Abstract
The present study was to evaluate the effects of dried Allium mongolicum Regel (AMR) powder and its water- and fat-soluble extracts (AWE and AFE) on the growth performance, serum metabolites, immune responses, antioxidant status, and meat quality of lambs. A total of 32 male small-tailed Han lambs (5 months old; initial body weight = 34.8 ± 0.40 kg) were used in a 60-d feeding experiment after a 15-d adaptation period. The lambs were randomly divided into 4 groups (n = 8) and fed a basal diet (control, CON group), the basal diet supplemented with dried AMR powder at 10 g/d per lamb (AMR group), the basal diet supplemented with AWE at 3.4 g/d per lamb (AWE group), or the basal diet supplemented with AFE at 2.8 g/d per lamb (AFE group). Blood samples were collected on d 0, 30, and 60 in the feeding experiment (n = 8). At the end of the experiment, the lambs were sacrificed and the longissimus dorsi muscles collected. Growth performance was not significantly affected by dietary supplementation of AMR, AWE and AFE (P > 0.05). However, significantly lower albumin (P = 0.006), total protein (P = 0.006), globin (P = 0.025), and blood urea nitrogen (P = 0.024) concentrations were observed in AFE group relative to CON and AMR groups. Similarly, a significantly lower lactate dehydrogenase activity (P = 0.018) was observed in AFE group relative to AWE group, but not in other groups (P > 0.05). In addition, significantly increasing trends in glutathione peroxidase (P = 0.06) in AMR, AWE, and AFE groups were observed relative to the control group. Furthermore, significantly lower drip loss (P = 0.011) across the treatment groups and cooking loss (P = 0.048) were observed in the AMR group relative to the control group. Taken together, these results indicate that AMR and its extracts had no significant effect on lamb growth performance, antioxidant status, and immune responses, but could significantly improve meat quality without the occurrence of pathological kidney and liver lesions.
Keywords: Allium mongolicum Regel, Growth performance, Serum metabolite, Immune response, Antioxidant status, Meat quality
1. Introduction
Previous studies have shown that naturally grazing sheep have more complex and diverse feeding behaviors than their barn-fed counterparts, and that this significantly affects the quality of animal products (Webb and Erasmus, 2013). These differences are reflected in the growth performance, serum metabolites, antioxidant status, immune responses, and meat quality of sheep (Dohme-Meier et al., 2014; Kumar et al., 2018; Watkins et al., 2014). One-third of the grasslands in China have been overgrazed since the 1970s, resulting in accelerated grassland degradation (Akiyama and Kawamura, 2007). To solve this challenge, the Chinese government established a grazing ban policy to protect grasslands in 2001. Consequently, farmers and farming firms had to change their sheep production systems from extensive grazing to intensive indoor feeding (Dong et al., 2007). However, these intensive production systems, which comprise the overuse of feed concentrate to improve production efficiency as well as a lack of physical activity, led to the production of animals with excessive fat deposition and thus decreased the immune function, resulted in poor meat quality, low feed efficiency, and reduced economic benefits (Feijó et al., 2015; Muñozosorio et al., 2016). Moreover, the early weaning, high metabolic rates, and transportation of sheep under intensive production systems can result in oxidative stress, leading to poor growth performance and increased susceptibility to diseases (Celi, 2011). Thus, numerous studies have explored the potential for supplementing ruminant diets with herbs and herbal extracts to improve growth performance, meat quality, antioxidant activity, and immunocompetence as well as to achieve a balance in metabolites in the serum (Castillo et al., 2012; Liu et al., 2018; Zhang et al., 2013).
Results from such studies have shown that natural herbal extracts can improve immune and antioxidant properties, as well as meat quality in ruminants (Shingfield et al., 2013). For example, Yang et al. (2010) found that supplementing a feedlot finishing diet with a small dose of cinnamaldehyde, a natural chemical compound found in the bark of cinnamon trees, improved average daily gain (ADG), feed efficiency, and carcass traits in 70 yearling steers. Amiri et al. (2019) found that supplementing the diet with onion (Allium cepa L.) extracts significantly improved the daily weight gain and feed intake of lambs. Moreover, a number of studies have reported that extracts from members of the genus Allium have had a protective effect on the livers of animals, by reducing the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in the serum (Javad et al., 2012; Kazemi et al., 2010; El Demerdash et al., 2005).
In the present study, we focused on Allium mongolicum Regel (AMR), a typical herb in the Liliaceae family, that grows in grassland regions in Inner Mongolia, Siberia, and northern China (Schmitt et al., 2005) that experience desertification. AMR is an excellent edible wild plant used for forage with aboveground parts used in Mongolian medicine (Obmann et al., 2010). Studies showed that dietary supplementation of AMR could significantly improve sheep meat quality and ADG (Liu et al., 2019). Its beneficial properties have been partly attributed to bioactive components in its extracts, including alkaloids, essential oils, flavones, and organic acids (Maisashvili et al., 2009). Previous studies have also shown the beneficial effects of AMR-derived bioactive components on antioxidant status, rumen fermentation, and ruminant production and performance (Bao, 2015). In addition, bioactive compounds from Allium plants have been shown to reduce parasitic infection-induced pathological tissue damage in the livers of animals (Eman et al., 2008; Javad et al., 2012). However, extracting a specific active component of AMR like flavonoids is complicated and time-consuming, which costs more than extracting water- and fat-soluble extracts (AWE and AFE). Futhermore, AWE and AFE contain many bioactive components. Therefore, dietary supplementation of AMR, AWE or AFE is feasible for wide application to the meat sheep-raising industry. To our knowledge, however, there is no literature that systematically compares the effects of AMR and its extract on growth performance, antioxidant status, immune responses, serum metabolites, and the meat quality of fattening lambs.
Based on previous studies related to AMR and other Allium plants, we hypothesized that supplementing the diet of lambs with AMR and its extracts could improve growth performance, antioxidant status, and meat quality, without predisposing the animals to liver lesions. The present study, therefore, aimed to evaluate the effects of AMR and its derivative extracts on growth performance, serum metabolites, antioxidant status, immune responses, and meat quality in lambs, and to establish a theoretical basis for the popularization and application of AMR and its extracts in the meat sheep-raising industry.
2. Materials and methods
The experimental protocol was approved by the Animal Care and Use Committee of the Inner Mongolia Agricultural University (Hohhot, China). The lamb feeding experiments were conducted at a company (Fuchuan, Bayannaoer, China), 40° 79′ N, 107° 42′ E, 1,038 m above sea level. This is a well-established company that adheres to the guidelines of the welfare standards of the Chinese Animal Care and Use Committee. Other procedures, including parameter measurements, were performed in the laboratory at the College of Animal Science of Inner Mongolia Agricultural University, China.
2.1. AMR powder and derivative extracts
2.1.1. Dried powder of AMR leaves
Dry leaf powder derived from AMR plants harvested in the middle of September was purchased from a company (Haohai Biological, Alxa League, China). Fresh AMR leaves were dried at 65 °C until a constant weight was obtained, and then ground to a powder using a grinder (Model 800C, Hongtaiyang Electromechanical Co., Ltd., Zhejiang, China) fitted with a 300-mesh (particle diameter 50 μm) sieve.
