Abstract
Anoctamin 1 (ANO1) or TMEM16A gene encodes a member of Ca2+ activated Cl– channels (CaCCs) that are critical for physiological functions, such as epithelial secretion, smooth muscle contraction and sensory signal transduction. The attraction and interest in ANO1/TMEM16A arise from a decade long investigations that abnormal expression or dysfunction of ANO1 is involved in many pathological phenotypes and diseases, including asthma, neuropathic pain, hypertension and cancer. However, the lack of specific modulators of ANO1 has impeded the efforts to validate ANO1 as a therapeutic target. This review focuses on the recent progress made in understanding of the pathophysiological functions of CaCC ANO1 and the current modulators used as pharmacological tools, hopefully illustrating a broad spectrum of ANO1 channelopathy and a path forward for this target validation.
KEY WORDS: Ca2+-activated Cl– channels (CaCCs), ANO1, TMEM16A, CaCCinh-A01, T16Ainh-A01, Drug target, Cancer, Cystic fibrosis
Abbreviations: Ang II, angiotensin II; ANO1, anoctamin-1; ASM, airway smooth muscle; BBB, blood–brain barrier; CaCCs, Ca2+ activated chloride channels; CAMK, Ca2+/calmodulin-dependent protein kinase; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; DRG, dorsal root ganglion; EGFR, epidermal growth factor receptor; ENaC, epithelial sodium channels; ER, endoplasmic reticulum; ESCC, esophageal squamous cell carcinoma; FRT, fisher rat thyroid; GI, gastrointestinal; GIST, gastrointestinal stromal tumor; GPCR, G-protein coupled receptor; HNSCC, head and neck squamous cell carcinoma; HTS, high-throughput screening; ICC, interstitial cells of Cajal; IPAH, idiopathic pulmonary arterial hypertension; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor κB; PAH, pulmonary arterial hypertension; PAR2, protease activated receptor 2; PASMC, pulmonary artery smooth muscle cells; PIP2, phosphatidylinositol 4,5-bisphosphate; PKD, polycystic kidney disease; TGF-β, transforming growth factor-β; VGCC, voltage gated calcium channel; VRAC, volume regulated anion channel; VSMC, vascular smooth muscle cells; YFP, yellow fluorescent protein
Graphical abstract
The abnormal expression or dysfunction of Ca2+ activated Cl– channel anoctamin 1 (ANO1) is involved in pathological phenotypes and diseases, suggesting that pharmacological modulation of ANO1 may serve as therapeutic potential and strategy.
1. Introduction
Ca2+-activated chloride channels (CaCCs) are a heterogeneous group of Cl– channels that can be synergistically activated by intracellular calcium and voltage. The CaCCs are found in almost all species ranging from invertebrates to mammals, and the ubiquitous expression of CaCCs indicates a variety of functions important for physiology, including regulation of epithelial Cl– secretion, excitability of neuronal and cardiac cells, smooth muscle contraction and nociception1, 2, 3.
A subtype of CaCCs was first described in Xenopus oocytes nearly 40 years ago and its molecular identity was debated until in 2008 when three independent laboratories reported that anoctamin-1 (ANO1) or transmembrane protein 16A (TMEM16A) underlies the molecular basis of a subgroup of CaCCs4, 5, 6. The Ano1/Tmem16a gene encodes a 986-amino-acid protein that belongs to anoctamin family consisting of 10 members (ANO1–ANO10) in mammals. ANO1, as a CaCC, is primarily expressed in epithelial cells, smooth muscle cells and sensory neurons. ANO2, also as a CaCC, is expressed in the olfactory sensory neurons7, photoreceptor synaptic terminals8, hippocampal pyramidal neurons9, thalamocortical neurons10, and inferior olive neurons11 in the brain. Other ANOs family members including ANO6 and ANO7 were onetime considered to be CaCCs12, but evidence shows that ANOs3–7 neither generate Ca2+-activated Cl– currents nor traffic into membrane, indicating that they are endoplasmic reticulum proteins13. More studies reveal that ANOs3–7 and ANO9 are linked to the Ca2+-dependent membrane phospholipid scramblases that are responsible for translocation of phospholipids14, 15, 16. It is generally accepted that ANO1 and ANO2 are two members of the CaCC subfamily, whereas ANO3–ANO10 have been debated for their functions as CaCCs or Ca2+-dependent membrane phospholipid scramblases, or other physiological proteins17,18. In addition, an integral membrane protein bestrophin-1 (BEST1) encoded by the BEST1 gene also functions as a CaCC. BEST1 is predominantly expressed in retinal pigment epithelium19, non-neuronal tissue, peripheral and central neurons20,21.
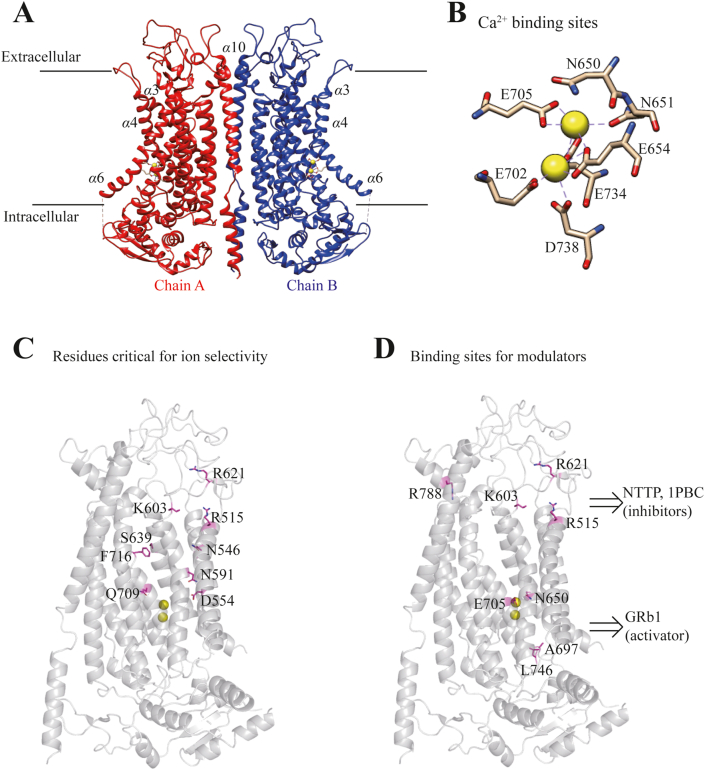
ANO1 was initially thought to have eight transmembrane domains, possessing multiple protein isoforms generated by alternative splicing of four segments (a, b, c, and d) located at the C-terminus and the first intracellular loop5. ANO1 splice variant lacking segment b, for instance, increases the calcium sensitivity, whereas deletion of the segment c decreases apparent Ca2+ sensitivity and increases voltage-dependent activity of ANO1 channel22,23. The X-ray structure of a Ca2+-activated lipid scramblase ANO1 from fungus Nectria haematococca (nhANO1) shows a homodimer with each subunit containing a ten-transmembrane α-helices24. Several critical amino acid residues for Ca2+ sensitivity and ion selectivity are subsequently found25,26. Recently, a high-resolution cryo-EM structure reveals an overall structure of mouse ANO1 similar to nhANO127,28, exhibiting two Ca2+ binding sites within the inner vestibule of the pore, and Ca2+ binding triggering conformational changes of α-helix rendering the pore conductive (Fig. 126, 27, 28, 29). The mechanisms underlying ANO1 activation and modulation are beginning to emerge. It has been shown that calmodulin, protons, cell volume and thermal stimuli can regulate the channel activation30,31, and phosphatidylinositol 4,5-bisphosphate (PIP2) regulates the activation and desensitization of ANO132,33.
Figure 1.
The molecular structure of ANO1/TMEM16A channel. (A) The Ca2+-bound structure of mANO1 channel in dimer (chains A and B), and 2 yellow filled circles for Ca2+ in each monomer containing 10 transmembrane α helices. (B) Ca2+ binding sites formed by residues N650, N651, E654 from α6, E702, E705 from α7, and E734, D738 from α828,27. (C) Residues critical for ion selectivity including R515 from α3, N546, D554 from α4, N591, V599 from α5, K603, R621 from α5–6 linker, S639 from α6, and Q709, F716 from α726,28. (D) Putative binding sites, R515 from α3, K603, R621 from α5–6 linker, and R788 from α8, for ANO1 inhibitors NTTP and 1PBC26; and N650 from α6, A697, E705 from α7, and L746 from α8 for ANO1 activator GRb129. The structure is regenerated based on the cryo-EM structure of ANO1 channel (PDB 5OYB)27. The residue number labeling is based on the sequence of mTMEM16A (ac) isoform (UniProt Q8BHY3.2).
ANO1 channel appears to be preferentially activated by local rise of intracellular Ca2+ release from the endoplasmic reticulum (ER) Ca2+ stores, which may represent a general mechanism of ANO1 activation34. ANO1 expression is regulated by multiple signaling cascade pathways such as mitogen-activated protein kinase (MAPK), nuclear factor κB (NF-κB) and transforming growth factor-β (TGF-β) in pathological functions35. Several recent reviews have substantially covered the molecular basis, structure and pathophysiological functions of ANO117,35, 36, 37. In this review we will mainly focus on the aspect of channelopathies and pharmacological validation of ANO1 as an emerging therapeutic target.
2. Pharmacological modulation of ANO1 channel
Validating ANO1 as a therapeutic target requires specific modulators that can serve as essential tools for understanding the channel pharmacology. Up to now, many ANO1 modulators have been reported, and unfortunately most of them are lack of potency and efficacy. Therefore, there exists a great need for discovery of more selective and potent modulators, inhibitors in particular, which can be used for ANO1 target validation.
2.1. Inhibitors
Some broad-spectrum blockers, such as NFA, FFA, DIDS, NPPB and 9AC, have been used as tools for understanding functions of CaCCs (Table 138, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84). These small molecules can block endogenous CaCCs in Xenopus laevis oocytes39,43,45,85 and are also known to non-specifically modulate other channels, such as inhibition of volume regulated anion channel (VRAC) and Kv4 by NFA and DIDS and activation of Ca2+ activated K+ channel by NFA and FFA or potentiation of TRPV1 channel by DIDS2,38. CaCCinh-A01 was identified to inhibit CaCC current in 2008 from screen of 50,000 compounds using human intestinal epithelial HT29 cell that highly express endogenous CaCCs without obvious effects on intracellular Ca2+, Ca2+/calmodulin-dependent protein kinase (CAMK) or cystic fibrosis transmembrane conductance regulator (CFTR)49. CaCCinh-A01 as an ANO1 inhibitor has been commonly used in many investigations for the role of ANO1 in interstitial cells of cajal (ICC), cardiac fibroblast and rod bipolar cells of retina86, 87, 88, and channelopathies, including cancer, hypertension, nociception, diarrhea and high glucose induced renal cyst growth51,53, 54, 55. CaCCinh-A01 is shown to inhibit cancer cell proliferation through increasing the ubiquitination of ANO1 and facilitating ER-associated, proteasomal degradation of ANO1, while other ANO1 inhibitors, such as T16Ainh-A01 and digallic acid, are shown to have no effect on cancer cell proliferation89, 90, 91. Similarly, CaCCinh-A01 inhibits proliferation of cardiac fibroblast, but not another ANO1 inhibitor T16Ainh-A0187. CaCCinh-A01 reduces upregulation of ANO1 expression and attenuates brain infarct size and neurological deficits after ischemic stroke whereas T16Ainh-A01 shows no effect56. That ANO1 protein expression is reduced by CaCCinh-A01 but not T16Ainh-A01 may partially explain why the two ANO1 inhibitors show different effects on cell proliferation and ischemic stroke. In addition, intrathecal injection of CaCCinh-A01 reduces tactile allodynia and thermal hyperalgesia and also decreases ANO1 upregulation after spinal nerve injury54.
Table 1.
Pharmacology of CaCC ANO1/TMEM16A inhibitors.
