Abstract
Traditional Chinese medicine (TCM) has been an indispensable source of drugs for curing various human diseases. However, the inherent chemical diversity and complexity of TCM restricted the safety and efficacy of its usage. Over the past few decades, the combination of liquid chromatography with mass spectrometry has contributed greatly to the TCM qualitative analysis. And novel approaches have been continuously introduced to improve the analytical performance, including both the data acquisition methods to generate a large and informative dataset, and the data post-processing tools to extract the structure-related MS information. Furthermore, the fast-developing computer techniques and big data analytics have markedly enriched the data processing tools, bringing benefits of high efficiency and accuracy. To provide an up-to-date review of the latest techniques on the TCM qualitative analysis, multiple data-independent acquisition methods and data-dependent acquisition methods (precursor ion list, dynamic exclusion, mass tag, precursor ion scan, neutral loss scan, and multiple reaction monitoring) and post-processing techniques (mass defect filtering, diagnostic ion filtering, neutral loss filtering, mass spectral trees similarity filter, molecular networking, statistical analysis, database matching, etc.) were summarized and categorized. Applications of each technique and integrated analytical strategies were highlighted, discussion and future perspectives were proposed as well.
KEY WORDS: Liquid chromatography−mass spectrometry, Qualitative analysis, Traditional Chinese medicine, Data acquisition, Data post-processing
Abbreviations: BS, background subtraction; CCS, collision cross section; CE, collision energy; CID, collision-induced dissociation; cMRM, conventional multiple reaction monitoring; DDA, data-dependent acquisition; DE, dynamic exclusion; DIA, data-independent acquisition; DIF, diagnostic ion filtering; DM, database matching; EL, exclusion list; EMS, enhanced mass spectrum; EPI, enhanced product ion; FS, full scan; HCD, high-energy C-trap dissociation; IDA, information dependent acquisition; IM, ion mobility; IPF, isotope pattern filtering; ISCID, in-source collision-induced dissociation; LC, liquid chromatography; LTQ-Orbitrap, linear ion-trap/orbitrap; MDF, mass defect filtering; MIM, multiple ion monitoring; MN, molecular networking; MRM, multiple reaction monitoring; MS, mass spectrometry; MTSF, mass spectral trees similarity filter; NL, neutral loss; NLF, neutral loss filtering; NLS, neutral loss scan; NRF, nitrogen rule filtering; PCA, principal component analysis; PIL, precursor ion list; PIS, precursor ion scan; PLS-DA, partial least square-discriminant analysis; QqQ, triple quadrupole; QSRR, quantitative structure retention relationship; Q-TRAP, hybrid triple quadrupole-linear ion trap; RT, retention time; SA, statistical analysis; sMRM, scheduled multiple reaction monitoring; TCM, traditional Chinese medicine; UHPLC, ultra-high performance liquid chromatography
Graphical abstract
This review summarized the mechanisms and applications of various data acquisition and data post-processing techniques of liquid chromatography−mass spectrometry (LC−MS) in qualitative analysis of traditional Chinese medicine (TCM).
1. Introduction
As the quintessence and treasure of China, traditional Chinese medicine (TCM) has taken on the responsibility to prevent and treat human diseases for millennia, and the excellent therapeutic effects of TCM have also been proved through the long historical clinical practice1. For this reason, TCM has always been inspiring scientists around the world and employed as an ideal library for development of drugs and dietary supplements2. However, disparate from mono-component Western medicines, the constituents of TCM are complex and yet undisclosed, thus leading to the vague mechanisms of action3 and hampering the worldwide adoption of TCMs, especially in the Western world. Therefore, it is of vital importance to conduct more in-depth research for a better understanding of the chemical complexity of TCM, especially those closely related with the pharmacological activities and toxicities. However, on the one hand, a large number of minor and trace components exhibiting predominant biological activities cannot be detected with an ordinary approach; on the other, the compounds in TCM are usually one or multiple types of secondary metabolites, and the metabolites of the same type are structurally similar and hard to differentiate because of the common biosynthetic pathways. Accordingly, the number and structural variety of metabolites constitute a great analytical challenge in terms of detection and identification.
Combining the separation capacity of liquid chromatography (LC), especially the ultra-high performance liquid chromatography (UPLC), and remarkable selectivity and sensitivity of mass spectrometry (MS), the hyphenated analytical platform (LC−MS) shows significant advantages in analyzing complex chemical systems and has become one of the most powerful tools for analysis of TCM4. In an endeavour to explore the structural variety of metabolites, the first step is to acquire as much LC−MS information as possible, and the second step is to convert LC−MS data into chemical information. Between these two steps, except for the stationary phase development of LC5 and hardware enhancement of MS instrumentations6, the fast-developing MS data acquisition and data post-processing techniques also play a pivotal role7,8.
Data acquisition methods by LC–MS can be roughly classified into data-independent acquisition (DIA) method and data-dependent acquisition (DDA) method. Typical DIA methods may provide production information in a non-selective manner without prior knowledge of the ions of interest. Featured with full coverage of MS1 and MS2 spectra in one injection, DIA methods maximally avoid the loss of MS information and possess satisfactory reproducibility and quantitative performance9. However, it is a daunting task to precisely assign the product ions generated by DIA methods to their precursor ions especially when the co-eluting compounds exist, due to the complex spectra and the lost link between MS1 and MS2, as well as requirements for more complicated deconvolution and data processing algorithms to further interpret the acquired MS data10. In a DDA method, a survey scan (usually full scan, FS) is first launched, and if certain criteria are met, further MS/MS (or MSn) scan will be automatically triggered7. The diverse criteria set up in DDA methods provide customized solutions, taking advantages of the intrinsic chemical characteristics and fragmentation behaviors of the target compounds. In contrast to the DIA data, the structural information could be easily elucidated from DDA data, especially with the help of data post-processing techniques.
Even though plenty of researches and applications of LC−MS techniques in TCM qualitative analysis have been reported, it is still short of relevant reviews, with only several categorizing different compound classes and citing some representative examples of analyzing these compounds in TCM and TCM prescriptions4,11, 12, 13, 14. Therefore, summary of universal and applicable techniques in TCM analysis are in urgent need. This review primarily focused on the applications of the advanced MS data acquisition and data post-processing techniques (Fig. 1), as well as integrated analytical strategies in qualitative analysis of TCM. Mechanisms of these techniques were firstly introduced, followed by representative applications. It is anticipated that this review would render a comprehensive reference for future research with regard to the metabolites identification in TCM complex systems.
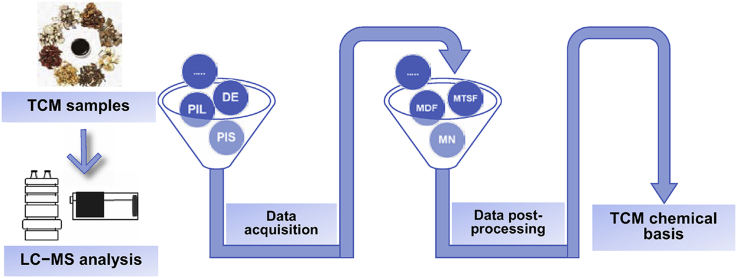
Figure 1.
Data acquisition and data post-processing techniques of LC−MS in TCM qualitative analysis. PIL, precursor ion list; DE, dynamic exclusion; PIS, precursor ion scan; NLS, neutral loss scan; MRM, multiple reaction monitoring; MDF, mass defect filtering; BS, background subtraction; IPF, isotope pattern filtering; DIF, diagnostic ion filtering; NLF, neutral loss filtering; MTSF, mass spectral trees similarity filter, MN: molecular networking; SA, statistical analysis; DM, database matching.
2. Data acquisition techniques
2.1. Data-independent acquisition techniques
Typical DIA modes include AIF (all ion fragmentation) mode of Orbitrap system (Thermo Fisher Scientific, CA, USA)15, MSE (elevated energy MS) and SONAR mode of Q-TOF system (Waters, MA, USA)16,17, MSALL and SWATH™ (sequential window acquisition of all theoretical mass spectra) mode of Triple TOF system (AB SCIEX, CA, USA)18,19. Among them, AIF acquisition mode allows all the precursor ions of defined range into the HCD (high-energy C-trap dissociation) cell for fragmentation15. MSALL is similar to MSE which involves two scan functions, one is at low collision energy (CE), collecting intact molecular ion information with a wide mass range. The other one obtains product ion data using higher CE (fixed or ramped), which is useful for structural elucidation16. However, even though the aforementioned DIA modes avoid missing MS/MS information of minor or trace components in TCM, the defects of complex MS/MS spectra resulting from co-eluting ions still restricted the prevalent use of DIA techniques in TCM qualitative analysis. Luckily, different manufacturers have been devoted to improving the performance of DIA modes through instrument enhancement. For example, newly-developed SWATH™ acquisition mode allows the fragmentation of all ions across a given mass range in sequential narrow selection windows, thus retaining the benefits of both selectivity and scan speed20. While SONAR acquisition mode operates in a similar way, when the resolving quadrupole slides over a selected mass range during each MS scan with collision cell alternating between high and low CE from scan to scan, and the software aligns to associate the precursor and product ions in different data channels. Besides, the application of ion mobility (IM) technique also helps obtain relatively clean and high-quality MS/MS spectra by enhanced separation of co-eluting ions based on their sizes, shapes and charge states21.
Different DIA-based strategies have been developed for TCM analysis. For example, MSALL was applied in identification of triterpene saponins in Acanthopanax senticosus leaves by simultaneously collecting all the precursor ions ([M+H]+, [M+NH4]+ and [M+Na]+) and product ions with ramped CE on an AB SCIEX QTOF system, while SWATH mode as a complementation for co-eluting ions22. To characterize lanostane-type triterpene acids in Poria cocos, UPLC−IM−MSE method was used to obtain multi-dimensional information including retention time, drift time and mass-to-charge ratio (m/z), where UPLC was for preliminary separation, whilst IM for identification of co-eluents by aligning precursor ions and corresponding product ions with the same drift time23.
2.2. Data-dependent acquisition techniques
Traditionally, in a DDA method, precursor ions were selected for fragmentation according to their signal abundances in MS1 scans. However, there could be a stochastic nature to data acquisition as TCM mixtures become more complex. In addition, only fragmentations of the top-n precursor ions were available. As a result, it often encounters obstacles in comprehensively detecting minor or trace components in TCM and systematically characterizing metabolites of the same type. Since the multiple types of constituents in a certain TCM are closely related to one or more common biosynthetic pathways, they could be structurally classified into several chemical families according to the same carbon skeletons or substructures with varieties in substitutional groups. Therefore, MS fragmentation patterns of chemically similar compounds usually displayed the same neutral losses, product ions, or other characters. Accordingly, various DDA techniques targeting one-type or multi-types of metabolites, and covering the trace metabolites have been developed.
2.2.1. Precursor ion list
In a precursor ion list (PIL) experiment, only (or preferred) target ions contained in the list can be scanned to trigger multistage fragmentations. PIL-based acquisition thus becomes a commonly used DDA technique with high selectivity, sensitivity and efficiency in discovery and characterization of target compounds. Unlike conventional DDA method that automatically fragments the top-n abundant ions, MS/MS or MSn data are acquired regardless of their peak abundances, overcoming the drawback of neglecting minor components in TCM extracts.
In a PIL-based acquisition, the key is to establish a sample-specific PIL, based on molecular features of m/z value or a combination with retention time24. One of the most widespread approach is collecting previous phytochemical reports for further structural prediction. With the loss or addition of different substitutional groups to certain core substructures in accordance with the biosynthetic pathways, the molecular formulas of these derivatives are predicted, correspondingly, molecular weights and theoretical parent ions can be calculated. PIL constructed in this way encompasses both known and predicted compounds, thus greatly increasing the coverage and improving the selectivity of detecting expected compounds in TCM. Another approach, is using various data mining techniques to explore the potential metabolites, such as mass defect filtering (MDF)25, 26, 27, neutral loss filtering (NLF)28,29 and molecular networking (MN)30. Their principles and applications in qualitative analysis of TCM are discussed in depth in Section 3.
For example, some compound-specific PILs were generated by summarizing the reported natural compounds, and applied in profiling of components in Carthamus tinctorius L. (safflower)31, Ligustri Lucidi Fructus32, etc. Besides, based on compound prediction, aglycones substituted by 2–7 hydroxyl and methoxyl groups were included in a PIL, achieving targeted screening of 135 polymethoxylated flavonoids when analyzing the sample of Citrus reticulata Blanco on a hybrid linear ion-trap/Orbitrap (LTQ-Orbitrap)33. Another PIL constructed by the arrangement of different substitutional groups to tanshinol was also used to characterize polymeric phenolic acids in Salvia miltiorrhiza34.
One successful application of generating PIL with data post-processing techniques is conducted by a FS-based untargeted metabolomics approach35. This method displays great advantages in analyzing complex systems, as no prior knowledge of the sample is necessary. Besides, separating a long PIL into several individual ion lists based on m/z value or time point can drastically avoid data loss and reduce duty cycle of the instumentation34. There is evidence showing that DDA triggered by time-staggered PIL exhibited superior selective advantages in activating MS/MS acquisition of co-eluting ions compared to the conventional DDA35. In summary, PIL-MSn, especially the time-triggered mode, displays excellent superiority in acquiring the fragmentation information of target ions, thereby enhancing the selective detection of target components in TCM.
2.2.2. Dynamic exclusion
Contrary to PIL−MS/MS technique which preferentially trigger MS/MS fragmentation of predefined ions in list, exclusion list (EL)-based acquisition methods improve the selectivity and sensitivity of characterization by entering an EL of masses that will be ignored for MSn analysis, exhibiting great advantages in removing background interfering ions and non-targeted ions, and showing potentials in exposing more novel compounds36. Except for abundant interfering ions, there will always be target components that completely overlap when analyzing TCM complex systems regardless of the optimized chromatographic separation conditions. Under these circumstances, only the top-n intense ions can be selected for fragmentation, and MS/MS information of less abundant co-eluting ions will escape the detection. Dynamic exclusion (DE) provides a solution for this problem by temporarily putting a mass over the defined threshold into an EL for a selected period of time after its MS/MS spectrum is acquired, enabling less abundant ions to be analyzed rather than repeated scans of the abundant ions37. For this reason, DE is frequently set to improve the detection coverage in TCM qualitative analysis, especially for detecting those less abundant co-eluting ions25,26,28,38.
The advantages of DE were fully illustrated by screening the polymethoxylated flavonoids from Citrus reticulata Blanco33. Compared with FS, FS-PIL and FS-DE on LTQ-Orbitrap, FS-PIL-DE acquisition was found to be most powerful in triggering MS/MS fragmentations. Its superiority in acquiring the MSn information was further confirmed by another study for screening indole alkaloids from Uncaria sinensis26.
Appropriate DE parameters are also essential for MS data acquisition. Because they are closely related to the chromatographic peak width, optimal chromatographic conditions should be investigated to minimize the differences in the peak widths of different retention times34. Besides, short repetition time and long exclusion time could minimize MS information loss of a complex system but potentially miss the fragmentation of the neighboring isomers. Therefore, a coordination of these two parameters is always a necessity for the better data quality.
2.2.3. Mass tag
In a mass tag dependent acquisition, the parent ions with certain mass differences were recognized as mass partners, and selected to trigger MSn fragmentation. The mass partners can be produced by introducing isotopically labeled compounds, such as chrysin-d0 and chrysin-d5 with a mass difference of 5 Da. The technique of mass tag can largely improve signal-to-noise ratios and data quality in detection of tagged compounds, including reactive metabolites39 and modified peptides40. Mass tag technique can be performed on LTQ-Orbitrap and applied to selectively detect phosphopeptides and N-glycopeptides with an in-source collision-induced dissociation (ISCID) energy on. After the m/z values of the charged ions in MS1 spectra were determined and converted into masses, mass partners that differed in the mass of defined mass tag and with intensity above the defined threshold, were selected to trigger MS2 acquisition with the ISCID off40.
Recently, expanding applications of mass tag can be found in TCM qualitative analysis, especially for targeted screening of components that can easily undergo in-source fragmentations. For example, malonylginsenosides from three Panax species were targeted screened out by defining mass tag of 43.9898 Da with an ISCID energy of 40 V, corresponding to the elimination of CO241. In addition, by applying ISCID of 50 V and mass tag of 46.0055 Da (referring to formic acid), neutral ginsenosides from three Panax species were also successfully classified and characterized42. In summary, mass tag-oriented acquisition has unique advantages in selectively screening the target components in TCM with a high coverage and introducing less false positives.
2.2.4. Precursor ion scan
Precursor ion scan (PIS) can be realized in a triple quadrupole (QqQ) mass spectrometer. Ions within a defined mass range are scanned in the first quadrupole (Q1), fragmented in the collision cell (q2), and then one specific product ion is selected in the third quadrupole (Q3)43. This acquisition mode allows selective screening of compounds with identical product ions, and is popular in metabolites identification44, lipidomics study45. Besides, PIS also shows great potential in characterization of various TCM components. Its advantage lies in requiring little prior-knowledge about the exact structure of compounds.
