Abstract
There are a large number of microorganisms in the porcine intestinal tract. These microorganisms and their metabolites contribute to intestinal mucosal immunity, which is of great importance to the health of the host. The host immune system can regulate the distribution and composition of intestinal microorganisms and regulate the homeostasis of intestinal flora by secreting a variety of immune effector factors, such as mucin, secretory immunoglobulin A (sIgA), regenerating islet-derived III (RegIII)γ, and defensin. Conversely, intestinal microorganisms can also promote the differentiation of immune cells including regulatory T cells (Treg) and Th17 cells through their specific components or metabolites. Studies have shown that imbalances in the intestinal flora can lead to bacterial translocation and compromised intestinal barrier function, affecting the health of the body. This review focuses on the composition of the pig intestinal flora and the characteristics of intestinal mucosal immunity, discusses the interaction mechanism between the flora and intestinal mucosal immunity, as well as the regulation through fecal microbiota transplantation (FMT), dietary nutritional composition, probiotics and prebiotics of pig intestinal microecology. Finally, this review provides insights into the relationship between intestinal microorganisms and the mucosal immune system.
Keywords: Gut microbe, Pig, Intestine, Mucosal immunity
1. Introduction
Modern pig production faces many persistent challenges, such as weaning diarrhea due to changes in diet and living environment (Markowiak and Slizewska, 2018), and the abuse of growth-promoting antibiotics in feed (Van Boeckel et al., 2019). In the past decade, through advancements in 16S rRNA sequencing and metagenomics analyses in addition to other microbiology technologies, it has become apparent that the homeostasis of intestinal flora plays an important role in host health (Isaacson and Kim, 2012; Nowland et al., 2019; Yin et al., 2020). Intestinal microorganisms metabolize dietary nutrients ingested by the host and ferment complex plant polysaccharides that cannot be directly utilized by the host, thus providing energy for the host. In addition, the microbiota has been shown to synthesize vitamin K, regulate choline metabolism, and stimulate the development and maturation of the intestinal immune system (Tremaroli and Backhed, 2012). Conversely, imbalances in the intestinal microflora have been associated with obesity, fatty liver, diabetes, inflammatory bowel disease, constipation, and other diseases (Portune et al., 2017; Sommer and Backhed, 2013; Yin et al., 2018).
There are a large number of immune tissues and cells in the intestinal tissue of pigs, including lymph nodes, Paneth cells, and innate lymphocytes (Kim and Ho, 2010; Pabst et al., 2016). Under normal physiological conditions, the intestinal immune system plays a vital role in the homeostasis of intestinal microorganisms (Thaiss et al., 2016). In piglets, incomplete development of intestinal organs, low autoimmunity, and drastic changes in food sources as a result of weaning quickly alter intestinal homeostasis, resulting in diarrhea. In finishing pigs with a more mature intestinal immune system, dietary changes do not have an obvious effect on intestinal dysfunction (Frese et al., 2015). During the period of intestinal flora colonization of piglets, transplantation of exogenous bacteria is able to increase the abundance of beneficial intestinal bacteria, significantly improving the intestinal barrier function of piglets, and ultimately alleviating the occurrence of weaning diarrhea (Cheng et al., 2018; Hu et al., 2018b).
An increasing number of studies on intestinal microecology and microflora have reported links between the immunity of intestinal microflora and intestinal mucosa health, as well as the entire body (JF and MT, 2020; Round and Mazmanian, 2009). Intestinal microorganisms can regulate the host intestinal epithelial barrier and immune barrier through flagellin, lipopolysaccharide (LPS) and metabolites, promote the development and maturity of the host immune system, and improve intestinal barrier function (Hooper et al., 2012; Powell et al., 2017; Sonnenberg and Artis, 2019). Here, the composition of intestinal microflora, as well as the composition and responses of the intestinal mucosal immune system in pigs are introduced, and the possible mechanism of the interaction between microflora and intestinal mucosal immunity will be discussed.
2. Composition of intestinal microorganisms in pigs
There are many types of microorganisms in the digestive tract of mammals, including bacteria, fungi, archaea, protozoa, and a number of viruses, with a total of at least 1014 (Clemente et al., 2012; Sommer and Backhed, 2013). Intestinal microorganisms are predominantly bacteria, consisting of thousands of species. Some of the most prominent genera include Firmicutes and Bacteriodetes, followed by Proteobacteria, Verrumicrobia, Actinobacteria (Donaldson et al., 2016; Human Microbiome Project, 2012). Newborn piglets gradually establish intestinal flora due to contact with sow vaginal and fecal microorganisms. These exposures come in the form of direct contact with the birth canal of sows during delivery, and sow feces during normal behaviors (Kim and Isaacson, 2015). During lactation, sow's milk provides nutritional advantages for the colonization of Lactobacillus, and establishes a milk-oriented microbiome (Frese et al., 2015). From 7 through to 21 d of age (birth to early weaning), more than 95% of the microorganisms in the intestines are strictly anaerobic (Firmicutes, Bacteriodetes, and Proteobacteria) (Slifierz et al., 2015). The abundance of Firmicutes (54.0%), Bacteroidetes (38.7%) and Proteobacteria (4.2%) at the phyla level changes to Bacteroidetes (59.6%), Firmicutes (35.8%) and Proteobacteria (1%) after weaning. This indicates that the phyla of microorganisms were essentially the same pre- and post-weaning, although the relative abundance changed significantly, where the proportion of Bacteroidetes increased (Alain et al., 2014).
The composition of organisms is affected by both dietary composition and weaning stress. It was reported that, especially after rapid weaning, the microbial diversity and the number of Lactobacillus bacteria decreased, while the abundance of Clostridium spp., Prevotella spp., and Escherichia coli, increased (Gresse et al., 2017). The abundance of bacteria in Bacteroides was the highest pre-weaning, whereas the highest levels of Prevotella and Clostridium were reported post-weaning. During the weaning transition period, a significant shift from Bacteroides to Prevotella was reported (Alain et al., 2014). It is possible that Bacteroides aid in the digestion of monosaccharides and oligosaccharides in breast milk pre-weaning, whereas Prevotella enhance the degradation of plant polysaccharides in feed, such as hemicellulose and xylan (Alain et al., 2014; Lamendella et al., 2011). Therefore, when the dietary composition of pigs is switched to plant carbohydrates, Prevotella-related microorganisms in the intestines proliferate (Slifierz et al., 2015).
Similar to other mammals, the intestinal microbial richness of pigs also varied significantly with the intestinal segment. The ileum is mainly colonized by Firmicutes and Proteobacteria, and the abundance of Bacteroidetes in the cecum and colon is significantly increased (Looft et al., 2014; Zhang et al., 2018). Furthermore, the microflora of the intestinal lumen is very different from that of the intestinal mucosa. The richness of Bacteroides in the lumen of the colon (40.09%) is much higher than that of the small intestine (1.69%). The genera found in the intestinal lumen primarily consist of Prevotellaceae, Ruminococcaceae, Lachnospiraceae and Veillonellaceae. Prevotellaceae, Enterobacteriaceae, Caulobacteraceae, Enterococcaceae, Xanthomonadaceae and Pseudomonadaceae dominate the intestinal mucosa (Zhang et al., 2018).
The composition of intestinal microorganisms in pigs is affected by diet, antibiotics, body weight, physiological status, genetics, and the living environment (Isaacson and Kim, 2012). Among these factors, diet is the most important. Corn fiber (neutral detergent fiber) contains plant lignin, hemicellulose, cellulose, and other structural components that pigs alone cannot digest. These nutrients enter the intestine and are fermented into bioavailable molecules by the colonic microbiota (Wang et al., 2019f). The intestinal flora regionally colonizes the gut in specific spaces suitable for their survival. The resulting interactions with the host form a dynamic yet balanced complex ecosystem.
3. Response mechanism of intestinal mucosal immune system
The mucosal immune system exists in various anatomical locations of the body, such as the respiratory, reproductive, or digestive tracts, and plays a complex role in defense of the host (Iijima et al., 2001). In the intestinal tract, the mucosal immune system plays an important role. Not only does it contribute to digestion and absorption of nutrients, it also serves as an important barrier for the body to resist pathogenic insult (Knoop and Newberry, 2018). The intestinal mucosal immune response is induced by the local mucosal tissues, where immunoreactive cells are stimulated by pathogens and other antigens. Mucosal immunity is characteristically defined by the production of secretory immunoglobulin A (sIgA) by plasma cells (Iijima et al., 2001). Intestinal epithelial cells (IEC) are the first line of defense against antigens within the intestinal lumen, and are composed of a variety of cell types (enterocytes, goblet cells, neuroendocrine cells, cluster cells, Paneth cells and Microfold cells). These different epithelial cell types cooperate to maintain intestinal homeostasis and promote host defenses (Allaire et al., 2018).
The intestinal mucosal immune system can be divided into induction and effector sites according to tissue structure and function. The induction site mainly refers to gut-associated lymphoid tissue (GALT), which is composed of highly organized lymphoid follicles known as Peyer's patches (PP), mesenteric lymph nodes (MLN), and isolated lymphoid follicles (ILF). The immune cells in the induction sites include Microfold cells and antigen presenting cells (APC), which uptake and present antigens to immune effector cells (Pabst and Rothkotter, 2006). Some types of APC include dendritic cells (DC), macrophages and IEC (Allaire et al., 2018). Effector sites are the diffuse distribution of lymphocytes in the epithelial layer and lamina propria, including intestinal mucosal intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL). The presentation of antigens by APC to these cells results in the production of antibodies and various immunomodulatory cytokine factors (Allaire et al., 2018; Castro and Arntzen, 1993; Nagura and Sumi, 1988; Pabst and Rothkotter, 2006).
The intestinal mucosal immune response is induced by the extraction of antigens from the induced site. The main induction site of the intestinal mucosal immune system is the PP, in which the antigen transporting cells (Microfold cells), make up the surface of the PP (Allaire et al., 2018). Histologically, Microfold cells are characterized by short microvilli, small cytoplasmic vesicles and a few lysosomes. These cells are capable of phagocytosing microorganisms and complex antigens, and can ingest and transport intraluminal antigens such as proteins and other small particles (viruses, parasites and microspheres) to APC in the subepithelial lymphoid tissue (Kobayashi et al., 2019; Neutra et al., 1996; Yamamoto et al., 2012). Next, the antigens are presented to antigen receptor molecules, and are finally presented to helper T lymphocytes. Helper T lymphocytes secrete a variety of lymphokines to induce B lymphocytes, which then differentiate and proliferate to produce a large amount of sIgA, which is released into the intestinal lumen, affecting its immunological properties (Iijima et al., 2001; Yamamoto et al., 2012).
