Abstract
A study was conducted with Hy-Line Brown laying hens to examine the effects of reduced protein diet, deficiency of arginine (Arg), and addition of crystalline Arg, citrulline (Cit) and guanidinoacetic acid (GAA) as substitutes for Arg. Hen performance, egg quality, serum uric acid, liver and reproductive organ weights, and energy and protein digestibility were measured using a completely randomized design with 5 treatments. Treatments were a standard diet (17% protein diet; SP), a reduced diet (13% protein diet deficient in Arg; RP) and RP with added Arg (0.35%, RP-Arg), GAA (0.46% equivalent to 0.35% Arg, RP-GAA) or Cit (0.35%, RP-Cit) to the level of SP. It was hypothesized that performance would decrease with Arg deficient RP diet and the addition of GAA or Cit in RP would allow birds to perform similar or greater than Arg-added RP treatment. The experiment was conducted from 20 to 39 wk of age but the treatment effect was seen only after 29 wk of age. The birds offered RP had reduced egg and albumin weights (P < 0.01), lower yolk color score (P < 0.01), lower protein intake and excretion (P < 0.01) than those offered SP. When Arg or Cit were added to RP to make them equivalent to SP, feed intake (FI) and egg production were not different than those of RP (P > 0.05). The birds offered RP-GAA decreased FI and egg production (P < 0.01) compared to those offered RP. The addition of Arg, Cit or GAA to the RP had no effect on egg quality parameters, protein and energy digestibilities (P > 0.05). However, birds offered the RP-Cit diet tended to have higher Haugh unit (P = 0.095) and lower shell breaking strength (P = 0.088) compared to all other treatments while those offered RP-GAA had higher energy digestibility (P < 0.05) than all other groups but RP. The limited performance response of hens fed RP with added Arg, GAA, or Cit may be due to deficiency of some other nutrients in RP such as phenylalanine, potassium or non-essential amino acids and other components of soybean meal in the diet.
Keywords: Arginine, Citrulline, Guanidinoacetic acid, Laying hen, Reduced protein
1. Introduction
Dietary crude protein (CP) reduction in laying hens may provide economic, environmental and welfare benefits to the poultry industry worldwide. Reduction of dietary CP with supplementation of crystalline amino acids (AA) brings the laying hen feed formulation closer to nutritional requirements and may allow for greater protein utilization and reduced N excretion while maintaining bird performance (de Carvalho et al., 2012; Laudadio et al., 2012).
Chickens have a high dietary requirement for arginine (Arg) because they have limited activity of key enzymes for de novo Arg synthesis (Sung et al., 1991) and therefore do not produce much, if any, endogenous Arg. Arginine plays an important role in various metabolic pathways including protein synthesis and immunity. Arginine is converted to nitric oxide (NO) at the macrophage level where it acts against invading pathogens (Jahanian, 2009) and is known to have a vasodilatory effect that may increase heat dissipation (Liu et al., 2019). Arginine supplementation has been extensively studied in broilers but reports are limited for laying hens. A recent study by Xia et al. (2017) showed that graded levels of Arg supplementation (from 0 to 0.88%) to a basal diet containing 0.66% Arg for laying ducks increased egg weight, yolk color score, yolk percentage and shell thickness. Guanidinoacetic acid (GAA) and citrulline (Cit) can spare Arg in chicken diets and they are all commercially available (Tamir and Ratner, 1963; DeGroot et al., 2018).
Guanidinoacetic acid is a precursor of creatine, which is formed from Arg and glycine in kidney via the activity of enzyme arginine–glycine amidinotransferase (Wu and Morris, 1998). The Arg-sparing effect of GAA has been demonstrated in broilers (Dilger et al., 2013; DeGroot et al., 2018). Furthermore, GAA supplementation has been found to increase the rates of fertilized eggs and sperm penetration in the perivitelline layer of broiler breeder hens (Sharideh et al., 2016), improve semen concentration, total sperm production, sperm forward motility and the fertility rate of broiler breeder roosters (Tapeh et al., 2017), reproductive parameters and performance of postnatal progeny in quails (Murakami et al., 2014). Similarly, Cit, a metabolite of Arg, has been found to have Arg-sparing effects in broilers although it is not incorporated into protein (Klose and Almquist, 1940; Tamir and Ratner, 1963). Arginine can be converted to Cit via enzyme nitric oxide synthase at macrophage level with nitric oxide is a co-product. Again, chickens can convert Cit to Arg under the successive actions of argininosuccinate synthetase and argininosuccinate lyase enzymes which is taken place in the kidney and other extrahepatic tissues (Jahanian, 2009; Fernandes and Murakami, 2010; Morris, 2016). Su and Austic (1999) showed that Cit can be converted to Arg at macrophage level when chickens are fed Arg-deficient diets. This study was undertaken to investigate the impact of Arg deficiency on laying performance, egg quality, serum uric acid (SUA) profile, the weight of liver and reproductive organs and nutrient digestibility in reduced protein diets for laying hens and the efficacy of adding it to the diet as either Arg, GAA or Cit.
2. Materials and methods
2.1. Experimental design and diets
The study was conducted at the University of New England Animal House facility, Armidale, NSW, Australia. All experimental procedures were approved by the UNE Animal Ethics Committee and met the requirements of the Australian Code of Practice for Care and Use of Animals for Scientific Purposes (NHMRC, 2013). A total of 125 Hy-line Brown pullets were obtained from Glendon Farm, Tamworth, NSW at 16 wk of age. The birds were fed a common commercial feed from 16 to 20 wk of age (Barastoc - Premium Top Layer Mash, CP: 16.5%, crude fat: 2.5%, crude fibre: 6%, salt: 0.3%, copper: 8.0 mg/kg, selenium: 0.3 mg/kg, calcium: 3.6%, Melbourne, VIC, Australia). Feed intake (FI) during this period was recorded and used to formulate the diets for the laying period according to Hy-Line Brown specifications (Hy-Line International, 2016). At 20 wk of age, birds were weighed and randomly allocated to 5 dietary treatments. Each treatment was replicated 25 times, with one bird per replicate. Birds were housed in individual cages (30 cm wide × 50 cm deep × 45 cm high) with one nipple drinker and one feed trough per bird. The average starting hen weights were similar between treatments (P > 0.05). Birds were housed in a curtain-sided house and feed and water were provided ad libitum throughout the study. The experimental period lasted for 21 wk until the hens were 40 wk of age. The lighting program of 16 h light:8 h dark was maintained throughout the study. An automatic timer was used to operate the lighting program in the layer house. Temperature and relative humidity inside the shed were measured at bird height and recorded automatically every 5 min by a recording thermometer/hygrometer alert (Temp Alert, FCC RoHS, 2011/65/EU, FCC: R17HE910, S4GEM35XB, US).
Diets were offered as mash and consisted of a standard protein diet (SP), a reduced protein diet deficient in Arg (RP) and RP supplemented with either Arg (0.35%, RP-Arg), GAA (0.46%, RP-GAA) or Cit (0.35%, RP-Cit) (Table 1, Table 2). Arginine, GAA and Cit were added on top of RP at the expense of wheat. Dry matter (DM), CP, crude fat, and an ash content of major ingredients including wheat, sorghum, soybean meal, canola meal, barley, and wheat millrun were analyzed before formulating the diet. Metabolizable energy and total and digestible AA used in the diet formulation were obtained from near-infra red reflectance spectroscopy (Foss NIR 6500, Denmark) standardized with Evonik AMINONIR Advanced calibration. The difference in CP levels between SP and RP diets was 4 percentage points. Levels of essential AA selected were based on Hy-Line Brown nutritional recommendation for the laying period based on average daily feed intake of 108 g/d (Hy-Line International, 2016). Levels of supplemental Cit were equivalent to Arg on a molar basis meanwhile GAA level was chosen based on finding that GAA has 77% Arg equivalence for feed conversion (Ringel et al., 2013). Dry matter, CP, crude fat, crude fiber, ash contents, and AA profiles of mixed SP and RP diets were analyzed by standard methods (AOAC, 1994). Guanidinoacetic acid level in the final mix was determined by validated procedures (Dobenecker and Braun, 2015). Supplemental Arg and Cit levels were quantified using a Waters AccQTag amino acid analysis methodology (Cohen, 2001) but adapted to run on an ultra-performance liquid chromatography system as described by Wheat et al. (2008). Specifically, samples (100 to 130 mg) were weighed in duplicate into hydrolysis vials and 5 mL of 20% HCl was added. The samples were then incubated at 110 °C for 24 h. After hydrolysis, the samples were derivatized using AccQTag reagents (Waters Corporation, Milford, MA, US). Samples were analyzed using a high-resolution reversed-phase column (BEH C18, 2.1 × 100 mm; 1.7 μm) on an ultra-performance liquid chromatography system with 12-min run time and an ultraviolet/visible light detector. The column temperature, detection wavelength, and flow rate employed were 57 °C, 260 nm and 0.55 mL/min, respectively.
Table 1.
Diet composition for experimental treatments (%, as-is basis).