Facilitated with PIS technique, target compounds that are expected to produce the same product ions can be screened out, such as the esters, saponins, flavonoids, etc. For example, benzoyl substituted diterpenoid alkaloids with diagnostic benzoyl ion at m/z 10546; caffeoylquinic acid derivatives with diagnostic product ion of m/z 191 referring to quinic acid anion47; oplopane-type sesquiterpenoids with ions at m/z 215 and 217, and bisabolane-type sesquiterpenoids with ions at m/z 229 and 23148. Besides, glycosyl-conjugated compounds could also be easily detected using characteristic ions corresponding to their core structures and sugar moieties. As an example, triterpene saponins in Glycyrrhiza yunnanensis with saccharide chains of glucuronic acid (GluA) and rhamnose (Rham) were detected using ions at m/z 351 [2GluA−H]− and 497 [2GluA+Rham−H]−49. Polyoxypregnane and its glycosides were identified from Marsdenia tenacissima (Roxb.) Wight et Arn., using characteristic ions at m/z 329 and m/z 273, corresponding to the aglycone core and sugar moiety at C-3 position, respectively50. And characteristic ions of 153+/151– were used to identify glycosyl-free flavonoids and then the related glycosyl-conjugated flavonoids were further searched using ions of the identified glycosyl-free flavonoids in PIS mode51.
PIS-information dependent acquisition (IDA)-enhanced product ion (EPI) mode implemented on a hybrid triple quadrupole-linear ion trap (QTRAP) mass spectrometer is more effective in rapid detection and characterization of certain types of analytes than experiments on QqQ, due to an additional MS/MS fragmentation. It was applied to rapidly identify phenolic constituents in Danhong injection using six diagnostic ions, they are m/z 197 ([M−H]−), 179 ([M–H–H2O]−), 135 ([M–H–H2O–CO2]−), 123 ([M–H–H2O–2CO]−) generated from danshensu-related compounds, 137 ([M–H]−) and 108 ([M–H–CHO]−) generated from protocatechualdehyde-related compounds52. Wider applications of PIS-IDA-EPI in TCM qualitative analysis include identification of naphthalenyl glycosides and amino naphthoquinones in Juglans cathayensis53, coumarins in Glehniae Radix54, phenylethanoid glycosides, iridoids, and lignans in Cistanche deserticola and C. tubulosa55, and so on.
2.2.5. Neutral loss scan
In the neutral loss scan (NLS) mode of QqQ and QTRAP, ions within a defined mass range are scanned in the first quadrupole (Q1), fragmented in the collision cell (q2), and then Q3 scan range shifts by Δm to a low mass, which corresponds to a specific neutral loss (NL) of every potential precursor ion43, e.g., 162 Da corresponding to glucose. It is widely used for specific screening of modified metabolites that can undergo neutral eliminations, and also highly selective and sensitive to profile and characterize various specific conjugated components in TCM, as well as discovery of new compounds.
Because O-glycosides can easily undergo the NL of sugars, NLS was used to profile glycosides with different aglycones in tobacco leaves56. NL-IDA-EPI scan mode was employed to screen glycosyl flavonoids in Astragali Radix with NL of 162 or 179 Da for screening of flavonoid aglycones that tended to form protonated or ammoniated glycosides, respectively57. Triterpene saponins in Glycyrrhiza yunnanensis were profiled using adducts-targeted NL-EPI by monitoring NL of 17 Da (referring to NH3) and 46 Da (referring to HCOOH) generated from their ammonium and formic acid adducts, respectively58. Sulfated flavonoids in Flaveria were detected by a NL of 80 Da (representing SO3) in negative mode59. And iridoids, lignans, and phenylethanoid glycosides in Cistanche deserticola and C. tubulosa were screened with NL of 162 Da (glucose residue or a C9H6O3 group), 146 Da (coumaroyl group) and 176 Da (feruloyl groups)55. In addition, NLS was also enabled in high-resolution LTQ-Orbitrap, as the exact mass of the neutral loss can be used to screen the targets more accurately. For instance, malonylginsenosides were screened from nine Ginseng extracts with NL set at 43.9898 Da, corresponding to loss of a CO2 unit from malonyl group41. Steroids substructures of dicarboxylic acid conjugated bufotoxins in Venenum bufonis were obtained by a NL-based MS3 acquisition in positive ion mode with neutral masses of seven dicarboxylic acids38.
2.2.6. Multiple reaction monitoring
Multiple reaction monitoring (MRM) mode is a typical scan mode operated in QqQ and QTRAP. By applying an appropriate CE, certain parent ions detected in Q1 cell can fragment in q2 cell and generate product ions passing through Q3 cell. Under optimal MS conditions, MRM-based acquisition, by simultaneously monitoring specific parent ion and corresponding product ion with the highest intensity, can drastically improve the selectivity and sensitivity of target components in TCM.
MRM scan mode was adopted to screen out 77 flavonoid glycosides in the flower of Carthamus tinctorius L.60, identify 80 triterpenes in Alismatis Rhizoma61, etc. MRM-IDA-EPI scan on a QTRAP mass spectrometer was also utilized to identify 27 major components (18 diterpenoids, 6 phenolic acids, and 3 flavonoids) in Isodon serra62, characterize 421 flavonoids in Astragali Radix57, verify the structures of all tentative curcuminoids in turmeric63, and screen ginsenosides in Ginseng, American Ginseng and their processed materials64.
However, the increasing number of analytes monitored in a single run will result in lower sensitivity of detection, because either the dwell time for each transition will be reduced and unable to accumulate sufficient signal for each transition, or the cycle time for each MS scan will increase, decreasing the data points of each peak65. Scheduled MRM (sMRM, or dynamic MRM) offers an option to increase the number of MRM transitions without compromising the data quality, by monitoring every sMRM transition in a defined range of retention time66. The superiority of sMRM over conventional MRM (cMRM) was proved by detection of components in a TCM mixture made up of eight commonly used TCM67, and more analytes could be detected using sMRM than cMRM method.
Multiple ion monitoring (MIM) mode could be considered as a special type of MRM mode, with a minimal collision energy applied in q2 cell, thus the product ion monitored in Q3 cell is identical to the parent ion in Q1 cell. In general, suitable ion transitions in an MRM method are designed based on the prior-knowledge of the fragment patterns of the parent ion, causing great challenges to screen unknown compounds. MIM method can overcome these limitations and be used to detect potential compounds regardless of their fragmentation patterns, thus playing an important role in targeted monitoring of compounds of interest in TCM, and can be served as a complementary approach for MRM68. For example, in order to identify naphthoquinones in Juglans cathayensis, MIM-EPI method was conducted based on 36 different molecular weights of all reported naphthoquinones from family Juglandaceae, stepwise MIM-EPI with a wide specified mass range (150.0–700.0 Da) was conducted for discovery of untargeted naphthoquinones, and segmented stepwise MIM-EPI with a narrow specified mass range was used for identification of certain types of naphthoquinones53. Another strategy integrating predefined MRM, step-wise MIM, and EPI scans was also proposed to universally screen the hydrophilic substances in Shenfu injection, and a total of 157 hydrophilic compounds were detected, 154 of which were identified as amino acids, nucleosides, organic acids, carbohydrates, etc.69.
3. Data post-processing techniques
Conventionally, the structures of compounds were manually elucidated based on their MS fragmentation patterns, which requires laborious spectral assignments and produces somewhat experience-based results. Similar to the DDA techniques, newly-developed data post-processing techniques take full advantages of the same neutral losses, product ions, or other characters produced by MS/MS fragmentation. In addition, the MS characters in FS, such as mass defect, can also be a clue for compound recognition. In contrast to the DDA techniques, different data post-processing techniques can be applied to the same MS data for different purposes without reacquisition of the MS data. For example, the MS data of Carthamus tinctorius L. acquired by LTQ-Orbitrap was explored for both the quinochalcone C-glycosides70 and flavonoid O-glycosides71 with versatile data mining strategies, showing the advantages of data post-processing techniques on recycling of MS data. In a word, various data post-processing techniques can greatly simplify the manual interpretation process of the MS data, providing more conveniences, evidences and higher accuracy for TCM qualitative analysis.
3.1. Mass defect filtering
The calculation of the mass defect is conducted by the mass difference between the exact mass and nominal integer mass of different elements, in this way, each molecule can be described with a unique exact mass and mass defect based on its elemental composition72. Therefore, structural analogues sharing similar core substructure with various chemical substituted groups will have very similar mass defects, and the limited differences are only corresponded to the different substituted groups. Based on this fact, mass defect filtering (MDF) technique is developed and used as a specific filtering criterion to selectively pick out certain compounds or compound classes in a complex mixture. For example, a target class of endogenous substances, or parent drug and its metabolites73,74 in a complex biological sample within an allowable mass defect range. In addition, MDF is also a powerful tool to expedite the screening process of different components contained in TCM and to generate cleaner profiles by providing more distinct and specific information75.
Classic MDF algorithms, including fixed MDF algorithms with a rectangular coverage area and linear gradient MDF algorithms with a parallelogram coverage area76, can be conveniently realized by some commercial software such as Peakview77, Metabolynx XS78, Metworks79 and MetID80. And both the mass range and the mass defect range can be defined for automatic exclusion of irrelevant ions from complex matrices. For example, a mass defect filter (mass range: 367.5 ± 65.5 Da; mass defect: 186 ± 40 mDa) was established using Metworks, which not only encompassed all free indole alkaloids in Uncaria rhynchophylla, but also distinguished indole alkaloids from triterpenic acids (mass range: 453–649 Da, mass defect: 333–413 mDa) and other components79. Besides, stepwise MDF approach which divides the mass defect range or mass range into multiple mass defect windows or mass windows were proved to improve the signal to noise ratio of the target peaks, and polymethoxylated flavonoids were successfully screened from the leaves of Citrus reticulata Blanco81. The strategy combining a single MDF window (mass range: 267–418 Da; mass defect: 0.04–0.12 Da) for methoxylated flavonoids, and multiple MDF windows (mass range/mass defect: 337–398 Da/0.08–0.12 Da; mass range/mass defect: 483–604 Da/0.10–0.18 Da; mass range/mass defect: 629–810 Da/0.13–0.23 Da) for three classes of chlorogenic acids was also successfully constructed and applied in Folium Artemisiae Argyi82.
However, because the distribution space of target compounds established by a classic MDF strategy usually covers a quite large area, increasing the possibility of false positives, several in-house polygonal MDF techniques have been developed to narrow down the distribution space and focus more on the target ions. A modified MDF strategy, termed as “five-point screening” MDF strategy was proposed to rapidly screen saponins in Panax notoginseng83. Every selected ion of notoginsenosides was distributed in the region depicted by five points (integer mass as x and decimal mass as y), and the “IF” function of the Microsoft excel platform was used to pick out every potential ion distributed in this region in the.xls documents. Compared with the classic MDF, this modified approach effectively removed more putative spaces and thus increasing the screening efficiency. Another polygonal MDF algorithm established by a relatively compact octagonal region was also used to screen target alkaloids in Uncaria sinensis, as well as enable three novelty levels classification: known, unknown-but-predicted, and unexpected26. The region established by eight vertexes were generated by a two-dimensional distribution plot of the mass range (Da) and the mass defect range (mDa) of the known and unknown-but-predicted molecules, and multiple “IF” equations representing the octagonal region were edited in Microsoft Excel to rapidly screen the alkaloid components. This modified MDF algorithm greatly enhanced the accuracy in screening target components, and more importantly, enabled the discovery of novel compounds.
Despite of the great efforts made in exploration of different MDF algorithms, including classic rectangular MDF and modified MDF algorithms, for reducing false positives and expanding the coverage, tedious data processing procedures are required to generate sample-specific target ions. To solve this problem, a raster-MDF screening strategy was implemented by a step-wise PIL with the parent mass width of ±55 mDa, and utilized for screening and characterization of indole alkaloids from Uncariae Ramulus Cum Unicis of multiple botanical origins84. This strategy not only comprehensively extended the coverage of potential indole alkaloids, but also excluded interferences of other components from the genus Uncaria.
3.2. Background subtraction
Background interferences are common in TCM analysis, which could be traced back to all steps of sample preparation procedures such as plasticizers from pipette tips and centrifuge tubes85, or just chemical noise resulting from mobile phases or buffers in LC−MS analytical systems86. To suppress interference of the background noise and obtain relatively clean spectra, BS technique is therefore introduced. It can be implemented manually87, directly in a commercial software (Waters Masslynx, Thermo Scientific Metworks26,88, etc.) by selecting a region with background noise and then subtracting from the original chromatogram, preparing blank samples in the same manner as the test samples for detection and removal of the background interferences, or utilizing some customized programs for further information89.
BS is a useful technique in elimination of background signals, as well as exposure of chemical constituents in TCM, especially those with a low abundance. To identify unknown compounds in Er-xian decoction, signals corresponding to the background interferences were checked manually and excluded from 533 candidate compounds, and finally 240 non-background compounds were extracted87. To comprehensively detect and characterize the chemical constituents in Arnebiae Radix, the obtained total ion chromatogram (TIC) was first processed with BS, and then MDF to filter the background-subtracted ion chromatogram, resulting in characterization of 96 compounds, including 30 with a low abundance88.
3.3. Isotope pattern filtering
Isotope pattern filtering (IPF) technique is specially developed for compounds containing elements of distinct isotopic distribution patterns in mass spectra, such as chlorine, bromine, iodine, etc. With the aid of IPF, compounds containing certain elements can be easily screened out. For instance, an ion with isotopic mass distance of 2 Da and equal isotopic abundance is most likely to correspond to a compound containing one bromine atom. Except for these natural stable isotopes, other isotope-labeled compounds such as 14C, 15N and 18O-labeled compounds are also widely used in tracing and detecting related metabolites90. Compared with traditional IPF implemented by commercially available software (e.g., Metworks), some newly-developed algorithms such as accurate-mass-based spectral-averaging IPF enhances the specificity by inspecting not only the M+2 isotopic ion and M molecular ion, but also the M+1 isotopic ion91.
In recent years, sulfurous compounds with very low abundance in TCM including Pueraria lobata (Willd.) Ohwi (Gegen) and Pueraria thomsonii (Fenge) were detected and characterized by a fine IPF technique92. Despite of the low abundance of 34S signal and the influence of 13C2+18O, this IPF strategy achieved screening of S-containing compounds by defining the accurate mass shift and relative abundance between M+2S (representing the M+2 isotopic ion of 12Cx+21Hy16Oz+134S) and M (representing the molecular ion) as well as M+2OC (representing the M+2 isotopic ion of 12Cx1Hy16Oz32S13C218O) and M in Compound Discoverer software.
3.4. Diagnostic ion filtering
Similar to PIS, diagnostic ion filtering (DIF) provides a criterion for rapid classification of the target compounds that produce the identical product ions, by extracting the relevant ions in the MS/MS or MSn channels. Whereas PIS can only be realized by triple quadrupole like low resolution mass spectrometry, DIF can be applied to high resolution data obtained by QTOF, LTQ-Orbitrap, and Q-Orbitrap, thus leading to high selectivity. In addition to the diagnostic ions, the high-resolution data and abundant fragmentation information drastically increase the identification confidence. DIF has been applied to characterize different types of chemical components in TCM, including saccharides and glycosides93, quinones94, phenylpropanoids82,95, flavonoids96, 97, 98, triterpenes61, triterpenoid saponins22,95,99,100, alkaloids101,102, other compounds such as physalins103, as well as multiple compound classes in the same sample104,105.
To get the diagnostic ions in abundance, different dissociation methods at different energies have been explored on LTQ-Orbitrap. For example, to selectively identify flavonoid O-glycosides from Carthamus tinctorius, combination of two fragmentation modes, CID and HCD, was used for obtaining complementary fragmentation information, including high-mass product ions produced from loss of sugars, low-mass product ions originated from aglycone moieties, as well as intensity ratios of radical aglycone ion species useful for precise elucidation of aglycones and glycosylation patterns71. DIF combined with enhanced separation strategy such as multidimensional LC and IM can greatly expand the peak capacity and enhance sensitivity of characterizing minor components with structural diversity in TCM. An LTQ-Orbitrap approach using DIF of m/z 119.05 (C8H7O−) was developed to characterize 163 quinochalcone C-glycosides in Carthamus tinctorius L. by combing offline two-dimensional LC separation70. Another multidimensional analytical strategy combining LC−IM−MSE and LC−IM−MS/MS were established to screen polycyclic polyprenylated acylphloroglucinols using diagnostic ions m/z 165.0182 or/and 177.0182, leading to the successful characterization of 140 targets in Garcinia oblongifolia106.
In addition, DIF has also significantly facilitated the rapid classification and structural elucidation of more complicated multi-components contained in TCM prescriptions, including ginsenosides and lignans in Shenmai injection107, phenolic acids, saponins, diarylhepatonoids and gingerol-related compounds in Guge Fengtong tablet108, flavonoids, ginkgolides, phenolic acids, tanshinones, and saponins in Yindan Xinnaotong soft capsule109, etc. In summary, DIF is an option to filter and classify target ions, as well as remove interfering ions from the raw LC−MS data.
