Abstract
This study examined the impacts of different fiber sources on growth, immune status and gut health in weaned piglets fed antibiotic-free diets. Sixty piglets (BW = 8.18 ± 1.35 kg) were assigned to 3 dietary treatments based on BW and gender in a randomized complete block design (5 replicates/treatment and 4 piglets [2 barrows and 2 gilts]/replicate): (1) an antibiotic-free diet (control, CON); (2) CON + 6% wheat bran (WB); (3) CON + 4% sugar beet pulp (SBP). Dietary WB supplementation tended to increase ADG compared with CON from d 1 to 14 (P = 0.051) and from d 1 to 28 (P = 0.099). Supplementation of WB increased (P < 0.05) G:F compared with CON and SBP from d 1 to 14 and from d 1 to 28. Compared with CON, the addition of WB reduced (P < 0.05) diarrhea rate from d 1 to 14 and tended (P = 0.054) to reduce diarrhea rate from d 1 to 28. The addition of WB decreased (P < 0.05) serum diamine oxidase activity on d 14, and up-regulated (P < 0.05) ileal mRNA levels of occludin on d 28 when compared with CON. Piglets fed WB showed decreased (P < 0.05) serum interleukin-6 levels compared to those fed SBP and decreased (P < 0.05) ileal interleukin-8 levels compared to those fed CON and SBP on d 28. Supplementation of WB increased (P < 0.05) serum levels of immunoglobulin A (IgA), IgG and IgM compared with SBP on d 14, and increased (P < 0.05) the levels of serum IgA and ileal sIgA compared with CON and SBP on d 28. Piglets fed WB showed an enhanced (P < 0.05) α-diversity of cecal microbiota than those fed SBP, while piglets fed SBP showed reduced (P < 0.05) α-diversity of cecal microbiota than those fed CON. Compared with CON, the addition of WB elevated (P < 0.05) the abundance of Lachnospira and cecal butyric acid level. Piglets fed WB also showed increased (P < 0.05) abundances of Lachnospira and unclassified_f_Lachnospiraceae compared with those fed SBP. Collectively, the supplementation of WB to antibiotic-free diets improved performance, immune responses, gut barrier function and microbiota compared with the CON and SBP fed piglets. Therefore, supplementing weaned piglets with WB was more effective than SBP.
Keywords: Antibiotic-free diet, Fiber source, Growth performance, Intestinal health, Weaned piglet
1. Introduction
The period around weaning is generally characterized by intestinal dysfunction with diarrhea and growth lag in weaned piglets (Pluske et al., 1997; O'Doherty et al., 2017). Supplementing subtherapeutic levels of antibiotics is a common practice to prevent diarrhea and promote growth (Cromwell, 2002). However, early-life exposure to antibiotics has adverse impacts on the development of immune system and gut microbiota, which eventually leads to elevated disease susceptibility during the growing and finishing phases (Schokker et al., 2015). Furthermore, antibiotic drug resistance due to the over use of antibiotics has become a public health concern (Phillips et al., 2004). Consequently, it is urgent that safer approaches are explored to improve growth and health in weaned piglets.
Dietary fiber is indigestible to mammalian endogenous enzymes, but can be degraded by bacteria in the hindgut, which generates short chain fatty acids (SCFA) that are beneficial to intestinal health (Grilli et al., 2016; Koh et al., 2016). According to its solubility, dietary fiber can be divided into soluble dietary fiber (SDF) and insoluble dietary fiber (IDF). SDF is rapidly fermented by bacteria into SCFA which contribute to gut microbiota, but it also increases digesta viscosity and reduces nutrient utilization (Wang et al., 2016; Dong et al., 2019). In contrast, IDF is slowly fermented and increases passage rate and fecal bulking (Molist et al., 2014). It has been shown that the effects of dietary fiber depend on its sources and physiochemical properties (Molist et al., 2014). To date, limited information is available concerning the comparative impacts of fiber sources on the performance of newly weaned piglets in the absence of antibiotics. The present experiment examined the influences of supplementing wheat bran (WB, IDF) and sugar beet pulp (SBP, SDF) to antibiotic-free diets on growth, immune responses, gut integrity and microbiota in weaned piglets.
2. Materials and methods
All animal procedures were approved by the Institutional Animal Care and Use Committee of China Agricultural University (Beijing, China).
2.1. Animals, diets and management
Sixty weaned piglets (weaned at 28 d, Duroc × Landrace × Yorkshire, BW = 8.18 ± 1.35 kg) were allocated to 3 dietary treatments based on BW and gender in a randomized complete block design (5 replicates/treatment and 4 piglets [2 barrows and 2 gilts]/replicate): (1) an antibiotic-free basal diet (control, CON); (2) basal diet + 6% WB; (3) basal diet + 4% SBP. Total dietary fiber (TDF, the sum of the dietary carbohydrates that are resistant to digestion by mammalian enzymes) content was equalized between the 2 fiber diets. The details of the nutrient composition of WB and SBP are presented in Table 1. Diets were formulated to meet or exceed the nutrient requirements for weaned piglets according to the NRC (2012) recommendations (Table 2).
Table 1.
Analyzed composition of wheat bran and sugar beet pulp (g/kg, as fed basis).
| Item | Wheat bran | Sugar beet pulp |
|---|---|---|
| Dry matter | 893.7 | 914.2 |
| Organic matter | 835.5 | 846.3 |
| Crude protein | 171.2 | 102.9 |
| Gross energy, MJ/kg | 17.01 | 15.62 |
| Neutral detergent fiber | 373.6 | 382.5 |
| Acid detergent fiber | 115.5 | 234.8 |
| Total dietary fiber | 445.7 | 616.9 |
| Soluble dietary fiber | 38.9 | 171.2 |
| Insoluble dietary fiber | 406.8 | 445.7 |
| Indispensable amino acids | ||
| Arginine | 10.2 | 6.0 |
| Histidine | 6.0 | 3.7 |
| Isoleucine | 6.1 | 4.3 |
| Leucine | 10.8 | 8.0 |
| Lysine | 8.8 | 8.3 |
| Methionine | 2.8 | 1.8 |
| Phenylalanine | 6.5 | 4.9 |
| Threonine | 7.1 | 5.4 |
| Tryptophan | 2.4 | 1.5 |
| Valine | 10.1 | 6.7 |
| Dispensable amino acids | ||
| Alanine | 11.0 | 6.1 |
| Aspartic acid | 15.2 | 8.7 |
| Cystine | 5.6 | 0.8 |
| Glutamic acid | 36.4 | 12.0 |
| Glycine | 10.9 | 4.9 |
| Proline | 4.5 | 3.8 |
| Serine | 9.1 | 5.9 |
| Tyrosine | 5.7 | 5.3 |
Table 2.