| Item | SP1 | RP2 | RP-Arg3 | RP-GAA3 | RP-Cit3 |
|---|---|---|---|---|---|
| Ingredients | |||||
| Wheat | 19.71 | 34.41 | 34.06 | 34.06 | 34.06 |
| Sorghum | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 |
| Soybean meal | 13.28 | – | – | – | – |
| Canola meal | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Barley | 5.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Wheat millrun | 8.57 | 5.00 | 5.00 | 5.00 | 5.00 |
| Canola oil | 2.99 | 0.69 | 0.69 | 0.69 | 0.69 |
| Limestone | 9.50 | 9.84 | 9.84 | 9.84 | 9.84 |
| Dicalcium phosphate | 1.78 | 1.86 | 1.86 | 1.86 | 1.86 |
| Salt | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 |
| Sodium bicarbonate | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Xylanase4 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Vitamin-mineral premix5 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Choline Cl 60% | 0.22 | 0.27 | 0.27 | 0.27 | 0.27 |
| l-Lys | 0.14 | 0.53 | 0.53 | 0.53 | 0.53 |
| dl-Met | 0.21 | 0.28 | 0.28 | 0.28 | 0.28 |
| l-Thr | 0.09 | 0.25 | 0.25 | 0.25 | 0.25 |
| l-Trp | – | 0.02 | 0.02 | 0.02 | 0.02 |
| l-Ile | – | 0.20 | 0.20 | 0.20 | 0.20 |
| l-Arg | – | – | 0.35 | – | – |
| GAA | – | – | – | 0.46 | – |
| l-Cit | – | – | – | – | 0.35 |
| l-Val | – | 0.16 | 0.16 | 0.16 | 0.16 |
| Pigment red | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 |
| Pigment yellow | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| Calculated composition | |||||
| Dry matter | 91.04 | 91.34 | 91.34 | 91.34 | 91.34 |
| AMEn, kcal/kg | 2750 | 2750 | 2750 | 2750 | 2750 |
| CP | 17.00 | 13.00 | 13.50 | 14.00 | 13.50 |
| Crude fat | 6.20 | 3.96 | 3.96 | 3.96 | 3.96 |
| Crude fiber | 3.30 | 2.97 | 2.97 | 2.97 | 2.97 |
| Ash | 4.81 | 4.12 | 4.12 | 4.12 | 4.12 |
| GAA | 0.00 | 0.00 | 0.00 | 0.46 | 0.00 |
| l-Cit | 0.00 | 0.00 | 0.00 | 0.00 | 0.35 |
| Dig.6 Arg | 0.90 | 0.54 | 0.89 | 0.54 | 0.54 |
| Dig.6 Lys | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 |
| Dig.6 Met | 0.44 | 0.46 | 0.46 | 0.46 | 0.46 |
| Dig.6 Cys | 0.25 | 0.22 | 0.22 | 0.22 | 0.22 |
| Dig.6 Met + Cys | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 |
| Dig.6 Trp | 0.19 | 0.15 | 0.15 | 0.15 | 0.15 |
| Dig.6 Ile | 0.59 | 0.57 | 0.57 | 0.57 | 0.57 |
| Dig.6 Thr | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Dig.6 Val | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 |
| Dig.6 Gly | 0.50 | 0.34 | 0.34 | 0.34 | 0.34 |
| Calcium | 4.10 | 4.22 | 4.22 | 4.22 | 4.22 |
| Available Phosphorus | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 |
| Sodium | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Potassium | 0.64 | 0.42 | 0.42 | 0.42 | 0.42 |
| Chloride | 0.28 | 0.36 | 0.36 | 0.36 | 0.36 |
| Choline, mg/kg | 2,000 | 2,000 | 2,000 | 2,000 | 2,000 |
| Linoleic acid | 2.10 | 1.57 | 1.57 | 1.57 | 1.57 |
| DEB7, mEq/kg | 166 | 118 | 118 | 118 | 118 |
GAA = guanidinoacetic acid; Cit = citrulline; CP = crude protein; AMEn = nitrogen corrected apparent metabolizable energy.
SP: standard protein diet with 17% CP.
RP: reduced protein diet with 13% CP.
l-Arg, GAA and l-Cit were added on top of the RP diets at 0.35%, 0.46% and 0.35% in diets, RP-Arg, RP-GAA and RP-Cit, respectively, to the level of Arg in SP.
Econase XT, 25, AB Vista.
Vitamin-mineral premix provided the following per kilogram diet: vitamin A, 10 MIU; vitamin D, 3 MIU; vitamin E, 20 g; vitamin K, 3 g; nicotinic acid, 35 g; pantothenic acid, 12 g; folic acid, 1 g; riboflavin, 6 g; cyanocobalamin, 0.02 g; biotin, 0.1 g; pyridoxine, 5 g; thiamine, 2 g; copper, 8 g as copper sulphate pentahydrate; cobalt, 0.2 g as cobalt sulphate 21%; molybdenum, 0.5 g as sodium molybdate; iodine, 1 g as potassium iodide 68%; selenium, 0.3 g as selenium 2%; iron, 60 g as iron sulphate 30%; zinc, 60 g as zinc sulphate 35%; manganese, 90 g as manganous oxide 60%; antioxidant, 20 g.
Digestible amino acid coefficients for raw ingredients were determined by Near-Infra Red spectroscopy (Foss NIR 6500, Denmark) standardized with Evonik AMINONIR Advanced calibration.
Dietary electrolyte balance (DEB) was calculated as 10,000 × (Na+ + K+ − Cl−).
Table 2.
Analyzed nutrient values of experimental diets (%, as-is basis)1.
| Nutrient composition | SP2 | RP3 | RP-Arg4 | RP-GAA4 | RP-Cit4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dry matter | 92.64 | – | 92.94 | – | 92.60 | – | 92.55 | – | 92.64 | – |
| Gross energy, kcal/kg | 3,734 | – | 3,446 | – | 3,559 | – | 3,495 | – | 3,523 | – |
| CP | 17.24 | – | 13.08 | – | 13.50 | – | 14.16 | – | 13.69 | – |
| Crude fat | 5.21 | – | 2.97 | – | 2.97 | – | 2.97 | – | 2.97 | – |
| Crude fiber | 5.32 | – | 9.70 | – | 9.70 | – | 9.70 | – | 9.70 | – |
| Ash | 17.08 | – | 16.15 | – | 14.67 | – | 16.46 | – | 15.36 | – |
| Calcium | 5.33 | – | 6.35 | – | 5.51 | – | 6.05 | – | 5.78 | – |
| Total phosphorus | 0.82 | – | 0.77 | – | 0.77 | – | 0.79 | – | 0.77 | – |
| GAA | 0.00 | – | 0.00 | – | 0.00 | – | 0.55 | – | 0.00 | – |
| Cit | 0.00 | – | 0.00 | – | 0.00 | – | 0.00 | – | 0.35 | – |
| Arg | 1.00 | (1.06) | 0.59 | (0.64) | 0.93 | (0.99) | 0.59 | (0.64) | 0.59 | (0.64) |
| Lys | 0.99 | (0.88) | 1.02 | (0.84) | 1.02 | (0.84) | 1.02 | (0.84) | 1.02 | (0.84) |
| Met | 0.46 | (0.48) | 0.52 | (0.49) | 0.52 | (0.49) | 0.52 | (0.49) | 0.52 | (0.49) |
| Cys | 0.32 | (0.31) | 0.26 | (0.26) | 0.26 | (0.26) | 0.26 | (0.26) | 0.26 | (0.26) |
| Met + Cys | 0.78 | (0.75) | 0.78 | (0.73) | 0.78 | (0.73) | 0.78 | (0.73) | 0.78 | (0.73) |
| Trp | 0.24 | (0.22) | 0.19 | (0.17) | 0.19 | (0.17) | 0.19 | (0.17) | 0.19 | (0.17) |
| His | 0.42 | (0.37) | 0.29 | (0.25) | 0.29 | (0.25) | 0.29 | (0.25) | 0.29 | (0.25) |
| Phe | 0.79 | (0.71) | 0.53 | (0.50) | 0.53 | (0.50) | 0.53 | (0.50) | 0.53 | (0.50) |
| Leu | 1.37 | (1.32) | 0.95 | (1.05) | 0.95 | (1.05) | 0.95 | (1.05) | 0.95 | (1.05) |
| Ile | 0.72 | (0.68) | 0.67 | (0.63) | 0.67 | (0.63) | 0.67 | (0.63) | 0.67 | (0.63) |
| Thr | 0.69 | (0.70) | 0.69 | (0.64) | 0.69 | (0.64) | 0.69 | (0.64) | 0.69 | (0.64) |
| Val | 0.84 | (0.84) | 0.79 | (0.78) | 0.79 | (0.78) | 0.79 | (0.78) | 0.79 | (0.78) |
| Gly | 0.72 | (0.63) | 0.51 | (0.44) | 0.51 | (0.44) | 0.51 | (0.44) | 0.51 | (0.44) |
| Tau | 0.14 | – | 0.15 | – | 0.15 | – | 0.15 | – | 0.15 | – |
| Ser | 0.67 | – | 0.44 | – | 0.44 | – | 0.44 | – | 0.44 | – |
| Glu | 3.43 | – | 2.66 | – | 2.66 | – | 2.66 | – | 2.66 | – |
| Pro | 1.09 | – | 0.90 | – | 0.90 | – | 0.90 | – | 0.90 | – |
| Ala | 0.85 | – | 0.60 | – | 0.60 | – | 0.60 | – | 0.60 | – |
| Tyr | 0.52 | – | 0.33 | – | 0.33 | – | 0.33 | – | 0.33 | – |
GAA = guanidinoacetic acid; Cit = citrulline; CP = crude protein.
Values of all the amino acids presented were total amino acids. Values in parentheses were calculated.
SP: standard protein diet with 17% CP.
RP: reduced protein diet with 13% CP.
l-Arg, GAA and l-Cit were added on top of the RP diets at 0.35%, 0.46% and 0.35% in diets, RP-Arg, RP-GAA and RP-Cit, respectively, to the level of Arg in SP.
2.2. Data collection
Eggs were collected, weighed, and counted daily. Feed consumption was measured weekly. Hen day egg production, egg mass, FI (g/d per hen), and feed conversion ratio (FCR) were calculated accordingly. Egg mass (g/d per hen) was calculated as hen day egg production multiplied by average egg weight of the hen. Besides, actual AA intake was calculated as by multiplying average FI (g/d per hen) by analyzed total AA of the diet. The FCR was calculated as the ratio of feed to egg mass. Individual hen weight was recorded at the beginning (wk 20), in the middle (wk 30), and at the end of the study (wk 40). At 24, 28, 32, 35, and 39 wk of age, fresh, clean, and normal-shape eggs were collected from all the hens for egg quality measurements. The measurement was performed within 3 h after collection. At wk 40, ten hens per treatment were randomly chosen and euthanized by electrical stunning and decapitated for sample collection. Blood samples were collected from the jugular vein after decapitation for uric acid assays. Then, birds were dissected to determine weights of liver, ovary (without yolky follicles) and oviduct, and oviduct length. Also, at wk 40, six hens per treatment (30 hens in total) with body weights (BW) close to average BW of the treatments were selected for measurements of gross energy (GE) and CP digestibility using total collection of excreta method. Birds were fed dietary treatments and excreta samples were collected from individual cages over 5 consecutive days (120 h). Excreta was collected twice daily starting from 8:00 and 16:00 after removing feathers and feed residues and stored at 4 °C. Collected excreta was then mixed thoroughly and sub-sampled. An aliquot was removed for DM determination using a forced air oven at 105 °C for around 48 h (to constant weight) and the remaining was stored at −20 °C until further analysis. Total feed consumption of individual hens in each treatment during the 5 d excreta collection was recorded for the determination of GE and CP intake.
