Abstract
Organic acids (OA) and their blends have been shown to positively affect performance and health of broilers. However, the data in the literature are not consistent. This study examined the potential of blended short-chain fatty acids (SCFA) with medium-chain fatty acids (MCFA) as alternatives to antibiotic growth promoters (AGP) on performance, health and welfare of broilers infected with necrotic enteritis (NE). The additives used were: A) a blend of SCFA, MCFA, and a phenolic compound (SMP); B) a blend of free and buffered SCFA with MCFA (SMF); C) a blend of free and buffered SCFA with a high concentration of MCFA (SHM). A total of 1,404 Ross 308 one-day-old male parental chicks were randomly distributed into 78-floor pens with 13 replicates of 18 birds each. Six treatments were the following: T1, unchallenged control (UCC); T2, challenged control (CHC); T3, challenged group plus zinc bacitracin (BAC); T4, challenged group plus additive SMP; T5, challenged group plus additive SMF; T6, challenged group plus additive SHM. Challenged birds were gavaged with Eimeria spp. on d 9 and Clostridium perfringens EHE-NE18 on d 14. Post NE challenge and cumulatively, BWG, FCR, and nutrient digestibility of birds were compromised (P < 0.05) by NE challenge indicating a successful induction of sub-clinical NE. Additive SHM had higher BWG compared to CHC and BAC groups (P = 0.001; d 10 to 24) but not different from SMP and SMF groups (P > 0.05). All the 3 additive groups had lower FCR compared to CHC (P = 0.001; d 0 to 35), and exhibited similar jejunal lesions (d 16) compared to BAC and apparent ileal protein digestibility (d 21) compared to UCC and BAC groups (P > 0.05). Birds in additive SHM group had a higher concentration of serum IgA compared to all groups (P = 0.001) except additive SMF (P > 0.05; d 21). All the additive groups had lower footpad dermatitis and hock burns compared to CHC (P < 0.05). The findings suggest the potential of blended OA as alternatives to BAC to protect broilers from NE indicated by improved FCR, immunity, digestibility, and bird welfare.
Keywords: Performance, Blended organic acid, Alternative to antibiotics, Necrotic enteritis, Broiler chicken
1. Introduction
In poultry, enteric diseases can reduce feed efficiency and enhance mortality, both of which dramatically increase the costs of production (Porter, 1998). Necrotic enteritis (NE), primarily caused by Clostridium perfringens, is one of the most severe and economically important enteric diseases in the modern poultry industry (Cooper et al., 2013), burdening a cost of US$6 billion to the global poultry industry each year (Wade and Keyburn, 2015). The signs of subclinical NE include a reduction in performance and feed efficiency, depression in feed ingestion and digestibility, diarrhoea, intestinal lesions, wet litter, disturbances of welfare, and a high mortality rate in case of the clinical form (Kaldhusdal et al., 2001; Immerseel et al., 2004). The subclinical form of NE is however more prevalent and of greater economic loss, as it reduces market weight by 12.9% and increases FCR by 10.9% without high mortality or obvious disease symptoms to be detected and treated (Skinner et al., 2010).
Antibiotic growth promoters (AGP) have long been used in poultry feed to control enteric diseases including NE (Van Immerseel et al., 2009; Moore, 2016). However, due to public health concern over the application of in-feed AGP and the development of AGP-resistant pathogens many countries have banned the application of in-feed AGP (Kocher and Choct, 2008) such as in the European Union (Castanon, 2007). The phasing-out and/or ban of in-feed AGP in the broiler industry have led to the emergence of enteric disorders, imbalance of gut microflora, and nutrient digestibility (Dibner and Richards, 2005). Thus, the performance and health of broilers are affected and the profitability in the industry is compromised. Moreover, it is critical to introduce an effective measure to minimise the occurrence of intestinal diseases in a post AGP era. As a result, the poultry industry has been actively seeking alternative means and strategies to improve gut health by controlling pathogenic organisms and enhancing immune responses. Among these alternatives, organic acids, probiotics, prebiotics, essential oils, phytogenic and herbal products have shown some promising effects (M'Sadeq et al., 2015; Khan and Iqbal, 2016; Adhikari et al., 2020) and thus received increased attention.
Organic acids (OA) have been used in the feed industry for decades to preserve feed and as alternatives to growth promoters to protect birds from diseases and improve performance. Literatures show that OA can improve feed efficiency and growth performance and have the potential to replace in-feed AGP (Adil et al., 2010; Polycarpo et al., 2017). However, several researchers did not observe significant performance improvement of broilers (Ao et al., 2009; Cengiz et al., 2012). The inconsistent results are reported in different studies due to the heterogeneity of experimental conditions, types of OA, the way of application such as a single or blended OA, diet composition, bird health, and disease status. Among the OA, short-chain fatty acids (SCFA) including propionic, formic and butyric acids (Khan and Iqbal, 2016), and medium-chain fatty acids (MCFA) including caproic, caprylic, capric and lauric acids derived from palm kernel or coconut oil have been reported to effectively improve intestinal health of broilers (Zentek et al., 2012; Onrust et al., 2018). Studies have indicated that the blend of OA containing MCFA positively affects intestinal health in piglets (Zentek et al., 2013; Kuang et al., 2015), broilers (Mathis et al., 2005; Nguyen et al., 2018; Sun et al., 2020), and laying hens (Lee et al., 2015). There is evidence suggesting that the beneficial effects of blended OA are higher when compared to single OA due to their synergistic effects on broiler intestinal health, performance, nutrients digestion, and absorption (Huang et al., 2015). However, the growth performance results from different studies supplemented with a blend of SCFA and MCFA are variable and the research concerning possible impact on immunity parameters and bird welfare under NE challenge conditions, are sparse.
It was hypothesised that blended OA that incorporate several ingredients in different combinations of SCFA, MCFA, and phenolic compounds may maintain a healthy gut environment for the optimal digestion and absorption of nutrients thereby enhancing performance under disease challenge. The current study was designed to investigate the effects of blended SCFA in combination with MCFA on performance, intestinal lesions, nutrient digestibility, immune responses, footpad health, and welfare of broilers subjected to sub-clinical necrotic enteritis challenge. The potential of blended feed additives to alleviate the impact of necrotic enteritis was compared against zinc bacitracin as an antibiotic growth promoter agent.
2. Materials and methods
2.1. Animal ethics
All the experimental procedures applied in this study were reviewed and approved by the Animal Ethics Committee of the University of New England, Armidale 2351, Australia (AEC18-057). All procedures involving live birds handling, management, and health care followed the regulations of laboratory animals used for scientific purposes and were implemented within the Code of Practice assigned by the Australian Bureau of Animal Health (NHMRC, 2013).
2.2. Blended feed additives
The current study was designed to evaluate 3 different commercial blended additives supplied by Trouw Nutrition (a Nutreco company, Selko B.V., Amersfoort, the Netherlands) in sub-clinically NE infected birds. The additives used and main ingredients were the following: A) a blend of SCFA, MCFA, slow-release lauric acid, target release butyrate, and a phenolic compound (SMP) with main ingredients: sodium and calcium salt of butyric acid, sorbic acid, acacia, pure distilled coconut/palm fatty acid, maltodextrin, starch from corn, vegetable oil from soy, vegetable fat from palm, a mixture of flavoring compounds, silicic acid, and sepiolite; B) a blend of free and buffered SCFA combined with MCFA (SMF) with main ingredients: formic acid, propionic acid, acetic acid, ammonium formate, citric acid, lactic acid, sorbic acid, silicic acid, and coconut/palm kernel fatty acid; C) a blend of free and buffered SCFA with a high concentration (3 ×) of MCFA (SHM) with main ingredients: formic acid, acetic acid, propionic acid, ammonium formate, sorbic acid, silicic acid, and pure distilled coconut/palm fatty acid.
