Abstract
Purpose
To evaluate the characteristics of urinary stone composition in a Korean population using a large database of stone composition.
Materials and Methods
From January 1, 2014, to June 30, 2019, a total of 33,078 urinary stone composition data were analyzed. Stone composition was classified into four main groups: calcium oxalate (CaOx), struvite, uric acid (UA), and calcium phosphate (CaP). We examined the relationship between stone composition and sex, age, geographic region, calendar month, and season.
Results
The CaOx group (46.41%) was the largest, followed by the struvite group (29.66%), UA group (19.61%), and CaP group (4.32%). The CaOx group tended to decrease with age, but the UA group increased with age. Also, the CaOx group had the highest percentage in summer and the lowest in spring (p<0.001). The struvite and CaP groups had higher percentages of females than males (struvite: 36.6% vs. 25.7%, p<0.001; CaP: 6.2% vs. 3.3%, p<0.001). Conversely, the UA stones were more common in males than in females (24.5% vs. 11.0%, p<0.001). The UA group had the lowest percentage in the capital region (p<0.001). The total male-to-female ratio decreased over time from 1.95:1 in 2014 to 1.67:1 in 2018 (p<0.001).
Conclusions
There were differences for each stone composition in the percentages according to sex, age, geographic region, month, and season. Identifying these differences based on the stone composition is vital for the treatment and prevention of urinary stones.
Keywords: Big data, Republic of Korea, Urolithiasis
Graphical Abstract
INTRODUCTION
Urolithiasis is one of the most common diseases, with a high incidence and prevalence among three significant urologic diseases. The urolithiasis prevalence rate is 7% to 13% in North America, 5% to 9% in Europe, and 1% to 5% in Asia. In addition to these geographical variations, there are considerable differences in urolithiasis prevalence associated with climate, diet, fluid intake, genetics, sex, occupation, and age. Moreover, the incidence and prevalence of urolithiasis have recently increased worldwide [1].
The recurrence rate of urolithiasis is 30% to 50% within 10 years after the first episode of disease [2]. Considering the direct cost of treatment of urolithiasis and the indirect damage of lost work can exceed five billion dollars in the United States; the monetary burden of recurrent urinary stone disease can play a significant role in the economy of each country [3]. The relatively high recurrence rate combined with the large monetary impact emphasize that the prevention of recurrence of urolithiasis in patients is crucial to the patient's quality of life and the economy of the country.
Knowledge of the urinary stone composition can help prevent future stone formation, and identification of stone composition facilitates choosing the necessary and specific treatment of patients with urinary stone disease. Thus, stone composition analysis should take place after the first episode of urinary stone disease. The European Association of Urology (EAU) and the American Urological Association (AUA) guidelines also suggest the need to analyze stone composition for the best treatment outcomes.
Western and Asian studies on stone composition have also been conducted [4,5]; for example, several studies regarding stone composition have been conducted in several Korean medical institutes [6,7]. However, there has been no large-scale study in Korea on whether stone composition has any connection to various factors like sex, age, region, year, month, and season. Recently, the prevalence of urolithiasis has been on the rise, possibly due to increased use of a western diet and a decreased level of physical activity in the Korean population [8]. In the current study, we analyzed the characteristics of stone composition using a large Korean database.
MATERIALS AND METHODS
1. Data collection
A total of 33,078 stones, submitted to Green Cross Laboratories (Yongin, Korea) from January 1, 2014 to June 30, 2019, were analyzed. A Fourier-transform infrared (FTIR) spectrometer was used to analyze stone composition. The study was performed with the approval of the Institutional Review Board of the Yonsei University Health System (approval number: 4-2020-0189). As this study was a retrospective study of anonymous data, informed consent was exempted.
2. Classification of stone composition
We classified stone composition into four main groups according to the Mayo Clinic classification [9]. Initially, we classified stone composition into six groups in the following order: 1) struvite group (stones including any struvite); 2) cystine group (stones including any cystine); 3) uric acid (UA) group (stones including any UA); 4) brushite group (stones including any brushite); 5) calcium oxalate (CaOx) group (stones including >50% CaOx); and 6) carbonate apatite (CA) group (stones including >50% CA). However, for efficient statistical analysis, <1% stone composition was excluded, and the CA and brushite groups were combined into the calcium phosphate (CaP) group. Finally, a total of 32,807 stones were classified into four main groups (CaOx, struvite, UA, and CaP).