2.3. Egg quality measurement
Eggshell and internal egg quality parameters were determined using TSS equipment (Technical Services and Supplies, Dunnington, York, UK). After egg weight was recorded, eggs were subjected to shell deformation and shell breaking strength measurement by TSS QC-SPA equipment (50 N load cell). Eggs were then broken and albumen height, Haugh unit, and yolk color score were determined by the TSS QCE-QCM equipment. Scoring of yolk color by the TSS QCE-QCM equipment was based on the yolk color fan scoring system of DSM (DSM Nutritional Products Europe Ltd., Basel, Switzerland). The egg yolk was collected by using filter round papers (diameter 90 mm, CAT No. 1541-090, Whatman, Buckinghamshire HP7 9NA, UK) and weighed. Eggshell residue was washed, dried thoroughly with weight measured using a Quintix513-1S balance (Sartorius Lab Instruments GmbH & Co. KG Goettingen, Germany). Shell thickness was determined on 3 pieces of each eggshell collected from the shell equator with intact shell membranes included. The value of shell thickness presented was an average of 3 measurements using a custom-built gauge based on a Mitutoyo Dial Comparator Gauge Model 2109-10 (Kawasaki, Japan). The weight of egg albumen was calculated by subtracting weights of egg yolk and shell from the intact egg weight. Percentages of eggshell, yolk, and albumen were obtained by dividing weights of these egg components for the total egg weight.
2.4. Analysis of serum uric acid
Blood was collected in Vacutainers (Becton, Dickinson U.K. Limited, Plymouth, UK) coated with silica and a polymer gel to separate serum, cooled to 4 °C, and centrifuged within 5 h after collecting. Blood samples were centrifuged at 3,000 × g at 4 °C for 10 min to separate serum that was then stored at −20 °C until further analysis. Serum uric acid level (SUA) was quantified in duplicate using an integrated chemistry analyzer (Reference number: DF77 [URCA Uric Acid], Siemens Dimension Xpand Plus, Siemens Healthcare, Newark, NJ, US) following the manufacturer's instruction.
2.5. Energy and protein digestibility
Excreta samples were freeze-dried (Christ Alpha 1-4 LDplus, Osterode am Harz, Germany) and ground to pass through a 0.5-mm screen. The protein content of feed and excreta were measured using the Dumas combustion method (Dumas, 1831) in a nitrogen analyzer (LECO Corporation, St Joseph, MI, US) with EDTA as a calibration standard. Gross energy was determined using a Parr adiabatic oxygen bomb calorimeter (Parr Instrument Co., Moline, IL, US), calibrated using benzoic acid as standard. Feed and freeze-dried excreta were oven-dried to constant weight (105 °C for approximately 24 h) for expression of GE and CP digestibility on a DM basis. Apparent protein and energy digestibility were calculated following equations described by Kong and Adeola (2014). In more detail, apparent protein digestibility was calculated by dividing average protein retained by average protein intake during 5-d excreta collection and multiply by 100. Similarly, apparent energy digestibility was calculated by dividing average energy retained by average energy intake during 5-d excreta collection and multiply by 100. Of which, protein and energy intake were calculated by multiplying average FI during 5-d excreta collection by CP and GE level of the feed, respectively. Protein retained was calculated by subtracting protein intake by average protein excreted through the excreta during 5-d excreta collection. Energy retained was calculated by subtracting energy intake by average energy excreted through the excreta during 5-d excreta collection. Amount of protein and energy excreted through the excreta were calculated by multiplying average excreta volume during 5-d excreta collection by CP and GE level of the excreta, respectively.
| Apparent protein digestibility (%) = (CPretained/CPintake) × 100 |
| Apparent energy digestibility (%) = (GEretained/GEintake) × 100 |
| CPintake (g/d) = CPfeed (%) × FI (g/d per hen) |
| GEintake (kcal/d) = GEfeed (kcal/g) × FI (g/d per hen) |
| CPretained (g/d) = CPintake – CPexcreta (%) × excreta volume (g/d per hen) |
| GEretained (kcal/d) = GEintake – GEexcreta (kcal/g) × excreta volume (g/d per hen) |
All data were calculated as per DM basis. Also, CP and GE intake, excretion, retained and digestibility were computed as per unit of BW to exclude the possible effect of growth rate on those variables.
2.6. Data analysis
The data was evaluated as a fixed effect model using the statistical model, Yij = μ + Ti + Ɛij, where Yij is the response expected independent variables, μ is overall mean, Ti is the effect of dietary treatment (i = 1, …, 5) and Ɛij is the random residual error ~ N(0, σ2e).
All data analyses were performed using R Commander (version 3.3.1, R Foundation for Statistical Computing, Vienna, Austria). Data were tested for normal distribution and equal variances between the dietary treatments. A quantile comparison plot was employed to check the data distribution, then a Levene's test was used to test the homogeneity of variances between the treatments. Depending on the results produced from the above 2 tests, either one-way ANOVA or the non-parametric ANOVA (Kruskal–Wallis test) was used to test statistical differences between the treatments. Then, Tukey's post hoc test was used to identify pairwise differences between the treatments. P-values were considered significant at ≤ 0.05.
3. Results
3.1. Air temperature, analyzed nutrient composition of the diets, actual amino acid intake and mortality rate
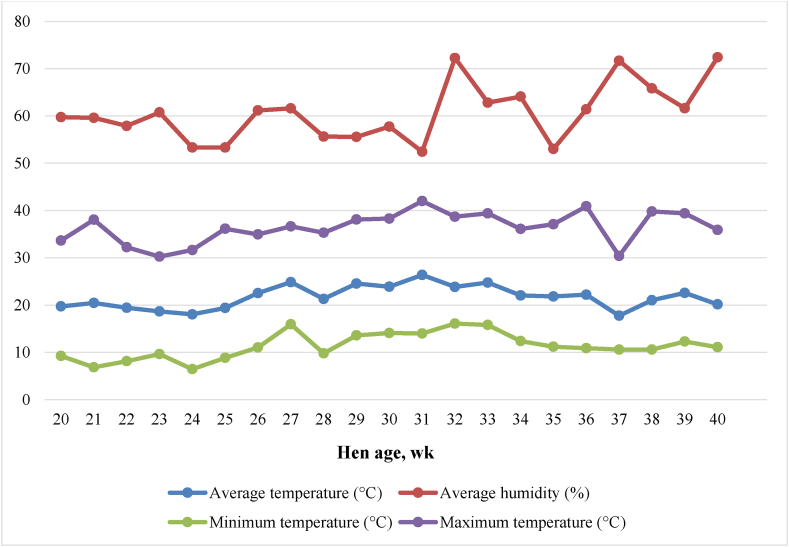
The temperature and relative humidity in the hen house recorded during the experiment are shown in Fig. 1. The average indoor temperature and relative humidity during the experimental period were 21.7 °C (ranging from 17.8 to 26.4 °C) and 60.7% (ranging from 52.4% to 72.4%), respectively. Maximum temperature ranged from 30.3 to 42.0 °C (average 36.4 °C) while minimum temperature ranged from 6.5 to 16.1 °C (average 11.4 °C). Thus, the minimum and maximum temperature observed in this study ranged from 19.8 to 31.2 °C (average 25.1 °C).
Fig. 1.
Temperature and relative humidity of the hen house during the study.
The analyzed nutritional compositions of final diets were close to the calculated values indicating formulation objectives were achieved. Also, a RP diet deficient in Arg with approximately 4% lower CP than the SP diet was obtained as expected. Levels of Arg and Cit added in the final mixes were similar while the GAA level was slightly higher as compared to the calculated ones (Table 1, Table 2). The results on actual AA intake showed that intakes of Arg, Lys, Met, Met + Cys, Thr, Trp, Ile, and Val in all RP diets were sufficient according to Hy-Line Brown nutritional recommendations (Hy-Line International, 2016), except for Arg in Arg-deficient RP treatment (Table 3). Intakes of other essential AA including Phe, His, Leu, and non-essential AA, otherwise, were lower in all RP diets compared to the SP, except for taurine. Birds in all treatments were visibly in good health with no mortality recorded throughout the study.
Table 3.
Actual amino acid intake of experimental treatments1.
| Nutrient, g/d | Week 20 to 29 |
Week 20 to 39 |
Hy-Line5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SP2 | RP3 | RP-Arg4 | RP-GAA4 | RP-Cit4 | SP | RP | RP-Arg | RP-GAA | RP-Cit | ||
| GAA | 0.00 | 0.00 | 0.00 | 0.63 | 0.00 | 0.00 | 0.00 | 0.00 | 0.54 | 0.00 | – |
| Cit | 0.00 | 0.00 | 0.00 | 0.00 | 0.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.38 | – |
| Arg | 1.17 | 0.69 | 1.09 | 0.67 | 0.69 | 1.18 | 0.67 | 1.04 | 0.58 | 0.64 | 0.92 |
| Lys | 1.16 | 1.20 | 1.20 | 1.16 | 1.19 | 1.17 | 1.15 | 1.14 | 1.00 | 1.11 | 0.90 |
| Met | 0.54 | 0.61 | 0.61 | 0.59 | 0.61 | 0.54 | 0.59 | 0.58 | 0.51 | 0.57 | 0.45 |
| Cys | 0.37 | 0.30 | 0.31 | 0.30 | 0.30 | 0.38 | 0.29 | 0.29 | 0.26 | 0.28 | – |
| Met + Cys | 0.91 | 0.91 | 0.92 | 0.89 | 0.91 | 0.92 | 0.88 | 0.87 | 0.77 | 0.85 | 0.81 |
| Trp | 0.28 | 0.22 | 0.22 | 0.22 | 0.22 | 0.28 | 0.21 | 0.21 | 0.19 | 0.21 | 0.19 |
| Ile | 0.84 | 0.79 | 0.79 | 0.76 | 0.78 | 0.85 | 0.76 | 0.75 | 0.66 | 0.73 | 0.66 |
| Thr | 0.81 | 0.81 | 0.81 | 0.79 | 0.80 | 0.81 | 0.78 | 0.77 | 0.68 | 0.75 | 0.69 |
| Val | 0.98 | 0.93 | 0.93 | 0.90 | 0.92 | 0.99 | 0.89 | 0.88 | 0.78 | 0.86 | 0.80 |
| His | 0.49 | 0.34 | 0.34 | 0.33 | 0.34 | 0.50 | 0.33 | 0.32 | 0.28 | 0.32 | – |
| Phe | 0.93 | 0.62 | 0.62 | 0.60 | 0.62 | 0.93 | 0.60 | 0.59 | 0.52 | 0.58 | – |
| Leu | 1.60 | 1.11 | 1.12 | 1.08 | 1.11 | 1.62 | 1.07 | 1.06 | 0.93 | 1.04 | – |
| Gly | 0.84 | 0.60 | 0.60 | 0.58 | 0.59 | 0.85 | 0.58 | 0.57 | 0.50 | 0.56 | – |
| Tau | 0.16 | 0.18 | 0.18 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.15 | 0.16 | – |
| Ser | 0.78 | 0.52 | 0.52 | 0.50 | 0.51 | 0.79 | 0.50 | 0.49 | 0.43 | 0.48 | – |
| Glu | 4.02 | 3.12 | 3.13 | 3.03 | 3.10 | 4.05 | 3.01 | 2.97 | 2.61 | 2.90 | – |
| Pro | 1.28 | 1.05 | 1.06 | 1.02 | 1.05 | 1.29 | 1.02 | 1.00 | 0.88 | 0.98 | – |
| Ala | 1.00 | 0.70 | 0.71 | 0.68 | 0.70 | 1.00 | 0.68 | 0.67 | 0.59 | 0.65 | – |
| Tyr | 0.61 | 0.39 | 0.39 | 0.38 | 0.38 | 0.61 | 0.37 | 0.37 | 0.32 | 0.36 | – |
GAA = guanidinoacetic acid; Cit = citrulline.
