Abstract
Transcellular permeation enhancers are known to increase the intestinal permeability of enalaprilat, a 349 Da peptide, but not hexarelin (887 Da). The primary aim of this paper was to investigate if paracellular permeability enhancers affected the intestinal permeation of the two peptides. This was investigated using the rat single-pass intestinal perfusion model with concomitant blood sampling. These luminal compositions included two paracellular permeation enhancers, chitosan (5 mg/mL) and ethylenediaminetetraacetate (EDTA, 1 and 5 mg/mL), as well as low luminal tonicity (100 mOsm) with or without lidocaine. Effects were evaluated by the change in lumen-to-blood permeability of hexarelin and enalaprilat, and the blood-to-lumen clearance of 51chromium-labeled EDTA (CLCr-EDTA), a clinical marker for mucosal barrier integrity. The two paracellular permeation enhancers increased the mucosal permeability of both peptide drugs to a similar extent. The data in this study suggests that the potential for paracellular permeability enhancers to increase intestinal absorption of hydrophilic peptides with low molecular mass is greater than for those with transcellular mechanism-of-action. Further, the mucosal blood-to-lumen flux of 51Cr-EDTA was increased by the two paracellular permeation enhancers and by luminal hypotonicity. In contrast, luminal hypotonicity did not affect the lumen-to-blood transport of enalaprilat and hexarelin. This suggests that hypotonicity affects paracellular solute transport primarily in the mucosal crypt region, as this area is protected from luminal contents by a constant water flow from the crypts.
KEY WORDS: Permeation enhancers, Absorption-modifying excipients, Oral peptide delivery, Intestinal permeability, Intestinal perfusion, Pharmaceutical development
Abbreviations: PE, permeation enhancer; CLCr-EDTA, clearance of 51Cr-EDTA; Peff, intestinal effective permeability; SDS, sodium dodecyl sulfate; SPIP, single-pass intestinal perfusion
Graphical abstract
Paracellular permeation enhancers increased the intestinal transport of the peptide drug hexarelin but there was no effect of transcellular permeation enhancers.
1. Introduction
Oral administration is preferred for systemically acting drugs because of its ease of intake and high patient compliance1. It also offers more flexibility in pharmaceutical formulation than other delivery routes and it comprises the bulk (62%) of total pharmaceutical products2. However, oral administration requires that the drug be chemically and metabolically stable in the highly dynamic gastrointestinal (GI) environment, and that it sufficiently permeates the intestinal membrane barrier. These features are typically associated with small molecules that are not substrates for various luminal digestive enzymes. Peptides, on the other hand, are larger and hydrophilic, resulting in low intestinal permeability. They are also substrates for luminal proteinases and peptidases, with very rapid luminal degradation rates3, 4, 5. Accordingly, albeit with a few exceptions, metabolic and/or permeation limitations hinder the development of oral pharmaceutical peptide products6. Nonetheless, there is a long and continuous interest in the oral administration route for peptides and other biologicals from the pharma industries, because these molecules are often superior to small molecules in their selectivity, potency, and safety7. For example, peptide drugs replace the physiological peptide hormones lacking in certain disorders, such as synthetic encephalin analogue in pain management and insulin in diabetes mellitus. However, many peptide drugs have a low permeation across cell membrane that prevents them from reaching and recognizing their intracellular targets8.
Lately, pharmacokinetic and pharmacodynamic properties of some peptides have been improved by making them cyclic, so that their properties becomes similar to many low-molecular-mass drugs9,10. Pharmaceutical strategies for increasing intestinal peptide absorption have also been investigated11. For instance, chemical stability can be increased by enteric coating that prevents pH denaturation in the stomach, and metabolic degradation can be reduced by coadministration of peptidase inhibitors12. Colonic targeting has also been investigated, as the large intestine has lower peptidase activity13. Nevertheless, none of these biopharmaceutical approaches for increasing intestinal peptide stability has resulted in any clinical product, indicating that luminal stability is less of a formulation issue than the low intestinal permeability of these polar and larger peptides.
New chemical synthetic methods have improved the biopharmaceutical pharmacokinetic properties of peptides; they have also improved the target specificity by applying amino acid or backbone modifications and incorporation of non-natural amino acids. Conjugation of molecules has been used to reduce clearance and prolong terminal half-life, and/or improve physicochemical properties and aqueous solubility6. However, the molecular structure of these pharmaceutical peptides cannot readily be changed to increase intestinal membrane permeability without affecting their pharmacological effect. One approach to enhance permeation is to make the mucosal membrane transiently more permeable to the peptide. This may be achieved by incorporating permeation enhancers (PE), also called absorption-modifying excipients, into the drug formulation14. A PE can be transcellular and/or paracellular, depending on which transport route it affects15. Such a formulation strategy was recently approved for human use, in which the oral bioavailability of the peptide drug, semaglutide, was increased by formulating it with the transcellular PE, SNAC [sodium N-(8-(2-hydroxybenzoyl)amino)caprylate]16.
However, clinical use of PE has been largely unsuccessful. Semaglutide/SNAC is only one of two approved permeation-enhancing drug products, despite the number of preclinical reports investigating this formulation strategy. There is still no validated and robust formulation framework that can be used for rational pharmaceutical development of an oral dosage for any given peptide12. As there is an absence of approved oral peptide PE formulations this research was focused on improving the mechanistic understanding by using a rat single-pass intestinal perfusion (SPIP) method. This study monitored the effect of transcellular PE on the permeability of one smaller (enalaprilat, 348 Da) and one larger (hexarelin, 887 Da) peptide drug, in vivo17. Permeability of enalaprilat was substantially increased by sodium dodecyl sulfate (SDS) and caprate, while the jejunal permeability of hexarelin was unaffected. This is surprising as the relative PE-induced increase in drug permeability is reversely proportional to basal permeability of low-molecular-mass (350 Da) model drugs (enalaprilat>atenolol>metoprolol>ketoprofen) in both the rat SPIP and intraintestinal bolus models18,19. Possibly the increased membrane fluidity induced by the surfactant-like transcellular PEs is insufficient to compensate for the thermodynamic cost of partitioning into, and across, the epithelial apical cell membrane for a large, hydrophilic peptide like hexarelin. However, it remains to be investigated in the SPIP model if paracellular PEs affect the absorption of enalaprilat and hexarelin differently than PEs acting mainly on the transcellular route.
