Abstract
Complement is known to play a role in ischemia and reperfusion injury (IRI). A general paradigm is that complement is activated by self-reactive natural IgM antibodies (nAbs), after they engage postischemic neoepitopes. However, a role for nAbs in lung transplantation (LTx) has not been explored. Using mouse models of LTx, we investigated the role of two postischemic neoepitopes, modified annexin IV (B4) and a subset of phospholipids (C2), in LTx. Antibody deficient Rag1−/−recipient mice were protected from LTx IRI. Reconstitution with either B4 or C2nAb restored IRI, with C2 significantly more effective than B4 nAb. Based on these information, we developed/ characterized a novel complement inhibitor composed of single-chain antibody (scFv) derived from the C2 nAb linked to Crry (C2scFv-Crry), a murine inhibitor of C3 activation. Using an allogeneic LTx, in which recipients contain a full nAb repertoire, C2scFv-Crry targeted to the LTx, inhibited IRI, and delayed acute rejection. Finally, we demonstrate the expression of the C2 neoepitope in human donor lungs, highlighting the translational potential of this approach.
Keywords: basic (laboratory) research / science, complement biology, immunobiology, innate immunity, lung (allograft) function / dysfunction, lung transplantation / pulmonology
1 |. INTRODUCTION
Primary graft dysfunction (PGD) causes early mortality after lung transplantation (LTx) and contributes to late graft failure.1–3 Although the pathogenesis of PGD is multifactorial, clear associations have been made between PGD and the severity of ischemia and reperfusion injury (IRI).1,2 The complement system plays a central role in IRI and has been associated with PGD. Elevated complement levels in LTx recipients early post- LTx, are associated with PGD development, and in the case of C3a, mortality.4,5 While it is clear that complement plays a role in IRI and PGD, the mechanisms that trigger complement activation are poorly understood.
Although not studied in LTx, recent evidence indicates a role for naturally occurring self-reactive IgM antibodies (nAb) in the induction of complement activation and propagation of IRI.6,7 Seminal studies demonstrated that a nAb reactive against non–muscle myosin reconstituted mesenteric IRI in otherwise protected antibody deficient mice.6 Subsequently, we and others, identified additional IgM specificities. These include an epitope recognized by B4 nAb, which binds modified murine annexin IV,8,9 and C2 nAb, which recognizes a subset of phospholipids in rodents and humans.10 Central to all of these pathogenic nAbs is that they are injury specific and recognize cryptic neoepitopes exposed by ischemic insult. Surprisingly, blocking an individual ischemic neoepitope can provide protection against IRI, even in the context of an entire poly-reactive nAb repertoire.11 These previous studies indicate the importance of self-reactive nAb in causing tissue injury and mark them as potential targets for therapeutic intervention.
It is currently unknown if the relative levels and specificities of post–ischemic neoepitope expression in various organs and tissues differ. In this regard, an nAb termed D5 that recognizes citrullinated proteins failed to reconstitute cerebral IRI,10 but reconstituted cardiac IRI in Rag1−/−mice (our unpublished data). In this study, we first investigated the postischemic expression of B4 and C2 neoepitopes (see above) in transplanted lungs, neoepitopes that were previously shown to be expressed in other ischemic tissues. Unlike cardiac and cerebral IRI,8,10,12 we found that C2 recognition played a more prominent role in LTx IRI than B4, and based on this finding we developed a novel bi-functional C2 epitope targeted complement inhibitor to localize IgM blockade and complement inhibition to a transplanted lung. We prepared a single-chain antibody (scFv) from C2 nAb, and linked it to the murine complement inhibitor Crry that inhibits all complement pathways at the C3 activation step. Using our novel constructs, we demonstrate an important role for IgM and complement in LTx IRI, and further demonstrate that lung-specific targeting of complement inhibition effectively ameliorates IRI. We also show that the neoepitope recognized by C2 nAb in mice is specifically expressed in human lungs pretransplant, establishing translational potential.
2 |. METHODS
2.1 |. Antibodies and recombinant proteins
C2 and B4 IgM hybridomas were isolated following fusion of spleen cells from unmanipulated wt C57BL/6 mice as described.13 C2 nAb recognizes a subset of phospholipids 10 and B4 a posttranslational modification of Annexin IV.13 The IgM mAb F632 recognizes 4-hydroxy- 3- nitrophenylacetyl (NP)-KLH,13 and was used as a control. For construction of the C2 scFv expressing plasmid, mRNA was prepared from the C2 hybridoma, and cDNA corresponding to mRNA was synthesized with primers for variable heavy (VH) and variable light (VL) chain domains. A linker sequence (G4S2)2 was added to 3’ end of the VH chain and linked to the 5’ end of the VL chain, and a CD5 signal peptide sequence and a His tag (6X) sequence was added to the 5’ end of the VH chain. For construction of the C2 scFv-Crry expression plasmid, the C2 scFv sequence was linked to the extracellular region of mouse Crry (residues 1–319 of mature protein, GenBank accession number NM013499) by overlapping PCR with the linker (G4S1)2, and OKT3 light chain signal peptide sequence added to the 5’ end of the VH chain. The construct was cloned into the pEE12.4 expression plasmid (Lonza) and expressed in Expi293 cells according to manufacturer’s instructions (Thermofisher). C2scFv-Crry was purified from culture supernatant using His60 Ni Superflow resin according to manufacturer’s instructions (TaKaRa). B4scFv-Crry was constructed and purified as previously described.8 Purity was >90% for both C2 scFv and C2 scFv-Crry as assessed by SDS-PAGE. All protein preparations were tested for binding to cardiolipin and for complement inhibitory activity (below) prior to use.
2.2 |. In vitro binding and complement inhibition assays
In vitro analysis of C2scFv and C2scFv-Crry binding was determined by ELISA using cardiolipin (Sigma Aldrich, MO) as described for C2 IgM nAb.10 In vitro complement inhibitory activity of C2scFv/C2scFv-Crry was determined as described.8,12
2.3 |. In vitro simulated cold storage and IRI
Mouse microvascular endothelial cells (Cedarlane, USA) were cultured to confluence in microtiter plates and exposed to simulated hyperoxemia, and reperfusion process of LTx, as described.14 Upon initiation of reperfusion cells were treated with either control, C2scFv, or C2scFv-Crry. After 6 hrs of reperfusion, media was removed and cells stained with anti-C3, IgM or IgG FITC labeled antibodies (Bethyl Laboratories, USA) and fluorescence assessed.
2.4 |. Animal models
Eight- to 12-week old C57BL/6-Rag1−/−, BALB/c and C57BL/6 mice were purchased from Charles River Laboratories and housed at the Medical University of South Carolina (MUSC). Left LTx’s were performed as described.15 For syngeneic transplant studies, the following experimental groups were used: 1. C57BL/6 donors and recipients; 2. Rag1−/− donors and recipients; 3. Rag1−/− donors and recipients were reconstituted with either 0.1 mg of B4 nAb, C2 nAb, or control F632 mAb. For allograft studies, Balb/c donor lungs were implanted into C57BL/6 recipients, and recipient groups treated with; 1. Vehicle control (PBS); 2. 0.17 mg C2scFv; 3. 0.4 mg C2scFv-Crry (molar equivalents), or 4. 0.4 mg B4scFv-Crry. Grafts were recovered at 48 hrs for IRI, and 7 days for rejection. All procedures were approved and conformed to the institutional animal care guidelines.
