Abstract
Selective serotonin reuptake inhibitors (SSRIs) are the first-line treatment for patients with unipolar depression, yet there is little guidance on which SSRI provides the most benefit to a patient, based on personal characteristics. In this work, we explore whether an individualized treatment strategy can be used by health-care providers to adapt their prescription pattern to reduce the risk of a severe depression-related outcome (SDO) when choosing between citalopram and fluoxetine, 2 commonly prescribed SSRIs. Our population-based cohort study used data from the Clinical Practice Research Datalink, the Hospital Episode Statistics repository, and the Office for National Statistics database in the United Kingdom to create a cohort of individuals diagnosed with depression who were prescribed citalopram or fluoxetine between April 1998 and December 2017. Patients were followed from treatment initiation until occurrence of the SDO outcome, treatment discontinuation, or end of study. To find an optimal treatment strategy, we used dynamic weighted survival modeling, considering patient features such as age, sex, body mass index, previous psychiatric diagnoses, and medications. Our findings suggest that using patient characteristics to tailor the antidepressant drug therapy is associated with an increase of 4 days in the median time to SDO (95% confidence interval: 2, 10 days).
Keywords: adaptive treatment strategy, citalopram, depression, dynamic weighted survival modeling, effect modification, fluoxetine, population-based cohort study, selective serotonin reuptake inhibitors
Abbreviations
- AD
antidepressant drug
- BMI
body mass index
- CI
confidence interval
- CPRD
Clinical Practice Research Datalink
- HES
hospital episode statistics
- ICD-10
International Classification of Diseases, Tenth Revision
- PY
person-year
- SDO
severe depression-related outcome
- SSRI
selective serotonin reuptake inhibitor
Editor’s note: An invited commentary on this article appears on page 1220, and the authors’ response appears on page 1223.
Depression is associated with significant functional impairment and disability (1–4). By 2030, it is estimated that unipolar major depression will be the world’s leading contributor to disease burden (5). Selective serotonin reuptake inhibitors (SSRIs), a class of antidepressant drugs (ADs) approved by the Food and Drug Administration in 1987 (6), are currently recommended as the first-line treatment option for unipolar depression (7).
Citalopram and fluoxetine are 2 SSRIs commonly prescribed in the United Kingdom (8–11), the United States (12), and Canada (13). These medications, respectively, entered the UK market in 1995 and 1989 and have remained highly prescribed since (9). Only a few studies have examined physician motivations for prescribing one of these drugs over the other (14, 15). The 2 SSRIs show similar effectiveness (11, 16–19) and have mild side effects (20, 21). Clinical recommendations for the prescription of the 2 drugs differ only due to the longer half-life of fluoxetine and its potential drug-drug interactions (22). It is not yet known whether sex, body mass index (BMI), and/or other patient characteristics modify the effectiveness of these 2 SSRIs. Such information could be useful for helping tailor their utilization according to the relevant patient characteristics. Importantly, not responding to first-line AD treatment is associated with greater risk of treatment failure or dropout (23, 24). It is therefore critical that the first treatment trial for an AD therapy works well for a patient.
In this work, we used adaptive treatment strategies (25–27) to build a rule that chooses between prescribing citalopram or fluoxetine as the first AD therapy in patients with a recent history of depression. The rule takes as input each patient’s personal characteristics, and the output is an optimal AD to prescribe, with the goal of delaying the time until severe depression-related outcomes (SDOs).
METHODS
The study protocol was approved by the Independent Scientific Advisory Committee of the United Kingdom Clinical Practice Research Datalink (CPRD) (protocol number 19_017R) and the Research Ethics Committee of the Jewish General Hospital (Montreal, Quebec, Canada).
Data source
We used data from the CPRD, one of the largest primary care databases of deidentified data on patients treated within a network of more than 700 general practitioner practices across the United Kingdom. The database contains data on more than 13 million patients (28), including information such as demographic characteristics, lifestyle factors, medical diagnoses coded using the Read Classification System (29), and referrals to specialists and hospitals. Every prescription written by a general practitioner is automatically recorded in the database based on the British National Formulary.
In this study, CPRD data were linked with the Hospital Episode Statistics (HES) repository and the Office for National Statistics mortality database. Currently, 50%–55% of the general practices enrolled with CPRD have consented to linkage to the HES repository. The HES repository contains information on diagnoses for each hospital stay, classified according to the International Classification of Diseases, Tenth Revision (ICD-10), coding frame. The Office for National Statistics mortality data were used to define dates and causes of death (as part of the study outcome), which were recorded using the ICD-10 coding frame.
Study population
We defined a cohort of new users of citalopram or fluoxetine between April 1, 1998, and December 31, 2017. Cohort entry corresponded to treatment initiation of citalopram or fluoxetine. At time of cohort entry, patients had to have at least 1 year of history in the CPRD, to allow a 1-year retrospective inspection period. We excluded patients under the age of 18 years along with those who used any AD in the year before cohort entry (these included SSRIs, tricyclic antidepressants, monoamine oxidase inhibitors, and other second-generation antidepressants). Patients with 2 prescriptions for different ADs on the date of cohort entry were also excluded. To confirm that patients had used one of the 2 study drugs specifically to treat depression, they had to have at least 1 record for a hospitalization for depression or 1 outpatient diagnosis code for depression in the year before cohort entry.