2.1.2. The water-soluble extract of dried AMR leaf powder (AWE)
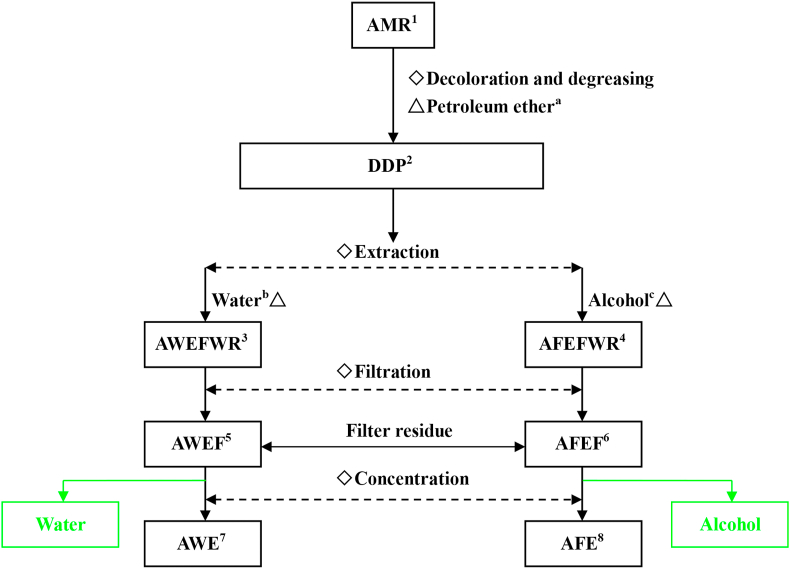
The extraction process is described as follows and is also outlined in Figure.
-
1)
Decoloration and degreasing
Figure.
Extraction processes for AWE and AFE. ◇, extraction processes; △, solvents. 1 AMR is the dried leaf powder of Allium mongolicum Regel; 2 DDP is decolored and degreased powder; 3 AWEFWR is AMR water-soluble extract fluid with residues; 4 AFEFWR is AMR fat-soluble extract fluid with residues; 5 AWEF is AWE fluid; 6 AFEF is AFE fluid; 7 AWE = AMR water-soluble extract; 8 AFE = AMR fat-soluble extract. a The ratio of AMR to petroleum ether is 1:5 (wt/vol). b The ratio of DDP to water is 1:20 (wt/vol). c Alcohol 75%; the ratio of DDP to alcohol is 1:30 (wt/vol). The green-labeled matter was thrown away.
To remove pigmented compounds, dry AMR powder was first mixed with petroleum ether (boiling point: 60 to 90 °C; Fuyu Chemical Co., Ltd., Shandong, China) at a ratio of 1:5 (wt/vol), and then the mixture as incubated for 24 h at room temperature (approximately 25 °C) on a shaker at a relative centrifugal force of 120 × g. The mixture was then stirred for 2 h, and the supernatant was collected for reuse. The precipitate, hereafter termed decolored and degreased powder, was dried for 2 h in a thermostat drier (101-3AB, Taisite Instrument Co., Ltd., Tianjin, China) at 65 °C.
-
2)
Extraction
The decolored powder was added to water at a ratio of 1:20 (wt/vol) and then was incubated for 8 h at 80 °C in a water bath kettle (HH-8, Guohua Electric Appliance Co., Ltd., Jiangsu, China).
-
3)
Filtration
The resulting fluid with residues from step 2 was subjected to vacuum filtration (SHB-III, Zhengzhou Greatwall Scientific Industrial and Trade Co., Ltd., Henan, China), which resulted a filtrate.
-
4)
Concentration
The resulting filtrate from step 3 was concentrated through a rotary evaporator (IKA HB10, IKA India Private Limited, Staufen, Germany), with centrifugation at 0.6 × g at 78 °C for 30 min (model: R1002B, Yamato Scientific Co., Ltd., Tokyo, Japan). And then, with minimal water, it was freeze-dried (CA301, Yamato Scientific) to remove any remaining water. We used ultra-performance liquid chromatography (UPLC; Shim-pack UFLC Shimadzu CBM30A)-electrospray ionization-tandem mass spectrometry (MS, Applied Biosystems 6500 Q TRAP, UPLC-ESI-MS/MS) system to identify the structures of the active ingredients of AWE, according to the method by Li et al. (2019). The results are shown in Table 1.
Table 1.
Yields and compound contents of AFE and AWE (%, DM basis).
| Item | AFE1 | AWE2 |
|---|---|---|
| Yield3 | 28.00 | 34.00 |
| Compound contents | ||
| Flavonoids | 26.43 | 21.72 |
| Organic acids and their derivatives | 18.57 | 21.77 |
| Nucleotides and their derivatives | 14.43 | 10.97 |
| Amino acids | 11.14 | 17.63 |
| Hydroxycinnamoyl derivatives | 4.43 | 6.18 |
| Amino acid derivatives | 3.14 | 3.73 |
| Phenol amine | 2.35 | 2.40 |
| Vitamins | 1.55 | 1.22 |
| Choline | 1.00 | 1.19 |
| Lipids | 7.22 | |
| Quinic acids and their derivatives | 1.09 | |
| Others | 9.74 | 12.10 |
AFE, the fat-soluble extract of Allium mongolicum Regel (AMR).
AWE, the water-soluble extract of AMR.
The yield was obtained from the dried powder of AMR.
2.1.3. The fat-soluble extract of dried AMR leaf powder (AFE)
The extraction process is described as follows and is also outlined in Figure.
-
1)
Decoloration and degreasing procedures were similar to those used for AWE.
-
2)
Extraction
A. mongolicum Regel decolored powder was mixed with 75% ethyl alcohol (80176961, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) at a ratio of 5:1 (vol/wt), and then the mixture was shaken with an ultrasonic cleaner (KQ-300DE, Kunshan Ultrasonic Instruments Co., Ltd., Jiangsu, China; 75 W, 15 min) and a fluid with residues was obtained.
-
3)
Filtration
The fluid from the mixture was retained, and the residue was extracted again as described in step 2. The resulting fluid from the first and second extractions was fat-soluble extract fluid (AFEF).
-
4)
Concentration
Ethyl alcohol in the AFEF was evaporated using a rotary evaporator (IKA HB10, IKA India Private Limited; 78 °C, 60 rpm) and the AFEF was freeze-dried (CA301, Yamato Scientific) to obtain AFE. The same UPLC-ESI-MS/MS system used for AWE was adopted to identify the structures in the active ingredients of AFE according to Li et al. (2019). The results are shown in Table 1.
2.2. Experimental animals and rearing
A total of 32 male small-tailed Han lambs (5 months of age; initial body weight = 34.8 ± 0.40 kg) were randomly divided into 4 equal groups to undergo a 75-d feeding period (comprising a 15-d adaptation period and a 60-d experimental period). Animals in the control group received a basal diet with no additives. Each animal in the AMR group received a basal diet supplemented with dried powder of AMR at 10 g/d. Each animal in the AWE group received the basal diet supplemented with AWE at 3.4 g/d. Each animal in the AFE group received the basal diet supplemented with AFE at 2.8 g/d. The 10 g of AMR, 3.4 g AWE, and 2.8 g AFE were each mixed with 100 g of basal diet concentrate. The mixtures were divided into 2 equal amounts, and administered to individual lambs twice a day to ensure that the supplements were completely consumed by each lamb (Du et al., 2019). The dosage of AMR dried powder added into the lamb diet was based on our previous studies (Liu et al., 2019; Du et al., 2019). The AWE and AFE doses added in the concentrate were calculated according to their rates of extraction from AMR, with 10 g of AMR containing 2.8 and 3.4 g of AFE and AWE, respectively. The total mixed ration (TMR) was supplied twice per day, at 06:00 and 18:00, in amounts that allowed 5% refusal (on a fresh basis). In addition, lambs were given free access to drinking water. Before the experiment, all animals were dewormed with albendazole and ivermectin tablets (Harbin Motian Veterinary Drug Co., Ltd., China) at a dose of 10.8 mg/kg of their living body weight. Each tablet contained 350 mg of albendazole and 10 mg of ivermectin. The animals were also vaccinated against foot-and-mouth disease, sheep pox, and peste des petits ruminants using the Foot and Mouth Disease Bivalent Vaccine (Inactivated, Type O, Strain OHM/02 + Type A, Strain AKT-Ⅲ) and Peste Des Petits Ruminants and Sheep Pox Vaccine (Strain Clone 9 + AV 41). These were provided by the Tecon Bio Co., Ltd. (Beijing, China) and Huapai Bio Co., Ltd. (Sichuan, China), respectively, with injections and operations strictly conducted in accordance with the instructions of the vaccines.