2.3. Animals and husbandry
A total of 1,404 one-day-old Ross 308 male parental chicks were obtained from Aviagen hatchery, Goulburn NSW, Australia. Chicks were weighed upon arrival and placed into their respective pens to have a similar pen body weight (±20 g/pen) across treatments based on a completely randomised design. Each of the 6 treatments had 13 replicate pens of 18 birds starting and 12 birds remaining at the end of the study. Birds were housed in floor pens (0.725 m × 1.175 m = 0.85 m2) with softwood shavings as litter to a depth of 8 cm. The house was environmentally controlled, and feed and water were provided ad libitum. Each pen was equipped with a tube feeder and 3 nipple drinkers. Overall, house conditions such as temperature, lighting, and moisture were controlled and maintained following Ross 308 guidelines (Aviagen, 2014).
2.4. Dietary treatments
The treatments in a completely randomised design comprised of 1 unchallenged group as control and 5 challenged groups to examine the additive effects as shown in Table 1. The treatments were T1,unchallenged control (UCC) without additives or in-feed zinc bacitracin (BAC); T2, challenged group (CHC), without additives or in-feed BAC; T3, challenged group plus in-feed BAC at 0.05 g/kg (50 part per million); T4, challenged group plus additive SMP at the levels of 1.5, 1.5, 0.5 g/kg feed in starter, grower and finisher phases, respectively; T5, challenged group plus additive SMF at the levels of 2.5, 2.0, 1.0 g/kg feed in starter, grower and finisher phases, respectively; T6, challenged group plus additive SHM at the levels of 2.0, 1.5, 1.0 g/kg feed in starter, grower and finisher phases, respectively. Diets were based on wheat and soybean meal and were supplemented with xylanase and phytase to meet the nutrient requirements of Ross 308 birds as shown in Table 2. Diets were cold pelleted and starter feed was further crumbled to maximise the feed intake. Diets were fed in 3 different phases: starter (d 0 to 10), grower (d 10 to 24), and finisher (d 24 to 35).
Table 1.
Treatments applied in the study.
| Treatments1 | Inclusion level instarter phase (d 0 to 10), g/kg |
Inclusion level in grower phase (d 10 to 24), g/kg |
Inclusion level in finisher phase (d 24 to 35), g/kg |
NE challenge2 |
|---|---|---|---|---|
| UCC | – | – | – | Non-challenged |
| CHC | – | – | – | Challenged |
| BAC | 0.05 | 0.05 | 0.05 | Challenged |
| SMP | 1.5 | 1.5 | 0.5 | Challenged |
| SMF | 2.5 | 2.0 | 1.0 | Challenged |
| SHM | 2.0 | 1.5 | 1.0 | Challenged |
UCC, unchallenged control; CHC, challenged control; BAC, zinc bacitracin; SMP, a blend of short-chain fatty acids (SCFA), medium-chain fatty acids (MCFA), and phenolic compound; SMF, a blend of buffered SCFA with MCFA; SHM, a blend of buffered SCFA with a high concentration of MCFA.
Necrotic enteritis (NE) challenged birds were gavaged with Eimeria spp. on d 9 and Clostridium perfringens on d 14.
Table 2.
Experimental diet composition and nutrients (as-fed basis, %).
| Item | Starter phase (d 0 to 10) |
Grower phase (d 10 to 24) |
Finisher phase (d 24 to 35) |
|---|---|---|---|
| Ingredients | |||
| Wheat | 62.2 | 65.1 | 67.9 |
| Soybean meal | 32.3 | 28.1 | 25.5 |
| Canola oil | 2.1 | 3.2 | 3.8 |
| Limestone | 1.1 | 1.1 | 1.0 |
| Dicalcium phosphate 18P/21Ca | 0.87 | 0.78 | 0.66 |
| Salt | 0.12 | 0.14 | 0.14 |
| Sodium bicarbonate | 0.15 | 0.13 | 0.13 |
| Vitamin premix1 | 0.09 | 0.09 | 0.09 |
| Mineral premix2 | 0.10 | 0.10 | 0.10 |
| Choline chloride 60% | 0.06 | 0.05 | 0.04 |
| l-lysine HCl | 0.31 | 0.24 | 0.22 |
| d, l-methionine | 0.28 | 0.21 | 0.19 |
| l-threonine | 0.10 | 0.06 | 0.05 |
| Phytase | 0.01 | 0.01 | 0.01 |
| Xylanase | 0.02 | 0.02 | 0.02 |
| Titanium di-oxide (TiO2) | – | 0.50 | – |
| Calculated nutrients3 | |||
| AME, kcal/kg | 3,025 | 3,120 | 3,200 |
| Crude protein | 23.5 | 21.7 | 20.8 |
| Crude fat | 3.60 | 4.73 | 5.36 |
| Crude fiber | 2.40 | 2.29 | 2.23 |
| Digestible Arg | 1.35 | 1.23 | 1.16 |
| Digestible Lys | 1.29 | 1.13 | 1.05 |
| Digestible Met | 0.59 | 0.5 | 0.46 |
| Digestible Met + Cys | 0.94 | 0.83 | 0.79 |
| Digestible Trp | 0.28 | 0.26 | 0.25 |
| Digestible Ile | 0.88 | 0.81 | 0.77 |
| Digestible Thr | 0.82 | 0.72 | 0.68 |
| Digestible Val | 0.95 | 0.88 | 0.84 |
| Calcium | 0.90 | 0.85 | 0.80 |
| Phosphorus available | 0.45 | 0.43 | 0.40 |
| Phosphorus total | 0.52 | 0.49 | 0.46 |
| Sodium | 0.16 | 0.16 | 0.16 |
| Chloride | 0.19 | 0.18 | 0.18 |
| Linoleic 18:2 | 1.28 | 1.56 | 1.73 |
| Choline, mg/kg | 1,700 | 1,600 | 1,500 |
| Analysed nutrients | |||
| Dry matter | 89.2 | 89.3 | 89.5 |
| Gross energy, kcal/kg | 3,915 | 4,004 | 4,108 |
| Crude protein | 23.6 | 21.9 | 20.9 |
AME = apparent metabolisable energy.
Vitamin premix provided the following per kilogram diet: vitamin A, 12 MIU; vitamin D, 5 MIU; cyanocobalamin, 0.016 mg; vitamin E, 75 mg; vitamin K, 3 mg; folic acid, 2 mg; riboflavin, 8 mg; nicotinic acid, 55 mg; pantothenic acid, 13 mg; pyridoxine, 5 mg; biotin, 0.25 mg; thiamine, 3 mg; and antioxidant ethoxyquin, 50 mg.
Mineral premix provided the following per kilogram diet: Cu (sulfate), 16 mg; Mn (sulfate), 60 mg; Mn (oxide), 60 mg; I (iodide), 0.125 mg; Se (selenite), 0.3 mg; Fe (sulfate), 40 mg; Zn (oxide and sulfate), 100 mg.
