Abstract
The objective of the study is to evaluate and compare the effects of betaine or glycine on carcass trait, meat quality and lipid metabolism of finishing Huan Jiang mini-pigs. Betaine called trimethylglycine is a methyl derivative of glycine, but few researches were conducted to compare the impact of dietary betaine and glycine on pigs. One hundred and forty-four Huan Jiang mini-pigs (body weight = 10.55 ± 0.15 kg; 70 d) were randomly divided to 3 treatment groups (basal diet, glycine or betaine). Results indicated that dietary betaine increased the average daily gain (ADG) and final weight (P < 0.05). Dietary glycine or betaine markedly reduced average backfat thickness (P < 0.05) and heightened lean percentage (P < 0.01) compared to the control group. Moreover, in comparison with the control group, betaine significantly improved the redness (a∗) and tenderness (shear force) of the longissimus dorsi (LD) muscle (P < 0.05), whereas glycine only raised the value of a∗ of the LD muscle (P < 0.05). These results showed that diet supplemented with 0.25% betaine and equimolar amounts of glycine could regulate cascass trait and meat quality of finishing Huan Jiang mini-pigs, and the effect of betaine was superior to that of glycine.
Keywords: Betaine, Glycine, Meat quality, Lipid metabolism, Huan jiang mini-pig
1. Introduction
Betaine, i.e., N,N,N-trimethylglycine, is a methyl derivative of glycine and a coproduct of extracting molasses from sugar beet (Cholewa et al., 2014). It is generally found in animals and plants and is a secondary metabolite of choline oxidative decomposition (Eklund et al., 2005). Betaine has many functions, mainly as a methyl donor involved in methionine-homocysteine cycle and as a penetrant to maintain body osmotic pressure balance (Martins et al., 2012; Albuquerque et al., 2017). Several researchers report that 0.25% betaine is moderate to increase lean percentage and reduce average backfat thickness and fat percentage in finishing pigs (Matthews et al., 2001; Siljander-Rasi et al., 2016; Li et al., 2017a). A previous study suggests that diet supplemented with betaine increases final weight and average daily gain (ADG) (Huang et al., 2006), although no difference is observed in other studies (Matthews et al., 2001; Martins et al., 2012). Another research shows that the average backfat thickness is lower in finishing pigs fed with betaine than that in the control group (Yu et al., 2004), but others indicate no effect noticed (Fernández-Fígares et al., 2008; Martins et al., 2012). In addition, some reports show that betaine increases lean percentage (Matthews et al., 2001; Huang et al., 2007), whereas others indicates that betaine has no effect on lean percentage (Rojas-Cano et al., 2011). It is also reported that intramuscular fat (IMF) increases in finishing pigs fed with betaine (Martins et al., 2012), but no significant difference is observed in the study of Rojas-Cano et al. (2011). These inconsistent results suggested that impact of betaine on growth performance and meat quality in finishing pigs is still ambiguous and needs further research.
Betaine is a methyl derivative of glycine. Glycine can improve muscle cell growth by enhancing protein synthesis and inhibiting protein degradation in C2C12 myoblasts (Sun et al., 2016). Some researches indicate that glycine enhances muscle protein mass as a functional amino acid in piglets (Wang et al., 2014; Liu et al., 2016b). However, there are few studies on the influence of glycine in finishing pigs. We consider whether glycine possesses a similar function to betaine in affecting carcass trait, meat quality and lipid metabolism in finishing pigs. Therefore, equimolar glycine and betaine are supplemented in the present experimental diets and compare the difference.
The Huan Jiang mini-pig is an indigenous pig breed in South China, with soft black skin and delicious meat, which is very popular in the local area. Due to its typical obese genotype, more and more attention is being paid to lipid metabolism research. Moreover, its physiological and anatomical structure is similar to human beings (Liu et al., 2015). Thus, Huan Jiang mini-pig was selected as an animal model in the present experiment.
2. Materials and methods
2.1. Animals and experiments
All of the experimental protocols were carried out in compliance with procedures approved by the Animal Ethics Committee of South China Agricultural University. A total of 144 Huan Jiang mini-pigs (neutered boars) with an average initial body weight of 10.55 ± 0.15 kg (mean ± SEM) were assigned to 3 treatment groups with a completely randomized design, with 8 replications of 6 pigs per replicate (pen). The treatment diets included a corn-soybean meal basal diet, which was supplemented with 0.25% betaine, and an equimolar amount of glycine (0.16%; Table 1). In addition, betaine was added in the form of betaine hydrochloride (0.35%, Shandong Xiangweisi Biotechnology Co., Ltd.). The basal diet was formulated to meet the nutritional requirement of finishing pigs according to Chinese National Feeding Standard for Swine (2004). All of the pigs were housed in pens of 2.0 m × 3.0 m with a concrete slotted floor with an area of 1.0 m × 3.0 m and had free access to water and diets throughout the whole experiment. The experimental period lasted for 60 d following a 3-d preliminary feeding period supplemented with the same basic diet.
Table 1.
Ingredients and nutrient levels of experimental diets (as-fed basis).1
| Item | Control | Glycine | Betaine |
|---|---|---|---|
| Ingredients, % | |||
| Corn | 65.00 | 65.20 | 64.71 |
| Soybean meal | 19.40 | 19.00 | 19.50 |
| Wheat bran | 12.46 | 12.50 | 12.30 |
| Lysine | 0.14 | 0.14 | 0.14 |
| CaHPO4 | 0.75 | 0.75 | 0.75 |
| Limestone | 0.95 | 0.95 | 0.95 |
| Salt | 0.30 | 0.30 | 0.30 |
| Premix2 | 1.00 | 1.00 | 1.00 |
| Betaine NaCl | 0.00 | 0.00 | 0.35 |
| Glycine | 0.00 | 0.16 | 0.00 |
| Nutrient, % | |||
| Digestible energy, MJ/kg | 13.62 | 13.59 | 13.58 |
| Crude protein3 | 15.07 | 15.08 | 15.06 |
| Lys | 0.75 | 0.74 | 0.75 |
| Met + Cys | 0.50 | 0.50 | 0.50 |
| Thr | 0.47 | 0.47 | 0.47 |
| Ca | 0.63 | 0.63 | 0.63 |
| Total P | 0.53 | 0.53 | 0.53 |
| Available P | 0.24 | 0.24 | 0.24 |
Basal diet formulated according to the Chinese National Feeding Standard for Swine.
Provided per kilogram of diet: Fe, 400 mg as FeSO4·7H2O; Cu, 19.8 mg as CuSO4·5H2O; I, 0.20 mg as KI; Se, 0.56 mg as NaSeO3; Zn, 359 mg as ZnSO4·7H2O; Mn, 10.2 mg as MnSO4·H2O; 5 mg vitamin K; 2 mg vitamin B1; 15 mg vitamin B2; 30 μg vitamin B12; 5,400 IU vitamin A; 110 IU vitamin D3; 18 IU vitamin E; 20 mg antioxidants.
Measured nutrient levels (DM basis).
2.2. Samples collection
At the end of the trial, all the pigs were fasted overnight (12 h), and one pig of each replicate with the average final body weight was selected (8 pigs of each group) to slaughter by electrical stunning and bleeding in a commercial abattoir. Before slaughter, blood samples were collected into 10-mL anticoagulant centrifuge tubes and placed at room temperature for 2 h, then centrifuged at 3,000 × g for 10 min at 4 °C. Serum was collected and stored at −80 °C until further analysis. The longissimus dorsi (LD) muscle between the 6th and 7th ribs was immediately excised and stored at −20 °C for determination of the chemical composition, or placed in liquid N2 and then stored at −80 °C for the analysis of quantitative real-time PCR.
