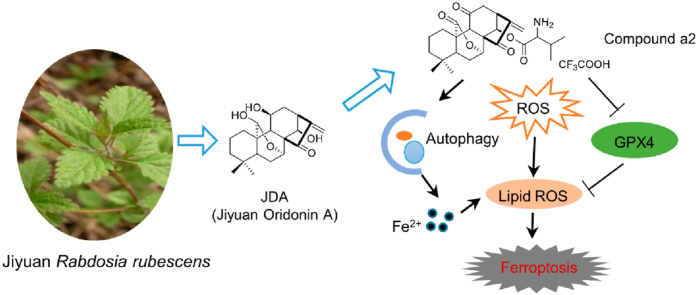
Abstract
Ferroptosis is a type of cell death accompanied by iron-dependent lipid peroxidation, thus stimulating ferroptosis may be a potential strategy for treating gastric cancer, therapeutic agents against which are urgently required. Jiyuan oridonin A (JDA) is a natural compound isolated from Jiyuan Rabdosia rubescens with anti-tumor activity, unclear anti-tumor mechanisms and limited water solubility hamper its clinical application. Here, we showed a2, a new JDA derivative, inhibited the growth of gastric cancer cells. Subsequently, we discovered for the first time that a2 induced ferroptosis. Importantly, compound a2 decreased GPX4 expression and overexpressing GPX4 antagonized the anti-proliferative activity of a2. Furthermore, we demonstrated that a2 caused ferrous iron accumulation through the autophagy pathway, prevention of which rescued a2 induced ferrous iron elevation and cell growth inhibition. Moreover, a2 exhibited more potent anti-cancer activity than 5-fluorouracil in gastric cancer cell line-derived xenograft mice models. Patient-derived tumor xenograft models from different patients displayed varied sensitivity to a2, and GPX4 downregulation indicated the sensitivity of tumors to a2. Finally, a2 exhibited well pharmacokinetic characteristics. Overall, our data suggest that inducing ferroptosis is the major mechanism mediating anti-tumor activity of a2, and a2 will hopefully serve as a promising compound for gastric cancer treatment.
KEY WORDS: Jiyuan Rabdosia rubescens, JDA derivative, Gastric cancer, Ferroptosis, ROS, GPX4, Ferrous iron, Autophagy
Abbreviations: 5-FU, 5-fluorouracil; CDX, cell line-derived xenograft; DCFH-DA, dichlorodihydro-fluorescein diacetate; DCM, dichloromethane; IKE, imidazole ketone erastin; JDA, Jiyuan oridonin A; KEGG, Kyoto Encyclopedia of Genes and Genomes; NAC, N-acetylcysteine; Papp, apparent permeability coefficient; PARP, poly ADP-ribose polymerase; PDX, patient-derived tumor xenograft; PK, pharmacokinetic; qRT-PCR, quantitative real time PCR; ROS, reactive oxygen species; RTV, relative tumor volume; Verp, verapamil
Graphical abstract
Natural product derivative, a2, exhibited anti-tumor activity by inducing ferroptosis through decreasing GPX4 and causing ferrous iron accumulation in gastric cancer cells.
1. Introduction
Gastric cancer is the fifth most frequent cancer and the third leading cause of death from cancer1. Limited therapeutic strategies, especially definite clinical used drugs, prompt the development of new agents against gastric cancer2. The Chinese medicinal herb Rabdosia rubescens has long been used to treat gastrointestinal tract diseases alone or together with other herbs. Jiyuan oridonin A (JDA) was first isolated and identified by our group, which was named as ent-kaurane diterpenoid (I) in the patent applied3. However, as in the case with oridonin4, low aqueous solubility and poor bioavailability significantly limit the clinical application of JDA, impelling scientists to optimize the structure of JDA.
To improve aqueous solubility and bioavailability, a series of JDA derivatives were designed and synthesized. Among them, compound a2, bearing a valine ester trifluoroacetate, exhibited excellent anti-proliferative activity against gastric cancer cells. However, the anti-cancer mechanism of a2 is unclear and needs to be further investigated.
A variety of studies have reported that ent-kaurane diterpenes exert anti-tumor effects through regulating cell cycle, apoptosis, autophagy, and metastasis5, 6, 7. While changes in P53, P21, CDK1/2, CDK2 and cyclin D1 contribute to cell cycle arrest, and the occurrence of apoptosis is associated with disordered expression of BAX, BCL-2, cytochrome c, caspase 3/7/9 and PARP. However, these reports mainly focused on the biological effects and the mechanism of action of ent-kaurane diterpenes remains unclear. With the development of molecular biology, specific targets of ent-kaurane diterpenes have emerged such as NLRP3, AKT, BCR-ABL and peroxiredoxin I8, 9, 10, 11. Therefore, there is still an urgent need to investigate the mechanism that mediates the anti-cancer activity of a2.
Ferroptosis is a form of regulated cell death that is characterized by large amounts of ferrous iron accumulation and lipid peroxidation. SLC7A11 and GPX4 are two key enzymes that repel the lipid reactive oxygen species (ROS) production by transporting l-cystine into cells and reducing lipid peroxides, respectively. Various compounds targeting SLC7A11 or GPX4 show anti-cancer activity12,13. Iron is a redox-active metal that is well regulated in cells, elevated levels of iron play a pivotal role in the induction of ferroptosis14,15. Thus, induction of ferroptosis is a novel method for the destruction of cancer cells12,16.
In this research, we show that one JDA derivative a2 could specifically inhibit the growth of gastric cancer cells. Mechanistically, a2 induced ferroptosis by decreasing GPX4 and causing ferrous iron accumulation. Furthermore, we found that prevention of ferroptosis strongly rescued the cell growth inhibited by a2. Importantly, a2 differentially prevents tumor growth in gastric cancer patient-derived tumor xenograft (PDX) models, and downregulation of GPX4 can predict the sensitivity of gastric cancer to a2, making a2 a promising therapeutic agent for gastric cancer.
2. Materials and methods
2.1. Reagents
Z-VAD-FMK, 3-methyladenine (3-MA), deferoxamine and RSL3 were obtained from MedChemExpress (NJ, USA); Z-LEHD-FMK and imidazole ketone erastin (IKE) were purchased from Selleck Chemicals (Houston, USA). Dichlorodihydro-fluorescein diacetate (DCFH-DA) was purchased from Beyotime Biotechnology (Shanghai, China). Antibodies against BCL-2, BAX, caspase 9, caspase 3, poly ADP-ribose polymerase (PARP) and β-actin were purchased from Cell Signaling Technology (Boston, MA, USA). Antibodies against SLC7A11 and GPX4 were obtained from Abcam (Cambridge, UK). Antibody against GAPDH was acquired from Goodhere Biological Technology (Hangzhou, China).
2.2. Chemical synthesis
JDA was isolated from extract of Jiyuan Rabdosia rubescens as described in patent17.
General procedure for preparation of JDAO: compound JDA (150 mg, 0.38 mmol) was dissolved in acetone (1 mL). After adding Jones reagent (50 μL), the mixture was stirred for 10 min at 0 °C. The mixture was diluted with water (50 mL) and then extracted with ethyl acetate (50 mL × 3). The extract was then washed with brine, saturated NaHCO3 solution, dried over anhydrous Na2SO4, filtered, and evaporated to generate JDAO (135 mg, 91%) without further purification.
General procedure for preparation of a2: JDAO (100 mg, 0.14 mmol) was mixed with N-Boc-l-valine (0.28 mmol), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (29 mg, 0.14 mmol), 4-dimethylaminopyridine (2 mg, 0.016 mmol) and diisopropylethylamine (26 μL, 0.14 mmol) in 3 mL of dichloromethane (DCM). And the reaction was stirred at room temperature for 1–2 h. After completion of the reaction as indicated by TLC, the resulting mixture was poured into DCM (30 mL), and washed with brine (10 mL × 3), and then dried over anhydrous Na2SO4 and concentrated under vacuum, and then purified by column chromatography to obtain corresponding product. The corresponding product was de-protected with excessive trifluoroacetic acid (1 mmol) in DCM (6 mL) at 0 °C and stirred for 30 min. After completion of the reaction as indicated by TLC, the reaction solution is concentrated under vacuum. And the residue was purified by crystallization with isopropyl ether to yield compound a2 as a white solid.
