Abstract
The objective of this study was to evaluate the effect of a product consisting of a combination of xylanase and xylo-oligosaccharide (STBIO) on performance and ileal digestibility of broiler chickens fed energy and amino acid (AA) deficient diets. Day-old male Ross 308 broiler chicks were randomly allocated to 8 pens per treatment, with 25 chicks per pen. Treatments based on wheat-corn-soybean meal diets were arranged in a 3 × 2 factorial design: a positive control that met or exceeded nutrient recommendations (PC), a negative control diet with a 50 kcal/kg apparent metabolizable energy (AME) reduction (NC1) and NC1 with a 3% reduction in AA content (NC2), each with or without supplementation of 100 g/t of the STBIO. Body weight gain (BWG), feed intake, feed conversion ratio corrected for mortality (FCR) and the European production efficiency factor (EPEF) were recorded from 0 to 42 d. On d 42, ileal samples were collected to determine dry matter (DM), organic matter (OM), ash, protein and energy digestibility. A significant interaction was observed for BWG and feed intake (P < 0.001). The energy and AA reduction reduced (P < 0.05) BWG when compared to the PC. The effect of STBIO on BWG was greater in NC1 (+451 g/bird) than in NC2 (+314 g/bird) or PC (+176 g/bird) diets (P < 0.05), and that in NC2 with STBIO was equal to that in PC without STBIO, and that in NC1 with STBIO was equal to that in PC with STBIO. No interactions were observed on the EPEF or FCR; however, STBIO improved EPEF (P < 0.001) and FCR (P < 0.001) irrespective of the energy reduction or AA density. The intake of digestible DM, OM, ash and energy for the finisher period was increased with STBIO supplementation (P < 0.01). A significant interaction was observed for the intake of digestible protein. NC1 and NC2 reduced the intake of digestible protein; however, when STBIO was supplemented, it was improved in both diets to similar levels to the PC. The stimbiotic supplementation improved performance of broiler chickens fed all diets, particularly those deficient in AME and AA.
Keywords: Xylanase, Fermentable oligosaccharide, Broiler, Stimbiotic
1. Introduction
Xylanase supplementation of poultry diets has become indispensable to optimize nutrient utilisation (Aftab and Bedford, 2018). The hydrolysis of soluble arabinoxylan (AX) to reduce digesta viscosity (Choct et al., 2004) coupled with degradation of feedstuff cell walls and the subsequent release of nutrients entrapped (Bedford, 2002) and provision of prebiotics are the historically suggested mechanisms of non-starch polysaccharide degrading enzyme activity. The degradation of cell walls has recently been questioned based in the lack of support from in vivo data (Aftab and Bedford, 2018; Bedford, 2018). Therefore, it seems plausible that the direct provision of AX breakdown products like xylo-oligosaccharides could be a key strategy to influence gut microbiota. The beneficial effects of xylo-oligosaccharides on broiler performance can be explained by the direct stimulation of lactate and butyrate producing bacteria, the former often cross-feeding butyrate producing bacteria (De Maesschalck et al., 2015). This hypothesis rests on the ability of xylo-oligosaccharides to act either directly as a prebiotic or as a signalling molecule to stimulate fibre-degrading bacteria. However, the amount of xylo-oligosaccharides generated by exogenous xylanases may not be sufficient to account for the increments in butyrate and other short-chain fatty acids (SCFA) concentrations noted. Thus, it is proposed that the small amount of xylo-oligosaccharides produced from xylanases are more likely acting as an activator of specific bacteria in the gastrointestinal tract (GIT) rather than directly as a quantitative prebiotic (Ribeiro et al., 2018). Also, the variability of the production of these oligosaccharides may be one of the reasons for the inconsistent results are found when providing carbohydrases for broilers. When other types of functional oligosaccharides are supplemented, the recommended dose normally exceeds 1 to 2 kg per tonne while xylo-oligosaccharides is bioactive at doses 10- to 100-fold lower (Vázquez et al., 2000). Consequently this work aims to introduce a new category of products termed “stimbiotic” which covers any product able to stimulate a fibre-degrading microbiome to increase fibre fermentability at doses which clearly are too low to contribute in a meaningful manner to SCFA content increments noted on their use (Bedford and Apajalahti, 2020). This suggests that the prebiotic concept that until now has been assigned to xylanase needs to be reconsidered. The objective of this study was to evaluate the effect of the stimbiotic on performance of broiler chickens fed apparent metabolizable energy (AME) and amino acid (AA) deficient diets.
2. Materials and methods
Husbandry, euthanasia methods, experimental procedures and biosafety precautions were approved by the Research Ethical Committee of the Faculty of Veterinary Medicine, Murcia University.
2.1. Birds and housing
A total of 1,200 male Ross 308 chicks (1-day-old) were purchased from a local hatchery (Avicola Levantina S.A., Spain). Upon arrival, birds were placed immediately in 48 floor pens in an environmentally controlled room, with 25 birds per pen. Each pen was equipped with a bell feeder and nipple drinkers, and wooden shavings (40% reutilized from the previous flock) were used as litter. Test diets and water were provided ad libitum throughout the trial. The room was preheated to 34 °C 2 d prior to the commencement of the study and kept at 32 °C for the first 2 d. Then room temperature was gradually decreased by 3 °C each week. From d 28 the temperature was kept at 22 °C until the end of the trial. For the first 10 d, 23 h of light were provided, and this was reduced to 18 h from d 11 onwards.
2.2. Experimental diets
Wheat, corn and soybean meal (Table 1) were used as primary ingredients to formulate the experimental diets that met or exceeded nutrient recommendations for broilers fed in 3 feeding phases: starter, from 0 to 14 d of age; grower, from 15 to 28 d of age; and finisher, from 29 to 42 d of age (FEDNA, 2008). The compositions of the experimental diets are shown in Table 2. Three basal diets were made: a positive control diet (PC), a negative control diet with a 50 kcal/kg apparent metabolizable energy (AME) reduction (NC1) and a negative control diet based on NC1 but with a further reduction in AA by 3% (NC2), each with or without supplementation of 100 g/t of the stimbiotic (Signis, AB Vista, Marlborough, UK). All diets contained phytase supplemented at 500 FTU/kg (Quantum Blue, AB Vista, Marlborough, UK; 5,000 FTU/g). Each diet was fed in mash form, and finisher diets contained 0.5% titanium dioxide (TiO2) as an indigestible marker.
Table 1.