Composition of the experimental diets (g/kg, as-fed basis).
| Item | Phase 1 (d 1 to 14) |
Phase 2 (d 15 to 28) |
||||
|---|---|---|---|---|---|---|
| CON | WB | SBP | CON | WB | SBP | |
| Corn | 568.6 | 514.5 | 525.2 | 636.4 | 583.0 | 594.0 |
| Soybean meal | 160.0 | 145.0 | 160.0 | 160.0 | 145.0 | 160.0 |
| Extruded soybean | 140.0 | 140.0 | 140.0 | 100.0 | 100.0 | 100.0 |
| Whey powder | 40.0 | 40.0 | 40.0 | 30.0 | 30.0 | 30.0 |
| Fish meal | 40.0 | 40.0 | 40.0 | 30.0 | 30.0 | 30.0 |
| Wheat bran | – | 60.0 | – | – | 60.0 | – |
| Sugar beet pulp | – | – | 40.0 | – | – | 40.0 |
| Soybean oil | 15.5 | 24.5 | 19.5 | 9.7 | 18.4 | 13.3 |
| Dicalcium phosphate | 10.0 | 8.8 | 10.4 | 7.6 | 6.3 | 7.6 |
| Limestone | 8.2 | 9.0 | 7.2 | 8.6 | 9.2 | 7.5 |
| Salt | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| l-lysine HCl, 78% | 4.5 | 4.8 | 4.5 | 4.5 | 4.8 | 4.4 |
| dl-methionine, 98% | 0.9 | 1.0 | 0.9 | 0.9 | 0.9 | 0.9 |
| l-threonine, 98% | 1.5 | 1.6 | 1.5 | 1.5 | 1.6 | 1.5 |
| l-tryptophan, 98% | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Premix1 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Chromium oxide | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Calculated composition | ||||||
| DE, Kcal/kg | 3542 | 3542 | 3542 | 3490 | 3490 | 3490 |
| NE, Kal/kg | 2657 | 2655 | 2650 | 2636 | 2634 | 2629 |
| Calcium | 8.0 | 8.0 | 8.0 | 7.0 | 7.0 | 7.0 |
| Available phosphorus | 4.0 | 4.0 | 4.0 | 3.3 | 3.3 | 3.3 |
| SID lysine | 13.5 | 13.5 | 13.5 | 12.3 | 12.3 | 12.3 |
| SID methionine | 3.9 | 3.9 | 3.9 | 3.6 | 3.6 | 3.6 |
| SID threonine | 7.9 | 7.9 | 7.9 | 7.3 | 7.3 | 7.3 |
| SID tryptophan | 2.2 | 2.2 | 2.2 | 2.0 | 2.0 | 2.0 |
| Analyzed composition | ||||||
| Crude protein | 196.4 | 196.0 | 196.7 | 180.6 | 180.2 | 180.9 |
| Total dietary fiber | 85.8 | 105.9 | 105.8 | 97.1 | 117.3 | 117.2 |
| Soluble dietary fiber | 10.4 | 11.5 | 15.6 | 11.2 | 12.3 | 16.4 |
| Insoluble dietary fiber | 75.4 | 94.4 | 90.2 | 85.9 | 105.0 | 100.8 |
CON = control; WB = wheat bran; SBP = sugar beet pulp; DE = digestible energy; NE = net energy; SID = standardized ileal digestible.
Premix provided the following per kilogram of diet: 12,000 IU vitamin A, 2,500 IU vitamin D3, 30 IU vitamin E, 12 μg vitamin B12, 3 mg vitamin K, 15 mg D-pantothenic acid, 40 mg nicotinic acid, 400 mg choline, 30 mg manganese, 80 mg zinc, 90 mg iron, 10 mg copper, 0.35 mg iodine, and 0.3 mg selenium.
Piglets were raised in fully slatted floor pens (2 m × 1.2 m) equipped with a stainless feeder and a nipple drinker in a temperature-controlled nursery room. The experiment was carried out in 2 phases (phase 1: d 1 to 14 and phase 2: d 15 to 28). Feed and water were given ad libitum during the whole period.
2.2. Growth performance and diarrhea rate
Individual pig weight and feed disappearance were recorded to determine the average daily gain (ADG), average daily feed intake (ADFI) and gain to feed ratio (G:F) on d 1, 14 and 28. Fecal score was evaluated daily at 08:00 by trained observers using the system described by Ma et al. (2019). Briefly, fresh excreta were graded as follows: 1 = hard feces; 2 = slightly soft feces; 3 = soft, partially formed feces; 4 = loose, semiliquid feces; and 5 = watery, mucous-like feces. The occurrence of diarrhea was defined as maintaining fecal scores of 4 or 5 for 2 consecutive days.
| Diarrhea rate (%) = (Number of diarrheic piglets × Diarrhea days)/(Total number of piglets × Total observational days) × 100. |
2.3. Sample collection
At the end of each phase, one pig from each pen (medium BW of the pen) was chosen for blood sample collection after overnight fasting. Blood samples (5 mL) were harvested from anterior vena cava into 10 mL vacutainer tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA), and centrifuged at 3,000 × g and 4 °C for 10 min to obtain serum. Then serum samples were frozen at −80 °C until analysis.
On d 28 after blood sampling and morning feeding, the same pig per pen was euthanized. Segments (2 cm) of the ileum were taken and fixed in 4% paraformaldehyde for further analysis. Ileal mucosa was scraped with a sterile glass slide and cecal content was collected into sterile tubes. Samples of ileal mucosa and cecal content were snap-frozen in liquid nitrogen, and then stored at −80 °C until analysis.
2.4. Chemical analysis
Ingredients and diets were ground through a 1-mm screen, and then analyzed for dry matter (DM; AOAC, 2007; method 930.15), crude protein (CP; AOAC, 2007; method 976.05) and ash (AOAC, 2007; method 942.15). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined using a fiber analyzer (Ankom Technology, Macedon, NY, USA) according to the method described by Van Soest et al. (1991). The gross energy (GE) was determined using an automatic adiabatic oxygen bomb calorimeter (Parr 6300 Calorimeter, Moline, IL, USA). TDF and IDF were analyzed using AOAC (2007) methods 985.29 and 991.43, respectively. SDF was calculated as the difference between TDF and IDF. Amino acids, except methionine, tryptophan and cystine, were assayed using ion-exchange chromatography with an automatic amino acid analyzer (L-8900, Automatic Amino Acid Analyzer; Hitachi, Tokyo, Japan) after hydrolyzing with 6 mol/L HCl at 110 °C for 24 h. Cystine was determined as cysteic acid and Met as methionine sulphone after peroxidation with performic acid and pre-column derivation using phenylisothiocyanate (L-8900, Automatic Amino Acid Analyzer; Hitachi, Tokyo, Japan). Tryptophan was determined after hydrolyzing with 4 mol/L LiOH at 110 °C for 22 h using high performance liquid chromatography (Agilent1200 Series; Aligent, Santa Clara, CA, USA).
2.5. Serum parameters analysis
Serum diamine oxidase (DAO) activity and endotoxin levels were analyzed by assay kits as directed by the manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Serum D-lactate levels were measured by an ELISA kit (Beijing Luyuan Byrd Biological Technology Company, Beijing, China) following the manufacturer's protocols.
Serum levels of interleukin-1β (IL-1β), IL-6, IL-8 and tumor necrosis factor α (TNF-α) were measured by commercial ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as recommended by the manufacturer. Serum levels of immunoglobulin A (IgA), IgG and IgM were analyzed using ELISA kits (Bethyl Laboratories, Texas, USA).
2.6. Intestinal morphology analysis
Ileal samples were removed from 4% paraformaldehyde, dehydrated in graded alcohol series, and embedded in paraffin wax. Five-μm thick sections were cut by a Leica RM 2155 microtome (Leica Microsystems, Wetzlar, Germany) and placed on a slide. Then, they were stained with hematoxylin and eosin and examined by a light microscope (CK40, Olympus, Tokyo, Japan) with a computerized image system. At least 10 intact villi and crypts of each sample were determined. The villus height to crypt depth ratio was then calculated.
