Abstract
Background
Diabetes mellitus (DM) may be associated with the etiology of rotator cuff disease; however, its effect on healing after surgical rotator cuff repair (RCR) is not well characterized. The purposes of this study are to analyze the association between DM and surgical RCR, the association between DM and revision RCR after RCR, and the association between DM and the cost of RCR.
Methods
A retrospective analysis of claims data of privately and publicly insured subjects from the Truven Health MarketScan database from 2008 to 2017 was conducted, collecting RCR cases and controls matched for age, sex, year of RCR, and first and last year in the database. Multivariable logistic regression models were used to compare DM incidence within the RCR and control groups after adjusting for all matching variables plus region, insurance plan type, tobacco use, and Charlson comorbidity index (CCI). Cox proportional hazard models were used to compare rates of revision RCR between DM and non-DM groups after adjusting for patient age, sex, year of RCR, plan type, and CCI. Generalized estimating equations were used to analyze RCR cost, and exponentiated regression coefficients were reported to represent cost ratios.
Results
The full analysis cohort consisted of 292,666 RCR cases and matched controls. The adjusted odds of having RCR surgery in diabetic patients was 48% higher (odds ratio = 1.48 [95% confidence interval {CI} 1.46 to 1.51], P < .001) than nondiabetics. DM was not significantly associated with revision RCR after RCR when adjusting for age, sex, region, plan type, tobacco use, year of RCR, and CCI (hazard ratio = 1.03, 95% CI 0.99 to 1.07, P = .17). Diabetes was associated with a higher cost of RCR by 3% (ratio = 1.03, 95% CI 1.02 to 1.03, P < .001).
Conclusions
Diabetic patients are at a higher risk of undergoing RCR surgery; however, there is no association between DM and subsequent rotator cuff revision surgery.
Keywords: Diabetes mellitus, Rotator cuff tear, Rotator cuff repair, MarketScan, Medical comorbidities
Rotator cuff disease is the most common shoulder disorder treated by orthopedic surgeons, with a prevalence of approximately 20.7% within the general population.28,34 Diabetes mellitus (DM) is also a common endocrine disease with an estimated prevalence of 9.3% worldwide.33 Arthroscopic rotator cuff repair (RCR) is rapidly rising in incidence.20 Failure of healing after RCR, however, has been reported in up to 90% of cases in the worst case scenarios.5,6,11,21 While initial tear size is reported to be the most significant factor that affects tendon healing,2 various other factors have been identified that influence rotator cuff tendon healing—including age, muscle fatty infiltration and atrophy, muscle-tendon unit retraction, smoking, osteoporosis, repair construct, rehabilitation, biology, and genetic predispositions.4,8,10,13,15,17,21,29, 30, 31, 32 Evidence also suggests that DM is associated with the etiology of rotator cuff tendinopathy and may also influence healing after RCR.3 Understanding the influence of DM on the pathogenesis of rotator cuff disease, as well as healing after RCR, may help surgeons in the development of prevention strategies as well as strategies to improve healing rates after RCR.
Several studies have identified DM as a risk factor for rotator cuff tendinopathy among both the working and general populations.16,19,23,24 Recent evidence showed that patients with diabetes had a 2.11-fold higher risk of rotator cuff disorders compared with those without diabetes,14 and a Finnish, population-based cross-sectional study showed that patients with insulin-dependent diabetes had an 8.8-fold increased risk for rotator cuff tendinitis.16 However, while these studies have addressed tendinopathy and tendinitis, it is unknown whether DM is a risk factor for rotator cuff tears and thus a risk factor for an increased incidence of surgical treatment in the form of RCR.27 Furthermore, while Cho et al demonstrated that sustained hyperglycemia increased the possibility of anatomic failure at the repaired rotator cuff, it remains unclear whether DM itself is a risk factor for revision RCR after RCR.3 A large retrospective database analysis of these relationships would be a favorable approach because it offers a very large, multiple-center, generalizable sample and the ability to correct for multiple comorbid factors.
Therefore, the purposes of this study are: to analyze (1) the association between DM and surgical RCR, (2) the association between DM and revision surgery post-RCR, and (3) the association between DM and the cost of RCR. We hypothesize that DM will associate with RCR, the need for revision surgery after RCR, and increased cost of RCR.
Materials and methods
Data source and sample selection
This retrospective study was conducted using the Truven Health MarketScan Commercial Claims Database of privately and publicly insured patients from 2008 to 2017. Truven MarketScan combines 2 separate databases, a commercial database and a supplemental Medicare database, and contains claims from 260 contributing employers, 40 health plans, and government and public organizations representing ∼161 million lives.9 This administrative claims database also includes a variety of fee-for-service, preferred provider organizations, and capitated health plans. It thus represents a large sample representative of the employed and insured United States population.