Predicted composition (as is) of wheat, corn and soybean meal used in the experimental diets (%).
| Item | Wheat | Corn | SBM | |
|---|---|---|---|---|
| Moisture | 9.99 | 11.60 | 9.46 | |
| Protein | 10.74 | 7.71 | 50.96 | |
| Fat | 2.19 | 3.45 | 1.97 | |
| Ash | 1.59 | 1.25 | 6.63 | |
| Starch | 61.84 | 64.71 | 5.06 | |
| Total NSP | 9.95 | 7.09 | 14.29 | |
| Insoluble NSP | 8.07 | 6.25 | 10.27 | |
| Soluble NSP | 1.88 | 0.84 | 4.02 | |
| Total A + X | 6.27 | 4.15 | 3.51 | |
| Total A + X insoluble | 5.16 | 3.70 | 2.66 | |
| Total A + X soluble | 1.11 | 0.45 | 0.85 | |
| Phytic P | 0.20 | 0.18 | 0.42 | |
| AME, kcal/kg | 3,154 | 3,278 | – | |
| Total lysine | 2.84 | |||
| Reactive lysine | 2.66 | |||
| SID Total lysine | 2.76 | |||
| SID Methionine | 0.64 | |||
| SID Cysteine | 0.64 | |||
| SID Threonine | 1.97 | |||
| SID Isoleucine | 2.05 | |||
| SID Leucine | 3.69 | |||
| SID Phenylalanine | 2.46 | |||
| SID Tyrosine | 1.71 | |||
| SID Histidine | 1.32 | |||
| Urease, U | 0.01 | |||
NSP = non-starch polysaccharides; AME = apparent metabolizable energy; A = arabinose; X = xylose; SID = standardized ileal digestibility.
Table 2.
Ingredients and calculated composition of the experimental diets (%, as is)1.
| Item | Starter (0 to 14 d) |
Grower (14 to 29 d) |
Finisher (29 to 42 d) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PC | NC1 | NC2 | PC | NC1 | NC2 | PC | NC1 | NC2 | |
| Ingredients | |||||||||
| Wheat | 30.00 | 30.00 | 30.00 | 35.00 | 35.00 | 35.00 | 40.00 | 40.00 | 40.00 |
| Corn | 30.35 | 31.41 | 31.21 | 35.07 | 36.14 | 35.97 | 33.51 | 34.49 | 35.33 |
| Soybean meal | 33.38 | 33.19 | 33.49 | 23.79 | 23.60 | 23.85 | 20.71 | 20.60 | 19.89 |
| Soya oil | 3.21 | 2.33 | 2.40 | 2.85 | 1.98 | 2.04 | 2.93 | 2.07 | 1.98 |
| Salt | 0.27 | 0.28 | 0.27 | 0.29 | 0.29 | 0.29 | 0.30 | 0.30 | 0.30 |
| Limestone | 1.10 | 1.10 | 1.10 | 1.06 | 1.07 | 1.06 | 0.94 | 0.94 | 0.94 |
| Monocalcium phosphate | 0.62 | 0.62 | 0.62 | 0.61 | 0.61 | 0.60 | 0.45 | 0.45 | 0.46 |
| Lysine HCl | 0.20 | 0.21 | 0.15 | 0.36 | 0.36 | 0.31 | 0.30 | 0.30 | 0.28 |
| dl-Methionine | 0.34 | 0.34 | 0.31 | 0.32 | 0.32 | 0.28 | 0.28 | 0.27 | 0.26 |
| Threonine | 0.06 | 0.06 | 0.03 | 0.12 | 0.12 | 0.09 | 0.11 | 0.11 | 0.10 |
| Valine | 0.04 | 0.04 | 0.00 | 0.12 | 0.12 | 0.08 | 0.06 | 0.06 | 0.05 |
| Vitamin & Mineral premix2 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Phytase3 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Calculated composition | |||||||||
| AME, kcal/kg | 3,050 | 3,000 | 3,000 | 3,100 | 3,050 | 3,050 | 3,125 | 3,075 | 3,075 |
| Crude protein | 22.50 | 22.50 | 22.50 | 19.00 | 19.00 | 19.00 | 17.79 | 17.81 | 17.50 |
| Calcium | 0.90 | 0.90 | 0.90 | 0.84 | 0.84 | 0.84 | 0.76 | 0.76 | 0.76 |
| Total P | 0.71 | 0.72 | 0.72 | 0.65 | 0.65 | 0.65 | 0.60 | 0.60 | 0.60 |
| Non-phytic P | 0.47 | 0.47 | 0.47 | 0.42 | 0.42 | 0.42 | 0.38 | 0.38 | 0.37 |
| Available P | 0.45 | 0.45 | 0.45 | 0.42 | 0.42 | 0.42 | 0.38 | 0.38 | 0.38 |
| Dig. Met + Cys | 0.89 | 0.89 | 0.86 | 0.80 | 0.80 | 0.77 | 0.74 | 0.74 | 0.72 |
| Dig. Lys | 1.22 | 1.22 | 1.18 | 1.10 | 1.10 | 1.07 | 0.98 | 0.98 | 0.95 |
| Dig. Tryp | 0.24 | 0.24 | 0.24 | 0.19 | 0.19 | 0.19 | 0.18 | 0.18 | 0.18 |
| Dig. Thr | 0.77 | 0.77 | 0.74 | 0.69 | 0.69 | 0.67 | 0.64 | 0.64 | 0.62 |
| Dig. Val | 0.94 | 0.94 | 0.91 | 0.85 | 0.85 | 0.82 | 0.75 | 0.75 | 0.73 |
| Sodium | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Chlorine | 0.26 | 0.26 | 0.25 | 0.30 | 0.31 | 0.30 | 0.30 | 0.30 | 0.30 |
Diets: PC, positive control diet; NC1, negative control 1, with a 50 kcal/kg apparent metabolizable energy (AME) reduction; NC2, negative control 2, with a 50 kcal/kg AME reduction and reducing amino acids by 3%.
Provided the following per kilogram of feed: vitamin A (E 672), 10,000 IU; vitamin D3 (E 671), 2,000 IU; vitamin E (a-tocopherol), 30.0 mg; vitamin K3, 2.0 mg; vitamin B1, 1.0 mg; vitamin B2, 5.0 mg; vitamin B6, 3.0 mg; vitamin B12, 12.0 μg; nicotinic acid, 40.0 mg; calcium pantothenate, 10.0 mg; folic acid, 1.0 mg; biotin, 0.1 mg; choline chloride, 400 mg; Cu (CuSO4·5H2O), 8.0 mg; Fe (FeCO3), 60.0 mg; I (IK), 2.0 mg; Mn (MnO), 70.0 mg; Se (Na2SeO3), 0.15 mg; Zn (ZnO), 32.0 mg.
Quantum Blue 5G, AB Vista.
2.3. Experimental procedures
Birds were weighted on a pen basis on d 0, 14, 28 and 42. Feed intake (FI) was determined by pen, mortality was checked daily, and the weights of dead birds were used to adjust the feed conversion ratio (FCR). On d 42, 4 broilers from each replicate were euthanatized by cervical dislocation, and the intestinal content was collected from the ileum, defined as the region from the Meckel's diverticulum to the ileocecal junction, by gently squeezing, pooled and frozen at −20 °C. Prior to analysis, ileal samples were freeze-dried and ground.