| Inhibitor | Structure | IC50 (μmol/L) | Test assay | Selectivity | Effect | Ref. |
|---|---|---|---|---|---|---|
| NFA | 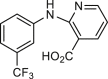 |
7–37 | Xenopus oocytes | KCa channel (+) | Anti-cancer, | 38, 39, 40, 41, 42 |
| mANO1-CHO | Kv4 channel (−) | Anti-asthma | ||||
| VRAC (−) | ||||||
| ANO6 (−) | ||||||
| [Ca2+]i (+) | ||||||
| FFA | 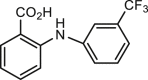 |
14–35 | Xenopus oocytes | KCa channel (+) | NR | 38,39 |
| mANO1-CHO | [Ca2+]i (+) | |||||
| NPPB |  |
22–68 | Xenopus oocytes | K+ channel (−) | Anti-cancer, | 38,39,43,44 |
| mANO1-CHO | [Ca2+]i (+) | Antinociception | ||||
| VRAC (−) | ||||||
| DIDS |  |
11–550 | Xenopus oocytes | Kv4 channel (−) | Anti-cancer, | 38,39,44, 45, 46, 47 |
| mANO1-CHO | VRAC (−) | Antinociception | ||||
| [Ca2+]i (+) | ||||||
| TRPV1 (+) | ||||||
| xBest2a (/) | ||||||
| Dichlorophen |  |
5.5 | ANO1-HEK293 | ANO2 (−) | Anti-asthma | 48 |
| CFTR (/) | ||||||
| ENaC (/) | ||||||
| Benzbromarone | 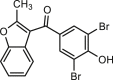 |
10 | ANO1-HEK293 | ANO2 (−) | Anti-asthma | 48 |
| CFTR (/) | ||||||
| ENaC (/) | ||||||
| CaCCinh-A01 | 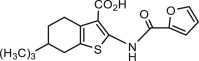 |
1–7.8 | HT-29 | hBest1 (–, 7 μmol/L) | Anti-cancer, | 38,47,49, 50, 51, 52, 53, 54, 55, 56 |
| hNO1-FRT | ANO2 (−) | Anti-hypertension, | ||||
| mANO1-CHO | CFTR (/) | Anti-diarrhea, | ||||
| VRAC (−) | Antinociception, | |||||
| [Ca2+]i (/) | Anti-high glucose induced renal cyst growth, | |||||
| xBest2a (/) | Anti-ischemic stroke induced BBB intergrity | |||||
| T16Ainh-A01 | 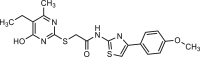 |
1 | hANO1-HEK293 | ANO2 (−) | Anti-cancer, | 38,47,50,52,54,57, 58, 59, 60 |
| CFTR (/) | Anti-hypertension, | |||||
| [Ca2+]i (/) | Anti-Asthma, | |||||
| hBest1 (/) | Antinociception, | |||||
| VGCC (–, 50 nmol/L) | Anti-eosinophilic esophagitis, | |||||
| xBest2a (/) | ||||||
| MONNA | 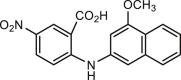 |
1.27 | hANO1-HEK293 | VRAC (−) | Anti-cancer, | 47,61, 62, 63, 64, 65 |
| xANO1 (0.08 μmol/L) | ANO2 (−) | Anti-itch, | ||||
| mBest1 (/) | Anti-hypertension, | |||||
| hBest1 (/) | Anti-polyfertilization | |||||
| mCLC2 (/) | ||||||
| hCFTR (/) | ||||||
| xBest2a (/) | ||||||
| Ani9 | 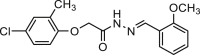 |
0.08 | hANO1-FRT | ANO2 (/) | Anti-cancer, | 47,57,65,66 |
| CFTR (/) | Anti-polyfertilization | |||||
| ENaC (/) | ||||||
| VRAC (/) | ||||||
| ANO6 (slower activation) | ||||||
| xBest2a (/) | ||||||
| 10bm | 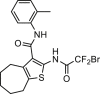 |
0.03 | hANO1-FRT | ANO2 (–, 0.4 μmol/L) | NR | 67 |
| CFTR (/) | ||||||
| Ani9-5f | 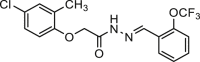 |
0.02 | hANO1-FRT | ANO2 (/) CFTR (/) | Anti-cancer | 68 |
| Dimer trans-ε-viniferin (TV) | 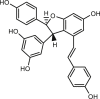 |
1.1 | HT-29 | CFTR (−) | Anti-diarrhea | 69 |
| Tetramer, γ-2-viniferin (RV) |  |
12.3 | HT-29 | CFTR (−) | Anti-diarrhea | 69 |
| Niclosamide |  |
0.1–2.4 | hANO1-HEK293T | CFTR (/) | Anti-asthma | 42,66,70 |
| ANO6 (−) | ||||||
| [Ca2+]i (−) | ||||||
| Tannic acid |  |
6–25 | hANO1-FRT | CFTR (/) | Anti-nociception, | 38,50,62,71, 72, 73 |
| mANO1-CHO | ENaC (/) | Anti-cancer, | ||||
| hBest1 (–, 15 μmol/L) | Anti-asthma | |||||
| ANO2 (−) | ||||||
| Eugenol |  |
150 | hANO1-FRT T84 cell |
CFTR (/) | Anti-diarrhea, | 74, 75, 76 |
| [Ca2+]i (/) | Local analgesia | |||||
| Nav1.8 (−) | ||||||
| TRPV1 (−) | ||||||
| Kv1.5 (−) | ||||||
| VGCC (−) | ||||||
| Galangin | 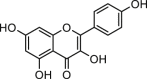 |
4.5–9.7 | mANO1-CHO | NR | Anti-cancer | 77,78 |
| Luteolin | 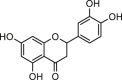 |
9.37 | hANO1-FRT | ANO2 (–, 120 μmol/L) | Anti-cancer | 77, 78, 79 |
| mANO1-CHO | L-type Ca2+ channel (−) | |||||
| [Ca2+]i (/) | ||||||
| Quercetin | 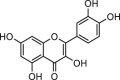 |
13.7 | mANO1-CHO | NKCC1 (+) | Anti-cancer, | 78,80 |
| Na+-K+-ATPase (+) | Attenuation of GI tract motility, | |||||
| CFTR (+) | Increasement of intestinal Cl– secretion | |||||
| K+ channel (+) | ||||||
| TRPM7 (−) | ||||||
| Idebenone | 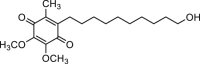 |
9.2 | hANO1-FRT | ANO2 (−) | Anti-cancer, | 81,82 |
| CFTR (/) | Anti-renal cyst | |||||
| [Ca2+]i (/) | ||||||
| Plumbagin | 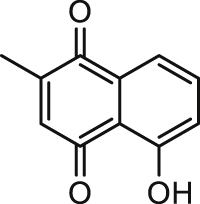 |
12.46 | hANO1-FRT | CFTR (−) | Anti-cancer, | 81,83 |
| K+ channel (/) | Anti-diarrhea | |||||
| Na+-K+-ATPase (/) | ||||||
| [Ca2+]i (/) | ||||||
| Avermectins | 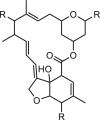 |
0.15–1.32 | mANO1-CHO | NR | Anti-cancer | 84 |
+, activation effect; –, inhibitory effect; /, no effect; NR, no report; NKCC, Na+/K+/Cl– co-transporter.
Since the identification of CaCCinh-A01, several ANO1 inhibitors have been screened out using the iodide-sensitive yellow fluorescent protein (YFP)-based high throughput screening (HTS) assay50,65,67. T16Ainh-A01 was reported to inhibit human ANO1 current expressed in Fisher rat thyroid (FRT) cells with an IC50 of 1 μmol/L and had no effect on CFTR current50. However, our previous study showed that T16Ainh-A01 at 10 μmol/L only inhibits mouse ANO1 current expressed in CHO cells about 28% at +80 mV38, and it may because that the efficacy of T16Ainh-A01 on ANO1 inhibition dependents on splice variants of ANO1 and intracellular calcium92. Nevertheless, T16Ainh-A01 has been used as a pharmacological probe to investigate the role of ANO1 in pancreatic ductal adenocarcinoma cells93, and also physiological and pathological functions of ANO1 in different tissues, including contraction of vesical smooth muscle, iodide release from thyrocyte, initial waveform modulation in retinal rod bipolar cell and melatonin secretion in pineal glands52,88,94, 95, 96 (Table 1). T16Ainh-A01 attenuates the interleukin-13 (IL-13) induced increase of ANO1 expression and secretion of mucin in human nasal polyp epithelial cells from chronic rhinosinusitis patients, cultured human bronchial epithelial cells and goblet cells in the guinea pig asthma model59,97, suggesting that T16Ainh-A01 may be useful for treatment of hypersecretion in asthma and other inflammatory airway diseases. In addition, several studies show that ANO1 inhibitor T16Ainh-A01 exerts therapeutic effect on cancer, neuropathic pain and eosinophilic esophagitis58,98, 99, 100.
MONNA, the most potent blocker of anthranilic acid derivatives, blocks xANO1 and hANO1 with IC50 of 0.08 and 1.27 μmol/L, respectively, without any effects on CFTR, CLC2 and BEST161. Similar to ANO1 inhibitors CaCCinh-A01 and T16Ainh-A01, MONNA concentration-dependently inhibits agonist-induced rodent vesical constriction through hyperpolarization of vesical smooth muscle cells52,63. MONNA also inhibits chloroquine-induced action potential discharge at itch nerve terminals and bouts of scratching64, suggesting a role of ANO1 in pruritus. MONNA has also been used to investigate the role of ANO1 in blocking poly-fertilization47.
Another small molecule Ani9 was also identified to inhibit hANO1 expressed in FRT cells with an IC50 of 0.08 μmol/L using YFP-HTS assay (Table 1). Ani9 belongs to acetamides and shows a relatively high selectivity on ANO1 over ANO2 without inhibitory effects on CFTR, VRAC, epithelial sodium channels (ENaC) and intracellular Ca2+ signaling at concentration of 30 μmol/L65, whereas Ani9 can inhibit inward current and slow the time-dependent activation of ANO666. The Ani9 derivative 5f shows more potent inhibitory effect on ANO1 with an IC50 of 20 nmol/L without activating on ANO268. Ani9 as a novel potent and selective ANO1 inhibitor has also been used in several investigations for the physiological and pathological functions of ANO1, including hypertension, polyspermy block and cancer47,57,63. As one of the 2-acylamino-cycloalkylthiophene-3-carboxylic acid arylamides, 10bm shows a potent inhibition on ANO1 current with an IC50 of 30 nmol/L and exhibits dose-dependent inhibition on isometric smooth muscle contraction. The 10bm compound also inhibits ANO2 with an IC50 of 0.4 μmol/L without effect on CFTR67. At present, synthesized ANO1 inhibitors are only used as tools for preclinical studies (Table 1).
Many natural products from diverse plants have been found to inhibit ANO1. Tannic acid presented in the green tea and red wine was identified as a blocker for both ANO1 and ANO2 without effect on CFTR or ENaC, showing an inhibitory effect on arterial smooth muscle contraction and intestinal Cl− secretion71. Like the synthesized small molecule ANO1 inhibitors, tannic acid has also been used as a pharmacological tool for investigations of the biophysical feature and physiological functions of ANO1101, 102, 103. Series of flavonoids were recently identified to inhibit ANO1, exerting anticancer effects77,78. Natural products or synthetic analogues of natural products, including idebenone with anticancer activity81, plumbagin with antidiarrhea83 and matrine with anti-lung adenocarcinoma activity104 have been shown to inhibit ANO1.
Several clinical drugs are recently found to have inhibitory effect on ANO1, including clarithromycin, benzbromarone, niclosamide, nitazoxanide and avermectins. Clarithromycin is a clinical antibiotic that was reported to decrease IL-13 induced ANO1 expression in goblet cells from asthma models105. Benzbromarone, a clinical drug for gout treatment, shows anti-asthmatic effect through inhibiting IL-13 induced mucin secretion and methacholine induced airway smooth muscle (ASM) contraction via the inhibition of ANO170,106. Niclosamide and nitazoxamide are clinical anthelmintics that as potent ANO1 inhibitors can block ASM depolarization and contraction70. Avermectins are a type of macrocyclic lactones that are widely used as pharmaceuticals against roundworms in humans and animals and also for crop protection. Several avermectins, including avermectin B1, ivermectin, doramectin, selamectin and moxidectin, exhibit inhibitory effects on cancer cell proliferation and migration through inhibition of ANO184. These drugs may have repurposing potential for dysfunctional ANO1 related diseases, such as asthma and hypertension.
It is noticeable that many compounds can act on ANO1 with limited selectivity, which imposes a big challenge for validation of ANO1 as a therapeutic target. The reasons for lack of identifying specific ANO1 modulators can be in multiple folds including chemical designs without guidance of target structure, use of an indirect YFP fluorescence-based HTS assay, and compound screens against cells expressing endogenous xANO1 in oocytes (for MONNA)61, hANO1 in HT29 cells (for CaCCinh-A01)49, or heterogeneous hANO1 in FRT cells (for T16Ainh-A01, Ani9 and 10bm)50,65,67. In addition, ANO1 and ANO2 subunits share 62% of sequence4, which also presents the selectivity challenge. The recent solved cryo-EM structure of ANO1 may help understand the biophysical features and physiological functions of the channel and also can greatly assist to develop more specific ANO1 modulators27,28. A pharmacophore model based on three-dimensional quantitative structure–activity relationship (3D-QSAR) for predicting best target and compound interactions also appears to be a promising tool for virtual screening and enhancing rational design for novel potent and specific ANO1 modulators107.
2.2. Activators
During the past almost 40 years since the identification of CaCC in 1982108, only a few activators of ANO1 have been reported. Two ANO1 activators Eact and Fact are the first discovered using an HTS approach109. Eact and Fact are two different classes of chemicals that activate ANO1 through different mechanisms (Table 266,109, 110, 111, 112, 113, 114, 115, 116, 117). Eact is an activator that induces ANO1 current in the absence of intracellular Ca2+ with an EC50 of 3 μmol/L. Several studies indicate that Eact might indirectly activate ANO1 through an intracellular Ca2+ increase110,111. Fact is a potentiator that increases ANO1 current in a Ca2+-dependent manner with an EC50 of 6 μmol/L. INO-4995 is an 1-O-octyl-2-O-butyryl-myo-inositol 3,4,5,6-tetrakisphosphate octakis(propionoxymethyl) ester directly activates overexpressed ANO1 current but without effect on endogenous ANO1 currents in Xenopus oocytes, human airways and colonic cells112.
Table 2.
Pharmacology of CaCC ANO1/TMEM16A Activators.
| Activator | Structure | EC50 (μmol/L) | Test assay | Selectivity | Effect | Ref. |
|---|---|---|---|---|---|---|
| Eact | 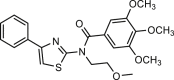 |
3 | hANO1-FRT | TRPV1 (+) TRPV4 (+) [Ca2+]i (+) ANO6 (+) |
Pain, Smooth muscle contraction |
66,109, 110, 111 |
| Fact | 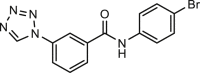 |
6 | hANO1-FRT | NR | NR | 109 |
| INO-4995 | 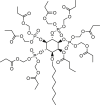 |
NR | hANO1-HEK293 | NR | NR | 112 |
| Resveratrol | 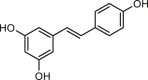 |
48 | mANO1-HEK293T | NR | Smooth muscle contraction |
113 |
| GRb1 | 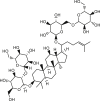 |
38.4 | mANO1-HEK293 | NR | Smooth muscle contraction |
114 |
| Cinnamaldehyde | 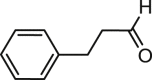 |
9.7 | ANO1-HEK293 | NR | Smooth muscle contraction |
66 |
| Chitosan oligosaccharides | 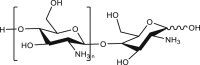 |
74.5 μg/mL | mANO1-HEK293 | NR | Smooth muscle contraction |
115 |
| ETX001 | NR | 116 nmol/L | hANO1-FRT sANO1-CHO |
[Ca2+]i (/) | Anti-CF | 116 |
| Melittin | C131H229N39O31 | 0.5–2 | hANO1-HEK293 | ANO6 (+) PLA2 (+) |
Phospholipid scrambling through ANO6 but not ANO1 |
66,117 |
+, activation effect; /, no effect; NR, no report.
A small molecule ANO1 potentiator ETX001 is recently shown to increase ANO1 current with an EC50 of 116 nmol/L without effect on intracellular Ca2+ signaling116 (Table 2). ETX001 enhances fluid secretory response in human bronchial epithelial cells from cystic fibrosis (CF) patients, and increases airway mucus clearance in sheep CF models. Several compounds from traditional Chinese medicines, including resveratrol113, ginsenoside Rb1 (GRb1)114, GRb229, cinnamaldehyde118 and chitosan oligosaccharides115, were recently reported to activate ANO1 channel although their mechanisms of action remain elusive. Activators of ANO1 can be useful for validating ANO1 as a therapeutic target for treatment of disorders associated with ANO1 hypofunction, including Sjogren's syndrome, cystic fibrosis lung disease, and dry eye syndrome.