3.5. Neutral loss filtering
Neutral loss filtering (NLF) works by extracting the predicted specific neutral loss between the daughter ions and parent ions. NLF is similar to NLS, and the advantage lies in circumventing the use of multiple injections because of the combination use of multiple filters to identify multiple targets. NLF is promising in untargeted characterization of endogenous modified metabolites with specific neutral loss fragments, such as acetylation, sulfation, glucuronidation, etc.110, and exclusive profiling of phase II metabolites such as GSH conjugates in drug metabolism studies111.
In analysis of TCM, NLF also finds its place in characterization of glycosidic compounds such as flavonoid glycosides71,109, alkaloid glycosides79 and triterpenoid saponins95, acylation compounds such as malonyl ginsenosides99 and diester-diterpenoid alkaloids112, as well as other compounds substituted with certain moieties such as methoxylated flavonoids82, etc. For example, the diester-diterpenoid alkaloids in Aconitum carmichaelii Debx. were determined by the diagnostic neutral losses of 60 28, 32, 18 and 122 Da, corresponding to AcOH, CO, MeOH, H2O and BzOH moieties, respectively112. Besides, NLF of the recognized sugars and acylation (GluA, Glc, Rha, Ace) was conducted for target characterization of flavonoid O-glycosides in Carthamus tinctorius, and some unknown ones (132.04, 86.00 and 71.98 Da for Xyl, Mal, and Oxa, respectively) were also detected and characterized71.
A software named Neutral Loss MS Finder was developed to extract the in-source NL110, and successfully applied in screening the target malonyl-ginsenosides from three Panax species29. Because of the in-source removal of both neutral CO2 and C3H2O3 groups, two NL filters (43.9898 and 86.0004 Da) were set to reduce the false positives and enhance the characterization accuracy, and ultimately, to produce the peak lists containing 156, 130 and 126 target malonyl-ginsenosides in three Panax species, respectively.
3.6. Mass spectral trees similarity filter
Mass spectral trees similarity filter (MTSF) is a data post-processing technique for rapid classification of the unknown compounds by comparing the similarity of mass spectral trees. Commercially available software such as Mass Frontier is usually used to construct mass spectral trees, using HRMS data as the stem of the tree, MS2 data as the bough, and multi-stage MSn data further extended as the branches. After importing MSn data of the template compounds into the software to build the library of MTSF, the similarity scores between the compounds detected in the sample and those in the library can be calculated, and unknown compounds can be classified and characterized by matching with those templates to obtain candidate structures.
Because the mass spectral trees of structural analogs usually exhibit high similarity scores, MTSF technique is widely applied for rapid identification of metabolites in a complicated biological matrix, due to the structural resemblance between the metabolites and corresponding prototype113. Meanwhile, it also reveals great potential to significantly accelerate and simplify the process of discovery and identification of various chemical components in TCM which share the same substructures as template compounds, including flavones, phenylpropanoids, and sphingolipids in Saussurea involucrata114, chlorogenic acids in Duhaldea nervosa115 and Lonicerae Japonicae Flos116, etc. In addition, for TCM prescriptions containing diverse types of compounds, MTSF can also efficiently fish potential components belonging to different compound classes, and rapidly identify both templated compounds and related compounds in Xiao-Xu-Ming decoction117, prenylflavonoid glycosides, alkaloids and phenylpropanoids in Er-xian decoction87. In a word, taking full advantages of MSn information, MTSF is a simple and efficient technique to fish unknown components in TCM complex systems, especially the structural analogs of the template compounds.
3.7. Molecular networking
Molecular networking (MN) uses a visualized computational strategy for comparison of the MS/MS spectral and calculation of the similarity degree between structural analogs. The major strength of this technique lies in requirements of no prior knowledge of the chemical structures for simultaneous exploration of up to millions (probably billions) of MS2 data118. Developed from early-used MATLAB scripts installed on computers for similarity computation, Global Natural Products Social Molecular networking (GNPS) (http://gnps.ucsd.edu), an open-access online platform, allows even more analysts with no professional bioinformatics background to take advantage of this advanced technique119. Data visualization can be performed either directly online, or offline with Cytoscape120 as well as other visualization tools, where similar MS/MS spectra can be grouped into clusters, and each node represents a unique MS/MS spectrum, connected with edges indicating the cosine similarity between corresponding MS/MS spectra. To help data interpretation, node size and color can also be tuned according to precursor ion intensity and sample origin. Moreover, GNPS can automatically retrieve detected MS/MS spectral against public libraries, allowing rapid identification of molecules that are similar to database records. In addition to known compounds, MN also allows for exploration and identification of potential unknown analogues or compound families based on their MS/MS spectra relatedness with any known compounds. Compared with MTSF, MN is suitable for more LC−MS platforms and attracts wider attentions.
Since the introduction of MN in metabolic profiling of live microbial colonies in 2012121, it has been successfully applied in multiple areas122, such as drug discovery and natural products dereplication123, including microbials124, 125, 126, 127, marine organisms128, 129, 130, fungi131, and plants132, 133, 134, 135, 136. In recent years, MN has also been used in TCM qualitative analysis. For example, offline two-dimensional separation integrated with MN was developed for dereplication and sorted-characterization of 229 bufadienolides in Venenum Bufonis, including two new subclasses137. Rapid recognition of 537 components in Lonicerae Japonicae Flos was also achieved with the help of MN, including a myriad of potential novel structures36. In addition, MN was also applied for identification and exploration of potential chemical markers of Aconiti Lateralis Radix Praeparata before and after processing based on both the compound clusters and node areas138.
3.8. Statistical analysis
In face of complex and large-scale mass spectral data, although several data post-processing techniques such as DIF, NLF, MDF, MTSF and MN have been utilized to facilitate the data interpretation process and enhance the identification accuracy of target compounds in complex samples, it's still a challenge in the aspect of rapid recognition and extraction of potential characteristic ions. Therefore, in order to save time and manpower to dig for attractive compounds, some advanced statistical analysis (SA) techniques have been developed based on the intrinsic relationships between compound structures and MS data. Targeted data with similar characteristics can be classified into the same groups by statistical methods, while compounds of different substructures in TCM are efficiently discriminated. Combined with some diagnostic tools, such as loading plot and variable importance in projection (VIP), rapid recognition of potential characteristic ions can be easily achieved.
SA technique was explored for rapid screening of the minor compounds in Danhong injection139. Firstly, a total of 7157 product ions were extracted from MS/MS spectra of 22 reference standards, and 4 filtering ions specific for compounds in Dan-shen, Hong-hua, and both herbs were selected from 195 common ions. Combined with another 6 diagnostic ions, 117 compounds were finally identified. Besides, SA was also utilized for discovery and global profiling of novel compounds from Curcuma longa140. After targeted detection of 846 terpecurcumins by 12 NL/PIS, principle component analysis (PCA) was used for discrimination of different structures and recognition of potential novel compounds by clustering similar NL/PIS patterns and differentiating distinct NL/PIS patterns. In another example of profiling phenylethanoid glycosides from Magnolia officinalis141, recognition models of partial least square-discriminant analysis (PLS-DA) were established to discriminate both the stereoisomers and the positional isomers using MS2 data of the reference standards, and product ions contributing the most to isomer recognition were picked out according to the ranking order of their VIP values. Finally, PCA analysis was conducted for discrimination of isomers in samples and verification of the selected discriminant ions.
3.9. Database matching
Since manual analysis of massive and informative data generated by LC−MS is a laborious task, which requires a tremendous amount of time and effort, and often generates non-reproducible results, database matching (DM) can significantly improve the efficiency via computer-aided extensive annotation of compounds in complex matrices, and is widely applied in identification of a variety of compounds. DM was accomplished by comparing the exact mass and fragments between the detected compounds and the recorded items in the database. These databases generally include chemical databases using accurate mass or molecular formula, and MS/MS spectral databases such as MassBank142, METLIN143, mzCloud144, HMDB, etc. Since the applications of some open-access online-databases in identification of compounds have already been described in several published reviews145, 146, 147, 148, this review mainly focuses on the in-house databases used in identification of TCM constituents.
Some intelligent software platforms from different manufacturers, including UNIFI23,149, 150, 151, 152, 153, 154, 155, 156, Progenesis QI157, MetWorks158, Compound Discoverer159, SURIUS160, MassHunter161 and PCDL Manager162, play an important part in alleviating the labor for mining structural information from large-scale datasets, by integrating various automated data processing steps. They usually provide accessibility to various online databases and allow creation of a customized database as well, facilitating automatic profiling and identification of chemical components in TCM and more complex TCM formulas155. For example, to rapidly elucidate structures of lanostane analogs and isomers in Poria cocos, LC−IM−MSE data were post-processed with the assistance of UNIFI software, a targeted compound database and a key MS database, resulting in identification of 121 lanostanes23. In a bid to expand the screening coverage of novel compounds in the leaves of P. notoginseng, a predicted metabolites screening approach was implemented in UNIFI, by using four modified groups and one or two steps of modification, increasing the theoretical coverage by 14 times, and finally 945 ginsenosides were discovered, including 662 potential novel ginsenosides154. Another strategy was developed to systematically profile lipids in three congeneric Panax species by searching full MS and fragments information against HMDB and LIPID MAPS, and collision cross section (CCS) information against Metabolic Profiling CCS Library (incorporated in Progenesis QI) and LipidCCS Predictor software157.
Besides, several self-developed automatic data-processing tools can also increase the chemical coverages of traditional databases by creation of in-house databases, enhancing the efficiency and reproducibility in characterization of compounds in TCM. FlavonQ was developed for characterization of flavone and flavonol glycosides by calculation and combination of the common substituted groups of the core structures, and the results obtained by FlavonQ proved to be consistent with those determined conventionally by a sophisticated chemist, but greatly facilitated the data interpretation process in flavonoid research163. Another custom-built software PlantMAT (Plant Metabolite Annotation Toolbox), was also developed for identification of saponins and glycosylated flavonoids by running through various glycosyl, and acyl groups in every possible combination164.
4. Integrated analytical strategies
In majority of the cases, single analytical method is insufficient for the comprehensive characterization of the complex constituents in TCM, since each technique has its own strength and limitations. Therefore, development of integrated analytical strategies is necessary for rapid, accurate and systematic chemical analysis of TCM. The integrated strategies can be developed by integration of different MS instrumentations, or integration of various data acquisition or/and data post-processing techniques, laying the solid foundation for interpretation of the chemical diversity of TCM.
High-resolution mass spectrometers (such as QTOF) with accurate mass measurement of ions are advantageous for untargeted qualitative analysis of TCM. However, extraction of corresponding precursor and product ions from the total ions chromatograms (TICs) could be time-consuming and labor-intensive. On the contrary, QTRAP-MS show its advantages in sensitive detection of target compounds especially co-eluted minor components in TCM by using multiple targeted scanning modes such as MRM, and an EPI scan can also be triggered to acquire fragmentations of the preferred precursor ions. Therefore, QTRAP could act as a complementary tool of QTOF despite of its low resolution. Combination of MS/MS functions of both QTOF and QTRAP have been applied in systematic characterization and targeted identification of triterpenes in Alismatis Rhizoma and the corresponding processed products61, curcuminoids in turmeric63, naphthoquinones in Juglans cathayensis dode53, as well as minor components in TCM formulas such as Baoyuan decoction155.
The integrated use of different DDA techniques is also powerful in identifying unknown trace compounds in TCM. Take QTRAP as an example, it possesses the scan capacities of both QqQ and linear ion trap (LIT), involving MIM, MRM, NLS, PIS, enhanced scans such as EPI and enhanced mass spectrum (EMS), as well as some combined functions, such as PIS-IDA-EPI. By the combined use of MIM-IDA-EPI and PIS-IDA-EPI modes, 41 coumarins were identified from Radix Glehniae54. Besides, integration of different triggered IDA-EPI scans, including those triggered by EMS, NLS, MRM, and PIS scans, lead to the detection of 513 components from Cistanche deserticola and C. tubulosa55. In the study of chemical profiling of Baoyuan decoction, the stepped MIM-EPI and predefined MRM-EPI data acquisition methods were established to pick out potential saponins based on the MS behaviors of authentic compounds, while MIM-EPI and PIS-EPI scanning modes were adapted to comprehensively recognize flavonoids based on various diagnostic product ions, and thus the co-eluted chromatographic peaks were easily distinguished and extracted155.
Compared with individual filtering techniques, integration of different data post-processing techniques also exhibits higher efficiency in classifying multi-type compounds, excluding interference ions, as well as detecting the minor or trace components in TCM. It is illustrated by a strategy integrating different filtering techniques, including MDF and nitrogen rule filtering (NRF) based on MS information, and DIF and NLF based on MS/MS information, to identify two types of components in Artemisiae Argyi Folium, including chlorogenic acids and methoxylated flavonoids82. Results showed that none of a single technique could totally eliminate the interfering ions, whereas the integration of MS and MS/MS-based filtering techniques exerted a complementary effect to remove more interfering ions and expose target compounds. In another example for rapid identification of chemical constituents in Yindan Xinnaotong soft capsule, MDF and DIF were applied to preliminarily classify detected compounds into different chemical families, while NLF were used for characterization of flavonoid glycosides and triterpene saponins substituted with certain sugar units109.
Intelligent DDA techniques allow targeted acquisition of MS/MS or MSn data, improving the sensitivity and selectivity in detection of attractive compounds, while various data post-processing techniques can further simplify the data interpretation process. Therefore, combined approaches which integrate targeted DDA and various data post-processing techniques have great advantages in detection and identification of target components in TCM. A strategy based on LTQ-Orbitrap following offline two-dimensional LC separation was established to systematically analyze five botanical origins of Uncariae Ramulus Cum Unicis, and lead to the ultimate characterization of 1227 indole alkaloids84. In the data acquisition process, a theoretical step-wise PIL (mass range: 310–950 Da; step size: 2 Da) and an optimal parent mass width (±55 mDa), was defined to selectively trigger fragmentations of potential alkaloids, namely step-wise PIL-based raster-MDF scan method. Additionally, different data post-processing techniques were also combinedly used to facilitate structural elucidation, including elemental composition analysis to remove false positives, DIF for subtype classification, DM and fragments annotation for structure characterization. In another example, 537 components were characterized in Lonicerae Japonicae Flos, including an enormous number of new structures36. To reduce the redundant scans and increase the selectivity, an EL was first generated by a two-step polygonal MDF and then used for triggering data dependent-MS/MS scan. To rapidly annotate the known compounds and elucidate those unreported ones, DM and MN were both adopted for the data interpretation process.
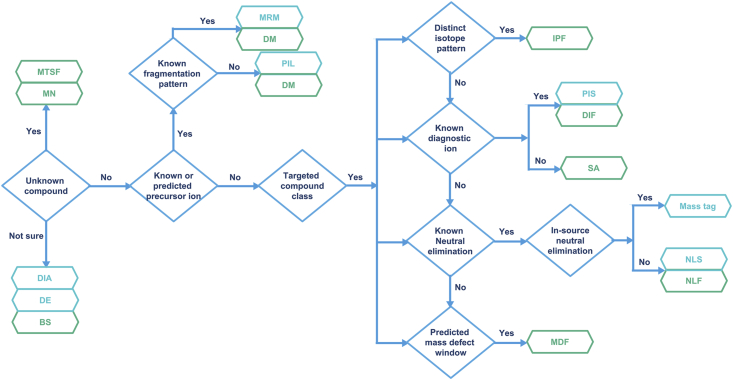
All the above-mentioned applications of LC−MS in TCM qualitative analysis were summarized in Table 1. Besides, the selection criteria of each data acquisition and data post-processing technique were also listed in Table 2, including the advantages, limitations, and scope of applications, providing valuable reference for future research on characterization and identification of chemical constituents in TCM. In addition, a decision tree in Fig. 2 also explained how to select appropriate data acquisition/post-processing methods based on the known chemical information, which may help the readers’ practical adoption of the above methods.
Table 1.
