Abstract
The 90-kiloDalton (kD) heat shock protein (Hsp90) is a ubiquitous, ATP-dependent molecular chaperone whose primary function is to ensure the proper folding of several hundred client protein substrates. Because many of these clients are overexpressed or become mutated during cancer progression, Hsp90 inhibition has been pursued as a potential strategy for cancer as one can target multiple oncoproteins and signaling pathways simultaneously. The first discovered Hsp90 inhibitors, geldanamycin and radicicol, function by competitively binding to Hsp90's N-terminal binding site and inhibiting its ATPase activity. However, most of these N-terminal inhibitors exhibited detrimental activities during clinical evaluation due to induction of the pro-survival heat shock response as well as poor selectivity amongst the four isoforms. Consequently, alternative approaches to Hsp90 inhibition have been pursued and include C-terminal inhibition, isoform-selective inhibition, and the disruption of Hsp90 protein−protein interactions. Since the Hsp90 protein folding cycle requires the assembly of Hsp90 into a large heteroprotein complex, along with various co-chaperones and immunophilins, the development of small molecules that prevent assembly of the complex offers an alternative method of Hsp90 inhibition.
Key words: Hsp90, Protein−protein interactions, Disruptors, Natural products, Small molecules, Peptidomimetics
Abbreviations: ADP, adenosine diphosphate; Aha1, activator of Hsp90 ATPase homologue 1; ATP, adenosine triphosphate; Cdc37, cell division cycle 37; CTD, C-terminal domain; Grp94, 94-kD glucose-regulated protein; Her-2, human epidermal growth factor receptor-2; hERG, human ether-à-go-go-related gene; HIF-1α, hypoxia-inducing factor-1α; HIP, Hsp70-interaction protein; HOP, Hsp70‒Hsp90 organizing protein; HSQC, heteronuclear single quantum coherence; Hsp90, 90-kD heat shock protein; MD, middle domain; NTD, N-terminal domain; PPI, protein−protein interaction; SAHA, suberoylanilide hydroxamic acid; SAR, structure–activity relationship; SUMO, small ubiquitin-like modifier; TRAP1, Hsp75tumor necrosis factor receptor associated protein 1; TROSY, transverse relaxation-optimized spectroscopy; TPR2A, tetratricopeptide-containing repeat 2A
Graphical abstract
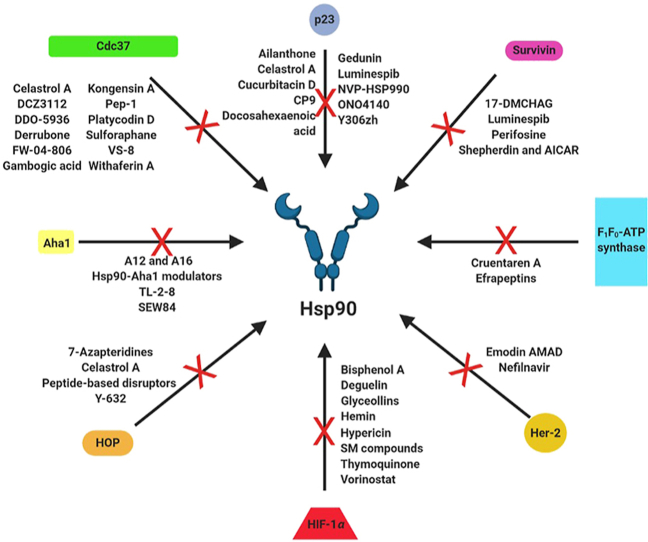
Many natural products and small molecules have been reported to disrupt protein–protein interactions (PPIs) between Hsp90 and its co-chaperones and client substrates. Such results offer support to the disruption of PPIs as an alternative strategy for selective inhibition of this molecular chaperone.
1. Protein folding: An introduction
Proteins are a class of biomolecules that perform a variety of biological functions and include enzyme catalysis, the regulation of genes, cellular transport, and facilitating the cellular response to environmental signals/stresses.
The proper conformation of a protein is crucial to its function, and the question of how a protein transforms from a simple peptide sequence into a complex three-dimensional structure has been extensively studied for over half a century1. It was initially thought that protein folding is a thermodynamic process in which the protein's amino acid composition aids enthalpic interactions2. However, some proteins require the assistance of molecular chaperones to transform linear polypeptides into biologically active and three-dimensional proteins to carry out their biological function. Hartl3 has defined molecular chaperones as “proteins that bind to and stabilize an otherwise unstable conformer of another protein—and, by controlled binding and release, facilitate its correct fate in vivo: be it folding, oligomeric assembly, transport to a particular subcellular compartment, or disposal by degradation.” The existence of molecular chaperones was first suggested by Fohlman and colleagues4 during their study with taipoxen, a sialo-glycoprotein neurotoxin that can be isolated from the venom of the Australian taipan (Oxyuranus s. scutellatus). Although their analysis found that taipoxen is comprised of three subunits (referred to as α, β, and γ), only the α subunit is toxic. Consequently, it was proposed that the role played by the other subunits is to increase α′s stability and molecular recognition. Another early discovery included nucleoplasmins, which are chaperones that stabilize histones and ensure proper interactions with DNA during the assembly of chromatin5. Later studies confirmed that molecular chaperones are conserved across all kingdoms of life and exist as many different families, which are often related by molecular weight.
2. The 90-kiloDalton (kD) heat shock protein (Hsp90)
Cellular stress can disrupt the proteostatic equilibrium and result in lethal outcomes. Because of the dynamic nature between interior and exterior cellular environments, cells have evolved to express chaperones that promote survival in response to various cellular insults such as acidosis, oxidative stress, and hypoxia. One important family of molecular chaperones are the heat shock proteins, which were first observed as a cellular response to high temperature. The heat shock response was first observed when Drosophila salivary glands were exposed to elevated temperatures, dinitrophenol or salicylate, which resulted in chromosomal puffing6, 7, 8. Tissières and colleagues9 correlated these puffs with the production of small proteins that would later come to be known as the heat shock proteins. The protein families are further subdivided by their molecular weight and include Hsp27, Hsp40, Hsp60, Hsp70, Hsp90, as well as other larger members.
The master regulator of the heat shock response is Hsp90, which is an ATP-dependent molecular chaperone that functions to fold nascent polypeptides into their active structures, as well as to facilitate the rematuration of protein aggregates/misfolded proteins and the processing of proteins via the ubiquitin‒proteasome pathway. Hsp90 is among the most abundant proteins in the cell and comprises 1%–2% of a cell's total protein content, which is increased to 4%–6% under stressful conditions10. In humans, Hsp90 exists as four isoforms: Hsp90α, Hsp90β, the 94-kD glucose-regulated protein (Grp94), and the Hsp75/tumor necrosis factor receptor associated protein 1 (TRAP-1). Although Hsp90α and Hsp90β are differentially expressed, with Hsp90α being inducible and Hsp90β being constitutively expressed, they are both cytosolic; meanwhile, Grp94 and TRAP-1 are localized to the endoplasmic reticulum and mitochondria, respectively11.
Hsp90 is a homodimer, and each monomer consists of four components; an N-terminal domain (NTD), a highly-charged linker region, a middle domain (MD), and a C-terminal domain (CTD)10. The N-terminal domain exhibits ATPase activity and is responsible for the generation of energy required for the proper folding of client protein substrates12. Unlike other ATP-dependent proteins like kinases, Hsp90 is a member of the gyrase, Hsp90, histidine kinase, MutL (GHKL) superfamily whose members contain an unusual Bergerat fold, a structural feature that forces ATP to bind in a “C-shaped” or bent conformation13. The middle domain contributes to ATPase activity by binding the γ-phosphate of ATP bound to the NTD14. In addition, the NTD facilitates the recognition and binding of client proteins and co-chaperones during the Hsp90-mediated protein folding cycle14. The CTD is responsible for homodimerization to yield the active form of Hsp9015. While the CTD also contains a nucleotide binding site, it does not hydrolyze ATP, but instead allosterically regulates the release of ADP from the NTD16. Thirdly, this domain contains a terminal MEEVD sequence that enables the docking of co-chaperones that contain a TPR (tetratricopeptide-containing repeats) domain17.
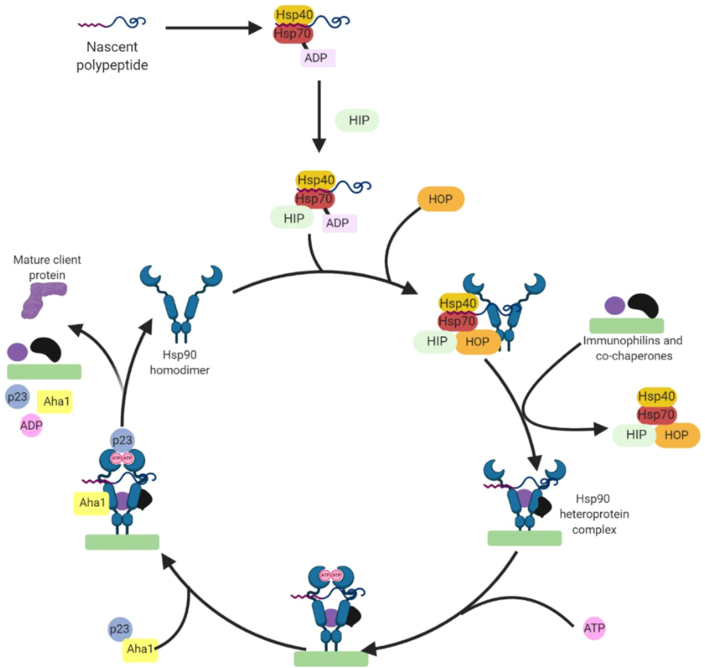
Although the Hsp90 folding cycle (Fig. 1) has been extensively studied, it is not yet completely understood. However, it requires the assembly of a heteroprotein complex that utilizes both co-chaperones and ancillary proteins to promote the protein folding process. New polypeptides that are produced by the ribosome can form a complex with Hsp40 and Hsp70 to prevent aggregation, as well as the Hsp70-interacting protein (HIP)18. The Hsp70‒Hsp90 organizing protein (HOP) associates with the complex19 to aid the transfer of client proteins from Hsp70 to Hsp9020. Hsp40, Hsp70, HIP and HOP dissociate under some conditions and are replaced by various immunophilins, co-chaperones, and other partner proteins to form the Hsp90 heteroprotein complex21. When ATP binds to the NTD binding site, Hsp90 shifts to a closed conformation22 and recruits p23 and the activator of Hsp90 ATPase homologue 1 (Aha1). Aha1 promotes the hydrolysis of ATP to provide the requisite energy necessary for the folding of the client protein, followed by a return to Hsp90's open conformation and regeneration of the homodimer23.
Figure 1.
The Hsp90 protein folding cycle.
3. Hsp90 as a target to treat cancer
In recent decades, Hsp90 gained widespread attention due to its potential as a target for the treatment of cancer. In 2000, Hanahan and Weinberg24 proposed a list of six characteristics exhibited by all cancers that include their ability to 1) produce their own growth signals, 2) exhibit insensitivity to anti-growth signals, 3) evade apoptosis, 4) increase angiogenesis, 5) induce telomerase levels, and 6) increase tissue invasion/metastasis. In 2011, they extended this list to include 7) the dysregulation of cellular energetics, 8) evasion of destruction by the immune system, 9) genome instability and mutations, and 10) tumor-promoting inflammation25. Since Hsp90 is responsible for the conformational maturation of ∼400 client protein substrates26, many of these are transcription factors, receptors, kinases, or oncoproteins that are overexpressed and/or mutated in cancer, which creates an oncogenic addiction to Hsp90 (Table 1)27, 28, 29. Consequently, Hsp90 inhibition represents an attractive strategy to treat cancer as multiple oncoproteins and pathways are simultaneously impacted.
Table 1.
A listing of Hsp90 clients implicated in Hanahan and Weinberg's hallmarks of cancer.
| Hallmarks of cancer | Implicated Hsp90 client proteins | Ref. |
|---|---|---|
| Self-production of growth signals | Raf-1, Akt, Her-2, Mek, Bcr-Abl, Xpo1 | 27,28 |
| Insensitivity to anti-growth signals | Plk, Wee 1, Myt1, Cdk4, Cdk6 | 27 |
| Evasion of apoptosis | Akt, p53, c-Met, Apaf-1, survivin, WT1 | 27, 28, 29 |
| Angiogenesis | Fak, Akt, HIF-1α, VEGFR, Flt-3, Tp73, Tbk1 | 27, 28, 29 |
| Replicative senescence | Telomerase, FoxM1, Ntrk1, Ntrk2, Ntrk3 | 27, 28, 29 |
| Tissue invasion/metastasis | c-Met, HIF-1α, Prmt5, Ikbka, Nuak2, MMP2 | 27, 28, 29 |
| Dysregulation of cellular energetics | Arnt, Arrb1, Arrb2, Hmga1 | 27,28 |
| Evasion of the immune response | Irak3 | 27,28 |
| Genome instability and mutations | Mafg, Nek8, Nek9, Nek11 | 27,28 |
| Tumor-promoting inflammation | IkbkA, IkbkB, IkbkG, IL-6, IL-8 | 27,28 |
One of the challenges associated with the creation of an effective chemotherapy is the ability to selectively kill cancer cells while minimizing harm to normal tissue. Most cancer drugs achieve some selectivity by exploiting the high rate of malignant cell growth as compared to normal cells. Unfortunately, this also means that fast-replicating healthy cells are often affected, which can lead to undesired side effects. The high abundance of Hsp90 in all cell types raises an additional similar concern with selectivity; however, experimental evidence has demonstrated that Hsp90 inhibitors accumulate more abundantly in cancerous cells as compared to healthy tissue30. An explanation for this observation was reported by Kamal and colleagues31, in which they suggest the Hsp90 heteroprotein complex to be exclusively found in tumor cells, while existing as an unassembled homodimer in normal tissue. Furthermore, the heteroprotein complex that exists in cancer cells is more active and exhibits a higher level of ATPase activity, which ultimately leads to a ∼200-fold higher affinity for inhibitors and/or ATP. Together, these cancer-derived data provide a foundation to develop Hsp90 inhibitors that can selectively target the molecular chaperone within this large therapeutic window.
One of the first strategies to target Hsp90 was the construction of molecules that compete with ATP for binding to the NTD. The first two Hsp90 inhibitors discovered were the natural products, geldanamycin and radicicol, which served as the basis for a number of compounds that underwent clinical evaluation. Unfortunately, none have been FDA-approved and most have failed32,33. The most likely explanation is that these compounds inhibit all four Hsp90 isoforms similarly, which is likely to manifest negative side effects that include cardiac, gastrointestinal, and ocular toxicities34. Specifically, formation of a functional hERG channel is heavily dependent on Hsp90α35, which highlights why compounds that target Hsp90α may cause cardiotoxicity. Furthermore, inhibition of Hsp90α may also induce ocular toxicity as it was recently reported that Hsp90α-deficient mice experienced retinal degradation, which led to blindness36. All four isoforms share at least 85% sequence identity in their NTD ATP-binding sites, with Hsp90α and Hsp90β exhibiting ∼95% sequence identity and differing by only two amino acids within the nucleotide-binding site37. However, Khandelwal and coworkers37 proved that even a difference of only two amino acids can be exploited to develop isoform-selective inhibitors.
A major disadvantage associated with the use of N-terminal inhibitors is induction of the pro-survival heat shock response29. When an inhibitor binds the NTD binding site, heat shock transcription factor 1 dissociates from Hsp90, trimerizes, undergoes phosphorylation, and enters the nucleus to promote expression of the heat shock proteins to facilitate cell survival38. Although complications related to dosing and toxicity have hindered the advancement of Hsp90 N-terminal inhibitors in the clinic, the same heat shock response that is detrimental to cancer, may prove advantageous for the treatment of neurodegenerative diseases such as Alzheimer's, Parkinson's and multiple sclerosis39. Neckers and colleagues40 discovered an alternative to N-terminal inhibition in 2000 via novobiocin, a coumarin antibiotic that induced the degradation of the oncogenic client proteins v-Src, Raf-1, and Erb 2 via inhibition of the CTD binding site. Because such binding does not induce the heat shock response41, the development of C-terminal inhibitors has become an alternative strategy for Hsp90 inhibition. Unfortunately, their Hsp90 C-terminal binding site remains poorly characterized and unconfirmed42.
4. Disruption of Hsp90 protein–protein interactions (PPIs)
While isoform-selective and C-terminal inhibition are promising strategies to target Hsp90, a third option is to prevent assembly of the functional heteroprotein complex. The Hsp90 protein folding cycle involves numerous proteins that associate with Hsp90 at different stages and appear to be dependent upon the substrate. In addition, Hsp90 is subject to post-translational modifications like phosphorylation, sumoylation and S-nitrosylation, which further attenuate its activity or affinity for clients/partner proteins43. Therefore, the design of inhibitors that disrupt Hsp90 PPIs represents a novel approach to treat cancer by “fine-tuning” Hsp90 inhibition instead of eliminating it. The following section highlights PPIs of interest, the molecules that have been discovered or developed to interfere with those interactions, and the results from such studies.
4.1. Hsp90 and activator of Hsp90 ATPase homologue 1 (Aha1)
Aha1 (Fig. 2) is a major co-chaperone whose interactions with Hsp90 are important for client protein maturation, as it enhances Hsp90's inherently low ATPase activity. Although the precise mechanism through which this stimulation occurs remains unknown, Oroz and colleagues44 used NMR studies to demonstrate that the increased activity results from the binding of Aha1's N-terminus with Hsp90's MD, while Hsp90's N-terminal domains remain flexible to allow their dimerization and the binding of ATP. More recently, cryo-electron microscopy studies performed by Liu and colleagues45 yielded multiple Hsp90‒Aha1 complexes and provided mechanistic insights into Hsp90's stimulation by Aha1. From these models, they proposed that the Aha1 NTD is recruited to the Hsp90 MD to induce a semi-closed state of the molecular chaperone. Steric clashes with the Aha1 CTD causes the Hsp90 NTD to undock from the MD, which leads to ATP binding, as well as rotation and dimerization of the two NTDs. Aha1 then rearranges to promote the asymmetric hydrolysis of both ATP molecules45.
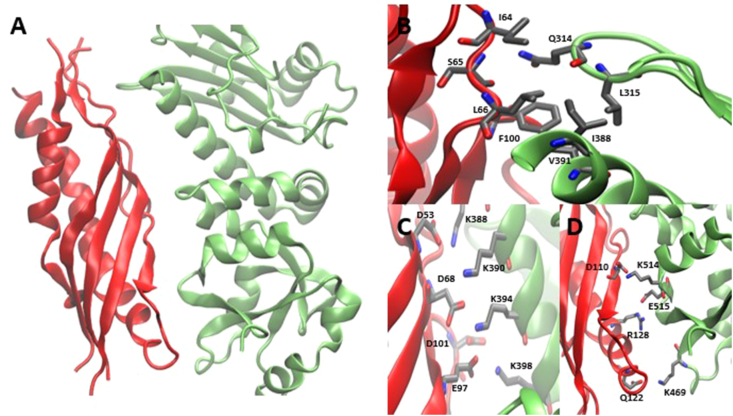
Figure 2.