2.3. Determination of growth performance
We analyzed growth performance for a period of 60 d, following a 15-d adaptation period. Feed was provided to the lambs and refusals were collected and weighed daily during the whole of the growth experiment. The dry matter intake (DMI) of a subsample of each was measured by method 934.01 (AOAC International, 1995), followed by determination of nutrient intake (crude protein, neutral detergent fiber, and acid detergent fiber), by calculating DMI as a percentage of a specific nutrient contained in diets. The body weight of each lamb was also recorded before morning feeding at 15-d intervals throughout the experimental period. Finally, ADG and feed conversion ratio (FCR) for each lamb were calculated.
2.4. Blood sampling and analysis
Blood was sampled from all lambs in the morning before feeding on d 0, 30, and 60 in the experimental period. Briefly, a 5-mL blood sample was collected from the jugular vein of each lamb using sterile vacuum glass test tubes (5 mL, Huabo Medical Instruments Co., Ltd., Nanjing, China) and a blood lancet (0.7 mm, Jiangxi Hongda Medical Equipment Co., Ltd., Jiangxi, China). To separate the serum, blood was centrifuged at 4,000 × g for 10 min then stored at −20 °C for later analysis. Serum glucose concentration, total protein, albumin, globin, and blood urea nitrogen (BUN) and enzyme activities, including ALT, AST, alkaline phosphatase (ALP), and lactate dehydrogenase, were analyzed using a Mindray fully automatic biochemical analyzer (BS-480, Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) and its corresponding detection kits, according to the manufacturer's instructions. Commercial diagnostic kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were also used to determine serum redox balance parameters, measured as concentrations, and to assess total superoxide dismutase (T-SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and malonaldehyde (MDA). Serum for the analysis of antioxidant activities was aliquoted into 4 sterile tubes (0.2 mL, Huabo Medical Instruments Co., Ltd., Nanjing, China) to avoid constant freeze–thawing. Immunoglobulins were measured through IgG/M/A ELISA kits (Beijing HuaYing Bioengineering Institute, Beijing, China) according to the manufacturer's instructions.
2.5. Determination of meat quality
At the end of the feeding experiment, 8 lambs from each group were sacrificed, following an 18-h fasting period by standard Halal procedures according to Santos et al. (2017). Their carcasses were stored at 4 ± 1 °C, and then specimens of the left lateral muscle longissimus dorsi, between the 12th and 13th ribs, were collected for evaluation.
2.5.1. pH determination
An analysis of pH was performed after 24 h of cooling as described previously (Mcgeehin et al., 2001). A pH-Star machine (PH-STAR, Matthaus, Pöttmes, Germany) was used to obtain the pH at 3 randomly selected positions in the M. longissimus dorsi, with the mean of pH values calculated to represent pH24 h.
2.5.2. Determination of drip loss
To determine drip loss, 1.5 cm of the longissimus dorsi muscle was weighed, placed in netting, and suspended in an inflated plastic bag. After a 24-h incubation at 4 °C, the samples were reweighed, and the drip loss was calculated as the weight loss as a percentage of the original weight of the sample (Honikel, 1998).
2.5.3. Determination of cooking loss
To determine cooking loss, a separate 1.5-cm section of the M. longissimus dorsi, from each carcass, was weighed, placed in a thin-walled plastic bag, and incubated for 1 h in a water bath maintained at 80 °C. The samples were removed from the water bath, cooled in cold water, blotted dry, and weighed. Cooking loss was calculated as the percentage difference in weight between the original and dried samples (Honikel, 1998).
2.5.4. Shear force
The shear force of M. longissimus dorsi (diameter: 1 cm; thickness: 1 cm) was measured using a tenderometer (C-LM3, Northeastern University, Shengyang, China). Briefly, 3 positions in the cooked meat were randomly selected, the shear force measured, and the mean of the 3 values calculated (Honikel, 1998).
2.5.5. Color
Three positions, across each sample, were randomly chosen and the lightness (L∗), yellowness (b∗), and redness (a∗) measured using a colorimeter (SMY-2000SF, SMY Science & Technology Co., Ltd., Beijing, China) and their averages calculated.
2.6. Chemical analysis of the total mixed ration
We determined the TMR for nitrogen (Method 990.03; AOAC International, 1995), ash (Method 942.05; AOAC International, 1995), and ether extract (Method 920.39; AOAC International, 1995). The TMR of crude protein was calculated as 6.25 × nitrogen content. Analysis of individual minerals (Ca and P) in the samples was performed according to methods described by Talapatra et al. (1940). In addition, neutral detergent fiber and acid detergent fiber were measured as described by Van Soest et al., (1991), using an ANKOM 200 Fiber Analyzer (A200, ANKOM Technology Corporation, New York, USA) and fiber filter bags (F57, ANKOM Technology Corporation, New York, USA). Results from these analyses are outlined in Table 2.
Table 2.
Ingredients and composition of the total mixed ration diets of experimental groups (DM basis).
| Item | Groups |
|||
|---|---|---|---|---|
| CON1 | AMR2 | AWE3 | AFE4 | |
| Ingredients, % | ||||
| Chinese wildrye grass hay | 32.00 | 32.00 | 32.00 | 32.00 |
| Alfalfa | 17.80 | 17.80 | 17.80 | 17.80 |
| Corn | 23.00 | 23.00 | 23.00 | 23.00 |
| Wheat bran | 2.87 | 2.87 | 2.87 | 2.87 |
| Sunflower meal cake | 16.90 | 16.09 | 16.90 | 16.90 |
| Pea straw | 2.45 | 2.45 | 2.45 | 2.45 |
| Pomace | 2.45 | 2.45 | 2.45 | 2.45 |
| Dicalcium phosphate | 0.73 | 0.73 | 0.73 | 0.73 |
| NaCl | 0.80 | 0.80 | 0.80 | 0.80 |
| Premix5 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient level6, % | ||||
| Metabolic energy, MJ/kg | 13.86 | |||
| Crude protein | 17.00 | |||
| Crude fat | 6.30 | |||
| Neutral detergent fiber | 37.90 | |||
| Acid detergent fiber | 29.60 | |||
| Calcium | 1.39 | |||
| Phosphorus | 0.51 | |||
CON = control; AMR = Allium mongolicum Regel; AWE = the water-soluble extract of AMR; AFE = the fat-soluble extract of AMR.
CON group was fed the basal diet.
AMR group was fed the basal diet supplemented with AMR at 10 g/d per lamb.
AWE group was fed the basal diet supplemented with AWE at 3.4 g/d per lamb.
AFE group was fed the basal diet supplemented with AFE at 2.8 g/d per lamb.
Provided the following per kilogram of diet: 25 mg of Fe (as ferrous sulfate), 29 mg of Zn (as zinc sulfate), 8 mg of Cu (as copper sulfate), 30 mg of Mn (as manganese), 0.04 mg of I (as potassium iodide), 0.1 mg of Co (as cobalt sulfate), 3,200 IU of vitamin A, 1,200 IU of vitamin D3, and 20 IU of vitamin E.