In the wall of the porcine small intestines, lymphoid tissue exists in the form of isolated lymphoid follicles, or as high tissue lymphoid follicles (such as PP) (Stokes, 2017). Under the regulation of the immune system, Microfold cells in the jejunum and ileum take up intestinal lumenal antigens (Rothkotter, 2009). In the porcine colon, the immune tissue is known as the lymphoglandular complex (Morfitt and Pohlenz, 1989). Compared with germ-free piglets, the proportion of B cells in the ileum of conventional piglets was higher, IgA+ B cells were more common, distributed evenly in the whole small intestine, and the ratio of effector to memory CD4+CD8+ αβ Th cells was significantly higher than that of germ-free piglets (Potockova et al., 2015). At birth, the mucosal immune system of piglets is underdeveloped, and the number of white blood cells in the lamina propria of the intestine is very small. Sow's milk is critical to the promotion and development of the neonatal immune system (Salmon et al., 2009). As the pigs age, the immune system will develop, in a manner that is driven by environment, diet, genotype, and intestinal microbes (Stokes, 2017).
4. Mucosal immune system regulates intestinal microbiota
4.1. Stratification and distribution
The intestinal immune system can limit direct contact between bacteria and the intestinal cell epithelium, thus reducing the possibility of pathological interactions. This occurs through 2 mechanisms. First, the intestinal barrier minimizes direct contact between intestinal microorganisms and the surface of epithelial cells. Second, the immune system limits bacteria to specific intestinal sites, reducing their exposure to the systemic immune system (Hooper et al., 2012; Yin et al., 2019). The regulation of the intestinal immune system on the stratification and distribution of microorganisms is accomplished via the combined action of epithelial cells, mucus, and antimicrobial peptides (Fig. 1) (Hooper, 2015; JF and MT, 2020).
Fig. 1.
Regulation of intestinal microorganisms by the mucosal immune system. The intestinal immune system regulates the stratification and distribution of intestinal microorganisms through the combined action of epithelial cells, mucus, and antimicrobial peptides. Goblet cells secrete mucus, limiting direct contact between microbes and the intestinal epithelium and preventing microbial translocation. Intestinal cells and goblet cells can secrete antibacterial proteins such as α-defensins and regenerating islet-derived III (RegIII)γ. Dendritic cells (DC) in the intestinal lamina propria can engulf some bacterial antigens that penetrate the mucous layer and present them in the mesenteric lymph nodes. DC induce B lymphocytes to differentiate into plasma cells that secrete large quantities of immunoglobulin A (IgA) into the intestinal cavity.
The goblet cells of the intestinal epithelium secrete mucus, which forms a layer of elastic gel that covers the epithelial surface, isolating the intestinal epithelium from the environment of the intestinal lumen. This greatly reduces the contact between the microbiota and the host tissue, protecting the parenchyma from luminal microorganisms and antigens, and preventing microbial translocation (Knoop and Newberry, 2018; Propheter et al., 2017). The layering of mucus over the small intestine is not obvious; there is only a thin and intimate mucus layer. In the colon, there are 2 structurally distinct mucus layers; the outer is loose, and the inner is adherent and tightly compact. The outer layer is where the symbiotic intestinal microflora colonize and interact with oligosaccharides on the mucin. The inner mucus layer is resistant to bacterial penetration, and has almost no bacterial colonization (Donaldson et al., 2016; Johansson et al., 2014; Kim and Ho, 2010). The outer mucus layer is colonized with B. acidifaciens, Bacteroides fragilis, Bifidobacteriaceae and Akkermansia muciniphila, inner mucus layer includes B. fragilis and Acinetobacter spp. (Donaldson et al., 2016).
The mucus secreted by intestinal goblet cells is mainly composed of mucin 2 (MUC2), intestinal trefoil factor 3 (TFF3), resistin-like molecule β (RELM β) and Fc-γ binding protein (Fc-γ BP) (Kim and Ho, 2010; Vaishnava et al., 2011). The MUC2 protein is the most abundant mucin in the intestinal tract. Goblet cells secrete mucin under the stimulation of many substances (including acetylcholine, histamine, and prostaglandin). The mucin is released to the external mucus layer, which competitively binds to the adhesin receptors of epithelial cells and inhibits the adhesion and colonization of pathogenic microorganisms in the intestinal tract (Knoop and Newberry, 2018). MUC2, Fc- γ BP, and TFF3 proteins then combine through covalent bonds to promote the secretion of soluble mucus, increase mucus viscosity, and function synergistically to protect and maintain the integrity of the intestinal mucosa (Yu et al., 2011). When the secretion of MUC2 and TFF3 decreases, harmful microorganisms can easily penetrate the mucus layer and colonize the intestinal epithelium, which results in epithelial cell damage (Lidell et al., 2006). RELM β is an antibacterial protein that limits the contact between Gram-negative bacteria and the surface of the colonic epithelium. In addition, RELM β can bind negatively charged lipids on the surface of bacterial cell membranes to form polymer pores to lyse bacterial cells. In mice lacking RELM β, the abundance of Proteus increased in the internal colonic mucus layer (Propheter et al., 2017).
In addition to goblet cells, other IEC secrete antibacterial proteins, among which Regenerating islet-derived III (RegIIIγ) and sIgA are essential in the isolation of microorganisms in the intestinal mucosa (Vaishnava et al., 2011). A C-type lectin, RegIIIγ is expressed by IEC, which forms a sterile isolation zone approximately 50 μm out from the intestinal epithelial tissue to restrict the contact between Gram-positive bacteria and the intestinal surface. In the intestine of RegIIIγ knockout mice, it was reported that the separation between intestinal mucosa and bacteria disappeared, which resulted in increased bacterial colonization of the intestinal epithelial surface (Vaishnava et al., 2011). The antibacterial mechanism of RegIII γ is mainly through binding to peptidoglycan on the surface of the Gram-positive bacterial cell wall to destroy the cell wall and membrane, resulting in cytoplasmic leakage and bacterial lysis (Cash et al., 2006). Plasma cells in the intestinal lamina propria produce sIgA, and are the main effector in the mucosal response. These immunoglobulins serve to neutralize bacterial toxins in the intestinal cavity and protect the mucosal surface, thus resisting pathogen infection and maintaining immune stability (JW et al., 1996; NJ et al., 2011; Pabst et al., 2016).
4.2. Composition of intestinal microorganisms
In recent years, it has been reported that antimicrobial peptides in the intestinal mucosal immune system play an important role in the regulation of the intestinal flora structure (Ganz, 1999; Ulm et al., 2012). At present, a variety of antimicrobial peptides have been identified in porcine intestines, including defensins, cathelicidins, hepcidin (liver expressed antimicrobial peptide-2), peptidoglycan recognition proteins (PGRP), liver expressed antimicrobial peptide-2 (LEAP-2), and NK-lysins (Sang and Blecha, 2009; Sang et al., 2006). The majority of recent studies have focused on the antibacterial mechanisms of defensin and cathelicidin proteins.
There are 3 classes of mammalian defensins, including α-, β-, and θ-defensins (Sang and Blecha, 2009). Secreted by neutrophils and IEC, defensins exhibit broad-spectrum antibacterial activity against Gram-positive and sexual bacteria, and in some cases, against fungi, viruses and protozoa (Selsted and Ouellette, 2005). At the time of publication, only porcine β-defensins (pBD) have been identified in pigs (Myer, 1982). The pBD contains 6 cysteine residues and can form 3 pairs of disulfide bonds, with pBD-1 and pBD-2 being the most studied (Yeaman and Yount, 2007). In vitro, pBD-1 showed broad-spectrum antibacterial activity when prepared in a 10 mM sodium phosphate buffer (pH7.4), and effectively killed pathogenic bacteria such as E. coli, Salmonella typhimurium, Listeria monocytogenes and Candida albicans (Shi et al., 1999). The treatment of S. typhimurium with a low dose of pBD-2 resulted in retraction of the bacterial cell membrane. When the concentration of pBD-2 is close to the minimum bactericidal concentration (MBC), it resulted in leakage of the bacterial cytoplasm and the complete dissolution of cells (EJ et al., 2008).
The family cathelicidin protein was the first class of mammalian antimicrobial peptides to be isolated (Sang and Blecha, 2009; Zhang et al., 2000). At present, 11 types of antimicrobial porcine cathelicidin peptides have been characterized, including amino acid peptide (PR-39) which is rich in proline and arginine, prophenin-1 (PF-1) that is rich in proline and phenylalanine, PG1 to PG-5, PF2 (rich in cysteine), and 3 porcine bone marrow antimicrobial peptides PMAP-23, PMAP-36, and PMAP-37 (Zanetti, 2005). Among these, PR-39 exhibits potent antibacterial effects on pathogenic gut bacteria, such as Enterococcus faecalis, E. coli and Bacillus subtilis (Veldhuizen et al., 2014). Lethal effect of PR-39 may be achieved by preventing protein synthesis and inducing the degradation of some proteins needed for DNA replication (Boman et al., 1993). PMAP-23 is a porcine antimicrobial peptide discovered in 1994 (Zanetti et al., 1994), which is rich in arginine and contains 2 α-helical regions. The C-terminal is lipophilic, can be inserted into cells to break cells, and has anti-Gram-positive and Gram-negative bacteria (Liu et al., 2020).
Although the above-described peptides exert antibacterial activity, the immunomodulatory and barrier protective effects of the antimicrobial peptides have not been thoroughly examined. At present, in order to reduce the use of antibiotics, antimicrobial peptides are intriguing and potential veterinary drugs for the prevention and treatment of diarrhea in piglets. The extraction and purification of natural antimicrobial peptides is very difficult, but can be obtained by artificial design, chemical synthesis or genetic engineering. It is also necessary to solve industrial bottlenecks such as poor stability in the gastrointestinal tract, low efficiency of oral absorption, and processing technology.
5. Regulation of intestinal microorganisms by the mucosal immune system
5.1. Intestinal lymphoid tissue development and epithelial barrier
In recent years, it has been demonstrated that intestinal flora is critical in the regulation of both the innate and adaptive immune systems (Honda and Littman, 2016; Thaiss et al., 2016). The germ-free mouse model is often used to explain how microflora in the digestive tract affects the development of the immune system. In the germ-free animal model, the intestinal immune system is immature, the intestinal lamina propria lacks plasma cells, and the number of T cells is strikingly low (Cebra, 1999). In addition, lymphoid follicles isolated from the small intestine of germ-free mice were not developed, and sIgA as well as CD8 α β IEL were lacking (Hooper et al., 2012). Compared with conventionally raised pigs, germ-free pigs have abnormal intestinal morphology, longer villi, lower crypt depth, lower crypt cell proliferation, and lower intestinal cell turnover (Willing and Van Kessel, 2007). In the preterm germ-free piglets, the mucus layer was thinner and the stratification was not obvious, the density of Lamina propria and submucosa cells of the ileum decreased, the fibrous tissue was loose and sparse, containing immature mesenchymal components, and the PP were smaller (Splichalova et al., 2018).
