Abstract
This study investigated the effects of different amounts of wheat aleurone (WA) (0, 15%, 30%) inclusion in gestation diets on the reproductive performance, postprandial satiety, stress status and stereotypic behaviors of sows. A total of 84 Landrace × Yorkshire sows (parity 4.87 ± 1.32) at breeding were randomly allotted to one of the three isoenergetic and isonitrogenous dietary treatments based on parity and body weight. The results showed that, compared with the control (0), sows fed the WA diet had a higher serum concentration of peptide YY (PYY) (P < 0.05) and glucagon like peptide-1 (GLP-1) (P < 0.05) and a lower concentration of saliva cortisol (P < 0.01). Importantly, compared with the control group, only the 15% WA group had a higher concentration of the total antioxidant capacity (T-AOC) (P < 0.05), lower proportions of sitting (P = 0.05) and stillbirth rates (P < 0.01). Accordingly, the production cost per piglet born alive ($ 6.9 vs. $ 7.6) or per piglet born healthy ($ 7.4 vs. $ 7.9) declined in the 15% WA group versus the control group. Overall, 15% WA inclusion in gestation diets contributed to enhancing postprandial satiety, alleviating stress status and decreasing stillbirth rate of sows. This study provides a reference for the application of WA as a partial substitute for conventional feed ingredients to improve sows’ reproductive performance.
Keywords: Wheat aleurone, Stillbirth rate, Stress, Postprandial satiety, Sow
1. Introduction
Generally, pregnant sows are fed a restricted diet during gestation to avoid excess weight gain and associated farrowing and locomotion problems (Meunier-Salaün et al., 2001). However, restricting pregnant sows’ diets is considered to cause hunger and has been raised as a welfare concern (Knudsen, 2019). Furthermore, insufficient satiety may result in the development of stress and abnormal behavior in gestating sows, increase their bodily injury, and produce a negative influence on their reproductive performance (Sekiguchi and Koketsu, 2004; Vanderhaeghe et al., 2013).
Inclusion of fiber in gestation diets is a method for promoting satiety and reducing apparent feeding motivation in sows without providing excess energy, thereby improving their welfare under feed restriction during pregnancy. Several studies have shown that fiber inclusion in gestation diets increases postprandial satiety as a consequence of gut-fill, delay of gastric emptying, and release of satiety-inducing gut peptides (Meunier-Salaün et al., 2001; Quesnel et al., 2009; Sun et al., 2015; Huang et al., 2020). However, a previous study indicated that the inclusion of dietary fiber in sows’ gestation diet produces no effects on stereotypic behaviors and stillbirth rate (Holt et al., 2006). This difference in research results may be attributable to the different type and amount of dietary fibers included in the diets (Jensen et al., 2012; Souza da Silva et al., 2013; Sun et al., 2015).
The wheat aleurone (WA) layer, also known as the outer endosperm, is the innermost layer of the wheat grain cortex for storage of minerals, vitamins, and bioactive phytochemicals (Brouns et al., 2012). WA seems to be the key component in the whole grain antioxidant potential, with ferulic acid as the main responsible compound (Brouns et al., 2012). The previous study showed that the contribution of ferulic acid to the antioxidant capacity was larger in the aleurone fractions (41% to 60%) than in the barn fractions (20% to 47%) (Mateo Anson et al., 2008), which may avoid the oxidative damage by the electron donation and the transfer of hydrogen atoms to free radicals (Graf, 1992). Moreover, WA contains 37.2% total dietary fiber, which can be regarded as a content source of dietary fiber. Studies in rats or in vitro have shown that WA contributes to increasing antioxidant capacity, decreasing pro-inflammatory factors or the risk of colon cancer, attenuating postprandial blood glucose level, and reducing symptoms of hypertension as well as hyperglycemia (Sagara et al., 2007; Anson et al., 2010; Stein et al., 2010; Brouns et al., 2012; Rosa et al., 2013; Malunga et al., 2017). However, the effects of including WA in gestation diets on the satiety and reproductive performance of sows have not been reported.
The purpose of this study is to investigate the effects of WA addition to the diets of sows in gestation on postprandial satiety, stress status and reproductive performance. The results will facilitate the understanding and development of effective sow gestation diets to improve their reproductive performance and welfare under feed restriction.
2. Materials and methods
All animal procedures in this study were conducted under the protocol approved by the South China Agricultural University Animal Care and Use (No. 20110107-1, Guangzhou, China).
2.1. Animals, experiment design and housing
A total of 84 mixed-parity Landrace × Yorkshire sows (parity 4.87 ± 1.32; backfat thickness 17.63 ± 3.97 mm, mean ± SD) were allotted to 1 of the following 3 dietary treatments based on parity and body weight, with 28 replicates per dietary treatment at breeding. Three isoenergetic and isonitrogenous gestation diets were formulated (Table 1): a control diet based on corn, wheat bran and soybean meal (control diet); the second diet included 15% wheat aleurone (15% WA diet); and the third diet included 30% wheat aleurone (30% WA diet). The WA was provided by Jiaxing Zhishifang Food Science and Technology Co., Ltd (Jiaxing, China). It was separated and extracted from wheat bran by the company using Vortex Cyclone Separation Technology.
Table 1.
Ingredients and nutrient composition of experimental gestation diets and lactation diets (%, as-fed basis).
| Item | Gestation diets1 |
Lactation diet | ||
|---|---|---|---|---|
| CON | 15%WA | 30%WA | ||
| Ingredients | ||||
| Corn | 61.6 | 60.1 | 54.5 | 61.0 |
| Soybean meal, 43% CP | 11.0 | 10.5 | 6.0 | 19.5 |
| Wheat bran | 20.0 | 0.0 | 0.0 | – |
| Wheat aleurone2 | – | 15.0 | 30.0 | – |
| Soybean hull | 3.0 | 10.0 | 5.0 | 5.0 |
| Wheat middlings | – | – | – | 1.5 |
| Corn meal | – | – | – | 0.4 |
| Fish meal, 67% CP | – | – | – | 1.0 |
| Extruded soybean | – | – | – | 5.5 |
| Soybean oil | – | – | – | 0.6 |
| Glucose | – | – | – | 1.3 |
| Dicalcium phosphate | 1.7 | 1.7 | 1.7 | 1.6 |
| Limestone | 1.0 | 1.0 | 1.0 | 0.9 |
| Salt | 0.5 | 0.5 | 0.5 | 0.5 |
| Lysine sulfate (70%) | 0.2 | 0.2 | 0.3 | 0.3 |
| Threonine | – | – | – | 0.1 |
| Methionine | – | – | – | 0.1 |
| Choline chloride | 0.1 | 0.1 | 0.1 | 0.1 |
| Sodium bicarbonate | 0.4 | 0.4 | 0.4 | 0.4 |
| Mildewcide | 0.1 | 0.1 | 0.1 | – |
| Premix3 | 0.4 | 0.4 | 0.4 | 0.4 |
| Nutrient composition | ||||
| Digestive energy, Mcal/kg | 2.99 | 3.03 | 2.99 | 3.30 |
| Crude protein | 13.32 | 13.34 | 13.35 | 17.61 |
| Ether extract | 3.15 | 3.01 | 3.32 | 4.05 |
| Crude fiber | 5.35 | 7.47 | 6.17 | 3.80 |
| Neutral detergent fiber | 17.02 | 18.54 | 19.13 | 12.26 |
| Calcium | 0.84 | 0.86 | 0.83 | 0.85 |
| Total phosphorus | 0.73 | 0.70 | 0.80 | 0.64 |
| Lys | 0.71 | 0.71 | 0.68 | 1.15 |
| Met | 0.23 | 0.23 | 0.21 | 0.34 |
| Thr | 0.48 | 0.47 | 0.43 | 0.78 |
| Trp | 0.17 | 0.16 | 0.16 | 0.19 |
CON, control diet group; 15% WA, 15% wheat aleurone diet group; 30% WA, 30% wheat aleurone diet group.