The enrollment data provided beneficiaries’ demographic data—including age, employment status, geographical region, and sex. These data also provided the beneficiaries’ insurance plan data, which included plan type and enrollment status. Medical service claims provide detailed inpatient and outpatient encounter information, including date and setting of service; provider type; plan- and patient-paid amounts; International Classification of Diseases, 9th and 10th revision, Clinical Modification (ICD-9-CM and ICD-10-CM) diagnosis and procedure codes; Current Procedural Terminology, 4th edition (CPT-4) codes; and Healthcare Common Procedure Coding System procedure codes.
Statistical analysis
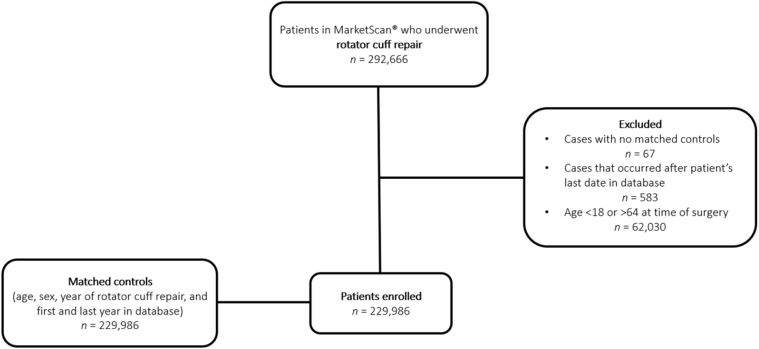
Patients with a diagnosis of rotator cuff tear (RCT) (ICD-9-CM: 726.10, 726.13, 727.61, 840.4; ICD-10-CM: M75.100, M75.110, M75.120, S43.429A) and surgical RCR (ICD-9-CM: 83.63 or CPT 29827, 23410, 23412, 23420) in any inpatient or outpatient service claim between January 1, 2008, and December 31, 2017, who were between 18 and 64 years of age were considered for inclusion in this analysis. The patient’s first claim date for RCR served as the index date, and the patient was followed up until the end of her or his continuous insurance enrollment. For purpose (1) of this study, matched controls without a history of RCT and subsequent RCR (1:1 on age ± 0 years, sex, year of RCR ± 0 years, and first and last year in the database ± 0 years) were obtained for comparison to patients with a history of RCT and subsequent RCR. Patients were stratified into 2 groups based on exposure to a diagnosis of DM: (1) patients with DM (ICD-9-CM: 250.x; ICD-10: E10, E11) and (2) patients without DM (Fig. 1).
Figure 1.
Flow diagram of study inclusion and exclusion.
For purpose (1) of this study, the primary outcome was RCT (ICD-9-CM: 726.10, 726.13, 727.61, 840.4; ICD-10-CM: M75.100, M75.110, M75.120, S43.429A) with accompanying RCR (CPT 29827, 23410, 23412, 23420). For purpose (2), the primary outcome was requiring a revision RCR (CPT 29827, 23410, 23412, 23420) after prior RCR. For purpose (3), total costs (gross payments to a provider for a service) 3 months post index procedure were compared between the 2 groups. The specific purpose of the cost analysis was to analyze costs related to surgery and it was felt that this period of time best reflected the time period during which surgical complications occur. Comorbidity was assessed using the Charlson comorbidity index (CCI). Inpatient diagnoses throughout the patient’s lifetime pre-index were used to construct the CCI for each patient. Comorbid tobacco use disorder (ICD-9-CM 305.1, V15.82; ICD-10: F17.x, 099.33, P04.2, P96.81, T65.2, Z57.31, Z71.6, Z72, Z77.2, Z87.8, Z72.0, Z87.891,F17.2) was examined as a potential risk factor. Demographic variables were extracted, including age, sex, insurance type, and region.
Univariable and multivariable logistic regressions were used to compare RCR outcome to the exposure of DM, where the multivariable model adjusted for all matching variables (age, sex, first and last year in the database) plus region, insurance plan type, tobacco use, and a modified CCI (calculated excluding points from diabetes to avoid over adjustment). Odds ratios (ORs) were reported with 95% confidence intervals (CIs) and P values. Model fit was examined using the Hosmer & Lemeshow goodness of fit test. C-statistics and R-squared were calculated for each model. Cumulative incidence of revision RCR over time were presented graphically using Kaplan-Meier plots stratified by exposure. Univariable and multivariable Cox proportional hazard models were used to compare revision RCR to the exposure of DM, where the multivariable model adjusted for patient age, sex, year of RCR, duration in the database, plan type, and CCI. Hazard ratios (HRs) were reported with 95% CIs and P values. Proportional hazard assumptions were examined visually by plotting Schoenfeld residuals against time.26 C-statistics and R-squared were calculated for each model.25 Univariable and multivariable models for RCR cost were constructed using Gamma regression obtained from generalized estimating equations. Statistical analyses were conducted using R v3.6.22 Statistical significance was evaluated at α = 0.05. All tests were 2 sided.