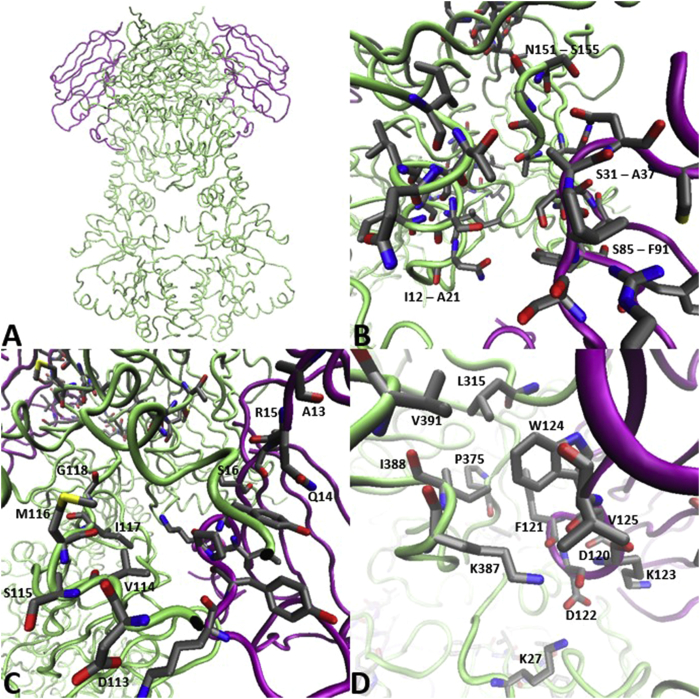
PPIs between Hsp90 middle domain and Aha1 (A) Co-crystal structure of Hsp90 (green) and Aha1 (red). (B) Hydrophobic interface between Hsp90 and Aha1, mediated by hydrogen bonding between Q314 of Hsp90 and the backbone of I64 and S65 on Aha1. (C) Hydrogen bonding and ionic interactions between Hsp90 lysines and Aha1 aspartic/glutamic acids. (D) Ion-pair interactions between K469, K514, and E515 on Hsp90 and D110, Q122, and R128 on Aha1 (PDB: 1USU46).
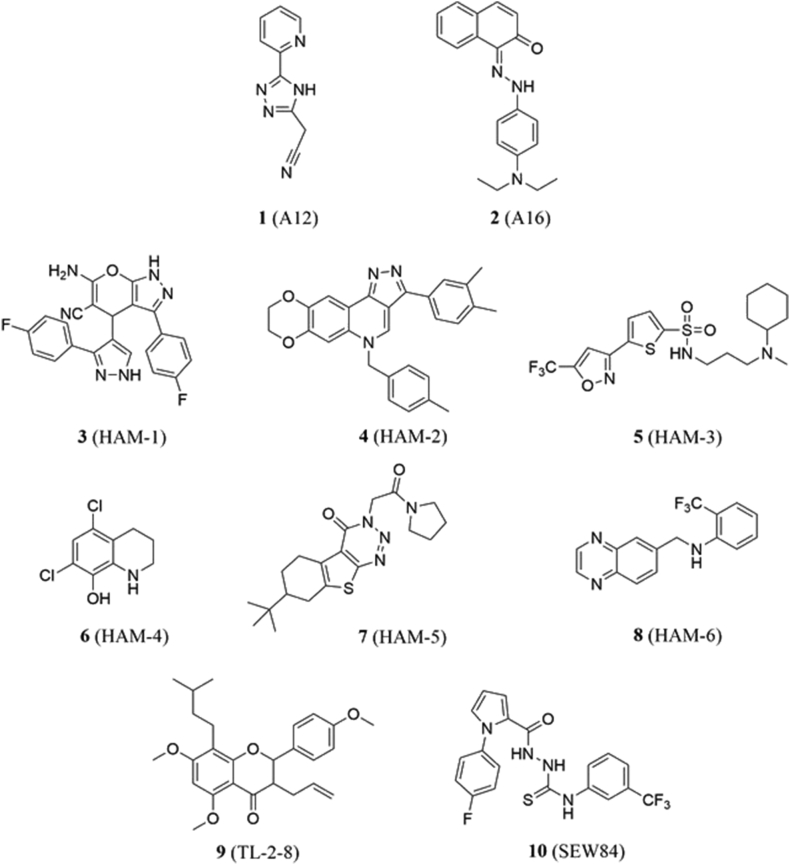
4.1.1. A12 and A16
In 2017, Ihrig and Obermann48 reported the disclosure of small molecules that disrupt interactions between Aha1 and Hsp90. Using an Alpha screening assay, they identified A12 and A16 (1 and 2, Fig. 3) from an initial pool of 16 compounds. As demonstrated by an iodide efflux assay, both compounds manifested IC50 values of 0.3 μmol/L at stabilizing CFTRΔF508, a mutated chloride channel whose degradation is implicated in cystic fibrosis. When used in combination with VX-809, a drug that promotes CFTRΔF508 trafficking to the cell surface47, A12 and A16 exhibited synergy, suggesting that disruption of the Aha1‒Hsp90 interaction could represent a viable treatment for cystic fibrosis48.
Figure 3.
Disruptors of Hsp90‒Aha1 PPIs.
4.1.2. Hsp90‒Aha1 modulators
During the same year, Stiegler and colleagues49 used a FRET-based assay to identify molecules that modulate Hsp90‒Aha1 interactions. Their scientific investigation led to the identification of 6 Hsp90‒Aha1 modulators (HAMs, 3–8, Fig. 3), three of which inhibited Aha1's stimulation of Hsp90 ATPase activity while the others enhanced it. The best inhibitor, HAM-1, resulted in 93 ± 1% inhibition at saturating concentrations with an apparent KD of 24 μmol/L, while NMR studies demonstrated it to bind the Hsp90 NTD and impair interactions with Aha1's C-terminus49.
4.1.3. TL-2-8
Another molecule that disrupts Hsp90‒Aha1 interactions was discovered by Liu and coworkers50, who demonstrated that TL-2-8 (9, Fig. 3), a derivative of quercetin, reduced the expression of PLK1, HSF1, Cdk 1, and cyclin D1 in MDA-MB-231 and MDA-MB-468 cancer cells in a concentration-dependent manner. The overexpression of Aha1 was found to rescue all four client proteins, which verifies the role played by Aha1 to accelerate protein folding.
4.1.4. SEW84
SEW84 (10, Fig. 3) was recently identified by Singh and colleagues51 as an inhibitor of Aha1-stimulated Hsp90 ATPase activity during a quinaldine red ATPase assay (IC50 = 0.3 μmol/L). 1H–15N-TROSY-HSQC spectroscopy revealed that SEW84 binds the Aha1 CTD with a KD of 1.74 μmol/L, thereby disrupting PPIs with Hsp90. SEW84's reported in vitro activities include inhibition of both wild-type and mutated variants of the androgen receptor, which is implicated in prostate cancer, as well as the clearance of phosphorylated-tau in HEK293 cells. The latter was also observed in rat cortical neurons and a transgenic mouse model, wherein similar results prevailed. Structure–activity relationship (SAR) analyses performed on SEW84 highlighted the importance of the trifluoromethyl and hydrazinecarbanothiamide moieties for activity, while meta- and para-substituents on the phenyl ring modulate activity51.
4.2. Hsp90 and cell division cycle 37 (Cdc37)
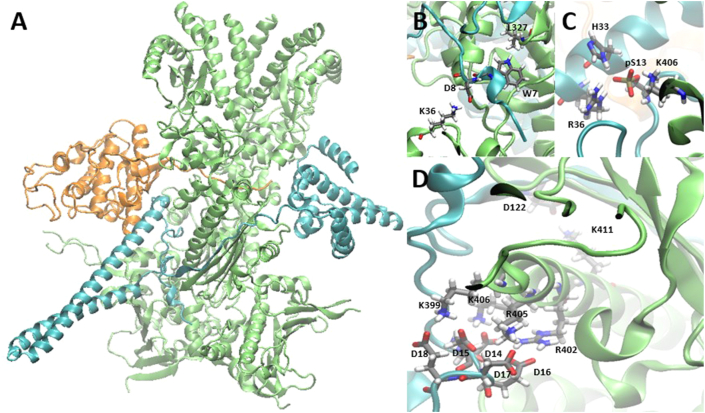
Cdc37 (Fig. 4), also known as p50, is a cell cycle protein that is also a major Hsp90 co-chaperone due to its participation in the folding of approximately 300 client proteins, many of which are kinases26. There are many structural features of Cdc37 that help to stabilize these substrates and facilitate their transfer to Hsp90, and the proposed mechanism is as follows. First, Cdc37 is phosphorylated by casein kinase 2 at Ser-13, which enables it to recognize and associate with client kinases52. The resulting complex then binds Hsp90, after which protein phosphatase 5 dephosphorylates Cdc37, which stabilizes the client protein and enables transfer to Hsp9053,54.
Figure 4.
PPIs between Hsp90 and Cdc37. (A) Co-crystal structure of Hsp90 (green), Cdc37 (cyan), and client kinase Cdk4 (gold). (B) Structural motif resembling Hsp90‒p23 PPIs. (C) The pS13 of Cdc37 facilitates both the protein's own stability and an interaction with K406 of Hsp90. (D) Numerous ionic interactions between Cdc37 aspartates and Hsp90 lysines/arginines (PDB ID: 5FWP55).
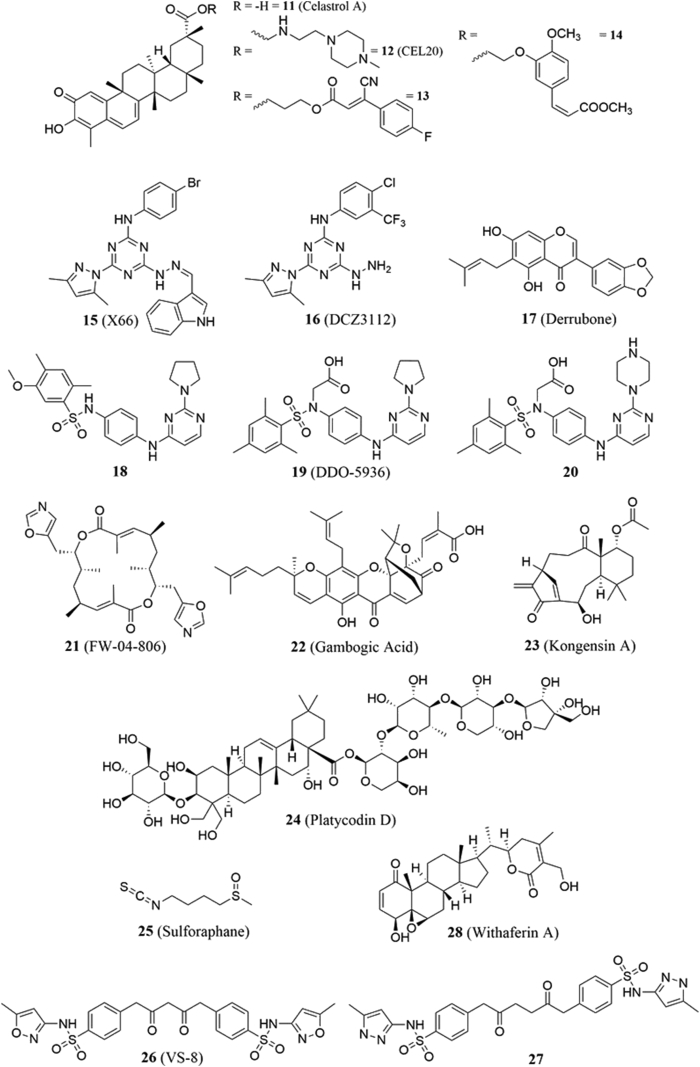
4.2.1. Celastrol A
Celastrol A (11, Fig. 5) is a pentacyclic quinone methide triterpene natural product isolated from Tripterygium wilfordii Hook F. It has been used in traditional Eastern medicine for inflammation and autoimmune disorders and has been investigated as a potential treatment for various inflammatory diseases and cancers. In 2008, Zhang and coworkers56 performed in silico molecular dynamics simulations and discovered a potential binding site on Hsp90 that blocked several key interactions with Cdc37. In vitro and in vivo studies demonstrated that the administration of celastrol to cells decreased levels of Akt and Cdk4 by 80% and 70%, respectively. In addition, celastrol exhibited antiproliferative activity (IC50 = 3 μmol/L) and induced apoptosis. It also displayed in vivo efficacy in a transgenic mice model of pancreatic cancer.
Figure 5.
Disruptors of Hsp90‒Cdc37 interactions.
In a follow-up study, the same group investigated the mechanism by which celastrol disrupts Hsp90‒Cdc37 interactions, and because it was found to protect Hsp90 from trypsin degradation, they determined that the molecule binds to Hsp90's CTD57. However, HSQC NMR studies performed by Sreeramulu and coworkers58 suggested the quinone portion of celastrol to act as a Michael acceptor for cysteine residues present in Cdc37, while its three saturated rings participate in hydrophobic interactions. Jiang and colleagues59 synthesized 23 ester and amide derivatives, and the most active derivative, CEL20 (12, Fig. 5) manifested an IC50 value of 4.71 ± 0.14 μmol/L against Panc-1 cells. Additional studies on this natural product involved the preparation of chimeras between celastrol and cinnamic acid60/ferulic acid61. The most potent derivatives obtained from these studies were 13 and 14 (Fig. 5), which were found to disrupt Hsp90‒Cdc37 PPIs more effectively than their parent compound. Hsp90 client substrates Akt and Cdk4 were degraded and a G0/G1 cell cycle arrest was observed along with the induction of apoptosis in A549 cancer cells. Celastrol's precise mechanism of action remains unclear, but such work demonstrates that the natural product and its derivatives exhibit utility as both a starting point for drug discovery and as a biological probe to further interrogate the nature of Hsp90‒Cdc37 PPIs.
4.2.2. DCZ3112
In 2016, Zhao and colleagues62 synthesized a triazine derivative known as X66 (15, Fig. 5) and reported its antitumor activity and mechanism of action via binding to the Hsp90 NTD (IC50 values against SkBr3, BT-474, A549, K562, and HCT-116 cell lines were 8.9, 7.1, 7.5, 8.6 and 6.7 μmol/L, respectively). In a subsequent study, the same group found the structurally related analog, DCZ3112 (16, Fig. 5), disrupts Hsp90‒Cdc37 interactions. DCZ3112 also induced cell cycle arrest and apoptosis in Her-2 positive breast cancer cells, which was observed when DCZ3112 was used individually or in combination with the anti-Her-2 antibodies, trastuzumab or pertuzumab. Furthermore, DCZ3112 was shown to overcome resistance to either antibody. Molecular docking studies suggest the compound operates via competitive binding to the Hsp90 NTD to displace Cdc3763.
4.2.3. DDO-5936
In 2019, Wang and colleagues64 used molecular dynamics simulations and mutagenesis studies to discover a novel binding interaction between Glu-47 and Gln-133 on Hsp90 and Arg-167 on Cdc37. Based on computational and biophysical assays, 18 (Fig. 5) was identified as a disruptor of Hsp90‒Cdc37 PPIs via binding to Hsp90 (KD = 21.1 μmol/L). Chemical optimization of this molecule led to DDO-5936 (19, Fig. 5), which exhibited improved solubility and binding (KD = 7.41 μmol/L). DDO-5936 was found to disrupt Hsp90‒Cdc37 PPIs against numerous cancer cell lines, and led to antiproliferative activity, G0/G1 cell cycle arrest, and the degradation of Hsp90 kinase clients in HCT116 cells without induction of the heat shock response. This activity was translated in vivo, as the administration of DDO-5936 in a HCT116 tumor xenograft mouse model led to reductions in the volume and growth of tumors, while manifesting little toxicity to normal tissues64. Although identification of a binding site enables DDO-5936 to stand out among the Hsp90‒Cdc37 PPI disruptors, its efficacy and drug-like properties are less than ideal. Replacement of the pyrrolidine with a piperazine yielded derivative 20 (Fig. 5) and resulted in improved binding affinity (KD = 0.50 μmol/L), inhibitory activity, stability in plasma and liver microsomes, and ultimately, oral activity65.
4.2.4. Derrubone
Derrubone (17, Fig. 5) is an isoflavenoid natural product that is isolated from the Indian tree, Derris robusta66, though its biological properties weren't fully revealed until 2007 when a luciferase refolding assay indicated that it was a potent inhibitor of Hsp90, resulting in an IC50 value of 0.23 ± 0.04 μmol/L. That same study found that derrubone induces the degradation of Her-2 in SkBr3 cells as well as the degradation of Raf-1, Akt, and ERα in MCF-7 cells in a dose-dependent manner. It also prevented geldanamycin from disrupting the Hsp90‒Cdc37‒HRI client protein complex, suggesting that derrubone stabilizes the heteroprotein complex and hinders progression through the protein folding cycle67. In a subsequent study, the same group synthesized derrubone analogues, which revealed a requirement for the prenyl side chain and substitution at the 3′-aryl position for activity68. In 2010, Mays and colleagues69 synthesized novobiocin‒derrubone chimeras that suggested the parent molecules exhibit different modes of binding. In 2014, Khalid and Paul70 performed docking studies and identified residues that form the Hsp90 C-terminal binding site, which led to the identification of leucine residues. At present, little work has continued toward elucidation of derrubone's precise mechanism of action.
4.2.5. FW-04-806
FW-04-806 (also known as conglobatin, 21, Fig. 5) is a bis-oxazolyl natural product isolated from Streptomyces FIM-04-806. Preliminary studies showed it to inhibit the growth of chronic myelocytic leukemia with an IC50 of 6.66 μg/mL71. An affinity-based screen found FW-04-806 to bind the Hsp90 NTD, but not alter ATP binding or Hsp90's ATPase activity. Pull-down experiments confirmed FW-04-806 disrupts Hsp90's interactions with Cdc37 and client proteins. In vitro studies revealed FW-04-806 could induce the degradation of Her-2, p-Her-2, Raf-1, Akt, and p-Akt levels in both SkBr3 and MCF-7 cells. In vivo studies revealed FW-04-806 exhibited efficacy in tumor xenograft models72. Further research showed that FW-04-806, either alone or in combination with the EGFR/Her-2 tyrosine kinase inhibitor, lapatinib, is effective against Her-2 positive breast cancer cells73.
4.2.6. Gambogic acid
(−)-Gambogic acid (22, Fig. 5) is a natural product found in Garcinia hanburyi (Hook F), a plant that has been commonly used in Southeast Asia for its medicinal properties. Like celastrol A, gambogic acid was among the natural products identified as an Hsp90 inhibitor based on the results from a luciferase refolding assay. It was found to disrupt Hsp90‒Cdc37 interactions as supported by the depletion of Cdc37-dependent client proteins. Surface plasmon resonance and molecular docking studies suggested it to bind the Hsp90 NTD without affecting ATP binding74. Zhang and colleagues75 also reported that gambogic acid could downregulate TNF-α/NF-κB signaling pathway in HeLa cells, which leads to apoptosis.
