Abstract
Obesity and its associated complications are highly related to a current public health crisis around the world. A growing body of evidence has indicated that G-protein coupled bile acid (BA) receptor TGR5 (also known as Gpbar-1) is a potential drug target to treat obesity and associated metabolic disorders. We have identified notoginsenoside Ft1 (Ft1) from Panax notoginseng as an agonist of TGR5 in vitro. However, the pharmacological effects of Ft1 on diet-induced obese (DIO) mice and the underlying mechanisms are still elusive. Here we show that Ft1 (100 mg/100 diet) increased adipose lipolysis, promoted fat browning in inguinal adipose tissue and induced glucagon-like peptide-1 (GLP-1) secretion in the ileum of wild type but not Tgr5−/− obese mice. In addition, Ft1 elevated serum free and taurine-conjugated bile acids (BAs) by antagonizing Fxr transcriptional activities in the ileum to activate Tgr5 in the adipose tissues. The metabolic benefits of Ft1 were abolished in Cyp27a1−/− mice which have much lower BA levels. These results identify Ft1 as a single compound with opposite activities on two key BA receptors to alleviate high fat diet-induced obesity and insulin resistance in mice.
KEY WORDS: Notoginsenoside Ft1, Obesity, Insulin resistance, Bile acids, TGR5, FXR, GLP-1, Metabolic disorders
Abbreviations: ANOVA, analysis of variance; AUC, area under the curve; BAs, bile acids; BAT, brown adipose tissue; cAMP, adenosine 3′,5′ cyclic monophosphate; DIO, diet-induced obesity; eWAT, epididymal white adipose tissue; FGF, fibroblast growth factor; Fxr, nuclear farnesoid X receptor; GLP-1, glucagon-like peptide-1; Tgr5, membrane-bound G protein-coupled receptor; HFD, high fat diet; GTT, glucose tolerance test; ITT, insulin tolerance test; iWAT, inguinal white adipose tissue; KO, knockout; Ft1, notoginsenoside Ft1; Ucp, uncoupling protein; Wt, wild-type
Graphical abstract
Notoginsenoside Ft1 activates Tgr5, but antagonizes Fxr in ileum to enhance bile acid synthesis, thereby imparts metabolic improvement in high fat diet induced obese mice.

1. Introduction
Obesity is a chronic and complex non-contagious medical condition characterized by excessive fat deposition in vital metabolic organs due to the disruption of lipid homeostasis1. It has been pathologically linked to a broad range of co-morbidities, including type 2 diabetes mellitus, non-alcoholic fatty liver diseases (NAFLD), hypertension, dyslipidemia, cardiovascular disease (CVD), as well as some cancers2, 3, 4, 5. Obesity has been escalating global epidemic and more than 650 million adults (age≥18 years) around the world are diagnosed with obesity disorder in 20166. The current conventional therapeutic options for the management of obesity include life-style interventions (exercise and diet) and pharmacotherapy. Recently, bariatric surgery becomes the most effective therapeutic intervention when all other treatments have failed7. However, surgery itself is invasive and has many side effects. Therefore, there is still an urgent need to develop innovative interventions and new policies to alleviate the detrimental effects of obesity by targeting metabolic regulators. Among the potential candidates, TGR5, a membrane-bound G protein-coupled bile acid (BA) receptor (also known as Gpbar-1), is a potent metabolic regulator and promising drug target8, 9, 10, 11, 12.
TGR5 is ubiquitously expressed in immune cells and tissues of multiple metabolic organs, including intestine, liver, adipose tissue and muscle13, and its involvement in various metabolic processes is crucial in regulation of the pathogenesis in metabolic disorders. Mechanistically, in brown adipose tissue (BAT) and muscle, the triggering of BA-induced TGR5 signaling promotes the production of energy expenditure through type 2 iodothyronine deiodinase (D2)-induced activation of thyroid hormone14. Interestingly, activation of TGR5 in enteroendocrine L-cells triggers the secretion of glucagon-like peptide-1 (GLP-1), which subsequently stimulates insulin secretion to alleviate glucose disorder, resulting in the improvement of pancreatic β-cell mass and glucose homeostasis9,15. Furthermore, activation of TGR5 in intestine also promotes the release of peptide tyrosine tyrosine (PYY) which attenuates food consumption rate16. On the other hand, TGR5 activation inhibits cytokine production by suppressing the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway in activated macrophages17. These characteristics of TGR5 highlight it as a potential metabolic drug target. Since BAs have been reported as endogenous TGR5 ligands11, cholic acid (CA)-derived 6α-ethyl-23(S)-methyl-CA (INT-777) was identified as a selective TGR5 agonist18. However, although INT-777 has been shown to exhibit potential therapeutic benefits in treating metabolic syndromes18, 19, 20, severe side-effects have limited its further application in clinical trials21, 22, 23, 24. Therefore, it is urgent to develop new TGR5 ligands with minimal adverse effects to treat obesity and its associated co-morbidities.
Radix Notoginseng, the dry roots of Panax notoginseng (Burk.) F.H. Chen (Araliaceae), has been used to cure trauma in traditional Chinese medicine for more than 2000 years25. One of the active compound notoginsenoside Ft1 (Ft1), separated from leaves and stems of P. notoginseng, is chemically a kind of saponin26. In the present study, we identified Ft1 as an agonist of the TGR5 by high-throughput screening of natural product libraries. We then evaluated the metabolic effects of Ft1 using diet-induced obese (DIO) model in mice. We also investigated the underlying mechanisms by which Ft1 improved lipid and glucose metabolism in DIO mice.
2. Materials and methods
2.1. Chemicals and reagents
Ft1 powder was provided by Shanghai R&D Center for Standardization of Chinese Medicines (Shanghai, China). Other chemicals including bile acids (BAs) were obtained from Sigma–Aldrich company (St. Louis, MO, USA), high fat diet (60 kcal% saturated HFD) and normal diet (chow diet, 10% kcal% derived from fat) were bought from Research Diet company (D12492 and D12450B, NJ, USA). FBS/DMEM medium were obtained from Gibco, TG and TC measurement kits were from Wako Life Sciences.
2.2. Construction of Tgr5 stable expressed HEK293 cells
The cell line HEK293 was cultured in an incubator with 37 °C and 5% CO2 by using Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum. A plasmid expressed mouse Tgr5 (pIRESneo3-mTgr5) was transfected into HEK293 cells with Fugene 6 reagent. Forty-eight hours later, cells were treated with 500 mg/mL G418 and resistant cells were selected from them as previously described27. Resistant clones were evaluated by reverse transcription polymerase chain reaction (RT-PCR).
2.3. TGR5 luciferase assay
To test whether Ft1 was a ligand of TGR5, the Luciferase Reporter Assay System (Promega Madison, WI, USA) was conducted as previously described27. The pCRE-luc reporter (50 ng), pCMV-galactosidase (5 ng) were transfected into Tgr5 stable expressed HEK293 cells by using Fugene 6 reagent (Promega) for 18 h. The next day, cells were incubated with 0.2% DMSO as vehicle, INT-777 as positive control and Ft1 as indicated concentration for 24 h. Luciferase and β-galactosidase activities were measured 6 h later by employing Luciferase Assay System (Promega) and Galacto-Star (Applied Biosystems) reagents, respectively. β-Galactosidase was used as an internal control for normalization.