Wheat aleurone contains gross energy 4.67 Mcal/kg, 21% crude protein, 37.2% total dietary fiber, 34.5% insoluble fiber, 2.7% soluble fiber, 8.7% crude fiber and 26.3% neutral detergent fiber. Wheat aleurone contains total ferulic acid 2,265 μg/g.
Provided the following per kilogram of diet: Fe 145 mg as ferrous sulfate; Zn 75 mg as zinc sulfate; Mn 50 mg (as MnO2); Cu 10.0 mg (as CuSO4·5H2O); Se 0.3 mg selenium selenite; I 0.25 mg as potassium iodide; Co 0.1 mg; vitamin A 7,500 IU; vitamin D3 4,992 IU; vitamin E 215.2 mg; vitamin C 200 mg; niacin 50 mg; riboflavin 22 mg; pyridoxine 8.5 mg; vitamin K 35.1 mg; folic acid 4.5 mg; ammonium 3.7 mg.
The sows were housed in individual stalls during gestation and the diets were supplied twice a day (07:00 and 17:00) as restricted-feeding from mating until the day of farrowing, with half of the daily feed given at each meal. Daily allowances during gestation are presented in Appendix Table 1. At day 109 of gestation, the sows were moved to farrowing rooms and kept in individual farrowing crates with stalls in pens to provide space for the pigs after birth. In contrast, all sows were fed ad libitum the same lactation diets (Table 1), which were supplied 4 times a day (07:00, 11:30, 15:30 and 18:00). Sows and piglets were given free access to drinking water, and piglets were not provided with creep feed during lactation. During the experimental period, sows with illness, serious lameness, death and reproductive failure, such as return to estrus, abortion or non-pregnant after breeding, were culled from further analysis (Appendix Table 2).
2.2. Performance measurement
Body weight and backfat thickness of sows were measured on day 0 and 109 of gestation and at weaning. Backfat thickness was measured at 65 mm on each side of the dorsal midline at the last rib level (P2) using ultrasound (Renco Lean-Meatier; Renco Corporation, Minneapolis, MN). At farrowing, the birthweight of piglets born alive and the number of total piglets born (which included live piglets, stillborn piglets, malformed piglets, and mummified fetuses) were recorded respectively. Cross-fostering was kept within diet treatments to adjust litter size to about 12.0 ± 0.5 piglets per sow within 48 h after parturition. Piglets were weaned at an average age of 22 ± 1 days. At weaning, the number of weaned piglets was recorded. Piglets were weighed within 24 h of birth (day 0), after cross-fostering (day 1), on day 7, 14, 21 and at weaning. The daily feed intake of sows during lactation was measured each morning by weighing daily feed refusals. After weaning, the sows were transferred to the gestation house. Estrous detection was done every day in the presence of a boar, and the time of estrus was recorded, which was used to calculate the wean-to-estrus interval.
2.3. Behavior determination
On day 71, 72 and 73 of gestation, 24 sows (8 replicates per dietary treatment) were observed successively by 2 video-cameras at 2 h after morning feedings. Each sow was observed for a total of 60 min (5 min × 12 sessions) within each observation period (Sun et al., 2015), and the final ethogram is provided in Appendix Table 3 (de Leeuw et al., 2008).
2.4. Blood sampling and saliva sampling
Twenty-four sows (8 sows per dietary treatment) were selected for blood sampling. This was done at intervals of the following: before feeding and 2 h after feeding on day 70 of gestation (G70d), before feeding on day 109 of gestation (G109d), and on day 0 of lactation and the day of weaning. Blood samples (10 mL) were collected from the ear vein of fasting sows into vacuum tubes (5 mL) and heparinized tubes (5 mL) by veterinarian (Tan et al., 2015b). Serum samples were obtained by centrifuging the blood samples in the vacuum tubes at 3,000 × g and 4 °C for 15 min, and stored at −80 °C for further analysis. Plasma samples were obtained by centrifuging the blood samples in the labeled heparinized tubes at 3,000 × g and 4 °C for 15 min, and stored at −80 °C for further analysis.
Before feeding on day 70 of gestation, 24 sows (8 sows per dietary treatment) were selected for saliva sampling. The saliva samples of sows (3 mL) were collected using a saliva collection kit provided by Guangdong Dream Biotechnology Co., Ltd (Guangzhou, China). The samples were centrifuged for 20 min at 2,000 × g and 4 °C for separation of supernatant, and stored at −80 °C for further analysis.
2.5. Fecal sampling
On day 60 of gestation, 18 Sows (6 sows per dietary treatment) were fed their respective experimental diets containing 0.3% chromic oxide as an exogenous marker. The experimental diets were fed for a 6-day adjustment period, followed by a 3-day collection period. Fecal samples were collected twice daily at 07:00 and 15:30, pooled, placed in plastic bags, and frozen at −20 °C. After sample collection, the fecal samples for each sow were thawed and pooled together, followed by drying in a forced-draft oven (65 °C) for 72 h, grinding through a 1-mm screen, and thorough mixing before a subsample was collected for chemical analysis. The digestibility of dietary nutrients was evaluated by the equation: nutrient digestibility = 1 − (TC × FN)/(FC × TN) (Zhao et al., 2018), where TC and TN are chromic oxide and nutrient contents in diets, respectively; FC and FN, the chromic oxide and nutrient content in feces, respectively. The apparent total tract digestibility of gross energy (GE), dry matter (DM), crude protein (CP) and neutral detergent fiber (NDF) in diets was calculated using the direct method (Adeola, 2000).
2.6. Chemical analysis and calculation
After grinding through a 1-mm screen, all samples were analyzed in duplicate. GE was determined by an automatic adiabatic oxygen bomb calorimeter (IKA-Werke GmbH & Co. Kg. Calorimeter system C200); DM by AOAC 2006, method 934.01; CP by AOAC 2006, method 990.03; crude fiber (CF) by AOAC 2000, method 978.10. NDF was analyzed according to the method described by Van Soest et al. (1991). The level of total ferulic acid in WA was measured by HPLC analysis as previously reported (Kim et al., 2006).
Serum pig peptide YY (PYY) was analyzed by the Pig PYY enzyme linked immunosorbent assay (ELISA) kit (CSB-EL019128PI, CUSABIO, Wuhan, Hubei, China). Serum glucagon like peptide-1 (GLP-1) was analyzed using the Pig GLP-1 ELISA kit (MM-37279O2, MEIMIAN, Yancheng, Jiangsu, China). Plasma cortisol was analyzed using the Pig Cortisol ELISA Kit (CSB-E06811p, CUSABIO, Wuhan, Hubei, China).