4.2.7. Kongensin A
Kongensin A (23, Fig. 5) is a diterpenoid natural product that was first isolated from the plant, Croton kongensis. Kongensin A first gained attention as an Hsp90 inhibitor during a high throughput screen that sought to identify compounds that exhibit anti-necroptotic activity. Li and coworkers76 elucidated the mechanism of action in which it forms a covalent linkage with Cys-420 in the Hsp90 MD, which disrupts interactions with Cdc37. Ultimately, this mechanism blocks RIP3-dependent necroptosis and induces apoptosis. While their work was performed in cancer cells, the authors propose that kongensin A could be used to treat inflammation, atherosclerosis, and/or ischemia‒reperfusion injury76.
4.2.8. Pep-1
In addition to small molecules, peptidomimetics that imitate residues on the partner protein represents another strategy to develop PPI disruptors that take advantage of the large, shallow surface areas that are common with PPIs. Using a combination of molecular dynamics simulations and MM-PBSA analyses, Wang and coworkers77 rationally designed a series of oligopeptides based on the Hsp90‒Cdc37 interface. Their best molecule, Pep-1 (Ac-KHFGMLRRWDD-NH2), was found to block Hsp90‒Cdc37 association by binding to the Hsp90 NTD with a calculated KD of 6.90 ± 0.9 μmol/L. It also inhibited Hsp90's ATPase activity and exhibited an IC50 of 3.0 ± 0.7 μmol/L. Two years later, the same group optimized Pep-1, which led to the truncated derivative Pep-5 (Ac-HFGMLRR-NH2). Using a pull-down assay, Pep-5 was shown to disrupt the Hsp90‒Cdc37 PPI, and isothermal titration calorimetry measured a slightly lower KD of 5.99 ± 0.8 μmol/L78. This was the first report of a synthetic polypeptide that was capable of disrupting Hsp90‒Cdc37 interactions, and thus providing another opportunity to target the molecular chaperone.
4.2.9. Platycodin D
Platycodin D (24, Fig. 5) is a saponin isolated from the Chinese herb Platycodonis Radix, and has exhibited immunoregulatory, anti-atherogenic, and anticancer activities79,80. It has also been shown to prevent cell adhesion, migration, invasion, and proliferation against numerous cancers81. In 2016, Li and colleagues82 proposed that Hsp90 was a potential target for the natural product, as decreased levels of the Hsp90-dependent clients, EGFR and Her-2, were observed upon the administration of platycodin D. In silico modeling studies suggest the natural product to hydrogen bond with residues on both proteins (Arg-32 and Phe-200 on Hsp90 and Asp-169 and Asp-170 on Cdc37), which was supported via immunoprecipitation assays. Using platycodin D in combination with the mTOR inhibitor, everolimus, the authors found the molecule to sensitize non-small cell lung cancer (NSCLC) cells to everolimus. Based on these data, the mechanism of action involves the modulation of Hsp90, which results in activation of the EGFR/IGF1R/Akt signaling pathway that normally renders mTOR inhibitors ineffective83. Additional studies to further deconvolute the mechanism of action for platycodin D are currently underway.
4.2.10. Sulforaphane
Sulforaphane (25, Fig. 5) is an antioxidant isothiocyanate present in broccoli and other cruciferous vegetables that has been studied as a potential treatment for pancreatic cancer84 and NSCLC85. The first report of Hsp90 as a potential target of sulforaphane occurred in 2009 when Gibbs and coworkers86 reported that inhibition of histone deacetylase 6 led to Hsp90 hyperacetylation, which in turn, destabilized the androgen receptor. A more direct effect on Hsp90 was reported by Li and colleagues87 when they used sulforaphane in combination with the geldanamycin derivative, 17-allylamino-17-demethoxygeldanamycin (17-AAG). Their data revealed that sulforaphane was able to sensitize Mia Paca-1 and Panc-1 pancreatic cells to treatment with 17-AAG, which led to enhanced degradation of Hsp90 client proteins in vitro and increased efficacy in pancreatic cancer xenograft mouse models. Immunoprecipitation assays were also used to confirm that sulforaphane disrupted Hsp90‒Cdc37 complex formation. The same researchers investigated the nature of this interaction via NMR studies and noted sulforaphane caused significant chemical shifts to several isoleucines on Hsp90, including Ile-74, Ile-75, Ile-43, and Ile-125. Interactions with the former two were further confirmed via an LC‒MS analysis which identified the Hsp90 NTD residues Ile-72 to Arg-81 (IDIIPNPQER) to be covalently labeled by sulforaphane. Meanwhile, shifts in the latter two were attributed to allosteric effects, which is significant since Ile-125 resides within the interface between the two proteins88.
4.2.11. VS-8
In 2017, Wang and colleagues89 sought to discover disruptors of Hsp90‒Cdc37 PPIs, which led to the development of a pharmacophore model, analog synthesis, and in vitro evaluation. Based on these efforts, VS-8 (26, Fig. 5) was identified by its ability to bind Hsp90 (KD = 80 μmol/L) and inhibit PPIs (IC50 = 77 μmol/L). Truncation of the central linker and replacement of the isoxazoles with N-methylpyrazoles produced 27 (Fig. 5). Not only did 27 exhibit better activity than VS-8 (KD = 40 μmol/L, IC50 = 27 μmol/L), but it also exhibited antiproliferative activity against MCF-7, SkBR3, and A549 cancer cells (IC50 = 26, 15, and 38 μmol/L, respectively) and induced the degradation of Hsp90 clients Akt and Cdk4 in SkBR3 cells. Although celastrol remained superior in these evaluations, VS-8 and 27 represent the first synthetic disruptors of this PPI.
4.2.12. Withaferin A
Withaferin A (28, Fig. 5) is a steroidal lactone found in Withania somnifera and exhibits both antitumor and antiangiogenic activities90,91. Withaferin A has been shown to inhibit NF-κB, resulting in the induction of apoptosis90,92. In 2010, Yu and colleagues93 found that the administration of withaferin A to pancreatic cells resulted in decreased proliferation and increased apoptosis via Hsp90 client protein degradation. A pull-down assay demonstrated that withaferin A binds the Hsp90 CTD via interactions with cysteine residues. Coimmunoprecipitation studies revealed that withaferin A disrupted the Hsp90‒Cdc37 complex in a dose-dependent manner. Molecular docking and molecular dynamics simulations performed by Grover and coworkers94 suggested that withaferin's disruption of the complex is thermodynamically favored. Initial SAR studies on withaferin A performed by Yokota and coworkers95 highlighted the importance of the unsaturated lactone and the 4-hydroxy-5,6-epoxy-2-en-1-one moiety for activity. Further SAR studies performed by Gu and colleagues96 confirmed the importance of the epoxide ring for its reactivity towards cysteine residues.
4.3. Hsp90 and F1F0-ATP synthase
F1F0-ATP synthase is a protein that is embedded in the eukaryotic inner mitochondrial membrane and generates ATP via the electrochemical proton gradient produced from oxidative phosphorylation. Previously, it had been shown to complex with Hsp60, but co-immunoprecipitations by Papathanassiu and coworkers97 discovered that the synthase also associates with Hsp90.
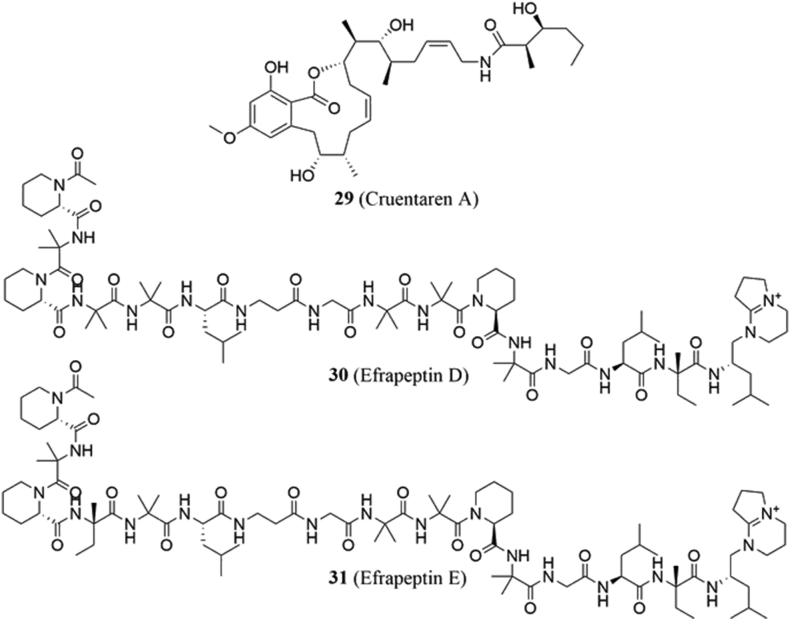
4.3.1. Cruentaren A
Cruentaren A (29, Fig. 6) is a benzolactone macrolide that can be isolated from the myxobacterium, Byssovorax cruenta98, and exhibits both antifungal99 and anticancer activities. The latter was reported by Kunze and coworkers99, after demonstrating that cruentaren A inhibited the growth of various cancers with IC50 values ranging from 0.1 to 1.0 ng/mL. Further studies by the same researchers determined that cruentaren A's mechanism of action involves selective inhibition of the catalytic F1 subunit100. In 2014, Hall and coworkers101 discovered that cruentaren A also modulated the Hsp90 protein folding machinery. Incubation of the natural product with MCF-7 cancer cells induced a dose-dependent decrease in the Hsp90 client proteins pAkt, Her-2 and Raf, as well as a disruption of F1F0–ATP synthase‒Hsp90α interactions that led to an increased nuclear localization of Hsp90α101. These data offer another approach to inhibit the Hsp90 heteroprotein complex without inducing the pro-survival heat shock response.
Figure 6.
Disruptors of Hsp90‒F1F0-ATP synthase PPIs.
4.3.2. Efrapeptins
Efrapeptins are a family of naturally occurring peptides that are known to inhibit the synthesis of ATP102. As part of the work demonstrating that F1F0-ATP synthase associates with Hsp90, Papathanassiu and coworkers97 utilized efrapeptins D and E as probes (30 and 31, Fig. 6) to assess this interaction. Not only did the peptides induce degradation of the complex, but they also induced the degradation of the Hsp90-dependent client proteins caspase-3, and p5397. In vitro, the peptides exhibited antiproliferative activities, and produced IC50 values ranging from 6 nmol/L–3.4 μmol/L. Synergy was also observed when these efrapeptins were used in combination with 2-deoxyglucose as a complementary method to inhibit glycolysis. However, in vivo studies using MCF-7 and MDA-MB-231 xenograft models found that the efrapeptins inhibited tumor growth on their own, while an antagonistic effect was observed in the combination studies. Suppression of Hsp90 chaperone activity is believed to be responsible for the paradoxical results; however, it is not uncommon for in vivo results to differ from in vitro studies103.
4.4. Hsp90 and human epidermal growth factor receptor-2 (Her-2)
Her-2 is a receptor-like glycoprotein and member of the ErbB family of receptor tyrosine kinases whose overexpression is commonly observed in highly advanced and metastatic cancers104. Consequently, it has become a promising target for the treatment of breast and ovarian cancers, which has resulted in currently available therapeutics such as lapatinib and herceptin. Several studies have revealed the role played by Hsp90 to stabilize Her-2105,106 via interactions between Hsp90 and Her-2's kinase domain107. Furthermore, Sidera and coworkers108 discovered a novel interaction between cell-surface Hsp90 and Her-2's extracellular domain that creates an additional opportunity to develop therapeutic that target this interaction.
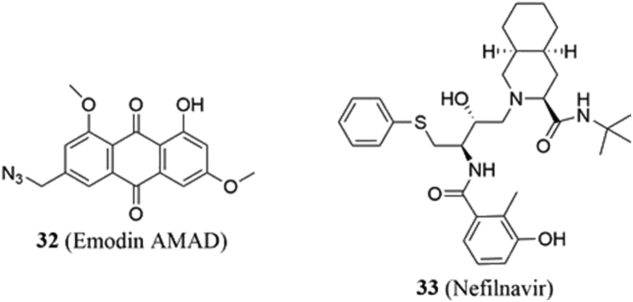
4.4.1. Emodin azide methyl anthraquinone derivative (AMAD)
In 2008, Yan and colleagues109 extracted an emodin AMAD (32, Fig. 7) from the giant knotweed rhizome and demonstrated that it induced apoptosis in MDA-MB-453 and Calu-3 cells. Three years later, they determined that emodin AMAD disrupts Hsp90‒Her-2 PPIs via hydrophobic, electrostatic, and hydrogen bonding interactions with each protein's nucleotide binding site, ultimately leading to Her-2 proteasomal degradation110. In addition, the administration of AMAD to MDA-MB-453 breast cancer cells induced G0/G1 cell cycle arrest as evidenced by reduced expression of the cell cycle proteins c-Myc, cyclin D1, Cdk4, and pRb111.
Figure 7.
Disruptors of Hsp90‒Her-2 PPIs.
4.4.2. Nefilnavir
Nefilnavir (33, Fig. 7) is an FDA-approved protease inhibitor that is used to treat HIV112. In addition to its antiviral activity, nefilnavir has been found to disrupt the PI3K and Akt signaling pathways, prompting investigation into its potential application as a chemotherapeutic113,114. In 2012, the molecule emerged as a hit from a screen of the Johns Hopkins Drug Library to identify genotype-selective anti-breast cancer drugs. From this screen, it was demonstrated that nefilnavir could selectively inhibit the growth of Her-2 positive breast cancers in vivo and cells resistant to trastuzumab and/or lapatinib in vitro115. However, the work of Soprano and coworkers116 suggested that the molecule may exhibit its anticancer activity via the production of reactive oxygen species.
Subsequent studies by Shim and colleagues115 indicated that nelfinavir binds the Hsp90 CTD, but in a manner different than novobiocin. This was further supported via molecular docking studies performed by Arodola and Soliman117 who proposed the potential repurposing of other HIV protease inhibitors as novel anticancer compounds as well.
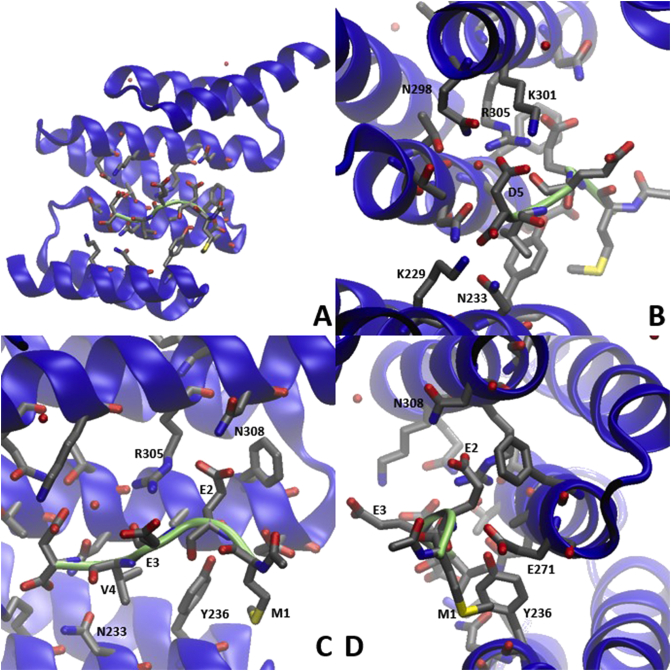
4.5. Hsp90 and hypoxia inducing factor 1α (HIF-1α)
Hypoxia inducing factor (HIF) is one of the major transcription factors deployed in response to hypoxia, wherein it promotes the transcriptional activation of genes associated with angiogenesis, oxygen consumption, increased rates of glycolysis, and metastasis118. The protein consists of a constitutively expressed β subunit and an oxygen-sensitive α subunit119, the latter of which is stabilized and regulated by Hsp90120.
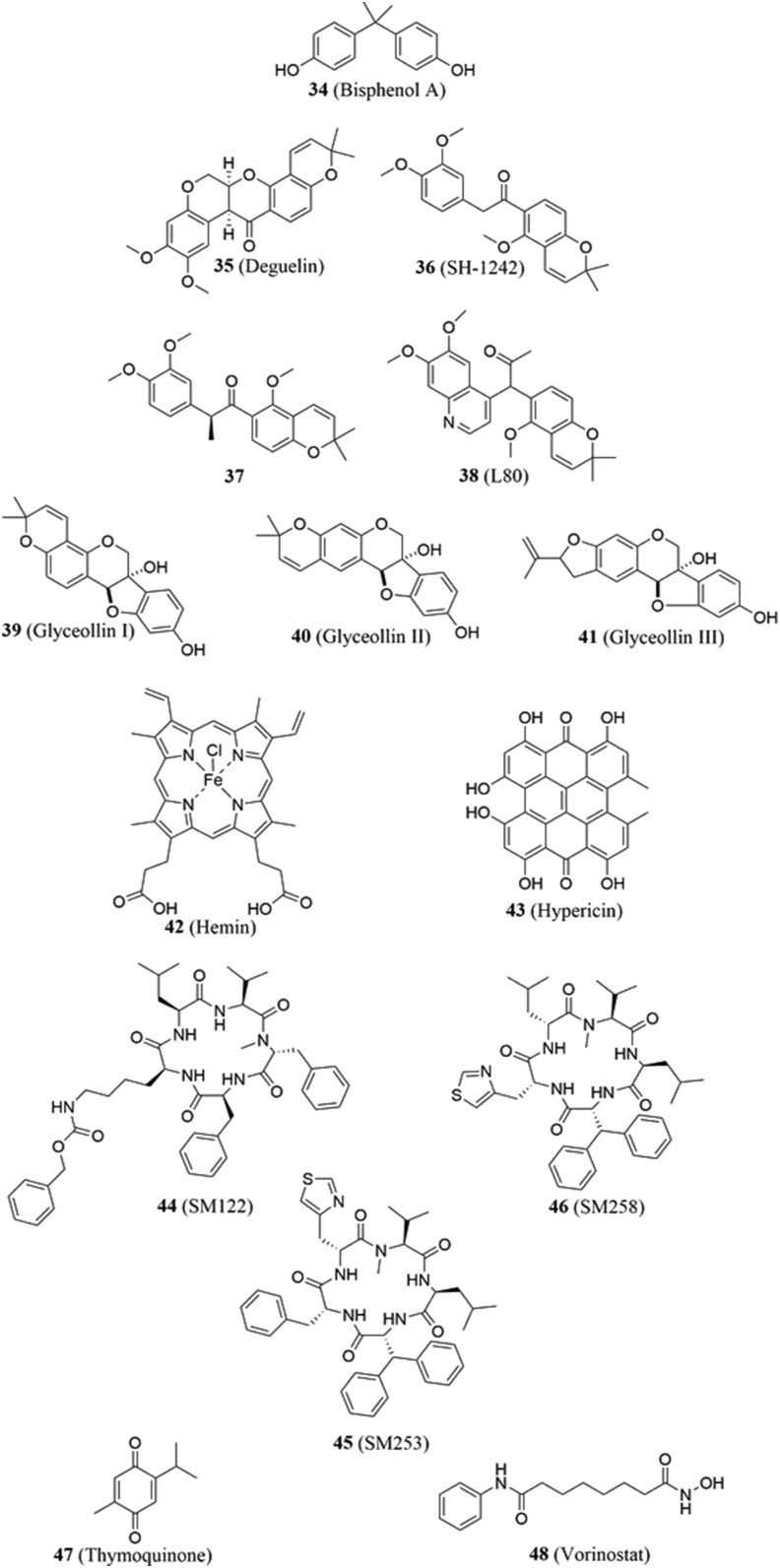
4.5.1. Bisphenol A
Bisphenol A (BPA, 34, Fig. 8) is commonly used in the manufacturing of plastics but has also gained notoriety in recent years as an endocrine disruptor121. Initial studies by Kubo and coworkers122 identified HIF-1α as a target of BPA, which results in its dissociation from Hsp90 and subsequent degradation. SAR studies found that although increased hydrophobicity on either side chain increased HIF-1α degradation, branched alkyl derivatives failed to exhibit activity123. Interestingly, BPA and its derivatives promoted HIF-1α degradation via the lysosome, rather than the ubiquitin‒proteasome pathway123.