2.4. Adenosine 3′,5′ cyclic monophosphate (cAMP) secretion measurement
Tgr5 stable expressed HEK293 cells were incubated with 0.2% DMSO as vehicle or Ft1 for 30 min in serum-free Krebs Ringer buffer supplemented with 100 mmol/L Ro 20–1274 and 500 mmol/L 3-isobutyl-1-methylxanthine (IBMX, Sigma) and cAMP concentration were assayed in lysates by employing cAMP-Glo Assay Kit (Promega). Finally, cAMP levels were calculated by GraphPad Prism software with a cAMP standard curve.
2.5. Measurement of GLP-1 release from NCI-H716 cells
NCI-H716 cells from the American Type Culture Collection (ATCC) were maintained in suspension culture as described by the previously published methods28. To evaluated Ft1 mediated GLP-1 secretion, the cells were plated into 24-well culture plates precoated with Matrigel incubated for 48 h. Then cells were incubated with the Krebs–Ringer bicarbonate buffer (128.8 mmol/L NaCl, 4.8 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 2.5 mmol/L CaCl2, 5 mmol/L NaHCO3, 10 mmol/L HEPES, and 0.2% bovine serum albumin, pH 7.4) containing Ft1 (1, 5 and 10 μmol/L) or INT-777 as a positive control (10 μmol/L). After incubating at 37 °C for 2 h, the supernatants were collected and GLP-1 was measured by a GLP-1 active ELISA kit (Millipore).
2.6. FXR transactivation assay
Caco-2 was differentiated as reported by Prof. Gonzalez's group29. Then Ft1 (10 μmol/L) supplemented with chenodeoxycholic acid (CDCA) (100 μmol/L) was exposed to differentiated Caco-2 cells for 24 h. All cell samples in each well were harvested for Realtime polymerase chain reaction (PCR) assay to determine mRNA levels of FXR target genes.
Next, the transactivation of human FXR induced by Ft1 was evaluated. HEK293T cells were cotransfected with EcRE-Luc, phFXR, phRXR and β-galactosidase for 6 h using Fugene 6 reagent (Promega) as previous reported30. Then, the medium was replenished with fresh medium and Ft1 supplemented with GW4064 (5 μmol/L) was exposed to transfected HEK293T for 24 h in indicated concentrations. After treatment, cells were lysed to evaluate luciferase and β-galactosidase activities by employing Luciferase Assay System (Promega) and Galacto-Star (Applied Biosystems) reagents, respectively. β-Galactosidase was used as an internal control for normalization.
2.7. Animals and experimental design
C57BL/6 wild type (Wt) mice and Cyp27a1 knockout (KO) mice (Cyp27a1−/−, stock number, B6.129-Cyp27a1tm1Elt/J) were obtained from the Jackson Laboratory (Bar Harbor, ME). Tgr5−/− mice in C57BL/6 background were kindly donated by Dr. Vassileva Galya as previously described31. Different kinds of genetic mice were kept at a pathogen-free animal facility under a standard 12 h:12 h light/dark cycle. All group mice were fed with standard chow diet and water ad libitum. NIH guidelines were followed by all procedures for the care and use of laboratory animals. The protocol of animal study has been accepted by City of Hope Institutional Animal Care and Use Committee (IACUC number: 13,004). The general procedures of animal study were described as below. Six-week old male mice were fed with high-fat diet (D12492, 60% kcal from fat Research Diets, NJ, USA) for 8 weeks before performing the studies. Next, the obese mice were randomly divided into HFD group, Ft1 high dose group (HFD + Ft1-H, 100 g high fat diet supplemented with 100 mg Ft1, 100 mg/100 g diet) and Ft1 low dose group (HFD + Ft1-L, 100 g high fat diet supplemented with 50 mg Ft1, 50 mg/100 g diet) (n = 8 per group). Mice fed with regular chow diet were used as control (n = 8). Bodyweight and food intake were recorded for 6 weeks. For all experiments, age and body weight matched animals were used. After mice were euthanatized by CO2, serum, liver, ileum, white adipose tissues (WAT) and BAT were collected and snap frozen in liquid nitrogen for RNA extracts, Western blot analysis or biochemistry studies.
2.8. Analysis of endocrine hormones and metabolites
The Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem Inc.) and portable glucose meter (Abbot Laboratories) were applied to measure fasting serum insulin levels and blood glucose level, respectively. At the 6th week following Ft1 treatment, all group mice were fasted for 14 h or 4 h and then intraperitoneally injected with d-glucose (2.0 g/kg bodyweight) or insulin (0.75 U/kg bodyweight) for glucose tolerance test (GTT) or insulin tolerance test (ITT), respectively. Blood from tails before and 15, 30, 60, or 120 min after the injection was collected for glucose levels test. 100 mg liver was homogenized in 1.0 mL PBS and 0.4 mL homogenate were used to extract hepatic lipids by using 3.2 mL CHCl3/CH3OH (chloroform/methanol, 2:1, v/v) mixture. Then, the lower organic phase was transferred and dried. The dried extract was resuspended in 1% Triton X-100 of absolute ethanol, and the triglyceride and cholesterol levels were measured with commercial kits (Wako Life Sciences). Serum glycerol was determined according to the instructions of commercial kits (Wako Life Sciences).
2.9. Measurement of GLP-1 release in vivo
Mice were orally treated with dipeptidyl-peptidase IV (DPP-IV) inhibitor sitagliptin (3 mg/kg) at 60 min before the gavage with d-glucose (2.0 g/kg). Blood was collected by retro-orbital puncture in different indicated time points for the determination of plasma GLP-1 level. Serum GLP-1 levels were measured follow the instruction of commercial kits (Millipore).
2.10. Indirect calorimetry
Oxymax lab animal monitoring system (Columbus Instruments, Columbus, OH, USA) was employed to determine energy expenditures. After receiving Ft1 for 6 weeks, each group mice were kept in the separated metabolic chamber for 24 h to adapt the environment. Oxygen consumption volume (VO2) and CO2 release were determined during a 24 h period. Respiratory quotient (RQ) equals volumes of CO2 production/volumes of O2 consumption. Results are exhibited for the last 12 h of the light cycle and 12 h of the dark cycle over both dark and light phases.
2.11. Cold tolerance test
Each group mice were maintained in individual cages with food, and water which were placed in a 4 °C room. The cold tolerance was measured by placing the mice at 4 °C room for 3 h. Mouse rectal body temperatures were measured using a Thermo Scan thermometer (PRO 4000, Braun) prior to or at 60, 120 and 180 min after cold exposure.
2.12. BAs composition analysis
BAs composition was detected by ultra performance liquid chromatography–mass spectrometry as previously reported32. Serum BAs were extracted using 75% methanol. Specifically, 150 μL methanol was added to 50 μL serum and mixed by vortexing. Next, samples were centrifuged at 20,000×g (4 °C) for 10 min. All the supernatant was separated and dried. Residues were reconstituted in 100 μL methanol–water (55:45, v/v; containing a mixture of 5 mmol/L ammonium acetate and 0.1% formic acid) before analysis.