The contents of total antioxidant capacity (T-AOC), malondialdehyde (MDA), 8-hydroxy-deoxyguanosine (8-OHdG), and protein carbonyl in the serum of G109d sows were determined using assay kits according to the manufacturers’ instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.7. Statistical analysis
The number of sows during the experimental period is shown in Appendix Table 2. Data are presented as means ± SEM. An individual sow was considered as the experimental unit in all statistical analyses. Before analysis, the data were tested for normality and homoscedasticity using the Kolmogorov–Smirnov and Levene tests (with the significance level set at 5%). Then the data were subjected to ANOVA using the GLM procedure of SAS 9.2 (SAS Institute Inc., Cary, NC, USA) in a completely randomized design. Repeated measures analysis of variance as implemented in the MIXED procedure of SAS was used to examine the responses of litter performance. This model included the effects of dietary treatment, parity of sows, number and birth weight of piglets, with dietary treatment considered as the main effect, and the other 3 as random effects. Regression analysis was performed to evaluate the linear and quadratic effects of different levels of WA. The stillbirth rate was calculated with the chi-square test. Differences were considered as statistically significant at P ≤ 0.05 and as a trend to significance at 0.05 < P < 0.10. Probability values ≤ 0.01 are considered as highly statistically significant.
3. Results
3.1. Sow and piglet performance
Table 2, Table 3, Table 4 show the effects of different amounts of WA inclusion in gestation diets on performance of sows and piglets.
Table 2.
Effects of different amounts of wheat aleurone inclusion in gestation diets on the digestibility of energy and nutrients.
| Item | Diet1 |
SEM |
P-value2 |
||||
|---|---|---|---|---|---|---|---|
| CON | 15%WA | 30%WA | Diet | Linear | Quadratic | ||
| No. of sows | 6 | 6 | 6 | ||||
| DM | 82.9 | 82.8 | 82.2 | 0.8 | 0.91 | 0.69 | 0.91 |
| GE | 85.7 | 84.7 | 84.3 | 0.7 | 0.72 | 0.42 | 0.72 |
| CP | 84.2 | 81.8 | 83.4 | 0.7 | 0.43 | 0.68 | 0.43 |
| NDF | 60.8 | 65.0 | 62.8 | 2.0 | 0.71 | 0.69 | 0.70 |
DM = dry matter; GE = gross energy; CP = crude protein; NDF = neutral detergent fiber.
CON, control diet group; 15% WA, 15% wheat aleurone diet group; 30% WA, 30% wheat aleurone diet group.
Linear (L) and quadratic (Q) effects of inclusion amounts of wheat aleurone were contrasted.
Table 3.
Effects of different amounts of wheat aleurone inclusion in gestation diets on body weight, backfat thickness, weaning to estrus interval and feed intake of sows during lactation.
| Item | Diet1 |
SEM |
P-value2 |
||||
|---|---|---|---|---|---|---|---|
| CON | 15%WA | 30%WA | Diet | Linear | Quadratic | ||
| No. of sows | 22 | 21 | 23 | ||||
| Average sow parity | 4.5 | 4.5 | 4.8 | ||||
| BW of sows, kg | |||||||
| Mating | 228.3 | 223.7 | 226.9 | 2.9 | 0.81 | 0.86 | 0.81 |
| Day 109 of gestation3 | 288.3 | 286.8 | 282.8 | 2.7 | 0.71 | 0.42 | 0.70 |
| Gain3 | 59.9 | 63.1 | 55.9 | 1.9 | 0.28 | 0.37 | 0.31 |
| Parturition | 260.9 | 260.4 | 256.9 | 2.7 | 0.81 | 0.55 | 0.81 |
| Weaning | 247.4 | 251.4 | 244.9 | 2.8 | 0.64 | 0.70 | 0.64 |
| Loss during lactation | 13.5 | 9.0 | 11.9 | 1.4 | 0.42 | 0.69 | 0.42 |
| Sow backfat thickness, mm | |||||||
| Mating3 | 17.5 | 17.5 | 17.2 | 0.5 | 0.92 | 0.82 | 0.97 |
| Day 109 of gestation3 | 17.9 | 18.2 | 18.2 | 0.6 | 0.73 | 0.86 | 0.98 |
| Weaning3 | 16.9 | 17.9 | 16.5 | 0.5 | 0.68 | 0.75 | 0.53 |
| Backfat gain during gestation3 | 0.4 | 0.8 | 1.0 | 0.6 | 0.72 | 0.70 | 0.93 |
| Backfat loss during lactation | 1.1 | 0.3 | 1.7 | 0.4 | 0.40 | 0.51 | 0.40 |
| Weaning to estrus interval3, d | 4.2 | 4.1 | 4.1 | 0.1 | 0.83 | 0.53 | 0.80 |
| Average daily feed intake4, kg | |||||||
| 1st week of lactation | 5.3 | 5.3 | 5.1 | 0.1 | 0.88 | 0.67 | 0.88 |
| 2 nd week of lactation | 7.2 | 7.0 | 7.2 | 0.1 | 0.69 | 0.77 | 0.69 |
| 3rd week of lactation3 | 7.8 | 7.5 | 7.6 | 0.1 | 0.59 | 0.40 | 0.55 |
| Mean of 1st week to 3rd week | 6.8 | 6.6 | 6.6 | 0.1 | 0.71 | 0.49 | 0.71 |
CON, control diet group; 15% WA, 15% wheat aleurone diet group; 30% WA, 30% wheat aleurone diet group.
Linear (L) and quadratic (Q) effects of inclusion amounts of wheat aleurone were contrasted.
Results were analyzed using the Kruskal–Wallis test.
Number of sows in CON, 15% WA and 30% WA is 19, 21 and 21, respectively.
Table 4.
Effects of different amounts of wheat aleurone inclusion in gestation diets on sows’ reproductive performance and litter performance.
