Abstract
Macrophages are typically identified as classically activated (M1) macrophages and alternatively activated (M2) macrophages, which respectively exhibit pro- and anti-inflammatory phenotypes, and the balance between these two subtypes plays a critical role in the regulation of tissue inflammation, injury, and repair processes. Recent studies indicate that tissue cells and macrophages interact via the release of small extracellular vesicles (EVs) in processes where EVs released by stressed tissue cells can promote the activation and polarization of adjacent macrophages which can in turn release EVs and factors that can promote cell stress and tissue inflammation and injury, and vice versa. This review discusses the roles of such EVs in regulating such interactions to influence tissue inflammation and injury in a number of acute and chronic inflammatory disease conditions, and the potential applications, advantage and concerns for using EV-based therapeutic approaches to treat such conditions, including their potential role of drug carriers for the treatment of infectious diseases.
KEY WORDS: Extracellular vesicles, Macrophage, Tissue injury, Inflammatory disease, Interaction loop, Stem cell, Sepsis, Targeted therapy
Abbreviations: ADSCs, adipose-derived stem cells; AKI, acute kidney injury; ALI, acute lung injury; AMs, alveolar macrophages; BMSCs, bone marrow stromal cells; CLP, cecal ligation and puncture; DSS, dextran sodium sulphate; EVs, extracellular vesicles; HSPA12B, heat shock protein A12B; HUCMSCs, human umbilical cord mesenchymal stem cells; IBD, inflammatory bowel disease; ICAM-1, intercellular adhesion molecule 1; IL-1β, interleukin-1β; iNOS, inducible nitrogen oxide synthase; KCs, Kupffer cells; KLF4, krüppel-like factor 4; LPS, lipopolysaccharides; MHC, major histocompatibility complex; MSCs, mesenchymal stromal cells; MVs, microvesicles; PEG, polyethylene glycol; PMFA, 5,7,30,40,50-pentamethoxyflavanone; PPARγ, peroxisome proliferator-activated receptor γ; SIRPα, signal regulatory protein α; TECs, tubular epithelial cells; TNF, tumor necrosis factor; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand
Graphical abstract
Macrophages and tissue cells can communicate via extracellular vehicles (EVs) to regulate tissue injury and tissue repair. Targeting these EV interactions mechanisms may lead to new therapeutic strategies for acute and chronic inflammatory conditions.
1. Introduction
Macrophages are myeloid linage cells involved in innate immune responses that have historically been viewed as terminally-differentiated phagocytic cells that primarily exhibit pro-inflammatory phenotypes1. Fate-mapping studies now indicate that macrophages involved in the regulation of tissue homeostasis have distinct origins, which we will herein refer to as tissue-resident and recruited macrophages2, 3, 4. Most tissue-resident macrophages derive from the yolk sac or fetal liver during embryonic development and migrate to their final site prior to the end of gestation, whereas recruited macrophages derive from circulating monocytes that continue to differentiate from hematopoietic stem cells during the organism's lifetime3, 4, 5. Monocytes that highly express Ly6c have pro-inflammatory and antimicrobial activity and accumulate at sites of inflammation, while monocytes with low levels of Ly6c expression are considered to be patrolling monocytes that crawl along and survey the vasculature and participate in early inflammatory and tissue repair responses6. Tissue-resident macrophages are generally considered to be responsible for maintaining homeostasis, but during tissue injury or infection they can initiate pro-inflammatory responses by secreting chemokines to drive the rapid influx of neutrophils and inflammatory leukocytes7. Alveolar macrophages are self-renewing, but decrease in other tissue-resident macrophage populations during infection or tissue injury are replaced by patrolling monocytes that enter the affected tissue and differentiate into macrophages8. The primary function of these recruited macrophages is to attenuate inflammation and promoted tissue repair by stimulating angiogenesis, inhibiting neutrophil recruitment, and clearing cell debris. Tissue-resident and recruited macrophages tend to exhibit functionally distinct phenotypes, but can sometimes be functionally interchangeable during certain inflammatory responses7,9, 10, 11. Both tissue-resident and recruited macrophages play important roles in regulating the development and resolution of pro-inflammatory tissue microenvironments, as described later in this review.
Macrophages, including both tissue-resident and recruited macrophages, are classified as belonging to two distinct functional subsets: classically activated (M1) macrophages, which are primarily associated with pro-inflammatory immune responses; and alternatively activated (M2) macrophages, which are primarily involved in tissue repair (Fig. 1)12. Nitric oxide (NO) released by M1 macrophages tends to inhibit cell proliferation and induce tissue damage, while ornithine secreted by M2 macrophages tends to promote cell proliferation and tissue repair responses12. M2 polarization appears to be the default option for tissue-resident macrophages, but M2 and M1 macrophages can interconvert in response to specific environmental stimuli12. M1 macrophages undergo polarization in response to lipopolysaccharides (LPS) and Toll-like receptor signaling to secrete an array of pro-inflammatory cytokines [e.g., interleukin (IL)-1β, IL-23, IL-12, IL-6, TNF-α] and chemokines (e.g., CXCL2, CCL8, CXCL4 and CCL5). M1 macrophages exhibit increased antigen presenting activity and pro-inflammatory phenotypes, and function to eliminate infections caused by bacterial, viral and fungal factors13,14. M2 macrophages are classified into three subpopulations (M2a, M2b and M2c) that arise in response to different stimuli. M2a macrophages are induced by exposure to IL-4 and IL-13, M2b macrophages by immune complex signalizing in conjunction with LPS or IL-1β, and M2c macrophage by anti-inflammatory stimuli, including glucocorticoids, IL-10 or TGF-β. All M2 macrophages express CD14 and secrete high levels of anti-inflammatory factors, including IL-10, arginase-1, CCL24, CCL22, and CCL1714,15. These cells play important roles in resolving inflammation at infection and tissue injury sites by suppressing pro-inflammatory immune responses and regulating tissue repair and angiogenesis.
Figure 1.
Macrophage polarization and phenotypes. Stimulation by TLR receptor signaling or by exposure to IL-4, IL-10, IL-13 and TGF-β induces macrophages to adopt M1 or M2 phenotypes, respectively, which causes these macrophage subtypes to secrete distinct sets of cytokines and chemokines that exert pro-inflammatory or anti-inflammatory effects.
Detecting differences in gene and protein expression is the simplest and most useful method to determine macrophage polarization states. For example, M1 macrophages are characterized by the abundant expression of the class II major histocompatibility complex (MHCII), MHCII-associated co-stimulatory molecules CD80/CD86, and CD11c and CCR2 after stimulation with IFN-γ or LPS. Similarly, macrophage expression of CD163, CD200R, and CD206 after treatment with IL-10 or IL-4 is considered a hallmark of M2 polarization14,16. Macrophage activation and polarization is mediated by different signaling pathways, and the presence of distinct regulatory molecules involved in the polarization to specific macrophage subtypes can be employed as macrophage phenotype markers. Mediators of TLR-dependent M1 macrophage polarization include the transcription factors activator protein-1 (AP-1), NF-κB, PU.1, STAT1, CCAAT/enhancer-binding protein α (C/EBP-α) and IRF5. Transcription factors promoting M2 activation include C/EBP-β, IRF4, STAT6, Krüppel-like factor 4 (KLF4) and peroxisome proliferator-activated receptor γ (PPARγ)14,17. As discussed in the following sections, important interactions between M1 and M2 macrophages and the respective cell types they regulate rely on the activity of EVs as molecular information carriers.
EVs are lipid bilayer-enclosed structures, formed either by outward budding of the plasma membrane or through an intracellular endocytic trafficking pathway18. EVs are typically classified into multiple sub-categories, with the most common classification scheme relying on differences of their diameters to segregate them three overlapping groups: exosomes (40–100 nm), microvesicles (MV; 100–1000 nm) and apoptotic bodies (50–5000 nm)19. Exosomes derive from the endosomal compartment, while MVs and apoptotic bodies bud from the plasma membrane, and each of these biogenesis processes is subject to different regulatory mechanisms. Given the potential for overlapping these groups during their characterization in the absence of additional information, this review will use “small EV” to refer to EVs populations that could contain exosomes and/or MVs to avoid classification errors in the absence of sufficient detail regarding EV isolation or characterization methods or data. Some small EVs, particularly exosomes, carry miRNAs, mRNAs, and proteins that can influence the inflammatory response20. Exosomes are produced by the endosomal compartments of most cells, including multiple cell types that play important roles in immune and pro-inflammatory responses, such as macrophages, dendritic cells, and T and B lymphocytes21. Such exosomes can travel throughout the body via the circulatory system and traverse the blood–brain barrier and other tissue to boundaries to be taken up by target cells to participate in numerous biological and pathological processes, including tissue damage and repair responses22. Small EVs, including exosomes, are also frequently produced in greater abundance stressed versus unstressed cells, including cells in tissues experiencing autoimmune responses21. Small EVs produced by malignant, infected or otherwise stressed cells and tissues may contain regulatory molecules that are critical for both local and systemic inflammatory responses by promoting the activation and secretion of pro-inflammatory cytokines23. Increasing effort is devoted to determining pro-inflammatory factors carried by EVs can regulate inflammatory reactions and could be altered for therapeutic purposes.
Small EVs released by immune cells can stimulate the adaptive immune response by carrying MHCII-antigen peptide complexes24, but the protective effects of these small EVs can be altered when their source cells are exposed to inflammatory stimuli25. For example, under hypertensive conditions exosomes secreted by macrophages stimulate the release inflammatory factors upon their uptake by endothelial cells26, while endothelial cell EVs can also modulate macrophage phenotypes24 in one example of an EV-based interaction loops between macrophages and host cells under pro-inflammatory conditions. EVs secreted by macrophages are also able to influence the phenotypes of adjacent macrophages to induce inflammatory tissue responses. For instance, native M1-derived exosomes can effectively shift recipient macrophages from a tumor permissive M2 to a tumor restrictive M1 phenotype27. Substantial literature has described interactions between exosomes and macrophages in autoimmune or metabolic diseases21,24. Small EVs secreted by macrophages and other cell types can act on their recipient cells to transmit regulation signals and effects through receptor-mediated endocytosis, receptor-mediated membrane fusion, and phagocytosis and by paracrine signaling upon the release of their cargoes to directly interact with specific receptors on the target cells22. Less is known about the specific EVs populations that are involved in some of these interactions given that EVs generated from the plasma membrane and endosome compartment may be difficult to distinguish since they are similar in size and may exhibit some degree of compositional overlap28.
Here, we discuss the roles macrophages play in some inflammation-related diseases, and how EVs secreted by pro-inflammatory M1 macrophages can induce tissue damage, while EVs released by stem cells can alter macrophage phenotypes to regulate anti-inflammatory M2 macrophage responses. Finally, we discuss the potential advantages of employing native or modified EVs as novel therapeutics for the treatment of infectious and inflammatory diseases, as well as additional questions and technical challenges that need to be addressed to allow the clinical translation of such applications.
2. EV-mediated interaction loops between macrophages and tissues in tissue damage
Macrophages can exhibit substantial heterogeneity and plasticity, displaying a continuum of phenotypes, with the extremes represented by the M1 and M2 states29. M1 macrophage subtypes predominate at the onset of tissue injury, and can secrete reactive oxygen species and other pro-inflammatory factors that can disrupt normal cell metabolism, induce apoptosis, and exacerbate tissue ischemia to ultimately worsen tissue damage. In the injury-resolution phase, however, M2 macrophages become predominant and produce a variety of effector molecules and growth factors that can regulate myofibroblast activation, stem and tissue progenitor cell differentiation, and angiogenesis30,31. Increasing evidence shows that macrophages take part in the regulation of tissue damage by releasing EVs with pro- or anti-inflammatory information to affect inflammation in their microenvironment, and that damaged tissue cells can release EVs to activate local macrophages to initiate these responses (Fig. 2), resulting in an EV interaction loop between macrophages and tissue cells during tissue injury.
Figure 2.
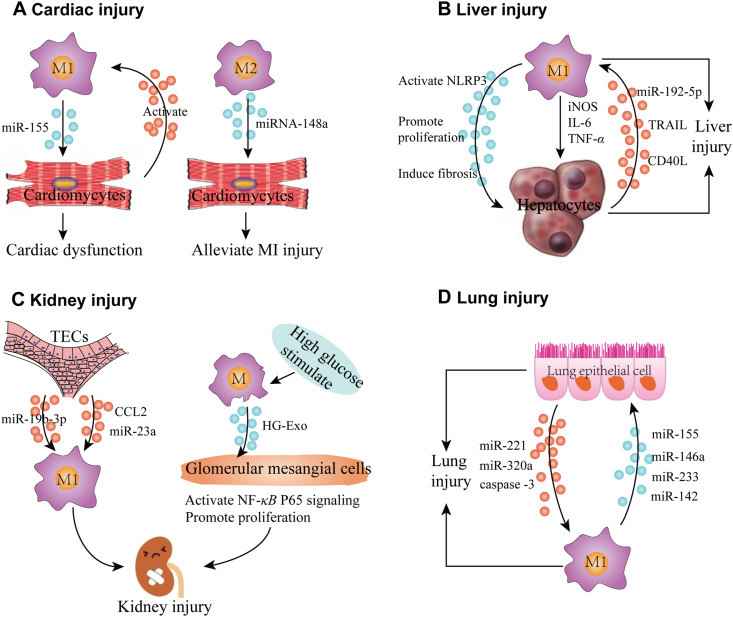
EV-mediated interactions between tissue macrophages and adjacent cells can induce tissue inflammation and injury. (A) Cardiac injury. M1 macrophages secrete extracellular vesicles (EVs) containing miR-155 that induce cardiomyocyte dysfunction, while EVs secreted by injured cardiomyocytes can activate macrophages. Conversely, exosomes secreted by M2 macrophages carry miRNA-148a, which can target cardiomyocytes and reduce myocardial ischemia/reperfusion injury. (B) Liver injury. EVs secreted by M1 macrophages can activate the hepatocyte NLRP3 inflammasome pathway and promote hepatocyte proliferation, liver fibrosis, and the secretion of inflammatory factors, including iNOS, IL-6 and TNF-α, to cause liver injury. Stressed hepatocytes can secrete EV containing miR-192-5p, TRAIL and CD40L to promote M1 macrophage polarization. (C) Kidney injury. Tubular epithelial cells (TECs) secrete EVs enriched with miR-19b-3p, CCL2 and miR-23a that promote M1 macrophage polarization to induce tubulointerstitial inflammation. Conversely, M1 macrophages stimulated by high glucose secrete EVs that can activate NF-κB P65 signaling and promote proliferation of glomerular mesangial cells to cause kidney injury. (D) Lung injury. EVs secreted by lung epithelial cells carry miR-221, miR320a and caspase-3 to promote alveolar macrophages to adopt an M1 phenotype, while M1 macrophage EVs containing miR-155, miR-146a, miR-233 and miR-142 induce lung epithelial cell injury.