2.4. Sample analysis
2.4.1. Enzyme activity
Diets were analysed for xylanase and phytase activity by ELISA method using Quantiplate Kits (Enzyme Services & Consultancy, Innovation & Technology Centre, Ystrad Mynach, UK) supplied by Envirologix (Enzyme Services & Consultancy, Innovation & Technology Centre, Ystrad Mynach, UK).
2.4.2. Nutrient analysis
The contents of dry matter (DM), ash, organic matter (OM), crude protein and energy were analysed in feed and ileal content. Additionally, ether extract was also analysed in feed. The analyses were performed according to AOAC Official (2006) Official Methods: Method 984.13 (crude protein), Method 920.39 (ether extract), Method 930.15 (DM), Method 942.05 (ash), and gross energy was determined by using an adiabatic bomb calorimeter (Parr 1356, Parr Instrument Company, Moline, Illinois, USA). Titanium (Ti) in feeds and ileal contents was determined by inductively coupled plasma optical emission spectrometry (ICP-OES).
2.4.3. Nutrient utilisation
Apparent ileal digestibility of DM, OM, ash, protein and energy were calculated using the Ti concentration in feed and ileal digesta as inert marker using the following equation:
where TiF is the concentration of the Ti in feed, NF is the nutrient content in feed, TiD is the concentration of Ti in ileal digesta, and ND is the nutrient content in ileal digesta.
2.5. Statistical analysis
Performance and ileal digestibility were subjected to two-way analysis of variance using JMP 14 Pro (SAS). Pen was the experimental unit for all measurements. The non-parametric Wilcoxon test was used for mortality rates comparison between experimental treatments. Means were separated only when the treatment P-value was significant and then by using the least significant difference (LSD) test. Statements of significance were based on P-value of equal to or less than 0.05. Additionally, a multivariate analysis between performance and the ileal digestibly was performed.
3. Results and discussion
In this study, broiler chickens fed 3 different types of diets, PC, NC1 or the NC2 based on wheat and corn, but differing in the AME and AA levels, were supplemented or not with the combination of xylanase and xylo-oligosaccharides (fed at stimbiotic doses). Analysed chemical composition and analysed enzyme activities of the experimental diets were all close to expected values (Table 3). Overall mortality was slightly higher than expected (6.79 ± 0.56)%, but it was not influenced by treatment (P = 0.900) (data not shown). Average body weight (BW) at 14, 29 and 42 d of age were 252, 963 and 1,919 g, respectively, approximately 52%, 45% and 39% below the breeder objectives. Several factors likely influenced the poor growth rates in this study: 1) a very light BW at 0 d of age, birds were on average 34.12 g, approximately 21% behind Ross 308 objectives for males (Ross, 2019); 2) feed was offered in mash form which will result in a reduced FI and thus lower BW gain (BWG) (Abdollahi and Ravindran, 2018); and 3) 40% of the litter was reutilised from the previous flock which was depopulated in June 2018. In addition, the high mortality rates observed could be also be linked to the litter reutilisation. The microbial analysis of the litter before the commencement of the study demonstrated that it was contaminated by total coliforms (3.4 × 103 CFU/g), Clostridium perfringens (2.4 × 103 CFU/g) and Enterococcus faecalis (1.3 × 104 CFU/g). The litter was stored during summer before being redistributed in the pens in September–October, which would have favoured the growth of these enteric pathogens thus challenging the animals to the threshold of the subclinical disease. The combination of the aforementioned factors clearly demonstrated that broilers do not perform to their genetic potential in a poor environment (Ritz et al., 2009), especially if they start with a low initial BW.
Table 3.
Analysed chemical composition and analysed enzyme activity of the experimental diets1.
| Item | Treatment2 | Diet3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Starter (0 to 14 d) |
Grower (14 to 29 d) |
Finisher (29 to 42 d) |
||||||||
| PC | NC1 | NC2 | PC | NC1 | NC2 | PC | NC1 | NC2 | ||
| Analysed chemical composition, % | ||||||||||
| Dry matter | 87.4 | 87.1 | 87.2 | 87.8 | 87.5 | 87.5 | 89.1 | 89.0 | 88.6 | |
| Ash | 5.4 | 5.4 | 5.4 | 4.8 | 4.7 | 4.6 | 4.8 | 4.8 | 4.7 | |
| Protein | 20.8 | 20.2 | 19.9 | 19.4 | 19.9 | 20.0 | 17.5 | 18.3 | 18.1 | |
| Ether extract | 5.2 | 4.3 | 4.4 | 4.8 | 4.2 | 4.5 | 5.0 | 4.6 | 4.6 | |
| Gross Energy, kcal/kg | 3,982 | 3,912 | 3,917 | 3,982 | 3,896 | 3,931 | 4,004 | 3,965 | 4,011 | |
| Analysed enzyme activity | ||||||||||
| Xylanase, BXU4/kg | -STBIO | <2,000 | <2,000 | <2,000 | <2,000 | <2,000 | <2,000 | <2,000 | <2,000 | <2,000 |
| +STBIO | 22,300 | 21,100 | 22,100 | 20,200 | 16,300 | 21,600 | 15,500 | 18,800 | 18,600 | |
| Phytase, FTU5/kg | -STBIO | 575 | 607 | 847 | 711 | 649 | 568 | 762 | 715 | 603 |
| +STBIO | 667 | 586 | 960 | 817 | 490 | 538 | 585 | 707 | 685 | |
Means were obtained from duplicates.
Treatment: (−) is the control treatment; (+) is the basal diet supplemented with 100 g/t of the stimbiotic, a combination of xylanase and xylo-oligosaccharide (STBIO).
Diet: PC, positive control diet; NC1, negative control 1, with a 50 kcal/kg AME reduction; NC2, negative control 2, with a 50 kcal/kg apparent metabolizable energy (AME) reduction and reducing amino acids by 3%.
One BXU is defined as the amount of enzyme that produces 1 nmol reducing sugars from birchwood xylan in 1 s at 50 °C and pH 5.3.
One FTU is defined as the amount of enzyme required to release 1 μmol of inorganic phosphorus per minute from sodium phytate at 37 °C and pH 5.5.
