then transferred to a reaction vessel and dried at 110 °C with a helium flow. Then a solution of bromopyridine precursor 19 (1.5 mg) in dry-DMSO (300 μL) was added, and the reaction vial containing precursor and dry [18F]F− was heated at 150 °C for 10 min before cooling to 60 °C. Then 6 mol/L HCl (500 μL) was added and heated at 100 °C (4 min) to remove the PMB group. After cooling to room temperature, 6 mol/L NaOH (500 μL) was added and the resulting mixture was purified through a semi-preparative HPLC (CAPCELL PAK C18 UG80, 5 μL, 250 mm × 10 mm) with an eluent of CH3CN/H2O (45/55, v/v) at a flow rate of 5.0 mL/min. A wavelength of 254 nm was used for the UV monitor. The radioactive [18F]14 fraction with a retention time of 9.5 min was collected with a flask containing ethanol (300 μL), Tween 80 (75 μL), and 25% ascorbic acid aqueous solution (0.1 mL). The mixture was then concentrated in vacuo and redissolved in 3 mL of saline containing 5% ethanol to obtain [18F]14. The chemical and radiochemical purity were measured by use of an analytical HPLC (OOF-4454-YO, Gemini 5 μm, 150 mm × 3 mm) with an eluent of CH3CN/H2O (40/60, v/v) at a flow rate of 1.0 mL/min. The retention time of [18F]14 was 5.2 min. The decay-corrected radiochemical yield of [18F]14 was determined to be 11% with good molar activity (up to 210 GBq/mmol) and high radiochemical purity (>99%).
4.3. Molecular docking studies
The procedure for molecular docking studies in this work was slightly modified from literature48. We first downloaded the crystal structure of soluble human MAGL with a resolution of 1.35 Å (PDB ID: 3PE6). The original ligand was re-docked into the binding site, and its binding pose from Autodock Vina exhibited a good overlapping with the original one. Compounds 14–17 were then docked into the aforementioned 3PE6 structure.
4.4. Activity-based protein profiling (ABPP)
The procedure of ABPP assay in this work was slightly modified from literatures41,56. In brief, 1 mg/mL membrane proteomes from mouse brain were first incubated at 37 °C together with a candidate MAGL inhibitor or DMSO as negative control for 30 min. FP-rhodamine was then added to give a final concentration of 0.5 μmol/L. After incubating at room temperature for another 15 min, 4 × SDS loading buffer was introduced to stop the reaction and the reaction mixture was separated with SDS–PAGE. A ChemiDoc MP system was used to visualize the samples by in-gel fluorescence scanning. For time-dependent studies, the membrane proteomes are preincubated at 37 °C with 1 μmol/L compound 14 for 30 min before incubation with FP-Rh at room temperature for different time (1–160 min) with a final concentration of 0.5 μmol/L. Herein the reversible MAGL inhibitor FEPAD50 was choosed as the positive control. Three parallel experiments were carried out and the data was indicated as an average of 3 runs. The intensity of DMSO-treated proteomes was normalized to 100% and the relative intensity of candidate MAGL inhibitors was acquired by comparison.
4.5. CB1 and CB2 binding assays
The profiles for CB1 and CB2 binding of 14 were obtained following literatures57,58 and the procedures were described on the Website (https://pdspdb.unc.edu/pdspWeb, assay protocol book). This experiment was supported by the National Institute of Mental Health's Psychoactive Drug Screening Program. In both CB1 and CB2 agonist assays, compound CP55940 was adopted as the positive control, while in CB1 and CB2 antagonist assays, Rimonabant and SR144528 was adopted as the positive control, respectively. Three to five parallel experiments were carried out and the data was indicated as an average of 3–5 runs.
4.6. Measurement of logD
The procedure for measuring the lipophilicity in this work was slightly modified from literatures40,41. In brief, to obtain the logD values, [18F]14, n-octanol (3 mL) and PBS (0.1 mol/L, 3 mL) was mixed in a centrifugal tube, and vortex was performed for 3 min followed by 5 min's centrifuge (∼14,000 rpm). Before use of PBS and n-octanol, pre-saturation with each other needs to be performed. PBS (500 μL) and n-octanol (50 μL) were then aliquoted and weighted. An autogamma counter (Cobra Model 5002/5003) was used to determine the radioactivity. The logD was calculated with Eq. (1):
| LogD = Log[(radioactivityn-octanol/weightn-octanol)/(radioactivityPBS/weightPBS)] | (1) |
Three parallel experiments were carried out and the data was indicated as an average of 3 runs.
