Abstract
Over the past decade, traditional Chinese medicine (TCM) has widely embraced systems biology and its various data integration approaches to promote its modernization. Thus, integrative pharmacology-based traditional Chinese medicine (TCMIP) was proposed as a paradigm shift in TCM. This review focuses on the presentation of this novel concept and the main research contents, methodologies and applications of TCMIP. First, TCMIP is an interdisciplinary science that can establish qualitative and quantitative pharmacokinetics–pharmacodynamics (PK–PD) correlations through the integration of knowledge from multiple disciplines and techniques and from different PK–PD processes in vivo. Then, the main research contents of TCMIP are introduced as follows: chemical and ADME/PK profiles of TCM formulas; confirming the three forms of active substances and the three action modes; establishing the qualitative PK–PD correlation; and building the quantitative PK–PD correlations, etc. After that, we summarize the existing data resources, computational models and experimental methods of TCMIP and highlight the urgent establishment of mathematical modeling and experimental methods. Finally, we further discuss the applications of TCMIP for the improvement of TCM quality control, clarification of the molecular mechanisms underlying the actions of TCMs and discovery of potential new drugs, especially TCM-related combination drug discovery.
KEY WORDS: Integrative pharmacology-based traditional Chinese medicine, PK–PD correlations, Big data, Mathematical modeling, Multidimensional association network
Graphical abstract
This review focuses on the presentation of the novel concept and the main research contents, methodologies and applications of integrative pharmacology-based traditional Chinese medicine.
1. Introduction
Traditional Chinese medicine (TCM) is one of the oldest traditional medical systems and is characterized by personalized, holistic, and multicomponent therapeutic strategies. TCM plays a crucial role in modern medical and health care with its unique theory and philosophy and has attracted worldwide attention in recent years1. TCM and natural plants are important sources of modern drugs, and approximately 45% of today's bestselling drugs originated from natural products or their derivatives2, including some modern “blockbuster” drugs such as artemisinin3, aspirin4, digitoxin5, and morphine6. Youyou Tu was inspired by the detailed methods of artemisia usage described in Ge Hong's ancient book to discover artemisinin (a famous antimalarial drug) and won the 2015 Nobel Prize in Physiology or Medicine. Characterized by its holistic concept, the TCM formula is the main form of its clinical application. Growing clinical evidence has shown that TCM has favorable clinical efficacy based on randomized double-blind experiments and placebo-controlled studies, such as with MaXingShiGan-YinQiaoSan, arsenic, and retinoic acid, and exerts definite curative effects against H1N1 influenza and acute promyelocytic leukemia7,8.
Over the past decades, TCM has been widely embraced by systems biology and its various data integration approaches to promote the modernization and internationalization of TCM. Recently, some novel concepts and methods have been proposed to systemically identify the active constituents and reveal the molecular basis of TCM in the treatment of various diseases or syndromes, such as network pharmacology of TCM (TCMNP)9,10, system pharmacology of TCM (TCMSP)11, Fangjiomics12, and chinmedomics13. However, the following accumulating problems have emerged. (1) The chemical profile of TCM formulas is greatly influenced by the different herb origins, production areas, harvest times, processing methods and preparation technologies, etc. However, a large amount of chemical information has been collected from chemical databases, which lack information on the qualitative identification and quantitative determination of TCM formulas. (2) It has widely been accepted that absorption, distribution, metabolism, excretion and pharmacokinetics (ADME/PK) are critical features for determining which chemical components are likely to be active and what kind of mode of action they may adopt to achieve their therapeutic effects. TCMNP and TCMSP only focus on direct action based on the absorbed prototype constituents interacting with therapeutic-related targets but often ignore regulation by the intestinal flora and constituent–constituent interactions. (3) A variety of ingredients in different amounts play a synergistic role in the treatment of various diseases. However, there is no appropriate method to determine the synergistic effects based on the multidimensional associations of multiple components, targets, pathways and bioactivities at the cellular, tissue and organ levels both in vitro and in vivo. Notably, there is poor consistency active substances between different in vitro and in vivo pharmacological studies, and there is also a lack of a dose–effect relationship between the multiple components of TCM and their bioactivities, which may hinder access to reliable and high-level experimental evidence to verify the pharmacological findings in vivo.
To address these problems, an integrated pharmacology-based research strategy, a paradigm shift in traditional Chinese medicine (TCMIP), was proposed and developed, which may inject new vitality into the modernization and internationalization of TCM14,15. In this review, we introduce the concept and routine research strategy of TCMIP, as well as summarize its recent developments. We also focus on the main research contents and data resources and methodologies of TCMIP in addition to its applications in the research field of TCM, its major challenges and future directions, which may create a paradigm shift in TCM studies.
2. The concept of TCMIP
TCMIP, under the guidance of TCM theories, is an interdisciplinary science that comprehensively explores the interactions between the multiple constituents of TCM and the body at multiple levels, such as the molecular, cellular, tissue, organ and animal levels16. TCMIP focuses on the construction and evaluation of the multidimensional associations among the chemical and ADME/PK profiles of TCM, the disease–syndrome–formula association network, and pharmacological actions to qualitatively and quantitatively assess the PK–PD correlations in vivo, which can be subsequently applied to identify the bioactive constituents, reveal the pharmacological mechanisms and elucidate the compatibility of TCM. The basic research framework of TCMIP is illustrated in Fig. 1. First, high-throughput and high-sensitivity analytical techniques in combination with chemical databases and virtual prediction methods are employed to fully characterize the chemical and ADME/PK profiles of TCM formulas. According to the ADME/PK properties, the active substances of TCM are divided into three forms: the absorbed prototypes (AP), the absorbed metabolites (AM) and the unabsorbed constituents (UAC). Second, TCMIP utilizes omics-based data and imbalanced biomolecular network analysis to comprehensively reveal the molecular basis associated with the initiation and progression of diseases and TCM syndromes. Then, three active TCM substances are associated with the therapeutic mechanisms through three modes of action, including the direct effects of the AP and AM interacting with the target network (PMTN), the indirect effects of the AP, AM and UAC regulating the gut microbiota (PMCGM), and the auxiliary effects of constituent–constituent interactions based on the actions of the AP and AM with ADME/PK-related enzymes or transporters (CCIs-PMPK). Third, a multidimensional association network is constructed for the qualitative PK–PD correlation to screen out key active constituents (KACs) of TCM, critical molecular targets (CMTs) and critical pharmacological effects (CPEs). Moreover, TCMIP characterizes the quantitative PK–PD correlations of the KACs of TCM and CPEs using in vitro PK–PD dynamic complex models (IV–PK/PD–DCMs) and AI-related algorithms. Finally, knock-in/out of constituents and genes will be utilized to validate the pharmacological actions of KACs and CMTs, as well as the synergetic mechanism of multiple KACs and CMTs.
Figure 1.
The basic research framework of TCMIP. Step 1: System characterization of the chemical profile of a TCM using LC–MS in combination with chemical databases. Step 2: Systemic identification of the ADME/PK profile of a TCM and uncovering the three action forms of the active substances of the TCM (AP, AM, UAC). Step 3: Comprehensive investigation of the therapeutic mechanisms based on the three action modes (PMTN, PMCGM, and CCIs-PMPK). Step 4: Establishment of the qualitative and quantitative PK–PD correlation by multidimensional association network and mathematical modeling. Step 5: Verification of the KACs and CMTs by knock-in/out of constituents and genes. AP: absorbed prototypes; AM: absorbed metabolites; UAC: unabsorbed constituents; PMTN: the direct interactions of AP and AM with the therapeutics target network; PMCGM: indirect effects of AP, AM and UAC regulating gut microbiota; CCIs-PMPK: auxiliary effects of constituent–constituent interactions based on AP and AM Action with ADME/PK-related enzymes or transporters; KACs: key active constituents; CMTs: critical molecular targets.
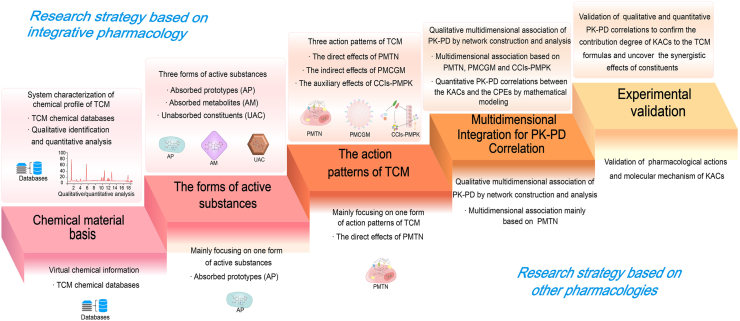
Therefore, TCMIP embodies the unity of system theory and reductionism in vivo and in vitro. TCMIP is also a database- and algorithm-dependent research strategy. Big data and artificial intelligence technologies will drive the rapid development of TCMIP. Moreover, there are several differences between TCMIP and similar concepts (TCMNP, TCMSP, etc.), as shown in Fig. 2. First, TCMIP focuses more on qualitative and quantitative analysis to characterize the reliable and precise chemical profiles of TCM, while TCMNP and TCMSP often obtain TCM chemical information from chemical databases. Second, TCMIP comprehensively elucidates the mechanism of TCM, including the three forms of active substances (AP, AM and UAC) and three modes of action of TCM (PMTN, PMCGM and CCIs-PMPK). In particular, gut microbiota and ADME/PK-related enzymes/transporters, in addition to the PMTN of AP and AM, are also important action modes of TCM based on PMCGM and CCIs-PMPK17,18. However, TCMNP and TCMSP mainly focus on active substances (AP) in their single form and single action modes (PMTN). In addition, the multidimensional association network in TCMIP has more complex interaction types, such as constituent–target interactions, constituent–gut microbiota interactions, constituent–constituent interactions, gut microbiota–target interactions, and target–target interactions. Additionally, in TCMIP, IV–PK/PD–DCMs and the knock-in/out of constituents are characteristically used as key techniques to establish the quantitative PK–PD correlation and verify the synergistic actions of multiple KACs of TCM.
Figure 2.
Comparison of TCMIP and other pharmacologies from the following aspects: chemical material basis, the forms of active substances, the action modes of TCM, multidimensional integration for PK–PD correlations, and experimental validation.
3. Main research contents of TCMIP
3.1. Systemic characterization of the chemical and ADME/PK profiles of TCM formulas and identification of the three forms of active substances
The chemical profile is the pharmacodynamic material basis of TCM and is a very complex chemical system including hundreds or thousands of known and unknown components, ranging from trace to very large amounts of ingredients and from small molecules to large molecules. After oral administration, the chemical constituents contained in TCM undergo a series of ADME/PK processes in vivo, which is critical to understand the pharmacodynamic material basis and the therapeutic mechanisms of TCM formulas. Recently, with the rapid development of chromatographic techniques and mass spectrometry, ultra-performance liquid chromatography coupled with high-resolution mass spectrometry (UPLC–HRMS) has been presented as a high-throughput and high-sensitivity platform to systemically characterize the chemical and ADME/PK profiles of TCMs. After oral administration, the chemical constituents contained in plasma, urine, feces and target organs are qualitatively identified and quantitatively determined at different times to obtain AP that are absorbed into the body, AM that are metabolized by the gut microbiota and cytochrome P450 (CYP450) enzymes, and UAC through excretion of feces. In particular, tissue and cellular pharmacokinetics are the key points that focus on ADME combinational models in vitro to prepare samples for ADME/PK profiling in accordance with the varieties and concentrations of AP and AM in vivo to reveal the molecular mechanism related to the ADME/PK process and constituent–constituent interactions of TCM.
However, current ADME/PK studies have paid insufficient attention to UAC and drug concentrations in target tissues and cells, including the varieties and concentrations of AP and AM. The complexity and microconstituents of TCMs present a major technological and economic challenge in ADME/PK studies due to the lack of chemical standards, inadaptability and time consumption of analytical technologies. Fortunately, in silico PK prediction provides a quick, inexpensive, and high-throughput technique that could be used to obtain the in vivo ADME/PK profiles of TCMs more conveniently. However, the existing in silico models have been established based on training datasets to predict the influence of selected physicochemical properties relevant to PK on the basis of the properties of a large number of individual components determined using high-throughput experimental assays19. The current in silico PK models are unsuitable for TCM-related research because of the multiple complex ingredient–ingredient interactions. Although we developed a TCM-ADMEpred method as a novel strategy for poly-pharmacokinetic prediction of TCM according to the theory that chemical components with similar structures often show similar pharmacokinetic properties, many open questions and unexplored limitations still need to be considered in future studies20.