Values of all amino acids presented were total amino acids.
SP: standard protein diet with 17% CP.
RP: reduced protein diet with 13% CP.
l-Arg, GAA and l-Cit were added on top of the RP diets at 0.35%, 0.46% and 0.35% in diets, RP-Arg, RP-GAA and RP-Cit, respectively, to the level of Arg in SP.
Total amino acid recommendation for commercial Hy-Line Brown layers (g/d) from 17 to 37 wk of age based on feed intake of 108 g/d (Hy-Line International, 2016).
3.2. Laying performance
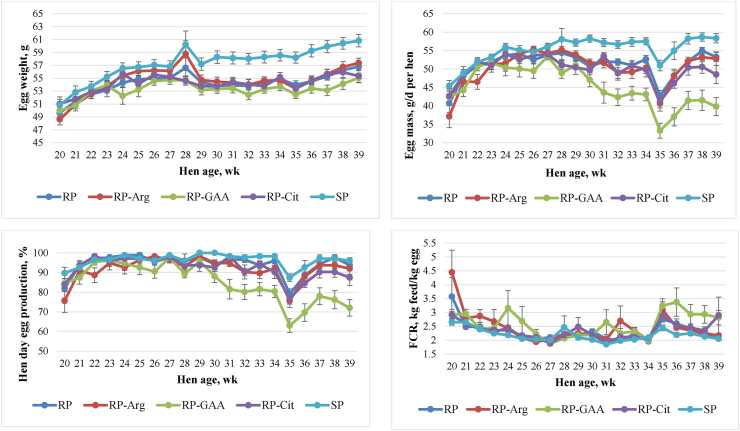
The laying performance including egg weight, egg mass, hen day egg production, FI and FCR from wk 20 to 39 are shown in Table 4 and Fig. 2. From wk 30 to 39 and throughout the entire study period (wk 20 to 39) birds offered SP had higher egg mass and egg weight compared to those offered RP and RP supplemented with either Arg, GAA or Cit (P < 0.05). The differences between egg mass and egg weight of SP and RP groups from wk 30 to 39 were 6.04 g (10.6%) and 4.20 g (7.1%) per d per hen, respectively. Birds offered SP also had the highest hen day egg production observed at all phases of the study (Table 4). Supplementation of either Arg or Cit to RP had no effect on egg mass, egg weight, hen day egg production, and FI while GAA in RP dramatically decreased egg mass, hen day egg production, and FI (P < 0.05, Table 4). Birds receiving the SP had higher FI and lower FCR than those offered the RP-GAA diet (P < 0.05) observed at both wk 20 to 29 and wk 20 to 39 whereas FI and FCR in RP, RP-Arg, and RP-Cit groups were similar to birds offered the SP (P > 0.05; Table 4). Also, GAA supplementation tended to decrease egg mass compared to other treatments in the first 10 wk of the study (P = 0.078, Table 4).
Table 4.
Laying performance of hens fed reduced protein diet from wk 20 to 39.
| Hen age, wk | Variable | SP1 | RP2 | RP-Arg3 | RP-GAA3 | RP-Cit3 | SEM | P-value |
|---|---|---|---|---|---|---|---|---|
| 20 to 29 | Egg mass, g/d per hen | 53.00 | 51.03 | 50.30 | 49.40 | 50.80 | 0.41 | 0.078 |
| Egg weight, g/egg | 55.41 | 53.83 | 54.55 | 53.25 | 53.8 | 0.28 | 0.133 | |
| Feed intake, g/d | 117.1 | 117.2 | 117.7 | 113.8 | 116.5 | 0.98 | 0.762 | |
| FCR, kg feed/kg egg | 2.202 | 2.300 | 2.330 | 2.308 | 2.302 | 0.02 | 0.333 | |
| Hen day egg production, % | 95.83 | 94.86 | 91.85 | 92.74 | 94.4 | 0.58 | 0.150 | |
| 30 to 39 | Egg mass, g/d per hen | 56.82c | 50.78b | 49.72b | 40.81a | 48.80b | 0.71 | <0.001 |
| Egg weight, g/egg | 59.00b | 54.80a | 55.10a | 53.54a | 54.65a | 0.35 | <0.001 | |
| Feed intake, g/d | 118.1b | 113.0b | 111.6b | 98.13a | 109.1b | 1.33 | <0.001 | |
| FCR, kg feed/kg egg | 2.089a | 2.237ab | 2.266ab | 2.472b | 2.261ab | 0.033 | 0.023 | |
| Hen day egg production, % | 96.29b | 92.74b | 90.17b | 76.19a | 89.20b | 1.02 | <0.001 | |
| 20 to 39 | Egg mass, g/d per hen | 53.85c | 50.90bc | 49.00ab | 45.10a | 49.80bc | 0.52 | <0.001 |
| Egg weight, g/egg | 57.50b | 54.31a | 54.82a | 53.40a | 54.23a | 0.31 | <0.001 | |
| Feed intake, g/d | 117.6b | 115.1b | 114.3ab | 106.0a | 112.8ab | 1.06 | 0.008 | |
| FCR, kg feed/kg egg | 2.145a | 2.269ab | 2.300ab | 2.390b | 2.282ab | 0.024 | 0.029 | |
| Hen day egg production, % | 96.06c | 93.80bc | 91.03b | 84.46a | 91.80bc | 0.65 | <0.001 |
GAA = guanidinoacetic acid; Cit = citrulline; FCR = feed to gain ratio.
a, b Differing superscripts indicate significant differences between means.
SP: standard protein diet with 17% CP.
RP: reduced protein diet with 13% CP.
l-Arg, GAA and l-Cit were added on top of the RP diets at 0.35%, 0.46% and 0.35% in diets, RP-Arg, RP-GAA and RP-Cit, respectively, to the level of Arg in SP.
Fig. 2.
Egg weight, egg mass, hen day egg production and feed to gain ratio (FCR) from 20 to 39 wk of age. The dot points represent means and error bars present standard errors of the means. SP: standard protein diet with 17% CP; RP: reduced protein diet with 13% CP. RP-Arg, RP-GAA and RP-Cit were RP diet with l-Arg (0.35%), guanidinoacetic acid (0.46% equivalent to 0.35% Arg) and l-citrulline (0.35%) supplemented, respectively, to the level of Arg in SP.
3.3. Egg quality
The total number of eggs collected from all the treatments for egg quality measurements at wk 24, 28, 32, 35, and 39 were 110, 111, 113, 98, and 106, respectively. Data on the effect of dietary treatments on egg quality and egg components are presented in Table 5, Table 6. Hens receiving SP had higher yolk color score, weights of albumen, shell, and yolk compared to those fed other diets (P < 0.05, Table 5, Table 6). Supplementation of either Arg, GAA, or Cit to RP tended to increase albumen weight from wk 20 to 29 (P = 0.076) but had no effect thereafter (P > 0.05, Table 6). Also, yolk color, shell weight, and yolk weight were unaffected by supplemental Arg, GAA, and Cit (P > 0.05, Table 5, Table 6). Birds offered the RP-Cit tended to have higher Haugh unit (P = 0.095) but lower shell breaking strength (P = 0.088) compared to the other treatment groups from wk 30 to 39. The shell thickness of the RP-Cit group was lower than those of the other groups (P < 0.05, Table 5) observed at different periods of the study (wk 20 to 29, wk 30 to 39, and wk 20 to 39). Birds fed the RP-GAA diet tended to have higher shell reflectivity (lighter color) than the other groups observed from wk 20 to 29 (P = 0.076, Table 5). There was no difference in albumen height, shell deformation, and percentages of egg components between the treatments in the entire study (P > 0.05, Table 5, Table 6).
Table 5.
Egg quality of hens fed reduced protein diets from wk 20 to 39.
| Hen age, wk | Variable | SP1 | RP2 | RP-Arg3 | RP-GAA3 | RP-Cit3 | SEM | P-value |
|---|---|---|---|---|---|---|---|---|
| 20 to 29 | Albumen height, mm | 8.34 | 8.35 | 8.50 | 7.74 | 8.37 | 0.08 | 0.167 |
| Haugh unit | 91.55 | 92.79 | 93.20 | 89.29 | 93.26 | 0.46 | 0.140 | |
| Yolk score | 11.30a | 10.84b | 10.81b | 10.52b | 10.75b | 0.05 | <0.001 | |
| Breaking strength, N | 47.83 | 45.66 | 45.50 | 46.70 | 46.16 | 0.46 | 0.547 | |
| Reflectivity, % | 24.30 | 24.48 | 24.04 | 26.19 | 23.56 | 0.31 | 0.076 | |
| Shell deformation, μm | 0.286a | 0.274a | 0.273a | 0.287a | 0.285a | 0.001 | 0.030 | |
| Shell thickness, mm | 0.410a | 0.403ab | 0.398ab | 0.398ab | 0.393b | 0.002 | 0.033 | |
| 30 to 39 | Albumen height, mm | 7.50 | 7.65 | 7.75 | 7.49 | 7.77 | 0.08 | 0.675 |
| Haugh unit | 86.58 | 88.32 | 89.15 | 87.84 | 89.39 | 0.44 | 0.095 | |
| Yolk score | 10.24a | 9.13b | 9.13b | 8.62b | 9.11b | 0.09 | <0.001 | |
| Breaking strength, N | 44.29 | 44.45 | 44.23 | 44.84 | 40.88 | 0.51 | 0.088 | |
| Reflectivity, % | 26.19 | 27.38 | 27.14 | 28.54 | 26.42 | 0.34 | 0.208 | |
| Shell deformation, μm | 0.265 | 0.265 | 0.265 | 0.267 | 0.258 | 0.002 | 0.690 | |
| Shell thickness, mm | 0.414a | 0.414a | 0.407ab | 0.405ab | 0.397b | 0.002 | 0.024 | |
| 20 to 39 | Albumen height, mm | 7.86 | 7.93 | 8.07 | 7.68 | 8.02 | 0.07 | 0.395 |
| Haugh unit | 88.71 | 90.10 | 90.86 | 88.89 | 91.01 | 0.38 | 0.105 | |
| Yolk score | 10.69a | 9.83b | 9.84b | 9.59b | 9.79b | 0.06 | <0.001 | |
| Breaking strength, N | 45.76 | 44.98 | 44.84 | 45.35 | 43.14 | 0.42 | 0.345 | |
| Reflectivity, % | 25.36 | 26.16 | 25.87 | 27.27 | 25.25 | 0.30 | 0.319 | |
| Shell deformation, μm | 0.274 | 0.268 | 0.268 | 0.276 | 0.270 | 0.002 | 0.334 | |
| Shell thickness, mm | 0.413a | 0.409ab | 0.403ab | 0.401ab | 0.396b | 0.002 | 0.010 |
GAA = guanidinoacetic acid; Cit = citrulline.
a, b Differing superscripts indicate significant differences between means.