2.13. Histological examination of liver and adipose tissues
When animal feeding was terminated, all mice were euthanized. Liver, BAT and WAT were fixed in 10% formalin, dehydrated and embedded in paraffin. Hematoxylin & eosin staining was performed for standard histological examination. 0.5% oil red O solution and Mayer's hematoxylin solution were used to stain frozen liver sections. Images were taken by employing Olympus BX51TF microscope (Olympus). Adipocyte size was quantified using Fiji Adiposoft software16 (Bethesda).
2.14. Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR)
The liver, inguinal white adipose tissue (iWAT) and BAT were collected at 6th weeks post Ft1 treatment and total RNA from those tissues or Caco-2 cell samples were extracted by using Trizol reagent (Molecular Research Center). After RNA quantification, 2 μg total RNA was transcripted reversely into cDNA by employing Superscript first-strand synthesis system (Invitrogen). Relative expressions of amplificants were measured with SYBR Green Supermix (Invitrogen) on an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems). Primer pairs used for gene determination were displayed in Supporting Information Tables S1 and S2. The relative mRNA levels of test genes were normalized with the internal control M36B4 or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) by using the Applied Biosystems software.
2.15. Western blot analysis
The iWAT protein was extracted with tissue lysis buffer (Pierce). Samples were performed with Western blot analysis by incubating antibodies against HSL (Cell Signaling, #4107), pHSL (Cell Signaling, #4126), phospho-protein kinase A (pPKA) substrate (Cell Signaling, #9624) and GAPDH (Cell Signaling, #5174) using standard methodology. Western blotting was conducted and quantified with ImageJ software package as previously described27.
2.16. Immunofluorescent staining
BAT and iWAT frozen sections were permeabilized by using PBS–0.05% Tx-100 solution for 30 min. Adipose tissue sections were incubated with uncoupling protein 1 (UCP1, abcam, #ab225490) as the primary antibodies and washed 3 times with 1% BSA for 10 min after blocking. Next, secondary anti-rabbit antibodies were used for immunofluorescence. Finally, all sections were incubated for 15 min with 4′,6-diamidino-2-phenylindole (DAPI) for nuclei staining. Sections were mounted with VECTASHIELD (Vector Laboratories) and covered with slips after washing for 3 times. Images were taken with a scanning microscope (EVOS FL Auto, Thermo Scientific).
2.17. Statistical analysis
All data are presented as mean ± standard deviation (SD) or mean ± standard error (of estimate mean value) (SEM). The differences among two groups were compared by using Student's t-test (unpaired, two tailed). The differences among multiple groups were compared by using one-way analysis of variance (ANOVA) with Dunnett's post hoc test, while those tests were conducted only if F achieved P < 0.05 and there was no significant variance inhomogeneity. Statistical analysis was undertaken only when each group size possesses a minimum of at n = 5 independent samples. Data were analyzed using Prism (GraphPad, San Diego, CA, USA) software. The threshold of statistical significance was set at P < 0.05.
3. Results
3.1. Ft1 ameliorates diet-induced obesity in mice
Tgr5 stable expression cell line was employed to whether Ft1 (Fig. 1A) was a TGR5 ligand in vitro. The results demonstrate that Ft1 activated TGR5 at 5 and 10 μmol/L test concentrations (Fig. 1B). Consistently, Ft1 at concentrations of 5 and 10 μmol/L remarkably stimulated cAMP secretion (Fig. 1C) and GLP-1 release in vitro (Supporting Information Fig. S1C) without any cytotoxicity (Fig. S1A and S1D). Since TGR5 regulates multiple signaling pathways to confer the metabolic homeostasis of lipids and glucose, we therefore investigated the pharmacological effects of Ft1 in DIO mice in vivo. A cohort of Tgr5−/− mice (KO) and wild-type mice were treated with HFD for eight weeks to induce diet-induced obesity. Then, HFD or HFD supplemented with Ft1 (100 mg/100 g diet or 50 mg/100 g diet) were fed for additional 6 weeks, while another cohort of KO and Wt mice were fed with a chow diet (chow) for the same duration. During the treatment, no obvious toxicity of Ft1 was observed. The Ft1-treated group showed similar food intake capacity compared to HFD group (Fig. 1E). Interestingly, although the bodyweight of mice fed with high dose of Ft1-mixed diet was heavier than that of chow-fed Wt mice, they exhibited less body-weight gain than HFD-fed mice over the course of experiments (Fig. 1D), suggesting that Ft1 conferred resistance to diet-induced obesity in mice (Fig. 1D). In contrast, Tgr5−/− mice treated with Ft1 at both high and low doses showed a similar body-weight gain compared to HFD-fed Tgr5−/− mice (Fig. 1D), indicating that Ft1 attenuated the HFD-induced obesity in a Tgr5-dependent manner. In addition, the liver and the WAT weights were determined and morphological changes were evaluated for the extent of lipid accumulation in different metabolic organs. The results reveal reductions in liver and bodyweight ratio (Fig. 1F), epididymal white adipose tissue (eWAT) weights (Supporting Information Fig. S2A), iWAT (Fig. 1G), adipocyte size of eWAT and iWAT (Fig. S2B), hepatic triglyceride (TG) content (Fig. S2C) and total cholesterol levels (TC) (Fig. S2D), following Ft1 treatment. As expected, these changes were not observed in Tgr5−/− mice with Ft1 treatment (Fig. 1D–G and Supporting Information Fig. S2A–S2D). Histopathological analysis indicates that Ft1 reduced hepatic steatosis and adipocyte hypertrophy in Wt mice (Figs. 1H and S2E), but not in Tgr5−/− mice (Figs. 1H and S2E). These results together demonstrate that Tgr5 is required to mediate Ft1-induced weight loss and fat mass decrease in diet-induced obesity.
Figure 1.
Ft1 improves body weight and hepatic steatohepatitis of DIO mice through activation of Tgr5. (A) Chemical structure of Ft1. (B) TGR5 luciferase reporter activities of Ft1. (C) cAMP elicited by Ft1 after binding to the TGR5 and intracellular cAMP levels were measured by luminescence. Wt and Tgr5−/− (KO) mice were fed with HFD to induce obesity, and then treated with Ft1 for 6 weeks. (D) Body weight and (E) food intake of Wt and Tgr5−/− mice after Ft1 treatment. (F) Liver/bodyweight ratio (G) iWAT weight and (H) oil red staining of liver sections and H&E staining of iWAT sections in both Wt and Tgr5−/− mice at 6th week after Ft1 treatment. Values are mean ± SD (n = 6 per group), ∗P < 0.05, ∗∗P < 0.01 vs. vehicle by two tailed Student's t test for panels A–C. Values are mean ± SEM (n = 8 per group), ∗P < 0.05, ∗∗P < 0.01 vs. HFD group by one-way ANOVA with Dunnett's post-test for panels D–G. Scale bar, 100 μm.