2.5 |. Graft outcomes and biodistribution
Histopathological, micro-computed tomography, arterial blood gas measurements, immunohistochemistry, and immunofluorescent staining and C2scFv-Crry biodistribution were assessed as outlined in the additional Data S1.
2.6 |. Human C2mAb immunostaining
Human lung tissues from the right upper and/or middle lobes were obtained from donor lungs which were not used during the lobar transplantation or donor lung volume reduction to fit the recipient thoracic cavity at University of Florida Lung transplant program, (UF Tissue/Databank IRB201501133). As a control nonischemic lung was procured from patients undergoing lung resection for lung tumor. See Table S1 for patient dynamics. To optimize C2 localization in human tissues we generated a chimeric C2 antibody. C2 was sequenced and cDNA made of the light chain and heavy chain on IgG1 backbone. C2-muIgG1 was transiently expressed in 0.1L HEK293 using the standard process at LakePharma (Hopkinton, MA). For immunostaining human lung frozen tissues were cut and stained with C2-muIgG1 and visualized with anti-mouse Alexa 555. To facilitate morphological detail sections were co-stained with an alexa444-labeled-pan-cytokeratin (Abcam, USA) and counterstained with diamidino-2-phenylindole.
2.7 |. Statistics
GraphPad Prism version 5.0 for Mac OS X (GraphPad, San Diego, CA) and SAS version 9.2 (SAS Institute Inc, Cary, NC) were used. Differences between various groups were compared by nonparametric Wilcoxon rank-sum or Mann-Whitney test, due to small sample sizes for some experiments. Three specific pairwise comparisons were made to analyze the key questions of the effect of C2scFv, C2scFv-Crry, and B4scFv-Crry on IRI outcome; specifically comparisons between control and C2scFv, control and C2scFv-Crry, control and B4scFv-Crry, and B4scFv-Crry and C2scFv-Crry. All analyses were two-sided and a p < .05 was deemed significant.
3 |. RESULTS
3.1 |. Natural IgM antibodies restore LTx IRI in Rag1−/−recipient mice
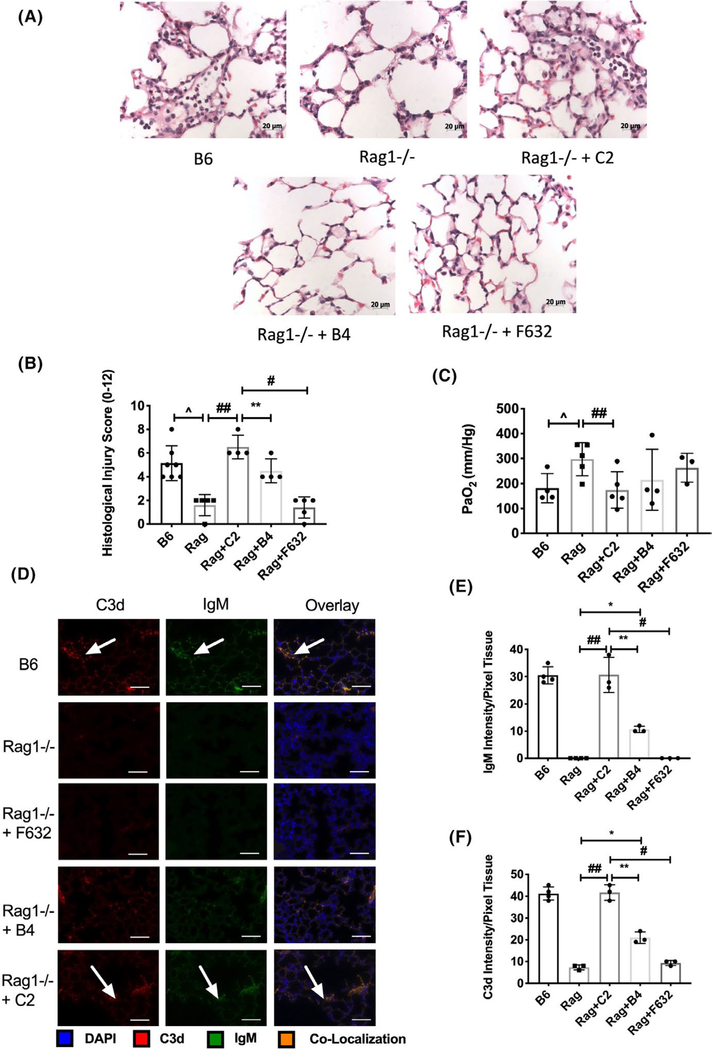
To investigate a role for nAb IgM, and specifically C2 and B4 neoepitopes in post-LTx IRI, we assessed lung IRI in wild type and Rag1−/−, and in Rag1−/− recipients reconstituted with C2, B4, or control IgM nAb. Compared to wild type, LTx IRI was significantly ameliorated in Rag1−/− recipients. However, IRI was fully restored in Rag1−/− recipients reconstituted with C2, and partially restored with B4 reconstitution. A control anti-NP IgM mAb, F632, had no effect on IRI (Figure 1A–C).
FIGURE 1.