The effects of experimental treatments on animal performance at the different phases or on the cumulative performance of the study are shown in Table 4, Table 5, respectively. Almost all performance parameters measured in all of the periods resulted in significant interactions (P < 0.05). This was mostly due to the differences noted among the PC and the NC1 and NC2 being minimised in the presence of the combination of xylanase and xylo-oligosaccharide (STBIO). During the starter phase, the FCR of birds fed the NC1 and NC2 diets was improved with the STBIO to values similar to that of the PC diet without STBIO. This was a result of an increase in FI but a proportionately greater increase in BWG. STBIO supplementation in PC diets did not improve performance (P > 0.05), but during the grower phase, STBIO increased BWG in PC diets (832 vs. 742 g; P < 0.05). Body weight gain of broilers fed NC1 and NC2 was reduced during the grower phase and was linked to a lower FI compared with PC diets (P < 0.05). The supplementation of NC1 and NC2 diets with STBIO increased 14- to 29-d BWG to equal that of the PC diets with STBIO or without STBIO, respectively (P < 0.05). Regardless of the diet, STBIO supplementation improved FCR from 1.627 to 1.514 (P < 0.05) during the grower phase. In the finisher phase, a significant interaction was observed on FI (P = 0.004). Feed intake of NC1 and NC2 diets was lower compared with PC diets (P < 0.05). Supplementation of the PC diets with STBIO tended (P > 0.05) to increase FI whereas it significantly increased FI (+295 and + 206 g, respectively) in the NC1 and NC2 diets to values equivalent to that of the PC with STBIO and non-supplemented PC diets, respectively. Body weight gain in the finisher phase was influenced by diet (P < 0.001) and treatment (P < 0.001). NC1 birds were 49 g lighter compared with the PC (P < 0.05) and NC2 birds and 83 g lighter than the NC1 birds (P < 0.05). STBIO supplementation increased BWG during the finisher phase compared to the non-supplemented group (1,034 vs. 918 g).
Table 4.
Effects of dietary treatments on body weight and performance of broiler chickens at the different phases of the study1.
| Diet2 | Treatment3 | BW, g |
0 to 14 d |
14 to 29 d |
29 to 42 d |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 d | 29 d | 42 d | BWG, g | FI, g | FCR4, g/g | BWG, g | FI, g | FCR, g/g | BWG, g | FI, g | FCR, g/g | ||
| PC | 277 | 1,064 | 2,100 | 242 | 360 | 1.485 | 787 | 1,196 | 1.526 | 1,036a | 1,898 | 1.837 | |
| NC1 | 259 | 995 | 1,982 | 225 | 336 | 1.509 | 736 | 1,133 | 1.563 | 987b | 1,805 | 1.830 | |
| NC2 | 235 | 879 | 1,783 | 201 | 315 | 1.581 | 644 | 1,038 | 1.623 | 904c | 1,675 | 1.855 | |
| -STBIO | 241 | 880 | 1,798 | 207 | 321 | 1.566 | 639 | 1,033 | 1.627a | 918b | 1,696 | 1.850 | |
| +STBIO | 273 | 1,078 | 2,112 | 239 | 353 | 1.485 | 805 | 1,212 | 1.514b | 1,034a | 1,889 | 1.832 | |
| PC | -STBIO | 284ab | 1,026b | 2,012b | 249ab | 363a | 1.453c | 742b | 1,169bc | 1.580 | 987 | 1,859bc | 1.889 |
| PC | +STBIO | 270bc | 1,102a | 2,188a | 236bc | 356ab | 1.518bc | 832a | 1,224ab | 1.472 | 1,086 | 1,937ab | 1.784 |
| NC1 | -STBIO | 231d | 841c | 1,757c | 197d | 311c | 1.581ab | 610c | 1,006d | 1.659 | 915 | 1,658d | 1.813 |
| NC1 | +STBIO | 287a | 1,148a | 2,207a | 253a | 362a | 1.437c | 861a | 1,261a | 1.467 | 1,059 | 1,953a | 1.847 |
| NC2 | -STBIO | 209e | 774d | 1,626d | 175e | 290c | 1.663a | 565c | 923e | 1.642 | 852 | 1,572d | 1.847 |
| NC2 | +STBIO | 261c | 984b | 1,940b | 227c | 340b | 1.500bc | 723b | 1,153c | 1.604 | 957 | 1,778c | 1.863 |
| SEM | 5 | 21 | 33 | 5 | 5 | 0.016 | 9 | 11 | 0.013 | 8 | 13 | 0.011 | |
| P-Diet | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.023 | <0.001 | <0.001 | 0.078 | <0.001 | <0.001 | 0.789 | |
| P-Treatment | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.007 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | 0.552 | |
| P-Interaction | <0.001 | <0.001 | <0.001 | 0.001 | 0.001 | 0.004 | 0.001 | <0.001 | 0.199 | 0.145 | 0.004 | 0.136 | |
BW = body weight; BWG = body weight gain; FI = feed intake; FCR = feed conversion ratio; SEM = standard error of the mean.
a – e Means with a different superscript letter differ (P < 0.05) based on t-test Student honestly significant difference test for the interaction.
Means were obtained from 8 replicate pens of 25 birds per replicate pen.
Diet: PC, positive control diet; NC1, negative control 1, with a 50 kcal/kg AME reduction; NC2, negative control 2, with a 50 kcal/kg apparent metabolizable energy (AME) reduction and reducing amino acids by 3%.
Treatment: (−) is the control treatment; (+) is the basal diet supplemented with 100 g/t of the stimbiotic, a combination of xylanase and xylo-oligosaccharide (STBIO).
Corrected for the weight of dead birds.
Table 5.
Effects of dietary treatments on cumulative performance of broiler chickens1.
| Diet2 | Treatment3 | 0 to 29 d |
0 to 42 d |
|||||
|---|---|---|---|---|---|---|---|---|
| BWG, g | FI, g | FCR4, g/g | BWG, g | FI, g | FCR, g/g | EPEF | ||
| PC | 1,029 | 1,547 | 1.506b | 2,066 | 3,445 | 1.670b | 282a | |
| NC1 | 960 | 1,470 | 1.548ab | 1,948 | 3,275 | 1.688ab | 260b | |
| NC2 | 845 | 1,353 | 1.612a | 1,749 | 3,028 | 1.735a | 220c | |
| -STBIO | 846 | 1,348 | 1.604a | 1,764 | 3,044 | 1.729a | 230b | |
| +STBIO | 1,044 | 1,565 | 1.506b | 2,078 | 3,455 | 1.667b | 278a | |
| PC | -STBIO | 991b | 1,514bc | 1.530 | 1,978b | 3,373b | 1.706 | 264 |
| PC | +STBIO | 1,068a | 1,580ab | 1.482 | 2,154a | 3,517a | 1.634 | 300 |
| NC1 | -STBIO | 807c | 1,316d | 1.638 | 1,722c | 2,974c | 1.730 | 224 |
| NC1 | +STBIO | 1,114a | 1,623a | 1.458 | 2,173a | 3,576a | 1.646 | 295 |
| NC2 | -STBIO | 740d | 1,213e | 1.646 | 1,592d | 2,785d | 1.751 | 201 |
| NC2 | +STBIO | 950b | 1,493c | 1.577 | 1,906b | 3,271b | 1.719 | 239 |
| SEM | 11 | 13 | 0.011 | 17 | 24 | 0.009 | 3 | |
| P-Diet | <0.001 | <0.001 | 0.016 | <0.001 | <0.001 | 0.046 | <0.001 | |
| P-Treatment | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.005 | <0.001 | |
| P-Interaction | <0.001 | <0.001 | 0.145 | 0.001 | <0.001 | 0.581 | 0.060 | |
BWG = body weight gain; FI = feed intake; FCR = feed conversion ratio; EPEF = European poultry efficiency factor; SEM = standard error of the mean.
a–e Means with a different superscript letter differ (P < 0.05) based on t-test Student honestly significant difference test for the interaction.