3. Division of geographic region
Geographic region was divided into four categories; namely, 1) the capital region (Seoul, Gyeonggi-do, and Incheon) in the midwestern area of the Korean Peninsula; 2) the Chungcheong region (Daejeon, Sejong, Chungcheongnam-do, and Chungcheongbuk-do) in the mid-southern area of the Korean Peninsula; 3) the Honam region (Gwangju, Jeollanam-do, and Jeollabuk-do) in the southwestern area of the Korean Peninsula; and 4) the Yeongnam region (Busan, Daegu, Ulsan, Gyeongsangnam-do, and Gyeongsangbuk-do) in the southeastern area of the Korean Peninsula. The Gangwon region was excluded because data were not collected by Green Cross Laboratories in this region.
4. Definition of season
The seasons were defined as spring (March, April, May), summer (June, July, August), autumn (September, October, November), and winter (December, January, February).
5. Statistical analysis
We examined the relationship between stone composition and sex, age, geographic region, season, and calendar month using chi-squared tests. We also analyzed the relationship between metabolic syndrome and the pattern of stone composition. Statistical analyses were completed using SPSS (IBM SPSS Statistics Subscription for Windows, Version: Build 1.0.0.1406.; IBM Corp., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
RESULTS
Overall, there were a total of 33,078 cases composed of 21,025 males and 12,053 females. The mean age of the patients was 56.55 years. The median weight of the stones was 45.6 mg (Table 1). The combinations of stone composition were very diverse and consisted of 99 types of stones. Pure, single-composition stones (homogeneous stones consisting 100% of one stone composition) occurred in 18,225 cases, which accounted for 55.10% of the total number of stones. CaOx monohydrate (CaOxMo) was the most common stone composition (32.33% of total stones), followed by UA (17.52%), CA (3.33%), and struvite (0.86%). There were 14,853 cases of mixed stones, which accounted for 44.90% of the total stones. Among mixed stones, there were 11,022 cases of two-composition stones (33.32% of total stones) and 3,831 cases of three-composition stones (11.60% of total stones) (Table 2). When sorted by the Mayo Clinic classification criteria, CaOx (46.26%) was noted most frequently, followed by struvite (29.55%), UA (19.54%), and CA (3.62%) (Table 3). When the stones were classified into the four main groups (<1% were excluded and CaP=CA+brushite), the total number of cases was reduced to 32,807. Of these cases, the CaOx group (46.41%) was the most frequent followed by the struvite group (29.66 %), the UA group (19.61%), and the CaP group (4.32%). The CaOx group was composed of 100% CaOxMo (70.23%), ≥50% CaOxMo (23.31%), and ≥50% CaOx dihydrate (CaOxDi) (6.46%). There was no 100% CaOxDi in the CaOx group. The struvite group was composed of 100% struvite (2.92%) and <100% struvite (97.08%). The UA group was composed of 100% UA (90.13%) and <100% UA (9.87%). The CaP group was composed of 100% carbonate apatite (77.77%), ≥50% carbonate apatite (6.42%), 100% brushite (7.27%), and <100% brushite (8.54%) (Table 4).
Table 1. Distribution of patients.
| Characteristic | Result | |
|---|---|---|
| Age (y), range | 0–98 | |
| Mean±standard deviation | 56.55±15.37 | |
| Median (interquartile range) | 58 (21) | |
| Sex, n (%) | 33,078 (100.0) | |
| Male | 21,025 (63.6) | |
| Female | 12,053 (36.4) | |
| Stone weight (mg) | ||
| Median (interquartile range) | 45.6 (155.52) | |
Table 2. Stone composition.
| Type | Total | Stone composition | Value |
|---|---|---|---|
| Pure | 18,225 (55.10) | Calcium oxalate monohydrate | 10,695 (32.33) |
| Uric acid | 5,798 (17.52) | ||
| Carbonate apatite | 1,102 (3.33) | ||
| Struvite | 284 (0.86) | ||
| Cystine | 109 (0.33) | ||
| Brushite | 103 (0.31) | ||
| Protein | 97 (0.29) | ||
| Ammonium urate | 34 (0.10) | ||
| Dihydroxyadenine | 3 (0.01) | ||
| Mixed | 14,853 (44.90) | Mixed type (2 compositions) | 11,022 (33.32) |
| Mixed type (3 compositions) | 3,831 (11.60) | ||
| Total | 33,078 (100.00) | 33,078 (100.00) |
Values are presented as number (%).