B4 and C2 IgM nAbs reconstitute lung transplant ischemia and reperfusion injury in otherwise protected Rag1−/− syngeneic recipients. Lungs from Rag1−/− donor mice were transplanted into WT recipients, or into Rag1−/− recipients with or without administration of IgM nAb as indicated. Syngeneic lung grafts were analyzed 24 hours posttransplant. A. Representative Hematoxylin and Eosin stained lung sections. Grafts from WT recipients show evidence of intra-and peri-vascular accumulation of neutrophils, fibrin deposition and alveolar thickening, all of which are reduced in Rag1−/− recipients. All injury characteristics are restored in Rag1−/− recipients treated with C2 nAb, and to a lesser extend with B4 nAb, but not with control F632 mAb. Scale bars =20 μm. B. Semi-quantitative histology injury scores (refer to methods for scoring system). Pairwise comparisons between Rag-B6 syngeneic control vs. Rag1−/−, Rag1−/− vs C2, B4, and F632 control were made using nonparametric analysis. Mean ± SEM, n = 4–7. C. Arterial blood partial pressure of oxygen (PaO2) was significantly impaired by reconstitution of C2 nAb. Pairwise comparisons between Rag-B6 syngeneic control vs. Rag1−/−, Rag1−/− vs C2, B4, and F632 control were made using nonparametric analysis. No significant differences could be determined in these other comparisons. Mean ± SEM, n = 3–5. D. Colocalization of IgM and C3d in syngeneic lung grafts following IgM nAb reconstitution of Rag1−/− recipient mice. Binding was assessed by immunofluorescence microscopy of sections of transplanted lungs isolated 24 hours posttransplant. IgM and C3d binding colocalized on the lung microvasculature and lung epithelial cells. Representative image, n = 3. Scale bar = 50 μm. E and F. Quantification of pixel fluorescence intensity normalized to tissue area demonstrating significant increase in IgM (D) and C3d (E) in C57BL/6 (B6) WT syngeneic recipients and C2 reconstituted Rag 1- /- recipients as compared to Rag1−/−, Rag1−/− + B4, and Rag 1−/− + F632. Pairwise comparisons ^p <.01 Rag1−/− vs B6 control, ##p < .01 Rag1−/− + C2 vs Rag 1−/−, **p < .01 Rag1−/− + C2 vs Rag1−/− + B4, #p < .001 Rag1−/− + C2 vs Rag1−/− + F632, *p < .01 Rag 1−/− vs Rag 1−/− + B4
Graft binding of C2 and B4 nAbs and complement activation was investigated by immunohistochemistry. C2 and B4 nAbs bound to epithelial and endothelial cells within lung grafts in reconstituted Rag1- /- recipients (Figure 1C). No IgM immunostaining was detected in grafts from Rag1−/− recipients treated with F623 control mAb (Figure 1C), or in native lungs (not shown). Automated quantification of immunofluorescent staining demonstrated a significant increase in IgM and C3d in C2 and B4 reconstituted recipients as compared to controls, and F632 reconstituted recipients (Figure 1D,E). Additionally, C2 IgM and C3d deposition was significantly greater than B4 (Figure 1D,E). Taken together, these data demonstrate that C2 epitope recognition appears to play a more prominent role in complement activation and lung IRI compared to B4 (Figure 1D,E). We therefore investigated whether the C2 epitope could be exploited as a therapeutic target for complement inhibition.
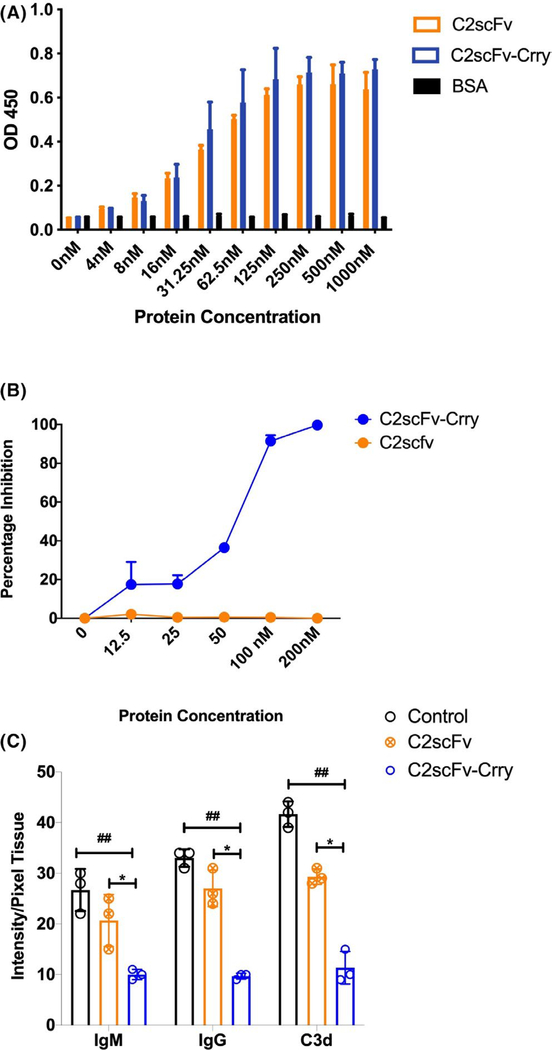
3.2 |. Preparation and in vitro characterization of C2 epitope-targeted scFv constructs
Our strategy for C2 epitope- targeted complement inhibition involved preparing a C2 scFv, derived from the parent IgM hybridoma, and linking C2scFv to Crry, a murine C3 complement inhibitor. The resultant construct, C2scFv-Crry, thus has the potential to not only target complement inhibition to the postischemic LTx, but also to inhibit the binding of C2-specific self-reactive pathogenic IgM to the reperfused graft. C2scFv and C2scFv-Crry retained parent IgM binding specificity, as demonstrated by its ability to bind directly to cardiolipin, a phospholipid we have shown to specifically bind C2 IgM (Figure 2A).10 C2scFv-Crry, unlike C2scFv, also demonstrated complement inhibitory activity (Figure 2B). Our in vivo Rag1−/− reconstitution data suggest that preexisting nAbs bind to ischemically exposed neoepitopes that drive complement-mediated graft injury. To explore the functionality of the C2 constructs, we utilized a model of simulated in vitro lung cold storage and reperfusion injury.15 To characterize the constructs, C2scFv or C2scFv-Crry was added to cultures of microvascular endothelial cells at the time of reperfusion. At 6 hrs postreperfusion, C2scFv and C2scFv-Crry significantly reduced IgM binding, suggesting that C2scFv interferes with IgM binding to injury-induced neoepitopes (Figure 2C). The bifunctionality of C2scFv-Crry resulted in a concomitant decrease in IgG and C3d deposition as compared to control or C2scFv.
FIGURE 2.
In vitro characterization of C2scFv and C2scFv-Crry. A. Phospholipid binding. Binding of C2scFv and C2scFv-Crry to cardiolipin-coated ELISA plate wells, a known ligand for C2 nAb IgM. BSA coated wells were used for specificity control. Mean ± SEM, n = 3. B. Complement inhibitor activity. Complement inhibitory activity of C2scFv and C2scFv-Crry as measured by zymosan assay. Increasing concentrations of the constructs were incubated with activated zymosan particles in mouse serum, and C3 deposition assayed by flow cytometry. Mean ± SD, n = 3. C. Ab and C3 cellular deposition after injury. Effect of C2scFv and C2scFv-Crry on IgM, IgG and C3d deposition on endothelial cells under conditions of simulated cold storage and reperfusion with pooled mouse serum. C2scFv-Crry significantly reduced binding of all molecules. ##p < .01 C2scFv-Crry vs Control and *p < .01 C2scFv-Crry vs C2scFv. Representative of n = 3 experiments
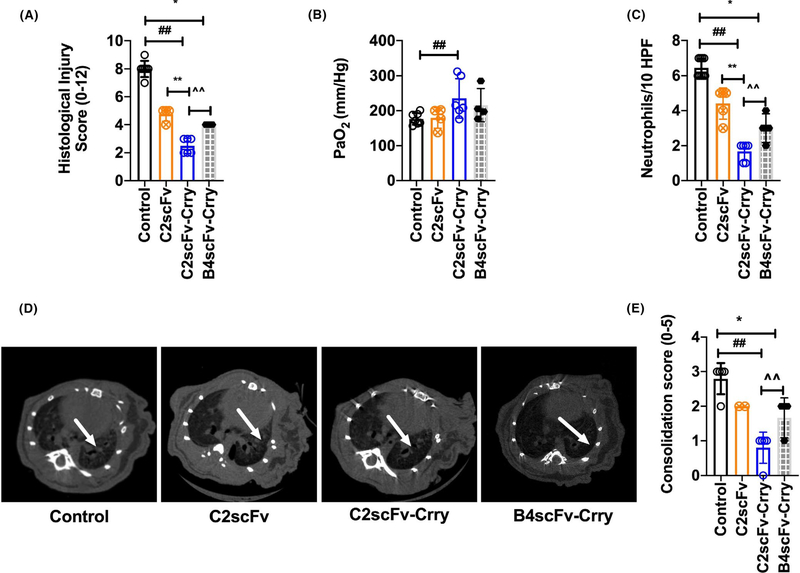
3.3 |. C2scFv- Crry significantly ameliorates lung allograft ischemia and reperfusion injury
Allograft recipients were treated with C2scFv, C2scFv-Crry or PBS vehicle immediately post-LTx, and graft injury assessed at 48 hrs post-LTx. Compared to controls, recipients treated with C2scFv or C2scFv-Crry had significantly reduced histological injury, although injury in C2scFv-Crry-treated animals was significantly less than that in animals treated with C2scFv (Figure 3A). C2scFv-Crry, but not C2scFv, also significantly improved oxygenation and reduced neutrophil infiltration compared to controls (Figure 3B,C). Micro-CT demonstrated that all LTx were aerated at 48 hrs post-LTx irrespective of treatment group. Allograft consolidation/opacification was significantly reduced in C2scFv-Crry treated compared to control and C2scFv 16 (Figure 4E).