3.2. Ft1 improves HFD-induced glucose disorder in mice
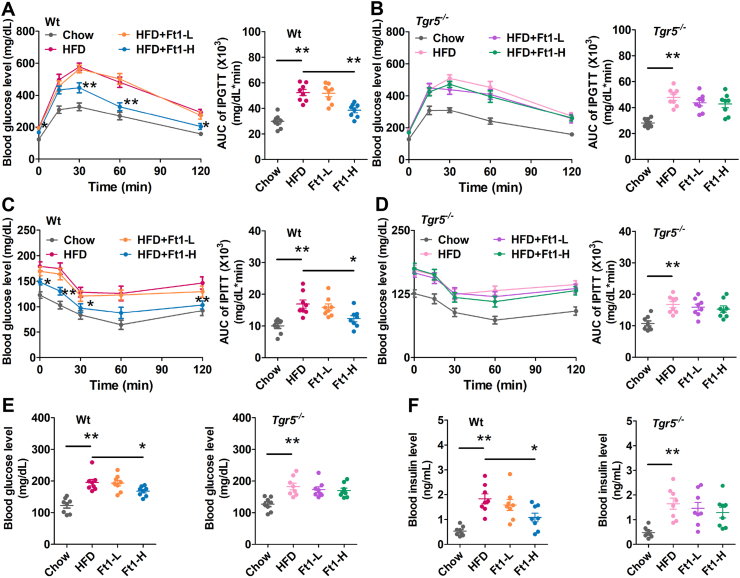
To determine the effects of Ft1 treatment on glucose metabolism, GTT and ITT were conducted in all experimental groups. The DIO mice showed reduced glucose tolerance and insulin resistance compared to those of chow-fed mice. Ft1 treatment significantly improved glucose tolerance (Fig. 2A) and insulin resistance (Fig. 2C) in Wt, but not in Tgr5−/− obese mice (Fig. 2B and D). Moreover, the increased fasting blood glucose (Fig. 2E) and insulin levels (Fig. 2F) in Wt obese mice were markedly reduced by Ft1 treatment, but not in Tgr5−/− mice (Fig. 2E and F). Taken together, these results indicate that Ft1 improves glucose metabolism in DIO mice in a Tgr5-dependent manner.
Figure 2.
Ft1 mediated improvement of glucose homeostasis in DIO mice is dependent on Tgr5. GTT at time point of 0–120 min and area under the curve (AUC) of (A) Wt and (B) Tgr5−/− mice after intraperitoneal (ip) injection with 2.0 g/kg d-glucose, ITT at time point 0–120 min and area under curve (AUC) of (C) Wt and (D) Tgr5−/− KO mice after ip injection with 0.75 U/kg insulin. (E) Fasting blood glucose levels and (F) blood insulin levels of Wt and Tgr5−/− mice at 6 weeks after Ft1 treatment. Values are mean ± SEM (n = 8 per group), ∗P < 0.05, ∗∗P < 0.01 vs. HFD group by one-way ANOVA with Dunnett's post-test.
3.3. Ft1 induces GLP-1 secretion and elevates energy expenditure of DIO mice by activating Tgr5
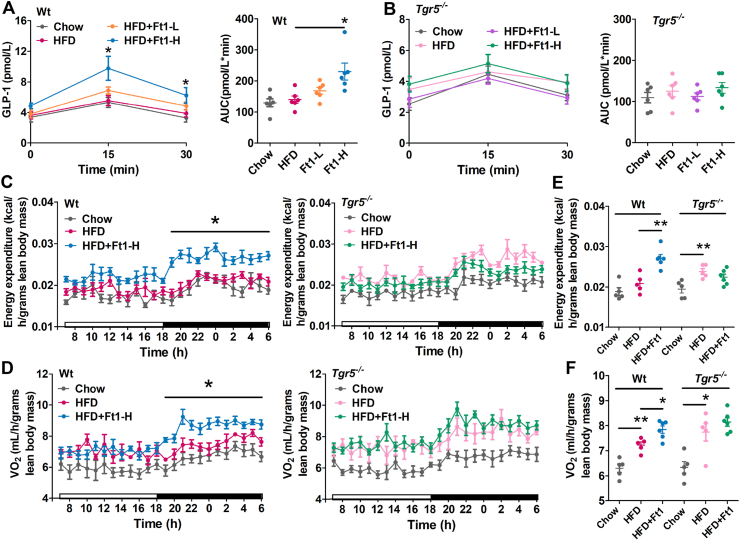
TGR5 plays a key role in modulating GLP-1 release in the intestine. Enhanced GLP-1secretion improves hyperglycemia by stimulating glucose-dependent secretion of insulin9. Previously, synthetic TGR5 agonist INT-777 has been shown to promote GLP-1 secretion in enteroendocrine L-cells, thereby improving the glucose tolerance in a Tgr5-dependent manner9. To test whether Ft1 could confer its metabolic benefits via Tgr5-induced GLP-1 secretion, oral glucose was loaded while tracing the plasma GLP-1 level. Notably, Ft1 treatment showed a strong increase of GLP-1 release in response to an oral glucose load (Fig. 3A). In Tgr5−/− mice, however, these responses were dramatically abolished (Fig. 3B), suggesting that Tgr5 is required to mediate GLP-1 secretion. In order to investigate the impact of Ft1 on energy metabolism, we analyzed the rates of oxygen consumption and energy expenditure in mice. In Ft1-treated Wt mice, energy expenditure (Fig. 3C) was dramatically enhanced in the dark phases. The oxygen consumption (Fig. 3D) was also augmented in the dark phase compared to those of untreated Wt mice. The average energy expenditure (Fig. 3E) and oxygen consumption (Fig. 3F) in 24 h were significantly increased after Ft1 treatment. Taken together, these results suggest that the increase in energy expenditure may contribute to the reduced bodyweight gain in Wt mice that received Ft1. However, the promotion of energy expenditure induced by Ft1 was abolished in Tgr5−/− mice.
Figure 3.
Ft1 induces GLP-1 secretion and increases energy expenditure of DIO mice by activation of Tgr5. GLP-1 production and the AUC of (A) Wt and (B) Tgr5−/− mice. (C) Energy expenditure over 24 h period, (D) O2 consumption over light phase and dark phase, (E) the average energy expenditure, and (F) the average oxygen consumption over 24 h period in Wt and Tgr5−/− mice at 6th week after Ft1 treatment. Values are mean ± SEM (n = 6 per group for panels A and B, n = 5 per group for panels C to F), ∗P < 0.05, ∗∗P < 0.01 vs. HFD group by one-way ANOVA with Dunnett's post-test.