4.7. Small-animal PET imaging studies
The procedure for PET imaging studies in this work was slightly modified from literatures40,41. An Inveon PET scanner (Siemens) was used to acquire PET scans and during the scan 1%–2% isoflurane/air (v/v) was used to keep the Sprague–Dawley rats under anesthesia. Intravenous injection of radioligand [18F]14 (ca. 0.5 mCi/150 μL) was performed by use of a preinstalled catheter and the dynamic PET images were then collected for 90 min in a 3D mode. For blocking experiments, intravenous injection of KML29 (3 mg/kg) or PF06795071 (3 mg/kg) was carried out 30 min before injecting [18F]14. As we described previously40,59,60, ASIPro VW software was used for the reconstruction of the dynamic PET images and the achievement of the volumes of interest, such as the whole brain and various brain regions. The radioactivity was indicated with SUV as Eq. (2):
| SUV = (Radioactivity per mL tissue/Injected radioactivity) × Body weight | (2) |
4.8. Ex vivo whole body biodistribution of [18F]14 in mice
The procedure for biodistribution experiments in this work was slightly modified from literatures40,41. In brief, [18F]14 (20 μCi/100 μL) was intravenously injected via the tail vein of CD-1 mice. At different time points (5, 15, 30 and 60 min) after injecting [18F]14, the mice were sacrificed and organs of interest were collected and weighted. An autogamma counter (Cobra Model 5002/5003) was used to determine the radioactivity in each organ. All experiments were repeated 4 times and the data was an average of 4 runs indicated as the percentage of injected dose per gram of wet tissue (%ID/g).
Acknowledgments
We thank Professor Thomas J. Brady (Nuclear Medicine and Molecular Imaging, Radiology, MGH and Harvard Medical School, USA) for helpful discussion. We also thank the National Institute of Mental Health's Psychoactive Drug Screening Program (NIMH PDSP; directed by Bryan L. Roth at the University of North Carolina at Chapel Hill and Jamie Driscoll at NIMH, USA) for in vitro screening. We also gratefully acknowledge the financial support from the NIH grants (DA038000 and DA043507 to S. H. L. and DA033760 to B. F. C.) and the Swiss National Science Foundation for a postdoctoral fellowship to Michael A. Schafroth (Grant No. P2EZP3_175137, Switzerland).
Author contributions
All the authors contributed to this manuscript and have approved its final version. Zhen Chen and Steven H. Liang designed the studyand wrote the manuscript. Zhen Chen, Wakana Mori, Jian Rong, Michael A. Schafroth, Tuo Shao, Richard S. Van, Daisuke Ogasawara, Tomoteru Yamasaki, Atsuto Hiraishi, Akiko Hatori, Jiahui Chen, Yiding Zhang, Kuan Hu, Masayuki Fujinaga, Jiyun Sun and Qingzhen Yu performed experiments. Thomas L. Collier, Yihan Shao, Benjamin F. Cravatt and Lee Josephson designed and guided experiments. Ming-Rong Zhang and Steven H. Liang conceived project and wrote the manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2021.01.021.
Contributor Information
Ming-Rong Zhang, Email: zhang.ming-rong@qst.go.jp.