FIGURE 3.
C2scFv-Crry reduces lung allograft ischemia and reperfusion injury and inflammation. Recipient mice were treated with PBS, C2scFv, C2scFv-Crry, or B4scFv-Crry and parameters of allograft injury and inflammation assessed at 48 hours posttransplant. A. Semi-quantitative histological assessment of injury (refer to methods for scoring system). Non–parametric two tailed analysis: PBS control vs. C2scFv (p = ns), ##PBS control vs. C2scFv-Crry (p < .004), and **C2scFv vs. C2scFv-Crry (p = ns), *PBS control vs. B4scFv-Crry (p = .01), ^^B4scFv-Crry vs. C2scFv-Crry (p = .04). B. Arterial blood partial pressure of oxygen (PaO2) is higher in mice treated with C2scFv-Crry. PBS control vs. C2scFv (p = ns), ##PBS control vs. C2scFv-Crry (p = .04), and **C2scFv vs. C2scFv-Crry (p = ns). *PBS control vs. B4scFv-Crry (p = ns), ^^B4scFv-Crry vs. C2scFv-Crry (p = ns). C. Neutrophil numbers were determined by image analysis of computerized randomly generated high-power fields. Neutrophil accumulation in transplanted lungs shows a significant decrease in neutrophil numbers in C2scFv and C2sFv-Crry-treated recipients as compared to PBS. PBS control vs. C2scFv (p = .001), ##PBS control vs. C2scFv-Crry (p = .001), and **C2scFv vs. C2scFv-Crry (p = .001). *PBS control vs. B4scFv-Crry (p = .01), ^^B4scFv-Crry vs. C2scFv-Crry (p = .01). D. Micro-CT scans were performed after lung transplantation. Transplanted left lung grafts are indicated with arrows (D). Allograft consolidation on micro-CT scan was graded from 1 to 5 (as described in the method section). Consolidation grades show significantly reduced consolidation in the C2scFv-Crry group compared to control and C2scFv-treated recipients. PBS control vs. C2scFv (p = ns), ##PBS control vs. C2scFv-Crry (p = .005), and **C2scFv vs. C2scFv-Crry (p = ns).*PBS control vs. B4scFv-Crry (p= .04), ^^B4scFv-Crry vs. C2scFv-Crry (p= .04). Representative of n = 2–5
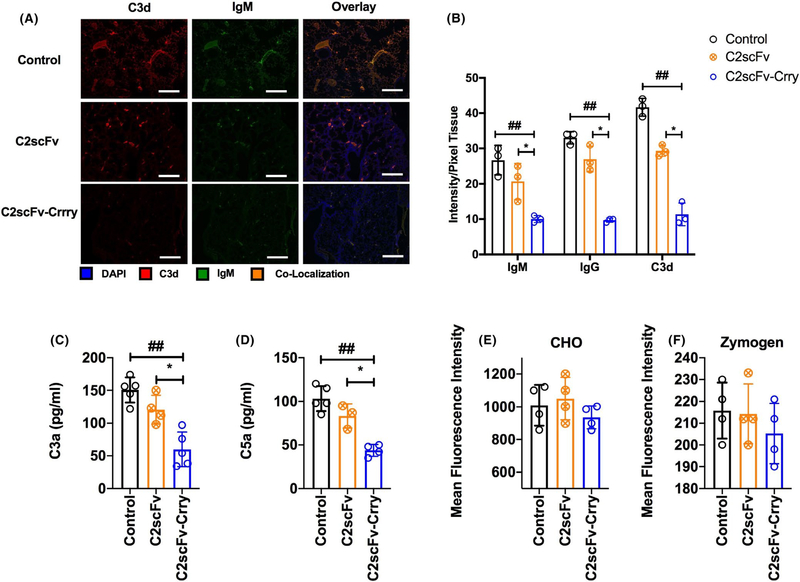
FIGURE 4.
C2scFv-Crry reduces IgM and complement deposition in transplanted lungs with minimal impact on systemic complement activity. All analyses at 48 h after transplant. A. Immunofluorescent localization of IgM, IgG and C3d in transplanted lungs. Representative images, n = 3. Scale bar 100 μm. B. Quantification of IgM, IgG and C3d by pixel fluorescence intensity normalized to tissue area. C2scFv-Crry treatment of recipients significantly reduced deposition of all molecules in transplanted lungs relative PBS and C2scFv treatment. For IgM, ##PBS control vs. C2scFv-Crry (p = .04), and *C2scFv vs. C2scFv-Crry (p = .01); for IgG, ##PBS control vs. C2scFv-Crry (p = .01), and*C2scFv vs. C2scFv-Crry (p = .001), and C3d; ##PBS control vs. C2scFv-Crry (p = .03), and*C2scFv vs. C2scFv-Crry (p = .01). No significant difference was seen between IgM, IgG, or C3d deposition in lungs from PBS- vs C2scFv-treated mice. Mean ± SEM, n = 3. C and D. C2scFv-Crry treatment significantly reduced lung allograft C3a and C5a levels as compared to PBS and C2scFv treatment. For C3a, ##PBS control vs. C2scFv-Crry (p = .002), and *C2scFv vs. C2scFv-Crry (p = .002); for C5a, ##PBS control vs. C2scFv-Crry (p = .01), and *C2scFv vs. C2scFv-Crry (p = .005). E and F. C2scFv-Crry did not significantly impact systemic complement activity as determined by CHO cell lysis assay (E) and zymozan C3d deposition assay (F), which measure classical and alternative pathway activity, respectively
We hypothesized that C2scFv would be the optimum nAb targeting strategy given C2 nAb ability to induce significantly more injury in our reconstitution experiments, as compared to B4 nAb. To further address this we utilized a B4scFv-Crry construct that we previously demonstrated to provide protection from IRI in a cardiac transplant model.8 While B4scFv-Crry also improved the histological score, micro-CT and reduced neutrophil influx, as compared to control (Figure 3A–E), C2scFv-Crry treatment was associated with significantly better outcomes than B4scFv-Crry-treated recipients when administered at an eqvilant dose, perhaps supporting improved lung targeting.
