ABSTRACT
Background
Previous studies have suggested that insufficient concentrations of vitamin D are associated with dental caries in primary teeth, but evidence remains inconclusive.
Objectives
We assessed the longitudinal associations between prenatal, perinatal, and early childhood serum 25-hydroxyvitamin D concentrations [25(OH)D] and the risk of dental caries in 6-year-old children.
Methods
This research was conducted within the Generation R Study, a large, multi-ethnic, prospective cohort study located in Rotterdam, the Netherlands. Dental caries were assessed in children using the decayed-missing-filled-primary teeth index at a mean age of 6.1 years (90% range, 4.8–9.1). We measured serum total 25(OH)D concentrations at 3 time points: prenatally (at 18–24 weeks of gestation), perinatally (at birth), and during early childhood (at age 6 years). We performed logistic regression analyses to determine the longitudinal association of serum 25(OH)D concentrations with caries risks in 5257 children. Additionally, we constructed a Genetic Risk Score (GRS) for the genetic predispositions to serum total 25(OH)D concentrations based on 6 vitamin D–related single nucleotide polymorphisms in a subsample of 3385 children.
Results
Children with severe prenatal and early childhood serum 25(OH)D deficiencies (<25 nmol/L) were more likely to be diagnosed with caries [OR, 1.56 (95% CI, 1.18–2.06) and 1.58 (95% CI, 1.10–2.25), respectively] than children with optimal concentrations (≥75 nmol/L). After adjustment for residuals of serum 25(OH)D concentrations at other time points, only the early childhood serum 25(OH)D concentration was inversely associated with the caries risk at 6 years (OR, 0.97; 95% CI, 0.95–0.98). However, our GRS analysis showed that children who are genetically predisposed to have lower serum 25(OH)D concentrations do not have a higher risk of developing caries in primary teeth.
Conclusions
Our study suggests a weak association between serum 25(OH)D concentrations and risks of caries in primary teeth. Based on our results, we do not recommend vitamin D supplementation for the prevention of dental caries in children.
Keywords: 25(OH)D, caries, primary teeth, pediatric dentistry, prevention, genetic risk score
Introduction
Dental caries are the most widespread noncommunicable disease in the world (1). Despite being preventable, the prevalence of caries in children remains high, reaching up to 85% (2). At the same time, several studies report serum 25-hydroxyvitamin D concentrations [25(OH)D] as being insufficient across different European pediatric populations (3). Through different pathways, 25(OH)D might play a role in dental caries. During dental development, 25(OH)D regulates calcium and phosphate ion concentrations in plasma and stimulates mineralization by binding to dental cells (4, 5). After the demineralization of tooth surfaces caused by acid-producing bacteria, 25(OH)D maintains sufficient levels of calcium and phosphates in saliva for remineralization, and it possesses lytic activity against cariogenic bacteria (6, 7). Therefore, insufficient serum concentrations of 25(OH)D may lead to both developmental defects in tooth structure and to an inadequate response to caries activity.
A possible benefit of vitamin D supplementation on caries was described almost a hundred years ago (8). However, inconclusiveness about the association between 25(OH)D and caries is still evident. Recent studies have suggested that low gestational serum 25(OH)D concentrations are associated with an increased risk of caries in offspring (9–11). Similarly, a cross-sectional study has shown that children with caries have lower serum 25(OH)D concentrations than children without caries (12). However, a study using genetic variants related to serum 25(OH)D concentrations in 5545 children suggested no causal effect of 25(OH)D on caries (13). Associations between gene polymorphisms of 25(OH)D receptors and caries differ based on the study population (14, 15). Genetic Risk Scores (GRS) in relation to caries in children have not yet been explored. Therefore, more research is necessary to understand the relationship between serum 25(OH)D and caries risks.
The present study assesses the longitudinal associations between prenatal, perinatal, and early childhood serum 25(OH)D concentrations and the risk of caries in primary teeth in a large, representative sample of 6-year-old children. We also use GRS to test whether serum 25(OH)D concentrations during early childhood influence the caries risk at age 6 years.
Methods
Study design and population
This study is part of the Generation R Study, a multi-ethnic, population-based, prospective cohort study of pregnant women and their children designed to identify environmental and genetic determinants of growth, development, and health from fetal life onwards, located in Rotterdam, the Netherlands (16). The Medical Ethics Committee of Erasmus Medical Center, Rotterdam, approved this study (MEC-2017-413), and informed consent was obtained from each participant.
Pregnant women who were living within the study area with an expected delivery date between April 2002 and January 2006 were eligible to participate in the Generation R Study. In total, 9749 pairs of mothers and live-birth children were enrolled in the study. We excluded mothers whose offspring died postnatally (n = 52), who withdrew from the study (n = 457), and who were lost to follow-up (n = 935). In cases of multiple births, we excluded the second twin and the second and third triplets (n = 109). After 5 years, 8196 mothers still agreed to participate in the study with their 6-year-old offspring. Next, 6603 mother-child pairs visited the research center. Mother-child pairs who did not visit the research center were excluded (n = 1593). Finally, 5257 children aged 6 years were assessed for caries. Children without suitable photos for caries assessment were excluded (n = 1346). The flowchart is provided in Supplemental Figure 1.
Data collection
Children visited the research center at a mean age of 6.1 years (90% range, 4.8–9.1 years) and intra-oral photographs were made of their dentition after they had brushed their teeth. From these photos, we obtained the decayed-missing-filled-primary teeth (dmft) index, which records the cumulative number of decayed teeth, teeth missing due to caries, and filled primary teeth, ranging from 0 to 20 (17). Trained nurses and dental students made the photographs using either the Poscam USB intra-oral (Digital Leader PointNix) or Sopro 717 autofocus camera (Acteon). One pediatric dentist judged the photographs and reevaluated 10% of them (intra-rater reliability k = 0.95), followed by a review of 10% of the photographs by a second calibrated pediatric dentist (inter-rater reliability k = 0.62). Scoring dental caries per tooth on intra-oral photographs has been shown to have high sensitivity and specificity (85.5 and 83.6%, respectively) when compared to an ordinary oral examination (18). If we were unable to score 1 or more teeth on the photographs, the dmft index was coded as missing. For the present analyses, results on the dmft index were categorized as either having (dmft > 0) or not having (dmft = 0) a caries diagnosis.