2.1. Cardiac injury
During the cardiac inflammation, circulating blood mononuclear cells are recruited into cardiac tissue and differentiate into macrophages that secrete exosomes that contribute to cardiac injury by attenuating the induction of angiogenesis and aggravating myocardial damage32,33. The contribution of macrophage-derived EVs to this process was identified by findings revealing that while pro-inflammatory miR-155 levels were elevated in macrophages and cardiac fibroblasts in injured cardiac tissue, only the cardiac macrophages expressed the miR-155 precursor required to generate miR-155 and that macrophage-derived exosomes contained miR-155 that could be transferred to cardiac fibroblasts34. Further, following myocardial infraction cardiac macrophages are reported to express the M1 macrophage markers CD86, CD11c and inducible NO synthase (iNOS) and release pro-inflammatory exosomes containing miR-155, and can transfer miR-155 to endothelial cells, leading to cardiac dysfunction and the inhibition of angiogenesis26. Conversely, exosomes derived from IL-4-induced M2 macrophages expressing the M2 macrophage markers CD163 and CD206 carry miRNA-148a, which can alleviate myocardial ischemia/reperfusion injury by inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway35. Finally, EVs secreted by neonatal cardiomyocytes are reported to polarize pro-inflammatory macrophages and exacerbate myocardial ischemia and its associated inflammatory responses36, suggesting that EV-regulated crosstalk between macrophages and cardiomyocytes may also contribute to cardiac injury. Further studies are required to determine the initiator and relative contribution of EVs from these cells during cardiac ischemia, although EVs derived from neonatal cardiomyocytes under normal and ischemic conditions appear to have similar polarizing affects, whether macrophages stimulate cardiomyocytes or whether cardiomyocytes activate macrophages first in this interaction loop. Additional studies are also necessary to evaluate whether adjusting the ratio of M1 and M2 macrophages in cardiac tissues using small EVs could serve as a therapeutic intervention during myocardial infarction.
2.2. Liver injury
Tissue-resident liver macrophages are referred to as Kupffer cells (KC)37, and several lines of evidence indicate that M1 KCs secrete pro-inflammatory factors that may induce liver damage, including reactive oxygen species, TNF-α, iNOS and IL-6. M2 KCs, conversely, release anti-inflammatory cytokines, which play important roles in liver repair38. In addition to KCs, circulating monocytes also have a profound impact on liver injury as they are recruited both during homeostasis and injury-associated inflammation37. Emerging evidence suggests that small EVs, especially exosomes, derived from the recruited macrophages that differentiate from these invasive monocytes, have an important role in mediating liver injury39. For example, hepatocytes are reported to take up LPS-induced macrophage-derived exosomes and subsequently activate the NLRP3 inflammasome pathway both in vitro and in vivo, resulting in liver injury39. Exosomes from LPS-treated THP-1 macrophages likewise promote hepatic stellate cell proliferation and induce hepatic fibrosis, a common pathological consequence of repeated liver injury40. These results indicate that exosomes secreted by pro-inflammatory KCs and by recruited macrophages alter the phenotypes of hepatic cells to promote hepatic tissue injury.
These interactions appear likely to be bidirectional, since hepatic cells can release small EVs that can activate or polarize macrophages in the hepatic microenvironment. For instance, after lipotoxic injury hepatocytes secrete exosomes that contain miR-192-5p, which can promote macrophages to undergo M1 polarization, while also releasing iNOS, IL-6 and TNF-α into the liver environment to aggravate hepatocyte cellular dysfunction41. Small EVs secreted by such hepatocytes contain tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which was found to promote an inflammatory phenotype in recipient macrophages42. Hepatocyte dysfunction and pro-inflammatory macrophages induced by EVs-based interactions between hepatocytes and macrophages thus appear to be an important pathological basis for nonalcoholic hepatitis. Other hepatocyte–macrophage interactions have also been demonstrated by exposing hepatocytes to alcohol, which release small EVs carrying CD40 ligand to promote macrophage activation, including the activation of primary murine KC and human monocyte-derived macrophages43. Thus, hepatocytes and macrophages can each secrete small EVs that can promote pro-inflammatory activity in the other cell type, with the potential for form a feedback loop to enhance and maintain hepatic inflammation leading to liver injury.
2.3. Kidney injury
Acute kidney injury (AKI) is a growing global health concern, and animal models demonstrate that macrophages are major contributors to the inflammatory response associated with AKI44. Macrophages from healthy kidneys primarily exhibit an M2-like phenotype, but M1 macrophages predominate shortly after acute kidney injury and act to aggravate renal inflammation, while M2 macrophages play a major role in the eventual attenuation of inflammation and tissue repair response. Both tissue-resident and invasive macrophage generated upon the differentiation of circulating monocytes following their tissue infiltration participate in these responses29. Tubular epithelial cells (TECs) are the most populous cell type in the kidney and have key roles in pathological kidney injury45. Tubulointerstitial inflammation is a common characteristic of many types of acute and chronic kidney injury, and several studies have demonstrated that TEC-derived exosomes communicate with kidney-resident macrophages to cause kidney damage. For instance, miR-19b-3p in TEC-derived exosomes internalized by primary macrophages and macrophage cell lines are reported to induce M1 polarization and trigger kidney inflammation45. A second study indicated that macrophage internalization of exosomes from BSA-treated TECs led to an increased inflammatory response and macrophage migration46. This study also found that TEC-derived exosomes enriched in CCL2 mRNA activate macrophages to subsequently induce interstitial inflammation46. Similarly, miR-23a-containing exosomes released by TECs exposed to hypoxic conditions were found to induce M1 macrophage polarization and tubulointerstitial inflammation by promoting local inflammatory effects47. In combination, these reports indicate that AKI-related inflammation is substantially regulated by communication between TECs and kidney-resident macrophages where TEC-derived exosomes induce M1 macrophage polarization and inflammation to cause or promote tissue injury.
Interactions between kidney-infiltrating macrophages and glomerular mesangial cells are also reported to promote kidney injury. Diabetes-associated chronic kidney inflammation is a consequence of the activation and proliferation of glomerular mesangial cells and the secretion of extracellular matrix and inflammatory cytokines in response to high glucose concentrations48. Exosomes from high glucose treated macrophages can transmit pro-inflammatory information between macrophages and glomerular mesangial cells48, and were found to activate kidney macrophages and accelerate kidney injury via the NF-κB P65 signaling pathway49. Thus, in tubulointerstitial inflammation-related kidney injury, TECs first release EVs to activate pro-inflammatory macrophages, while in diabetic nephropathy-related kidney injury, EVs secreted by pro-inflammatory macrophages induce pathological changes of glomerular mesangial cells. Both of these processes result in activation cascades which can develop into unchecked interaction loops. Approaches that employ modified small EVs to block the communication between macrophages and renal cells may thus prevent the development of renal tissue injury, and is worth further study.
2.4. Lung injury
Alveolar macrophages (AMs), unlike from KCs and other tissue-resident macrophages, are not replenished by in situ differentiation of invasive monocytes, so the maintenance of AM abundance requires self-renewal8. During the development of acute lung injury (ALI), AMs are pivotal factors in both the initiation and resolution of inflammatory lung responses. AMs are found to constantly communicate with alveolar epithelial cells, with macrophage–epithelial cell crosstalk occurring via epithelial cells-derived EVs and their constituent miRNAs50, where epithelial cells can secrete small EVs to activate pro-inflammatory macrophages. For instance, caspase-3 enriched small MVs derived from lung epithelial cells activate AMs and mediate lung inflammatory responses involved in lung injury, and exposure to caspase-3-deficient MVs can interfere with macrophage activation and reduce lung inflammation51. Epithelial cell-derived MV expression of miR-221 and miR-320a is also responsible for triggering macrophage-mediated pro-inflammatory effects52. EVs released by lung epithelial cells into the alveolar cavity are primarily MVs, with a lesser amount of exosomes, and few apoptotic bodies51,52. Similarly, macrophage-derived exosomes containing miR-155 and miR-146a induce pro-inflammatory cytokine expression in recipient epithelial cells to promote ALI progression53. Collectively, epithelial cell-derived MVs can promote macrophage-regulated lung inflammation, causing macrophage-derived EVs to continue to incite epithelial cell activation. Secreted interleukin (IL)-25 from lung epithelial cells can downregulate macrophage Rab27a and Rab27b expression, suppressing macrophage secretion of TNF-α-carrying exosomes and exosome-induced inflammatory effects54, in which alveolar epithelial cells negatively regulate the inflammatory responses of macrophages.
AM-derived MVs may also play a significant role in regulation of ALI associated inflammation, since in LPS-induced animal models of ALI, macrophages were the major source of MVs in bronchoalveolar lavage fluid55. These MVs were found to carry biologically active tumor necrosis factor (TNF) and play an important role in initiating ALI56. These MVs also contained miR-223 and miR-142 and were found to selectively target pulmonary macrophages57. Further study found that MVs released by AMs caused lung injury by activating the NLRP3 inflammatory pathway in adjacent macrophages57. Further, bronchoalveolar lavage fluid MVs loaded with miR-223/142 mimics were found to effectively target AMs and inhibit their activation and reduce lung inflammation57. Thus, using EVs to target the delivery of regulatory factors to pulmonary macrophages appears to represent a viable approach to prevent pulmonary injury in cases where there is an excessive inflammatory response, although it is possible that the opposite approach could be used in other conditions where a suboptimal inflammatory response was associated with chronic infection or other condition. EV-mediated interactions between macrophages and tissue cells in tissue injury are listed in Table 126,34, 35, 36,39, 40, 41, 42, 43,45, 46, 47, 48, 49,51,52,54, 55, 56, 57.
Table 1.
EV-mediated interactions between macrophages and tissue cells in tissue injury.