3.2. Comprehensive investigation of the three action modes of TCM in TCMIP
TCMIP utilizes omics-based data and imbalanced biomolecular network analysis to comprehensively reveal the molecular basis associated with the initiation and progression of diseases and TCM syndromes. In addition, TCMIP determines the efficacy of TCM formulas and the corresponding pharmacological mechanisms via multilevel network analysis and experimental validation. TCMIP will establish the in vivo relationship between the three forms of active substances contained in TCM (AP, AM, UAC) and the therapeutic molecular basis of TCM through the corresponding action modes of PMTN, PMCGM, and CCIs-PMPK. Various TCM formulas have different chemical bases, active substances, action modes and therapeutic molecular networks, which are characterized by the synergistic effects of TCM based on multiple components, modes and targets.
3.2.1. TCMIP reveals the mechanisms underlying the direct effects of PMTN in target tissues and cells
PMTN is the main mode of action of TCM, of which AP and AM directly interact with the corresponding therapeutic targets, including receptors, enzymes, ion channels, transporters, nucleic acids, etc.21, which further form a constituent–target regulatory network, as shown in Fig. 3. The main modes of constituent–target interactions are as follows22, 23, 24, 25, 26, 27: AP or AM combine with the binding sites of transporters, AP or AM bind with receptors, AP or AM are converted into active ingredients to exert their effects by enzymatic catalysis, AP or AM control the “gating” of ion channels, etc. In order to systemically verify the interaction between the constituents of TCMs (AP, AM) and the molecular targets of a disease/syndrome, the molecular characteristics of TCM therapeutics are revealed by omics, big data and artificial intelligence technologies. Moreover, using virtual prediction and experimental verification, constituent–target interactions can be obtained, and the composition, target, pathway and pharmacological action of the multidimensional complex association network can be further constructed. On this basis, TCMIP emphasizes the construction and analysis of the multidimensional complex association network of PMTN to identify candidate bioactive compounds and elucidate the mechanisms of action of TCM formulas.
Figure 3.
TCMIP uncovers the action modes of PMTN between the constituents of TCM (AP, AM) and the molecular targets of disease. ①: AP or AM as transport substrates combine with the binding sites of transporters to cause conformational changes and complete the process of absorption or expulsion. ②: AP or AM as ligands bind with receptors based on the following three steps: primary recognition of the receptor, orientation and change in the structural conformation, and physical binding. ③: AP or AM are converted into active ingredients to exert their effects by the substrate binding to the enzyme to convert the substrate into a product for release. ④: AP or AM control the “gating” of ion channels to open or close and influence the effects of inorganic ions. ⑤: AP or AM interfere with or block the synthesis of nucleic acids by bacteria, viruses and tumor cells to destroy their proliferation. PMTN: the direct interactions of AP and AM with the therapeutics target network; AP: absorbed prototypes; AM: absorbed metabolites.
However, there are still very few high-quality and high-level PMTN studies on TCM because of the following points. First, there are no in-depth studies on the ADME/PK of TCMs. Although TCMs have certain pharmacological effects in vivo after oral administration, the varieties and drug concentrations of the AP and AM in target tissues or cells are still unclear. Therefore, the existing PMTN studies are inconsistent with the active ingredients in vivo. In particular, in traditional TCM pharmacological research, crude herbal extracts are usually directly added to cell or organ culture systems for in vitro pharmacological evaluation, while the ADME/PK process is notably ignored, resulting in the active ingredients in the crude drugs not being the true effective components in vivo. In addition, constituent–target interactions are mainly based on virtual prediction without experimental verification. Furthermore, there is no dose–effect relationship between multiple components and the bioactivity of TCMs. Thus, we need to investigate the ADME/PK of TCMs in depth. Cellular pharmacokinetics has been proposed as a novel concept to reveal the intracellular localization and dynamic process of AP and AM to judge the possibility of the constituent–target interactions of TCMs in the future28. Additionally, the constituent–target interactions and regulatory mechanisms should be validated by high-throughput target fishing techniques or knock-in/out constituents or genes, etc. Moreover, in order to study the constituent–target interactions and dose–effect relationships between multiple constituents of TCMs and their bioactivities in depth, it is urgent to establish IV–PK/PD–DCMs in tissues and cells.
3.2.2. TCMIP elucidates the mechanisms underlying the indirect effects of PMCGM in the gut microbiota
The gut microbiota has gained increasing attention because it not only affects gastrointestinal physiology but also performs some basic functions in the immunological, metabolic, structural and neurological landscapes of the human body29. PMCGM promotes the paradigm shift from the direct effects of PMTN to the structural and functional correlations between different tissues and between tissues and their microenvironment, which is of great help to the pathophysiology of complex diseases and the treatment of TCM. Recently, PMCGM has been considered an important mode of TCM treatment through the regulation of the gut microbiota. As shown in Fig. 4, TCMIP elucidates the action approaches for PMCGM of the constituents (AP, AM and UAC) of the TCM–gut microbiota–host axis based on the following aspects30, 31, 32, 33: the constituents of TCM directly or indirectly regulate the composition of gut microbiota, directly or indirectly modulate the metabolism of the gut microbiota by altering the composition of microbiota, the metabolites of the gut microbiota enter into body and regulate host function, as well as regulation of the host intestinal barrier, etc.
Figure 4.
TCMIP elucidates the action approaches for PMCGM of the constituents (AP, AM and UAC) of TCM–gut microbiota–host. ①: The constituents of TCM directly regulate the composition of the gut microbiota by promoting the growth of beneficial microbiota or selectively inhibiting the growth of harmful microbiota. ②: The constituents of TCM indirectly regulate the composition of the gut microbiota by altering intermediate factors, such as the pH of the gastrointestinal tract. ③: The constituents of TCM modulate the metabolism of gut microbiota indirectly by altering the composition of the microbiota. ④: The constituents of TCM modulate the metabolism of the gut microbiota indirectly by means of increasing or reducing the activities of enzymes related to the gut microbiota. ⑤: The metabolites of the gut microbiota enter the body and regulate host function. ⑥: The TCM or gut microbiota regulate the host intestinal barrier to prevent gut microbiota or harmful substances from entering the body. PMCGM: indirect effects of AP, AM and UAC regulating gut microbiota; AP: absorbed prototypes; AM: absorbed metabolites; UAC: unabsorbed constituents.
TCMIP focuses on the investigation of the gut microbiota to understand the pharmacological effects and molecular basis of TCM therapeutics, deciphers the mystery of the oral bioavailability of TCMs, and explains the compatibility theory of TCM, etc.30. Growing evidence shows that TCMs may interact with various gut microbiota to exhibit their therapeutic effects on a variety of diseases, including diabetes, obesity, and cancer17,34,35. TCMIP is expected to become a new way to study the human gut microbiota by employing sequencing technology and metagenomic and bioinformatics methods36. Then, gut microbiota depletion and fecal transplantation strategies are utilized to confirm the relationship between the gut microbiota and the TCM28,29. However, it is very difficult to clarify the causal relationship between the constituents of the TCM and gut microbiota, and new virtual prediction and high-throughput experimental methods need to be developed. The interaction among TCMs, the gut microbiota and the host is a complex and dynamic process that provides a huge challenge for the establishment of simulation models in vitro. Thus, establishing PK–PD dynamic complex models of PMCGM in vitro may be an urgent task to evaluate the therapeutic effects of TCMs during the regulation of gut microbiota.
3.2.3. TCMIP clarifies the mechanisms underlying the auxiliary effects of CCIs-PMPK
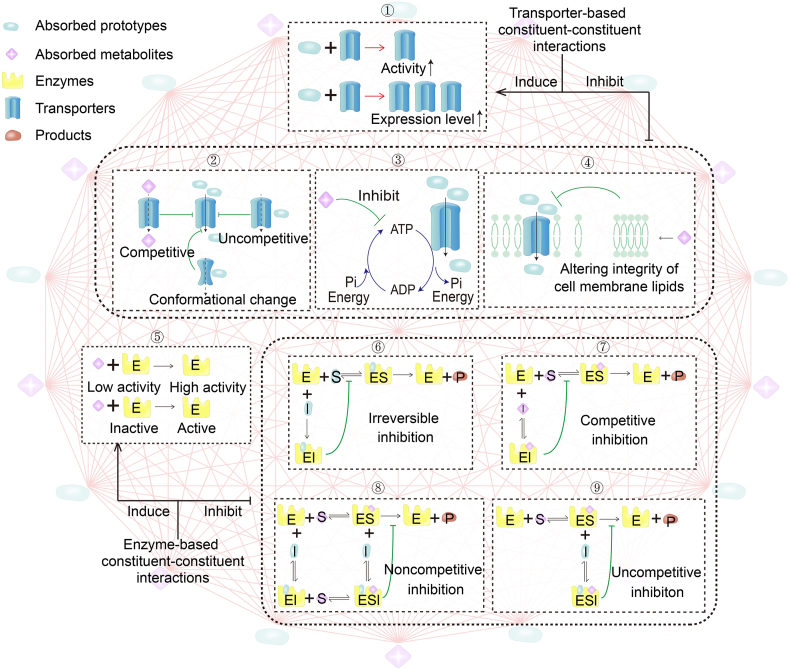
Although growing evidence shows that herb–herb interactions may be widespread in TCM formulas, CCIs-PMPK have received very little attention compared with PMTN and PMCGM37,38. At the micro level, CCIs-PMPK are the basis of herb–herb interactions and is able to alter the ADME properties of active or toxic constituents, including their oral bioavailability, protein binding capacity, blood–brain barrier permeability and half-life, etc., which may be associated with the risks and benefits of TCM formulas39. To the best of our knowledge, there are two types of transporter- and enzyme-based CCIs to indicate the existence of CCIs-PMPK40, as shown in Fig. 5. Transporter-based CCIs-PMPK are divided into activation or inhibition of activity or expression, blocking substrate binding site(s), inhibiting ATP hydrolysis, and altering the integrity of the cell membrane, etc. Enzyme-based CCIs-PMPK include irreversible inhibition, competitive inhibition, noncompetitive inhibition, uncompetitive inhibition to form a substrate–enzyme–inhibitor complex, etc.
Figure 5.
TCMIP clarifies the mechanisms of CCIs-PMPK based on the following aspects. Transporter-based CCIs-PMPK: ①: Activation or inhibition of the activity or expression of transport proteins; ②: Competitively, noncompetitively or allosterically blocking the substrate binding site(s); ③: Inhibiting ATP hydrolysis; and ④: Altering the integrity of the cell membrane. Enzyme-based CCIs-PMPK: ⑤: Irreversible inhibition by irreversible competition with the substrate for the same binding site(s); ⑥: Competitive inhibition through reversible binding of the inhibitor to the enzyme; ⑦: Noncompetitive inhibition by binding enzymes and substrates with two sequences to form substrate–enzyme–inhibitor complexes; ⑧: Uncompetitive inhibition by the enzyme–substrate complex binding to form a substrate–enzyme–inhibitor complex; and ⑨: Inhibiting the enzymes by uncompetitive inhibition. CCIs-PMPK: auxiliary effects of constituent–constituent interactions based on AP and AM action with ADME/PK-related enzymes or transporters.
In order to carry out CCI-PMPK studies, PK and PD experiments of different constituent–constituent and herb–herb combinations are first investigated to determine the ADME/PK profiles of KACs of the TCM and PD profiles related to the TCM therapeutic, confirm the existence of obvious constituent–constituent and herb–herb interactions, and establish the PK–PD correlation of the CCIs-PMPK. After that, efflux transporter and CYP450 enzymes are further employed to clarify the molecular mechanism of the constituent–constituent and herb–herb interactions. In particular, physiology-based pharmacokinetic (PBPK) modeling is an appropriate method for the high-throughput evaluation of membrane transporters and CYP450-mediated CCIs41. Transcriptome analysis has been applied to better understand CCIs and address the difficulties caused by the complex components of TCM formulas42. However, CCIs-PMPK in TCM are more complex than the study of the phenotypes and mechanisms of the CCIs, and the relationship between them can explain their efficacy or toxicity. Similar to PMCGM, virtual prediction methods and IV–PK/PD–DCMs of CCIs-PMPK will be developed for the high-throughput identification of CCIs and in-depth study of the relationship between CCIs and the benefits or risks of TCMs.
3.3. TCMIP constructs the qualitative association of PK–PD and identifies KACs, CMTs and CPEs by establishing a multidimensional network
The three types of active substances and the three action modes of TCM offer a good starting point for the establishment of the multidimensional association among the chemical and ADME/PK profiling of the TCM formulas, their regulatory targets and molecular profiling, pharmacological actions and clinical phenotypes, TCM theories, etc. There are many types of networks involved in the complex interactions between TCM formulas and the body, including constituent–constituent interaction networks, constituent–target interaction networks, constituent–gut microbiota interaction networks, protein–protein interaction networks, and gut microbiota–metabolite interaction networks, etc. On this basis, a multidimensional association complex network can be constructed from different standpoints, ranging from molecule–molecule interactions to cell–cell interactions, from unipartite networks to bipartite networks (constituent–target interactions) or even tripartite networks (constituent–protein–phenotype interactions). Thanks to the rapid advances of network science and high-throughput technologies, bioinformatics algorithms and computational biology approaches have driven the development of a complex multidimensional association network of TCMIP43. Network-based computational algorithms, such as network centrality, network controllability and network propagation, could be used to characterize the topological structure and heterogeneity of the network to better understand and simulate the dynamics and functions of a multidimensional association network. In particular, computational network-based approaches, such as deep-learning-based methods, show a strong capacity to extract hidden information and provide new insights. A multidimensional association network can be used to identify KACs of TCMs, mine CMTS-related diseases and reveal CPEs of TCM therapy44.