The nutrient level was the same in all groups. The digestible energy level was calculated (Liu et al., 2012), and others were measured.
2.7. Statistical analyses
Data on growth performance (ADG, FCR, and nutrient intake parameters), blood metabolites, immune responses, and antioxidant status were analyzed using linear mixed-effects models. Briefly, these models comprised treatment, period, and treatment × period as fixed factors, and lambs as a random factor to account for repeated measures over time on the same lamb (Littell et al., 1998). Means were estimated using least-square means, with statistical significance between groups determined using Tukey's test. Data on initial and final body weights, as well as meat quality, were analyzed using the general linear model, followed by Duncan's test. P < 0.05 was considered statistically significant, and P < 0.1 was used to indicate a trend.
3. Results
3.1. Growth performance
Initial body weight was not significantly different among the groups (P > 0.05; Table 3), whereas treatment and treatment × Period also had no effect (P > 0.05) on all parameters related to growth performance and feed nutrient intake. All parameters, except initial and final body weights, were significantly different during the experimental period (P < 0.05). In particular, we found significantly higher (P = 0.001) ADG on d 45, relative to those measured at other times. In addition, FCR were significantly different between d 45 and 30 (P = 0.045), and all parameters related to feed nutrient intake were significantly higher on d 45 relative to d 15 and 30 (P < 0.001).
Table 3.
Effects of dried leaf powder of AMR and its extracts on the growth performance and nutrient intake of lambs (n = 8).
| Item | Groups |
SEM | Period |
SEM |
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON1 | AMR2 | AWE3 | AFE4 | 15 d | 30 d | 45 d | 60 d | Treatment | Period | Treatment × Period | |||
| Initial body weight5, kg | 33.9 | 35.1 | 35.1 | 34.9 | 0.83 | – | – | – | – | – | 0.704 | – | – |
| Final body weight, kg | 43.2 | 44.4 | 45.2 | 45.0 | 1.21 | – | – | – | – | – | 0.646 | – | – |
| Average daily gain, g | 181 | 180 | 190 | 180 | 22.9 | 161b | 149b | 257a | 163b | 19.4 | 0.985 | 0.001 | 0.137 |
| Feed conversion ratio6 | 6.46 | 6.23 | 5.73 | 6.04 | 0.252 | 6.07ab | 6.68a | 5.37b | 6.33ab | 0.303 | 0.205 | 0.045 | 0.669 |
| Intake, g/d | |||||||||||||
| Dry matter | 1,110 | 1,133 | 1,116 | 1,134 | 24.9 | 1,083bc | 1,031c | 1,234a | 1,145ab | 28.6 | 0.871 | <0.001 | 0.227 |
| Crude protein | 170 | 175 | 172 | 175 | 3.9 | 166bc | 160c | 190a | 177ab | 4.5 | 0.792 | <0.001 | 0.175 |
| Neutral detergent fiber | 483 | 490 | 497 | 492 | 10.7 | 467bc | 453c | 542a | 503ab | 12.6 | 0.819 | <0.001 | 0.157 |
| Acid detergent fiber | 311 | 319 | 315 | 322 | 7.0 | 303bc | 293c | 348a | 323ab | 8.2 | 0.680 | <0.001 | 0.226 |
CON = control; AMR = Allium mongolicum Regel; AWE = the water-soluble extract of AMR; AFE = the fat-soluble extract of AMR.
a–c Within a row, means with different superscripts differ significantly (P < 0.05) as determined by Tukey's test.
CON group was fed the basal diet.
AMR group was fed the basal diet supplemented with AMR at 10 g/d per lamb.
AWE group was fed the basal diet supplemented with AWE at 3.4 g/d per lamb.
AFE group was fed the basal diet supplemented with AFE at 2.8 g/d per lamb.
Initial body weight is the body weight at the end of the15-day adaptation period.
Feed conversion ratio = Dry matter intake (g)/Average daily gain (g).
3.2. Concentrations of serum metabolites
The values of serum glucose, BUN, total protein, albumin, and globin ranged from 2.36 to 3.48 mmol/L, 4.90 to 6.12 mmol/L, 32.0 to 44.0 g/L, 16.0 to 21.3 g/L and 16.2 to 24.4 g/L, respectively. The treatment × period significantly affected total protein (P = 0.027) and globin (P = 0.021), but not glucose, albumin, and BUN (P > 0.05) levels. On the other hand, animals in the AFE group exhibited significantly lower albumin (P = 0.006), total protein (P = 0.006), globin (P = 0.025), and BUN (P = 0.024) concentrations relative to those in the control and AMR groups. However, the experimental period treatment did not affect (P > 0.05) the concentration of all the serum metabolites (Table 4). Additionally, the change in the ranges of ALT, AST, ALP, and lactate dehydrogenase (LDH) were 6.80 to 10.12, 60.5 to 74.2, 140 to 195, and 260 to 359 (U/L), respectively. The treatment × period had a significant effect on LDH (P = 0.025), but not ALT, AST, and ALP (P > 0.05). With regards to treatment, animals in the AFE group exhibited significantly lower LDH (P = 0.018) concentrations than those in the AWE group. Moreover, the period significantly affected ALT (P = 0.021), but not LDH, AST, and ALP (P > 0.05).
Table 4.
Effects of dried leaf powder of AMR and its extracts on serum metabolites and enzymes (n = 8).
| Item | Group |
SEM | Period |
SEM |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON1 | AMR2 | AWE3 | AFE4 | 0 d | 30 d | 60 d | Treatment | Period | Treatment × Period | |||
| Serum metabolites | ||||||||||||
| Glucose, mmol/L | 3.03 | 2.66 | 2.46 | 2.82 | 0.151 | 2.66 | 2.71 | 2.85 | 0.128 | 0.058 | 0.634 | 0.322 |
| Total protein, g/L | 41.6a | 41.0a | 40.1a | 33.9b | 1.63 | 40.0 | 40.1 | 37.4 | 1.31 | 0.006 | 0.362 | 0.027 |
| Albumin, g/L | 19.1a | 18.8a | 17.8ab | 16.6b | 0.54 | 18.8 | 18.0 | 17.5 | 0.55 | 0.006 | 0.253 | 0.169 |
| Globin, g/L | 22.5a | 22.2a | 22.3a | 17.3b | 1.37 | 21.3 | 22.1 | 19.8 | 0.98 | 0.025 | 0.214 | 0.021 |
| BUN, mmol/L | 5.87a | 5.97a | 5.62ab | 5.15b | 0.196 | 5.48 | 5.79 | 5.69 | 0.144 | 0.024 | 0.272 | 0.076 |
| Serum enzymes | ||||||||||||
| ALT, U/L | 8.43 | 7.16 | 7.87 | 7.95 | 0.707 | 7.73ab | 7.04b | 8.80a | 0.523 | 0.666 | 0.021 | 0.390 |
| AST, U/L | 67.5 | 64.1 | 64.7 | 55.8 | 4.19 | 60.3 | 59.9 | 68.9 | 3.15 | 0.235 | 0.205 | 0.102 |
| ALP, U/L | 190 | 171 | 167 | 143 | 14.8 | 153 | 170 | 181 | 11.0 | 0.173 | 0.235 | 0.076 |
| LDH, U/L | 347ab | 286ab | 356a | 272b | 22.3 | 304 | 309 | 334 | 18.5 | 0.018 | 0.577 | 0.025 |
CON = control; AMR = Allium mongolicum Regel; AWE = the water-soluble extract of AMR; AFE = the fat-soluble extract of AMR; BUN = blood urea nitrogen; ALT = alanine aminotransferase; AST = aspartate aminotransferase; ALP = alkaline phosphatase; LDH = lactate dehydrogenase.
a, b Within a row, means with different superscripts differ significantly (P < 0.05) as determined by Tukey's test.