(6R,6aS,9S,11bS,14R)-14-Hydroxy-4,4-dimethyl-8-methyleneoctahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalene-7,11,12(8H,11aH)-trione (JDAO), white solid, yield: 95%. m.p.:185.1–185.6 °C. IR (KBr, cm−1): 3565, 2960, 1760, 1733, 1644, 1380, 1105, 1042, 946, 718. 1H NMR (400 MHz, DMSO) δ 6.14 (s, 1H), 6.06 (s, 1H), 5.79 (s, 1H), 4.79–4.78 (m, 1H), 3.73 (s, 1H), 3.11–3.09 (d, J = 8.4 Hz, 1H), 2.96 (s, 1H), 2.91–2.84 (m, 2H), 2.70–2.63 (m, 1H), 2.55 (m, 1H), 1.80–1.73 (m, 1H), 1.62–1.56 (m, 2H), 1.37–1.34 (d, J = 11.2 Hz, 2H), 1.11–0.94 (m, 2H), 0.81 (s, 3H), 0.72 (s, 3H). 13C NMR (101 MHz, DMSO) δ 204.70, 200.62, 174.52, 148.36, 121.48, 73.07, 70.74, 58.73, 57.93, 46.71, 45.56, 43.18, 41.28, 40.09, 33.99, 30.75, 28.47, 23.55, 19.29, 18.40. HR-MS (ESI): m/z Calcd. for C20H25O5 [M+H]+: 345.1702, found 345.1690.
(6R,6aR,9S,11bS,14R)-4,4-Dimethyl-8-methylene-7,11,12-trioxododecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-14-yl valinate (a2), white solid, yield 81%, m.p.:183.1–183.9 °C. IR (KBr, cm−1): 3125, 2969, 1738, 1678, 1532, 1400, 1202, 1137, 1038, 722. 1H NMR (400 MHz, DMSO) δ 8.48 (s, 2H), 6.26 (s, 1H), 5.94 (m, 1H), 4.90–4.85 (m, 2H), 4.04 (m, 1H), 3.41–3.39 (dd, J = 12.0, 4.0 Hz, 1H), 3.30 (d, J = 8.1 Hz, 1H), 2.87–2.85 (m, 2H), 2.81–2.79(dd, J = 13.7, 8.5 Hz, 1H), 2.79–2.67 (m, 1H), 2.05–2.04 (m, 1H), 1.80–1.79 (m, 1H), 1.77–1.68 (m, 2H), 1.40–1.37 (m, 2H), 1.10–1.03 (m, 2H), 0.89 (s, 3H), 0.87 (s, 3H), 0.82 (s, 3H), 0.72 (s, 3H). 13C NMR (101 MHz, DMSO) δ 203.14, 198.01, 173.90, 167.92, 158.31, 158.00, 145.94, 123.17, 75.08, 72.16, 58.85, 56.19, 46.39, 45.27, 43.16, 38.46, 33.81, 30.48, 29.34, 28.11, 22.97, 18.92, 18.18, 18.12, 17.11. HR-MS (ESI): m/z Calcd. for C25H34NO6 [M+H]+: 444.2386, found 444.2381.
2.3. Cell lines and proliferation assay
Human gastric cancer cell lines HGC-27, MGC-803, BGC-823 and AGS, and human immortalized gastric mucosa epithelial cell line GES1 were purchased from Chinese Academy of Science Cell Bank (Shanghai, China). Gastric cancer cell lines SGC-7901 and MKN-45 were kindly provided by Ding Jian (Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China). Cell proliferation was measured by sulforhodamine B (SRB; Sigma–Aldrich, St. Louis, MO, USA) assay as described previously18.
| Relative cell growth = (ODtreated – ODstart) / (ODcontrol – ODstart) | (1) |
| Relative inhibitory rate (%) = (ODcontrol – ODtreated) / (ODcontrol – ODstart) × 100 | (2) |
ODstart means the optic density of cells at the moment of adding test compounds.
2.4. Measurement of cell cycle, cell apoptosis, mitochondrial membrane potential and intracellular ROS
Measurement of cell cycle, cell apoptosis, mitochondrial membrane potential and intracellular ROS have been described previously18.
2.5. RNA-seq analysis
MGC-803 cells seeded in 6-well plates were treated with a2 (10 μmol/L) for 24 h. Total RNA of cells were then extracted by TRIzol reagent (Solarbio Science & Technology Co., Beijing, China). cDNA library preparation, RNA sequencing, quality control and transcriptome profiling were performed by Novogene (Beijing, China). Briefly, total RNA were verified by agarose gel electrophoresis, Nanodrop, and Agilent 2100 analysis, following by cDNA library preparation with poly (A) selection and RNA sequencing with Illumina PE150. Raw data of RNA sequencing were aligned by Hisat2 and quantified by HTSeq, producing the data of sample read counts and FPKM. RNA-seq data was further analyzed by R packages DESeq2 and clusterProfiler to generate differentially expressed mRNAs and enriched pathways19,20.
2.6. Measurement of lipid peroxidation using C11-BODIPY581/591
Cells seeded in 6-well plates were treated with a2 for 6 h, cells were then collected and re-suspended in 500 μL PBS containing 2 μmol/L C11-BODIPY581/591 (Thermo Fisher Scientific, MA, USA) for 30 min at 37 °C. Subsequently, cells were measured by BD LSRFortessa flow cytometry (NJ, USA). Quantification of data was performed with Flowjo.
2.7. Quantitative real time PCR (qRT-PCR)
Total RNAs were extracted from cultured cells using the TRIzol reagent. cDNAs were generated by reverse transcription using HiScriptII Q RT SuperMix (Vazyme, Nanjing, China). qRT-PCR was performed with ChamQ Universal SYBR qPCR Master Mix (Vazyme) against specific genes. Relative quantity of gene expression was normalized to GAPDH and analyzed by QuantStudio™6 Flex Real-Time PCR System (Thermo Fisher Scientific). The gene-specific primer pairs, which were synthesized by GENEWIZ (Jiangsu, China), were as follows, respectively: GAPDH-F, 5′-GCACCGTCAAGGCTGAGAAC-3′; GAPDH-R, 5′-TGGTGAAGACGCCAGTGGA-3′; GPX4-F, 5′-GAGGCAAGACCGAAGTAAACTAC-3′; GPX4-R, 5′-CCGAACTGGTTACACGGGAA-3′; SLC7A11-F, 5′-GGTCCATTACCAGCTTTTGTACG-3′; SLC7A11-R, 5′-AATGTAGCGTCCAAATGCCAG-3′.
2.8. Transfection and generation of stable cell lines
Plasmid GPX4 (GV641) and lentivirus particles were generated by GeneChem Co. (Shanghai, China). Gastric cancer cells were seeded in 6-well plates and infected with viruses according to manufacturer's instruction, multiplicity of infection for MGC-803 and MKN-45 was 10 and 25, respectively. After 72 h, stably transfected cells were selected and maintained with puromycin (2.5 μg/mL).
2.9. RNA interference
Gastric cancer cells were seeded in 12-well plate, cells were then transfected with specific siRNAs using GP-transfect-Mate (GenePharma Co., Shanghai, China) according to manufacturer's protocols. After 12 h, cells were digested with trypsin and seeded in 96-well plate to test the anti-proliferative activity of compound a2. The targeted genes sense sequences were as follows, siSLC7A11#1: 5′- GCAGCUACUGCUGUGAUAUTT-3′, siSLC7A11#2: 5′-CCAUGAUUCAUGUCCGCAATT-3′. The negative control was provided by GenePharma Co.
2.10. Intracellular ferrous iron (Fe2+) determination
Cells seeded in 60-mm plates were treated with compounds for indicated time, cells were then collected and homogenized in iron assay buffer. Intracellular ferrous iron content was determined using the iron assay kit from Sigma–Aldrich (MAK025) according to instructions provided by manufacturer.
2.11. Gastric cancer cell line-derived xenograft (CDX) studies
All animal experiments were performed in accordance with relevant protocols and guidelines approved by animal care and committee of Zhengzhou University (Zhengzhou, China). Male nude mice (ages 6–8 weeks) used in the studies were purchased from Hunan SJA Laboratory Animal Co. (Changsha, China). Male nude mice were subcutaneously injected with MGC-803 cells into the right flank of mouse. Once the tumor volume reached 100–200 mm3, mice were randomly divided into 5 groups (6 mice/group) and administered with saline, a2 (5, 10, and 20 mg/kg), or 5-fluorouracil (5-FU, 15 mg/kg) once a day for 21 days via tail vein injection. Tumor volume (tumor length × width2/2) and body weight were recorded every two days. All mice were sacrificed after the final treatment, tumors were isolated and weighed, and liver, kidney, lung, heart, and spleen were collected to perform histopathological examination.