3.4 |. C2scFv- Crry inhibits lung injury without impacting systemic complement activity
At 48 hrs post-LTx, C2scFv treatment had no significant impact on IgM, IgG or C3d deposition in grafts compared to controls (Figure 4A,B). In contrast, C2scFv- Crry- treated recipients had significantly reduced IgM, IgG and C3d deposition compared to all other groups (Figure 4A,B). Increased C3a and C5a are associated with PGD.4 We therefore measured lung C3a and C5a levels in recipients. C2scFv-Crry, but not C2scFv, significantly reduced C3a and C5a in recipient lung homogenates.
Systemic complement inhibition has been shown to increase susceptibility to infection.17 We therefore assessed the effect of C2scFv and C2scFv-Crry on serum complement activity in recipients 48 hrs post-LTx. Using tests specific for either the classical or alternative complement pathways, we determined that neither C2scFv nor C2scFv-Crry had any measurable effect on serum complement activity (Figure 4E,F).
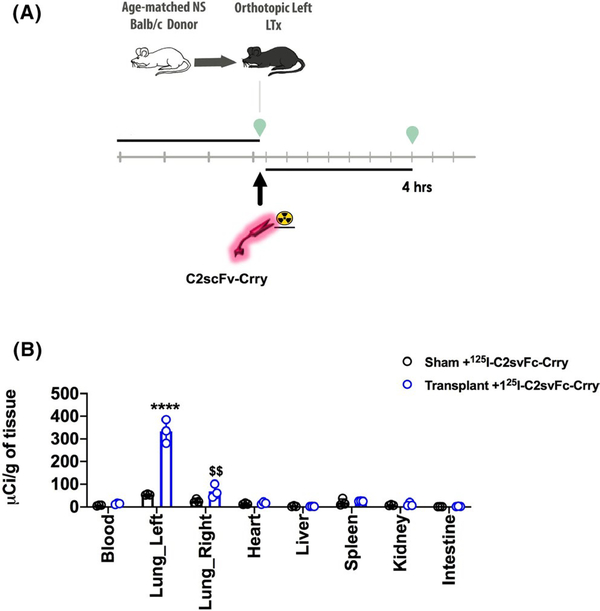
3.5 |. In vivo biodistribution of C2scFv-Crry
To explore ischemic/injury-specific targeting of C2scFv-Crry, we performed biodistribution studies.125I-labeled- C2scFv-Crry was administered to recipient mice immediately after LTx, and tissue distribution of radiolabel determined 4 hrs later (Figure 5A).125I-labeled- C2scFv-Crry localized primarily to the transplanted (left) lung (Figure 5B), with little to no radiolabel seen in peripheral organs. A relatively small amount of radiolabel localized to the right native lung, presumably associated with ventilator injury induced during the LTx process.
FIGURE 5.
C2scFv-Crry targets specifically to the transplanted lung. A. Schematic of the experimental procedure. B. Biodistribution of 125I-labeled C2scFv-Crry. Radiolabeled reagent was injected into recipient immediately after transplantation, and biodistribution of label determined 4 hrs later. Mean ± SEM, n = 3. C2scFv-Crry vs Sham control transplanted left lung ****p < .0001, C2scFv-Crry vs Sham control transplanted right lung $ $p < .01. Left and right lung vs all other organs C2scFv-Crry significantly increased
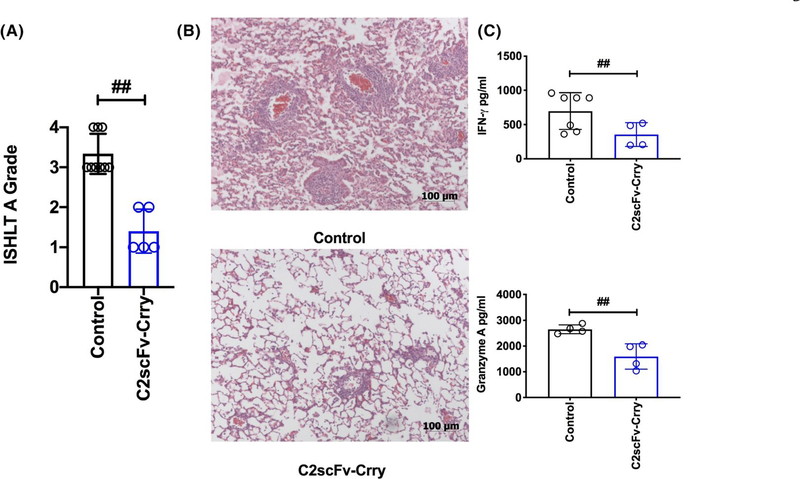
3.6 |. C2scFv-Crry significantly attenuates lung allograft rejection
To assess the impact of post–transplant C2scFv-Crry therapy on LTx acute rejection, we performed allogeneic LTx. Allografts generated robust grade 3–4 acute rejection 7 days post-LTx, as determined by the ISHLT Grade A score (Figure 6A,B). Treatment with C2scFv-Crry immediately post-LTx significantly reduced rejection grade (Figure 6A,B). To determine the impact of lung targeted complement inhibition on T cell allogenicity we performed mixed lymphocyte reaction analysis of recipient splenocytes at 7 days post-LTx. Analysis of recipient splenic T cell IFNγ and granzyme B production revealed that C2scFv-Crry therapy reduced systemic allogenicity (Figure 6C).
FIGURE 6.