It should be mentioned that the relatively wide distribution of ANO1 may impose a liability concern or a challenge in developing organ- or tissue-specific therapy. Nevertheless, development of targeted drug delivery systems such as tissue-specific or selective organ targeting nanoparticles and controlled release of therapeutic agents may circumvent potential ANO1-originated complications119. In addition, target-related complications can also be minimized thought means of different drug formulations, such as topical preparations, sublingual, buccal and rectal administrations.
3. Pathological functions and related diseases
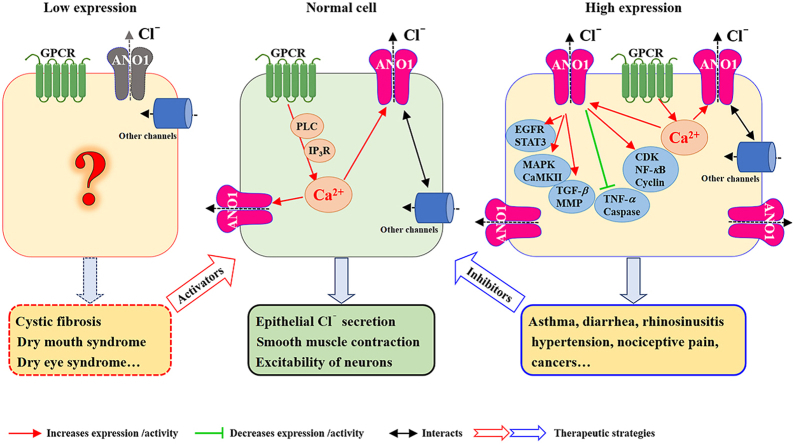
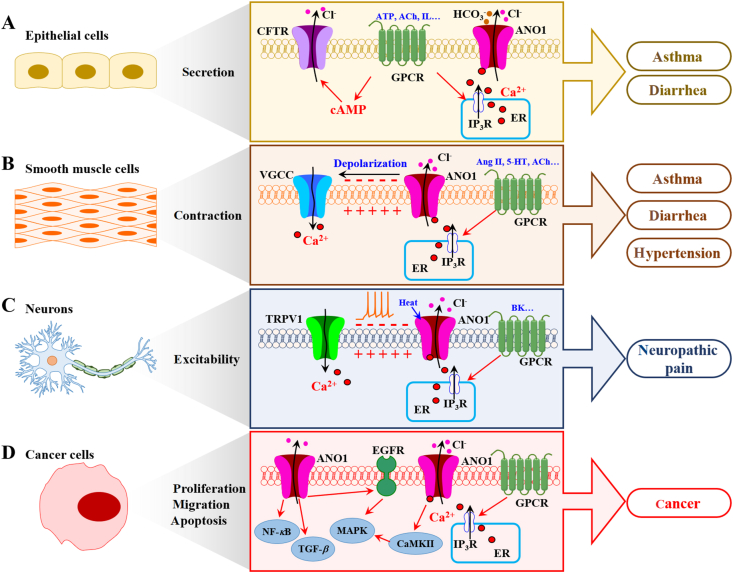
Since ANO1 was identified as a member of CaCCs in 2008, its investigations have been focused on the distribution and expression in numerous organs and tissues, and its roles in pathological conditions and diseases (Fig. 251,98,120,121).
Figure 2.
Distribution of ANO1/TMEM16A in different tissues and its role in diseases. (A) In epithelial cells, ANO1 activation contributes to electrolyte and music secretion. Activation of GPCRs causes an increase in intracellular cAMP and Ca2+, further inducing Cl− secretion through the activation of CFTR and ANO1. A crosstalk between CFTR and ANO1-dependent secretions also occurs for regulation of secretory signaling in the airway epithelia. ANO1 high expression or hyperactivity can cause inflammatory diseases such as asthma and diarrhea. (B) Outward flow of Cl− through the activation of ANO1 in smooth muscle cells causes depolarization and smooth muscle contraction. ANO1 high expression or hyperactivity is responsible for asthma diarrhea, and hypertension. (C) In DRG sensory neurons, activation of ANO1 by intracellular Ca2+ or heat causes Cl− efflux and increases neuronal excitability for induction of neuropathic pain. The functional coupling between TRPV1 and ANO1 is also involved in nociception. (D) In cancer cells, ANO1 upregulation promotes cell proliferation and migration, whereas ANO1 downregulation induces apoptosis through multiple signaling pathways, including EGFR/MAPK signaling pathway98, CaMKII/MAPK signaling pathway51, TGF-β signaling pathway120 and NF-κB signaling pathway121. Pharmacological activation of ANO1 by activators or potentiators may serve as a therapeutic strategy for treatment of CF, dry mouth and dry eye syndromes, and inhibition of ANO1 by inhibitors may be beneficial for ANO1 related channelopathies including asthma, diarrhea, hypertension, neuropathic pain and cancers.
3.1. Epithelial diseases
Epithelial cells are a group of tightly compressed cells that line the inside of all organs and function as a barrier between the inside and outside of an organ. Chloride ion is important for transepithelial secretion, whereas intracellular Ca2+ is one of two major signals to modulate Cl– secretion122,123, indicating an important role of Ca2+ activated Cl– channels in regulating epithelial secretion. ANO1 is observed in many epithelial tissues, including but not limited airway, bronchus, salivary glands, pancreatic ductal cells and intestinal epithelium1,4,124. Numerous literatures not only identify native ANO1 expressions in multiple epithelia tissues but also demonstrate the pathological functions of ANO1 important for the process of fluid and electrolyte secretion5,37,125, 126, 127. Here, we review several epithelial diseases involved in ANO1 dysfunction.
3.1.1. Asthma
Asthma is a prevalent inflammatory airway disease characterized by chronic inflammation, remodeling, and excessive constriction of the airway. The mechanism underlying asthma is complex and treatment for asthma is still challenging128. Hypersecretion of airway epithelium and hypercontraction of airway smooth muscle are two major contributions to asthma129. The Ca2+ activated Cl– channel ANO1 as an anion channel plays a critical role in airway transepithelial mucus function. Mucus secreted by goblet cells is composed of water and mucus protein, which requires anion channel activity to instill Cl– and HCO3– for ensurance of proper salination, hydration and pH of the mucus gel layers130. ANO1 is found robustly expressed in epithelial goblet cells and involved in mediating native Ca2+ dependent Cl– current131. ANO1 is also highly permeable to HCO3– at higher intracellular Ca2+ levels, likely contributing to mucus release by providing a secretory pathway for HCO3– that is an essential ingredient in mucus132,133. Downregulation of ANO1 markedly decreases epithelial mucus secretion126,127, suggesting ANO1 inhibition beneficial for asthma. ANO1 expression is upregulated by cytokines IL-4, IL-13 and T helper type 2 that are asthmatic biomarkers, and upregulated ANO1 colocalizes to the apical plasma membrane of goblet cells along with the mucin MUC5AC48,97,131. In addition, ANO1 is also found abundantly expressed in the smooth muscle cells and plays a role in airway hyperreactivity48. ANO1 expression is up-regulated both in epithelial cells and airway smooth muscle cells from asthmatic human patients and rodent models41,48,127. These investigations suggest that hyperactivity of ANO1 may underlie the pathogenesis of asthma, and inhibition of ANO1 channel activity may represent a therapeutic strategy for mucus hypersecretion in asthma.
The mechanism for ANO1 involving in asthma is unclear. A recent investigation shows that the GPCR/ANO1/VGCC axis contributes to the bronchial hyperresponsiveness in asthma134. The asthmatic inflammatory mediators, including 5-HT, histamine and thromboxane A2 binding to G-protein coupled receptors (GPCRs), activate IP3R to release Ca2+ from sarcoplasmic reticulum for activation of ANO1 and voltage gated calcium channel (VGCC) that leads to Ca2+ influx and subsequently depolarize the membrane potential and cause airway contraction both ex vivo ASM and in vivo asthmatic animals134. Downregulation of ANO1 by either pharmacological inhibitor T16Ainh-A01 or knockdown can inhibit the inflammatory mediator-induced Ca2+-activated Cl– current and ASM contractile response134. Several reports show the involvement of ANO1 in mucus production or secretion48,59,127, but the mechanistic insights into mucus production and secretion in airways and validation of the role ANO1 in asthma are required.
Huang and his colleges48 adopted a HTS assay and identified benzbromarone, a clinical drug for gout treatment, as an ANO1 blocker. Benzbromarone shows anti-asthmatic effect through inhibiting IL-13-induced mucin secretion and methacholine-induced ASM contraction via inhibition of ANO1 function70,106. In addition, several ANO1 inhibitors, such as NFA41, tannic acid73, T16Ainh-A0159, and niclosamide70 have been shown to exert anti-asthma effects. However, the anti-asthma effects observed using those ANO1 inhibitors need to be further confirmed in some degree because of their relatively low potency and poor selectivity.
3.1.2. Diarrhea
Diarrhea is characterized by an increase in the frequency of bowel movements with loose and watery stools and major causes of infectious secretory diarrhea include bacteria, viruses and parasites135. Intestinal Cl– channels are important for regulation of excessive mucus secretion induced by bacteria/virus-secreted enterotoxins through increasing apical membrane permeability, suggesting an involvement of Ca2+ activated Cl– channel ANO1 in pathology of diarrhea. There is an increasing evidence that ANO1 plays an important role in the regulation of intestinal epithelial secretion and the pathogenesis of diarrhea based on the following observations: 1) ANO1 is expressed in intestinal epithelia and ANO1 gene knockdown reduces Ca2+ activated Cl– secretion induced by muscarinic agonist carbochol126; 2) Ca2+-dependent Cl− secretion increases through enhancement of ANO1 expression or activation of ANO1 current in the distal colon in response to the rotavirus nonstructural glycoprotein NSP4 that causes infantile gastroenteritis136; and 3) ANO1 expression is upregulated in animal models of diarrhea137.
The increased activity of gastrointestinal (GI) tract is another important cause for diarrhea. ICC are mesenchymal cells located within the muscle layers in the GI tract and myenteric ICC serve as a pacemaker generating the bioelectrical slow wave potential that leads to contraction of GI smooth muscles in the GI tract138. Recent studies have found that ANO1 is also highly expressed in the ICC and mediates the slow wave current in the ICC4,139,140. The intracellular Ca2+ rise from intracellular stores and VGCC in the ICC causes the generation and propagation of pacemaker potential that is also amplified by activation of ANO1 channels138, suggesting that inhibition of ANO1 may reduce intestinal mobility for diarrhea. Indeed, pharmacological inhibition or gene silencing of ANO1 blocks slow wave in intestinal smooth muscle141,142. It is noted that several ANO1 inhibitors including T16Ainh-A01 and CaCCinh-A01 exhibit different potency on blocking gastric and small intestinal slow waves. For instance, CaCCinh-A01 blocks slow waves in the murine stomach at 5 μmol/L and the small intestine at more than 30 μmol/L143. The mechanism underlying these different sensitivities is not entirely clear, but several probabilities including other channels contributions, different splice variants and the local Ca2+ concentrations may contribute to these differences138. Nevertheless, both GI epithelium and ICC are synergistically involved in the pathogenesis of diarrhea and downregulation of ANO1 in either type of the two cells may help mitigate diarrhea.
Rotavirus has been known to cause severe diarrhea in infants and young children, whereas red wine extracts with alcohol-free and CaCCinh-A01 can prevent intestinal fluid loss in a neonatal mouse model of rotaviral diarrhea through inhibition of ANO1-mediated Ca2+-activated Cl– secretion53. However, red wine extracts and CaCCinh-A01 show no obvious effect on cholera toxin-induced diarrhea or CFTR current in cultured cells or intestinal absorption, suggesting the important role of ANO1 in diarrhea53. A recent study demonstrates that glucose and NSP4 synergistically increase the expression of ANO1 and Ca2+-activated Cl– secretory in the mouse model of diarrhea137. Suppressing ANO1 expression in apical membranes of colonic epithelium decreases Ca2+-activated Cl– secretion in chemical dextran sulfate sodium-induced chronic colitis in mice144. There are also observations that inhibition of ANO1 by non-specific ANO1 inhibitors including eugenol74, shikonin145, plumbagin83, resveratrol dimer trans-ε-viniferin (TV) and tetramer γ-2-viniferin (RV)69 can reduce water content in stools, but those observations should be confirmed with specific ANO1 inhibitors.
3.1.3. Cystic fibrosis
CF is an inherited disease of airway obstruction caused by mucus hypersecretion, mucus plugging and bronchoconstriction146. The dysfunction of CFTR Cl– channel is considered to be a major cause for CF as defective CFTR Cl– channel mutations are identified in CF patients147. It has been shown that small molecules can rescue dysfunctional CFTR mutation through increasing the number or open probability of CFTR Cl– channels. Unfortunately, these CFTR correctors or potentiators only exhibit limited efficacy for CF patients because of their multiple and different mutation sites of CFTR148. Therefore, an alternative strategy is CFTR-independent approach for treatment of CF by increasing the activity of ANO1 channel to promote mucus secretion149. This hypothesis is supported by observations that: 1) ANO1 is abundantly expressed in the airway goblet cells and upregulated in inflammatory conditions131; 2) overexpression of ANO1 in CF human bronchial epithelia suppresses proinflammatory cytokine IL-8 secretion150; 3) specific knockdown of ANO1 in respiratory airways eliminates Ca2+-and cAMP-activated Cl– secretion151; 4) Ano1 gene knockout mouse exhibits abnormal trachea morphology, and also mucus obstruction and defective mucociliary clearance that presenting with a CF-like lung phenotyp125,152.
Several studies demonstrate that ANO1 has a functional cross talk with CFTR through PSD-95/Dlg/AO-1 proteins151,153. Such an interaction between ANO1-mediated Ca2+-activated Cl– secretion and CFTR-mediated cAMP-dependent Cl– secretion in airway epithelial cells strongly overlaps through a cAMP sensor protein termed exchange protein directly activated by cAMP and Ca2+-sensitive adenylate cyclase type 1154. ANO1 expression and activity are deficient in CF patients and upregulation of ANO1 can improve mucus dynamics in CF mice155. These lines of evidence support the notion that ANO1 may represent an alternative therapeutic target to circumvent CFTR dysfunction in the airway epithelia of CF patients.
