Abstract
Serine and glycine are 2 of the first-affected nonessential amino acids in low crude protein (CP) diets for pigs. Therefore, we explored the effects of different dietary serine-to-glycine ratios on growth performance and lipid metabolism in growing-finishing pigs. A total of 160 crossbred healthy barrows, with a similar body weight of around 59.50 kg, were randomly allotted into 1 of 5 treatments (8 pens per treatment and 4 pigs per pen). The serine-to-glycine ratios of the 5 dietary treatments were as follows: diet A (NORMAL group), 1.18:1 (16% CP); diet B (LOW group), 1.2:1 (12% CP); diet C (S2G1 group), 2:1 (12% CP); diet D (S1G2 group), 1:2 (12% CP); and diet E (S1G1 group), 1:1 (12% CP).We found that the pigs fed a low CP diet (12% CP), when maintaining serine-to-glycine ratio at 1:2 and a total amount of 1.44%, had the same average daily gain as the pigs fed a normal CP diet (16% CP) (P > 0.05), but they had increased intramuscular fat (P < 0.05). Furthermore, they exhibited higher expression of genes involved in lipid oxidation (P < 0.05), which was regulated by modulating methylation levels in the promoters of acyl-CoA oxidase 1 (ACOX1) and acyl-CoA dehydrogenase medium chain (ACADM). When compared with the pigs fed a normal CP diet, these pigs had more oxidative myofibers (P < 0.05), which were regulated by AMPK-PGC-1α and Calcineurin-MEF2/NFAT pathways in a coordinated manner. Our findings suggested that a dietary serine-to-glycine ratio of 1:2 is beneficial for improving meat quality in pigs fed a low CP diet.
Keywords: AMP-activated protein kinase, Glycine, Lipid oxidation, Low-protein diet, Methylation, Serine
1. Introduction
Decreasing the crude protein (CP) level in pig diet has many beneficial effects, including reducing nitrogen excretion, feed cost, and the risk of gut disorders. However, low CP diets often result in impaired muscle protein synthesis and growth, because organisms adapt to chronic protein deficiency metabolically by reducing the protein synthesis rate (Duan et al., 2016). Nutritionally nonessential amino acids can become growth-limiting factors in low-CP diets. It has been reported that glycine and serine, as precursors of arginine and cysteine, respectively, would be 2 of the first-affected nonessential amino acids in protein deficiency, because their concentrations are reduced and endogenous metabolization processes (such as de novo synthesis) are slower without sufficient metabolic precursors when pigs are fed low-CP diets (Powell et al., 2011). The levels of these amino acids could be the limiting factor deciding the utilization efficiency of low-CP diets supplemented with amino acids.
Numerous studies have investigated the effects of dietary glycine and serine supplementation on growth performance of broiler chickens. For decades, most studies have established the performance-enhancing effects of glycine (Greene et al., 1960; Waterhouse and Scott 1961), and others suggested an equivalent effect of serine in fulfilling the function of glycine (Baker et al., 1968; Sugahara and Kandatsu 1976). For this reason, they claimed that dietary levels of both glycine and serine should be evaluated to determine the physiological value of a diet (Siegert et al., 2015). Recently, some studies have further shown performance-enhancing effects of glycine on broilers with reduced CP diets (Corzo et al., 2004; Dean et al., 2006). Surprisingly, a recent study indicated that the existing recommendations for glycine and serine levels for broilers, which have been used for almost 20 years, may be too low (Ospina-Rojas et al., 2013). However, studies on the influence of dietary glycine and serine on pigs are scarce, although these 2 amino acids are metabolically important. For instance, serine provides a one-carbon group for the methionine cycle and is a substrate for cysteine synthesis. Additionally, both glycine and serine are important substrates for the synthesis of glutathione, creatine, and protein.
Glycine can convert directly to serine and vice versa via serine hydroxy methyltransferase (SHMT). Glycine and serine play primary roles in protein synthesis and supporting proliferation. Additionally, they are important for the synthesis of glutathione, phospholipids, nucleotides, and other metabolites (Labuschagne et al., 2014). Although glycine and serine are metabolically related and share many functional roles, they cannot replace each other in diets. When serine is converted to glycine, one-carbon units are produced for methylation and building nucleotides, and the reaction in which glycine is converted to serine depletes the one-carbon pool (Labuschagne et al., 2014). Consequently, an imbalanced serine-to-glycine ratio in diets would cause imbalances in methylation reactions.
Previous studies have shown that either glycine or serine could be beneficial for intestinal health and improve growth performance in piglets (Li et al., 2016; Zhou et al., 2018b; Fan et al., 2019). However, no studies have examined the effects of glycine and/or serine on growth performance in growing-finishing pigs. Consequently, the aim of the current study was to explore the effects of dietary serine-to-glycine ratios on growth performance and other potential effects in the skeletal muscle, as well as the underlying mechanisms in growing-finishing pigs.
2. Materials and methods
The experimental protocol was approved by the Protocol Management and Review Committee of Institute of Subtropical Agriculture, Chinese Academy of Science (No. 20190045). All pigs were cared for and slaughtered according to the guidelines of Institute of Subtropical Agriculture on Animal Care (Changsha, China).
2.1. Animals and experiment diets
A total of 160 crossbred healthy barrows (Duroc × Landrace × Yorkshire), with a similar body weight (around 59.50 kg) were selected for the experiments. The pigs were randomly allotted into 1 of 5 treatments (8 pens per treatment and 4 pigs per pen). Diets were isoenergetic and met the nutritional needs for growing-finishing pigs according to the National Research Council except for protein content (Appendix Table 1). The serine-to-glycine ratios of the 5 dietary treatments were as follows: diet A (NORMAL group), 1.18:1 (16% CP, basal diet); diet B (LOW group), 1.2:1 (12% CP, low CP diet generated mostly by decreasing the content of soybean meal); diet C (S2G1 group), 2:1 (12% CP, low CP diet generated by decreasing the content of soybean meal and adding 0.49% serine and 0.08% glycine); diet D (S1G2 group), 1:2 (12% CP, low CP diet generated by decreasing the content of soybean meal and adding 0.57% glycine); and diet E (S1G1 group), 1:1 (12% CP, low CP diet generated by decreasing the content of soybean meal and adding 0.24% serine and 0.33% glycine). Pigs had ad libitum access to feed and water. The experimental period lasted for 43 days. Serine and glycine (purity ≥ 99.0%) were purchased from Aladdin Technology Co., LTD (Shanghai, China).
2.2. Sample collection
Feed intake was recorded every week and body weights were recorded at the start and the end of the experiment to calculate the feed to gain ratio. At the end of the experiment, 8 pigs were randomly chosen from each treatment and then slaughtered by electrical stunning (250 V, 0.5 A, for 5 to 6 s) and exsanguination. Immediately, samples of the longissimus muscle adjacent to the last rib from the left-half body, which is a predominantly fast-twitch muscle, were collected for the analysis of gene and protein expression, as well as transcriptomic study and pyrosequencing.