Microorganisms also play an important role in the regulation and repair of the physical intestinal barriers. For example, the probiotic E. coli strain Nissle 1917 enhances epithelial barrier function by up-regulating intestinal tight junction protein zonula occludens 1 (ZO-1), thus reducing intestinal epithelial permeability, and improves the incidence of gastrointestinal diseases (Ukena et al., 2007). Piglets fed Lactobacillus frumenti during d 6 to 20 before early weaning can significantly upregulate the expression of intestinal tight junction proteins (ZO-1, occludin and claudin-1), and had a significant increase in the levels of intestinal sIgA (Hu et al., 2018a).
Intestinal microbial pathogen associated molecular patterns (PAMP) such as LPS or flagellin can promote the secretion of the antibacterial protein RegIIIγ by IEC by activating the toll-like receptors-myeloid differentiation primary response 88 (TLR-MyD88) signaling pathway, which also appears to promote the repair of damaged IEC (Hooper et al., 2012; Vaishnava et al., 2011; Zhu et al., 2018). In addition, bacterial flagellin stimulates the production of interleukin (IL)-23 through the activation of TLR5, which is expressed on CD103+CD11b+ DC in the lamina propria. This in turn promotes the expression of IL-22 in innate lymphoid cells, which further stimulates the production of RegIIIγ (Kinnebrew et al., 2012; Macpherson and Ganal-Vonarburg, 2018). These examples illustrate the importance of microorganisms in inducing the development of the host immune system, but may represent only a small part of the many effects of the microflora on the host immune system. Moreover, current research has been limited to a small number of microorganisms, and the role of a large number of microbial populations in the intestinal barrier requires further exploration.
5.2. Regulation of T cell differentiation
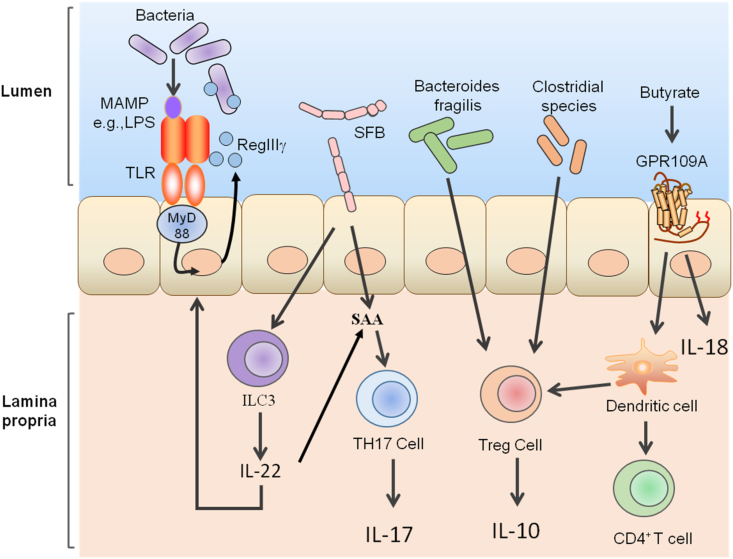
A large number of immune cells reside within the lamina propria of the intestinal tract. These cells include Th1 cells that produce interferon (IFN), Th17 cells that produce IL-17a, IL-17f and IL-22, innate lymphoid cells that produce various cytokines and Foxp3+ regulatory T cells (Treg) (Hooper et al., 2012). Under the action of intestinal microorganisms, pro-inflammatory and anti-inflammatory cells balance the responses, and jointly maintain the homeostasis of intestinal mucosa (Fig. 2).
Fig. 2.
Effect of intestinal microorganisms on the mucosal immune system. MAMP = microbe-associated molecular pattern; LPS = lipopolysaccharide. Intestinal microbial signals such as flagellin can promote the secretion of the antibacterial protein regenerating islet-derived III (RegIII)γ by intestinal epithelial cells through activation of the toll-like receptors myeloid differentiation primary response 88 (TLR-MyD88) signaling pathway. This promotes the repair of damaged intestinal epithelial cells. Segmented filamentous bacteria (SFB) can induce the expression of serum amyloid A (SAA) in the small intestine, thus promoting the differentiation of Th17 cells. In addition, SFB can induce intestinal innate lymphoid cell 3 (ILC3) to produce interleukin (IL)-22, induce epithelial expression of SAA, and promote the expression of IL-17 in Th17 cells. B. fragilis and Clostridial spp. Can induce regulatory T cells (Treg) differentiation. Butyrate, the product of bacteria fermentation of dietary fiber, induces the expression of IL-18 in intestinal epithelial cells (IEC) through G protein-coupled receptor 109 A (GPR109A) signaling. In addition, butyric acid can also promote the anti-inflammatory properties of colon dendritic cells through GPR109A signaling, enabling them to induce the differentiation of Treg cells and IL-10-producing CD4+ T cell cells.
A subgroup of effector helper T cells, Th17 cells, secrete cytokines such as IL-17, IL-22 and IFN-γ (Bellone et al., 2020). However, Th17 cells have been reported to contribute immunopathologic effects in many inflammatory diseases, but also serve to maintain the integrity of the intestinal barrier in a non-inflammatory manner (Lecuyer et al., 2014; Omenetti et al., 2019). The differentiation of Th17 cells in the lamina propria of the small intestine requires the participation of specific bacteria (Ivanov et al., 2008). Induction of Th17 cells by Citrobacter rodentium are inflammatory and mainly participate in aerobic glycolysis, which is usually related to inflammatory effector cells (Omenetti et al., 2019). The Th17 cells induced by segmented filamentous bacteria (SFB) are non-inflammatory and play an important role in the maintenance of intestinal homeostasis, the development of mucosal structure, the induction of IgA, and the maturation of postpartum intestinal immune function (Schnupf et al., 2013, 2017). Further, SFB can induce the expression of serum amyloid A (SAA) in the small intestine, and SAA also promotes the secretion of transforming growth factor (TGF)-β and IL-6 by DC. The synergistic effect of TGF-β and IL-6 is the key factor to initiating differentiation of Th17 cells (Ivanov et al., 2008). It has also been reported that SFB can transfer antigens to IEC and regulate host Th17 cell homeostasis through microbial adhesion-triggered endocytosis (Ladinsky et al., 2019).
Analysis by electron tomography showed that SFB could directly adhere to IEC, triggering the formation of endophagocytic vesicles at the adhesion site. These endocytic vesicles contained SFB cell wall derived bacterial proteins that induce the activation of Th17 cells. The endocytosed SFB proteins are then shuttled through the IEC endosomal lysosomal network, and induce the activation of lamina propria antigen-specific Th17 cells (Atarashi et al., 2015; Kumar et al., 2016; Ladinsky et al., 2019). This study suggests a new way for IEC to obtain antigens from intestinal symbionts and how microbial proteins communicate with the host to maintain mucosal T cell homeostasis. Although there are few Th17 cells in the lamina propria of germ-free mice (Ivanov et al., 2008), those inoculated with SFB exhibit increased differentiation and accumulation of Th17 cells, which results in increased numbers of Th1 cells (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009). Furthermore, SFB induces intestinal innate lymphoid cell 3 (ILC3) to produce IL-22, induces epithelial expression of SAA, and promotes Th17 cell differentiation and expression of IL-17 (Sano et al., 2016).
Regulatory T cells play an important role in adaptive immune tolerance (Russler-Germain et al., 2017). FOXP3+ regulatory T cells (FOXP3+ Treg) are a subtype of T cells that express CD4, CD25, and the transcription factor FOXP3. Although Treg cells expressing CD4 and Foxp3 are found in many organs throughout the body, the proportion is relatively high in the intestinal and colonic lamina propria (Honda and Littman, 2016), and is likely the key to regulating intestinal inflammation (Smith et al., 2013). Microorganisms can enhance the anti-inflammatory effect of the adaptive immune system by promoting the differentiation of Treg or through the induction of IL-10 expression (Russler-Germain et al., 2017). B. fragilis and clostridial species can induce Treg (Atarashi et al., 2011; JL and SK, 2010). B. fragilis exerts a beneficial immunomodulatory effect on the host immune system through capsular polysaccharide A (Deniz and Kasper, 2018), which acts on the heterodimer of TLR2/TLR1 on the surface of antigen-presenting cells, and the C-type lectin-like receptor Dectin-1. Binding to these receptors activates the PI3K signaling pathway, which results in the induction of host Treg to produce immunomodulatory cytokine IL-10 (Erturk-Hasdemir et al., 2019). Clostridium can induce colon Treg and play a central role in inhibiting inflammatory and hypersensitivity reactions (Atarashi et al., 2011). Colonization of gnotobiotic mice with a mixture of IV, XIVa and XVIII Clostridia strains isolated from mouse feces resulted in an increase in the number of Treg in the colonic lamina propria (Atarashi et al., 2013). At the same time, Clostridium strains were also demonstrated to promote the expression of IL-10 and cytotoxic T lymphocyte protein 4 in Treg cells. As a result, mice with high abundance of C. enterocolitica were resistant to colitis (Atarashi et al., 2011, 2013).
Metabolism by microbes in the intestinal lumen can produce butyrate, which has been demonstrated to promote the differentiation of Treg (Furusawa et al., 2013; N et al., 2013; Smith et al., 2013). Butyrate is produced by bacterial fermentation of dietary fiber, which is condensed from 2 acetyl-CoA molecules to acetoacetyl-CoA, and further reduced to butyryl-CoA. This molecule is then converted to butyrate via the phosphate transbutyrylase/butyrate kinase pathway (A et al., 2016; Liu et al., 2018). Butyrate can directly act on the immune cells in the intestinal mucosa, increase the number and activity of Treg, and inhibit the activity of neutrophils, macrophages, DC, and effector T cells (Gonçalves et al., 2018). In addition, it has been reported that butyrate promotes the anti-inflammatory properties of colon DC through G protein-coupled receptor 109 A (GPR109A) signaling, which induce the differentiation of Treg cells and CD4+ T cells. Butyrate also directly induces the expression of IL-18 in IEC through GPR109A signaling (Singh et al., 2014). Metabolomic analyses showed that the concentration of butyrate and the number of Treg cells in the colon were positively correlated. Similarly, it was demonstrated that butyrate induced Treg differentiation both in vitro and in vivo, and alleviated colitis in mice (Furusawa et al., 2013).
5.3. Regulation of flora metabolism nutrients on mucosal immunity
Dietary protein releases a large amount of tryptophan during digestion, and the intestinal flora converts it into indole and its derivatives (Roager and R, 2018; Zelante et al., 2013). Many of these indole derivatives are ligands of an aryl hydrocarbon receptor (AhR), such as indolealdehyde (IAld), indoleacetic acid (IAA), indolepropionic acid (IPA) and indole-3-acetaldehyde (IAAld) (Alexeev et al., 2018; Allison et al., 2018). Signaling by AhR is considered to be a key component of the barrier immune response, and is essential for intestinal epithelial renewal, maintenance of barrier integrity, and intestinal homeostasis (Lamas et al., 2018).