Patients were followed until an outcome of interest occurred, the administrative end of study (December 31, 2017), treatment discontinuation for citalopram or fluoxetine, switch to any AD other than the initiating drug, end of CPRD coverage, or nonsuicide death, whichever happened first.
Exposure
We used an as-treated exposure definition, where patients were considered exposed from treatment initiation to the end of continuous exposure, which was one of the main reasons for censoring patient follow-up time. For defining continuous exposure, all prescriptions for citalopram or fluoxetine and their duration in days were identified in the CPRD. Treatment discontinuation occurred whenever a subsequent prescription was issued more than 30 days after the end of the previous prescription for the same drug. Dosage information was not used in the exposure definition.
Outcomes
The primary outcome was the occurrence of an SDO, which was defined as a composite of hospitalization for depression, hospitalization for self-harm, or suicide. Admissions for depression were identified using ICD-10 codes in the primary diagnosis position in the HES repository, and suicides were identified in the Office for National Statistics database. Self-harm events were identified via ICD-10 codes in any diagnosis position in the HES repository (30), and consistent with the conclusions from an outcome validation study performed with CPRD data (31). The secondary outcome, which was studied in a sensitivity analysis, was defined as the composite of an SDO as defined above and any outpatient diagnosis for self-harm identified using medical codes in the CPRD.
Confounders
The following variables were considered as potential confounders of the association between the issued AD (citalopram or fluoxetine) and the SDO outcome: age, sex, interaction of age and sex, BMI, smoking, alcohol abuse, calendar year of cohort entry (categorized into 1998–2005, 2006–2011, 2012–2017), psychiatric disease history (including autism spectrum disorder, obsessive-compulsive disorder, bipolar disorder, schizophrenia, and anxiety or generalized anxiety disorder), any other psychotropic drug prescriptions (including benzodiazepines, other anxiolytics, barbiturates, and hypnotics), lipid-lowering drugs, the number of psychiatric admissions in the 6 months prior to cohort entry (including admissions for self-harm events), and, as a proxy for socioeconomic status, the Index of Multiple Deprivation (32, 33), which is a measure of relative deprivation for small areas in England. The Index of Multiple Deprivation was categorized into quintiles. All comorbidities were identified using ICD-10 codes in HES and Read codes in outpatient health records. Smoking and BMI were defined using any data in the 5 years before cohort entry. We measured any medication use in the year before cohort entry and defined other comorbidities using data recorded at any time before or at cohort entry. In the primary analysis and in a first sensitivity analysis, which used a broader outcome definition, age and BMI were divided by 10 and incorporated as such in the models. In a second sensitivity analysis, which used the same outcome definition as the primary analysis, age and BMI were categorized as follows: ages 18–24 years, 25–34 years, 35–44 years, 45–54 years, and ≥55 years and BMIs <25, 25–29, and ≥30.
We used multiple imputation (34) to account for missing values in the covariates BMI, smoking status, and Index of Multiple Deprivation. All the covariates listed in this section were used in imputation models, except for calendar year and the age and sex interaction term.
Statistical analysis
Baseline characteristics were described using the mean and standard deviation for continuous variables and using frequencies and percentages for categorical variables.
The rates of primary and secondary outcomes are presented separately according to treatment group. We assessed the crude probability of survival without an SDO in each treatment group using Kaplan-Meier curves. These curves were further stratified by sex, age category (<40, ≥40 years old; <65, ≥65 years old), BMI category (<25, ≥25), and year of treatment initiation (<2005, ≥2005). We used the R (R Foundation for Statistical Computing, Vienna, Austria) package developed by Le Borgne et al. (35) to compute the inverse probability of treatment weighted survival curves, which were adjusted for all confounders at baseline. We fitted a propensity score model for each imputed data set and averaged the coefficients from the 5 propensity score models to obtain one predicted probability of treatment (i.e., propensity score) that we used to construct weights and plot the adjusted Kaplan-Meier curves.
To estimate an optimal 1-stage treatment rule that answers the question, “Which of citalopram or fluoxetine should be prescribed as the first AD therapy in patients with a recent history of depression to increase their time to SDO?” we used a new statistical method called dynamic weighted survival modeling (DWSurv) (36, 37). The method uses an accelerated failure-time model for investigating associations between a survival-type outcome and covariates. The implementation of the accelerated failure-time model requires the user to identify confounders of the relationship between the exposure and the outcome, as well as a set of tailoring variables that will be used to adapt the prescription to the patient’s characteristics. One can also specify a set of other predictors of the outcome and a set of variables that could explain informative censoring of patient follow-up time if censoring is deemed to be informative. Once these sets are specified, the model is fitted. The fit provides estimates for effect modifications by the tailoring variables, which are used to define the optimal treatment rule. The fitted model can be used to predict the outcome (i.e., the log time to SDO) for a given combination of a patient’s characteristics.