Table 3. Stone composition by Mayo Clinic classification.
| Stone composition | Value |
|---|---|
| Calcium oxalate | 15,228 (46.26) |
| Struvite | 9,729 (29.55) |
| Uric acid | 6,433 (19.54) |
| Carbonate apatite | 1,193 (3.62) |
| Brushite | 224 (0.68) |
| Cystine | 115 (0.35) |
| Total | 32,922 (100.00) |
Values are presented as number (%).
Table 4. Four main stone composition groups (<1% were excluded).
| Stone composition | Value | Total (n=32,807) | |
|---|---|---|---|
| CaOx | 15,228 (46.41) | ||
| CaOxMo 100% | 10,695 (70.23) | ||
| CaOxMo ≥50% | 3,549 (23.31) | ||
| CaOxDi 100% | 0 (0.00) | ||
| CaOxDi ≥50% | 984 (6.46) | ||
| Total | 15,228 (100.00) | ||
| Struvite | 9,729 (29.66) | ||
| Struvite 100% | 284 (2.92) | ||
| Struvite <100% | 9,445 (97.08) | ||
| Total | 9,729 (100.00) | ||
| Uric acid | 6,433 (19.61) | ||
| Uric acid 100% | 5,798 (90.13) | ||
| Uric acid <100% | 635 (9.87) | ||
| Total | 6,433 (100.00) | ||
| CaP (Carbonate apatite, brushite) | 1,417 (4.32) | ||
| Carbonate apatite 100% | 1,102 (77.77) | ||
| Carbonate apatite ≥50% | 91 (6.42) | ||
| Brushite 100% | 103 (7.27) | ||
| Brushite <100% | 121 (8.54) | ||
| Total | 1,417 (100.00) | ||
Values are presented as number (%).
CaOx, calcium oxalate; CaOxMo, CaOx monohydrate; CaOxDi, CaOx dihydrate; CaP, calcium phosphate.
1. Sex
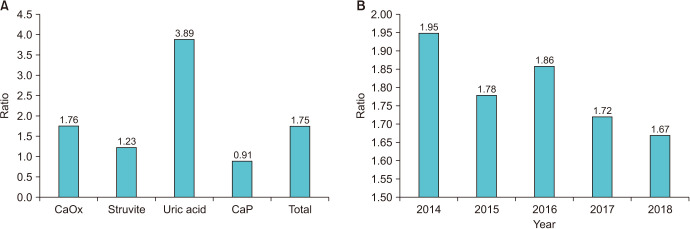
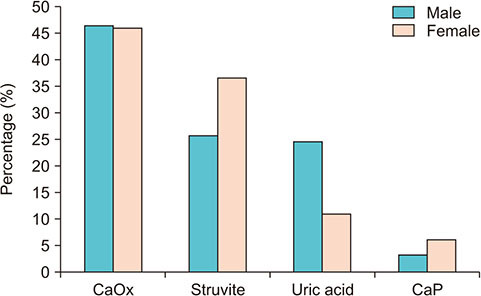
As shown in Fig. 1, females were more frequent than males in the struvite group (36.6% vs. 25.7%; p<0.001) and in the CaP group (6.2% vs. 3.3%; p<0.001), males were more frequent than females in the UA group (24.5% vs. 11.0%; p<0.001), and there was no difference in the percentage of each sex in the CaOx group (p=0.567). As shown in Fig. 2A, the male-to-female (M:F) ratio was 1.75 for a total of 32,807 stones. In addition, the highest M:F ratio was found in the UA group (3.89), followed by the CaOx group (1.76), struvite group (1.23), and CaP group (0.91). Interestingly, the total M:F ratio decreased over 4 years from 2014 (1.95:1) to 2018 (1.67:1) (p<0.001) (Fig. 2B).
Fig. 1. Percentage of stone composition by sex. Calcium oxalate (CaOx), p=0.567; struvite, p<0.001; uric acid, p<0.001; calcium phosphate (CaP), p<0.001.