| MΦ source | Stimulus | MΦ markers | EV origin | EV isolation | EVs characterization | EV marker analyzed | EVs function | Ref. |
|---|---|---|---|---|---|---|---|---|
| M1 MΦ | IFN-γ, TNF-α, IL-1β |
CD86, CD11c, iNOS | MΦ | UC | TEM (30–150 nm), NTA, WB (Alix, CD9, CD63, CD81 and calnexin) | miR-155 | Exacerbate myocardial infarction injury and inhibit cardiac angiogenesis | 26 |
| RAW264.7MΦ | ND | iNOS, CD68 | MΦ | UC | TEM (80–130 nm), WB (Alix, CD9, CD63) | miR-155 | Increase cardiomyocyte pyroptosis | 34 |
| M2 MΦ | IL-4 | CD68, CD163, CCL22 and PPARγ | MΦ | UC | TEM (mean 100 nm), NTA, WB (CD63, CD81, TSG101) | miR-148a | Reduce myocardial infarction injury | 35 |
| MΦ | EVs | p-p38 MAPR, iNOS | Neonatal cardiomyocytes & H9c2 cells | UC | TEM (50–200 nm), NTA, WB (D63, CD8, flotillin andcalnexin) | Cx43 | Increase macrophage phagocytic activity and resistance to oxidative damage | 36 |
| Raw264.7 MΦ | LPS | ND | MΦ | UC | TEM (112 nm), NTA, WB (CD9, CD63, CD81) | 22 NOD-like signaling pathway proteins | Cause hepatocyte damage and NLRPS inflammation activation | 39 |
| THP-1 MΦ | LPS | ND | THP-1 MΦ | ND | TEM (30–160 nm), DLS, WB (CD9, CD63) | miR-103-3p | Promote hepatic stellate cells proliferation and liver fibrosis | 40 |
| THP-1 MΦ | Exo | CD68, CD11b, CD86, iNOS, IL-6, TNF-α | Lipotoxic hepatocytes | ExoQuick-TC | TEM, NTA, WB (CD63, CD81) | miR-192-5p | Activate the pro-inflammatory macrophages and the progression of NAFLD | 41 |
| Mouse BMDMs | EVs | IL-6, IL-1β | Lipotoxic hepatocytes | ND | TEM (40–300 nm), NTA, WB (Alix, TSG101, cd63, ASGPR1, ARF6) | TRAIL | Activate M1 macrophages-associated inflammation in NASH | 42 |
| THP-1 MΦ | EVs | TNF-α, IL-6, IL-1β, NOS-2 | Hepatocytes | UC | TEM (110 nm), NTA, WB (TSG101, LAmp-1, CD63, RAB5) | CD40 ligand | Promote macrophage activation, contributing to inflammation in ALD | 43 |
| MΦ | Exo | MCP-1, IL-1β, IL-6 and TNF-α | Tubular EC | UC | TEM, NTA, WB (Alix, CD9, CD63) | miR-19b-3p | Lead to M1 macrophages | 45 |
| RAW264.7 MΦ | Exo | CCL2 | Tubular EC | UC | TEM, NTA, WB (Alix, CD9, CD63) | CCL2, TNF-α, IL6 | Activate the M1 macrophages and mediate renal inflammation | 46 |
| RAW264.7 MΦ | Exo | p-P65 | Hypoxic tubular EC | UC | TEM, NTA, WB (Alix, CD9, CD63) | miRNA-223a | Activate the M1 MΦs and tubulointerstitial inflammation | 47 |
| RAW264.7 MΦ | High glucose | CD63, TSG101, Calnexin | MΦs | ExoQuick-TC | TEM, WB (CD63, TSG101, calnexin) | TGF-β1/Smad3 | Activate glomerular mesangial cells | 48 |
| RAW264.7 MΦ | High glucose | p-P65 | MΦs | ExoQuick-TC | TEM, WB (CD63, TSG101, calnexin) | IL-1β, iNOS | Activate M1 MΦs and accelerate kidney injury | 49 |
| Mouse alveolar MΦ | EVs | MIP-2 | Lung EC | UC | TEM, DLS, flow cytometry (F4/80, CD11c) | Caspase-3 | Activate the alveolar MΦs via ROCK1 pathway | 51 |
| Mouse alveolar MΦ & THP-1 MΦ | MVs | TNF-α, IL-1β, IL-10 | Lung EC | UC | TEM, DLS, WB | miR-320a and miR-221 | Promote MΦ-regulated lung inflammatory responses | 52 |
| Mouse BMDMs | Exo | Rab27a, Rab37b | LPS-induced MΦs | UC | NTA, flow cytometry (CD63) | IL-25 | Promote neighboring MΦs to secrete TNF-α. | 54 |
| Mouse alveolar MΦ | LPS-induced ALI | TNF-α, IL-1β, IL-6 | Alveolar MΦs | UC | ELISA (TNF, IL-1β, IL-6), flow cytometry (CD11c) | ICAM-1 | Active epithelial cells and initiate ALI | 56 |
| Mouse alveolar MΦ | LPS-induced ALI | NLRP3, Asc | Alveolar MΦs | UC | TEM, NTA, flow cytometry (F4/80, CD11c), WB (CD40L) | miR-223 and miR-142 | Activate the inflammatory lung responses | 57 |
ALI, acute lung injury; BMDMs, bone marrow derived macrophages; DLS, dynamic light scattering; EC, epithelial cells; Exo, exosomes; EXO, exosomes; LPS, lipopolysaccharide; MΦ, macrophage; ND, not described; NTA, nanoparticle tracking analysis; TEC, tubular epithelial cells; TEM, transmission electron microscopy; UC, ultracentrifugation; WB, Western blot.
3. The role of macrophages and their EVs in inflammatory diseases
As discussed above, M1 macrophages release NO to inhibit cell proliferation during inflammatory responses, while M2 macrophages can secrete factors that induce cell proliferation to promote tissue repair. Similarly, small EVs secreted by M1 macrophages exhibit some of the same pro-inflammatory characteristics of their parental cells, while EVs secreted by M2 macrophages exhibit the ability to promote tissue repair. A major question is whether or not approaches that alter the phenotype of small EVs involved in pro-inflammatory interactions can attenuate the inflammatory responses and tissue injury to serve as potential therapeutic options. This section therefore discusses currently available evidence regarding the nature of EV-mediated regulated macrophage–tissue interactions in several importance inflammatory conditions, and whether these interactions and interventions differ in resolving and non-resolving inflammatory conditions. We discuss examples covering both acute inflammatory responses (AP, AKI, ALI, and sepsis) that can often resolve with supportive care or short-term interventions and non-resolving, chronic inflammatory conditions (IBD, obesity and diabetes) that are more difficult to attenuate and regress.
3.1. Inflammatory bowel diseases (IBDs)
Inflammatory bowel diseases (IBDs), which primarily represent ulcerative colitis and Crohn's disease, are generally considered to be immune homeostasis disorders affecting genetically susceptible individuals, and are characterized by prominent infiltration of the intestines by inflammatory cells such as lymphocytes, T cells, macrophages and neutrophils58. At present, the precise etiology of IBD is still unclear. Macrophages, as the most significant components of the intestinal mononuclear phagocyte system, have received much attention in the study of IBD development. Studies indicate that intestinal macrophages, predominantly M1 macrophages, recruit circulating mononuclear cells and other myeloid cells into the intestinal tract by secreting the chemokines CCL7 and CCL8, and that these recruited monocytes mechanism have pro-inflammatory properties58. However, both tissue-resident and recruited macrophages play key roles in intestinal inflammation in IBD, as they serve as a major source of chemokines that trigger further recruitment of pro-inflammatory monocytes, release IL-1β and TNF-α to induce inflammatory response, disrupt the epithelial barrier, and induce epithelial cell apoptosis and drive intestinal inflammation58,59. Thus, it has been proposed that attenuating monocyte recruitment or promoting M2 macrophage polarization could limit chronic inflammation in IBD. For example, the natural soya-based product genistein has been shown to promote M1 macrophages to adopt an M2 phenotype, and genistein treatment attenuates the severity of dextran sodium sulphate (DSS)-induced colitis60. The induction of M2 macrophages in the intestinal tract was likely IL-10 and TLR4 dependent, given that the effect disappeared in IL-10-, TLR4-and MyD88-deficient mice61.
Macrophages are thus likely to play a central role in IBD-related inflammation, and studies described below indicate that exosome stimulation is responsible for macrophage activation and polarization in IBD62. For example, RAW264.7 macrophages exhibited increases in M1 or M2 macrophage markers63, respectively, when treated with exosomes purified from the serum of DSS-induced or control C57BL/6 male mice. In-depth study found that serum exosomes of DSS-induced mice caused phosphorylation of p38 and ERK and TNF-α production62, and released TNF-α promoted M1 macrophage polarization to further aggravate the inflammatory response63. Small EVs with M1 polarizing activity are found in the peripheral circulation and the intestinal lumen of IBD patients, where small EVs are absorbed by intestinal macrophages, leading to increased IL-8 expression, the induction of M1 macrophage polarization, and ultimately colitis64. Polarization and stimulation of M1 macrophages by small EVs may thus be the major underlying mechanism behind propagation of local inflammation.
Exogenous administration of modified small EVs via the peripheral circulation may inhibit the polarization of M1 macrophages or increase the proportion of anti-inflammatory M2 macrophages to alleviate inflammation. For example, Pediococcus pentosaceus, a gut-resident bacteria, secrete small membrane vesicles that can promote M2 macrophage polarization and the production of myeloid-derived suppressor cells, and these vesicles have been shown to reduce colon inflammation in vivo and in vitro65. Several reports have also indicated that small EVs secreted by stem cells can also promote the M2 polarization of intestinal macrophages and alleviate colitis66, 67, 68. Finally, M2 macrophages also secrete small EVs that have therapeutic potential, since analysis of small EVs isolated from different M2 macrophage subtypes, found that M2b macrophage-derived exosomes could reduce the severity of DSS-induced colitis more effectively than those derived from M2a and M2c macrophages69. Following treatment with exosomes derived from M2b macrophages, mice with colitis revealed an increase in the number of anti-inflammatory regulatory T (Treg) cells and the level of IL-4 in their spleens, and significant suppression of key cytokines associated with colitis (IL-17A, IL-6, and IL-1β)69. In summary, these findings indicate that small EVs have potential to alter local or systematic macrophage M1/M2 macrophage balances and could thus represent an effective treatment strategy to alter and improve inflammation in IBD.
3.2. Aseptic inflammation: Pancreatitis
Acute pancreatitis (AP) is an inflammatory disorder of the pancreas characterized by infiltration of a large number of inflammatory cells or inflammatory factors, leading to the amplification of local and systemic inflammation. M1 macrophage infiltration and activation is a critical step during development of AP70. Because NLRP3 inflammasome activity and NF-κB activation induce the M1 macrophage phenotype and cytokine production, it is possible that prevention of pro-inflammatory M1 macrophage polarization and inhibition of oxidative stress-induced NF-κB and NLRP3 inflammasome responses in the pancreas could prevent or attenuate AP progression70.
Conversely, promoting M2 macrophage polarization could prevent AP or alleviate its inflammatory symptoms. After observing that carbon monoxide (CO) can modulate the macrophages to adopt an M2-like phenotype71, Taguchi et al.72 used nanotechnology-based CO-bound hemoglobin vesicles (CO-Hbv) to treat a mouse model of AP. They found that CO-Hbv treatment polarized macrophages toward an M2-like phenotype and inhibited neutrophil infiltration, eventually suppressing AP and pancreatitis-associated acute lung injury. Abdominal paracentesis drainage (APD) treatment has a beneficial effect on patients with severe AP before percutaneous catheter drainage, and Liu et al.73 determined that APD effect to improve the inflammatory environment of the peritoneal cavity in severe AP was mediated by polarizing peritoneal macrophages (PMs) towards the M2 phenotype, resulting in increased levels of anti-inflammatory mediators (IL-4 and IL-10) and decreased levels of pro-inflammatory mediators (IL-1β and L-selectin). In combination, these studies demonstrate that M1 macrophages can promote AP development, whereas M2-polarized macrophages can alleviate AP inflammation. Modifying macrophage polarization may thus represent a promising treatment for AP.
Exosomes appear to play an important role in AP. In vitro experiments have demonstrated that culture medium from taurolithocholate-activated AR42J pancreatic acinar cells can enhance macrophage NF-κB activation, but this effect disappears when using a supernatant fraction that lacks exosomes74. These results indicate that exosomes from pancreatic acinar cells play a key role in activating pro-inflammatory macrophages in pancreatitis. Further, in an AP rat model, miR-155 in plasma exosomes was found to induce pro-inflammatory activity in macrophages75. Thus, both pancreatic acinar cell-derived exosomes and circulating exosomes can activate recipient macrophages. However, few in vivo studies have reported that pancreatic acinar cells affect the phenotype of tissue-resident pancreatic macrophages via the secretion of small EVs to influence pancreatitis development. However, it has been reported that exosomes derived from pancreatic cancer cells can induce macrophages to adopt the M2 phenotype, which facilitates the migration, invasion, and epithelial-to-mesenchymal transition of pancreatic cancer cells76. MiR-501-3p expressed by M2 macrophage-derived exosomes can inhibit tumor suppressor TGFBR3 gene expression and facilitate the development of pancreatic ductal adenocarcinoma by activating the TGF-β signaling pathway77. It is therefore possible that, as with pancreatic cancer, there are EVs derived by pancreatic acinar cells that can promote the development of pancreatitis by communicating with adjacent macrophages. However, unlike in pancreatic cancer where M2 macrophages appear to promote cancer, M1 macrophages appear to promote pancreatitis73. Further research may be required and demonstrate the existence of an interaction loop between pancreatic cells and tissue-resident macrophages AP progression.
Severe AP develops rapidly and often causes multiple organ dysfunction. ALI is the most common extra-pancreatic complication associated with severe AP, and it is believed that pulmonary macrophages are involved in pancreatitis-associated ALI by releasing inflammatory mediators and recruiting neutrophils. Sabrina et al.78 have reported that AMs promote early inflammatory responses, while interstitial macrophages help resolve inflammation, and M1-like AMs are the dominant population in non-resolving lung inflammation79. A series of tracking experiments have demonstrated that circulating exosomes effectively reach the alveolar compartment and were internalized by macrophages in an AP rat model to polarize AMs towards a pro-inflammatory M1 phenotype80. Thus, circulating exosomes associated with AP appear to communicate with distant macrophages and to promote ALI via pro-inflammatory AM polarization.
3.3. Infection disease: Sepsis
Sepsis is a systemic inflammatory syndrome caused by an extreme response to pathogen-derived factors that can lead to multiple organ dysfunction and potentially death. Since macrophages play an important role in preventing microbial infection and regulating inflammation, macrophage polarization is thought to be a key process in the pathogenesis of sepsis. In bacterial septicemia progression, M1-polarized macrophages phagocytose bacteria and generate a series of pro-inflammatory cytokines that promote an innate immune response81, while in patients with severe sepsis, high circulating concentrations of M1-type cytokines correlate with increased mortality.
Approaches designed to attenuate M1 macrophage activity or induce M1 to M2 macrophage polarization have been studied and deemed successful as potential sepsis treatments. For example, treatment of a cecal ligation and puncture (CLP) mouse sepsis model with antibiotics and gold nanoparticles (AuNPs) that exhibit anti-inflammatory activity significantly attenuated CLP-induced bacterial sepsis, at least partially by inducing macrophage polarization toward an anti-inflammatory phenotype82. This process was measured by evaluating the decreased numbers of M1 macrophages and increased numbers of M2 macrophages in the spleens of these septic mice, as well as the altered cytokine production of bone-marrow-derived macrophages from these mice, which included reductions in pro-inflammatory factors and increases in anti-inflammatory factors82. PMFA (5,7,30,40,50-pentamethoxyflavanone) treatment was also found to promote macrophages to shift from an M1 to an M2 phenotype via effects on STAT1/STAT6 signaling. Intraperitoneal administration of PMFA to LPS- or CLP-induced mouse models of sepsis, significantly increased mouse survival rates and dramatically decreased pro-inflammatory cytokine levels81.