Lactobacillus spp. In the intestinal tract (such as Lactobacillus reuteri) can sense dietary tryptophan, rapidly promote its own proliferation, and produce the AhR ligand IAld, which activates the AhR-IL-22 axis. This pathway has been demonstrated to promote the colonization of a diverse flora, inhibiting colonization by C. albicans, and protecting mucous membrane from inflammation (Zelante et al., 2013). This study shows that the metabolites produced by intestinal flora metabolizing tryptophan are very important for balancing the intestinal immune system in mammals, and that intestinal flora can affect the symbiosis of host microorganisms through dietary tryptophan. In a recent study on pig production, fecal bacterial suspensions from healthy adult pigs were injected into newborn piglets, and it was found that it could regulate the diversity and composition of colonic flora in the piglets. It was also reported that the indole derivatives (IAA and IAId) produced by the colonic flora increased significantly. This in turn upregulation IL-22 expression and enhanced the activation of AhR in the piglet colonic mucosa to improve intestinal barrier function (S et al., 2018).
Butyrate also serves as the main energy source of IEC (A et al., 2016; T et al., 2012). Butyrate can inhibit the activity of histone deacetylase 3 (HDAC3), thus inducing metabolic changes of macrophages to increase the expression of antimicrobial peptides and enhance their bactericidal effect (J et al., 2019). In addition, urolithin A (UroA), produced by berry and pomegranate polyphenols is metabolized by intestinal flora, and can also activate AhR. It has been found that oral administration of UroA reduces systemic inflammation, increases epithelial tight junction protein expression, and prevents and relieves colitis in mice (Singh et al., 2019).
6. Regulation of microflora intervention on intestinal microecology of pigs
In pig production, healthy breeding is critical. Only when pigs have a healthy intestine can they grow efficiently and healthily. A healthy intestine is the sum of a balanced intestinal microecology and mucosal immunity.
6.1. Fecal microbiota transplantation (FMT)
FMT is one strategy to reconstruct the intestinal microecology, and is considered to be a breakthrough in recent years. This technology is not only widely studied in human medicine, but has been applied to pig production as well. An increasing number of studies have been conducted on flora transplantation from donor to recipient pigs. Here, we also list some studies on the effects of microbial intervention on intestinal mucosal immunity in pigs (Table 1). The fecal microorganisms of Chinese local Jinhua pigs were transferred to Duroc × Landrace × Yorkshire commercial piglets by FMT. It was found that the average daily gain of recipient pigs increased, rates of diarrhea decreased, intestinal morphology and physical barrier integrity improved, and the number of goblet cells, MUC2 expression, and immune-related receptor expression in the intestinal mucosa increased. High-throughput sequencing of 16S rDNA showed that the abundance of Prevotellaceae, Firmicutes, Ruminococcus and other microorganisms in the colon increased after transplantation (Hu et al., 2017). In a study by Cheng et al., the expression of 289 proteins in the intestinal mucosa of piglets receiving Jinhua pig FMT changed, and the expression of proteins related to inflammation in the intestinal mucosa decreased. The piglets were then infected with E. coli K88. Compared with the piglets that did not receive FMT, the abundance of beneficial bacteria such as Lactobacillus and Succinivibrio increased, the number of Enterobacteriaceae and Proteobacteria bacteria decreased, and the intestinal morphology and barrier integrity improved (Cheng et al., 2018). Furthermore, FMT enhanced the diversity and composition of colonic flora in piglets, which also resulted in increased levels of the tryptophan catabolite IAA in the colonic lumen, as well as IL-22 and AhR (Geng et al., 2018). Hu et al. transplanted healthy microorganisms from Jiangxiang pig feces to Landrace × Yorkshire piglets, which resulted in a significant reduction in diarrhea attributed to early weaning stress. The authors proposed a potential mechanism of this protection in which Lactobacillus gasseri LA39 and L. frumenti can secrete bacteriocin or gassericin A. These proteins bind to the host IEC membrane protein keratin 19, which appears to enhance intestinal water absorption, thus exerting the anti-diarrhea effect of FMT (Hu et al., 2018b).
Table 1.
Effects of microflora intervention on intestinal mucosal immunity in pigs.
| Age of pig | Breeds of pig | Microbial species | Mode of intervention | Design | Conclusions | Reference |
|---|---|---|---|---|---|---|
| 6 to 20 d | Landrace × Yorkshire crossbred piglets | Lactobacillus gasseri LA39 | Oral administration | Control group - oral PBS, LA39 - oral administrated of bacterial suspension (L. gasseri LA39 in PBS) | LA39 activates the oxidative phosphorylation pathway and increases the energy production in porcine intestinal epithelial cells | Hu et al. (2018c) |
| 6 to 20 d | Landrace × Yorkshire crossbred piglets | Lactobacillus frumenti | Oral gavage | Control group - sterile PBS, L. frumenti group - PBS suspension containing L. frumenti (108 CFU/mL) | The levels of intestinal sIgA, intestinal tight junction protein (including ZO-1, occludin and claudin-1) and interferon γ were significantly increased in L. frumenti group | Hu et al. (2018a) |
| 1 to 14 d | Duroc × Landrace × Yorkshire crossbred piglets | Chinese adult Jinhua pigs faecal microbiota suspension | FMT | FMT group - oral FMT, control group - orally inoculated with sterile PBS | FMT promotes IAA production in colonic lumen, enhance the activation of AhR and up-regulates IL-22 expression | Geng et al. (2018) |
| Days 70 and 100 of gestation | Large White × Landrace pregnant sows | Highly feed-efficient pigs faecal microbiota suspension | Gastric intubation | FMT group - FMT on d 70 and 100 of gestation | FMT group offspring have more goblet cells in the intestinal villi | McCormack et al. (2019) |
| 1 to 28 d | Large White × Landrace piglets | L. plantarum ZLP001 | Add freeze-dried L. plantarum ZLP001 to diet | Treatment group supplemented with freeze-dried L. plantarum ZLP001 | L. plantarum ZLP001 significantly enhanced expression of the 6 HDP in the jejunum | Wang et al. (2019c) |
| 1 to 20 d | Landrace × Yorkshire piglets | L. plantarum 299v | L. plantarum 299v dissolved in 2 mL of 0.1% peptone | Probiotic groups were given L. plantarum 299v dissolved in 2 mL of 0.1% peptone daily | Probiotic groups lower diarrhoea incidence, pBD2, pBD3 and ZO-1 expression increased in jejunum and ileum | Wang et al. (2019e) |
| 20 ± 2 d | The offspring of L 359 boars mated to Camborough females | Clostridium butyricum | Add C. butyricum to the pig diet | Add 1,250 × 108 CFU/kg, 2,500 × 108 CFU/kg and 3,500 × 108 CFU/kg C. butyricum to the diet of the treatment group | The depth of crypt and the width of villi increase with the dosage of C. butyricumto | Casas et al. (2020) |
| 1 to 3 d | Duroc × Landrace × Yorkshire piglets | Fecal microbiota from gestation sows combined with C. butyricum and Saccharomyces boulardii | Oral administration | FMT-CS group - gestation sows fecal microbiota combined with C. butyricum and S. boulardii | Early-life intervention significantly increased the alpha diversity of gut microbiota and plasma IL-22 and IL-17 | Xiang et al. (2020) |
| 1 to 6 d | Duroc × Landrace × Yorkshire piglets | Maternal FMT | Oral administration | FMT group-3 mL maternal fecal microbiota solution (>10 9 CFU/mL) | Increase of Blautia and decrease of C. sensu stricto in the relative abundances | Lin et al. (2018) |
| 1 to 2 d | Danish landrace × Large White × Duroc |
Colon microbiota suspension of suckling piglets | Oral + rectal administration or rectal FMT | Cesarean delivered preterm pigs were administered combined oral + rectal or rectal FMT | Only rectal FMT increased the stomach-to-colon pH gradient and resistance to mucosa bacterial adhesion | Brunse et al. (2019) |
| 1 to 10 d | Yorkshire piglets | Min sows (an indigenous pig breed in China) fecal microbiota suspension | Oral inoculation | Recipient group: oral FMT; control group: orally inoculated with sterile physiological saline | On d 21, the relative abundance of the Proteobacteria was reduced; the concentrations of IgM and IgG in the jejunal mucosa, and that of IgG in the ileal mucosa of the recipient group, were increased | Teng et al. (2020) |
PBS = phosphate buffer saline; sIgA = secretory immunoglobulin A; ZO-1 = zonula occludens 1; FMT = fecal microbiota transplantation; IAA = indoleacetic acid; AhR = aryl hydrocarbon receptor; HDP = host defense peptide; pBD = β-defensin; IgM = immunoglobulin M; IgG = immunoglobulin G.
These studies demonstrate the potential efficacy of FMT in pig production, and the important role of intestinal microorganisms in the health of the animals. Therefore, various techniques, including FMT, can be used to screen microorganisms that are beneficial to porcine intestinal health and promote the development of intestinal mucosal immunity. The rational tailoring of intestinal flora can be used to solve the common problems of diarrhea and abuse of anti-biologicals in breeding and production operations, and improve animal health.
The fecal suspensions used by FMT include bacteria, archaea, fungi, protozoa, viruses, cytokines and various metabolites. We do not know which microorganisms or which factors or products play a role in the treatment of which diseases. At present, all the microbiota in the faeces are transplanted across the board, which may not be the best way, and may bring the risk of unknown pathogenic microorganism infection. The selection of donors is particularly important in porcine FMT technology, and different donors may affect the efficacy of FMT technology. Donors must be strictly screened to minimize the risk of potential disease infection by the recipient. In addition, the pig manure suspension contains a large number of microorganisms and impurities, which may affect the efficacy of pig FMT technology. Therefore, how to highly purify fecal suspension without affecting the microbial activity in fecal suspension is worthy of further research and discussion.
6.2. Dietary nutritional composition
In previous research, the research hotspot of pig nutritional feed mainly focused on the regulation of nutrients on physiological function (Li et al., 2019). Along with the development of cross-disciplinary studies, this remains an important means to reshape intestinal health by changing the intestinal flora through nutritional intervention (mainly carbohydrates, amino acids and proteins) to regulate the imbalance of intestinal immunity in pigs.