Applications of LC−MS in TCM qualitative analysis.
| TCM sample | Mass analyzer | Data acquisition technique | Data post-processing technique | Characterization result | Ref. |
|---|---|---|---|---|---|
| Acanthopanax senticosus | Q-TOF | MSAll; SWATH | DIF | 89 triterpene saponins, along with 14 sapogenins, including 33 potentially new compounds and the first report of malonyl-saponin in genus Acanthopanax | 22 |
| Poria cocos | Q-TOF | IMS-MSE | DM; NLF | 121 lanostane-type triterpene acids, including three new compounds | 23 |
| Yi-Xin-Shu capsule | Q-TOF | PIL-MS/MS | DIF | 276 compounds, including 50 lignans, 30 tanshinones, 4 lactones, 18 flavones, 14 phenolicacids, 1 triterpenoid acid, 3 ophiopogonins, 7 astragalus saponins, and 149 ginsenosides | 24 |
| Erzhi pill | Q-Orbitrap | Full MS/PIL/dd-MS2; “If idle-pick others” (IIPO); DE | MDF; NLF; DIF | 146 components, including 25 triterpenes, 31 flavonoids, 46 iridoids, 16 phenylethanols, 3 coumarins, 12 phenols, and 13 others | 25 |
| Uncaria sinensis (stem, leaf, and flower) | LTQ-Orbitrap | PIL-DE-CID/MS2-HCD/MS3 | BS; MDF; DM | 158 potentially new alkaloids, including 10 unknown but predicted and 108 unexpected ones | 26 |
| Three different parts (the root, stem leaf, and flower bud) of Panax quinquefolius L. | Q-Orbitrap | Full MS/PIL/IIPO/dd-MS2; DE | MDF | 347 saponins (147 from root, 173 from stem leaf, and 195 from flower bud), and 157 thereof not ever-isolated from the Panax genus | 27 |
| Puerarin lobate (Gegen) | LTQ-Orbitrap | PIL; DE | NLF | 66 compounds, including 43 malonates of isoflavone glycoside | 28 |
| Panax ginseng, P. quinquefolius, and P. notoginseng | LTQ-Orbitrap | ISCID-FS; PIL-HCD-MS/MS; DE | NLF | 101 malonyl-ginsenosides, including 69 from P. ginseng, 52 from P. quinquefolius, and 44 from P. notoginseng | 29 |
| Eleutherococcus senticosus leaves | LTQ-Orbitrap | HCD-MS2; PIL-CID-MS4; FS-MS | MN; DM | 106 triterpene saponins including 49 potentially new ones | 30 |
| Carthamus tinctorius L. (safflower) | Q-Orbitrap | full MS/PIL/dd-MS2; IIPO; DE | Retention behavior; Reference compounds comparison; Elemental composition analysis; Fragmentation pathways interpretation; DM | 13 primary metabolites (1 nucleoside, 2 sugars, 5 organic alkali/acids, and 5 amino acids) and 135 secondary metabolites (97 quinochalcone C-glycosides and 38 flavonoids) | 31 |
| Ligustri Lucidi Fructus | Q-Orbitrap | Full MS/PIL/dd-MS2; IIPO; DE; Polarity switching | Reference compounds comparison; Elemental composition analysis; Fragmentation pathways analysis; DM | 158 components, including 137 in the negative mode and 21 in the positive mode | 32 |
| Citrus reticulata Blanco (leaves) | LTQ-Orbitrap | FS-PIL-DE | DIF | 135 polymethoxylated flavonoids, including 81 polymethoxyflavones, 54 polymethoxyflavanones or polymethoxychalcones | 33 |
| Salvia miltiorrhiza | LTQ-Orbitrap | PIL-MSn; DE | Predicted compounds filtering; Total ion chromatogram filtering; Fragment ion search | 190 polymeric phenolic acids, including 18 first detected ones | 34 |
| Lonicerae Japonicae Flos | Q-Obitrap | EL-dd-MS2 | DM; MN | A total of 537 compounds, including a large number of potential novel structures | 36 |
| Venenum bufonis | LTQ-Orbitrap | FS-DE-MS1; HCD-MS2 (−); CID-MS2 (−); CID-MS3 (−); Product ion-MS4 (−); CID-MS2 (+); NL-MS3 (+) | – | 78 dicarboxylic acid conjugated bufotoxins, including 68 new compounds, 25 types of substructure formulas and seven dicarboxylic acid side chains | 38 |
| Roots, leaves, and flower buds of Panax ginseng, P. quinquefolius, and P. notoginseng | LTQ-Orbitrap | ISCID-FS-MS1; Mass tag/CID-MS2; NLS/HCD-MS3; DE | – | 178 malonylginsenosides | 41 |
| Panax ginseng, Panax quinquefolius, and Panax notoginseng | LTQ-Orbitrap | ISCID-FS-MS1; Mass tag/CID-MS2; Product ion scan/CID-MS3; DE | NLF | 216 carboxyl-free ginsenosides | 42 |
| Processed Aconitum roots (Shengfuzi, Heishunpian, Baifupian, Zhichuanwu, and Zhicaowu) | QTOF; QqQ | PIS | – | 24 benzoyl-containing alkaloids | 46 |
| Cynara scolymus L. | Q-TRAP | PIS | – | 10 caffeoylquinic acid derivatives | 47 |
| Tussilago farfara L. (buds) | QqQ; QTOF | PIS; Product ion scan | – | 74 oplopane- and bisabolene- sesquiterpenoids, and isolation of 11 compounds | 48 |
| Glycyrrhiza yunnanensis | QqQ; QTOF | NLS; PIS | – | 50 triterpene saponins | 49 |
| Marsdenia tenacissima (Roxb.) Wight et Arn. | QqQ | PIS; FS; Product ion scan | DIF; NLF | 110 polyoxypregnane and its glycosides | 50 |
| Nicotiana tabacum | QqQ | Two-step PIS | – | 17 flavonoids, including 9 newly identified ones | 51 |
| Danhong injection | QTRAP | EMS-IDA-EPI; PIS-IDA-EPI | DM | 90 compounds, including 46 salvianolic acids and related phenolic compounds | 52 |
| Juglans cathayensis | QTRAP; QTOF | EMS-EPI; Stepwise MIM-EPI; PIS-EPI | NLF | 48 naphthoquinones including 24 novel ones | 53 |
| Radix Glehniae | QTRAP | MIM-IDA-EPI; PIS-IDA-EPI | – | 41 coumarins | 54 |
| Cistanche deserticola and C. tubulosa | QTRAP | EMS-IDA-EPI; Predefined MRM-IDA-EPI; PIS-IDA-EPI; NL-IDA-EPI | DM | 513 components detected, including 379 annotated compounds | 55 |
| Tobacco leaves | QqQ | NLS; Product ion scan | DM | 64 glycosides, including 39 glycosides linked with monosaccharides, 18 glycosides linked with disaccharides and 7 glycosides linked with trisaccharides | 56 |
| Astragali Radix (Huangqi) | QTRAP | MIM-EPI; PI-EPI; NL-EPI; MRM-EPI | Extracted ion chromatogram; DIF; NLF | 421 flavonoids | 57 |
| Astragali Radix | QTRAP | NL/PIS-IDA-enhanced resolution-EPI | – | 136 triterpenoid saponins | 58 |
| Flaveria (F. robusta, F. pringlei, F. linearis, F. anomala, F. australasica, and F. bidentis) seeds | QTRAP | NLS | – | 27 sulfated flavonoids | 59 |
| Carthamus tinctorius L. | Ion trap | FS; MSn; MRM | DIF; NLF | 77 flavonoid glycosides | 60 |
| Alismatis Rhizoma and processed Alismatis Rhizoma | QTOF; QTRAP | FS; MS/MS; MRM | DIF; NLF | 80 triterpenes including 14 novel compounds; 7 more triterpenes compounds in the processed Alismatis rhizoma | 61 |
| Isodon serra | QTRAP | MRM-IDA-EPI | – | 27 components, including eighteen diterpenoids, six phenolic acids, and three flavonoids | 62 |
| Turmeric | TripleTOF; QTRAP | CID-MS/MS; MRM-EPI | BS; DIF; Ring double bond equivalents calculation | 96 curcuminoids | 63 |
| Ginseng, American ginseng, and processed products | QTRAP | Step-wise MRM-IDA-EPI; sMRM | PCA | 221 ginsenosides, including 185 annotated ones | 64 |
| Shenfu injection | QTRAP | Predefined MRM-IDA-EPI; Step-wise MIM-IDA-EPI | DM | 157 detected hydrophilic compounds, and 154 of which were identified as amino acids, nucleosides, organic acid, carbohydrates, etc.; 40 primary hydrophilic and hydrophobic ingredients including 11 amino acids, 9 nucleosides, 9 aconite alkaloids, and 11 ginsenosides | 69 |
| Carthamus tinctorius L. | LTQ-Orbitrap | FS-MS1; CID-MS2; CID-MS3; DE-HCD-MS/MS | DIF; Ring double bond equivalent; Characteristic UV absorption; Diagnostic product ions analysis | 163 quinochalcone C-glycosides homologs, including 149 potential new ones | 70 |
| Carthamus tinctorius L. | LTQ-Orbitrap | CID-MS1-MS2-MS3; DE-HCD-MS1-MS2 | DIF; NLF; DM | 107 flavonoids, including 80 newly reported flavonoid O-glycosides | 71 |
| Ophiopogon japonicus | Q-TOF; IT-TOF | FS; MS1-MS2-MS3 | MDF | More than 50 ophiopogonins and 27 ophiopogonones | 77 |
| Yin Chen Si Ni Tang | Q-TOF | MSE | MDF | 62 Aconitum alkaloids | 78 |
| Uncaria rhynchophylla | LTQ-Orbitrap | DE-CID-MS4 | MDF; NLF; DIF; DM | 92 alkaloids (60 free alkaloids and 32 alkaloid O-glycosides), 56 of which were potential new alkaloids for the Uncaria genus | 79 |
| Processed Semen Strychni | IT-TOF | FS; MS2 and MS3 | MDF; DIF | 24 dihydroindole-type alkaloids, including 4 that were previously not described | 80 |
| Citrus reticulata Blanco | LTQ-Orbitrap | MS and MS/MS | MDF; DIF | 81 polymethoxylated flavonoids, including 50 polymethoxyflavones and 31 polymethoxyflavanones or polymethoxychalcones | 81 |
| Folium Artemisiae Argyi | Q-Orbitrap | FS; MS/MS | NRF; MDF; NLF; DIF | 16 methoxylated flavonoids and 55 chlorogenic acids analogues | 82 |
| Panax notoginseng (Sanqi) | Q-TOF | MS; Auto MS/MS; targeted MS/MS | MDF; Characteristic isotopic distribution | 234 ginsenosides including 67 potential new ones | 83 |
| Five botanical origins of Uncariae Ramulus Cum Unicis | LTQ-Orbitrap | DE-step-wise PIL-CID/MS2-HCD/MS3 | Elemental composition analysis; NLF; DIF; DM | 1227 indole alkaloids | 84 |
| Er-xian decoction | LTQ FT | FS; DE-CID-MS2; CID-MS3 | MTSF; Discriminant analysis; BS | 553 potential compounds and 66 candidates | 87 |
| Arnebiae Radix | Q-Orbitrap | Full MS; MS/MS | BS; DM; MDF | 96 compounds, 13 of which were confirmed by the reference standards, 30 with a low abundance, and 9 unknowns | 88 |
| Pueraria lobata (Willd.) Ohwi (Gegen) and Pueraria thomsonii (Fenge) | LTQ-Orbitrap | FS; DDA | IPF | 12 S-derivatives were found, 9 of which were tentatively identified | 92 |
| Forsythia suspensa | Q-TOF | MSE; CID-MS/MS | DIF | 8 potentially new C6–C2 glucoside conjugates | 93 |
| Zicao | Q-TOF | MS; MS/MS | BS; DIF; Characteristic product ion filtering | 58 compounds including 32 novel ones | 94 |
| Akebiae Fructus | Q-TOF | MSE | Adduct ion filtering; DIF; Characteristic ion filtering; NLF | 94 compounds (85 triterpenoid saponins and 9 chlorogenic acids), including 9 types of triterpenoid saponins and 2 types of chlorogenic acid; 50 newly discovered constituents | 95 |
| Spatholobus suberectus | Q-TOF | FS in both positive and negative modes; (−)-MS/MS | DM; DIF | 38 compounds, including 36 flavonoids and 2 non-flavonoid compounds | 96 |
| Scutellaria baicalensis | Q-Orbitrap | FS; MS/MS | DIF | 132 compounds, 59 of which were reported for the first time | 97 |
| Amphimas pterocarpoides | LTQ-Orbitrap | FS; MS/MS | DIF | 21 molecules, 17 of which were found to belong to the targeted chemical groups, including 8 isoflavones, 7 dihydroisoflavones and 2 isoflavone glycosides | 98 |
| Roots and berries of raw and steamed American ginseng (P. quinquefolius L.) | TOF; Q-TOF | FS; CID-MS/MS | DIF | 70 saponins | 99 |
| Fresh and processed ginseng | Q-TOF | FS; CID-MS/MS | DIF; NLF | 12 malonyl ginsenosides | 100 |
| Uncaria rhynchophylla | Q-TOF | MS; MS/MS | DIF | A total of 29 compounds, comprising 18 alkaloids, 6 flavonoids, and 5 quinic acids; 4 novel tetracyclic monoterpenoid oxindole alkaloids (TMOAs) | 101 |
| Lycoris spp. (Amaryllidaceae) | Q-TOF | MS; MS/MS | DIF | 37 lycorine-type alkaloids, including 16 previously undescribed compounds | 102 |
| Calyx of Physalis alkekengi L. var. franchetii (Mast.) Makino | Q-TOF | MS; MS/MS | DIF; NLF | 46 physalins, including 20 novel ones | 103 |
| Glechomae Herba | Q-TOF | MS; MS/MS | DIF; NLF | 120 compounds, including 10 chlorogenic acids, 10 gallic acids, 21 phenylpropionic acids and 77 flavonoids; 65 newly discovered constituents; 4 types of chlorogenic acids, 3 types of galloylglucoses, 3 types of phenylpropionic acid skeletons, and 5 types of flavonoid aglycone skeletons | 104 |
| Raw and processed pieces of Rheum palmatum L. | Q-TOF | (−)-MS; (−)-MS/MS | DM; DIF | 73 characteristic markers | 105 |
| Garcinia oblongifolia | QTOF | MS/MS; ISCID MS/MS; ISCID combined with TAP fragmentation; IM-MSE; IM-MS/MS | DIF | 140 potential polycyclic polyprenylated acylphloroglucinols (PPAPs), including 7 pairs coeluting isobaric PPAPs that were indistinguishable by conventional UHPLC−HRMS alone | 106 |
| Shengmai injection | IT-TOF | MS1, MS2, MS3 | DIF | More than 30 ginsenosides and 20 lignans | 107 |
| Guge Fengtong tablet | Q-TOF | FS | DIF | 47 components, including 18 phenolic acids, 8 saponins, 14 gingerol-related compounds, and 7 diarylhepatonoids | 108 |
| Yindan Xinnaotong soft capsule | Q-TOF; QqQ | FS; MRM | DM; DIF; MDF; NLF | 124 compounds, including 44 flavonoids, 31 phenolic acids, 20 tanshi-nones, 16 saponins, 6 ginkgolides, 5 sulfides, camphor and borneol | 109 |
| Roots of Aconitum carmichaelii Debx. | LTQ-Orbitrap | FS; CID-MS2-MS3 | NLF | 42 diester-diterpenoid alkaloids; 23 potential new compounds, including 16 short chain fatty acyls diester-diterpenoid alkaloids, 4 N-dealkyl diester-diterpenoid alkaloids and several isomers of aconitine, mesaconitine and hypaconitine | 112 |
| Saussurea involucrata | LTQ FT | DE-CID-MS3 | MTSF | 38 compounds, including 19 flavones, 11 phenylpropanoids and 8 sphingolipids; among them, 7 flavonoids, 8 phenylpropanoids and 8 sphingolipids were identified for the first time in Saussurea involucrate | 114 |
| Duhaldea nervosa (Wallich ex Candolle) A. Anderberg | LTQ-Orbitrap | FS; CID-MS2; DE | MTSF | 47 chlorogenic acids, including 19 monoacyl-quinic acids, 22 diacyl-quinic acids, and six triacyl-quinic acids | 115 |
| Flos Lonicerae Japonicae | LTQ-Orbitrap | FS; FS-PIL; MIM | DM; MTSF; DIF | 115 chlorogenic acids attributed to 18 categories, most of which were newly reported | 116 |
| Xiao-Xu-Ming decoction | LTQ FT | FS; DE-CID-MS2; CID-MS3 | MTSF | 68 compounds among 3362 detected compounds, including 14 templated compounds (reference compounds), 50 related compounds fished by MTSF technique, and 4 unrelated compounds identified by manual method | 117 |
| Venenum Bufonis | Q-TOF | Fast DDA | MN | 229 bufadienolides, including two subclasses of compounds (bufogeninsconjugated with carboxylic acid and N-heterocyclic bufogenins) which were found in Venenum Bufonis for the first time | 137 |
| Aconiti Lateralis Radix Praeparata and its processed products | Q-TOF | MSE | MN | 145 diterpenoid alkaloids, including 78 C19 (19 diester-diterpenoid alkaloids, 20 monoester-diterpenoid alkaloids, 7 lipo-diterpenoid alkaloids, 32 amine-diterpenoid alkaloids), 13 C20, and 54 other diterpenoid alkaloids | 138 |
| Danhong injection | Q-Orbitrap | FS | SA | 117 compounds, including 76 phenolic acids, 20 flavonoids, and 21 other compounds | 139 |
| Curcuma longa | QqQ | NL/PIS | SA | 846 terpecurcumins (terpene-conjugated curcuminoids), including a number of potentially novel compounds | 140 |
| Magnolia officinalis | Q-TOF | MS; MS/MS | DIF; NLF; SA | 87 phenylethanoid glycosides, including 14 isomers | 141 |
| Ziziphi Spinosae Semen and Ziziphi Mauritianae Semen | Q-TOF | MSE | DM; DIF; NLF | 60 target components in Ziziphi Spinosae Semen, including 27 flavonoids, 16 saponins, 10 alkaloids, 6 terpenes, and 1 other, and 53 nontarget components with 40 new deduced components; 132 chemical components in Ziziphi Mauritianae Semen, including 7 additional nontarget new components | 149 |
| Peanut stems and leaves | Q-TOF | MSE | DM | 283 chemical compounds, including 207 new compounds | 150 |
| White and red ginsengs | Q-TOF | IM-MSE; IM-MS/MS | DM | 201 ginsenosides, including 10 pairs of co-eluting isobaric ginseng saponins | 151 |
| Stems and leaves of Panax ginseng | LTQ-Orbitrap | DE-CID-MS3; DE-HCD-MS2 | NLF; DIF; DM | 646 ginsenosides, including 427 which have not been isolated from the genus of Panax L. | 152 |
| Five different parts (root, leaf, flower bud, berry, and seed) of Panax ginseng | Q-TOF | MSE | DM | 164 compounds | 153 |
| Panax notoginseng | Q-TOF | Fast DDA | DM; DIF; NLF | 945 ginsenosides, including 662 potentially novel ginsenosides | 154 |
| Baoyuan decoction | Q-TOF; QTRAP | FS-MS1; MSE; Predefined MRM-EPI, MIM-EPI, and PIS-EPI | DM; DIF | 236 compounds, including 139 saponins, 83 flavonoids, 6 procyanidins, 4 lignans, and 4 diterpenes | 155 |
| Paris polyphylla Smith var. yunnanensis (Franch.) Hand. -Mazz and Paris vietnamensis (Takht.) H. Li | Q-TOF | MSE | DM | 146 metabolites, including 42 potential new compounds | 156 |
| Panax ginseng, P. quinquefolius, and P. notoginseng | Q-TOF | MSE; HDMSE | DM | 24 potential lipid markers enabling the simultaneous differentiation of three Ginseng species | 157 |
| Cistanche deserticola, C. sinensis, and C. tubulosa | LTQ-Orbitrap | FS-HRMS; MS/MS; DE | DM; Parent mass modification search; DIF; MTSF | 69 phenylethanoid glycosides, including 17 new ones | 158 |
| Aconitum carmichaelii Debx. | Q-TOF | MS; MS/MS | DM | 148 lipo-alkaloids, including 93 potential new compounds and 38 compounds with oxygenated fatty acid moieties | 161 |
| Gelsemium elegans | Q-TOF | MS/MS | DM | 57 components, including 43 alkaloids, 9 iridoids, 2 steroids, 2 phenolic acids and 1 coumarin | 162 |
Table 2.