Circulating small EVs have been demonstrated to contribute to systemic signaling and immunomodulation during sepsis. Moreover, these small EVs can induce M1 or M2 macrophage polarization to aggravate or improve sepsis-associated phenotypes. For example, when small EVs derived from the plasma from septic shock patients was injected into animal tissues, there was a marked increase in NF-κB activation and NO synthesis, demonstrating a pro-inflammatory effect83. Conversely, another experiment found that human umbilical cord mesenchymal stem cells pretreated with interleukin-1β secreted miR-146a-containing exosomes that improved the phenotype of a mouse sepsis model by promoting M2 macrophage polarization84. Some EV-associated miRNAs are reported to regulate EV-induced cytokine production to contribute to pro-inflammatory or anti-inflammatory macrophage phenotypes activated by circulating exosomes in sepsis85. In vitro experiment have also shown that endothelial cell cultures release small EVs that carry heat shock protein A12B (HSPA12B) and when these EVs are taken up by LPS-stimulated macrophages they increase IL-10 and decrease TNF-α and IL-1β production, while the loss of endothelial HSPA12B expression aggravates inflammatory responses during sepsis86. This data suggests that specific modifications to small EV cargoes affecting known functional proteins or miRNAs can generate modified EVs that can employed to promote pro-inflammatory or an anti-inflammatory macrophage phenotypes.
Reducing macrophage secretion of small EVs or attenuating the uptake of these EVs could also have therapeutic potential. For instance, macrophages pre-treated with GW4869—an inhibitor of exosome biogenesis/release—and then challenged with LPS revealed impaired release of several pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) compared to cells treated with LPS alone87, suggesting that blocking the secretion of macrophage-derived exosomes in sepsis could reduce macrophage-triggered inflammatory responses. Macrophage-derived nanoparticles can also demonstrate anti-inflammatory properties in sepsis to prolong survival, by reducing pro-inflammatory and increasing anti-inflammatory gene expression in macrophages to reduce endothelial cell inflammation88. Although there are several known mediators of septic inflammation that could be targeted to downregulate inflammatory responses, macrophages-derived exosomes appear to be the most studied and thus the most likely to serve as the basis for a short-term clinical application.
Similar to previously mentioned examples in AKI and ALI, sepsis can also cause systemic organ damage and like pancreatitis-induced ALI, the polarization of M1 AMs is reported to contribute to ALI development in sepsis. One study has reported that serum exosomes from mice with sepsis-related ALI can deliver miR-155 to macrophages to promote their M1 polarization, stimulate NF-κB activation, induce the production of TNF-α and IL-6, and eventually cause ALI89. Evidence also suggests that activated or M1-polarized macrophages isolated from animals with sepsis-related inflammation can act as important factors in the renal injury of AKI animal models44. M1 macrophages were found to be activated by heparin-binding protein (HBP) in sepsis-induced AKI, and heparin-induced suppression of HBP reduced M1 macrophage activation, TNF-α and IL-6 secretion, and the severity of renal injury90. However, to date no studies appear to have reported what signals or factors activate kidney macrophages, although EVs are promising candidates for this mechanism, and we anticipate that future studies will support this hypothesis.
3.4. Inflammatory-related metabolic disease: Obesity and diabetes
Obesity is associated with chronic activation of inflammatory pathways, and obesity-associated insulin resistance is a central mechanism in metabolic diseases such as type 2 diabetes91,92. Emerging evidence has shown that obesity-induced inflammation is a result of elevated levels of circulating pro-inflammatory cytokines, such as TNF-α and IL-6, which negatively affect the insulin signaling cascade to promote insulin resistance93. Adipose tissue macrophages are the principal source of these inflammatory factors in obesity. Macrophages present in adipose tissue of lean individuals primarily exhibit an M2 phenotype, while macrophages in adipose tissue of obese individuals predominantly demonstrate an M1 phenotype94. An alternative M2 activation state maintained by STAT6 and PPARs has been detected in adipose tissue, in contrast to a classical M1 activation state driven by AP1, NF-κB, and other signal-dependent transcription factors95. Chemokines and cytokines secreted by adipose cells induce the recruitment and polarization of macrophages, which are important mediators of cell crosstalk leading to inflammation in adipose tissues.
EVs also play important roles in intercellular communication in adipose tissue, however, especially when one considers that exosome miRNAs have the ability to regulate TGF-β and WNT/β-catenin signaling in obesity-induced inflammation96. Human adipocyte derived EVs are also reported to have immunomodulatory effects on macrophages, and macrophages pre-treated with human adipocyte-derived EVs secrete small EVs that interfere with adipocyte insulin signaling97. This demonstrates that there is a reciprocal regulatory loop between adipocytes and macrophages that is mediated by EVs and which affects macrophage activation and adipocyte insulin signaling that leads to adipose tissue inflammation98. This behavior is demonstrated in animal models, where adipocytes can release EVs which act as “find me” signals to promote macrophage migration and infiltration99. Additionally, miR-155 in adipocyte-derived MVs induces M1 macrophage polarization, causing chronic inflammation and local insulin resistance100. Adipocyte-secreted exosomes also transport miR-34a into macrophages, thereby suppressing M2 polarization by repressing the expression of KLF4101. Adipocyte-derived small EVs can therefore regulate macrophage infiltration and the adipose tissue M1/M2 macrophage ratio to induce obesity-related inflammation. Alteration of the disease-promoting balance between M1 and M2 adipose tissue macrophages can also contribute to the regulation of adipose tissue inflammation and insulin resistance102. For example, Zhuge et al.103 used linaglipin, a strong DPP-4 inhibitor, to treat obesity, and found that it could reduce M1-polarized macrophage migration while inducing an M2-dominant macrophage shift within white adipose and liver tissues, thereby attenuating obesity-induced inflammation and insulin resistance.
Complementary to adipocyte mechanisms to activate macrophages, activated macrophages not only secrete inflammatory cytokines that provoke systemic and local inflammation but also release EVs to influence adipocyte activity. Exosomes from LPS-activated macrophages upregulate some of the pathways and metabolic processes related to inflammation onset in adipocytes20. These exosomes also exhibit increased levels of miR-29a, which can be transferred into hepatocytes, myocytes and adipocytes to impair the insulin sensitivity of these target cells in vitro and in vivo, ultimately promoting obesity-induced insulin resistance and the development of type 2 diabetes104. These results indicate that activated macrophages participate in a variety of inflammation-related metabolic pathways by releasing small EVs that promote local and systemic chronic inflammation. Altering the polarization of macrophages and thus the cargoes and activities of their small EVs may improve macrophage-mediated inflammation. Melatonin serves as an example for the potential of such an approach, since it alleviates adipose inflammation both by direct actions on adipocytes and indirect activity on macrophages mediate by adipocyte-derived EVs105.
Circulating EVs can thus polarize M1 macrophages to secrete small EVs that trigger pro-inflammatory responses (Fig. 3) associated with acute inflammatory responses (AP, AKI, ALI, and sepsis) that can resolve with supportive care or short-term interventions and non-resolving, chronic inflammatory conditions (IBD, obesity and diabetes) that are more difficult to attenuate and regress. Small EVs can, however, also carry protein and miRNA cargoes that limit attenuate acute inflammation and promote tissue repair or limit inflammation and tissue damage during chronic inflammation conditions. Better understanding of the roles that EVs play in regulating the polarization and exerting the regulatory effects of macrophages during the initiation and resolution of inflammatory responses and conditions may identify new therapeutic targets for intervention to limit excessive tissue injury. Approaches that target EV-mediated mechanisms involved in these processes may be more effective in limiting inflammation and tissue injury and have fewer side effects than approaches that attempt to attenuate macrophage accumulation or activity.
Figure 3.
M1 macrophage EV interactions in inflammatory diseases affecting different tissues. EVs in the peripheral circulation carry miR-155 that activates p38/ERK signaling pathways and M1 macrophage polarization. M1 macrophage EVs (M1-EVs) demonstrate multiple pro-inflammatory effects, as they: induce proinflammatory IL-1β and TNF-α secretion in colon tissue, and endothelial dysfunction, to promote inflammatory bowel diseases (IBDs); activate NF-κB and NLRP3 signaling in acinar cells to induce acute pancreatitis (AP); target lung and kidney tissues to induce acute lung injury (ALI) and kidney injury (AKI); carry miR-34a, which upregulates TGF-β and WNT/β-catenin signaling in adipocytes to promote in insulin resistance (IR). Colon cells and adipocytes experiencing inflammatory conditions also release EVs that can promote macrophage polarization to pro-inflammatory phenotypes.
4. MSC EVs promote macrophages to an anti-inflammatory M2 phenotype
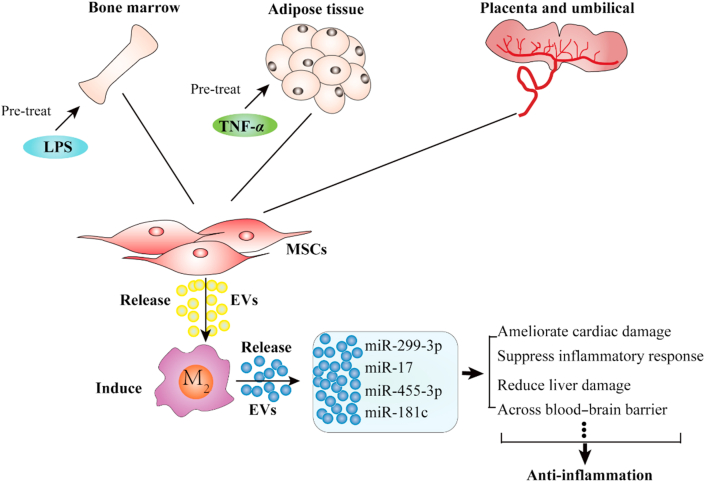
Given the ability of M2 macrophages to limit inflammation and tissue injury, several studies have examined the potential of different methods to stimulate M2 macrophages to release anti-inflammatory mediators in vitro and in vivo. Mesenchymal stromal cells (MSCs) have recently shown great potential as an alternative means to treat inflammatory disorders due to their immunomodulatory characteristics106, 107, 108. MSCs are mesodermal cells that can differentiate into chondrocytes, osteocytes, and adipocytes in vitro, and are present in multiple tissues including the umbilical cord, muscle, adipose and bone marrow109,110. While MSC-based products have been used in several clinical trials, various problems such as low transplanted cell viability, immunogenicity, poor homing to desired tissues, and safety issues have prevented MSCs from being used in clinical applications107,111. More recent research has therefore shifted from the use of MSCs to the use of MSC-derived EVs, which can effectively transfer MSC-derived functional molecules to target cells without the issues associated with direct MSC transfer. For example, the secreted apoptotic bodies from MSC carry the immunoregulatory factors of MSC, which are taken up by target cells by endocytosis or membrane fusion, and MVs or exosomes exert biological effects in a paracrine and endocrine manner112. Clinical trials have found that MSC-derived small EVs may offer specific advantages when compared to MSC cells due to their lower immunogenicity and greater safety profile, and MSC-EVs administration is also less likely to suffer from the pulmonary first pass effect109,113. More importantly, MSC-EVs have shown great potential in promoting M2 macrophage polarization in inflammatory related diseases113.
4.1. Bone marrow stromal cells (BMSCs)
Bone marrow stromal cell (BMSC)-derived small EVs (BMSC-EVs) have been reported to reduce colonic inflammation by downregulating inflammatory responses and maintaining intestinal barrier integrity. The beneficial effects of BMSC EVs are blocked by macrophage depletion, indicating that BMSC EVs exert anti-inflammatory through colonic macrophages114. Similarly, in an achilles tendon rupture model, Chamberlain et al.115 found that exosomes derived from macrophages exposed to BSMC exosomes (BSMC-Exo) promoted more regenerative healing responses than BSMC-Exo. In a second tendon rupture model study, local administration of BMSC-Exo at the injury site was also found to promote the formation of fibrocartilage by increasing M2 macrophage polarization and decreasing M1 macrophage levels, with beneficial effects on tissue repair and biomechanical properties at the injury site111. Finally, in myocardial ischemia mouse model, intramyocardial injection of BMSC-Exo could reduce infarct size, and its effects have been confirmed by modifying the polarization of M1 macrophages to M2 macrophages116,117. These examples illustrate that BMSC-Exo and BMSC-EV have broad effects to attenuate inflammation and promote tissue repair through M2 macrophage-dependent mechanisms.
BMSCs possess some characteristics of immune cells and short-term memory of environmental signals, as one group has demonstrated by showing that BSMC exhibit improved therapeutic efficacy in promoting skin flap survival after being pre-treated with LPS118. This discovery poses question of whether small EVs secreted by LPS-treated BMSCs have improved anti-inflammatory properties. A study by Ti et al.119 has reported that BSMC-Exo obtained after BSMCs were pre-conditioned with LPS (LPS-BSMC-Exo) were better than BMSC-Exo from untreated BSMCs at modulating the balance between macrophage phenotypes and promoting M2 macrophage activation due to their upregulation of anti-inflammatory cytokines, and that the let-7b/TLR4 pathway plays a critical role in this activity. Similarly, in an in vitro mouse model of myocardial injury, LPS-BSMC-Exo had superior therapeutic effects to attenuate post-infarction inflammation and cardiomyocyte apoptosis by regulating macrophage polarization than did BMSC-Exo from untreated cells116. These studies indicate that low concentration LPS-BMSC-Exo administration may be an alternative to BMSC therapy as cell-free treatment strategy to induce M2 macrophages.