2.12. Gastric cancer PDX studies
Fresh surgical tumor specimens of patients bearing gastric cancer were collected from the First Affiliated Hospital of Zhengzhou University. Before the tissue collection, the clinical protocol was approved by the ethics review board of the First Affiliated Hospital of Zhengzhou University. After examination by pathologists, tumor tissues resected from primary lesion were transferred to storage solution (RPMI 1640 medium supplemented with 2% FBS, and 5% amphotericin B). Tumors were dissected into 1 mm3 and subcutaneously implanted into NOD-SCID mice within 6 h. After tumor tissues were stably propagated for three passages, tumor tissues were used for evaluating the anti-tumor activity of a2 with the similar method described in gastric cancer CDX studies. Treatment duration of PDX mice varied from 15 to 36 days according to tumor volumes in control groups. The relative tumor volume (RTV) was determined according to Eq. (3):
| RTV = Vx/V0 | (3) |
Vx represented the tumor volume on Day x and V0 represented tumor volume on Day 0. The “treated/control” (T/C) ratio was determined according to Eq. (4):
| T/C (%) = RTVT/RTVC × 100 | (4) |
RTVT and RTVC represented the RTV of treated and control group, respectively.
2.13. Pharmacokinetic studies
Pharmacokinetic studies including Caco-2 permeability assay (n = 2), metabolic stability in liver microsomes (n = 2) and pharmacokinetic parameters in rats (n = 3) were conducted by Sundia MediTech Company (Shanghai, China).
2.14. Statistical analysis
The data are the means of at least three independent experiments and presented as mean ± standard deviation (SD). Statistical differences were assessed by one-way or two-way ANOVA analysis with GraphPad Software according to experiments. P < 0.05 was considered statistically significant. P < 0.05, *P< 0.01, **P 0.005, ***P 0.001.
3. Results
3.1. Compound a2 markedly inhibited the growth of gastric cancer cells
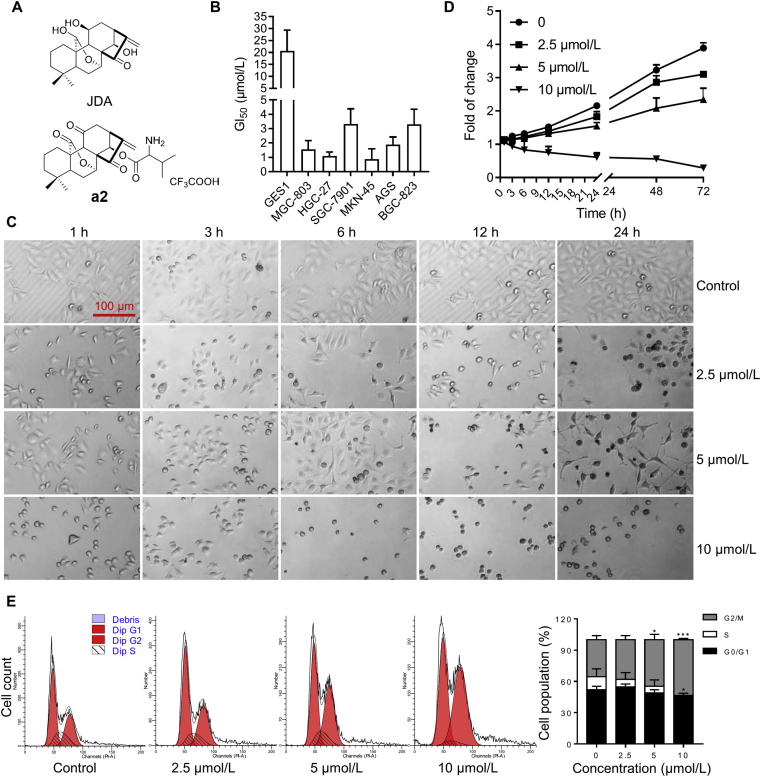
Inspired by the modification of oridonin with amino acid21, our group designed and synthesized a series of JDA derivatives (data not published) and a2 was selected for further investigation (Fig. 1A). To test the anti-proliferative effect of a2 in gastric cancer cells, the GI50 values of a2 were determined in the human gastric epithelial cell line GES1 and six gastric cancer cell lines MGC-803, HGC-27, SGC-7901, MKN-45, AGS and BGC-823. As shown in Fig. 1B, the GI50 values of a2 against gastric cancer cells ranged from 0.88 to 3.33 μmol/L, whereas the GI50 values of a2 in GES1 were at least five-fold higher than those in gastric cancer cells, suggesting that the a2 selectively inhibited the growth of gastric cancer cells. Next, MGC-803 and SGC-7901 cells were selected to make further investigation. We found a2 decreased the number of MGC-803 cells in time- and dose-dependent manner, and a2 induced morphologic changes of cells as early as 1 h after incubation at high concentrations (5 and 10 μmol/L, Fig. 1C and D). In addition, the relative cell growth of cells was gradually decreased after a2 treatment at 10 μmol/L compared with that of other cell groups, indicating that a2 led to cell death at high concentrations. Similar results were observed in MKN-45 cells (Supporting Information Fig. S1A and S1B).
Figure 1.
Compound a2 selectively inhibited the growth of gastric cancer cells. (A) Structural formulas of JDA and a2. (B) GI50 values of a2 in selected cells for 72 h. (C) MGC-803 cells were treated with a2 at indicated doses and then were imaged by the bright-field microscopy at specific point in time. (D) MGC-803 cells were treated with a2 at indicated concentrations for different time duration and then cell viability was detected by SRB staining. (E) MGC-803 cells were treated with a2 for 48 h, cells were then stained with PI and analyzed by flow cytometry and quantified. Data are presented as the mean ± SD (n = 3) from three independent experiments with biological duplicates in (B, D, E). Statistics differences were analyzed by two-way ANOVA analysis (E): ∗P < 0.05, ∗∗∗P < 0.005 vs. the control.
Given that a2 markedly suppressed cell growth, we tried to determine whether the cell cycle was affected by a2. The results showed that a2 significantly increased the cell population in the G2/M phase and reduced the number of cells in the S phase (Fig. 1E). Similar phenomenon was obtained in MKN-45 cells (Fig. S1C and S1D). Therefore, a2 showed significant anti-proliferative activity against gastric cancer cells and arrested them in the G2/M phase.
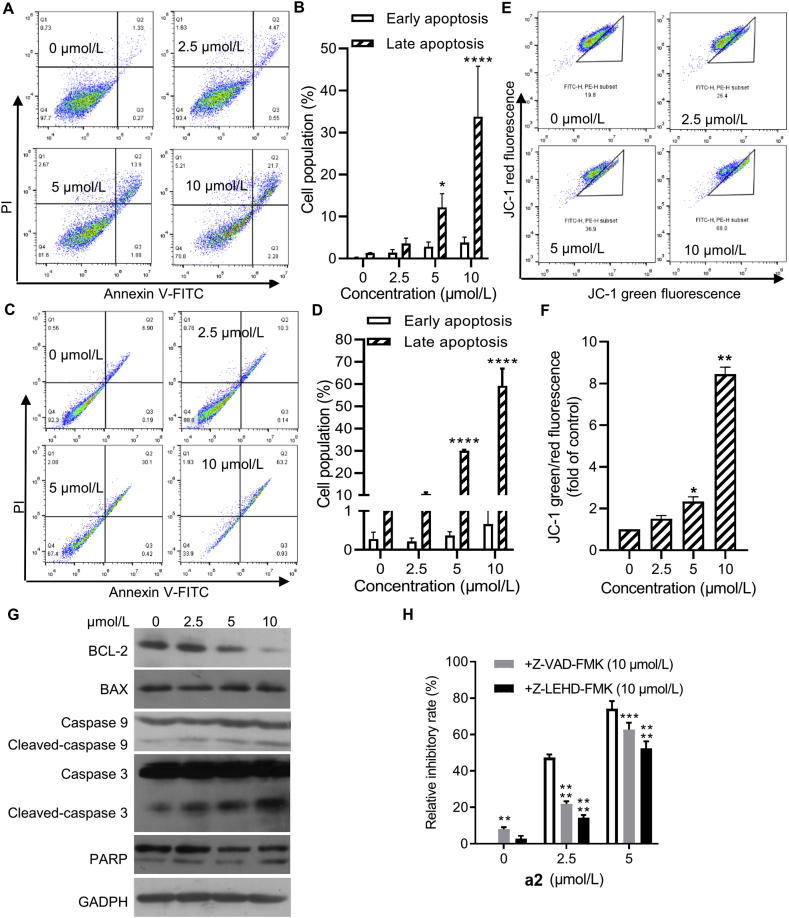
3.2. Compound a2 induced mitochondria-dependent apoptosis in gastric cancer cells
Considering that a2 caused cell death at high concentrations and that variety of ent-kaurane diterpenoids induce cell apoptosis, we examined whether a2 can induce cell apoptosis. As shown in Fig. 2A–D, a2 significantly elevated cell populations co-staining with PI and Annexin V-FITC in dose- and time-dependent manner, indicating that a2 induced apoptosis. Apoptosis induced by a2 was also observed in MKN-45 cells (Supporting Information Fig. S2A–S2D). Mitochondrial membrane potential was measured by JC-1 staining, loss of which marks the initiation of mitochondria-dependent apoptosis22. Compound a2 markedly induced the loss of mitochondrial potential, indicating that a2 caused mitochondrial injury (Fig. 2E and F, Fig. S2E and S2F). Apoptosis-related proteins were further examined. As shown in Fig. 2G and Fig. S2G, a2 dose-dependently decreased the levels of anti-apoptotic protein BCL-2 and elevated cleaved-caspase 9/3 and PARP proteins, confirming that a2 induced mitochondria-dependent apoptosis in gastric cancer cells.