2.7. Quantitative RT-PCR
Total RNA was isolated from ileal mucosal samples using Trizol reagent (Invitrogen, USA). The quantity and quality of RNA were analyzed by a spectrophotometer (NanoDrop ND-1000; Thermo Fisher Scientific, USA). The OD260:OD280 ratio ranging from 1.8 to 2.0 was considered acceptable. The integrity was analyzed by 1.0% agarose gel electrophoresis. Total RNA was subsequently reverse transcribed with a PrimeScript RT Reagent kit (TaKaRa, Dalian, China) according to the manufacturer's instructions. Real-time PCR was performed using a 2720 thermal cycler (Applied Biosystems, Foster City, CA, USA) with a volume of 10 μL containing 1 μL cDNA template, 5 μL SYBR Green mix, 0.2 μL ROX Reference Dye (50 times), and 0.2 μL each of forward and reverse primers. The thermal cycling conditions were as follows: predenaturation (10 s at 95 °C); 40 cycles of amplification (5 s at 95 °C and 20 s at 60 °C); melting curve construction (60 to 99 °C with heating rate of 0.1 °C/s and fluorescence measurements). To ensure the absence of contaminations, each quantitative RT-PCR run included a negative (water) control. glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was chosen as the reference gene. The primer sequences used are shown in Table 3. The fold changes of the gene mRNA levels were analyzed by the 2–ΔΔCt method normalized to GAPDH (Livak and Schmittgen, 2001).
Table 3.
Primer sequences for real-time polymerase chain reaction.
| Gene | Primer sequence (5′ to 3′) | Size, bp |
|---|---|---|
| ZO-1 | F: TCAAGGTCTGCCGAGACAAC | 140 |
| R: ATCACAGTGTGGTAAGCGCA | ||
| Claudin-1 | F: ACAGGAGGGAAGCCATTTTCA | 82 |
| R: TTTAAGGACCGCCCTCTCCC | ||
| Occludin | F: CAGGTGCACCCTCCAGATTG | 111 |
| R: TGGACTTTCAAGAGGCCTGG | ||
| GAPDH | F: GAAGGTCGGAGTGAACGGAT | 149 |
| R: CATGGGTAGAATCATACTGGAACA |
ZO-1 = Zonula occludens-1; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
2.8. Ileal cytokines and sIgA analysis
Ileal levels of cytokines (IL-1β, IL-6, IL-8 and TNF-α) and secretory immunoglobulin A (sIgA) were determined by ELISA kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) in accordance with the manufacturer's instructions. The protein concentration of ileal mucosal samples was measured by the bicinchoninic acid assay (Beyotime Biotechnology, Shanghai, China). Ileal mucosal levels of cytokines and sIgA were normalized to the protein concentration of each sample.
2.9. Cecal microbiota analysis
Total genomic DNA was extracted from cecal content samples by a Stool DNA Kit (Omega Bio-TEK, Norcross, GA, USA) as directed by the manufacturer. The sequencing process was conducted as previously described by Shang et al. (2019). Demultiplexing and quality-filtering of raw sequences were performed by QIIME (version 1.17). Then the remaining high-quality sequences were clustered into operational taxonomic units (OTU) at 97% similarity by UPARSE, and chimeric sequences were identified and removed by UCHIME. Each 16S rRNA gene sequence was taxonomically allocated on the basis of the silva (SSU128) 16S rRNA database by RDP Classifier (http://rdp.cme.msu.edu/) with a confidence threshold of 70%.
2.10. Cecal SCFA analysis
Cecal levels of SCFA were analyzed by a Dionex ICS-3000 Ion Chromatography system (Dionex, USA) as previously described (Shang et al., 2019). Samples of approximately 0.5 g were suspended in 8 mL ultrapure water, homogenized and centrifuged at 12,000 × g for 10 min to obtain the supernatant. The supernatant was then diluted 50 times and analyzed by a Dionex ICS-3000 Ion Chromatography system (Dionex, Sunnyvale, CA, USA). Cecal levels of SCFA were presented as mg/g sample weight.
2.11. Statistical analysis
Statistical analysis was performed using SAS 9.2 software (SAS Inst. Inc., Cary, NC, USA) with the pen or piglet as an experimental unit. Before analysis, all data were tested for normality using the Shapiro–Wilk test. Data that showed a non-normal distribution (Serum concentrations of TNF-α and IgA, ileal concentration of TNF-α and relative abundance of cecal microbial community) were analyzed by Kruskal–Wallis test. Other data that followed a normal distribution were analyzed with GLM procedures followed by Tukey's tests. Differences in diarrhea rate were tested by the χ2 contingency test. P < 0.05 was deemed as significant, and 0.05 < P < 0.10 was deemed as a tendency.
3. Results
3.1. Chemical composition of WB and SBP
There were no differences in the concentrations of DM and OM between WB and SBP (Table 1). However, the concentration of CP was greater in WB than in SBP. The concentration of NDF in WB was similar to that in SBP, whereas SBP contained more ADF and TDF than WB. The dietary fiber in WB was primarily composed of IDF, which accounted for about 91 percent of TDF. The concentration of SDF in SBP was about 4.4 times greater than that in WB, which indicates that SBP is a good source of SDF relative to WB.
3.2. Growth performance and diarrhea rate
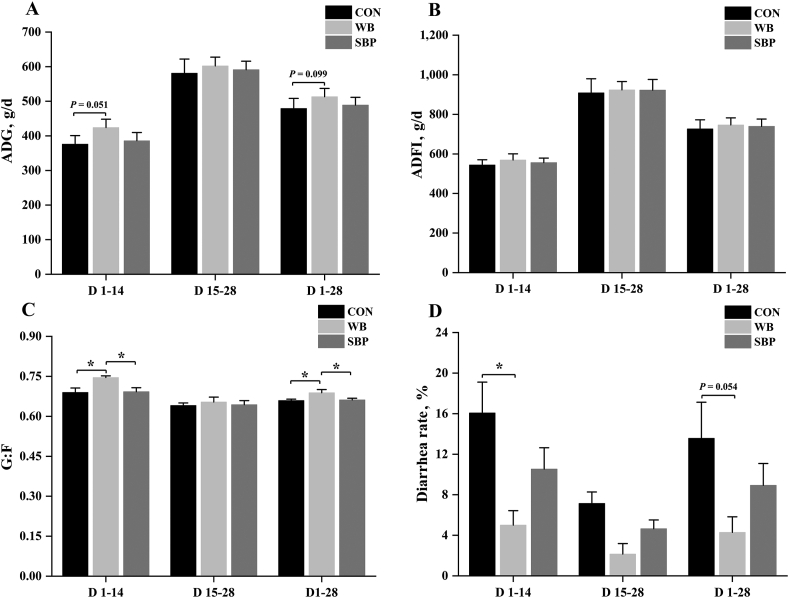
Dietary WB supplementation tended to increase ADG compared with CON during phase 1 (P = 0.051) and the whole experimental period (P = 0.099) (Fig. 1A). However, no significant differences were detected in ADFI among treatments (Fig. 1B). Inclusion of WB increased (P < 0.05) G:F compared with CON and SBP during phase 1 and the whole experimental period (Fig. 1C). In addition, compared with CON, the WB supplementation reduced (P < 0.05) the diarrhea rate during phase 1 and tended (P = 0.054) to decrease the diarrhea rate during the whole experimental period (Fig. 1D).
Fig. 1.
Effect of fiber sources on growth performance and diarrhea rate in weaned piglets: (A) Average daily gain (ADG), (B) Average daily feed intake (ADFI), (C) Gain:feed (G:F), (D) Diarrhea rate. CON, an antibiotic-free diet; WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp. Data are presented as means ± SEM, n = 5. Statistical trend at 0.05 ≤ P < 0.1 and ∗P < 0.05.