We measured serum 25(OH)D concentrations at 3 time points: prenatally (at 18–24 weeks of gestation using maternal venous blood); perinatally (at birth using umbilical cord blood); and during early childhood (in children aged 6, using venous blood). Details of the collection, logistics, and assessment of biological samples in the Generation R Study have been described elsewhere (16). We calculated the total serum 25(OH)D concentration as the sum of 25-hydroxyergocalciferol and 25-hydroxycholecalciferol concentrations using isotope dilution online solid-phase extraction LC-MS/MS (19). Maternal and umbilical cord blood samples were analyzed at the Queensland Brain Institute in Brisbane, Australia. Children's blood samples were analyzed at the Endocrine Laboratory of the Vrije Universiteit (VU) University Medical Centre, Amsterdam, the Netherlands. Cross-validation of the results between prenatal and early childhood serum 25(OH)D samples was performed for 31 samples, and showed an excellent correlation between both methods (r = 0.99; 95% CI, 0.98–0.99). We categorized serum 25(OH)D concentrations as severely deficient (<25 nmol/L), deficient (25 to <50 nmol/L), sufficient (50 to <75 nmol/L), and optimal (≥75 nmol/L), based on cutoff values used in previous studies (20).
DNA was isolated from umbilical cord blood or, in a small minority of the children, from blood samples. Participants were genotyped using Illumina 610 and 660W Quad platforms, and data were imputed to an HRC1.1 reference panel (16). Based on a recent genome-wide association study (GWAS), 6 single nucleotide polymorphisms (SNPs) are associated with serum 25(OH)D concentrations: 2 related to metabolism (GC/rs3755967 and CYP24A1/rs17216707) and 4 related to the synthesis of serum 25(OH)D (DHCR7/rs12785878, AMDHD1/rs10745742, SEC23A/rs8018720, and CYP2R1/rs10741657) (21). Genotypes for those SNPs were extracted from the Variant Call Format (VCF) files of all children with genotypic data available, using the VCF tool package, and were converted into scores of 0 [if child is homozygous for decreasing 25(OH)D alleles for the given SNP], 1 [if child is heterozygous for the given SNP], or 2 [if child is homozygous for increasing 25(OH)D alleles for the given SNP]. For every individual, we constructed 3 GRS results: a GRS2, based on the 2 metabolizing SNPs only; a GRS4, based on the 4 synthesizing SNPs only; and a GRS6, based on all 6 related SNPs. Each GRS was calculated by summing the score for each SNP only (GRS unweighted), and also by summing the score weighted for the effect size for its association with serum 25(OH)D (GRS weighted). The effect sizes of the SNPs for serum 25(OH)D concentrations are provided in the GWAS (22).
We selected several covariates as potential confounders based on previous literature (23–25). Using questionnaires, we collected data on maternal age, household income at enrollment and after the 6-year follow-up, use of folic acid supplements, parity, smoking during the second trimester, maternal educational level at enrollment and after the 6-year follow-up, the child's hours spent in front of a screen, the child's hours spent playing outside, and frequency of tooth brushing. Each child's sex and birthweight were collected from birth records. Mother and child BMIs (kg/m2) were calculated using anthropometric measurements during the visits to the research center, as well as the astronomical season of blood draw. We defined the ethnicities of the mother and child by the parents’ countries of birth, classified according to the Dutch Central Agency for Statistics (26).
Statistical analyses
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) for Windows, version 25.0 (IBM Corp.). First, we characterized our study population using descriptive statistics. Second, we performed binary logistic regression to examine the individual associations of prenatal, perinatal, and early childhood serum 25(OH)D concentrations, continuously and categorically, with caries diagnosed at age 6. We built a series of 3 models that were adjusted using covariates related to each time point according to the levels of influence on oral health outcomes in children (27). Model 1 was the basic model (i.e., sex, age, gestational age, and astronomical season of blood draw). Model 2 was additionally adjusted for socioeconomic status indicators (i.e., ethnicity, educational level, household income, and parity). Model 3 was additionally adjusted for lifestyle behavioral factors (i.e., BMI, smoking during the second trimester, use of folic acid supplements, low birthweight, hours spent in front of a screen, hours spent playing outside, and frequency of tooth brushing). Third, we used logistic regression with unexplained residuals to study the association of combined data on the serum 25(OH)D concentrations at the 3 time points with caries diagnosed at age 6 (28). Again, a series of 3 similarly adjusted models were built. To avoid collinearity with repeatedly assessed covariates, we only included the covariates related to early childhood. Finally, to illustrate the association between GRS and caries diagnosed at age 6 years, individuals were divided into 5 bins (GRS6, GRS4) and 3 bins (GRS2) based on their final GRS scores. This model was adjusted for the child's sex, age, BMI, and the first 10 principal components regarding differences due to ethnicity, and was tested against the middle bin using binary logistic regression.
To reduce potential bias due to missing data, we performed multiple imputation of missing covariates according to the fully conditional specification method (29). We used the exposure, all covariates, and the outcome as predictors for the imputation; however, the outcome was not imputed. We generated 5 imputed data sets, and the pooled effect estimates (ORs with 95% CIs) are presented. We evaluated whether there were different associations in boys and girls, as well as in different ethnicities, by first adding an interaction term between ethnicity and then adding an interaction term between sex with other covariates (i.e., astronomical season, BMI, maternal education, and household income) to the unadjusted logistic regression models. No interaction effects were present. For the regression analysis, we conducted sensitivity analyses to compare results between the original and the imputed data sets, and between the whole population and native Dutch participants only; for the GRS analysis, the sensitivity analysis was only performed for comparisons between the whole population and European participants only. For all analyses, a P value < 0.05 was considered statistically significant. The presented study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist (30).