The objective of this study was to investigate the time-course effects of permeation enhancers on the peptides enalaprilat and hexarelin. Five luminal compositions, reported to increase paracellular permeability in the rat SPIP model, were tested20,21. These compositions included two paracellular PEs, chitosan (5 mg/mL) and ethylenediaminetetraacetate (EDTA, 1 and 5 mg/mL), as well as low luminal tonicity (100 mOsm) with or without lidocaine. Lidocaine was included because it potentiates the permeability enhancing effect of luminal hypotonicity21. Effects were evaluated in the rat small intestine by the change in lumen-to-blood permeability of hexarelin and enalaprilat, and blood-to-lumen clearance of 51chromium-labeled EDTA (CLCr-EDTA), the clinical marker for mucosal barrier integrity.
2. Materials and methods
2.1. Active pharmaceutical ingredients and excipients, and other chemicals
The molecular structures and some physicochemical properties of enalaprilat and hexarelin are presented in Fig. 1 and Table 122,23. Enalaprilat (Lot. No. 1235274), EDTA (Lot. No. E9884), lidocaine hydrochloride (Lot. No. PHR1257), hexarelin (Lot. No. 80666), Pefabloc SC (Lot. No. 76307), bovine albumin (Lot. No. 05470), and thiobutabarbital sodium salt hydrate (Inactin, Lot. No. T133) were purchased from Sigma–Aldrich (St. Louis, US). Sodium phosphate dibasic dihydrate (Na2HPO4·2H2O), potassium dihydrogen phosphate (KH2PO4), sodium hydroxide (NaOH), and sodium chloride (NaCl) were purchased from Merck KGaA (Darmstadt, Germany). 51Cr-EDTA was purchased from PerkinElmer Life Sciences (Boston, MA).
Figure 1.
Molecular structures of two peptide drugs: hexarelin and enalaprilat.
Table 1.
| Substance (BCS class) | MM (Da) | pKa | PSA | HBA/HBD | LogP | LogD7.4 | LogD6.5 |
|---|---|---|---|---|---|---|---|
| Enalaprilat (III) | 348 | 3.17b/7.84a | 102.1 | 6/3 | −0.13 | −1.0 | −1.0 |
| Hexarelin (III) | 887 | – | 300 | 9/11 | 0.73 | −2.26 | −3.40 |
HBA/HBD, hydrogen bond acceptor/donor; LogD7.4/6.5, n-octanol−water partition coefficient at pH 7.4/6.5; LogP, n-octanol−water coefficient; MM, molar mass; pKa, dissociation constant; PSA, polar surface area.
Acid.
Base.
2.2. Study formulations
Seven different phosphate buffer perfusates were prepared—five at pH 7.4 (50 mmol/L), and two at pH 6.5 (8 mmol/L, Table 2). The five perfusates at pH 7.4 all contained 100 μmol/L enalaprilat and 90 μmol/L hexarelin. The two perfusates at pH 6.5 contained 200 μmol/L of enalaprilat and 180 μmol/L hexarelin instead. This was to enable plasma quantification of hexarelin in rat with our validated bioanalytical method, as the small intestinal permeability (and therefore plasma concentrations) of hexarelin reduces with pH17.
Table 2.
Intestinal luminal composition of the seven perfusates investigated. The entering concentrations of enalaprilat, hexarelin, EDTA, and chitosan are presented, and so are the entering osmolarity and pH. There were two control solutions and five test solutions.
| Perfusate composition | pH | Osmolarity (mOsm) | Enalaprilat/hexarelin concentration (μmol/L) |
|---|---|---|---|
| Control 1 pH 6.5 | 6.5 | 290 | 200/180 |
| Control 2 pH 7.4 | 7.4 | 290 | 100/90 |
| EDTA 1 mg/mL | 7.4 | 290 | 100/90 |
| EDTA 5 mg/mL | 7.4 | 290 | 100/90 |
| Chitosan 5 mg/mL | 6.5 | 290 | 200/180 |
| 100 mOsm | 7.4 | 100 | 100/90 |
| 100 mOsm + Lidocaine 1 mg/mL | 7.4 | 100 | 100/90 |
Of the seven perfusates that all contained both enalaprilat and hexarelin, there were two isotonic (290 mOsm) control solutions that contained only buffer (1 at pH 6.5 and 1 at 7.4), and five test solutions (4 at pH 6.5 and 1 at pH 7.4) with compositions known to increase paracellular permeability20,21. Three of the five test solutions contained PEs: EDTA at 1 and 5 mg/mL, and chitosan at 5 mg/mL (these perfusate concentrations corresponds to oral doses of 0.2 and 1.0 g of a PE administered with 200 mL water)18. Two of the test solutions were hypotonic (100 mOsm), with or without lidocaine at 1 mg/mL; lidocaine is previously shown to potentiate the increase in paracellular permeability induced by hypotonicity21. All test solutions were at pH 7.4, except chitosan that was at pH 6.5 as it precipitates above this pH at luminally effective concentrations. All seven perfusion solutions contained the serine protease inhibitor, Pefabloc SC, at 0.3 mg/mL, as it completely inhibits the intestinal degradation of hexarelin in rat23,24.
The perfusion solutions (100 mL) were prepared as described earlier18. There was no incompatibility, degradation, or binding to glass/plastic of any of the study drugs in solution (pH 6.5, 37 °C) during four 4 h (before:after, hexarelin: 29.9 vs. 33.9 μmol/L, enalaprilat 83.9 vs. 84.8 μmol/L). After addition of all perfusate constituents (i.e., salt, PE, water) osmolarity was determined by freezing point depression using a Micro Osmometer (Model 3MO; Advanced Instruments, Needham Heights, USA).
2.3. Animals and study design
The surgical procedure and experimental setup of the rat SPIP experiments has been previously described in detail18. The study was approved by the local ethics committee for animal research (no: C64/16) in Uppsala, Sweden. In short, male Wistar Han rats (strain 273) from Charles River Co. (Germany), weight 299–395 g, were used. On the study day, the rats were anesthetized using an intraperitoneal injection of a 5% (w/v) inactin solution (180 mg/kg). Body temperature was maintained at 37.5 ± 0.5 °C. Systemic arterial blood pressure was continuously recorded by connecting the arterial catheter to a transducer operating a PowerLab system (AD Instruments, Hastings, UK) to validate the condition of the animal.