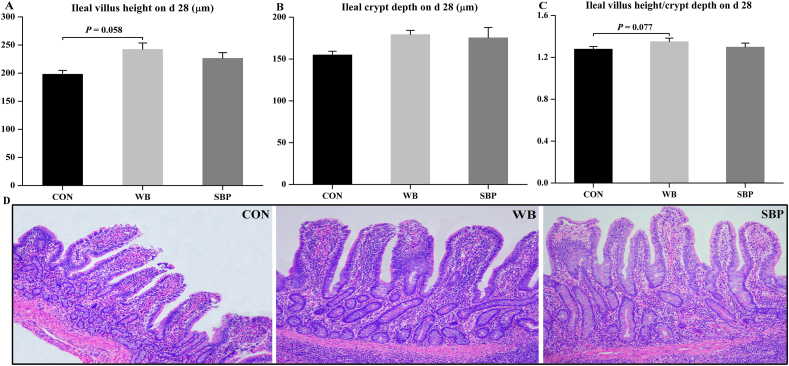
3.3. Ileal morphology
Dietary WB supplementation tended to increase ileal villus height (P = 0.058) and villus height/crypt depth (P = 0.077) compared with CON (Fig. 2A and C). No difference in ileal crypt depth was observed among the three dietary treatments (Fig. 2B).
Fig. 2.
Effect of fiber sources on ileal morphology in weaned piglets: (A) Ileal villus height on d 28, (B) Ileal crypt depth on d 28, (C) Ileal villus height/crypt depth on d 28, (D) hematoxylin-eosin staining of ileum morphology (40× ). CON, an antibiotic-free diet; WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp. Data are presented as means ± SEM, n = 5. Statistical trend at 0.05 ≤ P < 0.1.
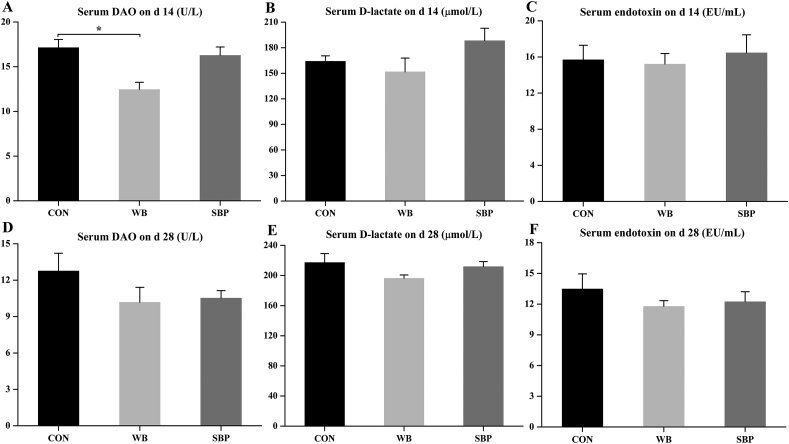
3.4. Serum DAO, D-lactate and endotoxin
Piglets fed WB showed lower (P < 0.05) serum DAO levels when compared with those fed CON on d 14 (Fig. 3A). No differences were observed on d 28 (Fig. 3D). No significant differences were found in serum levels of D-lactate and endotoxin on d 14 and d 28 (Fig. 3B, C, E and F).
Fig. 3.
Effect of fiber sources on serum levels of DAO, D-lactate and endotoxin in weaned piglets: (A to C) Serum levels of DAO, D-lactate and endotoxin on d 14, (D to F) Serum levels of DAO, D-lactate and endotoxin on d 28. CON, an antibiotic-free diet; WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp. DAO = diamine oxidase. Data are presented as means ± SEM, n = 5. ∗P < 0.05.
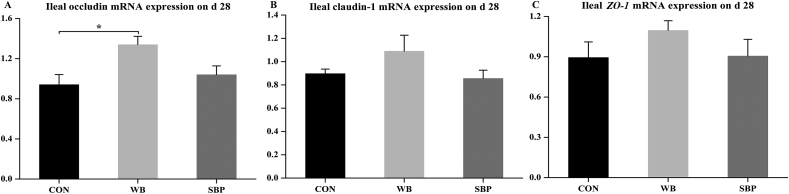
3.5. Ileal mRNA levels of tight junction proteins
Piglets fed WB had greater (P < 0.05) mRNA levels of occludin than those fed CON (Fig. 4A). However, dietary treatments did not influence ileal mRNA levels of claudin-1 or zonula occludens-1 (ZO-1; Fig. 4B and C).
Fig. 4.
Effect of fiber sources on ileal mRNA levels of tight junction proteins in weaned piglets: (A) Ileal occludin mRNA levels on d 28, (B) Ileal claudin-1 mRNA levels on d 28, (C) Ileal ZO-1 mRNA levels on d 28. CON, an antibiotic-free diet; WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp. ZO-1 = Zonula occludens-1. Data are presented as means ± SEM, n = 5. Statistical trend at 0.05 ≤ P < 0.1 and ∗P < 0.05.
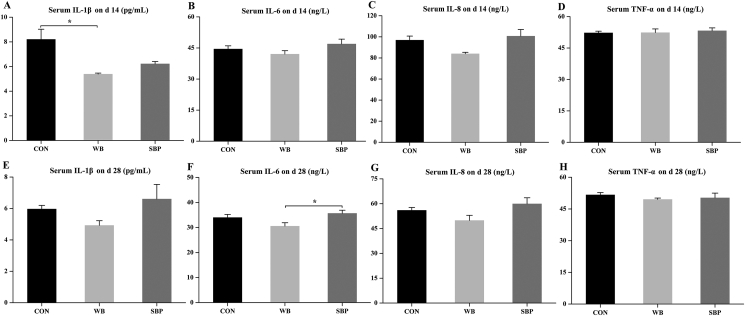
3.6. Serum inflammatory cytokines
On d 14, dietary WB supplementation reduced (P < 0.05) the serum IL-1β level compared with CON (Fig. 5A), but had no effect on serum IL-6, IL-8 and TNF-α levels (Fig. 5B–D). On d 28, piglets fed WB had a lower (P < 0.05) serum IL-6 level when compared with those fed SBP (Fig. 5E). Dietary treatments did not affect serum IL-1β, IL-8 and TNF-α levels on d 28 (Fig. 5F–H).
Fig. 5.
Effect of fiber sources on serum inflammatory cytokines in weaned piglets: (A to D) Serum levels of IL-1β, IL-6, IL-8 and TNF-α on d 14, (E to H) Serum levels of IL-1β, IL-6, IL-8 and TNF-α on d 28. CON, an antibiotic-free diet; WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp. IL = interleukin; TNF = tumor necrosis factor. Data are presented as means ± SEM, n = 5. ∗P < 0.05.