C2scFv-Crry ameliorates acute cellular rejection measured at day 7 posttransplant. A. Histopathological lung graft analysis (refer to methods for scoring system). All graft samples demonstrated characteristic perivascular immune infiltrates, but pairwise comparisons of ISHLT A grades demonstrated that acute rejection is significantly reduced in C2scFv-Crry-treated recipients. ##PBS control vs. C2scFv-Crry (p = .0005). B. Representative hematoxylin and eosin stained lung sections, depicting data shown in (A). C. Splenic immune cells from each recipient were isolated at 7 days posttransplant and co-incubated with donor splenocytes. The mixed lymphocyte reactions show a significant reduction in systemic allogenicity as demonstrated by a reduction in T cell IFNγ and Granzyme A production in C2scFv-Crry at day 7 posttransplant. IFNγ ##PBS control vs. C2scFv-Crry (p = .048), and Granzyme A ##PBS control vs. C2scFv-Crry (p = .006)
3.7 |. C2 epitope is present in human donor lungs pretransplant
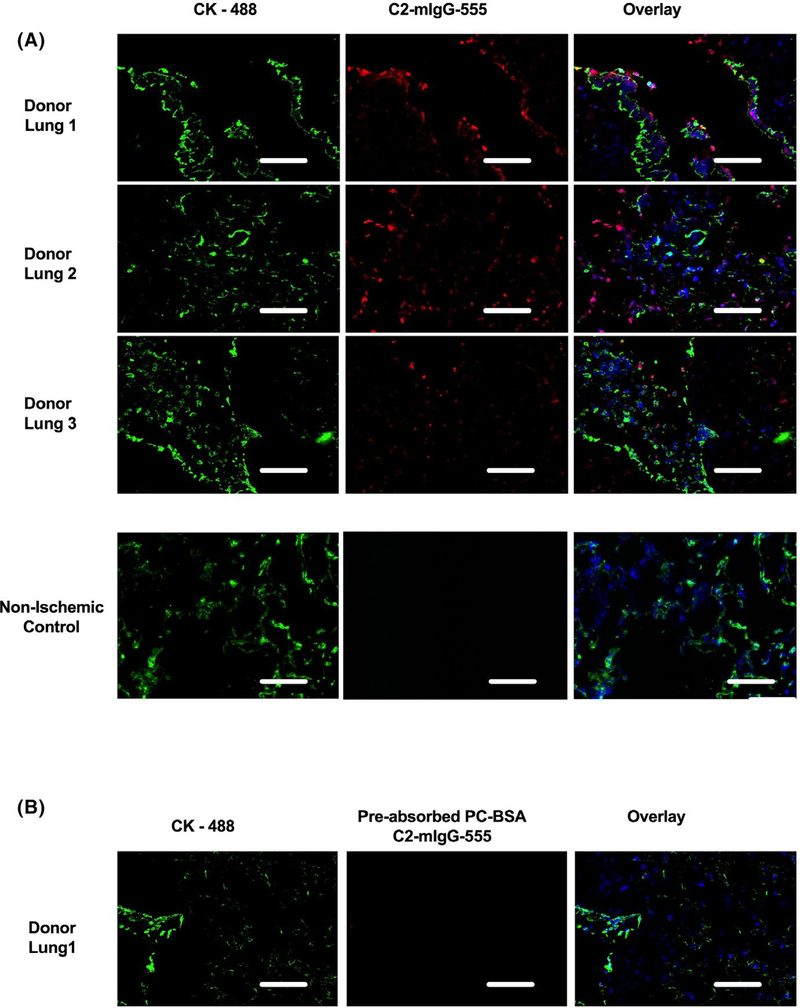
Finally, for translational significance we investigated whether there is a similar C2 recognition system in humans. We utilized a recombinant chimeric C2 antibody prepared by linking C2 nAb variable region to mouse IgG1 Fc to facilitate detection and visualization using an anti-mouse IgG secondary antibody. Lung samples were collected from donor lungs, trimmed to enable reduced size lobar LTx, and lung tissues from non–ischemic control lungs (from lung tumor resections). There was extensive binding of C2 to the lung epithelial and endothelial cells within the parenchyma and large airway (Figure 7A) of the ischemic donor lungs that was absent in nonischemic controls. We analyzed samples from three donor lungs and three nonischemic controls, and immunostained three lung tissue blocks per patient. Positive staining in donor lungs was abolished by preincubation of the C2-IgG chimeric antibody with PCA-BSA, a phospholipid-protein complex previously shown to bind C2 (Figure 7B), thus confirming the specificity of C2 binding.
FIGURE 7.
C2 epitope is present in ischemic human donor lungs. A. Immunofluorescent localization of C2 antigen using a chimeric C2-mIgG antibody for detection. Lung tissues were collected from donor lungs (n = 3), trimmed to enable reduced size lobar LTx and lung tissues from non–ischemic control lungs (n = 3, from lung tumor resections). C2 epitope staining with C2-mIgG-Alexa 555 (Red) and Pan cytokeratin (green) used for tissue architecture, demonstrated the presence of the C2 epitope (red) dispersed throughout the lung tissues from ischemic donor lungs, but were not present in nonischemic controls (representative of n = 3). B. Specificity of the C2-mIgG-555 antibody was confirmed by preincubating the antibody with PC-BSA, a known binding epitope of C2, which resulted in loss of C2 epitope staining in the donor lung samples (refer to methods for details). Representative images from 18 samples from six different patients. Scale bar 100 μm
4 |. DISCUSSION
IRI is a significant risk factor associated with the development of PGD, and here we describe a mechanism for LTx IRI mediated by nAb recognition of ischemic neoepitopes and subsequent activation of complement. We describe the development and characterization of a novel bi-functional construct that exploits this self-reactive nAb recognition system to target a complement inhibitor specifically to post–ischemic transplanted lung.
Multiple specificities of self-reactive nAbs that pathogenically engage ischemic or injured cells have been identified, but it is not clear whether there are differences in epitope expression or nAb engagement in different tissues. The postischemic neoepitopes expressed in mice and recognized by B4 and C2 IgM nAbs have been identified as modified annexin IV,13 and a subset of phospholipids,10 respectively. Their expression posttransplant has only been shown in a cardiac transplantation model, where both epitopes appeared to be similarly expressed in terms of IgM binding and B4 vs. C2 reconstitution of cardiac IRI in Rag1−/− mice.8 In contrast to this finding, while we found a similar distribution of B4 and C2 nAb binding in transplanted lungs of Rag1−/− recipients, the level of IRI was significantly greater in C2 compared to B4 reconstituted recipients. Whether this was related to quatitative differences in neoepitope exposure in the lung as compared to the heart, or whether there are temporal differences in the exposure of B4 or C2 epitopes associated with the propogation of injury, is not clear and warrants further investigation. Nevertheless given that C2 binds, activates complement, and in this post–transplant IRI paradigm most effectively reconstitutes injury, we exploited C2 epitope exposure for the targeted delivery of a complement inhibitor directly and specifically to the lung posttransplant. To note, the B4 and C2 neoepitopes have been identified in other tissues in models of warm IRI, and have been reviewed.18
Given the polyreactivity and various specificities of nAbs that have been described, it is somewhat surprising that previous studies have shown that blockade of a single nAb specificity can provide protection from IRI. For example, peptide mimics of post–ischemically expressed nonmuscle myosin,11 and recombinant annexin IV,10 can each protect against IRI. There is currently no explanation for why blockade of a single Ab specificity in wild type mice that contain a full natural Ab repertoire is protective. Blocking single nAb specificities with blocking peptides is an attractive therapeutic approach, but there are a number of drawbacks. The strategy would require a systemic effect and, potentially, a high dose of blocking agent. It is also not known if there are significant variations in the natural Ab repertoire, particularly as they relate to binding in different organs. The redundancy in this recognition system would also appear to make an approach that blocks a single Ab specificity suboptimal. Preventing the binding of pathogenic IgM by targeting neoepitopes expressed on the postischemic organ/tissue thus seems a preferable approach, although the concern of redundancy remains. We therefore generated C2scFv and linked it to Crry, an inhibitor of complement that functions at the central C3 activation step. The advantage of this is two fold; firstly, in the event of insufficient blockade of pathogenic IgM binding by scFv, it would still function as a targeting vehicle, and localized inhibition of complement activation would be maintained. Secondly, recent studies have shown that a second wave of nAbs can be formed in response to lung injury caused by IRI.19 In this second wave, injury to the lung liberates lung peptides, such as Collagen V, which stimulate B1 cells, the B cells responsible of nAb production, to release collagen V nAb. These novel observations were made in a human PGD cohort study where a small group of eight patients were shown to be collagen V antibody negative prior to transplantation, but within 24 hrs posttransplant were positive and had severe grade 3 PGD posttransplant. As a means to explain the phenomenon the authors stimulated B1 cells with collagen V peptides and demonstrated that collagen V antibody production was contact dependant, with antibodies secreted 4 hrs poststimulation.19
In a previous study we characterized B4scFv and B4scFv-Crry constructs in a model of cardiac transplantation,8 and here show that B4scFv-Crry, at eqivalent dose to C2scFv-Crry, provides some protection in the lung, albeit less than that seen with C2scFv-Crry. We showed in the heart that B4scFv alone had a modest, but significant therapeutic effect on various outcomes measured at 48 hrs posttransplant, although B4scFv-Crry provided optimal protection.8 In contrast, in the current LTx studies, C2scFv alone did not provide a significant level of protection in any outcome measure at 48 hrs posttransplant, and the inclusion of Crry in the construct was required for protection. Possible explantaions for this include increased involvement of other complement activation pathways in lung IRI, such as the alternative pathway, graft recognition by alloreactive Abs, or the presence of a second wave of nAb released in response to injury.