Selection criteria of data acquisition and post-processing techniques in TCM qualitative analysis.
| Technique | Advantage | Limitation | Scope of application | |
|---|---|---|---|---|
| DIA | DIA | Full coverage of MS1 and MS2 information | Loss link between precursor and product ions | Acquisition of MS/MS information of minor or trace compounds |
| DDA | PIL | Screening of only (or preferred) target ions in the list | Miss of novel compounds with new sub-structures or substituted groups | Target compounds with predictable molecular weights and/or m/z values |
| DE | Detection of less abundant co-eluting ions | Quality loss of MS2 information of abundant ions | MS/MS information of both abundant and less abundant compounds in complex samples | |
| Mass tag | Selective screening of compounds that undergo in-source fragmentations | Not applicable to relatively stable compounds that cannot generate certain in-source fragmentation patterns | Compounds that can undergo certain in-source fragmentations with ISCID energy | |
| PIS | Requirements of little prior-knowledge about the exact structure of target compounds | Miss of compounds without selected product ions | Compounds with known specific product ions | |
| NLS | Targeted screening of modified or conjugated compounds that undergo neutral eliminations | Only compounds with certain neutral eliminations | Compounds with certain neutral eliminations | |
| MRM | High selectivity and sensitivity in screening target compounds | Low resolution and unable to screen unknown compounds without information of parent and product ions | Target compounds with known parent and product ions | |
| Data post-processing | MDF | Selective screening of certain compounds or compound classes and remove of interferences | Requirements of a carefully-designed appropriate MDF region | Compounds with predictable mass range and mass defect range |
| BS | Elimination of background signals, and exposure of target compounds | Poor selectivity of target compounds | Severe background interferences | |
| IPF | Specific screening of compounds with certain elements | Relatively narrow application scope | Compounds with distinct isotope pattern | |
| DIF | Rapid screening of target compounds that produce identical product ions | Miss of compounds without selected product ions | Compounds with known product ion | |
| NLF | Compounds that undergo neutral eliminations | Only compounds with certain neutral eliminations | Compounds with specific neutral loss | |
| MTSF | Rapid classification and characterization of unknown compounds | Need of MSn information for both template compounds and target compounds | Target compounds sharing the same sub-structures and similar MSn mass spectra as template compounds | |
| MN | Rapid classification and characterization of compounds without prior knowledge of the chemical structures | Manual interpretation of detailed structure information of unknown compounds based on the related known compounds | Compounds with MS/MS spectra | |
| SA | Rapid exploration of intrinsic relationships between compound structures and MS data | Further characterization and identification of compounds | Recognition of potential characteristic ions, discrimination of different structures and isomers | |
| DM | Alleviation of labor for mining structural information from large-scale datasets | Limited records in databases and requirements of further manual confirmation | Automatic search and annotation of compounds through on-line or in-house databases | |
Figure 2.
A decision tree that represents a possible way to select an LC–MS based data acquisition/post-processing strategy.
5. Discussion and future perspectives
Despite of the continuous developments of advanced data acquisition and post-processing techniques in TCM qualitative analysis, there still remains drastic challenges to be solved to achieve the ultimate goal of comprehensive chemical profiling and annotation. Firstly, it is virtually impossible to simultaneously detect all the components in one experiment, due to the selectivity and priority of the analytical system. Therefore, arrays of separation techniques differing from conventional reversed-phase LC were developed and combined in either on-line mode or off-line mode165, including hydrophilic interaction liquid chromatography36,152,154, supercritical fluid chromatography137, etc. Besides, IM23,106,157 was also adopted to improve the MS performance. Secondly, to make the full use of typical DIA methods featured with minimum loss of MS information and good reproducibility, more complicated deconvolution and data processing techniques should be developed to further interpret the DIA data. Thirdly, manual annotation and interpretation of the MS data is still a dominant method used, which is labor-intensive and somewhat subjective. Databases of MS spectra of TCM ingredients combined with intelligent software may be an option. Though database search is anticipated to play a pivotal role in matching the known compounds, however, the comprehensive TCM databases of the known compounds are still in serious shortage, let alone the potentially new structures. Therefore, de novo annotation software, especially those considering the biosynthetic pathways may help increase the identification credibility. Fourthly, ambiguous differentiation and identification of the isomers hinder the further application of the acquired data. Common isomer identification strategies involve two aspects. One is associated with the liquid chromatographic separation by comparing the difference of retention time (RT), and various retention time prediction strategies have also been proposed to improve the identification reliability, including logKow (logP) prediction166, ACD/ChromGenius software prediction167, and some quantitative structure retention relationship (QSRR) models such as linear solvation energy relationship, multiple linear regression168, Kernel-based partial least-squares169, artificial neural network170, OPERA-RT model171, etc. The other one is based on the MS information, including fragmentation patterns and intensity of product ions. Besides, SA methods have been applied for recognition of isomers141, and some advanced techniques based on other physicochemical principles have also been explored. For example, the characteristic CE (CE50)172 values and optimal CE (OCE) values which corresponded to the dissociation of chemical bonds were proved to be helpful in isomeric differentiation173,174. In addition, some cutting-edge instrumentations such as LC–infrared ion spectroscopy MS have been introduced and applied to distinguish closely related isomers175, 176, 177.
In summary, based on the recent development and bottlenecks of LC−MS in TCM qualitative analysis, following suggestions are tentatively proposed for the future research. Firstly, because the bioactive components contained in TCM are usually secondary metabolites, which share similar substructures due to the common biosynthetic pathways, in order to achieve targeted characterization of different classes of compounds, more efficient and specific data acquisition and post-processing techniques based on their chemical characteristics, MS fragmentation patterns and other properties are in urgent demand. Next, resorting to the theory of big data analytics and rapid developments of computer science as well as mathematical statistics methods, exploration of high-throughput, high coverage and automatic data analysis strategies become possible. By developing computer and software-aided automatic data processing workflows, MS data interpretation efficiency of TCM complex systems will be dramatically improved. Last but not least, utilization of integrated analytical strategies, combing the advantages of various MS instrumentations, data acquisition and data post-processing techniques, are helpful for comprehensive and systematic analysis of chemical components of TCM.
6. Conclusions
TCM is gaining more and more global attentions because of its effectiveness in preventing and treating human diseases. LC−MS is one of the most powerful tools for TCM qualitative analysis. In this review, the basic principles and applications of a variety of advanced LC−MS-based data acquisition and post-processing techniques were summarized and illustrated for rapid and efficient characterization and identification of various chemical constituents in TCM. These data acquisition techniques include PIL, DE and mass tag based on MS information, PIS, NLS and MRM based on MS/MS information, and data post-processing techniques include MDF, BS, IPF, DIF, NLF, MTSF, MN, SA and DM, each one is indispensable with its unique superiority. Therefore, a brief introduction and applications of the integrated analytical strategies in TCM qualitative analysis were delivered. At last, discussions on the obstacles, possible solutions and future trends of TCM analysis were also provided, laying foundations for future research on the chemical basis of TCM.
Acknowledgments
This work was financially supported by National Key R&D Program of China (2018YFC1707900, 2019YFC1711000, and 2019YFC1711400), National Natural Science Foundation of China (82003938), State Key Program of National Natural Science Foundation of China (81530095), and Qi-Huang Scholar of National Traditional Chinese Medicine Leading Talents Support Program (2018).
Author contributions
Yang Yu wrote the paper. Changliang Yao edited the paper. De-an Guo conceived the review topic and edited the paper.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Luo L., Jiang J.W., Wang C., Fitzgerald M., Hu W.F., Zhou Y.M. Analysis on herbal medicines utilized for treatment of COVID-19. Acta Pharm Sin B. 2020;10:1192–1204. doi: 10.1016/j.apsb.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey A.L. Natural products in drug discovery. Drug Discov Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J., Jiang P., Zhang W.D. Molecular networks for the study of TCM pharmacology. Briefings Bioinf. 2010;11:417–430. doi: 10.1093/bib/bbp063. [DOI] [PubMed] [Google Scholar]
- 4.Wu H.F., Guo J., Chen S.L., Liu X., Zhou Y., Zhang X.P. Recent developments in qualitative and quantitative analysis of phytochemical constituents and their metabolites using liquid chromatography−mass spectrometry. J Pharmaceut Biomed Anal. 2013;72:267–291. doi: 10.1016/j.jpba.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Jin H.L., Liu Y.F., Guo Z.M., Wang J.X., Zhang X.L., Wang C.R. Recent development in liquid chromatography stationary phases for separation of Traditional Chinese Medicine components. J Pharmaceut Biomed Anal. 2016;130:336–346. doi: 10.1016/j.jpba.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Holčapek M., Jirásko R., Lísa M. Recent developments in liquid chromatography−mass spectrometry and related techniques. J Chromatogr A. 2012;1259:3–15. doi: 10.1016/j.chroma.2012.08.072. [DOI] [PubMed] [Google Scholar]
- 7.Ma S.G., Chowdhury S.K. Data acquisition and data mining techniques for metabolite identification using LC coupled to high-resolution MS. Bioanalysis. 2013;5:1285–1297. doi: 10.4155/bio.13.103. [DOI] [PubMed] [Google Scholar]
- 8.Stavrianidi A. A classification of liquid chromatography mass spectrometry techniques for evaluation of chemical composition and quality control of traditional medicines. J Chromatogr A. 2020;1609:460501. doi: 10.1016/j.chroma.2019.460501. [DOI] [PubMed] [Google Scholar]
- 9.Doerr A. DIA mass spectrometry. Nat Methods. 2015;12 35–35. [Google Scholar]
- 10.Zhou J.T., Li Y.H., Chen X., Zhong L.J., Yin Y.X. Development of data-independent acquisition workflows for metabolomic analysis on a quadrupole-orbitrap platform. Talanta. 2017;164:128–136. doi: 10.1016/j.talanta.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 11.Yang M., Sun J.H., Lu Z.Q., Chen G.T., Guan S.H., Liu X. Phytochemical analysis of traditional Chinese medicine using liquid chromatography coupled with mass spectrometry. J Chromatogr A. 2009;1216:2045–2062. doi: 10.1016/j.chroma.2008.08.097. [DOI] [PubMed] [Google Scholar]
- 12.Li M., Hou X.F., Zhang J., Wang S.C., Fu Q., He L.C. Applications of HPLC/MS in the analysis of traditional Chinese medicines. J Pharm Anal. 2011;1:81–91. doi: 10.1016/S2095-1779(11)70015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinmann D., Ganzera M. Recent advances on HPLC/MS in medicinal plant analysis. J Pharmaceut Biomed Anal. 2011;55:744–757. doi: 10.1016/j.jpba.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Ganzera M., Sturm S. Recent advances on HPLC/MS in medicinal plant analysis—an update covering 2011−2016. J Pharmaceut Biomed Anal. 2018;147:211–233. doi: 10.1016/j.jpba.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Sentandreu E., Peris-Díaz M.D., Sweeney S.R., Chiou J., Muñoz N., Tiziani S. A survey of orbitrap all ion fragmentation analysis assessed by an R MetaboList package to study small-molecule metabolites. Chromatographia. 2018;81:981–994. [Google Scholar]
- 16.Bateman K.P., Castro-Perez J., Wrona M., Shockcor J.P., Yu K., Oballa R. MSE with mass defect filtering for in vitro and in vivo metabolite identification. Rapid Commun Mass Spectrom. 2007;21:1485–1496. doi: 10.1002/rcm.2996. [DOI] [PubMed] [Google Scholar]
- 17.Gethings L.A., Richardson K., Wildgoose J., Lennon S., Jarvis S., Bevan C.L. Lipid profiling of complex biological mixtures by liquid chromatography/mass spectrometry using a novel scanning quadrupole data-independent acquisition strategy. Rapid Commun Mass Spectrom. 2017;31:1599–1606. doi: 10.1002/rcm.7941. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X.C., Chen Y.P., Subramanian R. Comparison of information-dependent acquisition, SWATH, and MSAll techniques in metabolite identification study employing ultrahigh-performance liquid chromatography−quadrupole time-of-flight mass spectrometry. Anal Chem. 2014;86:1202–1209. doi: 10.1021/ac403385y. [DOI] [PubMed] [Google Scholar]
- 19.van der Laan T., Boom I., Maliepaard J., Dubbelman A.C., Harms A.C., Hankemeier T. Data-independent acquisition for the quantification and identification of metabolites in plasma. Metabolites. 2020;10:514. doi: 10.3390/metabo10120514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopfgartner G., Tonoli D., Varesio E. High-resolution mass spectrometry for integrated qualitative and quantitative analysis of pharmaceuticals in biological matrices. Anal Bioanal Chem. 2012;402:2587–2596. doi: 10.1007/s00216-011-5641-8. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann A., Butcher P., Maden K., Walker S., Widmer M. Practical application of in silico fragmentation based residue screening with ion mobility high-resolution mass spectrometry. Rapid Commun Mass Spectrom. 2017;31:1147–1157. doi: 10.1002/rcm.7890. [DOI] [PubMed] [Google Scholar]
- 22.Xia Y.G., Gong F.Q., Guo X.D., Song Y., Li C.X., Liang J. Rapid screening and characterization of triterpene saponins in Acanthopanax senticosus leaves via untargeted MSAll and SWATH techniques on a quadrupole time of flight mass spectrometry. J Pharmaceut Biomed Anal. 2019;170:68–82. doi: 10.1016/j.jpba.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Feng G.F., Zheng Y., Sun Y., Liu S., Pi Z.F., Song F.R. A targeted strategy for analyzing untargeted mass spectral data to identify lanostane-type triterpene acids in Poria cocos by integrating a scientific information system and liquid chromatography−tandem mass spectrometry combined with ion mobility spectrometry. Anal Chim Acta. 2018;1033:87–99. doi: 10.1016/j.aca.2018.06.048. [DOI] [PubMed] [Google Scholar]
- 24.Wang H.P., Chen C., Liu Y., Yang H.J., Wu H.W., Xiao H.B. Identification of the chemical constituents of Chinese medicine Yi-Xin-Shu capsule by molecular feature orientated precursor ion selection and tandem mass spectrometry structure elucidation. J Separ Sci. 2015;38:3687–3695. doi: 10.1002/jssc.201500698. [DOI] [PubMed] [Google Scholar]