Ingredients were analysed using near-infrared spectroscopy (NIRS, Evonik AminoProx, Germany).
2.5. Necrotic enteritis challenge
Previously reported NE challenge model (Wu et al., 2014; Rodgers et al., 2015) was performed in this study where Eimeria spp. were used as a predisposing factor and C. perfringens as a primary causative agent to induce NE. In brief, challenged birds were given 1 mL per os field strains of Eimeria spp. oocysts consisting of E. acervulina (5,000), E. maxima (5,000), and E. brunetti (2,500) on d 9 (Eimeria Pty Ltd., Werribee, VIC, Australia). On d 14, challenged birds were gavaged with 1 mL per os approximately 1 × 108 CFU/mL of C. perfringens EHE-NE18 strain (CSIRO Livestock, Geelong, VIC, Australia). Simultaneously, unchallenged birds were gavaged with 1 mL per os phosphate-buffered saline on d 9 and sterile medium on d 14.
2.6. Performance measurement and sampling
Pen weights were measured on d 0, 10, 24, and 35. Feed refusal was weighed on d 10, 24, and 35. Mortalities were recorded daily and totalled for each period. Necropsies were performed to determine the cause of bird death. On d 16 and 21 of the study, 2 birds per pen were randomly chosen, weighed and stunned by an electric stunner (JF poultry equipment, Weltevreden Park, South Africa) and decapitated for blood collection. Blood serum and ileal digesta samples were taken and preserved appropriately for the lab analysis.
2.7. Intestinal lesion scores
Two birds/pen were randomly selected from each pen and euthanised to perform intestinal lesion scoring of duodenum, jejunum, and ileum on d 16, based on a previously reported lesion scoring system that ranges from 0 to 6 (Keyburn et al., 2006; Shojadoost et al., 2012).
2.8. Diet and digesta analysis
The diet samples and ileal digesta samples collected on d 21 were freeze-dried, ground to pass through a 0.5-mm sieve and then analysed for nitrogen (N) using a combustion analyser (LECO Corp., St. Joseph, MI). Gross energy (GE) contents of diets and ileal digesta samples were measured in duplicates by using an adiabatic bomb calorimeter (IKA, Werke C7000, GMBH, and Co., Staufen, Germany) with benzoic acid as a calibration standard. Titanium dioxide (TiO2) was determined in diets and digesta samples in duplicate following Short et al. (1996) by the colorimetric method.
Apparent ileal digestibility (AID) of CP and GE was calculated using the following equation, where TiO2 was used as an indigestible marker:
2.9. ELISA analyses
The total antibody titre concentrations of immunoglobulin A (IgA) in birds serum samples collected on d 16 and 21 were determined using ELISA assays. Blood samples were taken by the decapitation of broilers after being stunned. Blood was kept at room temperature for 3 h to allow clotting, and centrifuged (3,000 × g for 10 min) to separate serum. All serum samples were immediately frozen at −20 °C until ELISA assays were performed. Serum IgA concentrations were measured using chicken-specific ELISA reagents according to the manufacturer's instructions (Abnova chicken ELISA assays, Taiwan). The concentrations of antibodies were calculated from the standard curve using standard serum samples that were included on each plate.
2.10. Litter quality, footpad dermatitis and hock burn scores
On d 35, litter structure (quality) per pen was scored by visual inspection using a scale ranging from 0 to 3 following a standard scoring method as described by Kheravii et al. (2017). The 4-point scales were instructed as follows: 0 = dry litter; 1 = slightly caked/moist litter; 2 = more caked/moist litter; 3 = wet litter. Visual observation was done in 4 different points of each pen and the average was taken to calculate the litter scores per pen. On d 35, footpad dermatitis (FPD) and hock burn (HB) scores were evaluated in all birds from each pen by visually inspecting and scoring following the methods described by Allain et al. (2009). The 10-point scale scoring was done based on the severity of lesions in footpad and hock where 0 indicated healthy birds without lesions and 9 appeared as most severe and macroscopic lesions. The scoring was performed by 2 experienced personnel who were not aware of the study design and pen arrangements.
2.11. Data analysis
All the data derived in this study were checked for normal distribution prior to performing statistical analysis. Data with normal distribution were subjected to one-way ANOVA analysis as a completely randomized design, using General Linear Model procedure of SAS 9.3 package (Guide, 2010). Pen was the study unit and the values presented in the tables are means with a pooled standard error of the mean (SEM) (n = 78). When a significant effect of treatment was detected, the differences between means were separated by least significant difference test. Performance data were analysed for the treatment effect and corrected with mortality. The intestinal lesion scores data were analysed by the nonparametric Kruskal–Wallis test as the data were not normally distributed. Significant values were declared if P < 0.05, and 0.05 < P < 0.10 was considered a tendency.
3. Results
3.1. Performance
The effects of blended additives on growth performance are presented in Table 3. In the starter phase (d 0 to 10), birds treated with all the 3 additives had higher BWG compared to CHC group (P = 0.016) but were not different from the UCC group (P > 0.05). Birds fed additives SMP and SMF had higher BWG compared to BAC group (P = 0.016). Birds fed additive SHM had lower FCR compared to UCC, CHC and BAC groups (P = 0.046) but were not different from additives SMP and SMF groups (P > 0.05). Feed intake and livability were not different among treatment groups (P > 0.05).
Table 3.
Effects of additives and NE challenge on growth performance of broilers at different phases.1
| Item | UCC | NE challenged2 |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| CHC | BAC | SMP | SMF | SHM | ||||
| Starter phase (d 0 to 10) | ||||||||
| BWG, g | 240abc | 234c | 237bc | 246a | 246a | 243ab | 2.9 | 0.016 |
| FI, g | 293 | 284 | 291 | 294 | 298 | 287 | 4.0 | 0.154 |
| FCR | 1.216ab | 1.214ab | 1.229a | 1.194bc | 1.193bc | 1.181c | 0.011 | 0.046 |
| Livability, % | 100 | 100 | 100 | 100 | 100 | 100 | 0.0 | 1.000 |
| Grower phase (d 10 to 24) | ||||||||
| BWG, g | 1,113a | 798cd | 772d | 820bc | 812bc | 843b | 11.4 | 0.001 |
| FI, g | 1,439a | 1,167cd | 1,139d | 1,219b | 1,187bc | 1,217b | 13.5 | 0.001 |
| FCR | 1.292c | 1.463ab | 1.475a | 1.489a | 1.462ab | 1.443b | 0.010 | 0.001 |
| Livability, % | 99.1 | 99.1 | 97.9 | 98.7 | 100 | 99.1 | 0.641 | 0.325 |
| Finisher phase (d 24 to 35) | ||||||||
| BWG, g | 1,280 | 1,278 | 1,290 | 1,275 | 1,272 | 1,267 | 18.2 | 0.966 |
| FI, g | 2,033a | 2,024ab | 1,925c | 1,954bc | 1,925c | 1,946c | 26.5 | 0.009 |
| FCR | 1.590a | 1.580a | 1.493c | 1.532b | 1.513bc | 1.536b | 0.010 | 0.001 |
| Livability, % | 97.4 | 98.1 | 98.7 | 100 | 98.1 | 98.7 | 0.97 | 0.546 |
| Overall period (d 0 to 35) | ||||||||
| BWG, g | 2,633a | 2,310b | 2,300b | 2,342b | 2,331b | 2,353b | 23.5 | 0.001 |
| FI, g | 3,764a | 3,476b | 3,356c | 3,469b | 3,407bc | 3,451b | 32.3 | 0.001 |
| FCR | 1.430d | 1.504a | 1.459c | 1.481b | 1.461c | 1.466bc | 0.006 | 0.001 |
| Livability, % | 97.4 | 97.8 | 97.0 | 98.7 | 98.7 | 98.3 | 0.91 | 0.705 |
NE = necrotic enteritis; BWG = body weight gain; FI = feed intake; FCR = feed conversion ratio.
a – d Values in a row with no common superscripts differ significantly (P < 0.05). Mean values are based on 18 birds per replicate and 13 replicates per treatment.