2.3. Carcass trait analysis
The left side of the carcass was weighed and then split up into skeletal muscle and fat as previously described (Martins et al., 2012). Loin-eye area and average backfat thickness (3rd to 4th lumbar vertebra, 10th to 11th, and the last rib) were measured.
2.4. Meat quality assessment
The pH value, color, shear force, water-holding capacity, cooking loss and IMF content of the LD muscle were measured to evaluate pork quality. A hand-held pH meter (pH-STAR, SFK-Technology, Denmark) was used to determine the pH values at 45 min and 24 h postmortem. The LD muscle was given 10 to 15 min for the color to develop after cutting. Subsequently, meat color including lightness (L∗), redness (a∗) and yellowness (b∗) was measured using a hand-held colorimeter (CR-410, Minolta Camera, Co., Osaka, Japan). Water-holding capacity, cooking loss and shear force were determined as described in our previous study (Li et al., 2018). Shear force was measured using a texture analyzer (TA-tx2i Texture Analyzer, Stable Micro Systems, Godalming, UK) equipped with a Warner–Bratzler shear force device using cooked and cooled samples.
2.5. Serum biochemical indexes measurements
Alanine aminotransferase, aspartate aminotransferase), total protein, albumin, urea nitrogen, creatinine, blood ammonia in serum were measured with automatic biochemical analyzer (Beckman CX4; Beckman Coulter, Germany) and commercial kits (Leadman Biotech Limited, Beijing, China) as specified by the manufacturer.
2.6. Measurement of free amino acids in serum and LD muscle
The free amino acid profile in serum was measured as previously described (Hu et al., 2019). Firstly, 1 mL of 8% 5-sulfosalicylic acid was added to tube including 1 mL of serum, mixed, stood for 15 min, and then centrifuged at 9,390 × g at room temperature for 10 min. Subsequently, 0.22 μm membrane was used to filter the supernatant before measurement. The free amino acid concentrations in LD muscle were determined as our previous studies outlined (Duan et al., 2016b; Liu et al., 2016a). Approximately 0.5 g of freeze-dried muscle sample was homogenized in 10 mL of 0.01 mol/L hydrochloric acid and shaken for 30 min. Then the solution added up to 25 mL with 0.01 mol/L hydrochloric acid. After centrifuging at 13,520 × g at room temperature for 10 min, 1 mL of the supernatant was mixed with 1 mL of 8% 5-sulfosalicylic acid and centrifuged again. One milliliter of the supernatant was chosen to filter with 0.22 μm membrane before analysis. All samples were determined by an ion-exchange AA analyzer (L8800, Hitachi, Tokyo, Japan).
2.7. Measurement of fatty acid composition and IMF in LD muscle
The IMF content was measured using the Soxhlet extraction method as previously outlined (Duan et al., 2016a). The free fatty acid concentrations were measured as described in detail (Li et al., 2017a). The fatty acid composition of LD muscle was measured as previously described (Li et al., 2015). Lipids were extracted from the LD muscle samples by the benzene-petroleum ether (1:1, vol/vol) procedure. Fatty acid methyl esters were prepared using 0.4 mol/L KOH/methanol solution, then determined by an Agilent 6890N gas chromatographer equipped with a flame ionization detector (Agilent Technologies) and A CP-Sil 88 fused silica open tubular capillary column (100 m × 0.25 nm; Chrompack). The method of gas chromatography analysis was as follows: original temperature was set at 45 °C for 4 min, raised to 175 °C at 13 °C/min, held at 175 °C for 27 min, warmed from 175 to 215 °C at 4 °C/min, and then held at 215 °C for 35 min. The injector and detector temperatures were stabilized at 250 °C. The carrier gas was hydrogen and the flow rate was set at 30 mL/min. The concentration of individual fatty acids was quantified by the precise standard and expressed as a percentage of the total fatty acids.
2.8. Quantitative real-time PCR analysis
The quantitative real-time PCR was conducted as described in our previous study (Zhong et al., 2019). The TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to isolate RNA from LD muscle tissues (Invitrogen, Carlsbad, CA, US) following the manufacturer's protocol. Quantitative real-time PCR was performed with an ABI 7900HT Real-time PCR system (Applied Biosystems, Branchburg, NJ) to determine relative mRNA expression levels of the selected genes.
Primers of the target genes were designed using Primer 5.0 software (Table 2). To normalize the mRNA expression levels of target genes, the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was selected as an internal control. The procedures of quantitative real-time PCR were set as follows: incubated at 95 °C for 10 min, denatured at 95 °C for 15 s with 40 cycles, annealed at 60 °C for 60 s, and extended at 72 °C for 30 s. The mRNA expression levels of target genes in arbitrary unit were acquired from the value of the threshold cycle (Ct) of the real-time PCR as related to that of internal control using the comparative 2−ΔΔCt method (Duan et al., 2019).
Table 2.
Primers used for quantitative real-time PCR.
| Genes | Primers | Sequences (5′ to 3′) | Product size, bp |
|---|---|---|---|
| ACC | Forward | TTCCAGGCACAGTCCTTAGG | 161 |
| Reverse | TCATCCAACACGAGCTCAGT | ||
| FAS | Forward | CTACCTTGTGGATCACTGCATAGA | 114 |
| Reverse | GGCGTCTCCTCCAAGTTCTG | ||
| CPT1B | Forward | ATGGTGGGCGACTAACT | 321 |
| Reverse | TGCCTGCTGTCTGTGAG | ||
| HSL | Forward | GCAGCATCTTCTTCCGCACA | 195 |
| Reverse | AGCCCTTGCGTAGAGTGACA | ||
| FAT/CD36 | Forward | CTGGTGCTGTCATTGGAGCAGT | 160 |
| Reverse | CTGTCTGTAAACTTCCGTGCCTGTT | ||
| FATP1 | Forward | GGAGTAGAGGGCAAAGCAGG | 208 |
| Reverse | AGGTCTGGCGTGGGTCAAAG | ||
| PPARγ | Forward | AGGGCCAAGGATTCATGACA | 248 |
| Reverse | GTGGTTCAACTTGAGCTGCA | ||
| SREBP1c | Forward | GCGACGGTGCCTCTGGTAGT | 218 |
| Reverse | CGCAAGACGGCGGATTTA | ||
| MyHC I | Forward | GGCCCCTTCCAGCTTGA | 63 |
| Reverse | TGGCTGCGCCTTGGTTT | ||
| MyHC IIa | Forward | TTAAAAAGCTCCAAGAACTGTTTCA | 100 |
| Reverse | CCATTTCCTGGTCGGAACTC | ||
| MyHC IIx | Forward | AGCTTCAAGTTCTGCCCCACT | 76 |
| Reverse | GGCTGCGGGTTATTGATGG | ||
| MyHC IIb | Forward | CACTTTAAGTAGTTGTCTGCCTTGAG | 80 |
| Reverse | GGCAGCAGGGCACTAGATGT | ||
| GAPDH | Forward | AAGGAGTAAGAGCCCCTGGA | 140 |
| Reverse | TCTGGGATGGAAACTGGAA |
ACC = acetyl-CoA carboxylase α; FAS = fatty acid synthase; CPT1B = carnitine palmitoyl transferase 1B; HSL = hormone-sensitive lipase; FAT/CD36 = fatty acid translocase; FATP1 = fatty acid transport protein 1; PPARγ = peroxisome proliferator-activated receptor γ; SREBP1c = sterol regulatory element binding protein-1c; MyHC = myosin heavy chain; GAPDH = Glyceraldehyde-3-phosphate dehydrogenase.