Dietary fiber is the substrate of microbial fermentation in the pig intestinal tract. The fiber used in pig feed mainly includes corn fiber, soybean fiber and wheat bran. Different types of fiber have different effects on the composition of pig intestinal microflora and short-chain fatty acids (SCFA) due to different water-soluble water binding capacity. Highly diversified fiber diets can induce the increase of bacterial diversity (Van Nevel et al., 2006). Zhang et al. found that alfalfa diet increased the number of Clostridium cluster XIVb and Sporobacter termitidis in the cecum compared to the pure cellulose diet (Zhang et al., 2016). Long-term intake of fiber (maize fiber, soybean fiber, wheat bran fiber and pea fiber) with different fiber diets has different effects on intestinal bacterial composition and mucosal digestive physiology of (fattening pigs) in fattening pigs. Among them, wheat bran fiber and pea fiber could promote the morphology of porcine intestinal mucosa and the expression of disaccharidase and glucose transporter, while feeding soybean fiber damaged the digestive physiological structure of porcine intestinal mucosa (Chen et al., 2014).
Amino acid metabolism determines the activation and function of immune cells, such as the proliferation of immune cells and the secretion of cytokines, which is closely related to the fate of immune cells (Li et al., 2019). Arginine is the precursor of nitric oxide synthesis, which can promote macrophages and neutrophils to resist pathogen infection. L-arginine can reduce the expression of TLR4 induced by LPS, inhibit the activation of TLR4/nuclear factor κB (NFκB) and mitogen-activated protein kinase (MAPK) signal pathways, attenuate the inflammatory response induced by LPS, and stimulate the expression of pBD-1 in porcine epithelium by activating the target of rapamycin (mTOR) pathway in mammals (Lan et al., 2020). Adding 1.0% L-arginine to the diet of piglets could increase the serum insulin concentration and villus height of jejunum and ileum (Zheng et al., 2018). The above research results partially indicate that changes in dietary nutrients can affect intestinal health by changing microbial metabolism.
6.3. Probiotics and prebiotics
The feed industry has prohibited using antibiotics as a feed additive, and people's expectations for probiotic additives are increasing. As a microbial agent, probiotics help maintain the integrity and stability of the intestinal mucosa, the homeostasis of the flora, and promote intestinal immunity. Saccharomyces boulardii is widely used to relieve acute intestinal inflammation and acute diarrhea (Badia et al., 2012; Chang et al., 2017). Lactobacillus that relieves acute and chronic diarrhea and promotes intestinal development (Lee et al., 2009), and Clostridium butyricum can regulate intestinal immunity (Li et al., 2018). Studies have shown that Enterococcus faecium Strain NCIMB 10415 can reduce the abundance of E. coli virulence factors and reduce the number of pathogenic E. coli adhering to intestinal mucosa (Bednorz et al., 2013). C. butyricum increased the levels of intestinal tight junction proteins (ZO-1, claudin-3 and occluding) in pigs infected with enterotoxigenic E. coli K88, decreased the levels of IL-1β and IL-18 in the intestines, and increased the level of IL-10 (Li et al., 2018). The addition of C. butyricum and E. faecalis to the diet of weaned piglets can improve growth performance, which may regulate intestinal morphology by activating the TLR4-mediated MyD88-dependent signaling pathway (Wang et al., 2019d).
As a substitute for antibiotics, prebiotics are more and more widely used in the field of animal breeding. Prebiotics refers to a class of substances that cannot be digested by the host itself, which can selectively promote the growth or activity of specific bacteria in the intestinal tract, thereby promoting the health of the host, such as functional oligosaccharides, polysaccharides, polyols, protein hydrolysates, and plant extracts (Markowiak and Slizewska, 2018). Oligosaccharide prebiotics such as fructo-oligosaccharides can improve the intestinal mucosal barrier in suckling piglets (Schokker et al., 2018). Resistant starch increases the concentration of butyrate in the colon of weaned piglets and promotes intestinal immunity (Trachsel et al., 2019). At present, there are many studies on the application of prebiotics in pigs. Table 2 lists the experimental examples of the effects of prebiotics on pigs. Supplementation of 1,000 mg/kg chlorogenic acid to basal diet improves the intestinal barrier of weaned piglets by inhibiting the TLR4/NFκB signal pathway and activating the Nuclear factor erythropoietin-2-related factor 2/heme oxygenase 1 (NRF2/HO-1) signal pathway (Chen et al., 2018). Le Bourgot et al. (2017) reported that the addition of short-chain fructo-oligosaccharides to the diet of pregnant sows could increase the secretion of IFN-γ and IL-4 in the ileum of piglets, increase the number of cecal goblet cells and increase the content of IgA in serum and ileal mucosa. Wang et al. showed that feeding galactose oligosaccharides to piglets during lactation increased total SCFA concentration, and promoted the gene expression of inflammatory cytokines (IL-8 and IL-10) and tight junction proteins (ZO-1 and claudin) in the colon (Wang et al., 2019a). The utilization degree of different prebiotics by pig intestinal flora is different. There are differences in the degree of proliferation of beneficial bacteria and the degree of inhibition of harmful bacteria, and a lot of research needs to be done in its practical application.
Table 2.
Examples of trials regarding the effect of prebiotics on pig.
| Age of pig | Breeds of pig | Prebiotics | Main outcome | Reference |
|---|---|---|---|---|
| 2 to 14 d | Offspring of Topigs sows | FOS | Piglets supplemented with FOS had numerically greater villi and deeper crypts, modulates the bacterial colonization of the gut and the intestinal development | Schokker et al. (2018) |
| 21 to 42 d | Offspring of Large White crossbred sows | RPS | RPS intake increases the content of anaerobic bacteria in feces, and the concentration of butyrate in the intestine, and increases the content of regulatory T cells in the cecum | Trachsel et al. (2019) |
| 4 months | Large White male growing pigs | TGS | TGS down-regulated the caecal expression of zonula occludens-1 and mucin 2 and of genes within the toll-like receptor 4 and nuclear factor κB pro-inflammatory signaling cascade | Metzler-Zebeli et al. (2018) |
| 23 ± 2 d | Duroc × Landrace Large × White barrow | YG | YG group up-regulated the expression of occludin m-RNA in the duodenum and jejunum mucosa, increased the relative abundance of Lactobacillus, and decreased the acetate content | Qin et al. (2019) |
| From d 86 of gestation to d 20 of postpartum (sows); 7 to 35 d (piglets) | Landrace × Yorkshire sows and their offspring (Duroc × Landrace Yorkshire) | MOS | sow diet MOS decreased proinflammatory cytokines IL-2 and IL-4 concentrations in piglet serum, Piglets diet MOS decreased the contents of IL-2, IL-4 and interferon γ while increased anti-inflammatory cytokine IL-10 content in serum | Duan et al. (2019) |
| 21 to 35 d | Huanjiang mini-piglets | SBOS | SBOS supplementation increased elevated the numbers of beneficial intestinal bacteria, also increased the concentration of short-chain fatty acids in the intestinal lumen, and it reduced the numbers of bacteria with pathogenic potential (e.g., E. coli, Clostridium, and Streptococcus) | Zhou et al. (2014) |
| 28 d | Duroc × Landrace × Yorkshire piglets | AMSLF | AMSLF supplementation significantly decreased diarrheal incidence in piglets, 7.50% AMSLF group having higher IL-2 and TNF-α levels than the other treatment groups | Che et al. (2019) |
| First week after birth | Duroc × Landrace × Large White piglets | GOS | On d 8 and 21 after GOS intervention, ileal microbiota composition was significantly enriched in Lactobacillus and unclassified Lactobacillaceae, and the expression of anti-inflammatory cytokines and antibacterial peptides increased | Tian et al. (2019) |
| Approximately 8 weeks | Yorkshire-Landrace pigs | Inulin | Inulin supplementation up-regulated Th2-related immune genes (IL-13, IL-5), and suppressed Th1-related pro-inflammatory genes (interferon γ, IL-1A, IL-8) in the colon | Myhill et al. (2018) |
FOS = fructooligosaccharides; RPS = resistant potato starch; TGS = transglycosylated starch; YG = yeast glycoprotein; MOS = mannan oligosaccharide; SBOS = soybean oligosaccharides; AMSLF = Astragalus membranaceus fiber; GOS = galacto-oligosaccharide.
The combination of probiotics and prebiotics in the form of synergism is called synbiotics. Synbiotics have the characteristics of both probiotics and prebiotics. Probiotics can promote the balance of intestinal microecology, and prebiotics can provide nutritional substrates for probiotics, thus improving the viability of probiotics in the gastrointestinal tract (Vrese and Schrezenmeir, 2008). Many studies have shown that specific synbiotic combinations can have beneficial effects on the host. For example, the compound addition of probiotics and prebiotics can better improve the intestinal barrier function and health status of pigs. Adding 5% corn husk 108 CFU/g C. butyricum and can improve the growth performance of weaned piglets and reduce the mortality of piglets. It is further revealed that C. butyricum can improve the intestinal health of piglets by optimizing the structure of microbial flora and improving the production and utilization efficiency of acetate and butyrate (Zhao et al., 2018). The combined use of 0.2% Lactobacillus plantarum ZLP001 and 0.5% FOS in the diet of piglets could significantly increase the abundance of Lactobacillus, reduce the number of Enterobacteriaceae, and increase the concentration of serum INF-γ and IgG, showing a good synergistic effect (Wang et al., 2019b).
In the preparation of synbiotics, the selection of probiotics and prebiotics and their effects on intestinal microorganisms are very important. There are great differences in the methods, doses and duration of symbiotic studies. At present, it has gradually become a convenient method to study intestinal microflora and their metabolites to evaluate the effect of synbiotics by simulating the intestinal environment by fermentation in vitro.
7. Future prospects and challenges
Intestinal flora contributes to the development of and regulation of the intestinal mucosal immune system. Evidence also suggests that gut flora also plays a direct protective effect against pathogenic bacteria. At present, only a small number of intestinal bacteria have been studied with regards to their effects on the differentiation of immune cells. The data on most microorganisms are not clear about the differentiation of immune cells. In addition, the intestinal microflora also includes viruses, phages and eukaryotic microorganisms. It is well established that these organisms co-evolve with the host immune system, and the interaction between them and the immune system requires further in-depth analyses. Beyond interactions with the host immune system, in-depth studies on metabolic utilization pathways, immune regulation, and regulation of gene expression by intestinal flora through modern nutrition are likely to be hot fields of future discovery. Understanding the different types of intestinal microorganisms and their regulatory mechanisms, especially those between intestinal microflora and mucosal immunity, is of great significance to understanding optimum nutrition for various livestock industries, particularly pig production.
According to existing literature reports, there are still some challenges to promoting mucosal immunity through intestinal microorganisms. First of all, living intestinal microorganisms are different from any drug, and their particularity comes from the fact that the bacteria themselves are living cells. In the actual production and application, how many live bacteria can reach the intestinal tract? How can we better maintain the number of living bacteria by changing the dosage form, or adding some protective agents or microbial coating technology, so as to promote the colonization of microorganisms in the intestinal tract? In addition, with the application of microfluidic chip technology in biomedicine, the construction of the pig intestinal system in vitro plays an important role in realizing the cultivation of pig intestinal flora and studying the influence of different microorganisms and metabolites on intestinal mucosal immunity (Jalili-Firoozinezhad et al., 2019; Shah et al., 2016).