However, the complexities of TCMs pose a great challenge to TCMIP research on multidimensional associations. The existing methods of multidimensional association networks are mainly suitable for PMTN patterns and rarely involve PMCGM and CCIs-PMPK. Therefore, new network-based computational algorithms and approaches have been developed for PMCGM and CCIs-PMPK. In addition, the multidimensional association network involves multiple links of the PK–PD correlation without effective correlations and cross-validation computational and experimental methods. Thus, some cross-validation methods, such as IV–PK/PD–DCMs, will be developed into functional universal methods for the analysis and validation of multidimensional association networks, systemically revealing the role of TCMs ranging from the macro to micro level.
3.4. TCMIP establishes quantitative PK–PD correlations between the KACs of TCM formulas and the CPEs of the corresponding therapeutics
On the basis of a multidimensional association network, quantitative PK–PD correlations are an effective method to characterize the correlation, dynamic and quantitative description between the KACs of the TCM formulas and the CPEs of the therapeutics. Recently, quality markers (Q-markers), pharmacokinetic markers (PK-markers) and biomarkers have been deemed the KACs of TCM formulas and the CPEs of therapeutics and have become the current frontier topics of quality control, therapeutic evaluation, mechanistic exploration and synergistic effects of TCM formulas45, 46, 47. Thus, it is of great significance to establish quantitative PK–PD correlations between KACs and CPEs, which can improve the quality control level of TCMs, improve the curative effects, control the risks, and promote personalized medication, etc.
In the present review, we propose the following workflow to establish a quantitative PK–PD correlation between KACs and CPEs, as shown in Fig. 6. First, IV–PK/PD–DCMs will be developed with good stability, high sensitivity, high intelligence and high consistency to the human body, which reflects the mechanism of internal and external correlation of system. A number of TCM products of different quality can be used to obtain different ADME/PK profiles and different molecular and pharmacological profiles using IV–PK/PD–DCMs. After the standardized pretreatment of these PK and PD data, the multi-index weight of each index contribution is calculated by using all standardized data, and the quantitative PK–PD correlation between the KACs and CPEs is preliminarily constructed based on mathematical modeling methods, such as gray relational analysis, artificial neural networks, support vector machines, and genetic algorithms. In particular, the concept of optimal design should be introduced into quantitative PK–PD correlation models to achieve effective sampling to minimize the parameter estimation error. Finally, samples with knockout/in KACs were obtained to further verify the biological activity and the contribution that can be attributed to the whole activity. Through PK–PD modeling, the overall evaluation of a TCM can be performed, and a number of quantitative indicators closely related to efficacy can be determined to clarify the material basis of the biological effects from an overall perspective of TCM.
Figure 6.
Establishment of the quantitative PK–PD correlation from the following aspects. First, a large number of TCM samples with different qualities were designed to obtain PK or PD values in vivo and in vitro with significant differences. Second, all these data were preprocessed (data standardization) in order to eliminate the incompatibility of different indexes due to different dimensions, and then all of these standardized data were used to construct the PK–PD correlation model by using machine learning, such as ANNs, SVMs, genetic algorithms or a combination method. Third, through PK–PD modeling, the overall evaluation of a TCM can be performed, and a number of quantitative and qualitative indicators closely related to the efficacy can be determined to clarify the material basis of the biological effects for the whole drug. Fourth, knockout/in of constituents will be carried out to verify the biological activity and confirm the contribution of the KACs to the overall activity. ANNs: artificial neural networks; SVMs: support vector machines; KACs: key active constituents.
4. Current data resources, computational models and experimental methods for TCMIP
Facing the complexity of the interactions between the TCM formulas and the human body, TCMIP integrates the existing data resources, computational models and experimental methods. In this section, the data resources, computational algorithms and tools as well as experimental methods of TCMIP are summarized based on the following five aspects: (1) chemical profiling of TCMs; (2) ADME/PK profiling of TCMs; (3) the three action modes of TCMs; (4) multidimensional association networks of qualitative PK–PD correlation; and (5) quantitative PK–PD correlations (Table 1).
Table 1.
Current data resources, computational models and experimental methods for TCMIP.
| Research content | Main source and database | Main algorithms and computational software | Main experimental method | Limitation |
|---|---|---|---|---|
| System characterization of the chemical profile of TCM |
2. PubChem (https://pubchem.ncbi.nlm.nih.gov/)
2. ChEMBL (v27) (https://www.ebi.ac.uk/chembl/) 3. TCMSP (v2.3) (http://tcmspw.com/tcmsp.php) 4. BATMAN-TCM (http://bionet.ncpsb.org/batman-tcm/index.php) 5. TCMID (v2.03) (http://www.megabionet.org/tcmid/) 6. TCM-Mesh (http://mesh.tcm.microbioinformatics.org/) 7. CHEM-TCM (http://www.chemtcm.com/)
2. Wiley MSforID 3. NIST (v2018) (http://chemdata.nist.gov/) |
/ | HPLC, 2D-LC, GC, MS, NMR, LC–MS, GC–MS, MS–NMR, UV, IR |
|
| System characterization of the ADME/PK profile of TCM |
2. TCMSP |
3. VolSurf+ 4. ADMET Predictor 5. SwissADME 6. DataWarrior 7. ACD/Percepta, 8. MetaDrug.
2. Phoenix WinNonlin 3. ADAPT 4. Kinetica 5. MONOLIX |
|
|
| Analyzing and validating the three action modes of TCM |
2. STITCH (v5.0) (http://stitch.embl.de/) 3. TTD (v2020) (http://db.idrblab.net/ttd/) 4. BindingDB (v2020) (http://www.bindingdb.org/bind/index.jsp)
6. TCM-Mesh
2. MaxQuant (v1.6.3.0) (https://www.maxquant.org/) 3. UniProt (https://sparql.uniprot.org/) 4. TCGA (https://cancergenome.nih.gov/abouttcga/overview) 5. ICGC (v2020) (https://icgc.org/) 6. KEGG (https://www.genome.jp/kegg/) 7. Reactome (https://reactome.org)
2. Gmrepo (https://gmrepo.humangut.info) 3. GgutMEGA (http://gutmega.omicsbio.info) 4. BIO-ML
2. Zinc (http://zinc.docking.org/)
|
3. FlexX 4. Glide 5. GOLD 6. LigandFit 7. Surflex 8. Similarity ensemble approach 9. SuperPred 10. SwissTargetPrediction 11. ChemMapper 12. INVDOCK 13. TarfisDock 14. DDI-CPI 15. IDTarget
2. ChemBio3D
2. Micromedex® Healthcare Series 3. Drug Interactions Facts® 4. Lexi-Interact® 5. Pharmavista® 6. EpocratesRx® 7. MediQ® 8. Drug interaction checker®
|
|
|
| Construction and analysis of a multidimensional association network |
2. OMIM (https://omim.org/) 3. MalaCards (https://www.malacards.org/) 4. DisGeNET (https://www.disgenet.org/)
2. HAPPI (http://bio.informatics.iupui.edu/HAPPI/) 3. HINT (v2020) (http://hint.yulab.org) 4. OPHID (v2.9) (http://ophid.utoronto.ca) 5. MINT (v2012) (https://mint.bio.uniroma2.it/) 6. HPRD (http://hprd.org/index_html) 7. DIP (http://dip.doe-mbi.ucla.edu) 8. PDZBase (http://icb.med.cornell.edu/services/pdz/start) 9. IntAct (https://www.ebi.ac.uk/intact/)
4. BATMAN-TCM 5. TCM-Mesh
|
2. NAVIGaTOR 3. Matascape 4. Pajek
2. Molecular Complex Detection 3. Random walks with restart 4. Hyperlink-Induced Topic Search 5. Ingenuity Pathway Analysis 6. ScaffoldGraph 7. Network propagation 8. Shortest path 9. Dijkstra algorithm
2. Node compactness analysis 3. Node mediation analysis |
/ |
|
| Establishment and validation of a quantitative PK–PD correlation | / |
2. Least angle regression 3. Gray relational analysis 4. Canonical correlation analysis 5. Least squares support vector machines 6. Hierarchical clustering analysis |
|
|
BATMAN-TCM, bioinformatics analysis tool for molecular mechanism of traditional Chinese medicine; BBB, blood–brain barrier; BIO-ML, Broad Institute-Open Biome Microbiome Library; CHEM-TCM, chemical database-traditional Chinese medicine; DAVID, Database For Annotation, Visualization And Integrated Discovery; DIP, Database of Interacting Proteins; ETCM, Encyclopedia of Traditional Chinese Medicine; GC, gas chromatography; Gmrepo, Data Repository for Gut Microbiota; GO, Gene Ontology; gutMEGA, Gut MEtaGenome Atlas; HAPPI, human annotated protein–protein interaction; HINT, high-quality INTeractomes; HPLC, high-performance liquid chromatography; HPO, Human Phenotype Ontology; HPRD, Human Protein Reference Database; ICGC, International Cancer Genome Consortium; IR, infrared spectroscopy; KEGG, Kyoto Encyclopedia of Genes and Genomes; MINT, Molecular Interaction Database; MS, mass spectrometry; NIST, National Institute of Standards and Technology; NMR, nuclear magnetic resonance; OMIM, Online Mendelian Inheritance in Man; OPHID, Online Predicted Human Interaction Database; PDB, Protein Data Bank; TCGA, The Cancer Genome Atlas; TCMID, Traditional Chinese Medicine Integrated Database; TCMSP, Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform; TD-LC, two-dimensional LC; TTD, Therapeutic Target Database; UV, ultraviolet spectroscopy; VMH, Virtual Metabolic Human.
4.1. Chemical profiling of TCMs
4.1.1. Data resources
ChemSpider48 and PubChem49 are commonly used chemical component databases that contain the molecular structures, physicochemical properties and spectral information of chemical components. However, the chemical components of TCMs collected from foreign databases are limited. Currently, there are some comprehensive TCM databases that can be used to obtain chemical information on TCM components, such as encyclopedia of traditional Chinese medicine (ETCM)50, ChEMBL51, TCMSP11, bioinformatics analysis tool for molecular mechanism of traditional Chinese medicine (BATMAN-TCM)52, traditional Chinese medicine integrated database (TCMID), TCM-Mesh and chemical database-traditional Chinese medicine (CHEM-TCM). ETCM is a comprehensive database established by our team that contains 402 herbal medicines, 3959 formulas and 7284 ingredients contained in the Pharmacopoeia of the People's Republic of China (2015 version) as well as information from the Fourth National Survey on Chinese Materia Medica Resources. The chemical information could cross search with that of CHEMBL and PubChem. In particular, the ETCM database evaluates the druggability of each constituent by calculating the pharmacokinetic parameters according to the models in the Pipeline Pilot absorption, distribution, metabolism, excretion and toxicity (ADMET) collection50. However, these databases also have some limitations. In these databases, some chemical components do not have 3D structures, which makes further experiments, such as molecular docking, inconvenient. Moreover, these databases rarely provide information on the metabolites of the prototype components after metabolism in vivo. In addition, we need to enrich the database of TCM ingredients and establish good cross-references among the databases.
4.1.2. Analytical methods
In recent years, a variety of technologies, such as liquid chromatography-mass spectrometry (LC–MS), high-performance liquid chromatography (HPLC), gas chromatography (GC), mass spectrometry (MS), nuclear magnetic resonance (NMR), ultraviolet spectroscopy (UV) and infrared spectroscopy (IR), have been developed and applied to describe the chemical profiles of TCMs. In particular, LC–MS seems to be the most promising technology53. LC–MS is suitable for the identification of alkaloids, flavonoids, terpenes, fatty acids, organic acids, polysaccharides and other TCM constituents54, 55, 56, 57. The combination of ultrahigh-performance liquid chromatography (UHPLC) and high-resolution mass spectrometry (HRMS), such as UHPLC coupled with quadrupole time-of-flight tandem mass spectrometry (UHPLC–Q-TOF-MS/MS) and UHPLC–Q Exactive hybrid quadrupole-orbitrap high-resolution accurate mass spectrometry (UHPLC–Q-Orbitrap HRMS), facilitates the high-throughput detection of multiple components in TCMs. In addition, the combined strategy of NMR and LC–MS is a powerful tool for structural identification and promotes the discovery of new active components in TCMs57, 58, 59. Volatile components, such as the volatiles of olive oil and the volatile oil of turmeric, are more suitable for detection by GC–MS60,61. The structures of complex polysaccharides can be analyzed and characterized by chemical and instrumental methods, such as Fourier transform infrared spectroscopy (FT-IR), HPLC, gel permeation chromatography, monosaccharide composition, methylation analysis, and NMR62, 63, 64.