BMSC-EV exposure should, in theory, be capable of inducing both M1 and M2 macrophage polarization. The literature we retrieved for this review indicated only that BMSC-EV promoted the polarization and activity of M2 macrophages. However, it is possible that pre-treatment of BSMCs with alternate stimuli could alter BSMC-Exo cargoes to promote pro-inflammatory responses in recipient cells, and is worth investigation.
4.2. Adipose stem cells
Although BMSC-derived small EVs are reported useful in tissue repair and disease treatment, the BSMC isolation procedure is complex and painful120. Adipose-derived stem cells (ADSCs), also known as adipose-derived stromal cells, are MSCs derived from the stromal vascular fraction of adipose tissue. Evidence suggests that adipose tissue can provide up to 500-fold more MSCs than an equivalent amount of bone marrow aspirate, and ADSCs have therefore emerged as a stable source of MSCs due to their greater relative abundance, high yield upon isolation, ease of collection via a less invasive procedure that has fewer ethical issues120,121. Due to the relatively simple procedure involved in ASDC isolation their ability of SADCs to differentiate into other tissue types of the mesoderm (e.g., bone, cartilage and muscle), ADSCs have been studied in and proven useful in a wide variety of applications122, such as potential therapies for arthritis, colitis, and autoimmune diabetes123. ADSCs have shown the same immunoregulation and regeneration capabilities as other MSCs, and small EVs secreted by ADSCs (ADSC-EV) appear to exert the same functions as ADSCs.
ADSC-EV, like BMSC-EV, has been found to promote the polarization and activation of M2 macrophages. Exosomes derived from ADSCs reduce adipose inflammation and obesity by interacting with macrophages to induce anti-inflammatory M2 phenotypes, in which activated M2 macrophages exert immunosuppressive and anti-inflammatory effects123,124. ADSC-EVs have also been shown to promote the polarization and activation of M2 macrophages to ameliorate inflammation in lung epithelial cells treated with an inflammatory factor125. While the mechanisms responsible for the activity of ADSC-EVs are not fully understood, one study indicates that the ability of these EVs to induce M2 macrophage polarization and limit inflammatory responses is mediated by inhibiting autophagy, a process that may shift macrophage polarization toward the M1 phenotype126.
Several proteins and miRNAs in ADSC-derived exosomes can also activate M2 macrophage signaling pathways. For example, miR-17-enriched EVs secreted by ADSCs can suppress NLRP3 inflammasome activation by targeting TXNIP to contribute to the reduction in inflammatory factors secreted by macrophages127. Further, reduced expression of regulatory factors in target cells and tissue that leads to inflammation can be countered by the administration of modified ADSC-EVs that carry these factors. Lung tissue of asthmatic mice exhibits a marked downregulation of the circular RNA mmu_circ_0001359 and treatment of asthmatic mice with ADSC-EVs loaded with this RNA has been reported to reduce airway remodeling and promote the polarization of AM to an M2 phenotype to reduce the release of inflammatory factors128. In another example, tissue from the brains of patients and animals exposed to acute ischemic shock was found to exhibit reduced expression of microRNA miR-30d-5p, while treatment with ADSC-EVs produced by ADSCs expressing an miR-30d-5p mimic vector could effectively promote the M2 polarization of microglia and reduce the cerebral injury area at the site of infarction126. These studies indicate that ADSC can be readily modified in vitro to express small RNAs to produce EVs with therapeutic effects. Similar direct therapeutic approaches may also be possible for other inflammatory conditions where the downregulation of specific factors lead to enhanced, aberrant or dysregulated inflammation. Due to the high abundance of ADSCs in their source tissue relative to other MSCs, and the relative simplicity of the isolation and extraction processes required to produce ADSC isolates, ADSCs are likely to become the primary source of anti-inflammatory EVs employed to promote M2 macrophage polarization in the treatment of inflammatory disease when and such applications are approved for therapeutic applications.
4.3. Human umbilical cord mesenchymal stem cells (HUCMSCs)
MSCs derived from the human umbilical cord (HUCMSCs) can be obtained from the amniotic membrane, cord-lining, Wharton's jelly, or peri-vascular region129. HUCMSCs are isolated using a painless collection procedure and have rapid self-renewal properties, unlike BMSCs129. In addition, pre-clinical trials have found that HUCMSCs applications have fewer side effects and are generally safer than other MSC treatments129. Thus, HUCMSCs are a promising source of MSCs due to their relative abundance, non-invasive collection, convenient and inexpensive isolation procedure, low immunogenicity and high gene transfection efficiency130. Evidence shows that HUCMSCs exert their therapeutic effects primarily through EV-mediated paracrine effects. In many cases of inflammation-related diseases and tissue damage repair110,131, HUCMSC EVs exhibit all of the advantages of HUCMSCs without their disadvantages, and are easier to maintain in a bioactive state during storage and transport132. HUCMSC EVs are acquired by extensive in vitro expanding HUCMSCs; are convenient to extract, store and transport; and exhibit lower immunogenicity and better biocompatibility than other macrophage-polarizing exosomes133. Multiple studies have now shown that HUCMSC EVs exhibit strong potential for use in “cell-free therapy” approaches110.
HUCMSC EVs are reported to reduce inflammation by targeting macrophages, promoting M2 macrophage polarization, and enhancing the recruitment of M2 macrophages to several organs and tissues, including the islets, liver, fat, and muscle134. HUCMSC exosomes have been shown to effectively trigger bone marrow-derived macrophages to undergo a shift from M1 to M2 polarization in vitro, to possess characteristic anti-inflammatory effects, and to improve functional recovery in mice with spinal cord injuries by downregulating inflammatory cytokines (e.g., TNF-α, MIP-1α, IL-6 and IFN-γ135). Systemic administration of HUCMSC exosomes has also been shown to reverse inflammation related to burn injuries in rats, by limiting macrophage-based inflammatory reactions136. HUCMSC exosome miR-181c played a pivotal role in attenuating burn-associated inflammation in this study, since HUCMSC exosomes that overexpressed miR-181c were more effective at suppressing TLR4 signaling in burned rats to reduce inflammation136. Similarly, another group examined the ability of HUCMSC exosome miR-455-3p, which was induced by IL-6 treatment, to inhibit IL-6 signaling by suppressing PI3K expression137, and found that miR-455-3p-enriched exosomes inhibited LPS-challenged macrophage activation and cytokine production. Thus, like ADSCs cells, it is feasible to modify HUCMSCs in vitro to obtain EVs enriched in specific regulatory miRNAs for targeted treatment of selected inflammatory conditions.
HUCMSCs, unlike BMSCs and ADSCs, have innate hepatogenic properties, however, and HUCMSC therapy can be used to directly treat acute liver injury without requiring that HUCSMCs undergo differentiation or manipulation prior to their administeration138, raising the question as to whether or not unmodified HUCMSC EVs exhibit similar tissue repair activity. One study has reported that unmodified HUCMSC exosomes can indeed efficiently accumulate in the liver to alleviate acute liver failure by reducing macrophage NLRP3 inflammasome activity139. However, a second study has shown that exosomes derived from HUCMSC treated with TNF-α exhibit enhanced therapeutic effects to attenuate liver damage140, finding that miRNA-299-3p is upregulated in TNF-α-stimulated HUCMSCs and selectively packaged into exosomes to exert anti-inflammatory functions in exosome treatments140. Thus, HUCMSC-EVs appear to have a unique advantage for the treatment of liver inflammation and injury.
HUCMSC exosomes have also been reported to penetrate the blood–brain barrier and modulate the activation of microglia in mouse brains to alleviate neuroinflammation131, which is a critical advantage for exosome-based applications intended to treat neurodegenerative conditions, such as Alzheimer's disease, and other neurological pathologies, since the blood–brain barrier poses a formidable barrier for most therapeutic approaches.
Most stem cells appear to produce EVs that can promote anti-inflammatory M2 macrophage polarization, regardless of whether the stem cells derive from bone marrow, adipose, or umbilical cord tissue, and LPS-pretreatment of many of these cells has been reported to enhance the anti-inflammatory activity of their EVs. However, one report indicates that LPS-pretreated periodontal ligament stem cells secrete small EVs that can induce M1 macrophage polarization141. Various therapeutic effects mediated by stem cell derived EVs on macrophage polarization are listed in the following Table 2114, 115, 116, 117,119,123,125, 126, 127, 128,135, 141. The ability of stem cells and their EVs to induce M1 or M2 macrophage polarization may thus depend on the source tissue and original microenvironment of the stem cells, which may affect their relative differentiation state, as well as conditions and stimuli these cells encounter during isolation and cell culture expansion. The ability of MSC EVs to promote M2 macrophage polarization appears to have significant potential utility for applications designed to attenuate inflammatory conditions and promote tissue repair, however, there is also a concern for potential negative consequences due to off-target treatment effects. For example, M2 macrophages present within a tumor microenvironment can accelerate tumor progression by promoting cell proliferation and metastasis, and MSC EV administration during non-small cell lung cancer has been shown promoting the growth and mobility of cancer cells by polarizing M2 macrophages142.
Table 2.
Macrophage polarization effects of EVs derived from stem cell from different tissue sources.
| MSC source | Stimulus | Species; model | Macrophage polarization effect | Effects/mechanism | Therapeutic effect | Ref. |
|---|---|---|---|---|---|---|
| Bone marrow | No | Mouse; IBD (DSS & TNBS induced) | M2b macrophage polarization | Induction of IL-10 production | Suppressed inflammatory responses | 114 |
| Bone marrow | No | Mouse; Achilles tendon rupture | M2 macrophage polarization | Increase the number of endothelial cells and reduce type I collagen | Improved mechanical function & angiogenesis; reduced inflammation | 115 |
| Bone marrow | LPS | Mouse; myocardial infarction | M2 macrophage polarization | Depress NF-κB signaling pathway and activate AKT1/AKT2 signaling pathway | Reduced post-infarction cardiomyocyte apoptosis & inflammation | 116 |
| Bone marrow | No | Mouse; myocardial ischemia/reperfusion injury | M2 macrophage polarization | miR-183 targeting of the TLR4 pathway | Reduced infarct size & inflammation | 117 |
| Bone marrow | LPS | Rat; diabetic cutaneous wound healing | M2 macrophage activation | Let-7b regulation of the TLR4/NF-κB/STAT3/AKT signaling pathway | Reduced inflammation & enhance cutaneous wound healing | 119 |
| Adipose tissue | No | Mouse; Obesity | M2 macrophage polarization | Arginase-1 activation of STAT3 transcription. | Greater insulin sensitivity & reduced adipose inflammation & obesity | 123 |
| Adipose tissue | No | Mouse; Aspergillus protease-treated MLE12 epithelial cells | M2 macrophage activation | Reduce eotaxin and IL-25, increase TGF-β and IL-10 | Reduced Th2-mediated inflammation | 125 |
| Adipose tissue | miR-30d-5p mimic | Rat; acute ischemic stroke | Promote M2 microglia/macrophage polarization | Suppress autophagy | Reduced cerebral injury area following infarction | 126 |
| Adipose tissue | LPS and d-galactosamine | Macrophages | Suppress M1 macrophage activation | miR-17 suppression of TXNIP/NLRP3 pathway | Reduce inflammatory factor secretion | 127 |
| Adipose tissue | No | Mouse; ovalbumin-induced asthma | M1 to M2 macrophage conversion | miR-183-5p sponging to enhance FoxO1-mediated M2 macrophage activation | Reduced airway remodeling | 128 |
| Human umbilical cord tissue | No | Mouse; spinal cord injury | Drive BMDM from M1 to M2 polarization | Inflammatory cytokine downregulation | Improved resolution of spinal cord injury | 135 |
| Human umbilical cord tissue | No | Rat; burn healing | Inhibit secretion of pro-inflammatory factors | miR-181c downregulation of TLR4 signaling pathway | Reduced burn-induced inflammation | 136 |
| Human umbilical cord tissue | IL-6 | Mouse; chemically induced liver injury | Inhibit macrophage activation and cytokine production | miR-455-3p targeting of PI3K signaling | Reduced macrophage infiltration & improved liver histology | 137 |
| Human umbilical cord tissue | No | Mouse; acute liver injury | ND | Reduced inflammation | Accelerated resolution of acute liver injury | 138 |
| Human umbilical cord tissue | No | Mouse; acute liver injury & LPS-stimulated RAW264.7 macrophages | Inhibit activation of the NLRP3 pathway | Reduced expression of the NLRP3 inflammasome and its regulated inflammatory factors | Enhanced liver tissue repair | 139 |
| Human umbilical cord tissue | TNF-α | Mouse; acute liver injury & LPS-stimulated RAW264.7 macrophages | Inhibit activation of the NLRP3 pathway | miRNA-299-3p reduction of proinflammatory cytokines and inhibition of the NLRP3 pathway | Reduced liver damage | 140 |
| Periodontal ligament | LPS | THP-1 macrophages | M1 macrophage polarization | Increased expression of M1-associated cytokines | M1 macrophage polarization | 141 |
IBD, Inflammatory bowel diseases; DSS, dextran sodium sulfate; TNBS, 2,4,6-trinitrobenenesulfonic acid solution; LPS, lipopolysaccharide; ND, not described; BMDMs, bone marrow derived macrophages.