For the SPIP experiment, the abdomen was opened along the midline and a jejunal segment of 10–15 cm was cannulated, covered with polyethylene wrap, and placed on the outside of the abdomen. This avoids intestinal folding resulting in luminal pressure build-up, without affecting normal permeation parameters25. The bile duct was cannulated to avoid pancreaticobiliary secretion into the duodenum. After completion of surgery, 51Cr-EDTA was administered intravenously as a bolus of 75 μCi (0.4 mL), followed by a continuous intravenous infusion at a rate of 50 μCi per hour (1 mL/h) for the duration of the experiment. During the first 30 min following surgery, each small intestinal segment was single-passed perfused with 37 °C phosphate buffered saline (6 mmol/L, pH 6.5 or 7.4). This allowed cardiovascular, respiratory, and intestinal functions to stabilize, and to achieve stable 51Cr-EDTA activity in the blood plasma. The length of the proximal small intestinal segment was measured after the jejunal cannulation, and its wet tissue weight was measured after the experiment. The single-pass perfusion rate was at all times 0.2 mL/min (maintained by peristaltic pump, Gilson Minipuls 3, Le Bel, France).
Each perfusion experiment was divided into two parts. In the first part, the small intestinal segment was perfused with the isotonic control buffer solution (containing both peptide drugs at the same time, enalaprilat and hexarelin) during 60 min. In the second part of the experiment, the segment was perfused during 75 min with one each of the five test solutions (containing both peptide drugs at the same time, and one PE or 100 mOsm±lidocaine). All five test solutions are previously shown to increase paracellular intestinal permeability in rat18,20,21.
The five experiments were designed such that each rat served as its own control. The experimental period started with a rapid filling (<30 s) of the whole proximal segment of small intestine with the perfusate (about 1.5 mL for a 10-cm segment). The intestinal segment and perfusion solutions were kept at 37 °C and all perfusate leaving the segment was collected and weighed at each 15-min intervals. Previous investigations using this SPIP model without pharmaceutical excipients or PEs show that the integrity of the intestinal barrier is unaffected during at least 150 min, as there is no change in epithelial transport for a range of compounds: 51Cr-EDTA, enalaprilat, atenolol, phenol red, acyclovir, and ketoprofen26.
Blood samples of <0.3 mL (300 μL Li-heparin Sarstedt tubes) were collected from the femoral artery for a maximum volume of 4 mL during each experiment. Sampled blood volumes were replaced by an equivalent volume of saline (0.9% NaCl) solution with 70 mg/mL bovine serum albumin. Blood was sampled at 15-min intervals for 135 min (7 samples), put on ice, and centrifuged (5000×g, 3 min at 4 °C) within 10 min. 100 μL of the plasma was transferred to 0.5-mL micro tubes and stored at −80 °C until analysis. The bioanalytical ultra-performance liquid chromatography-tandem mass spectrometry (UHPLC–MS/MS) method for quantifying enalaprilat and hexarelin in plasma is described elsewhere17 and was used with slight modifications. The changes were: i) the analytical column was a Luna Omega Polar C18 (100 mm × 21 mm length × inner diameter, particle diameter 1.6 μm); and ii) the initial gradient was 2.0% B for 0.50 min, a linear increase to 90% B in 3.10 min, constant at 90% B for 0.80 min, then back to 2.0% B and constant again at 2.0% B for 1.50 min. The mobile phase constituents were: (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. The concentration intervals in the calibration curve for the LC–MS/MS analytical method were 0.5–587 nmol/L and 1.5–113 nmol/L for enalaprilat and hexarelin, respectively. The accuracy was between 85% and 93% for hexarelin and between 101% and 105% for enalaprilat. The calibration curves were evaluated based on the back-calculated values, which were within ± 15% of their nominal values except at LLOQ where they were within ± 20% of the nominal value. The inter-day precision was in the range of 8.0%–9.7% for enalaprilat and 8.0%–15% for hexarelin.
2.4. Determination of blood-to-lumen jejunal 51Cr-EDTA clearance (CLCr-EDTA) and jejunal effective permeability (Peff)
A detailed description of the calculation of blood-to-lumen CLCr-EDTA and lumen-to-blood effective permeability (Peff) can be found in Dahlgren et al. 202017. In short, CLCr-EDTA was calculated based on the transport of 51Cr-EDTA from the blood to the lumen per 100 g jejunal tissue, while Peff was calculated by relating the absorption rate from the lumen to the central circulation per jejunal surface are using the deconvolution method27,28.
2.5. Statistical analysis
The sample size in each study group was six rats, on the basis of previous perfusion studies18,29. Peff and CLCr-EDTA values are expressed as mean ± standard deviation (SD) or standard error of the mean (SEM). The Peff and CLCr-EDTA ratio between the 45-min control and 60-min test periods in the rat perfusion studies are also presented (Eq. (1)).
| (1) |
The ratio was compared using the paired student's t-test with the Benjamini–Hochberg multiple t-test correction. Multiple comparisons between groups were performed using a one-way ANOVA with a post-hoc Tukey's multiple comparison test. Log transformation of values was performed when the original measured data were heteroscedastic and not normally distributed; this was investigated using the Bartlett test. Differences were considered to be statistically significant when the P-value was smaller than 0.05 (P < 0.05).
3. Results
3.1. Plasma concentration–time profiles of enalaprilat and hexarelin
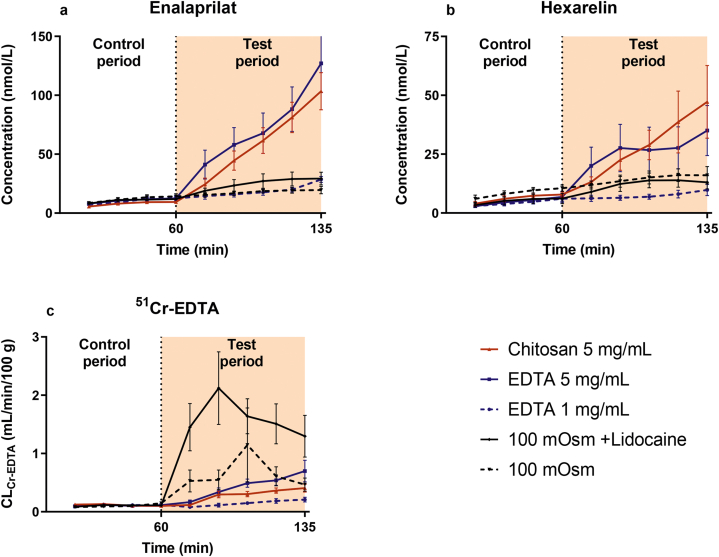
The mean (±SEM) plasma concentration–time profiles of enalaprilat and hexarelin following the jejunal single-pass perfusions of the control solution (0–60 min) and five test solutions (60–135 min) are presented in Fig. 2a and b. These plasma data were used to determine baseline (control period) and test period Peff values of enalaprilat and hexarelin, and their Peff ratios (test vs. control period) using equation 1. There was a consistent increase in plasma concentration–time profile compared to the control period of both enalaprilat and hexarelin during the 75-min test period for EDTA and chitosan at 5 mg/mL. In contrast, there was no increase in the test period for EDTA at 1 mg/mL, or for the two perfusion solutions with hypotonicity (100 mOsm), with or without lidocaine (Fig. 2a and b).