All the covariates included in our models were defined at cohort entry, because we aimed for an optimal treatment rule that allowed a decision at the time of the first AD treatment choice. We used the same set of variables to adjust for confounding and for informative censoring. We did not include in the outcome model any outcome predictors other than the potential confounders listed above, and we chose as tailoring variables the following variables: age, sex, BMI, smoking status, a composite indicator of psychiatric history (of autism spectrum disorder, obsessive-compulsive disorder, bipolar disorder, and schizophrenia diagnostics), an indicator variable for anxiety or generalized anxiety disorder, and the number of psychiatric admissions or hospitalizations for self-harm in the 6 months before cohort entry. In a third sensitivity analysis, we incorporated concomitant psychotherapy in the sets of tailoring and confounder variables. Psychotherapy, defined using any medical or procedural codes in the year before cohort entry, was not included in the main analysis because it was deemed to be poorly recorded given the nature of the database, which contains records from general practices. In a fourth sensitivity analysis, we restricted the study cohort to the patients who initiated one of the 2 study drugs after 2003, the year when both study drugs’ patents had expired and generic versions started being available in Europe.
We present the average coefficients of the tailoring variables in the outcome model (over the 5 imputed data sets and corresponding analyses), along with their 95% bootstrap confidence intervals. We used 500 bootstrap samples to compute the confidence intervals based on the bootstrap percentiles. For each bootstrap sample, we estimated an imputation model and used this model to construct 5 completed data sets. We performed the analyses on all 5 imputed data sets and used the average over those 5 completed data sets as that bootstrap estimate. We further compared the estimated optimal treatment and the actual received treatment for each patient and computed the average proportion of patients for whom they both agreed over the 5 analyses corresponding to each imputed data set. To assess the improvement in outcomes due to receiving the optimal therapy, we computed the predicted time to SDO in different subgroups of the study cohort, under the optimal treatment and under the treatment they actually received and for each imputed data set. We report the median (taken over the 5 analyses corresponding to 5 imputed data sets) of the median time to SDO and used 500 bootstrap samples, as described above, to assess the variation around that estimate. SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina), was used to manage all databases and to produce analytical data sets. All statistical analyses were performed using R, version 3.6.1 (R Foundation for Statistical Computing).
RESULTS
From the CPRD, we identified 736,324 patients with a first prescription for citalopram or fluoxetine between 1998 and 2017 (Figure 1). After exclusions, the final cohort comprised 246,503 patients, of whom 137,791 (56%) initiated citalopram and 108,712 initiated fluoxetine. The average time between the diagnosis of depression and cohort entry was 9 days for citalopram users, and 7 days for fluoxetine users.
Figure 1.
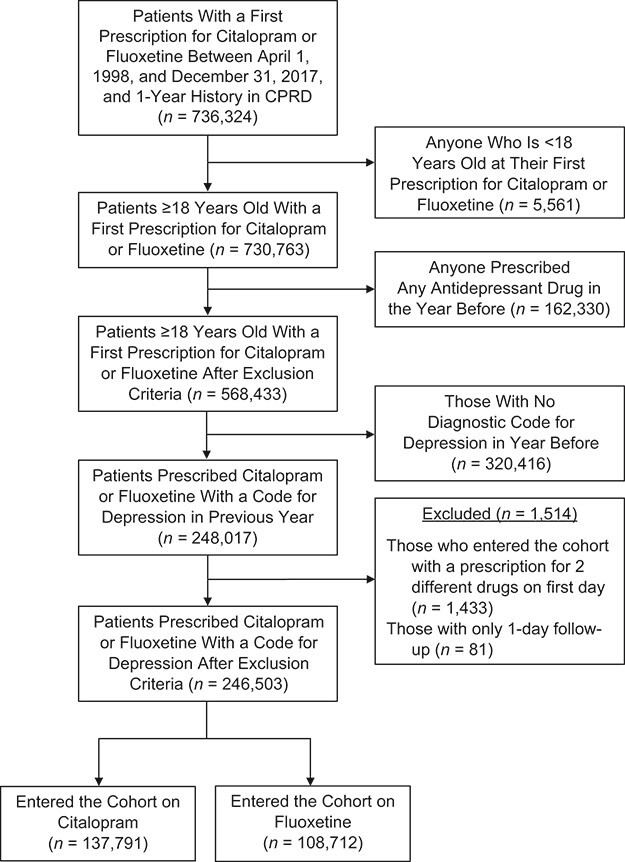
Flowchart of the study cohort, Clinical Practice Research Datalink (CPRD), United Kingdom, 1998–2017.