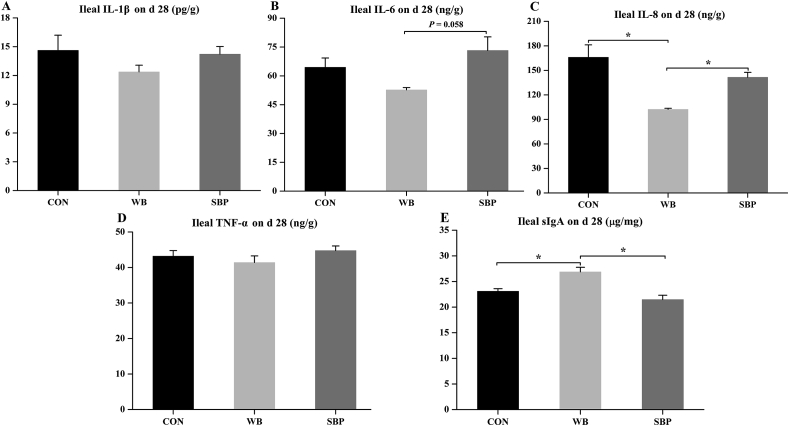
3.7. Ileal mucosal inflammatory cytokines and sIgA
Ileal levels of IL-1β and TNF-α were not altered by dietary treatments (Fig. 6A and D). However, dietary WB supplementation tended (P = 0.058) to decrease IL-6 level compared with SBP (Fig. 6B). Moreover, piglets fed WB had a lower (P < 0.05) ileal IL-8 level and a greater (P < 0.05) ileal sIgA level compared with those fed the other 2 diets (Fig. 6C and E).
Fig. 6.
Effect of fiber sources on ileal inflammatory cytokines and sIgA in weaned piglets: (A to E) Ileal levels of IL-1β, IL-6, IL-8, TNF-α and sIgA on d 28. CON, an antibiotic-free diet; WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp. IL = interleukin; TNF = tumor necrosis factor; sIgA = secretory immunoglobulin A. Data are presented as means ± SEM, n = 5. Statistical trend at 0.05 ≤ P < 0.1; ∗P < 0.05.
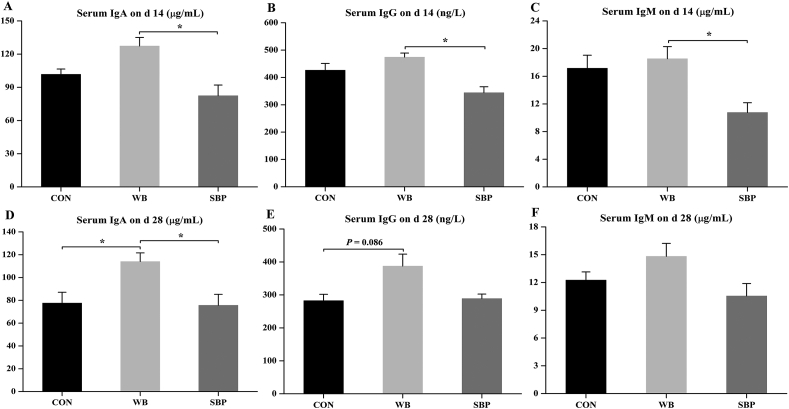
3.8. Serum immunoglobulins
Piglets fed WB had greater (P < 0.05) serum levels of IgA, IgG and IgM compared with those fed SBP on d 14 (Fig. 7A–C). In addition, inclusion of WB increased (P < 0.05) the serum IgA level compared with the other 2 diets on d 28 (Fig. 7D). Moreover, there was a tendency (P = 0.086) for an increased serum IgG level in WB than that in CON on d 28 (Fig. 7E). However, no difference was found in the serum IgM level among treatments on d 28 (Fig. 7F).
Fig. 7.
Effect of fiber sources on serum immunoglobulins in weaned piglets: (A to C) Serum levels of IgA, IgG and IgM on d 14, (D to F) Serum levels of IgA, IgG and IgM on d 28. CON, an antibiotic-free diet; WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp; Ig = immunoglobulin; Data are presented as means ± SEM, n = 5. Statistical trend at 0.05 ≤ P < 0.1 and ∗P < 0.05.
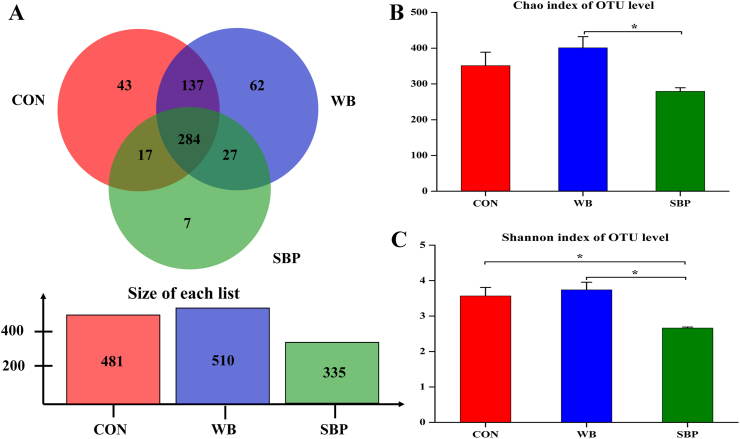
3.9. Cecal microbiota
As shown in Fig. 8A, there were 481, 510 and 335 OTU generated from piglets in CON, WB and SBP. The Venn analysis identified 284 shared OTU among the three treatments and 43, 62 and 7 unique OTU in CON, WB and SBP, respectively. Piglets fed WB showed enhanced (P < 0.05) α-diversity than those fed SBP, as indicated by increased Chao and Shannon indexes (Fig. 8B and C). Moreover, the inclusion of SBP reduced (P < 0.05) α-diversity compared with those fed CON, as indicated by a decreased Shannon index (Fig. 8C).
Fig. 8.
Venn diagram and α-diversity of cecal microbial community in weaned piglets: (A) Venn diagram, (B) Chao index, (C) Shannon index. CON, an antibiotic-free diet. WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp. OTU = operational taxonomic units. Data are presented as means ± SEM, n = 4. ∗P < 0.05.
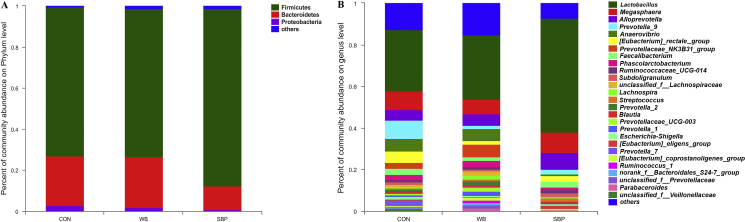
At the phylum level, Firmicutes and Bacteroidetes were the 2 main phyla of bacteria in all groups, which accounted for more than 96% (Fig. 9A). At the genus level, Lactobacillus, Megasphaera, Alloprevotella, Prevotella_9, Anaerovibrio [Eubacterium]_rectale_group, Prevotellacese_NK3B31_group, Faecalibacterium, Phascolarctobacterium and Ruminococcacese_UCG_014 were predominant (Fig. 9B).
Fig. 9.
Effect of fiber sources on cecal microbial composition in weaned piglets: (A) Cecal microbial composition at the phylum level, (B) Cecal microbial composition at the genus level. CON, an antibiotic-free diet; WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp. n = 4.
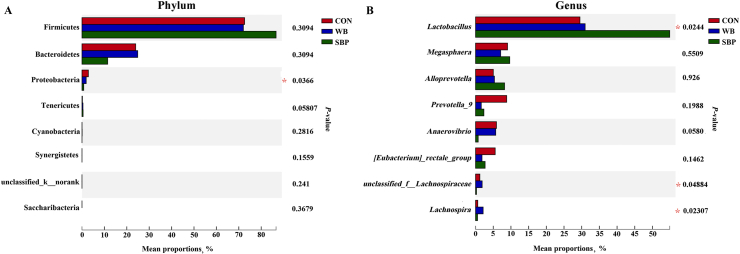
Differential analysis of cecal microbial community in weaned piglets are shown in Fig. 10. At the phylum level, piglets in SBP showed a lower (P < 0.05) abundance of Proteobacteria than those in CON (Fig. 10A). At the genus level, the inclusion of WB significantly increased (P < 0.05) the abundance of Lachnospira compared with CON (Fig. 10B). Meanwhile, the abundances of Lactobacillus were enriched (P < 0.05) by SBP supplementation compared with CON. Piglets in WB had greater (P < 0.05) abundances of Lachnospira and unclassified_f_Lachnospiraceae, but a decreased (P < 0.05) abundance of Lactobacillus compared to those fed SBP.