Figure 2.
Compound a2 induced mitochondria-dependent apoptosis in gastric cancer cells. MGC-803 cells were treated with a2 at indicated concentrations for 24 h (A and B) and 48 h (C and D), cells were then stained by PI and Annexin V-FITC and analyzed by flow cytometry. (E and F) MGC-803 cells were treated with a2 for 24 h, cells were then stained by JC-1 and analyzed by flow cytometry. (G) MGC-803 cells were treated with a2 for 24 h with illustrated concentrations, indicated proteins were tested by Western blot. (H) MGC-803 cells were treated with a2 alone or in combination with the indicated agents for 72 h, cell viability was then tested by SRB assay. Data are presented as the mean ± SD (n = 3) from three independent experiments with biological duplicates in (B, D, F, H). Statistics differences were analyzed by two-way ANOVA analysis (B, D, H) or one-way ANOVA analysis (F): ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.001 vs. the control (B, D, F). ∗∗P < 0.01, ∗∗∗P < 0.005, ∗∗∗∗P < 0.001 vs. the a2 treated samples (H).
To explore the contribution of apoptosis to the anti-tumor activity of a2, the pan-caspase inhibitor Z-VAD-FMK or selective caspase 9 inhibitor Z-LEHD-FMK were utilized to investigate the role of apoptosis. In MGC-803 cells, the presence of Z-VAD-FMK and Z-LEHD-FMK partially antagonized anti-proliferative activity of a2 (Fig. 2H). However, combination of a2 with Z-VAD-FMK or Z-LEHD-FMK caused equivalent relative inhibitory rate with that of a2 alone in MKN-45 cells, suggesting that a2 inhibited cell growth independent of apoptosis (Fig. S2H). Thus, a2 can induce apoptosis in both MGC-803 and MKN-45 cells, whereas cell growth inhibited by a2 partially relied on apoptosis only in MGC-803 cells.
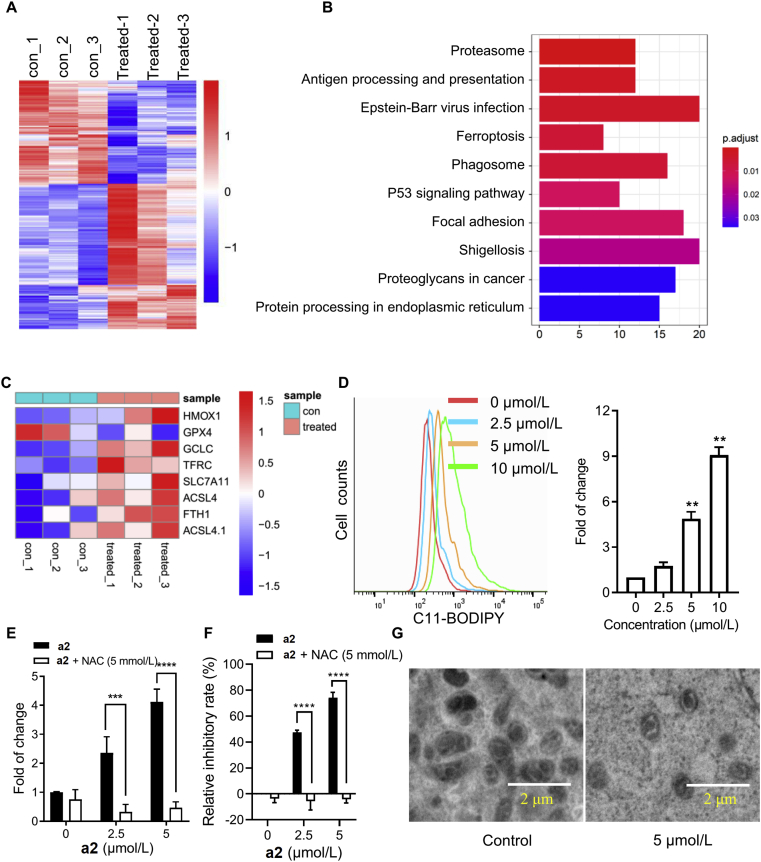
3.3. Identification of ferroptosis induced by compound a2 in gastric cancer cells
To further explore the mechanism mediating the anti-cancer effect of a2, mRNAs extracted from control and treated MGC-803 cells were analyzed for differentially expressed genes. As shown in Fig. 3A, there were 560 significant differentially expressed mRNAs affected by a2 in MGC-803 cells (Supporting Information Table S1). Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of these 560 mRNAs showed that the genes were mainly enriched in proteasome, antigen processing and presentation, ferroptosis, phagosome, etc (Fig. 3B). Given that ferroptosis is a type of programmed cell death initiated by large amounts of ROS and most of ent-kaurane diterpenes caused ROS elevation in cancer cells, we further presented the ferroptosis-related mRNAs affected by a2. Compound a2 affected mRNAs encoding ferroptosis-related proteins in a seemingly paradoxical manner: the mRNA encoding key anti-ferroptosis kinase GPX4 was decreased, whereas a2 elevated the mRNA encoding the anti-ferroptosis protein SLC7A11 (Fig. 3C). To explore whether a2 induces ferroptosis, lipid ROS were determined with C11-BODIPY581/591. Compound a2 dose-dependently elevated lipid ROS production in both MGC-803 and MKN-45 cells, confirming that a2 induced cell ferroptosis (Fig. 3D and Supporting Information Fig. S3A). In addition, total ROS were measured, elevation of which will initiate ferroptosis. Compound a2 markedly elevated the level of ROS, and ROS scavenger N-acetylcysteine (NAC) almost totally reversed ROS accumulation triggered by a2 (Fig. 3E and Fig. S3B). In accordance with that of ROS change, NAC can dramatically rescue cell growth prevented by a2 (Fig. 3F and Fig. S3C). Furthermore, transmission electron microscopy examination showed that mitochondria became smaller and with increased membrane density in a2-treated cells compared with those in control cells (Fig. 3G). Thus, a2 can induce ferroptosis in gastric cancer cells.
Figure 3.