SP: standard protein diet with 17% CP.
RP: reduced protein diet with 13% CP.
l-Arg, GAA and l-Cit were added on top of the RP diets at 0.35%, 0.46% and 0.35% in diets, RP-Arg, RP-GAA and RP-Cit, respectively, to the level of Arg in SP.
Table 6.
Egg components of hens fed reduced protein diets from wk 20 to 39.
| Hen age, wk | Variable | SP1 | RP2 | RP-Arg3 | RP-GAA3 | RP-Cit3 | SEM | P-value |
|---|---|---|---|---|---|---|---|---|
| 20 to 29 | Albumen weight, g | 38.15 | 35.93 | 37.10 | 36.63 | 36.63 | 0.26 | 0.076 |
| Yolk weight, g | 13.13 | 13.03 | 13.17 | 12.83 | 13.01 | 0.08 | 0.684 | |
| Shell weight, g | 5.54a | 5.22b | 5.26ab | 5.24b | 5.19b | 0.03 | 0.008 | |
| Albumen weight, % | 67.09 | 66.26 | 66.73 | 66.92 | 66.81 | 0.15 | 0.470 | |
| Yolk weight, % | 23.16 | 24.07 | 23.74 | 23.46 | 23.74 | 0.13 | 0.228 | |
| Shell weight, % | 9.70 | 9.66 | 9.51 | 9.62 | 9.46 | 0.05 | 0.573 | |
| 30 to 39 | Albumen weight, g | 38.99a | 36.15b | 36.23b | 35.51b | 35.85b | 0.28 | <0.001 |
| Yolk weight, g | 14.82a | 13.92b | 13.81b | 13.16b | 13.60b | 0.10 | <0.001 | |
| Shell weight, g | 5.72a | 5.44ab | 5.35b | 5.23b | 5.17b | 0.04 | <0.001 | |
| Albumen weight, % | 65.45 | 65.08 | 65.37 | 65.87 | 65.63 | 0.14 | 0.476 | |
| Yolk weight, % | 24.92 | 25.09 | 24.92 | 24.44 | 24.91 | 0.13 | 0.597 | |
| Shell weight, % | 9.63 | 9.83 | 9.74 | 9.70 | 9.46 | 0.05 | 0.260 | |
| 20 to 39 | Albumen weight, g | 38.64a | 36.06b | 36.50b | 36.26b | 36.18b | 0.25 | 0.004 |
| Yolk weight, g | 14.13a | 13.55ab | 13.54ab | 12.99b | 13.34b | 0.08 | <0.001 | |
| Shell weight, g | 5.64a | 5.35b | 5.32b | 5.22b | 5.18b | 0.03 | <0.001 | |
| Albumen weight, % | 66.11 | 65.56 | 65.89 | 66.52 | 66.12 | 0.13 | 0.245 | |
| Yolk weight, % | 24.21 | 24.67 | 24.47 | 23.88 | 24.41 | 0.12 | 0.289 | |
| Shell weight, % | 9.66 | 9.77 | 9.65 | 9.62 | 9.47 | 0.05 | 0.374 |
GAA = guanidinoacetic acid; Cit = citrulline.
a, b Differing superscripts indicate significant differences between means.
SP: standard protein diet with 17% CP.
RP: reduced protein diet with 13% CP.
l-Arg, GAA and l-Cit were added on top of the RP diets at 0.35%, 0.46% and 0.35% in diets, RP-Arg, RP-GAA and RP-Cit, respectively, to the level of Arg in SP.
3.4. Hen body weight, internal organ weight and serum uric acid level
Hens offered SP had significantly higher BW than those offered the other treatment diets at wk 40 (P < 0.001). The BW at wk 40 were similar for RP, RP-Arg, and RP-Cit groups while those fed RP-GAA had lower BW than the other groups (P < 0.05, Table 7). Similar tendency on hen BW were found on wk 30 (P = 0.076, Table 7).
Table 7.
Hen weight, internal organ weight and serum uric acid level.
| Hen age, wk | Variable | SP 1 | RP2 | RP-Arg3 | RP-GAA3 | RP-Cit3 | SEM | P-value |
|---|---|---|---|---|---|---|---|---|
| 20 | Hen BW, g | 1,924 | 1,941 | 1,941 | 1,939 | 1,932 | 13.24 | 0.994 |
| 30 | Hen BW, g | 2,015 | 1,988 | 1,936 | 1,875 | 1,947 | 16.54 | 0.076 |
| 40 | Hen BW, g | 2,078c | 1,974bc | 1,893b | 1,699a | 1,908b | 20.88 | <0.001 |
| 40 | Liver weight, g | 41.75 | 42.08 | 38.84 | 35.06 | 38.42 | 1.30 | 0.432 |
| 40 | Liver weight per BW, g/kg | 19.55 | 20.79 | 21.15 | 19.59 | 21.15 | 0.56 | 0.806 |
| 40 | Ovary weight, g | 42.53b | 41.49b | 36.54ab | 27.98a | 35.14ab | 1.38 | 0.003 |
| 40 | Ovary weight per BW, g/kg | 19.91ab | 20.53b | 19.82ab | 15.61a | 19.32ab | 0.57 | 0.041 |
| 40 | Oviduct weight, g | 70.00 | 54.48 | 64.38 | 56.61 | 64.55 | 2.39 | 0.228 |
| 40 | Oviduct weight per BW, g/kg | 32.55 | 27.17 | 35.12 | 32.65 | 35.90 | 1.26 | 0.208 |
| 40 | Oviduct length, cm | 67.50 | 66.60 | 67.10 | 58.75 | 66.20 | 1.11 | 0.062 |
| 40 | Oviduct length per BW, cm/kg | 31.75 | 33.06 | 36.67 | 33.47 | 37.61 | 0.77 | 0.071 |
| 40 | Serum uric acid, mg/dL | 3.744b | 2.698ab | 2.849ab | 2.547a | 2.665ab | 0.114 | 0.043 |
GAA = guanidinoacetic acid; Cit = citrulline; BW = body weight.
a, b Differing superscripts indicate significant differences between means.
SP: standard protein diet with 17% CP.
RP: reduced protein diet with 13% CP.
l-Arg, GAA and l-Cit were added on top of the RP diets at 0.35%, 0.46% and 0.35% in diets, RP-Arg, RP-GAA and RP-Cit, respectively, to the level of Arg in SP.
Absolute and relative ovary weights of birds fed the RP-GAA diet were lower than those fed the other diets at wk 40 (P < 0.05, Table 7). Ovary weight of birds fed the SP diet was not different from those fed RP, RP-Arg, and RP-Cit diets. The shortest and longest relative oviduct length (per unit of BW) were observed in birds fed the SP and RP-Cit diet, respectively (P = 0.071). The shortest absolute oviduct length was found in hens fed the RP-GAA diet. Weights of liver and oviduct were unaffected by dietary treatments at wk 40 (Table 7). The reduction of dietary CP resulted in decreased SUA levels between RP and SP on wk 40 (P < 0.05) with the lowest value observed in RP-GAA group (Table 7).
3.5. Apparent protein and energy digestibility
Reduction in CP level tended to decrease excreta moisture at wk 40 (P = 0.062, Table 8). Birds fed the SP diet had higher CP and GE intake and higher CP and GE in excreta than those fed the other diets (P < 0.05). Absolute CP and GE retentions were not different between the dietary treatments (P > 0.05). Birds receiving SP diet tended to have reduced relative apparent CP digestibility compared to those fed the RP diet at wk 40 (P = 0.070). The consumed, excreted, and retained levels of CP and GE in birds fed the RP diet were similar to those fed RP-Arg, RP-Cit or RP-GAA. Gross energy intake and excretion in birds fed the RP-GAA diet at wk 40 were lowest but their relative apparent GE (P < 0.01) and CP digestibility (P = 0.070) were highest compared to the other groups (Table 8).
Table 8.
Protein and energy digestibility by total collection method at wk 401.
| Item | SP2 | RP3 | RP-Arg4 | RP-GAA4 | RP-Cit4 | SEM | P-value |
|---|---|---|---|---|---|---|---|
| Excreta moisture, % | 67.53 | 60.11 | 67.90 | 59.52 | 60.74 | 1.29 | 0.062 |
| Protein digestibility | |||||||
| CP intake, g/d | 26.93b | 17.76a | 19.08ab | 18.66ab | 19.21ab | 1.12 | 0.043 |
| CP intake per unit of BW, g/d | 13.45 | 8.84 | 9.38 | 11.31 | 9.32 | 0.64 | 0.105 |
| CP excreted, g/d | 9.22b | 5.68a | 6.38a | 5.76a | 6.39a | 0.31 | 0.043 |
| CP excreted per unit of BW, g/d | 4.59b | 2.83a | 3.19a | 3.48a | 3.06a | 0.15 | <0.001 |
| CP retained, g/d | 17.71 | 12.07 | 12.73 | 12.89 | 12.83 | 1.01 | 0.386 |
| CP retained per unit of BW, g/d | 8.86 | 6.01 | 6.29 | 7.83 | 6.26 | 0.57 | 0.511 |
| CP apparent digestibility, % | 63.53 | 67.08 | 66.06 | 66.95 | 66.09 | 1.39 | 0.980 |
| CP apparent digestibility per unit of BW, % | 31.71 | 33.38 | 32.42 | 40.91 | 32.03 | 1.22 | 0.070 |
| Energy digestibility | |||||||
| GE intake, kcal/d | 583.3b | 467.8ab | 503.3ab | 386.9a | 494.5ab | 21.75 | 0.049 |
| GE intake per unit of BW, kcal/d | 291.3 | 232.9 | 247.4 | 233.3 | 239.8 | 11.44 | 0.452 |
| GE excreted, kcal/d | 89.54b | 68.31ab | 74.16ab | 60.14a | 73.36ab | 3.01 | 0.028 |
| GE excreted per unit of BW, kcal/d | 44.60 | 33.93 | 37.02 | 35.89 | 35.10 | 1.41 | 0.105 |
| GE retained, kcal/d | 493.8 | 399.5 | 428.3 | 326.8 | 421.1 | 20.84 | 0.121 |
| GE retained per unit of BW, kcal/d | 246.7 | 198.9 | 210.9 | 197.4 | 204.7 | 11.03 | 0.616 |
| GE apparent digestibility, % | 83.64 | 84.98 | 84.85 | 84.58 | 84.82 | 0.63 | 0.971 |
| GE apparent digestibility per unit of BW, % | 41.68a | 42.30ab | 41.49a | 51.60b | 40.99a | 1.19 | 0.019 |
GAA = guanidinoacetic acid; Cit = citrulline; CP = crude protein; BW = body weight; GE = gross energy.
a, b Differing superscripts indicate significant differences between means.