Patients entering the study with a prescription for citalopram were similar to those with a prescription for fluoxetine: mean age was 43.4 versus 40.7 years, and 36.4% versus 33.9% were men, respectively (Table 1). The 2 groups differed only with respect to calendar year at cohort entry and diagnostic of anxiety or generalized anxiety disorder (Table 1).
Table 1.
Baseline Characteristics According to Exposure Group, Clinical Practice Research Datalink, United Kingdom, 1998–2017
| AD Prescription at Cohort Entry | ||||
|---|---|---|---|---|
| Variable | Citalopram (n = 137,791) | Fluoxetine (n = 108,712) | ||
| No. | % | No. | % | |
| Agea, years | 43.4 (18.2) | 40.7 (16.3) | ||
| Age group, years | ||||
| 18–24 | 20,665 | 15.0 | 17,950 | 16.5 |
| 25–34 | 31,382 | 22.8 | 27,248 | 25.1 |
| 35–44 | 29,927 | 21.7 | 25,564 | 23.5 |
| 45–54 | 22,309 | 16.2 | 17,523 | 16.1 |
| ≥55 | 33,508 | 24.3 | 20,427 | 18.8 |
| Female sex | 87,561 | 63.6 | 71,826 | 66.1 |
| Year of cohort entry | ||||
| 1998–2005 | 40,077 | 29.1 | 56,502 | 52.0 |
| 2006–2011 | 65,297 | 47.4 | 38,780 | 35.7 |
| 2012–2017 | 32,417 | 23.5 | 13,430 | 12.4 |
| Comorbidities | ||||
| Smoking status | ||||
| Ever | 70,033 | 50.8 | 52,910 | 48.7 |
| Never | 46,795 | 34.0 | 34,010 | 31.3 |
| Unknown (further multiply imputed) | 20,963 | 15.2 | 21,792 | 20.1 |
| BMIb | ||||
| <18.5 | 2,296 | 1.7 | 1,725 | 1.6 |
| 18.5–24.9 | 37,957 | 27.5 | 29,652 | 27.3 |
| ≥25.0 | 50,285 | 36.5 | 38,538 | 35.4 |
| Unknown (further multiply imputed) | 47,253 | 34.3 | 38,797 | 35.7 |
| Index of Multiple Deprivation (quintile) | ||||
| 1 (least deprived) | 26,592 | 19.3 | 19,922 | 18.3 |
| 2 | 27,454 | 19.9 | 21,026 | 19.3 |
| 3 | 28,410 | 20.6 | 22,874 | 21.0 |
| 4 | 27,999 | 20.3 | 22,536 | 20.7 |
| 5 (most deprived) | 27,182 | 19.7 | 22,188 | 20.4 |
| Missing (further multiply imputed) | 154 | 0.1 | 166 | 0.2 |
| No. of psychiatric admissions or hospitalizations for self-harm in previous 6 monthsa | 0.046 (0.274) | 0.030 (0.478) | ||
| 0 | 132,749 | 96.3 | 106,329 | 97.8 |
| 1 | 4,203 | 3.1 | 1,980 | 1.8 |
| 2–5 | 814 | 0.6 | 387 | 0.4 |
| >5 | 25 | 0.0 | 16 | 0.0 |
| Alcohol abuse | 11,008 | 8.0 | 7,130 | 6.6 |
| Psychiatric diagnosis | ||||
| Schizophrenia | 1,968 | 1.4 | 1,177 | 1.1 |
| Bipolar disorder | 1,033 | 0.8 | 720 | 0.7 |
| Anxiety/generalized anxiety disorder | 41,979 | 30.5 | 24,019 | 22.1 |
| Autism spectrum disorder | 209 | 0.2 | 135 | 0.1 |
| Obsessive-compulsive disorder | 736 | 0.5 | 618 | 0.6 |
| Drug use | ||||
| Lipid-lowering drugs | 10,487 | 7.6 | 5,385 | 5.0 |
| Antipsychotics | 16,078 | 11.7 | 11,499 | 10.6 |
| Benzodiazepine or other psychotropic drugs | 27,169 | 19.7 | 18,579 | 17.1 |
Abbreviations: AD, antidepressant drug; BMI, body mass index.
a Values are expressed as mean (standard deviation).
b Weight (kg)/height (m)2.
In the primary analysis, patients who entered the cohort with a prescription for citalopram presented with 1,371 SDOs (26% depression, 71% self-harm, 4% suicide) over a total follow-up time of 80,907 person-years (PYs), yielding an SDO rate of 1.69 per 100 PY. The median follow-up time in the citalopram group was 103 days (interquartile range, 58–234); after restricting to those who experienced an SDO during follow-up, the median follow-up time was 45 days (interquartile range, 17–124). Patients who entered the cohort with a prescription for fluoxetine presented with 920 events (23% depression, 74% self-harm, and 4% suicide) over a total follow-up time of 54,781 PY, yielding an SDO rate of 1.67 per 100 PY. The median follow-up time in the fluoxetine group was 93 days (interquartile range, 60–204); in those who experienced an SDO during follow-up, the median follow-up time was 47 days (interquartile range, 18–114). After adding outpatient codes for self-harm (sensitivity analysis 1), the SDO rates were 2.45 per 100 PY in the citalopram group and 2.62 in the fluoxetine group (data not shown).