Figure 2.
The mean ± SEM rat plasma concentration–time profiles (n = 6) of (a) enalaprilat and (b) hexarelin, and (c) the blood-to-lumen clearance of 51Cr-EDTA (CLCr-EDTA), after intestinal perfusions of a control solution for 60 min, followed by a 75-min perfusion of any of five test solutions with constituents reported to increase paracellular permeability. There were three isotonic (290 mOsm) test solutions containing EDTA at 1 and 5 mg/mL, and chitosan at 5 mg/mL, and two hypotonic test solutions (100 mOsm) with or without lidocaine. All perfusates were at pH 7.4, except chitosan (pH 6.5). The control solution and all test formulations contained 100 μmol/L enalaprilat and 90 μmol/L hexarelin, except chitosan for which the concentration of both drugs were doubled (plasma values with the chitosan solution were normalized to the lower drug concentration).
3.2. Blood-to-lumen jejunal 51Cr-EDTA clearance profiles
The mean (±SEM) CLCr-EDTA over time following the jejunal single-pass perfusions of the control solution (0–60 min) and five test solutions (60–135 min) are presented in Fig. 2c. There was a consistent increase in CLCr-EDTA during the 75-min test period for the two paracellular PEs: EDTA at 1 and 5 mg/mL, and chitosan at 5 mg/mL. Hypotonicity at 100 mOsm, both with and without lidocaine, increased CLCr-EDTA sharply at the beginning of the 75-min test period. This was followed by a 50% recovery in CLCr-EDTA during the last 30 min of the 75-min test period for 100 mOsm without lidocaine, and during the last 45 min for 100 mOsm with lidocaine (1 mmol/L, Fig. 2c).
3.3. Lumen-to-blood effective jejunal permeability of enalaprilat and hexarelin
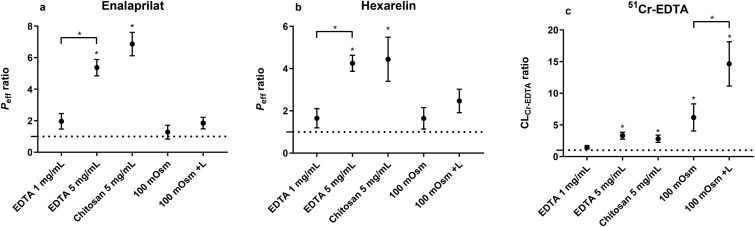
The absolute mean (±SD) Peff values of enalaprilat and hexarelin, with or without PEs, are presented in Table 3. The mean (±SEM) Peff ratios of enalaprilat and hexarelin between the control and test period for the five test formulations are shown in Fig. 3a and b. The Peff ratio (baseline vs. test) for enalaprilat and hexarelin increased significantly with EDTA and chitosan at 5 mg/mL. There was also a significantly higher Peff ratio for enalaprilat and hexarelin when the EDTA concentration increased from 1 to 5 mg/mL. There was no increase in Peff ratios for enalaprilat and hexarelin with EDTA at 1 mg/mL or with the two hypotonic (100 mOsm) perfusion solutions with or without lidocaine (Fig. 3a and b).
Table 3.
The absolute effective permeability of enalaprilat and hexarelin in the control period (buffer at pH 7.4 and 6.5), and the five different test periods: three isotonic (290 mOsm) test solutions containing EDTA at 1 and 5 mg/mL, and chitosan at 5 mg/mL, and two hypotonic test solutions (100 mOsm) with or without lidocaine (L).
| Perfusate composition | Effective permeability ( × 10−4 cm/s)a |
|
|---|---|---|
| Enalaprilat | Hexarelin | |
| Control 1 pH 6.5 | 0.009 ± 0.003 | 0.018 ± 0.0.6 |
| Control 2 pH 7.4 | 0.010 ± 0.005 | 0.016 ± 0.01 |
| EDTA 1 mg/mL | 0.014 ± 0.003 | 0.017 ± 0.008 |
| EDTA 5 mg/mL | 0.069 ± 0.036 | 0.068 ± 0.055 |
| Chitosan 5 mg/mL | 0.057 ± 0.024 | 0.082 ± 0.064 |
| 100 mOsm | 0.010 ± 0.005 | 0.032 ± 0.017 |
| 100 mOsm+L | 0.017 ± 0.009 | 0.029 ± 0.015 |
Data are mean ± SD. All test perfusates were at pH 7.4, except chitosan (pH 6.5). n = 6 for all groups except Control at pH 7.4 where n = 23.
Figure 3.
The mean ± SEM lumen-to-blood intestinal effective permeability (Peff) ratio of (a) enalaprilat and (b) hexarelin, and (c) the blood-to-lumen 51Cr-EDTA clearance (CLCr-EDTA) ratio, after the intestinal perfusions of a control solution for 60 min, followed by a 75-min perfusion of any of five test solutions with constituents reported to increase paracellular permeability. There were three isotonic (290 mOsm) test solutions containing EDTA at 1 and 5 mg/mL, and chitosan at 5 mg/mL, and two hypotonic test solutions (100 mOsm) with or without lidocaine (L). All perfusates were at pH 7.4, except chitosan (pH 6.5). A ∗ represents a significant increase in Peff or CLCr-EDTA ratio.
3.4. Blood-to-lumen CLCr-EDTA
The mean (±SEM) CLCr-EDTA ratios (equation 1) between the control and test period for the five test formulations are shown in Fig. 3c. The CLCr-EDTA ratio increased significantly for all test solutions except EDTA at 1 mg/mL. The CLCr-EDTA ratio also increased significantly by adding lidocaine at 1 mmol/L to the 100 mOsm luminal solution (i.e., hypotonicity with lidocaine>hypotonicity).