Statistical analysis of GE intake and GE retained were performed on log transformed data whereas values were actual data.
SP: standard protein diet with 17% CP.
RP: reduced protein diet with 13% CP.
l-Arg, GAA and l-Cit were added on top of the RP diets at 0.35%, 0.46% and 0.35% in diets, RP-Arg, RP-GAA and RP-Cit, respectively, to the level of Arg in SP.
4. Discussion
The indoor temperature within 14 to 28 °C has been reported to increase feed efficiency, BW and BWG (Abbas et al., 2011) while those above 30 °C might associated with heat stress in laying hens (Kilic and Simsek, 2013). The average inside temperature in the current study (21.7 °C) was within the above range although average maximum temperature (36.4 °C) was outside the thermal comfort range. It has been suggested that diurnal fluctuating temperatures have minor effects on laying hen performance compared to constant high temperatures. Hens are able to tolerate to short term increases daily temperatures with little impact on production (Al-Saffar and Rose, 2002; Uyanga et al., 2020). The overall laying performance in the current study from wk 20 to 39 in hens fed the SP treatment, were similar to Hy-Line standards (Hy-Line International, 2016). The current vs. Hy-Line values were as follows: FI, 118 vs. 109 g/d; FCR, 2.15 vs. 2.06 kg feed/kg egg; hen day egg production, 96.1% vs. 95.5%, respectively. Thus, housing temperature did not affect laying performance in the current study.
Feeding reduced protein diets did not affect laying performance during the first 10 wk of the study; however, lower egg production and egg weight were observed during the subsequent 10 wk. Hens receiving RP diets also had lower yolk color score, shell thickness, and absolute weight of albumen, shell, and yolk than those of SP fed birds in the current study. Eggs laid by RP hens weighed less compared to those of SP hens in the current study thus lower weights of different egg components were understandable. The findings of this study were in agreement with those previously reported showing that reduced protein results in low egg weight (Novak et al., 2006; Khajali et al., 2007; Shim et al., 2013; Lieboldt et al., 2015; Dong et al., 2017). Egg production can be maintained for short periods with reduced protein but can lead to reduced performance in the long-term (Blair et al., 1999; Keshavarz and Austic, 2004; Novak et al., 2006; Khajali et al., 2008). Khajali et al. (2008) suggested that the tendency for reduced egg production in hens fed reduced CP diets might be attributed to a reduction in BW as a result of a gradual decrease in body protein reserves. The current findings were in agreement with those found by Khajali et al. (2008) and other research groups (Shim et al., 2013; Lieboldt et al., 2015). Noticeably, Sohail et al. (2002) illustrated that egg weight was significantly influenced by essential AA thus removal of these AA may result in decreased egg weight within 2 wk. The RP diet used in the current study was deficient in Arg. Arginine is necessary for protein synthesis to maintain hens body protein and their egg production; therefore, the reduction in egg weight of RP fed birds compared to those of the SP group in this study was understandable. Besides, the deficiency of Arg in the RP diet used in this study might cause an Arg: Lys imbalance and further reduce hens laying performance (Knight et al., 1994; Balnave and Brake, 2002).
No effects on FI, egg weight, egg mass, hen day egg production were observed when Arg and Cit were added to the Arg-deficient RP diet however the addition of GAA reduced FI, egg mass, and hen day egg production from wk 30 onwards. Albumen height, Haugh unit, yolk color score, shell weight, yolk weight and shell quality were unaffected by supplemental Arg, GAA, and Cit in the current study. The results of the current study were supported by Novak et al. (2006), who reported that egg weight was decreased significantly in hens receiving low protein diets even when crystalline AA (Met, Lys, Trp, and Thr) were added in the diet. This may be due to the marginal deficiencies of other AA (Penz and Jensen, 1991). Reductions of dietary CP level decreased the intake of nonessential AA including Glu, Cys, and Gly in the current study. When an essential AA is deficient, other essential AA may be degraded or converted for nonessential purposes, resulting in depressed protein synthesis, and therefore egg production (Kadowaki and Kanazawa, 2003; Novak et al., 2006). In the current study, diets were formulated to meet the Hy-Line Brown nutritional recommendations (Hy-Line International, 2016) for digestible Lys, Met, Met + Cys, Thr, Trp, Arg, Ile, and Val. The intakes of these AA on a daily basis were sufficient according to Hy-Line Brown recommendations except for Arg in RP treatment. Daily AA consumption in the current study showed lower intakes of Phe, His, Gly and Leu in the RP, RP-Arg, RP-GAA and RP-Cit treatments compared to the SP. It is possible that the lack of response to Arg, GAA and Cit in RP diet in the present study could have been caused by a deficiency of Phe, His, Leu, Gly or other non-essential AA as a result of the exclusion of soybean meal from the RP formulation. When CP is lowered by 4 percentage points and supplemented with crystalline AA as in the current study, the least cost formulation has excluded soybean meal. The exclusion of soybean meal also reduces potassium and dietary electrolyte balance (DEB) that may further decrease laying performance in the RP fed birds as compared to those fed the SP diet (Gezen et al., 2005; Abdallah et al., 2010; Hilliar et al., 2019).
The FI level used to determine AA requirements in the formulation was 108 g/d being lower than the actual FI of 115 g/d in the hens fed the RP diet. However, the daily intake of Arg in hens fed the RP treatment was still deficient compared to recommendations (Hy-Line International, 2016). When Arg was supplemented to the RP diet, consumption levels of essential AA including Arg, Met, Lys, Thr, Met + Cys, Val, Ile and Trp met the daily consumption requirement but FI, egg weight, egg mass, and hen day egg production between RP and RP-Arg treatment were still not different. Thus, it is possible that daily consumption of Arg from the RP diet was less important than consumption of other AA such as Leu, His, Phe or Gly. It has been reported that the limiting AA order in corn-soybean meal based 14% protein diet for laying hens was Met, Lys, Thr, Trp, Ile and Val (Da Silva, 2012). To our knowledge, order of importance for other essential AA such as Arg, Leu, His and Phe in layer hens diet especially reduced protein wheat-based diets has not been reported in the literature.
Guanidinoacetic acid supplementation to the RP Arg-deficient diet reduced FI, egg mass, hen day egg production and increased FCR from wk 30 while it did not affect egg quality during the study. This is similar to results reported by Khakran et al. (2018) showing that graded levels of GAA supplementation (0, 0.057%, 0.114%, and 0.171%) did not affect FI, egg mass, and hen day egg production while egg weight was reduced in hens fed 0.171% GAA diet from wk 31 to 42. Hens fed RP-GAA diet in the current study had lower BW, lower absolute and relative ovary weight, and lower absolute oviduct length than those of other groups. This might explain the decrease in laying performance and suggest possible toxicity in RP-GAA treatment at the levels fed. The recommended inclusion level of GAA in feed range from 0.06 to 0.12%; however, the safety level of GAA has not been tested (EFSA, 2009). In the current study, GAA level was selected based on finding that GAA has 77% Arg equivalence for feed conversion (Ringel et al., 2013). Recently, based on results from various publications, Khajali et al., (2020) concluded in their review that an Arg-sparing capacity of GAA in chickens can range from 75 to 149%. It is possible that the selected level of GAA in the current study was overestimated and might further increase the toxicity of GAA in RP-GAA group. The formation of creatine from GAA produces S-adenosylhomocysteine as a co-product, which is then hydrolyzed to form homocysteine (Selhub, 1999; Fowler, 2005). Elevated homocysteine levels as a result of GAA supplementation have been reported in humans (Ostojic et al., 2013) and rat (Stead et al., 2001) and may lead to neurotoxicity and depression (Bhatia and Singh, 2015). In a similar manner, the high dose of GAA used in the current study might cause excessive homocysteine production thus decrease growth and performance in RP-GAA fed birds. In addition, it has been reported that the long-term supplementation of GAA might cause a deficiency in methyl donors for protein synthesis as they are required for converting GAA to creatine (Walker, 1979; Ibrahim et al., 2019).
In the current study, supplementation of either Arg, GAA, or Cit to the RP diet slightly increased albumen weight from wk 20 to 29 with no change thereafter. Higher albumen proportion in hens fed Arg-sufficient low CP diet (11.9% CP, 100% Arg of National Research Council (NRC) 1994 recommended level) compared to birds offered Arg-deficient low CP diet was reported by Lieboldt et al., (2015). Physiologically, egg yolk contains higher levels of Arg than that of the albumen (Bergquist, 1987); however, variation in percentages of yolk and/or albumen following dietary Arg or CP supply were not observed in the present study. This indicates that the synthesis of yolk protein was not selectively limited by an inadequate provision of Arg or CP in the hen diets. Regarding internal egg quality, birds fed RP-Cit tended to have higher Haugh unit but lower shell breaking strength and lower shell thickness compared to other treatment groups in the current study. Carvalho et al. (2018) pointed out that dietary AA requirement for egg production, eggshell, and egg internal quality could vary considerably. This fact might explain the contradictory effects of Cit supplementation on egg quality parameters in the current study. Consideration of those facts may be important for egg producers to maximize the farm profit.
Absolute and relative weights of liver, ovary, and oviduct, as well as oviduct length, were generally unaffected by dietary CP levels or Arg supplementation in the present study. The results on organ weight in the current study were closely associated with laying performance and egg quality data, and were in agreement with those previously reported (Basiouni et al., 2006; Khalaji et al., 2013; Yang et al., 2016).