3.4. Ft1 promotes lipolysis and thermogenesis by activating Tgr5 in adipose tissues
To determine the potential mechanism governing the Tgr5-dependent decrease of weight loss perceived after Ft1 treatment, we examined a potential TGR5–cAMP–PKA pathway in inducing lipolysis in iWAT. The results demonstrate that Ft1 strongly increased the protein levels of PKA substrates and increased phosphorylation of HSL (Fig. 4A). Meanwhile, the serum glycerol level was significantly increased after Ft1 administration (Fig. 4B), suggesting that lipolysis was elevated in iWAT. We then measured the mRNA levels of thermogenesis-associated genes in iWAT and BAT. The results show that Ft1 significantly increased the levels of mRNAs encoding Ucp1, peroxisome proliferator-activated receptor-γ coactivator-1α (Pgc1a), transcription factor PR domain containing 16 (Prdm16), and Kelch like family member 13 (Klhl13) in iWAT of Wt mice, but did not affect the mRNA levels of cytochrome c oxidase polypeptide 7A1 (Cox7a1), solute carrier family 27 member 1 (Slc27a1), tumor necrosis factor receptor superfamily member 5 (Cd40) and tumor necrosis factor receptor superfamily member 9 (Cd137) (Fig. 4C). Meanwhile, Ft1 significantly up-regulated the mRNA levels of Pgc1a, Ucp1, Ucp3, muscle-type carnitine palmitoyltransferase 1 (Cpt1b) and type 2 iodothyronine deiodinase (Dio2) rather than straight-chain acyl-CoA oxidase1 (Acox1) in the BAT (Fig. 4D) of Wt mice following Ft1 treatment. A similar profile of energy expenditure-associated gene expressions in both iWAT and BAT were observed in KO mice fed with or without Ft1. Immunofluorescence analysis further confirmed that UCP1 expression in both iWAT and BAT of Wt mice were increased after Ft1 administration. In contrast, no further increase was observed in Tgr5−/− mice fed with Ft1-mixed diet (Fig. 4E), suggesting that Ft1 might confer the increase in energy expenditure in DIO mice via Tgr5 activation in adipose tissues.
Figure 4.
Ft1 promotes lipolysis and thermogenesis in DIO mice through activation of Tgr5. (A) The protein amounts of PKA substrates, HSL and phosphorylation of HSL in inguinal fat of Wt and Tgr5−/− mice after Ft1 administration. (B) Blood glycerol level of Wt and Tgr5−/− mice after Ft1 treatment. The mRNA levels of energy expenditure associated genes in (C) iWAT and (D) BAT of Wt and Tgr5−/− mice after Ft1 treatment. (E) UCP1 immunofluorescence staining of iWAT and BAT sections from Wt and Tgr5−/− mice treated with Ft1. Blue, DAPI; Green, UCP1. Scale bar, 100 μm. Values are mean ± SEM (n = 6 per group for panels A, C and D, n = 8 for panel B), ∗P < 0.05, ∗∗P < 0.01 vs. HFD group by one-way ANOVA with Dunnett's post-test.
3.5. Ft1 as a FXR antagonist enhances hepatic BA synthesis in vivo
To test whether Ft1 could activate Tgr5 directly to mediate the metabolic effects in DIO mice, we examined the tissue/organ distribution patterns of Ft1 in Wt mice. The results show that Ft1 was mainly distributed in various parts of the intestine tissues, including the ileum and colon (Supporting Information Fig. S3). The content of Ft1 was low in serum as well as the major metabolic organs, including liver, iWAT, eWAT, BAT and muscle (Fig. S3). These results indicate that Ft1 might mainly act in the intestine, but not in other metabolic organs. Because BAs are the endogenous TGR5 ligands, we thus measured the levels of BAs after Ft1 treatment to answer how Ft1 treatment could activate Tgr5 in adipose tissues. Intriguingly, most serum tauro-conjugated and unconjugated BA levels were strongly increased in both Wt and Tgr5−/− mice (Fig. 5A and B), indicating that Ft1 increased BA levels in a Tgr5-independent manner. Although it has been reported that TGR5 activation might affect BA synthesis, TGR5-specific agonist INT777 did not show significant impact on BA production, whereas FXR and TGR5 dual agonists could decrease BA secretion into the intestine33. To address the question of why BA levels were elevated after Ft1 treatment, the expressions of Fxr and its target genes in ileum and liver were determined. The results showed that Fxr transcriptional activity was suppressed as indicated by the reduced expressions of Fxr target genes, including intestinal bile acid-binding protein (Ibabp), the small heterodimer partner (Shp) and fibroblast growth factor 15 (Fgf15) in the ileum (Fig. 5C). We also observed a down-regulated FGF15 protein level in serum (Fig. 5D), which might result in the upregulated levels of hepatic Cyp7a1 and Cyp27a1 genes (Fig. 5E), two key enzymes of BA synthesis in the liver. Consistently, the results of luciferase assay confirm that the transactivation of FXR was significantly suppressed in a dose-dependent manner by Ft1 treatment (Fig. 5F). Consistently, when Caco-2 cells were treated with Ft1 for 24 h, we observed a significant decrease in the mRNA expressions of FXR target genes, including SHP, FGF19 and intestinal bile acid-binding protein (IBABP) (Fig. 5G–I).
Figure 5.
Ft1 is an FXR antagonist and enhances hepatic bile acids synthesis in DIO mice. (A) Taurine-conjugated BAs, glycine-conjugated BAs, unconjugated BAs and (B) total BAs in serum of Wt and Tgr5−/− mice after Ft1 treatment. (C) Relative mRNA expression of Fxr and its target genes in ileum (D) serum FGF15 amounts and (E) relative mRNA expressions of Fxr and its target genes in liver of Wt and Tgr5−/− mice after Ft1 treatment. (F) Luciferase activity were assayed (G) SHP (H) FGF19 and (I) IBABP mRNA expressions of differentiated Caco2 cells after treatment with 100 μmol/L CDCA with 10 μmol/L Ft1, expression was normalized to GAPDH mRNA. Values are mean ± SD (n = 6 per group), ∗∗P < 0.01 compared to vehicle, #P < 0.05 compared to GW4064 by two tailed Student's t test for panels F–I. Values are mean ± SEM (n = 6 per group), ∗P < 0.05, ∗∗P < 0.01 vs. HFD group by one-way ANOVA with Dunnett's post-test for panels A–E.
3.6. The metabolic benefits conferred by Ft1 are abolished in Cyp27a1−/− mice
To further test whether the metabolic benefits exerted by Ft1 were indeed mediated by BA elevation, we examined the metabolic effects of Ft1 in Cyp27a1−/− mice with a markedly reduced BA pool. Wt and Cyp27a1−/− mice were fed with HFD, and then treated with Ft1-mixed diet. Ft1 had no effect on food intake rate in both Wt and Cyp27a1−/− mice compared to that of HFD fed group (Fig. 6A). As expected, Ft1 failed to induce further weight loss in Cyp27a1−/− mice (Fig. 6A). At 6 weeks after Ft1 treatment, we assessed the weight of eWAT (Fig. S4A) and iWAT (Fig. 6B), adipocyte size of eWAT and iWAT (Fig. S4C), as well as measured liver/body weight ratio (Fig. 6B). We also performed a histopathological analysis for adipocyte hypertrophy (Fig. 6D and Fig. S4D). As expected, the values of these parameters from HFD group were similar to those of Ft1 treatment group of Cyp27a1−/− mice. Ft1 could not further improve the metabolic phenotypes, including hepatosteatosis in Cyp27a1−/− mice (Figs. 6B, C, and S4D). Similar results were also observed for fasting blood glucose level, glucose toleranceand insulin sensitivity (Figs. S4B and 6E and F). Altogether, these results confirm that Cyp27a1 deletion abolished the metabolic effects of Ft1 due to a markedly lower level of BA pool.