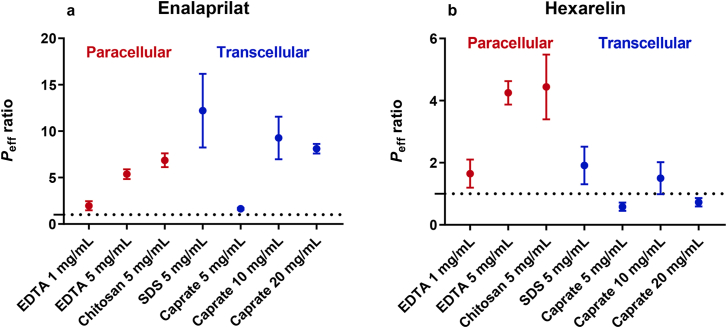
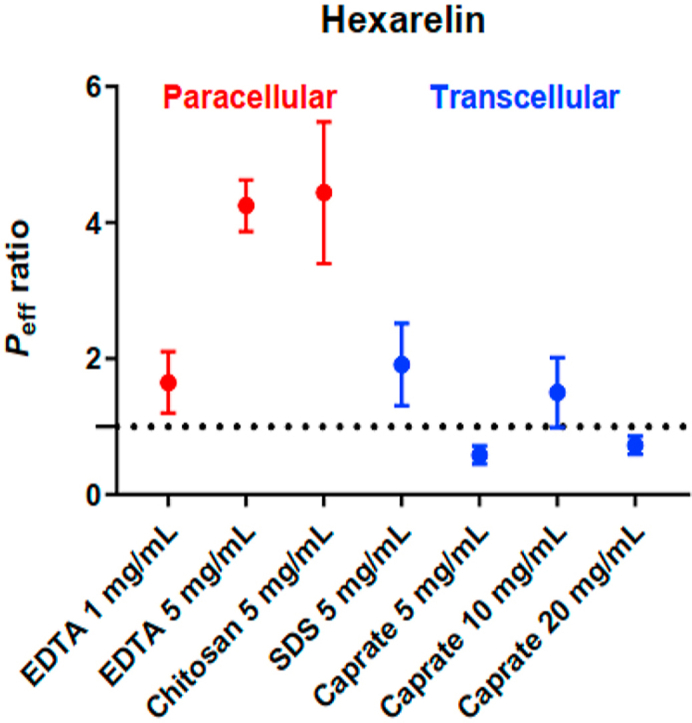
3.5. Transcellular versus paracellular intestinal permeation enhancers
Fig. 4 compares the effect of paracellular and transcellular permeation enhancers on the small intestinal permeability of enalaprilat and hexarelin17. Both types of permeation enhancers increased the jejunal permeability of enalaprilat to similar extents. In contrast, only the small intestinal permeability of hexarelin was increased by the permeation enhancers mainly acting on the paracellular transport route.
Figure 4.
Comparison of the effect of paracellular (red) and transcellular (blue) permeation enhancers on the lumen-to-blood intestinal effective permeability (Peff) ratio of (a) enalaprilat and (b) hexarelin, after the intestinal perfusions of a control solution for 60 min, followed by a 75-min perfusion of test solutions with permeation enhancers. The paracellular permeation enhancer data for EDTA and chitosan were from this study. The transcellular permeation enhancer data was recently published by us17.
4. Discussion
Intestinal singe-pass perfusion systems are commonly applied for various types of drug transport and absorption investigations10,30,31. Our laboratory has extensively investigated the in vivo effect of permeation enhancers (PE) on transport of model drugs and marker compounds with molecular masses ranging from 250 to 900 Da in the small and large intestine14,17, 18, 19, 20,26,29,32. In this sequence of papers, the effect of a range of transcellular and paracellular PEs were initially examined with both the established rat single-pass intestinal perfusion (SPIP) model18, and with the in vivo relevant rat and dog intraintestinal bolus absorption models19. A follow-up rat SPIP study investigating time dependence in PE effects showed that the lower effect in the rat bolus model compared to the SPIP model was attributed to time to maximum effect of the PE (not maximum effect)26. More specifically, the onset time of the PE on the intestinal mucosa needed to be shorter than the intestinal transit rate, which is identified as a crucial in vivo finding. We have also shown that physiological parameters, such as enteric neural activity, can have a substantial impact on results obtained from PE investigations using the rat SPIP model29. Furthermore, regional intestinal investigations showed that PE effects on drug permeability were similar in the small and large intestine, while mucosal injury was greater in the large intestine. This indicates permeation enhancing oral or rectal drug delivery systems targeting the large intestine might be less appropriate20. Finally, we have shown that the effect of surfactant-based PEs (caprate and SDS) was related to the free dissolved fraction (non-particulate or non-solubilized in micelles) in the intestinal lumen and not to the total dose17,32, and that the same transcellular PEs had no effect on the transport of larger peptides17. All these experimental studies clearly demonstrate the importance of considering in vivo system with neural and endocrine functions and feed-back systems. The present study is a continuation of these in vivo investigations of PEs, where the main objective was to investigate the effect of paracellular enhancement of the transport of peptides of different sizes.
Pharmaceutical peptides are typically rapidly degraded in the gastrointestinal (GI) lumen and have low and highly variable intestinal permeability. This results in a low and variable rate and extent of intestinal absorption33. As a consequence of these drug delivery hurdles, there are only two approved, orally-administered, and systemically-acting peptide drugs, desmopressin and semaglutide. They are approved as they have very useful pharmacodynamic properties, despite the low bioavailability of the given oral dose (desmopressin, 0.1%; semaglutide, 0.4%–1.0% with SNAC) and high interindividual variability34,35. Semaglutide, a modified GLP-1 agonist (4113 Da) has a very long terminal half-life in plasma (∼1 week) and is significantly more potent than the endogenous GLP-136,37. In addition, oral semaglutide is included in a drug product containing excipients that increase its luminal stability as well as its intestinal permeability. It is also the first product on the market using a PE to increase oral drug absorption. The manufacturer claims that the mechanism for permeation enhancement is the insertion of the PE (SNAC) in the intestinal epithelium, thereby fluidizing the lipid cell membrane to increase transcellular drug transport16. The approval of oral semaglutide has spurred interest in developing other oral formulations that increase the safe transport of peptides across the GI epithelium38.
Transcellular PEs also tend to be more potent than paracellular ones, as seen for the model compounds atenolol, enalaprilat, FD-4/10, and 51Cr-EDTA14,39. The rat SPIP model illustrates this well: SDS and caprate increase intestinal permeability more than permeation enhancers primarily reported to increase paracellular transport, like chitosan and EDTA18,20. Drug transport through the paracellular route is restricted because of the very low surface area available and the complex sequence of transport barriers that need to be crossed, such as tight junctions (zonula occludens), adherens junctions (zonula adherens) and desmosomes (macula adherens)1,40. We have also observed that the increase in small intestinal permeability is reversely proportional to the basal permeability of a range of low molecular mass (230–350 Da) model drugs (enalaprilat>atenolol>metoprolol>ketoprofen) in both the rat SPIP and intraintestinal bolus models18,19. This relationship, and the greater potency of transcellular PEs, support their use for increasing intestinal absorption of bulky and hydrophilic peptides.