The SUA level is considered to be inversely correlated to the net protein utilization and reflects the relative equivalence between protein intake, utilization, and degradation (Robin et al., 1987). In the present study, the reduction of CP level resulted in a decrease of wk 40 SUA levels in experimental hens with the lowest value observed in RP-GAA group. Feeding high CP diets have been reported to increase SUA levels in broiler chickens (Namroud et al., 2008; Hilliar et al., 2019). The higher SUA levels observed in high CP fed birds are associated with excessive intake of AA in the form of intact protein (Hilliar et al., 2019). Also, de Carvalho et al. (2015) suggested that an appropriate level of dietary Arg can boost protein anabolism; meanwhile, an overdose may cause Arg:Lys imbalance that may increase arginase activity in the kidney with increased Arg oxidization into uric acid. Yuan et al. (2015) examined the effects of supplementing the graded level of Arg on SUA level in laying hens fed 17% CP diet. The SUA level of hens fed 1.27% Arg was lowest; however, when supplemental Arg increased from 1.27% to 1.66%, the SUA level was increased (Yuan et al., 2015). In the current study, Arg was supplemented as per nutrient requirements for the Hy-Line Brown laying hens (Hy-Line International, 2016) thus SUA levels were maintained at low levels in RP diets with different sources of Arg added.
The results of the current study showed that hens fed RP diets had increased protein digestibility with reduced excreta moisture and protein excretion to the environment compared to SP. This result was consistent with the results on SUA level in this study, and were supported by previous reports (Keshavarz and Austic, 2004; Roberts et al., 2007; Alagawany et al., 2011, 2014; Huang et al., 2011; Zeweil et al., 2011). The higher relative apparent GE and CP digestibility in RP-GAA fed birds may suggest the capacity of birds to use nutrients more efficiently under stress/toxicity conditions.
5. Conclusions
The present findings confirm the beneficial effects of reduced protein diets to increase protein digestibility and reduce protein excretion; however, it suggests that reduction of 4 percentage points CP from 17% to 13% is excessive in practical diets based on wheat and sorghum for laying hens. The lack of effects of Arg, GAA, and Cit supplementation on laying performance in RP diets was possibly due to excessive reduction of dietary CP resulting in the deficiency of some essential and non-essential AA and other components of soybean meal in the diets. Citrulline showed more positive effects than the other sources of Arg on internal egg quality in laying hens while GAA was ineffective. Further investigation is warranted to study the effects of different Arg sources in diets with a more moderate CP reduction.
Author contributions
Hiep Thi Dao: conceptualization, methodology, formal analysis, validation, writing original, review, statistics and editing; Nishchal K. Sharma: review, editing and validation; Emma J. Bradbury: review and editing; Robert A. Swick: conceptualization, review and editing, supervision, project administration, resources.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors would like to acknowledge and thank Australian Egg Corporation Limited for their financial support for this study. We thank Agricultural Experiment Station Chemical Laboratories, University of Missouri–Columbia; AMINO lab, Evonik Singapore and Germany, and Australian Proteome Analysis Facility, University of Macquarie, Australia for feed analysis. Also, the authors would like to thank Mr. Jonathon Clay working at Science and Technology School, and the Poultry Research and Teaching Unit, the University of New England, Australia for their help during the experiment and laboratory analysis.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abbas T.E., Yousuf M.M., Ahmed M.E., Hassabo A.A. Effect of fluctuating ambient temperature on the performance of laying hens in the closed poultry house. Res Opin Anim Vet Sci. 2011;1(4):254–257. [Google Scholar]
- Abdallah A.G., Khalil H., El-Sheikh A.M.H., El-Gabry H.E., Hanafy M.M., Mohamed M.S. Impacts of dietary electrolyte balance on egg production, shell quality, hatchability and some physiological parameters of local laying hens reared in moderate weather. Egypt J Nutr Feeds. 2010;13(1):107–122. [Google Scholar]
- Alagawany M., El-Hindawy M.M., Ali A.A., Soliman M.M. Protein and total sulfur amino acids relationship effect on performance and some blood parameters of laying hens. Egypt J Nutr Feeds. 2011;14:477–487. [Google Scholar]
- Alagawany M., El-Hack M.E.A., Laudadio V., Tufarelli V. Effect of low-protein diets with crystalline amino acid supplementation on egg production, blood parameters and nitrogen balance in laying Japanese quails. Avian Biol Res. 2014;7:235–243. [Google Scholar]
- Al-Saffar A.A., Rose S.P. Ambient temperature and the egg laying characteristics of laying fowl. World’s Poult Sci J. 2002;58:317–331. [Google Scholar]
- Aoac . Association of Official Analytical Chemists; Washington, D.C: 1994. Official methods of analysis. [Google Scholar]
- Balnave D., Barke J. Re-evaluation of the classical dietary arginine:lysine interaction for modern poultry diets: a review. World’s Poult Sci J. 2002;58:275–289. [Google Scholar]
- Basiouni G., Najib H., Zaki M.M., Al-Ankari A.S. Influence of extra supplementation with arginine and lysine on overall performance, ovarian activities and humoral immune response in local Saudi hens. Int J Poultry Sci. 2006;5:441–448. [Google Scholar]
- Bergquist J.C. In: Geflügel. Scholtyssek S., editor. Verlag Eugen Ulmer; Stuttgart, Germany: 1987. p. 38. [Google Scholar]
- Bhatia P., Singh N. Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fund Clin Pharmacol. 2015;29(6):522–528. doi: 10.1111/fcp.12145. [DOI] [PubMed] [Google Scholar]
- Blair R., Jacob J.P., Ibrahim S., Wang P.A. Quantitative assessment of reduced-protein diets and supplements to improve nitrogen utilization. J Appl Poultry Res. 1999;8:25–47. [Google Scholar]
- Carvalho T.S.M., Sousa L.S., Nogueira F.A., Vaz D.P., Saldanha M.M., Triginelli M.V. Digestible methionine+ cysteine in the diet of commercial layers and its influence on the performance, quality, and amino acid profile of eggs and economic evaluation. Poultry Sci. 2018;97:2044–2052. doi: 10.3382/ps/pey036. [DOI] [PubMed] [Google Scholar]
- Cohen S.A. Amino acid analysis using precolumn derivatisation with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. In: Cooper C., Packer N., Williams K., editors. Methods in molecular biology. Humana Press; Totowa, NJ: 2001. pp. 39–47. [DOI] [PubMed] [Google Scholar]
- Da Silva J.H.V. A determination of order of limiting amino acids in a low crude protein diet for laying hens. Poultry Sci. 2012;91(Suppl. 1):113. [Google Scholar]
- de Carvalho F.B., Stringhini J.H., Matos M.S., Jardim F., Café M.B., Leandro N.M. Performance and nitrogen balance of laying hens fed increasing levels of digestible lysine and arginine. Rev Bras Zootec. 2012;41:2183–2188. [Google Scholar]
- de Carvalho F.B., Stringhini J.H., Matos M.S., Café M.B., Leandro N., Gomes N.A. Egg quality of hens fed different digestible lysine and arginine levels. Rev Bras Zootec. 2015;17:63–68. [Google Scholar]
- DeGroot A.A., Braun U., Dilger R.N. Efficacy of guanidinoacetic acid on growth and muscle energy metabolism in broiler chicks receiving arginine-deficient diets. Poultry Sci. 2018;97:890–900. doi: 10.3382/ps/pex378. [DOI] [PubMed] [Google Scholar]
- Dilger R.N., Bryant-Angeloni K., Payne R.L., Lemme A., Parsons C.M. Dietary guanidino acetic acid is an efficacious replacement for arginine for young chicks. Poultry Sci. 2013;92:171–177. doi: 10.3382/ps.2012-02425. [DOI] [PubMed] [Google Scholar]
- Dobenecker B., Braun U. Creatine and creatinine contents in different diet types for dogs - effects of source and processing. J Anim Physiol Anim Nutr. 2015;99:1017–1024. doi: 10.1111/jpn.12383. [DOI] [PubMed] [Google Scholar]
- Dong X.Y., Azzam M.M.M., Zou X.T. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poultry Sci. 2017;96:3654–3663. doi: 10.3382/ps/pex185. [DOI] [PubMed] [Google Scholar]
- Dumas J.B.A. Procedes de l’analyse organique. Ann Chem Phys. 1831;247:198–213. [Google Scholar]
- EFSA (the European Food Safety Authority) Scientific opinion of the panel on additives and products or substances used in animal feed (FEEDAP) on a request from the European Commision on the safety and efficacy of CreAminoTM (guanidinoacetic acid) as feed additive for chickens for fattening. EFSA J. 2009;988:1–30. [Google Scholar]
- Fernandes J.I.M., Murakami A.E. Arginine metabolism in uricotelic species. Acta Sci Anim Sci. 2010;32(4):357–366. [Google Scholar]
- Fowler B. Homocysteine: overview of biochemistry, molecular biology, and role in disease process. Semin Vasc Med. 2005;5:77–86. doi: 10.1055/s-2005-872394. [DOI] [PubMed] [Google Scholar]
- Gezen S.S., Eren M., Deniz G. The effect of different dietary electrolyte balances on eggshell quality in laying hens. Revue Méd Vét. 2005;156(10):491–497. [Google Scholar]
- Hilliar M., Huyen N., Girish C.K., Barekatain R., Wu S., Swick R.A. Supplementing glycine, serine, and threonine in low protein diets for meat type chickens. Poultry Sci. 2019;98(12):6857–6865. doi: 10.3382/ps/pez435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.H., Kemp C., Fisher C. Proceedings of 22nd Australian poultry science symposium. Sydney, Australia. 2011. Effects of nutrition on water intake and litter moisture in broiler chickens; pp. 26–31. [Google Scholar]
- Hy-Line International Management guide for Hy-Line Brown commercial layers. 2016. https://www.hyline.com/filesimages/Hy-Line-Products/Hy-Line-Product-PDFs/Brown/BRN%20COM%20ENG.pdf
- Ibrahim D., El Sayed R., Abdelfattah-Hassan A., Morshedy A.M. Creatine or guanidinoacetic acid? Which is more effective at enhancing growth, tissue creatine stores, quality of meat, and genes controlling growth/myogenesis in Mulard ducks. J Appl Anim Res. 2019;47(1):159–166. [Google Scholar]