Complement inhibition has been previously explored in LTx in rodent models and in clinical trials. We have previously shown that direct nebulization of C3a receptor antagonist (C3aRA) prior to transplantation reduces lung injury and delays the onset of acute rejection.20 This is in keeping with the studies presented herein where C2scFv-Crry significantly inhibits all measured outcomes of IRI, and further delay rejection onset and alter systemic alloimmunity. While we did not perform a side by side analysis, there are major potential advantages of C2scFv-Crry compared to C3aRA as a therapeutic approach; whereas C3aRA only inhibits C3a signaling, C2scFv-Crry will inhibit both C3 and C5 converatse activity, thus inhibiting the generation of all bioactive complement activation products, namely C3 opsonins, C3a, C5a and the cytolytic membrane attack complex. This is likely an important consideration given the key roles that C3 and C5 activation fragments play in PGD and adaptive alloimmune responses.21 To note, the human structural and functional analogue of Crry is CR1, and soluble (untargeted) forms of CR1 have been shown to be safe in clinical trials, although high dosing of this untargeted inhibitor was required to maintain inhibition of serum complement 22(see also below).
To date, three clinical trials have explored short-term perioperative complement inhibition in LTx. Administration of TP10 (soluble CR1) led to early extubation in a significantly higher proportion of LTx patients in a small randomized control trial.22,23 C1 esterase inhibitor (C1-INH) has been used to ameliorate PGD, and while C1-INH had acceptable outcomes in terms of PGD, overall survival in treated patients was lower compared to controls.24 Another study used C1-INH in two patients refractory to standard of care antibody mediated rejection (AMR) therapies.25 C1-INH therapy demonstrated a rapid clinical and radiological improvement, but there were concerns over associated infection risks.25 Importantly, the above therapeutic strategies require systemic inhibition of complement for efficacy. As reported in the AMR study above, a potential problem in the translation of a complement inhibitory strategy to the clinic, especially in the transplant setting where the patient is heavily immunocompromised, is the additional immunosuppressive effect of systemic complement inhibition. This highlights an important advantage of a targeted approach. We have shown previously,8,12,26 and as demonstrated here, targeting a complement inhibitor to sites of complement activation significantly improves bioavailability and efficacy, thus reducing required dose, while obviating the need for systemic complement inhibition. We have also previously shown that unlike systemic complement inhibition, targeted complement inhibition does not increase host susceptibility to infection.26
Our first generation targeted complement inhibitors comprised constructs that linked a complement inhibitor to a fragment of complement receptor 2, that binds C3d, a complement activation product deposited at sites of complement activation.17 There are a number of potential advantages with the targeting strategy described here for ameliorating post–ischemic lung injury. First, it will target the proximal event in complement activation. Second, the targeting vehicle itself contributes to therapeutic activity, at least early in the injury cycle, by blocking the binding of complement activating pathogenic IgM. This will in turn also reduce the binding of the complement recognition molcules C1q and MBL, which can impact inflammation, endothelial activation, cell trafficking 27–29 and have recently been associated with PGD.5 Finally, neoepitope targeting may be less immunosuppressive since not all sites of infection and C3 deposition will be targeted with complement inhibition.
It is well documented that complement activation fragments play key roles in driving and skewing T cell immune activation.30–33 Rejection in the lung allograft is unique, in so much as immune activation and priming for rejection appears to occur locally within the lung itself,34 and as such we hypothesize that reduction in local lung allograft complement activation fragments, C3a and C5a (Figure 4A–D), likely alters complement-mediated T cell activation. Our acute rejection studies and T cell alloimmune MLR data indicate that preventing early graft complement activation reduces IRI, and as such has an impact on the T cell alloimmune response. Altering local lung complement activation levels may have a myriad of potential effects on dendritic cells, macrophages and T cell priming, given published literature,32,35–37 and therefore we believe a detailed and extensive study is required to dissect out the mechanism/s by which local complement inhibition delays the onset of rejection.
In summary, we have identified pathophysiologically important neoepitopes expressed in the lung posttransplant, and demonstrate that recognition of these neoepitopes by nAbs promotes lung IRI. Based on these findings, we developed and characterized a therapeutic that exploits posttransplant expression of one of these neoepitopes, C2, in the lung. Our C2scFv-Crry construct targeted specifically to the transplanted lung and provided effective protection from complement activation and IRI, while having minimal effect on systemic complement levels. We additionally demonstrated that the C2 neoepitope is expressed in ischemic human lungs that were used for transplantation, indicating a potential path for direct clinical translation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Q32 Bio, Cambridge, MA, for providing the C2-mouse IgG1 chimeric antibody that was used for immunostaining human samples. These studies were supported by grants from the NIH (HL140470-0181 to CA, 1U01 AI132894-01 to CA/ ST, RO1DK102912 to ST, R01AR51749 to VMH, HL116656 and HL135227 to EC), Lee Patterson Allen Foundation Award (CA and SN), NIH Institutional Postdoctoral Training Grant, NIH-HL-007260 (KP) and South Carolina Clinical & Translational Research Institute, Medical University of South Carolina’s CTSA, NIH/NCATS Grant Number UL1TR000062.