2.3. Body fat and intramuscular fat content
Fat ratio was calculated as the ratio of fat weight to total body weight. Back fat was measured at the first, tenth, and last ribs, as well as the last lumbar vertebrae, and backfat thickness was defined as the average of the four sites. Crude fat on the longissimus dorsi muscle obtained between the thirteenth and fourteenth ribs from the left-half carcasses were extracted by Soxhlet extraction and intramuscular fat content was defined as the ratio of crude fat content to the weight of muscle sample.
2.4. Transcriptomic study
Samples of the longissimus dorsi muscle were used for total RNA isolation. After qualification, samples were submitted for differential gene expression (DGE) experiments by Novogene Bioinformatics Technology Co. Ltd (Beijing, China) on an Illumina HiSeq platform, and 125/150 bp paired-end reads were generated. Total reads were mapped to the Sus scrofa genome in Ensembl (http://www.ensembl.org/sus_scrofa/Info/Index) using Bowtie v2.2.3 and TopHat v2.0.12 software. HTSeq v0.6.1 was used to count the read numbers mapped to each gene. DESeq R package (1.18.0) was used for DGE analysis. To control the false discovery rate, the resulting P-values were adjusted using Benjamini and Hochberg's approach. DGE were confirmed when log2 fold change >1 and adjusted P-value < 0.05 were found by DESeq.
2.5. Pyrosequencing
Pyrosequencing was performed as previously reported (Cao et al., 2018). Briefly, genomic DNA was extracted from samples of longissimus dorsi muscle and then modified by sodium bisulfate using the EZ DNA Methylation-Gold Kit D5005 (ZYMO Research, Beijing, China). Specific regions in the promoters of fatty acid synthase (FAS), acyl-CoA oxidase 1 (ACOX1), acyl-CoA dehydrogenase medium chain (ACADM), and myocyte enhancer factor 2D (MEF2D) were amplified, respectively, using a PCR kit (Sangon Biotech, Shanghai, China). The PCR was performed under the following cycling conditions: 3 min at 95 °C; then 35 cycles of 25 s at 94 °C, 25 s at 58 °C, 25 s at 72 °C; and 5 min at 72 °C. Then, the single-stranded (ss) DNA isolated from the PCR products was used as a template in the pyrosequencing PCR to analyze CpG sites. Target sequences for detecting DNA methylation and primer sequences for pyrosequencing PCR are presented in Appendix Table 2, and the target sequences in promoters of FAS, ACOX1, ACADM, and MEF2D are presented in Appendix Table 3.
2.6. RT-qPCR analysis
RT-qPCR analysis was performed to validate the results of transcriptome sequencing and determine myosin heavy chain gene expression. Total RNA was isolated from longissimus dorsi muscle samples using an RNA Extraction Kit (Takara, Beijing, China). An RT reagent Kit with gDNA Eraser (Takara) was used for reverse transcription. Target gene transcript levels were normalized to levels of GAPDH and β-actin. RT-qPCR was performed as reported previously (He et al., 2018a, 2018b). Briefly, the reaction mixture had a total volume of 10 μL containing 1 μL DNA, 5 μL SYBR Green mix (Takara), 3 μL deionized H2O, 0.2 μL ROX, and 0.4 μL each of forward and reverse primers. Fold differences in mRNA levels were calculated using the 2−ΔΔCt method. All primers used in the reaction are shown in Appendix Table 4.
2.7. Protein quantification by the Wes Simple Western System
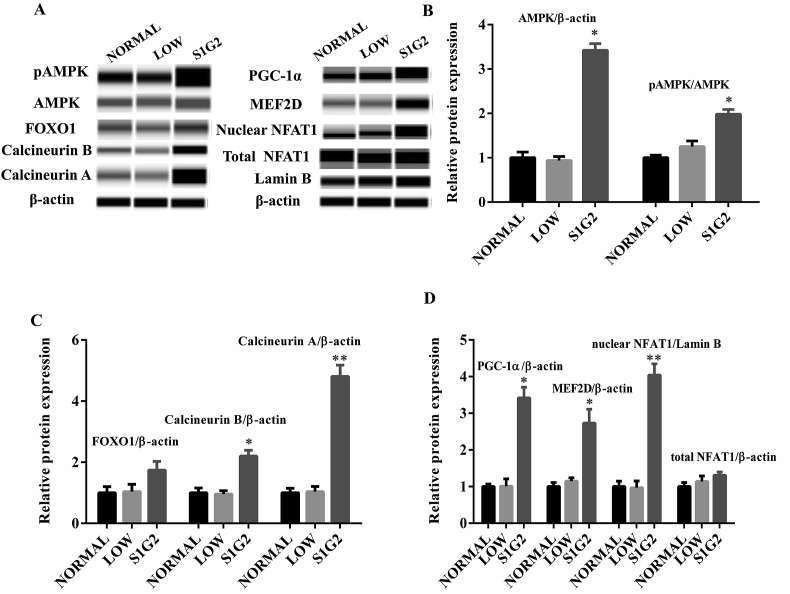
Protein quantification was performed with the Wes Simple Western System (Proteinsimple, San Jose, CA, USA). Proteins extracted from longissimus dorsi muscle samples were mixed with fluorescent standards, Master Mix, dithiothreitol, and Simple Western Sample Buffer (Proteinsimple) and then were loaded into Wes 25-well plates. Primary antibodies against AMP-activated protein kinase (AMPK) and myocyte enhancer factor 2D (MEF2D) were purchased from Bioss, Beijing, China; antibodies against β-actin, phopho-AMPK, forkhead transcription factor 1 (FOXO1), slow skeletal myosin heavy chain (SSMHC), fast skeletal myosin heavy chain (FSMHC), Calcineurin A, Calcineurin B, peroxisome-proliferator-activated receptor coactivator (PGC-1α), and nuclear factor of activated T cells-1 (NFAT1) were purchased from Abcam, Cambridge, MA, USA. The appropriate secondary antibodies, stacking and separation gel matrix were added according to the manufacturer's instructions. Protein bands were obtained using the “gel view” function of the Protein Simple software (Proteinsimple).
2.8. Statistical analysis
Data were analyzed by one-way ANOVA using the SAS 8.2 software package, followed by a Duncan's multiple-range test. The results were considered to be statistically significant at P < 0.05.
3. Results
3.1. Growth performance
As presented in Table 1, pigs in the NORMAL and S1G2 diet groups had a significantly higher average daily weight gain (ADG) than pigs in the LOW diet group, but no significant difference in ADG was observed among pigs in the LOW, S2G1, and S1G1 diet groups. No difference was observed in average daily feed intake among the 5 diet treatment groups. However, when compared with pigs in the NORMAL diet group, pigs in the LOW, S2G1, and S1G2 diet groups exhibited higher feed to gain ratios.
Table 1.