Author contributions
Yanhua Huang: conceptualization, review. Jie Peng: conceptualization, investigation, review. Yimei Tang: conceptualization, data curation, formal analysis, visualization, writing.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was financially supported by the Guangdong Basic and Applied Basic Research Foundation (2021A1515012417) and Research Fund of Zhongkai University of Agriculture and Engineering (KA190577872).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- A K., F D.V., P K.-D., F B. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Alain B.P.E., Chae J.P., Balolong M.P., Bum Kim H., Kang D.K. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J Gen Appl Microbiol. 2014;60:140–146. doi: 10.2323/jgam.60.140. [DOI] [PubMed] [Google Scholar]
- Alexeev E.E., Lanis J.M., Kao D.J., Campbell E.L., Kelly C.J., Battista K.D., Gerich M.E., Jenkins B.R., Walk S.T., Kominsky D.J. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am J Pathol. 2018;188(5):1183–1194. doi: 10.1016/j.ajpath.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire J.M., Crowley S.M., Law H.T., Chang S.-Y., Ko H.-J., Vallance B.A. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Allison A., Julien P., Harry S. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Arpaia N., Campbell C., Fan X., Dikiy S., Van Der Veeken J., Deroos P. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Ando M., Kamada N., Nagano Y., Narushima S., Suda W., Imaoka A., Setoyama H., Nagamori T. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell. 2015;163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Oshima K., Suda W., Nagano Y., Nishikawa H., Fukuda S., Saito T., Narushima S., Hase K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia R., Zanello G., Chevaleyre C., Lizardo R., Meurens F., Martinez P., Brufau J., Salmon H. Effect of Saccharomyces cerevisiae var. Boulardii and beta-galactomannan oligosaccharide on porcine intestinal epithelial and dendritic cells challenged in vitro with Escherichia coli F4 (K88) Vet Res. 2012;43:4. doi: 10.1186/1297-9716-43-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednorz C., Guenther S., Oelgeschlager K., Kinnemann B., Pieper R., Hartmann S., Tedin K., Semmler T., Neumann K., Schierack P. Feeding the probiotic Enterococcus faecium strain NCIMB 10415 to piglets specifically reduces the number of Escherichia coli pathotypes that adhere to the gut mucosa. Appl Environ Microbiol. 2013;79:7896–7904. doi: 10.1128/AEM.03138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone M., Brevi A., Huber S. Microbiota-propelled T helper 17 cells in inflammatory diseases and cancer. Microbiology and molecular biology reviews : MMBR (Microbiol Mol Biol Rev) 2020;84 doi: 10.1128/MMBR.00064-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H.G., Agerberth B., Boman A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect Immun. 1993;61:2978–2984. doi: 10.1128/iai.61.7.2978-2984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunse A., Martin L., Rasmussen T.S., Christensen L., Skovsted Cilieborg M., Wiese M., Khakimov B., Pieper R., Nielsen D.S., Sangild P.T. Effect of fecal microbiota transplantation route of administration on gut colonization and host response in preterm pigs. ISME J. 2019;13:720–733. doi: 10.1038/s41396-018-0301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas G.A., Blavi L., Cross T.L., Lee A.H., Swanson K.S., Stein H.H. Inclusion of the direct-fed microbial Clostridium butyricum in diets for weanling pigs increases growth performance and tends to increase villus height and crypt depth, but does not change intestinal microbial abundance. J Anim Sci. 2020;98 doi: 10.1093/jas/skz372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash H.L., Whitham C.V., Behrendt C.L., Hooper L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro G.A., Arntzen C.J. Immunophysiology of the gut: a research frontier for integrative studies of the common mucosal immune system. Am J Physiol. 1993;265:G599–G610. doi: 10.1152/ajpgi.1993.265.4.G599. [DOI] [PubMed] [Google Scholar]
- Cebra J.J. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69:1046S–1051S. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- Chang C., Wang K., Zhou S.N., Wang X.D., Wu J.E. Protective effect of Saccharomyces boulardii on deoxynivalenol-induced injury of porcine macrophage via attenuating p38 MAPK signal pathway. Appl Biochem Biotechnol. 2017;182:411–427. doi: 10.1007/s12010-016-2335-x. [DOI] [PubMed] [Google Scholar]
- Che D., Adams S., Wei C., Gui-Xin Q., Atiba E.M., Hailong J. Effects of Astragalus membranaceus fiber on growth performance, nutrient digestibility, microbial composition, VFA production, gut pH, and immunity of weaned pigs. MicrobiologyOpen. 2019;8 doi: 10.1002/mbo3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Mao X.B., Che L.Q., Yu B., He J., Yu J., Han G.Q., Huang Z.Q., Zheng P., Chen D.W. Impact of fiber types on gut microbiota, gut environment and gut function in fattening pigs. Animal Feed ence & Technology. 2014;195:101–111. [Google Scholar]
- Chen J., Yu B., Chen D., Huang Z., Mao X., Zheng P., Yu J., Luo J., He J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J Nutr Biochem. 2018;59:84–92. doi: 10.1016/j.jnutbio.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Cheng S., Ma X., Geng S., Jiang X., Li Y., Hu L., Li J., Wang Y., Han X. vol. 3. mSystems; 2018. (Fecal microbiota transplantation beneficially regulates intestinal mucosal autophagy and alleviates gut barrier injury). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniz E.H., Kasper D.L. Finding a needle in a haystack: Bacteroides fragilis polysaccharide A as the archetypical symbiosis factor. Ann N Y Acad Sci. 2018;1417 doi: 10.1111/nyas.13660. [DOI] [PubMed] [Google Scholar]
- Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Tian G., Chen D., Huang L., Zhang D., Zheng P., Mao X., Yu J., He J., Huang Z. Mannan oligosaccharide supplementation in diets of sow and (or) their offspring improved immunity and regulated intestinal bacteria in piglet1. J Anim Sci. 2019;97:4548–4556. doi: 10.1093/jas/skz318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ej V., M R., Ea C., A, v.D., Hp H. Porcine beta-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol Immunol. 2008;45:386–394. doi: 10.1016/j.molimm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Erturk-Hasdemir D., Oh S.F., Okan N.A., Stefanetti G., Gazzaniga F.S., Seeberger P.H., Plevy S.E., Kasper D.L. Proceedings of the national academy of sciences of the United States of America. 2019. Symbionts exploit complex signaling to educate the immune system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese S.A., Parker K., Calvert C.C., Mills D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. 2015;3:28. doi: 10.1186/s40168-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V., Rakotobe S., Lecuyer E., Mulder I., Lan A., Bridonneau C., Rochet V., Pisi A., De Paepe M., Brandi G. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins and host defense. Science. 1999;286:420–421. doi: 10.1126/science.286.5439.420. [DOI] [PubMed] [Google Scholar]
- Geng S., Cheng S., Li Y., Wen Z., Ma X., Jiang X., Wang Y., Han X. Faecal microbiota transplantation reduces susceptibility to epithelial injury and modulates tryptophan metabolism of the microbial community in a piglet model. J Crohns Colitis. 2018;12:1359–1374. doi: 10.1093/ecco-jcc/jjy103. [DOI] [PubMed] [Google Scholar]
- Gonçalves P., Araújo J.R., Di Santo J.P. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:558–572. doi: 10.1093/ibd/izx029. [DOI] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- Hooper L.V. Epithelial cell contributions to intestinal immunity. Adv Immunol. 2015;126:129–172. doi: 10.1016/bs.ai.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Chen L., Zheng W., Shi M., Liu L., Xie C., Wang X., Niu Y., Hou Q., Xu X. Lactobacillus frumenti facilitates intestinal epithelial barrier function maintenance in early-weaned piglets. Front Microbiol. 2018;9:897. doi: 10.3389/fmicb.2018.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Ma L., Nie Y., Chen J., Zheng W., Wang X., Xie C., Zheng Z., Wang Z., Yang T. A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe. 2018;24:817–832. doi: 10.1016/j.chom.2018.11.006. e818. [DOI] [PubMed] [Google Scholar]
- Hu J., Ma L., Zheng W., Nie Y., Yan X. Lactobacillus gasseri LA39 activates the oxidative phosphorylation pathway in porcine intestinal epithelial cells. Front Microbiol. 2018;9:3025. doi: 10.3389/fmicb.2018.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Geng S., Li Y., Cheng S., Fu X., Yue X., Han X. Exogenous fecal microbiota transplantation from local adult pigs to crossbred newborn piglets. Front Microbiol. 2017;8:2663. doi: 10.3389/fmicb.2017.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima H., Takahashi I., Kiyono H. Mucosal immune network in the gut for the control of infectious diseases. Rev Med Virol. 2001;11:117–133. doi: 10.1002/rmv.307. [DOI] [PubMed] [Google Scholar]
- Isaacson R., Kim H.B. The intestinal microbiome of the pig. Anim Health Res Rev. 2012;13:100–109. doi: 10.1017/S1466252312000084. [DOI] [PubMed] [Google Scholar]
- Ivanov, Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Frutos Rde L., Manel N., Yoshinaga K., Rifkin D.B., Sartor R.B., Finlay B.B., Littman D.R. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J S., S P., M C., Kc R.-A., I A., F F., A C., Ne I., Dgw J., E P. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50:432–445. doi: 10.1016/j.immuni.2018.12.018. e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili-Firoozinezhad S., Gazzaniga F.S., Calamari E.L., Camacho D.M., Fadel C.W., Bein A., Swenor B., Nestor B., Cronce M.J., Tovaglieri A. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nature biomedical engineering. 2019;3:520–531. doi: 10.1038/s41551-019-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jf B., Mt A. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol. 2020;17(5):263–278. doi: 10.1038/s41575-019-0261-4. [DOI] [PubMed] [Google Scholar]