2.9. Statistical analysis
To assess influence of treatments, all data in the current study was analyzed by one-way analysis of variance (ANOVA) using the SAS 8.2 software (Cary, NC, USA) followed by Duncan's multiple comparison test. Means and standard error of mean (SEM) were adopted to express results. P < 0.05 was considered statistically significant and 0.05 < P < 0.10 was considered a trend toward significance.
3. Results
3.1. Growth performance
As shown in Table 3, final weight (P = 0.08) and ADG (P = 0.06) of the pigs fed with 0.25% betaine were higher than those of control group, but no difference was observed between the glycine and the control groups. In addition, no significant difference was noticed in the average daily feed intake (ADFI) and the ratio of feed intake to body weight gain (Feed:Gain) among the 3 treatment groups (P > 0.05).
Table 3.
Impact of diet supplemented with betaine or glycine on growth performance of finishing Huan Jiang mini-pigs.
| Item | Control | Glycine | Betaine | SEM | P-value |
|---|---|---|---|---|---|
| Initial weight, kg | 10.59 | 10.55 | 10.53 | 0.15 | 0.97 |
| Final weight, kg | 30.21b | 31.39ab | 32.29a | 0.32 | 0.08 |
| ADG, g/d | 326.90b | 347.02ab | 360.49a | 1.24 | 0.06 |
| ADFI, g/d | 972.35 | 999.74 | 979.35 | 3.12 | 0.74 |
| Feed:Gain ratio1 | 2.98 | 2.89 | 2.80 | 0.17 | 0.26 |
ADG = average daily gain; ADFI = average daily feed intake.
a, b Within a row, values with different superscripts differ significantly at P < 0.05 and a trend toward significance at P < 0.10. Data are expressed as means ± SEM, n = 8.
Feed:Gain ratio = the ratio of feed intake to body weight gain.
3.2. Carcass trait and meat quality
Betaine and glycine both decreased the average backfat thickness (P < 0.05) and increased lean percentage (P < 0.01) when compared to the control group. The 45 min pH value, 24 h pH value, L∗ value, b∗ value, water-holding capacity, and cooking loss had no significant difference among the 3 treatment groups (P > 0.05). Nevertheless, a∗ value was higher in the betaine and the glycine groups than the control group (P = 0.04). Moreover, the shear force showed a notable decrease in the betaine group compared to the other 2 groups (P = 0.01; Table 4).
Table 4.
Impact of diet supplemented with betaine or glycine on carcass trait and meat quality of finishing Huan Jiang mini-pigs.
| Item | Control | Glycine | Betaine | SEM | P-value |
|---|---|---|---|---|---|
| Average backfat thickness, mm | 19.64a | 18.00b | 18.09b | 0.40 | 0.05 |
| Loin eye area, mm2 | 893.60 | 931.81 | 897.70 | 3.65 | 0.78 |
| Lean percentage, % | 38.11b | 40.69a | 40.87a | 0.41 | <0.01 |
| pH45 min | 6.29 | 6.31 | 6.39 | 0.17 | 0.73 |
| pH24 h | 5.46 | 5.48 | 5.34 | 0.12 | 0.13 |
| L∗ (lightness) | 45.16 | 44.57 | 45.14 | 0.44 | 0.76 |
| a∗ (redness) | 15.77b | 16.71a | 16.78a | 0.30 | 0.04 |
| b∗ (yellowness) | 4.71 | 4.35 | 4.42 | 0.20 | 0.12 |
| Water-holding capacity, % | 20.24 | 19.68 | 18.41 | 0.68 | 0.67 |
| Cooking loss, % | 58.34 | 56.93 | 56.73 | 0.43 | 0.12 |
| Shear force, N | 46.77a | 45.08a | 39.37b | 0.74 | 0.01 |
a,b Within a row, values with different superscripts differ significantly at P < 0.05 and a trend toward significance at P < 0.10. Data are expressed as means ± SEM, n = 8.
3.3. Serum biochemical indexes
The concentrations of serum metabolites with different dietary treatments are presented in Table 5. Diet supplemented with betaine or glycine did not affect the concentrations of alanine aminotransferase, aspartate aminotransferase, total protein, albumin, urea nitrogen, creatinine, and blood ammonia in serum of the pigs (P > 0.05).
Table 5.
Impact of diet supplemented with betaine or glycine on serum metabolites of finishing Huan Jiang mini-pigs.
| Item | Control | Glycine | Betaine | SEM | P-value |
|---|---|---|---|---|---|
| ALT, U/L | 62.67 | 63.69 | 68.61 | 0.89 | 0.22 |
| AST, U/L | 62.10 | 65.00 | 65.00 | 1.08 | 0.81 |
| TP, g/L | 74.55 | 74.66 | 74.97 | 0.50 | 0.93 |
| ALB, g/L | 49.21 | 48.34 | 47.64 | 0.44 | 0.21 |
| NH3, μmol/L | 206.72 | 207.08 | 210.52 | 1.24 | 0.84 |
| BUN, mmol/L | 4.43 | 4.77 | 4.98 | 0.24 | 0.13 |
| CREA, μmol/L | 69.00 | 70.60 | 73.00 | 0.70 | 0.21 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; TP = total protein; ALB = albumin; NH3 = ammonia; BUN = urea nitrogen; CREA = creatinine.
Data are expressed as means ± SEM, n = 8.
3.4. Free amino acid profiles in serum and skeletal muscle
As can be seen from Table 6, the concentration of essential amino acids in serum did not change significantly (P > 0.05). An obvious increase was observed in glycine concentration in the glycine group compared to the other 2 groups (P < 0.05). Moreover, the cysteine concentration in the pigs fed with glycine or betaine was lower than the control group (P < 0.01).
Table 6.
Impact of dietary betaine on serum free amino acids of finishing Huan Jiang mini-pigs (μg/mL).
| Item | Control | Glycine | Betaine | SEM | P-value |
|---|---|---|---|---|---|
| EAA | |||||
| Lysine | 22.37 | 22.84 | 22.83 | 0.34 | 0.59 |
| Methionine | 4.14 | 4.21 | 4.27 | 0.18 | 0.69 |
| Valine | 33.55 | 33.95 | 33.62 | 0.59 | 0.96 |
| Isoleucine | 15.63 | 15.95 | 15.02 | 0.38 | 0.37 |
| Leucine | 25.03 | 24.93 | 25.08 | 0.49 | 0.99 |
| Phenylalanine | 12.90 | 12.35 | 13.00 | 0.27 | 0.12 |
| Histidine | 13.25 | 13.40 | 13.48 | 0.44 | 0.96 |
| Threonine | 17.92 | 17.92 | 17.97 | 0.46 | 1.00 |
| NEAA | |||||
| Alanine | 26.05 | 26.24 | 24.87 | 0.54 | 0.53 |
| Aspartic acid | 3.04 | 3.23 | 3.15 | 0.18 | 0.42 |
| Glutamic acid | 34.44 | 34.35 | 35.14 | 0.71 | 0.93 |
| Arginine | 33.35 | 33.48 | 33.07 | 0.53 | 0.95 |
| Glycine | 40.11b | 46.49a | 41.37b | 0.64 | <0.01 |
| Serine | 8.32 | 8.63 | 8.90 | 0.30 | 0.39 |
| Tyrosine | 11.51 | 11.27 | 11.72 | 0.34 | 0.68 |
| Cysteine | 5.72a | 4.37b | 4.18b | 0.30 | <0.01 |
| Proline | 17.27 | 17.63 | 17.58 | 0.35 | 0.78 |
| Total EAA | 144.78 | 145.56 | 145.27 | 0.80 | 0.96 |
| Total NEAA | 179.82 | 185.69 | 179.98 | 0.83 | 0.11 |
| Total AA | 324.60 | 331.25 | 325.25 | 0.92 | 0.18 |
EAA = essential amino acids; NEAA = non-essential amino acids.
a,b Within a row, values with different superscripts differ significantly at P < 0.05 and a trend toward significance at P < 0.10. Data are expressed as means ± SEM, n = 8.