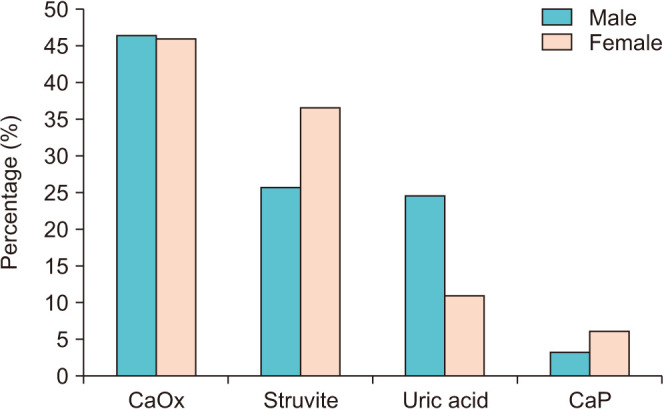
Fig. 2. Male-to-female ratio by (A) stone composition and (B) year. CaOx, calcium oxalate; CaP, calcium phosphate.
2. Age
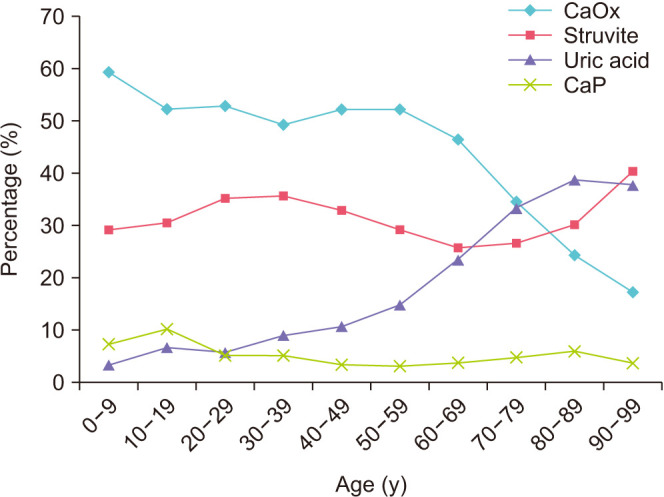
The incidence of urolithiasis was the highest in 50–59-year-old patients. The influence of age on stone composition is shown in Fig. 3. The age group with the highest percentage of CaOx stone composition was 0–9 years old (p<0.001). The percentage of CaOx stone composition then decreased with increasing age. The age group with the highest percentage of struvite stone composition was 90–99 years old (p<0.001). The percentage of UA stone composition increased with age and was the highest in 80–89-year-olds (p<0.001). The age group with the highest percentage of CaP stone composition was 10–19-year-olds (p<0.001).
Fig. 3. Percentage of stone composition by age. Calcium oxalate (CaOx), p<0.001; struvite, p<0.001; uric acid, p<0.001; calcium phosphate (CaP), p<0.001.
3. Region
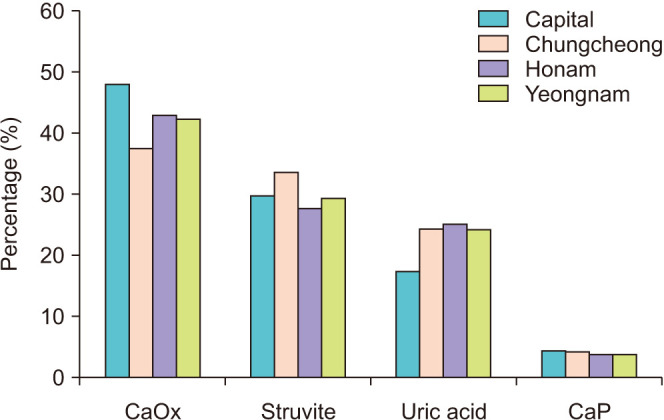
The influence of geographic region on stone composition is shown in Fig. 4. The area with the highest percentage of CaOx group members was the capital region (p<0.001). The area with the highest percentage of struvite stone composition was the Chungcheong region (p<0.001), and the area with the highest percentage of the UA stone composition was the Honam region (p<0.001). The percentage of the UA stone composition in the capital region tended to be lower than in other areas (p<0.001). Interestingly, UA is associated with metabolic syndrome [10] and, according to Metabolic Syndrome Fact Sheet in Korea 2018 [11], the capital region tends to have a lower incidence of metabolic syndrome than the Chungcheong, Honam, and Yeongnam regions. These findings are in keeping with the lower rate of UA stone composition in the capital region. In the CaP group, there was no difference in the percentage of each region (p=0.200).