- 25.Fu L.L., Ding H., Han L.F., Jia L., Yang W.Z., Zhang C.X. Simultaneously targeted and untargeted multicomponent characterization of Erzhi Pill by offline two-dimensional liquid chromatography/quadrupole-Orbitrap mass spectrometry. J Chromatogr A. 2019;1584:87–96. doi: 10.1016/j.chroma.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Pan H.Q., Yang W.Z., Yao C.L., Shen Y., Zhang Y.B., Shi X.J. Mass defect filtering-oriented classification and precursor ions list-triggered high-resolution mass spectrometry analysis for the discovery of indole alkaloids from Uncaria sinensis. J Chromatogr A. 2017;1516:102–113. doi: 10.1016/j.chroma.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Wang H.D., Zhang C.X., Zuo T.T., Li W.W., Jia L., Wang X.Y. In-depth profiling, characterization, and comparison of the ginsenosides among three different parts (the root, stem leaf, and flower bud) of Panax quinquefolius L. by ultra-high performance liquid chromatography/quadrupole-Orbitrap mass spectrometry. Anal Bioanal Chem. 2019;411:7817–7829. doi: 10.1007/s00216-019-02180-8. [DOI] [PubMed] [Google Scholar]
- 28.Yang M., Zhou Z., Yao S., Li S.R., Yang W.Z., Jiang B.H. Neutral loss ion mapping experiment combined with precursor mass list and dynamic exclusion for screening unstable malonyl glucoside conjugates. J Am Soc Mass Spectrom. 2016;27:99–107. doi: 10.1007/s13361-015-1240-9. [DOI] [PubMed] [Google Scholar]
- 29.Shi X.J., Yang W.Z., Qiu S., Yao C.L., Shen Y., Pan H.Q. An in-source multiple collision-neutral loss filtering based nontargeted metabolomics approach for the comprehensive analysis of malonyl-ginsenosides from Panax ginseng, P. quinquefolius, and P. notoginseng. Anal Chim Acta. 2017;952:59–70. doi: 10.1016/j.aca.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Ge Y.W., Zhu S., Yoshimatsu K., Komatsu K. MS/MS similarity networking accelerated target profiling of triterpene saponins in Eleutherococcus senticosus leaves. Food Chem. 2017;227:444–452. doi: 10.1016/j.foodchem.2017.01.119. [DOI] [PubMed] [Google Scholar]
- 31.Lu J.X., Zhang C.X., Hu Y., Zhang M.H., Wang Y.N., Qian Y.X. Application of multiple chemical and biological approaches for quality assessment of Carthamus tinctorius L. (safflower) by determining both the primary and secondary metabolites. Phytomedicine. 2019;58:152826. doi: 10.1016/j.phymed.2019.152826. [DOI] [PubMed] [Google Scholar]
- 32.Li M.R., Wang X.Y., Han L.F., Jia L., Liu E.W., Li Z. Integration of multicomponent characterization, untargeted metabolomics and mass spectrometry imaging to unveil the holistic chemical transformations and key markers associated with wine steaming of Ligustri Lucidi Fructus. J Chromatogr A. 2020;1624:461228. doi: 10.1016/j.chroma.2020.461228. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J.Y., Wang Z.J., Zhang Q., Wang F., Ma Q., Lin Z.Z. Rapid screening and identification of target constituents using full scan-parent ions list-dynamic exclusion acquisition coupled to diagnostic product ions analysis on a hybrid LTQ-Orbitrap mass spectrometer. Talanta. 2014;124:111–122. doi: 10.1016/j.talanta.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y., Feng Z.J., Yang M., Zhou Z., Han S.M., Hou J.J. Rapid profiling of polymeric phenolic acids in Salvia miltiorrhiza by hybrid data-dependent/targeted multistage mass spectrometry acquisition based on expected compounds prediction and fragment ion searching. J Separ Sci. 2018;41:1888–1895. doi: 10.1002/jssc.201701134. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Feng R., Wang R., Yang F., Li P., Wan J.B. Enhanced MS/MS coverage for metabolite identification in LC−MS-based untargeted metabolomics by target-directed data dependent acquisition with time-staggered precursor ion list. Anal Chim Acta. 2017;992:67–75. doi: 10.1016/j.aca.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 36.Pan H.Q., Zhou H., Miao S., Cao J.Y., Liu J.M., Lan L. An integrated approach for global profiling of multi-type constituents: comprehensive chemical characterization of Lonicerae Japonicae Flos as a case study. J Chromatogr A. 2020;1613:460674. doi: 10.1016/j.chroma.2019.460674. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z.Q. Automated precursor ion exclusion during LC−MS/MS data acquisition for optimal ion identification. J Am Soc Mass Spectrom. 2012;23:1400–1407. doi: 10.1007/s13361-012-0401-3. [DOI] [PubMed] [Google Scholar]
- 38.Bi Q.R., Hou J.J., Yang M., Shen Y., Qi P., Feng R.H. A strategy combining higher energy C-trap dissociation with neutral loss- and product ion-based MSn acquisition for global profiling and structure annotation of fatty acids conjugates. J Am Soc Mass Spectrom. 2017;28:443–451. doi: 10.1007/s13361-016-1558-y. [DOI] [PubMed] [Google Scholar]
- 39.Yan Z.Y., Caldwell G.W., Maher N. Unbiased high-throughput screening of reactive metabolites on the linear ion trap mass spectrometer using polarity switch and mass tag triggered data-dependent acquisition. Anal Chem. 2008;80:6410–6422. doi: 10.1021/ac800887h. [DOI] [PubMed] [Google Scholar]
- 40.Hsiao H.H., Urlaub H. Pseudo-neutral-loss scan for selective detection of phosphopeptides and N-glycopeptides using liquid chromatography coupled with a hybrid linear ion-trap/orbitrap mass spectrometer. Proteomics. 2010;10:3916–3921. doi: 10.1002/pmic.201000290. [DOI] [PubMed] [Google Scholar]
- 41.Shi X.J., Yang W.Z., Huang Y., Hou J.J., Qiu S., Yao C.L. Direct screening of malonylginsenosides from nine Ginseng extracts by an untargeted profiling strategy incorporating in-source collision-induced dissociation, mass tag, and neutral loss scan on a hybrid linear ion-trap/Orbitrap mass spectrometer coupled to ultra-high performance liquid chromatography. J Chromatogr A. 2018;1571:213–222. doi: 10.1016/j.chroma.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 42.Yang W.Z., Shi X.J., Yao C.L., Huang Y., Hou J.J., Han S.M. A novel neutral loss/product ion scan-incorporated integral approach for the untargeted characterization and comparison of the carboxyl-free ginsenosides from Panax ginseng, Panax quinquefolius, and Panax notoginseng. J Pharm Biomed Anal. 2020;177:112813. doi: 10.1016/j.jpba.2019.112813. [DOI] [PubMed] [Google Scholar]
- 43.Marcos J., Pozo O.J. Current LC−MS methods and procedures applied to the identification of new steroid metabolites. J Steroid Biochem Mol Biol. 2016;162:41–56. doi: 10.1016/j.jsbmb.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Tang C.M., Tang C.X., Zhan W., Du J., Wang Z.F., Peng X.Z. Strategies for ascertaining the interference of phase II metabolites co-eluting with parent compounds using LC–MS−MS. J Separ Sci. 2013;36:2584–2592. doi: 10.1002/jssc.201300235. [DOI] [PubMed] [Google Scholar]
- 45.Chao H.C., Chen G.Y., Hsu L.C., Liao H.W., Yang S.Y., Wang S.Y. Using precursor ion scan of 184 with liquid chromatography−electrospray ionization-tandem mass spectrometry for concentration normalization in cellular lipidomic studies. Anal Chim Acta. 2017;971:68–77. doi: 10.1016/j.aca.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 46.Gao W., Liu X.G., Liu L., Li P., Yang H. Targeted profiling and relative quantification of benzoyl diterpene alkaloids in Aconitum roots by using LC−MS/MS with precursor ion scan. J Separ Sci. 2018;41:3515–3526. doi: 10.1002/jssc.201800149. [DOI] [PubMed] [Google Scholar]
- 47.Shen Q., Lu Y.B., Dai Z.Y., Cheung H.Y. Precursor ion scan driven fast untargeted screening and semi-determination of caffeoylquinic acid derivatives in Cynara scolymus L. Food Chem. 2015;166:442–447. doi: 10.1016/j.foodchem.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 48.Song K., Ha I.J., Kim Y.S. A strategy for identification and structural characterization of oplopane- and bisabolane-type sesquiterpenoids from Tussilago farfara L. by multiple scan modes of mass spectrometry. J Chromatogr A. 2019;1602:188–198. doi: 10.1016/j.chroma.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Ji S., Wang Q., Qiao X., Guo H.C., Yang Y.F., Bo T. New triterpene saponins from the roots of Glycyrrhiza yunnanensis and their rapid screening by LC/MS/MS. J Pharmaceut Biomed Anal. 2014;90:15–26. doi: 10.1016/j.jpba.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 50.Wu X., Zhu L., Ma J., Ye Y., Lin G. Adduct ion-targeted qualitative and quantitative analysis of polyoxypregnanes by ultra-high pressure liquid chromatography coupled with triple quadrupole mass spectrometry. J Pharmaceut Biomed Anal. 2017;145:127–136. doi: 10.1016/j.jpba.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 51.Li Y., Pang T., Shi J.L., Lu X.P., Deng J.H., Lin Q. Liquid chromatography with mass spectrometry method based two-step precursor ion scanning for the structural elucidation of flavonoids. J Separ Sci. 2014;37:3067–3073. doi: 10.1002/jssc.201400720. [DOI] [PubMed] [Google Scholar]
- 52.Li C., Yang J.H., Tong X., Zhao C., He Y., Wan H.T. Precursor ion scan enhanced rapid identification of the chemical constituents of Danhong injection by liquid chromatography–tandem mass spectrometry: an integrated strategy. J Chromatogr A. 2019;1602:378–385. doi: 10.1016/j.chroma.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 53.Gan Y., Zhang Y., Li A.Q., Song C.W., Chen C., Xu Y. A novel four-step approach for systematic identification of naphthoquinones in Juglans cathayensis dode using various scan functions of liquid chromatography−tandem mass spectrometry along with data mining strategies. Phytochem Anal. 2015;26:413–422. doi: 10.1002/pca.2575. [DOI] [PubMed] [Google Scholar]
- 54.Yang W., Ye M., Liu M., Kong D.Z., Shi R., Shi X.W. A practical strategy for the characterization of coumarins in Radix Glehniae by liquid chromatography coupled with triple quadrupole-linear ion trap mass spectrometry. J Chromatogr A. 2010;1217:4587–4600. doi: 10.1016/j.chroma.2010.04.076. [DOI] [PubMed] [Google Scholar]
- 55.Song Y.L., Song Q.Q., Li J., Zhang N., Zhao Y.F., Liu X. An integrated strategy to quantitatively differentiate chemome between Cistanche deserticola and C. tubulosa using high performance liquid chromatography-hybrid triple quadrupole-linear ion trap mass spectrometry. J Chromatogr A. 2016;1429:238–247. doi: 10.1016/j.chroma.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 56.Ding L., Wang X.Y., Wang S., Yu J.J., Qin Y.Q., Zhang X.B. Systematic screening and characterization of glycosides in tobacco leaves by liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry using neutral loss scan and product ion scan. J Separ Sci. 2015;38:4029–4035. doi: 10.1002/jssc.201500760. [DOI] [PubMed] [Google Scholar]
- 57.Yan Z.X., Lin G., Ye Y., Wang Y.T., Yan R. A generic multiple reaction monitoring based approach for plant flavonoids profiling using a triple quadrupole linear ion trap mass spectrometry. J Am Soc Mass Spectrom. 2014;25:955–965. doi: 10.1007/s13361-014-0863-6. [DOI] [PubMed] [Google Scholar]
- 58.Yan Z.X., Lin G., Ye Y., Wang Y.T., Yan R. Triterpenoid saponins profiling by adducts-targeted neutral loss triggered enhanced resolution and product ion scanning using triple quadrupole linear ion trap mass spectrometry. Anal Chim Acta. 2014;819:56–64. doi: 10.1016/j.aca.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 59.Kleinenkuhnen N., Büchel F., Gerlich S.C., Kopriva S., Metzger S. A novel method for identification and quantification of sulfated flavonoids in plants by neutral loss scan mass spectrometry. Front Plant Sci. 2019;10:885. doi: 10.3389/fpls.2019.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin Y., Xiao Y.S., Zhang F.F., Xue X.Y., Xu Q., Liang X.M. Systematic screening and characterization of flavonoid glycosides in Carthamus tinctorius L. by liquid chromatography/UV diode-array detection/electrospray ionization tandem mass spectrometry. J Pharmaceut Biomed Anal. 2008;46:418–430. doi: 10.1016/j.jpba.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 61.Li S., Jin S.N., Song C.W., Jia S.L., Zhang Y., Feng Y.L. The strategy for establishment of the multiple reaction monitoring based characteristic chemical profile of triterpenes in Alismatis Rhizoma using two combined tandem mass spectrometers. J Chromatogr A. 2017;1524:121–134. doi: 10.1016/j.chroma.2017.09.057. [DOI] [PubMed] [Google Scholar]
- 62.Liu P.W., Du Y.F., Zhang X.W., Sheng X.N., Shi X.W., Zhao C.C. Rapid analysis of 27 components of Isodon serra by LC–ESI-MS–MS. Chromatographia. 2010;72:265–273. [Google Scholar]
- 63.Jin S.N., Song C.W., Jia S.L., Li S., Zhang Y., Chen C. An integrated strategy for establishment of curcuminoid profile in turmeric using two LC−MS/MS platforms. J Pharmaceut Biomed Anal. 2017;132:93–102. doi: 10.1016/j.jpba.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 64.Song Y.L., Zhang N., Shi S.P., Li J., Zhao Y.F., Zhang Q. Homolog-focused profiling of ginsenosides based on the integration of step-wise formate anion-to-deprotonated ion transition screening and scheduled multiple reaction monitoring. J Chromatogr A. 2015;1406:136–144. doi: 10.1016/j.chroma.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 65.Yan Z.X., Li T.X., Lv P., Li X., Zhou C., Yang X.H. Sensitive and reliable multianalyte quantitation of herbal medicine in rat plasma using dynamic triggered multiple reaction monitoring. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;928:22–31. doi: 10.1016/j.jchromb.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y., Uboh C.E., Soma L.R., Li X.Q., Guan F.Y., You Y.W. Efficient use of retention time for the analysis of 302 drugs in equine plasma by liquid chromatography−MS/MS with scheduled multiple reaction monitoring and instant library searching for doping control. Anal Chem. 2011;83:6834–6841. doi: 10.1021/ac2016163. [DOI] [PubMed] [Google Scholar]
- 67.Song Q.Q., Song Y.L., Zhang N., Li J., Jiang Y., Zhang K.R. Potential of hyphenated ultra-high performance liquid chromatography-scheduled multiple reaction monitoring algorithm for large-scale quantitative analysis of traditional Chinese medicines. RSC Adv. 2015;5:57372–57382. [Google Scholar]
- 68.Yao M., Ma L., Humphreys W.G., Zhu M. Rapid screening and characterization of drug metabolites using a multiple ion monitoring-dependent MS/MS acquisition method on a hybrid triple quadrupole-linear ion trap mass spectrometer. J Mass Spectrom. 2008;43:1364–1375. doi: 10.1002/jms.1412. [DOI] [PubMed] [Google Scholar]
- 69.Song Y.L., Zhang N., Shi S.P., Li J., Zhang Q., Zhao Y.F. Large-scale qualitative and quantitative characterization of components in Shenfu injection by integrating hydrophilic interaction chromatography, reversed phase liquid chromatography, and tandem mass spectrometry. J Chromatogr A. 2015;1407:106–118. doi: 10.1016/j.chroma.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 70.Yang W.Z., Si W., Zhang J.X., Yang M., Pan H.Q., Wu J. Selective and comprehensive characterization of the quinochalcone C-glycoside homologs in Carthamus tinctorius L. by offline comprehensive two-dimensional liquid chromatography/LTQ-Orbitrap MS coupled with versatile data mining strategies. RSC Adv. 2016;6:495–506. [Google Scholar]
- 71.Yao C.L., Yang W.Z., Si W., Shen Y., Zhang N.X., Chen H.L. An enhanced targeted identification strategy for the selective identification of flavonoid O-glycosides from Carthamus tinctorius by integrating offline two-dimensional liquid chromatography/linear ion-trap-Orbitrap mass spectrometry, high-resolution diagnostic product ions/neutral loss filtering and liquid chromatography-solid phase extraction-nuclear magnetic resonance. J Chromatogr A. 2017;1491:87–97. doi: 10.1016/j.chroma.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 72.Sleno L. The use of mass defect in modern mass spectrometry. J Mass Spectrom. 2012;47:226–236. doi: 10.1002/jms.2953. [DOI] [PubMed] [Google Scholar]
- 73.Zhu M.S., Ma L., Zhang D.L., Ray K., Zhao W.P., Humphreys W.G. Detection and characterization of metabolites in biological matrices using mass defect filtering of liquid chromatography/high resolution mass spectrometry data. Drug Metab Dispos. 2006;34:1722–1733. doi: 10.1124/dmd.106.009241. [DOI] [PubMed] [Google Scholar]
- 74.Zhang H.Y., Zhang D.L., Ray K., Zhu M.S. Mass defect filter technique and its applications to drug metabolite identification by high-resolution mass spectrometry. J Mass Spectrom. 2009;44:999–1016. doi: 10.1002/jms.1610. [DOI] [PubMed] [Google Scholar]
- 75.Gu Z.M., Wang L.Q., Wu J. Mass defect filter—a new tool to expedite screening and dereplication of natural products and generate natural product profiles. Nat Prod J. 2011;1:135–145. [Google Scholar]