Some CFTR-independent drug candidates have been developed for potential CF therapy. Denufosol, a P2Y2 receptor agonist, promotes airway epithelial chloride secretion through activating CaCCs with P2Y receptors156. Unfortunately, denufosol failed in phase III trial because of its short half-life in vivo147,156,157. Recently, an ANO1 activator, ET000516-A-2 from pre-clinical study is expected to be evaluated in CF patients147. Another novel ANO1 potentiator ETX001 developed by Enterprise Therapeutics (Brighton, UK) was recently reported that it can enhance fluid secretion and improve mucociliary clearance in both primary CF bronchial epithelial cells and sheep model of CF-like airway disease116. These two candidates may bring us an exciting prospect for potential development of alternative CF therapy in the clinic.
3.1.4. Other epithelial diseases
A recent study shows that ANO1 is overexpressed in mouse pancreatic tissue of acute pancreatitis model158. ANO1 promotes the pathogenesis of acute pancreatitis through activating the IP3R/Ca2+/NF-κB/IL-6 pathway, and inhibition of ANO1 by T16Ainh-A01 reduces the pancreatic damage in acute pancreatitis mice158. ANO1 is also likely involved in other epithelial diseases, such as polycystic kidney disease (PKD), diabetic nephropathy and pulmonary fibrosis. The PKD is characterized by multiple bilateral renal cysts that gradually enlarge and can lead to a decline in renal function. ANO1 is found in human kidney and renal epithelial cell lines, and its expression is upregulated in the forskolin induced renal cyst model, autosomal dominant PKD patients and high-fat diet/streptozotocin-induced diabetic nephropathy mice55,159,160, indicating a role of ANO1 in kidney disease.
ANO1 promotes renal cyst growth via induction of Cl– secretion and proliferation of cyst lining epithelium159. Inhibition of ANO1 by pharmacological inhibitors or gene knockdown significantly decreases glucose dependent cyst growth, reduces nephron numbers and also causes albuminuria and tubular damage55,159,161. Mechanistically, ANO1 drives the growth of renal cysts through enhancing Ca2+ release from IP3R sensitive Ca2+ stores162 and lipid peroxidation also promotes renal cyst growth through activating ANO182. In diabetic nephropathy, ANO1 deletion alleviates renal injury in diabetic mice through increasing nephrin expression, reducing the expression level of apoptosis related factors and also suppressing the activation of P38/JNK signaling pathway160.
On the contrary, ANO1 activation aggravates renal injury by activating P38/JNK signaling pathway to promote podocyte apoptosis in diabetic nephropathy mice160 and exacerbates inflammation via activating the TGF-β-SMAD3 pathway163. Downregulation of ANO1 by shRNA can inhibit apoptosis and promote the proliferation of lung fibroblasts in mouse model of idiopathic pulmonary fibrosis163. These observations suggest that inhibition of ANO1 may hold therapeutic potential for kidney and other epithelium-originated diseases.
3.2. Cancers
Epithelial cancer is also known as carcinoma that arises from epithelial tissues. Prior to identification as a CaCC, ANO1 was known as TMEM16A first described in 2003164. ANO1 was also named as DOG1 (gastrointestinal stromal tumor 1), TAOS2 (tumor amplified and overexpressed sequence 2) and ORAOV2 (oral cancer overexpressed 2) because of its overexpression in these cancers35. Investigations from ours and others show that ANO1 is overexpressed and involved in the pathogenesis of cancers especially originated from epithelial cancers, such as gastrointestinal stromal tumor (GIST)165, head and neck squamous cell carcinoma (HNSCC)166, prostate cancer46, lung cancer167, colon cancer90, ovarian cancer168, breast cancer51, liver cancer169, gastric cancer120, esophageal cancers170, pancreatic adenocarcinoma171, salivary gland carcinoma172 and glioblastoma121 (Table 340,46,51,57,62,91,93,98,120,121,165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187).
Table 3.
ANO1 modulation, expression and function in cancer cell lines, xenograft tumors and human cancer tissues.
| Cancer type | High expression |
Cell assay |
Inhibition |
Signaling pathways | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cell line | Human tissue | Clinical implication | Proliferation/viability | Migration/invasion | Tool | Xenograft tumor | |||
| Breast cancer | ZR75-1, HCC1954, MDA-MB-415 |
+ | Poor Prognosis (+) | + | NR | shRNA/CaCCinh-A01 | Tumor growth (−) | 11q13 amplification, Cl– channel activity, Apoptosis, EGFR, CAMKII, AKT, MAPK | 51 |
| YMB-1 | NR | NR | + | NR | siRNA/NFA | NR | Epigenetic regulation | 40 | |
| YMB-1, MDA-MB-453 |
NR | NR | NR | NR | siRNA/T16inh-A01 | NR | AKT, STAT3 | 173 | |
| SKBR3 | NR | Improved response to biological therapies (−) | + | NR | siRNA/T16Ainh-A01 CaCCinh-A01 |
NR | EGFR, HER2, STAT3 | 174 | |
| MCF-7, T47D |
+ | Shorter overall survival in ER + patients (+) | + | NR | shRNA/T16Ainh-A01 | Tumor growth (−) | EGFR/STAT3 signaling | 175 | |
| HNSCC | UM-SCC1, T24 |
+ | Poor prognosis (+) | + | NR | shRNA/T16Ainh-A01 | Tumor growth (−) | MAPK, Ki67 | 176 |
| HEp-2, SCC-25 |
+ | A marker for distal metastasis (+) | No effect | + | siRNA/NFA, DIDS, Fluoxetine |
NR | 11q13 amplification | 166 | |
| UM-SCC1, T24 |
High in primary tumor and low in metastatic tumor | A biomarker for metastasis (−) | NR | – | shRNA | Tumor growth (−), Metastatic development (+) |
Promoter methylation, E-cadherin |
177 | |
| FaDu | NR | Poor prognosis (+) | + | NR | shRNA/CaCCinh-A01 | Tumor growth (−) | 11q13 amplification, Cl– channel activity, Apoptosis, EGFR, CAMKII, AKT, MAPK | 51 | |
| Cal-33, OSC19, UM-SCC-1, FaDu |
NR | Increased efficacy of biologic therapies (−) | OSC19 (+) | NR | siRNA/T16Ainh-A01 CaCCinh-A01 |
ANO1-overexpressing tumors were heavier than control tumors | EGFR, HER2, STAT3 | 174 | |
| OSC19, FaDu, UM-SCC-1 |
Positively correlated with tumor size | Recurrence of cancer (+) | + | NR | shRNA | ANO1-overexpressing tumors were greater than control tumors | ERK, BIM, Apoptosis | 178 | |
| ESCC | KYSE30, KYSE510 |
+ | Lymph node metastasis and advanced clinical stage (+) | + | NR | siRNA | NR | 11q13 amplification | 170 |
| KYSE410, KYSE30 |
+ | Poor prognosis (+) Advanced stage (+) |
+ | + | shRNA | NR | TGF-β signaling, cell cycle | 179 | |
| Prostate cancer | PC-3, LNCap, RWPE1 |
+ | NR | + | NR | siRNA/T16Ainh-A01, CaCCinh-A01, MONNA, tannic acid |
Tumor growth (−) | ERK, AKT | 62 |
| PC-3, LNCaP |
NR | NR | + | NR | siRNA/NFA | NR | Epigenetic regulation | 40 | |
| LNCaP, PC-3 |
+ | Clinical TNM stage (+) Gleason score (+) |
+ | + | shRNA/DIDS | Tumor growth (−) | NR | 46 | |
| PC-3 | NR | NR | + | NR | shRNA siRNA/CaCCinh-A01, T16Ainh-A01, Ani9 |
Tumor growth (−) | TNF-α signaling, apoptosis | 57 | |
| Gastric cancer | AGS, BGC823 |
+ | Poor overall survival (+) TNM (+) Lymphnode metastasis (+) |
No effect | + | shRNA | NR | TGF-β, E-cadherin | 180 |
| AGS, BGC823 | Negatively related with miR381 | Poor prognosis (+) | No effect | + | siRNA | NR | Regulated by miR-381, TGF-β, E-cadherin | 120 | |
| AGS, SGC7901 | + | TNM stage (+) | NR | + | siRNA | Tumor metastasis (−) | Regulated by SP1 through MLL1 and H3K4 trimethylation | 181 | |
| HCC | SMMC7721 | + | NR | + | + | siRNA | Tumorigenicity (−) | MAPK signaling, cell cycle | 169 |
| HepG2, SMMC7721 | + | Tumor grade (+) | + | + | shRNA | Tumor growth (−) | PI3K/AKT-MAPK signaling pathway, apoptosis, cell cycle | 182 | |
| Glioma | U87MG | + | Tumor grade (+) | + | + | siRNA | NR | NF-κB signaling | 121 |
| U251, U87MG | NR | NR | NR | + | shRNA/T16Ainh-A01 | NR | CaMKII-β | 183 | |
| Lung cancer | GLC82, NCI-H520 | + | NR | + | + | shRNA | Tumor growth (−) | NR | 167 |
| H1299 | NR | NR | + | + | shRNA/T16Ainh-A01 | Tumor growth (−) | EGFR/MAPK signaling | 98 | |
| Pancreatic adenocarcinoma | BxPC-3, AsPC-1, Capan-1 |
NR | NR | No effect | + | siRNA/T16Ainh-A01, CaCCinh-A01, NS3728 | NR | NR | 93 |
| AsPC-1 | + | Poor prognosis (+) Biomarker (+) |
NR | + | shRNA | NR | Ligand-dependent EGFR signaling | 171 | |
| GIST | GIST-T1, GIST-882 | NR | NR | No effect | NR | shRNA/T16Ainh-A01, NFA, NPPB | Tumor growth (−) | IGFBP-5, no effect on KIT | 165 |
| NR | Cancer and PBMCs | Biomarkers (+) Tumor size (+) |
NR | NR | NR | NR | NR | 184 | |
| GIST-T1, GIST882 | NR | NR | + | NR | T16Ainh-A01 CaCCinh-A01 |
NR | Cell cycle | 91 | |
| Salivary gland carcinoma | NR | + | NR | NR | NR | NR | NR | NR | 172 |
| Ovarian cancer | SKOV3 | Cancer and PBMCs | Pathologic stage and differentiation (+) | + | + | siRNA | Tumor growth (−) | PI3K/AKT signaling | 168 |
| Colorectal cancer | SW620 | NR | NR | + | + | shRNA | NR | MAPK signaling, cell cycle | 185 |
| DLD-1, HCT116 | Liver metastasis cancer tissue | Poor prognosis (+) | + | + | siRNA | NR | Regulated by miR-132 | 186 | |
| SW480 | + | Poor prognosis (+) | NR | + | siRNA | NR | EGFR signaling, regulated by miR-144 | 187 | |
+, positive effect; –, negative effect; NR, no report. HCC, hepatocellular carcinoma; PBMCs, peripheral blood mononuclear cells; TNM, tumor, lymph nodes and metastasis.
ANO1 upregulation in many kinds of cancers is related to its gene location at chromosome 11q13 that is frequently amplified in many malignant tumors51,164 and amplification of 11q13 is associated with the increase of ANO1 gene copy numbers166. Overexpression of ANO1 promotes proliferation and migration in multiple cancer cell lines51,169,179, and ANO1 upregulation is associated with lower overall survival in patients with breast cancer, pancreatic cancer or gastric cancer51,171,180 (Table 3). ANO1 mRNA is also highly expressed in the blood of GIST patients184 and patients with epithelial ovarian cancer, and the expression level of ANO1 mRNA decreases after surgical removal of tumors168, suggesting that detection of ANO1 gene in blood may serve as a biomarker for early diagnosis of cancer.
Multiple signaling pathways have been shown to be involved in ANO1 modulation in cancer development (Table 3). In breast cancer and HNSCC, downregulation of ANO1 by knockdown or pharmacological inhibition inhibits cancer cell proliferation, induces apoptosis and reduces tumor growth through reducing epidermal growth factor receptor (EGFR) cell signaling pathways51. Studies have also shown that ANO1 can affect the progression of intestinal cancer, liver cancer, and pancreatic cancer through the EGFR pathway169,171,187, as well as the modulation of glioma by NF-κB signaling121. In prostate cancer, ANO1 can regulate TNF-α signaling to contribute to cell growth and apoptosis57. In epithelial ovarian cancer, silencing ANO1 can suppress cancer cell proliferation, migration and invasion as well as the growth of xenograft tumors through inactivation of PI3K/AKT cell signaling pathway168. In gastric cancer, ANO1 overexpression can promote tumor invasion and predict poor prognosis through affecting TGF-β signaling function120,180, that is also the target for ANO1 regulating cell proliferation, migration and invasion in esophageal squamous cell carcinoma (ESCC)179. In glioblastoma, ANO1 expression is regulated by CaMKII-β and suppression of CaMKII-β inhibits ANO1 mediated glioblastoma development183, and CaMKII also plays a role in ANO1-mediated tumorigenic properties of HNSCC and breast cancer51. It appears that ANO1 can regulate different signaling pathways in the same type of tumor or the same signaling pathway in different types of tumor.
Inhibition of ANO1 by gene silencing or pharmacological means can suppress cancer cell proliferation and migration, invasion and tumor growth62,175,181. Small molecules, such as CaCCinh-A01, T16Ainh-A01, MONNA and Ani9, as well as natural products including avermectins and flavonoids exhibit anti-cancer effects through inhibition of ANO1 activity57,62,78,84, suggesting that ANO1 may serve as a potential drug target for cancer therapy.
3.3. Hypertension
Hypertension or high blood pressure is a common condition in which the long-term force of the blood against the blood vessels is high enough that it may increase the risk of heart diseases and brain stroke. ANO1 is expressed in various smooth muscle cells of arteries and veins188,189, suggesting a role of ANO1 in regulation of vasoconstriction. Activation of Ca2+-activated Cl– current can lead to Cl– efflux after Ca2+ influx and membrane depolarization, thus resulting in vasoconstriction and increase of blood pressure. Indeed, ANO1 expression and activity are upregulated in murine pulmonary arterial myocytes induced by chronic hypoxia, which contributes to pulmonary hypertension60. Similar results were also observed in monocrotaline-induced pulmonary hypertension rats188, spontaneously hypertension rats63, idiopathic pulmonary arterial hypertension patients190,191, and high-flow-induced pulmonary arterial hypertension (PAH) rats192. Conversely, cell-specific knockout of ANO1 reduces blood pressure and attenuates hypertension in mice and spontaneously hypertensive rats193, 194, 195.