CON group was fed the basal diet.
AMR group was fed the basal diet supplemented with AMR at 10 g/d per lamb.
AWE group was fed the basal diet supplemented with AWE at 3.4 g/d per lamb.
AFE group was fed the basal diet supplemented with AFE at 2.8 g/d per lamb.
3.3. Blood antioxidant levels
The treatment × period showed significant differences with regards to T-SOD, MDA, and GSH-Px among the various time points (P < 0.001). With regards to treatment, T-SOD, MDA, and CAT showed no significant differences (P > 0.05) across AMR, AWE, and AFE groups, although we observed an increasing trend in GSH-Px across each of the groups relative to the control, albeit with no statistical significance (P = 0.06, Table 5).
Table 5.
Effects of dried leaf powder of AMR and its extracts on serum antioxidant activities (n = 8).
| Item | Group |
SEM | Period |
SEM |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON1 | AMR2 | AWE3 | AFE4 | 0 d | 30 d | 60 d | Treatment | Period | Treatment × Period | |||
| T-SOD, U/mL | 174 | 165 | 174 | 173 | 4.0 | 188a | 176b | 150c | 3.4 | 0.288 | <0.001 | 0.323 |
| MDA, nmol/mL | 1.29 | 1.29 | 1.74 | 1.27 | 0.159 | 1.49b | 0.48c | 2.23a | 0.064 | 0.111 | <0.001 | 0.431 |
| CAT, U/mL | 2.71 | 2.31 | 2.00 | 2.26 | 0.374 | 2.25 | 2.04 | 2.67 | 0.265 | 0.537 | 0.334 | 0.673 |
| GSH-Px, U/mL | 128 | 178 | 158 | 193 | 21.4 | 116c | 158b | 220a | 11.9 | 0.060 | <0.001 | 0.246 |
CON = control; AMR = Allium mongolicum Regel; AWE = the water-soluble extract of AMR; AFE = the fat-soluble extract of AMR; T-SOD = total superoxide dismutase; MDA = malonaldehyde; CAT = catalase; GSH-Px = glutathione peroxidase.
a, b, c Within a row, means with different superscripts differ significantly (P < 0.05) as determined by Tukey's test.
CON group was fed the basal diet.
AMR group was fed the basal diet supplemented with AMR at 10 g/d per lamb.
AWE group was fed the basal diet supplemented with AWE at 3.4 g/d per lamb.
AFE group was fed the basal diet supplemented with AFE at 2.8 g/d per lamb.
3.4. Immune responses
Treatment and treatment × period had no significant effects on IgA, IgG, and IgM (P > 0.05; Table 6). However, the levels of all measured immunoglobulins significantly differed among days, with significantly higher IgA (P = 0.006), IgG (P = 0.010), and IgM (P = 0.017) recorded on d 60 relative to d 0. However, these levels were not significantly different relative to those observed on d 30.
Table 6.
Effects of dried leaf powder of AMR and its extracts on serum immune parameter activities (n = 8).
| Item | Group |
SEM | Period |
SEM |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON1 | AMR2 | AWE3 | AFE4 | 0 d | 30 d | 60 d | Treatment | Period | Treatment × Period | |||
| IgA, g/L | 0.65 | 0.64 | 0.62 | 0.62 | 0.017 | 0.60b | 0.63ab | 0.65a | 0.014 | 0.518 | 0.006 | 0.560 |
| IgG, g/L | 16.94 | 16.76 | 15.84 | 15.67 | 0.594 | 15.37b | 16.61ab | 16.92a | 0.460 | 0.339 | 0.010 | 0.916 |
| IgM, g/L | 1.04 | 1.02 | 0.95 | 0.93 | 0.050 | 0.92b | 0.99ab | 1.04a | 0.039 | 0.391 | 0.017 | 0.535 |
CON = control; AMR = Allium mongolicum Regel; AWE = the water-soluble extract of AMR; AFE = the fat-soluble extract of AMR.
a, b Within a row, means with different superscripts differ significantly (P < 0.05) as determined by Tukey's test.
CON group was fed the basal diet.
AMR group was fed the basal diet supplemented with AMR at 10 g/d per lamb.
AWE group was fed the basal diet supplemented with AWE at 3.4 g/d per lamb.
AFE group was fed the basal diet supplemented with AFE at 2.8 g/d per lamb.
3.5. Meat quality
We found a decreasing trend in shear force in the AMR and AWE groups, relative to those in the other groups, although this difference was not significant (P = 0.096). Similarly, a significantly lower drip loss (P = 0.011) was recorded in AMR, AWE, and AFE groups relative to the control (Table 7). We also observed significantly lower cooking loss (P = 0.048) in the AMR, relative to the control group, whereas the other meat quality parameters resulted in no significant difference among experimental groups (P > 0.05; Table 7).
Table 7.
Effects of dried leaf powder of AMR and its extracts on meat quality (n = 8).
| Item | Group |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON1 | AMR2 | AWE3 | AFE4 | |||
| Shear force, kg | 3.72 | 3.10 | 3.09 | 3.88 | 0.151 | 0.096 |
| Drip loss, % | 7.21a | 4.17b | 4.31b | 5.05b | 0.473 | 0.011 |
| Cooking loss, % | 54.2a | 39.9b | 45.7ab | 45.0ab | 1.14 | 0.048 |
| pH 24 h | 5.49 | 5.50 | 5.45 | 5.55 | 0.022 | 0.570 |
| L∗24 h | 34.9 | 35.5 | 36.1 | 35.4 | 0.57 | 0.927 |
| a∗24 h | 28.0 | 26.9 | 26.4 | 28.5 | 0.38 | 0.189 |
| b∗24 h | 7.64 | 8.42 | 7.94 | 8.91 | 0.401 | 0.713 |
CON = control; AMR = Allium mongolicum Regel; AWE = the water-soluble extract of AMR; AFE = the fat-soluble extract of AMR; L∗ = lightness; a∗ = redness; b∗ = yellowness.
a, b Within a row, means with different superscripts differ significantly (P < 0.05) as determined by Tukey's test.
CON group was fed the basal diet.
AMR group was fed the basal diet supplemented with AMR at 10 g/d per lamb.
AWE group was fed the basal diet supplemented with AWE at 3.4 g/d per lamb.
AFE group was fed the basal diet supplemented with AFE at 2.8 g/d per lamb.
4. Discussion
Previous studies have reported the presence of various active components, including flavonoids and polysaccharides, in AMR leaves (Du et al., 2019; Liu et al., 2019). In the present study, different solvents allowed the extraction of 34% and 28% solvent extracts of AWE and AFE, respectively, from AMR as DM. The identification of active ingredients by structure analysis for AWE and AFE indicated the presence of flavonoids, organic acids, nucleotides, amino acids and their derivatives, and hydroxycinnamoyl derivatives, which are the dominant substances (Table 2). Furthermore, results from previous studies have indicated that the largest difference in the bioactive components between AWE and AFE is that AWE is enriched with polysaccharides, whereas AFE is rich in flavonoids (Liu et al., 2019; Hu et al., 2011).