Results
The association of primary RCR in patients with DM
The full analysis cohort consisted of 229,986 RCR cases and their matched controls (Table I). Owing to matching on age, sex and duration in the database, the average age was 54 years (SD = 7.7), there were 42.3% females, and the duration in the database was 4.4 years (SD = 2.7) for subjects in both the groups. Among the RCR cases, 17.9% (41,157/229,986) were diagnosed with DM, as compared to 13% (29,927/229,986) in the control group. Tobacco abuse made up 18.8% (43,223/229,986) of the RCR cases as opposed to 5.6% (12,773/229,986) of the matched controls.
Table I.
Patient demographics stratified by RCR status, in which age, sex, and duration in the database were used to select the matched controls.
| Variable | RCR cases (N = 229,986) | Controls (N = 229,986) | P value |
|---|---|---|---|
| Diabetes | 41,157 (17.9%) | 29,927 (13%) | <.001† |
| CCI: mean (SD) | 0.5 (1.0) | 0.4 (1.1) | |
| Tobacco abuse | 43,223 (18.8%) | 12,773 (5.6%) | <.001† |
| Age: mean (SD) | 54.0 (7.7) | 54.0 (7.7) | 1.00∗ |
| Sex: female | 97,368 (42.3%) | 97,368 (42.3%) | 1.00† |
| Duration (yrs) in database: mean (SD) | 4.4 (2.7) | 4.4 (2.7) | 1.00∗ |
| Region: North Central | 52,530 (22.8%) | 48,588 (21.7%) | <.001† |
| Northeast | 40,504 (17.6%) | 40,966 (18.3%) | |
| South | 92,096 (40%) | 97,119 (43.4%) | |
| West | 39,107 (17%) | 34,963 (15.6%) | |
| Unknown | 5749 (2.5%) | 1969 (0.9%) | |
| Plan type: comprehensive | 6661 (3.1%) | 8074 (3.7%) | <.001† |
| EPO | 4053 (1.9%) | 3502 (1.6%) | |
| HMO | 23,186 (10.8%) | 30,195 (13.8%) | |
| POS | 16,040 (7.5%) | 20,693 (9.5%) | |
| PPO | 146,286 (68.2%) | 133,125 (60.9%) | |
| POS with capitation | 1426 (0.7%) | 1495 (0.7%) | |
| CDHP | 10,122 (4.7%) | 14,826 (6.8%) | |
| HDHP | 6705 (3.1%) | 6612 (3%) | |
| Employment status: active full time | 75,953 (33%) | 119,688 (53.5%) | <.001† |
| Active part time | 1311 (0.6%) | 3121 (1.4%) | |
| Early retiree | 17,154 (7.5%) | 20,846 (9.3%) |
POS, point of service; PPO, preferred provider organization; CDHP, consumer-directed health plan; HDHP, high-deductible health plan.
T-test.
Chi-squared test.
Table II (N = 433,001) shows the odds ratio of undergoing RCR in the univariate and multivariate models. The odds of undergoing RCR is 48% higher in patients with DM (OR = 1.48 [95% CI 1.46 ∼ 1.51], P < .001) than nondiabetic patients after adjusting for age, sex, region, insurance plan type, occupation, tobacco use, the number of years in the database, year, and CCI (calculated excluding diabetes). The association between diabetes and RCR was less than the association between tobacco abuse and RCR, which had an odds ratio of 3.91 ([95% CI 3.86 ∼ 4.02], P < .001) in the multivariate model.
Table II.
Univariable and multivariable logistic regression model results comparing RCR to diabetes and other characteristics.
| Variable | OR† (95% CI) | P value | OR‡ (95% CI) | P value |
|---|---|---|---|---|
| Diabetes | 1.46 (1.43, 1.48) | <.001 | 1.48 (1.46, 1.51) | <.001 |
| Tobacco abuse | 3.94 (3.86, 4.02) | <.001 | 3.91 (3.82, 3.99) | <.001 |
| CCI∗ | 1.02 (1.02, 1.03) | <.001 | 0.95 (0.94, 0.96) | <.001 |
| Age (in 10 yrs) | 1.00 (0.99, 1.01) | 1.00 | 0.97 (0.96, 0.98) | <.001 |
| Sex: male | 1.00 (0.99, 1.01) | 1.00 | 0.96 (0.95, 0.97) | <.001 |
| Years in database | 1.00 (1.00, 1.00) | 1.00 | 1.01 (1.01, 1.01) | <.001 |
| Year of RCR | 1.00 (1.00, 1.00) | 1.00 | 0.99 (0.98, 0.99) | <.001 |
CI, confidence interval; CCI, Charlson comorbidity index; OR, odds ratio; RCR, rotator cuff repair.