Fig. 10.
Differential analysis of cecal microbial community in weaned piglets: (A) Differential analysis at the phylum level, (B) Differential analysis at the genus level. CON, an antibiotic-free diet; WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp. n = 4. ∗P < 0.05.
3.10. Cecal short chain fatty acids
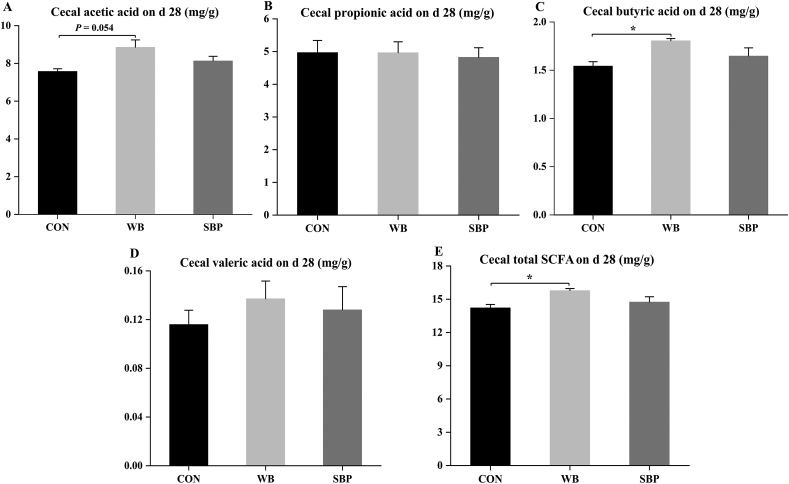
Piglets in WB tended (P = 0.054) to have greater cecal acetic acid. They also had greater (P < 0.05) cecal butyric acid and total SCFA than those in CON on d 28 (Fig. 11). There was no significant difference in cecal propionic acid and valeric acid levels among treatments.
Fig. 11.
Effects of fiber sources on cecal short chain fatty acids (SCFA) in weaned piglets: (A to E) Cecal levels of acetic acid, propionic acid, butyric acid, valeric acid and total SCFA on d 28. CON, an antibiotic-free diet; WB, CON +6% wheat bran; SBP, CON +4% sugar beet pulp; SCFA, short chain fatty acids; Data are presented as means ± SEM, n = 4. Statistical trend at 0.05 ≤ P < 0.1 and ∗P < 0.05.
4. Discussion
In this study, the WB supplementation tended to increase ADG during phase 1 and the whole experimental period compared with CON, whereas the SBP supplementation did not. Furthermore, the WB supplementation increased G:F during phase 1 and the whole experimental period compared with CON and SBP. These results indicated that the impact of dietary fiber depends on its source, and WB seemed to be more effective than SBP in improving growth performance. This is supported by Hedemann et al. (2006), who demonstrated that piglets fed soluble fiber (pectin) showed lower ADG and G:F compared with piglets fed insoluble fiber (barley hulls). Our results were also consistent with previous studies reporting that the inclusion of WB increased piglet ADG (Schedle et al., 2008). In contrast, supplementation of SBP decreased piglet ADG (Wang et al., 2016). The possible reason is that soluble fiber (mainly pentin) from SBP could increase digesta viscosity and reduce nutrient utilization, thereby impairing growth performance (Flis et al., 2017). This study also found that although the WB supplementation improved growth performance during the first 2 wk (d 1 to 14) after weaning, these significant effects were no longer observed during the second 2 wk (d 15 to 28). This suggests that the effects of dietary fiber depend not only on fiber source, but also the physiological status of the pig (Jarrett and Ashworth, 2018).
Post-weaning diarrhea is considered to be the major reason for reduced growth performance during the weaning period (Heo et al., 2015). Our results showed that piglets fed WB had the lowest diarrhea rate from d 1 to 14, which may explain the improved growth performance. Similarly, Chen et al. (2020) demonstrated that insoluble fiber (lignocellulose) decreased the diarrhea rate during the first 2 weeks post-weaning, while soluble fiber (pectin) did not. The lower diarrhea rate in piglets fed WB may be associated with the improved intestinal function due to WB supplementation. Another possibility was that WB could block Escherichia coli attachment to the intestinal mucosa (González-Ortiz et al., 2014). Previous studies regarding the effects of dietary fiber on diarrhea rate have been inconsistent. For example, Mateos et al. (2006) reported that the inclusion of oat hulls decreased diarrhea rate of piglets, while Weber et al. (2008) showed that feeding DDGS increased the expression of proinflammatory cytokines in intestinal tissue, which may induce diarrhea rate. Berrocoso et al. (2015) observed that the inclusion of fiber (wheat middlings, straw, soybean hulls or SBP) increased post-weaning diarrhea in piglets reared under optimal hygienic conditions, however, when the pigs were reared under poor hygienic conditions, no negative effects of supplemental fiber on growth performance were detected. Discrepancies for the inconsistent results may be attributed to the sources and levels of additional fiber and experimental conditions (Pascoal et al., 2012).
The changes in growth performance and diarrhea rate may be associated with changes in the piglet's intestinal function. Therefore, we further evaluated the effects of dietary fiber on intestinal function to explore the underlying mechanisms.
Weaning stress generally induces marked changes in intestinal structure, such as villus atrophy and crypt hyperplasia, and these changes in turn result in diarrhea and growth lag in weaned piglets (Pluske et al., 1997). In this study, dietary WB supplementation tended to increase villus height and the villus height to crypt depth ratio in the ileum compared with CON, which was indicative of better function and maturity of the intestine. The increased villus height induced by WB supplementation could increase the surface area for the absorption of nutrients, thereby increasing growth rate in piglets. Similarly, Hedemann et al. (2006) demonstrated that piglets offered high insoluble fiber diets showed improved intestinal morphology than those offered pectin-containing diets. The possible reason is that soluble fiber could increase digesta viscosity, which in turn increases the rate of villus cell losses, leading to villus atrophy (Montagne et al., 2003).
The intestinal epithelial barrier includes a lining of enterocytes and intercellular multiprotein complexes (such as ZO-1, claudin and occludin), which plays a crucial role in preventing pathogenic bacteria, antigens and toxins from entering into the systemic circulation via the gut lumen(Chen et al., 2018; Li et al., 2019). The DAO, D-lactate and endotoxins in serum are useful markers of intestinal permeability (Xiong et al., 2019). The present study showed that WB supplementation reduced the serum DAO activity compared with CON on d 14, suggesting decreased intestinal permeability and enhanced intestinal barrier function. Our results regarding the mRNA levels of tight junction proteins demonstrated that the inclusion of WB enhanced ileal mRNA levels of occludin, which, once again, indicate improved intestinal barrier function. Taken together, these results suggested that the inclusion of WB could relieve the gut barrier dysfunction that results from weaning stress. The improved gut barrier function may be attributed to the modulation of the gut microbiota and its metabolites SCFA by WB (Desai et al., 2016). The enhanced barrier function may therefore prevent the invasion of pathogenic bacteria and antigens, thereby decreasing the diarrhea rate and improving growth in weaned piglets. Similarly, Chen et al. (2013) demonstrated that WB fiber up-regulated ileal mRNA levels of occludin and ZO-1 in weaned piglets, while maize fiber, pea fiber and soybean fiber had no effects, which indicates that fiber sources could affect gut barrier function.