Means were obtained from 8 replicate pens of 25 birds per replicate pen.
Diet: PC, positive control diet; NC1, negative control 1, with a 50 kcal/kg AME reduction; NC2, negative control 2, with 50 kcal/kg apparent metabolizable energy (AME) reduction and reducing amino acids by 3%.
Treatment: (−) is the control treatment; (+) is the basal diet supplemented with 100 g/tone of the stimbiotic (STBIO) a combination of xylanase and xylo-oligosaccharide.
Corrected for the weight of dead birds.
A significant interaction was observed on BWG (P < 0.0001) and FI (P = 0.0001) of birds from 0 to 29 d of age. In this period, the STBIO supplementation of NC1 improved BWG numerically beyond that of the supplemented PC diets. NC2 diets supplemented with the STBIO had BWG comparable to that of the PC diet without the STBIO (950 vs. 991 g, respectively; P > 0.10), suggesting the reduction in AA and AME could be compensated for with the stimbiotic. The effects on BWG with STBIO supplementation is associated with the higher FI observed in the supplemented compared to the non-supplemented diets, especially in the NC1 and NC2 diets (+307 and 280 g, respectively). Diet and treatment influenced 29-d FCR (P = 0.016 and P = 0.001, respectively). Energy reduction, increased 29-d FCR from 1.506 to 1.548 (P > 0.05) and the additional reduction of 3% AA further increased FCR to 1.612, this being significant compared with the PC diet (P < 0.05). The STBIO supplementation, regardless of the diet, improved FCR from 1.604 to 1.506 (P = 0.001). The cumulative results considering all 3 periods showed a significant interaction on the 42-d BWG (P = 0.001) and FI (P < 0.001). In general, a positive response in BWG was observed with STBIO supplementation of all diets; however, it is interesting to highlight the significant increase in BWG of NC1 supplemented birds compared with the PC non-supplemented birds (2,173 vs 1,978 g, respectively; P < 0.05) and also the significant increase in BWG of NC2 supplemented birds, which was similar to the non-supplemented PC birds (1,906 vs 1,978 g, respectively; P > 0.05). The cumulative 42-d FI was lower in NC1 and even lower in NC2 diets compared with PC diets, being restored to higher levels than the non-supplemented PC diets in PC and NC1 STBIO treatments (P < 0.05). No interactions were observed on the EPEF or FCR. Energy reduction and further AA reduction resulted in impaired FCR (P = 0.046) and EPEF (P < 0.001) values. STBIO improved EPEF (230 vs. 278; P < 0.001) and FCR (1.766 vs. 1.608 g/g; P < 0.001) irrespective of the energy reduction or AA density.
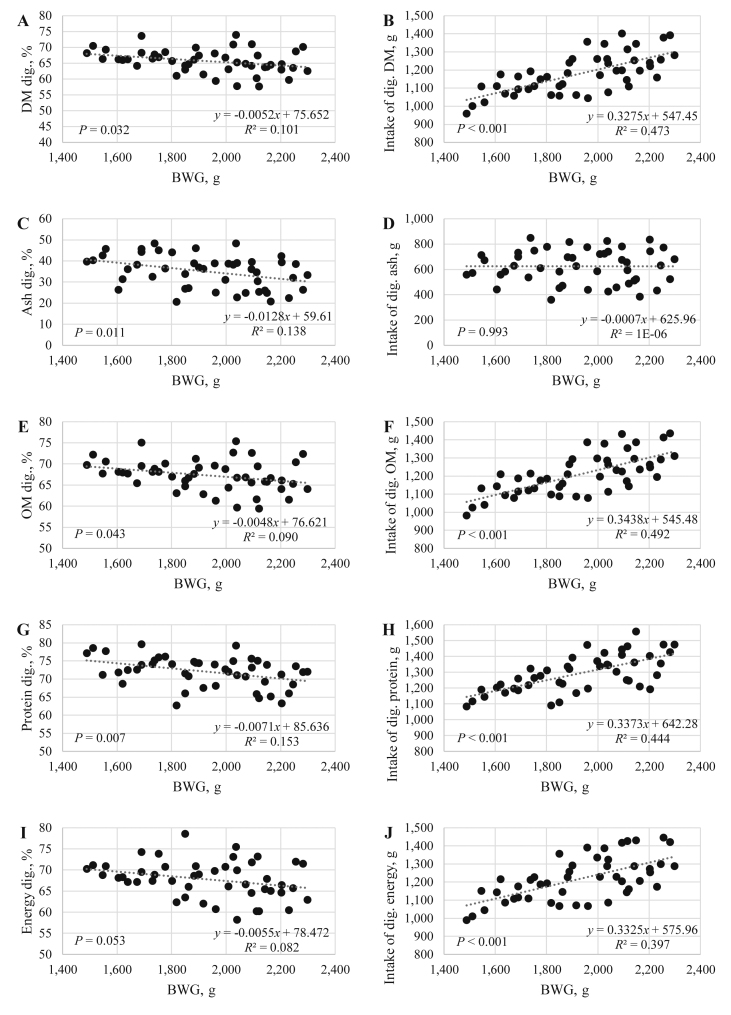
In this study, the digestibility of DM, ash, OM, protein and energy at d 42 are presented in Table 6. No significant interactions, diet or treatment effects on the digestibility of any nutrient measured were noted with the exception of a diet effect on ash digestibility (P < 0.001). The further reduction of AA in the NC2 resulted in higher ash digestibility coefficients when compared to NC1 and PC diets (41.04 vs. 30.65 vs. 33.70, respectively). Digestibility is not the result of a cumulative effect of a treatment, as the samples are only collected over a limited portion of the entire experimental period. As shown in this study, improvements in animal performance were not correlated with the digestibilities determined. Previous research on particle size and feed presentation support this finding, as it was shown that growth rate and efficiency of broilers was not a related to nutrient digestibility per se, but rather to digestible nutrient intake which actually drives performance (Abdollahi et al., 2014). In some studies, a treatment can have a far greater effect on FI than it does on nutrient digestibility, as was the case in this study. As shown in Fig. 1 and Table 7, overall BWG was positively correlated with the intake of digestible DM (r = 0.688; P < 0.001), OM (r = 0.702; P < 0.001), protein (r = 0.666; P < 0.001) and energy (r = 0.630; P < 0.001). However, the regression coefficients between BWG and digestibility data were all negative. These results demonstrate the lack of relevance of the digestibility coefficients measured at one single point in describing potential effects on performance without consideration of intake as well (Bedford and Walk, 2017; Walk and Bedford, 2020). The correction of the digestibility coefficients by the intake makes a better correlation of the digestible nutrient with performance as seen in this study. Particular care must be taken if digestibility trials are used in isolation without considering the intake and postabsorptive effects (Bedford and Cowieson, 2019). Moreover, in the ileal methods the samples are collected in a single point which may not represent the digestibility of nutrient at time points before or after that selected. A significant interaction was observed on the intake of digestible protein (P = 0.023). In the PC diets, the STBIO did not influence the intake of digestible protein, but in NC1 and NC2, the STBIO increased these values to similar levels observed in the PC diets. Almost all of these effects were driven by intake and not digestibility. The NC1 and NC2 diets reduced the intake of digestible DM (P = 0.004), ash (P = 0.008), OM (P = 0.002) and energy (P = 0.002) as expected. STBIO supplementation improved the intake of digestible DM (P < 0.001), ash (P = 0.047), OM (P < 0.001) and energy (P = 0.001) but again this was achieved principally by stimulating intake.