The survival curves for time to SDO varied across the 2 treatment groups (Figure 2 and Web Appendix 1, available at https://doi.org/10.1093/aje/kwaa260). The citalopram group showed faster times to SDO both marginally and after stratification by sex (Web Figure 1A–B), by BMI (Web Figure 2A–B), in younger patients (Web Figure 3A–B), and in patients who entered the study before 2005 (Web Figure 4). We found the opposite relationship in older patients (Web Figure 5A–B) and in those initiating treatment after 2005 (Web Figure 6). Adjusting the survival curves for confounders did not change the results.
Figure 2.
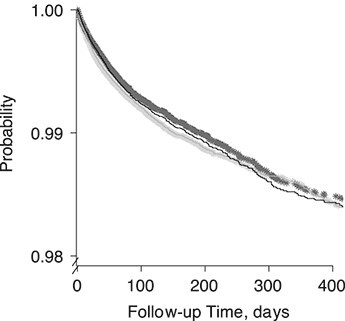
Probability of remaining free of a severe depression-related outcome, stratified by selective serotonin reuptake inhibitor, Clinical Practice Research Datalink, United Kingdom, 1998–2017. The cross-dotted lines are the crude Kaplan-Meier estimates, and the full curves are adjusted for confounder variables at baseline via an inverse probability of treatment weight (fluoxetine users, black; citalopram users, gray).
In the primary analysis, the recommended adaptive treatment rule for a given patient can be stated as:
Treat patient with citalopram if −0.50 + 0.01 × (Age/10) + 0.14 × (BMI/10) + 0.13 × (Indicator Male) + 0.15 × (Ever-Smoker Indicator) + 0.41 × (Other Psychiatric Diagnostic) − 0.26 × (Anxiety or Generalized Anxiety Disorder) − 0.04 × (Number of Psychiatric Admissions or Hospitalizations for Self-Harm in Previous 6 Months) > 0,
where Other Psychiatric Diagnostic refers to any previous diagnostic for autism spectrum disorder, obsessive-compulsive disorder, bipolar disorder, or schizophrenia. None of the coefficients in the dynamic weighted survival model were significant (Table 2). Similar results were found in all 4 sensitivity analyses (Web Tables 1–4 in Web Appendix 2).
Table 2.
Coefficients for the Optimal 1-Stage Treatment Rule for Citalopram Versus Fluoxetine in the Primary Analysis, Clinical Practice Research Datalink, United Kingdom, 1998–2017
| Variable in the Decision Rule | Coefficient | 95% CI |
|---|---|---|
| Intercept | −0.50 | −1.06, 0.18 |
| Agea | 0.01 | −0.07, 0.09 |
| Male sex | 0.13 | −0.12, 0.38 |
| BMIa | 0.14 | −0.07, 0.30 |
| Ever smoking | 0.15 | −0.10, 0.39 |
| Psychiatric diseaseb | 0.41 | −0.07, 0.82 |
| Anxiety or generalized anxiety disorder | −0.26 | −0.56, 0.01 |
| No. of psychiatric admissions or hospitalizations for self-harm events in previous 6 months | −0.04 | −0.16, 0.16 |
Abbreviations: BMI, body mass index; CI, confidence interval.
a For each 10-unit change.
b Autism spectrum disorder, obsessive-compulsive disorder, bipolar disorder, or schizophrenia.
Comparing the optimal treatment and the actual received treatment, 21% of the patients who entered the cohort on fluoxetine received their optimal therapy, against 75% for those who entered the cohort on citalopram. These proportions respectively ranged between 15%–29% and 67%–81% in sensitivity analyses (Web Tables 5–7 in Web Appendix 3). The patients who did not receive the optimal therapy according to our rule might have benefited from receiving the other treatment under study: Using the estimated optimal therapy was predicted to lead to a median increase of 4 days in the time-to-SDO relative to using the actual received therapy (95% confidence interval (CI): 2, 10; for sensitivity analysis 1, 95% CI: 2, 8; for sensitivity analysis 2, 95% CI: 3, 10; for sensitivity analysis 3, 95% CI: 3, 10; for sensitivity analysis 4, 95% CI: 2, 11 days). When we restricted the cohort to those patients using fluoxetine for whom citalopram was the optimal decision, we observed a gain of 8 days in median time to SDO under the optimal treatment decision (95% CI: 4, 20). In those using citalopram but for whom fluoxetine was the optimal treatment decision, we observed a gain of 6 days in median time to SDO under the optimal therapy (95% CI: 2, 15 days) (Table 3). The sensitivity analyses led to similar results (Web Tables 8–10 in Web Appendix 4).
Table 3.