Identification of ferroptosis induced by compound a2 in gastric cancer cells. (A) Heatmap of differentially expressed mRNAs after incubation of MGC-803 cells with a2 (10 μmol/L) for 24 h (RNA-seq, n = 3), P < 0.05, basemean of read > 100. (B) KEGG pathways of differentially expressed mRNAs in a2 treated vs. control MGC-803 cells. Bar color represents statistical significance of the enrichment, length of bar indicates gene number. (C) Heatmap of differentially expressed mRNAs in ferroptosis pathway in a2 treated versus control MGC-803 cells. (D) MGC-803 cells were treated with a2 for 6 h, cells were then stained by C11-BODIPY581/591 and analyzed by flow cytometry. (E) MGC-803 cells were treated with a2 in the absence or presence of NAC for 24 h, cells were then stained with DCFH-DA and analyzed by flow cytometry. (F) MGC-803 cells were treated with a2 in the absent or present of NAC for 72 h, relative inhibitory rates of compounds were determined with SRB assay. (G) MGC-803 cells were treated with a2 for 6 h, cells were then observed by transmission electron microscopy. Data are presented as the mean ± SD (n = 3) from three independent experiments with biological duplicates in (D and F). Statistics differences were analyzed by one-way ANOVA analysis (D) or two-way ANOVA analysis (E and F): ∗∗P < 0.01 vs. the control (D). ∗∗∗P < 0.005, ∗∗∗∗P < 0.001 vs. the a2 treated samples (E and F).
3.4. Compound a2 induced ferroptosis via decreasing GPX4
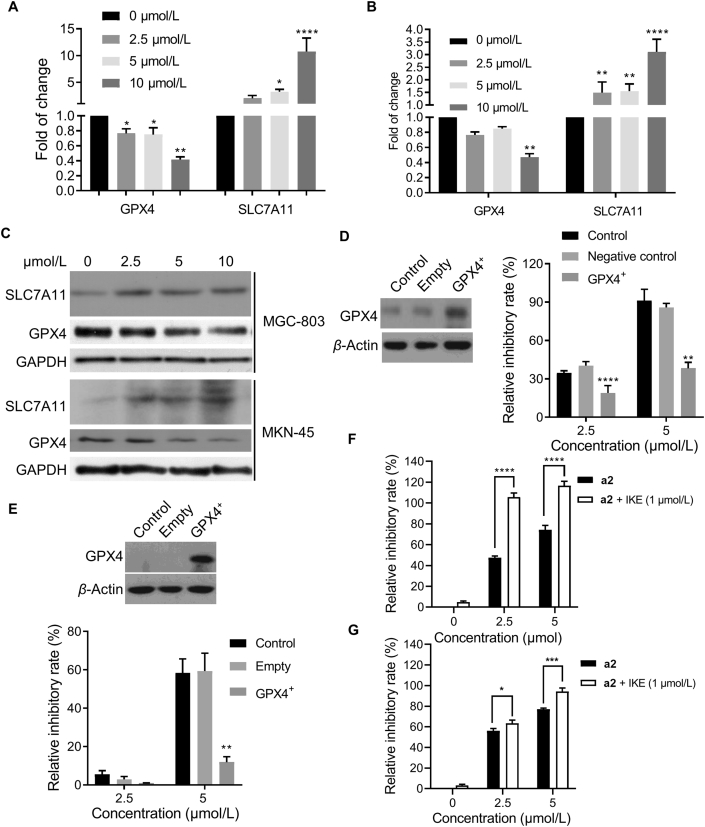
SLC7A11 and GPX4 are two important proteins that negatively regulate ferroptosis by importing l-cystine and catalyzing the reduction of lipid peroxides, respectively23. However, a2 induced the opposite alteration of SLC7A11 and GPX4 in MGC-803 cells (Fig. 3C). qRT-PCR analysis confirmed that a2 dose-dependently reduced the mRNA level of GPX4 and increased the mRNA level of SLC7A11 in both MGC-803 and MKN-45 cells (Fig. 4A and B). The protein levels of GPX4 and SLC7A11 affected by a2 were consistent with that of the changes in mRNAs (Fig. 4C). To investigate the biological function of GPX4 in a2-induced cell arrest, GPX4 was overexpressed in gastric cancer cells. GPX4 overexpression significantly reversed the cell growth inhibited by a2 in both MGC-803 and MKN-45 cells (Fig. 4D and E). Next, we found GPX4 inhibitor RSL3 and a2 exerted additive anti-proliferative activity against MGC-803 cells at the low concentration (Supporting Information Fig. S4B), indicating that both RSL3 and a2 exerted anti-cancer activity through inhibiting GPX4. In addition, we measured the expression level of GPX4 in a panel of cell lines. Concerning GI50 value of a2 in different cell lines, we found that the cell lines with high expressed GPX4 were more sensitive to a2 (Fig. S4A and Fig. 1B).
Figure 4.
Compound a2 induced ferroptosis via decreasing GPX4. MGC-803 (A) and MKN-45 (B) cells were treated with a2 at indicated doses for 24 h, indicated mRNAs were then determined by qRT-PCR. (C) MGC-803 and MKN-45 cells were treated with a2 for 24 h, indicated proteins were measured. GPX4 were overexpressed in MGC-803 (D) and MKN-45 (E) cells, relative inhibitory rates of a2 in indicated cells for 24 h were tested. MGC-803 (F) and MKN-45 (G) cells were treated with a2 alone or combined with IKE for 72 h, cells were then examined by SRB assay. Data are presented as the mean ± SD (n = 3) from three independent experiments with biological duplicates in (A–B, D–G). Statistics differences were analyzed by one-way ANOVA analysis (A and B) or two-way ANOVA analysis (D–G): ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.001 vs. the control (A–B, D–E). ∗P < 0.05, ∗∗∗P < 0.005, ∗∗∗∗P < 0.001 vs. the a2 treated samples (F and G).
We then tested the biological function of SLC7A11 in anti-tumor activity of a2 with the specific system xc– inhibitor IKE. IKE alone had no effect on cell growth, whereas the combination of IKE and a2 significantly augmented the relative inhibitory rate induced by a2 alone in both MGC-803 and MKN-45 cells, indicating that elevated SLC7A11 antagonized a2-induced cell growth inhibition (Fig. 4F and G). In addition, a2 showed higher relative inhibition rate in MGC-803 cells with reduced expression of SLC7A11 (Fig. S4C and S4D), suggesting that decreasing of SLC7A11 enhanced the response of cells to a2. We also examined the expression level of SLC7A11, and found no observed relationship between SLC7A11 protein level and GI50 values of a2 (Fig. S4A and Fig. 1B). Together, these data supported that a2 inhibited cell growth by decreasing GPX4 expression, while elevated SLC7A11 antagonized anti-proliferative activity of a2.
3.5. Compound a2 induced cell ferroptosis by causing ferrous iron (Fe2+) accumulation
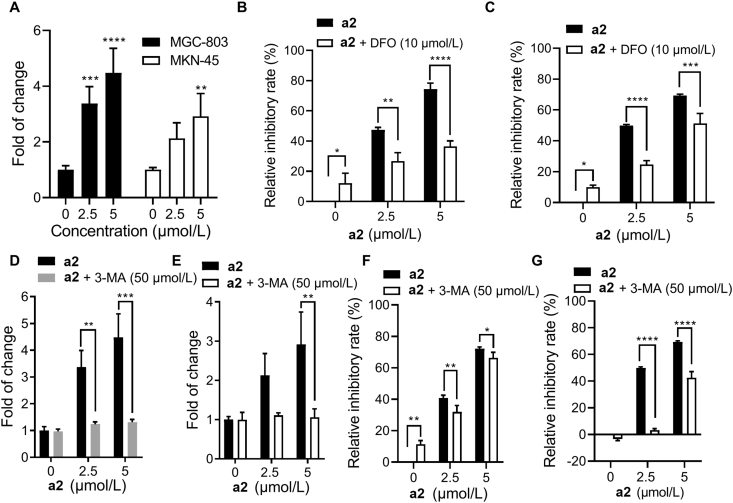
Given that ferroptosis is a form of regulated cell death driven by iron-dependent lipid peroxidation, we tested the ferrous iron content in the presence of a2. As shown in Fig. 5A, a2 significantly increased ferrous iron accumulation in both MGC-803 and MKN-45 cells. In accordance with that, iron chelater deferoxamine can markedly reverse the cell growth prevented by a2 in gastric cancer cells (Fig. 5B and C). Considering that autophagy can cause excessive extracellular iron by degrading the iron storage protein ferritin, an inhibitor of autophagy 3-MA was utilized to explore the function of autophagy in a2-induced ferrous iron accumulation24,25. As expected, ferrous iron accumulation triggered by a2 could be completely prevented by 3-MA in both MGC-803 and MKN-45 cells, indicating that a2 induced an excess of ferrous iron through autophagy (Fig. 5D and E). Consistent with this, a2 can dose-dependently induce the expression of LC3B (Supporting Information Fig. S5), and the relative inhibitory rate induced by the combination of a2 and 3-MA was significantly lower than that of a2 alone (Fig. 5F and G). Taken together, a2 induced cell growth inhibition through autophagy-dependent ferrous iron accumulation.