- Jahanian R. Immunological responses as affected by dietary protein and arginine concentrations in starting broiler chicks. Poultry Sci. 2009;88:1818–1824. doi: 10.3382/ps.2008-00386. [DOI] [PubMed] [Google Scholar]
- Kadowaki M., Kanazawa T. Amino acids as regulators of proteolysis. J Nutr. 2003;133(6):2052S–2056S. doi: 10.1093/jn/133.6.2052S. [DOI] [PubMed] [Google Scholar]
- Keshavarz K., Austic R.E. The use of low-protein, low-phosphorous, amino acid and phytase supplemented diets on laying hen performance and nitrogen and phosphorous excretion. Poultry Sci. 2004;83:75–83. doi: 10.1093/ps/83.1.75. [DOI] [PubMed] [Google Scholar]
- Khajali F., Faraji M., Karimi Dehkordi S. Effects of reduced-protein diets at constant total sulfur amino acids:lysine ratio on pullet development and subsequent laying hen performance. Am J Anim Vet Sci. 2007;2:89–92. [Google Scholar]
- Khajali F., Khoshouie E.A., Dehkordi S.K., Hematian M. Production performance and egg quality of Hy-line W36 laying hens fed reduced-protein diets at a constant total sulfur amino acid: lysine ratio. J Appl Poultry Res. 2008;17:390–397. [Google Scholar]
- Khajali F., Lemme A., Rademacher-Heilshorn M. Guanidinoacetic acid as a feed supplement for poultry. World’s Poult Sci J. 2020;76:1–22. doi: 10.1080/00439339.2020.1716651. [DOI] [Google Scholar]
- Khakran G., Chamani M., Foroudi F., Sadeghi A.A., Afshar M.A. Effect of guanidine acetic acid addition to corn-soybean meal based diets on productive performance, blood biochemical parameters and reproductive hormones of laying hens. Kafkas Univ Vet Fak Derg. 2018;24:99–105. [Google Scholar]
- Khalaji S., Zaghari M., Ganjkhanloo M., Ghaziani F. Arginine, soy isoflavone and hydroxypropylmethylcellulose have protective effects against obesity in broiler breeder hens fed on high-energy diets. Br Poultry Sci. 2013;54:766–779. doi: 10.1080/00071668.2013.843070. [DOI] [PubMed] [Google Scholar]
- Kilic I., Simsek E. The effects of heat stress on egg production and quality of laying hens. J Anim Vet Adv. 2013;12(1):42–47. [Google Scholar]
- Klose A.A., Almquist H.J. The ability of citrulline to replace arginine in the diet of the chick. J Biol Chem. 1940;135:153–155. [Google Scholar]
- Knight C.D., Wuelling C.W., Atwell C.A., Dibner J.J. Effect of intermittent periods of high environmental temperature on broiler performance responses to sources of methionine activity. Poultry Sci. 1994;73:627–639. doi: 10.3382/ps.0730627. [DOI] [PubMed] [Google Scholar]
- Kong C., Adeola O. Evaluation of amino acid and energy utilization in feedstuff for swine and poultry diets. Asian-Australas J Anim Sci. 2014;27(7):917–925. doi: 10.5713/ajas.2014.r.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudadio V., Passantino L., Perillo A., Lopresti G., Passantino A., Khan R.U. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poultry Sci. 2012;91:265–270. doi: 10.3382/ps.2011-01675. [DOI] [PubMed] [Google Scholar]
- Lieboldt M.A., Halle I., Frahm J., Schrader L., Weigend S., Preisinger R. Effects of long-term graded L-arginine supply on growth development, egg laying and egg quality in four genetically diverse purebred layer lines. J Poult Sci. 2015;53 doi: 10.2141/jpsa.0150067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., de Ruyter E.M., Athorn R.Z., Brewster C.J., Henman D.J., Morrison R.S. Effects of L-citrulline supplementation on heat stress physiology, lactation performance and subsequent reproductive performance of sows in summer. J Anim Physiol Anim Nutr. 2019;103:251–257. doi: 10.1111/jpn.13028. [DOI] [PubMed] [Google Scholar]
- Morris S.M., Jr. Arginine metabolism revisited. J Nutr. 2016;146(12):2579S–2586S. doi: 10.3945/jn.115.226621. [DOI] [PubMed] [Google Scholar]
- Murakami A.E., Rodrigueiro R.J.B., Santos T.C., Ospina-Rojas I.C., Rademacher M. Effects of dietary supplementation of meat-type quail breeders with guanidinoacetic acid on their reproductive parameters and progeny performance. Poultry Sci. 2014;93:2237–2244. doi: 10.3382/ps.2014-03894. [DOI] [PubMed] [Google Scholar]
- Namroud N.F., Shivazad M., Zaghari M. Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level, and excreta characteristics of broiler chicks. Poultry Sci. 2008;87:2250–2258. doi: 10.3382/ps.2007-00499. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) 9th rev. ed. National Academy Press; Washington, DC: 1994. Nutrient requirements of poultry. [Google Scholar]
- NHMRC (The National Health and Medical Research Council) Australian code of practice for the care and use of animals for scientific purposes. 8th ed. The National Health and Medical Research Council; Australia: 2013. [Google Scholar]
- Novak C., Yakout H.M., Schedeler E. The effect of dietary protein level and total amino acid:lysine ratio on egg production parameters and egg yield in Hy-Line W-98 hens. Poultry Sci. 2006;85:2195–2206. doi: 10.1093/ps/85.12.2195. [DOI] [PubMed] [Google Scholar]
- Ostojic S.M., Niess B., Stojanovic M., Obrenovic M. Creatine metabolism and safety profiles after six-week oral guanidinoacetic acid administration in healthy humans. Int J Med Sci. 2013;10(2):141. doi: 10.7150/ijms.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penz A.M., Jr., Jensen L.S. Influence of protein concentration, amino acid supplementation and daily time of access to high or low-protein diets on egg weight and components in laying hens. Poultry Sci. 1991;70:2460–2466. doi: 10.3382/ps.0702460. [DOI] [PubMed] [Google Scholar]
- Ringel J., Rademacher M., Elwert C. Proceedings of 19th European symposium on poultry nutrition. Potsdam; Germany: 2013. Arginine sparing effect of guanidinoacetic acid in broilers. [Google Scholar]
- Roberts S.A., Xin H., Kerr B.J., Russell J.R., Bregendahl K. Effects of dietary fiber and reduced crude protein on nitrogen balance and egg production in laying hens. Poultry Sci. 2007;86:1716–1725. doi: 10.1093/ps/86.8.1716. [DOI] [PubMed] [Google Scholar]
- Robin J.P., Cherel Y., Girard H., Geloen A., Lemaho Y. Uric acid and urea in relation to protein catabolism in long-term fasting geese. J Comp Physiol B. 1987;157:491–499. doi: 10.1007/BF00691834. [DOI] [PubMed] [Google Scholar]
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Sharideh H., Esmaeile Neia L., Zaghari M., Zhandi M., Akhlaghi A., Lotfi L. Effect of feeding guanidinoacetic acid and L-arginine on the fertility rate and sperm penetration in the perivitelline layer of aged broiler breeder hens. J Anim Physiol Anim Nutr. 2016;100:316–322. doi: 10.1111/jpn.12372. [DOI] [PubMed] [Google Scholar]
- Shim M.Y., Song E., Billard L., Aggrey S.E., Pesti G.M., Sodsee P. Effects of balanced dietary protein levels on egg production and egg quality parameters of individual commercial layers. Poultry Sci. 2013;92:2687–2696. doi: 10.3382/ps.2012-02569. [DOI] [PubMed] [Google Scholar]
- Sohail S.S., Bryant M.M., Roland D.A. Influence of supplemental lysine, isoleucine, threonine, tryptophan and total sulfur amino acids on egg weight of Hy-Line W36 hens. Poultry Sci. 2002;81:1038–1044. doi: 10.1093/ps/81.7.1038. [DOI] [PubMed] [Google Scholar]
- Stead L.M., Au K.P., Jacobs R.L., Brosnan M.E., Brosnan J.T. Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am J Physiol Endocrinol Metab. 2001;281:E1095–E1100. doi: 10.1152/ajpendo.2001.281.5.E1095. [DOI] [PubMed] [Google Scholar]
- Su C.L., Austic R.E. The recycling of L-citrulline to L-arginine in a chicken macrophage cell line. Poultry Sci. 1999;78:353–355. doi: 10.1093/ps/78.3.353. [DOI] [PubMed] [Google Scholar]
- Sung Y.J., Hotchkiss J.H., Austic R.E., Dietert R.R. L-Arginine-dependent production of a reactive nitrogen intermediate by macrophages of a uricotelic species. J Leukoc Biol. 1991;50:49–56. doi: 10.1002/jlb.50.1.49. [DOI] [PubMed] [Google Scholar]
- Tamir H., Ratner S. A study of ornithine, citrulline and arginine synthesis in growing chicks. Arch Biochem Biophys. 1963;102:259–269. doi: 10.1016/0003-9861(63)90179-6. [DOI] [PubMed] [Google Scholar]
- Tapeh R.S., Zhandi M., Zaghari M., Akhlaghi A. Effects of guanidinoacetic acid diet supplementation on semen quality and fertility of broiler breeder roosters. Theriogenology. 2017;89:178–182. doi: 10.1016/j.theriogenology.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Uyanga V.A., Jiao H., Zhao J., Wang X., Lin H. Dietary L-Citrulline supplementation modulates nitric oxide synthesis and anti-oxidant status of laying hens during summer season. J Anim Sci Biotechnol. 2020;11 doi: 10.21203/rs.3.rs-22779/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J.B. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Area Mol Biol. 1979;50:177–242. doi: 10.1002/9780470122952.ch4. [DOI] [PubMed] [Google Scholar]
- Wheat T.E., Grumbach E.S., Mazzeo J.R. Waters Corporation; Milford, MA: 2008. UPLC amino acid analysis solution (Application note No. 720001683en) [Google Scholar]
- Wu G.Y., Morris S.M. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Fouad A.M., Chen W., Ruan D., Wang S., Fan Q. Estimation of dietary arginine requirements for Longyan laying ducks. Poultry Sci. 2017;96:144–150. doi: 10.3382/ps/pew205. [DOI] [PubMed] [Google Scholar]
- Yang H., Ju X., Wang Z., Yang Z., Lu J., Wang W. Effects of arginine supplementation on organ development, egg quality, serum biochemical parameters, and immune status of laying hens. Rev Bras Ciência Avícola. 2016;18:181–186. [Google Scholar]
- Yuan C., Bu X.C., Yan H.X., Lu J.J., Zou X.T. Dietary L-arginine levels affect the liver protein turnover and alter the expression of genes related to protein synthesis and proteolysis of laying hens. Poultry Sci. 2015;95:261–267. doi: 10.3382/ps/pev314. [DOI] [PubMed] [Google Scholar]
- Zeweil H.S., Abdalah A.A., Ahmed M.H., Ahmed M.R.S. Effect of different levels of protein and methionine on performance of Baheij laying hens and environmental pollution. Egypt Poult Sci. 2011;31:621–639. [Google Scholar]