Nevertheless, the analysis of the chemical profiles of TCMs are not an easy task, especially in the study of TCM prescriptions, and the diversity of ingredients and mutual influence between multiple components lead to difficulty in component analysis. In particular, the identification of unknown compounds has baffled researchers. Therefore, the penetration of multiple disciplines and technologies is required to ensure the establishment of the chemical profile of TCMs.
4.2. ADME/PK profiling of TCMs
4.2.1. Data resources
ETCM is a comprehensive TCM database that contains information on ADMET BB, ADMET solubility, ADMET hepatotoxicity probability, and ADMET CYP2D6 probability of its ingredients. The TCMSP database can also be used to query oral bioavailability (OB), half-life (HL), Caco-2, blood–brain barrier (BBB), etc. These databases make it easier for researchers to understand the AMDE properties of TCMs. Moreover, we should be aware that these databases contain a limited number of components and ADME parameters. Moreover, the ADME parameters provided by these databases are calculated based on certain models that need to be verified by in vivo experiments. Therefore, the experimental information needs to be integrated into the database to ensure that the information provided by the database is consistent with the process of a drug in vivo.
4.2.2. Computational algorithms and tools
In the study of PK, ADME parameters can be simulated in silico according to drug likeness. The commonly used prediction software packages include GastroPlus™65,66, QikProp67, VolSurf+68, etc. GastroPlus™ is pharmacokinetic and pharmacodynamic (PK/PD) simulation software based on physiological models that can simulate intravenous, oral, eye, nasal and pulmonary administration routes and is known as the gold standard among similar software programs. For constituents with clear structures and efficacies, HPLC and LC–MS are usually used to detect the drug concentration in the blood at different time points in vivo, and then the ADME parameters are fitted according to the PK fitting software NONMEM69, Phoenix Winnonlin70, adapt71, etc. In addition to calculating the pharmacokinetic parameters, NONMEM can also estimate errors, especially to separate individual errors from total errors. However, what draws more of our attention is that TCMs have the characteristics of complex multiple components and targets, which is quite different from the traditional PK model of a single component, and most of the PK curves of TCM components do not conform to the conventional compartment model. Therefore, it is necessary to establish a model that is more suitable for the multicomponent interaction of TCMs.
4.2.3. Experimental methods
Numerous tissue- and cell-based in vitro models of ADME already exist, including the Caco-2 cell model72, primary hepatocyte model73, drug–plasma protein binding model, BBB osmotic model74,75, and MDCK-MDR1 cell model76,77. Additionally, the organ-on-a-chip system is based on microfluidic technology and aims to simulate the main physiological characteristics or functions of human organs and faithfully reflect human body functions in vitro78. To date, organ chips such as intestinal79, liver80, kidney81, heart82, and lung83 chips have been successfully constructed. The drug concentration in vivo is determined by the content changes of the active ingredients in the TCM in vivo, and analytical techniques usually include HPLC, GC, LC–MS, and GC–MS. However, the prescription is the main clinical application form of TCMs, and biological effect methods are often used to study prescriptions with unclear ingredients. Moreover, isotope tracer technology is often used to study tissue distribution and PK84, which is characterized by high sensitivity, location observation of drug distribution and the determination of drug target organs.
Although there are many experimental models and analytical techniques, ADME research in TCM is not an easy task. First, it is necessary to establish a model that fits the human body system to study the multiple constituent ADME. Second, studies on PK and PD should be combined and PK markers that can represent the overall efficacy of TCM should be chosen.
4.3. Analysis and validation of the three action modes of TCM
4.3.1. Data resources
4.3.1.1. PMTN
Target databases include DrugBank85, STITCH86, Therapeutic Target Database (TTD)87, and BindingDB88, and the comprehensive TCM databases include ETCM, CHEM-TCM, BARMAN-TCM and TCM-Mesh. In the ETCM database, target information was collected from the predictions of MedChem Studio (version 3.0), and these targets were obtained through DrugBank. At the biological level, omics techniques (genomics, transcriptomics, proteomics, metabolomics, etc.) are often used to research drug targets. MetaboAnalyst89, MaxQuant90, UniProt91 The Cancer Genome Atlas (TCGA)92, and the International Cancer Genome Consortium (ICGC)93 are websites and database resources related to omics research. Additionally, Databases For Annotation, Visualization And Integrated Discovery (DAVID)94, Gene Ontology (GO)95, Kyoto Encyclopedia of Genes and Genomes (KEGG)96 and Reactome97 are often used for pathway and enrichment analyses.
4.3.1.2. PMCGM
Virtual Metabolic Human (VMH) is a metabolic database of human and intestinal flora that can be combined with human and intestinal flora to reconstruct a metabolic model for large-scale, multi-omics research on diet, metabolism and disease. The data repository for gut microbiota (GMrepo) database is a database of human intestinal metagenomics that achieves data sorting by manually managing the corresponding human host macro data (age, gender, country, etc.). This database covers 58,903 human gut runs/samples from 253 projects, which are related to 92 human phenotypes, and users can easily access the collected data98. The gut MEGA database collects published human quantitative gut microbial metagenomic data99. In addition, the Broad Institute-OpenBiome Microbiome Library (BIO-ML) is a comprehensive database consisting of 7758 isolates of intestinal bacteria, 3632 genome sequences and longitudinal multiomics data100.
4.3.2. Computational algorithms and tools
4.3.2.1. PMTN
Currently, commonly used molecular docking software includes AutoDock, eHiTS, FlexX, Glide, GOLD, LigandFit, and Surflex, among which AutoDock is the most cited free software101. The structures of most small molecules can be obtained from the PubChem and Zinc databases. Receptor protein structures can be obtained through the Protein Data Bank (PDB) database. In addition, reverse virtual screening based on reverse molecular docking can also be used to predict drug targets. Importantly, we should note that computer simulation docking data cannot completely replace experimental data. A high docking score and high affinity do not necessarily mean that a ligand has a better effect, as this needs to be verified by biological experiments.
4.3.2.2. CCIs-PMPK
The current drug interaction software packages used at home and abroad include Micromedex® Drug-Reax, Micromedex® Healthcare Series, Drug Interactions Facts®, Lexi-Interact®, Pharmavista®, EpocratesRx®, MediQ®, and Drug interaction checker®102. However, inconsistent standards exist between these databases, and the analysis results obtained by using different database software programs are different. Therefore, it is essential to be cautious when using CCI results as clinical decisions. In addition, it is necessary to establish a multiconstituent CCI model for the complex system of TCMs.
4.3.3. Experimental methods
4.3.3.1. PMTN
Target fishing has the characteristics of high throughput, high speed and high specificity, and it has a wide range of target screening for TCMs. Small molecules are immobilized on a chip, interact with the protein lysate, and the eluent is detected by MS, thereby fishing out the targets of small molecules. However, some proteins with weak binding forces are unavoidably and often missed in vitro, resulting in false negatives. Moreover, in vitro research is different from the real environment of an organism, which may lead to error in reporting. Chemical proteomics uses synthetic chemical methods to generate probes, which can be used to analyze the protein targets of small molecules in cells. Importantly, it can also be administered to cells in situ, which has significant biological advantages103. However, this technology is not perfect, and nonspecific binding may occur. How to deal with promiscuity and background binders is a problem that needs to be solved. In the experiment, multiple methods are needed to achieve complementary effects. In addition, the results to be verified in organisms in order to determine the targets of the small molecules.
4.3.3.2. PMCGM
The most commonly used methods for detecting intestinal flora include 16S rDNA sequencing, metagenomic blood sequencing and metabolomics. 16S rDNA sequencing can accurately quantify all bacterial species in the intestinal microbes, metagenomic sequencing can discover the enrichment of important coding genes or pathways, and metabolomics is related to the phenotype, which can directly reflect the changes in intestinal microbes. Therefore, the combined use of 16S rDNA sequencing, metagenomic sequencing, and metabonomics can overcome the limitations of single omics research to a certain extent104. In animal intestinal flora research experiments, the consumption of intestinal flora can be realized by using spectrum antibiotics, and a “sterile” animal model can be established105. Additionally, the most commonly used methods of administration, such as gavage and enema, can be used for bacterial transplantation. Moreover, molecular probe technology and gene chip technology can also be used to carry out research on intestinal flora.
4.3.3.3. CCIs-PMPK
Some extracorporeal liver systems can be used to evaluate the interaction between constituents, such as reconstituted microsome systems, recombinant human CYP enzymes, and hepatocytes prepared from human liver tissues. In addition, the in vitro system of membrane vesicles, bidirectional transport assays with cell-based systems, and uptake assays with cell-based systems can be used to study transporter-mediated CCI. Commonly used cell models for studying drug transporters (P-gp, BCRP, OAT1/3, OCT2, etc.) are Caco-2 cells, Madin–Darby canine kidney cells, Lilly Laboratory cancer porcine kidney 1 cells, Chinese hamster ovary cells, human embryonic kidney 293 cells, etc.106.
4.4. Multidimensional association network of qualitative PK–PD correlations
4.4.1. Data resources
Disease databases, including Human Phenotype Ontology (HPO)107, Online Mendelian Inheritance In Man (OMIM)108, MalaCards109 and DisGeNET110, protein interaction databases, including String111, Human Annotated Protein–Protein Interaction (HAPPI)112, High-Quality INTeractomes (HINT)113, Online Predicted Human Interaction Database (OPHID)114, Molecular Interaction Database (MINT)115, Human Protein Reference Database (HPRD)116, Database Of Interacting Proteins (DIP)117, PDZBase118 and IntAct119, and other databases, including GO, DAVID, KEGG, and Reactome, are commonly used databases for the construction of biological networks. Some TCM comprehensive resource databases, such as ETCM, TCMSP, and TCMID, can also be used to construct TCM networks, especially the ETCM database. The protein–protein interaction data in ETCM are collected from five molecular interaction databases, including Reactome, HPRD, MINT, IntAct and DIP. The ETCM database provides a platform for establishing a biological network of formulas, herbs, ingredients, targets, diseases, and pathways, and users can easily build biological networks according to their own needs.
4.4.2. Network construction
In the construction of the network, the involved algorithms include the network module search algorithm and the node sorting algorithm in the biological network. Among them, the restricted neighborhood search clustering (RNSC)120 algorithm is a clustering algorithm based on the cost function. Random walks with restart (RWR)121 is an algorithm for the random selection of nodes. In addition, there are other algorithms, such as Ingenuity Pathway Analysis (IPA)122 and Network Propagation (NP)123. Cytoscape124, Matascape125, NAVIGaTOR, and Pajek are often used for the visual analysis of complex networks. Users can integrate biological networks with various molecular data, such as gene expression in a visual environment, and link these networks to functional annotation databases.
4.4.3. Network analysis
Common network analysis technologies include network node centrality analysis, module analysis, global topological attribute analysis, comparison and similarity analysis, dynamic analysis, etc.126,127. Node connectivity, node compactness, and node mediation are all topological characteristic indicators used to evaluate the centrality of the node network. The nodes with three characteristic values that are all greater than the median of the corresponding characteristic values of all nodes in the network are selected as the core nodes in the network. These indicators can be obtained through TCMIP v2.0 (http://www.tcmip.cn/TCMIP/index.php/Home/Login/login.html)50, NAViGaTOR, TCMSP and TCMIP. Additionally, we can build a custom multidimensional network based on the following principles in TCMIP V2.0: retain the TCM components with good drug properties; retain the TCM components with a large number of corresponding core network targets; retain the core network targets to be verified and the path nodes that are significantly involved; and retain the core targets closely related to the key pathogenic links of the target disease. All of the above screening is based on the topological importance of the core network targets. Through these methods, the complex network is simplified, and the readability of the network is improved.
However, we cannot ignore some existing problems. The accuracy and comprehensiveness of most biological networks and network analysis algorithms have some limitations. In addition, most existing biological networks are undirected networks, from which it is impossible to know the mode of action between neighboring molecules. Information on the type and direction of the interaction needs to be further improved.