Growth performance of the growing-finishing pigs.1
| Index | NORMAL | LOW | S2G1 | S1G2 | S1G1 |
|---|---|---|---|---|---|
| IBW, kg | 59.2 ± 1.2 | 59.6 ± 1.4 | 59.7 ± 1.3 | 59.7 ± 0.9 | 59.4 ± 1.2 |
| FBW, kg | 96.0 ± 2.6 | 93.2 ± 2.9 | 93.7 ± 2.4 | 95.7 ± 3.0 | 95.1 ± 3.3 |
| ADG, g | 855 ± 17a | 777 ± 15b | 791 ± 21ab | 839 ± 31a | 831 ± 29ab |
| ADFI, kg | 2.22 ± 0.09 | 2.25 ± 0.08 | 2.32 ± 0.09 | 2.42 ± 0.06 | 2.34 ± 0.07 |
| F:G | 2.59 ± 0.08b | 2.89 ± 0.08a | 2.94 ± 0.11a | 2.90 ± 0.14a | 2.82 ± 0.06ab |
IBW = initial body weight; FBW = final body weight; ADG = average daily weight gain; ADFI = average daily feed intake; F:G = the ratio of feed to gain.
a, bMean values within a row with unlike superscript letters were significantly different (P < 0.05). n = 8.
NORMAL, pigs fed a normal crude protein (16%) diet; LOW, pigs fed a low crude protein (12%) diet generated by decreasing the content of soybean meal; S2G1, pigs fed a low crude protein (12%) diet with serine-to-glycine ratio 2:1, generated by decreasing the content of soybean meal and adding 0.49% serine and 0.08% glycine; S1G2, pigs fed a low crude protein (12%) diet with serine-to-glycine ratio 1:2, generated by decreasing the content of soybean meal and adding 0.57% glycine; S1G1, pigs fed a low crude protein (12%) diet with serine-to-glycine ratio 1:1, generated by decreasing the content of soybean meal and adding 0.24% serine and 0.33% glycine.
3.2. Body fat and intramuscular fat content
As presented in Table 2, pigs in the S1G2 and S2G1 diet groups had significantly higher intramuscular fat content when compared with pigs in the NORMAL diet group. Pigs in the S1G2 diet group had significantly higher fat rate when compared with pigs in the NORMAL diet group. However, no significant difference in backfat thickness was observed among pigs in the 5 diet groups.
Table 2.
Body fat content of the growing-finishing pigs.1
| Index | NORMAL1 | LOW2 | S2G13 | S1G24 | S1G15 |
|---|---|---|---|---|---|
| Body fat ratio, % | 2.18 ± 0.15b | 2.48 ± 0.29ab | 2.16 ± 0.26b | 2.97 ± 0.25a | 2.10 ± 0.14b |
| Backfat thickness, mm | 20.96 ± 0.97 | 22.51 ± 0.77 | 22.84 ± 1.04 | 23.53 ± 1.40 | 20.80 ± 0.89 |
| Intramuscular fat, % | 1.83 ± 0.23bc | 2.64 ± 0.27ab | 3.22 ± 0.25a | 2.92 ± 0.38a | 2.50 ± 0.34ab |
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05). n = 8.
NORMAL, pigs fed a normal crude protein (16%) diet.
LOW, pigs fed a low crude protein (12%) diet generated by decreasing the content of soybean meal.
S2G1, pigs fed a low crude protein (12%) diet with serine-to-glycine ratio 2:1, generated by decreasing the content of soybean meal and adding 0.49% serine and 0.08% glycine.
S1G2, pigs fed a low crude protein (12%) diet with serine-to-glycine ratio 1:2, generated by decreasing the content of soybean meal and adding 0.57% glycine.
S1G1, pigs fed a low crude protein (12%) diet with serine-to-glycine ratio 1:1, generated by decreasing the content of soybean meal and adding 0.24% serine and 0.33% glycine.
3.3. Transcriptome analysis
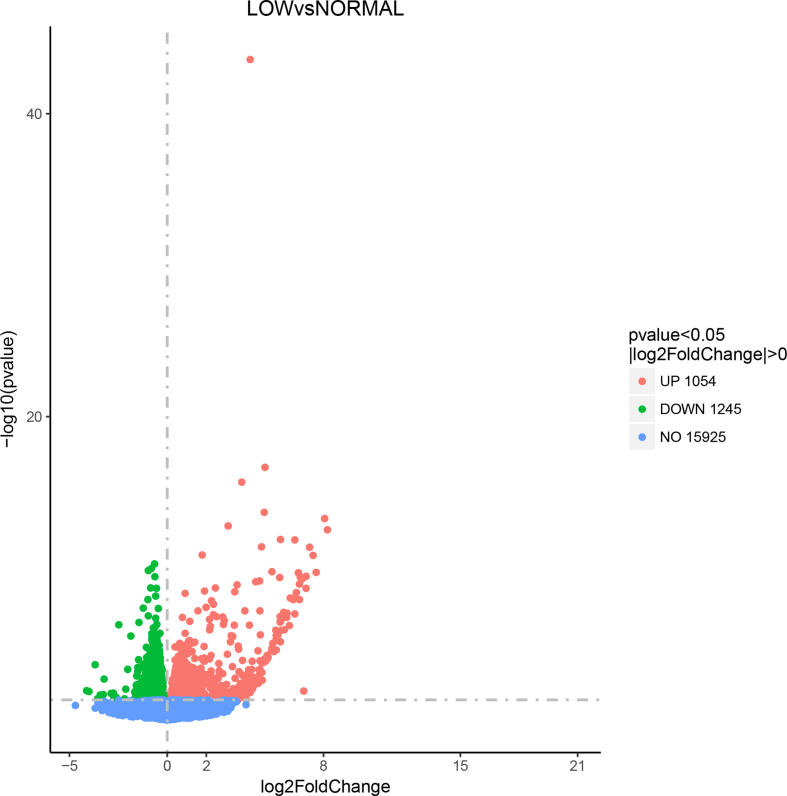
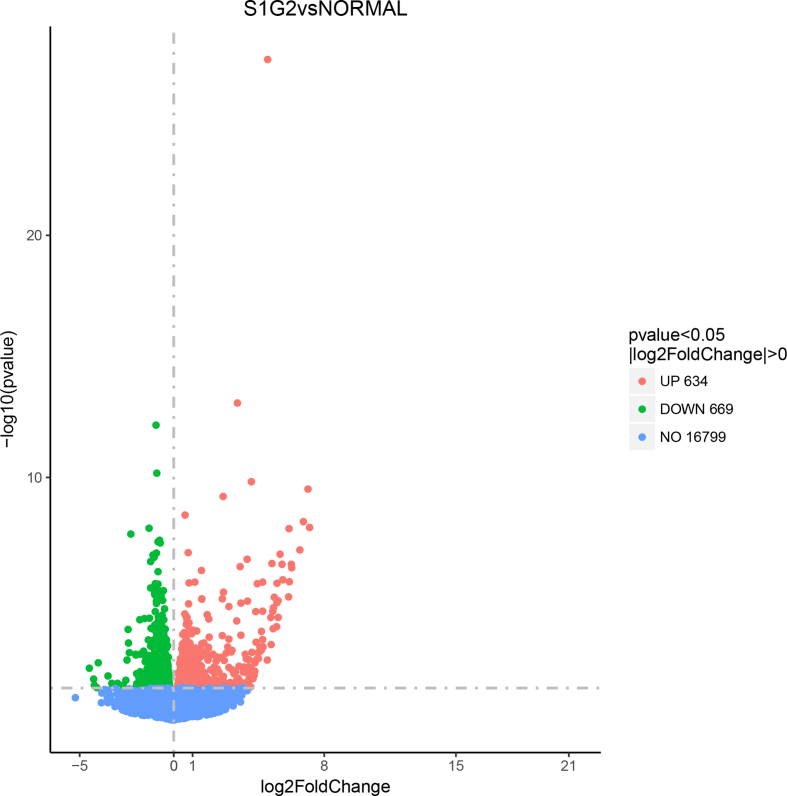
To explore why pigs in the S1G2 diet group had more intramuscular fat, we performed transcriptome analysis in longissimus dorsi muscle samples from pigs in different diet treatment groups. The temporally differentially expressed genes (DEG) with a log2 ratio > 0.5 (P > 0.009, false discovery rate < 0.02) between libraries are presented in Appendix Figure Figs1 and Figs2. The data for the LOW diet group versus the NORMAL diet group produced 2,299 DEG, including 1,054 upregulated DEG and 1,245 downregulated DEG. The data for the S1G2 diet group versus the NORMAL diet group produced 1,303 DEG, including 634 upregulated DEG and 669 downregulated DEG.