Table 6.
Effects of dietary treatments on the ileal digestibility coefficients of broiler chickens at d 42 and on the intake of digestible nutrients during the finisher period.1.
| Diet2 | Treatment3 | Nutrient utilisation, % |
Intake of digestible nutrients4, g |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry matter | Ash | Organic matter | Protein | Energy | Dry matter | Ash | Organic matter | Protein | Energy | ||
| PC | 64.56 | 33.70b | 66.25 | 70.84 | 67.65 | 1,226a | 641a | 1,258a | 1,344 | 1,283a | |
| NC1 | 65.62 | 30.65b | 67.35 | 71.52 | 66.94 | 1,183a | 549b | 1,215a | 1,290 | 1,207b | |
| NC2 | 67.01 | 41.04a | 68.45 | 73.96 | 68.95 | 1,121b | 686a | 1,145b | 1,237 | 1,154b | |
| -STBIO | 66.06 | 35.13 | 67.70 | 72.90 | 68.69 | 1,118b | 590b | 1,146b | 1,234 | 1,164b | |
| +STBIO | 65.40 | 35.13 | 67.00 | 71.32 | 67.00 | 1,235a | 660a | 1,266a | 1,347 | 1,266a | |
| PC | -STBIO | 64.11 | 31.09 | 65.91 | 71.94 | 68.14 | 1,192 | 578 | 1,225 | 1,336a | 1,265 |
| PC | +STBIO | 65.02 | 36.32 | 66.59 | 69.74 | 67.16 | 1,260 | 703 | 1,290 | 1,352a | 1,301 |
| NC1 | -STBIO | 66.14 | 32.75 | 67.79 | 71.97 | 67.57 | 1,095 | 540 | 1,123 | 1,192b | 1,119 |
| NC1 | +STBIO | 65.10 | 28.55 | 66.91 | 71.08 | 66.31 | 1,271 | 558 | 1,307 | 1,389a | 1,295 |
| NC2 | -STBIO | 67.93 | 41.56 | 69.40 | 74.78 | 70.37 | 1,067 | 652 | 1,091 | 1,173b | 1,107 |
| NC2 | +STBIO | 66.08 | 40.52 | 67.51 | 73.15 | 67.52 | 1,175 | 720 | 1,200 | 1,301a | 1,200 |
| SEM | 0.54 | 1.14 | 0.53 | 0.61 | 0.64 | 16 | 19 | 16 | 17 | 18 | |
| P-Diet | 0.203 | <0.001 | 0.268 | 0.100 | 0.458 | 0.004 | 0.008 | 0.002 | 0.008 | 0.002 | |
| P-Treatment | 0.551 | 0.998 | 0.525 | 0.197 | 0.203 | <0.001 | 0.047 | <0.001 | <0.001 | 0.001 | |
| P-Interaction | 0.573 | 0.133 | 0.627 | 0.902 | 0.825 | 0.185 | 0.441 | 0.140 | 0.023 | 0.128 | |
a, b Means with a different superscript letter differ (P < 0.05) based on t-test Student honestly significant difference test for the interaction.
Data are means of 4 birds per pen with 8 pens per treatment.
Diet: PC, positive control diet; NC1, negative control 1, with a 50 kcal/kg AME reduction; NC2, negative control 2, with a 50 kcal/kg apparent metabolizable energy (AME) reduction and reducing amino acids by 3%.
Treatment: (−) is the control treatment; (+) is the basal diet supplemented with 100 g/t of the stimbiotic, a combination of xylanase and xylo-oligosaccharide (STBIO).
Based on the overall intake during the finisher period (29 to 42 d).
Fig. 1.
Relationship between broilers' performance measured as the overall body weight gain (BWG) from d 0 to d 42 of age. The digestibility coefficients for DM (A), intake of digestible DM (B), ash (C), intake of digestible ash (D), OM (E), intake of digestible OM (F), protein (G), intake of digestible protein (H), energy (I) and intake of digestible energy (J).
Table 7.
Multivariate analysis between the ileal digestibility coefficients of broiler chickens at d 42 and the intake of digestible nutrients during the finisher period with body weight gain (BWG)1.
| Item | Dry matter dig. | Ash dig. | Organic matter dig. | Protein dig. | Energy dig. | Intake of dig. dry matter | Intake of dig. Ash | Intake of dig. organic matter | Intake of dig. Protein | Intake of dig. Energy | BWG 0 to 42 d |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry matter dig. | 1.000∗ | ||||||||||
| Ash dig. | 0.619∗ | 1.000∗ | |||||||||
| Organic matter dig. | 0.997∗ | 0.558∗ | 1.000∗ | ||||||||
| Protein dig. | 0.782∗ | 0.648∗ | 0.761∗ | 1.000∗ | |||||||
| Energy dig. | 0.856∗ | 0.548∗ | 0.854∗ | 0.718∗ | 1.000∗ | ||||||
| Intake of dig. dry matter | 0.312∗ | 0.017 | 0.329∗ | 0.089 | 0.290 | 1.000∗ | |||||
| Intake of dig. ash | 0.514∗ | 0.905∗ | 0.456∗ | 0.503∗ | 0.467∗ | 0.377∗ | 1.000∗ | ||||
| Intake of dig. organic matter | 0.288 | −0.033 | 0.308∗ | 0.062 | 0.269 | 0.999∗ | 0.329∗ | 1.000∗ | |||
| Intake of dig. protein | 0.177 | 0.035 | 0.184 | 0.256 | 0.210 | 0.911∗ | 0.397∗ | 0.906∗ | 1.000∗ | ||
| Intake of dig. energy | 0.285 | 0.021 | 0.301∗ | 0.110 | 0.437∗ | 0.943∗ | 0.362∗ | 0.942∗ | 0.887∗ | 1.000∗ | |
| BWG (0 to 42 d) | −0.317∗ | −0.371∗ | −0.300∗ | −0.392∗ | −0.287 | 0.688∗ | −0.001 | 0.702∗ | 0.666∗ | 0.630∗ | 1.000∗ |
∗P < 0.05.