A recent study shows that upregulation of ANO1 depolarizes pulmonary artery smooth muscle cells (PASMC) membrane potential, contributing vasoconstriction and the increased pulmonary vascular resistance in PAH rats190. Conversely, pharmacological inhibition or gene silencing of ANO1 reverse the membrane depolarizes of PASMC. For instance, a specific ANO1 inhibitor MONNA hyperpolarizes the rat coronary artery smooth muscle cell membrane potential and increases coronary flow63. Vascular smooth muscle cells (VSMC) are the stromal cells of the vascular wall and are responsible for regulating arterial tone, and blood pressure. Overexpression of ANO1 in healthy donor PASMC promotes the cell proliferation and produces an idiopathic pulmonary arterial hypertension (IPAH)-like phenotype. Pharmacological inhibition of ANO1 may reverse vasoconstriction and remodeling of pulmonary arteries in IPAH190,196. These investigations suggest the involvement of ANO1 in VSMC contraction and vascular remodeling for hypertension.
Circulating angiotensin II (Ang II) as a major contributor of the renin–angiotensin system is upregulated during hypertension, and Ang II is frequently used to establish hypertension models. It has been shown that Ang II significantly enhances ANO1 expression in human umbilical vein endothelial cells and endothelial-specific ANO1 knockout significantly reduces Ang II-induced hypertension through ROS signaling pathway, whereas endothelial-specific transgenic ANO1 shows the opposite effect195. These studies suggest that inhibition of ANO1 function may be beneficial for hypertension, and ANO1 inhibitors, including T16Ainh-A01, MONNA, and Ani9 can inhibit agonist-induced vesical constriction and cause vasorelaxation63,94.
3.4. Nociception
Nociception is a perception in response to painful or harmful stimuli, such as heat, cold, mechanical and chemical stimulus in the environment. Dorsal root ganglion (DRG) is a cluster of sensory neurons in the dorsal root of spinal nerves responsible for pain signal transmission197. ANO1 is mainly expressed in small diameter DRG neurons that are intimately involved in nociception4,198, suggesting a modulatory role of ANO1 in pain sensation. It has been shown that an inflammatory mediator bradykinin, released from damaged tissues or applied exogenously can activate ANO1 through B2 receptors and PLC pathway, subsequently depolarizing membrane potential and markedly stimulating firings in DRG neurons, and pharmacological inhibition of ANO1 attenuates pain behaviors44. ANO1 expression is upregulated in the spinal cord and DRG neurons after spinal nerve injury, suggesting the involvement of ANO1 in development of neuropathic pain54. Protease activated receptor 2 (PAR2), also known as G-protein coupled receptor 11, has been identified to be involved in the pathogenesis of pain199. PAR2 and ANO1 are co-localized in DRG neurons, and their expressions are increased in rat pain model of chronic constriction injury200.
ANO1 can be activated by temperatures over 44 °C, and silencing of ANO1 in DRG neurons significantly reduces nociceptive behavior in thermal pain model198, inflammation and nerve-injury induced hyperalgesia or allodynia54,201. Thus, downregulation of ANO1 activity may present a potential therapeutic strategy for neuropathic pain. ANO1 as a potential target for pain is evidenced by observations that inhibition of ANO1 by small molecules NPPB, NFA, T16Ainh-A01 or CaCCinh-A01 reduces capsaicin-induced inward current and action potential firing, and as well as pain-related behaviors99,100. Again, these ANO1 inhibitors are not specific and a key question such as whether ANO1 directly affects the excitability of nociceptors should be addressed.
3.5. Others
A recent study shows that ANO1 is expressed in mouse brain endothelial cells where ANO1 expression is upregulated after ischemic stroke induced by the middle cerebral artery occlusion56. Targeting ANO1 with inhibitor CaCCinh-A01 or silencing attenuates blood–brain barrier (BBB) breakdown after ischemic stroke through decreasing intracellular adhesion molecule-1 via NF-κB signaling pathway, suggesting that downregulation of ANO1 protects BBB disruption after ischemia stroke. It is supported by another study that ANO1 inhibitor T16Ainh-A01 or siRNA inhibits proliferation and migration of brain capillary endothelial cells that comprise BBB202. It would be interesting to see more studies that are designed to validate ANO1 as a therapeutic target for brain stroke.
4. Summary and perspectives
The CaCC ANO1 channel is expressed in a wide variety of epithelial cells, smooth muscle cells and neurons. Although abnormal expression or dysfunction of ANO1 is involved in the pathology of many diseases, validating ANO1 as a therapeutic target still presents a big challenge. While a significant progress has been made for the distribution, expression, structure and pathophysiological functions of ANO1, there still exists an urgent need for selective modulators of the channel for target validation. Current available ANO1 modulators are also in preclinical stage without any treatments ready for clinical utility, which highlights the much-needed efforts in understanding the channel pharmacology and validation of ANO1 as therapeutic target. The recent structure of ANO1 solved by single-particle cryo-electron microscopy can provide a valuable model for the design of more potent and selective ANO1 modulators that can be used to help validate this emerging target for therapeutic potential of diseases, including cancer, inflammatory epithelial diseases, neuropathic pain and hypertension and cystic fibrosis lung disease.
Acknowledgments
We thank Dr. Xiaowen Tang (School of Pharmacy, Qingdao University Medical College, Qingdao, China) for his help in Fig. 1. This work was supported by grants from the National Natural Science foundation of China (81573410), the Ministry of Science and Technology of China (2018ZX09711001-004-006), Shandong Provincial Medicine and Health Technology Development Plan (2019WS145, China), Medical Research Guidance Plan of Qingdao Municipal Health Committee (2019-WJZD091, China) and Qingdao Excellent Young Medical Talent Training Project.
Author contributions
Yani Liu, Zongtao Liu, and KeWei Wang wrote and edited the manuscript. Yani Liu and KeWei Wang contributed to manuscript revision and discussion of the content.
Conflicts of interest
The authors declare that there is no conflict of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Yani Liu, Email: liuyani@qdu.edu.cn.
KeWei Wang, Email: wangkw@qdu.edu.cn.
References
- 1.Oh U., Jung J. Cellular functions of TMEM16/anoctamin. Pflügers Archiv. 2016;468:443–453. doi: 10.1007/s00424-016-1790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartzell C., Putzier I., Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 3.Ji Q., Guo S., Wang X., Pang C., Zhan Y., Chen Y. Recent advances in TMEM16A: structure, function, and disease. J Cell Physiol. 2019;234:7856–7873. doi: 10.1002/jcp.27865. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y.D., Cho H., Koo J.Y., Tak M.H., Cho Y., Shim W.S. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 5.Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder B.C., Cheng T., Jan Y.N., Jan L.Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephan A.B., Shum E.Y., Hirsh S., Cygnar K.D., Reisert J., Zhao H. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci U S A. 2009;106:11776–11781. doi: 10.1073/pnas.0903304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stohr H., Heisig J.B., Benz P.M., Schoberl S., Milenkovic V.M., Strauss O. TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci. 2009;29:6809–6818. doi: 10.1523/JNEUROSCI.5546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W.C., Xiao S., Huang F., Harfe B.D., Jan Y.N., Jan L.Y. Calcium-activated chloride channels (CaCCs) regulate action potential and synaptic response in hippocampal neurons. Neuron. 2012;74:179–192. doi: 10.1016/j.neuron.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha G.E., Lee J., Kwak H., Song K., Kwon J., Jung S.Y. The Ca2+-activated chloride channel anoctamin-2 mediates spike-frequency adaptation and regulates sensory transmission in thalamocortical neurons. Nat Commun. 2016;7:13791. doi: 10.1038/ncomms13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Zhang Z., Xiao S., Tien J., Le S., Le T. Inferior olivary TMEM16B mediates cerebellar motor learning. Neuron. 2017;95:1103–11011 e4. doi: 10.1016/j.neuron.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiber R., Uliyakina I., Kongsuphol P., Warth R., Mirza M., Martins J.R. Expression and function of epithelial anoctamins. J Biol Chem. 2010;285:7838–7845. doi: 10.1074/jbc.M109.065367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duran C., Qu Z., Osunkoya A.O., Cui Y., Hartzell H.C. ANOs 3–7 in the anoctamin/Tmem16 Cl– channel family are intracellular proteins. Am J Physiol Cell Physiol. 2012;302:C482–C493. doi: 10.1152/ajpcell.00140.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki J., Umeda M., Sims P.J., Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki J., Fujii T., Imao T., Ishihara K., Kuba H., Nagata S. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem. 2013;288:13305–13316. doi: 10.1074/jbc.M113.457937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gyobu S., Miyata H., Ikawa M., Yamazaki D., Takeshima H., Suzuki J. A role of TMEM16E carrying a scrambling domain in sperm motility. Mol Cell Biol. 2016;36:645–659. doi: 10.1128/MCB.00919-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamaleddin M.A. Molecular, biophysical, and pharmacological properties of calcium-activated chloride channels. J Cell Physiol. 2018;233:787–798. doi: 10.1002/jcp.25823. [DOI] [PubMed] [Google Scholar]
- 18.Maniero C., Scudieri P., Haris Shaikh L., Zhao W., Gurnell M., Galietta L.J.V. ANO4 (Anoctamin 4) is a novel marker of Zona glomerulosa that regulates stimulated aldosterone secretion. Hypertension. 2019;74:1152–1159. doi: 10.1161/HYPERTENSIONAHA.119.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marmorstein A.D., Marmorstein L.Y., Rayborn M., Wang X., Hollyfield J.G., Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2000;97:12758–12763. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh S.J., Lee C.J. Distribution and function of the bestrophin-1 (Best1) channel in the brain. Exp Neurobiol. 2017;26:113–121. doi: 10.5607/en.2017.26.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartzell H.C., Qu Z., Yu K., Xiao Q., Chien L.T. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev. 2008;88:639–672. doi: 10.1152/physrev.00022.2007. [DOI] [PubMed] [Google Scholar]
- 22.Ferrera L., Caputo A., Ubby I., Bussani E., Zegarra-Moran O., Ravazzolo R. Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem. 2009;284:33360–33368. doi: 10.1074/jbc.M109.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Q., Yu K., Perez-Cornejo P., Cui Y., Arreola J., Hartzell H.C. Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc Natl Acad Sci U S A. 2011;108:8891–8896. doi: 10.1073/pnas.1102147108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunner J.D., Lim N.K., Schenck S., Duerst A., Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature. 2014;516:207–212. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- 25.Tien J., Peters C.J., Wong X.M., Cheng T., Jan Y.N., Jan L.Y. A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. Elife. 2014;3 doi: 10.7554/eLife.02772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters C.J., Yu H., Tien J., Jan Y.N., Li M., Jan L.Y. Four basic residues critical for the ion selectivity and pore blocker sensitivity of TMEM16A calcium-activated chloride channels. Proc Natl Acad Sci U S A. 2015;112:3547–3552. doi: 10.1073/pnas.1502291112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulino C., Kalienkova V., Lam A.K.M., Neldner Y., Dutzler R. Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature. 2017;552:421–425. doi: 10.1038/nature24652. [DOI] [PubMed] [Google Scholar]
- 28.Dang S., Feng S., Tien J., Peters C.J., Bulkley D., Lolicato M. Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature. 2017;552:426–429. doi: 10.1038/nature25024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo S., Chen Y.F., Shi S., Pang C.L., Wang X.Z., Zhang H.L. The molecular mechanism of ginsenoside analogs activating TMEM16A. Biophys J. 2020;118:262–272. doi: 10.1016/j.bpj.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma K., Wang H., Yu J., Wei M., Xiao Q. New insights on the regulation of Ca2+-activated chloride channel TMEM16A. J Cell Physiol. 2017;232:707–716. doi: 10.1002/jcp.25621. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Zhang H., Men H., Du Y., Xiao Z., Zhang F. Volume-regulated Cl– current: contributions of distinct Cl– channels and localized Ca2+ signals. Am J Physiol Cell Physiol. 2019;317:C466–C480. doi: 10.1152/ajpcell.00507.2018. [DOI] [PubMed] [Google Scholar]
- 32.Le S.C., Jia Z., Chen J., Yang H. Molecular basis of PIP2-dependent regulation of the Ca2+-activated chloride channel TMEM16A. Nat Commun. 2019;10:3769–3780. doi: 10.1038/s41467-019-11784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu K., Jiang T., Cui Y., Tajkhorshid E., Hartzell H.C. A network of phosphatidylinositol 4,5-bisphosphate binding sites regulates gating of the Ca2+-activated Cl– channel ANO1 (TMEM16A) Proc Natl Acad Sci U S A. 2019;116:19952–19962. doi: 10.1073/pnas.1904012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin X., Shah S., Du X., Zhang H., Gamper N. Activation of Ca2+ -activated Cl– channel ANO1 by localized Ca2+ signals. J Physiol. 2016;594:19–30. doi: 10.1113/jphysiol.2014.275107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crottes D., Jan L.Y. The multifaceted role of TMEM16A in cancer. Cell Calcium. 2019;82:102050–102060. doi: 10.1016/j.ceca.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi S., Pang C., Guo S., Chen Y., Ma B., Qu C. Recent progress in structural studies on TMEM16A channel. Comput Struct Biotechnol J. 2020;18:714–722. doi: 10.1016/j.csbj.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rottgen T.S., Nickerson A.J., Rajendran V.M. Calcium-activated Cl– channel: insights on the molecular identity in epithelial tissues. Int J Mol Sci. 2018;19:1432–1444. doi: 10.3390/ijms19051432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y., Zhang H., Huang D., Qi J., Xu J., Gao H. Characterization of the effects of Cl– channel modulators on TMEM16A and bestrophin-1 Ca2+ activated Cl– channels. Pflügers Archiv. 2015;467:1417–1430. doi: 10.1007/s00424-014-1572-5. [DOI] [PubMed] [Google Scholar]