The unbolded variables were used to match the cases and controls.
N = 433,001.
CCI calculated excluding points from diabetes (approximately cci-1 for those with diabetes).
From univariable models.
From multivariable model.
The association of revision RCR in patients with DM
Table III shows the characteristics of RCR patients by DM status. Patients with DM had a mean age of 56.3 ± 6.2 as compared to 53.5 ± 7.9 of the nondiabetic RCR cases. 42.9% (17,665/41,157) of patients with DM were female, while 42.2% (79,703/188,829) of the nondiabetic RCR cases were female. Mean duration (years) in the database was 4.7 ± 2.7 for the diabetic RCR patients, as compared to 4.3 ± 2.7 for the nondiabetic RCR patients. 30.3% (12,461/41,157) of the diabetic RCR patients were active full-time employees, compared to 33.6% (63,492/188,829) of the nondiabetic RCR patients. 67% (25,717/41,157) of the diabetic RCR patients has a preferred provider organization insurance plan, compared to 68.5% (120,569/188,829) of the nondiabetic RCR cases. Postoperative infections in the DM group occurred at a rate of 0.7% (289/41,157) as compared to 0.4% (848/18,829). Mean CCI was 1.5 ± 1.2 in the diabetic RCR patients, while CCI was 0.3 ± 0.7 in the nondiabetic RCR patients. Tobacco abuse in the DM group was 19.6% (8069/41,157) as compared to 18.6% (35,154/188,829) of nondiabetic RCR patients.
Table III.
Demographics of RCR patients stratified by DM status.
| Variable | DB = yes (N = 41,157) | DB = no (N = 188,829) | P value |
|---|---|---|---|
| Revision RCR | 3172 (7.7%) | 13,944 (7.4%) | .024∗ |
| Infection | 289 (0.7%) | 848 (0.4%) | <.001∗ |
| Age: mean (SD) | 56.3 (6.2) | 53.5 (7.9) | <.001† |
| Sex: female | 17,665 (42.9%) | 79,703 (42.2%) | .008∗ |
| Duration (yrs) in database: mean (SD) | 4.7 (2.7) | 4.3 (2.7) | |
| CCI: mean (SD) | 1.5 (1.2) | 0.3 (0.7) | |
| Tobacco abuse | 8069 (19.6%) | 35,154 (18.6%) | <.001∗ |
| Payment: median (IQR) | 11,855.6 (8650.7, 17,244.8) | 11,495.0 (8432.3, 16,498.5) | <.001‡ |
CCI, Charlson comorbidity index; IQR, interquartile range; RCR, rotator cuff repair; SD, standard deviation.
Chi-squared test.
T-test.
Wilcoxon rank-sum test.
Table IV (N = 214,479) shows the hazard ratio of undergoing a subsequent RCR after primary RCR. Although DM status is significantly associated with revision RCR in the univariable model (HR = 1.07, 95% CI 1.03 ∼ 1.11, P < .001), the effect is no longer significant when adjusting for age, sex, region, plan type, tobacco use, year of RCR, and CCI (HR = 1.03, 95% CI 0.99∼1.07, P = .17). Both CCI and tobacco abuse were associated with revision RCR, with ORs of 1.15 (95% CI 1.11 ∼ 1.19, P < .001) and 1.04 (95% CI 1.02 ∼ 1.06, P < .001) respectively.
Table IV.
Univariable and multivariable Cox proportional hazards results comparing revision RCR to diabetes and other characteristics among RCR patients.
| Variable | HR† (95% CI) | P value | HR‡ (95% CI) | P value |
|---|---|---|---|---|
| Diabetes | 1.07 (1.03, 1.11) | <.001 | 1.03 (0.99, 1.07) | .17 |
| Tobacco abuse | 1.15 (1.11, 1.19) | <.001 | 1.15 (1.11, 1.19) | <.001 |
| CCI∗ | 1.05 (1.03, 1.07) | <.001 | 1.04 (1.02, 1.06) | <.001 |
| Sex: male | 1.04 (1.02, 1.06) | <.001 | 1.05 (1.02, 1.07) | <.001 |
| Age (in 10 yrs) | 1.02 (0.99, 1.06) | .12 | 1.03 (1.00, 1.06) | .06 |
| Year of RCR | 1.04 (1.03, 1.05) | <.001 | 1.04 (1.03, 1.04) | <.001 |
CI, confidence interval; CCI, Charlson comorbidity index; HR, hazard ratio; RCR, rotator cuff repair.
N = 214,479.