Results
In total, 5257 mother-child pairs participated in this study (Supplemental Figure 1). The mothers had a mean age of 30.6 years (SD, 5.1 years) at pregnancy. Most of them had Dutch or Western ethnicity and were highly educated. Of all prenatal serum 25(OH)D concentration samples, 22.8% were severely 25(OH)D-deficient (<25 nmol/L), 26.4% were vitamin D–deficient (25 to <50 nmol/L), 24.6% had sufficient concentrations (50 to <75 nmol/L), and 26.2% had optimal concentrations (≥75 nmol/L). Notably, nearly half of the umbilical cord samples had serum 25(OH)D concentrations considered severely deficient (<25 nmol/L). At the caries assessment, children had a mean age of 6.1 years (90% range, 4.8–9.1 years). Most of them were living in households with a high income, brushed their teeth at least twice a day, and had early childhood serum 25(OH)D concentrations considered sufficient (50 to <75 nmol/L). The prevalence of caries (dmft > 0) in this sample was 31.7% (n = 1664), similar to the prevalence in the pediatric population of the Netherlands (31). Complete sample characteristics for the 3 time points are presented in Table 1. Within the whole data set, missing values per covariate ranged from 0 to 53%. Only 2 covariates measured at early childhood had more than 50% missing values (i.e., hours spent in front of a screen, 50.0%; and hours spent playing outside, 53.0%). There were no missing values in the caries assessment (Supplemental Table 1).
TABLE 1.
Prenatal, perinatal, and early childhood sample characteristics1
| n | Values | |
|---|---|---|
| Prenatal | ||
| Maternal age, years | 5257 | 30.6 ± 5.1 |
| Gestational age at intake, weeks | 4747 | 13.9 (5.9–39.2) |
| Ethnicity | 5128 | — |
| Native Dutch/Western | 62.8 | |
| Pre-pregnancy BMI, kg/m2 | 3901 | 22.6 (14.3–50.2) |
| Smoking during pregnancy, yes | 4544 | 25.6 |
| Use of folic acid supplements, yes | 3609 | 75.2 |
| Parity | 5082 | — |
| Primipara | — | 56.0 |
| Educational level at enrolment | 4782 | — |
| >12 years | — | 46.9 |
| Household income at enrolment | 3956 | — |
| ≥2200 EUR | — | 58.3 |
| Astronomical season of blood draw | 3998 | — |
| Spring | — | 27.9 |
| Summer | — | 22.2 |
| Autumn | — | 25.3 |
| Winter | — | 24.6 |
| Serum 25(OH)D, nmol/L | 3998 | 50.6 (0–161.9) |
| 25(OH)D status | 3998 | — |
| Severely deficient (<25 nmol/L) | — | 22.8 |
| Deficient (25 to <50 nmol/L) | — | 26.4 |
| Sufficient (50 to <75 nmol/L) | — | 24.6 |
| Optimal (≥75 nmol/L) | — | 26.2 |
| Perinatal | ||
| Gestational age at birth, weeks | 5220 | 40.1 (25.2–43.5) |
| Sex, female | 5327 | 49.9 |
| Low birthweight | 5247 | 5.1 |
| Astronomical season of blood draw | 2879 | — |
| Spring | — | 25.9 |
| Summer | — | 26.7 |
| Autumn | — | 23.1 |
| Winter | — | 24.3 |
| Serum 25(OH)D, nmol/L | 2879 | 28.9 (0.1–144.8) |
| 25(OH)D status | 2879 | — |
| Severely deficient (<25 nmol/L) | — | 43.1 |
| Deficient (25 to <50 nmol/L) | — | 35.8 |
| Sufficient (50 to <75 nmol/L) | — | 16.8 |
| Optimal (≥75 nmol/L) | — | 4.2 |
| Early childhood | ||
| Age, years | 5257 | 6.17 (4.8–9.1) |
| Ethnicity2 | 4987 | — |
| Native Dutch/Western | — | 67.1 |
| Mean maternal age at the 6-year follow-up, years | 5165 | 37.1 ± 4.9 |
| BMI, kg/m2 | 5257 | 15.8 (11.9–29.1) |
| Maternal educational level at the 6-year follow-up,years | 4461 | — |
| >12 years | — | 56.3 |
| Household income at the 6-year follow-up | 4224 | — |
| ≥2400 EUR | — | 68.4 |
| Screen time, ≥2 h/d | 2627 | 19.6 |
| Outdoor playing, ≥ 2h/d | 2470 | 22.4 |
| Tooth brushing frequency, ≥2 t/d | 3311 | 51.2 |
| Astronomical season of blood draw | 3347 | — |
| Spring | — | 28.7 |
| Summer | — | 26.2 |
| Autumn | — | 23.5 |
| Winter | — | 21.6 |
| Serum 25(OH)D, nmol/L | 3347 | 65.7 ± 28.4 |
| 25(OH)D status | 3347 | — |
| Severely deficient (<25 nmol/L) | — | 5.9 |
| Deficient (25 to <50 nmol/L) | — | 23.2 |
| Sufficient (50 to <75 nmol/L) | — | 36.5 |
| Optimal (≥75 nmol/L) | — | 34.4 |
| Genetic data available, yes | 3350 | 63.7 |
| Caries diagnosed at age 6 | 5257 | — |
| Caries (dmft > 0) | — | 31.7 |
Values are numbers and percentages for categorical variables; means ± SDs for continuous variables with a normal distribution; and medians (90% range) for continuous variables with a skewed distribution. dmft, decayed-missing-filled-primary teeth; EUR, Euro; 25(OH)D, 25-hydroxyvitamin D.