4.1. Growth performance
To date, only a handful of studies have described the effects of AMR and its extracts on growth performance in ruminants. Conversely, the effects of extracts from other Allium plants, such as garlic and onion, on ruminant and non-ruminant growth performance have been extensively studied. For example, Elkatcha et al. (2016) reported that the addition of garlic extracts to lamb diets did not significantly improve feed efficiency, which is consistent with our results. This may be attributed to the fact that garlic and AMR, both members of Allium, contain similar compositions and amounts of bioactive components. However, the findings of the present study contrasted with the results of a previous study at our laboratory (Du et al., 2019), in which adding AMR to TMR significantly improved the ADG of lambs. The differences may be attributed to differences in the ages of the experimental animals. The lambs were 6 months old in the former study, but only 5 months old in this study. In the present study, we found a significantly higher ADG and FCR on d 45 relative to the other time points, indicating a superior physiological status on d 45. Similar findings have been reported. Salem et al. (2011) reported that nutrient intakes, including DM, organic matter, crude protein, and acid detergent fiber as well as ADG were highest on d 43 in a 77-d experimental period. Their study also indicated a general rise, then a decrease in the aforementioned parameters, which was comparable to our findings.
4.2. Serum metabolite concentrations
To our knowledge, this is the first study evaluating the effects of AMR and its extracts on serum metabolites in lambs. Here, all serum metabolites were within reference ranges (Radostits et al., 2000; Harith and Badawi, 2015), suggesting that the selected lambs were in good health. Especially, albumin and globulin are considered the 2 major protein components of serum, owing to their diagnostic significance, with liver-derived albumin primarily responsible for the oncotic pressure in plasma. In the present study, we found significantly lower concentrations of albumin, globulin, and total proteins in animals from the AFE group, relative to those in the control group, whereas those in the AMR and AWE groups were not significantly different to the control. Braun et al. (2010) demonstrated that concentrations of total protein higher than 65 g/L were highly indicative of chronic inflammation, and albumin would be lowered to more than 15 g/L in case of infection by blood-spoiling parasites. In the current study, the recorded values of these parameters were within the recommended healthy ranges, further confirming that the lambs were healthy during the experimental period with diets containing AMR and its extracts. Blood urea nitrogen levels are an indicator of kidney function. In the present study, the BUN values of the groups were within the normal range, which indicated that kidney function was not significantly impaired by dietary AMR and its extracts. Furthermore, because BUN is the metabolic outcome of protein and amino acids, it is always considered as an indicator of protein utilization (Fang et al., 2019). A study demonstrated that a lower concentration of BUN enhanced feed conversion and protein synthesis in vivo (Pereira et al., 2017). In the present study, we recorded lower BUN concentrations in the AWE and AFE groups, relative to the control, with significant differences between the AFE and control groups. These results suggested that bioactive components (mainly flavonoids) from AFE may improve the efficiency of protein utilization.
Our results further revealed that serum enzymes AST, ALT, ALP, and LDH were within normal physiological ranges, indicating normal serum activity and function. Therefore, no issues or pathological organ lesions occurred (Van Putten et al., 2013; Mahmood et al., 2013). AST and ALT activities are considered important indicators of hepatocellular necrosis. In particular, severe viral hepatitis, drugs, and toxins generate chronic liver disease and increase the activities of serum AST and ALT (McLaughlin et al., 1993). In the present study, we found no significant differences in activities of AST and ALT across the AMR, AWE, AFE, and control groups, indicating no damage to liver tissue or function (Thapa and Walia, 2007). A previous study also found neither liver lesions nor health issues when Allium plants (garlic) were used as additives in lamb feedlot diets (Elkatcha et al., 2016). High levels of ALP have been reported in cholestatic disorders. In the present study, we found no significant differences between experimental groups and the control with regards to ALP activity, indicating that the lambs were unlikely to have cholestatic disorders (Thapa and Walia, 2007). In addition, previous studies have shown that hemolysis, as well as muscle and hepatocellular damage, can result in increased LDH activity (Latimer et al., 2003), with an increase in this activity considered an indicator of the abovementioned conditions. In the present study, we recorded a significantly lower LDH activity in the AFE group relative to the AWE group, although this was not significantly lower than in the other groups. However, the concentrations across all groups remained within normal clinical ranges. Overall, these results indicated that AMR and its extracts do not contribute to pathological kidney and liver lesions.
4.3. Serum antioxidant levels
Endogenous antioxidant enzymes, including T-SOD and GSH-Px, are considered to be the main contributors to intracellular defense against oxidative stress. In fact, serum GSH-Px activity not only contributes to oxidative defense in animal tissues but also serves as an indicator of oxidative stress (Celi, 2011; Miller et al., 1993; Tüzün et al., 2002). Our results revealed an increasing trend of GSH-Px activity in the supplementation groups, although this was not significantly different from that in the control group. Polysaccharides and flavonoids comprise the main bioactive components in the AMR. Polysaccharides are the main bioactive component in AWE, and flavonoids are the main bioactive component in AFE. These bioactive components may increase GSH-Px activity (Liu et al., 2019). It was evident that dietary supplementation of AMR did not significantly affect MDA, SOD, or CAT in the serum of meat sheep, which is consistent with reports related to the effects of AMR on antioxidant activities by Ding et al. (2018). However, other plants rich in flavonoids and polysaccharides, and their water- and fat-soluble extracts, have been shown to have contrasting effects on serum SOD, CAT, and MDA activities in ruminants relative to this study (Amiri et al., 2019). This discrepancy may be attributed to a number of reasons, key among them being the structures and compositions of flavonoids and polysaccharides in AMR and other plants (Bors et al., 1990; Fraga et al., 2010).
4.4. Immune responses
Immunoglobulins IgA, IgG, and IgM provide defenses for all the tissues reached by blood and help protect against blood-borne infections, septicemia, and the spread of microorganisms by neutralizing them when they enter the circulation system (Roomruangwong et al., 2017). In the present study, our results indicated that AMR and its extracts had no significant effect on IgG, IgA, and IgM. These results were in contrast to the findings by Muqier (2016), and the reasons for this need further investigation. Nevertheless, the 2 experiments indicate that the above immune parameter values are likely to increase with an increase in the age of lambs.
4.5. Meat quality
Our results showed that meat color (L∗, a∗, and b∗) did not significantly differ across groups. However, results on meat color from studies on the effects of dietary supplementation of plants belonging to the Allium genus have been inconsistent. For example, Amiri et al. (2020) reported that the dietary supplementation of onion (A. cepa) could significantly increase the lightness (L∗) of lamb meat, which is different from our results. A possible reason for the different results could be the different structures and contents of active components contained in AMR and onion.
Moreover, we found significantly lower drip loss in the AMR, AWE, and AFE groups relative to the control group, indicating that bioactive components of AMR, AWE, and AFE have the potential to improve meat quality by decreasing drip loss. The AMR group showed significantly lower cooking loss than that of the control group, which was in contrast with the findings of Ding et al. (2018), who found that dietary AMR (10 g/d per sheep) did not have a significant effect on the drip and cooking loss of Duhan crossed lambs. The difference between these results may be attributed to the different lamb breeds studied, although this remains to be confirmed experimentally. Strangely, dietary AWE and AFE did not significantly decrease the cooking loss like AMR. The reasons may be that AMR contains both AWE and AFE, and the active substances may have a combination, synergistic or dependent effect on meat quality. In addition, shear force in the AMR and AWE groups revealed a decreasing trend relative to that in the control and AFE groups. The results may indicate that the polysaccharides in AWE exhibit a major influence on the shear force relative to flavonoids in AFE. In conclusion, the use of AMR and its extracts as dietary additives can improve the meat quality of small-tailed Han lambs.