Correlation coefficients between the overall BWG (0 to 42 d), the nutrient and energy utilisation percentages measured at 42 d of age and the intake of digestible nutrients and energy measured for the finisher period (29 to 42 d).
Wheat and corn were used in similar proportions throughout all the dietary phases and the stimbiotic clearly showed benefits in performance and on the intake of digestible nutrients and energy. Many studies have demonstrated positive outcomes when xylanase is supplemented in wheat (Bedford and Autio, 1996; Bedford and Schulze, 1998; González-Ortiz et al., 2016a, 2019) and corn-based diets (Masey-O'Neill et al., 2012) suggesting utility of this enzyme is possible in both cereals. However, in this study xylanase was evaluated in combination with xylo-oligosaccharides on performance and its relationship with ileal digestibility of broiler chickens fed AME and AA deficient diets.
Digesta viscosity was not measured in this study, but the content of soluble AX in wheat and corn used was in the low-normal rank for wheat (1.107% as is) and high normal-rank for corn (0.447% as is) according to previous data from the literature (Bach Knudsen, 1997; Gomes et al., 2020). This suggests that viscosity may not have been a significant issue in this study. A reduction of 150 or 100 kcal/kg are often recommended by feed enzyme suppliers when high or low viscosity diets are used. However, a more conservative energy (50 kcal/kg) and energy plus 3% AA reduction was evaluated in this study which allowed the STBIO to restore performance and intake of digestible nutrients and energy to that of the PC.
Recently another mechanism has been suggested that could explain the benefits observed particularly in non-viscous diets. The abrasion of the endosperm cell walls reducing the lag time for bacterial attachment, thus accelerating AX digestion and the release of xylo-oligosaccharides and arabinoxylo-oligosaccharides (AXOS) in the distal sections of the GIT are the new mechanisms proposed (Bedford, 2018). The AX are the main non-starch polysaccharide components in the cell walls of cereals (Bach Knudsen, 2014) and provide ample substrate for production of AXOS from these substrates (Dale et al., 2019; Morgan et al., 2019). However, the ability of xylanase to produce xylo-oligosaccharides in the GIT takes, may vary with cereal species and sample and is difficult to determine in situ (Lee et al., 2017; Morgan et al., 2019) and therefore direct provision of xylo-oligosaccharides in the diet may accelerate the evolution of a more fibrolytic microbiota. Bautil et al. (2019) observed significant limitations in hind gut fermentation capacity of young broilers, but as the bird grows it develops a more complex and mature microbiota capable of production of the enzymes necessary to breakdown fibre (Bedford, 2018; Bedford and Apajalahti, 2018). Xylo-oligosaccharides and AXOS once produced are fermented to SCFA by specific groups of beneficial bacteria. De Maesschalck et al. (2015) observed that xylo-oligosaccharides directly stimulate lactate producing bacteria in the lower small intestine and consequently lactate being further fermented to butyrate in the large intestine through butyrate producing bacteria. Moreover, xylo-oligosaccharides supplementation of weaned piglets increased xylanase and cellulase activity in different sections of the GIT digesta (Marinho et al., 2007), suggesting this oligosaccharide stimulates xylanolytic and cellulolytic bacteria to increase endogenous enzyme production.
It is unlikely that the xylo-oligosaccharides produced from xylanases or directly supplemented at the levels employed here can quantitatively be responsible to the increased fermentation noted in many studies. They are not used in the same quantity as other prebiotics (e.g., fructo-oligosaccharide, galacto-oligosaccharide, and mannan-oligosaccharide) and thus cannot be responsible for the incremental SCFA production noted. Supplementation of xylo-oligosaccharides in broilers at 0.1 g/kg and in piglets at 0.2 g/kg improved performance while energetically 0.1 g of xylo-oligosaccharides only contributes with 0.3 kcal/kg of energy to the diet. Therefore, the authors suggest that it may be a stimbiotic response given the low doses (just a few grams) needed to stimulate fibre fermentability as proposed by Bedford (2018) and defined in Bedford and Apajalahti (2020).
Higher butyric acid concentrations in the digesta of broiler chickens have been reported when xylanase has been administered and this has been linked to better animal performance in several studies (González-Ortiz et al., 2016b; Lee et al., 2017; Masey-O'Neill et al., 2014), suggesting the development of a fibre fermenting microbiome. Any increase in the concentration of butyric acid is of particular interest as it is the preferred energy source for colonocytes and has an important influence on the integrity of the epithelial cells (Lopetuso et al., 2013). The birds in this study faced subclinical health challenges but the combination of xylanase and xylo-oligosaccharides appears to be an interesting strategy to restore animal performance likely by accelerating the establishment of a specific microbiota to enhance fibre digestion rates.
4. Conclusions
Xylanase coupled with STBIO supplementation improved broiler performance and intake of digestible nutrients and energy of broiler chickens fed all diets, particularly those deficient in energy and AA. When added to energy and energy plus AA deficient diets, the stimbiotic recovered performance and the intake of digestible nutrients and energy to similar levels to the PC. These results suggest that the combination of xylanase and xylo-oligosaccharides may reduce viscosity, improve nutrient absorption and stimulate the microbial communities in the gut to more efficiently degrade fibre and produce short-chain fatty acids, improving performance.
Author contributions
Gemma González-Ortiz: conceptualization writing - original draft, writing - review & editing; Tiago T. dos Santos: supervision, conceptualization; Michael R. Bedford: supervision, conceptualization.
Conflict of interest
Gemma González-Ortiz, Tiago T. dos Santos and Michael R. Bedford work for AB Vista, who supplied the xylanase and fermentable oligosaccharide (Signis) used in this trial.