UCC, unchallenged control; CHC, challenged control; BAC, zinc bacitracin; SMP, a blend of short-chain fatty acids (SCFA), medium-chain fatty acids (MCFA) and phenolic compound; SMF, a blend of buffered SCFA with MCFA; SHM, a blend of buffered SCFA with a high concentration of MCFA.
NE challenged birds were gavaged with Eimeria spp. on d 9 and C. perfringens on d 14.
In the grower phase (d 10 to 24), the NE challenge reduced BWG and FI, and increased FCR (P = 0.001) compared to the UCC group. Birds fed additives (SMP, SMF, and SHM) had higher BWG and FI compared to the BAC group but lower BWG than the UCC group (P = 0.001). Improved FCR and higher BWG were observed in the additive SHM group compared to CHC and BAC groups (P = 0.001) but not different from additives SMP and SMF groups (P > 0.05).
In the finisher phase (d 24 to 35), the deleterious effect of the NE challenge on BWG was not observed. Birds fed additives (SMP, SMF, and SHM) had lower FCR compared to UCC and CHC groups (P = 0.001).
Considering the entire study period (d 0 to 35), birds infected with NE had lower BWG compared to the UCC group (P = 0.001) but no difference was observed among the challenged groups (P > 0.05). Birds fed additives (SMP, SMF and SHM) had lower FCR compared to the CHC group (P = 0.001). Additives SMF and SHM had similar FCR compared to the BAC group although had higher FCR compared to the UCC group (P = 0.001). Livability was not different between treatment groups during this period (P > 0.05).
3.2. Lesion scores
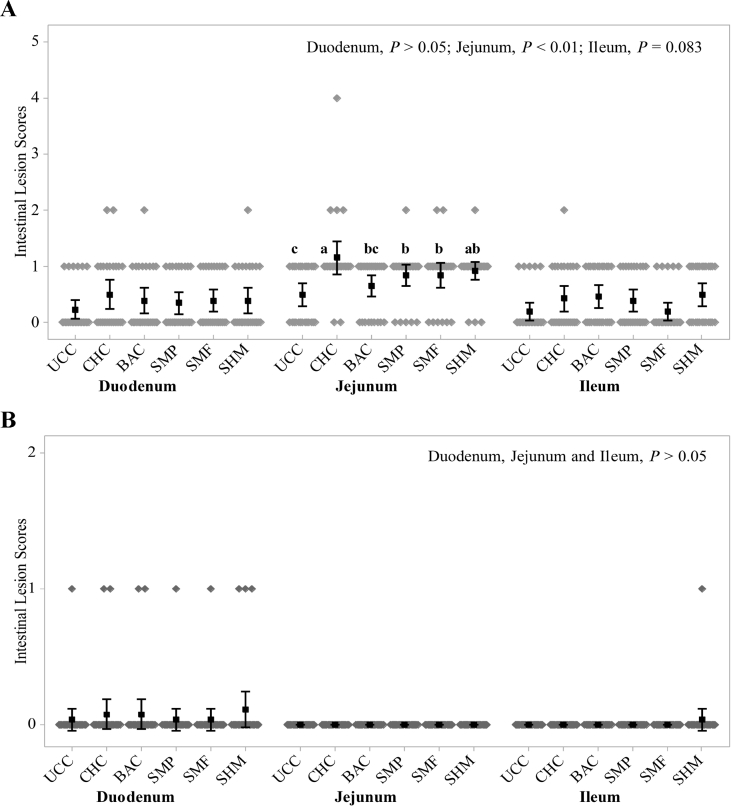
On d 16, NE challenge increased jejunal lesion score compared to UCC group (P < 0.01; Fig. 1A). Birds fed additives SMP and SMF had lower jejunal lesion scores compared to CHC group (P < 0.01). Birds treated with additives (SMP, SMF, and SHM) had similar jejunal lesion scores compared to the BAC group (P > 0.05). No difference was observed in duodenal and ileal lesion scores between different treatment groups (P > 0.05) although there was a tendency in ileal lesion scores (P = 0.083) to be lower in UCC (0.192) and additive SMF (0.192) groups compared to CHC (0.423), BAC (0.461), SMP (0.384) and SHM (0.500) treatment groups. The NE challenge effect on intestinal lesions disappeared on d 21 (Fig. 1B) in jejunum and ileum (P > 0.05). There was no difference in duodenal lesion scores between different treatment groups on d 21 (P > 0.05).
Fig. 1.
Effects of additives and necrotic enteritis (NE) challenge on intestinal lesion scores: (A) d 16, (B) d 21. UCC, unchallenged control; CHC, challenged control; BAC, zinc bacitracin; SMP, a blend of short-chain fatty acids (SCFA), medium-chain fatty acids (MCFA) and phenolic compound; SMF, a blend of buffered SCFA with MCFA; SHM, a blend of buffered SCFA with a high concentration of MCFA. a – c Values in a row with no common superscripts differ significantly (P < 0.05). Mean values are based on 2 birds per replicate and 13 replicates per treatment.
3.3. Serum immunoglobulins
The effects of dietary treatments on serum immunoglobulins of birds are shown in Table 4. On d 16, NE infected groups had higher levels of serum IgA compared to UCC group (P = 0.033). On d 21, all challenged groups, except CHC, had higher levels of serum IgA compared to UCC group (P = 0.001). Birds fed additive SHM had higher levels of serum IgA compared to SMP and BAC groups (P = 0.001), and similar IgA levels compared to the additive SMF group (P > 0.05).
Table 4.
Effects of additives and NE challenge on serum IgA of broilers on day 16 and 21.1
| Item | UCC | NE challenged2 |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| CHC | BAC | SMP | SMF | SHM | ||||
| IgA, mg/mL | ||||||||
| d 16 | 0.18b | 0.27a | 0.32a | 0.30a | 0.32a | 0.30a | 0.032 | 0.033 |
| d 21 | 0.17c | 0.22bc | 0.25b | 0.23b | 0.26ab | 0.31a | 0.019 | 0.001 |
NE = necrotic enteritis; IgA = immunoglobulin A.
a – cValues in a row with no common superscripts differ significantly (P < 0.05). Mean values are based on 2 birds per replicate and 13 replicates per treatment.
UCC, unchallenged control; CHC, challenged control; BAC, zinc bacitracin; SMP, a blend of short-chain fatty acids (SCFA), medium-chain fatty acids (MCFA) and phenolic compound; SMF, a blend of buffered SCFA with MCFA; SHM, a blend of buffered SCFA with a high concentration of MCFA.
NE challenged birds were gavaged with Eimeria spp. on d 9 and C. perfringens on d 14.