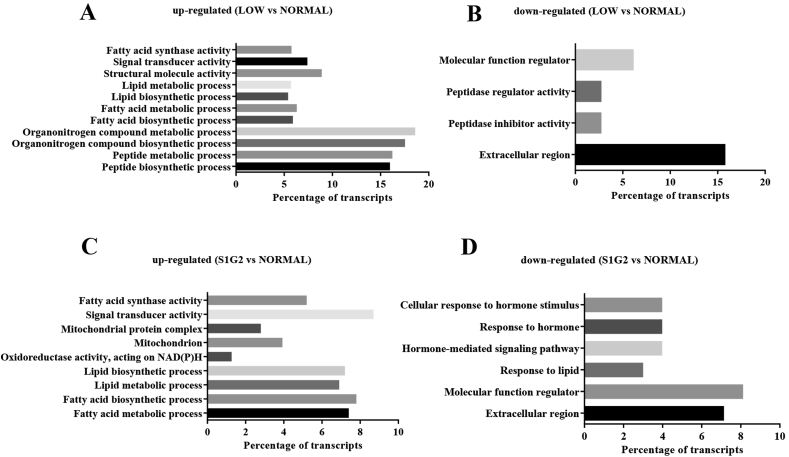
We next performed a gene ontology (GO) enrichment analysis to identify overrepresented biological processes, molecular functions, and cellular components corresponding to the list of DEG (Fig. 1). The upregulated DEG (LOW diet group versus NORMAL diet group) were significantly enriched in 11 GO terms principally including biosynthesis and metabolism of lipids and peptides, and the downregulated DEG were significantly enriched in 4 GO terms including molecular function regulator, peptidase regulator, and inhibitor activity, as well as extracellular region. The upregulated DEG (S1G2 diet group versus NORMAL diet group) were significantly enriched in 9 GO terms principally including lipid biosynthesis and metabolism and mitochondrial function, and the downregulated DEG were significantly enriched in 6 GO terms including cellular response to hormone stimulus, response to hormone, hormone-mediated signaling pathway, response to lipid, molecular function regulator, and extracellular region.
Fig. 1.
The GO analysis for differentially expressed genes. (A) Up-regulated GO terms between LOW and NORMAL groups. (B) Down-regulated GO terms between LOW and NORMAL groups. (C) Up-regulated GO terms between S1G2 and NORMAL groups. (D) Down-regulated GO terms between S1G2 and NORMAL groups. NORMAL, pigs fed a normal CP (16%) diet; LOW, pigs fed a low CP (12%) diet generated by decreasing the content of soybean meal; S1G2, pigs fed a low CP (12%) diet with serine-to-glycine ratio 1:2, generated by decreasing the content of soybean meal and adding 0.57% glycine. GO = gene ontology; CP = crude protein.
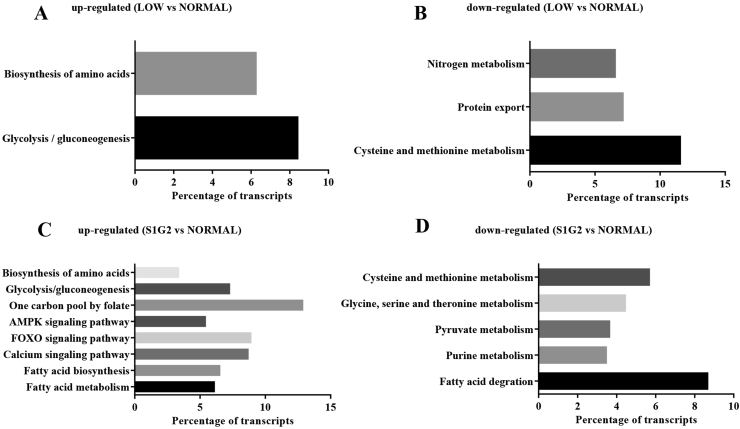
Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolic pathway analysis provided additional information on possible functional pathways that the DEG are involved in (Fig. 2). The results showed that the largest upregulated functional pathway was glycolysis/gluconeogenesis, followed by biosynthesis of amino acids in the DEG (LOW diet group versus NORMAL diet group). Nitrogen metabolism, protein export, as well as cysteine and methionine metabolism pathways were important downregulated pathways detected in the DEG (LOW diet group versus NORMAL diet group). When comparing the S1G2 diet group with the NORMAL diet group, the upregulated DEG were principally related to glycolysis/gluconeogenesis, biosynthesis of amino acids, one carbon pool by folate, AMPK, FOXO and calcium signaling pathway, as well as fatty acid biosynthesis and metabolism, and the downregulated DEG were mostly related to cysteine and methionine metabolism, glycine, serine and threonine metabolism, pyruvate metabolism, purine metabolism, and fatty acid degradation.
Fig. 2.
The KEGG pathway analysis for differentially expressed genes. (A) Up-regulated pathways between LOW and NORMAL group. (B) Down-regulated pathways between LOW and NORMAL group. (C) Up-regulated pathways between S1G2 and NORMAL group. (D) Down-regulated pathways between S1G2 and NORMAL group. NORMAL, pigs fed a normal CP (16%) diet; LOW, pigs fed a low CP (12%) diet generated by decreasing the content of soybean meal; S1G2, pigs fed a low CP (12%) diet with serine-to-glycine ratio 1:2, generated by decreasing the content of soybean meal and adding 0.57% glycine. KEGG = Kyoto Encyclopedia of Genes and Genomes. CP = crude protein; AMPK = adenosine monophosphate-activated protein kinase; FOXO = forkhead transcription factor O.