The free amino acid profiles in LD muscle are presented in Table 7. A significant increase was noticed in methionine and threonine concentrations in the glycine group and the betaine group (P < 0.01). Serine concentration showed an increasing trend in the glycine and the betaine group compared to the control group (P < 0.10). In addition, the cysteine concentration in the control group was higher than the other 2 groups (P < 0.01). The essential amino acids (EAA), non-essential amino acids (NEAA) and total amino acids (TAA) did not change among the 3 treatment groups (P > 0.05).
Table 7.
Impact of diet supplemented with betaine on free amino acids of longissimus dorsi muscle in finishing Huan Jiang mini-pigs (μg/g).
| Item | Control | Glycine | Betaine | SEM | P-value |
|---|---|---|---|---|---|
| EAA | |||||
| Lysine | 81.64 | 86.17 | 82.82 | 1.01 | 0.59 |
| Methionine | 59.05b | 63.03a | 63.88a | 0.58 | <0.01 |
| Valine | 111.79 | 104.28 | 98.02 | 1.23 | 0.14 |
| Isoleucine | 90.14 | 87.93 | 87.25 | 0.94 | 0.75 |
| Leucine | 129.57 | 124.85 | 119.14 | 1.25 | 0.34 |
| Phenylalanine | 93.57 | 92.81 | 94.65 | 0.70 | 0.70 |
| Histidine | 62.01 | 64.35 | 62.10 | 0.84 | 0.70 |
| Threonine | 71.09b | 84.28a | 81.46a | 0.96 | <0.01 |
| NEAA | |||||
| Alanine | 437.75 | 494.87 | 488.10 | 2.63 | 0.15 |
| Aspartic acid | 250.48 | 261.96 | 274.28 | 2.04 | 0.45 |
| Glutamic acid | 201.78 | 196.43 | 198.52 | 1.56 | 0.89 |
| Arginine | 58.51 | 59.20 | 57.03 | 0.70 | 0.61 |
| Glycine | 281.43 | 317.01 | 292.62 | 2.65 | 0.52 |
| Serine | 62.38 | 70.59 | 70.39 | 0.97 | 0.10 |
| Tyrosine | 83.91 | 74.98 | 79.93 | 0.96 | 0.12 |
| Cysteine | 21.63a | 18.55b | 18.17b | 0.48 | <0.01 |
| Proline | 113.85 | 116.51 | 113.53 | 0.92 | 0.69 |
| Total EAA | 698.86 | 707.73 | 689.33 | 2.10 | 0.65 |
| Total NEAA | 1,511.72 | 1,610.10 | 1,592.55 | 3.83 | 0.29 |
| Total AA | 2,210.58 | 2,317.83 | 2,281.88 | 4.08 | 0.35 |
EAA = essential amino acids; NEAA = non-essential amino acids.
a,b Within a row, values with different superscripts differ significantly at P < 0.05 and a trend toward significance at P < 0.10. Data are expressed as means ± SEM, n = 8.
3.5. Fatty acid profile in skeletal muscle
The free fatty acid (FFA) and IMF content in the glycine and the betaine groups were lower than that of the control group (P < 0.01). Diet supplemented with betaine and glycine greatly altered the fatty acid composition of LD muscle (Table 8). Betaine and glycine decreased the concentration of saturated fatty acid (SFA, P < 0.05), whereas the concentration of polyunsaturated fatty acid (PUFA) and PUFA-to-SFA ratio markedly increased relative to the control group (P < 0.01). The concentrations of C18:3n3 and C20:4n6 were higher in the glycine and betaine groups when compared to the control group (P < 0.01). Furthermore, diet supplemented with glycine and betaine did not affect the value of monounsaturated fatty acid (MUFA, P > 0.05).
Table 8.
Impact of diet supplemented with betaine on fatty acid composition and intramuscular fat content of longissimus dorsi muscle in finishing Huan Jiang mini-pigs (% of total fatty acids).
| Item | Control | Glycine | Betaine | SEM | P-value |
|---|---|---|---|---|---|
| FFA, mmol/g protein | 0.43a | 0.23b | 0.23b | 0.10 | <0.01 |
| IMF, % | 4.45a | 3.91b | 3.99b | 0.19 | <0.01 |
| C14:0 | 1.19a | 0.87b | 0.93b | 0.12 | <0.01 |
| C14:1 | 0.05b | 0.05a | 0.05b | 0.03 | 0.04 |
| C15:0 | 0.09 | 0.09 | 0.10 | 0.04 | 0.55 |
| C16:0 | 24.44 | 22.94 | 23.17 | 0.35 | 0.02 |
| C16:1 | 3.26 | 2.90 | 2.86 | 0.22 | 0.16 |
| C17:0 | 0.84 | 0.74 | 0.82 | 0.12 | 0.27 |
| C18:0 | 12.85 | 12.95 | 13.13 | 0.27 | 0.68 |
| C18:1n9t | 0.13a | 0.12b | 0.12b | 0.03 | <0.01 |
| C18:1n9c | 32.21 | 31.53 | 32.00 | 0.41 | 0.66 |
| C18:2n6c | 15.98b | 17.02a | 16.58ab | 0.32 | 0.10 |
| C20:0 | 0.22a | 0.18b | 0.19ab | 0.06 | 0.02 |
| C18:3n6 | 0.15 | 0.16 | 0.16 | 0.05 | 0.38 |
| C20:1 | 0.75 | 0.70 | 0.75 | 0.08 | 0.16 |
| C18:3n3 | 0.30b | 0.33a | 0.33a | 0.05 | 0.05 |
| C20:2 | 0.40 | 0.44 | 0.43 | 0.08 | 0.43 |
| C20:3n6 | 0.63 | 0.65 | 0.65 | 0.07 | 0.59 |
| C20:4n6 | 6.00b | 7.43a | 7.14a | 0.27 | <0.05 |
| C20:5n3 | 0.23 | 0.20 | 0.21 | 0.07 | 0.27 |
| C22:6n3 | 0.23 | 0.25 | 0.23 | 0.08 | 0.75 |
| SFA1 | 39.63a | 37.77b | 38.33b | 0.37 | 0.01 |
| MUFA2 | 36.41 | 35.31 | 35.77 | 0.43 | 0.43 |
| PUFA3 | 23.93b | 26.48a | 25.73a | 0.39 | <0.01 |
| PUFA:SFA ratio | 0.61b | 0.70a | 0.67a | 0.07 | <0.01 |
FFA = free fatty acid; IMF = intramuscular fat; SFA = saturated fatty acid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid.
a,b Within a row, values with different superscripts differ significantly at P < 0.05 and a trend toward significance at P < 0.10. Data are expressed as means ± SEM, n = 8.
SFA = C14:0 + C15:0 + C16:0 + C17:0 + C18:0 + C20:0.
MUFA = C14:1 + C16:1 + C18:1n9t + C18:1n9c + C20:1.
PUFA = C18:2n6c + C18:3n6 + C18:3n3 + C20:2 + C20:3n6 + C20:4n6 + C20:5n3 + C22:6n3.