- 39.Oh S.J., Park J.H., Han S., Lee J.K., Roh E.J., Lee C.J. Development of selective blockers for Ca2+-activated Cl channel using Xenopus laevis oocytes with an improved drug screening strategy. Mol Brain. 2008;1:14–24. doi: 10.1186/1756-6606-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuba S., Niwa S., Muraki K., Kanatsuka S., Nakazono Y., Hatano N. Downregulation of Ca2+-activated Cl– channel TMEM16A by the inhibition of histone deacetylase in TMEM16A-expressing cancer cells. J Pharmacol Exp Therapeut. 2014;351:510–518. doi: 10.1124/jpet.114.217315. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C.H., Li Y., Zhao W., Lifshitz L.M., Li H., Harfe B.D. The transmembrane protein 16A Ca2+-activated Cl– channel in airway smooth muscle contributes to airway hyperresponsiveness. Am J Respir Crit Care Med. 2013;187:374–381. doi: 10.1164/rccm.201207-1303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabrita I., Benedetto R., Schreiber R., Kunzelmann K. Niclosamide repurposed for the treatment of inflammatory airway disease. JCI Insight. 2019;4 doi: 10.1172/jci.insight.128414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu G., Hamill O.P. NPPB block of Ca2+-activated Cl– currents in Xenopus oocytes. Pflügers Archiv. 1992;420:227–229. doi: 10.1007/BF00374996. [DOI] [PubMed] [Google Scholar]
- 44.Liu B., Linley J.E., Du X., Zhang X., Ooi L., Zhang H. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl– channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu Z., Hartzell H.C. Functional geometry of the permeation pathway of Ca2+-activated Cl-channels inferred from analysis of voltage-dependent block. J Biol Chem. 2001;276:18423–18429. doi: 10.1074/jbc.M101264200. [DOI] [PubMed] [Google Scholar]
- 46.Liu W., Lu M., Liu B., Huang Y., Wang K. Inhibition of Ca2+-activated Cl– channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012;326:41–51. doi: 10.1016/j.canlet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Wozniak K.L., Phelps W.A., Tembo M., Lee M.T., Carlson A.E. The TMEM16A channel mediates the fast polyspermy block in Xenopus laevis. J Gen Physiol. 2018;150:1249–1259. doi: 10.1085/jgp.201812071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang F., Zhang H., Wu M., Yang H., Kudo M., Peters C.J. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci U S A. 2012;109:16354–16359. doi: 10.1073/pnas.1214596109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De La Fuente R., Namkung W., Mills A., Verkman A.S. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol Pharmacol. 2008;73:758–768. doi: 10.1124/mol.107.043208. [DOI] [PubMed] [Google Scholar]
- 50.Namkung W., Phuan P.W., Verkman A.S. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286:2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Britschgi A., Bill A., Brinkhaus H., Rothwell C., Clay I., Duss S. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci U S A. 2013;110:E1026–E1034. doi: 10.1073/pnas.1217072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boedtkjer D.M., Kim S., Jensen A.B., Matchkov V.M., Andersson K.E. New selective inhibitors of calcium-activated chloride channels-T16A(inh)-A01, CaCC(inh)-A01 and MONNA—what do they inhibit?. Br J Pharmacol. 2015;172:4158–4172. doi: 10.1111/bph.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ko E.A., Jin B.J., Namkung W., Ma T., Thiagarajah J.R., Verkman A.S. Chloride channel inhibition by a red wine extract and a synthetic small molecule prevents rotaviral secretory diarrhoea in neonatal mice. Gut. 2014;63:1120–1129. doi: 10.1136/gutjnl-2013-305663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pineda-Farias J.B., Barragan-Iglesias P., Loeza-Alcocer E., Torres-Lopez J.E., Rocha-Gonzalez H.I., Perez-Severiano F. Role of anoctamin-1 and bestrophin-1 in spinal nerve ligation-induced neuropathic pain in rats. Mol Pain. 2015;11:41–54. doi: 10.1186/s12990-015-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraus A., Schley G., Kunzelmann K., Schreiber R., Peters D.J., Stadler R. Glucose promotes secretion-dependent renal cyst growth. J Mol Med (Berl) 2016;94:107–117. doi: 10.1007/s00109-015-1337-4. [DOI] [PubMed] [Google Scholar]
- 56.Liu P.Y., Zhang Z., Liu Y., Tang X.L., Shu S., Bao X.Y. TMEM16A inhibition preserves blood–brain barrier Integrity after ischemic stroke. Front Cell Neurosci. 2019;13:360–372. doi: 10.3389/fncel.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song Y., Gao J., Guan L., Chen X., Gao J., Wang K. Inhibition of ANO1/TMEM16A induces apoptosis in human prostate carcinoma cells by activating TNF-alpha signaling. Cell Death Dis. 2018;9:703–716. doi: 10.1038/s41419-018-0735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanoni S., Zeng C., Marella S., Uddin J., Wu D., Arora K. Identification of anoctamin 1 (ANO1) as a key driver of esophageal epithelial proliferation in eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145:239–254 e2. doi: 10.1016/j.jaci.2019.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kondo M., Tsuji M., Hara K., Arimura K., Yagi O., Tagaya E. Chloride ion transport and overexpression of TMEM16A in a guinea-pig asthma model. Clin Exp Allergy. 2017;47:795–804. doi: 10.1111/cea.12887. [DOI] [PubMed] [Google Scholar]
- 60.Sun H., Xia Y., Paudel O., Yang X.R., Sham J.S. Chronic hypoxia-induced upregulation of Ca2+-activated Cl− channel in pulmonary arterial myocytes: a mechanism contributing to enhanced vasoreactivity. J Physiol. 2012;590:3507–3521. doi: 10.1113/jphysiol.2012.232520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh S.J., Hwang S.J., Jung J., Yu K., Kim J., Choi J.Y. MONNA, a potent and selective blocker for transmembrane protein with unknown function 16/anoctamin-1. Mol Pharmacol. 2013;84:726–735. doi: 10.1124/mol.113.087502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cha J.Y., Wee J., Jung J., Jang Y., Lee B., Hong G.S. Anoctamin 1 (TMEM16A) is essential for testosterone-induced prostate hyperplasia. Proc Natl Acad Sci U S A. 2015;112:9722–9727. doi: 10.1073/pnas.1423827112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Askew Page H.R., Dalsgaard T., Baldwin S.N., Jepps T.A., Povstyan O., Olesen S.P. TMEM16A is implicated in the regulation of coronary flow and is altered in hypertension. Br J Pharmacol. 2019;176:1635–1648. doi: 10.1111/bph.14598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ru F., Sun H., Jurcakova D., Herbstsomer R.A., Meixong J., Dong X. Mechanisms of pruritogen-induced activation of itch nerves in isolated mouse skin. J Physiol. 2017;595:3651–3666. doi: 10.1113/JP273795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seo Y., Lee H.K., Park J., Jeon D.K., Jo S., Jo M. Ani9, a novel potent small-molecule ANO1 inhibitor with negligible effect on ANO2. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Centeio R., Cabrita I., Benedetto R., Talbi K., Ousingsawat J., Schreiber R. Pharmacological inhibition and activation of the Ca2+ activated Cl– channel TMEM16A. Int J Mol Sci. 2020;21:2557–2573. doi: 10.3390/ijms21072557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Truong E.C., Phuan P.W., Reggi A.L., Ferrera L., Galietta L.J.V., Levy S.E. Substituted 2-acylaminocycloalkylthiophene-3-carboxylic acid arylamides as inhibitors of the calcium-activated chloride channel transmembrane protein 16A (TMEM16A) J Med Chem. 2017;60:4626–4635. doi: 10.1021/acs.jmedchem.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seo Y., Kim J., Chang J., Kim S.S., Namkung W., Kim I. Synthesis and biological evaluation of novel Ani9 derivatives as potent and selective ANO1 inhibitors. Eur J Med Chem. 2018;160:245–255. doi: 10.1016/j.ejmech.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Yu B., Jiang Y., Zhang B., Yang H., Ma T. Resveratrol dimer trans-epsilon-viniferin prevents rotaviral diarrhea in mice by inhibition of the intestinal calcium-activated chloride channel. Pharmacol Res. 2018;129:453–461. doi: 10.1016/j.phrs.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Miner K., Labitzke K., Liu B., Wang P., Henckels K., Gaida K. Drug repurposing: the anthelmintics niclosamide and nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways. Front Pharmacol. 2019;10:51–84. doi: 10.3389/fphar.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Namkung W., Thiagarajah J.R., Phuan P.W., Verkman A.S. Inhibition of Ca2+-activated Cl– channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. Faseb J. 2010;24:4178–4186. doi: 10.1096/fj.10-160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X., Zhang H., Zhou N., Xu J., Si M., Jia Z. Tannic acid modulates excitability of sensory neurons and nociceptive behavior and the ionic mechanism. Eur J Pharmacol. 2015;764:633–642. doi: 10.1016/j.ejphar.2015.06.048. [DOI] [PubMed] [Google Scholar]
- 73.Gallos G., Remy K.E., Danielsson J., Funayama H., Fu X.W., Chang H.Y. Functional expression of the TMEM16 family of calcium-activated chloride channels in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2013;305:L625–L634. doi: 10.1152/ajplung.00068.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao Z., Namkung W., Ko E.A., Park J., Tradtrantip L., Verkman A.S. Fractionation of a herbal antidiarrheal medicine reveals eugenol as an inhibitor of Ca2+-activated Cl– channel TMEM16A. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li H.Y., Park C.K., Jung S.J., Choi S.Y., Lee S.J., Park K. Eugenol inhibits K+ currents in trigeminal ganglion neurons. J Dent Res. 2007;86:898–902. doi: 10.1177/154405910708600918. [DOI] [PubMed] [Google Scholar]
- 76.Park C.K., Kim K., Jung S.J., Kim M.J., Ahn D.K., Hong S.D. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain. 2009;144:84–94. doi: 10.1016/j.pain.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Seo Y., Ryu K., Park J., Jeon D.K., Jo S., Lee H.K. Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X., Li H., Zhang H., Liu Y., Huo L., Jia Z. Inhibition of transmembrane member 16A calcium-activated chloride channels by natural flavonoids contributes to flavonoid anticancer effects. Br J Pharmacol. 2017;174:2334–2345. doi: 10.1111/bph.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang M., Zhou Y., Wan L.L., Ye J.Z., Lu H.L., Huang X. Luteolin suppresses colonic smooth muscle motility via inhibiting L-type calcium channel currents in mice. Gen Physiol Biophys. 2020;39:49–58. doi: 10.4149/gpb_2019045. [DOI] [PubMed] [Google Scholar]
- 80.Yu B., Jiang Y., Jin L., Ma T., Yang H. Role of quercetin in modulating chloride transport in the intestine. Front Physiol. 2016;7:549–560. doi: 10.3389/fphys.2016.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seo Y., Park J., Kim M., Lee H.K., Kim J.H., Jeong J.H. Inhibition of ANO1/TMEM16A chloride channel by idebenone and its cytotoxicity to cancer cell lines. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schreiber R., Buchholz B., Kraus A., Schley G., Scholz J., Ousingsawat J. Lipid peroxidation drives renal cyst growth in vitro through activation of TMEM16A. J Am Soc Nephrol. 2019;30:228–242. doi: 10.1681/ASN.2018010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu B., Zhu X., Yang X., Jin L., Xu J., Ma T. Plumbagin prevents secretory diarrhea by inhibiting CaCC and CFTR channel activities. Front Pharmacol. 2019;10:1181–1194. doi: 10.3389/fphar.2019.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang X., Zhang G., Zhai W., Zhao Z., Wang S., Yi J. Inhibition of TMEM16A Ca2+-activated Cl– channels by avermectins is essential for their anticancer effects. Pharmacol Res. 2020;156:104763. doi: 10.1016/j.phrs.2020.104763. [DOI] [PubMed] [Google Scholar]
- 85.White M.M., Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2+-activated Cl– channels in Xenopus oocytes. Mol Pharmacol. 1990;37:720–724. [PubMed] [Google Scholar]
- 86.Fedigan S., Bradley E., Webb T., Large R.J., Hollywood M.A., Thornbury K.D. Effects of new-generation TMEM16A inhibitors on calcium-activated chloride currents in rabbit urethral interstitial cells of Cajal. Pflügers Archiv. 2017;469:1443–1455. doi: 10.1007/s00424-017-2028-5. [DOI] [PubMed] [Google Scholar]
- 87.Tian X.Q., Ma K.T., Wang X.W., Wang Y., Guo Z.K., Si J.Q. Effects of the calcium-activated chloride channel inhibitors T16Ainh-A01 and CaCCinh-A01 on cardiac fibroblast function. Cell Physiol Biochem. 2018;49:706–716. doi: 10.1159/000493036. [DOI] [PubMed] [Google Scholar]
- 88.Paik S.S., Park Y.S., Kim I.B. Calcium- and voltage-dependent dual gating ANO1 is an intrinsic determinant of repolarization in rod bipolar cells of the mouse retina. Cells. 2020;9:543–554. doi: 10.3390/cells9030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bill A., Hall M.L., Borawski J., Hodgson C., Jenkins J., Piechon P. Small molecule-facilitated degradation of ANO1 protein: a new targeting approach for anticancer therapeutics. J Biol Chem. 2014;289:11029–11041. doi: 10.1074/jbc.M114.549188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guan L., Song Y., Gao J., Gao J., Wang K. Inhibition of calcium-activated chloride channel ANO1 suppresses proliferation and induces apoptosis of epithelium originated cancer cells. Oncotarget. 2016;7:78619–78630. doi: 10.18632/oncotarget.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frobom R., Sellberg F., Xu C., Zhao A., Larsson C., Lui W.O. Biochemical inhibition of DOG1/TMEM16A achieves antitumoral effects in human gastrointestinal stromal tumor cells in vitro. Anticancer Res. 2019;39:3433–3442. doi: 10.21873/anticanres.13489. [DOI] [PubMed] [Google Scholar]