Comparison of the Median Predicted Time to Severe Depression-Related Outcome (Bootstrap Interquartile Range) Under Different Scenarios, According to the Dynamic Weighted Survival Model, Clinical Practice Research Datalink, United Kingdom, 1998–2017
| Scenario | Median (IQR) |
|---|---|
| Everyone treated with the treatment they received | 45 (43–48) |
| Everyone treated with citalopram | 48 (45–52) |
| Everyone treated with fluoxetine | 43 (40–47) |
| Everyone treated with the optimal treatment according to the decision rule | 49 (48–54) |
| Among fluoxetine users for whom citalopram is optimal | |
| Everyone treated with fluoxetine | 42 (38–43) |
| Everyone treated with citalopram | 50 (48–55) |
| Among citalopram users for whom fluoxetine is optimal | |
| Everyone treated with citalopram | 42 (39–44) |
| Everyone treated with fluoxetine | 48 (46–53) |
Abbreviation: IQR, interquartile range.
DISCUSSION
In this retrospective cohort study, we used electronic health record data from 1998 to 2017 and built an optimal decision rule for patients suffering from depression that could inform the choice between prescribing citalopram and fluoxetine. That rule is the result of focusing only on the treatment-related parameters in our time-to-SDO model (i.e., the treatment coefficient and the interactions with the tailoring variables included in the model). To build our treatment rule, we selected a set of tailoring variables that could potentially be effect modifiers for the associations between these antidepressant drugs and the SDO outcome. All the variables that we chose to incorporate in the decision rule are easily and quickly measured by the physician to inform treatment choice.
In the main analysis, none of the assessed patient characteristics (age, sex, BMI, smoking status, psychiatric disorders, anxiety or generalized anxiety disorder, and the prior number of psychiatric admissions) were statistically significant effect modifiers for the 2 ADs compared. However, using a combination of all those variables in a personalized approach to treatment choice was predicted to increase the median time to SDO by 4 days (95% bootstrap CI: 2, 10). Furthermore, the sensitivity analyses showed that our results are robust to covariate and outcome definition.
Our study is, to our knowledge, the first to assess simultaneous antidepressant effect modification by multiple patient characteristics and to build an optimal treatment strategy that chooses between prescribing citalopram or fluoxetine for patients diagnosed with depression. While other studies have compared the efficacy and side effects of citalopram and fluoxetine (10, 11, 15, 16, 19, 20, 38), only a few studies have discussed potential for SSRI effect modification by characteristics like age or sex (39, 40); these 2 studies focused on the SSRI antidepressant class more broadly and did not provide any rule to choose between 2 or more ADs within that class. While the rule we built is associated with a statistically significantly increased time to SDO, it is not clear whether a 4-day difference is clinically meaningful. That result emphasizes the need for further research with more power to detect effect modification or to affirm the lack of clinically meaningful tailoring. Other clinical characteristics (such as genetic traits) and their effect modification should be explored. Finally, an analysis of a larger data set could allow the development of separate rules for hospitalization for depression, hospitalization for self-harm, or suicide. This could provide more specific insights on effect modifications.
Strengths and limitations
Our study has several strengths. First, it used the CPRD, which contains information on medical diagnoses and any drug prescribed by a general practitioner. These data were linked with the HES repository and the Office for National Statistics mortality database, enabling access to important information on inpatient diagnoses and cause of death for defining the outcome. Second, a validated definition of the primary outcome was used, which made it more robust to misspecification. Moreover, a sensitivity analysis using a broader definition for the composite outcome, which also included outpatient diagnostics for self-harm, showed results consistent with the primary analysis. Third, we used dynamic weighted survival modeling, a novel and useful tool for estimating adaptive treatment strategies in settings with censored survival outcomes. Finally, the dynamic weighted survival model accounted for many available potential confounders, as well as for informative censoring due to covariates at baseline.
Limitations of this study include the potential for unmeasured confounders. First, our study database did not include information on the severity of depression. We attempted to account for disease severity by using proxies such as the number of psychiatric admissions or hospitalizations for self-harm in the 6 months preceding cohort entry. Second, we had no information on race or ethnicity, which could be associated with antidepressant treatment indication and depression outcomes (41, 42). More research is needed to assess differences in the two study drugs’ heterogeneity across ethnicities. Third, we had no information on expectations and preference of treatment in this study. Furthermore, we were likely unable to capture the full information on concomitant psychotherapy: Only 0.8% of the patients in the study cohort (59% citalopram, 41% fluoxetine) had a record for psychotherapy in the year before cohort entry. This might suggest that the psychotherapy information was poorly recorded, which might reflect the clinical nature of health records in CPRD and the fact that psychotherapy must be provided by a third party, or it could mean that patients did not have adequate access to psychotherapy or preferred pharmacotherapy. Other limitations of this study include the lack of information on reasons for treatment discontinuation and not having access to recorded indication for AD prescriptions. We tried to address the latter by including in the study cohort only those patients who had a confirming diagnosis of depression.