Western: Dutch, European, American Western, Asian Western, and Oceania. Non-Western: Moroccan, Turkish, Surinamese, Antillean, African, Cape Verdean, Indonesian, Asian non-Western, and American non-Western.
Associations between 25(OH)D concentrations and caries diagnosis
The associations between prenatal, perinatal, and early childhood serum 25(OH)D concentrations with caries diagnosed at the age of 6 are shown in Table 2. Continuously assessed serum 25(OH)D concentrations were inversely associated with caries diagnosed at all 3 time points (Model 3). In the categorical assessments, children with severely deficient and deficient prenatal serum 25(OH)D concentrations (<50 nmol/L) had higher odds of being diagnosed with caries at the age of 6 [Model 3: OR, 1.56 (95% CI, 1.18–2.06) and 1.23 (95% CI, 1.00–1.51), respectively] than children with optimal serum 25(OH)D concentrations (≥75 nmol/L). Similarly, children who had severe early childhood deficiency of serum 25(OH)D (<25 nmol/L) were more likely to have been diagnosed with caries (Model 3: OR, 1.58; 95% CI, 1.10–2.25) as compared to children with optimal serum 25(OH)D concentrations at the age of 6. The results from the logistic regression with unexplained residuals analyses are presented in Table 3. Only the early childhood 25(OH)D concentration, independent from prenatal and perinatal serum 25(OH)D concentrations, remained inversely associated with caries diagnoses in 6-year-old children (Model 3: OR, 0.97; 95% CI, 0.95–0.98).
TABLE 2.
Associations of prenatal, perinatal, and early childhood serum 25(OH)D concentrations with caries diagnosed at the age of 6 years (n = 5257)1
| Categorical analysis | |||||
|---|---|---|---|---|---|
| Continuous analysis Per 10 nmol/L | ≥75 nmol/L (optimal) | 50 to <75 nmol/L (sufficient) | 25 to <50 nmol/L (deficient) | <25 nmol/L (severely deficient) | |
| Prenatal | — | n = 1362 | n = 1275 | n = 1402 | n = 1218 |
| Caries diagnosis (yes) | 1664 | 296 | 322 | 457 | 589 |
| Model 12 | 0.988 (0.985–0.991)3 | Ref. | 1.16 (0.96–1.41) | 1.64 (1.36–1.97)3 | 2.78 (2.23–3.47)3 |
| Model 24 | 0.993 (0.990–0.996)3 | Ref. | 1.08 (0.88–1.32) | 1.32 (1.08–1.61)3 | 1.79 (1.37–2.36)3 |
| Model 35 | 0.995 (0.992–0.998)3 | Ref. | 1.06 (0.86–1.30) | 1.23 (1.00–1.51)3 | 1.56 (1.18–2.06)3 |
| Perinatal | 1664 | n = 244 | n = 896 | n = 1885 | n = 2232 |
| Caries diagnosis (yes) | — | 53 | 201 | 500 | 910 |
| Model 16 | 0.983 (0.979–0.987)3 | Ref. | 1.10 (0.56–2.18) | 1.37 (0.69–2.73) | 2.41 (1.30–4.44)3 |
| Model 27 | 0.992 (0.988–0.997)3 | Ref. | 1.09 (0.55–2.14) | 1.21 (0.62–2.38) | 1.56 (0.86–2.83) |
| Model 38 | 0.995 (0.991–0.998)3 | Ref. | 1.10 (0.56–2.15) | 1.20 (0.63–2.26) | 1.42 (0.80–2.53) |
| Early childhood | 1664 | n = 1551 | n = 1889 | n = 1376 | n = 441 |
| Caries diagnosis (yes) | — | 387 | 564 | 504 | 209 |
| Model 19 | 0.989 (0.986–0.992)3 | Ref. | 1.32 (1.09–1.60)3 | 1.87 (1.47–2.37)3 | 2.81 (2.07–3.82)3 |
| Model 210 | 0.995 (0.992–0.999)3 | Ref. | 1.16 (0.93–1.45) | 1.31 (0.98–1.77) | 1.62 (1.13–2.30)3 |
| Model 311 | 0.997 (0.995–1.000)3 | Ref. | 1.15 (0.92–1.43) | 1.29 (0.96–1.73) | 1.58 (1.10–2.25)3 |
Values are pooled ORs with 95% CIs for the associations of 25(OH)D status with caries diagnosis (yes/no) using logistic regression models. Ref, reference; 25(OH)D, 25-hydroxyvitamin D.
2Prenatal Model 1 is adjusted for the astronomical season of blood draw, maternal age at enrollment, gestational age at intake, and child's sex.
3Indicates significance at an α level of 0.05.
Prenatal Model 2 includes the variables in Prenatal Model 1 and is additionally adjusted for maternal ethnicity, educational level and household income at enrollment, and parity.
Prenatal Model 3 includes the variables in Prenatal Model 2 and is additionally adjusted for pre-pregnancy BMI, smoking during the second trimester, and use of folic acid supplements.
Perinatal Model 1 is adjusted for astronomical season of blood draw, maternal age at enrollment, gestational age at birth, child's sex.
Perinatal Model 2 includes the variables in Perinatal Model 15 and is additionally adjusted for maternal ethnicity, educational level and household income at enrollment, and parity.
Perinatal Model 3 includes the variables in Perinatal Model 26 and is additionally adjusted for pre-pregnancy BMI, smoking during the second trimester, use of folic acid supplements, and low birthweight.
Early Childhood Model 1 is adjusted for the astronomical season of blood draw, child's age, and child's sex.
Early Childhood Model 2 includes the variables in Early Childhood Model 18 and is additionally adjusted for child's ethnicity, maternal age, educational level, and household income at the 6-year follow-up.
Early Childhood Model 3 includes the variables in Early Childhood Model 29 and is additionally adjusted for child's BMI, hours spent playing outside, hours spent in front of a screen, and frequency of tooth brushing.
TABLE 3.