Steven H. Liang, Email: liang.steven@mgh.harvard.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Di Marzo V., Bisogno T., De Petrocellis L. Endocannabinoids and related compounds: walking back and forth between plant natural products and animal physiology. Chem Biol. 2007;14:741–756. doi: 10.1016/j.chembiol.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Ahn K., McKinney M.K., Cravatt B.F. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gil-Ordóñez A., Martín-Fontecha M., Ortega-Gutiérrez S., López-Rodríguez M.L. Monoacylglycerol lipase (MAGL) as a promising therapeutic target. Biochem Pharmacol. 2018;157:18–32. doi: 10.1016/j.bcp.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 4.Grabner G.F., Zimmermann R., Schicho R., Taschler U. Monoglyceride lipase as a drug target: at the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther. 2017;175:35–46. doi: 10.1016/j.pharmthera.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanda P.K., Gao Y., Mark L., Btesh J., Strassle B.W., Lu P. Monoacylglycerol lipase activity Is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- 6.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 7.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 8.Stella N., Schweitzer P., Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 9.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 10.Chevaleyre V., Takahashi K.A., Castillo P.E. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 11.Chiurchiù V., Battistini L., Maccarrone M. Endocannabinoid signalling in innate and adaptive immunity. Immunology. 2015;144:352–364. doi: 10.1111/imm.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long J.Z., Li W., Booker L., Burston J.J., Kinsey S.G., Schlosburg J.E. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long J.Z., Nomura D.K., Cravatt B.F. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomura D.K., Long J.Z., Niessen S., Hoover H.S., Ng S.W., Cravatt B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nomura D.K., Lombardi D.P., Chang J.W., Niessen S., Ward A.M., Long J.Z. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011;18:846–856. doi: 10.1016/j.chembiol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomura D.K., Morrison B.E., Blankman J.L., Long J.Z., Kinsey S.G., Marcondes M.C. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye L., Zhang B., Seviour E.G., Tao K.X., Liu X.H., Ling Y. Monoacylglycerol lipase (MAGL) knockdown inhibits tumor cells growth in colorectal cancer. Cancer Lett. 2011;307:6–17. doi: 10.1016/j.canlet.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Chen R., Zhang J., Wu Y., Wang D., Feng G., Tang Y.P. Monoacylglycerol lipase is a therapeutic target for Alzheimer's disease. Cell Rep. 2012;2:1329–1339. doi: 10.1016/j.celrep.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung K.-M., Clapper J.R., Fu J., D'Agostino G., Guijarro A., Thongkham D. 2-Arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metabol. 2012;15:299–310. doi: 10.1016/j.cmet.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Z., Mulvihill M.M., Mukhopadhyay P., Xu H., Erdélyi K., Hao E. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology. 2013;144:808–817. doi: 10.1053/j.gastro.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costola-de-Souza C., Ribeiro A., Ferraz-de-Paula V., Calefi A.S., Aloia T.P.A., Gimenes-Júnior J.A. Monoacylglycerol lipase (MAGL) inhibition attenuates acute lung injury in mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griebel G., Pichat P., Beeske S., Leroy T., Redon N., Jacquet A. Selective blockade of the hydrolysis of the endocannabinoid 2-arachidonoylglycerol impairs learning and memory performance while producing antinociceptive activity in rodents. Sci Rep. 2015;5:7642. doi: 10.1038/srep07642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulvihill M.M., Nomura D.K. Therapeutic potential of monoacylglycerol lipase inhibitors. Life Sci. 2013;92:492–497. doi: 10.1016/j.lfs.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long J.Z., Jin X., Adibekian A., Li W., Cravatt B.F. Characterization of tunable piperidine and piperazine carbamates as inhibitors of endocannabinoid hydrolases. J Med Chem. 2010;53:1830–1842. doi: 10.1021/jm9016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang J.W., Niphakis M.J., Lum K.M., Cognetta A.B., Wang C., Matthews M.L. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol. 2012;19:579–588. doi: 10.1016/j.chembiol.