| Item | Diet1 |
SEM |
P-value2 |
||||
|---|---|---|---|---|---|---|---|
| CON | 15%WA | 30%WA | Diet | Linear | Quadratic | ||
| No. of sows | 22 | 21 | 23 | ||||
| Average sow parity | 4.5 | 4.5 | 4.8 | ||||
| Total piglets born3 | 15.6 | 16.1 | 15.3 | 0.5 | 0.92 | 0.79 | 0.78 |
| Piglets born alive3 | 13.3 | 14.8 | 13.7 | 0.4 | 0.53 | 0.67 | 0.30 |
| Piglets stillborn3 | 2.0 | 1.1 | 1.3 | 0.2 | 0.14 | 0.12 | 0.08 |
| Litter weight alive at parturition3, kg | 18.7 | 20.0 | 18.6 | 0.5 | 0.45 | 0.94 | 0.47 |
| Average weight of piglets born alive3, kg | 1.4 | 1.4 | 1.4 | 0.03 | 0.66 | 0.68 | 0.85 |
| No. of pigs per litter3,4 | |||||||
| After cross-foster | 12.1 | 11.7 | 11.5 | 0.1 | 0.29 | 0.11 | 0.26 |
| On day 7 | 11.4 | 10.9 | 10.9 | 0.1 | 0.20 | 0.16 | 0.25 |
| On day 14 | 11.0 | 10.5 | 10.7 | 0.2 | 0.42 | 0.60 | 0.54 |
| On day 21 | 10.6 | 10.4 | 10.4 | 0.2 | 0.53 | 0.61 | 0.84 |
| Piglet mean BW4, kg | |||||||
| After cross-foster | 1.6 | 1.5 | 1.5 | 0.04 | 0.95 | 0.78 | 0.95 |
| On day 7 | 3.0 | 3.0 | 3.0 | 0.1 | 0.87 | 0.95 | 0.97 |
| On day 14 | 5.0 | 4.9 | 4.8 | 0.1 | 0.29 | 0.44 | 0.74 |
| On day 21 | 6.7 | 6.7 | 6.6 | 0.1 | 0.54 | 0.28 | 0.53 |
| Piglet average daily gain4, g/d | |||||||
| Day 1 to 73 | 203.3 | 214.4 | 199.1 | 5.7 | 0.49 | 0.70 | 0.56 |
| Day 7 to 14 | 269.8 | 271.5 | 259.2 | 6.0 | 0.58 | 0.49 | 0.71 |
| Day 14 to 21 | 260.4 | 260.7 | 240.7 | 6.3 | 0.34 | 0.14 | 0.25 |
| Day 1 to 21 | 248.6 | 247.2 | 237.4 | 4.4 | 0.46 | 0.24 | 0.76 |
| Litter weight4, kg | |||||||
| After cross-foster | 18.7 | 18.2 | 17.4 | 0.4 | 0.42 | 0.19 | 0.42 |
| On day 7 | 33.5 | 32.8 | 34.0 | 0.8 | 0.64 | 0.40 | 0.66 |
| On day 14 | 52.0 | 52.6 | 52.4 | 1.2 | 0.92 | 0.51 | 0.80 |
| On day 21 | 70.1 | 70.5 | 69.0 | 1.7 | 0.62 | 0.38 | 0.68 |
CON, control diet group; 15% WA, 15% wheat aleurone diet group; 30% WA, 30% wheat aleurone diet group.
Linear (L) and quadratic (Q) effects of inclusion amounts of wheat aleurone were contrasted.
Results were analyzed using the Kruskal–Wallis test. Values are means ± Pooled SEM.
Number of sows in CON, 15% WA and 30% WA is 19, 21 and 21, respectively.
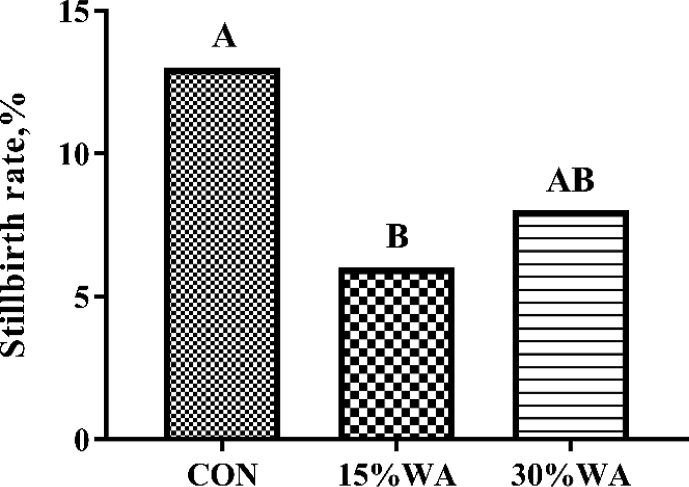
The three dietary treatments showed no difference (P > 0.05) in the body weight and backfat thickness of sows at mating, on day 109 of gestation, at farrowing and at weaning, nor in average daily feed intake during overall lactation (P > 0.05). Additionally, WA inclusion in sows’ gestation diets showed no influence (P > 0.05) on the body weight or backfat thickness gain during gestation or loss during lactation, or weaning-to-estrus interval of sows (Table 3). However, sows fed 15% WA diet showed significantly lower stillbirth rate than those fed the control diet (P < 0.01) (Fig. 1).
Fig. 1.
Effects of different amounts of wheat aleurone inclusion in gestation diets on stillbirth rate. CON, control diet group; 15% WA, 15% wheat aleurone diet group; 30% WA, 30% wheat aleurone diet group. Stillbirth rate was analyzed using the Chi-square test. Different capital letters represent the extremely significant difference at P < 0.01.
When compared with the control (0), WA inclusion (15% and 30%) showed no obvious impact (P > 0.05) on the numbers of total piglets born, piglets born alive, piglet stillbirth and pigs per litter at birth and day 7, 14 and 21 of lactation. Meanwhile, the three treatments exhibited no difference in the litter weight, average piglet weight and average daily gain of suckling piglets during overall lactation (P > 0.05) (Table 4).
3.2. Behavior determinations
Table 5 shows the effects of WA inclusion on the behavior of pregnant sows. The sows in the 3 treatments exhibited no differences (P > 0.05) in physical activity (standing, lying, kneeling and position change), drinking behavior, sham-chewing behavior, sniffing and licking behavior (de Leeuw et al., 2008). However, compared with the control (0), the 15% WA group showed a significant decline (P = 0.05) in the proportion of sitting behavior of sows as well as 86% and 67% decrease (P > 0.05) in the time spent on sham-chewing behavior (1.8% vs. 13.1%) and sniffing and licking behavior (0.6% vs. 1.9%) of sows (Table 5). The frequency of position change behavior was twice lower in the 15% treatment than in the control (P > 0.05) (Table 5).
Table 5.
Effects of different amounts of wheat aleurone inclusion in gestation diets on behavior of pregnant sows.1
| Item | Diet2 |
SEM |
P-value3 |
||||
|---|---|---|---|---|---|---|---|
| CON | 15%WA4 | 30%WA | Diet | Linear | Quadratic | ||
| No. of sows | 8 | 6 | 8 | ||||
| Lying | 91.9 | 93.3 | 90.2 | 2.0 | 0.57 | 0.74 | 0.70 |
| Standing | 7.7 | 6.7 | 5.9 | 1.5 | 0.89 | 0.62 | 0.89 |
| Sitting | 0.5 | 0.0 | 3.9 | 1.0 | 0.05 | 0.15 | 0.23 |
| Drinking, times | 2.0 | 2.0 | 2.4 | 1.0 | 0.72 | 0.76 | 0.94 |
| Sham-chewing | 13.1 | 1.8 | 4.2 | 2.9 | 0.22 | 0.19 | 0.25 |
| Sniffing, licking | 1.9 | 0.6 | 2.5 | 0.6 | 0.48 | 0.73 | 0.53 |
| Position change, frequency | 3.6 | 1.7 | 4.1 | 0.5 | 0.25 | 0.7 | 0.17 |
Results are presented as the proportion of observation time. Results were analyzed using the Kruskal–Wallis test.
CON, control diet group; 15% WA, 15% wheat aleurone diet group; 30% WA, 30% wheat aleurone diet group.
Linear (L) and quadratic (Q) effects of inclusion amounts of wheat aleurone were contrasted.
Two sows with serious lameness were culled.