Figure 8.
Disruptors of Hsp90‒HIF-1α PPIs.
4.5.2. Deguelin
Deguelin (35, Fig. 8) is a rotenoid that can be extracted from members of the Fabaceae (legume) family and displays chemoprotective behavior against skin and breast tumor models124. In addition, it has been shown to induce cell cycle arrest and apoptosis in malignant human bronchial epithelial cells and NSCLC125, the latter study of which involved disruption of the PI3K/Akt signaling pathway. Oh and coworkers126 reported that deguelin targets Hsp90, interacts with its N-terminal ATP binding site and induces the ubiquitin-mediated degradation of client proteins, including HIF-1α and pAkt. Chang and coworkers127 provided evidence that deguelin binds the Hsp90 CTD. Although deguelin has been reported to be well-tolerated and exhibit no adverse effects after a 4- or 19-week treatment128, Caboni and coworkers129 observed that subcutaneous administration in rats caused them to develop a Parkinson's-like syndrome.
Research has been conducted to investigate structure–activity relationships for deguelin. In 2012, Chang and colleagues127 synthesized several derivatives of deguelin and reported that both methoxy groups, the cis conformation, oxygenation at carbon-7, and the 2,2-dimethyl-2H-chromene moiety are essential for activity. Truncation of the B and/or C rings led to compounds that exhibited comparable activity to the parent compound. Two derivatives, 36 and 37 (Fig. 8), were the most efficient at suppressing both HIF-1α and angiogenesis127. Further studies on the synthesis and biological evaluation of truncated derivatives have gained additional attention in recent years130, 131, 132. Two derivatives that emerged from these studies are SH-1242 and L80 (36 and 38, Fig. 8). SH-1242 is a derivative with truncation to both the B and C rings. In 2014, Jo and coworkers133 reported that SH-1242 was able to reduce hypoxia-mediated retinal neovascularization in a diabetic mouse model via destabilization of HIF-1α. Since then, SH-1242 has undergone pre-clinical and pharmacokinetic studies134,135. L80 is a derivative that contains a C ring truncation. In 2015, Lee and coworkers136 reported the synthesis and evaluation of L80 against NSCLC. Their results showed that L80 suppressed proliferation, angiogenesis, metastasis and displayed reduced toxicity to healthy cells as compared to the natural product136. Co-precipitation and molecular docking studies support L80 to bind the Hsp90 CTD136. L80 has also been found to inhibit metastasis in triple negative breast cancer137. There is also a report of a novobiocin‒deguelin chimera that exhibits cytotoxic and antiangiogenic properties against NSCLC138, suggesting that their binding sites may be near one another or overlap.
4.5.3. Glyceollins
Glyceollins (39–41, Fig. 8) are a family phytoalexins found in soybeans that have been shown to exhibit anticancer activity139, 140, 141. In 2014, Lee and colleagues142 investigated the glyceollins’ mechanism of action and observed decreased levels of HIF-1α in MKN1, SNU668, and MDA-MB-321 cells after administration. They proposed two potential mechanisms; 1) inhibition of the PI3K/Akt/mTOR signaling pathway or 2) binding to the Hsp90 NTD binding site142. Hsp90 binding was supported by immunoprecipitation studies and molecular docking. In vivo, the natural products were shown to reduce the tumor size in a xenograft mouse model for lung cancer142.
4.5.4. Hemin
Hemin (42, Fig. 8) is an iron-containing porphyrin that has been used to treat porphyria attacks143. However, a handful of studies have suggested that these molecules exhibit protective effects against mutagenesis and carcinogenesis via the promotion of oxidative stress144,145. In 2012, Lee and coworkers146 discovered that hemin and other protoporphyrins could induce the proteasomal degradation of HIF-1α by interfering with its binding to Hsp90. This inhibitory activity resulted in a reduction of angiogenesis and the suppression of HCT116 cell proliferation and migration146. In vitro immunoprecipitation studies confirmed disruption, but determined that the addition of ATP could reverse this observation, suggesting that the porphyrins interact with Hsp90's nucleotide binding site146. While further studies are needed to determine how the porphyrin ring structure and chelated metal impact inhibitory activities, the data demonstrate protoporphyrins as novel inhibitors of Hsp90 PPIs.
4.5.5. Hypericin
Hypericin (43, Fig. 8) is a perihydroxylated perylene quinone whose anticancer properties are believed to occur via the disruption of multiple signaling pathways related to tumor proliferation147 and angiogenesis148. In 2004, Blank and coworkers149 reported that hypericin inhibited murine breast and squamous cell carcinoma tumor metastasis in vivo. In addition, they observed that hypericin promoted poly-ubiquitinylation of Hsp90149, while Barliya and coworkers150 demonstrated that poly-ubiquitinylation negatively impacted its interactions with HIF-1α, as Hsp90 was unable to transport HIF-1α to the nucleus.
4.5.6. SM compounds
The SM compounds (SM122, SM253, and SM258, 44–46, Fig. 8) are three sansalvamide A analogues developed by Kataria and colleagues152 as Hsp90 C-terminal inhibitors that disrupt Hsp90‒HIF-1α PPIs without inducing the heat shock response. The molecules had previously been shown to bind the Hsp90 CTD151 and were subsequently evaluated against hypoxic colorectal, prostate, and breast cancers. Those studies demonstrated that the compounds reduced HIF-1α mRNA levels, which resulted in decreased expression. As anticipated, the compounds also induced apoptosis and decreased angiogenesis without induction of the heat shock response152.
4.5.7. Thymoquinone
Thymoquinone (47, Fig. 8) is a natural product that is present in black cumin. While it has been reported to exhibit diverse activities such as anti-microbial and anti-diabetic activities153, it also inhibits angiogenesis, proliferation, and the metastasis of cancer cells154. Because of HIF-1α′s implication in those processes, Lee and coworkers155 hypothesized that thymoquinone's mechanism of action involved HIF-1α. Thymoquinone induced a decrease in HIF-1α levels in hypoxic renal cancer cells via a proteasome-mediated pathway155, leading researchers to suspect that thymoquinone disrupted interactions between HIF-1α and Hsp90. This was confirmed when a combination of thymoquinone and geldanamycin did not lead to a further reduction in HIF-1α levels154. Subsequent studies with Caki-1 and A498 cancer cells demonstrated thymoquinone's ability to selectively target hypoxic cancer cells over normal tissue155.
4.5.8. Vorinostat
Vorinostat (suberoylanilide hydroxamic acid (SAHA), 48, Fig. 8) is a histone deacetylase inhibitor that is approved to treat cutaneous T-cell lymphoma156. It has been shown to inhibit hypoxia signaling pathways along with a decrease in HIF-1α levels; however, its precise mechanism of action remained unclear157, 158, 159. In 2017, Zhang and colleagues160 proposed that SAHA increases the amount of acetylated Hsp90, which leads to a lower affinity for client proteins, while simultaneously inhibiting chaperone function. Co-immunoprecipitation experiments demonstrated that SAHA decreased the affinity of HIF-1α for both Hsp90 and the nuclear karyopherin importin, as well as inducing its ubiquitinylation and proteasomal degradation.
4.6. Hsp90 and the Hsp70‒Hsp90 organizing protein (HOP)
The Hsp70‒Hsp90 organizing protein (HOP) is an essential component of the Hsp90-mediated protein folding cycle as it bridges both proteins and ensures the transfer of nascent polypeptides from Hsp70 to Hsp90. The CTD of Hsp90 contains a pentapeptide MEEVD sequence that is responsible for binding proteins with tetratricopeptide repeat (TPR) domains. The interaction between Hsp90 and HOP occurs within its TPR2A domain (Fig. 9). It was determined that high affinity between these two proteins is maintained by a combination of electrostatic, hydrogen bonding, and hydrophobic interactions, which gives rise to a “carboxylic clamp”161,162. The necessity of this PPI for proper Hsp90 activity provides yet another opportunity to inhibit the molecular chaperone.
Figure 9.
PPIs between Hsp90 and HOP. (A) Co-crystal structure of HOP (blue) with the Hsp90 C-terminal MEEVD sequence (green). (B)‒(D) Depictions of the electrostatic, hydrogen bonding, and hydrophobic interactions between residues from the viewpoints of the MEEVD C-terminus, a 90° rotation, and the MEEVD N-terminus, respectively (PDB: 1ELR163).
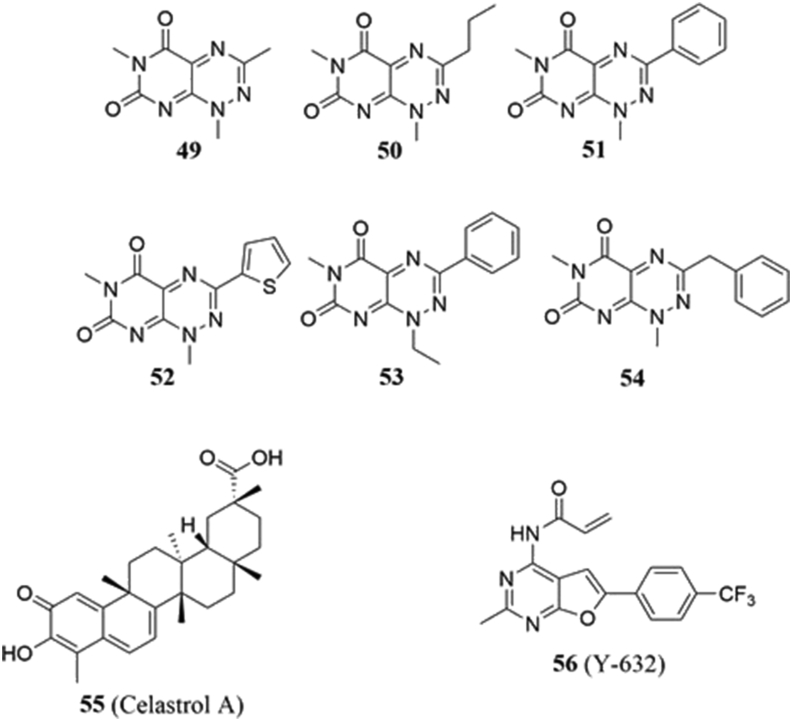
4.6.1. 7-Azapteridines
7-Azapteridines (49–54, Fig. 10) are compounds identified as novel Hsp90‒HOP PPI inhibitors during a high-throughput Alpha Screen developed by Yi and Regan164. The screen initially yielded 149 compounds that manifested IC50 values ≤10 μmol/L, while follow-up assays eliminated the false positives to ultimately reveal three molecules164. 49 was chosen for further investigation, and fluorescence polarization and isothermal titration calorimetry confirmed it to disrupt PPIs by binding to HOP's TPR2A domain with an apparent KD of 16 μmol/L. Overall, the compounds induced the death of BT474 cells; however, they displayed modest selectivity over non-malignant MCF-12F cells. The 7-azapteridines also led to a reduction of Her-2 levels in BT474 and SkBR3 cells after 6 h, but this led to an increase in Her-2 levels after 12 h. Further studies are needed to resolve the mechanism of action manifested by these novel inhibitors.
Figure 10.
Disruptors of Hsp90‒HOP PPIs.
In 2011, the same researchers identified 50 and discovered its antimigratory and antiproliferative activity against breast cancer cell lines, including the highly drug-resistant MDA-MB-468 and MDA-MD-231 cells, manifesting IC50 values of 2 and 1.75 μmol/L, respectively165. When used in combination with the known N-terminal inhibitors 17-AAG, PU-H71 or NVP-AUY922, the cytotoxic activity exhibited by 50 was enhanced. Similarly, the administration of 50 also led to a decrease in the levels of Hsp90-dependent client proteins Cdc37, Cdk4, Raf-1, JNK1/2, p38 and HSF-1, but did not alter Hsp70 mRNA expression, indicating that it did not induce the heat shock response.
4.6.2. Celastrol A
In 2010, Zhang and colleagues166 reported that celastrol (55, Fig. 10) regulates multiple transcription factors in a dose-dependent manner, but to a different extent in MCF-7, HepG2, and THP-1 cells. Co-immunoprecipitations studies with MCF-7 whole cell lysates revealed a reduction in HOP bound to Hsp90, suggesting that celastrol can disrupt the stability of other client proteins as well166.
4.6.3. Peptide-based disruptors
In 2011, Horibe and coworkers167 synthesized a peptidomimetic based on the TPR2A domain of HOP (KAYARIGNSYFK). The inclusion of a lysine and arginine at the first and fifth positions, respectively, recreated a crucial hydrogen bonding interaction with the Hsp90 MEEVD sequence. The peptide, referred to as the “Antp-TPR peptide,” was found to bind Hsp90 with a KD of 4.43 μmol/L and exhibited antiproliferative activity against breast, pancreatic, renal, gastric, lung, and prostate cancer cell lines manifesting IC50 values in the range of 19.4–65.9 μmol/L. Furthermore, “Antp-TPR peptide” exhibited efficacy in vivo against BXPC3 pancreatic xenograft mouse models, which resulted in a significant decrease in tumor size.
Another peptide inhibitor reported by Gupta and coworkers168 was developed in silico. The authors performed molecular docking studies between Hsp90α and HOP to identify residues that are most likely to participate in PPIs. A series of ten peptides was rationally designed and then docked to Hsp90α, with the best (PEP7) exhibiting a strong predicted docking energy. PEP7 (INSAYKFKYARG), which required further refinement due to its potential to form amyloid plaques, yielded 5 derivatives. PEP73 (INSAYKLKYARG) was tentatively the best compound identified based upon docking scores. However, the results have yet to be validated in vitro, and there have been no follow-up studies reported thus far.
4.6.4. Y-632
Y-632 (56, Fig. 10) is a pyrimidine that was identified as an Hsp90 inhibitor by Wang and coworkers169 in 2016. Y-632 induced the proteasome-mediated degradation of several Hsp90-dependent client proteins in SkBr3, A-431, MCF-7, and SNU-5 cancer cell lines, which led to induction of G0/G1 cell cycle arrest and apoptosis169. Y-632's mechanism of action was discovered after it failed to inhibit the ATPase activity of Hsp90, and surface plasmon resonance studies demonstrated that it doesn't bind the chaperone directly169. The first indication that Y-632 disrupted Hsp90‒HOP interactions came during a luciferase refolding assay and immunoprecipitation experiments in which HOP was the only co-chaperone downregulated168. Glutathione reversed the anticancer activities manifested by Y-632, suggesting that Y-632 disrupts Hsp90 PPIs with HOP via the oxidation of cysteines, and this is supported by the presence of its α,β-unsaturated amide moiety that can serve as a Michael acceptor. In addition, Y-632 was shown to be effective against imatinib-resistant cells and to inhibit their growth.
4.7. Hsp90 and survivin
Survivin is a member of the inhibitors of apoptosis family and is universally overexpressed in cancer170. In addition to its ability to prevent apoptosis, it also serves as a major mitotic regulator171. Because of its overexpression and diverse function, survivin has been implicated to confer chemotherapy and radiotherapy resistance in cancer cells172. Fortugno and coworkers173 were the first to report that survivin binds Hsp90; however, it does not depend upon Hsp90 to achieve its conformational maturity, but instead, relies upon Hsp90 to stabilize its fully assembled form. The interface between the two resides at the Hsp90 NTD and survivin region comprised of Lys-79–Lys-90. However, mutagenesis studies reinforced the importance of His-80 and Cys-84 to maintain these interactions174. Consequently, these residues serve as the basis for molecules that were specifically designed to disrupt this PPI.
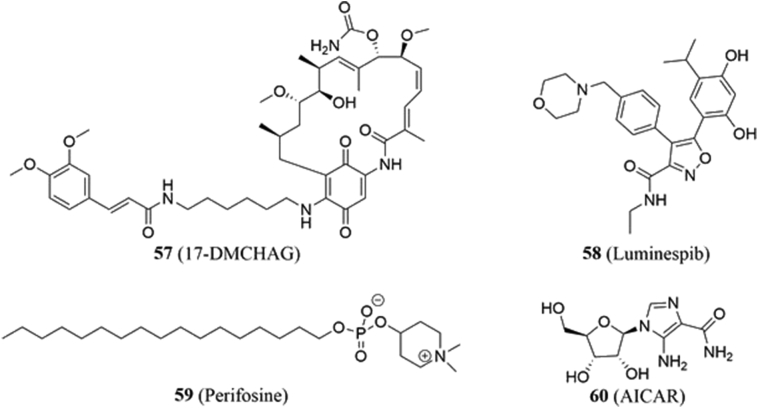
4.7.1. 17-DMCHAG
17-(6-(3,4-Dimethoxycinnamamido)hexylamino)-17-demethoxy-geldanamycin (17-DMCHAG, 57, Fig. 11) is a geldanamycin analogue prepared by Wang and coworkers175 that was evaluated for anticancer activity against prostate cancer cells. 17-DMCHAG selectively induced apoptosis in malignant cells versus normal RWPE-1 cells and inhibited cellular migration of metastatic cancer cells. Co-immunoprecipitation studies and Western blots revealed 17-DMCHAG to promote Hsp90 client protein degradation. However, the compound stood out by its abilities to potently disrupt androgen receptor signaling and to downregulate survivin levels, suggesting that 17-DMCHAG may disrupt interactions with Hsp90. Unlike geldanamycin and its first derivatives, 17-DMCHAG does not appear to induce the heat shock response, and instead, suppressed the tumor growth of DU-145 and LNCaP xenografts without organ damage or toxicity. Overall, these data provide further support for 17-DMCHAG as a potential treatment for prostate cancer.
Figure 11.
Disruptors of Hsp90‒survivin PPIs.
4.7.2. Luminespib (NVP-AUY922)
Luminespib (NVP-AUY922, 58, Fig. 11) is an isoxazole-based inhibitor of Hsp90 ATPase activity that is currently being investigated in at least 13 clinical trials176,177. Liu and coworkers178 sought to determine its effectiveness against papillary thyroid carcinoma (PTC). In vitro studies using IHH4 and K1 PTC cells demonstrated that luminespib inhibited cell proliferation and induced cell death via apoptosis178. It was also demonstrated that luminespib lowered survivin levels via disruption of the Hsp90‒survivin complex.