Acknowledgments
The authors acknowledge the support of Jaime Sanchez and Marta Garcia of Imasde Agroalimentaria S.L (Spain) during the performance of the study and the data and information transfer.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abdollahi M.R., Ravindran V. Book Pelleting modern broiler feeds: why, what and how. FEDNA; Madrid, Spain: 2018. Pelleting modern broiler feeds: why, what and how; pp. 261–270. [Google Scholar]
- Abdollahi M.R., Ravindran V., Svihus B. Influence of feed form on performance, ileal nutrient digestibility and energy utilisation in broiler starters fed sorghum-based diets. Livest Prod Sci. 2014;165:80–86. [Google Scholar]
- Aftab U., Bedford M.R. The use of NSP enzymes in poultry nutrition: myths and realities. World’s Poult Sci J. 2018;74(2):277–286. [Google Scholar]
- AOAC. Official . 2006. Methods of analysis of AOAC international 18th. Gaithersburg (Maryland), USA. [Google Scholar]
- Bach Knudsen K.E. Carbohydrate contents of plant materials used in animal feeding. Anim Feed Sci Technol. 1997;67:319–338. [Google Scholar]
- Bach Knudsen K.E. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poultry Sci. 2014;93(9):2380–2393. doi: 10.3382/ps.2014-03902. [DOI] [PubMed] [Google Scholar]
- Bautil A., Verspreet J., Buyse J., Goos P., Bedford M.R., Courtin C.M. Age-related arabinoxylan hydrolysis and fermentation in the gastrointestinal tract of broilers fed wheat-based diets. Poultry Sci. 2019;98(10):4606–4621. doi: 10.3382/ps/pez159. [DOI] [PubMed] [Google Scholar]
- Bedford M.R. The role of carbohydrases in feedstuff digestion. In: McNab J.M., Boorman K.N., editors. Poultry feedstuffs: supply, composition and nutritive value. CABI Publishing; Oxon, United Kingdom: 2002. pp. 319–336. [Google Scholar]
- Bedford M.R. The evolution and application of enzymes in the animal feed industry: the role of data interpretation. Br Poultry Sci. 2018;59(5):486–493. doi: 10.1080/00071668.2018.1484074. [DOI] [PubMed] [Google Scholar]
- Bedford M.R., Apajalahti J. Exposure of a broiler to a xylanase for 35d increases the capacity of cecal microbiome to ferment soluble xylan. Poultry Science Association 107th Annual Meeting. 2018;97(E-Supplement 1):98–99. [Google Scholar]
- Bedford M.R., Apajalahti J. The role of feed enzymes in maintaining poultry intestinal health. J Sci Food Agric. 2020 doi: 10.1002/jsfa.11670. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford M.R., Autio K. Microscopic examination of feed and digesta from wheat-fed broiler chickens and its relation to bird performance. Poultry Sci. 1996;75(Supp 1):1. [Google Scholar]
- Bedford M.R., Cowieson A.J. Matrix values for exogenous enzymes and their application in the real world. J Appl Poultry Res. 2019;29(1):15–22. [Google Scholar]
- Bedford M.R., Schulze H. Exogenous enzymes for pigs and poultry. Nutr Res Rev. 1998;11(1):91–114. doi: 10.1079/NRR19980007. [DOI] [PubMed] [Google Scholar]
- Bedford M.R., Walk C.L. vol. 2. Animal breeding and nutrition University of Nottingham; UK: 2017. The use of exogenous enzymes to improve feed efficiency in pigs. (Wiseman J. Achieving sustainable production of pig meat). Burleigh Dodds. [Google Scholar]
- Choct M., Kocher A., Waters D.L., Pettersson D., Ross G. A comparison of three xylanases on the nutritive value of two wheats for broiler chickens. Br J Nutr. 2004;92(1):53–61. doi: 10.1079/BJN20041166. [DOI] [PubMed] [Google Scholar]
- Dale T., Brameld J.M., Parr T., Bedford M.R. BSAS Annual Conference; Edinburgh, UK: 2019. Differential effects of fibrolytic enzymes on the in vitro release of xylobiose from different cereal types; p. 20. [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl Environ Microbiol. 2015;81(17):5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedna . España; Madrid: 2008. Necesidades nutricionales para avicultura: pollos de carne y aves de puesta. [Google Scholar]
- Gomes G.A., Dos Santos T.T., Piotrowski C., Garcia R.S. 31st annual Australian poultry science symposium. 2020. Development of near infrared calibrations for determination of non-starch polysaccharide content in feedstuff; pp. 54–57. [Google Scholar]
- González-Ortiz G., Olukosi O., Bedford M.R. Evaluation of the effect of different wheats and xylanase supplementation on performance, nutrient and energy utilisation in broiler chicks. Animal Nutr. 2016;2(3):173–179. doi: 10.1016/j.aninu.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ortiz G., Vienola K., Apajalahti J., Bedford M.R. vol. 128. 10th Symposium INRA-Rowett; 2016. Xylanase supplementation influences performance and intestinal fermentation in broiler chickens. (Gut microbiology). [Google Scholar]
- González-Ortiz G., Dos Santos T.T., Vienola K., Vartiainen S., Apajalahti J., Bedford M.R. Response of broiler chickens to xylanase and butyrate supplementation. Poultry Sci. 2019;89(9):3914–3925. doi: 10.3382/ps/pez113. [DOI] [PubMed] [Google Scholar]
- Lee S.A., Apajalahti J., Vienola K., González-Ortiz G., Fontes C.M.G.A., Bedford M.R. Age and dietary xylanase supplementation affects ileal sugar residues and short chain fatty acid concentration in the ileum and caecum of broiler chickens. Anim Feed Sci Technol. 2017;234:29–42. [Google Scholar]
- Lopetuso L.R., Scaldaferri F., Petito V., Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5(1):23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho M.C., Lordelo M.M., Cunha L.F., Freire J.P.B. Microbial activity in the gut of piglets: I. Effect of prebiotic and probiotic supplementation. Livest Sci. 2007;108(1):236–239. [Google Scholar]
- Masey-O'Neill H.V., Liu N., Wang J.P., Diallo A., Hill S. Effect of xylanase on performance and apparent metabolisable energy in starter broilers fed diets containing one maize variety harvested in different regions of China. AJAS (Asian-Australas J Anim Sci) 2012;25(4):515–523. doi: 10.5713/ajas.2011.11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masey-O'Neill H.V., Singh M., Cowieson A.J. Effects of exogenous xylanase on performance, nutrient digestibility, volatile fatty acid production and digestive tract thermal profiles of broilers fed on wheat- or maize-based diet. Br Poultry Sci. 2014;55(3):351–359. doi: 10.1080/00071668.2014.898836. [DOI] [PubMed] [Google Scholar]
- Morgan N.K., Choct M., Wallace A., Hawkins K.L., Wu S.B., Bedford M.R. Book In vitro evaluation of xylo-oligosaccharide production from different batches of wheat with and without xylanase. University of Syndey; Syndey, Australia: 2019. In vitro evaluation of xylo-oligosaccharide production from different batches of wheat with and without xylanase; pp. 59–60. [Google Scholar]
- Ribeiro T., Cardoso V., Ferreira L.M.A., Lordelo M.M.S., Coelho E., Moreira A.S.P., Domingues M.R.M., Coimbra M.A., Bedford M.R., Fontes C.M.G.A. Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poultry Sci. 2018;97(12):4330–4341. doi: 10.3382/ps/pey336. [DOI] [PubMed] [Google Scholar]
- Ritz C.W., Fairchild B.D., Lacy M.P. The bulletin 1267 of the cooperative extension of the university of Georgia. 2009. Litter quality and broiler performance. [Google Scholar]
- Ross Aviagen. Book Ross 308 performance objectives. 2019. 308 performance objectives. [Google Scholar]
- Vázquez M.J., Alonso J.L., Domínguez H., Parajó J.C. Xylo-oligosaccharides: manufacture and applications. Trends Food Sci Technol. 2000;11:387–393. [Google Scholar]
- Walk C.L., Bedford M.R. Application of exogneous enzyes: is digestibility and appropriate response variable? Anim Prod Sci. 2020;60(8):993–998. doi: 10.1071/AN19437. [DOI] [Google Scholar]