- 76.Mortishire-Smith R.J., Castro-Perez J.M., Yu K., Shockcor J.P., Goshawk J., Hartshorn M.J. Generic dealkylation: a tool for increasing the hit-rate of metabolite rationalization, and automatic customization of mass defect filters. Rapid Commun Mass Spectrom. 2009;23:939–948. doi: 10.1002/rcm.3951. [DOI] [PubMed] [Google Scholar]
- 77.Xie T., Liang Y., Hao H.P., A J.Y., Xie L., Gong P. Rapid identification of ophiopogonins and ophiopogonones in Ophiopogon japonicus extract with a practical technique of mass defect filtering based on high resolution mass spectrometry. J Chromatogr A. 2012;1227:234–244. doi: 10.1016/j.chroma.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 78.Yan G.L., Sun H., Sun W.J., Zhao L., Meng X.C., Wang X.J. Rapid and global detection and characterization of aconitum alkaloids in Yin Chen Si Ni Tang, a traditional Chinese medical formula, by ultra performance liquid chromatography-high resolution mass spectrometry and automated data analysis. J Pharmaceut Biomed Anal. 2010;53:421–431. doi: 10.1016/j.jpba.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Pan H.Q., Yang W.Z., Zhang Y.B., Yang M., Feng R.H., Wu W.Y. An integrated strategy for the systematic characterization and discovery of new indole alkaloids from Uncaria rhynchophylla by UHPLC/DAD/LTQ-Orbitrap-MS. Anal Bioanal Chem. 2015;407:6057–6070. doi: 10.1007/s00216-015-8777-0. [DOI] [PubMed] [Google Scholar]
- 80.Tian J.X., Tian Y., Xu L., Zhang J., Lu T., Zhang Z.J. Characterisation and identification of dihydroindole-type alkaloids from processed semen strychni by high-performance liquid chromatography coupled with electrospray ionisation ion trap time-of-flight mass spectrometry. Phytochem Anal. 2014;25:36–44. doi: 10.1002/pca.2457. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J.Y., Wang F., Zhang H., Lu J.Q., Qiao Y.J. Rapid identification of polymethoxylated flavonoids in traditional Chinese medicines with a practical strategy of stepwise mass defect filtering coupled to diagnostic product ions analysis based on a hybrid LTQ-orbitrap mass spectrometer. Phytochem Anal. 2014;25:405–414. doi: 10.1002/pca.2508. [DOI] [PubMed] [Google Scholar]
- 82.Ren D.B., Ran L., Yang C., Xu M.L., Yi L.Z. Integrated strategy for identifying minor components in complex samples combining mass defect, diagnostic ions and neutral loss information based on ultra-performance liquid chromatography-high resolution mass spectrometry platform: Folium Artemisiae Argyi as a case study. J Chromatogr A. 2018;1550:35–44. doi: 10.1016/j.chroma.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 83.Lai C.J.S., Tan T., Zeng S.L., Qi L.W., Liu X.G., Dong X. An integrated high resolution mass spectrometric data acquisition method for rapid screening of saponins in Panax notoginseng (Sanqi) J Pharmaceut Biomed Anal. 2015;109:184–191. doi: 10.1016/j.jpba.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 84.Pan H.Q., Yao C.L., Yang W.Z., Yao S., Huang Y., Yb Zhang. An enhanced strategy integrating offline two-dimensional separation and step-wise precursor ion list-based raster-mass defect filter: characterization of indole alkaloids in five botanical origins of Uncariae Ramulus Cum Unicis as an exemplary application. J Chromatogr A. 2018;1563:124–134. doi: 10.1016/j.chroma.2018.05.066. [DOI] [PubMed] [Google Scholar]
- 85.Keller B.O., Sui J., Young A.B., Whittal R.M. Interferences and contaminants encountered in modern mass spectrometry. Anal Chim Acta. 2008;627:71–81. doi: 10.1016/j.aca.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 86.Windig W., Phalp J.M., Payne A.W. A noise and background reduction method for component detection in liquid chromatography−mass spectrometry. Anal Chem. 1996;68:3602–3606. [Google Scholar]
- 87.Wang C.H., Zhang J.L., Wu C.S., Wang Z. A multiple-dimension liquid chromatography coupled with mass spectrometry data strategy for the rapid discovery and identification of unknown compounds from a Chinese herbal formula (Er-xian decoction) J Chromatogr A. 2017;1518:59–69. doi: 10.1016/j.chroma.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 88.Feng J.J., Yu P.F., Zhou Q., Tian Z.H., Sun M.J., Li X.L. An integrated data filtering and identification strategy for rapid profiling of chemical constituents, with Arnebiae Radix as an example. J Chromatogr A. 2020;1629:461496. doi: 10.1016/j.chroma.2020.461496. [DOI] [PubMed] [Google Scholar]
- 89.Zeng S.L., Duan L., Chen B.Z., Li P., Liu E.H. Chemicalome and metabolome profiling of polymethoxylated flavonoids in Citri Reticulatae Pericarpium based on an integrated strategy combining background subtraction and modified mass defect filter in a Microsoft Excel Platform. J Chromatogr A. 2017;1508:106–120. doi: 10.1016/j.chroma.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 90.Guo J., Zhang M., Elmore C.S., Vishwanathan K. An integrated strategy for in vivo metabolite profiling using high-resolution mass spectrometry based data processing techniques. Anal Chim Acta. 2013;780:55–64. doi: 10.1016/j.aca.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 91.Zhu P.J., Tong W., Alton K., Swapan C. An accurate-mass-based spectral-averaging isotope-pattern-filtering algorithm for extraction of drug metabolites possessing a distinct isotope pattern from LC−MS data. Anal Chem. 2009;81:5910–5917. doi: 10.1021/ac900626d. [DOI] [PubMed] [Google Scholar]
- 92.Yang M., Zhou Z., Guo D.A. A strategy for fast screening and identification of sulfur derivatives in medicinal Pueraria species based on the fine isotopic pattern filtering method using ultra-high-resolution mass spectrometry. Anal Chim Acta. 2015;894:44–53. doi: 10.1016/j.aca.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 93.Xia Y.G., Liang J., Guo X.D., Sun H.M., Kuang H.X. UPLC-QTOF-MSE-based diagnostic product ion filtering to unveil unstable C6-C2 glucoside conjugates in Forsythia suspensa. J Mass Spectrom. 2017;52:848–859. doi: 10.1002/jms.4030. [DOI] [PubMed] [Google Scholar]
- 94.Liao M., Li A.Q., Chen C., Ouyang H., Zhang Y., Xu Y. Systematic identification of shikonins and shikonofurans in medicinal Zicao species using ultra-high performance liquid chromatography quadrupole time of flight tandem mass spectrometry combined with a data mining strategy. J Chromatogr A. 2015;1425:158–172. doi: 10.1016/j.chroma.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 95.Ma B.L., Yang S.L., Li J.M., Ouyang H., He M.Z., Feng Y.L. A four-step filtering strategy based on ultra-high-performance liquid chromatography coupled to quadrupole-time-of-flight tandem mass spectrometry for comprehensive profiling the major chemical constituents of Akebiae Fructus. Rapid Commun Mass Spectrom. 2019;33:1464–1474. doi: 10.1002/rcm.8480. [DOI] [PubMed] [Google Scholar]
- 96.Cheng X.L., Wan J.Y., Li P., Qi L.W. Ultrasonic/microwave assisted extraction and diagnostic ion filtering strategy by liquid chromatography-quadrupole time-of-flight mass spectrometry for rapid characterization of flavonoids in Spatholobus suberectus. J Chromatogr A. 2011;1218:5774–5786. doi: 10.1016/j.chroma.2011.06.091. [DOI] [PubMed] [Google Scholar]
- 97.Qiao X., Li R., Song W., Miao W.J., Liu J., Chen H.B. A targeted strategy to analyze untargeted mass spectral data: rapid chemical profiling of Scutellaria baicalensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J Chromatogr A. 2016;1441:83–95. doi: 10.1016/j.chroma.2016.02.079. [DOI] [PubMed] [Google Scholar]
- 98.Tchoumtchoua J., Njamen D., Mbanya J.C., Skaltsounis A.L., Halabalaki M. Structure-oriented UHPLC−LTQ Orbitrap-based approach as a dereplication strategy for the identification of isoflavonoids from Amphimas pterocarpoides crude extract. J Mass Spectrom. 2013;48:561–575. doi: 10.1002/jms.3167. [DOI] [PubMed] [Google Scholar]
- 99.Qi L.W., Wang H.Y., Zhang H., Wang C.Z., Li P., Yuan C.S. Diagnostic ion filtering to characterize ginseng saponins by rapid liquid chromatography with time-of-flight mass spectrometry. J Chromatogr A. 2012;1230:93–99. doi: 10.1016/j.chroma.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 100.Wan J.Y., Fan Y., Yu Q.T., Ge Y.Z., Yan C.P., Alolga R.N. Integrated evaluation of malonyl ginsenosides, amino acids and polysaccharides in fresh and processed ginseng. J Pharmaceut Biomed Anal. 2015;107:89–97. doi: 10.1016/j.jpba.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 101.Xie S.L., Shi Y.Y., Wang Y., Wu C.Y., Liu W.Y., Feng F. Systematic identification and quantification of tetracyclic monoterpenoid oxindole alkaloids in Uncaria rhynchophylla and their fragmentations in Q-TOF-MS spectra. J Pharmaceut Biomed Anal. 2013;81–82:56–64. doi: 10.1016/j.jpba.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 102.Li A.Q., Du Z.F., Liao M., Feng Y.L., Ruan H.L., Jiang H.L. Discovery and characterisation of lycorine-type alkaloids in Lycoris spp. (Amaryllidaceae) using UHPLC−QTOF−MS. Phytochem Anal. 2019;30:268–277. doi: 10.1002/pca.2811. [DOI] [PubMed] [Google Scholar]
- 103.Huang C., Xu Q.M., Chen C., Song C.W., Xu Y., Xiang Y. The rapid discovery and identification of physalins in the calyx of Physalis alkekengi L.var.franchetii (Mast.) Makino using ultra-high performance liquid chromatography-quadrupole time of flight tandem mass spectrometry together with a novel three-step data mining strategy. J Chromatogr A. 2014;1361:139–152. doi: 10.1016/j.chroma.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 104.Li J.M., Wen Q., Feng Y.L., Zhang J., Luo Y., Tan T. Characterization of the multiple chemical components of Glechomae Herba using ultra high performance liquid chromatography coupled to quadrupole-time-of-flight tandem mass spectrometry with diagnostic ion filtering strategy. J Separ Sci. 2019;42:1312–1322. doi: 10.1002/jssc.201801212. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y., Li L., Xiao Y.Q., Yao J.Q., Li P.Y., Yu D.R. Global metabolite profiling and diagnostic ion filtering strategy by LC−QTOF MS for rapid identification of raw and processed pieces of Rheum palmatum L. Food Chem. 2016;192:531–540. doi: 10.1016/j.foodchem.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 106.Zhang H., Zheng D., Li H.H., Wang H., Tan H.S., Xu H.X. Diagnostic filtering to screen polycyclic polyprenylated acylphloroglucinols from Garcinia oblongifolia by ultrahigh performance liquid chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry. Anal Chim Acta. 2016;912:85–96. doi: 10.1016/j.aca.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 107.Zheng C.N., Hao H.P., Wang X., Wu X.L., Wang G.J., Sang G.W. Diagnostic fragment-ion-based extension strategy for rapid screening and identification of serial components of homologous families contained in traditional Chinese medicine prescription using high-resolution LC−ESI−IT−TOF/MS: Shengmai injection as an example. J Mass Spectrom. 2009;44:230–244. doi: 10.1002/jms.1502. [DOI] [PubMed] [Google Scholar]
- 108.Zeng S.L., Liu X.G., Lai C.J.S., Liu E.H., Li P. Diagnostic ion filtering strategy for chemical characterization of Guge Fengtong Tablet with high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Chin J Nat Med. 2015;13 doi: 10.1016/S1875-5364(15)30031-5. 390–400. [DOI] [PubMed] [Google Scholar]
- 109.Pang H.Q., An H.M., Yang H., Wu S.Q., Fan J.L., Mi L. Comprehensive chemical profiling of Yindan Xinnaotong soft capsule and its neuroprotective activity evaluation in vitro. J Chromatogr A. 2019;1601:288–299. doi: 10.1016/j.chroma.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 110.Dai W.D., Yin P.Y., Zeng Z.D., Kong H.W., Tong H.W., Xu Z.L. Nontargeted modification-specific metabolomics study based on liquid chromatography−high-resolution mass spectrometry. Anal Chem. 2014;86:9146–9153. doi: 10.1021/ac502045j. [DOI] [PubMed] [Google Scholar]
- 111.Brink A., Fontaine F., Marschmann M., Steinhuber B., Cece E.N., Zamora I. Post-acquisition analysis of untargeted accurate mass quadrupole time-of-flight MSE data for multiple collision-induced neutral losses and fragment ions of glutathione conjugates. Rapid Commun Mass Spectrom. 2014;28:2695–2703. doi: 10.1002/rcm.7062. [DOI] [PubMed] [Google Scholar]
- 112.Zhang J., Huang Z.H., Qiu X.H., Yang Y.M., Zhu D.Y., Xu W. Neutral fragment filtering for rapid identification of new diester-diterpenoid alkaloids in roots of Aconitum carmichaeli by ultra-high-pressure liquid chromatography coupled with linear ion trap-orbitrap mass spectrometry. PLoS One. 2012;7:e52352. doi: 10.1371/journal.pone.0052352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jin Y., Wu C.S., Zhang J.L., Li Y.F. A new strategy for the discovery of epimedium metabolites using high-performance liquid chromatography with high resolution mass spectrometry. Anal Chim Acta. 2013;768:111–117. doi: 10.1016/j.aca.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 114.Jia Z.X., Wu C.S., Jin H.T., Zhang J.L. Identification of the chemical components of Saussurea involucrata by high-resolution mass spectrometry and the mass spectral trees similarity filter technique. Rapid Commun Mass Spectrom. 2014;28:2237–2251. doi: 10.1002/rcm.7014. [DOI] [PubMed] [Google Scholar]
- 115.Liu L.H., Zhang J.Y., Zheng B.J., Guan Y., Wang L.T., Chen L. Rapid characterization of chlorogenic acids in Duhaldea nervosa based on ultra-high-performance liquid chromatography-linear trap quadropole-Orbitrap-mass spectrometry and mass spectral trees similarity filter technique. J Separ Sci. 2018;41:1764–1774. doi: 10.1002/jssc.201701047. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J.Y., Wang Z.J., Li Y., Liu Y., Cai W., Li C. A strategy for comprehensive identification of sequential constituents using ultra-high-performance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometer, application study on chlorogenic acids in Flos Lonicerae Japonicae. Talanta. 2016;147:16–27. doi: 10.1016/j.talanta.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 117.Wang C.H., Wu C.S., Qin H.L., Zhang J.L. Rapid discovery and identification of 68 compounds in the active fraction from Xiao-Xu-Ming decoction (XXMD) by HPLC–HRMS and MTSF technique. Chin Chem Lett. 2014;25:1648–1652. [Google Scholar]
- 118.Garg N., Kapono C.A., Lim Y.W., Koyama N., Vermeij M.J.A., Conrad D. Mass spectral similarity for untargeted metabolomics data analysis of complex mixtures. Int J Mass Spectrom. 2015;377:719–727. doi: 10.1016/j.ijms.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang M.X., Carver J.J., Phelan V.V., Sanchez L.M., Garg N., Peng Y. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat Biotechnol. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Watrous J., Roach P., Alexandrov T., Heath B.S., Yang J.Y., Kersten R.D. Mass spectral molecular networking of living microbial colonies. Proc Natl Acad Sci U S A. 2012;109:E1743–E1752. doi: 10.1073/pnas.1203689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Quinn R.A., Nothias L.F., Vining O., Meehan M., Esquenazi E., Dorrestein P.C. Molecular networking as a drug discovery, drug metabolism, and precision medicine strategy. Trends Pharmacol Sci. 2017;38:143–154. doi: 10.1016/j.tips.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 123.Allard P.M., Péresse T., Bisson J., Gindro K., Marcourt L., Pham V.C. Integration of molecular networking and in-silico MS/MS fragmentation for natural products dereplication. Anal Chem. 2016;88:3317–3323. doi: 10.1021/acs.analchem.5b04804. [DOI] [PubMed] [Google Scholar]