4.7.3. Perifosine
Perifosine (59, Fig. 11) is an alkyl phospholipid drug candidate that has been investigated in clinical trials as a treatment for multiple cancers, including neuroblastoma179, colorectal cancer180, and metastatic melanoma181. While its primary mechanism involves interactions with the plasma membrane and the inactivation of Akt182, it has been proposed to display activity through other pathways as well. In 2013, Yao and coworkers183 administered the molecule to osteosarcoma cells and found that perifosine inhibited the growth, promoted apoptosis, and sensitized cells to doxorubicin. In addition to Akt inhibition, the authors noted that perifosine induced the degradation of survivin, which co-immunoprecipitation studies indicate is due to interference with the Hsp90‒survivin complex.
4.7.4. Shepherdin and AICAR
Shepherdin (KHSSGCAFLIVK) is a peptidomimetic and one of the first disruptors of Hsp90‒survivin PPIs identified. The molecule represents the minimum sequence required for survivin to bind Hsp90 and was designed by Plescia and colleagues174 to recreate this interaction. In vitro studies revealed shepherdin to selectively target carcinomas while leaving human fibroblasts unaffected. Shepherdin also reduced in vivo tumor size in both PC3 and MCF-7 xenograft mouse models without any detrimental side effects or toxicity. It was reported that shepherdin also suppressed ATP binding to Hsp90, promoted the degradation of other Hsp90 clients, and induced cell death via both apoptopic and non-apoptopic pathways. Such data suggests that shepherdin exhibits anticancer activity through multiple pathways. Shepherdin has since been assessed against numerous cancers including retinoblastoma184, leukemia185, and gallbladder carcinoma186.
In 2006, the same researchers discovered 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR, 60, Fig. 11), a small molecule that behaves similarly to shepherdin. AICAR was developed following a molecular docking and dynamics screen187. In silico modeling suggested that AICAR binds the Hsp90 N-terminal ATP binding site, but in a manner different than other Hsp90 N-terminal inhibitors. Such binding was confirmed via an ELISA screen. Like shepherdin, AICAR inhibits Hsp90 function, which led to the selective induction of apoptosis in cancer cell lines and the degradation of survivin along with other client proteins. In silico modeling and NMR studies suggested that both shepherdin and AICAR depend upon their imidazole rings for establishing hydrophobic interactions with a nonpolar patch of Hsp90 that is comprised of Ala-55, Ile-96, and Met-98188. Despite its potential as a lead compound for inhibitor development, no medicinal chemistry studies pertaining to AICAR have been reported. Instead, AICAR has been used as a probe for biological investigation of various pathways189,190.
4.8. Hsp90 and p23
p23 is one of the many co-chaperones that associates with Hsp90 during the protein folding cycle. Upon transfer of the client protein to Hsp90 from the Hsp40/Hsp70/HIP/HOP complex, p23 is recruited to Hsp90 to inhibit Hsp90's ATPase activity and to stabilize its interactions with the client substrate. Ali and colleagues23 were among the first to solve a co-crystal structure of the Hsp90‒p23 complex in yeast, indicating that p23 binds to a cavity formed by the NTDs of the Hsp90 dimer in an ATP-dependent manner (Fig. 12). However, NMR studies by Martinez-Yamout and coworkers191 suggest that Aha1, which is known to bind Hsp90's MD46, competes with p23 to achieve this. The precise location of p23 binding to Hsp90 thus remains unclear. p23 has been implicated to play a key role in metastasis and advanced malignancy192, and therefore, disruption of Hsp90‒p23 PPIs has become an attractive therapeutic strategy.
Figure 12.
PPIs between Hsp90 and p23. (A) Co-crystal structure of yeast Hsp90 (green) and p23/Sba1 (purple). (B)‒(D) Residues within the Hsp90 NTD and MD that interact with p23/Sba1 (PDBID: 2CG923).
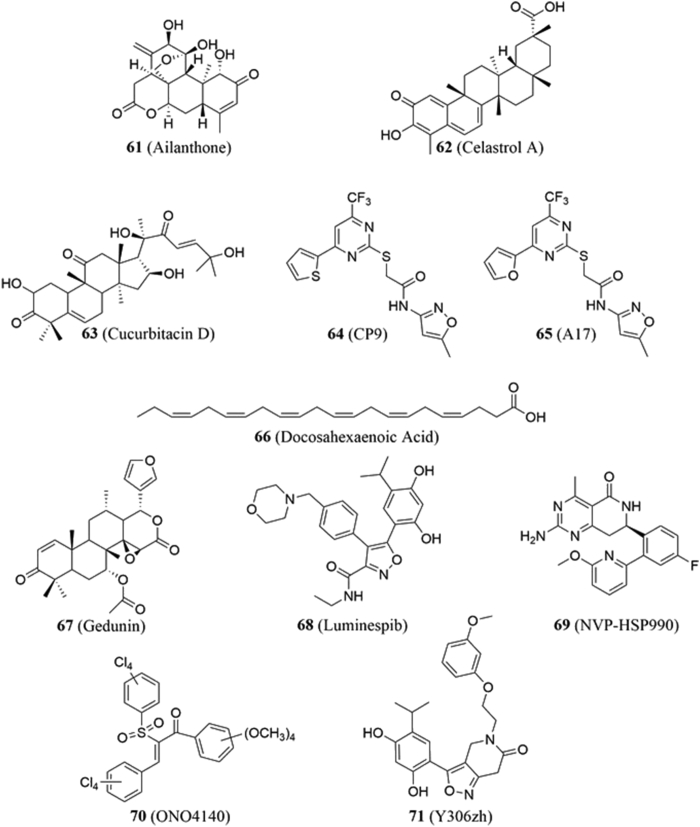
4.8.1. Ailanthone
Ailanthone (61, Fig. 13) is a quassinoid natural product isolated from the Chinese tree of heaven (Ailanthus altissima)193 that exhibits anticancer activity against numerous cancer types, including bladder194, leukemia195, and hepatocellular carcinoma196. Though it has been reported to induce G0/G1 cell cycle arrest197 and interfere with various oncogenic and tumor-suppressant miRNAs198,199, the work of He and colleagues200 discovered that ailanthone inhibits p23. During their studies of castration-resistant prostate cancer, the authors found that ailanthone inhibited tumor growth and metastasis via degradation of the androgen receptor. This degradation was caused by ailanthone's ability to prevent p23's binding to Hsp90, which was further supported when p23 knockdown reduced ailanthone inhibition and p23 overexpression rescued ailanthone-hindered proliferation200. Molecular docking studies suggest a potential binding site for ailanthone on p23 in a pocket that is formed by residues Trp-8, Pro-87, Arg-93, Lys-95, Ser-100, and Val-101. This proposed binding site differs from that ascribed to celastrol201, suggesting that the two might display synergistic activities against p23.
Figure 13.
Disruptors of Hsp90‒p23 PPIs.
4.8.2. Celastrol A
Despite uncertainty regarding the mechanism of action manifested by celastrol (62, Fig. 13) and its ability to promote the degradation of Hsp90-dependent client proteins, its ability to disrupt p23 has been well-defined. p23 was first identified as a target of celastrol by Chadli and coworkers201 who reported that the compound induced fibrillization of the co-chaperone. This aggregation was supported by 1H–15N heteronuclear single quantum coherence spectroscopy and detection of the fibrils via electron microscopy201. Although it has been hypothesized that celastrol reacts with cysteine residues to form a covalent linkage202, mutagenic studies with p23 indicate they are not required for formation of the fibrils201. Instead, the NMR spectra of the mutants suggest celastrol to bind the same hydrophobic pocket on p23 as Hsp90.
4.8.3. Cucurbitacin D
Cucurbitacins are a family of triterpenoids that are isolated from the fruit of the Cucurbitaceae family and other related species and are known to display a variety of biological activities, including anticancer and antimetastatic properties203. In 2015, Hall and coworkers204 isolated cucurbitacin D (63, Fig. 13) and its isomer, 3-epi-isocucurbitacin D, from Cucurbita texana and synthesized derivatives to assess their biological properties. With few exceptions, all the evaluated cucurbitacins manifested IC50 values in the nanomolar range against MCF-7 breast cancer cells. The proposed mechanism of action involved a reaction between the electrophilic α,β-unsaturated carbonyl and nucleophilic residues. The compounds induced the degradation of Hsp90 client proteins in a dose-dependent manner and without induction of the heat shock response, indicating that they act through a mechanism different than Hsp90 N-terminal inhibition. However, co-immunoprecipitation experiments suggest that only cucurbitcain D demonstrated its antiproliferative activity through disruption of the Hsp90‒p23‒Cdc37 complex.
4.8.4. CP9
CP9 (N-(5-methylisoxazol-3-yl)-2-[4-(thiophen-2-yl)-6-(trifluoromethyl)pyrimidin-2-ylthio]acetamide, 64, Fig. 13) was found to disrupt Hsp90‒p23 interactions in a high throughput screen developed by Chan and colleagues205. A competitive binding assay determined that CP9 binds in a manner similar to 17-AAG and displayed antiproliferative activity, as well as enabled glucose metabolism and thymidine kinase function across multiple cancer cell lines, while exhibiting no significant effect on normal embryonic mouse fibroblasts. In addition, similar results were obtained in a 293 T xenograft mouse study. SAR studies on CP9 yielded the derivative A17 (65, Fig. 13), which exhibits superior activity and is believed to result from increased hydrophobicity.
4.8.5. Docosahexaenoic acid (DHA)
Docosahexaenoic acid (66, Fig. 13) is an omega-3 polyunsaturated fatty acid found in fish oil that has been proposed to improve outcomes in cancer patients either on its own206 or as a supplement to chemotherapy and/or radiotherapy regiments207. To determine how DHA elicits its anticancer effects, Mouradian and coworkers208 administered DHA to A549 lung and BT-474 breast cancer cells, which resulted in decreased cellular ATP and disruption of Hsp90‒p23 interactions. It was also observed that DHA decreased levels of the Hsp90 clients, HIF-1α and Her-2. These data indicate that DHA manifests its activity through regulation of the Hsp90 heteroprotein complex and its ATPase activity, while also highlighting the potential influence of a patient's diet on treatment outcome.
4.8.6. Gedunin
Gedunin (67, Fig. 13) is a tetranortriterpenoid natural product that can be isolated from the mahogany family of plants and has been reported to exhibit antiproliferative activity against colon209 and ovarian cancer cell lines210. Patwardhan and colleagues211 sought to determine whether the compound could serve as an inhibitor of Hsp90. In vitro and in vivo analyses demonstrated that gedunin could target p23 and inhibit its ability to chaperone citrate synthase and various steroid receptors. In addition, gedunin caused destabilization of the Hsp90‒p23 complex, induced apoptosis in cancer cells, and enabled cleavage of p23 by caspase-7211. Molecular docking studies identified a potential binding site for gedunin, which highlighted Thr-90 and Lys-95 as critical for hydrogen bonds, while Ala-94 mediated a hydrophobic interaction. The proposed binding site was supported via mutagenic studies; however, deletion of the p23 C-terminal residues also support its role to stabilize this interaction.
SAR studies on this compound resulted in the synthesis of 19 derivatives. Although none of the derivatives were more potent than the natural product, important information about the structure was still obtained212. The 7-position was found to be sensitive to steric bulk, but not electronic properties. In addition, the α,β-unsaturated carbonyl of the A-ring does not serve as a Michael acceptor. In 2018, Pinkerton and coworkers213 reported a synthesis that allows for diversification of the BCD ring system of gedunin, and through this route, they generated molecules which were found equipotent as gedunin at inhibiting p23 function.
4.8.7. Luminespib (NVP-AUY922)
In addition to the disruption of PPIs between Hsp90 and survivin, luminespib (68, Fig. 13) has been found to destabilize the Hsp90‒p23 complex. While luminespib has received attention as a potential treatment for a variety of cancers, Jensen and colleagues214 investigated it specifically for activity against breast cancer. In vitro results found that luminespib potently inhibited the growth of multiple breast cancer cell lines and manifested an average GI50 of 5.4 nmol/L, while growth inhibition of several breast tumors was reported to have an average GI50 value of 191 nmol/L. Unfortunately, one of the tumors exhibited drug resistance. Immunoprecipitation studies supported the hypothesis that the molecule interferes with the Hsp90‒p23 complex and provides support for the potential use of NVP-AUY922 for the treatment of breast cancer.
4.8.8. NVP-HSP990
NVP-HSP990 (69, Fig. 13) is a novel dihydropyridopyrimidinone Hsp90 inhibitor that binds the N-terminal ATP binding site. In 2012, Menezes and colleagues215 found that it inhibited Hsp90α, Hsp90β, and Grp94 by manifesting IC50 values of 0.6, 0.8 and 8.5 nmol/L, respectively. In addition, such inhibition resulted in degradation of the Hsp90‒p23 complex in both a time- and dose-dependent manner215. In vivo studies showed that NVP-HSP990 suppressed the growth of various cancer cell lines, and this translated well against gastric, breast, AML, and NSCLC xenograft models. In addition, NVP-HSP990 did not exhibit hepatotoxicity, suggesting minimal chance for drug–drug interactions. SAR studies performed by McBride and coworkers216 determined the importance of the para-fluorine on ring C to maintain interactions with the Hsp90 binding pocket. Derivatives wherein the ortho-pyridine was replaced with either a pyrazine or a thiazole exhibited comparable activity, but the parent molecule exhibited a more favorable half-life216. A phase I clinical trial has indicated that NVP-HSP990 is well-tolerated, although neurological toxicities may limit the maximum-tolerated dosage to 50 mg per week217.
4.8.9. ONO4140
ONO4140 (70, Fig. 13) is a novel Hsp90 inhibitor that was identified by Eachkoti and colleagues218 during a luciferase refolding screening assay. ONO4140 exhibited low GI50 values against BT-474 (invasive ductal breast carcinoma), DU-145 (prostrate carcinoma), and K562 (Bcr-Abl positive CML) cell lines. ONO4140 also induced the degradation of Hsp90-dependent client proteins, Her-2 and Akt, and induced the expression of Hsp70, which provided evidence for the mechanism of action for ONO4140 as Hsp90 inhibition. At higher concentrations, ONO4140 disrupted the Hsp90‒p23 PPI as confirmed by co-immunoprecipitation experiments.
4.8.10. Y306zh
Y306zh (71, Fig. 13) is a novel Hsp90 inhibitor identified by Xue and colleagues219 as a potential treatment for pancreatic cancer. During the elucidation of the mechanism of action, it was determined that Y306zh blocks Hsp90‒p23 association by competing with ATP for binding to the NTD, while manifesting an IC50 of 85 nmol/L219. In vitro analysis found Y306zh to induce G2/M cell cycle arrest in pancreatic cell lines and inhibit tumor growth in a Mia-paca 2 xenograft mouse model without affecting normal cells.
5. Conclusions and perspectives
Hsp90 is an ATP-dependent molecular chaperone whose primary function is to maintain cellular proteostasis by folding ∼400 client substrates, restoring damaged/denatured proteins, solubilizing protein aggregates, and promoting protein turnover via the ubiquitin‒proteasome pathway. Because many of its client proteins are implicated in the development and progression of cancer, Hsp90 inhibition has been pursued as a chemotherapeutic strategy to disrupt multiple oncogenic pathways simultaneously.
The abundance of Hsp90, its higher affinity for ATP, and its enhanced ATPase activity in cancer cells relative to normal tissue support the development of inhibitors that selectively target malignant cells. Despite evidence that demonstrates the effectiveness of Hsp90 N-terminal inhibitors to treat cancer in vitro and in vivo, there are no FDA-approved Hsp90 inhibitors. The first Hsp90 inhibitors discovered were geldanamycin, radicicol and their derivatives, which have in many cases failed during clinical evaluation due to unanticipated issues related to dosing and toxicity. Unfortunately, NTD-inhibition results in induction of the heat shock response, which leads to the overexpression of pro-survival proteins that contradict inhibitory activity. The latter appears to be related to the inhibitors’ lack of selectivity among the four isoforms. Potential solutions include C-terminal or isoform-selective inhibition, however, each of them comes with their own challenge. For example, the binding pocket for C-terminal inhibitors has not been fully elucidated, and the high sequence identity among the isoforms in the NTD makes selective inhibition very difficult.
A third approach to modulate the Hsp90 machinery involves disruption of protein−protein interactions between Hsp90 and various co-chaperones/client proteins. Some co-chaperones such as HOP, p23 and Aha1 are essential for progression through the protein folding cycle; and therefore, disruption of these interactions represents an alternative approach toward Hsp90 inhibition. Roughly 400 client proteins rely upon Hsp90 to attain conformational maturity, and this process also requires the recruitment of other immunophilins and co-chaperones as well. Since co-chaperones associate with Hsp90 at different stages during the protein folding cycle, they can be exploited to target a more refined set of substrates. For instance, the folding of kinase clients is facilitated by Cdc37, and consequently, disruption of Hsp90−Cdc37 PPIs are likely to prove effective for the treatment of kinase-driven cancers. Other proteins like survivin and HIF-1α are not clients, but depend upon Hsp90 for stability. As a result of Hsp90 inhibition, they are degraded and ultimately, can lead to cell death.
The discovery and development of small molecule inhibitors has been the primary approach toward Hsp90 inhibition, although peptidomimetics of certain PPIs have also been pursued. Much like early Hsp90 inhibitors, many of these molecules have proven to be efficacious in vitro and in vivo and remain at various stages of the drug development process. Many of these inhibitors are derived from natural products, although some were discovered from high throughput screens. Other compounds are known Hsp90 inhibitors whose biological outcomes are better understood; however, the exact mechanism manifested by some of these compounds remains undetermined.
Although Hsp90 inhibition has been heavily pursued as a promising approach to treat cancer for the last decade, the molecular chaperone remains a challenge. In this article, the disruption of Hsp90 PPIs is presented and provides insights into the potential promise of targeting Hsp90 PPIs as a viable and complementary approach to Hsp90 N-terminal inhibition.
Acknowledgments
The graphical abstract and Fig. 1 were produced using BioRender.com. All crystal structures were obtained from the Protein Data Bank and rendered using the Visual Molecular Dynamics software220. Financial support comes from the National Institutes of Health (CA213566, USA).
Author contributions
Brian Blagg proposed the idea and revised/edited the manuscript. Michael Serwetnyk wrote the manuscript.