5. Conclusions
In summary, our results indicated that supplementing lamb diets with AMR and its extracts does not significantly affect growth performance and immune responses. Specifically, AMR and its extracts did not disturb liver functions, as evidenced by the values of serum enzymes measured. By comparison, AFE had a tendency to improve serum GSH-Px activity and could significantly decrease the concentration of BUN. However, all of AMR and its extracts can improve meat quality in lambs by reducing drip loss. Taken together, these findings indicate that AMR and its extracts are green dietary additives with a potential to improve meat quality in intensive sheep production systems.
Author contributions
He Ding and Wangjing Liu participated in the experimental design, analyzed the data and wrote the manuscript. He Ding, Wangjing Liu and Hongxi Du carried out the animal feeding experiment and were responsible for recording the original data. Khas-Erdene provided advice on the appropriate data analysis methods. Changjin Ao conceived the study. All authors have read and approved the manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by the National Natural Science Regional Fund Project of China (30360075) and the Inner Mongolia Agricultural University “Double First Class” talent cultivation program (NDSC2018-03). The authors thank Fuchuan Inner Mongolia Farming Polytron Technologies, Inc. for providing access to the experimental site.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Akiyama T., Kawamura K. Grassland degradation in China: methods of monitoring, management and restoration. Grassl Sci. 2007;53:1–17. [Google Scholar]
- Amiri M., Jelodar G.A., Erjaee H., Nazifi S. The effects of different doses of onion (Allium cepa. L) extract on leptin, ghrelin, total antioxidant capacity, and performance of suckling lambs. Comp Clin Pathol. 2019;28:391–396. [Google Scholar]
- Amiri M., Jelodar G.A., Saeed Nazifi, Omidi A. Onion (Allium cepa. L) extract supplementation improved meat quality, oxidative stability and some hematological parameters in sucking lambs. Agric J. 2020;15:91–96. [Google Scholar]
- AOAC International . AOAC International; Rockville, Maryland: 1995. Official methods of analysis of AOAC international. [Google Scholar]
- Bao L.L. Inner Mongolia Agricultural University; 2015. The influence of allium mongilicum regel flavonoids on the rumen microbial fermentation, rumen environment parameters and rumen cellulose degradation in sheep. [Master's Dissertation] [Google Scholar]
- Bors W., Heller W., Michel C., Saran M. Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol. 1990;186:343–355. doi: 10.1016/0076-6879(90)86128-i. [DOI] [PubMed] [Google Scholar]
- Braun J.P., Trumel C., Bézille P. Clinical biochemistry in sheep: a selected review. Small Rumin Res. 2010;92:10–18. [Google Scholar]
- Castillo C., Benedito J.L., Vázquez P., Pereira V., Méndez J., Sotillo J., Hernández J. Effects of supplementation with plant extract product containing carvacrol, cinnamaldehyde and capsaicin on serum metabolites and enzymes during the finishing phase of feedlot-fed bull calves. Anim Feed Sci Technol. 2012;171:246–250. [Google Scholar]
- Celi P. Oxidative stress in ruminants. J Neurophysiol. 2011;110:2887–2894. [Google Scholar]
- Ding H., Liu W., Ao C., Khas E. Effects of dietary Allium mongolicum Regel leaves or probiotic complexes on mutton eating quality and antioxidant activities of DuHan sheep. J Anim Sci. 2018;96(Suppl 3):279–280. [Google Scholar]
- Dohme-Meier F., Kaufmann L.D., Görs S., Junghans P., Metges C.C., Dorland H.A.V., Bruckmaier R.M., Münger A. Comparison of energy expenditure, eating pattern and physical activity of grazing and zero-grazing dairy cows at different time points during lactation. Livest Sci. 2014;162:86–96. [Google Scholar]
- Dong S.K., Gao H.W., Xu G.C., Hou X.Y., Long R.J., Kang M.Y., Lassoie J.P. Farmer and professional attitudes to the large-scale ban on livestock grazing of grasslands in China. Environ Conserv. 2007;34:246–254. [Google Scholar]
- Du H.X., Erdene K., Chen S.Y., Saruli, Bao Z.B., Zhao Y.X., Wang C.F., Zhao G.F., Ao C.J. Correlation of the rumen fluid microbiome and the average daily gain with a dietary supplementation of Allium mongolicum Regel extracts in sheep. J Anim Sci. 2019;97:2865–2877. doi: 10.1093/jas/skz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Demerdash F.M., Yousef M.I., El Naga N.I. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Elkatcha M.I., Soltan M.A., Essi M.S. Effect of Pediococcus spp. supplementation on growth performance, nutrient digestibility and some blood serum biochemical changes of fattening lambs. Alex J Vet Sci. 2016;49:44–54. [Google Scholar]
- Eman M., Elgharieb H.H., Rahman M.A. Parasitological and clinico-pathological studies on some herbal preparations in mice experimentally infected with schistoma mansoni. Egypt J Comp Path & Clin Path. 2008;21:269–299. [Google Scholar]
- Fang L.H., Jin Y.H., Jeong J.H., Jeong J.H., Hong J.S., Kim Y.Y. Effects of dietary energy and protein levels on reproductive performance in gestating sows and growth of their progeny. J Anim Sci Technol. 2019;61:154–162. doi: 10.5187/jast.2019.61.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijó J.O., Schneider A., Schmitt E., Brauner C.C., Martins C.F., Barbosaferreira M., Pino F.A.B.D., Junior S.P.F., Rabassa V.R., Corrêa M.N. Prepartum administration of recombinant bovine somatotropin (rBST) on adaptation to subclinical ketosis of the ewes and performance of the lambs. Arq Bras Med Vet Zootec. 2015;67:103–108. [Google Scholar]
- Fraga C.G., Galleano M., Verstraeten S.V., Oteiza P.I. Basic biochemical mechanisms behind the health benefts of polyphenols. Mol Aspect Med. 2010;31:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Harith A.H., Badawi H.N. Determination of serum proteins and glucose concentrations in clinically normal and anemic awassi sheep. World's Vet J. 2015;6:1–6. [Google Scholar]
- Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Hu R.P., Du L., Ao C.J., Zhang X.F., Deng F. Studies on purified with SephadexG—100 column chromatography and molecular weight measured of Allium mongolium Regel polysaccharides. Sci Technol Eng. 2011;12:1671–1815. [Google Scholar]
- Javad H., Seyed-Mostafa H., Farhad O., Mehdi M., Ebrahim A.O., Naber R.G., Ramin G.S., Behrooz H. Hepatoprotective effects of hydroalcoholic extract of Allium hirti-folium (Persian shallot) in diabetic rats. J Basic Clin Physiol Pharmacol. 2012;23:83–87. doi: 10.1515/jbcpp-2012-0017. [DOI] [PubMed] [Google Scholar]
- Kazemi S., Asgary S., Moshtaghian J., Rafi eian M., Adelnia A., Shamsi F. Liver-protective effects of hydroalcoholic extract of Allium hirtifolium Bioss. in rats with alloxan-induced diabetes mellitus. ARYA Atheroscler. 2010;6:11–15. [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Mendiratta S.K., Agrawal R.K., Sharma H., Singh B.P. Anti-oxidant and anti-microbial properties of lamb nuggets incorporated with blends of essential oils. J Food Sci Technol. 2018;55:821–832. doi: 10.1007/s13197-017-3009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer K.S., Mahaffey E.A., Prasse K.W. Duncan and prasse's veterinary laboratory medicine: clinical pathology. Vet Clin Pathol. 2003;32:218–225. [Google Scholar]