Figure 6.
The metabolic benefits induced by Ft1 are lost in Cyp27a1−/− mice. Wt and Cyp27a1−/− mice were fed with HFD to lead obesity, and then treated with Ft1 for additional 6 weeks. (A) Body weight and food intake of Wt and Cyp27a1−/− mice after Ft1 treatment. (B) Liver/bodyweight ratio, iWAT weight of both Wt and Tgr5−/− mice at 6th week after Ft1 treatment. (C) Oil red staining of liver sections and (D) H&E staining of iWAT sections from Wt and Tgr5−/− mice at 6 weeks after Ft1 treatment. (E) IPGTT curve during 0–120 min and AUC of Wt and Cyp27a1−/− mice after ip injection with 2.0 g/kg d-glucose. (F) IPITT curve during 0–120 min and AUC of Wt and Cyp27a1−/− mice after ip injection with 0.75 U/kg insulin. Values are mean ± SEM (n = 8 per group), ∗P < 0.05, ∗∗P < 0.01 vs. HFD group by one-way ANOVA with Dunnett's post-test. Scale bar, 100 μm.
To verify whether the endogenous BAs were the key mediators for activation of TGR5 signaling following Ft1 treatment, we then evaluated the effects of GLP-1 secretion and thermogenesis in Cyp27a1−/− mice treated with Ft1. The data show that Ft1 could still promote the GLP-1 secretion after oral glucose load in both Wt and Cyp27a1−/− mice (Fig. 7A), indicating that Ft1 could directly promote GLP-1 secretion due to its high distribution in the intestine. However, Ft1 administration failed to increase the serum levels of most of the BA species in Cyp27a1−/− mice (Fig. 7B). Furthermore, the augmentation of pHSL-induced lipolysis as well as serum glycerol level in iWAT after Ft1 treatment were not detected in Cyp27a1−/− mice (Fig. 7C and D), suggesting that Ft1 induced lipolysis through BAs. Besides, Ft1 increased the body temperature of Wt mice rather than Cyp27a1−/− mice after cold exposure for 3 h (Fig. 7E). The upregulated expressions of thermogenesis-associated genes after Ft1 treatment were abolished in Cyp27a1−/− mice (Fig. 7F), further confirming that Ft1 conferred the increased energy expenditure in DIO mice through BA-dependent Tgr5 activation in adipose tissues.
Figure 7.
Ft1 mediated thermogenesis is abolished in Cyp27a1−/− mice. (A) GLP-1 release at indicated time point and AUC of Wt and Cyp27a1−/− mice. (B) BAs profile in WT and Cyp27a1−/− mice after Ft1 treatment. (C) The protein amounts of HSL and phosphorylation of HSL in inguinal fat of Wt and Tgr5−/− mice after Ft1 administration. (D) Blood glycerol level of Wt and Cyp27a1−/− mice after Ft1 treatment. (E) Cold intolerance in Wt and Cyp27a1−/− mice was measured before and at 1, 2, and 3 h after cold exposure (4 °C), mouse rectal body temperatures were measured using a ThermoScan thermometer. (F) Energy expenditure associated genes in iWAT of Wt and Cyp27a1−/− mice after Ft1 treatment. Values are mean ± SEM (n = 6 per group for panels A to E, n = 5 for panel F), ∗P < 0.05, ∗∗P < 0.01 vs. HFD group by one-way ANOVA with Dunnett's post-test.
4. Discussion
Functional activation of TGR5 promotes rapid intracellular cAMP production, leading to sequential downstream reactions to regulate various metabolic processes34. Our previous findings demonstrated that vertical sleeve gastrectomy, which is the most effective approach for morbid obesity, imparts its metabolic benefits through BA-mediated TGR5 activation34. TGR5 is a promising drug target to develop new therapeutic agents for treating obesity and associated co-morbidities. We screened a series of natural products from compound libraries and identified Ft1 as a potent TGR5 ligand in vitro. Although in vivo animal data demonstrated that Ft1 enhanced GLP-1 secretion through intestinal Tgr5 activation, it did not activate Tgr5 directly in adipose tissue. Instead, Ft1 acts as a Fxr antagonist in the intestine, which led to increased hepatic BA production and serum BA levels to activate Tgr5 in adipose tissues.
BAs are synthesized from cholesterol in the liver parenchymal cells35. During digestion, they act as surfactants to emulsify dietary fat and facilitate intestinal absorption of lipids, cholesterol, and a number of vitamins36. Currently, the functions of BAs are revealed as signaling molecules that impart systemic changes in metabolism via targeting nuclear receptor FXR37,38 and cell surface receptor TGR539. FXR is the primary BA receptor to control BA synthesis in the liver. TGR5 is also considered to modulate hepatic BA production to some extent. Tgr5 gene ablation significantly increases taurocholic acid (TCA) and taurodeoxycholic acid (TDCA) levels and blunts the generation of tauro α-muricholic acid (TαMCA) and tauro β-muricholic acid (TβMCA) in both free-fed and fasting states compared to Wt mice, which are consistent with our data40. However, in the present study, we observed that Ft1 alters BA production mainly through the suppression of intestinal Fxr. The downregulation of intestinal Fxr target genes and the reduced serum level of FGF15 support this hypothesis. TGR5 has been demonstrated as an endogenous BA receptor by Dr. Tanaka's group41. In this context, taurolithocholic acid (TLCA) and lithocholic acid (LCA) are the most potent Tgr5 ligands, CA, DCA, CDCA and their tauro-conjugated products also show TGR5 activation effects42. Currently, TGR5 has been manifested as the downstream effector in BA signaling pathway to regulate various metabolic processes13. Pharmacological activation of TGR5 by agonists or genetic overexpression of the receptor itself imparts beneficial effects on the regulation of glucose and lipid metabolism9,43. However, under normal conditions, serum BA levels are typically low. Here we demonstrate that Ft1 can significantly enhance hepatic BA production and increase serum BA levels to exert metabolic benefits in DIO mice via Tgr5 activation.