- Jl R., Sk M. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E., Gustafsson J.K., Holmen-Larsson J., Jabbar K.S., Xia L., Xu H., Ghishan F.K., Carvalho F.A., Gewirtz A.T., Sjovall H. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jw B., M S.-P., Aa K., Hb G. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science (New York, NY) 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- Kim H.B., Isaacson R.E. The pig gut microbial diversity: understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet Microbiol. 2015;177:242–251. doi: 10.1016/j.vetmic.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnebrew M.A., Buffie C.G., Diehl G.E., Zenewicz L.A., Leiner I., Hohl T.M., Flavell R.A., Littman D.R., Pamer E.G. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop K.A., Newberry R.D. Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018;11:1551–1557. doi: 10.1038/s41385-018-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Takahashi D., Takano S., Kimura S., Hase K. The roles of peyer's patches and Microfold cells in the gut immune system: relevance to autoimmune diseases. Front Immunol. 2019;10:2345. doi: 10.3389/fimmu.2019.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Monin L., Castillo P., Elsegeiny W., Horne W., Eddens T., Vikram A., Good M., Schoenborn A.A., Bibby K. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity. 2016;44:659–671. doi: 10.1016/j.immuni.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky M.S., Araujo L.P., Zhang X., Veltri J., Galan-Diez M., Soualhi S., Lee C., Irie K., Pinker E.Y., Narushima S. Endocytosis of commensal antigens by intestinal epithelial cells regulates mucosal T cell homeostasis. Science. 2019;363 doi: 10.1126/science.aat4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas B., Natividad J.M., Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11 doi: 10.1038/s41385-018-0019-2. [DOI] [PubMed] [Google Scholar]
- Lamendella R., Domingo J.W., Ghosh S., Martinson J., Oerther D.B. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol. 2011;11:103. doi: 10.1186/1471-2180-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Dou X., Li J., Yang Y., Xue C., Wang C., Gao N., Shan A. l-Arginine ameliorates lipopolysaccharide-induced intestinal inflammation through inhibiting the TLR4/NF-kappaB and MAPK pathways and stimulating beta-defensin expression in vivo and in vitro. J Agric Food Chem. 2020;68:2648–2663. doi: 10.1021/acs.jafc.9b07611. [DOI] [PubMed] [Google Scholar]
- Le Bourgot C., Le Normand L., Formal M., Respondek F., Blat S., Apper E., Ferret-Bernard S., Le Huerou-Luron I. Maternal short-chain fructo-oligosaccharide supplementation increases intestinal cytokine secretion, goblet cell number, butyrate concentration and Lawsonia intracellularis humoral vaccine response in weaned pigs. Br J Nutr. 2017;117:83–92. doi: 10.1017/S0007114516004268. [DOI] [PubMed] [Google Scholar]
- Lecuyer E., Rakotobe S., Lengline-Garnier H., Lebreton C., Picard M., Juste C., Fritzen R., Eberl G., McCoy K.D., Macpherson A.J. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity. 2014;40:608–620. doi: 10.1016/j.immuni.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Lee D.Y., Seo Y.S., Rayamajhi N., Kang M.L., Lee S.I., Yoo H.S. Isolation, characterization, and evaluation of wild isolates of Lactobacillus reuteri from pig feces. J Microbiol. 2009;47:663–672. doi: 10.1007/s12275-009-0124-8. [DOI] [PubMed] [Google Scholar]
- Li H.H., Li Y.P., Zhu Q., Qiao J.Y., Wang W.J. Dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88. J Appl Microbiol. 2018;125:964–975. doi: 10.1111/jam.13936. [DOI] [PubMed] [Google Scholar]
- Li R., Hou G., Jiang X., Song Z., He X. Different dietary protein sources in low protein diet regulate colonic microbiota and barrier function in a piglet model. Food & Function. 2019;10 doi: 10.1039/c9fo01154d. [DOI] [PubMed] [Google Scholar]
- Lidell M.E., Moncada D.M., Chadee K., Hansson G.C. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci USA. 2006;103:9298–9303. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Wan J., Su Y., Zhu W. Effects of early intervention with maternal fecal microbiota and antibiotics on the gut microbiota and metabolite profiles of piglets. Metabolites. 2018;8 doi: 10.3390/metabo8040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang J., He T., Becker S., Zhang G., Li D., Ma X. Butyrate: a double-edged sword for health? Advances in Nutrition. 2018;9:21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Shen T., Chen L., Zhou J., Wang C. Analogs of the cathelicidin-derived antimicrobial peptide PMAP-23 exhibit improved stability and antibacterial activity. Probiotics and antimicrobial proteins. 2020;13(1):273–286. doi: 10.1007/s12602-020-09686-z. [DOI] [PubMed] [Google Scholar]
- Looft T., Allen H.K., Cantarel B.L., Levine U.Y., Bayles D.O., Alt D.P., Henrissat B., Stanton T.B. Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. 2014;8:1566–1576. doi: 10.1038/ismej.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson A.J., Ganal-Vonarburg S.C. Checkpoint for gut microbes after birth. Nature. 2018;560:436–438. doi: 10.1038/d41586-018-05861-z. [DOI] [PubMed] [Google Scholar]
- Markowiak P., Slizewska K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018;10:21. doi: 10.1186/s13099-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack U.M., Curiao T., Metzler-Zebeli B.U., Wilkinson T., Reyer H., Crispie F., Cotter P.D., Creevey C.J., Gardiner G.E., Lawlor P.G. Improvement of feed efficiency in pigs through microbial modulation via fecal microbiota transplantation in sows and dietary supplementation of inulin in offspring. Appl Environ Microbiol. 2019;85 doi: 10.1128/AEM.01255-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Zebeli B.U., Newman M.A., Grull D., Zebeli Q. Consumption of transglycosylated starch down-regulates expression of mucosal innate immune response genes in the large intestine using a pig model. Br J Nutr. 2018;119:1366–1377. doi: 10.1017/S0007114518001113. [DOI] [PubMed] [Google Scholar]
- Morfitt D.C., Pohlenz J.F. Porcine colonic lymphoglandular complex: distribution, structure, and epithelium. Am J Anat. 1989;184:41–51. doi: 10.1002/aja.1001840105. [DOI] [PubMed] [Google Scholar]
- Myer M.S. The presence of Paneth cells confirmed in the pig. Onderstepoort J Vet Res. 1982;49:131–132. [PubMed] [Google Scholar]
- Myhill L.J., Stolzenbach S., Hansen T.V.A., Skovgaard K., Stensvold C.R., Andersen L.O., Nejsum P., Mejer H., Thamsborg S.M., Williams A.R. Mucosal barrier and Th2 immune responses are enhanced by dietary inulin in pigs infected with trichuris suis. Front Immunol. 2018;9:2557. doi: 10.3389/fimmu.2018.02557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagura H., Sumi Y. Immunological functions of the gut--role of the mucosal immune system. Toxicol Pathol. 1988;16:154–164. doi: 10.1177/019262338801600208. [DOI] [PubMed] [Google Scholar]
- Neutra M.R., Frey A., Kraehenbuhl J.P. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- Nj M., N R., B C. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowland T.L., Plush K.J., Barton M., Kirkwood R.N. Development and function of the intestinal microbiome and potential implications for pig production. Animals : an open access journal from MDPI. 2019;9 doi: 10.3390/ani9030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenetti S., Bussi C., Metidji A., Iseppon A., Lee S., Tolaini M., Li Y., Kelly G., Chakravarty P., Shoaie S. The intestine harbors functionally distinct homeostatic tissue-resident and inflammatory Th17 cells. Immunity. 2019;51:77–89. doi: 10.1016/j.immuni.2019.05.004. e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O., Cerovic V., Hornef M. Secretory IgA in the coordination of establishment and maintenance of the microbiota. Trends Immunol. 2016;37(5):287–296. doi: 10.1016/j.it.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Pabst R., Rothkotter H.J. Structure and function of the gut mucosal immune system. Adv Exp Med Biol. 2006;579:1–14. doi: 10.1007/0-387-33778-4_1. [DOI] [PubMed] [Google Scholar]
- Portune K.J., Benítez-Páez A., Del Pulgar E.M., Cerrudo V., Sanz Y. Gut microbiota, diet, and obesity-related disorders-The good, the bad, and the future challenges. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201600252. [DOI] [PubMed] [Google Scholar]
- Potockova H., Sinkorova J., Karova K., Sinkora M. The distribution of lymphoid cells in the small intestine of germ-free and conventional piglets. Dev Comp Immunol. 2015;51:99–107. doi: 10.1016/j.dci.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Powell N., Walker M.M., Talley N.J. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol. 2017;14:143–159. doi: 10.1038/nrgastro.2016.191. [DOI] [PubMed] [Google Scholar]
- Propheter D.C., Chara A.L., Harris T.A., Ruhn K.A., Hooper L.V. Resistin-like molecule beta is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proc Natl Acad Sci USA. 2017;114:11027–11033. doi: 10.1073/pnas.1711395114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Ji W., Wang J., Li B., Hu J., Wu X. Effects of dietary supplementation with yeast glycoprotein on growth performance, intestinal mucosal morphology, immune response and colonic microbiota in weaned piglets. Food & function. 2019;10:2359–2371. doi: 10.1039/c8fo02327a. [DOI] [PubMed] [Google Scholar]
- Roager H.M., R L.T. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9:3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkotter H.J. Anatomical particularities of the porcine immune system-a physician's view. Dev Comp Immunol. 2009;33:267–272. doi: 10.1016/j.dci.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russler-Germain E.V., Rengarajan S., Hsieh C.S. Antigen-specific regulatory T-cell responses to intestinal microbiota. Mucosal Immunol. 2017;10:1375–1386. doi: 10.1038/mi.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S G., S C., Y L., Z W., X M., X J., Y W., X H. Faecal microbiota transplantation reduces susceptibility to epithelial injury and modulates tryptophan metabolism of the microbial community in a piglet model. Journal of Crohn's & colitis. 2018;12:1359–1374. doi: 10.1093/ecco-jcc/jjy103. [DOI] [PubMed] [Google Scholar]
- Salmon H., Berri M., Gerdts V., Meurens F. Humoral and cellular factors of maternal immunity in swine. Dev Comp Immunol. 2009;33:384–393. doi: 10.1016/j.dci.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Sang Y., Blecha F. Porcine host defense peptides: expanding repertoire and functions. Dev Comp Immunol. 2009;33:334–343. doi: 10.1016/j.dci.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Sang Y., Ramanathan B., Minton J.E., Ross C.R., Blecha F. Porcine liver-expressed antimicrobial peptides, hepcidin and LEAP-2: cloning and induction by bacterial infection. Dev Comp Immunol. 2006;30:357–366. doi: 10.1016/j.dci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Sano T., Huang W., Hall J.A., Yang Y., Chen A., Gavzy S.J., Lee J.Y., Ziel J.W., Miraldi E.R., Domingos A.I. An IL-23r/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell. 2016;164:324. doi: 10.1016/j.cell.2015.12.047. [DOI] [PubMed] [Google Scholar]