It has been shown that dietary fiber has immunomodulatory properties (Trachsel et al., 2019). The current study demonstrated that the inclusion of WB reduced serum IL-1β levels compared with CON on d 14, and decreased serum IL-6 levels compared with SBP on d 28. The results from ileal tissue also showed that piglets in WB had the lowest serum IL-8 level. These inflammatory cytokines have been previously documented as disrupting intestinal barrier function (Bruewer et al., 2003). Therefore, the decreased inflammatory cytokines in piglets fed WB may partly contribute to the improved gut barrier function. Additionally, our results demonstrated that dietary WB supplementation increased serum IgA, IgG and IgM levels, compared with SBP on d 14, and increased the ileal sIgA level compared with the other 2 treatments. Immunoglobulins are crucial parts of the humoral immunity, and they also have an impact on immune regulation (Berkman et al., 1988). Secretory IgA is the predominant antibody in mucosal secretions and is essential in preventing pathogens from adhering to epithelial mucosa (Molnar et al., 2018). Increased secretion of immunoglobulins can, in turn, reinforce the mucosal barrier on the extraepithelial side, which may be one reason that explains the enhanced gut barrier function observed in pigs offered WB (Sánchez de Medina et al., 2014). One possible reason for the beneficial impacts of WB on immune responses is that WB derived arabinoxylans could directly increase the activation potential of immune cells and enhance the humoral and cell-mediated immunity (Mendis et al., 2016). Another possible reason is due to the indirect effects of WB on gut microbiota and their metabolites (Holscher, 2017). These findings, when taken together, indicated that the inclusion of WB could enhance the immune response of piglets by modulating the production of cytokines and antibodies.
The intestinal microbiota is a complicated ecosystem that has a significant impact on gastrointestinal function, immune function, and health of the host (Xiong et al., 2019). Alpha-diversity is defined as the diversity of organisms within one site or one sample. Generally, a high α-diversity is considered to be beneficial for the maintenance of gut immune homeostasis (Ciocan et al., 2018). Our results showed that the inclusion of WB enhanced α-diversity, as indicated by the increased Chao and Shannon indexes, may contribute towards maintaining gut immune homeostasis in piglets. Meanwhile, the relative abundance of unclassified_f_Lachnospiraceae, and Lachnospira significantly increased in piglets fed WB compared with SBP. Lachnospiraceae is shown to be a butyrate producing bacteria with various effects on host energy regulation and barrier function (Lin et al., 2018). Lachnospira is a genus of anaerobic polysaccharide degrading bacteria, which can help to maintain gut homeostasis and epithelial integrity due to its ability to synthesize butyrate (Vital et al., 2014). The present study showed that cecal level of butyric acid was increased by WB supplementation when compared with CON, which was consistent with the increased abundances of unclassified_f_Lachnospiraceae and Lachnospira. Butyric acid has been shown to possess various beneficial properties, including promotion of intestinal development, reinforcement of barrier function, alleviation of inflammatory responses and regulation of intestinal microbiota (Bedford and Gong, 2018). As a consequence, the improved microbial composition and subsequent increased butyric acid production may partly explain the improved immune responses and enhanced barrier function in piglets fed WB. Taken together, these results showed that the supplementation of WB could improve gut microbiota by altering gut microbial diversity and composition, as well as their metabolites. Another finding of our study was that supplementation of SBP enriched the abundances of Lactobacillus. It is well known that Lactobacillus is a kind of probiotic bacteria with beneficial roles in protecting against pathogens, maintaining gut barrier function and reducing diarrhea (Sun and Kim, 2019). However, this study failed to observe the positive effects of SBP on gut immunity and barrier function as well as diarrhea rate, which indicates its mode of action may not only be through the modulation of gut microbiota. Pectin, the main component of SBP, has been shown to increase digesta viscosity, but impair intestinal architecture and the mucus layer in young piglets, which may be a reason for the undesirable effects of SBP (Hedemann et al., 2006; Święch et al., 2012). Unexpectedly, the alternation in microbial composition induced by SBP had no effects on the concentrations of SCFA. We speculated that SBP could increase digesta viscosity and allow more nutrients (such as protein) to become available to gut microbiota, which may interfere with fiber fermentation and therefore SCFA production. However, the exact mechanism behind this process needs further investigation.
5. Conclusion
In conclusion, this study found that the supplementation of WB to antibiotic-free diets improved performance, immune responses, gut barrier function and microbiota compared with the CON and SBP fed piglets. Therefore, supplementing weaned piglets with WB was more effective than SBP.
Author contributions
Qinghui Shang: Conceptualization, Validation, Formal analysis, Investigation, Writing-Original Draft. Hansuo Liu: Investigation, Data Curation. Di Wu: Investigation, Visualization. Shad Mahfuz: Writing-Reviewing and Editing. Xiangshu Piao: Supervision, Funding acquisition.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (31772612) and the Beijing Municipal Natural Science Foundation (6202019).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- AOAC . 18th ed. Association of Official Analytical Chemists; Arlington, VA: 2007. Official methods of analysis. [Google Scholar]
- Bedford A., Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim Nutr. 2018;4:151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman S.A., Lee M.L., Gale R.P. Clinical uses of intravenous immunoglobulins. Semin Hematol. 1988;25:140–158. [PubMed] [Google Scholar]
- Berrocoso J.D., Menoyo D., Guzman P., Saldana B., Camara L., Mateos G.G. Effects of fiber inclusion on growth performance and nutrient digestibility of piglets reared under optimal or poor hygienic conditions. J Anim Sci. 2015;93:3919–3931. doi: 10.2527/jas.2015-9137. [DOI] [PubMed] [Google Scholar]
- Bruewer M., Luegering A., Kucharzik T., Parkos C.A., Madara J.L., Hopkins A.M., Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- Chen H., Mao X., He J., Yu B., Huang Z., Yu J., Zheng P., Chen D. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br J Nutr. 2013;110:1837–1848. doi: 10.1017/S0007114513001293. [DOI] [PubMed] [Google Scholar]
- Chen J., Yu B., Chen D., Huang Z., Mao X., Zheng P., Yu J., Luo J., He J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J Nutr Biochem. 2018;59:84–92. doi: 10.1016/j.jnutbio.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Chen T., Chen D., Tian G., Zheng P., Mao X., Yu J., He J., Huang Z., Luo Y., Luo J., Yu B. Effects of soluble and insoluble dietary fiber supplementation on growth performance, nutrient digestibility, intestinal microbe and barrier function in weaning piglet. Anim Feed Sci Technol. 2020;260:114335. [Google Scholar]