4.5. Quantitative PK–PD correlations
4.5.1. IV–PK/PD–DCMs in tissues and cells
There have been in vitro PK–PD models: a dialysis/diffusion closed model to study the antibacterial activity128 and pharmacodynamics of the cerebrospinal fluid drug concentration and that in the central nervous system for neurological drug evaluation129 and the intestinal absorption–vascular activity combination model established by our group to study the vasodilator activity. Nevertheless, conventional models are limited due to their lack of physiological relevance, and in vitro models based on microfluidics have become a potential solution130. For example, the toxicity of anticancer drugs can be tested by the connection between liver, tumor and bone marrow cell lines using microfluidic technology131. Based on the concept of TCMIP, we propose an in vitro PK–PD study with microfluidics as the key technology (Fig. 7). In the entire microfluidic device, the liquid flow can be controlled by a circulating pump, which is set between the chips to control the liquid flow and direction between the chips. With this device, a system of “drugs–gut microbiota metabolism–intestinal absorption–liver metabolism–drug transport–target tissue” is established to simulate the process of the drug in the human body. In the whole system, the key part is that the type and concentration of drug components obtained by the microfluidic device is consistent with the corresponding animal samples. Moreover, the efficacy index and effect intensity of the microfluidic samples are also consistent with those of the in vivo samples. Finally, based on an in vitro microfluidic system, the effects of the drug on the target are studied. However, we should know that although we already have such an idea and the corresponding technology, there is still a long way to go to truly simulate the drug process in the human body, which requires joint efforts of multiple disciplines and continuous technological innovation.
Figure 7.
Construction of the IV–PK/PD–DCM via microfluidic-based chip technology in combination with the intestinal flora, intestinal cells, liver drug enzyme system and tissue distribution that simulate the drug ADME/PK and pharmacodynamic process. IV–PK/PD–DCM is utilized to obtain TCM administration samples in vitro with a consistent composition and concentration of target tissues or cells in vivo and to evaluate the bioactivities of a TCM in vitro more scientifically. IV–PK/PD–DCM: in vitro PK–PD dynamic complex models.
4.5.2. Computational algorithms and tools
Common PK–PD models include the linear model, log-linear model, maximum effect model (Emax), sigmoid Emax model, and β-function model. Moreover, the connection mode of PK–PD can be divided into four attributes132. NONMEM is a powerful tool for nonlinear mixed effects modeling and pharmacokinetic and pharmacodynamic data simulation and has been used in the PK–PD study of intranasal and intravenous dexmedetomidine133. In addition, the ANN modeling method can be used to establish a PK–PD prediction model134,135. These models are more suitable for the study of a single active constituent. In the TCM system, there is a complex relationship between multiple constituents and multiple effects, where the constituents also interact with each other. Therefore, it is necessary to develop models in accordance with the characteristics of TCM.
4.5.3. Validation of the key active constituents
Gene knockout is a mature technology that can be used to study key targets136. Under the influence of gene knockout, constituent knockout technology has been gradually introduced into the study of the effective material basis of TCM137. Its research methods include chromatography knockout and antibody knockout138,139. The chromatographic knockout method mainly relies on the collection and separation technology of the preparative liquid chromatography system. It has the advantages of simplicity, rapidity, and a wide application range, but it has higher requirements for the separation effects of preparation instruments. The principle of the antibody knockout method is similar to that of gene knockout, which can specifically knock out an active ingredient in the extract. This method is suitable for polar and macromolecular compounds and has high selectivity, but its operation is complicated, and its scope of application is narrow. In the future, more experiments are needed to improve constituent knockout technology.
5. Applications of TCMIP
5.1. Quality marker identification for improving TCM quality control
TCM quality control guarantees safety and efficacy for clinical use. However, there are many challenges in quality control, including the use of the same marker ingredients to evaluate the quality of different herbs, the lack of an effective quality traceability system from medicinal material to product, and the lack of correlation between multiple marker ingredients and the safety and efficacy of a TCM. In recent years, we proposed the Q-marker as a novel concept for quality evaluation and standard elaboration of TCM140,141. A constituent can be defined as a Q-marker when it possesses specificity, abundance, inherent chemical compounds in the products, appropriate PK properties, and ameliorative effects. Notably, Q-markers may contribute greatly to the therapeutic effects or safety of TCM prescriptions; they are often closely related to the pharmacological mechanisms used to establish the qualitative and quantitative correlation between the quality standard of a TCM and its clinical efficacy. Thus, Q-markers may become a new direction in the quality development of TCMs and a breakthrough in the modernization and internationalization of TCMs142.
TCMIP is a systematic approach to screen out Q-markers of TCM based on the following steps. (1) Comprehensive comparison of the chemical profiles of different herbs and TCM formulas to identify the characteristic components. (2) Establishment of the quality traceability system to screen out the stabilizing and inherent ingredients from crude herbal drugs or their products. (3) Characterization of the ADME/PK profiles of herbal and TCM formulas to discover the potential KACs in vivo. (4) Establishment and validation of the qualitative and quantitative PK-PD correlations between KACs and the corresponding bioactivities. (5) Optimization of the minimum combination of the KACs of TCM and establishment of the quality control methods and quality standards of TCMs. According to the above research strategy, several TCMIP-based investigations have been carried out to identify the potential Q-markers of various TCM formulas, such as the Yuanhu Zhitong prescription (YZP)143 and Xin-Su-Ning capsules144.
YZP is a classic Chinese patent medicine (CPM) that contains Angelicae Dahuricae Radix and Corydalis Rhizome for the treatment of gastralgia, costalgia, headache, and dysmenorrhea. The quality of YZP produced by different pharmaceutical companies is quite different due to the different origins, harvest times, pretreatments, manufacturing processes and dosage forms of the medicinal materials used. Based on our research over the last several years, we utilized the TCMIP approach to systematically identify Q-markers and establish a quality standard for YZP. First, the chemical constituents of YZP were characterized by chemical fingerprinting and multicomponent quantitative determination145,146. Second, the intestinal absorption of the YZP constituents was analyzed in vitro, as well as serum pharmacochemistry and pharmacokinetic evaluation, which revealed the ADME/PK profile and identified potential KACs in YZP in vivo147, 148, 149. Third, a computational constituent–target network was constructed, and its molecular basis mainly involved opioid receptors, dopamine receptors, cation channels, GABA-A receptors, benzodiazepine receptors, etc., which indicated that YZP had analgesic, antianxiety, antidepression, vasodilatation and other activities150. Based on this, seven constituents were qualitatively identified as Q-markers of YZT based on their high abundance, specific presence in the individual herbs and final product, appropriate drug-like properties, and critical role in the bioactivity of the mixture of YZT constituents. Finally, data mining methods, such as grey relational analysis (GRA) and least squares support vector machine (LS-SVM), were utilized to precisely characterize the quantitative correlation between the identified Q-markers of the YZTs and their efficacy. Three Q-markers were chosen as a minimum combination to control the quality of the YZTs, indicating that the determined Q-markers were suitable for quality control use of this TCM formulation and may represent a widely applicable, low-cost, rapid, simple TCM quality control method143.
Buchang Naoxintong capsule (BNC) is a well-known TCM prescription for the treatment of cardiovascular and cerebrovascular diseases. We first developed qualitative and quantitative analytical methods for the rapid high-throughput screening of the preliminary chemical profile of BNC. Then, based on text mining and predictions of the intestinal microbial metabolism and oral bioavailability of these components, the interaction network between the BNC components and their therapeutic molecular targets was constructed. According to their high abundance in the products, appropriate drug properties and importance to the biological activity, amygdalin and paeoniflorin were identified as KACs of BNC. Finally, based on the middle cerebral artery occlusion (MCAO) model, the neuroprotective effects of amygdalin and paeoniflorin were evaluated. The results showed that amygdalin and paeoniflorin could significantly reduce the cerebral infarction volume and improve the neurological function score. Interestingly, we also demonstrated that amygdalin exerted a partial anticerebral ischemia effect by interacting with the glucocorticoid receptor NR3C1 and serpin family C member 1 (SERPINC1)151. Therefore, KACs should be an important basis of Q-markers, which can be qualitatively and quantitatively related to the bioactivities of TCMs.
5.2. Clarification of the molecular mechanisms of TCM therapeutics and the promotion of accurate clinical applications
For a long time, TCM theories have provided guidance for the clinical applications of TCM based on syndrome differentiation and treatment. However, with the development of molecular biology and systems biology, under the promotion of molecular bases, accurate clinical application will become the future development trend of TCM. CPMs, as drugs approved by the National Medical Products Administration (NMPA), have a large market share and high clinical value. Increasing evidence has shown that some CPMs have been investigated in randomized double-blind experiments and placebo-controlled studies, which shows that many CPMs have clear clinical efficacy and advantages for the treatment of complex diseases152,153. In previous studies, we employed a TCMIP approach to reveal the molecular mechanism of CPM therapeutics and to screen out KACs and CMTs, such as Yuanhu Zhitong tablets (YZTs)150, Guanxinjing capsule154, and Quanduzhong capsules (QDZJNs)155. For example, 18 AP and 13 AM of YZTs in vivo were virtually calculated to potentially bind to the opioid receptor, dopamine receptor, cation channel, GABA-A receptor, and benzodiazepine receptor, which suggested that YZTs might have analgesic and antidepressive action. Then, mouse hot plate and writhing tests proved that YZTs exhibited a significant analgesic effect with a dose–effect relationship156. Additionally, the forced swimming test and the tail suspension test demonstrated that YZTs had a significant decrease in diving compared to controls and possessed obvious antidepressive activity. Furthermore, the total alkaloids of YZTs were confirmed as the main active constituents of the antidepressive effect in a chronic unpredictable mild stress (CUMS) rat model using 1H NMR-based metabonomics157. This evidence indicated that the optimal indications of YZTs would be chronic pain with depression. As an NMPA-approved drug, QDZJN is widely used to control blood pressure and protect renal function. In our study, the potential targets of QDZJN were specifically expressed in the kidneys and were involved in the inflammatory response, blood pressure regulation, LPS response and hypoxia. Based on network robustness assessment, it was suggested that QDZJN may have a greater impact on the glomerular network after clearing its potential targets, which provided more evidence for the precise clinical applications of QDZJN against hypertensive nephropathy155.
6. Limitations and future prospects
TCMIP has emerged as a novel approach to elucidate qualitative and quantitative PK–PD correlations by integrating the knowledge and key techniques of multiple disciplines. TCMIP puts forward the three forms of active substances and the corresponding action modes of TCMs, the dynamic development of PK and PD profiles of TCMs with various treatment time points and action spaces, as well as the relationship between macroscopic and microscopic, in vivo and in vitro, PK and PD, etc. Although great progress has been made in its methodology and applications, there are several problems and limitations of current TCMIP research. First, the development of TCMIP needs high-quality data and efficient algorithms. Although there have been several TCM-related databases and research platforms, comprehensive and structured TCM-related data ranging from ancient TCM literature to current clinical medical records, from clinical trials to basic research data involving drugs, and from TCM syndromes and symptoms to complex molecular bases are urgently needed. Moreover, efficient algorithms that are in accordance with the complex characteristics of TCM are lacking. Second, there is a lack of mature in vitro and in vivo pharmacological technologies to verify the qualitative and quantitative PK–PD correlations between multiple KACs of TCMs, and CMTs and CPEs of TCM therapeutics. Third, it is difficult to carry out research on PMCGM and CCIs-PMPK due to the lack of proper calculation and experimental techniques.
To overcome the above bottlenecks in the TCMIP research field, it is necessary to establish a high quality, comprehensive, structured and ecological big data TCM platform by systematically sorting ancient books and documents of TCM theories, dynamically collecting current clinical medical records of famous TCM doctors, and arranging overall information on the basic characteristics and related molecular properties for TCM syndromes, TCM formulas, Chinese herbal medicines, etc. In addition, we should develop a series of innovative algorithms and experimental methods to comprehensively investigate the three action modes of TCMs (PMTN, PMCGM, CCIs-PMPK) in TCMIP, such as TCM-related ADME/PK virtual prediction models, high-throughput prediction and determination of the interactions between the multiple constituents of TCM and gut microbiota, and high-throughput prediction and determination of ADME/PK enzyme or transporter CCIs. In particular, IV–PK/PD–DCMs will be developed as an intelligent platform for in vitro pharmacokinetic and pharmacodynamic evaluations and molecular mechanism research. Moreover, systemically uncovering the active constituents and therapeutic basis of TCM formulas at the molecular level should be carried out based on the guidance of TCM theories in order to enhance the level of quality control, personalized treatment and precise clinical applications of TCM. Notably, TCMIP must strengthen the correlation and transformation with clinical trials of TCMs. Taken together, TCMIP is still in its infancy, and advancements in this field will undoubtedly elicit a conceptual change in combination drug discovery and make an important contribution to the modernization and globalization of TCM.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81830111 and 81774201), National Key Research and Development Program of China (2017YFC1702104 and 2017YFC1702303), the Youth Innovation Team of Shaanxi Universities and Shaanxi Provincial Science and Technology Department Project (No. 2016SF-378, China), the Fundamental Research Funds for the Central public Welfare Research Institutes (ZXKT17058, China), the National Science and Technology Major Project of China (2019ZX09201005-001-003). The funding agencies had no role in the study design, the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication.