- 92.Sung T.S., O'Driscoll K., Zheng H., Yapp N.J., Leblanc N., Koh S.D. Influence of intracellular Ca2+ and alternative splicing on the pharmacological profile of ANO1 channels. Am J Physiol Cell Physiol. 2016;311:C437–C451. doi: 10.1152/ajpcell.00070.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sauter D.R.P., Novak I., Pedersen S.F., Larsen E.H., Hoffmann E.K. ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC) Pflügers Archiv. 2015;467:1495–1508. doi: 10.1007/s00424-014-1598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davis A.J., Shi J., Pritchard H.A., Chadha P.S., Leblanc N., Vasilikostas G. Potent vasorelaxant activity of the TMEM16A inhibitor T16A(inh)-A01. Br J Pharmacol. 2013;168:773–784. doi: 10.1111/j.1476-5381.2012.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Twyffels L., Strickaert A., Virreira M., Massart C., Van Sande J., Wauquier C. Anoctamin-1/TMEM16A is the major apical iodide channel of the thyrocyte. Am J Physiol Cell Physiol. 2014;307:C1102–C1112. doi: 10.1152/ajpcell.00126.2014. [DOI] [PubMed] [Google Scholar]
- 96.Yamamura H., Nishimura K., Hagihara Y., Suzuki Y., Imaizumi Y. TMEM16A and TMEM16B channel proteins generate Ca2+-activated Cl– current and regulate melatonin secretion in rat pineal glands. J Biol Chem. 2018;293:995–1006. doi: 10.1074/jbc.RA117.000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y., Wang X., Wang H., Jiao J., Li Y., Fan E. TMEM16A-mediated mucin secretion in IL-13-induced nasal epithelial cells from chronic rhinosinusitis patients. Allergy Asthma Immunol Res. 2015;7:367–375. doi: 10.4168/aair.2015.7.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu C., Zhang R., Jiang D. TMEM16A as a potential biomarker in the diagnosis and prognosis of lung cancer. Arch Iran Med. 2019;22:32–38. [PubMed] [Google Scholar]
- 99.Takayama Y., Uta D., Furue H., Tominaga M. Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc Natl Acad Sci U S A. 2015;112:5213–5218. doi: 10.1073/pnas.1421507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deba F., Bessac B.F. Anoctamin-1 Cl– channels in nociception: activation by an N-aroylaminothiazole and capsaicin and inhibition by T16A[inh]-A01. Mol Pain. 2015;11:55–69. doi: 10.1186/s12990-015-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cruz-Rangel S., De Jesus-Perez J.J., Contreras-Vite J.A., Perez-Cornejo P., Hartzell H.C., Arreola J. Gating modes of calcium-activated chloride channels TMEM16A and TMEM16B. J Physiol. 2015;593:5283–5298. doi: 10.1113/JP271256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crutzen R., Virreira M., Markadieu N., Shlyonsky V., Sener A., Malaisse W.J. Anoctamin 1 (Ano1) is required for glucose-induced membrane potential oscillations and insulin secretion by murine beta-cells. Pflügers Archiv. 2016;468:573–591. doi: 10.1007/s00424-015-1758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao da Y., Zhang B.L., Leung M.C., Au S.C., Wong P.Y., Shum W.W. Coupling of TRPV6 and TMEM16A in epithelial principal cells of the rat epididymis. J Gen Physiol. 2016;148:161–182. doi: 10.1085/jgp.201611626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guo S., Chen Y., Pang C., Wang X., Shi S., Zhang H. Matrine is a novel inhibitor of the TMEM16A chloride channel with antilung adenocarcinoma effects. J Cell Physiol. 2019;234:8698–8708. doi: 10.1002/jcp.27529. [DOI] [PubMed] [Google Scholar]
- 105.Hara K., Kondo M., Tsuji M., Takeyama K., Tamaoki J. Clarithromycin suppresses IL-13-induced goblet cell metaplasia via the TMEM16A-dependent pathway in guinea pig airway epithelial cells. Respir Investig. 2019;57:79–88. doi: 10.1016/j.resinv.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 106.Danielsson J., Perez-Zoghbi J., Bernstein K., Barajas M.B., Zhang Y., Kumar S. Antagonists of the TMEM16A calcium-activated chloride channel modulate airway smooth muscle tone and intracellular calcium. Anesthesiology. 2015;123:569–581. doi: 10.1097/ALN.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee Y.H., Yi G.S. Prediction of novel anoctamin1 (ANO1) inhibitors using 3D-QSAR pharmacophore modeling and molecular docking. Int J Mol Sci. 2018;19:3204–3221. doi: 10.3390/ijms19103204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 109.Namkung W., Yao Z., Finkbeiner W.E., Verkman A.S. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. Faseb J. 2011;25:4048–4062. doi: 10.1096/fj.11-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu S., Feng J., Luo J., Yang P., Brett T.J., Hu H. Eact, a small molecule activator of TMEM16A, activates TRPV1 and elicits pain- and itch-related behaviours. Br J Pharmacol. 2016;173:1208–1218. doi: 10.1111/bph.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Genovese M., Borrelli A., Venturini A., Guidone D., Caci E., Viscido G. TRPV4 and purinergic receptor signalling pathways are separately linked in airway epithelia to CFTR and TMEM16A chloride channels. J Physiol. 2019;597:5859–5878. doi: 10.1113/JP278784. [DOI] [PubMed] [Google Scholar]
- 112.Tian Y., Schreiber R., Wanitchakool P., Kongsuphol P., Sousa M., Uliyakina I. Control of TMEM16A by INO-4995 and other inositolphosphates. Br J Pharmacol. 2013;168:253–265. doi: 10.1111/j.1476-5381.2012.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chai R., Chen Y., Yuan H., Wang X., Guo S., Qi J. Identification of resveratrol, an herbal compound, as an activator of the calcium-activated chloride channel, TMEM16A. J Membr Biol. 2017;250:483–492. doi: 10.1007/s00232-017-9975-9. [DOI] [PubMed] [Google Scholar]
- 114.Guo S., Chen Y., Pang C., Wang X., Qi J., Mo L. Ginsenoside Rb1, a novel activator of the TMEM16A chloride channel, augments the contraction of guinea pig ileum. Pflügers Archiv. 2017;469:681–692. doi: 10.1007/s00424-017-1934-x. [DOI] [PubMed] [Google Scholar]
- 115.Guo S., Wang H., Pang C., Ren X., Li J., Wang X. Entering the spotlight: chitosan oligosaccharides as novel activators of CaCCs/TMEM16A. Pharmacol Res. 2019;146:104323. doi: 10.1016/j.phrs.2019.104323. [DOI] [PubMed] [Google Scholar]
- 116.Danahay H.L., Lilley S., Fox R., Charlton H., Sabater J., Button B. TMEM16A potentiation: a novel therapeutic approach for the treatment of cystic fibrosis. Am J Respir Crit Care Med. 2020;201:946–954. doi: 10.1164/rccm.201908-1641OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schreiber R., Ousingsawat J., Wanitchakool P., Sirianant L., Benedetto R., Reiss K. Regulation of TMEM16A/ANO1 and TMEM16F/ANO6 ion currents and phospholipid scrambling by Ca2+ and plasma membrane lipid. J Physiol. 2018;596:217–229. doi: 10.1113/JP275175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang Y., Guo S., Ren S., Chen Y., Zhan Y., An H. The natural compound cinnamaldehyde is a novel activator of calcium-activated chloride channel. J Membr Biol. 2018;251:747–756. doi: 10.1007/s00232-018-0052-9. [DOI] [PubMed] [Google Scholar]
- 119.Li C., Wang J., Wang Y., Gao H., Wei G., Huang Y. Recent progress in drug delivery. Acta Pharm Sin B. 2019;9:1145–1162. doi: 10.1016/j.apsb.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cao Q., Liu F., Ji K., Liu N., He Y., Zhang W. MicroRNA-381 inhibits the metastasis of gastric cancer by targeting TMEM16A expression. J Exp Clin Cancer Res. 2017;36:29–44. doi: 10.1186/s13046-017-0499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu J., Liu Y., Ren Y., Kang L., Zhang L. Transmembrane protein with unknown function 16A overexpression promotes glioma formation through the nuclear factor-kappaB signaling pathway. Mol Med Rep. 2014;9:1068–1074. doi: 10.3892/mmr.2014.1888. [DOI] [PubMed] [Google Scholar]
- 122.Willumsen N.J., Boucher R.C. Activation of an apical Cl– conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am J Physiol. 1989;256:C226–C233. doi: 10.1152/ajpcell.1989.256.2.C226. [DOI] [PubMed] [Google Scholar]
- 123.Gray M.A., Winpenny J.P., Porteous D.J., Dorin J.R., Argent B.E. CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic CF mouse. Am J Physiol. 1994;266:C213–C221. doi: 10.1152/ajpcell.1994.266.1.C213. [DOI] [PubMed] [Google Scholar]
- 124.Kunzelmann K., Tian Y., Martins J.R., Faria D., Kongsuphol P., Ousingsawat J. Anoctamins. Pflugers Arch. 2011;462:195–208. doi: 10.1007/s00424-011-0975-9. [DOI] [PubMed] [Google Scholar]
- 125.Rock J.R., O'Neal W.K., Gabriel S.E., Randell S.H., Harfe B.D., Boucher R.C. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl– secretory channel in mouse airways. J Biol Chem. 2009;284:14875–14880. doi: 10.1074/jbc.C109.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ousingsawat J., Martins J.R., Schreiber R., Rock J.R., Harfe B.D., Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J Biol Chem. 2009;284:28698–28703. doi: 10.1074/jbc.M109.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Benedetto R., Cabrita I., Schreiber R., Kunzelmann K. TMEM16A is indispensable for basal mucus secretion in airways and intestine. Faseb J. 2019;33:4502–4512. doi: 10.1096/fj.201801333RRR. [DOI] [PubMed] [Google Scholar]
- 128.Holgate S.T., Wenzel S., Postma D.S., Weiss S.T., Renz H., Sly P.D. Asthma. Nat Rev Dis Primers. 2015;1:15025. doi: 10.1038/nrdp.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Papi A., Brightling C., Pedersen S.E., Reddel H.K. Asthma. Lancet. 2018;391:783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 130.Sala-Rabanal M., Yurtsever Z., Berry K.N., Brett T.J. Novel roles for chloride channels, exchangers, and regulators in chronic inflammatory airway diseases. Mediat Inflamm. 2015;2015:497387. doi: 10.1155/2015/497387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Scudieri P., Caci E., Bruno S., Ferrera L., Schiavon M., Sondo E. Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia. J Physiol. 2012;590:6141–6155. doi: 10.1113/jphysiol.2012.240838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jung J., Nam J.H., Park H.W., Oh U., Yoon J.H., Lee M.G. Dynamic modulation of ANO1/TMEM16A HCO3– permeability by Ca2+/calmodulin. Proc Natl Acad Sci U S A. 2013;110:360–365. doi: 10.1073/pnas.1211594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gorrieri G., Scudieri P., Caci E., Schiavon M., Tomati V., Sirci F. Goblet cell hyperplasia requires high bicarbonate transport to support mucin release. Sci Rep. 2016;6:36016. doi: 10.1038/srep36016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang P., Zhao W., Sun J., Tao T., Chen X., Zheng Y.Y. Inflammatory mediators mediate airway smooth muscle contraction through a G protein-coupled receptor-transmembrane protein 16A-voltage-dependent Ca2+ channel axis and contribute to bronchial hyperresponsiveness in asthma. J Allergy Clin Immunol. 2018;141:1259–1268. doi: 10.1016/j.jaci.2017.05.053. e11. [DOI] [PubMed] [Google Scholar]
- 135.Walker C.L.F., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ousingsawat J., Mirza M., Tian Y., Roussa E., Schreiber R., Cook D.I. Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption. Pflügers Archiv. 2011;461:579–589. doi: 10.1007/s00424-011-0947-0. [DOI] [PubMed] [Google Scholar]
- 137.Yin L., Menon R., Gupta R., Vaught L., Okunieff P., Vidyasagar S. Glucose enhances rotavirus enterotoxin-induced intestinal chloride secretion. Pflügers Archiv. 2017;469:1093–1105. doi: 10.1007/s00424-017-1987-x. [DOI] [PubMed] [Google Scholar]
- 138.Sanders K.M. Spontaneous electrical activity and rhythmicity in gastrointestinal smooth muscles. Adv Exp Med Biol. 2019;1124:3–46. doi: 10.1007/978-981-13-5895-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu M.H., Kim T.W., Ro S., Yan W., Ward S.M., Koh S.D. A Ca2+-activated Cl– conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gomez-Pinilla P.J., Gibbons S.J., Bardsley M.R., Lorincz A., Pozo M.J., Pasricha P.J. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hwang S.J., Blair P.J., Britton F.C., O'Driscoll K.E., Hennig G., Bayguinov Y.R. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malysz J., Gibbons S.J., Saravanaperumal S.A., Du P., Eisenman S.T., Cao C. Conditional genetic deletion of Ano1 in interstitial cells of Cajal impairs Ca2+ transients and slow waves in adult mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2017;312:G228–G245. doi: 10.1152/ajpgi.00363.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hwang S.J., Basma N., Sanders K.M., Ward S.M. Effects of new-generation inhibitors of the calcium-activated chloride channel anoctamin 1 on slow waves in the gastrointestinal tract. Br J Pharmacol. 2016;173:1339–1349. doi: 10.1111/bph.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rottgen T.S., Nickerson A.J., Minor E.A., Stewart A.B., Harold A.D., Rajendran V.M. Dextran sulfate sodium-induced chronic colitis attenuates Ca2+-activated Cl– secretion in murine colon by downregulating TMEM16A. Am J Physiol Cell Physiol. 2018;315:C10–C20. doi: 10.1152/ajpcell.00328.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang Y., Yu B., Yang H., Ma T. Shikonin inhibits intestinal calcium-activated chloride channels and prevents rotaviral diarrhea. Front Pharmacol. 2016;7:270–278. doi: 10.3389/fphar.2016.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ratjen F., Bell S.C., Rowe S.M., Goss C.H., Quittner A.L., Bush A. Cystic fibrosis. Nat Rev Dis Primers. 2015;1:15010. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Strug L.J., Stephenson A.L., Panjwani N., Harris A. Recent advances in developing therapeutics for cystic fibrosis. Hum Mol Genet. 2018;27:R173–R186. doi: 10.1093/hmg/ddy188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu Y., Wang K. Exploiting the diversity of ion channels: modulation of ion channels for therapeutic indications. Handb Exp Pharmacol. 2019;260:187–205. doi: 10.1007/164_2019_333. [DOI] [PubMed] [Google Scholar]