Fig. 4. Percentage of stone composition by region. Calcium oxalate (CaOx), p<0.001; struvite, p<0.001; uric acid, p<0.001; calcium phosphate (CaP), p=0.200.
4. Year
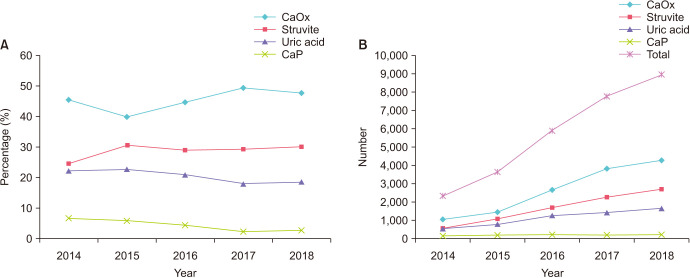
As shown in Fig. 5A, the percentage of UA group members decreased from 22.4% in 2014 to 18.7% in 2018 (p<0.001). The percentage of CaP group members also recently decreased from 7.0% in 2014 to 3.0% in 2018 (p<0.001). The percentage of CaOx and struvite group members showed no tendency to increase or decrease over the 2014–2018 time-frame. As shown in Fig. 5B, the number of stone analyses per year tended to increase from 2014 (2,352 cases) to 2018 (8,943 cases).
Fig. 5. Percentage of stone composition (A) and number of stone analysis (B) by year. (A) Calcium oxalate (CaOx), p<0.001; struvite, p<0.001; uric acid, p<0.001; calcium phosphate (CaP), p<0.001.
5. Month
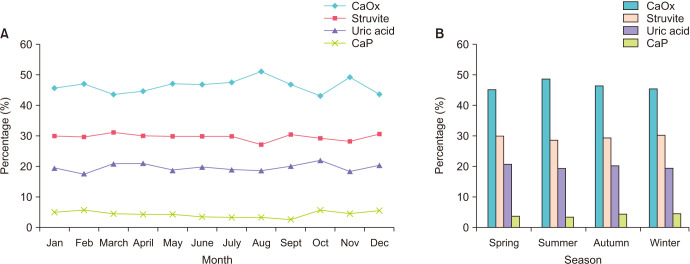
The trends in stone composition by month are shown in Fig. 6A. Among all the groups of different stone compositions, the CaOx group had the highest percentage in August (50.9%) and the lowest percentage in October (43.2%) (p<0.001). The UA group had the highest percentage in October (21.9%) and the lowest percentage in February (17.5%) (p<0.002). The CaP group was the highest in February (5.8%) and the lowest in September (2.6%) (p<0.001). There was no difference in the percentage by month in the struvite group (p=0.116).
Fig. 6. Percentage of stone composition by month (A) and percentage of stone composition by season (B). (A) Calcium oxalate (CaOx), p<0.001; struvite, p=0.116; uric acid, p<0.002; calcium phosphate (CaP), p<0.001. (B) CaOx, p<0.001; struvite, p=0.114; uric acid, p=0.200; CaP, p<0.001.
6. Season
The influence of season on number of stone analyses based on stone composition is shown in Fig. 6B. The number of stone analysis cases was the highest in summer (8,083 cases) and the lowest in winter (6,308 cases). The CaOx group had the highest percentage in summer (48.7%) and the lowest percentage in spring (45.2%) (p<0.001). The CaP group had the highest percentage in winter (4.7%) and the lowest percentage in summer (3.3%) (p<0.001). In the struvite group and the UA group, there were no differences in the percentage by season in the struvite group (p=0.114) and the UA group (p=0.200).
DISCUSSION
The importance of urinary stone composition analysis has been on the rise due to the worldwide increase in prevalence of urinary stones in recent years. Historically, several methods have been used for the analysis of the compositions of urinary stones; namely, infrared spectroscopy, X-ray diffraction, and chemical analysis. Chemical analysis is no longer recommended [12]. FTIR is currently the accepted method of choice for stone analysis [13]. Stone analysis is recommended for all first instances of urinary stones. It is also essential to re-analyze the stone composition when stones recur during preventative pharmacotherapy, soon after active removal of urinary stones, or when stones recur after a long absence because there is a possibility that stone compositions have changed [14,15]. Knowledge of urinary stone composition assists in reducing stone recurrence by appropriate dietary modification and pharmacotherapy.