Logistic regression analysis with unexplained residuals for the association of prenatal, perinatal and early childhood serum 25(OH)D concentrations in relation to caries diagnosed at the age of 6 (n = 5257)1
| Caries diagnosed, OR (95% CI) | |
|---|---|
| Prenatal (n = 1664/5257) | |
| Model 12 | 0.97 (0.96–0.98)3 |
| Model 24 | 0.98 (0.97–0.99)3 |
| Model 35 | 0.98 (0.97–1.00) |
| Perinatal (n = 1664/5257) | |
| Model 12 | 0.95 (0.93–0.97)3 |
| Model 24 | 0.97 (0.95–0.99)3 |
| Model 35 | 0.98 (0.96–1.00) |
| Early childhood (n = 1664/5257) | |
| Model 12 | 0.94 (0.93–0.95)3 |
| Model 24 | 0.96 (0.95–0.97)3 |
| Model 35 | 0.97 (0.95–0.98)3 |
Values are pooled ORs with 95% CIs for associations of prenatal, perinatal, and early childhood serum 25(OH)D (per 10 nmol/L) with caries diagnosed (yes/no) using logistic regression with unexplained residuals. 25(OH)D, 25-hydroxyvitamin D.
Model 1 is adjusted for maternal age at enrollment, gestational age at intake, gestational age at birth, child's age, sex, astronomical season of blood draw, and standard residuals.
3Indicates significance at an α level of 0.05.
Model 2 includes the variables in Model 1 and is additionally adjusted for child's ethnicity, maternal educational level and household income at 6-year follow-up, and parity.
Model 3 includes the variables in Model 2 and is additionally adjusted for pre-pregnancy BMI, child's BMI, smoking during the seconnd trimester, use of folic acid supplements, hours spent in front of a screen, hours spent playing outside, and frequency of tooth brushing.
Association between 25(OH)D GRS and caries diagnosis
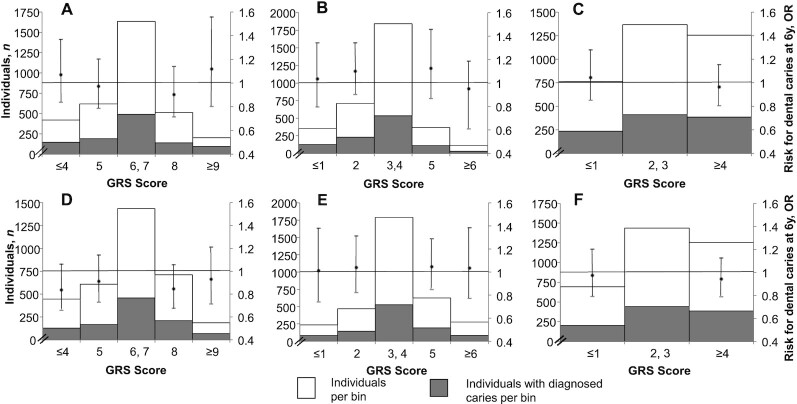
Serum 25(OH)D concentrations increased by 3.87 nmol/L for each additional increasing 25(OH)D allele scored in the unweighted GRS (Supplemental Figure 2). None of the GRS results were associated with caries diagnoses in 6-year-old children. The effect estimates for the weighted and unweighted GRS results were similar (Figure 1; Supplemental Table 4).
FIGURE 1.
Association of 25(OH)D genetic risk score with caries diagnosed at age 6 years using a logistic regression analyses (n = 3385). The x-axis represents the GRS’ score numbers, which subsequently were grouped into bins (i.e., categories). The left y-axis shows the number of individuals: the white area represents the total number of individuals per bin, and the gray area represents the number of individuals diagnosed with caries within each bin. The right y-axis shows the OR, and the dot represents the effect estimate with 95% CI. Each upper panel represents an unweighted GRS: (A) GRS6 based on all 25(OH)D-related SNPs; (B) GRS4 based on 25(OH)D synthesis-related SNPs; and (C) GRS2 based on 25(OH)D metabolism-related SNPs. Each lower panel represents a weighted GRS: (D) GRS6 based on all 25(OH)D-related SNPs; (E) GRS4 based on 25(OH)D synthesis-related SNPs; and (F) GRS2 based on 25(OH)D metabolism-related SNPs. No significant association was found between any GRS and caries diagnosed at age 6 years. GRS, Genetic Risk Score; GRS2, Genetic Risk Score for the 2 metabolizing single nucleotide polymorphisms; GRS4, Genetic Risk Score for the 4 synthetizing single nucleotide polymorphisms; GRS6, Genetic Risk Score for all single nucleotide polymorphisms; SNP, single nucleotide polymorphism; 25(OH)D, 25-hydroxyvitamin D; .
Discussion
We found a significant longitudinal association between low early childhood serum 25(OH)D concentrations and caries diagnosed in 6-year-old children. However, our GRS analysis suggests that children who are genetically predisposed to have lower serum 25(OH)D concentrations do not have a higher risk of developing caries in primary teeth.
Two previous observational studies showed that low prenatal serum 25(OH)D concentrations were strongly associated with caries in children (9, 10). In our logistic regression analyses, we found similar effect estimates, regardless of the dissimilar methodology. These previous studies partially explained the association based on the correlation between maternal and fetal serum 25(OH)D concentration (28). Thus, insufficient serum 25(OH)D during pregnancy could negatively impact the enamel constitution of primary teeth in the offspring, as dental calcification starts between gestational weeks 13 and 16 (32, 33). However, another recent review highlights that the fetal calcium concentration is primarily regulated by the fetal parathyroid hormone and parathyroid hormone-related peptide rather than by maternal serum 25(OH)D concentrations (34). Therefore, the biological mechanism by which higher maternal gestational serum 25(OH)D concentrations could reduce caries risks in offspring is still unknown. We performed a logistic regression with unexplained residuals analysis, which suggests that there is no association between prenatal serum 25(OH)D deficiency and caries experience in 6-year-old children. Unmeasured serum 25(OH)D concentrations during early childhood or unconsidered lifestyle factors related to serum 25(OH)D concentrations and caries, such as an unhealthy diet or phosphorus intake, might explain the results from previous research (35).