3.6. The relative mRNA expression levels of myosin heavy chain (MyHC) and the genes related to lipid metabolism
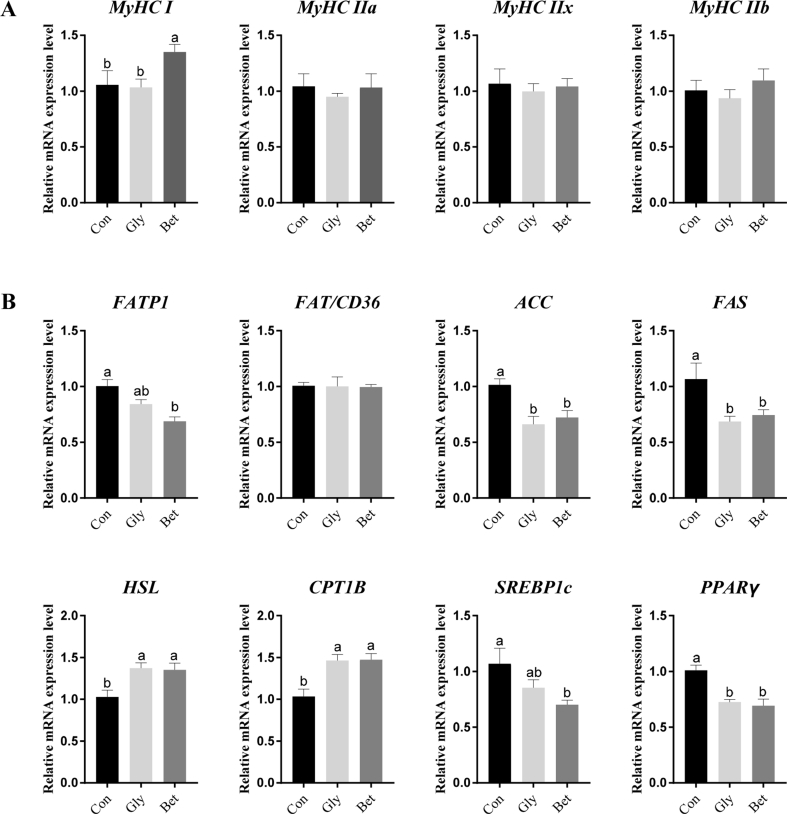
The gene expression level of MyHC I was markedly up-regulated in LD muscle in the betaine group (P < 0.05, Fig. 1A). However, there was no pronounced difference in the mRNA expression levels of MyHC IIa, MyHC IIb, and MyHC IIx among the 3 dietary groups (P > 0.05).
Fig. 1.
Betaine promoted the synthesis of type I muscle fibers in longissimus dorsi muscle; betaine (Bet) and glycine (Gly) inhibited fat synthesis and promoted fat catabolism in longissimus dorsi muscle. (A) The relative mRNA expression levels of the key genes related to skeletal muscle fiber type including myosin heavy chain (MyHC) I, MyHC IIa, MyHC IIb, and MyHC IIx (n = 8). (B) The relative mRNA expression levels of the key genes related to lipid metabolism including fatty acid transport protein 1 (FATP1), fatty acid translocase (FAT/CD36), acetyl-CoA carboxylase α (ACC), fatty acid synthase (FAS), hormone-sensitive lipase (HSL), carnitine palmitoyl transferase 1B (CPT1B), sterol regulatory element binding protein-1c (SREBP1c), and peroxisome proliferator-activated receptor γ (PPARγ) (n = 8). a, b Bars with different letters differ significantly at P < 0.05 and a trend toward significance at P < 0.10. Data are expressed as means ± SEM.
The mRNA expression levels of the genes involved in muscle lipid metabolism: Lipid intake includes fatty acid translocase (FAT/CD36) and fatty acid transport protein 1 (FATP1), lipogenesis includes acetyl-CoA carboxylase α (ACC) and fatty acid synthase (FAS), lipolysis includes hormone-sensitive lipase (HSL) and fatty acid oxidation includes carnitine palmitoyl transferase 1B (CPT1B), and transcription factors includes sterol regulatory element binding protein-1c (SREBP1c) and peroxisome proliferator-activated receptor γ (PPARγ). As shown in Fig. 1B, in comparison with the control group, betaine lowered the relative mRNA expression levels of FATP1 and SREBP1c, whereas no significant differences were observed in the value of FAT/CD36 in the present study (P > 0.05). Additionally, betaine and glycine both down-regulated the mRNA expression levels of ACC, FAS, and PPARγ (P < 0.05), and the value of HSL and CPT1B were up-regulated (P < 0.05).
4. Discussion
The present study was designed to investigate and compare the effects of betaine or glycine on carcass trait, meat quality and lipid metabolism in finishing Huang Jiang mini-pigs. Our results indicated that betaine increased final weight and ADG, but had no impact on ADFI and Feed:Gain ratio. The addition of glycine did not improve the growth performance parameters of the pigs. Additionally, betaine or glycine both decreased average backfat thickness and betaine also increased lean percentage. It was consistent with previous studies (Matthews et al., 2001; Huang et al., 2007). The reason might be that betaine increased the synthesis of proteins and the rate of cell proliferation by regulating the response of various cells to hypertonicity (Petronini et al., 1992), or increased sarcoplasmic osmolality to increase muscle mass (Courtenay et al., 2000). Glycine also increased lean percentage, probably because it enhanced the amino acid metabolism cycle, which promoted protein synthesis of skeletal muscle (Wang et al., 2014; Liu et al., 2016b; Sun et al., 2016).
As mentioned earlier, people are keen on high-quality meat, especially excellent tenderness and juiciness (Mehta et al., 2013). Some experiments were conducted to evaluate effect of betaine on pork quality (Overland et al., 1999; Matthews et al., 2001; Martins et al., 2012), whereas the results were not consistent. It is reported that betaine does not influence L∗, a∗ and b∗ values of pork (Martins et al., 2012; Matthews et al., 2001). In our study, a∗ value of the betaine group was markedly increased in LD muscle. The reason for the discrepancy might be that the amount of dietary betaine addition was different. Furthermore, glycine also increased a∗ value of the LD muscle in the present experiment. Another finding of our study was that the dietary betaine supplementation decreased shear force, and thus improved pork tenderness. The reduction in shear force value might be related to the increase in protein content (Hwang et al., 2010). However, no changes were observed in serum metabolites of the pigs fed with betaine or glycine. The latest study indicates that specific amino acids could enhance protein synthesis and promote skeletal muscle growth through activating the key signaling pathway (Cholewa et al., 2014; Hu et al., 2019). In the present experiment, betaine or glycine partly changed the free amino acid profiles in serum and LD muscle. The content of free cysteine in serum and LD muscle was observed a reduction in the betaine and glycine groups, and free methionine in LD muscle was increased in the betaine group compared to the control group. Homocysteine is an intermediate in the synthesis of methionine and cysteine (Ganguly and Alam, 2015). Betaine participates in the methionine-homocysteine cycle, and as a methyl donor to promote homocysteine re-methylation to methionine (Schwab et al., 2006; Cholewa et al., 2014). This explained the increase of free methionine and the decrease of free cysteine. Additionally, betaine and glycine both boosted the concentration of threonine in LD muscle. A previous study indicates that threonine is converted to glycine by threonine aldolase (Joshi et al., 2010). When there is enough glycine in the body, threonine aldolase activity is inhibited, and the threonine concentration is increased. Interestingly, dietary glycine supplementation increased the content of serum glycine, but betaine had no effect. It is suggested that their physiological functions are different. Betaine is mainly involved in methyl metabolism, and glycine is primarily participated in protein synthesis in the body (Martins et al., 2012; Wang et al., 2014; Liu et al., 2016b; Sun et al., 2016).