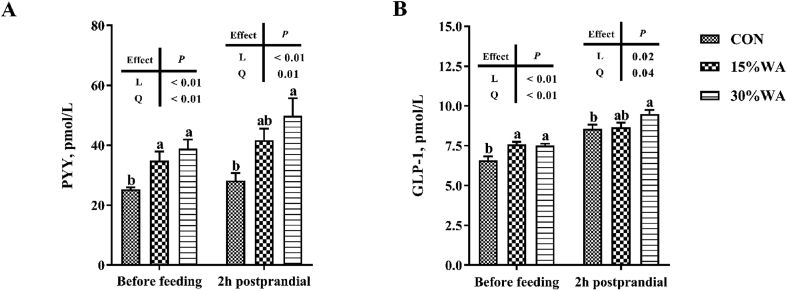
3.3. Serum concentration of PYY and GLP-1
Figure 2 shows the effects of different amounts of WA inclusion on the serum concentration of PYY and GLP-1 of sows at day 70 of gestation. The sows’ serum concentrations of pre-prandial and 2 h postprandial PYY and GLP-1 of sows on day 70 of gestation were shown to increase significantly, linearly and quadratically (P < 0.05) with increasing WA amount.
Fig. 2.
Effects of different amounts of wheat aleurone inclusion in gestation diets on serum concentration of peptide YY (PYY) and glucagon like peptide-1 (GLP-1) on sows on day 70 of gestation. CON, control diet group; 15% WA, 15% wheat aleurone diet group; 30% WA, 30% wheat aleurone diet group. Values are means ± SEM, n = 6 to 8. Linear (L) and quadratic (Q) effects of the inclusion amounts of wheat aleurone were contrasted. Different lowercase letters represent significant difference at P < 0.05.
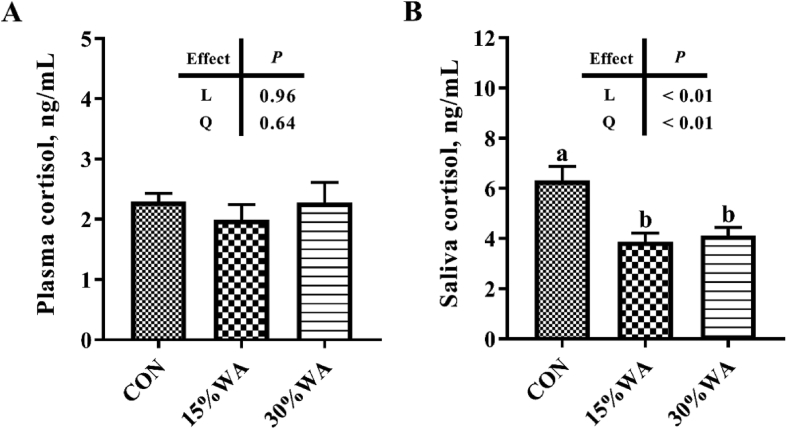
3.4. Plasma and saliva concentration of cortisol
Figure 3 shows the effects of different amounts of WA inclusion on plasma and saliva concentrations of cortisol of sows on day 70 of gestation. The 3 groups exhibited no differences (P > 0.05) in the plasma concentrations of pre-prandial cortisol of sows on day 70 of gestation (Fig. 3A). However, the saliva concentrations of pre-prandial cortisol of sows on day 70 of gestation were shown to decrease significantly, linearly and quadratically (P < 0.01) with increasing WA addition (Fig. 3B).
Fig. 3.
Effects of different amounts of wheat aleurone inclusion in gestation diets on plasma and saliva concentration of cortisol on sows on day 70 of gestation. CON, control diet group; 15% WA, 15% wheat aleurone diet group; 30% WA, 30% wheat aleurone diet group. Values are means ± SEM, n = 7. Linear (L) and quadratic (Q) effects of inclusion amounts of wheat aleurone were contrasted. Different lowercase letters represent significant difference at P < 0.05.
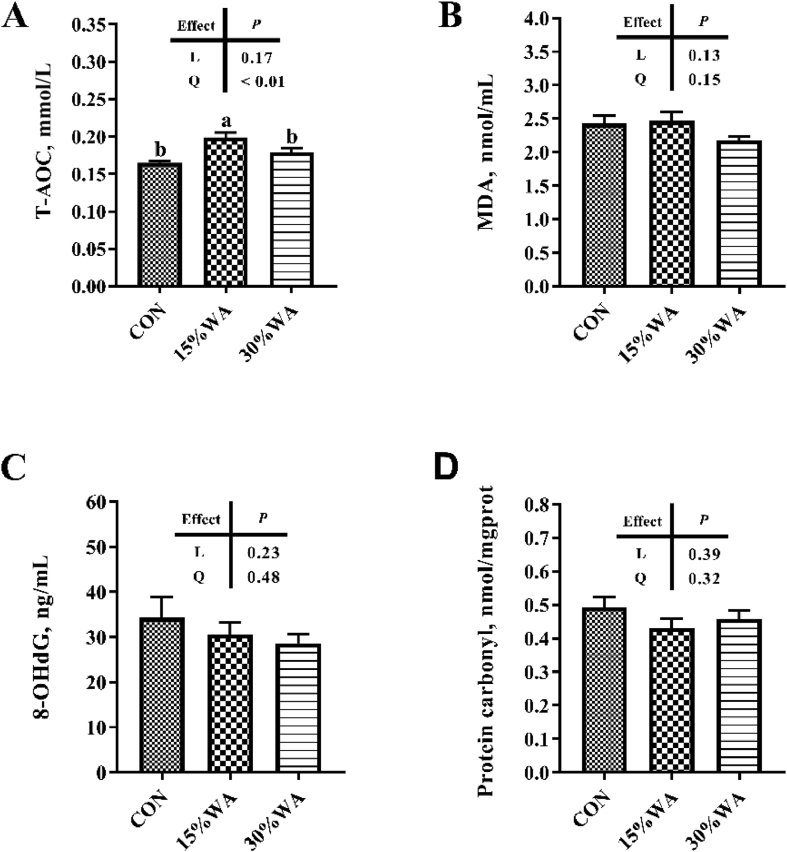
3.5. Concentration of serum oxidative stress parameter of G109d sows
Figure 4 shows the effects of different amounts of WA inclusion on the serum levels of T-AOC, MDA, 8-OHdG and protein carbonyl of sows on day 109 of gestation. The 3 groups exhibited no obvious differences (P > 0.05) in serum concentrations of MDA, 8-OHdG and protein carbonyl. However, the 15% treatment showed higher concentrations of sows’ serum T-AOC than the control or 30% treatment (P < 0.05) (Fig. 4).
Fig. 4.
Effects of different amounts of wheat aleurone inclusion in gestation diets on serum levels of T-AOC(A), MDA(B), 8-OHdG (C) and protein carbonyl of G109d sows; T-AOC = total antioxidant capacity; MDA = malondialdehyde; 8-OHdG = 8-hydroxy-deoxyguanosine. CON, control diet group; 15% WA, 15% wheat aleurone diet group; 30% WA, 30% wheat aleurone diet group. Values are means ± SEM, n = 8. Linear (L) and quadratic (Q) effects of inclusion amounts of wheat aleurone were contrasted. Different lowercase letters represent significant difference at P < 0.05.