Stone compositions can be classified as non-infectious (CaOx, CaP, and UA), infectious (struvite, CA, and ammonium urate), genetic (cystine, xanthine, and 2,8-dihydroxyadenine), and medication-induced [12]. In the current study, there were 99 types of urinary stones (including mixed stones), so it was difficult to analyze them. To achieve efficient statistical analyses, the stone composition was analyzed based on the Mayo Clinic classification system with minor modifications. We chose the Mayo Clinic classification of stone analysis because it has the clearest criteria for classifying stone compositions. Our analyses differed slightly from the Mayo Clinic classification in one study in which brushite and CA were combined (CaP group), which resulted in exclusion of <1% of urinary stones.
In pure-composition stones, the struvite group was the lowest percentage among the four main groups (0.86%). However, the struvite group became the second largest after it was reclassified according to the Mayo Clinic classification criteria. The reason for this difference was that there were only 284 cases (2.92%) of pure struvite stones, but mixed struvite stones accounted for 9,445 cases (97.08%) of the total struvite group. As in other studies, CaOx had the largest percentage (46.41%) in our study, but our proportion of CaOx stones was lower than that of the Mayo Clinic (67%). In the Mayo Clinic study [9], CaOx (67%) is the most common, followed by hydroxyapatite (16%), UA (8%), struvite (3%), brushite (0.9%), and cystine (0.35%). In a Chinese study [16], the order of compositions is CaOx (65.9%), carbapatite (15.6%), urate (12.4%), struvite (2.7%), and brushite (1.7%).
Stones are more predominant in males than in females. However, there is controversy about the change in the gap between males and females. One study shows a decrease from 1.7 in 1997 to 1.3 in 2002 [16], but another study shows an increase from 1.86:1 to 2.7:1 [4]. In our study, the M:F ratio declined from 2014 (1.95:1) to 2018 (1.67:1). The probable reason for this result is that obesity is on the rise in both males and females in Korea [17], but the effect of obesity related to stone formation may be more significant in females than in males because females have a higher percent body fat even with the same body mass index as males [18].
Generally, in CaOx and UA groups, the ratio in males is higher than in females. The reason why CaOx is more common in males is possibly because of a greater tendency for oversaturated urine for CaOx [19] and diet tendencies (higher protein intake in males) [20]. Also, UA is more common in males because males show lower urine pH and lower glomerular filtration rates than females. Another factor could be that female hormones (estrogen) improve the efficiency of the kidneys when UA is removed and keep a lower UA concentration in the blood [21]. In the struvite and CaP groups, the ratio in females was higher than in males. This may be related to the fact that females have more risk of urinary tract infections [22]. If infected with urea-splitting bacteria, the bacterial enzyme splits urea, causing the formation of ammonium and bicarbonate, which are responsible for an increased urinary pH. As urinary pH increases, the solubility of phosphoric acid decreases, resulting in the formation of new infected stones or the growth of existing stones [23].
Similar to other studies, the number of middle-aged (50–59 years) patients with stones was the highest. The percentage of patients with CaOx stones declined with age. In contrast, the percentage of patients with UA stones increased with age. The UA increase could be associated with decreasing urine pH and increasing UA excretion [24] related to obesity, insulin resistance, and diabetes that are common with aging [10,24,25].
In this study, the number of stone analyses by year increased from 2014 (2,352 cases) to 2018 (8,943 cases). Recently, a Korean study using the National Health Insurance Service sample cohort dataset showed that the incidence of urinary stones has been on the rise every year. The reason for this increase has been suggested to be the result of increased comorbidities along with lifestyle changes (Western dietary habits and a decreased level of physical activity) [8]. Also, it is believed that the number of stone analyses has increased recently due to the increase in the number of retrograde intrarenal surgeries in South Korea (the number of flexible ureteroscopic lithotripsy increased by 16-fold from 2009 to 2016) [26].
By season, urinary stones were most common in summer and least common in winter. In warm weather, urinary supersaturations increase due to unconscious water loss [9]. The trend of global warming is likely to lead to the movement and expansion of areas that increase the risk of stone formation [27]. The temperature on the Korean Peninsula also shows long-term warming trends with more frequent hot events [28].