- Li J., Yang P., Yang Q., Gong X.W., Ma H.C., Dang K., Chen G.H., Gao X.L. Feng baili analysis of flavonoid metabolites in buckwheat leaves using UPLC-ESI-MS/MS. Molecules. 2019;24:1–13. doi: 10.3390/molecules24071310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell R.C., Henry P.R., Ammerman C.B. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- Liu H.W., Zhao J.S., Li K., Deng W. Effects of chlorogenic acids-enriched extract from Eucommia ulmoides leaves on growth performance, stress response, antioxidant status and meat quality of lambs subjected or not to transport stress. Anim Feed Sci Technol. 2018;238:47–56. [Google Scholar]
- Liu J., Diao Q.Y., Zhao Y.G., Jiang C.G., Den K.D., Li L.Y., Tu Y. Prediction of nutrient digestibility and energy concentrations using chemical compositions in meat sheep feeds. Chin J Anim Veter Sci. 2012;8:60–68. [Google Scholar]
- Liu W.J., Ding H., Khas-erdene, Chen R.W., Qier M., Ao C.J. Effects of flavonoids from Allium mongolicum Regel as a dietary additive on meat quality and composition of fatty acids related to flavor in lambs. Can J Anim Sci. 2019;1:1–9. [Google Scholar]
- Mahmood M., Al-Hadithy H.A., Al-Badawi N.M. Estimation of serum liver enzymes activities in awassi sheep. Iraqi J Vet Sci. 2013;37:115–120. [Google Scholar]
- Maisashvili M.R., Gvazava L.N., Kuchukhidze J.K. Flavonoids and coumarins from Allium rotundum. Chem Nat Compd. 2009;45:87–88. [Google Scholar]
- Mcgeehin B., Sheridan J.J., Butler F. Factors affecting the pH decline in lamb after slaughter. Meat Sci. 2001;58:79–84. doi: 10.1016/s0309-1740(00)00134-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin C., Byatt J., Hedrick H., Veenhuizen J., Curran D., Hintz R., Hartnell G., Kasser T., Collier R.J., Baile C. Performance, clinical chemistry, and carcass responses of finishing lambs to recombinant bovine somatotropin and bovine placental lactogen. J Anim Sci. 1993;71:3307–3318. doi: 10.2527/1993.71123307x. [DOI] [PubMed] [Google Scholar]
- Miller J.K., Brzezinskaslebodzinska E., Madsen F.C. Oxidative stress, antioxidants, and animal function. J Dairy Sci. 1993;76:2812–2823. doi: 10.3168/jds.S0022-0302(93)77620-1. [DOI] [PubMed] [Google Scholar]
- Muñozosorio G.A., Aguilarcaballero A.J., Sarmientofranco L.A., Wurzinger M., Gutiérrezreynoso G.A. The effect of two housing systems on productive performance of hair-type crossbreed lambs in sub-humid tropics of Mexico. J Appl Anim Res. 2016;45:1–5. [Google Scholar]
- Muqier . Inner Mongolia Agricultural University; 2016. The effects of flavonoids from Allium Mongolicum Regel on antioxidant capacity, immune function and its mechanism in meat sheep. [Doctoral Dissertation] [Google Scholar]
- Obmann A., Tsendayush D., Thalhammer T., Zehl M., Vo T.P.N., Purevsuren S., Natsagdorj D., Narantuya S., Kletter C., Glasl S. Extracts from the Mongolian traditional medicinal plants Dianthus versicolor FISCH and Lilium pumilum D elile stimulate bile flow in an isolated perfused rat liver model. J Ethnopharmacol. 2010;131:555–561. doi: 10.1016/j.jep.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Pereira E.S., Campos A.C.N., Castelo-Branco K.F., Bezerra L.R., Gadelha C.R.F., Silva L.P., Pereira M.W.F., Oliveira R.L. Impact of feed restriction, sexual class and age on the growth, blood metabolites and endocrine responses of hair lambs in a tropical climate. Small Rumin Res. 2017;158:9–14. [Google Scholar]
- Radostits O.M., Gay C.C., Blood D.C., Hinchcliff K.W. 9th ed. Saunders; Spain: 2000. Veterinary medicine. [Google Scholar]
- Roomruangwong C., Kanchanatawan B., Sirivichayakul S., Anderson G., Carvalho A., Duleu S., Geffard M., Maes M. IgA/IgM responses to gram-negative bacteria are not associated with perinatal depression, but with physio-somatic symptoms and activation of the tryptophan catabolite pathway at the end of term and postnatal anxiety. CNS Neurol Disord - Drug Targets. 2017;16:472–483. doi: 10.2174/1871527316666170407145533. [DOI] [PubMed] [Google Scholar]
- Salem A.Z.M., Olivares M., Lopez S., Gonzalez-Ronquillo M., Rojo R., Camacho L.M. Effect of natural extracts of salix babylonica and leucaena leucocephala on nutrient digestibility and growth performance of lambs. Anim Feed Sci Technol. 2011;170:27–34. [Google Scholar]
- Santos V.A., Silva A.O., Cardoso J.V., Silvestre A.J., Silva S.R., Martins C. Genotype and sex effects on carcass and meat quality of suckling kids protected by the pgi “cabrito de barroso”. Meat Sci. 2017;75:725–736. doi: 10.1016/j.meatsci.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Schmitt B., Schulz H., Storsberg J., Keusgen M. Chemical characterization of Allium ursinum L. depending on harvesting time. J Agric Food Chem. 2005;53:7288–7294. doi: 10.1021/jf0504768. [DOI] [PubMed] [Google Scholar]
- Shingfield K.J., Bonnet M., Scollan N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Anim Int J Anim Biosci. 2013;7:132–162. doi: 10.1017/S1751731112001681. [DOI] [PubMed] [Google Scholar]
- Talapatra S.K., Ray S.C., Sen K.C. Analysis of mineral constituents of biological materials. Indian J Vet Sci Anim Husb. 1940;10:243–247. [Google Scholar]
- Thapa B.R., Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74:663–671. doi: 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- Tüzün A., Erdil A., Inal V., Aydin A., Bağci S., Yeşilova Z., Sayal A., Karaeren N., Dağalp K. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem. 2002;35:569–572. doi: 10.1016/s0009-9120(02)00361-2. [DOI] [PubMed] [Google Scholar]
- Van Putten M., Hulsker M., Young C., Nadarajah V.D., Heemskerk H., van der Weerd L., Hoen P.A., van Ommen G.J., Aartsma-Rus A.M. Low dystrophin levels increase survival and improve muscle pathology and function in dystrophin/utrophin double-knockout mice. Faseb J. 2013;27:2484–2495. doi: 10.1096/fj.12-224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Watkins P.J., Kearney G., Rose G., Allen D., Ball A.J., Pethick D.W., Warner R.D. Effect of branched-chain fatty acids, 3-methylindole and 4-methylphenol on consumer sensory scores of grilled lamb meat. Meat Sci. 2014;96:1088–1094. doi: 10.1016/j.meatsci.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Webb E.C., Erasmus L.J. The effect of production system and management practices on the quality of meat products from ruminant livestock. S Afr J Anim Sci. 2013;43:413–423. [Google Scholar]
- Yang W.Z., Ametaj B.N., Benchaar C., He M.L., Beauchemin K.A. Cinnamaldehyde in feedlot cattle diets: intake, growth performance, carcass characteristics, and blood metabolites. J Anim Sci. 2010;88:1082–1092. doi: 10.2527/jas.2008-1608. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Luo H., Chen Y., Yan L., Chang Y., Jiao L., Liu K. Effects of liquorice extract on the pH value, temperature, drip loss, and meat color during aging of longissimus dorsi muscle in tan sheep. Small Rumin Res. 2013;113:98–102. [Google Scholar]