Author contributions
Haiyu Xu, Yanqiong Zhang, Ping Wang, Junhong Zhang and Hong Chen wrote the article. Yanqiong Zhang and Junhong Zhang are responsible for sorting out the table. Hong Chen, Luoqi Zhang, Xia Du, Chunhui Zhao and Dan Wu participated in the drawing of the illustrations. Changxiao Liu, Haiyu Xu, Feng Liu and Hongjun Yang conceived the article.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Haiyu Xu, Email: hyxu@icmm.ac.cn.
Changxiao Liu, Email: liuchangxiao@163.com.
References
- 1.Tian J., Shi J., Zhang X., Wang Y. Herbal therapy: a new pathway for the treatment of Alzheimer's disease. Alzheimer's Res Ther. 2010;2:30. doi: 10.1186/alzrt54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patridge E., Gareiss P., Kinch M.S., Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today. 2016;21:204–207. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Tu Y.Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17:1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- 4.Newman D.J., Cragg G.M., Snader K.M. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 5.Williams J.F., Potter R.D. The effect of chronic digitoxin administration on the contractile state of normal and nonfailing hypertrophied myocardium. J Clin Invest. 1975;56:71–78. doi: 10.1172/JCI108081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hans B.S., Grabley R. Thiericke drug discovery from nature 1999 springer verlag cambridge. J Plant Physiol. 2000;156:141. [Google Scholar]
- 7.Zhu H.H., Huang X.J. Oral arsenic and retinoic acid for non-high-risk acute promyelocytic leukemia. N Engl J Med. 2014;371:2239–2241. doi: 10.1056/NEJMc1412035. [DOI] [PubMed] [Google Scholar]
- 8.Cheng C.W., Wu T.X., Shang H.C., Li Y.P., Altman D.G., Moher D. CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration (simplified Chinese version) Ann Intern Med. 2017;167:W21–W34. doi: 10.7326/IsTranslatedFrom_M17-2977_2. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J., Jiang P., Zhang W. Molecular networks for the study of TCM pharmacology. Brief Bioinform. 2010;11:417–430. doi: 10.1093/bib/bbp063. [DOI] [PubMed] [Google Scholar]
- 10.Li S., Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11:110–120. doi: 10.1016/S1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- 11.Ru J., Li P., Wang J., Zhou W., Li B., Huang C. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminf. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Liu J., Cheng Y., Wang Y. Fangjiomics: in search of effective and safe combination therapies. J Clin Pharmacol. 2011;51:1132–1151. doi: 10.1177/0091270010382913. [DOI] [PubMed] [Google Scholar]
- 13.Wang X., Zhang A., Sun H. Future perspectives of Chinese medical formulae: chinmedomics as an effector. Omics. 2012;16:414–421. doi: 10.1089/omi.2011.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H.Y., Yang H.J. Integrative pharmacology: new paradigm of modernization of Chinese medicine. China J Chin Mater Med. 2014;39:357–362. [PubMed] [Google Scholar]
- 15.Wang P., Tang S.H., Su J., Zhang J.Q., Cui R.Y., Xu H.Y. Modern research progress of traditional Chinese medicine based on integrative pharmacology. China J Chin Mater Med. 2018;43:1297–1302. doi: 10.19540/j.cnki.cjcmm.2018.0052. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C.H., Li S., Zhang J.H., Huang Y.Y., Zhang L.Q., Zhao F. Current state and future perspective of cardiovascular medicines derived from natural products. Pharmacol Ther. 2020;216:107698. doi: 10.1016/j.pharmthera.2020.107698. [DOI] [PubMed] [Google Scholar]
- 17.Xu J., Lian F., Zhao L., Zhao Y., Chen X., Zhang X. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015;9:552–562. doi: 10.1038/ismej.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C.X., Yi X.L., Si D.Y., Xiao X.F., He X., Li Y.Z. Herb–drug interactions involving drug metabolizing enzymes and transporters. Curr Drug Metabol. 2011;12:835–849. doi: 10.2174/138920011797470083. [DOI] [PubMed] [Google Scholar]
- 19.Hou T., Wang J. Structure–ADME relationship: still a long way to go?. Expert Opin Drug Met. 2008;4:759–770. doi: 10.1517/17425255.4.6.759. [DOI] [PubMed] [Google Scholar]
- 20.Wang P., Li K., Tao Y., Li D., Zhang Y., Xu H. TCM-ADMEpred: a novel strategy for poly-pharmacokinetics prediction of traditional Chinese medicine based on single constituent pharmacokinetics, structural similarity, and mathematical modeling. J Ethnopharmacol. 2019;236:277–287. doi: 10.1016/j.jep.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Gashaw I., Ellinghaus P., Sommer A., Asadullah K. What makes a good drug target?. Drug Discov Today. 2011;16:1037–1043. doi: 10.1016/j.drudis.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Diallinas G. Dissection of transporter function: from genetics to structure. Trends Genet. 2016;32:576–590. doi: 10.1016/j.tig.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Petukh M., Stefl S., Alexov E. The role of protonation states in ligand–receptor recognition and binding. Curr Pharmaceut Des. 2013;19:4182–4190. doi: 10.2174/1381612811319230004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guryanov I., Fiorucci S., Tennikova T. Receptor–ligand interactions: advanced biomedical applications. Mater Sci Eng C Mater Biol Appl. 2016;68:890–903. doi: 10.1016/j.msec.2016.07.072. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R., Wong K. High performance enzyme kinetics of turnover, activation and inhibition for translational drug discovery. Expet Opin Drug Discov. 2017;12:17–37. doi: 10.1080/17460441.2017.1245721. [DOI] [PubMed] [Google Scholar]
- 26.Gutteridge A., Thornton J. Conformational change in substrate binding, catalysis and product release: an open and shut case?. FEBS Lett. 2004;567:67–73. doi: 10.1016/j.febslet.2004.03.067. [DOI] [PubMed] [Google Scholar]
- 27.Takakura Y. Nucleic acid drug delivery and targeting. Pharm Res. 2011;28:691–693. doi: 10.1007/s11095-011-0394-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Zhou F., Lu M., Ji W., Niu F., Zha W. Pharmacokinetics–pharmacology disconnection of herbal medicines and its potential solutions with cellular pharmacokinetic-pharmacodynamic strategy. Curr Drug Metabol. 2012;13:558–576. doi: 10.2174/1389200211209050558. [DOI] [PubMed] [Google Scholar]
- 29.Adak A., Khan M.R. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76:473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng W., Ao H., Peng C., Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 31.An X., Bao Q., Di S., Zhao Y., Zhao S., Zhang H. The interaction between the gut microbiota and herbal medicines. Biomed Pharmacother. 2019;118:109252. doi: 10.1016/j.biopha.2019.109252. [DOI] [PubMed] [Google Scholar]
- 32.Bron P.A., Kleerebezem M., Brummer R.J., Cani P.D., Mercenier A., MacDonald T.T. Can probiotics modulate human disease by impacting intestinal barrier function?. Br J Nutr. 2017;117:93–107. doi: 10.1017/S0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burge K., Gunasekaran A., Eckert J., Chaaban H. Curcumin and intestinal inflammatory diseases: molecular mechanisms of protection. Int J Mol Sci. 2019;20:1912. doi: 10.3390/ijms20081912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhi C., Huang J., Wang J., Cao H., Bai Y., Guo J. Connection between gut microbiome and the development of obesity. Eur J Clin Microbiol Infect Dis. 2019;38:1987–1998. doi: 10.1007/s10096-019-03623-x. [DOI] [PubMed] [Google Scholar]
- 35.Lv J., Jia Y., Li J., Kuai W., Li Y., Guo F. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10:415. doi: 10.1038/s41419-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lepage P., Leclerc M.C., Joossens M., Mondot S., Blottière H.M., Raes J. A metagenomic insight into our gut's microbiome. Gut. 2013;62:146–158. doi: 10.1136/gutjnl-2011-301805. [DOI] [PubMed] [Google Scholar]
- 37.Zhi H., Deng Y., Yan B., Li Z., Han S., Zhang Y. Study on the herb–herb interaction of Danqi Tongmai Tablet based on the pharmacokinetics of twelve notoginsenoides in acute myocardial ischemia and sham rats. J Pharmaceut Biomed Anal. 2019;166:52–65. doi: 10.1016/j.jpba.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 38.Shen J., Mo X., Tang Y.P., Zhang L., Pang H.Q., Qian Y.F. Analysis of herb–herb interaction when decocting together by using ultra-high-performance liquid chromatography–tandem mass spectrometry and fuzzy chemical identification strategy with poly-proportion design. J Chromatogr A. 2013;1297:168–178. doi: 10.1016/j.chroma.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z., Shang H., Li Y., Zhang C., Dong Y., Cui T. Transporters (OATs and OATPs) contribute to illustrate the mechanism of medicinal compatibility of ingredients with different properties in yuanhuzhitong prescription. Acta Pharm Sin B. 2020;10:1646–1657. doi: 10.1016/j.apsb.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Meng Q., Yang M., Liu D., Hou X., Tang L. Current trends in drug metabolism and pharmacokinetics. Acta Pharm Sin B. 2019;9:1113–1144. doi: 10.1016/j.apsb.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taskar K.S., Pilla Reddy V., Burt H., Posada M.M., Varma M., Zheng M. Physiologically-based pharmacokinetic models for evaluating membrane transporter mediated drug–drug interactions: current capabilities, case studies, future opportunities, and recommendations. Clin Pharmacol Ther. 2020;107:1082–1115. doi: 10.1002/cpt.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen H.Y., Qu Z.P., Harata-Lee Y., Cui J., Aung T.N., Wang W. A new strategy for identifying mechanisms of drug–drug interaction using transcriptome analysis: compound kushen injection as a proof of principle. Sci Rep. 2019;9:15889. doi: 10.1038/s41598-019-52375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C., Ma Y.F., Zhao J., Ruth Nussinov, Zhang Y.C., Cheng F.X. Computational network biology: data, models, and applications. Phys Rep. 2020;846:1–66. [Google Scholar]
- 44.Eraslan G., Avsec Ž., Gagneur J., Theis F.J. Deep learning: new computational modelling techniques for genomics. Nat Rev Genet. 2019;20:389–403. doi: 10.1038/s41576-019-0122-6. [DOI] [PubMed] [Google Scholar]
- 45.Yang W., Zhang Y., Wu W., Huang L., Guo D., Liu C. Approaches to establish Q-markers for the quality standards of traditional Chinese medicines. Acta Pharm Sin B. 2017;7:439–446. doi: 10.1016/j.apsb.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu T., Yang J., Gao X., Chen P., Du F., Sun Y. Plasma and urinary tanshinol from Salvia miltiorrhiza (Danshen) can be used as pharmacokinetic markers for cardiotonic pills, a cardiovascular herbal medicine. Drug Metab Dispos. 2008;36:1578–1586. doi: 10.1124/dmd.108.021592. [DOI] [PubMed] [Google Scholar]
- 47.Huang Z.Q., Fan X.M., Wang Y.M., Liang Q.L., Tong X.L., Bai Y. A new method to evaluate the dose–effect relationship of a TCM formula Gegen Qinlian Decoction: "Focus" mode of integrated biomarkers. Acta Pharmacol Sin. 2017;38:1141–1149. doi: 10.1038/aps.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pence H.E., Williams A. ChemSpider: an online chemical information resource. J Chem Educ. 2010;87:1123–1124. [Google Scholar]