CCI calculated excluding points from diabetes (approximately cci-1 for those with diabetes).
From univariable models.
From multivariable model.
The cost of RCR in patients with DM
Table V (N = 214,365) demonstrates ratios in RCR cost for DM and other patient characteristics. In the univariable model, DM status was associated with a higher cost of RCR by 7% (95% CI 1.06 ∼ 1.08, P < .001). In the multivariate model, however, DM status was associated with a higher cost of RCR by 3% (95% CI 1.02 ∼ 1.03, P < .001). Cost of RCR also varied significantly by region (Table V). When compared to a comprehensive insurance plan, the insurance plan type of point of service, preferred provider organization, consumer-directed health plan, and high-deductible health plan were associated with a higher cost of RCR by 12% (95% CI 1.10 ∼ 1.15, P < .001), 9% (95% CI 1.07 ∼ 1.11, P < .001), 9% (95% CI 1.07 ∼ 1.12, P < .001), and 7% (95% CI 1.04 ∼ 1.10, P < .001) respectively. Tobacco abuse and CCI were also associated with a higher cost of RCR of 2% (95% CI 1.01 ∼ 1.03, P < .001) and 9% (95% CI 1.08 ∼ 1.09, P < .001) respectively.
Table V.
Univariable and multivariable gamma regression results comparing RCR cost to diabetes and other characteristics among RCR patients.
| Variable | Ratio in cost∗ (95% CI) | P value∗ | Ratio in cost† (95% CI) | P value† |
|---|---|---|---|---|
| Diabetes | 1.07 (1.06, 1.08) | <.001 | 1.03 (1.02, 1.04) | <.001 |
| Age (in 10 yrs) | 1.03 (1.03, 1.03) | <.001 | 1.01 (1.01, 1.02) | <.001 |
| Sex: male | 1.02 (1.02, 1.03) | <.001 | 1.03 (1.02, 1.03) | <.001 |
| Region: Northeast vs. North Central | 0.96 (0.95, 0.96) | <.001 | 1.00 (0.99, 1.01) | .91 |
| South vs. North Central | 0.95 (0.94, 0.95) | <.001 | 0.94 (0.93, 0.95) | <.001 |
| West vs. north Central | 1.06 (1.05, 1.07) | <.001 | 1.06 (1.05, 1.07) | <.001 |
| Unknown vs. north Central | 0.82 (0.81, 0.84) | <.001 | 0.83 (0.82, 0.85) | <.001 |
| Insurance plan: EPO vs. comprehensive | 0.97 (0.94, 1.00) | .022 | 0.98 (0.95, 1.00) | .10 |
| HMO vs. comprehensive | 0.94 (0.92, 0.96) | <.001 | 0.97 (0.95, 0.99) | .002 |
| POS vs. comprehensive | 1.08 (1.06, 1.11) | <.001 | 1.12 (1.10, 1.15) | <.001 |
| PPO vs. comprehensive | 1.06 (1.04, 1.08) | <.001 | 1.09 (1.07, 1.11) | <.001 |
| POS with capitation vs. comprehensive | 0.89 (0.85, 0.94) | <.001 | 0.93 (0.89, 0.98) | .002 |
| CDHP vs. comprehensive | 1.08 (1.06, 1.11) | <.001 | 1.09 (1.07, 1.12) | <.001 |
| HDHP vs. comprehensive | 1.08 (1.06, 1.11) | <.001 | 1.07 (1.04, 1.10) | <.001 |
| Tobacco abuse | 1.04 (1.03, 1.05) | <.001 | 1.02 (1.01, 1.03) | <.001 |
| Year of RCR | 1.04 (1.04, 1.04) | <.001 | 1.04 (1.04, 1.04) | <.001 |
| CCI | 1.09 (1.09, 1.10) | <.001 | 1.09 (1.08, 1.09) | <.001 |
CI, confidence interval; CCI, Charlson comorbidity index; RCR, rotator cuff repair; POS, point of service; PPO, preferred provider organization; CDHP, consumer-directed health plan; HDHP, high-deductible health plan.
N = 214,365.
From univariable models.
From multivariable model.
Discussion
In patients with DM, the odds of undergoing RCR surgery was 48% higher when compared with their nondiabetic counterparts, after adjusting for various factors. While the odds of undergoing subsequent surgical revision RCR was significantly associated with DM in the univariate model, after correcting for age, sex, region, plan type, tobacco use, year of RCR, and CCI there was no association. In the cost analysis, DM significantly associated with a 3% increase in all billable costs 3 months after RCR; however, this effect likely is not consequential and only significantly different due to the size of the cohort.