3.4. Verification of changes of gene expression in transcriptomic analysis
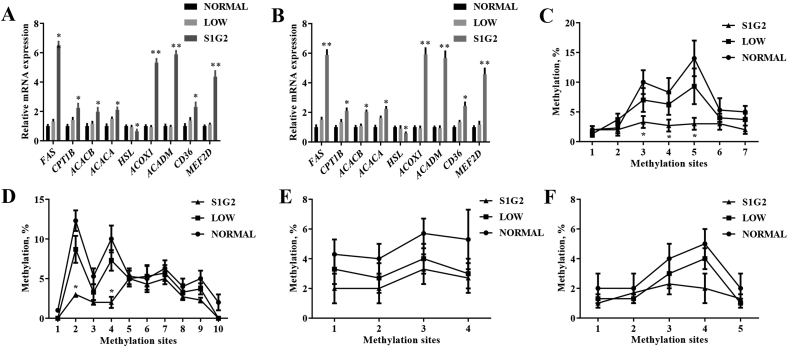
To verify the results of RNA-sequencing, 9 DEG closely involved in regulating lipid metabolism, namely FAS, carnitine palmitoyltransferase 1b (CPT1B), acetyl-CoA carboxylase beta (ACACB), acetyl-CoA carboxylase alpha (ACACA), lipase E, hormone sensitive type (HSL), ACOX1, ACADM, CD36 molecule (CD36), and MEF2D were selected for RT-PCR analysis. As shown in Fig. 3A and B, the results of gene expression by RT-PCR agreed with the transcriptomics results.
Fig. 3.
Differentially expressed genes involved in lipid metabolism and status of promoter DNA methylation. (A) Relative expression of differentially expressed genes according to RNA-seq sequencing (n = 3). (B) Verification of transcriptomics quantification by qRT-PCR (n = 8). The methylation status of representative CpG sites from promoter sequence of (C) ACOX1, (D) ACADM, (E) FAS and (F) MEF2D genes, respectively (n = 3). NORMAL, pigs fed a normal CP (16%) diet; LOW, pigs fed a low CP (12%) diet generated by decreasing the content of soybean meal; S1G2, pigs fed a low CP (12%) diet with serine-to-glycine ratio 1:2, generated by decreasing the content of soybean meal and adding 0.57% glycine. CP = crude protein. FAS = Fatty acid synthase; CPT1B = Carnitine palmityl transferase 1B; ACACB = acetyl-CoA carboxylase beta; ACACA = acetyl-CoA carboxylase alpha; HSL = lipase E, hormone sensitive type; ACOX1 = acyl-CoA oxidase 1; ACADM = acyl-CoA dehydrogenase medium chain; MEF2D = myocyte enhancer factor 2D. Values are expressed as means ± standard deviations. Mean values were significantly different among groups (∗P < 0.05, ∗∗P < 0.01).
3.5. Promoter DNA methylation of key genes
To further verify whether the significantly upregulated genes were regulated by DNA methylation, we further analyzed promoter DNA methylation of genes including FAS, ACOX1, ACADM, and MEF2D. As shown in Fig. 3C and D, the methylation levels of the promoter sequences of 2 genes (ACOX1 and ACADM) were highly different depending on diet. For the promoter sequence of the ACOX1 gene, CpG sites 3, 4, and 5 were significantly less methylated in S1G2 diet groups than in the NORMAL and LOW groups. For the promoter sequence of the ACADM gene, CpG sites 2 and 4 were significantly less methylated in S1G2 diet groups than in NORMAL and LOW groups. No significant difference in the methylation levels of the promoter sequences of FAS and MEF2D were observed (Fig. 3E and F).
3.6. Changes of fiber type of the longissimus dorsi muscle
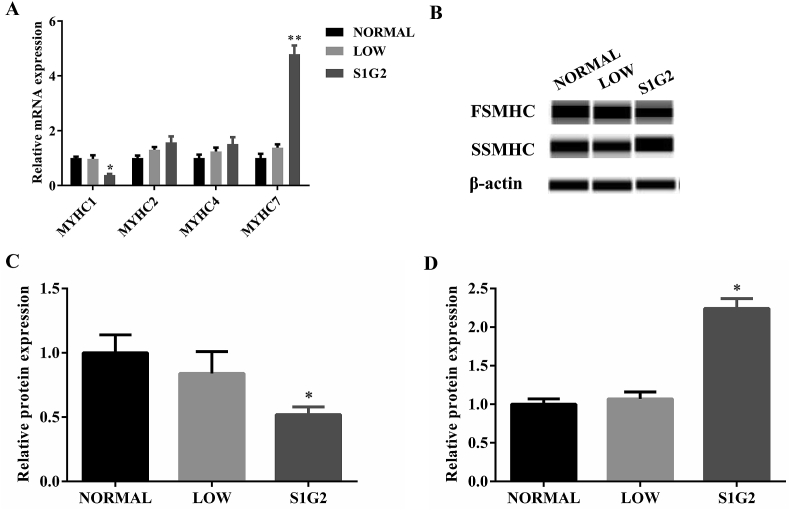
To further explore whether the increased intramuscular fat was accompanied by muscle fiber type transition, we analyzed gene and protein expression of myosin heavy chain. The results showed that gene expression of myosin heavy chain 1 (MYHC1) was significantly decreased whereas MYHC7 expression was significantly increased in the longissimus dorsi muscle of pigs in the S1G2 diet group when compared with pigs in NORMAL and LOW diet groups (Fig. 4A). Additionally, no changes in gene expression of MYHC2 and MYHC4 were observed among the three diet treatment groups. Moreover, protein expression of FSMHC was significantly decreased whereas SSMHC expression significantly increased in the longissimus dorsi muscles of pigs in the S1G2 diet group when compared with pigs in the NORMAL and LOW diet groups (Fig. 4B–D).
Fig. 4.
Expression of myosin heavy chain. (A) Relative mRNA expression of myosin heavy chain (n = 8). (B) Protein expression of myosin heavy chain. Relative protein expression of (C) FSMHC and (D) SSMHC calculated according to the results of (B) (n = 3). NORMAL, pigs fed a normal CP (16%) diet; LOW, pigs fed a low CP (12%) diet generated by decreasing the content of soybean meal; S1G2, pigs fed a low CP (12%) diet with serine-to-glycine ratio 1:2, generated by decreasing the content of soybean meal and adding 0.57% glycine. CP = crude protein. MYHC = myosin heavy chain; FSMHC = fast myosin skeletal heavy chain; SSMHC = slow myosin skeletal heavy chain. Values are expressed as means ± standard deviations. Mean values were significantly different among groups (∗P < 0.05, ∗∗P < 0.01).
3.7. Expression of proteins involved in the regulation of muscle fiber transition
To further explore changes of proteins involved in regulating the muscle fiber transition based on the KEGG metabolic pathway analysis, we measured the protein expression of AMPK, FOXO1, and Calcineurins, as well as their downstream targets. The results showed that expression of AMPK, phospho-AMPK, Calcineurin A and B, PGC-1α, MEF2D, and nuclear NAFT1 were significantly increased in longissimus dorsi muscles of pigs in the S1G2 diet group when compared with pigs in NORMAL and LOW diet groups (Fig. 5). However, the increase of FOXO1 protein expression did not reach statistical significance.