3.4. Apparent ileal gross energy and crude protein digestibility
The effects of blended additives on apparent ileal GE and CP digestibility determined on d 21 are presented in Table 5. The unchallenged group had higher apparent ileal GE and CP digestibility compared to CHC group (P = 0.001). Birds fed additive SHM had higher apparent ileal GE digestibility compared to the CHC group (P = 0.001) but were not different from UCC and BAC groups (P > 0.05). Birds fed additive SMF and SHM had higher apparent ileal CP digestibility compared to the CHC group (P = 0.019). However, the apparent ileal CP digestibility of additive groups (SMP, SMF and SHM) were similar to UCC and BAC groups (P > 0.05).
Table 5.
Effects of additives and necrotic enteritis (NE) challenge on apparent ileal digestibility of broilers on d 21 (%).1
| Item | UCC | NE challenged2 |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| CHC | BAC | SMP | SMF | SHM | ||||
| Gross energy | 74.4a | 69.6c | 73.7ab | 70.1c | 71.3bc | 73.9a | 0.009 | 0.001 |
| Crude protein | 85.0a | 81.3b | 84.0a | 82.9ab | 83.7a | 84.7a | 0.008 | 0.019 |
a – cValues in a row with no common superscripts differ significantly (P < 0.05). Mean values are based on 2 birds per replicate and 7 replicates per treatment.
UCC, unchallenged control; CHC, challenged control; BAC, zinc bacitracin; SMP, a blend of short-chain fatty acids (SCFA), medium-chain fatty acids (MCFA) and phenolic compound; SMF, a blend of buffered SCFA with MCFA; SHM, a blend of buffered SCFA with a high concentration of MCFA.
NE challenged birds were gavaged with Eimeria spp. on d 9 and C. perfringens on d 14.
3.5. Litter scores, footpad dermatitis and hock burn scores
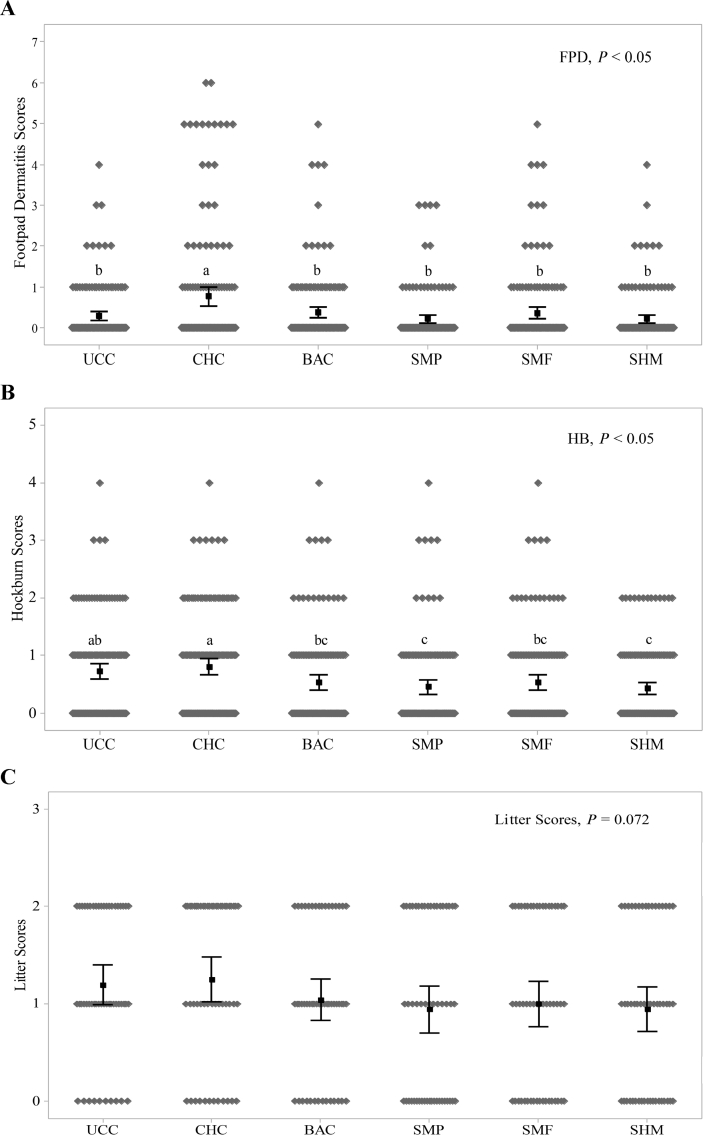
The effects of blended additives on FPD, HB and litter scores of broilers are shown in Fig. 2A, B, and C, respectively. Birds in the UCC group had lower FPD scores than those in the CHC group (P = 0.032). Birds fed additives (SMP, SMF, and SHM) had lower FPD scores compared to those in the CHC group (P = 0.032) but not different from BAC group (P > 0.05). Interestingly, no difference of HB scores between UCC and CHC groups was observed, whereasall additive (SMP, SMF and SHM) and BAC groups had lower HB scores compared to the CHC groups (P = 0.030). Moreover, additive SMP and SHM groups had lower HB scores compared to UCC group (P = 0.030) but not different from additive SMF and BAC groups (P > 0.05). Litter scores were not significantly different between treatment groups but there was a tendency (P = 0.072) for birds fed additives SMP (0.94), SMF (1.00), SHM (0.94) and BAC (1.03) to have a lower score compared to UCC (1.19) and CHC (1.25) groups.
Fig. 2.
Effects of additives and necrotic enteritis (NE) challenge on bird welfare on d 35: (A) footpad dermatitis scores, (B) hock burn scores, and (C) litter scores. UCC, unchallenged control; CHC, challenged control; BAC, zinc bacitracin; SMP, a blend of short-chain fatty acids (SCFA), medium-chain fatty acids (MCFA) and phenolic compound; SMF, a blend of buffered SCFA with MCFA; SHM, a blend of buffered SCFA with a high concentration of MCFA. a-c Values in a row with no common superscripts differ significantly (P < 0.05). Mean values are based on all birds or pens per treatment and 13 replicates per treatment.
4. Discussion
Necrotic enteritis is a frequent and devastating disease among enteric bacterial diseases prevalent in the highly productive broiler industry, particularly in the post-antibiotic era. Globally, the search to find effective alternatives to in-feed AGP is still ongoing. The current study investigated the potential of blended additives that incorporate several ingredients to mitigate the deleterious effects of sub-clinical NE of broiler chickens. The reduced BWG and FI, impaired FCR, lower digestibility, and presence of mild lesions and low mortality (1%) observed in NE infected groups, illustrate a successful introduction of sub-clinical NE. The findings of this study further support the hypothesis that dietary supplementation with blended SCFA in combination with MCFA improves feed efficiency, apparent ileal digestibility of nutrients, immunity, footpad health, and bird welfare of broilers under NE challenged condition.