- 49.Wang Y., Xiao J., Suzek T.O., Zhang J., Wang J., Bryant S.H. PubChem: a public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009;37:W623–W633. doi: 10.1093/nar/gkp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu H.Y., Zhang Y.Q., Liu Z.M., Chen T., Lv C.Y., Tang S.H. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019;47:D976–D982. doi: 10.1093/nar/gky987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendez D., Gaulton A., Bento A.P., Chambers J., De Veij M., Félix E. ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res. 2019;47:D930–D940. doi: 10.1093/nar/gky1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z., Guo F., Wang Y., Li C., Zhang X., Li H. BATMAN-TCM: a bioinformatics analysis tool for molecular mechanism of traditional Chinese medicine. Sci Rep. 2016;6:21146. doi: 10.1038/srep21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang W., Qiao X., Li K., Fan J., Bo T., Guo D.A. Identification and differentiation of Panax ginseng, Panax quinquefolium, and Panax notoginseng by monitoring multiple diagnostic chemical markers. Acta Pharm Sin B. 2016;6:568–575. doi: 10.1016/j.apsb.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu S., Li J., Guo L., Di C., Qin X., Li Z. Integrated liquid chromatography–mass spectrometry and nuclear magnetic resonance spectra for the comprehensive characterization of various components in the Shuxuening injection. J Chromatogr A. 2019;1599:125–135. doi: 10.1016/j.chroma.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Yang L., Liu R.H., He J.W. Rapid Analysis of the chemical compositions in Semiliquidambar cathayensis Roots by ultra high-performance liquid chromatography and quadrupole time-of-flight tandem mass spectrometry. Molecules. 2019;24:4098. doi: 10.3390/molecules24224098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong Y., Ruan J., Ding Z., Zhao W., Hao M., Zhang Y. Phytochemistry and comprehensive chemical profiling study of flavonoids and phenolic acids in the aerial parts of Allium Mongolicum Regel and their intestinal motility evaluation. Molecules. 2020;25:577. doi: 10.3390/molecules25030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao C.L., Pan H.Q., Wang H., Yao S., Yang W.Z., Hou J.J. Global profiling combined with predicted metabolites screening for discovery of natural compounds: characterization of ginsenosides in the leaves of Panax notoginseng as a case study. J Chromatogr A. 2018;1538:34–44. doi: 10.1016/j.chroma.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 58.Sun C., Liu F., Sun J., Li J., Wang X. Optimisation and establishment of separation conditions of organic acids from Usnea longissima Ach. by pH-zone-refining counter-current chromatography: discussion of the eluotropic sequence. J Chromatogr A. 2016;1427:96–101. doi: 10.1016/j.chroma.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 59.Qiu S., Yang W.Z., Shi X.J., Yao C.L., Yang M., Liu X. A green protocol for efficient discovery of novel natural compounds: characterization of new ginsenosides from the stems and leaves of Panax ginseng as a case study. Anal Chim Acta. 2015;893:65–76. doi: 10.1016/j.aca.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 60.Stilo F., Liberto E.R.S., Tao Q., Bicchi C., Cordero C. Untargeted and targeted fingerprinting of extra virgin olive oil volatiles by comprehensive two-dimensional gas chromatography with mass spectrometry: challenges in long-term studies. J Agric Food Chem. 2019;67:5289–5302. doi: 10.1021/acs.jafc.9b01661. [DOI] [PubMed] [Google Scholar]
- 61.Chen Z., Quan L., Zhou H., Zhao Y., Chen P., Hu L. Screening of active fractions from Curcuma Longa Radix isolated by HPLC and GC–MS for promotion of blood circulation and relief of pain. J Ethnopharmacol. 2019;234:68–75. doi: 10.1016/j.jep.2018.09.035. [DOI] [PubMed] [Google Scholar]
- 62.Li C.Y., Chen H.Y., Liu W.P., Rui W. Multi-fingerprint profiling combined with chemometric methods for investigating the quality of Astragalus polysaccharides. Int J Biol Macromol. 2019;123:766–774. doi: 10.1016/j.ijbiomac.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 63.Liu J., Shang F., Yang Z., Wu M., Zhao J. Structural analysis of a homogeneous polysaccharide from Achatina fulica. Int J Biol Macromol. 2017;98:786–792. doi: 10.1016/j.ijbiomac.2017.01.149. [DOI] [PubMed] [Google Scholar]
- 64.Zhao K., Li B., He D., Zhao C., Shi Z., Dong B. Chemical characteristic and bioactivity of hemicellulose-based polysaccharides isolated from Salvia miltiorrhiza. Int J Biol Macromol. 2020;165:2475–2483. doi: 10.1016/j.ijbiomac.2020.10.113. [DOI] [PubMed] [Google Scholar]
- 65.Kuentz M., Nick S., Parrott N., Röthlisberger D. A strategy for preclinical formulation development using GastroPlus as pharmacokinetic simulation tool and a statistical screening design applied to a dog study. Eur J Pharmaceut Sci. 2006;27:91–99. doi: 10.1016/j.ejps.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Sjögren E., Thörn H., Tannergren C. In silico modeling of gastrointestinal drug absorption: predictive performance of three physiologically based absorption models. Mol Pharm. 2016;13:1763–1778. doi: 10.1021/acs.molpharmaceut.5b00861. [DOI] [PubMed] [Google Scholar]
- 67.Shaikh S., Dhavan P., Pavale G., Ramana M.M.V., Jadhav B.L. Design, synthesis and evaluation of pyrazole bearing α-aminophosphonate derivatives as potential acetylcholinesterase inhibitors against Alzheimer's disease. Bioorg Chem. 2020;96:103589. doi: 10.1016/j.bioorg.2020.103589. [DOI] [PubMed] [Google Scholar]
- 68.Hosey C.M., Benet L.Z. Predicting the extent of metabolism using in vitro permeability rate measurements and in silico permeability rate predictions. Mol Pharm. 2015;12:1456–1466. doi: 10.1021/mp500783g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carmichael S.J., Charles B., Tett S.E. Population pharmacokinetics of hydroxychloroquine in patients with rheumatoid arthritis. Ther Drug Monit. 2003;25:671–681. doi: 10.1097/00007691-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 70.Huang X., Zhang S., Ma Y., Yang H., He C., Tian R. Bioequivalence of two quetiapine extended release tablets in Chinese healthy volunteers under fasting and fed conditions and effects of food on pharmacokinetic profiles. Drug Des Dev Ther. 2019;13:255–264. doi: 10.2147/DDDT.S182965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Serrano-Rodríguez J.M., Mengual C., Quirós-Carmona S., Fernández J., Domínguez J.M., Serrano-Caballero J.M. Comparative pharmacokinetics and a clinical laboratory evaluation of intravenous acetaminophen in Beagle and Galgo Español dogs. Vet Anaesth Analg. 2019;46:226–235. doi: 10.1016/j.vaa.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 72.Ingels F., Deferme S., Destexhe E., Oth M., van den Mooter G., Augustijns P. Simulated intestinal fluid as transport medium in the Caco-2 cell culture model. Int J Pharm. 2002;232:183–192. doi: 10.1016/s0378-5173(01)00897-3. [DOI] [PubMed] [Google Scholar]
- 73.LeCluyse E.L. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharmaceut Sci. 2001;13:343–368. doi: 10.1016/s0928-0987(01)00135-x. [DOI] [PubMed] [Google Scholar]
- 74.Jiao Q., Wang R., Jiang Y., Liu B. Study on the interaction between active components from traditional Chinese medicine and plasma proteins. Chem Cent J. 2018;12:48. doi: 10.1186/s13065-018-0417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaisar M.A., Sajja R.K., Prasad S., Abhyankar V.V., Liles T., Cucullo L. New experimental models of the blood–brain barrier for CNS drug discovery. Expert Opin Drug Discov. 2017;12:89–103. doi: 10.1080/17460441.2017.1253676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y., Zeng S. Advances in the MDCK-MDR1 cell model and its applications to screen drug permeability. Acta Pharm Sin. 2008;43:559–564. [PubMed] [Google Scholar]
- 77.da Silva Junior J.B., Dezani T.M., Dezani A.B., dos Reis Serra C.H. Evaluating potential P-gp substrates: main aspects to choose the adequate permeability model for assessing gastrointestinal drug absorption. Mini Rev Med Chem. 2015;15:858–871. doi: 10.2174/1389557515666150511152705. [DOI] [PubMed] [Google Scholar]
- 78.Bhatia S.N., Ingber D.E. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 79.Kim H.J., Huh D., Hamilton G., Ingber D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 80.Yeon J.H., Na D., Park J.K. Hepatotoxicity assay using human hepatocytes trapped in microholes of a microfluidic device. Electrophoresis. 2010;31:3167–3174. doi: 10.1002/elps.201000122. [DOI] [PubMed] [Google Scholar]
- 81.Qu Y., An F., Luo Y., Lu Y., Liu T., Zhao W. A nephron model for study of drug-induced acute kidney injury and assessment of drug-induced nephrotoxicity. Biomaterials. 2018;155:41–53. doi: 10.1016/j.biomaterials.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 82.Agarwal A., Goss J.A., Cho A., McCain M.L., Parker K.K. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13:3599–3608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huh D., Matthews B.D., Mammoto A., Montoya-Zavala M., Hsin H.Y., Ingber D.E. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brunner M., Langer O., Dobrozemsky G., Müller U., Zeitlinger M., Mitterhauser M. [18F]Ciprofloxacin, a new positron emission tomography tracer for noninvasive assessment of the tissue distribution and pharmacokinetics of ciprofloxacin in humans. Antimicrob Agents Chemother. 2004;48:3850–3857. doi: 10.1128/AAC.48.10.3850-3857.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wishart D.S., Knox C., Guo A.C., Shrivastava S., Hassanali M., Stothard P. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34:D668–D672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szklarczyk D., Santos A., von Mering C., Jensen L.J., Bork P., Kuhn M. Stitch 5: augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016;44:D380–D384. doi: 10.1093/nar/gkv1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y., Zhang S., Li F., Zhou Y., Zhang Y., Wang Z. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020;48:D1031–D1041. doi: 10.1093/nar/gkz981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gilson M.K., Liu T., Baitaluk M., Nicola G., Hwang L., Chong J. BindingDB in 2015: a public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016;44:D1045–D1053. doi: 10.1093/nar/gkv1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chong J., Xia J. Using MetaboAnalyst 4.0 for metabolomics data analysis, interpretation, and integration with other omics data. Methods Mol Biol. 2020;2104:337–360. doi: 10.1007/978-1-0716-0239-3_17. [DOI] [PubMed] [Google Scholar]
- 90.Tyanova S., Temu T., Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc. 2016;11:2301–2319. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- 91.Consortium. U UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hutter C., Zenklusen J.C. The Cancer Genome Atlas: creating lasting value beyond its data. Cell. 2018;173:283–285. doi: 10.1016/j.cell.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 93.Siebert R., Gerhäuser C., Simon R., Wagener R., Weber U.D., Sauter G. International cancer genome Consortium (ICGC) Med Genet-Berlin. 2016;28:416–423. [Google Scholar]
- 94.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 95.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du J., Yuan Z., Ma Z., Song J., Xie X., Chen Y. KEGG-PATH: Kyoto encyclopedia of genes and genomes-based pathway analysis using a path analysis model. Mol Biosyst. 2014;10:2441–2447. doi: 10.1039/c4mb00287c. [DOI] [PubMed] [Google Scholar]
- 97.Fabregat A., Jupe S., Matthews L., Sidiropoulos K., Gillespie M., Garapati P. The reactome pathway knowledgebase. Nucleic Acids Res. 2018;46:D649–D655. doi: 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu S., Sun C., Li Y., Wang T., Jia L., Lai S. GMrepo: a database of curated and consistently annotated human gut metagenomes. Nucleic Acids Res. 2020;48:D545–D553. doi: 10.1093/nar/gkz764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Q., Yu K., Li S., Zhang X., Zhao Q., Zhao X. gutMEGA: a database of the human gut MEtaGenome Atlas. Brief Bioinform. 2021;22:bbaa082. doi: 10.1093/bib/bbaa082. [DOI] [PubMed] [Google Scholar]
- 100.Poyet M., Groussin M., Gibbons S.M., Avila-Pacheco J., Jiang X., Kearney S.M. A library of human gut bacterial isolates paired with longitudinal multiomics data enables mechanistic microbiome research. Nat Med. 2019;25:1442–1452. doi: 10.1038/s41591-019-0559-3. [DOI] [PubMed] [Google Scholar]
- 101.Plewczynski D., Łaźniewski M., Augustyniak R., Ginalski K. Can we trust docking results? Evaluation of seven commonly used programs on PDBbind database. J Comput Chem. 2011;32:742–755. doi: 10.1002/jcc.21643. [DOI] [PubMed] [Google Scholar]
- 102.Roblek T., Vaupotic T., Mrhar A., Lainscak M. Drug–drug interaction software in clinical practice: a systematic review. Eur J Clin Pharmacol. 2015;71:131–142. doi: 10.1007/s00228-014-1786-7. [DOI] [PubMed] [Google Scholar]
- 103.Wright M.H., Sieber S.A. Chemical proteomics approaches for identifying the cellular targets of natural products. Nat Prod Rep. 2016;33:681–708. doi: 10.1039/c6np00001k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thingholm L.B., Rühlemann M.C., Koch M., Fuqua B., Laucke G., Boehm R. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe. 2019;26:252–264. doi: 10.1016/j.chom.2019.07.004. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qi X., Yun C., Sun L., Xia J., Wu Q., Wang Y. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25:1225–1233. doi: 10.1038/s41591-019-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sudsakorn S., Bahadduri P., Fretland J., Lu C. 2020 FDA drug–drug interaction guidance: a comparison analysis and action plan by pharmaceutical industrial scientists. Curr Drug Metabol. 2020;21:403–426. doi: 10.2174/1389200221666200620210522. [DOI] [PubMed] [Google Scholar]