Fig. 5.
Expression of proteins involved in AMPK, calcineurin and FOXO signaling pathway. (A) Protein qualification by the Wes Simple Western System. (B) Relative protein expression of AMPK and pAMPK. (C) FOXO1, calcineurin A and calcineurin B. (D) PGC-1α, MEF2D, nuclear NFAT1 and total NFAT1, calculated according to the results of (A) (n = 3). Values are expressed as means ± standard deviations. Mean values were significantly different among groups (∗P < 0.05, ∗∗P < 0.01). NORMAL, pigs fed a normal CP (16%) diet; LOW, pigs fed a low CP (12%) diet generated by decreasing the content of soybean meal; S1G2, pigs fed a low CP (12%) diet with serine-to-glycine ratio 1:2, generated by decreasing the content of soybean meal and adding 0.57% glycine. CP = crude protein.
4. Discussion
There is increasing attention on the potential beneficial effects of glycine and serine because these amino acids lie at a key pivot point in intermediary metabolism, although they have long been considered as nutritionally non-essential amino acids. The current study showed that pigs fed a low-CP diet with 0.48% serine and 0.4% glycine had lower ADG and higher feed to gain ratio when compared with pigs fed the normal-CP diet. Surprisingly, when fed the low-CP diet with 0.48% serine and 0.96% glycine, pigs had the same ADG as pigs fed the normal CP diet, although with a higher feed to gain ratio. However, when feeding the low CP diet with the same total amount of serine and glycine (serine plus glycine = 1.44%) but different serine-to-glycine ratios (2:1 or 1:1), pigs had lower ADG and higher feed to gain ratio. These results suggested that maintaining the dietary serine-to-glycine ratio at 1:2 with a total amount of 1.44% in low-CP diets would benefit growth performance in growing-finishing pigs.
Fortunately, we unexpectedly found that the lipid contents of pigs in the S1G2 group were significantly changed, with a higher body fat rate and increased backfat thickness. Most importantly, the intramuscular fat was also increased. This result indicated that feeding pigs with the low-CP diet with 0.48% serine and 0.96% glycine may exert beneficial effects on meat quality, as intramuscular fat is one of the major characteristics of meat quality. Lipid metabolism including fatty acid biosynthesis, fatty acid oxidation, and lipid transportation regulate fat content in the skeletal muscle. We used a transcriptional analysis of gene expression and found that most DEG between pigs in the NORMAL group and pigs in the S1G2 group were enriched in lipid biosynthetic and metabolic processes as found by GO enrichment analysis. These results indicated that lipid metabolism was changed in the skeletal muscle of pigs in the S1G2 group. We further identified 8 DEG involved in lipid metabolism. Levels of 5 genes (CPT1B, ACACB, ACACA, ACOX1, and ACADM) involved in fatty acid oxidation, one gene (FAS) involved in fatty acid synthesis, and one gene (CD36) involved in fatty acid transportation were increased, whereas one gene (HSL) involved in lipolysis was decreased according to the transcriptomic analysis results and verified by RT-qPCR analysis. These changes suggested that a dietary serine-to-glycine ratio at 1:2 with a total amount of 1.44% in low-CP diets not only increased fatty acid deposition, but also increased fatty acid utilization in the skeletal muscle. Nevertheless, these changes ultimately resulted in net lipid accumulation in the skeletal muscle.
Epigenetic modifications including DNA methylation using methyl groups supplied by dietary nutrients, play an important role in regulating the transcription of genes involved in lipid metabolism (Zhao et al., 2018). Serine is critically important for maintaining whole-body methylation potential by supporting homeostasis of the methionine cycle, not only by providing one-carbon units, but also by increasing de novo ATP synthesis (Zhou et al., 2017a, 2017b). Our previous studies showed that dietary serine not only affected the ratio of S-adenosylmethionine to S-adenosylhomocysteine, which reflects methylation capacity, but also influenced the DNA methylation level of the genome (Zhou et al., 2017a). Importantly, dietary serine affects methylation status of CpG sites in the promoters of certain genes (Cao et al., 2018; Zhou et al., 2018a). Glycine is also closely involved in methylation reactions. Glycine can provide one-carbon units through the glycine cleavage system and promote the formation of methylene tetrahydrofolate from tetrahydrofolate (Newman and Maddocks 2017). In addition, the conversion of glycine to serine by SHMT1/2 can deplete the one-carbon pool (Labuschagne et al., 2014).
The balance of serine and glycine may determine the maintenance of whole-body methylation potential. Consequently, dietary serine and glycine concentrations and their ratio may regulate the expression of genes involved in fatty acid metabolism by affecting methylation in the promoters of certain genes. This speculation was confirmed by our finding of hypomethylation at certain CpG sites in the promoter of ACOX1 and ACADM genes. However, it has been suggested that dietary supply of methyl donors generally results in global DNA methylation, yet does not necessarily result in changes of methylation on the promoters of all functional genes (Cai et al., 2014; Zhou et al., 2015). In the present study, we did not observe changes in the methylation status of CpG sites in the promoter of FAS and MEF2D, although their mRNA levels were significantly increased. Presumably, the expression of FAS and MEF2D, which are downstream of the AMPK signaling pathway, may be regulated by the activation of AMPK. However, the mechanism underlying the effects of dietary glycine and serine balance on DNA methylation of certain gene promoters is difficult to define. Previous studies observed both hypomethylation and hypermethylation effects of dietary methyl donors on different gene promoters in the same experiments (Cai et al., 2014).
Myofiber type is one of the primary factors that determine meat quality, as it is closely associated with intramuscular fat content (Guo et al., 2011). The increased intramuscular fat content in pigs fed a low-CP diet maintaining the serine-to-glycine ratio at 1:2 and a total amount of 1.44% may be accompanied with changes in the skeletal muscle fiber type. MYHC is modulated at the transcriptional level in the pig skeletal muscle (Choi and Kim 2009). In the present study, we found increased gene expression of MYHC7 but decreased expression of MYHC1 in pigs in the S1G2 group. These results suggested that these pigs had more oxidative fibers and fewer glycolytic fibers. Previous studies showed that the molecular identification of myofiber types based on the results of MYHC expression was more credible and precise than the results of immunohistochemical staining (Park et al., 2009). Nevertheless, our protein expression results further confirmed that slow skeletal myosin heavy chain levels were increased and fast myosin skeletal heavy chain levels were decreased in pigs fed a low-CP diet maintaining the serine-to-glycine ratio at 1:2 and a total amount of 1.44%. These results suggested that the increased intramuscular fat observed in this study may also be a result of the formation of slow-twitch muscle fibers, as previous studies showed that slow-twitch muscle fibers contain more lipids than fast-twitch muscle fibers (Karlsson et al., 1999; Guo et al., 2011).