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afzal O., Kumar S., Kumar R., Firoz A., Jaggi M., Bawa S. Docking based virtual screening and molecular dynamics study to identify potential monoacylglycerol lipase inhibitors. Bioorg Med Chem Lett. 2014;24:3986–3996. doi: 10.1016/j.bmcl.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 27.Korhonen J., Kuusisto A., van Bruchem J., Patel J.Z., Laitinen T., Navia-Paldanius D. Piperazine and piperidine carboxamides and carbamates as inhibitors of fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) Bioorg Med Chem. 2014;22:6694–6705. doi: 10.1016/j.bmc.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Brindisi M., Maramai S., Gemma S., Brogi S., Grillo A., Di Cesare Mannelli L. Development and pharmacological characterization of selective blockers of 2-arachidonoyl glycerol degradation with efficacy in rodent models of multiple sclerosis and pain. J Med Chem. 2016;59:2612–2632. doi: 10.1021/acs.jmedchem.5b01812. [DOI] [PubMed] [Google Scholar]
- 29.Butler C.R., Beck E.M., Harris A., Huang Z., McAllister L.A., Am Ende C.W. Azetidine and piperidine carbamates as efficient, covalent inhibitors of monoacylglycerol lipase. J Med Chem. 2017;60:9860–9873. doi: 10.1021/acs.jmedchem.7b01531. [DOI] [PubMed] [Google Scholar]
- 30.Cisar J.S., Weber O.D., Clapper J.R., Blankman J.L., Henry C.L., Simon G.M. Identification of ABX-1431, a selective inhibitor of monoacylglycerol lipase and clinical candidate for treatment of neurological disorders. J Med Chem. 2018;61:9062–9084. doi: 10.1021/acs.jmedchem.8b00951. [DOI] [PubMed] [Google Scholar]
- 31.McAllister L.A., Butler C.R., Mente S., O'Neil S.V., Fonseca K.R., Piro J.R. Discovery of trifluoromethyl glycol carbamates as potent and selective covalent monoacylglycerol lipase (MAGL) inhibitors for treatment of neuroinflammation. J Med Chem. 2018;61:3008–3026. doi: 10.1021/acs.jmedchem.8b00070. [DOI] [PubMed] [Google Scholar]
- 32.Hernández-Torres G., Cipriano M., Hedén E., Björklund E., Canales Á., Zian D. A reversible and selective inhibitor of monoacylglycerol lipase ameliorates multiple sclerosis. Angew Chem Int Ed. 2014;53:13765–13770. doi: 10.1002/anie.201407807. [DOI] [PubMed] [Google Scholar]
- 33.Granchi C., Rizzolio F., Palazzolo S., Carmignani S., Macchia M., Saccomanni G. Structural optimization of 4-chlorobenzoylpiperidine derivatives for the development of potent, reversible, and selective monoacylglycerol lipase (MAGL) inhibitors. J Med Chem. 2016;59:10299–10314. doi: 10.1021/acs.jmedchem.6b01459. [DOI] [PubMed] [Google Scholar]
- 34.Aghazadeh Tabrizi M., Baraldi P.G., Baraldi S., Ruggiero E., de Stefano L., Rizzolio F. Discovery of 1,5-diphenylpyrazole-3-carboxamide derivatives as potent, reversible, and selective monoacylglycerol lipase (MAGL) inhibitors. J Med Chem. 2018;61:1340–1354. doi: 10.1021/acs.jmedchem.7b01845. [DOI] [PubMed] [Google Scholar]
- 35.Aida J., Fushimi M., Kusumoto T., Sugiyama H., Arimura N., Ikeda S. Design, synthesis, and evaluation of piperazinyl pyrrolidin-2-ones as a novel series of reversible monoacylglycerol lipase inhibitors. J Med Chem. 2018;61:9205–9217. doi: 10.1021/acs.jmedchem.8b00824. [DOI] [PubMed] [Google Scholar]
- 36.Ametamey S.M., Honer M., Schubiger P.A. Molecular imaging with PET. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 37.Willmann J.K., van Bruggen N., Dinkelborg L.M., Gambhir S.S. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 38.Miller P.W., Long N.J., Vilar R., Gee A.D. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 39.Wang L., Mori W., Cheng R., Yui J., Rotstein B., Fujinaga M. A novel class of sulfonamido [11C-carbonyl]-labeled carbamates and ureas as radiotracers for monoacylglycerol lipase. J Nucl Med. 2016;57:4. [Google Scholar]
- 40.Wang L., Mori W., Cheng R., Yui J., Hatori A., Ma L. Synthesis and preclinical evaluation of sulfonamido-based [11C-carbonyl]-carbamates and ureas for imaging monoacylglycerol lipase. Theranostics. 2016;6:1145–1159. doi: 10.7150/thno.15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng R., Mori W., Ma L., Alhouayek M., Hatori A., Zhang Y. In vitro and in vivo evaluation of 11C-labeled azetidinecarboxylates for imaging monoacylglycerol lipase by PET imaging studies. J Med Chem. 2018;61:2278–2291. doi: 10.1021/acs.jmedchem.7b01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z., Mori W., Deng X., Cheng R., Ogasawara D., Zhang G. Design, synthesis and evaluation of reversible and irreversible monoacylglycerol lipase positron emission tomography (PET) tracers using a ‘tail switching’ strategy on a piperazinyl azetidine skeleton. J Med Chem. 2019;62:3336–3353. doi: 10.1021/acs.jmedchem.8b01778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C., Placzek M.S., Van de Bittner G.C., Schroeder F.A., Hooker J.M. A novel radiotracer for imaging monoacylglycerol lipase in the brain using positron emission tomography. ACS Chem Neurosci. 2016;7:484–489. doi: 10.1021/acschemneuro.5b00293. [DOI] [PubMed] [Google Scholar]