- Schnupf P., Gaboriau-Routhiau V., Cerf-Bensussan N. Host interactions with Segmented Filamentous Bacteria: an unusual trade-off that drives the post-natal maturation of the gut immune system. Semin Immunol. 2013;25:342–351. doi: 10.1016/j.smim.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Schnupf P., Gaboriau-Routhiau V., Sansonetti P.J., Cerf-Bensussan N. Segmented filamentous bacteria, Th17 inducers and helpers in a hostile world. Curr Opin Microbiol. 2017;35:100–109. doi: 10.1016/j.mib.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Schokker D., Fledderus J., Jansen R., Vastenhouw S.A., de Bree F.M., Smits M.A., Jansman A. Supplementation of fructooligosaccharides to suckling piglets affects intestinal microbiota colonization and immune development. J Anim Sci. 2018;96:2139–2153. doi: 10.1093/jas/sky110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M.E., Ouellette A.J. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- Shah P., Fritz J.V., Glaab E., Desai M.S., Greenhalgh K., Frachet A., Niegowska M., Estes M., Jager C., Seguin-Devaux C. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat Commun. 2016;7:11535. doi: 10.1038/ncomms11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Zhang G., Wu H., Ross C., Blecha F., Ganz T. Porcine epithelial beta-defensin 1 is expressed in the dorsal tongue at antimicrobial concentrations. Infect Immun. 1999;67:3121–3127. doi: 10.1128/iai.67.6.3121-3127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., Thangaraju M., Prasad P.D., Manicassamy S., Munn D.H. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Chandrashekharappa S., Bodduluri S.R., Baby B.V., Hegde B., Kotla N.G. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10:89. doi: 10.1038/s41467-018-07859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifierz M.J., Friendship R.M., Weese J.S. Longitudinal study of the early-life fecal and nasal microbiotas of the domestic pig. BMC Microbiol. 2015;15:184. doi: 10.1186/s12866-015-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly Y.M., Glickman J.N., Garrett W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F., Backhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Sonnenberg G.F., Artis D. Novel connections and precision approaches. Nat Rev Immunol. 2019;19:75–76. doi: 10.1038/s41577-018-0114-3. [DOI] [PubMed] [Google Scholar]
- Splichalova A., Slavikova V., Splichalova Z., Splichal I. Preterm life in sterile conditions: a study on preterm, germ-free piglets. Front Immunol. 2018;9:220. doi: 10.3389/fimmu.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C.R. The development and role of microbial-host interactions in gut mucosal immune development. J Anim Sci Biotechnol. 2017;8:12. doi: 10.1186/s40104-016-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T L., J L., Y L., N X., H S., K X., C Y., C W. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation. 2012;35:1676–1684. doi: 10.1007/s10753-012-9484-z. [DOI] [PubMed] [Google Scholar]
- Teng T., Gao F., He W., Fu H., Guo J., Bai G., Shi B. An early fecal microbiota transfer improves the intestinal conditions on microflora and immunoglobulin and antimicrobial peptides in piglets. J Agric Food Chem. 2020;68:4830–4843. doi: 10.1021/acs.jafc.0c00545. [DOI] [PubMed] [Google Scholar]
- Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- Tian S., Wang J., Yu H., Wang J., Zhu W. Changes in ileal microbial composition and microbial metabolism by an early-life galacto-oligosaccharides intervention in a neonatal porcine model. Nutrients. 2019;11 doi: 10.3390/nu11081753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel J., Briggs C., Gabler N.K., Allen H.K., Loving C.L. Dietary resistant potato starch alters intestinal microbial communities and their metabolites, and markers of immune regulation and barrier function in swine. Front Immunol. 2019;10:1381. doi: 10.3389/fimmu.2019.01381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Ukena S.N., Singh A., Dringenberg U., Engelhardt R., Seidler U., Hansen W., Bleich A., Bruder D., Franzke A., Rogler G. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PloS One. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm H., Wilmes M., Shai Y., Sahl H.G. Antimicrobial host defensins - specific antibiotic activities and innate defense modulation. Front Immunol. 2012;3:249. doi: 10.3389/fimmu.2012.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S., Yamamoto M., Severson K.M., Ruhn K.A., Yu X., Koren O., Ley R., Wakeland E.K., Hooper L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel T.P., Pires J., Silvester R., Zhao C., Song J., Criscuolo N.G., Gilbert M., Bonhoeffer S., Laxminarayan R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365 doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- Van Nevel C.J., Dierick N.A., Decuypere J.A., De Smet S.M. In vitro fermentability and physicochemical properties of fibre substrates and their effect on bacteriological and morphological characteristics of the gastrointestinal tract of newly weaned piglets. Arch Anim Nutr. 2006;60:477–500. doi: 10.1080/17450390600973659. [DOI] [PubMed] [Google Scholar]
- Veldhuizen E.J., Schneider V.A., Agustiandari H., van Dijk A., Tjeerdsma-van Bokhoven J.L., Bikker F.J., Haagsman H.P. Antimicrobial and immunomodulatory activities of PR-39 derived peptides. PloS One. 2014;9 doi: 10.1371/journal.pone.0095939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrese M., Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Advances in biochemical engineering/biotechnology. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- Wang J., Tian S., Yu H., Wang J., Zhu W. Response of colonic mucosa-associated microbiota composition, mucosal immune homeostasis, and barrier function to early life galactooligosaccharides intervention in suckling piglets. J Agric Food Chem. 2019;67:578–588. doi: 10.1021/acs.jafc.8b05679. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang S., Liu H., Zhang D., Wang Y., Ji H. Effects of oligosaccharides on the growth and stress tolerance of Lactobacillus plantarum ZLP001 in vitro, and the potential synbiotic effects of L. plantarum ZLP001 and fructo-oligosaccharide in post-weaning piglets1. J Anim Sci. 2019;97:4588–4597. doi: 10.1093/jas/skz254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhang W., Wang S., Liu H., Zhang D., Wang Y., Ji H. Swine-derived probiotic Lactobacillus plantarum modulates porcine intestinal endogenous host defense peptide synthesis through TLR2/MAPK/AP-1 signaling pathway. Front Immunol. 2019;10:2691. doi: 10.3389/fimmu.2019.02691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Chen G., Cao G., Xu Y., Wang Y., Yang C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, intestinal structure, and inflammation in lipopolysaccharide-challenged weaned piglets. J Anim Sci. 2019;97:4140–4151. doi: 10.1093/jas/skz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Sun Q., Qi R., Wang J., Qiu X., Liu Z., Huang J. Effects of Lactobacillus plantarum on the intestinal morphology, intestinal barrier function and microbiota composition of suckling piglets. J Anim Physiol Anim Nutr. 2019;103:1908–1918. doi: 10.1111/jpn.13198. [DOI] [PubMed] [Google Scholar]
- Wang X., Tsai T., Deng F., Wei X., Chai J., Knapp J., Apple J., Maxwell C.V., Lee J.A., Li Y. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome. 2019;7:109. doi: 10.1186/s40168-019-0721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing B.P., Van Kessel A.G. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J Anim Sci. 2007;85:3256–3266. doi: 10.2527/jas.2007-0320. [DOI] [PubMed] [Google Scholar]
- Xiang Q., Wu X., Pan Y., Wang L., Cui C., Guo Y., Zhu L., Peng J., Wei H. Early-Life intervention using fecal microbiota combined with probiotics promotes gut microbiota maturation, regulates immune system development, and alleviates weaning stress in piglets. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Pascual D.W., Kiyono H. M cell-targeted mucosal vaccine strategies. Curr Top Microbiol Immunol. 2012;354:39–52. doi: 10.1007/82_2011_134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M.R., Yount N.Y. Unifying themes in host defence effector polypeptides. Nat Rev Microbiol. 2007;5:727–740. doi: 10.1038/nrmicro1744. [DOI] [PubMed] [Google Scholar]
- Yin J., Li Y., Han H., Chen S., Gao J., Liu G., Wu X., Deng J., Yu Q., Huang X. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res. 2018;65 doi: 10.1111/jpi.12524. [DOI] [PubMed] [Google Scholar]
- Yin J., Li Y., Han H., Ma J., Liu G., Wu X., Huang X., Fang R., Baba K., Bin P. Administration of exogenous melatonin improves the diurnal rhythms of the gut microbiota in mice fed a high-fat diet. mSystems. 2020;5 doi: 10.1128/mSystems.00002-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y.B., de Jonge H.R., Wu X., Yin Y.L. Enteroids for nutritional studies. Mol Nutr Food Res. 2019;63 doi: 10.1002/mnfr.201801143. [DOI] [PubMed] [Google Scholar]
- Yu H., He Y., Zhang X., Peng Z., Yang Y., Zhu R., Bai J., Tian Y., Li X., Chen W. The rat IgGFcgammaBP and Muc2 C-terminal domains and TFF3 in two intestinal mucus layers bind together by covalent interaction. PloS One. 2011;6 doi: 10.1371/journal.pone.0020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7:179–196. [PubMed] [Google Scholar]
- Zanetti M., Storici P., Tossi A., Scocchi M., Gennaro R. Molecular cloning and chemical synthesis of a novel antibacterial peptide derived from pig myeloid cells. J Biol Chem. 1994;269:7855–7858. [PubMed] [Google Scholar]
- Zelante T., Iannitti R.G., Cunha C., De?Luca A., Giovannini G., Pieraccini G., Zecchi R., D'Angelo C., Massi-Benedetti C., Fallarino F. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang G., Ross C.R., Blecha F. Porcine antimicrobial peptides: new prospects for ancient molecules of host defense. Vet Res. 2000;31:277–296. doi: 10.1051/vetres:2000121. [DOI] [PubMed] [Google Scholar]
- Zhang L., Mu C., He X., Su Y., Mao S., Zhang J., Smidt H., Zhu W. Effects of dietary fibre source on microbiota composition in the large intestine of suckling piglets. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw138. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu W., Lee Y.K., Xie J., Zhang H. Spatial heterogeneity and Co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front Microbiol. 2018;9:48. doi: 10.3389/fmicb.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Liu P., Wu Y., Guo P., Liu L., Ma N., Levesque C., Chen Y., Zhao J., Zhang J. Dietary fiber increases butyrate-producing bacteria and improves the growth performance of weaned piglets. J Agric Food Chem. 2018;66:7995–8004. doi: 10.1021/acs.jafc.8b02545. [DOI] [PubMed] [Google Scholar]
- Zheng P., Song Y., Tian Y., Zhang H., Yu B., He J., Mao X., Yu J., Luo Y., Luo J. Dietary arginine supplementation affects intestinal function by enhancing antioxidant capacity of a nitric oxide-independent pathway in low-birth-weight piglets. J Nutr. 2018;148:1751–1759. doi: 10.1093/jn/nxy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.L., Kong X.F., Lian G.Q., Blachier F., Geng M.M., Yin Y.L. Dietary supplementation with soybean oligosaccharides increases short-chain fatty acids but decreases protein-derived catabolites in the intestinal luminal content of weaned Huanjiang mini-piglets. Nutr Res (NY) 2014;34:780–788. doi: 10.1016/j.nutres.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Zhu H., Wang H., Wang S., Tu Z., Zhang L., Wang X., Hou Y., Wang C., Chen J., Liu Y. Flaxseed oil attenuates intestinal damage and inflammation by regulating necroptosis and TLR4/NOD signaling pathways following lipopolysaccharide challenge in a piglet model. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201700814. [DOI] [PubMed] [Google Scholar]