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Number: AI132894-01, HL140470-0181, RO1DK102912, R01AR51749, HL116656 and HL135227
Abbreviations:
- AMR
antibody mediated rejection
- C1- INH
C1 esterase inhibitor
- C3aRA
C3a receptor antagonist
- IRI
ischemia and reperfusion injury
- LTx
lung transplantation
- MUSC
Medical University of South Carolina
- nAbs
natural IgM antibodies
- NP
nitrophenylacetyl
- PGD
Primary graft dysfunction
- scFv
single-chain antibody
- VH
variable heavy
- VL
variable light
Footnotes
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. ST and VMH are consultants for Q32 Bio, a company developing complement inhibitors. ST, CA, VMH, and LK are inventors on a licensed patent for targeting constructs based on natural antibodies. The other authors have no conflicts to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Cantu E, Diamond JM, Suzuki Y, et al. Quantitative evidence for revising the definition of primary graft dysfunction after lung transplant. Am J Respir Crit Care Med. 2018;197(2):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porteous MK, Lee JC, Lederer DJ, et al. Clinical risk factors and prognostic model for primary graft dysfunction after lung transplantation in patients with pulmonary hypertension. Ann Am Thorac Soc. 2017;14(10):1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porteous MK, Diamond JM, Christie JD. Primary graft dysfunction: lessons learned about the first 72 h after lung transplantation. Curr Opin Organ Transplant. 2015;20(5):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah RJ, Emtiazjoo AM, Diamond JM, et al. Plasma complement levels are associated with primary graft dysfunction and mortality after lung transplantation. Am J Respir Crit Care Med. 2014;189(12):1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni HS, Ramphal K, Ma L, et al. Local complement activation is associated with primary graft dysfunction after lung transplantation. JCI Insight. 2020;5(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Austen WG, Chiu I, et al. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2004;101(11):3886–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M, Michael LH, Grosjean SA, et al. The role of natural IgM in myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2006;41(1):62–67. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson C, Qiao F, Yang X, et al. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury. Circulation. 2015;131(13):1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alawieh A, Elvington A, Zhu H, et al. Modulation of post-stroke degenerative and regenerative processes and subacute protection by site-targeted inhibition of the alternative pathway of complement. J Neuroinflammation. 2015;12:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elvington A, Atkinson C, Kulik L, et al. Pathogenic natural antibodies propagate cerebral injury following ischemic stroke in mice. J Immunol. 2012;188(3):1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas MS, Alicot EM, Schuerpf F, et al. Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc Res. 2010;87(4):618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson C, He S, Morris K, et al. Targeted complement inhibitors protect against posttransplant cardiac ischemia and reperfusion injury and reveal an important role for the alternative pathway of complement activation. J Immunol. 2010;185(11):7007–7013. [DOI] [PubMed] [Google Scholar]
- 13.Kulik L, Fleming SD, Moratz C, et al. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182(9):5363–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao W, Zhao J, Kim H, et al. alpha1-Antitrypsin inhibits ischemia reperfusion-induced lung injury by reducing inflammatory response and cell death. J Heart Lung Transplant. 2014;33(3):309–315. [DOI] [PubMed] [Google Scholar]
- 15.Patel KJ, Cheng QI, Stephenson S, et al. Emphysema-associated Autoreactive Antibodies Exacerbate Post-Lung Transplant Ischemia-Reperfusion Injury. Am J Respir Cell Mol Biol. 2019;60(6):678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida M, Oishi H, Martinu T, et al. Pentraxin 3 deficiency enhances features of chronic rejection in a mouse orthotopic lung transplantation model. Oncotarget. 2018;9(9):8489–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson C Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J Clin Invest. 2005;115(9):2444–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomlinson S, Thurman JM. Tissue-targeted complement therapeutics. Mol Immunol. 2018;102:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaffiri L, Shah RJ, Stearman RS, et al. Collagen type-V is a danger signal associated with primary graft dysfunction in lung transplantation. Transpl Immunol. 2019;56:101224. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Q, Patel K, Lei B, et al. Donor pretreatment with nebulized complement C3a receptor antagonist mitigates brain-death induced immunological injury post-lung transplant. Am J Transplant. 2018;18(10):2417–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chun N, Horwitz J, Heeger PS. Role of complement activation in allograft inflammation. Curr Transplant Rep. 2019;6(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamora MR, Davis RD, Keshavjee SH, et al. Complement inhibition attenuates human lung transplant reperfusion injury: a multicenter trial. Chest. 1999;116(1 Suppl):46S. [DOI] [PubMed] [Google Scholar]
- 23.Keshavjee S, Davis RD, Zamora MR, et al. A randomized, placebo-controlled trial of complement inhibition in ischemia-reperfusion injury after lung transplantation in human beings. J Thorac Cardiovasc Surg. 2005;129(2):423–428. [DOI] [PubMed] [Google Scholar]
- 24.Sommer W, Tudorache I, Kühn C, et al. C1-esterase-inhibitor for primary graft dysfunction in lung transplantation. Transplantation. 2014;97(11):1185–1191. [DOI] [PubMed] [Google Scholar]
- 25.Parquin F, Cuquemelle E, Camps E, et al. C1-esterase inhibitor treatment for antibody-mediated rejection after lung transplantation: two case reports. Eur Respir J. 2020. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson C, Song H, Lu B, et al. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J Clin Invest. 2005;115(9):2444–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu ZH, Li C, Chen JW, et al. C1q/TNF-related protein 1 promotes endothelial barrier dysfunction under disturbed flow. Biochem Biophys Res Commun. 2017;490(2):580–586. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Tan A, Yang R, et al. C1ql1/Ctrp14 and C1ql4/Ctrp11 promote angiogenesis of endothelial cells through activation of ERK1/2 signal pathway. Mol Cell Biochem. 2017;424(1–2):57–67. [DOI] [PubMed] [Google Scholar]
- 29.Megyeri M, Jani PK, Kajdácsi E, et al. Serum MASP-1 in complex with MBL activates endothelial cells. Mol Immunol. 2014;59(1):39–45. [DOI] [PubMed] [Google Scholar]
- 30.Lalli PN, Zhou W, Sacks S, Medof ME, Heeger PS. Locally produced and activated complement as a mediator of alloreactive T cells. Front Biosci (Schol Ed). 2009;1:117–124. [DOI] [PubMed] [Google Scholar]
- 31.Strainic MG, Liu J, Huang D, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28(3):425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raedler H, Yang M, Lalli PN, Medof ME, Heeger PS. Primed CD8(+) T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant. 2009;9(8):1784–1795. [DOI] [PubMed] [Google Scholar]
- 33.Raedler H, Heeger PS. Complement regulation of T-cell alloimmunity. Curr Opin Organ Transplant. 2011;16(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krupnick AS, Lin X, Li W, et al. Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest. 2014;124(3):1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horwitz JK, Chun NH, Heeger PS. Complement and Transplantation: From New Mechanisms to Potential Biomarkers and Novel Treatment Strategies. Clin Lab Med. 2019;39(1):31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieyra M, Leisman S, Raedler H, et al. Complement regulates CD4 T-cell help to CD8 T cells required for murine allograft rejection. Am J Pathol. 2011;179(2):766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwan W-H, Hashimoto D, Paz-Artal E, et al. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest. 2012;122(6):2234–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.