Meat quality, especially tenderness, is extremely associated with muscle fiber type composition (Ryu and Kim, 2005; Maltin et al., 2007). According to the major subtype of MyHC in adult mammalian skeletal muscles, muscle fibers are divided into 4 types, namely type I, type IIa, type IIx, and type IIb, which influence many aspects of meat quality (Ali et al., 2008; Choi and Kim, 2009; Joo et al., 2013). In the present study, the relative mRNA expression level of MyHC I in LD muscle was higher in the betaine group than the other 2 groups. The results suggested that betaine could induce the conversion of type II fibers into type I fibers, thus improve muscle type composition. It has been pointed out that type I fiber is related to meat tenderness (Maltin et al., 2010; Lee et al., 2010), and this could explain that betaine reduced shear forces of the finishing mini-pigs in our study. Additionally, MyHC I fiber contains more myoglobin and thus improves meat color (Joo et al., 2013), which also could explain the increase in a∗ value of the pork in the present experiment.
It is indicated that IMF content is directly correlative with meat quality, particularly juiciness and flavor (Jeremiah et al., 2003; Wood et al., 2008; Hocquette et al., 2009; Li et al., 2017b). Previous studies reported that betaine could increase IMF content (Martins et al., 2012; Madeira et al., 2016; Albuquerque et al., 2017), but others pointed out no effect was obtained (Rojas-Cano et al., 2011). In the current trial, IMF content of the betaine and the glycine groups was reduced but remained at a fairly high level (Wood et al., 2008). This result might be associated with fatty acid metabolism in skeletal muscle. The available evidence indicates that FFA composition plays a quite momentous role in flavor generation and nutritional value (Wood et al., 2003). Fatty acid saturation could affect fat hardness, which in turn affects meat quality (Perry et al., 1998). An earlier research reported that the intake of a lower level of SFA with an increased ratio of PUFA to SFA could reduce the risk of coronary heart disease (Hu et al., 1999). It can be seen from the current study that betaine and glycine decreased the percentage of SFA, and at the same time increased the content of PUFA and the ratio of PUFA to SFA.
As mentioned above, the IMF content was decreased in the betaine and glycine groups. Whether the decrease related to the expression levels of the genes involved in lipid metabolism remains to be learned. Lipid uptake is mainly regulated by fatty acid transporters, such as FAT/CD36 and FATP1 (Koonen et al., 2005; Nickerson et al., 2009), and overexpression of which could promote intracellular fatty acid uptake (Ibrahimi et al., 1999; Sebastián et al., 2009). In the present study, betaine significantly down-regulated the mRNA expression level of FATP1, indicating that betaine might reduce the uptake of fatty acid. It is well known that ACC and FAS are the rate-limiting enzymes for fatty acid de novo synthesis (Liu et al., 1994; Smith et al., 2003). The nuclear transcription factors including SREBP1c and PPARγ are extremely associated with lipogenic genes (Farmer, 2005; Li et al., 2017b). SREBP1c can up-regulate the expression level of ACC (Stoeckman and Towle, 2002), whereas PPARγ suppresses HSL gene expression level (Grindflek et al., 2000). In the present study, the mRNA expression levels of ACC, FAS, SREBP1c, and PPARγ were all decreased significantly in the finishing mini-pigs fed betaine or glycine (Du et al., 2018). However, these results were inconsistent with previous research which dietary supplementation with betaine increased the mRNA levels of ACC and PPARγ in LD muscle (Li et al., 2017a). The conflict can be explained due to different breed, gender, feeding cycles and experiment stages, etc. Meanwhile, HSL and CPT1B were the rate-limiting enzymes of lipid catabolism (Mersmann, 1998; Peffer et al., 2005). Between them, the role of HSL is to hydrolyze triglycerides, and CPT1B is responsible for transporting fatty acids into mitochondria for oxidation. Previous experiments showed that betaine could enhance the mRNA expression levels of HSL and CPT1B (Li et al., 2017a; Albuquerque et al., 2017), which were also observed in our study. The results indicated that betaine heightened the catabolism of fatty acids in skeletal muscle tissue. In view of the foregoing, the reduction of IMF content and FFA in finishing pigs fed with betaine and glycine might be that dietary betaine and glycine both inhibited the uptake and synthesis of fatty acids and enhanced the catabolism of fatty acids in LD muscle tissue.
5. Conclusion
In summary, diets supplemented with 0.25% betaine could improve growth performance, carcass trait, pork quality and lipid metabolism of Huan Jiang finishing mini-pigs, and equimolar amounts of glycine could also improve meat quality and lipid metabolism of the finishing pigs to some extent, but was inferior to betaine. Therefore, we consider that betaine could partially replace glycine in finishing pig feeding. Besides, this study will also provide reference value for the further application of betaine in animal husbandry and human nutrition.
Author contributions
Yinzhao Zhong and Zhaoming Yan performed the sample measurements. Yinzhao Zhong, Bo Song and Changbing Zhen conducted the animal feeding and sampling. Yehui Duan, Jinping Deng and Xiangfeng Kong analyzed data and revised the manuscript. Fengna Li designed the trial and provided financial support. All authors have read and agreed the final manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgement
This study was financially supported by the National Key R & D Program (2018YFD0500405), the National Nature Science Foundation of China (31972582, U19A2037), the Changsha Natural Science Funds for Distinguished Young Scholar (kq2009020), Special funds for the construction of innovative provinces in Hunan Project (2019NK2193, 2019RS3022), Open Fund of Key Laboratory of Agro-ecological Processes in Subtropical Region, Chinese Academy of Sciences (ISA2020203), the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (XDA24030204), the Science and technology projects of Changsha City (kq1801059), Hunan Province Key Laboratory of Animal Nutritional Physiology and Metabolic Process (2018TP1031), the General Program of National Natural Science Foundation of China (31872985), and the Earmarked Fund for China Agriculture Research System (CARS-35).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Albuquerque A., Neves J.A., Redondeiro M., Laranjo M., Félix M.R., Freitas A., Tirapicos J.L., Martins J.M. Long term betaine supplementation regulates genes involved in lipid and cholesterol metabolism of two muscles from an obese pig breed. Meat Sci. 2017;124:25–33. doi: 10.1016/j.meatsci.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Ali M.S., Yang H.S., Jeong J.Y., Moon S.H., Hwang Y.H., Park G.B., Joo S.T. Effect of chilling temperature of carcass on breast meat quality of duck. Poultry Sci. 2008;87:1860–1867. doi: 10.3382/ps.2007-00194. [DOI] [PubMed] [Google Scholar]