- 107.Köhler S., Carmody L., Vasilevsky N., Jacobsen J.O.B., Danis D., Gourdine J.P. Expansion of the human phenotype ontology (HPO) knowledge base and resources. Nucleic Acids Res. 2019;47:D1018–D1027. doi: 10.1093/nar/gky1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amberger J.S., Hamosh A. Searching Online Mendelian Inheritance in Man (OMIM): a knowledgebase of human genes and genetic phenotypes. Curr Protoc Bioinformatics. 2017;58:1. doi: 10.1002/cpbi.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rappaport N., Twik M., Plaschkes I., Nudel R., Iny Stein T., Levitt J. MalaCards: an amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017;45:D877–D887. doi: 10.1093/nar/gkw1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piñero J., Ramírez-Anguita J.M., Saüch-Pitarch J., Ronzano F., Centeno E., Sanz F. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48:D845–D855. doi: 10.1093/nar/gkz1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen J.Y., Pandey R., Nguyen T.M. HAPPI-2: a comprehensive and high-quality map of human annotated and predicted protein interactions. BMC Genom. 2017;18:182. doi: 10.1186/s12864-017-3512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Das J., Yu H. HINT: high-quality protein interactomes and their applications in understanding human disease. BMC Syst Biol. 2012;6:92. doi: 10.1186/1752-0509-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown K.R., Jurisica I. Online predicted human interaction database. Bioinformatics. 2005;21:2076–2082. doi: 10.1093/bioinformatics/bti273. [DOI] [PubMed] [Google Scholar]
- 115.Licata L., Briganti L., Peluso D., Perfetto L., Iannuccelli M., Galeota E. MINT, the molecular interaction database: 2012 update. Nucleic Acids Res. 2012;40:D857–D861. doi: 10.1093/nar/gkr930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Keshava Prasad T.S., Goel R., Kandasamy K., Keerthikumar S., Kumar S., Mathivanan S. Human protein reference database—2009 update. Nucleic Acids Res. 2009;37:D767–D772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salwinski L., Miller C.S., Smith A.J., Pettit F.K., Ju Bowie, Eisenberg D. The database of interacting proteins: 2004 update. Nucleic Acids Res. 2004;32:D449–D451. doi: 10.1093/nar/gkh086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beuming T., Skrabanek L., Niv M.Y., Mukherjee P., Weinstein H. PDZBase: a protein–protein interaction database for PDZ-domains. Bioinformatics. 2005;21:827–828. doi: 10.1093/bioinformatics/bti098. [DOI] [PubMed] [Google Scholar]
- 119.Kerrien S., Aranda B., Breuza L., Bridge A., Broackes-Carter F., Chen C. The IntAct molecular interaction database in 2012. Nucleic Acids Res. 2012;40:D841–D846. doi: 10.1093/nar/gkr1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brohée S., van Helden J. Evaluation of clustering algorithms for protein–protein interaction networks. BMC Bioinf. 2006;7:488. doi: 10.1186/1471-2105-7-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blatti C., Sinha S. Characterizing gene sets using discriminative random walks with restart on heterogeneous biological networks. Bioinformatics. 2016;32:2167–2175. doi: 10.1093/bioinformatics/btw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krämer A., Green J., Pollard J., Jr., Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cowen L., Ideker T., Raphael B.J., Sharan R. Network propagation: a universal amplifier of genetic associations. Nat Rev Genet. 2017;18:551–562. doi: 10.1038/nrg.2017.38. [DOI] [PubMed] [Google Scholar]
- 124.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A.H., Tanaseichuk O. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ghalmane Z., Cherifi C., Cherifi H., Hassouni M.E. Centrality in complex networks with overlapping community structure. Sci Rep. 2019;9:10133. doi: 10.1038/s41598-019-46507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bröhl T., Lehnertz K. Centrality-based identification of important edges in complex networks. Chaos. 2019;29 doi: 10.1063/1.5081098. [DOI] [PubMed] [Google Scholar]
- 128.Siopi M., Siafakas N., Vourli S., Zerva L., Meletiadis J. Optimization of polyene-azole combination therapy against aspergillosis using an in vitro pharmacokinetic–pharmacodynamic model. Antimicrob Agents Chemother. 2015;59:3973–3983. doi: 10.1128/AAC.05035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Caruso A., Alvarez-Sánchez R., Hillebrecht A., Poirier A., Schuler F., Lavé T. PK/PD assessment in CNS drug discovery: prediction of CSF concentration in rodents for P-glycoprotein substrates and application to in vivo potency estimation. Biochem Pharmacol. 2013;85:1684–1699. doi: 10.1016/j.bcp.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 130.Lee S.H., Choi N., Sung J.H. Pharmacokinetic and pharmacodynamic insights from microfluidic intestine-on-a-chip models. Expert Opin Drug Metab Toxicol. 2019;15:1005–1019. doi: 10.1080/17425255.2019.1700950. [DOI] [PubMed] [Google Scholar]
- 131.Sung J.H., Kam C., Shuler M.L. A microfluidic device for a pharmacokinetic–pharmacodynamic (PK–PD) model on a chip. Lab Chip. 2010;10:446–455. doi: 10.1039/b917763a. [DOI] [PubMed] [Google Scholar]
- 132.Derendorf H., Meibohm B. Modeling of pharmacokinetic/pharmacodynamic (PK/PD) relationships: concepts and perspectives. Pharm Res. 1999;16:176–185. doi: 10.1023/a:1011907920641. [DOI] [PubMed] [Google Scholar]
- 133.Li A., Yuen V.M., Goulay-Dufaÿ S., Sheng Y., Standing J.F., Kwok P.C.L. Pharmacokinetic and pharmacodynamic study of intranasal and intravenous dexmedetomidine. Br J Anaesth. 2018;120:960–968. doi: 10.1016/j.bja.2017.11.100. [DOI] [PubMed] [Google Scholar]
- 134.Haidar S.H., Johnson S.B., Fossler M.J., Hussain A.S. Modeling the pharmacokinetics and pharmacodynamics of a unique oral hypoglycemic agent using neural networks. Pharm Res. 2002;19:87–91. doi: 10.1023/a:1013611617787. [DOI] [PubMed] [Google Scholar]
- 135.Yamamura S. Clinical application of artificial neural network (ANN) modeling to predict pharmacokinetic parameters of severely ill patients. Adv Drug Deliv Rev. 2003;55:1233–1251. doi: 10.1016/s0169-409x(03)00121-2. [DOI] [PubMed] [Google Scholar]
- 136.Brommage R., Powell D.R., Vogel P. Predicting human disease mutations and identifying drug targets from mouse gene knockout phenotyping campaigns. Dis Model Mech. 2019;12 doi: 10.1242/dmm.038224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cui W.B., Li A.P., Cui T., Yang L., Qin X.M. Research progress on identification of pharmacodynamic substance basis of traditional Chinese medicine based on target constituent knock-out/knock-in technology. China J Chin Mater Med. 2020;45:1279–1286. doi: 10.19540/j.cnki.cjcmm.20191221.304. [DOI] [PubMed] [Google Scholar]
- 138.Zhao Y., Feng H., Shan W., Cheng J., Wang X., Zhao Y. Development of immunoaffinity chromatography to specifically knockout baicalin from Gegenqinlian Decoction. J Separ Sci. 2015;38:2746–2752. doi: 10.1002/jssc.201500168. [DOI] [PubMed] [Google Scholar]
- 139.Xiao S., Kexuan Z., Qiang Z., Yunan Z., Center R. Application of ingredient knock-out technology in pharmacodynamic material basis research of traditional Chinese medicine. Moder Tradit Chin Med Mater Med World Sci Technol. 2016;9:1563–1566. [Google Scholar]
- 140.Liu C.X., Chen S.L., Xiao X.H., Zhang T.J., Liao M.L. A new concept on quality marker of Chinese materia medica: quality control for Chinese medicinal products. Chin Tradit Herb Drugs. 2016;47:1443–1457. [Google Scholar]
- 141.Liu C.X., Cheng Y.Y., Guo D.A., Zhang T.J., Li Y.Z., Hou W.B. A new concept on quality marker for quality assessment and process control of Chinese medicines. Chin Herb Med. 2017;9:3–13. [Google Scholar]
- 142.Xu H.Y., Hou W.B., Li K., Shen Y., Tang S.H., Guo F.F. Discovery and application of quality marker of traditional Chinese medicine based on integrative pharmacology. Chin J Exp Tradit Med Form. 2019;25:9–16. [Google Scholar]
- 143.Li K., Li J., Su J., Xiao X., Peng X., Liu F. Identification of quality markers of Yuanhu Zhitong tablets based on integrative pharmacology and data mining. Phytomedicine. 2018;44:212–219. doi: 10.1016/j.phymed.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 144.Guo R., Zhang X., Su J., Xu H., Zhang Y., Zhang F. Identifying potential quality markers of Xin-Su-Ning capsules acting on arrhythmia by integrating UHPLC–LTQ-Orbitrap, ADME prediction and network target analysis. Phytomedicine. 2018;44:117–128. doi: 10.1016/j.phymed.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 145.Wang H., Sun H., Zhang A., Li Y., Wang L., Shi H. Rapid identification and comparative analysis of the chemical constituents and metabolites of Phellodendri amurensis cortex and Zhibai dihuang pill by ultra-performance liquid chromatography with quadrupole TOF-MS. J Separ Sci. 2013;36:3874–3882. doi: 10.1002/jssc.201300794. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Y.C., Xu H.Y., Chen X.M., D S Study on the application of intestinal absorption in vitro coupled with bioactivity assessment in Yuanhu Zhitong preparation. J Med Plants Res. 2012;6:1941–1947. [Google Scholar]
- 147.Zhang Y.C., Xu H.Y., Chen X.M., Chen C., Wang H.J., Meng F.Y. Simultaneous quantification of 17 constituents from Yuanhu Zhitong tablet using rapid resolution liquid chromatography coupled with a triple quadrupole electrospray tandem mass spectrometry. J Pharmaceut Biomed. 2011;56:497–504. doi: 10.1016/j.jpba.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 148.Tao Y., Xu H.Y., Wang S.S., Wang B., Zhang Y.C., Wang W.H. Identification of the absorbed constituents after oral administration of Yuanhu Zhitong prescription extract and its pharmacokinetic study by rapid resolution liquid chromatography/quadrupole time-of-flight. J Chromatogr B. 2013;935:1–9. doi: 10.1016/j.jchromb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 149.Wang P., Zhang T.L., Yu G.H., Li M.J., Su J., Zhang J.Q. Poly-pharmacokinetic strategy-delineated metabolic fate of bioactive compounds in a traditional Chinese medicine formula, Yuanhu Zhitong tablets, using parallel reaction monitoring mode. Phytomedicine. 2019;53:53–61. doi: 10.1016/j.phymed.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 150.Xu H.Y., Tao Y., Lu P., Wang P., Zhang F.B., Yuan Y. A computational drug–target network for yuanhu zhitong prescription. Evid Based Complement Alternat Med. 2013;2013:658531. doi: 10.1155/2013/658531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu H.Y., Yang S., Zhang Y.Q., Jia Q., Li D.F., Zhang Y. Identification of key active constituents of Buchang Naoxintong capsules with therapeutic effects against ischemic stroke by using an integrative pharmacology-based approach. Mol Biosyst. 2016;12:233–245. doi: 10.1039/c5mb00460h. [DOI] [PubMed] [Google Scholar]
- 152.Wang Y., Wang X., Wang J., Li B., Yu R., Hu Y. Tongmai Yangxin intervening in myocardial remodeling after PCI for coronary heart disease: study protocol for a double-blind, randomized controlled trial. Trials. 2020;21:287. doi: 10.1186/s13063-020-4208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xu L., Zhang J., Li J., Lv L., Zhang Z., Wang F. Clinical study on post evaluation after listing of Qizhi Weitong granules: study protocol clinical trial (SPIRIT compliant) Medicine (Baltim) 2020;99 doi: 10.1097/MD.0000000000019758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang Y.Q., Guo Q.Y., Li Q.Y., Ren W.Q., Tang S.H., Wang S.S. Main active constituent identification in Guanxinjing capsule, a traditional Chinese medicine, for the treatment of coronary heart disease complicated with depression. Acta Pharmacol Sin. 2018;39:975–987. doi: 10.1038/aps.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo F., Zhang W., Su J., Xu H., Yang H. Prediction of drug positioning for Quan-Du-Zhong Capsules against hypertensive nephropathy based on the robustness of disease network. Front Pharmacol. 2019;10:49. doi: 10.3389/fphar.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li J.F., Wang P., Zhai X., Xiao X.F., Zhang Y., Li D.F. Study on analgesic effect and dose–effect relationship of Yuanhuzhitong prescrption on mice model of pain. Res Pract Chin Med. 2017;31:21–24. [Google Scholar]
- 157.Wu H.W., Wang P., Liu M.T., Tang L.Y., Fang J., Zhao Y. A 1H-NMR-based metabonomic study on the anti-depressive effect of the total alkaloid of Corydalis Rhizoma. Molecules. 2015;20:10047–10064. doi: 10.3390/molecules200610047. [DOI] [PMC free article] [PubMed] [Google Scholar]