Conflicts of interest
The authors have no conflict of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Dill K.A., MacCallum J.L. The protein-folding problem, 50 years on. Science. 2012;338:1042–1046. doi: 10.1126/science.1219021. [DOI] [PubMed] [Google Scholar]
- 2.Anfinsen C.B., Haber E., Sela M., White F.H., Jr. The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proc Natl Acad Sci U S A. 1961;47:1309–1314. doi: 10.1073/pnas.47.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartl F.U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 4.Fohlman J., Eaker D., Karlsson E., Thesleff S. Taipoxin, an extremely potent presynaptic neurotoxin from the venom of the Australian snake taipan (Oxyuranus s. scutellatus) Eur J Biochem. 1976;68:457–469. doi: 10.1111/j.1432-1033.1976.tb10833.x. [DOI] [PubMed] [Google Scholar]
- 5.Laskey R.A., Honda B.M., Mills A.D., Finch J.T. Nucleosomes are assembled by an acidic protein that binds histones and transfers them to DNA. Nature. 1978;275:416–420. doi: 10.1038/275416a0. [DOI] [PubMed] [Google Scholar]
- 6.Ritossa F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- 7.Ritossa F. New puffs induced by temperature shock, DNP, and salicilate in salivary chromosomes of Drosophila melanogaster. Drosoph Inf Serv. 1963;37:122–123. [Google Scholar]
- 8.Ritossa F. Experimental activation of specific loci in polytene chromosomes of Drosophila. Exp Cell Res. 1964;35:601–607. doi: 10.1016/0014-4827(64)90147-8. [DOI] [PubMed] [Google Scholar]
- 9.Tissières A., Mitchel H.K., Tracy U.M. Protein synthesis in salivary glands of Drosophila melanogaster: Relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly A., Blagg B.S.J. Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide binding pocket. Curr Med Chem. 2008;15:2702–2717. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maloney A., Workman P. Hsp90 as a new therapeutic target for cancer therapy: The story unfolds. Expet Opin Biol Ther. 2002;2:3–24. doi: 10.1517/14712598.2.1.3. [DOI] [PubMed] [Google Scholar]
- 12.Panaretou B., Prodromou C., Roe S.M., O'Brien R., Ladbury J.E., Piper P.W. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta R., Inouye M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem Sci. 2000;25:24–28. doi: 10.1016/s0968-0004(99)01503-0. [DOI] [PubMed] [Google Scholar]
- 14.Meyer P., Prodromou C., Hu B., Vaughan C., Roe M.S., Panaretou B. Structural and functional analysis of the middle segment of Hsp90: Implications for ATP hydrolysis and client protein and cochaperone interactions. Mol Cell. 2003;11:647–658. doi: 10.1016/s1097-2765(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 15.Pearl L.H., Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 16.Söti C., Rácz A., Csermely P. A nucleotide-dependent molecular switch controls ATP binding at the C-terminal domain of Hsp90: N-terminal nucleotide binding unmasks a C-terminal binding pocket. J Biol Chem. 2002;277:7066–7075. doi: 10.1074/jbc.M105568200. [DOI] [PubMed] [Google Scholar]
- 17.Prodromou C., Siligardi G., O'Brien R., Woolfson D.N., Regan L., Panaretou B. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. EMBO J. 1999;18:754–762. doi: 10.1093/emboj/18.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter S., Buchner J. Molecular chaperones—cellular machines for protein folding. Angew Chem Int Ed Engl. 2002;41:1098–1113. doi: 10.1002/1521-3773(20020402)41:7<1098::aid-anie1098>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Murphy P.J.M., Kanelakis K.C., Galigniana M.D., Morishima Y., Pratt W.B. Stoichiometry, abundance, and functional significance of the Hsp90/Hsp70-based multiprotein chaperone machinery in reticulocyte lysate. J Biol Chem. 2001;276:30092–30098. doi: 10.1074/jbc.M103773200. [DOI] [PubMed] [Google Scholar]
- 20.Caplan A.J., Mandal A.K., Theodoraki M.A. Molecular chaperones and protein kinase quality control. Trends Cell Biol. 2007;17:87–92. doi: 10.1016/j.tcb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Kosano H., Stensgard B., Charlesworth M.C., McMahon N., Toft D. The assembly of progesterone receptor−Hsp90 complexes using purified proteins. J Biol Chem. 1998;273:32973–32979. doi: 10.1074/jbc.273.49.32973. [DOI] [PubMed] [Google Scholar]
- 22.Prodromou C., Panaretou B., Chohan S., Siligardi G., O'Brien R., Ladbury J.E. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J. 2000;19:4383–4392. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali M.M.U., Roe S.M., Vaughan C.K., Meyer P., Panaretou B., Piper P.W. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Taipale M., Krykbaeva I., Koeva M., Kayatekin C., Westover K.D., Karras G.I. Quantitative analysis of Hsp90-client, interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blagg B.S.J., Kerr T.D. Hsp90 inhibitors: Small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med Res Rev. 2006;26:310–338. doi: 10.1002/med.20052. [DOI] [PubMed] [Google Scholar]
- 28.Vartholomaiou E., Echeverría P.C., Picard D. Unusual suspects in the twilight zone between the Hsp90 interactome and carcinogenesis. Adv Cancer Res. 2016;129:1–30. doi: 10.1016/bs.acr.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Whitesell L., Lindquist S.L. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 30.Chiosis G., Neckers L. Tumor selectivity of Hsp90 inhibitors: The explanation remains elusive. ACS Chem Biol. 2006;1:279–284. doi: 10.1021/cb600224w. [DOI] [PubMed] [Google Scholar]
- 31.Kamal A., Thao L., Sensintaffar J., Zhang L., Boehm M.F., Fritz L.C. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 32.Bhat R., Tummalapalli S.R., Rotella D.P. Progress in the discovery and development of heat shock protein 90 (Hsp90) inhibitors. J Med Chem. 2014;57:8718–8728. doi: 10.1021/jm500823a. [DOI] [PubMed] [Google Scholar]
- 33.Khandelwal A., Crowley V.M., Blagg B.S.J. Natural product inspired N-terminal Hsp90 inhibitors: from bench to bedside?. Med Res Rev. 2016;36:92–118. doi: 10.1002/med.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong D.S., Banerji U., Tavana B., George G.C., Aaron J., Kurzrock R. Targeting the molecular chaperone heat shock protein 90 (HSP90): Lessons learned and future directions. Cancer Treat Rev. 2013;39:375–387. doi: 10.1016/j.ctrv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Peterson L.B., Eskew J.D., Vielhauer G.A., Blagg B.S.J. The hERG channel is dependent upon the Hsp90α isoform for maturation and trafficking. Mol Pharm. 2012;9:1841–1846. doi: 10.1021/mp300138n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y., Zheng X., Ding Y., Zhou M., Wei Z., Liu T. The molecular chaperone Hsp90α deficiency causes retinal degeneration by disrupting Golgi organization and vesicle transportation in photoreceptors. J Mol Cell Biol. 2020;12:216–229. doi: 10.1093/jmcb/mjz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khandelwal A., Kent C.N., Balch M., Peng S., Mishra S.J., Deng J. Structure-guided design of an Hsp90β N-terminal isoform-selective inhibitor. Nat Commun. 2018;9:425. doi: 10.1038/s41467-017-02013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garg G., Khandelwal A., Blagg B.S.J. Anticancer inhibitors of Hsp90 function: Beyond the usual suspects. Adv Cancer Res. 2016;129:51–88. doi: 10.1016/bs.acr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson L.B., Blagg B.S.J. To fold or not to fold: Modulation and consequences of Hsp90 inhibition. Future Med Chem. 2009;1:267–283. doi: 10.4155/fmc.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcu M.G., Chadli A., Bouhouche I., Catelli M., Neckers L.M. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 41.Marcu M.G., Schulte T.W., Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst. 2000;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 42.Sgobba M., Forestiero R., Degliesposti G., Rastelli G. Exploring the binding site of C-terminal Hsp90 inhibitors. J Chem Inf Model. 2010;50:1522–1528. doi: 10.1021/ci1001857. [DOI] [PubMed] [Google Scholar]
- 43.Hall J.A., Forsberg L.K., Blagg B.S.J. Alternative approaches to Hsp90 modulation for the treatment of cancer. Future Med Chem. 2014;6:1587–1605. doi: 10.4155/fmc.14.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oroz J., Blair L.J., Zweckstetter M. Dynamic Aha1 co-chaperone binding to human Hsp90. Protein Sci. 2019;28:1545–1551. doi: 10.1002/pro.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y., Sun M., Myasnikov A.G., Elnatan D., Delaeter N., Nguyenquang M. Cryo-EM structures reveal a multistep mechanism of Hsp90 activation by co-chaperone Aha1. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.06.30.180695v1 Available from: [Google Scholar]
- 46.Meyer P., Prodromou C., Liao C., Hu B., Roe S.M., Vaughan C.K. Structural basis for recruitment of the ATPase activator Aha1 to the Hsp90 chaperone machinery. EMBO J. 2004;23:511–519. doi: 10.1038/sj.emboj.7600060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuk K., Taylor-Cousar J.L. Lumacaftor and ivacaftor in the management of patients with cystic fibrosis: Current evidence and future prospects. Ther Adv Respir Dis. 2015;9:313–326. doi: 10.1177/1753465815601934. [DOI] [PubMed] [Google Scholar]
- 48.Ihrig V., Obermann W.M.J. Identifying inhibitors of the Hsp90−Aha1 protein complex, a potential target to drug cystic fibrosis, by alpha technology. SLAS Discov. 2017;22:923–928. doi: 10.1177/2472555216688312. [DOI] [PubMed] [Google Scholar]
- 49.Stiegler S.C., Rübbelke M., Korotkov V.S., Weiwad M., John C., Gunter F. A chemical compound inhibiting the Aha1−Hsp90 chaperone complex. J Biol Chem. 2017;292:17073–17083. doi: 10.1074/jbc.M117.797829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu H., Jiang X., Guo Y., Sun F., Kou X., Bao Y. The flavonoid TL-2-8 induces cell death and immature mitophagy in breast cancer cells via abrogating the function of the AHA1/Hsp90 complex. Acta Pharmacol Sin. 2017;38:1381–1393. doi: 10.1038/aps.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh J.K., Hutt D.M., Tait B., Guy N.C., Sivils J.C., Ortiz N.R. Management of Hsp90-dependent protein folding by small molecules targeting the Aha1 co-chaperone. Cell Chem Biol. 2020;27:292–305. doi: 10.1016/j.chembiol.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao J., Prince T., Hartson S.D., Matts R.L. Phosphorylation of serine 13 is required for the proper function of the Hsp90 co-chaperone, Cdc37. J Biol Chem. 2003;278:38117–38120. doi: 10.1074/jbc.C300330200. [DOI] [PubMed] [Google Scholar]
- 53.Oberoi J., Dunn D.M., Woodford M.R., Mariotti L., Scuhlman J., Bourboulia D. Structural and functional basis of protein phosphatase 5 substrate specificity. Proc Natl Acad Sci U S A. 2016;113:9009–9014. doi: 10.1073/pnas.1603059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li T., Jiang H., Tong Y., Lu J. Targeting the Hsp90−Cdc37−client protein interaction to disrupt Hsp90 chaperone machinery. J Hematol Oncol. 2018;11:59. doi: 10.1186/s13045-018-0602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verba K.A., Wang R.Y., Arakawa A., Liu Y., Shirouzu M., Yokoyama S. Atomic structure of Hsp90−Cdc37−Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science. 2016;352:1542–1547. doi: 10.1126/science.aaf5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang T., Hamza A., Cao X., Wang B., Yu S., Zhan C. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol Canc Therapeut. 2008;7:162–170. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 57.Zhang T., Li Y., Yu Y., Zou P., Jiang Y., Sun D. Characterization of celastrol to inhibit Hsp90 and Cdc37 interaction. J Biol Chem. 2009;284:35381–35389. doi: 10.1074/jbc.M109.051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sreeramulu S., Gande S.L., Göbel M., Schwalbe H. Molecular mechanism of inhibition of the human protein complex Hsp90−Cdc37, a kinome chaperone−cochaperone, by triterpene celastrol. Angew Chem Int Ed Engl. 2009;48:5853–5855. doi: 10.1002/anie.200900929. [DOI] [PubMed] [Google Scholar]
- 59.Jiang F., Wang H., Bao Q., Wang L., Jin Y., Zhang Q. Optimization and biological evaluation of celastrol derivatives as Hsp90−Cdc37 interaction disruptors with improved druglike properties. Bioorg Med Chem. 2016;24:5431–5439. doi: 10.1016/j.bmc.2016.08.070. [DOI] [PubMed] [Google Scholar]
- 60.Li N., Xu M., Wang B., Shi Z., Zhao Z., Tang Y. Discovery of novel celastrol derivatives as Hsp90−Cdc37 interaction disruptors with antitumor activity. J Med Chem. 2019;62:10798–10815. doi: 10.1021/acs.jmedchem.9b01290. [DOI] [PubMed] [Google Scholar]
- 61.Xu M., Li N., Zhao Z., Shi Z., Sun J., Chen L. Design, synthesis, and antitumor evaluation of novel celastrol derivatives. Eur J Med Chem. 2019;174:265–276. doi: 10.1016/j.ejmech.2019.04.050. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Z.X., Zhu J.M., Quan H.T., Wang G.M., Li B., Zhu W.L. X66, a novel N-terminal heat shock protein 90 inhibitor, exerts antitumor effects without induction of heat shock response. Oncotarget. 2016;7:29648–29663. doi: 10.18632/oncotarget.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X., Liu P., Wang Q., Li Y., Fu L., Fu H. DCZ3112, a novel Hsp90 inhibitor, exerts potent antitumor activity against HER2-positive breast cancer through disruption of Hsp90−Cdc37 interaction. Canc Lett. 2018;434:70–80. doi: 10.1016/j.canlet.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 64.Wang L., Zhang L., Li L., Jiang J., Zheng Z., Shang J. Small-molecule inhibitor targeting the Hsp90−Cdc37 protein−protein interaction in colorectal cancer. Sci Adv. 2019;5 doi: 10.1126/sciadv.aax2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L., Jiang J., Zhang L., Zhang Q., Zhou J., Li L. Discovery and optimization of small molecules targeting the protein−protein interaction of heat shock protein 90 (Hsp90) and cell division cycle 37 as orally active inhibitors for the treatment of colorectal cancer. J Med Chem. 2020;63:1281–1297. doi: 10.1021/acs.jmedchem.9b01659. [DOI] [PubMed] [Google Scholar]
- 66.East A.J., Ollis W.D., Wheeler R.E. Natural occurrence of 3-aryl-4-hydroxycoumarins. Part I. Phytochemical examination of Derris robusta(roxb.) benth. J Chem Soc C. 1969;3:365–374. [Google Scholar]
- 67.Hadden M.K., Galam L., Gestwicki J.E., Matts R.L., Blagg B.S.J. Derrubone, an inhibitor of the Hsp90 folding machinery. J Nat Prod. 2007;70:2014–2018. doi: 10.1021/np070190s. [DOI] [PubMed] [Google Scholar]
- 68.Hastings J.M., Hadden M.K., Blagg B.S.J. Synthesis and evaluation of derrubone and select analogues. J Org Chem. 2008;73:369–373. doi: 10.1021/jo702366g. [DOI] [PubMed] [Google Scholar]
- 69.Mays J.R., Hill S.A., Moyers J.T., Blagg B.S.J. The synthesis and evaluation of flavone and isoflavone chimeras of novobiocin and derrubone. Bioorg Med Chem. 2010;18:249–266. doi: 10.1016/j.bmc.2009.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khalid S., Paul S. Identifying a C-terminal ATP binding sites-based novel Hsp90-inhibitor in silico: A plausible therapeutic approach in Alzheimer's disease. Med Hypotheses. 2014;83:39–46. doi: 10.1016/j.mehy.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 71.Wei H., Wei J., Fu H., Hong C., Xu J., Hong J. Structure and identification and anti-tumor activity research of FW-04-806. Chin J Antibiot. 2011;36:502–508. [Google Scholar]
- 72.Huang W., Ye M., Zhang L., Wu Q., Zhang M., Xu J. FW-04-806 inhibits proliferation and induces apoptosis in human breast cancer cells by binding to N-terminus of Hsp90 and disrupting Hsp90−Cdc37 complex formation. Mol Canc. 2014;13:150–162. doi: 10.1186/1476-4598-13-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang W., Wu Q., Zhang M., Kong Y., Cao P., Zheng W. Novel Hsp90 inhibitor FW-04-806 displays potent antitumor effects in HER2-positive breast cancer cells as a single agent or in combination with lapatinib. Canc Lett. 2015;356:862–871. doi: 10.1016/j.canlet.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 74.Davenport J., Manjarrez J.R., Peterson L., Krumm B., Blagg B.S.J., Matts R.L. Gambogic acid, a natural product inhibitor of Hsp90. J Nat Prod. 2011;74:1085–1092. doi: 10.1021/np200029q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L., Yi Y., Chen J., Sun Y., Guo Q., Zheng Z. Gambogic acid inhibits Hsp90 and deregulates TNF-α/NF-κB in HeLa cells. Biochem Biophys Res Commun. 2010;403:282–287. doi: 10.1016/j.bbrc.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 76.Li D., Li C., Li L., Chen S., Wang L., Li Q. Natural product kongensin A is a non-canonical HSP90 inhibitor that blocks RIP3-dependent necroptosis. Cell Chem Biol. 2016;23:257–266. doi: 10.1016/j.chembiol.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 77.Wang L., Bao Q., Xu X., Jiang F., Gu K., Jiang Z. Discovery and identification of Cdc37-derived peptides targeting the Hsp90−Cdc37 protein−protein interaction. RSC Adv. 2015;5:96138–96145. [Google Scholar]
- 78.Wang L., Li L., Fu W., Jiang Z., You Q., Xu X. Optimization and bioevaluation of Cdc37-derived peptides: An insight into Hsp90−Cdc37 protein−protein interaction modulators. Bioorg Med Chem. 2017;25:233–240. doi: 10.1016/j.bmc.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 79.Li T., Xu W.S., Wu G.S., Chen X.P., Wang Y.T., Lu J.J. Platycodin D induces apoptosis, and inhibits adhesion, migration and invasion in HepG2 hepatocellular carcinoma cells. Asian Pac J Cancer Prev APJCP. 2014;15:1745–1749. doi: 10.7314/apjcp.2014.15.4.1745. [DOI] [PubMed] [Google Scholar]