This study demonstrated that DM is associated with a higher risk of RCR. This finding is consistent with other large population-based studies. Lin et al reported a 47% higher risk of rotator cuff disease among individuals with DM, which is consistent with our finding of 48% increased odds.14 Our findings showed that DM significantly associated with rotator cuff disease in both men and women, while another reported that this effect was only present in men.23 A Finnish study demonstrated that patients with DM had an 8.8-fold increased risk of developing rotator cuff tendinitis.16 The study only included patients between the ages of 30-64, however, while our study included patients between the ages of 18-64, which may bias our results towards the null hypothesis. Overall, our results are consistent with the current literature and provide generalizable results for adult patients within the United States. These results further support the hypothesis that rotator cuff tears are a metabolic disease, in which tendon degeneration occurs partially secondary to systemic factors. This hypothesis further supported by recent studies demonstrating an increased incidence of rotator cuff disease in patients with hyperlipidemia.7,14,35
This study also demonstrated that DM does not significantly associate with rotator cuff revision surgery when adjusting for age, sex, region, plan type, tobacco use, year of RCR, and CCI. To our knowledge, this is the first database study in a general population analyzing the effect of DM on RCR failure and reoperation. Cho et al performed a retrospective analysis of 64 diabetic patients with medium- to large-sized tears with supraspinatus fatty infiltration and showed that patients with DM had a 21.5% increased rate of RCR failure. The study also reported a higher rate of revision RCR among patients with poorly-controlled hyperglycemia when compared to patients with controlled HbA1c levels.3 Our study does not control for tear size or for glycemic control, and our analysis does not measure RCR failure, only subsequent revision RCR, which is an imperfect correlate with failure. However, it does control for various comorbid factors and offers a much larger sample size. Having a diagnosis of DM alone, may not predispose patients to subsequent reoperation. Based upon these findings, the medical comorbidity of DM alone should not be used as a contraindication to RCR.
Finally, this study reported that DM significantly associates with a higher cost in health-care expenses 3 months post-RCR. Other studies have analyzed RCR cost and the patient-, as well as, physician-specific variables that affect cost1,12,18; however, to the best of our knowledge, this study is the first to analyze the effect of DM on RCR cost. While an effect was demonstrated when controlling for region, insurance type, CCI, age, sex, and date of RCR, the effect (3%) is not large enough to be clinically significant, and thus likely does not reflect an important or explainable difference. Our analysis is limited in that it reports all health-care costs within 3 months from the index date. It may be that a more comprehensive cost-analysis would arrive at a different finding.
This study has limitations that are inherent to a claims-based database analysis. Information is limited to diagnosis and procedure codes entered by individual physicians’ offices without consideration for standard diagnostic criteria. The presenting symptoms, chronicity, or the size of the rotator cuff tear cannot be ascertained from the database. Therefore, we are unable to comment on the appropriateness of the procedure performed. Our study only evaluated individuals between the ages of 18 and 64, which excludes older subjects who may have different rates of healing post-RCR. Also, our study does not differentiate between type 1 vs type 2 DM, well-controlled from poorly controlled DM, DM without complications and DM with complications. The database limits us in how the patients are adhering to their medications; thus, interpretations on the isolated effect of hyperglycemia on rotator cuff tendinopathy cannot be distinguished. Future studies analyzing the effects of healing post-RCR in insulin-dependent versus insulin-independent DM are warranted. Our study characterized tobacco use disorder using a broad range of ICD-9-CM and ICD-10-CM billings codes, which may overreport smoking habits in the population. Additionally, laterality could not be ascertained from the data; thus, the odds of revision surgery may not be representative of the ipsilateral shoulder. Furthermore, many inherent factors can increase the risk of RCR failure, such as rotator cuff tear size and fatty atrophy. Additionally, both the ICD-10-CM codes for atraumatic and traumatic RCT were utilized, limiting our analysis in that not all tears included were degenerative. We did not subdivide between rotator cuff tear types and between diabetes types as we were concerned that the coding data may not be accurate at this level of granularity. While specific demographics and other comorbid conditions were controlled for in the multivariate analyses, many other factors were not as this information is not reported to the database. Finally, MarketScan does not include patients who have noncommercial insurances such as Medicaid or a significant portion of Medicare, which includes those who are unemployed. Therefore, there may be inherent socioeconomic biases in the data. However, a large administrative database such as MarketScan can provide insight into nationwide practice patterns and raise questions for further study.
Conclusions
Patients with DM are at a higher risk of undergoing RCR surgery; however, there is no association between diabetes and subsequent rotator cuff revision surgery.
Disclaimers:
Funding: This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR002538 (formerly 5UL1TR001067-05, 8UL1TR000105 and UL1RR025764). We thank the Surgical Population Analysis Research Core, University of Utah, for its role in facilitating data collection, database management, and analysis.