- Choi Y.M., Kim B.C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest Sci. 2009;122:105–118. [Google Scholar]
- Cholewa J.M., Guimaraes-Ferreira L., Zanchi N.E. Effects of betaine on performance and body composition: a review of recent findings and potential mechanisms. Amino Acids. 2014;46:1785–1793. doi: 10.1007/s00726-014-1748-5. [DOI] [PubMed] [Google Scholar]
- Courtenay E.S., Capp M.W., Anderson C.F., Record M.T., Jr. Vapor pressure osmometry studies of osmolyte protein interactions: implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry-US. 2000;2000:4455–4471. doi: 10.1021/bi992887l. [DOI] [PubMed] [Google Scholar]
- Du J., Shen L., Tan Z., Zhang P., Zhao X., Xu Y., Gan M., Yang Q., Ma J., Jiang A., Tang G., Jiang Y., Jin L., Li M., Bai L., Li X., Wang J., Zhang S., Zhu L. Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients. 2018;10:131. doi: 10.3390/nu10020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Duan Y., Li F., Li Y., Guo Q., Ji Y., Tan B., Li T., Yin Y. Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs. Amino Acids. 2016;48:2131–2144. doi: 10.1007/s00726-016-2223-2. [DOI] [PubMed] [Google Scholar]
- Duan Y., Guo Q., Wen C., Wang W., Li Y., Tan B., Li F., Yin Y. Free amino acid profile and expression of genes implicated in protein metabolism in skeletal muscle of growing pigs fed low-protein diets supplemented with branched-chain amino acids. J Agric Food Chem. 2016;64:9390–9400. doi: 10.1021/acs.jafc.6b03966. [DOI] [PubMed] [Google Scholar]
- Duan Y., Zhong Y., Xiao H., Zheng C., Song B., Wang W., Guo Q., Li Y., Han H., Gao J., Xu K., Li T., Yin Y., Li F., Yin J., Kong X. Gut microbiota mediates the protective effects of dietary β-hydroxy-β-methylbutyrate (HMB) against obesity induced by high-fat diets. FASEB J. 2019;33:10019–10033. doi: 10.1096/fj.201900665RR. [DOI] [PubMed] [Google Scholar]
- Eklund M., Bauer E., Wamatu J., Mosenthin R. Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev. 2005;18:31–48. doi: 10.1079/NRR200493. [DOI] [PubMed] [Google Scholar]
- Farmer S.R. Regulation of PPARγ activity during adipogenesis. Int J Obes. 2005;29:S13–S16. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- Fernández-Fígares I., Conde-Aguilera J.A., Nieto R., Lachica M., Aguilera J.F. Synergistic effects of betaine and conjugated linoleic acid on the growth and carcass composition of growing Iberian pigs. J Anim Sci. 2008;86:102–111. doi: 10.2527/jas.2006-0230. [DOI] [PubMed] [Google Scholar]
- Ganguly P., Alam S.F. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindflek E., Sundvold H., Lien S., Rothschild M.F. Rapid communication: physical and genetic mapping of the peroxisome proliferator activated receptor γ (PPARγ) gene to porcine chromosome 13. J Anim Sci. 2000;2000:1391–1392. doi: 10.2527/2000.7851391x. [DOI] [PubMed] [Google Scholar]
- Hocquette J.F., Gondret F., Baéza E., Médale F., Jurie C., Pethick D.W. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal. 2009;4:303–319. doi: 10.1017/S1751731109991091. [DOI] [PubMed] [Google Scholar]
- Hu C.J., Li F.N., Duan Y.H., Zhang T., Li H.W., Yin Y.L., Wu G.Y., Kong X.F. Dietary supplementation with arginine and glutamic acid alters the expression of amino acid transporters in skeletal muscle of growing pigs. Amino Acids. 2019;51:1081–1092. doi: 10.1007/s00726-019-02748-9. [DOI] [PubMed] [Google Scholar]
- Hu F.B., Stampfer M.J., Manson J.E., Ascherio A., Colditz G.A., Speizer F.E., Hennekens C.H., Willent W.C. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–1008. doi: 10.1093/ajcn/70.6.1001. [DOI] [PubMed] [Google Scholar]
- Huang Q.C., Xu Z.R., Han X.Y., Li W.F. Changes in hormones, growth factor and lipid metabolism in finishing pigs fed betaine. Livest Sci. 2006;105:78–85. [Google Scholar]
- Huang Q.C., Xu Z.R., Han X.Y., Li W.F. Effect of betaine on growth hormone pulsatile secretion and serum metabolites in finishing pigs. J Anim Physiol An N. 2007;91:85–90. doi: 10.1111/j.1439-0396.2006.00644.x. [DOI] [PubMed] [Google Scholar]
- Hwang Y.H., Hur S.J., Park G.B., Joo S.T. Effects of dietary glycine betaine on blood characteristics and pork quality. J Muscle Foods. 2010;2008:87–101. [Google Scholar]
- Ibrahimi A., Bonen A., Blinn W.D., Hajri T., Li X., Zhong K. Cameron, R., Abumrad, N. A. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem. 1999;274:26761–26766. doi: 10.1074/jbc.274.38.26761. [DOI] [PubMed] [Google Scholar]
- Jeremiah L.E., Gibson L.L., Aalhus J.L., Dugan M.E.R. Assessment of palatability attributes of the major beef muscles. Meat Sci. 2003;65:949–958. doi: 10.1016/S0309-1740(02)00307-8. [DOI] [PubMed] [Google Scholar]
- Joo S.T., Kim G.D., Hwang Y.H., Ryu Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95:828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Joshi V., Joung J.G., Fei Z.J., Jander G. Interdependence of threonine, methionine and isoleucine metabolism in plants: accumulation and transcriptional regulation under abiotic stress. Amino Acids. 2010;2010:933–947. doi: 10.1007/s00726-010-0505-7. [DOI] [PubMed] [Google Scholar]
- Koonen D.P.Y., Glatz J.F.C., Bonen A., Luiken J.J.F.P. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. BBA-Mol Cell Biol L. 2005;1736:163–180. doi: 10.1016/j.bbalip.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Joo S.T., Ryu Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010;86:166–170. doi: 10.1016/j.meatsci.2010.04.040. [DOI] [PubMed] [Google Scholar]
- Li F., Duan Y., Li Y., Tang Y., Geng M., Oladele O.A., Kim S.W., Yin Y. Effects of dietary n-6:n-3 PUFA ratio on fatty acid composition, free amino acid profile and gene expression of transporters in finishing pigs. Br J Nutr. 2015;113:739–748. doi: 10.1017/S0007114514004346. [DOI] [PubMed] [Google Scholar]
- Li S., Wang H., Wang X., Wang Y., Feng J. Betaine affects muscle lipid metabolism via regulating the fatty acid uptake and oxidation in finishing pig. J Anim Sci Biotechnol. 2017;8:72. doi: 10.1186/s40104-017-0200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu Y., Li F., Lin Q., Dai Q., Sun J., Huang X., Chen X., Yin Y. Effects of dietary ramie powder at various levels on carcass traits and meat quality in finishing pigs. Meat Sci. 2018;143:52–59. doi: 10.1016/j.meatsci.2018.04.019. [DOI] [PubMed] [Google Scholar]
- Li Y., Wei H., Li F., Duan Y., Guo Q., Yin Y. Effects of low-protein diets supplemented with branched-chain amino acid on lipid metabolism in white adipose tissue of piglets. J Agric Food Chem. 2017;65:2839–2848. doi: 10.1021/acs.jafc.7b00488. [DOI] [PubMed] [Google Scholar]