The factors involved in regulating adaptive changes of muscle fibers include (a) signals such as calcium signaling, (b) energy state (such as AMPK, Sirt1, and FOXO), and (c) transcriptional regulation (such as PGC-1α, myostatin, and NFκB) (Blaauw et al., 2013). According to the results of KEGG metabolic pathway analysis, we focused on AMPK, FOXO, and calcium signaling pathways. The AMPK pathway is the most important signal that senses actual energy levels and energy stores. There is a strong interaction between AMPK and PGC-1α. AMPK activation by phosphorylation upregulates PGC-1α, promoting transcriptional regulation of its target genes, and subsequently results in slow muscle properties (Jager et al., 2007). Our results showed increased expression of phosphorylated AMPK and PGC-1α, confirming that the AMPK-PGC-1α signaling pathway plays a role in forming slow-twitch muscle fibers in the longissimus muscle of pigs fed a low-CP diet maintaining the serine-to-glycine ratio at 1:2 and a total amount of 1.44%. It is not surprising that dietary serine and glycine activated the AMPK signaling pathway because previous studies showed that either serine or glycine could affect AMPK activity (Sun et al., 2016; Zhou et al. 2017b, 2018a). However, our results in pigs were opposite to those of a previous study which showed an inhibitory effect of glycine on AMPK activity in the C2C12 cell line (Sun et al., 2016). We presumed that the effects of these 2 amino acids on AMPK activity may differ in vivo and in vitro. Most importantly, the serine-to-glycine ratio and the total amount of these 2 amino acids may be critical factors that determine their effects on AMPK activity.
Calcineurin plays a fundamental role in regulating the formation of slow-twitch muscle fibers. The transcription factors NFAT and MEF2, which are both downstream targets of Calcineurin, are also pivotal in regulating fiber-type specification (Crabtree and Schreiber 2009). Activation of MEF2 and translocation of NFAT from the cytoplasm to the nucleus promote the transition of fiber type from slow to fast (Wu et al., 2000; Tothova et al., 2006). In the present study, we observed increased expression of Calcineurin A and B, MEF2D, and nuclear NFAT1 in the longissimus muscles of pigs fed a low-CP diet maintaining a serine-to-glycine ratio at 1:2 and a total amount of 1.44%, which was in line with the results of KEGG metabolic pathway analysis. Because MEF2 is also known to interact with PGC-1α (Akimoto et al., 2004), we presumably suggested that the AMPK-PGC-1α and Calcineurin-MEF2/NFAT pathways may regulate changes in skeletal muscle fiber type in a coordinated manner. The expression of FOXO1 did not change, so likely excludes the involvement of this pathway in regulating myofiber transition.
In conclusion, we explored the effects of various dietary serine-to-glycine ratios on growth performance of growing-finishing pigs and found that the pigs fed a low-CP diet, when maintaining the serine-to-glycine ratio at 1:2 and a total amount of 1.44%, had the same ADG as the pigs fed a normal-CP diet. Importantly, we found that the pigs in the S1G2 treatment group had increased intramuscular fat in the longissimus muscle. Transcriptome analysis and pyrosequencing showed that these pigs had higher expression of genes (ACOX1 and ACADM) involved in fatty acid oxidation, which was regulated by epigenetically modulating the methylation level in the promoters of these 2 genes. Furthermore, we found that these pigs had more slow-twitch muscle fibers, which were regulated by the AMPK-PGC-1α and Calcineurin-MEF2/NFAT pathways in a coordinated manner. Our findings are beneficial for understanding the molecular mechanisms underlying how the serine-to-glycine ratio and content affect meat quality in growing-finishing pigs, and may shed new light on the application of low-CP diets as a nutrition strategy for pig production when dietary protein is restricted.
Author contributions
Xihong Zhou: Conceptualization, Methodology, Writing – original draft. Yonghui Liu: Investigation, Resources. Lingyu Zhang: Investigation, Resources. Xiangfeng Kong: Writing – review & editing. Fengna Li: Conceptualization, Writing – review & editing, Supervision.
Conflic of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgement
This work was financially supported by National Key Research and Development Program of China (2018YFD0500405), Youth Innovation Promotion Association CAS, Open Fund of Key Laboratory of Agro-ecological Processes in Subtropical Region, Chinese Academy of Sciences (ISA2018304) and the earmarked fund for China Agriculture Research System (CARS-35).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2020.08.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- Akimoto T., Sorg B.S., Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-gamma coactivator-1 alpha promoter activity in skeletal muscles of living mice. Am J Physiol Cell Physiol. 2004;287(3):C790–C796. doi: 10.1152/ajpcell.00425.2003. [DOI] [PubMed] [Google Scholar]
- Baker D.H., Sugahara M., Scott H.M. Glycine-serine interrelationship in chick nutrition. Poultry Sci. 1968;47(4):1376–1377. doi: 10.3382/ps.0471376. [DOI] [PubMed] [Google Scholar]
- Blaauw B., Schiaffino S., Reggiani C. Mechanisms modulating skeletal muscle phenotype. Comp Physiol. 2013;3(4):1645–1687. doi: 10.1002/cphy.c130009. [DOI] [PubMed] [Google Scholar]
- Cai D.M., Jia Y.M., Song H.G., Sui S.Y., Lu J.Y., Jiang Z., Zhao R.Q. Betaine supplementation in maternal diet modulates the epigenetic regulation of hepatic gluconeogenic genes in neonatal piglets. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0105504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G.T., Tao F., Xin L., Li Z.M., Zhou X.H. Effects of maternal serine supplementation on high-fat diet-induced oxidative stress and epigenetic changes in promoters of glutathione synthesis-related genes in offspring. J Funct Foods. 2018;47:316–324. [Google Scholar]
- Choi Y.M., Kim B.C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest Sci. 2009;122(2–3):105–118. [Google Scholar]
- Corzo A., Kidd M.T., Burnham D.J., Kerr B.J. Dietary glycine needs of broiler chicks. Poultry Sci. 2004;83(8):1382–1384. doi: 10.1093/ps/83.8.1382. [DOI] [PubMed] [Google Scholar]
- Crabtree G.R., Schreiber S.L. SnapShot: Ca2+-calcineurin-NFAT signaling. Cell. 2009;138(1) doi: 10.1016/j.cell.2009.06.026. 210, 210 e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D.W., Bidner T.D., Southern L.L. Glycine supplementation to low protein, amino acid-supplemented diets supports optimal performance of broiler chicks. Poultry Sci. 2006;85(2):288–296. doi: 10.1093/ps/85.2.288. [DOI] [PubMed] [Google Scholar]