A recent study suggests that perinatal serum 25(OH)D concentrations below 30 nmol/L are associated with caries in 3-year-old Alaska native children (11). Adequate serum 25(OH)D shortly before birth may compensate for the reduction on enamel thickness due to physiological lower enamel secretion after birth, by stimulating dental cells (36). Our logistic regression results show a significant association between the continuous perinatal serum 25(OH)D concentration and caries at age 6. However, the association is no longer present when serum 25(OH)D is analyzed using clinical categories, nor when it is analyzed using logistic regression with unexplained residuals. Limitations to define clinical categories and to measure insufficient levels perinatally in our sample challenge the understanding of the importance of perinatal serum 25(OH)D concentrations.
Many studies have been conducted concerning serum 25(OH)D during early childhood and caries during childhood. A Canadian case-control study suggested that 3- to 4-year-old children with caries were significantly more likely to have insufficient serum 25(OH)D concentrations (<75 nmol/L) (37). Although that study's methodology differs from ours, our findings are similar. Even the logistic regression with unexplained residuals analysis suggests that serum 25(OH)D concentrations during early childhood may be critical for caries risks. Higher concentrations of early childhood serum 25(OH)D may improve immunity against cariogenic bacteria and remineralization after the effect of acids (6, 7). Nonetheless, the study also showed that children with caries were more likely to have lower calcium and albumin concentrations, as well as poorer general health (12). Therefore, the present association might still be confounded by unmeasured lifestyle factors or other health conditions. In our analyses, we adjusted for hours spent in front of a screen and hours spent playing outside as proxies for lifestyle factors; however, further adjustments for general health status are challenging.
The latest meta-analysis about serum 25(OH)D and caries based on controlled clinical trials (CCTs) mentioned vitamin D supplementation as a promising caries-preventive agent in children (38). However, the included 24 CCTs presented significant heterogeneity, and there was evidence of publication bias; thus, the finding had a low certainty. In order to mirror the advantages of a randomized controlled trial, we performed a GRS analysis, where the sum of 25(OH)D-related alleles is used to indicate the genetic predisposition for a higher or lower 25(OH)D concentration (20). Our GRS analysis suggests no significant association between increasing 25(OH)D concentrations and lower odds of caries risk in 6-year-old children. An explanation might be the modest overall heritability of 25(OH)D concentrations (21). Age, BMI, the astronomical season of blood draw, dietary and supplemental intakes of serum 25(OH)D, region of residence, and ethnicity might explain approximately 18% of the overall variance in serum 25(OH)D concentrations according to genetic studies (39). Thus, a large proportion of serum 25(OH)D concentration variability is determined by environmental and genetic factors that are still unidentified. Similar results were previously presented by the Avon Longitudinal Study of Parents and Children (ALSPAC) study, with Mendelian randomization based on 5545 European children. We share the approach of SNPs known to be associated with serum 25(OH)D concentrations; however, we provide further estimates based on biological pathways and different ancestries, in addition to the longitudinal assessment of serum 25(OH)D and the caries risk. The metabolizing GRS suggests an inverse, nonsignificant trend. Therefore, we could not show convincingly whether the biological pathways related to the metabolism of serum 25(OH)D could be the link between 25(OH)D and caries.
Methodological considerations
We have been the first to assess the role of serum 25(OH)D on primary dentition from tooth development until caries onset, and to explore the associations between prenatal, perinatal, and early childhood serum 25(OH)D concentrations simultaneously, as well as genetically, with caries in primary teeth within a large, multi-ethnic, population-based, prospective study.
We examined 25(OH)D concentrations using blood samples, which is the reference standard to determine overall 25(OH)D. Further, we analyzed serum 25(OH)D concentrations continuously and categorically. Interpreting the results from the continuous analysis needs attention. As seen in the results, there is evidence of a protective effect, which is diluted when analyzed categorically. This suggests that serum 25(OH)D might have a threshold effect rather than a continuous effect. Nonetheless, there is still no consensus for clinical cutoffs to define deficiency during pregnancy and birth, as the production of serum 25(OH)D during this period is not yet fully understood (40). Additionally, concentrations of serum 25(OH)D might vary, and 1 measurement might not be sufficient to assess the early childhood 25(OH)D status. However, evidence suggests that serum 25(OH)D concentrations do not vary largely from infancy to school-age (40).
We addressed potential residual confounding using GRS (20). Confounding is minimized by using genetic variants, which are reliably measured, reproducible, and randomly inherited. Our GRS was shown to be a reliable instrument, as it closely mirrors a causal association and correlates with early childhood serum 25(OH)D concentrations (Supplemental Figure 2). While our selected SNPs were extracted from a GWAS performed in adults, further research is required to identify relevant SNPs during childhood. Given the prevalence of caries and the strength of the association between the GRS and early childhood serum 25(OH)D concentrations, this study had sufficient size to detect an effect. However, genetically determined changes in exposure levels are usually small; thus, this study might have been underpowered (41).
As in other prospective studies, there was loss to follow-up, and measurements of serum 25(OH)D and caries were not available for the whole population intended. To avoid selection bias, we performed multiple imputation for missing data, and ran sensitivity analyses. Finally, the reference standard for caries assessment is visual and tactile examination, while in our study we used intra-oral photographs, which might affect the caries assessment and may have resulted in a nondifferential measurement error, leading to information bias. Nevertheless, this method has been validated for its use in cohort studies (18).