As the intestinal permeability of peptides is very low, a higher proportional PE-induced increase in their permeability is expected compared to similar molecules with high basal permeability. Therefore, it is surprising that a recent rat SPIP study shows a substantial increase in the permeability of enalaprilat induced by transcellular PEs, but no effect on hexarelin. This is unexpected given that the basal permeability of hexarelin is > 10-fold lower than that of enalaprilat (0.0019 ± 0.0013 vs. 0.013 ± 0.009 × 10−4 cm/s)17. We speculated that the membrane fluidizing effect of SDS and caprate is insufficient to accommodate any transcellular transport of the larger peptide, hexarelin. In other words, the thermodynamic cost of partitioning into, and across, the SDS and caprate fluidized epithelial cell membrane is still too large for hexarelin compared to enalaprilat41. If this assumption is true, it may partly explain why PE strategies for increasing oral peptide delivery and absorption have largely failed to translate from preclinical evaluation to clinical product. However, it is possible that paracellular permeation enhancers would still be effective at increasing the permeability of hexarelin. This is because paracellular PEs primarily take into consideration the hydrodynamic diameter of the molecule in relation to the size of the paracellular pores, as opposed to lipoidal translocation with transcellular PEs. We tested this hypothesis in this paper to better understand how to improve GI peptide absorption38.
Indeed, both paracellular PEs—EDTA at 5 mg/mL and chitosan at 1 and 5 mg/mL—increased jejunal permeability of hexarelin over time. This change was irrespective of their different mechanisms of action. The cations in chitosan interact with the epithelial membrane causing structural reorganization of tight junction proteins, whereas EDTA binds extracellular Ca2+ thereby limiting the impact of this ion on tight junction regulation39,42. It is interesting to note that despite the generally lower effect of paracellular permeation enhancers compared to transcellular ones, only the former had any impact on the epithelial transport of hexarelin14,39. Paracellular enhancers may consequently improve oral peptide delivery more than transcellular ones. However, it cannot be excluded that this effect is limited to hexarelin. Therefore, model peptides varying in molecular mass, hydrodynamic size, and other physicochemical properties must be investigated to further develop oral strategies to deliver peptides. It may also be that each oral peptide delivery system with a PE needs to be carefully designed and titrated. For instance, semaglutide with a SNAC dose of 300 mg was effective in humans, while a higher SNAC dose of 600 mg had no effect16.
Perfusion of the intestinal segment with a hypotonic solution (100 mOsm) increased CLCr-EDTA 6-fold in this study. This increase in paracellular permeability is likely due to a physiological feedback mechanism that moderates paracellular interstitium-to-lumen flux of osmolytes in response to luminal hypotonicity43, 44, 45. Luminal hypotonicity induces release of serotonin by the enterochromaffin cells in the intestinal mucosa and activates cholinergic efferent neurons involved in regulating jejunal mucosal permeability and secretion. Support for this view is the previous finding that luminal serotonin receptor antagonists, and nicotinic acetylcholine receptor antagonists, significantly reduce or abolish the hypotonicity-induced increase in duodenal mucosal paracellular permeability45, 46, 47.
The hypotonicity-induced increase in mucosal permeability and osmolarity-adjusting capability may be enhanced by lidocaine, an amide-type of local anaesthetic. Adding lidocaine to the luminal hypotonic perfusate increased the permeability from six-fold to 15-fold in the present study, in line with similar increases shown previously21. The mechanism for the potentiating effect of lidocaine is presently not known. However, it is most likely not associated with blockade of voltage-gated Na+ channels, as a selective blocker of this channel (tetrodoxin) inhibits the effect of hypotonicity46. It is also known that the local anaesthetic effects of lidocaine are rapid and of medium duration when administered either as a parenteral or topical formulation48. Luminal hypotonicity, with or without lidocaine, resulted in a sharp increase in CLCr-EDTA followed by a recovery of about 50% during the intestinal hypotonicity exposure. Further, unlike the two paracellular permeation enhancers, the increase in CLCr-EDTA induced by hypotonicity, with or without lidocaine, did not increase permeability of enalaprilat and hexarelin. This is in stark contrast to previous rat SPIP studies investigating paracellular and transcellular PEs. In those studies, there was a linear correlation between an increase in CLCr-EDTA and model drug permeability18,29. A possible explanation for this is that luminal hypotonicity affects the physiological regulation of paracellular permeability primarily in the mucosal crypts; these crypts are largely unavailable to luminal contents and drugs, as there is a constant outward water flow to protect stem cells in this mucosal region49. This would explain why hypotonicity increase blood-to-lumen CLCr-EDTA—but not lumen-to-blood drug permeability—whereas chitosan and EDTA affect paracellular permeability in the whole epithelium, and consequently transport of solutes in both directions.
5. Conclusions
In conclusion, this rat SPIP study showed that two permeation enhancers with mainly paracellular mechanism-of-action increased the mucosal permeability of two peptide drugs, enalaprilat and hexarelin. This is in contrast to a previous SPIP study in which transcellular permeation enhancers only increased the small intestinal permeability of enalaprilat. This suggests that paracellular enhancers may be more effective for oral delivery of smaller peptides when used in conjunction with novel, absorption-enhancing drug delivery systems. Further, mucosal blood-to-lumen transport of 51Cr-EDTA increased with the two paracellular permeation enhancers and with luminal hypotonicity. However, luminal hypotonicity did not affect the lumen-to-blood transport of enalaprilat and hexarelin. This suggests that hypotonicity affects paracellular solute transport only in the mucosal crypts, a mucosal area not accessible to luminal contents due to a constant, outward flow of water.