Figure 5.
Compound a2 induced ferroptosis through accumulation of ferrous iron. (A) Ferrous iron contents were measured in MGC-803 and MKN-45 cells treated with a2 for 24 h. Relative inhibitory effects of a2 without or with deferoxamine on MGC-803 (B) and MKN-45 (C) cells (72 h). MGC-803 (D) and MKN-45 (E) cells were treated with a2 alone or combined with 3-MA for 24 h, ferrous iron contents were then examined. MGC-803 (F) and MKN-45 (G) cells were treated with indicated compounds for 72 h, relative inhibitory rates were tested. Data are presented as the mean ± SD (n = 3) from three independent experiments with biological duplicates in (A–G). Statistics differences were analyzed by two-way ANOVA analysis: ∗∗P < 0.05, ∗∗∗P < 0.005, ∗∗∗∗P < 0.001 vs. the control (A). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.005, ∗∗∗∗P < 0.001 vs. the a2 treated samples (B–G).
3.6. Compound a2 inhibited tumor growth in both the CDX and PDX models of gastric cancer, and downregulation of GPX4 indicated the sensitivity of PDX models to a2
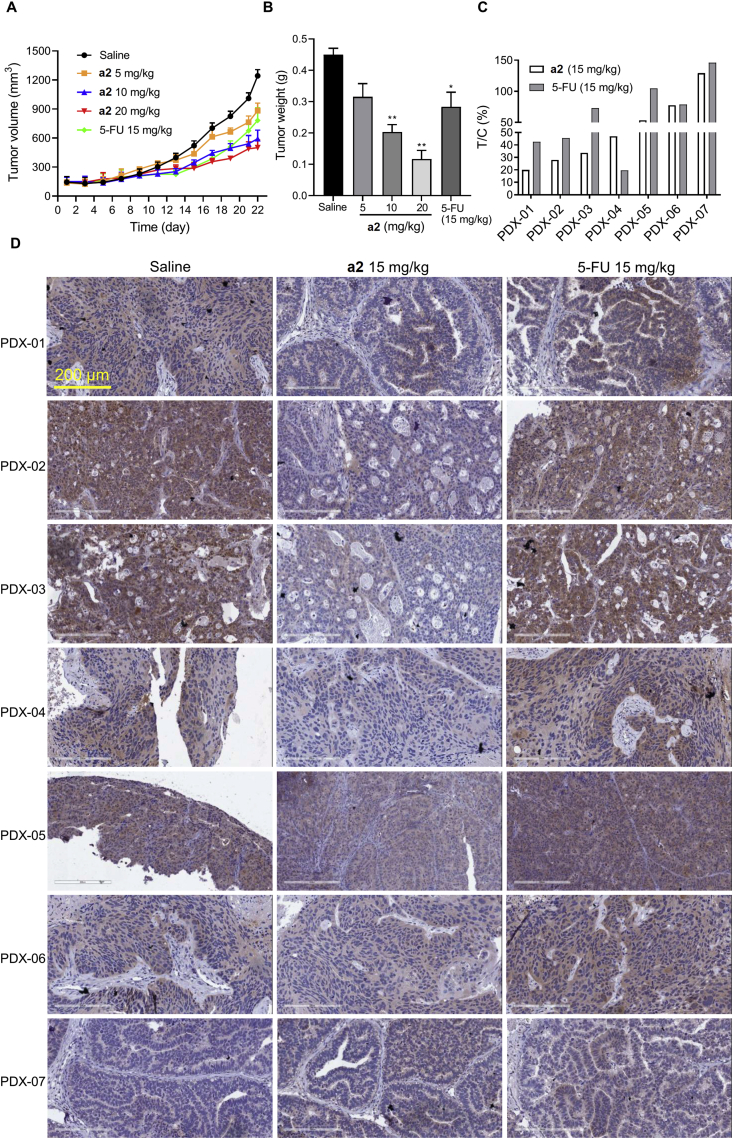
Based on the fact that a2 markedly inhibited the growth of gastric cancer cells, we further measured the anti-tumor efficacy of a2 in MGC-803 CDX mice models. As shown in Fig. 6A and B and Supporting Information Fig. S6A, a2 dose- and time-dependently inhibited tumor growth; the anti-tumor efficacy of a2 at 10 or 20 mg/kg were more potent than that of the positive control drug 5-FU. In addition, a2 had fewer effect on mouse body weight compared to 5-FU (Fig. S6B). We also performed pathologic examination of the main organs and hematology test to evaluate the adverse effects of a2 in mice. Compared with the saline group, groups with a2 and 5-FU showed no observed adverse effects on the heart, liver, spleen, lung, and kidney (Fig. S6C). Furthermore, a2 and 5-FU had minor effects on the number of white blood cells, red blood cells, hemoglobin and platelets compared to the saline (Fig. S6D). Thus, a2 exhibited strong anti-tumor efficacy with minor adverse effects in CDX mice models of gastric cancer.
Figure 6.
Compound a2 inhibited tumor growth in both CDX and PDX models of gastric cancer. (A) Male mice bearing MGC-803 xenograft were treated with indicated agents once a day for 21 days, tumor volume was measured periodically. Data are presented as the mean ± SD (n = 6). (B) Average tumor weights with indicated agents at the end of treatment. Data are presented as the mean ± SD (n = 6). Statistics differences were analyzed by one-way ANOVA analysis: ∗P < 0.05, ∗∗P < 0.01 vs. the saline group. (C) Tumor growth inhibition T/C ratio of indicated compounds were measured at the end of treatment in mice bearing specific gastric cancer patient derived xenografts. (D) Expression of GPX4 in tumor tissues from PDX modes was determined by IHC.
As a2 inhibited tumor growth with minor adverse effects, we next measured the anti-tumor activity of a2 in gastric cancer PDX models. As shown in Fig. 6C, a2 inhibited tumor growth with a range of tumor growth inhibition T/C ratios from 19.9% to 129.1%, indicating that the tumors in PDX models displayed diverse sensitivity to a2. The respective tumor volume and time curve of a2 in different gastric cancer PDX mice models were supplied in Supporting Information Fig. S7. We further measured the expression of GPX4 at the end of treatment, and found that a2 decreased expression of GPX4 in sensitive PDX models including PDX-01, PDX-02, and PDX-03, whereas GPX4 displayed resistant to a2 in resistant PDX models, which was in consistent with previous data that a2 induced ferroptosis by downregulating GPX4 (Fig. 6D). In contrast, the anti-tumor activity of 5-FU in PDX models showed no relationship with GPX4 expression. Therefore, these results indicated that a2 differentially inhibited tumor growth in PDX models and that downregulation of GPX4 can be a biomarker to indicate the sensitivity of a2 against tumors.
3.7. Compound a2 exhibited good pharmacokinetic characteristics
The excellent anti-tumor activity of a2 encouraged us to evaluate its pharmacokinetic parameters. The Caco-2 cell permeability assay was used to measure the human intestinal absorption of a2. As shown in Table 1, the apparent permeability coefficient (Papp) value of propranolol was 28.31, which was a highly membrane permeable compound. The Papp (A‒B) values of atenolol and digoxin, which are poorly permeable agents, were both less than 1. The Papp (A‒B) value of tested a2 was 16.08, which is between 28.31 and 0.13, suggesting that a2 was a moderately permeable compound against the Caco-2 monolayer from A to B. Papp (B‒A)/Papp (A‒B) is also called the efflux ratio and can quantify the levels of active efflux. A compound with an efflux ratio greater than 2 is subject to active efflux. The efflux ratios of atenolol and digoxin were 9.8 and 196.6, respectively, which represented active efflux. The Papp (B‒A)/Papp (A‒B) value of a2 was 0.2, suggesting that a2 will not undergo active efflux.
Table 1.