Conclusion
In this study, we used patient characteristics that are commonly available in electronic health record data to build a prescribing rule that chooses between citalopram and fluoxetine in patients with depression. In the primary analysis, we found no important effect modifier for the association between either of the 2 SSRIs and time to SDO. However, when applied, the estimated optimal rule we developed increased the time to SDO by 4 days (95% CI: 2, 10). Our findings suggest that accounting for patient characteristics to choose between citalopram and fluoxetine in the treatment of depression might, to a small extent, affect the risk of SDO. Further work in additional cohorts is needed to assess the generalizability of these findings.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montréal, Québec, Canada (Janie Coulombe, Erica E. M. Moodie, Christel Renoux); Biostatistics Unit, Kaiser Permanente Washington Health Research Institute, Seattle, Washington, United States (Susan M. Shortreed); Biostatistics Department, University of Washington, Seattle, Washington, United States (Susan M. Shortreed); Centre for Clinical Epidemiology, Lady Davis Institute for Medical Research, Jewish General Hospital, Montréal, Québec, Canada (Christel Renoux); and Department of Neurology and Neurosurgery, McGill University, Montréal, Québec, Canada (Christel Renoux).
This work was funded by an Innovative Ideas Award from Healthy Brains for Healthy Lives (#HBHL 1c-II-11). E.E.M.M. acknowledges salary support from a senior chercheur-boursier salary award from the Fonds de recherche du Québec–Santé. S.M.S. acknowledges that research reported in this publication was supported by the National Institute of Mental Health (award R01 MH114873). C.R. acknowledges a chercheur-boursier salary award from the Fonds de recherche du Québec–Santé.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
S.M.S. has worked on grants awarded to Kaiser Permanente Washington Health Research Institute by Bristol Meyers Squibb and by Pfizer. She was also a co-investigator on grants awarded to Kaiser Permanente Washington Health Research Institute from Syneos Health, who represented a consortium of pharmaceutical companies carrying out Food and Drug Administration–mandated studies regarding the safety of extended-release opioids. The other authors report no conflicts.
REFERENCES
- 1. Löwe B, Spitzer RL, Williams JB, et al. Depression, anxiety and somatization in primary care: syndrome overlap and functional impairment. Gen Hosp Psychiatry. 2008;30(3):191–199. [DOI] [PubMed] [Google Scholar]
- 2. McKnight PE, Kashdan TB. The importance of functional impairment to mental health outcomes: a case for reassessing our goals in depression treatment research. Clin Psychol Rev. 2009;29(3):243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Judd LL, Akiskal HS, Zeller PJ, et al. Psychosocial disability during the long-term course of unipolar major depressive disorder. Arch Gen Psychiatry. 2000;57(4):375–380. [DOI] [PubMed] [Google Scholar]
- 4. Jaeger J, Berns S, Uzelac S, et al. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006;145(1):39–48. [DOI] [PubMed] [Google Scholar]
- 5. Lépine J-P, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7(suppl 1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334–385. [DOI] [PubMed] [Google Scholar]
- 8. Coupland C, Hill T, Morriss R, et al. Antidepressant use and risk of adverse outcomes in people aged 20-64 years: cohort study using a primary care database. BMC Med. 2018;16(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCrea RL, Sammon CJ, Nazareth I, et al. Initiation and duration of selective serotonin reuptake inhibitor prescribing over time: UK cohort study. Brit J Psychiatry. 2016;209(5):421–426. [DOI] [PubMed] [Google Scholar]
- 10. Mines D, Hill D, Yu H, et al. Prevalence of risk factors for suicide in patients prescribed venlafaxine, fluoxetine, and citalopram. Pharmacoepidemiol Drug Saf. 2005;14(6):367–372. [DOI] [PubMed] [Google Scholar]
- 11. Rubino A, Roskell N, Tennis P, et al. Risk of suicide during treatment with venlafaxine, citalopram, fluoxetine, and dothiepin: retrospective cohort study. BMJ. 2007;334(7587):242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Milea D, Verpillat P, Guelfucci F, et al. Prescription patterns of antidepressants: findings from a US claims database. Curr Med Res Opin. 2010;26(6):1343–1353. [DOI] [PubMed] [Google Scholar]
- 13. Kennedy SH, Lam RW, Morris B, et al. Clinical guidelines for depressive disorders—summary of recommendations relevant to family physicians. Can Fam Physician. 2003;49:489–491. [PMC free article] [PubMed] [Google Scholar]
- 14. Simon G. Choosing a first-line antidepressant: equal on average does not mean equal for everyone. JAMA. 2001;286(23):3003–3004. [DOI] [PubMed] [Google Scholar]
- 15. Marken PA, Munro JS. Selecting a selective serotonin reuptake inhibitor: clinically important distinguishing features. Prim Care Companion J Clin Psychiatry. 2000;2(6):205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patris M, Bouchard JM, Bougerol T, et al. Citalopram versus fluoxetine: a double-blind, controlled, multicentre, phase III trial in patients with unipolar major depression treated in general practice. Int Clin Psychopharmacol. 1996;11(2):129–136. [PubMed] [Google Scholar]
- 17. RTI-UNC Evidence-based Practice Center . Comparative Effectiveness of Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression. (Comparative Effectiveness Review no. 7) (Agency for Healthcare Research and QualityPublication no. 07-EHC007-EF) (Contract no. 290-02-0016). https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/antidepressants-2007_research.pdf. Accessed September 28, 2020.