Strategies that employ EVs isolated from different MSCs to induce M2 macrophage polarization to limit tissue inflammation and promote tissue repair (Fig. 4) have been attempted for various inflammatory disease conditions. MSC-derived EVs can promote AMs to adopt M2 phenotypes and attenuate tissue damage in clinically relevant lung injury models143, and to reduce hypoxia-ischemic injury in neonatal mice by modulating microglia/macrophage polarization144. HUCMSCs seem to be the best overall source of anti-inflammatory EVs among the MSCs discussed herein for the reasons listed above. EVs of each of these MSC types, however, have shown considerable ability to inhibit tissue inflammation and injury, and their relative merits as therapeutic approaches designed to treat specific inflammatory conditions should be carefully considered in future studies.
Figure 4.
Extracellular vesicles (EVs) secreted by mesenchymal stromal cells (MSCs) from bone marrow, and adipose and umbilical cord tissue induce M2 macrophage polarization via specific miRNA cargoes to regulate an anti-inflammatory effects.
5. Potential application of macrophage-derived EVs for inflammatory disease treatment
Conventional drug-loaded nanoparticles have, to a degree, demonstrated potential utility for infectious disease treatment, but such nanoparticles are often difficult to manufacture, can be rapidly cleared from the circulation by the mononuclear phagocyte system, and may cause undesirable side effects such as organ toxicity and the activation of immune responses145, 146, 147. Decorating nanoparticles with a polyethylene glycol (PEG) corona is thus sometimes used to reduce the recognition of nanoparticles by the mononuclear phagocyte system to avoid their rapid clearance from the circulation. However, small EVs have emerged as a better means for drug delivery than PEGylated nanoformulations, due to the low immunogenicity, high biocompatibility, and high efficacy of these EVs22, although exosome PEGylation can also reduce mononuclear phagocyte system recognition after administration to mice to improve their time in circulation147. Small EVs contain a variety of adhesion molecules with their lipid bilayer that can interact with cell membrane factors to promote their interaction and uptake by specific cell types, and thus small EVs can have specific cell tropisms according to their surface display of specific adhesion molecules, which is reflective of their specific cell origin145. Nanoparticles are usually of abiotic origin, however, and thus lack these cell interaction mechanisms. Studies have also indicated that small EVs are taken up by their recipient cells about 30 times more efficiently than synthetic nanoparticles145. This suggests that drugs loaded into small EVs can be more efficiently delivered to target cells at therapeutic quantities, demonstrating a major advantage of small EVs-based drug delivery systems over common synthetic nanocarriers. Taken together, these characteristics imply that small EVs-based treatment approaches are likely to be more effective than those that employ synthetic nanoparticles.
Many challenges still need to be solved to allow the clinical translation of approaches that employ small EVs for therapeutic applications, especially for infectious disease applications. First, standard procedures for small EV isolation must be established to allow the consistent isolation of high purity small EV preparations. Several methods are currently used for small EV isolation, including immunoaffinity, density gradient, and ultracentrifugation isolation approaches, but each method has drawbacks that limit utility for clinical applications19. For example, immunoaffinity separations have low recovery rates; density gradient procedures are labor-intensive and are not amenable to automation; and ultracentrifugation approaches are time-consuming19,22, while the performance of commercial kits requires further improvement. Small EV isolation methods thus require additional improvement to allow consistent large-scale isolation of high-purity EVs that will be required for clinical applications.
Second, additional studies are required to determine which cells (e.g., macrophages or MSCs) are the most appropriate source for the small EVs used in a therapeutic application, which may vary by the specific application. Small EVs tend to have characteristics of their parent cells. EVs derived from M1 macrophages have elevated potential to activate inflammatory responses via boosting a Th1 and Th17 response, while EVs derived from M2 macrophages are generally able to attenuate inflammation via a mechanism bypassing a Th1 and Th17 response148, while small EVs secreted by MSCs have been shown to suppress pro-inflammatory responses and stimulate cell proliferation in clinical trials for several diseases149. Approaches employing all these small EVs have lower therapeutic risk and superior safety profiles than cell-based therapy approaches employing their parental cells150,151. Beyond their ability to mediate pro- or anti-inflammatory responses, small EVs derived from these different cell types may exhibit distinct cell targeting capacities or other functionalities. Macrophages secrete exosomes that express CD47 receptors that interact with signal regulatory protein α (SIRPα) to produce a “don't eat me” signal upon their interaction with phagocytes147. Macrophage-derived exosomes express intercellular adhesion molecule 1 (ICAM-1), enhancing their interaction with blood–brain barrier endothelial cells152 and transit through the blood–brain barrier. Similarly, the innate ability of HUCMSC EVs to accumulate in the liver and attenuate hepatic tissue inflammation may make them the best candidates for small EV therapeutics when designing applications to treat liver inflammation and injury.
Finally, the ability to modify the cargoes of small therapeutic EVs by loading small molecules may represent an additional consideration. Many different approaches have been described to load small molecules into EVs, either directly or indirectly, including electroporation, sonication, incubation19, and these exhibit different loading efficiencies, and may cause off-target effects22. Go et al.153 have achieved remarkable results in the treatment of inflammation induced by Gram-negative bacteria by loading dexamethasone into monocyte-derived EVs by sonication, illustrating that it is feasible to directly load antibiotics directly into macrophages-derived EVs to produce effective therapeutics for infectious disease treatment. In a related study, Yang et al.154 used an incubation approach to load the antibiotic linezolid into exosomes derived from mouse RAW264.7 macrophages, and found that this exosome-encapsulated linezolid formulation had a better inhibitory effect on intracellular methicillin-resistant Staphylococcus aureus infections in vitro and in vivo that direct linezolid treatment, without any sign of macrophage cytotoxicity. These studies indicate that antibiotic-loaded macrophage-derived small EVs may be more effective therapeutics than direct treatment, particularly for intracellular microbial infections. Macrophage-derived EVs may be particularly useful for such applications, since macrophage cell lines can serve as stable sources of small EV preparations and are amenable to manipulations that promote the selective delivery of specific therapeutic molecules to desired target cells or tissues, while the inherent anti-inflammatory properties of such EVs may amplify desired therapeutic effects.
Finally, differences in stimuli encountered during cell isolation or culture or EV processing can alter the characteristics and function of small EV preparations produced from the same tissues or cells. Strictly controlling the consistency of small EVs therefore requires considerable attention to detail, and may be especially challenging when using stem cell populations. Additional studies are required to establish standardized procedures for EV isolation and manipulation for the various cell types under investigation. Further experiments are also needed to verify the roles of these EVs in specific processes pertaining to various categories of inflammatory disease.
6. Conclusion and perspectives
This review summarizes the specific effects of macrophages, MSCs and their small EVs in several inflammatory disease conditions, particularly in regard to inflammatory responses that lead to local or remote tissue damage, and how EV-based interaction loops between pro-inflammatory macrophages and tissues play a vital role in disease progression. Evidence now indicates that altering the interactions between macrophages and their target cells in tissues affected by inflammatory conditions via administration of native or exogenously modified EVs preparations can be a promising approach to attenuate tissue inflammation and injury. Consideration must be paid to the relative stage of the tissue injury/repair process or the severity of the inflammatory response, however, since both pro- and anti-inflammatory macrophage activities are required at different stages of the resolution of infection or tissue injury. In the early stages of some inflammatory responses, M1 macrophages release regulatory factors and recruit cells required to initiate innate and adaptive immune responses to limit and resolve local infections, while later in such infections or during injury responses M2 macrophages are needed promote tissue repair by limiting inflammation, clearing cell debris, and promoting the cell proliferation to replace cell deficits. Small EVs secreted by MSCs mentioned in this paper can polarize M2 macrophages, and EVs released by these cells have been shown to have important actions in the resolution of inflammatory responses and tissue repair, and may serve as therapeutics to improve the control of dysregulated or excessive inflammation that may cause tissue injury.
Several questions remain to be answered about the potential small EV therapeutics, including the best time to employ them and what their side effects may be when used in different situations.
Over-activation of M1 macrophages is expected to promote systemic inflammatory responses, potentially including atherosclerosis155. M2 macrophages appear to be the “default” form for most tissue-resident macrophages, and appear to be required to control systemic inflammation. However, M2 macrophage EVs can also facilitate tumor progression by promoting tumor cell proliferation and invasion156. Thus, the decision to use potential future EV applications to resolve inflammatory conditions or treat certain infections will need to consider multiple factors to achieve maximum efficacy and reduce potential side effects.
In addition to these considerations, further studies are also required to establish standardized conditions for EV isolation and modification procedures, since such differences may alter the characteristics and innate functionality of therapeutic small EV preparations. Macrophage-derived EVs appear to have potential as drug carriers for the treatment of infectious disease. However, similar studies have not yet demonstrated that such EVs can be used to deliver anti-inflammatory drugs to attenuate inflammatory disease conditions, although this is likely to be future applications, particularly when considering the innate anti-inflammatory characteristics of M2 macrophages within the topic aseptic inflammation.
Acknowledgments
Tony Y. Hu thanks the support from Weatherhead Presidential Endowment Fund (USA).
Author contributions
Qian Hu and Jesse K. Fletcher drafted the main part of the manuscript. Wenfu Tang and Christopher Lyon edited the manuscript and contributed by iteratively reviewing and improving the manuscript. Meihua Wan and Tony Y. Hu provided oversight of drafting the manuscript, and made substantive intellectual contributions and improvements. All authors read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Meihua Wan, Email: wanmh@scu.edu.cn.
Tony Y. Hu, Email: tonyhu@tulane.edu.
References
- 1.van Furth R., Cohn Z.A., Hirsch J.G., Humphrey J.H., Spector W.G., Langevoort H.L. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- 2.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz C., Gomez Perdiguero E., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 4.Ovchinnikov D.A. Macrophages in the embryo and beyond: much more than just giant phagocytes. Genesis. 2008;46:447–462. doi: 10.1002/dvg.20417. [DOI] [PubMed] [Google Scholar]
- 5.Epelman S., Lavine K.J., Randolph G.J. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kratofil R.M., Kubes P., Deniset J.F. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol. 2017;37:35–42. doi: 10.1161/ATVBAHA.116.308198. [DOI] [PubMed] [Google Scholar]
- 7.Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai S.M., Sheng J., Gupta P., Renia L., Duan K., Zolezzi F. Organ-specific fate, recruitment, and refilling dynamics of tissue-resident macrophages during blood-stage malaria. Cell Rep. 2018;25:3099–3109. doi: 10.1016/j.celrep.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Davies L.C., Taylor P.R. Tissue-resident macrophages: then and now. Immunology. 2015;144:541–548. doi: 10.1111/imm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zigmond E., Samia-Grinberg S., Pasmanik-Chor M., Brazowski E., Shibolet O., Halpern Z. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014;193:344–353. doi: 10.4049/jimmunol.1400574. [DOI] [PubMed] [Google Scholar]
- 11.Minutti C.M., Knipper J.A., Allen J.E., Zaiss D.M. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3–11. doi: 10.1016/j.semcdb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Mills C.D. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 13.Varol C., Mildner A., Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–675. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 14.Juhas U., Ryba-Stanisławowska M., Szargiej P., Myśliwska J. Different pathways of macrophage activation and polarization. Postepy Hig Med Dosw. 2015;69:496–502. doi: 10.5604/17322693.1150133. [DOI] [PubMed] [Google Scholar]
- 15.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.A., Mardani F. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 16.Ambarus C.A., Krausz S., van Eijk M., Hamann J., Radstake T.R., Reedquist K.A. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Murray P.J. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 18.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 19.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 20.De Silva N., Samblas M., Martínez J.A., Milagro F.I. Effects of exosomes from LPS-activated macrophages on adipocyte gene expression, differentiation, and insulin-dependent glucose uptake. J Physiol Biochem. 2018;74:559–568. doi: 10.1007/s13105-018-0622-4. [DOI] [PubMed] [Google Scholar]