It is widely accepted that the OA and their blends have the potential to improve performance and reduce the impact of diseases of broilers. The OA which are naturally occurring weak acids and commonly used through water or feed have been known as acidifiers that enable the reduction of certain bacterial pathogens (Cherrington et al., 1991; Van Immerseel et al., 2006; Gharib Naseri et al., 2012). The OA ameliorate the conditions of the intestinal gut via reducing pH that promoting digestive enzyme activity and nutrient digestibility, creating stability of the gut microbial inhabitants and enhancing the growth of beneficial bacteria by being bactericidal and bacteriostatic to the pathogenic bacteria (Papatsiros et al., 2013). Hence, the OA can have a beneficial effect on the performance of the birds. The results obtained in the present study showed that birds fed additives had a lower FCR in the overall study period (d 0 to 35) compared to the CHC group possibly due to the increased CP digestibility and intestinal health status. It can be supported by a review compiled with different study findings reported that diet supplemented with OA has a significant impact on feed efficiency and protein digestibility and the correlation between them (Khan and Iqbal, 2016). Higher feed efficiency and reduced NE effects indicated by intestinal lesions, apparent ileal nutrients digestibility, and immune responses may have a positive impact on footpad health and litter quality that were observed in the study. It is apparent that wet or poor quality litter is associated with NE (Williams, 2005; Sharma et al., 2018) and wet litter increases the incidence of FPD (Cengiz et al., 2011; Kheravii et al., 2017). Further, researchers also reported that the prolonged contact of footpads, hocks with poor quality litter can increase the incidence of skin inflammation and necrosis (Martland, 1985; Michel et al., 2012). Moreover, higher apparent ileal CP digestibility could have had a significant effect on feed efficiency. These findings were also supported by the fact that blended additive groups had similar lesion scores comparable to the BAC group whereas the CHC group had the highest jejunal lesions. In earlier reports, birds fed similar additives had intestinal lesions not different between additives and BAC groups (Sun et al., 2020). These support the findings of the current study with blended additives showing positive effects on intestinal health. Moreover, the higher BWG, better or similar FCR in birds fed blended additives compared to birds fed BAC during the challenged period (d 10 to 24) further confirms the protective effects of the additives, used in this study, against subclinical NE. Cumulatively, it can be suggested that the blended additives supported the performance and reduced the NE challenge effects similar to that of the BAC which are in agreement with previous studies (Polycarpo et al., 2017; Nguyen et al., 2018; McKnight et al., 2020).
The combination of various OA can affect bird performance, intestinal health, digestion, and absorption of nutrients differently (Huang et al., 2015; Adhikari et al., 2020). It might be due to different modes of action of individual additives in the blend and their synergistic effects. In the current study, either significantly or numerically improved BWG, FCR, serum IgA, and apparent ileal GE digestibility observed in birds fed additive SHM during the challenge period (d 10 to 24) indicating that additive SHM performed to a greater extent when compared to additive SMP and SMF blends. The synergistic effects of SCFA with a high concentration of MCFA may have played a role. Moreover, higher IgA in the broilers fed additive SHM on d 21 indicates that the birds were more immune protective against NE when supplied with SCFA with a high concentration of MCFA. It can be speculated that the elevation of serum IgA concentrations might be related to the potential boost of B and T lymphocytes but further investigations are needed to examine specific cell-mediated immune responses to confirm this hypothesis. In fact, an earlier study in pigs stated that OA enhanced immune status and lymphocyte responses in the gut (Lee et al., 2007) which may indicate the possibility of such a response in chickens. Overall, these findings suggest that combinations of additives with a high concentration of MCFA should have better-modulated gut health and immunity response of the chickens leading to improved performance under disease challenge conditions such as NE.
The efficacy of OA in diets as a potential antibiotic alternative for improving the digestibility of nutrients has been previously reported in pigs and poultry (Khan and Iqbal, 2016; Pearlin et al., 2020). Organic acids play multifunctional roles to improve digestion and absorption of nutrients through gastric pH reduction, the increase of nutrients retention time, mucosal morphology alteration, stimulation of pancreatic enzymes secretions, and the modulation of intermediary metabolic pathways as substrates (Partanen and Mroz, 1999). It is speculated that nutrient retention in the birds supplemented with buffered or coated OA can be increased. It might be due to the prevention of dissociation of OA in the upper part of the digestive tract which helped to enhance their bioavailability towards the distal part of the digestive tract and modulate intestinal microflora and enhanced proliferation of epithelial cells (Hu and Guo, 2007; Smulikowska et al., 2009). Moreover, pH decreasing abilities and inhibitory effects of OA on pathogenic bacteria might be able to reduce their metabolic needs, thereby increased nutrient availability for the host (Adil et al., 2010). In the current study, the increased protein and energy digestibility in birds fed blended additives revealed the beneficial effects of a blend of SCFA with MCFA on nutrient retention. These findings are in agreement with previous studies reporting that the OA supplementation in diets improves protein and energy digestibility of broilers (Pirgozliev et al., 2008; Fascina et al., 2012) compared to the broilers fed the diets without OA supplementation. In contrast with our results, however, previous studies reported that the blended SCFA in combination with MCFA had no effects on CP and GE digestibility of broilers (Nguyen et al., 2018), laying hens (Lee et al., 2015) and pigs (Upadhaya et al., 2016). Variation in nutrients digestibility outcomes observed in studies supplemented with OA might be due to the single or different combinations of OA, doses and symbiotic effects of OA, bird health status, challenged conditions, bird genetics, and management. The higher apparent ileal energy digestibility in birds fed additive SHM compared to birds fed additives SMP and SMF, may suggest synergistic effects of SCFA with a high concentration of MCFA. It is apparent that nutrient digestibility has a significant impact on feed efficiency (Pelicia et al., 2004). Therefore, it can be speculated that the improved FCR might be due to the better digestibility and stronger immune responses against the disease. This is supported by several studies (Rafacz-Livingston et al., 2005; Nezhad et al., 2011) reporting that one of the possible mechanisms of improving FCR in birds fed OA is increasing the digestibility of nutrients by improving dry matter and protein retention and mineral absorption, and utilisation.
Litter quality, FPD, and HB scores are good indicators for animal management and health status, and key parameters to measure birds’ welfare when additives are used to combat the NE challenge. There are several factors associated with wet litter and the incidence of FPD such as ingredients and diet composition (Youssef et al., 2012; Cengiz et al., 2013), litter type and quality (Bilgili et al., 2009; Kheravii et al., 2017), intestinal diseases (Kaldhusdal and Hofshagen, 1992; Sharma et al., 2018), stocking density and other management measures i.e. ventilation (Thaxton et al., 2006). The current study showed that birds fed the blended additives and BAC had significantly reduced FPD and HB scores. The additive application also led to a tendency to reduce litter scores. Reduced litter scores suggested the possible improvement in litter quality which had a significant effect on FPD and HB scores and this was indicated by correlations where a positive correlation between FPD or HB and litter scores were observed in previous studies (Michel et al., 2012; Kheravii et al., 2017). Good litter quality and low FPD, and HB scores suggest that the dietary supplementation of a blend of SCFA with MCFA reduced the incidence of NE, improved bird health condition and better bird welfare.
To conclude, the current findings suggest that the blended SCFA in combination with a high concentration of MCFA (additive SHM) was more effective in alleviating the impact of sub-clinical NE as indicated by higher BWG, boosted serum IgA and higher GE digestibility during the challenge period (d 10 to 24), albeit all the blended additives improved overall FCR, apparent ileal CP digestibility, footpad health, and bird welfare. These results also demonstrated the potential of blended organic acids as alternatives to in-feed AGP (Zinc bacitracin) of broilers to alleviate the detrimental impact of enteritis challenges such as NE.