- 80.Xie Y., Sun H.X., Li D. Platycodin D is a potent adjuvant of specific cellular and humoral immune responses against recombinant hepatitis B antigen. Vaccine. 2009;27:757–764. doi: 10.1016/j.vaccine.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 81.Chun J., Kim Y.S. Platycodin D inhibits migration, invasion, and growth of MDA-MB-231 human breast cancer cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem Biol Interact. 2013;205:212–221. doi: 10.1016/j.cbi.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 82.Li T., Chen X., Chen X., Ma D., Leung C.H., Lu J.J. Platycodin D potentiates proliferation inhibition and apoptosis induction upon AKT inhibition via feedback blockade in non-small cell lung cancer cells. Sci Rep. 2016;6:37997–38007. doi: 10.1038/srep37997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li T., Chen X., Dai X., Wei B., Weng Q., Chen X. Novel Hsp90 inhibitor platycodin D disrupts Hsp90/Cdc37 complex and enhances the anticancer effect of mTOR inhibitor. Toxicol Appl Pharmacol. 2017;330:65–73. doi: 10.1016/j.taap.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 84.Hutzen B., Willis W., Jones S., Cen L., Deangelis S., Fuh B. Dietary agent, benzyl isothiocyanate inhibits signal transducer and activator of transcription 3 phosphorylation and collaborates with sulforaphane in the growth suppression of PANC-1 cancer cells. Cancer Cell Int. 2009;9:24–30. doi: 10.1186/1475-2867-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen C., Yu Z., Chuang Y., Huang R., Wang T.V. Sulforaphane attenuates EGFR signaling in NSCLC cells. J Biomed Sci. 2015;22:38–46. doi: 10.1186/s12929-015-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gibbs A., Schwartzman J., Deng V., Alumkal J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc Natl Acad Sci U S A. 2009;106:16663–16668. doi: 10.1073/pnas.0908908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y., Zhang T., Schwartz S.J., Sun D. Sulforaphane potentiates the efficacy of 17-allylamino 17-demethoxygeldanamycin against pancreatic cancer through enhanced abrogation of Hsp90 chaperone function. Nutr Cancer. 2011;63:1151–1159. doi: 10.1080/01635581.2011.596645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Y., Karagöz G.E., Seo Y.H., Zhang T., Jiang Y., Yu Y. Sulforaphane inhibits pancreatic cancer through disrupting Hsp90−p50Cdc37 complex and direct interactions with amino acids residues of Hsp90. J Nutr Biochem. 2012;23:1617–1626. doi: 10.1016/j.jnutbio.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L., Li L., Zhou Z., Jiang Z., You Q., Xu X. Structure-based virtual screening and optimization of modulators targeting Hsp90−Cdc37 interaction. Eur J Med Chem. 2017;136:63–73. doi: 10.1016/j.ejmech.2017.04.074. [DOI] [PubMed] [Google Scholar]
- 90.Mohan R., Hammers H.J., Bargagna-Mohan P., Zhan X.H., Herbstritt C.J., Ruiz A. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 91.Yang H., Shi G., Dou Q.P. The tumor proteasome is a primary target for the natural anticancer compound withaferin A isolated from “Indian winter cherry”. Mol Pharmacol. 2007;71:426–437. doi: 10.1124/mol.106.030015. [DOI] [PubMed] [Google Scholar]
- 92.Srinivasan S., Ranga R.S., Burikhanov R., Han S.S., Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 93.Yu Y., Hamza A., Zhang T., Gu M., Zou P., Newman B. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem Pharmacol. 2010;79:542–551. doi: 10.1016/j.bcp.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grover A., Shandilya A., Agrawal V., Pratik P., Bhasme D., Bisaria V.S. Hsp90/Cdc37 chaperone/co-chaperone complex, a novel junction anticancer target elucidated by the mode of action of herbal drug Withaferin A. BMC Bioinf. 2011;12(Suppl 1):S30. doi: 10.1186/1471-2105-12-S1-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yokota Y., Bargagna-Mohan P., Ravindranath P.P., Kim K.B., Mohan R. Development of withaferin A analogs as probes of angiogenesis. Bioorg Med Chem Lett. 2006;16:2603–2607. doi: 10.1016/j.bmcl.2006.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gu M., Yu Y., Gunaherath G.M.K.B., Gunatilaka A.A.L., Li D., Sun D. Structure−activity relationship (SAR) of withanolides to inhibit Hsp90 for its activity in pancreatic cancer cells. Invest N Drugs. 2014;32:68–74. doi: 10.1007/s10637-013-9987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Papathanassiu A.E., MacDonald N.J., Bencsura A., Vu H.A. F1F0-ATP synthase functions as a co-chaperone of Hsp90−substrate protein complexes. Biochem Biophys Res Commun. 2006;345:419–429. doi: 10.1016/j.bbrc.2006.04.104. [DOI] [PubMed] [Google Scholar]
- 98.Jundt L., Steinmetz H., Luger P., Weber M., Kunze B., Reichenbach H. Isolation and structure elucidation of cruentarens A and B—novel members of the benzolactone class of ATPase inhibitors from the Myxobacterium Byssovorax cruenta. Eur J Org Chem. 2006;22:5036–5044. [Google Scholar]
- 99.Kunze B., Steinmetz H., Höfle G., Huss M., Wieczorek H., Reichenbach H. Cruentaren, a new antifungal salicylate-type macrolide from Byssovorax cruenta (Myxobacteria) with inhibitory effect on mitochondrial ATPase activity. J Antibiot. 2006;59:664–668. doi: 10.1038/ja.2006.89. (Tokyo) [DOI] [PubMed] [Google Scholar]
- 100.Kunze B., Sasse F., Wieczorek H., Huss M. Cruentaren A, a highly cytotoxic benzolactone from Myxobacteria is a novel selective inhibitor of mitochondrial F1-ATPases. FEBS Lett. 2007;581:3523–3527. doi: 10.1016/j.febslet.2007.06.069. [DOI] [PubMed] [Google Scholar]
- 101.Hall J.A., Kusuma B.R., Brandt G.E.L., Blagg B.S.J. Cruentaren A binds F1F0 ATP synthase to modulate the Hsp90 protein folding machinery. ACS Chem Biol. 2014;9:976–985. doi: 10.1021/cb400906e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cross R.L., Kohlbrenner W.E. The mode of inhibition of oxidative phosphorylation by efrapeptin (A23871). Evidence for an alternating site mechanism for ATP synthesis. J Biol Chem. 1978;253:4865–4873. [PubMed] [Google Scholar]
- 103.Papathanassiu A.E., MacDonald N.J., Emlet D.R., Vu H.A. Antitumor activity of efrapeptins, alone or in combination with 2-deoxyglucose, in breast cancer in vitro and in vivo. Cell Stress Chaperones. 2011;16:181–193. doi: 10.1007/s12192-010-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iqbal N., Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. doi: 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu W., Mimnaugh E., Rosser M.F.N., Nicchitta C., Marcu M., Yarden Y. Sensitivity of mature ErbB2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- 106.Xu W., Mimnaugh E.G., Kim J., Trepel J.B., Neckers L.M. Hsp90, not Grp94, regulates the intracellular trafficking and stability of nascent ErbB2. Cell Stress Chaperones. 2002;7:91–96. doi: 10.1379/1466-1268(2002)007<0091:hngrti>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Citri A., Kochupurakkal B.S., Yarden Y. The achilles heel of ErbB-2/HER2: Regulation by the Hsp90 chaperone machine and potential for pharmacological intervention. Cell Cycle. 2004;3:50–60. [PubMed] [Google Scholar]
- 108.Sidera K., Gaitanou M., Stellas D., Matsas R., Patsavaoudi E. A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J Biol Chem. 2008;283:2031–2041. doi: 10.1074/jbc.M701803200. [DOI] [PubMed] [Google Scholar]
- 109.Yan Y., Su X., Liang Y., Zhang J., Shi C., Lu Y. Emodin azide methyl anthraquinone derivative triggers mitochondrial-dependent cell apoptosis involving in caspase-8-mediated Bid cleavage. Mol Cancer Therapeut. 2008;7:1688–1697. doi: 10.1158/1535-7163.MCT-07-2362. [DOI] [PubMed] [Google Scholar]
- 110.Yan Y., Zheng L., Zhang X., Chen L., Singh S., Wang F. Blockade of Her 2/neu binding to Hsp90 by emodin azide methyl anthraquinone derivative induces proteasomal degradation of Her2/neu. Mol Pharm. 2011;8:1687–1697. doi: 10.1021/mp2000499. [DOI] [PubMed] [Google Scholar]
- 111.Yan Y., Fu L., Zhang W., Ma H., Ma C., Liang Y. Emodin azide methyl anthraquinone derivative induced G0/G1 arrest in HER2/neu-overexpressing MDA-MB-453 breast cancer cells. J BUON. 2014;19:650–655. [PubMed] [Google Scholar]
- 112.James J.S. Nelfinavir (Viracept) approved: fourth protease inhibitor available. AIDS Treat News. 1997;1997:1–2. [PubMed] [Google Scholar]
- 113.Yang Y., Ikezoe T., Nishioka C., Bandobashi K., Takeuchi T., Adachi Y. NFV, an HIV-1 protease inhibitor, induces growth arrest, reduced Akt signaling, apoptosis and docetaxel sensitisation in NSCLC cell lines. Br J Cancer. 2006;95:1653–1662. doi: 10.1038/sj.bjc.6603435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang W., Mikochik P.J., Ra J.H., Lei H., Flaherty K.T., Winkler J.D. HIV protease inhibitor nelfinavir inhibits growth of human melanoma cells by induction of cell cycle arrest. Cancer Res. 2007;67:1221–1227. doi: 10.1158/0008-5472.CAN-06-3377. [DOI] [PubMed] [Google Scholar]
- 115.Shim J.S., Rao R., Beebe K., Neckers L., Han I., Nahta R. Selective inhibition of HER2-positive breast cancer cells by the HIV protease inhibitor nelfinavir. J Natl Cancer Inst. 2012;104:1576–1590. doi: 10.1093/jnci/djs396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Soprano M., Sorriento D., Rusciano M.R., Maione A.S., Limite G., Forestieri P. Oxidative stress mediate the antiproliferative effects of nelfinavir in breast cancer cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arodola O.A., Soliman M.E.S. Could the FDA-approved anti-HIV PR inhibitors be promising anticancer agents? An answer from enhanced docking approach and molecular dynamics analyses. Drug Des Dev Ther. 2015;9:6055–6065. doi: 10.2147/DDDT.S87653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Semenza G.L. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 119.Semenza G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Minet E., Mottet D., Michel G., Roland I., Raes M., Remacle J. Hypoxia-induced activation of HIF-1: Role of HIF-1α−Hsp90 interaction. FEBS Lett. 1999;460:251–256. doi: 10.1016/s0014-5793(99)01359-9. [DOI] [PubMed] [Google Scholar]
- 121.Wang H.F., Liu M., Li N., Luo T., Zheng L.P., Zeng X.H. Bisphenol A impairs mature sperm function by a CatSper-relevant mechanism. Toxicol Sci. 2016;152:145–154. doi: 10.1093/toxsci/kfw070. [DOI] [PubMed] [Google Scholar]
- 122.Kubo T., Maezawa N., Osada M., Katsumura S., Funae Y., Imaoka S. Bisphenol A, an environmental endocrine-disrupting chemical, inhibit hypoxic response via degradation of hypoxia-inducible factor 1α (HIF-1α): Structural requirement of bisphenol A for degradation of HIF-1α. Biochem Biophys Res Commun. 2004;318:1006–1011. doi: 10.1016/j.bbrc.2004.04.125. [DOI] [PubMed] [Google Scholar]
- 123.Kobayashi Y., Oguro A., Imaoka S. Bisphenol A and its derivatives induce degradation of HIF-1 alpha via the lysosomal pathway in human hepatocarcinoma cell line, Hep3B. Biol Pharm Bull. 2018;41:374–382. doi: 10.1248/bpb.b17-00693. [DOI] [PubMed] [Google Scholar]
- 124.Udeani G.O., Gerhauser C., Thomas C.F., Moon R.C., Kosmeder J.W., Kinghorn A.D. Cancer chemopreventive activity mediated by deguelin, a naturally occurring rotenoid. Cancer Res. 1997;57:3424–3428. [PubMed] [Google Scholar]
- 125.Lee H.Y. Molecular mechanisms of deguelin-induced apoptosis in transformed human bronchial epithelial cells. Biochem Pharmacol. 2004;68:1119–1124. doi: 10.1016/j.bcp.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 126.Oh S.H., Woo J.K., Yazici Y.D., Myers J.N., Kim W.Y., Jin Q. Structural basis for depletion of heat shock protein 90 client proteins by deguelin. J Natl Cancer Inst. 2007;99:949–961. doi: 10.1093/jnci/djm007. [DOI] [PubMed] [Google Scholar]
- 127.Chang D., An H., Kim K., Kim H.H., Jung J., Lee J.M. Design, synthesis, and biological evaluation of novel deguelin based heat shock protein 90 (HSP90) inhibitors targeting proliferation and angiogenesis. J Med Chem. 2012;55:10863–10884. doi: 10.1021/jm301488q. [DOI] [PubMed] [Google Scholar]
- 128.Lee H.Y., Oh S.H., Woo J.K., Kim W.Y., Van Pelt C.S., Price R.E. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J Natl Cancer Inst. 2005;97:1695–1699. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 129.Caboni P., Sherer T.B., Zhang N., Taylor G., Na H.M., Greenamyre J.T. Rotenone, deguelin, their metabolites, and the rat model of Parkinson's disease. Chem Res Toxicol. 2004;17:1540–1548. doi: 10.1021/tx049867r. [DOI] [PubMed] [Google Scholar]
- 130.Kim H.S., Hong M., Ann J., Yoon S., Nguyen C., Lee S. Synthesis and biological evaluation of C-ring truncated deguelin derivatives as heat shock protein 90 (HSP90) inhibitors. Bioorg Med Chem. 2016;24:6082–6093. doi: 10.1016/j.bmc.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 131.Kim H.S., Hoang V., Hong M., Kim K.C., Ann J., Nguyen C. Investigation of B,C-ring truncated deguelin derivatives as heat shock protein 90 (HSP90) inhibitors for use as anti-breast cancer agents. Bioorg Med Chem. 2019;27:1370–1381. doi: 10.1016/j.bmc.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 132.Yao H., Xu F., Wang G., Xie S., Li W., Yao H. Design, synthesis, and biological evaluation of truncated deguelin derivatives as Hsp90 inhibitors. Eur J Med Chem. 2019;167:485–498. doi: 10.1016/j.ejmech.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 133.Jo D.H., An H., Chang D., Baek Y., Cho C.S., Jun H.O. Hypoxia-mediated retinal neovascularization and vascular leakage in diabetic retina is suppressed by HIF-1α destabilization by SH-1242 and SH-1280, novel Hsp90 inhibitors. J Mol Med (Berl) 2014;92:1083–1092. doi: 10.1007/s00109-014-1168-8. [DOI] [PubMed] [Google Scholar]
- 134.Lee S., Min H., Choi H., Bae S.Y., Park K.H., Hyun S.Y. Deguelin analogue SH-1242 inhibits Hsp90 activity and exerts potent anticancer efficacy with limited neurotoxicity. Cancer Res. 2016;76:686–699. doi: 10.1158/0008-5472.CAN-15-1492. [DOI] [PubMed] [Google Scholar]
- 135.Jeong Y.S., Baek M., Lee S., Kim M.S., Maeng H.J., Lee J.H. Development and validation of analytical method for SH-1242 in the rat and mouse plasma by liquid chromatography/tandem mass spectroscopy. Molecules. 2020;25:531–542. doi: 10.3390/molecules25030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee S., Min H., Choi H., Kim H.S., Kim K., Park S. Synthesis and evaluation of a novel deguelin derivative, L80, which disrupts ATP binding to the C-terminal domain of heat shock protein 90. Mol Pharmacol. 2015;88:245–255. doi: 10.1124/mol.114.096883. [DOI] [PubMed] [Google Scholar]
- 137.Cho T.M., Kim J.Y., Kim Y.J., Sung D., Oh E., Jang S. C-terminal HSP90 inhibitor L80 elicits anti-metastatic effects in triple-negative breast cancer via STAT3 inhibition. Cancer Lett. 2019;447:141–153. doi: 10.1016/j.canlet.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 138.Hyun S.Y., Le H.T., Nguyen C., Yong Y., Boo H., Lee H.J. Development of a novel Hsp90 inhibitor NCT-50 as a potential anticancer agent for the treatment of non-small cell lung cancer. Sci Rep. 2018;8:13924. doi: 10.1038/s41598-018-32196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Darvill A.G., Albersheim P. Phytoalexins and their elicitors: A defense against microbial infections in plants. Annu Rev Plant Physiol. 1984;35:243–275. [Google Scholar]
- 140.Salvo V.A., Boué S.M., Fonseca J.P., Elliott S., Corbitt C., Collins-Burow B.M. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res. 2006;12:7159–7164. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 141.Burow M.E., Boue S.M., Collins-Burow B.M., Melnik L.I., Duong B.N., Carter-Wientjes C.H. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J Clin Endocrinol Metab. 2001;86:1750–1758. doi: 10.1210/jcem.86.4.7430. [DOI] [PubMed] [Google Scholar]
- 142.Lee S., Jee J., Bae J., Liu K., Lee Y.M. A group of novel HIF-1α inhibitors, glyceollins, blocks HIF-1α synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 binding. J Cell Physiol. 2014;230:853–862. doi: 10.1002/jcp.24813. [DOI] [PubMed] [Google Scholar]
- 143.Tsiftsoglou A.S., Tsamadou A.I., Papadopoulou L.C. Heme as key regulator of major mammalian cellular functions: Molecular, cellular, and pharmacological aspects. Pharmacol Ther. 2006;111:327–345. doi: 10.1016/j.pharmthera.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 144.Park J.H., Lee C.K., Hwang Y.S., Park K.K., Chung W.Y. Hemin inhibits cyclooxygenase-2 expression through nuclear factor-kappa B activation and ornithine decarboxylase expression in 12-O-tetradecanoylphorbol-13-acetate-treated mouse skin. Mutat Res. 2008;642:68–73. doi: 10.1016/j.mrfmmm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 145.Chung W.Y., Lee J.M., Lee W.Y., Surh Y.J., Park K.K. Protective effects of hemin and tetrakis(4-benzoic acid)porphyrin on bacterial mutagenesis and mouse skin carcinogenesis induced by 7,12-dimethylbenz[a]anthracene. Mutat Res. 2000;472:139–145. doi: 10.1016/s1383-5718(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 146.Lee J.M., Lee W.H., Kay H.Y., Kim E., Moon A., Kim S.G. Hemin, an iron-binding porphyrin, inhibits HIF-1α induction through its binding with heat shock protein 90. Int J Cancer. 2012;130:716–727. doi: 10.1002/ijc.26075. [DOI] [PubMed] [Google Scholar]
- 147.Blank M., Mandel M., Keisari Y., Meruelo D., Lavie G. Enhanced ubiquitinylation of heat shock protein 90 as a potential mechanism of mitotic cell death in cancer cells induced with hypericin. Cancer Res. 2003;63:8241–8247. [PubMed] [Google Scholar]
- 148.Lavie G., Mandel M., Hazan S., Barliya T., Blank M., Grunbaum A. Anti-angiogenic activities of hypericin in vivo: Potential for ophthalmologic applications. Angiogenesis. 2005;8:35–42. doi: 10.1007/s10456-005-3828-3. [DOI] [PubMed] [Google Scholar]
- 149.Blank M., Lavie G., Mandel M., Hazan S., Orenstein A., Meruelo D. Antimetastatic activity of the photodynamic agent hypericin in the dark. Int J Cancer. 2004;111:596–603. doi: 10.1002/ijc.20285. [DOI] [PubMed] [Google Scholar]
- 150.Barliya T., Mandel M., Livnat T., Weinberger D., Lavie G. Degradation of HIF-1 alpha under hypoxia combined with induction of Hsp90 polyubiquitination in cancer cells by hypericin: A unique cancer therapy. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Adri V.C., Alexander L.D., Johnson V.A., McAlpine S.R. Macrocycles that inhibit the binding between heat shock protein 90 and TPR-containing proteins. ACS Chem Biol. 2011;6:1357–1366. doi: 10.1021/cb200203m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kataria N., Martinez C., Kerr B., Zaiter S.S., Morgan M., McAlpine S.R. C-terminal HSP90 inhibitors block the HIF-1 hypoxic response by degrading HIF-1α through the oxygen-dependent degradation pathway. Cell Physiol Biochem. 2019;53:480–495. doi: 10.33594/000000152. [DOI] [PubMed] [Google Scholar]