Permeability coefficients of the compounds a2.
| Test sample | Direction |
Papp (10−6 cm/s) |
Papp (B‒A)/Papp (A‒B) |
|---|---|---|---|
| Mean ± RSD | |||
| Propranolol | A‒B | 28.31 ± 0.43 | 0.7 |
| B‒A | 18.5 ± 0.3 | ||
| Atenolol | A‒B | 0.23 ± 0.02 | 9.8 |
| B‒A | 2.2 ± 0.1 | ||
| Digoxin | A‒B | 0.13 ± 0.02 | 196.6 |
| B‒A | 24.7 ± 0.1 | ||
| a2 | A‒B | 16.08 ± 1.01 | 0.2 |
| B‒A | 3.3 ± 0.3 |
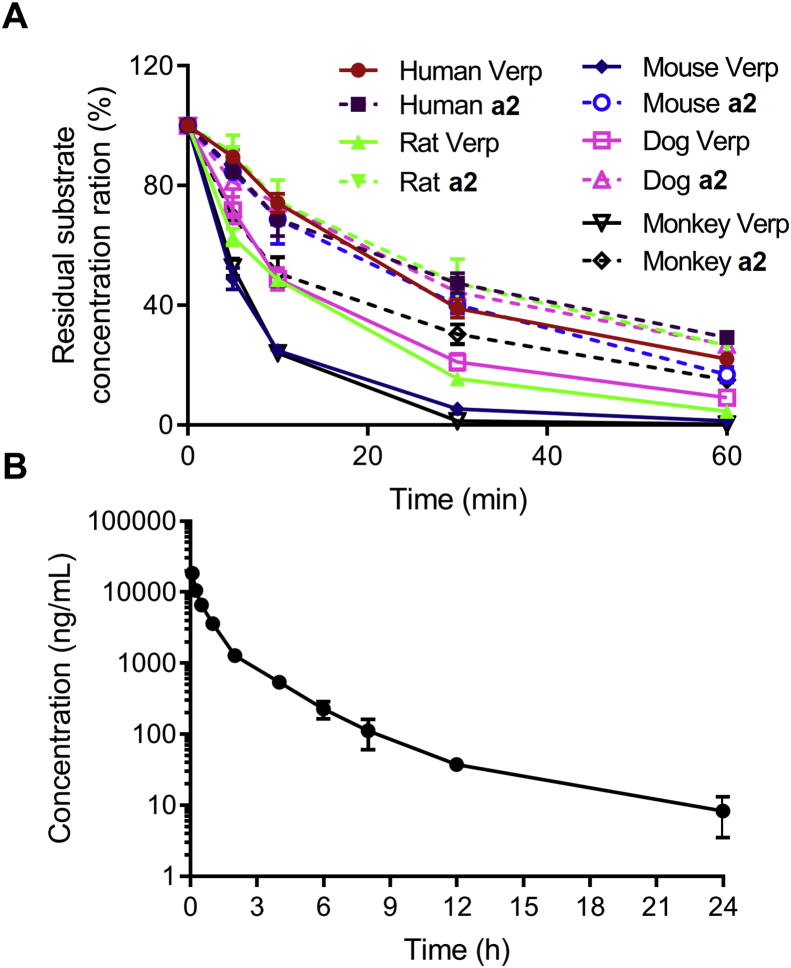
Metabolic stability plays an important role in substance clearance, thus we used liver microsomes from human, rat, mouse, dog and monkey to measure the in vitro intrinsic clearance of a2. As shown in Fig. 7A and Table 2, the concentration–time curve of the positive control substance, verapamil, varied among different species according to the duration of metabolism, suggesting that Clhuman < Cldog < Clrat < Clmouse < Clmonkey. Compared with verapamil, a2 displayed an undifferentiated efficacy of metabolism in different species, with t1/2 values ranging from 23.5 to 34.9 min. Thus, a2 exhibited strong microsomal metabolic stability with minor species diversity.
Figure 7.
Pharmacokinetic characteristics of compound a2. (A) Microsomal metabolic stability of a2 (1 μmol/L) in liver microsomes from indicated species. Verapamil (Verp) was selected as a positive control. Data are presented as the mean ± SD (n = 2). (B) Mean plasma concentration–time profile of a2 after an intravenous administration of a2 (20 mg/kg) to male SD rats. Data are presented as the mean ± SD (n = 3).
Table 2.
The t1/2 of compound a2 and verapamil in liver microsomes of different species.
| Species |
t1/2 (min) |
CL (μL/min/mg) |
||
|---|---|---|---|---|
| a2 | Verapamil | a2 | Verapamil | |
| Human | 34.9 | 26.9 | 99.4 | 128.8 |
| Rat | 31.2 | 13.7 | 111.2 | 252.7 |
| Mouse | 23.7 | 10 | 146.3 | 347.3 |
| Dog | 32.2 | 17.8 | 107.7 | 194.8 |
| Monkey | 23.5 | 4.8 | 147.7 | 725.7 |
We further evaluated the pharmacokinetic (PK) parameters of a2 in male SD rats, the mean plasma concentration–time curve and corresponding PK parameters of a2 were shown in Fig. 7B and Table 3. The Cmax value of a2 was 18,319 ng/mL. The half-life and mean residence time were 3.93 and 1.54 h, respectively. According to a previous report, the t1/2 value of 5-FU upon intraperitoneal injection in mice is 1.36 h, which is shorter than that of a226. Therefore, these data indicated that a2 had superior pharmacokinetic characteristics.
Table 3.
Main pharmacokinetic parameters of a2 in rats.
| PK parameter | a2 |
|---|---|
| Cmax (ng/mL) | 18319.0 ± 1422.0 |
| t1/2 (h) | 3.93 ± 0.48 |
| AUClast (h·ng/mL) | 14804.9 ± 1322.1 |
| AUCINF_pred (h·ng/mL) | 14840.8 ± 1328.4 |
| CL_pred (L/h/kg) | 1.65 ± 0.23 |
| Vz-pred (L/kg) | 7.73 ± 1.46 |
| MRTlast (h) | 1.54 ± 0.13 |
4. Discussion
JDA was first isolated and identified from Jiyuan R. rubescens early in 2011 by our group, after which several JDA analogues showed anti-tumor activity against esophageal and gastric cancer cells3,27,28. However, poorly known mechanism of action and bioavailability hinder the clinical development of compound JDA. In this study, we found a novel JDA derivative, a2, selectively inhibited the proliferation of gastric cancer cells. Induction of ferroptosis by downregulating GPX4 and ferrous iron accumulation mainly contributed to the anti-tumor activity of a2 in gastric cancer cells. Results from the CDX and PDX models of gastric cancer further confirmed the anti-tumor efficacy of a2, and the downregulation of GPX4 predicted the sensitivity of tumors to a2 in gastric cancer PDX models. Importantly, a2 exhibited superior PK characteristics. Therefore, the excellent anti-tumor activity and PK characteristics of a2 make it a promising anti-tumor agent for gastric cancer treatment.
Our results indicated that a2 inhibited cell cycle at the G2/M phase, which was in accordance with previous reports that JDA analogues (jaridonin and JDA-202) induce the G2/M arrest in esophageal cancer cells8,27. We also observed that a2 caused mitochondria-dependent apoptosis in gastric cancer cells. However, prevention of apoptosis only partially antagonized the anti-proliferative activity of a2 only in MGC-803 cells and not in MKN-45 cells. These results suggested that a2 may inhibit cell growth through different mechanisms in different gastric cancer cell lines. And apoptosis may be an alternative or secondary event caused by a2 and contributed little to the anti-proliferative activity of a2.
Ferroptosis is a recently discovered form of programmed cell death executed by the interaction of ROS and ferrous iron, resulting in lipid ROS burst and cell death14,29. RNA-seq analysis of MGC-803 cells treated with a2 indicated that significantly differentiated mRNAs enriched in ferroptosis, lipid peroxidation assay and transmission electron microscope data confirmed that a2 induced ferroptosis in both MGC-803 and MKN-45 cells. GPX4 is an enzyme that catalyzes the reduction of lipid peroxides and plays an essential role in regulation of ferroptosis, and inhibition of GPX4 is a promising strategy to treat refractory cancer30,31. Compound a2 downregulated expression of GPX4 and overexpression of GPX4 markedly attenuated the anti-proliferative activity of a2 in both MGC-803 and MKN-45 cells, suggesting that downregulation of GPX4 played a critical role in a2-induced cell growth inhibition. Consistent with this, the expression of GPX4 was only inhibited by a2 in sensitive gastric cancer PDX models, making GPX4 downregulation a promising biomarker. We also found that a2 upregulated SLC7A11 and inhibiting SLC7A11 with specific SLC7A11 inhibitor or specific siRNAs enhanced the anti-proliferative activity of a2. It has been reported that JDA analogues jaridonin and JDA-202 cause upregulation of P53, and P53 can inhibit ferroptosis by repression of the SLC7A118,27,32. In contrast, a2 had no effect on the mRNA and protein levels of P53 in MGC-803 cells (data not shown), suggesting that a2 exerted anti-tumor activity with new mechanism.