In conclusion, we found weak evidence for an association between serum 25(OH)D concentrations and caries risks in 6-year-old children. Our logistic regression with unexplained residuals and the GRS analyses suggest that previous studies might suffer from confounding by environmental and/or lifestyle behavioral factors that are still unidentified. We advise further causal research on the relationships between 25(OH)D concentrations and caries risks, using more robust instrumental variables and constructed with additional SNPs, SNPs assessed during childhood, and SNPs identified in different ancestries.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge the contributions of the participants, general practitioners, hospitals, midwives, and pharmacies in Rotterdam, The Netherlands. The Generation R Study was conducted by the Erasmus Medical Center, Rotterdam, The Netherlands, in close collaboration with the School of Law and Faculty of Social Sciences of Erasmus University, Rotterdam, the Municipal Health Service, Rotterdam area, the Rotterdam Homecare Foundation, and the Stichting Trombosedienst & Artsen laboratorium Rijnmond, Rotterdam. In addition, we acknowledge the reviewers’ contribution to the revised manuscript.
The authors’ responsibilities were as follows – CLAN, JTvdT, KT, EBW, FR, TV, LK: designed the research; OG, KT, TV: provided essential databases; CLAN, OG, KT, LK: performed the statistical analysis; CLAN, LK: conducted the research, wrote the manuscript, and had primary responsibility for the final content; and all authors: revised the manuscript and read and approved the final manuscript.
Notes
The Erasmus Medical Center, Rotterdam; Erasmus University, Rotterdam; and the Netherlands Organization for Health Research and Development made the first phase of the Generation R Study financially possible. For caries assessment, we received an additional and unrestricted grant from GABA, Therwil, Switzerland. The vitamin D assay was supported by the Australian National Health and Medical Research Council (NHMRC APP1062846).
The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author disclosures: FR and KT are supported by the Netherlands Scientific Organization (NWO) and ZonMW (NW O/ZONMW-VIDI-0 16-136-367). CLAN received a grant from the Chilean National Commission for Scientific and Technological Research (CONICYT-PCHA/Magister en el extranjero/2017-73180047) for tuition fees, living expenses, and research consumables. All the other authors report no conflict of interest.
Supplemental Figures 1–3 and Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CCT, controlled clinical trials; dmft, decayed-missing-filled-primary teeth; GRS, Genetic Risk Score; GRS2, Genetic Risk Score for the 2 metabolizing single nucleotide polymorphisms; GRS4, Genetic Risk Score for the 4 synthetizing single nucleotide polymorphisms; GRS6, Genetic Risk Score for all single nucleotide polymorphisms; GWAS, genome-wide association study; SNP, single nucleotide polymorphisms; VCF, Variant Call Format; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Constanza L Andaur Navarro, The Generation R Study Group, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Oral & Maxillofacial Surgery, Erasmus University Medical Center, Rotterdam, The Netherlands.
Olja Grgic, The Generation R Study Group, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Oral & Maxillofacial Surgery, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
Katerina Trajanoska, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
Justin T van der Tas, The Generation R Study Group, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Oral & Maxillofacial Surgery, Erasmus University Medical Center, Rotterdam, The Netherlands.
Fernando Rivadeneira, The Generation R Study Group, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
Eppo B Wolvius, The Generation R Study Group, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Oral & Maxillofacial Surgery, Erasmus University Medical Center, Rotterdam, The Netherlands.
Trudy Voortman, The Generation R Study Group, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Epidemiology, Erasmus University Medical Center, Rotterdam, The Netherlands.
Lea Kragt, The Generation R Study Group, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Oral & Maxillofacial Surgery, Erasmus University Medical Center, Rotterdam, The Netherlands.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, Carter A, Casey DC, Charlson FJ, Chen AZet al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet North Am Ed. 2016;388(10053):1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anil S, Anand PS. Early childhood caries: Prevalence, risk factors, and prevention. Front Pediatr. 2017;5:157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, Hojsak I, Mihatsch W, Molgaard C, Shamir Ret al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013;56(6):692–701. [DOI] [PubMed] [Google Scholar]
- 4. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. [DOI] [PubMed] [Google Scholar]
- 5. Berdal A, Papagerakis P, Hatton D, Bailleul-Forestier I, Davideau J. Ameloblast and odontoblast, a target-cells for 1,25-dihydroxyvitamin D3: A review. Int J Dev Biol. 1995;39(1):257–62. [PubMed] [Google Scholar]
- 6. Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet North Am Ed. 2007;369(9555):51–9. [DOI] [PubMed] [Google Scholar]
- 7. Saputo S, Faustoferri RC. Vitamin D compounds are bactericidal against streptococcus. Antimicrob Agents Chemother. 2018;62(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mellanby M, Pattison C. The action of vitamin D in preventing the spread and promoting the arrest of caries in children. BMJ. 1928;2:1079–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schroth RJ, Lavelle C, Tate R, Bruce S, Billings RJ, Moffatt MEK. Prenatal vitamin D and dental caries in infants. Pediatrics. 2014;133(5):e1277–84. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka K, Hitsumoto S, Miyake Y, Okubo H, Sasaki S, Miyatake N, Arakawa M. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann Epidemiol. 2015;25(8):620–5. [DOI] [PubMed] [Google Scholar]