- 153.Darakhshan S., Pour A.B., Colagar A.H., Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacol Res. 2015;95–96:138–158. doi: 10.1016/j.phrs.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 154.Imran M., Rauf A., Khan I.A., Shahbaz M., Qaisrani T.B., Fatmawati S. Thymoquinone: A novel strategy to combat cancer: A review. Biomed Pharmacother. 2018;106:390–402. doi: 10.1016/j.biopha.2018.06.159. [DOI] [PubMed] [Google Scholar]
- 155.Lee Y., Kim G., Park E., Oh T., Lee S., Kan S. Thymoquinone selectively kills hypoxic renal cancer cells by suppressing HIF-1α-mediated glycolysis. Int J Mol Sci. 2019;20:1092–1104. doi: 10.3390/ijms20051092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marks P.A. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 157.Qian D.Z., Kachhap S.K., Collis S.J., Verheul H.M., Carducci M.A., Atadja P. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1α. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 158.Hutt D.M., Roth D.M., Vignaud H., Cullin C., Bouchecareilh M. The histone deacetylase inhibitor, Vorinostat, represses hypoxia-inducible factor 1 alpha expression through translational inhibition. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kong X., Lin Z., Liang D., Fath D., Sang N., Caro J. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1α. Mol Cell Biol. 2006;26:2019–2028. doi: 10.1128/MCB.26.6.2019-2028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang C., Yang C., Feldman M.J., Wang H., Pang Y., Maggio D.M. Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha. Oncotarget. 2017;8:56110–56125. doi: 10.18632/oncotarget.18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kajander T., Sachs J.N., Goldman A., Regan L. Electrostatic interactions of Hsp-organizing protein tetratricopeptide domains with Hsp70 and Hsp90: Computational analysis and protein engineering. J Biol Chem. 2009;284:25364–25374. doi: 10.1074/jbc.M109.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Adão R., Zanphorlin L.M., Lima T.B., Sriranganadane D., Dalhström K.M., Pinheiro G.M.S. Revealing the interaction mode of the highly flexible Sorghum bicolor Hsp70/Hsp90 organizing protein (Hop): A conserved carboxylate clamp confers high affinity binding to Hsp90. J Proteomics. 2019;191:191–201. doi: 10.1016/j.jprot.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 163.Scheufler C., Brinker A., Bourenkov G., Pegoraro S., Moroder L., Bartunik H. Structure of TPR domain−peptide complexes: Critical elements in the assembly of the Hsp70‒Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- 164.Yi F., Regan L. A novel class of small molecule inhibitors of Hsp90. ACS Chem Biol. 2008;3:645–654. doi: 10.1021/cb800162x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pimienta G., Herbert K.M., Regan L. A compound that inhibits the HOP−Hsp90 complex formation and has unique killing effects in breast cancer cell lines. Mol Pharm. 2011;8:2252–2261. doi: 10.1021/mp200346y. [DOI] [PubMed] [Google Scholar]
- 166.Zhang D., Xu L., Cao F., Wei T., Yang C., Uzan G. Celastrol regulates multiple nuclear transcription factors belonging to HSP90's clients in a dose- and cell type-dependent way. Cell Stress Chaperons. 2010;15:939–946. doi: 10.1007/s12192-010-0202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Horibe T., Kohno M., Haramoto M., Ohara K., Kawakami K. Designed hybrid TPR peptide targeting Hsp90 as a novel anticancer agent. J Transl Med. 2011;9:8. doi: 10.1186/1479-5876-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gupta U.K., Mahanta S., Paul S. In silico design of small peptide-based Hsp90 inhibitor: A novel anticancer agent. Med Hypotheses. 2013;81:853–861. doi: 10.1016/j.mehy.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 169.Wang W., Liu Y., Zhao Z., Xie C., Xu Y., Hu Y. Y-632 inhibits heat shock protein 90 (Hsp90) function by disrupting the interaction between Hsp90 and Hsp70/Hsp90 organizing protein, and exerts antitumor activity in vitro and in vivo. Cancer Sci. 2016;107:782–790. doi: 10.1111/cas.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aliteri D.C. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 171.Cheung C.H., Chen H.H., Kuo C.C., Chang C.Y., Coumar M.S., Hsieh H.P. Survivin counteracts the therapeutic effect of microtubule destabilizers by stabilizing tubulin polymers. Mol Cancer. 2009;8:43. doi: 10.1186/1476-4598-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zaffroni N., Daidone M.G. Survivin expression and resistance to anticancer treatments: Perspectives for new therapeutic interventions. Drug Resist Updates. 2002;5:65–72. doi: 10.1016/s1368-7646(02)00049-3. [DOI] [PubMed] [Google Scholar]
- 173.Fortugno P., Beltrami E., Plescia J., Fontana J., Pradhan D., Marchisio P.C. Regulation of survivin function of Hsp90. Proc Natl Acad Sci U S A. 2003;100:13791–13796. doi: 10.1073/pnas.2434345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Plescia J., Salz W., Xia F., Pennati M., Zaffaroni N., Daidone M.G. Rational design of shepherdin, a novel anticancer agent. Cancer Cell. 2005;7:457–468. doi: 10.1016/j.ccr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 175.Wang J., Li Z., Lin Z., Zhao B., Wang Y., Peng R. 17-DMCHAG, a new geldanamycin derivative, inhibits prostate cancer cells through Hsp90 inhibition and survivin downregulation. Cancer Lett. 2015;362:83–96. doi: 10.1016/j.canlet.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 176.Whitesell L., Lin N.U. HSP90 as a platform for the assembly of more effective cancer chemotherapy. Biochim Biophys Acta. 2012;1823:756–766. doi: 10.1016/j.bbamcr.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 177.Doi T., Onozawa Y., Fuse N., Yoshino T., Yamazaki K., Watanabe J. Phase I dose-escalation study of the Hsp90 inhibitor AUY922 in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;74:629–636. doi: 10.1007/s00280-014-2521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu J., Sun W., Dong W., Wang Z., Qin Y., Zhang T. HSP90 inhibitor NVP-AUY922 induces cell apoptosis by disruption of the survivin in papillary thyroid carcinoma cells. Biochem Biophys Res Commun. 2017;487:313–319. doi: 10.1016/j.bbrc.2017.04.056. [DOI] [PubMed] [Google Scholar]
- 179.Kushner B.H., Cheung N.V., Modak S., Becher O.J., Basu E.M., Roberts S.S. A phase I/Ib trial targeting the Pi3k/Akt pathway using perifosine: Long-term progression-free survival of patients with resistant neuroblastoma. Int J Cancer. 2017;140:480–484. doi: 10.1002/ijc.30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bendell J.C., Nemunaitis J., Vukelja S.J., Hagenstad C., Campos L.T., Hermann R.C. Randomized placebo-controlled phase II trial of perifosine plus capecitabine as second- or third-line therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2011;29:4394–4400. doi: 10.1200/JCO.2011.36.1980. [DOI] [PubMed] [Google Scholar]
- 181.Ernst D.S., Eisenhauer E., Wainman N., Davis M., Lohmann R., Baetz T. Phase II study of perifosine in previously untreated patients with metastatic melanoma. Invest N Drugs. 2005;23:569–575. doi: 10.1007/s10637-005-1157-4. [DOI] [PubMed] [Google Scholar]
- 182.Fei H.R., Chen G., Wang J.M., Wang F.Z. Perifosine induces cell cycle arrest and apoptosis in human hepatocellular carcinoma cell lines by blockade of Akt phosphorylation. Cytotechnology. 2010;62:449–460. doi: 10.1007/s10616-010-9299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yao C., Wei J., Wang Z., Ding H., Li D., Yan S. Perifosine induces cell apoptosis in human osteosarcoma cells: New implication for osteosarcoma therapy? Cell Biochem Biophys. 2013;65:217–227. doi: 10.1007/s12013-012-9423-5. [DOI] [PubMed] [Google Scholar]
- 184.Venkatesan N., Kanwar J.R., Deepa P.R., Navaneethakrishnan S., Joseph C., Krishnakumar S. Targeting HSP90/Survivin using a cell permeable structure based peptide-mimetic shepherdin in retinoblastoma. Chem Biol Interact. 2016;252:141–149. doi: 10.1016/j.cbi.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 185.Gyurkocza B., Plescia J., Raskett C.M., Garlick D.S., Lowry P.A., Carter B.Z. Antileukemic activity of shepherdin and molecular diversity of Hsp90 inhibitors. J Natl Cancer Inst. 2006;98:1068–1077. doi: 10.1093/jnci/djj300. [DOI] [PubMed] [Google Scholar]
- 186.Zhu A., Ren Y., Wang N., Jin Q., Zhang D., Yang G. Adeno-associated virus mediated gene transfer of Shepherdin inhibits gallbladder carcinoma growth in vitro and in vivo. Gene. 2015;572:87–94. doi: 10.1016/j.gene.2015.06.080. [DOI] [PubMed] [Google Scholar]
- 187.Meli M., Pennati M., Curto M., Daidone M.G., Plescia J., Toba S. Small-molecule targeting of heat shock protein 90 chaperone function: Rational identification of a new anticancer lead. J Med Chem. 2006;49:7721–7730. doi: 10.1021/jm060836y. [DOI] [PubMed] [Google Scholar]
- 188.Tomaselli S., Meli M., Plescia J., Zetta L., Altieri D.C., Colombo G. Combined in silico and experimental approach for drug design: The binding mode of peptidic and non-peptidic inhibitors to Hsp90 N-terminal domain. Chem Biol Drug Des. 2010;76:382–391. doi: 10.1111/j.1747-0285.2010.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guzman J.R., Fukuda S., Pelus L.M. Inhibition of caspase-3 by Survivin prevents Wee 1 kinase degradation and promotes cell survival by maintaining phosphorylation of p34Cdc2. Gene Ther Mol Biol. 2009;13B:264–273. [PMC free article] [PubMed] [Google Scholar]
- 190.Shin E.J., Choi H., Sung M.J., Park J.H., Chung M., Chung S. Anti-tumour effects of beta-sitosterol are mediated by AMPK/PTEN/HSP90 axis in AGS human gastric adenocarcinoma cells and xenograft mouse models. Biochem Pharmacol. 2018;152:60–70. doi: 10.1016/j.bcp.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 191.Martinez-Yamout M.A., Venkitakrishnan R.P., Preece N.E., Kroon G., Wright P.E., Dyson H.J. Localization of sites of interaction between p23 and Hsp90 in solution. J Biol Chem. 2006;281:14457–14464. doi: 10.1074/jbc.M601759200. [DOI] [PubMed] [Google Scholar]
- 192.Cano L.Q., Lavery D.N., Sin S., Spanjaard E., Brooke G.N., Tilman J.D. The co-chaperone p23 promotes prostate cancer motility and metastasis. Mol Oncol. 2015;9:295–308. doi: 10.1016/j.molonc.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Heisy R.M. Identification of an allelopathic compound from Ailanthus altissima (Simaroubaceae) and characterization of its herbicidal activity. Am J Bot. 1996;83:192–200. [Google Scholar]
- 194.Daga M., Pizzimenti S., Dianzani C., Cucci M.A., Cavalli R., Grattarola M. Ailanthone inhibits cell growth and migration of cisplatin resistant bladder cancer cells through downregulation of Nrf 2, YAP, and c-Myc expression. Phytomedicine. 2019;56:156–164. doi: 10.1016/j.phymed.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 195.Zhang Y., Zhang C., Min D. Ailanthone up-regulates miR-449a to restrain acute myeloid leukemia cells growth, migration and invasion. Exp Mol Pathol. 2019;108:114–120. doi: 10.1016/j.yexmp.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 196.Zhuo Z., Hu J., Yang X., Chen M., Lei X., Deng L. Ailanthone inhibits Huh 7 cancer cell growth via cell cycle arrest and apoptosis in vitro and in vivo. Sci Rep. 2015;5:16185. doi: 10.1038/srep16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang R., Lu Y., Li H., Sun L., Yang N., Zhao M. Antitumor activity of the Ailanthus altissima bark phytochemical ailanthone against breast cancer MCF-7 cells. Oncol Lett. 2018;15:6022–6028. doi: 10.3892/ol.2018.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang P., Sun D., Jiang F. Ailanthone promotes human vestibular schwannoma cell apoptosis and autophagy by downregulation of miR-21. Oncol Res. 2018;26:941–948. doi: 10.3727/096504018X15149775533331. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 199.Hou S., Cheng Z., Wang W., Wang X., Wu Y. Ailanthone exerts an antitumor function on the development of human lung cancer by upregulating microRNA-195. J Cell Biochem. 2019;120:10444–10451. doi: 10.1002/jcb.28329. [DOI] [PubMed] [Google Scholar]
- 200.He Y., Peng S., Wang J., Chen H., Cong X., Chen A. Ailanthone targets p23 to overcome MDV3100 resistance in castration-resistant prostate cancer. Nat Commun. 2016;7:13122. doi: 10.1038/ncomms13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chadli A., Felts S.J., Wang Q., Sullivan W.P., Botuyan M.V., Fauq A. Celastrol inhibits Hsp90 chaperoning of steroid receptors by inducing fibrillization of the co-chaperone p23. J Biol Chem. 2010;285:4224–4231. doi: 10.1074/jbc.M109.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Abbas S., Bhoumik A., Dahl R., Vasile S., Krajewski S., Cosford N.D.P. Preclinical studies of celastrol and acetyl isogambogic acid in melanoma. Clin Cancer Res. 2007;13:6769–6778. doi: 10.1158/1078-0432.CCR-07-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen X., Bao J., Guo J., Ding Q., Lu J., Huang M. Biological activities and potential molecular targets of cucurbitacins: A focus on cancer. Anti Cancer Drugs. 2012;23:777–787. doi: 10.1097/CAD.0b013e3283541384. [DOI] [PubMed] [Google Scholar]
- 204.Hall J.A., Seedarla S., Rice N., Kopel L., Halaweish F., Blagg B.S.J. Cucurbitacin D is a disruptor of the HSP90 chaperone machinery. J Nat Prod. 2015;78:873–879. doi: 10.1021/acs.jnatprod.5b00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chan C.T., Reeves R.E., Geller R., Yaghoubi S.S., Hoehne A., Solow-Cordero D.E. Discovery and validation of small-molecule heat-shock protein 90 inhibitors through multimodality molecular imaging in living subjects. Proc Natl Acad Sci U S A. 2012;109:E2476–E2485. doi: 10.1073/pnas.1205459109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.D'Eliseo D., Di Renzo L., Santoni A., Velotti F. Docosahexaenoic acid (DHA) promotes immunogenic apoptosis in human multiple myeloma cells, induces autophagy and inhibits STAT3 in both tumor and dendritic cells. Genes Cancer. 2017;8:426–437. doi: 10.18632/genesandcancer.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.de Aguiar Pastore Silva J., de Souza Fabre M.E., Waitzberg D.L. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: A systematic review. Clin Nutr. 2015;34:359–366. doi: 10.1016/j.clnu.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 208.Mouradian M., Ma I.V., Vicente E.D., Kikawa K.D., Pardini R.S. Docosahexaenoic acid-mediated inhibition of heat shock protein 90–p23 chaperone complex and downstream client proteins in lung and breast cancer. Nutr Cancer. 2017;69:92–104. doi: 10.1080/01635581.2017.1247886. [DOI] [PubMed] [Google Scholar]
- 209.Uddin S.J., Nahar L., Shilpi J.A., Shoeb M., Borkowski T., Gibbons S. Gedunin, a limonoid from Xylocarpus granatum, inhibits the growth of CaCo-2 colon cancer cell line in vitro. Phytother Res. 2007;21:757–761. doi: 10.1002/ptr.2159. [DOI] [PubMed] [Google Scholar]
- 210.Kamath S.G., Chen N., Xiong Y., Wenham R., Apte S., Humphrey M. Gedunin, a novel natural substance, inhibits ovarian cancer cell proliferation. Int J Gynecol Cancer. 2009;19:1564–1569. doi: 10.1111/IGC.0b013e3181a83135. [DOI] [PubMed] [Google Scholar]
- 211.Patwardhan C.A., Fauq A., Peterson L.B., Miller C., Blagg B.S.J., Chadli A. Gedunin inactivates the co-chaperone p23 protein causing cancer cell death by apoptosis. J Biol Chem. 2013;288:7313–7325. doi: 10.1074/jbc.M112.427328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Brandt G.E.L., Schmidt M.D., Prisinzano T.E., Blagg B.S.J. Gedunin, a novel Hsp90 inhibitor: semisynthesis of derivatives and preliminary structure−activity relationship. J Med Chem. 2008;51:6495–6502. doi: 10.1021/jm8007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pinkerton D.M., Chow S., Eisa N.H., Kainth K., Vanden Berg T.J., Burns J.M. Synthesis of the seco-limonoid BCD ring system identifies a Hsp90 chaperon machinery (p23) inhibitor. Chemistry. 2019;25:1451–1455. doi: 10.1002/chem.201805420. [DOI] [PubMed] [Google Scholar]
- 214.Jensen M.R., Schoepfer J., Radimerski T., Massey A., Guy C.T., Brueggen J. NVP-AUY922: A small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res. 2008;10:R33. doi: 10.1186/bcr1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Menezes D.L., Taverna P., Jensen M.R., Abrams T., Stuart D., Karen G. The novel oral Hsp90 inhibitor NVP-HSP990 exhibits potent and broad-spectrum antitumor activities in vitro and in vivo. Mol Cancer Therapeut. 2012;11:730–739. doi: 10.1158/1535-7163.MCT-11-0667. [DOI] [PubMed] [Google Scholar]
- 216.McBride C.M., Levine B., Xia Y., Bellamacina C., Machajewski T., Gao Z. Design, structure−activity relationship, and in vivo characterization of the development candidate NVP-HSP990. J Med Chem. 2014;57:9124–9129. doi: 10.1021/jm501107q. [DOI] [PubMed] [Google Scholar]
- 217.Spreafico A., Delord J.P., Mattos-Arruda L., Berge Y., Rodon J., Cottura E. A first-in-human phase I, dose-escalation, multicentre study of HSP990 administered orally in adult patients with advanced solid malignancies. Br J Cancer. 2015;112:650–659. doi: 10.1038/bjc.2014.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Eachkoti R., Reddy M.V.R., Lieu Y.K., Cosenza S.C., Reddy E.P.K. Identification and characterization of a novel heat shock protein 90 inhibitor ONO4140. Eur J Cancer. 2014;50:1982–1992. doi: 10.1016/j.ejca.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xue N., Jin J., Lilu D., Yan R., Zhang S., Yu X. Antiproliferative effect of HSP90 inhibitor Y306zh against pancreatic cancer is mediated by interruption of AKT and MAPK signaling pathways. Curr Cancer Drug Targets. 2014;14:671–683. doi: 10.2174/1568009614666140908101523. [DOI] [PubMed] [Google Scholar]
- 220.Humphrey W., Dalke A., Schulten K. VMD: Visual molecular dynamics. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]