Author contributions
David Dahlgren, Markus Sjöblom, Hans Lennernäs designed the research. David Dahlgren, Tobias Olander, Markus Sjöblom, Mikael Hedeland, Hans Lennernäs carried out the experiments and performed data analysis. David Dahlgren, Tobias Olander, Markus Sjöblom, Mikael Hedeland, Hans Lennernäs participated part of the experiments. Mikael Hedeland provided quality control. David Dahlgren and Hans Lennernäs wrote the manuscript. David Dahlgren, Tobias Olander, Markus Sjöblom, Mikael Hedeland, Hans Lennernäs revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Dahlgren D., Roos C., Johansson P., Lundqvist A., Tannergren C., Abrahamsson B. Regional intestinal permeability in dogs: biopharmaceutical aspects for development of oral modified-release dosage forms. Mol Pharm. 2016;13:3022–3033. doi: 10.1021/acs.molpharmaceut.6b00515. [DOI] [PubMed] [Google Scholar]
- 2.Zhong H., Chan G., Hu Y., Hu H., Ouyang D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics. 2018;10:263. doi: 10.3390/pharmaceutics10040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krondahl E., Orzechowski A., Ekström G., Lennernäs H. Rat jejunal permeability and metabolism of μ-selective tetrapeptides in gastrointestinal fluids from humans and rats. Pharm Res. 1997;14:1780–1785. doi: 10.1023/a:1012144232666. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Yadav V., Smart A.L., Tajiri S., Basit A.W. Toward oral delivery of biopharmaceuticals: an assessment of the gastrointestinal stability of 17 peptide drugs. Mol Pharm. 2015;12:966–973. doi: 10.1021/mp500809f. [DOI] [PubMed] [Google Scholar]
- 5.Taki Y., Sakane T., Nadai T., Sezaki H., Amidon G.L., Langguth P. Gastrointestinal absorption of peptide drug: quantitative evaluation of the degradation and the permeation of metkephamid in rat small intestine. J Pharmacol Exp Therapeut. 1995;274:373–377. [PubMed] [Google Scholar]
- 6.Lau J.L., Dunn M.K. Therapeutic peptides: historical perspectives, current development trends, and future directions. Bioorg Med Chem. 2018;26:2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 7.Muheem A., Shakeel F., Jahangir M.A., Anwar M., Mallick N., Jain G.K. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharmaceut J. 2016;24:413–428. doi: 10.1016/j.jsps.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee A.C.-L., Harris J.L., Khanna K.K., Hong J.H. A comprehensive review on current advances in peptide drug development and design. Int J Mol Sci. 2019;20:2383. doi: 10.3390/ijms20102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewitt W.M., Leung S.S., Pye C.R., Ponkey A.R., Bednarek M., Jacobson M.P. Cell-permeable cyclic peptides from synthetic libraries inspired by natural products. J Am Chem Soc. 2015;137:715–721. doi: 10.1021/ja508766b. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher-Klinger A., Fanous J., Merzbach S., Weinmüller M., Reichart F., Räder A.F. Enhancing oral bioavailability of cyclic RGD hexa-peptides by the lipophilic prodrug charge masking approach: redirection of peptide intestinal permeability from a paracellular to transcellular pathway. Mol Pharm. 2018;15:3468–3477. doi: 10.1021/acs.molpharmaceut.8b00466. [DOI] [PubMed] [Google Scholar]
- 11.Brayden D.J., Hill T., Fairlie D., Maher S., Mrsny R. Systemic delivery of peptides by the oral route: formulation and medicinal chemistry approaches. Adv Drug Deliv Rev. 2020;157:2–36. doi: 10.1016/j.addr.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Drucker D.J. Advances in oral peptide therapeutics. Nat Rev Drug Discov. 2019:1–13. doi: 10.1038/s41573-019-0053-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang J., Yadav V., Smart A.L., Tajiri S., Basit A.W. Stability of peptide drugs in the colon. Eur J Pharmaceut Sci. 2015;78:31–36. doi: 10.1016/j.ejps.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Dahlgren D., Sjöblom M., Lennernäs H. Intestinal absorption-modifying excipients: a current update on preclinical in vivo evaluations. Eur J Pharm Biopharm. 2019;142:411–420. doi: 10.1016/j.ejpb.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Park K. Rational design of agents to transiently increase paracellular permeability. J Control Release. 2015;210:246. doi: 10.1016/j.jconrel.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Buckley S.T., Bækdal T.A., Vegge A., Maarbjerg S.J., Pyke C., Ahnfelt-Rønne J. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10:eaar7047. doi: 10.1126/scitranslmed.aar7047. [DOI] [PubMed] [Google Scholar]
- 17.Dahlgren D., Sjöblom M., Hedeland M., Lennernäs H. The in vivo effect of transcellular permeation enhancers on the intestinal permeability of two peptide drugs enalaprilat and hexarelin. Pharmaceutics. 2020;12:99. doi: 10.3390/pharmaceutics12020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahlgren D., Roos C., Lundqvist A., Langguth P., Tannergren C., Sjöblom M. Preclinical effect of absorption modifying excipients on rat intestinal transport of five model compounds and the intestinal barrier marker 51Cr-EDTA. Mol Pharm. 2017;14:4243–4251. doi: 10.1021/acs.molpharmaceut.7b00353. [DOI] [PubMed] [Google Scholar]
- 19.Dahlgren D., Roos C., Johansson P., Tannergren C., Lundqvist A., Langguth P. The effects of three absorption-modifying critical excipients on the in vivo intestinal absorption of six model compounds in rats and dogs. Int J Pharm. 2018;547:158–168. doi: 10.1016/j.ijpharm.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Dahlgren D., Cano-Cebrián M.-J., Olander T., Hedeland M., Sjöblom M., Lennernas H. Regional intestinal drug permeability and effects of permeation enhancers in rat. Pharmaceutics. 2020;12:242. doi: 10.3390/pharmaceutics12030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nylander O., Sjöblom M. Modulation of mucosal permeability by vasoactive intestinal peptide or lidocaine affects the adjustment of luminal hypotonicity in rat duodenum. Acta Physiol. 2007;189:325–335. doi: 10.1111/j.1748-1716.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 22.Winiwarter S., Bonham N.M., Ax F., Hallberg A., Lennernäs H., Karlén A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J Med Chem. 1998;41:4939–4949. doi: 10.1021/jm9810102. [DOI] [PubMed] [Google Scholar]
- 23.Fagerholm U., Sjöström B., Sroka-Markovic J., Wijk A., Svensson M., Lennernäs H. The effect of a drug-delivery system consisting of soybean phosphatidyl choline and medium-chain monoacylglycerol on the intestinal permeability of hexarelin in the rat. J Pharm Pharmacol. 1998;50:467–473. doi: 10.1111/j.2042-7158.1998.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 24.Westberg C., Benkestock K., Fatouros A., Svensson M., Sjöström B. Hexarelin-evaluation of factors influencing oral bioavailability and ways to improve absorption. J Pharm Pharmacol. 2001;53:1257–1264. doi: 10.1211/0022357011776540. [DOI] [PubMed] [Google Scholar]