3.6. Economic benefit analysis
Table 6 shows the economic benefit analysis results of different amounts of WA inclusion in the gestation diets. The production cost per piglet born alive ($ 6.9 vs. $ 7.6) or per piglet born healthy ($7.4 vs. $7.9) was shown to decline in the 15% WA group versus the control group.
Table 6.
Economic benefit analysis of different amounts of wheat aleurone inclusion in the gestation diets.
| Item | Diet1 |
||
|---|---|---|---|
| CON | 15%WA | 30%WA | |
| Feed price, $/1,000 kg | 373.1 | 373.8 | 390.2 |
| Feed consumption during gestation per sow, kg | 271.0 | 271.0 | 271.0 |
| Feed cost per sow, $ | 101.1 | 101.3 | 105.7 |
| Piglets born alive | 13.3 | 14.8 | 13.7 |
| Production cost per piglet born alive, $ | 7.6 | 6.9 | 7.7 |
| Piglets born healthy | 12.8 | 13.6 | 12.9 |
| Production cost per piglet born healthy, $ | 7.9 | 7.4 | 8.2 |
CON, control diet group; 15% WA, 15% wheat aleurone diet group; 30% WA, 30% wheat aleurone diet group.
4. Discussion
In the last 2 decades, with genetic improvement and an increase in the total number of piglets born per litter, the number of stillborn piglets has also increased, representing a major economic cost to the pig industry (Lewis and Hermesch, 2013; Yang et al., 2019). In the present study, the 15% WA treatment group showed a decline in the number and the rate of stillborn piglets, which agreed with the result reported by Feyera et al. who showed that the supplemented dietary fiber reduced the proportion of stillborn piglets (Feyera et al., 2017). Additionally, it also increased economic benefits. As shown in Table 6, the production cost per piglet born alive ($ 6.9 vs. $ 7.6) or per piglet born healthy ($ 7.4 vs. $ 7.9) was lower in the 15% WA group than the control group, which is of high realistic significance to the pig production industry, especially at a time when the outbreak of African swine fever in China has caused a sharp decline in the number of pigs.
It is worth noting that different amounts of WA inclusion in the gestation diets of sows did not affect the nutrient digestibility, thus leading to no weight changes during pregnancy. This suggests that WA can serve as a fiber source for long-term bulk addition to pregnant sow diets to replace part of the protein and energy feed ingredients.
As reported previously, an increased stillbirth rate was positively correlated with a higher oxidative stress status in late pregnancy of sows (Zhao et al., 2013; Wang et al., 2019a). There is an increased systemic oxidative stress during late gestation of sows (Tan et al., 2015a; Wang et al., 2019a), which can alter maternal parameters important for neonatal survival, such as colostrum production and immunoglobulin content, as well as maternal care to the newborns (Merlot et al., 2013). In this study, sows fed 15% WA diet showed higher concentrations of serum T-AOC on day 109 of gestation than those fed the control diet, thus enhancing the antioxidant capacity of pregnant sows and contributing to the low stillbirth rate in the 15% WA group. Besides, our results showed that, despite no statistical difference, the 15% WA group was also lower than the control group in the serum protein carbonyl concentration, which could contribute to the decreased stillbirth rate in 15% WA group. This was consistent with a previous report showing that the number of stillborn piglets was positively correlated with protein carbonyl concentration on day 90 of gestation (Zhao et al., 2013). Eight-hydroxy-deoxyguanosine is the major marker for oxidative damage to nucleic acids, which was reported to be negatively correlated with the litter weight of sows (Zhao et al., 2013). In the present study, the 3 groups showed no difference in the level of 8-OHdG, which agreed with no change in the litter weight of the 3 dietary treatments.
Studies have shown that the active components of WA have certain antioxidant capacity (Brouns et al., 2012). Ferulic acid can mitigate oxidative stress by electron donation and hydrogen atom transfer to free radicals (Graf, 1992). However, the amount of ferulic acid is not directly proportional to the body's antioxidant capacity. Higher amounts of ferulic acid may have no effect on the oxidative stress state of the body, and may even exacerbate oxidative stress (Wang et al., 2019b). This may explain why the 30% WA group had no effect on the antioxidant capacity of pregnant sows.
The stillbirth rate was reported to increase with higher levels of cortisol in pregnant sheep (Antolic et al., 2019). In our study, the WA group had lower saliva concentrations of pre-prandial cortisol than the control diet group, which contributed to the reduction or downward trend for the stillbirth rate in the 15% and 30% WA groups, respectively. One point we must emphasize is that the saliva cortisol content can reliably reflect the activity of the hypothalamic–pituitary–adrenal axis (HPA-axis), which is a more practical method than blood collection in stress research as it can avoid elevated cortisol levels induced by blood collection stress (Cook et al., 1996). The results indicated that feeding sows with WA can decrease the stress levels of pregnant sows, which in turn may reduce the stillbirth rate.
A growing number of studies have indicated that animal welfare is impaired in many conditions and should be improved (Sapkota et al., 2016; Greenwood et al., 2019; Maes et al., 2019). Gestating sows tend to suffer insufficient satiety frequently due to feed restriction (De Leeuw et al., 2005; Schneider et al., 2007; de Leeuw et al., 2008; Greenwood et al., 2019). The insufficient satiety of sows can be reflected by their stereotypical behaviors (often referred to as sham-chewing behavior, sniffing behavior and licking behavior) and physical activities (standing, lying, sitting, kneeling and position change) (de Leeuw et al., 2008). Our results showed that sows fed the 15% WA diet had a decrease in the proportion of sitting behaviors, indicative of greater postprandial satiety. Previous studies have shown that dietary supplementation of WA could delay the postprandial peaks of glucose and insulin in horses (Delesalle et al., 2015), which was confirmed by Quemeneur et al. (2020), who reported that the increase in the plasmatic concentration of glucose following the meal was lower and delayed with the diet supplemented with 4-g WA per kg in the growing pigs. Moreover, previous studies have shown that a reduction in stereotypical behavior is associated with an improvement in the body's status of oxidative stress (Ben-Azu et al., 2016; Nadeem et al., 2019; Zhang et al., 2019), which agreed with the current study by an increase in the concentrations of serum T-AOC and a decrease in the proportion of sitting behaviors for the 15% WA group versus the control group.
Previous studies have revealed GLP-1 and PYY as important regulators of satiety (Gibbons et al., 2013; Schueler et al., 2013; Tan et al., 2016a), both of which affect satiety by reducing gut motility, delaying gastric emptying and slowing transit time to enhance digestion and nutrient absorption, thereby reducing appetite (Sleeth et al., 2010). In our study, compared with the control group, the 15% WA group significantly increased serum concentrations of pre-prandial PYY and GLP-1 of sows, coupled with an increased tendency in the serum concentrations of 2 h postprandial PYY on day 70 of gestation. These results indicated that feeding sows with 15% WA increased the levels of serum satiety hormone of pregnant sows, which may contribute to reducing abnormal behavior and enhancing postprandial satiety. It has been reported that the microbiota of rodents and humans in the distal part of the gastrointestinal tract can ferment dietary fiber to produce short-chain fatty acids (SCFA), which could activate the gene expression of free fatty acid receptors FFA2 and FFA3 in L-cells and promote the production of GLP-1 and PYY (Byrne et al., 2015). WA is a source of insoluble fermentable dietary fiber (Mcintosh et al., 2001), which may promote the generation of more GLP-1 and PYY by producing more SCFA than the control group. In this study, we did not detect the plasma concentrations of postprandial SCFA of pregnant sows, but previous studies have indicated that, compared with the control, inclusion of dietary fiber remarkably increased the plasma concentrations of 4 h postprandial acetic acid, propionic acid, butyric acid and total SCFA of sows on late gestation (Sun et al., 2015; Tan et al., 2016b, Tan et al., 2018).