- Ciocan D., Rebours V., Voican C.S., Wrzosek L., Puchois V., Cassard A., Perlemuter G. Characterization of intestinal microbiota in alcoholic patients with and without alcoholic hepatitis or chronic alcoholic pancreatitis. Sci Rep-Uk. 2018;8:1–12. doi: 10.1038/s41598-018-23146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell G.L. Why and how antibiotics are used in swine production. Anim Biotechnol. 2002;13:7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., Pudlo N.A., Kitamoto S., Terrapon N., Muller A. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Chen L., Gutin B., Zhu H. Total, insoluble, and soluble dietary fiber intake and insulin resistance and blood pressure in adolescents. Eur J Clin Nutr. 2019;73:1172–1178. doi: 10.1038/s41430-018-0372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flis M., Sobotka W., Antoszkiewicz Z. Fiber substrates in the nutrition of weaned piglets-a review. Ann Anim Sci. 2017;17:627–644. [Google Scholar]
- González-Ortiz G., Pérez J.F., Hermes R.G., Molist F., Jiménez-Díaz R., Martín-Orúe S.M. Screening the ability of natural feed ingredients to interfere with the adherence of enterotoxigenic Escherichia coli (ETEC) K88 to the porcine intestinal mucus. Br J Nutr. 2014;111:633–642. doi: 10.1017/S0007114513003024. [DOI] [PubMed] [Google Scholar]
- Grilli E., Tugnoli B., Foerster C.J., Piva A. Butyrate modulates inflammatory cytokines and tight junctions components along the gut of weaned pigs. J Anim Sci. 2016;94:433–436. [Google Scholar]
- Hedemann M.S., Eskildsen M., Lærke H.N., Pedersen C., Lindberg J.E., Laurinen P., Knudsen K.E. Intestinal morphology and enzymatic activity in newly weaned pigs fed contrasting fiber concentrations and fiber properties. J Anim Sci. 2006;84:1375–1386. doi: 10.2527/2006.8461375x. [DOI] [PubMed] [Google Scholar]
- Heo J.M., Kim J.C., Yoo J., Pluske J.R. A between-experiment analysis of relationships linking dietary protein intake and post-weaning diarrhea in weanling pigs under conditions of experimental infection with an enterotoxigenic strain of Escherichia coli. Anim Sci J. 2015;86:286–293. doi: 10.1111/asj.12275. [DOI] [PubMed] [Google Scholar]
- Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microb. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett S., Ashworth C.J. The role of dietary fibre in pig production, with a particular emphasis on reproduction. J Anim Sci Biotechnol. 2018;9:1–11. doi: 10.1186/s40104-018-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., B Ckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Li R., Hou G., Jiang X., Song Z., Fan Z., Hou D., He X. Different dietary protein sources in low protein diets regulate colonic microbiota and barrier function in a piglet model. Food Funct. 2019;10:6417–6428. doi: 10.1039/c9fo01154d. [DOI] [PubMed] [Google Scholar]
- Lin Z., Ye W., Zu X., Xie H., Li H., Li Y., Zhang W. Integrative metabolic and microbial profiling on patients with Spleen-yang-deficiency syndrome. Sci Rep-Uk. 2018;8:1–11. doi: 10.1038/s41598-018-24130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma X.K., Shang Q.H., Wang Q.Q., Hu J.X., Piao X.S. Comparative effects of enzymolytic soybean meal and antibiotics in diets on growth performance, antioxidant capacity, immunity, and intestinal barrier function in weaned pigs. Anim Feed Sci Technol. 2019;248:47–58. [Google Scholar]
- Mateos G.G., Martin F., Latorre M.A., Vicente B., Lazaro R. Inclusion of oat hulls in diets for young pigs based on cooked maize or cooked rice. Anim Sci. 2006;82:57–63. [Google Scholar]
- Mendis M., Leclerc E., Simsek S. Arabinoxylans, gut microbiota and immunity. Carbohydr Polym. 2016;139:159–166. doi: 10.1016/j.carbpol.2015.11.068. [DOI] [PubMed] [Google Scholar]
- Molist F., Van Oostrum M., Pérez J.F., Mateos G.G., Nyachoti C.M., Van Der Aar P.J. Relevance of functional properties of dietary fibre in diets for weanling pigs. Anim Feed Sci Technol. 2014;189:1–10. [Google Scholar]
- Molnar D.S., Granger D.A., Shisler S., Eiden R.D. Prenatal and postnatal cigarette and cannabis exposure: effects on secretory immunoglobulin A in early childhood. Neurotoxicol Teratol. 2018;67:31–36. doi: 10.1016/j.ntt.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. 2003;108:95–117. [Google Scholar]
- NRC . 11th ed. National Academy Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- O'Doherty J.V., Bouwhuis M.A., Sweeney T. Novel marine polysaccharides and maternal nutrition to stimulate gut health and performance in post-weaned pigs. Anim Prod Sci. 2017;57:2376. [Google Scholar]
- Pascoal L.A.F., Thomaz M.C., Watanabe P.H., Ruiz U.D.S., Ezequiel J.M.B., Amorim A.B., Daniel E., Masson G.C.I. Fiber sources in diets for newly weaned piglets. Rev Bras Zootec. 2012;41:636–642. [Google Scholar]
- Phillips I., Casewell M., Cox T., De Groot B., Friis C., Jones R., Nightingale C., Preston R., Waddell J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J Antimicrob Chemother. 2004;53:28–52. doi: 10.1093/jac/dkg483. [DOI] [PubMed] [Google Scholar]
- Pluske J.R., Hampson D.J., Williams I.H. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 1997;51:215–236. [Google Scholar]
- Sánchez de Medina F., Romero-Calvo I., Mascaraque C., Martínez-Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm Bowel Dis. 2014;20:2394–2404. doi: 10.1097/MIB.0000000000000204. [DOI] [PubMed] [Google Scholar]
- Schedle K., Plitzner C., Ettle T., Zhao L., Domig K.J., Windisch W. Effects of insoluble dietary fibre differing in lignin on performance, gut microbiology, and digestibility in weanling piglets. Arch Anim Nutr. 2008;62:141–151. doi: 10.1080/17450390801892617. [DOI] [PubMed] [Google Scholar]
- Schokker D., Zhang J., Vastenhouw S.A., Heilig H.G., Smidt H., Rebel J.M., Smits M.A. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PloS One. 2015;10 doi: 10.1371/journal.pone.0116523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Q., Liu H., Liu S., He T., Piao X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets. J Anim Sci. 2019;97:4922–4933. doi: 10.1093/jas/skz278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.Y., Kim I.H. Evaluation of an emulsifier blend on growth performance, nutrient digestibility, blood lipid profiles, and fecal microbial in growing pigs fed low energy density diet. Livest Sci. 2019;227:55–59. [Google Scholar]
- Święch E., Tuśnio A., Taciak M., Boryczka M., Buraczewska L. The effects of pectin and rye on amino acid ileal digestibility, threonine metabolism, nitrogen retention, and morphology of the small intestine in young pigs. J Anim Feed Sci. 2012;21:89–106. [Google Scholar]
- Trachsel J.M., Briggs C., Gabler N.K., Allen H.K., Loving C.L. Dietary resistant potato starch alters intestinal microbial communities and metabolites, and markers of immune regulation and barrier function. Front Immunol. 2019;10:1381. doi: 10.3389/fimmu.2019.01381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest P.V., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Vital M., Howe A.C., Tiedje J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. mBio. 2014;5:e889. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.F., Beltranena E., Zijlstra R.T. Diet nutrient digestibility and growth performance of weaned pigs fed sugar beet pulp. Anim Feed Sci Technol. 2016;211:145–152. [Google Scholar]
- Weber T.E., Ziemer C.J., Kerr B.J. Effects of adding fibrous feedstuffs to the diet of young pigs on growth performance, intestinal cytokines, and circulating acute-phase proteins. J Anim Sci. 2008;86:871–881. doi: 10.2527/jas.2007-0330. [DOI] [PubMed] [Google Scholar]
- Xiong W., Ma H., Zhang Z., Jin M., Wang J., Xu Y., Wang Z. Icariin enhances intestinal barrier function by inhibiting NF-κB signaling pathways and modulating gut microbiota in a piglet model. RSC Adv. 2019;9:37947–37956. doi: 10.1039/c9ra07176h. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]