- 21.Xu H., Jia S., Xu H. Potential therapeutic applications of exosomes in different autoimmune diseases. Clin Immunol. 2019;205:116–124. doi: 10.1016/j.clim.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Hu Q., Su H., Li J., Lyon C., Tang W., Wan M. Clinical applications of exosome membrane proteins. Precis Clin Med. 2020;3:54–66. doi: 10.1093/pcmedi/pbaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cypryk W., Nyman T.A., Matikainen S. From inflammasome to exosome-does extracellular vesicle secretion constitute an inflammasome-dependent immune response?. Front Immunol. 2018;9:2188. doi: 10.3389/fimmu.2018.02188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalyfa A., Kheirandish-Gozal L., Gozal D. Exosome and macrophage crosstalk in sleep-disordered breathing-induced metabolic dysfunction. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19113383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald M.K., Tian Y., Qureshi R.A., Gormley M., Ertel A., Gao R. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain. 2014;155:1527–1539. doi: 10.1016/j.pain.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S., Chen J., Shi J., Zhou W., Wang L., Fang W. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol. 2020;115:22. doi: 10.1007/s00395-020-0781-7. [DOI] [PubMed] [Google Scholar]
- 27.Nie W., Wu G., Zhang J., Huang L.L., Ding J., Jiang A. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl. 2020;59:2018–2022. doi: 10.1002/anie.201912524. [DOI] [PubMed] [Google Scholar]
- 28.Wan M., Ning B., Spiegel S., Lyon C.J., Hu T.Y. Tumor-derived exosomes (TDEs): how to avoid the sting in the tail. Med Res Rev. 2020;40:385–412. doi: 10.1002/med.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen T., Cao Q., Wang Y., Harris D.C.H. M2 macrophages in kidney disease: biology, therapies, and perspectives. Kidney Int. 2019;95:760–773. doi: 10.1016/j.kint.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 30.Vannella K.M., Wynn T.A. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79:593–617. doi: 10.1146/annurev-physiol-022516-034356. [DOI] [PubMed] [Google Scholar]
- 31.Pinto A.R., Godwin J.W., Rosenthal N.A. Macrophages in cardiac homeostasis, injury responses and progenitor cell mobilisation. Stem Cell Res. 2014;13:705–714. doi: 10.1016/j.scr.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Frangogiannis N.G. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol. 2015;30:240–245. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epelman S., Lavine K.J., Beaudin A.E., Sojka D.K., Carrero J.A., Calderon B. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C., Zhang C., Liu L., Xi A., Chen B., Li Y. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol Ther. 2017;25:192–204. doi: 10.1016/j.ymthe.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Y., Wang S., Chang S., Ren D., Shali S., Li C. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway. J Mol Cell Cardiol. 2020;142:65–79. doi: 10.1016/j.yjmcc.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Almeida Paiva R., Martins-Marques T., Jesus K., Ribeiro-Rodrigues T., Zuzarte M., Silva A. Ischaemia alters the effects of cardiomyocyte-derived extracellular vesicles on macrophage activation. J Cell Mol Med. 2019;23:1137–1151. doi: 10.1111/jcmm.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y.Y., Li X.F., Meng X.M., Huang C., Zhang L., Li J. Macrophage phenotype in liver injury and repair. Scand J Immunol. 2017;85:166–174. doi: 10.1111/sji.12468. [DOI] [PubMed] [Google Scholar]
- 38.Dey A., Allen J., Hankey-Giblin P.A. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol. 2014;5:683. doi: 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang G., Jin S., Ling X., Li Y., Hu Y., Zhang Y. Proteomic profiling of LPS-Induced macrophage-derived exosomes indicates their involvement in acute liver injury. Proteomics. 2019;19 doi: 10.1002/pmic.201800274. [DOI] [PubMed] [Google Scholar]
- 40.Chen L., Yao X., Yao H., Ji Q., Ding G., Liu X. Exosomal miR-103-3p from LPS-activated THP-1 macrophage contributes to the activation of hepatic stellate cells. Faseb J. 2020;34:5178–5192. doi: 10.1096/fj.201902307RRR. [DOI] [PubMed] [Google Scholar]
- 41.Liu X.L., Pan Q., Cao H.X., Xin F.Z., Zhao Z.H., Yang R.X. Lipotoxic hepatocyte-derived exosomal microRNA 192-5p activates macrophages through Rictor/Akt/Forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology. 2020;72:454–469. doi: 10.1002/hep.31050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirsova P., Ibrahim S.H., Krishnan A., Verma V.K., Bronk S.F., Werneburg N.W. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 2016;150:956–967. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma V.K., Li H., Wang R., Hirsova P., Mushref M., Liu Y. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64:651–660. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huen S.C., Cantley L.G. Macrophages in renal injury and repair. Annu Rev Physiol. 2017;79:449–469. doi: 10.1146/annurev-physiol-022516-034219. [DOI] [PubMed] [Google Scholar]
- 45.Lv L.L., Feng Y., Wu M., Wang B., Li Z.L., Zhong X. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2020;27:210–226. doi: 10.1038/s41418-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lv L.L., Feng Y., Wen Y., Wu W.J., Ni H.F., Li Z.L. Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol. 2018;29:919–935. doi: 10.1681/ASN.2017050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z.L., Lv L.L., Tang T.T., Wang B., Feng Y., Zhou L.T. HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019;95:388–404. doi: 10.1016/j.kint.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Q.J., Zhu M., Xu X.X., Meng X.M., Wu Y.G. Exosomes from high glucose-treated macrophages activate glomerular mesangial cells via TGF-β1/Smad3 pathway in vivo and in vitro. Faseb J. 2019;33:9279–9290. doi: 10.1096/fj.201802427RRR. [DOI] [PubMed] [Google Scholar]
- 49.Zhu M., Sun X., Qi X., Xia L., Wu Y. Exosomes from high glucose-treated macrophages activate macrophages andinduce inflammatory responses via NF-κB signaling pathway in vitro and in vivo. Int Immunopharm. 2020;84:106551. doi: 10.1016/j.intimp.2020.106551. [DOI] [PubMed] [Google Scholar]
- 50.Lee H., Abston E., Zhang D., Rai A., Jin Y. Extracellular vesicle: an emerging mediator of intercellular crosstalk in lung inflammation and injury. Front Immunol. 2018;9:924. doi: 10.3389/fimmu.2018.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moon H.G., Cao Y., Yang J., Lee J.H., Choi H.S., Jin Y. Lung epithelial cell-derived extracellular vesicles activate macrophage-mediated inflammatory responses via ROCK1 pathway. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee H., Zhang D., Zhu Z., Dela Cruz C.S., Jin Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep. 2016;6:35250. doi: 10.1038/srep35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan Z., Bedi B., Sadikot R.T. Bronchoalveolar lavage exosomes in lipopolysaccharide-induced septic lung injury. J Vis Exp. 2018;21:57737. doi: 10.3791/57737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z.G., Scott M.J., Brzóska T., Sundd P., Li Y.H., Billiar T.R. Lung epithelial cell-derived IL-25 negatively regulates LPS-induced exosome release from macrophages. Mil Med Res. 2018;5:24. doi: 10.1186/s40779-018-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye C., Li H., Bao M., Zhuo R., Jiang G., Wang W. Alveolar macrophage - derived exosomes modulate severity and outcome of acute lung injury. Aging (Albany NY) 2020;12:6120–6128. doi: 10.18632/aging.103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soni S., Wilson M.R., O'Dea K.P., Yoshida M., Katbeh U., Woods S.J. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax. 2016;71:1020–1029. doi: 10.1136/thoraxjnl-2015-208032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang D., Lee H., Wang X., Groot M., Sharma L., Dela Cruz C.S. A potential role of microvesicle-containing miR-223/142 in lung inflammation. Thorax. 2019;74:865–874. doi: 10.1136/thoraxjnl-2018-212994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones G.R., Bain C.C., Fenton T.M., Kelly A., Brown S.L., Ivens A.C. Dynamics of colon monocyte and macrophage activation during colitis. Front Immunol. 2018;9:2764. doi: 10.3389/fimmu.2018.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lissner D., Schumann M., Batra A., Kredel L.I., Kuhl A.A., Erben U. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis. 2015;21:1297–1305. doi: 10.1097/MIB.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abron J.D., Singh N.P., Price R.L., Nagarkatti M., Nagarkatti P.S., Singh U.P. Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Che X., Park K.C., Park S.J., Kang Y.H., Jin H.A., Kim J.W. Protective effects of guggulsterone against colitis are associated with the suppression of TREM-1 and modulation of macrophages. Am J Physiol Gastrointest Liver Physiol. 2018;315:G128–g139. doi: 10.1152/ajpgi.00027.2018. [DOI] [PubMed] [Google Scholar]
- 62.Wong W.Y., Lee M.M., Chan B.D., Kam R.K., Zhang G., Lu A.P. Proteomic profiling of dextran sulfate sodium induced acute ulcerative colitis mice serum exosomes and their immunomodulatory impact on macrophages. Proteomics. 2016;16:1131–1145. doi: 10.1002/pmic.201500174. [DOI] [PubMed] [Google Scholar]
- 63.Liu R., Tang A., Wang X., Chen X., Zhao L., Xiao Z. Inhibition of incRNA NEAT1 suppresses the inflammatory response in IBD by modulating the intestinal epithelial barrier and by exosome-mediated polarization of macrophages. Int J Mol Med. 2018;42:2903–2913. doi: 10.3892/ijmm.2018.3829. [DOI] [PubMed] [Google Scholar]
- 64.Mitsuhashi S., Feldbrugge L., Csizmadia E., Mitsuhashi M., Robson S.C., Moss A.C. Luminal extracellular vesicles (EVs) in inflammatory bowel disease (IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm Bowel Dis. 2016;22:1587–1595. doi: 10.1097/MIB.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alpdundar Bulut E., Bayyurt Kocabas B., Yazar V., Aykut G., Guler U., Salih B. Human gut commensal membrane vesicles modulate inflammation by generating M2-like macrophages and myeloid-derived suppressor cells. J Immunol. 2020;205:2707–2718. doi: 10.4049/jimmunol.2000731. [DOI] [PubMed] [Google Scholar]
- 66.An J.H., Li Q., Ryu M.O., Nam A.R., Bhang D.H., Jung Y.C. TSG-6 in extracellular vesicles from canine mesenchymal stem/stromal is a major factor in relieving DSS-induced colitis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0220756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao L., Xu H., Wang G., Liu M., Tian D., Yuan Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int Immunopharm. 2019;72:264–274. doi: 10.1016/j.intimp.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 68.An J.H., Li Q., Bhang D.H., Song W.J., Youn H.Y. TNF-α and INF-γ primed canine stem cell-derived extracellular vesicles alleviate experimental murine colitis. Sci Rep. 2020;10:2115. doi: 10.1038/s41598-020-58909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang R., Liao Y., Wang L., He P., Hu Y., Yuan D. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 2019;10:2346. doi: 10.3389/fimmu.2019.02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han X., Ni J., Wu Z., Wu J., Li B., Ye X. Myeloid-specific dopamine D2 receptor signalling controls inflammation in acute pancreatitis via inhibiting M1 macrophage. Br J Pharmacol. 2020;177:2991–3008. doi: 10.1111/bph.15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Otterbein L.E., Bach F.H., Alam J., Soares M., Tao Lu H., Wysk M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 72.Taguchi K., Nagao S., Maeda H., Yanagisawa H., Sakai H., Yamasaki K. Biomimetic carbon monoxide delivery based on hemoglobin vesicles ameliorates acute pancreatitis in mice via the regulation of macrophage and neutrophil activity. Drug Deliv. 2018;25:1266–1274. doi: 10.1080/10717544.2018.1477860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu R.H., Wen Y., Sun H.Y., Liu C.Y., Zhang Y.F., Yang Y. Abdominal paracentesis drainage ameliorates severe acute pancreatitis in rats by regulating the polarization of peritoneal macrophages. World J Gastroenterol. 2018;24:5131–5143. doi: 10.3748/wjg.v24.i45.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y., Wang H., Lu M., Qiao X., Sun B., Zhang W. Pancreatic acinar cells employ miRNAs as mediators of intercellular communication to participate in the regulation of pancreatitis-associated macrophage activation. Mediat Inflamm. 2016;2016:6340457. doi: 10.1155/2016/6340457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jimenez-Alesanco A., Marcuello M., Pastor-Jimenez M., Lopez-Puerto L., Bonjoch L., Gironella M. Acute pancreatitis promotes the generation of two different exosome populations. Sci Rep. 2019;9:19887. doi: 10.1038/s41598-019-56220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X., Luo G., Zhang K., Cao J., Huang C., Jiang T. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Res. 2018;78:4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 77.Yin Z., Ma T., Huang B., Lin L., Zhou Y., Yan J. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-beta signaling pathway. J Exp Clin Cancer Res. 2019;38:310. doi: 10.1186/s13046-019-1313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gea-Sorli S., Guillamat R., Serrano-Mollar A., Closa D. Activation of lung macrophage subpopulations in experimental acute pancreatitis. J Pathol. 2011;223:417–424. doi: 10.1002/path.2814. [DOI] [PubMed] [Google Scholar]
- 79.Sun K., He S.B., Qu J.G., Dang S.C., Chen J.X., Gong A.H. IRF5 regulates lung macrophages M2 polarization during severe acute pancreatitis in vitro. World J Gastroenterol. 2016;22:9368–9377. doi: 10.3748/wjg.v22.i42.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonjoch L., Casas V., Carrascal M., Closa D. Involvement of exosomes in lung inflammation associated with experimental acute pancreatitis. J Pathol. 2016;240:235–245. doi: 10.1002/path.4771. [DOI] [PubMed] [Google Scholar]
- 81.Feng L., Song P., Zhou H., Li A., Ma Y., Zhang X. Pentamethoxyflavanone regulates macrophage polarization and ameliorates sepsis in mice. Biochem Pharmacol. 2014;89:109–118. doi: 10.1016/j.bcp.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 82.Taratummarat S., Sangphech N., Vu C.T.B., Palaga T., Ondee T., Surawut S. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiol. 2018;18:85. doi: 10.1186/s12866-018-1227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mastronardi M.L., Mostefai H.A., Meziani F., Martinez M.C., Asfar P., Andriantsitohaina R. Circulating microparticles from septic shock patients exert differential tissue expression of enzymes related to inflammation and oxidative stress. Crit Care Med. 2011;39:1739–1748. doi: 10.1097/CCM.0b013e3182190b4b. [DOI] [PubMed] [Google Scholar]