- 124.Yang J.Y., Sanchez L.M., Rath C.M., Liu X., Boudreau P.D., Bruns N. Molecular networking as a dereplication strategy. J Nat Prod. 2013;76:1686–1699. doi: 10.1021/np400413s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Crusemann M., O'Neill E.C., Larson C.B., Melnik A.V., Floros D.J., da Silva R.R. Prioritizing natural product diversity in a collection of 146 bacterial strains based on growth and extraction protocols. J Nat Prod. 2017;80:588–597. doi: 10.1021/acs.jnatprod.6b00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Purves K., Macintyre L., Brennan D., Hreggviðsson Gó, Kuttner E., Ásgeirsdóttir M.E. Using molecular networking for microbial secondary metabolite bioprospecting. Metabolites. 2016;6:2. doi: 10.3390/metabo6010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trivella D.B.B., de Felicio R. The tripod for bacterial natural product discovery: genome mining, silent pathway induction, and mass spectrometry-based molecular networking. mSystems. 2018;3 doi: 10.1128/mSystems.00160-17. e00160–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Philippus A.C., Zatelli G.A., Wanke T., Gabriela de A., Barros M., Kami S.A., Lhullier C. Molecular networking prospection and characterization of terpenoids and C15-acetogenins in Brazilian seaweed extracts. RSC Adv. 2018;8:29654–29661. doi: 10.1039/c8ra02802h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grauso L., Yegdaneh A., Sharifi M., Mangoni A., Zolfaghari B., Lanzotti V. Molecular networking-based analysis of cytotoxic saponins from sea cucumber Holothuria atra. Mar Drugs. 2019;17:86. doi: 10.3390/md17020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ding C.Y.G., Pang L.M., Liang Z.X., Goh K.K.K., Glukhov E., Gerwick W.H. MS/MS-based molecular networking approach for the detection of aplysiatoxin-related compounds in environmental marine cyanobacteria. Mar Drugs. 2018;16:505. doi: 10.3390/md16120505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hou X.M., Li Y.Y., Shi Y.W., Fang Y.W., Chao R., Gu Y.C. Integrating molecular networking and 1H NMR to target the isolation of chrysogeamides from a library of marine-derived Penicillium fungi. J Org Chem. 2019;84:1228–1237. doi: 10.1021/acs.joc.8b02614. [DOI] [PubMed] [Google Scholar]
- 132.Olivon F., Apel C., Retailleau P., Allard P.M., Wolfender J.L., Touboul D. Searching for original natural products by molecular networking: detection, isolation and total synthesis of chloroaustralasines. Organic Chemistry Frontiers. 2018;5:2171–2178. [Google Scholar]
- 133.Caesar L.K., Kellogg J.J., Kvalheim O.M., Cech R.A., Cech N.B. Integration of biochemometrics and molecular networking to identify antimicrobials in Angelica keiskei. Planta Med. 2018;84:721–728. doi: 10.1055/a-0590-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Raheem D.J., Tawfike A.F., Abdelmohsen U.R., Edrada-Ebel R., Fitzsimmons-Thoss V. Application of metabolomics and molecular networking in investigating the chemical profile and antitrypanosomal activity of British bluebells (Hyacinthoides non-scripta) Sci Rep. 2019;9:2547. doi: 10.1038/s41598-019-38940-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Oliveira G.G., Carnevale Neto F., Demarque D.P., de Sousa Pereira-Junior J.A., Sampaio Peixoto Filho R.C., de Melo S.J. Dereplication of flavonoid glycoconjugates from Adenocalymma imperatoris-maximilianii by untargeted tandem mass spectrometry-based molecular networking. Planta Med. 2017;83:636–646. doi: 10.1055/s-0042-118712. [DOI] [PubMed] [Google Scholar]
- 136.Abbas-Mohammadi M., Moridi Farimani M., Salehi P., Nejad Ebrahimi S., Sonboli A., Kelso C. Acetylcholinesterase-inhibitory activity of Iranian plants: combined HPLC/bioassay-guided fractionation, molecular networking and docking strategies for the dereplication of active compounds. J Pharmaceut Biomed Anal. 2018;158:471–479. doi: 10.1016/j.jpba.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 137.Wei W.L., Hou J.J., Yao C.L., Bi Q.R., Wang X., Li Z.W. A high-efficiency strategy integrating offline two-dimensional separation and data post-processing with dereplication: characterization of bufadienolides in Venenum Bufonis as a case study. J Chromatogr A. 2019;1603:179–189. doi: 10.1016/j.chroma.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 138.Lei H.B., Zhang Y.H., Ye J., Cheng T.F., Liang Y.L., Zu X.P. A comprehensive quality evaluation of Fuzi and its processed product through integration of UPLC−QTOF/MS combined MS/MS-based mass spectral molecular networking with multivariate statistical analysis and HPLC−MS/MS. J Ethnopharmacol. 2021;266:113455. doi: 10.1016/j.jep.2020.113455. [DOI] [PubMed] [Google Scholar]
- 139.Xu L.L., Shang Z.P., Bo T., Sun L., Guo Q.L., Qiao X. Rapid quantitation and identification of the chemical constituents in Danhong Injection by liquid chromatography coupled with orbitrap mass spectrometry. J Chromatogr A. 2019;1606:460378. doi: 10.1016/j.chroma.2019.460378. [DOI] [PubMed] [Google Scholar]
- 140.Qiao X., Lin X.H., Ji S., Zhang Z.X., Bo T., Guo D.A. Global profiling and novel structure discovery using multiple neutral loss/precursor ion scanning combined with substructure recognition and statistical analysis (MNPSS): characterization of terpene-conjugated curcuminoids in Curcuma longa as a case study. Anal Chem. 2016;88:703–710. doi: 10.1021/acs.analchem.5b02729. [DOI] [PubMed] [Google Scholar]
- 141.Xue Z.Z., Lai C.J.S., Kang L.P., Kotani A., Hakamata H., Jing Z.X. Profiling and isomer recognition of phenylethanoid glycosides from Magnolia officinalis based on diagnostic/holistic fragment ions analysis coupled with chemometrics. J Chromatogr A. 2020;1611:460583. doi: 10.1016/j.chroma.2019.460583. [DOI] [PubMed] [Google Scholar]
- 142.Horai H., Arita M., Kanaya S., Nihei Y., Ikeda T., Suwa K. MassBank: a public repository for sharing mass spectral data for life sciences. J Mass Spectrom. 2010;45:703–714. doi: 10.1002/jms.1777. [DOI] [PubMed] [Google Scholar]
- 143.Smith C.A., O'Maille G., Want E.J., Qin C., Trauger S.A., Brandon T.R. METLIN: a metabolite mass spectral database. Ther Drug Monit. 2005;27:747–751. doi: 10.1097/01.ftd.0000179845.53213.39. [DOI] [PubMed] [Google Scholar]
- 144.Fabresse N., Larabi I.A., Stratton T., Mistrik R., Pfau G., Lorin de la Grandmaison G. Development of a sensitive untargeted liquid chromatography-high resolution mass spectrometry screening devoted to hair analysis through a shared MS2 spectra database: a step toward early detection of new psychoactive substances. Drug Test Anal. 2019;11:697–708. doi: 10.1002/dta.2535. [DOI] [PubMed] [Google Scholar]
- 145.Barlow D.J., Buriani A., Ehrman T., Bosisio E., Eberini I., Hylands P.J. In-silico studies in Chinese herbal medicines' research: evaluation of in-silico methodologies and phytochemical data sources, and a review of research to date. J Ethnopharmacol. 2012;140:526–534. doi: 10.1016/j.jep.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blazenovic I., Kind T., Ji J., Fiehn O. Software tools and approaches for compound identification of LC−MS/MS data in metabolomics. Metabolites. 2018;8:31. doi: 10.3390/metabo8020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gil de la Fuente A., Grace Armitage E., Otero A., Barbas C., Godzien J. Differentiating signals to make biological sense—a guide through databases for MS-based non-targeted metabolomics. Electrophoresis. 2017;38:2242–2256. doi: 10.1002/elps.201700070. [DOI] [PubMed] [Google Scholar]
- 148.Johnson S.R., Lange B.M. Open-access metabolomics databases for natural product research: present capabilities and future potential. Front Bioeng Biotechnol. 2015;3:22. doi: 10.3389/fbioe.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang F.X., Li M., Qiao L.R., Yao Z.H., Li C., Shen X.Y. Rapid characterization of Ziziphi Spinosae Semen by UPLC/Qtof MS with novel informatics platform and its application in evaluation of two seeds from Ziziphus species. J Pharmaceut Biomed Anal. 2016;122:59–80. doi: 10.1016/j.jpba.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 150.Deng L., Shi A.M., Liu H.Z., Meruva N., Liu L., Hu H. Identification of chemical ingredients of peanut stems and leaves extracts using UPLC−QTOF−MS coupled with novel informatics UNIFI platform. J Mass Spectrom. 2016;51:1157–1167. doi: 10.1002/jms.3887. [DOI] [PubMed] [Google Scholar]
- 151.Zhang H., Jiang J.M., Zheng D., Yuan M., Wang Z.Y., Zhang H.M. A multidimensional analytical approach based on time-decoupled online comprehensive two-dimensional liquid chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry for the analysis of ginsenosides from white and red ginsengs. J Pharmaceut Biomed Anal. 2019;163:24–33. doi: 10.1016/j.jpba.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 152.Qiu S., Yang W.Z., Shi X.J., Yao C.L., Yang M., Liu X. A green protocol for efficient discovery of novel natural compounds: characterization of new ginsenosides from the stems and leaves of Panax ginseng as a case study. Anal Chim Acta. 2015;893:65–76. doi: 10.1016/j.aca.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 153.Qiu S., Yang W.Z., Yao C.L., Qiu Z.D., Shi X.J., Zhang J.X. Nontargeted metabolomic analysis and “commercial-homophyletic” comparison-induced biomarkers verification for the systematic chemical differentiation of five different parts of Panax ginseng. J Chromatogr A. 2016;1453:78–87. doi: 10.1016/j.chroma.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 154.Yao C.L., Pan H.Q., Wang H., Yao S., Yang W.Z., Hou J.J. Global profiling combined with predicted metabolites screening for discovery of natural compounds: characterization of ginsenosides in the leaves of Panax notoginseng as a case study. J Chromatogr A. 2018;1538:34–44. doi: 10.1016/j.chroma.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 155.Ma X.L., Guo X.Y., Song Y.L., Qiao L.R., Wang W.G., Zhao M.B. An integrated strategy for global qualitative and quantitative profiling of traditional Chinese medicine formulas: Baoyuan Decoction as a case. Sci Rep. 2016;6:38379. doi: 10.1038/srep38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kang L.P., Huang Y.Y., Zhan Z.L., Liu D.H., Peng H.S., Nan T.G. Structural characterization and discrimination of the Paris polyphylla var. yunnanensis and Paris vietnamensis based on metabolite profiling analysis. J Pharmaceut Biomed Anal. 2017;142:252–261. doi: 10.1016/j.jpba.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 157.Shi X.J., Yang W.Z., Qiu S., Hou J.J., Wu W.Y., Guo D.A. Systematic profiling and comparison of the lipidomes from Panax ginseng, P. quinquefolius, and P. notoginseng by ultrahigh performance supercritical fluid chromatography/high-resolution mass spectrometry and ion mobility-derived collision cross section measurement. J Chromatogr A. 2018;1548:64–75. doi: 10.1016/j.chroma.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 158.Zhang J.Y., Li C., Che Y.Y., Wu J.R., Wang Z.J., Cai W. LTQ-Orbitrap-based strategy for traditional Chinese medicine targeted class discovery, identification and herbomics research: a case study on phenylethanoid glycosides in three different species of Herba Cistanches. RSC Adv. 2015;5:80816–80828. [Google Scholar]
- 159.Chen X.F., Wu Y.L., Chen C., Gu Y.Q., Zhu C.Y., Wang S.P. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm Sin B. 2020;11:222–236. doi: 10.1016/j.apsb.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Luo S.F., Guo L.B., Sheng C.M., Zhao Y.M., Chen L., Li C.F. Rapid identification and isolation of neuraminidase inhibitors from mockstrawberry (Duchesnea indica Andr.) based on ligand fishing combined with HR-ESI-Q-TOF-MS. Acta Pharm Sin B. 2020;10:1846–1855. doi: 10.1016/j.apsb.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liang Y., Wu J.L., Leung E.L., Zhou H., Liu Z.Q., Yan G.Y. Identification of oxygenated fatty acid as a side chain of lipo-alkaloids in Aconitum carmichaelii by UHPLC–Q-TOF-MS and a database. Molecules. 2016;21:437. doi: 10.3390/molecules21040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu Y.C., Xiao S., Yang K., Ling L., Sun Z.L., Liu Z.Y. Comprehensive identification and structural characterization of target components from Gelsemium elegans by high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry based on accurate mass databases combined with MS/MS spectra. J Mass Spectrom. 2017;52:378–396. doi: 10.1002/jms.3937. [DOI] [PubMed] [Google Scholar]
- 163.Zhang M.L., Sun J.H., Chen P. FlavonQ: an automated data processing tool for profiling flavone and flavonol glycosides with ultra-high-performance liquid chromatography-diode array detection-high resolution accurate mass-mass spectrometry. Anal Chem. 2015;87:9974–9981. doi: 10.1021/acs.analchem.5b02624. [DOI] [PubMed] [Google Scholar]
- 164.Qiu F., Fine D.D., Wherritt D.J., Lei Z., Sumner L.W. PlantMAT: a metabolomics tool for predicting the specialized metabolic potential of a system and for large-scale metabolite identifications. Anal Chem. 2016;88:11373–11383. doi: 10.1021/acs.analchem.6b00906. [DOI] [PubMed] [Google Scholar]
- 165.Zhou W.J., Liu Y.M., Wang J.X., Guo Z.M., Shen A.J., Liu Y.F. Application of two-dimensional liquid chromatography in the separation of traditional Chinese medicine. J Separ Sci. 2020;43:87–104. doi: 10.1002/jssc.201900765. [DOI] [PubMed] [Google Scholar]
- 166.Bade R., Bijlsma L., Sancho J.V., Hernández F. Critical evaluation of a simple retention time predictor based on LogKow as a complementary tool in the identification of emerging contaminants in water. Talanta. 2015;139:143–149. doi: 10.1016/j.talanta.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 167.Tyrkkö E., Pelander A., Ojanperä I. Prediction of liquid chromatographic retention for differentiation of structural isomers. Anal Chim Acta. 2012;720:142–148. doi: 10.1016/j.aca.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Q.Q., Huo M.Q., Zhang Y.L., Qiao Y.J., Gao X.Y. A strategy to improve the identification reliability of the chemical constituents by high-resolution mass spectrometry-based isomer structure prediction combined with a quantitative structure retention relationship analysis: phthalide compounds in Chuanxiong as a test case. J Chromatogr A. 2018;1552:17–28. doi: 10.1016/j.chroma.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 169.Falchi F., Bertozzi S.M., Ottonello G., Ruda G.F., Colombano G., Fiorelli C. Kernel-based, partial least squares quantitative structure-retention relationship model for UPLC retention time prediction: a useful tool for metabolite identification. Anal Chem. 2016;88:9510–9517. doi: 10.1021/acs.analchem.6b02075. [DOI] [PubMed] [Google Scholar]
- 170.Munro K., Miller T.H., Martins C.P., Edge A.M., Cowan D.A., Barron L.P. Artificial neural network modelling of pharmaceutical residue retention times in wastewater extracts using gradient liquid chromatography-high resolution mass spectrometry data. J Chromatogr A. 2015;1396:34–44. doi: 10.1016/j.chroma.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 171.McEachran A.D., Mansouri K., Newton S.R., Beverly B.E.J., Sobus J.R., Williams A.J. A comparison of three liquid chromatography (LC) retention time prediction models. Talanta. 2018;182:371–379. doi: 10.1016/j.talanta.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nagy T., Kuki Á Antal B., Nagy L., Purgel M., Sipos A. Chiral differentiation of the noscapine and hydrastine stereoisomers by electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2015;50:240–246. doi: 10.1002/jms.3527. [DOI] [PubMed] [Google Scholar]
- 173.Song Y.L., Song Q.Q., Liu Y., Li J., Wan J.B., Wang Y.T. Integrated work-flow for quantitative metabolome profiling of plants, Peucedani Radix as a case. Anal Chim Acta. 2017;953:40–47. doi: 10.1016/j.aca.2016.11.066. [DOI] [PubMed] [Google Scholar]
- 174.Song Q.Q., Li J., Huo H.X., Cao Y., Wang Y.T., Song Y.L. Retention time and optimal collision energy advance structural annotation relied on LC−MS/MS: an application in metabolite identification of an antidementia agent namely echinacoside. Anal Chem. 2019;91:15040–15048. doi: 10.1021/acs.analchem.9b03720. [DOI] [PubMed] [Google Scholar]
- 175.Martens J., Berden G., Gebhardt C.R., Oomens J. Infrared ion spectroscopy in a modified quadrupole ion trap mass spectrometer at the FELIX free electron laser laboratory. Rev Sci Instrum. 2016;87:103108. doi: 10.1063/1.4964703. [DOI] [PubMed] [Google Scholar]
- 176.Kranenburg R.F., van Geenen F., Berden G., Oomens J., Martens J., van Asten A.C. Mass-spectrometry-based identification of synthetic drug isomers using infrared ion spectroscopy. Anal Chem. 2020;92:7282–7288. doi: 10.1021/acs.analchem.0c00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Martens J., van Outersterp R.E., Vreeken R.J., Cuyckens F., Coene K.L.M., Engelke U.F. Infrared ion spectroscopy: new opportunities for small-molecule identification in mass spectrometry—a tutorial perspective. Anal Chim Acta. 2020;1093:1–15. doi: 10.1016/j.aca.2019.10.043. [DOI] [PubMed] [Google Scholar]