Author contributions
Alip Kumar: animal trial, feed formulation, laboratory experiment, statistical analysis, study design and writing; Mehdi Toghyani: study design, feed formulation, animal trial, data evaluation, manuscript review; Sarbast K. Kheravii: data evaluation, manuscript review; Lane Pineda: Methodology, data evaluation, manuscript review; Yanming Han: methodology, data evaluation, manuscript review; Robert A. Swick: study design, data evaluation, manuscript review; Shu-Biao Wu: coordination, study design, data collection, statistical analyses, critical review of manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was financially supported by Trouw Nutrition, a Nutreco company, The Netherlands. The authors would also like to acknowledge the technical assistance provided by Shuyu Song, Leanne Lisle, Dr Nishchal K Sharma, Jonathon Clay, Danielle Smith and David Trenerry. The authors thank Prof. Robert Moore for providing Clostridium perfringens EHE-18 and Ms. Petrina Young for providing Eimeria oocysts.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adhikari P., Kiess A., Adhikari R., Jha R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J Appl Poultry Res. 2020;29:515–534. [Google Scholar]
- Adil S., Banday T., Bhat G.A., Mir M.S., Rehman M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet Med Int. 2010:479485. doi: 10.4061/2010/479485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain V., Mirabito L., Arnould C., Colas M., Le Bouquin S., Lupo C. Skin lesions in broiler chickens measured at the slaughterhouse: relationships between lesions and between their prevalence and rearing factors. Br Poultry Sci. 2009;50:407–417. doi: 10.1080/00071660903110901. [DOI] [PubMed] [Google Scholar]
- Ao T., Cantor A., Pescatore A., Ford M., Pierce J., Dawson K. Effect of enzyme supplementation and acidification of diets on nutrient digestibility and growth performance of broiler chicks. Poultry Sci. 2009;88:111–117. doi: 10.3382/ps.2008-00191. [DOI] [PubMed] [Google Scholar]
- Aviagen . Aviagen Ltd.; Newbridge, Scotland, UK: 2014. Ross broiler management handbook. [Google Scholar]
- Bilgili S., Hess J., Blake J., Macklin K., Saenmahayak B., Sibley J. Influence of bedding material on footpad dermatitis in broiler chickens. J Appl Poultry Res. 2009;18:583–589. [Google Scholar]
- Castanon J. History of the use of antibiotic as growth promoters in European poultry feeds. Poultry Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Cengiz O., Hess J., Bilgili S. Effect of bedding type and transient wetness on footpad dermatitis in broiler chickens. J Appl Poultry Res. 2011;20:554–560. [Google Scholar]
- Cengiz O., Hess J., Bilgili S. Effect of protein source on the development of footpad dermatitis in broiler chickens reared on different flooring types. Archiv Geflügelkunde. 2013;77:166–170. [Google Scholar]
- Cengiz O., Koksal B., Tatli O., Sevim O., Avci H., Epikmen T. Influence of dietary organic acid blend supplementation and interaction with delayed feed access after hatch on broiler growth performance and intestinal health. Vet Med (Praha) 2012:57. [Google Scholar]
- Cherrington C., Hinton M., Mead G., Chopra I. Organic acids: chemistry, antibacterial activity and practical applications. Adv Microb Physiol. 1991;32:87–108. doi: 10.1016/s0065-2911(08)60006-5. [DOI] [PubMed] [Google Scholar]
- Cooper K.K., Songer J.G., Uzal F.A. Diagnosing clostridial enteric disease in poultry. J Vet Diagn Invest. 2013;25:314–327. doi: 10.1177/1040638713483468. [DOI] [PubMed] [Google Scholar]
- Dibner J., Richards J. Antibiotic growth promoters in agriculture: history and mode of action. Poultry Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Fascina V.B., Sartori J.R., Gonzales E., Carvalho F.B.d., Souza I.M.G.P.d., Polycarpo GdV. Phytogenic additives and organic acids in broiler chicken diets. Rev Centro Am Odontol. 2012;41:2189–2197. [Google Scholar]
- Gharib Naseri K., Rahimi S., Khaki P. Comparison of the effects of probiotic, organic acid and medicinal plant on Campylobacter jejuni challenged broiler chickens. J Agri Sci Tech-IRAN. 2012;14:1485–1496. [Google Scholar]
- Guide S.U.s. SAS Institute. Inc; Cary, NC, USA: 2010. Statistic (Version 9.3) [Google Scholar]
- Hu Z., Guo Y. Effects of dietary sodium butyrate supplementation on the intestinal morphological structure, absorptive function and gut flora in chickens. Anim Feed Sci Technol. 2007;132:240–249. [Google Scholar]
- Huang C., Song P., Fan P., Hou C., Thacker P., Ma X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J Nutr. 2015;145:2774–2780. doi: 10.3945/jn.115.217406. [DOI] [PubMed] [Google Scholar]
- Immerseel F.V., Buck J.D., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Hofshagen M. Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poultry Sci. 1992;71:1145–1153. doi: 10.3382/ps.0711145. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Schneitz C., Hofshagen M., Skjerve E. Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Dis. 2001:149–156. [PubMed] [Google Scholar]
- Keyburn A.L., Sheedy S.A., Ford M.E., Williamson M.M., Awad M.M., Rood J.I. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.H., Iqbal J. Recent advances in the role of organic acids in poultry nutrition. J Appl Anim Res. 2016;44:359–369. [Google Scholar]
- Kheravii S., Swick R.A., Choct M., Wu S.-B. Potential of pelleted wheat straw as an alternative bedding material for broilers. Poultry Sci. 2017;96:1641–1647. doi: 10.3382/ps/pew473. [DOI] [PubMed] [Google Scholar]
- Kocher A., Choct M. Rural Industries Research and Development Corporation; Canberra, Australia: 2008. Improving broiler chicken performance. [Google Scholar]
- Kuang Y., Wang Y., Zhang Y., Song Y., Zhang X., Lin Y. Effects of dietary combinations of organic acids and medium chain fatty acids as a replacement of zinc oxide on growth, digestibility and immunity of weaned pigs. Anim Feed Sci Technol. 2015;208:145–157. [Google Scholar]
- Lee D., Liu S., Chen Y., Wang R., Lin S., Weng C. Effects of diets supplemented with organic acids and nucleotides on growth, immune responses and digestive tract development in weaned pigs. J Anim Physiol Anim Nutr. 2007;91:508–518. doi: 10.1111/j.1439-0396.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- Lee S.I., Kim H.S., Kim I. Microencapsulated organic acid blend with MCFAs can be used as analternative to antibiotics for laying hens. Turk J Vet Anim Sci. 2015;39:520–527. [Google Scholar]
- M'Sadeq S.A., Wu S., Swick R.A., Choct M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim Nutr. 2015;1:1–11. doi: 10.1016/j.aninu.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martland M. Ulcerative dermatitis dm broiler chickens: the effects of wet litter. Avian Pathol. 1985;14:353–364. doi: 10.1080/03079458508436237. [DOI] [PubMed] [Google Scholar]
- Mathis G., Van Dam J., Corujo F., Hofacre C. Effect of an organic acids and medium-chain fatty acids containing product in feed on the course of artificial necrotic enteritis infection in broiler chickens. Proc Europ Symp Poult Nutr. 2005;15:372–374. [Abstr.] [Google Scholar]