It is well documented that traditional Chinese herbal medicines are generally administered orally, because many constituents could not be absorbed into peripheral blood circulation due to the low bioavailability44. For instance, the bioavailability of berberine is less than 1%, but it still significantly imparts metabolic improvement of non-alcoholic fatty liver diseases (NAFLD) animal models by directly affecting gut microbiota and intestinal-liver signaling45. Another particular example is the saponins, which are the major active compounds from ginseng and P. notoginseng. It has been reported that deglycosylation and hydroxylation are the major metabolic pathway for saponins in the intestine46. Aglycone of Ft1 is protopanaxatriol (PPT), which did not show effects on TGR5 activation (Fig. S1B). Therefore, the direct target tissue of Ft1 may be the intestine rather than other metabolic tissues. The intestine has been considered as a major endocrinal organ and immune organrather than an organ just for digestion and absorption47,48. Multiple functional metabolites can be produced in the intestine and absorbed into blood circulation to execute the metabolic functions. For example, short chain fatty acids SCFA and branched chain amino acid (BCAA) are now known as functional metabolites to regulate metabolism49,50. Intestine also produces a variety of gut hormones including FGF15, GLP-1, polypeptide YY (PYY) and glucose-dependent insulinotropic peptide (GIP) to regulate different metabolic functions51. Here we showed that Ft1 upregulated expressions of Cyp7a1 and Cyp27a1 in liver, two key enzymes in the classic and alternative pathways of BA synthesis by suppressing the transcriptionl activation of intestinal Fxr. Indeed, the inhibition of intestinal Fxr conferred metabolic benefit for HFD-induced obese and insulin resistance mice29,52,53. Similarly, the metabolic disorders of HFD-fed hamsters were improved through Tβ-MCA mediated suppression of intestinal Fxr following gut microbiota depletion54. It is noteworthy that the diabetic mouse treated with hyocholicacid (HCA) promotes GLP-1 secretion and improve glucose homeostasis by activating Tgr5 and inhibiting Fxr55, indicating that it might be a promising approach to develop dual TGR5 agonist and FXR antagonist for treatment of diabetes. Our data also has proved that Ft1 as a single compound with opposite activities on two key BA receptors alleviated high fat diet-induced obesity and insulin resistance in mice.
5. Conclusions
Our present studies demonstrated that Ft1 is a TGR5 agonist but FXR antagonist to alleviate high fat diet-induced obesity and insulin resistance in mice. On one hand, Ft1 activates intestinal Tgr5 to enhance intestinal GLP-1 release. On the other hand, Ft1 increases the hepatic BA production by suppressing intestinal Fxr. The elevated serum BA levels subsequently activate Tgr5 in adipose tissues to increase energy expenditure, thereby conferring beneficial metabolic effects in obese mice (Fig. 8). Ft1 thus may represent an innovative compound with opposing effects on two key BA receptors and may be a potential leading compound for anti-diabetes drug development.
Figure 8.
Mechanisms of Ft1 on improvement of obesity and insulin resistance. Ft1 activates intestinal Tgr5 to enhance intestinal GLP-1 release and improves glucose homeostasis. Furthermore, Ft1 increases the hepatic BA production by suppressing intestinal Fxr–Fgf15 axis. The elevated serum BA levels subsequently activate Tgr5 in adipose tissues to increase energy expenditure, thereby conferring beneficial metabolic effects in obese mice.
Acknowledgments
This work is financially sponsored by Shanghai Pujiang Program (17PJ1408800, China) and the Natural Science Foundations of China to Lili Ding (81773961), Zhengtao Wang (81920108033) and Yingbo Yang (81703682). It is also financially supported by the National S&T Major Special Projects of China (No. 2017ZX09309006) to Li Yang and Interdisciplinary Program of Shanghai Jiao Tong University to Qiaoling Yang (YG2019QNA03, China). This work is partially supported by R01DK124627, George Schaeffer fund and John Hench fund (USA) to Wendong Huang. We thank Tongxi Zhuang and Suzhou BioNovoGene Metabolomics Platform (Suzhou, China) for providing material to draw the graphical abstract.
Author contributions
Wendong Huang, Li Yang, Lili Ding and Zhengtao Wang designed the experiments and secured funding. Lili Ding, Qiaoling Yang, Eryun Zhang, Siming Sun, Yangmeng Wang and Linshan Jiang performed the experiments. Lili Ding, Yingbo Yang, Tong Tian, Zhengcai Ju and Xunjiang Wang analyzed the data. Lili Ding wrote the manuscript. Wendong Huang provided editing and final approval of the manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.03.038.
Contributor Information
Wendong Huang, Email: whuang@coh.org.
Li Yang, Email: yangli7951@hotmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sikaris K.A. The clinical biochemistry of obesity. Clin Biochem Rev. 2004;25:165–181. [PMC free article] [PubMed] [Google Scholar]
- 2.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 3.Fruh S.M. Obesity: risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017;29:S3–S14. doi: 10.1002/2327-6924.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakhani H.V., Sharma D., Dodrill M.W., Nawab A., Sharma N., Cottrill C.L. Phenotypic alteration of hepatocytes in non-alcoholic fatty liver disease. Int J Med Sci. 2018;15:1591–1599. doi: 10.7150/ijms.27953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dădârlat-Pop A., Sitar-Tăut A., Zdrenghea D., Caloian B., Tomoaia R., Pop D. Profile of obesity and comorbidities in elderly patients with heart failure. Clin Interv Aging. 2020;15:547–556. doi: 10.2147/CIA.S248158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . World Health Organization; 2020. Obesity and overweight.https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight Available from: [Google Scholar]
- 7.Cefalu W.T., Bray G.A., Home P.D., Garvey W.T., Klein S., Pi-Sunyer F.X. Advances in the science, treatment, and prevention of the disease of obesity: reflections from a Diabetes Care Editors' Expert Forum. Diabetes Care. 2015;38:1567–1582. doi: 10.2337/dc15-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X.S., Lou G.Y., Meng Z.P., Huang W.D. TGR5: a novel target for weight maintenance and glucose metabolism. Exp Diabetes Res. 2011;2011:853501. doi: 10.1155/2011/853501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velazquez-Villegas L.A., Perino A., Lemos V., Zietak M., Nomura M., Pols T.W.H. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat Commun. 2018;9:245. doi: 10.1038/s41467-017-02068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 12.Li T., Chiang J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duboc H., Taché Y., Hofmann A.F. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe M., Houten S.M., Mataki C., Christoffolete M.A., Kim B.W., Sato H. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 15.Kuhre R.E., Holst J.J., Kappe C. The regulation of function, growth and survival of GLP-1-producing L-cells. Clin Sci (Lond) 2016;130:79–91. doi: 10.1042/CS20150154. [DOI] [PubMed] [Google Scholar]
- 16.Bala V., Rajagopal S., Kumar D.P., Nalli A.D., Mahavadi S., Sanyal A.J. Release of GLP-1 and PYY in response to the activation of G protein-coupled bile acid receptor TGR5 is mediated by Epac/PLC-ε pathway and modulated by endogenous H2S. Front Physiol. 2014;5:420. doi: 10.3389/fphys.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y.D., Chen W.D., Yu D., Forman B.M., Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54:1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellicciari R., Gioiello A., Macchiarulo A., Thomas C., Rosatelli E., Natalini B. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J Med Chem. 2009;52:7958–7961. doi: 10.1021/jm901390p. [DOI] [PubMed] [Google Scholar]