- 44.Ahamed M., Attili B., van Veghel D., Ooms M., Berben P., Celen S. Synthesis and preclinical evaluation of [11C]MA-PB-1 for in vivo imaging of brain monoacylglycerol lipase (MAGL) Eur J Med Chem. 2017;136:104–113. doi: 10.1016/j.ejmech.2017.04.066. [DOI] [PubMed] [Google Scholar]
- 45.Yamasaki T., Mori W., Zhang Y., Hatori A., Fujinaga M., Wakizaka H. First demonstration of in vivo mapping for regional brain monoacylglycerol lipase using PET with [11C]SAR127303. NeuroImage. 2018;176:313–320. doi: 10.1016/j.neuroimage.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L., Butler C.R., Maresca K.P., Takano A., Nag S., Jia Z. Identification and development of an irreversible monoacylglycerol lipase (MAGL) positron emission tomography (PET) radioligand with high specificity. J Med Chem. 2019;62:8532–8543. doi: 10.1021/acs.jmedchem.9b00847. [DOI] [PubMed] [Google Scholar]
- 47.Hattori Y., Aoyama K., Maeda J., Arimura N., Takahashi Y., Sasaki M. Design, synthesis, and evaluation of (4R)-1-{3-[2-(18F)fluoro-4-methylpyridin-3-yl]phenyl}-4-[4-(1,3-thiazol-2-ylcarbonyl)piperazin-1-yl]pyrrolidin-2-one ([18F]T-401) as a novel positron-emission tomography imaging agent for monoacylglycerol lipase. J Med Chem. 2019;62:2362–2375. doi: 10.1021/acs.jmedchem.8b01576. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z., Mori W., Fu H., Schafroth M.A., Hatori A., Shao T. Design, synthesis, and evaluation of 18F-labeled monoacylglycerol lipase inhibitors as novel positron emission tomography probes. J Med Chem. 2019;62:8866–8872. doi: 10.1021/acs.jmedchem.9b00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schalk-Hihi C., Schubert C., Alexander R., Bayoumy S., Clemente J.C., Deckman I. Crystal structure of a soluble form of human monoglyceride lipase in complex with an inhibitor at 1.35 Å resolution. Protein Sci. 2011;20:670–683. doi: 10.1002/pro.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z., Cheng R., Yang J., Shao T., Vasdev N., Ran C. A novel 18F-labeled MAG lipase biomarker for differentiating brown and white adipose tissue in the lipid network. J Nucl Med. 2018;59:262. [Google Scholar]
- 51.OECD . OECD Publishing; 1995. Test No. 107: partition coefficient (n-octanol/water): Shake Flask method.https://www.oecd-ilibrary.org/environment/test-no-107-partition-coefficient-n-octanol-water-shake-flask-method_9789264069626-en Available from: [Google Scholar]
- 52.Waterhouse R.N. Determination of lipophilicity and its use as a predictor of blood−brain barrier penetration of molecular imaging agents. Mol Imaging Biol. 2003;5:376–389. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Patel S., Gibson R. In vivo site-directed radiotracers: a mini-review. Nucl Med Biol. 2008;35:805–815. doi: 10.1016/j.nucmedbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Pike V.W. Considerations in the development of reversibly binding PET radioligands for brain imaging. Curr Med Chem. 2016;23:1818–1869. doi: 10.2174/0929867323666160418114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baell J.B., Holloway G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 56.Cravatt B.F., Wright A.T., Kozarich J.W. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 57.Besnard J., Ruda G.F., Setola V., Abecassis K., Rodriguiz R.M., Huang X.P. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kroeze W.K., Sassano M.F., Huang X.P., Lansu K., McCorvy J.D., Giguère P.M. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol. 2015;22:362–369. doi: 10.1038/nsmb.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L., Yui J., Wang Q., Zhang Y., Mori W., Shimoda Y. Synthesis and preliminary PET imaging studies of a FAAH radiotracer ([11C]MPPO) based on α-ketoheterocyclic scaffold. ACS Chem Neurosci. 2016;7:109–118. doi: 10.1021/acschemneuro.5b00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Z., Mori W., Zhang X., Yamasaki T., Dunn P.J., Zhang G. Synthesis, pharmacology and preclinical evaluation of 11C-labeled 1,3-dihydro-2H-benzo[d]imidazole-2-ones for imaging γ8-dependent transmembrane AMPA receptor regulatory protein. Eur J Med Chem. 2018;157:898–908. doi: 10.1016/j.ejmech.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.