- Liu C.Y., Grant A.L., Kim K.H., Mill S.E. Porcine somatotropin decreases acetyl-CoA carboxylase gene expression in porcine adipose tissue. Domest Anim Endocrinol. 1994;11:125–132. doi: 10.1016/0739-7240(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Liu Y., Kong X., Jiang G., Tan B., Deng J., Yang X., Li F., Xiong X., Yin Y. Effects of dietary protein/energy ratio on growth performance, carcass trait, meat quality, and plasma metabolites in pigs of different genotypes. J Anim Sci Biotechnol. 2015;6:36. doi: 10.1186/s40104-015-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Kong X., Li F., Tan B., Li Y., Duan Y., Yin Y., He J., Hu C., Blachier F., Wu G. Co-dependence of genotype and dietary protein intake to affect expression on amino acid/peptide transporters in porcine skeletal muscle. Amino Acids. 2016;48:75–90. doi: 10.1007/s00726-015-2066-2. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang X., Wu H., Chen S., Zhu H., Zhang J., Hou Y., Hu C.A., Zhang G. Glycine enhances muscle protein mass associated with maintaining Akt-mTOR-FOXO1 signaling and suppressing TLR4 and NOD2 signaling in piglets challenged with LPS. Am J Physiol-Reg I. 2016;311:R365–R373. doi: 10.1152/ajpregu.00043.2016. [DOI] [PubMed] [Google Scholar]
- Madeira M.S., Rolo E.A., Alfaia C.M., Pires V.R., Luxton R., Doran O., Bessa R.J.B., Prates J.A.M. Influence of betaine and arginine supplementation of reduced protein diets on fatty acid composition and gene expression in the muscle and subcutaneous adipose tissue of cross-bred pigs. Br J Nutr. 2016;115:937–950. doi: 10.1017/S0007114515005218. [DOI] [PubMed] [Google Scholar]
- Maltin C., Balcerzak D., Tilley R., Delday M. Determinants of meat quality: tenderness. Proc Nutr Soc. 2007;62:337–347. doi: 10.1079/pns2003248. [DOI] [PubMed] [Google Scholar]
- Maltin C.A., Sinclair K.D., Warriss P.D., Grant C.M., Porter A.D., Delday M.I., Warkup C.C. The effects of age at slaughter, genotype and finishing system on the biochemical properties, muscle fibre type characteristics and eating quality of bull beef from suckled calves. Anim Sci. 2010;66:341–348. [Google Scholar]
- Martins J.M., Neves J.A., Freitas A., Tirapicos J.L. Effect of long-term betaine supplementation on chemical and physical characteristics of three muscles from the Alentejano pig. J Sci Food Agric. 2012;92:2122–2127. doi: 10.1002/jsfa.5595. [DOI] [PubMed] [Google Scholar]
- Matthews J.O., Southern L.L., Higbie A.D., Persica M.A., Bidner T.D. Effects of betaine on growth, carcass characteristics, pork quality, and plasma metabolites of finishing pigs. J Anim Sci. 2001;79:722–728. doi: 10.2527/2001.793722x. [DOI] [PubMed] [Google Scholar]
- Mehta N., Ahlawat S.S., Sharma D.P., Dabur R.S. Novel trends in development of dietary fiber rich meat products—a critical review. Journal of Food Sci Tech-Brazil. 2013;52:633–647. doi: 10.1007/s13197-013-1010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersmann H.J. Lipoprotein and Hormone-Sensitive Lipases In porcine adipose tissue. J Anim Sci. 1998:1396–1404. doi: 10.2527/1998.7651396x. [DOI] [PubMed] [Google Scholar]
- Nickerson J.G., Alkhateeb H., Benton C.R., Lally J., Nickerson J., Han X.-X., Wilson M.H., Jain S.S., Snook L.A., Glatz J.F.C., Chabowski A., Luiken J.J.F.P., Bonen A. Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J Biol Chem. 2009;284:16522–16530. doi: 10.1074/jbc.M109.004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overland M., Rorvik K.A., Skrede A. Effect of trimethylamine oxide and betaine in swine diets on growth performance, carcass characteristics, nutrient digestibility, and sensory quality of pork. J Anim Sci. 1999;1999:2143–2153. doi: 10.2527/1999.7782143x. [DOI] [PubMed] [Google Scholar]
- Peffer P.L., Lin X., Odle J. Hepatic β-oxidation and carnitine palmitoyltransferase I in neonatal pigs after dietary treatments of clofibric acid, isoproterenol, and medium-chain triglycerides. Am J Physiol-Reg I. 2005;288:R1518–R1524. doi: 10.1152/ajpregu.00822.2004. [DOI] [PubMed] [Google Scholar]
- Perry D., Nicholls P.J., Thompson J.M. The effect of sire breed on the melting point and fatty acid composition of subcutaneous fat in steers. J Anim Sci. 1998;1998:87–95. doi: 10.2527/1998.76187x. [DOI] [PubMed] [Google Scholar]
- Petronini P.G., De Angelis E.M., Borghetti P., Borghetti A.F., Wheeler K.P. Modulation by betaine of cellular responses to osmotic stress. Biochem J. 1992;1991:69–73. doi: 10.1042/bj2820069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Cano M.L., Lara L., Lachica M., Aguilera J.F., Fernandez-Figares I. Influence of betaine and conjugated linoleic acid on development of carcass cuts of Iberian pigs growing from 20 to 50 kg body weight. Meat Sci. 2011;88:525–530. doi: 10.1016/j.meatsci.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Ryu Y.C., Kim B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005;71:351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Schwab U., Törrönen A., Meririnnr E., Saarinen M. Orally administered betaine has an acute and dose-dependent effect on serum betaine and plasma homocysteine concentrations in healthy humans. J Nutr. 2006;136:34–38. doi: 10.1093/jn/136.1.34. [DOI] [PubMed] [Google Scholar]
- Sebastián D., Guitart M., García-Martínez C., Mauvezin C., Orellana-Gavaldà J.M., Serra D., Gómez-Foix A.M., Hegardt F.G., Asins G. Novel role of FATP1 in mitochondrial fatty acid oxidation in skeletal muscle cells. J Lipid Res. 2009;50:1789–1799. doi: 10.1194/jlr.M800535-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siljander-Rasi H., Peuranen S., Tiihonen K., Virtanen E., Kettunen H., Alaviuhkola T., Simmins P.H. Effect of equi-molar dietary betaine and choline addition on performance, carcass quality and physiological parameters of pigs. Anim Sci. 2016;76:55–62. [Google Scholar]
- Smith S., Witkowski A., Joshi A.K. Structural and functional organization of the animal fatty acid synthase. Prog Lipid Res. 2003;42:289–317. doi: 10.1016/s0163-7827(02)00067-x. [DOI] [PubMed] [Google Scholar]
- Stoeckman A.K., Towle H.C. The role of SREBP-1c in nutritional regulation of lipogenic enzyme gene expression. J Biol Chem. 2002;277:27029–27035. doi: 10.1074/jbc.M202638200. [DOI] [PubMed] [Google Scholar]
- Sun K., Wu Z., Ji Y., Wu G. Glycine regulates protein turnover by activating protein kinase B/mammalian target of rapamycin and by inhibiting MuRF1 and atrogin-1 gene expression in C2C12 myoblasts. J Nutr. 2016;146:2461–2467. doi: 10.3945/jn.116.231266. [DOI] [PubMed] [Google Scholar]
- Wang W., Wu Z., Lin G., Hu S., Wang B., Dai Z., Wu G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J Nutr. 2014;144:1540–1548. doi: 10.3945/jn.114.194001. [DOI] [PubMed] [Google Scholar]
- Wood J.D., Enser M., Fisher A.V., Nute G.R., Sheard P.R., Richardson R.I., Hughes S.I., Whittington F.M. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Wood J.D., Richardson G.R., Nute A.V., Fisher M.M., Campo E., Kasapidou P.R., Sheard M.E. Effects of fatty acids on meat quality: a review. Meat Sci. 2003;66:21–32. doi: 10.1016/S0309-1740(03)00022-6. [DOI] [PubMed] [Google Scholar]
- Yu D.Y., Xu Z.R., Li W.F. Effects of betaine on growth performance and carcass characteristics in growing pigs. J Anim Sci. 2004;17:1700–1704. [Google Scholar]
- Zhong Y., Song B., Zheng C., Li F., Kong X., Duan Y., Deng J. alpha-Ketoisocaproate and beta-hydroxy-beta-methyl butyrate regulate fatty acid composition and lipid metabolism in skeletal muscle of growing pigs. J Anim Physiol An N. 2019;103:846–857. doi: 10.1111/jpn.13077. [DOI] [PubMed] [Google Scholar]