- Duan Y.H., Guo Q.P., Wen C.Y., Wang W.L., Li Y.H., Tan B., Li F.N., Yin Y.L. Free amino acid profile and expression of genes implicated in protein metabolism in skeletal muscle of growing pigs fed low-protein diets supplemented with branched-chain amino acids. J Agric Food Chem. 2016;64(49):9390–9400. doi: 10.1021/acs.jafc.6b03966. [DOI] [PubMed] [Google Scholar]
- Fan X.X., Li S., Wu Z.L., Dai Z.L., Li J., Wang X.L., Wu G.Y. Glycine supplementation to breast-fed piglets attenuates post-weaning jejunal epithelial apoptosis: a functional role of CHOP signaling. Amino Acids. 2019;51(3):463–473. doi: 10.1007/s00726-018-2681-9. [DOI] [PubMed] [Google Scholar]
- Greene D.E., Scott H.M., Johnson B.C. A need for Glycine in crystalline amino acid diets. Poultry Sci. 1960;39(2):512–514. [Google Scholar]
- Guo J., Shan T., Wu T., Zhu L.N., Ren Y., An S., Wang Y. Comparisons of different muscle metabolic enzymes and muscle fiber types in Jinhua and Landrace pigs. J Anim Sci. 2011;89(1):185–191. doi: 10.2527/jas.2010-2983. [DOI] [PubMed] [Google Scholar]
- He L., Wu J., Tang W., Zhou X., Lin Q., Luo F., Yin Y., Li T. Prevention of oxidative stress by alpha-ketoglutarate via activation of CAR signaling and modulation of the expression of key antioxidant-associated targets in vivo and in vitro. J Agric Food Chem. 2018;66(43):11273–11283. doi: 10.1021/acs.jafc.8b04470. [DOI] [PubMed] [Google Scholar]
- He L.Q., Zhang H.W., Zhou X.H. Weanling offspring of dams maintained on serine-deficient diet are vulnerable to oxidative stress. Oxid Med Cell Longev. 2018:8026496. doi: 10.1155/2018/8026496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S., Handschin C., St-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1 alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson A.H., Klont R.E., Fernandez X. Skeletal muscle fibres as factors for pork quality. Livest Prod Sci. 1999;60(2–3):255–269. [Google Scholar]
- Labuschagne C.F., van den Broek N.J.F., Mackay G.M., Vousden K.H., Maddocks O.D.K. Serine, but not Glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 2014;7(4):1248–1258. doi: 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- Li W., Sun K.J., Ji Y., Wu Z.L., Wang W.W., Dai Z.L., Wu G.Y. Glycine regulates expression and distribution of claudin-7 and ZO-3 proteins in intestinal porcine epithelial cells. J Nutr. 2016;146(5):964–969. doi: 10.3945/jn.115.228312. [DOI] [PubMed] [Google Scholar]
- Newman A.C., Maddocks O.D.K. Serine and functional metabolites in cancer. Trends Cell Biol. 2017;27(9):645–657. doi: 10.1016/j.tcb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Ospina-Rojas I.C., Murakami A.E., Moreira I., Picoli K.P., Rodrigueiro R.J.B., Furlan A.C. Dietary glycine plus serine responses of male broilers given low-protein diets with different concentrations of threonine. Br Poultry Sci. 2013;54(4):486–493. doi: 10.1080/00071668.2013.794257. [DOI] [PubMed] [Google Scholar]
- Park S.K., Gunawan A.M., Scheffler T.L., Grant A.L., Gerrard D.E. Myosin heavy chain isoform content and energy metabolism can be uncoupled in pig skeletal muscle. J Anim Sci. 2009;87(2):522–531. doi: 10.2527/jas.2008-1269. [DOI] [PubMed] [Google Scholar]
- Powell S., Bidner T.D., Payne R.L., Southern L.L. Growth performance of 20-to 50-kilogram pigs fed low-crude-protein diets supplemented with histidine, cystine, glycine, glutamic acid, or arginine. J Anim Sci. 2011;89(11):3643–3650. doi: 10.2527/jas.2010-3757. [DOI] [PubMed] [Google Scholar]
- Siegert W., Ahmadi H., Rodehutscord M. Meta-analysis of the influence of dietary glycine and serine, with consideration of methionine and cysteine, on growth and feed conversion of broilers. Poultry Sci. 2015;94(8):1853–1863. doi: 10.3382/ps/pev129. [DOI] [PubMed] [Google Scholar]
- Sugahara M., Kandatsu M. Protein-metabolism in domestic-fowl .5. Glycine serine interconversion in rooster. Agr Biol Chem Tokyo. 1976;40(5):833–837. [Google Scholar]
- Sun K., Wu Z., Ji Y., Wu G. Glycine regulates protein turnover by activating protein kinase B/mammalian target of rapamycin and by inhibiting MuRF1 and atrogin-1 gene expression in C2C12 myoblasts. J Nutr. 2016;146(12):2461–2467. doi: 10.3945/jn.116.231266. [DOI] [PubMed] [Google Scholar]
- Tothova J., Blaauw B., Pallafacchina G., Rudolf R., Argentini C., Reggiani C., Schiaffino S. NFATc1 nucleocytoplasmic shuttling is controlled by nerve activity in skeletal muscle. J Cell Sci. 2006;119(Pt 8):1604–1611. doi: 10.1242/jcs.02875. [DOI] [PubMed] [Google Scholar]
- Waterhouse H., Scott H.M. Effect of different proteins and protein levels on Glycine need of chick fed purified diets. Poultry Sci. 1961;40(5):1160–1165. [Google Scholar]
- Wu H., Naya F.J., McKinsey T.A., Mercer B., Shelton J.M., Chin E.R., Simard A.R., Michel R.N., Bassel-Duby R., Olson E.N., Williams R.S. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 2000;19(9):1963–1973. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Yang S., Jia Y., Sun B., He B., Zhao R. Maternal betaine supplementation attenuates glucocorticoid-induced hepatic lipid accumulation through epigenetic modification in adult offspring rats. J Nutr Biochem. 2018;54:105–112. doi: 10.1016/j.jnutbio.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Zhou X.H., Chen J.Q., Chen J., Wu W.C., Wang X.X., Wang Y.Z. The beneficial effects of betaine on dysfunctional adipose tissue and N6-methyladenosine mRNA methylation requires the AMP-activated protein kinase alpha 1 subunit. J Nutr Biochem. 2015;26(12):1678–1684. doi: 10.1016/j.jnutbio.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Zhou X.H., He L.Q., Wu C.R., Zhang Y.M., Wu X., Yin Y.L. Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol Nutr Food Res. 2017;61(11):1700262. doi: 10.1002/mnfr.201700262. [DOI] [PubMed] [Google Scholar]
- Zhou X.H., He L.Q., Zuo S.N., Zhang Y.M., Wan D., Long C.M., Huang P., Wu X., Wu C.R., Liu G., Yin Y.L. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. BBA-Mol Basis Dis. 2018;1864(2):488–498. doi: 10.1016/j.bbadis.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Zhou X.H., Zhang Y.M., He L.Q., Wan D., Liu G., Wu X., Yin Y.L. Serine prevents LPS-induced intestinal inflammation and barrier damage via p53-dependent glutathione synthesis and AMPK activation. J Funct Foods. 2017;39:225–232. [Google Scholar]
- Zhou X.H., Zhang Y.M., Wu X., Wan D., Yin Y.L. Effects of dietary serine supplementation on intestinal integrity, inflammation and oxidative status in early-weaned piglets. Cell Physiol Biochem. 2018;48(3):993–1002. doi: 10.1159/000491967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.