- McKnight L., Page G., Han Y. Effect of replacing in-feed antibiotics with synergistic organic acids, with or without trace minerals and/or water acidification, on growth performance and health of broiler chickens under a Clostridium perfringens type A challenge. Avian Dis. 2020 doi: 10.1637/aviandiseases-D-19-00101. [DOI] [PubMed] [Google Scholar]
- Michel V., Prampart E., Mirabito L., Allain V., Arnould C., Huonnic D. Histologically-validated footpad dermatitis scoring system for use in chicken processing plants. Br Poultry Sci. 2012;53:275–281. doi: 10.1080/00071668.2012.695336. [DOI] [PubMed] [Google Scholar]
- Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016:1–22. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- Nezhad Y.E., Gale-Kandi J.G., Farahvash T., Yeganeh A. Effect of combination of citric acid and microbial phytase on digestibility of calcium, phosphorous and mineralization parameters of tibia bone in broilers. Afr J Biotechnol. 2011;10:15089–15093. [Google Scholar]
- Nguyen D.H., Lee K.Y., Mohammadigheisar M., Kim I.H. Evaluation of the blend of organic acids and medium-chain fatty acids in matrix coating as antibiotic growth promoter alternative on growth performance, nutrient digestibility, blood profiles, excreta microflora, and carcass quality in broilers. Poultry Sci. 2018;97:4351–4358. doi: 10.3382/ps/pey339. [DOI] [PubMed] [Google Scholar]
- NHMRC . 8th ed. 2013. Australian code for the care and use of animals for scientific purposes. [Google Scholar]
- Onrust L., Van Driessche K., Ducatelle R., Schwarzer K., Haesebrouck F., Van Immerseel F. Valeric acid glyceride esters in feed promote broiler performance and reduce the incidence of necrotic enteritis. Poultry Sci. 2018;97:2303–2311. doi: 10.3382/ps/pey085. [DOI] [PubMed] [Google Scholar]
- Papatsiros V., Katsoulos P.-D., Koutoulis K., Karatzia M., Dedousi A., Christodoulopoulos G. Alternatives to antibiotics for farm animals. CAB Rev. 2013;8:1–15. [Google Scholar]
- Partanen K.H., Mroz Z. Organic acids for performance enhancement in pig diets. Nutr Res Rev. 1999;12:117–145. doi: 10.1079/095442299108728884. [DOI] [PubMed] [Google Scholar]
- Pearlin B.V., Muthuvel S., Govidasamy P., Villavan M., Alagawany M., Ragab Farag M. Role of acidifiers in livestock nutrition and health: a review. J Anim Physiol Anim Nutr. 2020;104:558–569. doi: 10.1111/jpn.13282. [DOI] [PubMed] [Google Scholar]
- Pelicia K., Mendes A., Saldanha E., Pizzolante C., Takahashi S., Garcia R. Probiotic and prebiotic utilization in diets for free-range broiler chickens. Braz J Poult Sci. 2004;6:99–104. [Google Scholar]
- Pirgozliev V., Murphy T., Owens B., George J., McCann M. Fumaric and sorbic acid as additives in broiler feed. Res Vet Sci. 2008;84:387–394. doi: 10.1016/j.rvsc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Polycarpo G.V., Andretta I., Kipper M., Cruz-Polycarpo V.C., Dadalt J.C., Rodrigues P.H.M. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poultry Sci. 2017;96:3645–3653. doi: 10.3382/ps/pex178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R.E.J. Bacterial enteritides of poultry. Poultry Sci. 1998;77:1159–1165. doi: 10.1093/ps/77.8.1159. [DOI] [PubMed] [Google Scholar]
- Rafacz-Livingston K., Parsons C., Jungk R. The effects of various organic acids on phytate phosphorus utilization in chicks. Poultry Sci. 2005;84:1356–1362. doi: 10.1093/ps/84.9.1356. [DOI] [PubMed] [Google Scholar]
- Rodgers N.J., Swick R.A., Geier M.S., Moore R.J., Choct M., Wu S.-B. A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015;59:38–45. doi: 10.1637/10774-011614-reg.1. [DOI] [PubMed] [Google Scholar]
- Sharma N.K., Choct M., Wu S.B., Swick R.A. Necrotic enteritis challenge and high dietary sodium level affect odorant composition or emission from broilers. Poultry Sci. 2018;97:39–46. doi: 10.3382/ps/pex257. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short F., Gorton P., Wiseman J., Boorman K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim Feed Sci Technol. 1996;59:215–221. [Google Scholar]
- Skinner J.T., Bauer S., Young V., Pauling G., Wilson J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 2010;54:1237–1240. doi: 10.1637/9399-052110-Reg.1. [DOI] [PubMed] [Google Scholar]
- Smulikowska S., Czerwinski J., Mieczkowska A., Jankowiak J. The effect of fat-coated organic acid salts and a feed enzyme on growth performance, nutrient utilization, microflora activity, and morphology of the small intestine in broiler chickens. J Anim Feed Sci. 2009;18:478–489. [Google Scholar]
- Sun Y., Han Y., Chen J., Ni A., Jiang Y., Li Y. Effect of replacing in-feed antibiotics with synergistic organic acids on growth performance, health, carcass, and immune and oxidative status of broiler chickens under Clostridium perfringens type A challenge. Avian Dis. 2020 doi: 10.1637/aviandiseases-D-19-00101. [DOI] [PubMed] [Google Scholar]
- Thaxton J., Dozier W., III, Branton S., Morgan G., Miles D., Roush W. Stocking density and physiological adaptive responses of broilers. Poultry Sci. 2006;85:819–824. doi: 10.1093/ps/85.5.819. [DOI] [PubMed] [Google Scholar]
- Upadhaya S.D., Lee K.Y., Kim I.H. Effect of protected organic acid blends on growth performance, nutrient digestibility and faecal microflora in growing pigs. J Appl Anim Res. 2016;44:238–242. [Google Scholar]
- Van Immerseel F., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Russell J., Flythe M., Gantois I., Timbermont L., Pasmans F. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 2006;35:182–188. doi: 10.1080/03079450600711045. [DOI] [PubMed] [Google Scholar]
- Wade B., Keyburn A. The true cost of necrotic enteritis. Poultry World. 2015;31:16–17. [Google Scholar]
- Williams R. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Wu S.-B., Stanley D., Rodgers N., Swick R.A., Moore R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet Microbiol. 2014;169:188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Youssef I., Beineke A., Rohn K., Kamphues J. Influences of increased levels of biotin, zinc or mannan-oligosaccharides in the diet on foot pad dermatitis in growing turkeys housed on dry and wet litter. J Anim Physiol Anim Nutr. 2012;96:747–761. doi: 10.1111/j.1439-0396.2010.01115.x. [DOI] [PubMed] [Google Scholar]
- Zentek J., Buchheit-Renko S., Manner K., Pieper R., Vahjen W. Intestinal concentrations of free and encapsulated dietary medium-chain fatty acids and effects on gastric microbial ecology and bacterial metabolic products in the digestive tract of piglets. Arch Anim Nutr. 2012;66:14–26. doi: 10.1080/1745039x.2011.644916. [DOI] [PubMed] [Google Scholar]
- Zentek J., Ferrara F., Pieper R., Tedin L., Meyer W., Vahjen W. Effects of dietary combinations of organic acids and medium chain fatty acids on the gastrointestinal microbial ecology and bacterial metabolites in the digestive tract of weaning piglets. J Anim Sci. 2013;91:3200–3210. doi: 10.2527/jas.2012-5673. [DOI] [PubMed] [Google Scholar]