Conflicts of interest: Peter Chalmers is a paid consultant for Arthrex and Mitek, is paid speaker for Depuy, serves on the editorial board for the Journal of Shoulder and Elbow Surgery, receives intellectual property royalties from Depuy, and has received other support from Tornier. Karch Smith, JJ Horns, Chong Zhang, and Angela Presson certify that they have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, and so forth) that might pose a conflict of interest in connection with the submitted article. Jim Hotaling is a paid consultant for Turtle health, holds educational/research grants from Boston Scientific and Endo Pharmaceuticals; owns equity in StreamDx, Andro360, Advanced Conceptions (early stage startups unrelated to this work) and serves on the editorial board of Fertility & Sterility, Journal of Assisted Reproduction & Genetics, International Journal of Impotence Research. He holds multiple NIH grants unrelated to this work. Robert Tashjian is a paid consultant for Zimmer/Biomet, Wright Medical and Depuy-Mitek; has stock in Conextions, INTRAFUSE, Genesis and KATOR; receives intellectual property royalties from Wright Medical, Shoulder Innovations, and Zimmer/Biomet; receives publishing royalties from Springer and the Journal of Bone and Joint Surgery, and serves on the editorial board for the Journal of Shoulder and Elbow Arthroplasty and the Journal of the American Academy of Orthopaedic Surgeons.
Footnotes
No institutional review board approval was necessary due to the data being de-identified.
References
- 1.Ardeljan A., Palmer J., Drawbert H., Ardeljan A., Vakharia R.M., Roche M.W. Partial thickness rotator cuff tears: Patient demographics and surgical trends within a large insurance database. J Orthop. 2020;17:158–161. doi: 10.1016/j.jor.2019.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedeir Y., Jimenez A., Grawe B. Recurrent tears of the rotator cuff: Effect of repair technique and management options. Orthop Rev. 2018;10:7593. doi: 10.4081/or.2018.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho N.S., Moon S.C., Jeon J.W., Rhee Y.G. The influence of diabetes mellitus on clinical and structural outcomes after arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:991–997. doi: 10.1177/0363546514565097. [DOI] [PubMed] [Google Scholar]
- 4.Chung S., Oh J., Gong H., Kim J.Y., Kim S. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39:2099–2107. doi: 10.1177/0363546511415659. [DOI] [PubMed] [Google Scholar]
- 5.Frank J., ElAttrache N., Dines J., Blackburn A., Crues J., Tibone J. Repair Site Integrity After Arthroscopic Transosseous-Equivalent Suture-Bridge Rotator Cuff Repair. Am J Sports Med. 2008;36:1496–1503. doi: 10.1177/0363546507313574. [DOI] [PubMed] [Google Scholar]
- 6.Galatz L., Ball C., Teefey S., Middleton W., Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Garcia G.H., Liu J.N., Wong A., Cordasco F., Dines D.M., Dines J.S. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086–2090. doi: 10.1016/j.jse.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Gulotta L., Nho S., Dodson C., Adler R., Altchek D., Macgillivray J. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II-prognostic factors for clinical and radiographic outcomes. J Shoulder Elbow Surg. 2011;20:941–946. doi: 10.1016/j.jse.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Hansen L. The Truven Health Marketscan Databases for life sciences researchers. 2017. https://truvenhealth.com/Portals/0/Assets/2017-MarketScan-Databases-Life-Sciences-Researchers-WP.pdf Available at: Accessed October 15, 2020.