5. Conclusions
In this study, feeding sows with 15% WA was shown to decrease the stillbirth rate, alleviate stress status and enhance postprandial satiety of pregnant sows. Importantly, 15% WA inclusion during gestation could reduce the production cost per piglet born alive or per piglet born healthy. This study facilitates the application of dietary fiber to partially replace conventional feed ingredients to improve the reproductive performance of sows and reduce the production cost.
Author contributions
Chengquan Tan: Conceptualization, Methodology, Writing - Review & Editing, Project administration. Jinping Deng: Methodology, Investigation, Writing - Original Draft. Chuanhui Cheng: Investigation, Data Curation, Writing - Original Draft. Haoyuan Yu: Investigation, Data Curation. Shuangbo Huang: Investigation. Xiangyu Hao: Investigation. Jianzhao Chen: Investigation. Jiansen Yao: Resources. Jianjun Zuo: Resources.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China, China (31872985), the National Key Research and Development Program of China, China (2018YFD0500600), and the China Scholarship Council. China (201907630006).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2020.06.015.
Contributor Information
Jianjun Zuo, Email: zuoj@scau.edu.cn.
Chengquan Tan, Email: tanchengquan@scau.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adeola O. CRC Press; 2000. Digestion and balance techniques in pigs, Swine nutrition; pp. 923–936. [Google Scholar]
- Anson N.M., Havenaar R., Bast A., Haenen G.R. Antioxidant and anti-inflammatory capacity of bioaccessible compounds from wheat fractions after gastrointestinal digestion. J Cereal Sci. 2010;51(1):110–114. [Google Scholar]
- Antolic A., Richards E.M., Wood C.E., Keller-Wood M. A transcriptomic model of postnatal cardiac effects of prenatal maternal cortisol excess in sheep. Front Physiol. 2019;10:816. doi: 10.3389/fphys.2019.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns F., Hemery Y., Price R., Anson N.M. Wheat aleurone: separation, composition, health aspects, and potential food use. Crit Rev Food Sci Nutr. 2012;52(6):553–568. doi: 10.1080/10408398.2011.589540. [DOI] [PubMed] [Google Scholar]
- Byrne C.S., Chambers E.S., Morrison D.J., Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes. 2015;39(9):1331–1338. doi: 10.1038/ijo.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N.J., Schaefer A.L., Lepage P., Jones S.M. Salivary vs. serum cortisol for the assessment of adrenal activity in swine. Can J Anim Sci. 1996;76(3):329–335. [Google Scholar]
- De Leeuw J., Jongbloed A., Spoolder H., Verstegen M. Effects of hindgut fermentation of non-starch polysaccharides on the stability of blood glucose and insulin levels and physical activity in empty sows. Livest Prod Sci. 2005;96(2):165–174. [Google Scholar]
- de Leeuw J.A., Bolhuis J.E., Bosch G., Gerrits W.J. Effects of dietary fibre on behaviour and satiety in pigs. Proc Nutr Soc. 2008;67(4):334–342. doi: 10.1017/S002966510800863X. [DOI] [PubMed] [Google Scholar]
- Delesalle C., Popovic A., Hespel P., de Oliveira J., Duchateau L., De Bruijn M. 2015. Effect of aleurone supplementation on postprandial glucose and insulin response in horses. Paper presented at ENUTRACO , 3–7 Septembre 2015, Bingen, Germany. [Google Scholar]
- Feyera T., Højgaard C.K., Vinther J., Bruun T.S., Theil P.K. Dietary supplement rich in fiber fed to late gestating sows during transition reduces rate of stillborn piglets. J Anim Sci. 2017;95(12):5430–5438. doi: 10.2527/jas2017.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons C., Caudwell P., Finlayson G., Webb D.L., Hellstrom P.M., Naslund E. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J Clin Endocrinol Metab. 2013;98(5):E847–E855. doi: 10.1210/jc.2012-3835. [DOI] [PubMed] [Google Scholar]
- Graf E. Antioxidant potential of ferulic acid. Free Radical Biol Med. 1992;13(4):435–448. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- Greenwood E.C., Dickson C.A., van Wettere W.H. Feeding strategies before and at mixing: the effect on sow aggression and behavior. Animals. 2019;9(1):23. doi: 10.3390/ani9010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J.P., Johnston L.J., Baidoo S.K., Shurson G.C. Effects of a high-fiber diet and frequent feeding on behavior, reproductive performance, and nutrient digestibility in gestating sows. J Anim Sci. 2006;84(4):946–955. doi: 10.2527/2006.844946x. [DOI] [PubMed] [Google Scholar]
- Huang S., Wei J., Yu H., Hao X., Zuo J., Tan C. Effects of dietary fiber sources during gestation on stress status, abnormal behaviors and reproductive performance of sows. Animals. 2020;10(1):141. doi: 10.3390/ani10010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M.B., Pedersen L.J., Theil P.K., Yde C.C., Bach Knudsen K.E. Feeding motivation and plasma metabolites in pregnant sows fed diets rich in dietary fiber either once or twice daily. J Anim Sci. 2012;90(6):1910–1919. doi: 10.2527/jas.2010-3289. [DOI] [PubMed] [Google Scholar]
- Kim K.H., Rong T., Yang R., Cui S.W. 2006. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95(3):466–473. [Google Scholar]
- Knudsen K.B. vol. 6. Wageningen Academic Publishers; 2019. pp. 87–96. (Nutritional modulation to improve health and welfare, Poultry and pig nutrition: challenges of the 21st century). [Google Scholar]
- Lewis C.R., Hermesch S. Genetic parameters and phenotypic trends in the mean and variability of number of stillborn piglets and changes in their relationships with litter size and gestation length. Anim Prod Sci. 2013;53(5):395–402. [Google Scholar]
- Maes D.G., Dewulf J., Piñeiro C., Edwards S., Kyriazakis I. A critical reflection on intensive pork production with an emphasis on animal health and welfare. J Anim Sci. 2019 doi: 10.1093/jas/skz362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malunga L.N., Izydorczyk M., Beta T. Antiglycemic effect of water extractable arabinoxylan from wheat aleurone and bran. J Nutr Metabol. 2017;2017:5784759. doi: 10.1155/2017/5784759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Anson N., Robin V.D.B., Havenaar R., Bast A., Guido R.M.M.H. Ferulic acid from aleurone determines the antioxidant potency of wheat grain (Triticum aestivum L.) J Agric Food Chem. 2008;56(14):5589–5594. doi: 10.1021/jf800445k. [DOI] [PubMed] [Google Scholar]
- Mcintosh G.H., Royle P.J., Pointing G. Wheat aleurone flour increases cecal b-glucuronidase activity and butyrate concentration and reduces colon adenoma burden in azoxymethane-treated rats. J Nutr. 2001;131(1):127. doi: 10.1093/jn/131.1.127. [DOI] [PubMed] [Google Scholar]