- 19.Guo C., Chen W.D., Wang Y.D. TGR5, not only a metabolic regulator. Front Physiol. 2016;7:646. doi: 10.3389/fphys.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hegade V.S., Speight R.A., Etherington R.E., Jones D.E. Novel bile acid therapeutics for the treatment of chronic liver diseases. Therap Adv Gastroenterol. 2016;9:376–391. doi: 10.1177/1756283X16630712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorucci S., Distrutti E., Ricci P., Giuliano V., Donini A., Baldelli F. Targeting FXR in cholestasis: hype or hope. Expert Opin Ther Targets. 2014;18:1449–1459. doi: 10.1517/14728222.2014.956087. [DOI] [PubMed] [Google Scholar]
- 22.Lavoie B., Balemba O.B., Godfrey C., Watson C.A., Vassileva G., Corvera C.U. Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 receptors and activation of KATP channels. J Physiol. 2010;588:3295–3305. doi: 10.1113/jphysiol.2010.192146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stepanov V., Stankov K., Mikov M. The bile acid membrane receptor TGR5: a novel pharmacological target in metabolic, inflammatory and neoplastic disorders. J Recept Signal Transduct Res. 2013;33:213–223. doi: 10.3109/10799893.2013.802805. [DOI] [PubMed] [Google Scholar]
- 24.Fryer R.M., Ng K.J., Nodop Mazurek S.G., Patnaude L., Skow D.J., Muthukumarana A. G protein-coupled bile acid receptor 1 stimulation mediates arterial vasodilation through a K(Ca)1.1 (BK(Ca))-dependent mechanism. J Pharmacol Exp Therapeut. 2014;348:421–431. doi: 10.1124/jpet.113.210005. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q.S. Pharmacological studies on notoginseng saponins isolated from the fibrous root of Panax notoginseng. Bull Chin Mater Med. 1987;12:45–47. [PubMed] [Google Scholar]
- 26.Chen J.T., Li H.Z., Wang D., Zhang Y.J., Yang C.R. New dammarane monodesmosides from the acidic deglycosylation of notoginseng-leaf saponins. Helv Chim Acta. 2006;89:1442–1448. [Google Scholar]
- 27.Lou G.Y., Ma X.X., Fu X.H., Meng Z.P., Zhang W.Y., Wang Y.D. GPBAR1/TGR5 mediates bile acid-induced cytokine expression in murine Kupffer cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reimer R.A., Darimont C., Gremlich S., Nicolas-Metral V., Ruegg U.T., Mace K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology. 2001;142:4522–4528. doi: 10.1210/endo.142.10.8415. [DOI] [PubMed] [Google Scholar]
- 29.Jiang C.T., Xie C., Lv Y., Li J., Krausz K.W., Shi J.M. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding L.L., Zhang B.F., Li J.M., Yang L., Wang Z.T. Beneficial effect of resveratrol on α-naphthyl isothiocyanate-induced cholestasis via regulation of the FXR pathway. Mol Med Rep. 2018;17:1863–1872. doi: 10.3892/mmr.2017.8051. [DOI] [PubMed] [Google Scholar]
- 31.Vassileva G., Golovko A., Markowitz L., Abbondanzo S.J., Zeng M., Yang S.J. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J. 2006;398:423–430. doi: 10.1042/BJ20060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L., Xiong A.Z., He Y.Q., Wang Z.Y., Wang C.H., Wang Z.T. Bile acids metabonomics study on the CCl4- and α-naphthylisothiocyanate-induced animal models: quantitative analysis of 22 bile acids by ultraperformance lipid chromatography-mass spectrometry. Chem Res Toxicol. 2008;21:2280–2288. doi: 10.1021/tx800225q. [DOI] [PubMed] [Google Scholar]
- 33.Donepudi A.C., Boehme S., Li F., Chiang J.Y. G protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis. Hepatology. 2017;65:813–827. doi: 10.1002/hep.28707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pols T.W., Noriega L.G., Nomura M., Auwerx J., Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 2011;54:1263–1272. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang J.Y. Regulation of bile acid synthesis. Front Biosci. 1998;3:d176–d193. doi: 10.2741/a273. [DOI] [PubMed] [Google Scholar]
- 36.Monte M.J., Marin J.J., Antelo A., Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15:804–816. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H.B., Chen J., Hollister K., Sowers L.C., Forman B.M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 38.Parks D.J., Blanchard S.G., Bledsoe R.K., Chandra G., Consler T.G., Kliewer S.A. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 39.Staels B., Fonseca V.A. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009;32 Suppl 2:S237–S245. doi: 10.2337/dc09-S355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maruyama T., Tanaka K., Suzuki J., Miyoshi H., Harada N., Nakamura T. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191:197–205. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- 41.Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 42.Ding L.L., Yang L., Wang Z.T., Huang W.D. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 2015;5:135–144. doi: 10.1016/j.apsb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finn P.D., Rodriguez D., Kohler J., Jiang Z., Wan S., Blanco E. Intestinal TGR5 agonism improves hepatic steatosis and insulin sensitivity in Western diet-fed mice. Am J Physiol Gastrointest Liver Physiol. 2019;316:G412–G424. doi: 10.1152/ajpgi.00300.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suroowan S., Mahomoodally M.F. Herbal medicine of the 21st century: a focus on the chemistry, pharmacokinetics and toxicity of five widely advocated phytotherapies. Curr Top Med Chem. 2019;19:2718–2738. doi: 10.2174/1568026619666191112121330. [DOI] [PubMed] [Google Scholar]
- 45.Yan T.T., Yan N.N., Wang P., Xia Y.L., Hao H.P., Wang G.J. Herbal drug discovery for the treatment of nonalcoholic fatty liver disease. Acta Pharm Sin B. 2020;10:3–18. doi: 10.1016/j.apsb.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He Y., Hu Z.Y., Li A.R., Zhu Z.Z., Yang N., Ying Z.X. Recent advances in biotransformation of saponins. Molecules. 2019;24:2365. doi: 10.3390/molecules24132365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chassaing B., Kumar M., Baker M.T., Singh V., Vijay-Kumar M. Mammalian gut immunity. Biomed J. 2014;37:246–258. doi: 10.4103/2319-4170.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinagoga K.L., Wells J.M. Generating human intestinal tissues from pluripotent stem cells to study development and disease. EMBO J. 2015;34:1149–1163. doi: 10.15252/embj.201490686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tajiri K., Shimizu Y. Branched-chain amino acids in liver diseases. Transl Gastroenterol Hepatol. 2018;3:47. doi: 10.21037/tgh.2018.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi X.Y., Yun C.Y., Sun L.L., Xia J.L., Wu Q., Wang Y. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25:1225–1233. doi: 10.1038/s41591-019-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin A.M., Sun E.W., Keating D.J. Mechanisms controlling hormone secretion in human gut and its relevance to metabolism. J Endocrinol. 2019;244:R1–R15. doi: 10.1530/JOE-19-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun L.L., Xie C., Wang G., Wu Y., Wu Q., Wang X.M. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24:1919–1929. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez F.J., Jiang C.T., Patterson A.D. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology. 2016;151:845–859. doi: 10.1053/j.gastro.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun L.L., Pang Y.Y., Wang X.M., Wu Q., Liu H.Y., Liu B. Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters. Acta Pharm Sin B. 2019;9:702–710. doi: 10.1016/j.apsb.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng X.J., Chen T.L., Jiang R.Q., Zhao A.H., Wu Q., Kuang J.L. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2021;33 doi: 10.1016/j.cmet.2020.11.017. 791-803.e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.