- 149.Quesada R., Dutzler R. Alternative chloride transport pathways as pharmacological targets for the treatment of cystic fibrosis. J Cyst Fibros. 2020;19(Suppl 1):S37–S41. doi: 10.1016/j.jcf.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 150.Veit G., Bossard F., Goepp J., Verkman A.S., Galietta L.J., Hanrahan J.W. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell. 2012;23:4188–4202. doi: 10.1091/mbc.E12-06-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Benedetto R., Ousingsawat J., Wanitchakool P., Zhang Y., Holtzman M.J., Amaral M. Epithelial chloride transport by CFTR requires TMEM16A. Sci Rep. 2017;7:12397. doi: 10.1038/s41598-017-10910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.He M., Wu B., Ye W., Le D.D., Sinclair A.W., Padovano V. Chloride channels regulate differentiation and barrier functions of the mammalian airway. Elife. 2020;9 doi: 10.7554/eLife.53085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kunzelmann K., Ousingsawat J., Cabrita I., Dousova T., Bahr A., Janda M. TMEM16A in cystic fibrosis: activating or inhibiting?. Front Pharmacol. 2019;10:3–20. doi: 10.3389/fphar.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lerias J., Pinto M., Benedetto R., Schreiber R., Amaral M., Aureli M. Compartmentalized crosstalk of CFTR and TMEM16A (ANO1) through EPAC1 and ADCY1. Cell Signal. 2018;44:10–19. doi: 10.1016/j.cellsig.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 155.Sonneville F., Ruffin M., Coraux C., Rousselet N., Le Rouzic P., Blouquit-Laye S. MicroRNA-9 downregulates the ANO1 chloride channel and contributes to cystic fibrosis lung pathology. Nat Commun. 2017;8:710–720. doi: 10.1038/s41467-017-00813-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Accurso F.J., Moss R.B., Wilmott R.W., Anbar R.D., Schaberg A.E., Durham T.A. Denufosol tetrasodium in patients with cystic fibrosis and normal to mildly impaired lung function. Am J Respir Crit Care Med. 2011;183:627–634. doi: 10.1164/rccm.201008-1267OC. [DOI] [PubMed] [Google Scholar]
- 157.Ratjen F., Durham T., Navratil T., Schaberg A., Accurso F.J., Wainwright C. Long term effects of denufosol tetrasodium in patients with cystic fibrosis. J Cyst Fibros. 2012;11:539–549. doi: 10.1016/j.jcf.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 158.Wang Q., Bai L., Luo S., Wang T., Yang F., Xia J. TMEM16A Ca2+-activated Cl– channel inhibition ameliorates acute pancreatitis via the IP3R/Ca2+/NFkappaB/IL-6 signaling pathway. J Adv Res. 2020;23:25–35. doi: 10.1016/j.jare.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Buchholz B., Faria D., Schley G., Schreiber R., Eckardt K.U., Kunzelmann K. Anoctamin 1 induces calcium-activated chloride secretion and proliferation of renal cyst-forming epithelial cells. Kidney Int. 2014;85:1058–1067. doi: 10.1038/ki.2013.418. [DOI] [PubMed] [Google Scholar]
- 160.Lian H., Cheng Y., Wu X. TMEM16A exacerbates renal injury by activating P38/JNK signaling pathway to promote podocyte apoptosis in diabetic nephropathy mice. Biochem Biophys Res Commun. 2017;487:201–208. doi: 10.1016/j.bbrc.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 161.Schenk L.K., Buchholz B., Henke S.F., Michgehl U., Daniel C., Amann K. Nephron-specific knockout of TMEM16A leads to reduced number of glomeruli and albuminuria. Am J Physiol Ren Physiol. 2018;315:F1777–F1786. doi: 10.1152/ajprenal.00638.2017. [DOI] [PubMed] [Google Scholar]
- 162.Cabrita I., Buchholz B., Schreiber R., Kunzelmann K. TMEM16A drives renal cyst growth by augmenting Ca2+ signaling in M1 cells. J Mol Med (Berl) 2020;98:659–671. doi: 10.1007/s00109-020-01894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dai W.J., Qiu J., Sun J., Ma C.L., Huang N., Jiang Y. Downregulation of microRNA-9 reduces inflammatory response and fibroblast proliferation in mice with idiopathic pulmonary fibrosis through the ANO1-mediated TGF-beta-Smad3 pathway. J Cell Physiol. 2019;234:2552–2565. doi: 10.1002/jcp.26961. [DOI] [PubMed] [Google Scholar]
- 164.Katoh M., Katoh M. FLJ10261 gene, located within the CCND1-EMS1 locus on human chromosome 11q13, encodes the eight-transmembrane protein homologous to C12orf3, C11orf25 and FLJ34272 gene products. Int J Oncol. 2003;22:1375–1381. [PubMed] [Google Scholar]
- 165.Simon S., Grabellus F., Ferrera L., Galietta L., Schwindenhammer B., Muhlenberg T. DOG1 regulates growth and IGFBP5 in gastrointestinal stromal tumors. Cancer Res. 2013;73:3661–3670. doi: 10.1158/0008-5472.CAN-12-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ayoub C., Wasylyk C., Li Y., Thomas E., Marisa L., Robe A. ANO1 amplification and expression in HNSCC with a high propensity for future distant metastasis and its functions in HNSCC cell lines. Br J Cancer. 2010;103:715–726. doi: 10.1038/sj.bjc.6605823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jia L., Liu W., Guan L., Lu M., Wang K. Inhibition of calcium-activated chloride channel ANO1/TMEM16A suppresses tumor growth and invasion in human lung cancer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu Z., Zhang S., Hou F., Zhang C., Gao J., Wang K. Inhibition of Ca2+-activated chloride channel ANO1 suppresses ovarian cancer through inactivating PI3K/Akt signaling. Int J Cancer. 2019;144:2215–2226. doi: 10.1002/ijc.31887. [DOI] [PubMed] [Google Scholar]
- 169.Deng L., Yang J., Chen H., Ma B., Pan K., Su C. Knockdown of TMEM16A suppressed MAPK and inhibited cell proliferation and migration in hepatocellular carcinoma. OncoTargets Ther. 2016;9:325–333. doi: 10.2147/OTT.S95985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shi Z.Z., Shang L., Jiang Y.Y., Hao J.J., Zhang Y., Zhang T.T. Consistent and differential genetic aberrations between esophageal dysplasia and squamous cell carcinoma detected by array comparative genomic hybridization. Clin Cancer Res. 2013;19:5867–5878. doi: 10.1158/1078-0432.CCR-12-3753. [DOI] [PubMed] [Google Scholar]
- 171.Crottes D., Lin Y.T., Peters C.J., Gilchrist J.M., Wiita A.P., Jan Y.N. TMEM16A controls EGF-induced calcium signaling implicated in pancreatic cancer prognosis. Proc Natl Acad Sci U S A. 2019;116:13026–13035. doi: 10.1073/pnas.1900703116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chenevert J., Duvvuri U., Chiosea S., Dacic S., Cieply K., Kim J. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol. 2012;25:919–929. doi: 10.1038/modpathol.2012.57. [DOI] [PubMed] [Google Scholar]
- 173.Fujimoto M., Kito H., Kajikuri J., Ohya S. Transcriptional repression of human epidermal growth factor receptor 2 by ClC-3 Cl–/H+ transporter inhibition in human breast cancer cells. Cancer Sci. 2018;109:2781–2791. doi: 10.1111/cas.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kulkarni S., Bill A., Godse N.R., Khan N.I., Kass J.I., Steehler K. TMEM16A/ANO1 suppression improves response to antibody-mediated targeted therapy of EGFR and HER2/ERBB2. Genes Chromosomes Cancer. 2017;56:460–471. doi: 10.1002/gcc.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang H., Yao F., Luo S., Ma K., Liu M., Bai L. A mutual activation loop between the Ca2+-activated chloride channel TMEM16A and EGFR/STAT3 signaling promotes breast cancer tumorigenesis. Cancer Lett. 2019;455:48–59. doi: 10.1016/j.canlet.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 176.Duvvuri U., Shiwarski D.J., Xiao D., Bertrand C., Huang X., Edinger R.S. TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012;72:3270–3281. doi: 10.1158/0008-5472.CAN-12-0475-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shiwarski D.J., Shao C., Bill A., Kim J., Xiao D., Bertrand C.A. To "grow" or "go": TMEM16A expression as a switch between tumor growth and metastasis in SCCHN. Clin Cancer Res. 2014;20:4673–4688. doi: 10.1158/1078-0432.CCR-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Godse N.R., Khan N., Yochum Z.A., Gomez-Casal R., Kemp C., Shiwarski D.J. TMEM16A/ANO1 inhibits apoptosis via downregulation of bim expression. Clin Cancer Res. 2017;23:7324–7332. doi: 10.1158/1078-0432.CCR-17-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yu Y., Cao J., Wu W., Zhu Q., Tang Y., Zhu C. Genome-wide copy number variation analysis identified ANO1 as a novel oncogene and prognostic biomarker in esophageal squamous cell cancer. Carcinogenesis. 2019;40:1198–1208. doi: 10.1093/carcin/bgz077. [DOI] [PubMed] [Google Scholar]
- 180.Liu F., Cao Q.H., Lu D.J., Luo B., Lu X.F., Luo R.C. TMEM16A overexpression contributes to tumor invasion and poor prognosis of human gastric cancer through TGF-beta signaling. Oncotarget. 2015;6:11585–11599. doi: 10.18632/oncotarget.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zeng X., Pan D., Wu H., Chen H., Yuan W., Zhou J. Transcriptional activation of ANO1 promotes gastric cancer progression. Biochem Biophys Res Commun. 2019;512:131–136. doi: 10.1016/j.bbrc.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 182.Zhang C., Liu J., Han Z., Cui X., Peng D., Xing Y. Inhibition of TMEM16A suppresses growth and induces apoptosis in hepatocellular carcinoma. Int J Clin Oncol. 2020;25:1145–1154. doi: 10.1007/s10147-020-01653-6. [DOI] [PubMed] [Google Scholar]
- 183.Sim K.M., Lee Y.S., Kim H.J., Cho C.H., Yi G.S., Park M.J. Suppression of CaMKIIbeta inhibits ANO1-mediated glioblastoma progression. Cells. 2020;9:1079–1095. doi: 10.3390/cells9051079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li H., Wu A., Zhu W., Hou F., Cheng S., Cao J. Detection of ANO1 mRNA in PBMCs is a promising method for GISTs diagnosis. Sci Rep. 2019;9:9525. doi: 10.1038/s41598-019-45941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sui Y., Sun M., Wu F., Yang L., Di W., Zhang G. Inhibition of TMEM16A expression suppresses growth and invasion in human colorectal cancer cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mokutani Y., Uemura M., Munakata K., Okuzaki D., Haraguchi N., Takahashi H. Down-regulation of microRNA-132 is associated with poor prognosis of colorectal cancer. Ann Surg Oncol. 2016;23:599–608. doi: 10.1245/s10434-016-5133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jiang Y., Cai Y., Shao W., Li F., Guan Z., Zhou Y. MicroRNA144 suppresses aggressive phenotypes of tumor cells by targeting ANO1 in colorectal cancer. Oncol Rep. 2019;41:2361–2370. doi: 10.3892/or.2019.7025. [DOI] [PubMed] [Google Scholar]
- 188.Forrest A.S., Joyce T.C., Huebner M.L., Ayon R.J., Wiwchar M., Joyce J. Increased TMEM16A-encoded calcium-activated chloride channel activity is associated with pulmonary hypertension. Am J Physiol Cell Physiol. 2012;303:C1229–C1243. doi: 10.1152/ajpcell.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Davis A.J., Forrest A.S., Jepps T.A., Valencik M.L., Wiwchar M., Singer C.A. Expression profile and protein translation of TMEM16A in murine smooth muscle. Am J Physiol Cell Physiol. 2010;299:C948–C959. doi: 10.1152/ajpcell.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Papp R., Nagaraj C., Zabini D., Nagy B.M., Lengyel M., Skofic Maurer D. Targeting TMEM16A to reverse vasoconstriction and remodelling in idiopathic pulmonary arterial hypertension. Eur Respir J. 2019;53:1800965. doi: 10.1183/13993003.00965-2018. [DOI] [PubMed] [Google Scholar]
- 191.Allawzi A.M., Vang A., Clements R.T., Jhun B.S., Kue N.R., Mancini T.J. Activation of anoctamin-1 limits pulmonary endothelial cell proliferation via p38-mitogen-activated protein kinase-dependent apoptosis. Am J Respir Cell Mol Biol. 2018;58:658–667. doi: 10.1165/rcmb.2016-0344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shang L., Wang K., Liu D., Qin S., Huang J., Zhao Y. TMEM16A regulates the cell cycle of pulmonary artery smooth muscle cells in high-flow-induced pulmonary arterial hypertension rat model. Exp Ther Med. 2020;19:3275–3281. doi: 10.3892/etm.2020.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang B., Li C., Huai R., Qu Z. Overexpression of ANO1/TMEM16A, an arterial Ca2+-activated Cl– channel, contributes to spontaneous hypertension. J Mol Cell Cardiol. 2015;82:22–32. doi: 10.1016/j.yjmcc.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 194.Heinze C., Seniuk A., Sokolov M.V., Huebner A.K., Klementowicz A.E., Szijarto I.A. Disruption of vascular Ca2+-activated chloride currents lowers blood pressure. J Clin Invest. 2014;124:675–686. doi: 10.1172/JCI70025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ma M.M., Gao M., Guo K.M., Wang M., Li X.Y., Zeng X.L. TMEM16A contributes to endothelial dysfunction by facilitating Nox2 NADPH oxidase-derived reactive oxygen species generation in hypertension. Hypertension. 2017;69:892–901. doi: 10.1161/HYPERTENSIONAHA.116.08874. [DOI] [PubMed] [Google Scholar]
- 196.Zeng J.W., Chen B.Y., Lv X.F., Sun L., Zeng X.L., Zheng H.Q. Transmembrane member 16A participates in hydrogen peroxide-induced apoptosis by facilitating mitochondria-dependent pathway in vascular smooth muscle cells. Br J Pharmacol. 2018;175:3669–3684. doi: 10.1111/bph.14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tracey W.D., Jr. Nociception. Curr Biol. 2017;27:R129–R133. doi: 10.1016/j.cub.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 198.Cho H., Yang Y.D., Lee J., Lee B., Kim T., Jang Y. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci. 2012;15:1015–1021. doi: 10.1038/nn.3111. [DOI] [PubMed] [Google Scholar]
- 199.Bao Y., Hou W., Hua B. Protease-activated receptor 2 signalling pathways: a role in pain processing. Expert Opin Ther Targets. 2014;18:15–27. doi: 10.1517/14728222.2014.844792. [DOI] [PubMed] [Google Scholar]
- 200.Zhang M., Gao C.X., Wang Y.P., Ma K.T., Li L., Yin J.W. The association between the expression of PAR2 and TMEM16A and neuropathic pain. Mol Med Rep. 2018;17:3744–3750. doi: 10.3892/mmr.2017.8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee B., Cho H., Jung J., Yang Y.D., Yang D.J., Oh U. Anoctamin 1 contributes to inflammatory and nerve-injury induced hypersensitivity. Mol Pain. 2014;10:5–13. doi: 10.1186/1744-8069-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Suzuki T., Yasumoto M., Suzuki Y., Asai K., Imaizumi Y., Yamamura H. TMEM16A Ca2+-Activated Cl– channel regulates the proliferation and migration of brain capillary endothelial cells. Mol Pharmacol. 2020;98:61–71. doi: 10.1124/mol.119.118844. [DOI] [PubMed] [Google Scholar]