- Merlot E., Quesnel H., Prunier A. Prenatal stress, immunity and neonatal health in farm animal species. Anim : Int J Anim Biosci. 2013;7(12):2016–2025. doi: 10.1017/S175173111300147X. [DOI] [PubMed] [Google Scholar]
- Meunier-Salaün M., Edwards S., Robert S. Effect of dietary fibre on the behaviour and health of the restricted fed sow. Anim Feed Sci Technol. 2001;90:53–69. [Google Scholar]
- Quemeneur K., Montagne L., Gall M.L., Lechevestrier Y., Labussiere E. Relation between feeding behaviour and energy metabolism in pigs fed diets enriched in dietary fibre and wheat aleurone. Animal. 2020;14(3):508–519. doi: 10.1017/S1751731119002246. [DOI] [PubMed] [Google Scholar]
- Quesnel H., Meunier-Salaun M.C., Hamard A., Guillemet R., Etienne M., Farmer C. Dietary fiber for pregnant sows: influence on sow physiology and performance during lactation. J Anim Sci. 2009;87(2):532–543. doi: 10.2527/jas.2008-1231. [DOI] [PubMed] [Google Scholar]
- Rosa N.N., Dufour C., Lullien-Pellerin V., Micard V. Exposure or release of ferulic acid from wheat aleurone: impact on its antioxidant capacity. Food Chem. 2013;141(3):2355–2362. doi: 10.1016/j.foodchem.2013.04.132. [DOI] [PubMed] [Google Scholar]
- Sagara M., Mori M., Mori H., Tsuchikura S., Yamori Y. Effect of dietary wheat aleurone on blood pressure and blood glucose and its mechanisms in obese spontaneously hypertensive rats: preliminary report on comparison with a soy diet. Clin Exp Pharmacol Physiol. 2007;34(s1):S37–S39. [Google Scholar]
- Sapkota A., Marchant-Forde J.N., Richert B.T., Lay D.C. Including dietary fiber and resistant starch to increase satiety and reduce aggression in gestating sows. J Anim Sci. 2016;94(5):2117–2127. doi: 10.2527/jas.2015-0013. [DOI] [PubMed] [Google Scholar]
- Schneider J., Tokach M., Dritz S., Nelssen J., DeRouchey J., Goodband R. Effects of feeding schedule on body condition, aggressiveness, and reproductive failure in group-housed sows. J Anim Sci. 2007;85(12):3462–3469. doi: 10.2527/jas.2007-0345. [DOI] [PubMed] [Google Scholar]
- Schueler J., Alexander B., Hart A.M., Austin K., Larson-Meyer D.E. Presence and dynamics of leptin, GLP-1, and PYY in human breast milk at early postpartum. Obesity. 2013;21(7):1451–1458. doi: 10.1002/oby.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi T., Koketsu Y. Behavior and reproductive performance by stalled breeding females on a commercial swine farm. J Anim Sci. 2004;82(5):1482–1487. doi: 10.2527/2004.8251482x. [DOI] [PubMed] [Google Scholar]
- Souza da Silva C., Bolhuis J.E., Gerrits W.J., Kemp B., van den Borne J.J. Effects of dietary fibers with different fermentation characteristics on feeding motivation in adult female pigs. Physiol Behav. 2013;110–111:148–157. doi: 10.1016/j.physbeh.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Stein K., Borowicki A., Scharlau D., Glei M. Fermented wheat aleurone induces enzymes involved in detoxification of carcinogens and in antioxidative defence in human colon cells. Br J Nutr. 2010;104(8):1101–1111. doi: 10.1017/S0007114510001881. [DOI] [PubMed] [Google Scholar]
- Sun H.Q., Tan C.Q., Wei H.K., Zou Y., Long G., Ao J.T. Effects of different amounts of konjac flour inclusion in gestation diets on physio-chemical properties of diets, postprandial satiety in pregnant sows, lactation feed intake of sows and piglet performance. Anim Reprod Sci. 2015;152:55–64. doi: 10.1016/j.anireprosci.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Tan C., Wei H., Sun H., Ao J., Long G., Jiang S. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. BioMed Res Int. 2015;2015:525218. doi: 10.1155/2015/525218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C., Wei H., Sun H., Long G., Ao J., Jiang S. Effects of supplementing sow diets during two gestations with konjac flour and Saccharomyces boulardii on constipation in peripartal period, lactation feed intake and piglet performance. Anim Feed Sci Technol. 2015;210:254–262. [Google Scholar]
- Tan C., Wei H., Zhao X., Xu C., Zhou Y., Peng Y. Soluble fiber with high water-binding capacity, swelling capacity, and fermentability reduces food intake by promoting satiety rather than satiation in rats. Nutrients. 2016;8(10):615. doi: 10.3390/nu8100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C., Wei H., Ao J., Long G., Peng J. Inclusion of konjac flour in the gestation diet changes the gut microbiota, alleviates oxidative stress, and improves insulin sensitivity in sows. Appl Environ Microbiol. 2016;82(19):5899–5909. doi: 10.1128/AEM.01374-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.Q., Sun H.Q., Wei H.K., Tan J.J., Long G., Jiang S.W. Effects of soluble fiber inclusion in gestation diets with varying fermentation characteristics on lactational feed intake of sows over two successive parities. Animal. 2018;12(7):1388–1395. doi: 10.1017/S1751731117003019. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghe C., Dewulf J., de Kruif A., Maes D. Non-infectious factors associated with stillbirth in pigs: a review. Anim Reprod Sci. 2013;139(1–4):76–88. doi: 10.1016/j.anireprosci.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Wang H., Hu C., Cheng C., Cui J., Ji Y., Hao X. Unraveling the association of fecal microbiota and oxidative stress with stillbirth rate of sows. Theriogenology. 2019;136:131–137. doi: 10.1016/j.theriogenology.2019.06.028. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang W., Wang R., Meng Z., Duan Y., An X. Dietary supplementation of ferulic acid improves performance and alleviates oxidative stress of lambs in a cold environment. Can J Anim Sci. 2019;99(4):705–712. [Google Scholar]
- Yang Y., Hu C.J., Zhao X., Xiao K., Deng M., Zhang L. Dietary energy sources during late gestation and lactation of sows: effects on performance, glucolipid metabolism, oxidative status of sows, and their offspring. J Anim Sci. 2019;97(11):4608–4618. doi: 10.1093/jas/skz297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Ping L., Yi W., Guo P., Ling L., Ning M. Dietary fiber increases the butyrate-producing bacteria and improves growth performance of weaned piglets. J Agric Food Chem. 2018;66(30):7995–8004. doi: 10.1021/acs.jafc.8b02545. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Flowers W.L., Saraiva A., Yeum K.J., Kim S.W. Effect of social ranks and gestation housing systems on oxidative stress status, reproductive performance, and immune status of sows. J Anim Sci. 2013;91(12):5848–5858. doi: 10.2527/jas.2013-6388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.