Compound a2 caused accumulation of ferrous iron, which interacts with ROS to produce large amounts of lipid ROS. Iron homeostasis plays an essential role in cell survival and is well regulated by multiple pathways33. Inhibition of autophagy antagonized ferrous iron accumulation and cell growth inhibition caused by a2, which was in accordance with previous report that ent-kaurane diterpenoid oridonin induces autophagy in P53-mutated colorectal cancer cells34.
5. Conclusions
Taken together, we found a novel JDA derivative, a2, which showed excellent anti-tumor efficacy with minor adverse effects in both gastric cancer cells and mouse models. Compound a2 selectively inhibited tumor growth in gastric cancer PDX models that in which GPX4 expression can be suppressed. It is well known that gastric cancer a kind of cancers with highly heterogeneity with diverse molecular features. Downregulation of GPX4 can thus be a biomarker to indicate the anti-tumor activity of a2 in gastric cancer.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81773562, 82020108030, and U1904163), National Key Research and Development Project (No. 2018YFE0195100, China), and the Science and Technology Program of Henan Province (No. 202102310152, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.05.006.
Contributor Information
Yichao Xu, Email: xuyc2017@zzu.edu.cn.
Dequan Yu, Email: dqyu@imm.ac.cn.
Hongmin Liu, Email: liuhm@zzu.edu.cn.
Author contributions
Yichao Xu, Dequan Yu, Hongmin Liu conceived and designed all the experiments and revised the manuscript. Ying Liu conducted the majority experiments, analyzed data and wrote original draft. Zan Song conducted the animal assays. Yajie Liu and Xubin Ma carried out the ROS determination and ferrous iron assay. Wang Wang and Yu Ke isolated JDA and synthesized compound a2.
Conflicts of interest
The authors declare no conflicts of interest.
Appendix A Supporting information
The following are the Supporting data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 3.Liu H.M., Zhu C.G., Wang Q.D., Ke Y., Liu Z.Z., Yan X.B. 2011 Dec 27. inventors. Novel ent-kaurene diterpene compound and its derivatives, their preparation and their use. US patent US8084430B2. [Google Scholar]
- 4.Zhang Y., Wang S., Dai M., Nai J., Zhu L., Sheng H. Solubility and bioavailability enhancement of oridonin: a review. Molecules. 2020;25:332–355. doi: 10.3390/molecules25020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarwar M.S., Xia Y.X., Liang Z.M., Tsang S.W., Zhang H.J. Mechanistic pathways and molecular targets of plant-derived anticancer ent-kaurane diterpenes. Biomolecules. 2020;10:144–161. doi: 10.3390/biom10010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J.N., Zhang Z.R., Che Y., Yuan Z.Y., Lu Z.L., Li Y. Acetyl-macrocalin B, an ent-kaurane diterpenoid, initiates apoptosis through the ROS-p38-caspase 9-dependent pathway and induces G2/M phase arrest via the Chk1/2-Cdc25C-Cdc2/cyclin B axis in non-small cell lung cancer. Cancer Biol Ther. 2018;19:609–621. doi: 10.1080/15384047.2018.1449613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Li N., Shi J., Ahmed T., Liu H., Guo J. Involvement of glutathione depletion in selective cytotoxicity of oridonin to p53-mutant esophageal squamous carcinoma cells. Front Oncol. 2019;9:1525–1535. doi: 10.3389/fonc.2019.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi X.J., Ding L., Zhou W., Ji Y., Wang J., Wang H. Pro-apoptotic effects of JDA-202, a novel natural diterpenoid, on esophageal cancer through targeting peroxiredoxin I. Antioxidants Redox Signal. 2017;27:73–92. doi: 10.1089/ars.2016.6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H., Weng H., Dong B., Zhao P., Zhou H., Qu L. Oridonin triggers chaperon-mediated proteasomal degradation of BCR-ABL in leukemia. Sci Rep. 2017;7:41525–41537. doi: 10.1038/srep41525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He H.B., Jiang H., Chen Y., Ye J., Wang A.L., Wang C. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9:2550–2556. doi: 10.1038/s41467-018-04947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song M., Liu X., Liu K., Zhao R., Huang H., Shi Y. Targeting AKT with oridonin inhibits growth of esophageal squamous cell carcinoma in vitro and patient-derived xenografts in vivo. Mol Cancer Therapeut. 2018;17:1540–1553. doi: 10.1158/1535-7163.MCT-17-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su Y., Zhao B., Zhou L., Zhang Z., Shen Y., Lv H. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127–136. doi: 10.1016/j.canlet.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Koppula P., Zhuang L., Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2020 doi: 10.1007/s13238-020-00789-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battaglia A.M., Chirillo R., Aversa I., Sacco A., Costanzo F., Biamonte F. Ferroptosis and cancer: mitochondria meet the "iron maiden" cell death. Cells. 2020;9:1505–1531. doi: 10.3390/cells9061505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassannia B., Vandenabeele P., Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Yin X., He W., Xue W., Zhang J., Huang Y. SUV39H1 deficiency suppresses clear cell renal cell carcinoma growth by inducing ferroptosis. Acta Pharm Sin B. 2021;11:406–419. doi: 10.1016/j.apsb.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L.M., Li G.Y., Ding L.S., Yang L.B., Zhao Y., Pu J.X. ent-Kaurane diterpenoids from isodon nervosus. J Nat Prod. 2008;71:684–688. doi: 10.1021/np800027a. [DOI] [PubMed] [Google Scholar]
- 18.Wang S., Ma X.B., Yuan X.H., Yu B., Xu Y.C., Liu H.M. Discovery of new [1,2,4]triazolo[1,5-a]pyrimidine derivatives that kill gastric cancer cells via the mitochondria pathway. Eur J Med Chem. 2020;203:112630–112642. doi: 10.1016/j.ejmech.2020.112630. [DOI] [PubMed] [Google Scholar]
- 19.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550–571. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu G.C., Wang L.G., Han Y.Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun P.Y., Wu G.L., Qiu Z.J., Chen Y.J., inventors . 2017 Jan 04. l-Alanine-(14-oridonin) ester trifluoroacetate as well as preparation method and application. Chinese patent CN104017000B. [Google Scholar]
- 22.Green D.R., Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., Kuang F., Kroemer G., Klionsky D.J., Kang R., Tang D. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem Biol. 2020;27:420–435. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alu A., Han X., Ma X., Wu M., Wei Y., Wei X. The role of lysosome in regulated necrosis. Acta Pharm Sin B. 2020;10:1880–1903. doi: 10.1016/j.apsb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith T., Affram K., Nottingham E.L., Han B., Amissah F., Krishnan S. Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer. Sci Rep. 2020;10:16989–17003. doi: 10.1038/s41598-020-73218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y.C., Ke Y., Zi X., Zhao W., Shi X.J., Liu H.M. Jaridonin, a novel ent-kaurene diterpenoid from Isodon rubescens, inducing apoptosis via production of reactive oxygen species in esophageal cancer cells. Curr Cancer Drug Targets. 2013;13:611–624. doi: 10.2174/15680096113139990030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Y.C., Su N., Shi X.J., Zhao W., Ke Y., Zi X. Jaridonin-induced G2/M phase arrest in human esophageal cancer cells is caused by reactive oxygen species-dependent Cdc2-tyr15 phosphorylation via ATM-Chk1/2-Cdc25C pathway. Toxicol Appl Pharmacol. 2015;282:227–236. doi: 10.1016/j.taap.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hangauer M.J., Viswanathan V.S., Ryan M.J., Bole D., Eaton J.K., Matov A. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang L., Hickman J.H., Wang S.J., Gu W. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle. 2015;14:2881–2885. doi: 10.1080/15384101.2015.1068479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogdan A.R., Miyazawa M., Hashimoto K., Tsuji Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci. 2016;41:274–286. doi: 10.1016/j.tibs.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao Z., Xie F., Li M., Liang Z., Xu W., Yang J. Oridonin induces autophagy via inhibition of glucose metabolism in p53-mutated colorectal cancer cells. Cell Death Dis. 2017;8:e2633. doi: 10.1038/cddis.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.