- 11. Singleton R, Day G, Thomas T, Schroth R, Kleijka J, Lenaker D, Berner J. Association of maternal vitamin D deficiency with early childhood caries. J Dent Res. 2019;98(5):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schroth RJ, Rabbani R, Loewen G, Moffatt ME. Vitamin D and dental caries in children. J Dent Res. 2016;95(2):173–9. [DOI] [PubMed] [Google Scholar]
- 13. Dudding T, Thomas SJ, Duncan K, Lawlor DA, Timpson NJ. Re-examining the association between vitamin D and childhood caries. PLOS One. 2015;10(12):e0143769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Izakovicova Holla L, Borilova Linhartova P, Kastovsky J, Bartosova M, Musilova K, Kukla L, Kukletova M. Vitamin D receptor Taq I gene polymorphism and dental caries in Czech children. Caries Res. 2017;51(1):7–11. [DOI] [PubMed] [Google Scholar]
- 15. Kong YY, Zheng JM, Zhang WJ, Jiang QZ, Yang XC, Yu M, Zeng SJ. The relationship between vitamin D receptor gene polymorphism and deciduous tooth decay in Chinese children. BMC Oral Health. 2017;17(1):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IJzendoorn MH, de Jongste JC, Klaver CCW, van der Lugt A, Mackenbach JPet al. The Generation R Study: Design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization . Oral health surveys: Basic method. 5th edition. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 18. Elfrink ME, Veerkamp JSJ, Aartman IHA, Moll HA, Ten Cate JM. Validity of scoring caries and primary molar hypomineralization (DMH) on intraoral photographs. Eur Arch Paediatr Dent. 2009;10(Suppl):5–10. [DOI] [PubMed] [Google Scholar]
- 19. Vogeser M. Quantification of circulating 25-hydroxyvitamin D by liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol. 2010;121(3–5):565–73. [DOI] [PubMed] [Google Scholar]
- 20. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcome. Am J Clin Nutr. 2006;84(1):18–28. [DOI] [PubMed] [Google Scholar]
- 21. Jiang X, O'Reilly PF, Aschard H, Hsu YH, Richards JB, Dupuis J, Ingelsson E, Karasik D, Pilz S, Berry Det al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat Commun. 2018;9(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet. 2018;19(9):581–90. [DOI] [PubMed] [Google Scholar]
- 23. Vinkhuyzen AAE, Eyles EW, Burne TH, Blanken LM, Kruithof CJ, Verhulst F, Jaddoe VWV, Tiemeier H, McGrath JJ. Prevalence and predictors of vitamin D deficiency based on maternal mid-gestation and neonatal cord bloods: The Generation R Study. J Steroid Biochem Mol Biol. 2016;164:161–7. [DOI] [PubMed] [Google Scholar]
- 24. Voortman T, van den Hooven EH, Heijboer AC, Hofman A, Jaddoe VWV, Franco OH. Vitamin D deficiency in school-age children is associated with sociodemographic and lifestyle factors. J Nutr. 2015;145(4):791–8. [DOI] [PubMed] [Google Scholar]
- 25. van der Tas JT, Kragt L, Veerkamp JJS, Jaddoe VWV, Moll HA, Ongkosuwito EM, Elfrink MEC, Wolvius EB. Ethnic disparities in dental caries among six-year-old children in the Netherlands. Caries Res. 2016;50(5):489–97. [DOI] [PubMed] [Google Scholar]
- 26. Centraal Bureau voor de Statistiek . Jaarrapport integratie 2016. The Hague (Netherlands): Centraal Bureau voor de Statistiek;2016. [Google Scholar]
- 27. Fisher-Owens SA, Gansky SA, Platt L, Weintraub JA, Soobader MJ, Bramleet MD, Newacheck PW. Influences on children's oral health: A conceptual model. Pediatrics. 2007;120(3):e510–20. [DOI] [PubMed] [Google Scholar]
- 28. Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol. 2005;58(12):1320–4. [DOI] [PubMed] [Google Scholar]
- 29. Sterne JAC, Carlin WJB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ. 2009;338:2393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. [DOI] [PubMed] [Google Scholar]
- 31. Schuller AA, Vermaire JH, Verrips GHW. Kies-voor-Tandenonderzoek 2017: Cariëservaring bij 5-jarigen in Nederland. NTvT. 2019;7-8:399–407. [DOI] [PubMed] [Google Scholar]
- 32. Karras SN, Shah I, Petroczi A, Goulis DG, Bili H, Papadopoulou F, Harizopoulou V, Tarlatzis BC, Naughton DP. An observational study reveals that neonatal vitamin D is primarily determined by maternal contributions: Implications of a new assay on the roles of vitamin D forms. Nutr J. 2013;12:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lunt RC, Law DB. A review of the chronology of calcification of deciduous teeth. J Am Dent Assoc. 1974;89(3):599–606. [DOI] [PubMed] [Google Scholar]
- 34. Kovacs CS. Bone development and mineral homeostasis in the fetus and neonate: Roles of the calciotropic and phosphotropic hormones. Physiol Rev. 2014;94(4):1143–218. [DOI] [PubMed] [Google Scholar]
- 35. Goodson JM, Shi P, Mumena CH, Haq A, Razzaque MS. Dietary phosphorus burden increases cariogenesis independent of vitamin D uptake. J Steroid Biochem Mol Biol. 2017;167:33–8. [DOI] [PubMed] [Google Scholar]
- 36. Żądzińska E, Kurek M, Borowska-Strugińska B, Lorkiewicz W, Rosset I, Sitek A. The effect of the season of birth and of selected maternal factors on linear enamel thickness in modern human deciduous incisors. Arch Oral Biol. 2013;58(8):951–63. [DOI] [PubMed] [Google Scholar]
- 37. Schroth RJ, Levi JA, Sellers EA, Friel J, Kliewer E, Moffatt MEK. Vitamin D status of children with severe early childhood caries: A case-control study. BMC Pediatr. 2013;13:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hujoel PP. Vitamin D and dental caries in controlled clinical trials: Systematic review and meta-analysis. Nutr Rev. 2013;71(2):88–97. [DOI] [PubMed] [Google Scholar]
- 39. Hiraki LT, Major JM, Chen C, Cornelis MC, Hunter DJ, Rimm EB, Simon KC, Weinstein SJ, Purdue MP, Yu Ket al. Exploring the genetic architecture of circulating 25-hydroxyvitamin D. Genet Epidemiol. 2013;37(1):92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papapetrou PD. The interrelationship of serum 1, 25-dihydroxyvitamin D, 25-hydroxyvitamin D and 24, 25-dihydroxyvitamin D in pregnancy at term: A meta-analysis. Hormones (Athens). 2010;9(2):136–44. [DOI] [PubMed] [Google Scholar]
- 41. Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171(8):903–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.