- 25.Dahlgren D., Roos C., Peters K., Lundqvist A., Tannergren C., Sjögren E. Evaluation of drug permeability calculation based on luminal disappearance and plasma appearance in the rat single-pass intestinal perfusion model. Eur J Pharm Biopharm. 2019;142:31–37. doi: 10.1016/j.ejpb.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Dahlgren D., Roos C., Lundqvist A., Tannergren C., Sjöblom M., Sjögren E. Time-dependent effects on small intestinal transport by absorption-modifying excipients. Eur J Pharm Biopharm. 2018;132:19–28. doi: 10.1016/j.ejpb.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Nylander O., Kvietys P., Granger D.N. Effects of hydrochloric acid on duodenal and jejunal mucosal permeability in the rat. Am J Physiol Gastrointest Liver Physiol. 1989;257:G653–G660. doi: 10.1152/ajpgi.1989.257.4.G653. [DOI] [PubMed] [Google Scholar]
- 28.Sjögren E., Dahlgren D., Roos C., Lennernas H. Human in vivo regional intestinal permeability: quantitation using site-specific drug absorption data. Mol Pharm. 2015;12:2026–2039. doi: 10.1021/mp500834v. [DOI] [PubMed] [Google Scholar]
- 29.Dahlgren D., Roos C., Lundqvist A., Tannergren C., Sjöblom M., Sjögren E. Effect of absorption-modifying excipients, hypotonicity, and enteric neural activity in an in vivo model for small intestinal transport. Int J Pharm. 2018;549:239–248. doi: 10.1016/j.ijpharm.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 30.Lozoya-Agullo I., Gonzalez-Alvarez I., Zur M., Fine-Shamir N., Cohen Y., Markovic M. Closed-loop doluisio (colon, small intestine) and single-pass intestinal perfusion (colon, jejunum) in rat—biophysical model and predictions based on Caco-2. Pharm Res. 2018;35:2. doi: 10.1007/s11095-017-2331-z. [DOI] [PubMed] [Google Scholar]
- 31.Yang G., Wu F., Chen M., Jin J., Wang R., Yuan Y. Formulation design, characterization, and in vitro and in vivo evaluation of nanostructured lipid carriers containing a bile salt for oral delivery of gypenosides. Int J Nanomed. 2019;14:2267. doi: 10.2147/IJN.S194934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roos C., Dahlgren D., Sjögren E., Sjöblom M., Hedeland M., Lennernäs H. Effects of absorption-modifying excipients on jejunal drug absorption in simulated fasted and fed luminal conditions. Eur J Pharm Biopharm. 2019;142:387–395. doi: 10.1016/j.ejpb.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Tyagi P., Pechenov S., Subramony J.A. Oral peptide delivery: translational challenges due to physiological effects. J Control Release. 2018;287:167–176. doi: 10.1016/j.jconrel.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Elliott W., Chan J. Semaglutide tablets (Rybelsus) Intern Med Alert. 2019:41. [Google Scholar]
- 35.Fjellestad-Paulsen A., Höglund P., Lundin S., Paulsen O. Pharmacokinetics of 1-deamino-8-D-arginine vasopressin after various routes of administration in healthy volunteers. Clin Endocrinol (Oxf) 1993;38:177–182. doi: 10.1111/j.1365-2265.1993.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 36.Juul K.V., Bichet D.G., Nørgaard J.P. Desmopressin duration of antidiuretic action in patients with central diabetes insipidus. Endocrine. 2011;40:67–74. doi: 10.1007/s12020-011-9492-z. [DOI] [PubMed] [Google Scholar]
- 37.Marbury T.C., Flint A., Jacobsen J.B., Karsbøl J.D., Lasseter K. Pharmacokinetics and tolerability of a single dose of semaglutide, a human glucagon-like peptide-1 analog, in subjects with and without renal impairment. Clin Pharmacokinet. 2017;56:1381–1390. doi: 10.1007/s40262-017-0528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abramson A., Halperin F., Kim J., Traverso G. Quantifying the value of orally delivered biologic therapies: a cost-effectiveness analysis of oral semaglutide. J Pharmacol Sci. 2019;108:3138–3145. doi: 10.1016/j.xphs.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maher S., Mrsny R.J., Brayden D.J. Intestinal permeation enhancers for oral peptide delivery. Adv Drug Deliv Rev. 2016;106:277–319. doi: 10.1016/j.addr.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Garcia M.A., Nelson W.J., Chavez N. Cell–cell junctions organize structural and signaling networks. Cold Spr Harb Perspect Biol. 2018;10:a029181. doi: 10.1101/cshperspect.a029181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awoonor-Williams E., Rowley C.N. Molecular simulation of nonfacilitated membrane permeation. Biochim Biophys Acta Biomembr. 2016;1858:1672–1687. doi: 10.1016/j.bbamem.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Schipper N.G., Olsson S., Hoogstraate J.A., Vårum K.M., Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs 2: mechanism of absorption enhancement. Pharm Res. 1997;14:923–929. doi: 10.1023/a:1012160102740. [DOI] [PubMed] [Google Scholar]
- 43.Pihl L., Wilander E., Nylander O. Comparative study of the effect of luminal hypotonicity on mucosal permeability in rat upper gastrointestinal tract. Acta Physiol. 2008;193:67–78. doi: 10.1111/j.1748-1716.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- 44.Lambert G., Chang R.-T., Xia T., Summers R., Gisolfi C. Absorption from different intestinal segments during exercise. J Appl Physiol. 1997;83:204–212. doi: 10.1152/jappl.1997.83.1.204. [DOI] [PubMed] [Google Scholar]
- 45.Nylander O., Pihl L., Perry M. Hypotonicity-induced increases in duodenal mucosal permeability facilitates adjustment of luminal osmolality. Am J Physiol Gastrointest Liver Physiol. 2003;285:G360–G370. doi: 10.1152/ajpgi.00428.2002. [DOI] [PubMed] [Google Scholar]
- 46.Nylander O., Pihl L. Luminal hypotonicity increases duodenal mucosal permeability by a mechanism involving 5-hydroxytryptamine. Acta Physiol. 2006;186:45–58. doi: 10.1111/j.1748-1716.2005.01507.x. [DOI] [PubMed] [Google Scholar]
- 47.Sedin J., Sjöblom M., O Nylander The selective cyclooxygenase-2 inhibitor parecoxib markedly improves the ability of the duodenum to regulate luminal hypertonicity in anaesthetized rats. Acta Physiol. 2012;205:433–451. doi: 10.1111/j.1748-1716.2012.02411.x. [DOI] [PubMed] [Google Scholar]
- 48.Conklin K.A. Pharmacology of local anesthetics. AANA J (Am Assoc Nurse Anesth) 1987;55:36. [PubMed] [Google Scholar]
- 49.Fihn B.M., Sjöqvist A., Jodal M. Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterology. 2000;119:1029–1036. doi: 10.1053/gast.2000.18148. [DOI] [PubMed] [Google Scholar]