- 18. RTI-UNC Evidence-based Practice Center . Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: an Update of the 2007 Comparative Effectiveness Review. (Effective Health Care Program, Comparative Effectiveness Review, no. 46) (Agency for Healthcare Research and Qualitypublication no. 12-EHC012-EF) (Contract no. 290-2007-10056-I). https://www.diva-portal.org/smash/get/diva2:810275/FULLTEXT01.pdf. Accessed September 28, 2020.
- 19. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–758. [DOI] [PubMed] [Google Scholar]
- 20. Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bauer M, Monz BU, Montejo AL, et al. Prescribing patterns of antidepressants in Europe: results from the Factors Influencing Depression Endpoints Research (FINDER) study. Eur Psychiatry. 2008;23(1):66–73. [DOI] [PubMed] [Google Scholar]
- 22. National Institute for Clinical Excellence et al. Depression in Adults: Recognition and Management (CG90). London, UK: NICE; 2009. [PubMed] [Google Scholar]
- 23. Zilcha-Mano S, Keefe JR, Chui H, et al. Reducing dropout in treatment for depression: translating dropout predictors into individualized treatment recommendations. J Clin Psychiatry. 2016;77(12):E1584–E1590. [DOI] [PubMed] [Google Scholar]
- 24. Perlman K, Benrimoh D, Israel S, et al. A systematic meta-review of predictors of antidepressant treatment outcome in major depressive disorder. J Affect Disord. 2019;243:503–515. [DOI] [PubMed] [Google Scholar]
- 25. Krakow EF, Hemmer M, Wang T, et al. Tools for the precision medicine era: how to develop highly personalized treatment recommendations from cohort and registry data using Q-learning. Am J Epidemiol. 2017;186(2):160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moodie EE, Richardson TS, Stephens DA. Demystifying optimal dynamic treatment regimes. Biometrics. 2007;63(2):447–455. [DOI] [PubMed] [Google Scholar]
- 27. Wallace MP, Moodie EE. Personalizing medicine: a review of adaptive treatment strategies. Pharmacoepidemiol Drug Saf. 2014;23(6):580–585. [DOI] [PubMed] [Google Scholar]
- 28. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chisholm J. The Read clinical classification. BMJ. 1990;300(6732):1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Montastruc F, Nie R, Loo S, et al. Association of aripiprazole with the risk for psychiatric hospitalization, self-harm, or suicide. JAMA Psychiatry. 2019;76(4):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomas KH, Davies N, Metcalfe C, et al. Validation of suicide and self-harm records in the Clinical Practice Research Datalink. Br J Clin Pharmacol. 2013;76(1):145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deas I, Robson B, Wong C, et al. Measuring neighbourhood deprivation: a critique of the Index of Multiple Deprivation. Environ Plann C Gov Policy. 2003;21(6):883–903. [Google Scholar]
- 33. Department of Communities and Local Government . English Indices of Deprivation: Consultation. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/8551/1524728.pdf. Accessed June 29, 2020.
- 34. Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473–489. [Google Scholar]
- 35. Le Borgne F, Giraudeau B, Querard AH, et al. Comparisons of the performance of different statistical tests for time-to-event analysis with confounding factors: practical illustrations in kidney transplantation. Stat Med. 2016;35(7):1103–1116. [DOI] [PubMed] [Google Scholar]
- 36. Simoneau G, Moodie EE, Nijjar JS, et al. Estimating optimal dynamic treatment regimes with survival outcomes. JASA. 2019;115(531):1531–1539. [Google Scholar]
- 37. Simoneau G, Moodie EE, Azoulay L, et al. Adaptive treatment strategies with survival outcomes: an application to the treatment of type 2 diabetes using a large observational database. Am J Epidemiol. 2020;189(5):461–469. [DOI] [PubMed] [Google Scholar]
- 38. Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years' follow-up: population based cohort study. BMJ. 2018;361:k1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thase ME, Entsuah R, Cantillon M, et al. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health. 2005;14(7):609–616. [DOI] [PubMed] [Google Scholar]
- 40. Olivier J, Blom T, Arentsen T, et al. The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(6):1400–1408. [DOI] [PubMed] [Google Scholar]
- 41. Blanco C, Patel SR, Liu L, et al. National trends in ethnic disparities in mental health care. Med Care. 2007;45(11):1012–1019. [DOI] [PubMed] [Google Scholar]
- 42. Simpson SM, Krishnan LL, Kunik ME, et al. Racial disparities in diagnosis and treatment of depression: a literature review. Psychiatry Q. 2007;78(1):3–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