- 10.Kluczynski M., Isenburg M., Marzo J., Bisson L. Does early versus delayed active range of motion affect rotator cuff healing after surgical repair?: a systematic review and meta-analysis. Am J Sports Med. 2015;44:785–791. doi: 10.1177/0363546515582032. [DOI] [PubMed] [Google Scholar]
- 11.Lafosse L., Brzoska R., Toussaint B., Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2008;90:275–286. doi: 10.2106/JBJS.H.00388. [DOI] [PubMed] [Google Scholar]
- 12.Li L., Bokshan S.L., Ready L.V., Owens B.D. The primary cost drivers of arthroscopic rotator cuff repair surgery: a cost-minimization analysis of 40,618 cases. J Shoulder Elbow Surg. 2019;28:1977–1982. doi: 10.1016/j.jse.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Liem D., Lichtenberg S., Magosch P., Habermeyer P. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J Bone Joint Surg Am. 2007;89:1770–1776. doi: 10.2106/JBJS.F.00749. [DOI] [PubMed] [Google Scholar]
- 14.Lin T., Lin C.-H., Chang C.-L., Chi C.-H., Chang S.-T., Sheu W. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med. 2015;43:2126–2132. doi: 10.1177/0363546515588173. [DOI] [PubMed] [Google Scholar]
- 15.Millett P., Warth R., Dornan G., Lee J., Spiegl U. Clinical and structural outcomes after arthroscopic single-row versus double-row rotator cuff repair: a systematic review and meta-analysis of level I randomized clinical trials. J Shoulder Elbow Surg. 2014;23:586–597. doi: 10.1016/j.jse.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Miranda H., Viikari-Juntura E., Heistaro S., Heliövaara M., Riihimäki H. A population study on differences in the determinants of a specifc shoulder disorder versus non-specific shoulder pain without clinical findings. Am J Epidemiol. 2005;161:847–855. doi: 10.1093/aje/kwi112. [DOI] [PubMed] [Google Scholar]
- 17.Neyton L., Godenèche A., Nové-Josserand L., Carrillon Y., Cléchet J., Hardy M. Arthroscopic suture-bridge repair for small to medium size supraspinatus tear: healing rate and retear pattern. Arthrosc J Arthrosc Relat Surg. 2012;29:10–17. doi: 10.1016/j.arthro.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson J.A., Searle H.K.C., MacDonald D., McBirnie J. Cost-effectiveness and satisfaction following arthroscopic rotator cuff repair: does age matter? Bone Joint J. 2019;101-B:860–866. doi: 10.1302/0301-620X.101B7.BJJ-2019-0215.R1. [DOI] [PubMed] [Google Scholar]
- 19.Northover J., Lunn P., Clark D., Phillipson M. Risk factors for the development of rotator cuff disease. Int J Shoulder Surg. 2007:1. doi: 10.4103/0973-6042.34025. [DOI] [Google Scholar]
- 20.Paloneva J., Lepola V., Äärimaa V., Joukainen A., Ylinen J., Mattila V. Increasing incidence of rotator cuff repairs—A nationwide registry study in Finland. BMC Musculoskelet Disord. 2015;16:189. doi: 10.1186/s12891-015-0639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascal B., Brassart N., Watkinson D., Carles M., Hatzidakis A., Krishnan S. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. doi: 10.2106/JBJS.D.02035. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team . R Found. Stat. Comput.; Vienna Austria: 2020. R: a language and environment for statistical computing.https://www.R-project.org/ Available at: [Google Scholar]
- 23.Rechardt M., Shiri R., Karppinen J., Jula A., Heliövaara M., Viikari-Juntura E. Lifestyle and metabolic factors in relation to shoulder pain and rotator cuff tendinitis: a population-based study. BMC Musculoskelet Disord. 2010;11:165. doi: 10.1186/1471-2474-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roquelaure Y., Bodin J., Ha C., Petit Le A., Descatha A., Chastang J.-F. Personal, biomechanical, and psychosocial risk factors for rotator cuff syndrome in a working population. Scand J Work Environ Health. 2011;37:502–511. doi: 10.5271/sjweh.3179. [DOI] [PubMed] [Google Scholar]
- 25.Royston P. Explained variation for survival models. Stata J. 2006;6:83–96. doi: 10.1177/1536867X0600600105. [DOI] [Google Scholar]
- 26.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 27.Shah K., Clark B., Mcgill J., Mueller M. Upper extremity impairments, pain and disability in patients with diabetes mellitus. Physiotherapy. 2015;101:147–154. doi: 10.1016/j.physio.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tashjian R. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31:589–604. doi: 10.1016/j.csm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Tashjian R., Hollins A., Kim H.-M., Teefey S., Middleton W., Steger-May K. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38:2435–2442. doi: 10.1177/0363546510382835. [DOI] [PubMed] [Google Scholar]
- 30.Tashjian R., Hung M., Burks R., Greis P. Influence of preoperative musculotendinous junction position on rotator cuff healing using single-row technique. Arthrosc J Arthrosc Relat Surg. 2013;29:1748–1754. doi: 10.1016/j.arthro.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Tashjian R.Z., Granger E.K., Zhang Y., Teerlink C.C., Cannon-Albright L.A. Identification of a genetic variant associated with rotator cuff repair healing. J Shoulder Elbow Surg. 2016;25:865–872. doi: 10.1016/j.jse.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Vavken P., Sadoghi P., Palmer M., Rosso C., Mueller M., Szöllösy G. Platelet-rich plasma reduces retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med. 2015;43:3071–3076. doi: 10.1177/0363546515572777. [DOI] [PubMed] [Google Scholar]
- 33.Williams R., Colagiuri S., Chan J., Gregg E., Ke C., Lim L.-L. 9th Edition. 2019. IDF Atlas. [Google Scholar]
- 34.Yamamoto A., Takagishi K., Osawa T., Yanagawa T., Nakajima D., Shitara H. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2009;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y., Qu J. The effects of hyperlipidemia on rotator cuff diseases: a systematic review. J Orthop Surg. 2018;13:204. doi: 10.1186/s13018-018-0912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]