- 84.Song Y., Dou H., Li X., Zhao X., Li Y., Liu D. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1beta-primed mesenchymal stem cells against sepsis. Stem Cell. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 85.Wisler J.R., Singh K., McCarty A.R., Abouhashem A.S.E., Christman J.W., Sen C.K. Proteomic pathway analysis of monocyte-derived exosomes during surgical sepsis identifies immunoregulatory functions. Surg Infect. 2020;21:101–111. doi: 10.1089/sur.2019.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tu F., Wang X., Zhang X., Ha T., Wang Y., Fan M. Novel role of endothelial derived exosomal HSPA12B in regulating macrophage inflammatory responses in polymicrobial sepsis. Front Immunol. 2020;11:825. doi: 10.3389/fimmu.2020.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Essandoh K., Yang L., Wang X., Huang W., Qin D., Hao J. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta. 2015;1852:2362–2371. doi: 10.1016/j.bbadis.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molinaro R., Pasto A., Corbo C., Taraballi F., Giordano F., Martinez J.O. Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale. 2019;11:13576–13586. doi: 10.1039/c9nr04253a. [DOI] [PubMed] [Google Scholar]
- 89.Jiang K., Yang J., Guo S., Zhao G., Wu H., Deng G. Peripheral circulating exosome-mediated delivery of miR-155 as a novel mechanism for acute lung inflammation. Mol Ther. 2019;27:1758–1771. doi: 10.1016/j.ymthe.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X., Lu Z., Song C., Chen P., He L., Jin Q. Activation of M1 macrophages in sepsis-induced acute kidney injury in response to heparin-binding protein. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Russo L., Lumeng C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–417. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ying W., Lee Y.S., Dong Y., Seidman J.S., Yang M., Isaac R. Expansion of islet-resident macrophages leads to inflammation affecting beta cell proliferation and function in obesity. Cell Metabol. 2019;29:457–474. doi: 10.1016/j.cmet.2018.12.003. e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lauterbach M.A., Wunderlich F.T. Macrophage function in obesity-induced inflammation and insulin resistance. Pflügers Archiv. 2017;469:385–396. doi: 10.1007/s00424-017-1955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Appari M., Channon K.M., McNeill E. Metabolic regulation of adipose tissue macrophage function in obesity and diabetes. Antioxidants Redox Signal. 2018;29:297–312. doi: 10.1089/ars.2017.7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 96.Ferrante S.C., Nadler E.P., Pillai D.K., Hubal M.J., Wang Z., Wang J.M. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77:447–454. doi: 10.1038/pr.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kranendonk M.E., Visseren F.L., van Balkom B.W., Nolte-'t Hoen E.N., van Herwaarden J.A., de Jager W. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity. 2014;22:1296–1308. doi: 10.1002/oby.20679. [DOI] [PubMed] [Google Scholar]
- 98.Gao X., Salomon C., Freeman D.J. Extracellular vesicles from adipose tissue-a potential role in obesity and type 2 diabetes?. Front Endocrinol. 2017;8:202. doi: 10.3389/fendo.2017.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eguchi A., Mulya A., Lazic M., Radhakrishnan D., Berk M.P., Povero D. Microparticles release by adipocytes act as "find-me" signals to promote macrophage migration. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y., Mei H., Chang X., Chen F., Zhu Y., Han X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J Mol Cell Biol. 2016;8:505–517. doi: 10.1093/jmcb/mjw040. [DOI] [PubMed] [Google Scholar]
- 101.Pan Y., Hui X., Hoo R.L.C., Ye D., Chan C.Y.C., Feng T. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129:834–849. doi: 10.1172/JCI123069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiong X.Q., Geng Z., Zhou B., Zhang F., Han Y., Zhou Y.B. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism. 2018;83:31–41. doi: 10.1016/j.metabol.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 103.Zhuge F., Ni Y., Nagashimada M., Nagata N., Xu L., Mukaida N. DPP-4 Inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating M1/M2 macrophage polarization. Diabetes. 2016;65:2966–2979. doi: 10.2337/db16-0317. [DOI] [PubMed] [Google Scholar]
- 104.Liu T., Sun Y.C., Cheng P., Shao H.G. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem Biophys Res Commun. 2019;515:352–358. doi: 10.1016/j.bbrc.2019.05.113. [DOI] [PubMed] [Google Scholar]
- 105.Liu Z., Gan L., Zhang T., Ren Q., Sun C. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res. 2018;64 doi: 10.1111/jpi.12455. [DOI] [PubMed] [Google Scholar]
- 106.Kotas M.E., Matthay M.A. Mesenchymal stromal cells and macrophages in sepsis: new insights. Eur Respir J. 2018;51 doi: 10.1183/13993003.00510-2018. [DOI] [PubMed] [Google Scholar]
- 107.Regmi S., Pathak S., Kim J.O., Yong C.S., Jeong J.H. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol. 2019;98:151041. doi: 10.1016/j.ejcb.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 108.Shi Y., Wang Y., Li Q., Liu K., Hou J., Shao C. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 109.Zheng G., Huang R., Qiu G., Ge M., Wang J., Shu Q. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018;374:1–15. doi: 10.1007/s00441-018-2871-5. [DOI] [PubMed] [Google Scholar]
- 110.Yin S., Ji C., Wu P., Jin C., Qian H. Human umbilical cord mesenchymal stem cells and exosomes: bioactive ways of tissue injury repair. Am J Transl Res. 2019;11:1230–1240. [PMC free article] [PubMed] [Google Scholar]
- 111.Shi Y., Kang X., Wang Y., Bian X., He G., Zhou M. Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Mon Int Med J Exp Clin Res. 2020;26 doi: 10.12659/MSM.923328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harrell C.R., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8 doi: 10.3390/cells8121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harting M.T., Srivastava A.K., Zhaorigetu S., Bair H., Prabhakara K.S., Toledano Furman N.E. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cell. 2018;36:79–90. doi: 10.1002/stem.2730. [DOI] [PubMed] [Google Scholar]
- 114.Liu H., Liang Z., Wang F., Zhou C., Zheng X., Hu T. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 2019;4 doi: 10.1172/jci.insight.131273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chamberlain C.S., Clements A.E.B., Kink J.A., Choi U., Baer G.S., Halanski M.A. Extracellular vesicle-educated macrophages promote early achilles tendon healing. Stem Cell. 2019;37:652–662. doi: 10.1002/stem.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu R., Zhang F., Chai R., Zhou W., Hu M., Liu B. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J Cell Mol Med. 2019;23:7617–7631. doi: 10.1111/jcmm.14635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao J., Li X., Hu J., Chen F., Qiao S., Sun X. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205–1216. doi: 10.1093/cvr/cvz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu G.Y., Liu Y., Lu Y., Qin Y.R., Di G.H., Lei Y.H. Short-term memory of danger signals or environmental stimuli in mesenchymal stem cells: implications for therapeutic potential. Cell Mol Immunol. 2016;13:369–378. doi: 10.1038/cmi.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ti D., Hao H., Tong C., Liu J., Dong L., Zheng J. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seo Y., Shin T.H., Kim H.S. Current strategies to enhance adipose stem cell function: an update. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20153827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baer P.C., Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cell Int. 2012;2012:812693. doi: 10.1155/2012/812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Argentati C., Morena F., Bazzucchi M., Armentano I., Emiliani C., Martino S. Adipose stem cell translational applications: from bench-to-bedside. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19113475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao H., Shang Q., Pan Z., Bai Y., Li Z., Zhang H. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67:235–247. doi: 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- 124.Heo J.S., Choi Y., Kim H.O. Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cell Int. 2019;2019:7921760. doi: 10.1155/2019/7921760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cho K.S., Kang S.A., Kim S.D., Mun S.J., Yu H.S., Roh H.J. Dendritic cells and M2 macrophage play an important role in suppression of Th2-mediated inflammation by adipose stem cells-derived extracellular vesicles. Stem Cell Res. 2019;39:101500. doi: 10.1016/j.scr.2019.101500. [DOI] [PubMed] [Google Scholar]
- 126.Jiang M., Wang H., Jin M., Yang X., Ji H., Jiang Y. Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. 2018;47:864–878. doi: 10.1159/000490078. [DOI] [PubMed] [Google Scholar]
- 127.Liu Y., Lou G., Li A., Zhang T., Qi J., Ye D. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine. 2018;36:140–150. doi: 10.1016/j.ebiom.2018.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shang Y., Sun Y., Xu J., Ge X., Hu Z., Xiao J. Exosomes from mmu_circ_0001359-modified ADSCs attenuate airway remodeling by enhancing FoxO1 signaling-mediated M2-like macrophage activation. Mol Ther Nucleic Acids. 2020;19:951–960. doi: 10.1016/j.omtn.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ding D.C., Chang Y.H., Shyu W.C., Lin S.Z. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 130.Hoffmann A., Floerkemeier T., Melzer C., Hass R. Comparison of in vitro-cultivation of human mesenchymal stroma/stem cells derived from bone marrow and umbilical cord. J Tissue Eng Regen Med. 2017;11:2565–2581. doi: 10.1002/term.2153. [DOI] [PubMed] [Google Scholar]
- 131.Ding M., Shen Y., Wang P., Xie Z., Xu S., Zhu Z. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in alzheimer's disease. Neurochem Res. 2018;43:2165–2177. doi: 10.1007/s11064-018-2641-5. [DOI] [PubMed] [Google Scholar]
- 132.Mao F., Wu Y., Tang X., Kang J., Zhang B., Yan Y. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. BioMed Res Int. 2017;2017:5356760. doi: 10.1155/2017/5356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yaghoubi Y., Movassaghpour A., Zamani M., Talebi M., Mehdizadeh A., Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. doi: 10.1016/j.lfs.2019.116733. [DOI] [PubMed] [Google Scholar]
- 134.Deng M., Tan J., Hu C., Hou T., Peng W., Liu J. Modification of PLGA scaffold by MSC-derived extracellular matrix combats macrophage inflammation to initiate bone regeneration via TGF-β-induced protein. Adv Healthc Mater. 2020 doi: 10.1002/adhm.202000353. [DOI] [PubMed] [Google Scholar]
- 135.Sun G., Li G., Li D., Huang W., Zhang R., Zhang H. HUCMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204. doi: 10.1016/j.msec.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 136.Li X., Liu L., Yang J., Yu Y., Chai J., Wang L. Exosome derived from human umbilical cord mesenchymal stem cell mediates miR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016;8:72–82. doi: 10.1016/j.ebiom.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shao M., Xu Q., Wu Z., Chen Y., Shu Y., Cao X. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11:37. doi: 10.1186/s13287-020-1550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burra P., Arcidiacono D., Bizzaro D., Chioato T., Di Liddo R., Banerjee A. Systemic administration of a novel human umbilical cord mesenchymal stem cells population accelerates the resolution of acute liver injury. BMC Gastroenterol. 2012;12:88. doi: 10.1186/1471-230X-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jiang L., Zhang S., Hu H., Yang J., Wang X., Ma Y. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem Biophys Res Commun. 2019;508:735–741. doi: 10.1016/j.bbrc.2018.11.189. [DOI] [PubMed] [Google Scholar]
- 140.Zhang S., Jiang L., Hu H., Wang H., Wang X., Jiang J. Pretreatment of exosomes derived from hUCMSCs with TNF-α ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci. 2020;246:117401. doi: 10.1016/j.lfs.2020.117401. [DOI] [PubMed] [Google Scholar]
- 141.Kang H., Lee M.J., Park S.J., Lee M.S. Lipopolysaccharide-preconditioned periodontal ligament stem cells induce M1 polarization of macrophages through extracellular vesicles. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19123843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ren W., Hou J., Yang C., Wang H., Wu S., Wu Y. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J Exp Clin Canc Res. 2019;38:62. doi: 10.1186/s13046-019-1027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morrison T.J., Jackson M.V., Cunningham E.K., Kissenpfennig A., McAuley D.F., O'Kane C.M. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196:1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xin D., Li T., Chu X., Ke H., Yu Z., Cao L. Mesenchymal stromal cell-derived extracellular vesicles modulate microglia/macrophage polarization and protect the brain against hypoxia-ischemic injury in neonatal mice by targeting delivery of miR-21a-5p. Acta Biomater. 2020;113:597–613. doi: 10.1016/j.actbio.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 145.Kim M.S., Haney M.J., Zhao Y., Mahajan V., Deygen I., Klyachko N.L. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim M.S., Haney M.J., Zhao Y., Yuan D., Deygen I., Klyachko N.L. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14:195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 148.Du T., Yang C.L., Ge M.R., Liu Y., Zhang P., Li H. M1 macrophage derived exosomes aggravate experimental autoimmune neuritis via modulating Th1 response. Front Immunol. 2020;11:1603. doi: 10.3389/fimmu.2020.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ela S., Mager I., Breakefield X.O., Wood M.J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 150.Fujita Y., Kadota T., Araya J., Ochiya T., Kuwano K. Clinical application of mesenchymal stem cell-derived extracellular vesicle-based therapeutics for inflammatory lung diseases. J Clin Med. 2018;7 doi: 10.3390/jcm7100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y., Zhao M., Liu S., Guo J., Lu Y., Cheng J. Macrophage-derived extracellular vesicles: diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020;11:924. doi: 10.1038/s41419-020-03127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yuan D., Zhao Y., Banks W.A., Bullock K.M., Haney M., Batrakova E. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Go G., Lee J., Choi D.S., Kim S.S., Gho Y.S. Extracellular vesicle-mimetic ghost nanovesicles for delivering anti-inflammatory drugs to mitigate gram-negative bacterial outer membrane vesicle-induced systemic inflammatory response syndrome. Adv Healthc Mater. 2019;8 doi: 10.1002/adhm.201801082. [DOI] [PubMed] [Google Scholar]
- 154.Yang X., Shi G., Guo J., Wang C., He Y. Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus. Int J Nanomed. 2018;13:8095–8104. doi: 10.2147/IJN.S179380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang C., Han J., Wu Y., Li S., Wang Q., Lin W. Exosomal MALAT1 derived from oxidized low-density lipoprotein-treated endothelial cells promotes M2 macrophage polarization. Mol Med Rep. 2018;18:509–515. doi: 10.3892/mmr.2018.8982. [DOI] [PubMed] [Google Scholar]
- 156.Guan H., Peng R., Fang F., Mao L., Chen Z., Yang S. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. J Cell Physiol. 2020;235:9729–9742. doi: 10.1002/jcp.29784. [DOI] [PubMed] [Google Scholar]