There are four limitations of this study. First, the Gangwon region was excluded because there were no urinary stones analyzed in this area by Green Cross Laboratories. Second, it is impossible to distinguish whether a particular stone occurred for the first time or was a recurrence. Third, there was no information regarding the location of stones within the urinary tract. Finally, the prevalence rate is unknown because there is no exact denominator. However, it is meaningful that this is the first large-scale urinary stone analysis study in Korea.
CONCLUSIONS
The total number of stone analyses by year tended to increase. There were differences in the percentage according to sex, age, geographic region, month, and season for each stone composition. In CaOx and UA groups, the ratio was higher in males. In struvite and CaP groups, the ratio was higher in females. The percentage of patients with UA stone composition increased with age, but the percentage of those with CaOx stone composition decreased. Similar to the low incidence of metabolic syndrome in the capital region, UA in the capital region tended to be lower than other areas. The number of urinary stones was the highest in summer and lowest in winter. Identifying these differences based on the stone composition is essential for the treatment and prevention of urinary stones.
ACKNOWLEDGMENTS
We appreciate Green Cross Laboratories for providing a large database of stone composition.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Ill Young Seo and Joo Yong Lee.
- Data acquisition: Hae Do Jung.
- Statistical analysis: Hae Do Jung and Joo Yong Lee.
- Data analysis and interpretation: Hae Do Jung and Joo Yong Lee.
- Drafting of the manuscript: Hae Do Jung.
- Critical revision of the manuscript: Ill Young Seo and Joo Yong Lee.
- Administrative, technical, or material support: Ill Young Seo.
- Supervision: Joo Yong Lee.
- Approval of the final manuscript: Ill Young Seo and Joo Yong Lee.
References
- 1.Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World J Urol. 2017;35:1301–1320. doi: 10.1007/s00345-017-2008-6. [DOI] [PubMed] [Google Scholar]
- 2.Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med. 1989;111:1006–1009. doi: 10.7326/0003-4819-111-12-1006. [DOI] [PubMed] [Google Scholar]
- 3.Saigal CS, Joyce G, Timilsina AR. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int. 2005;68:1808–1814. doi: 10.1111/j.1523-1755.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 4.Knoll T, Schubert AB, Fahlenkamp D, Leusmann DB, Wendt-Nordahl G, Schubert G. Urolithiasis through the ages: data on more than 200,000 urinary stone analyses. J Urol. 2011;185:1304–1311. doi: 10.1016/j.juro.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 5.Ye Z, Zeng G, Yang H, Li J, Tang K, Wang G, et al. The status and characteristics of urinary stone composition in China. BJU Int. 2020;125:801–809. doi: 10.1111/bju.14765. [DOI] [PubMed] [Google Scholar]
- 6.Jeong JY, Doo SW, Yang WJ, Lee KW, Kim JM. Differences in urinary stone composition according to body habitus. Korean J Urol. 2011;52:622–625. doi: 10.4111/kju.2011.52.9.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang PM, Seo WI, Kang DI. Analysis of urinary stone composition: a retrospective single center study during the last five years (2009-2013) Korean J Urogenit Tract Infect Inflamm. 2014;9:44–49. [Google Scholar]
- 8.Tae BS, Balpukov U, Cho SY, Jeong CW. Eleven-year cumulative incidence and estimated lifetime prevalence of urolithiasis in Korea: a National Health Insurance Service-National Sample Cohort based study. J Korean Med Sci. 2018;33:e13. doi: 10.3346/jkms.2018.33.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieske JC, Rule AD, Krambeck AE, Williams JC, Bergstralh EJ, Mehta RA, et al. Stone composition as a function of age and sex. Clin J Am Soc Nephrol. 2014;9:2141–2146. doi: 10.2215/CJN.05660614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abate N, Chandalia M, Cabo-Chan AV, Jr, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 11.Korean Society of Cardiometabolic Syndrome. Metabolic syndrome fact sheet in Korea 2018. Incheon: Korean Society of Cardiometabolic Syndrome; 2018. [Google Scholar]
- 12.Türk C, Petŕík A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. 2016;69:475–482. doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 13.Mandel NS, Mandel IC, Kolbach-Mandel AM. Accurate stone analysis: the impact on disease diagnosis and treatment. Urolithiasis. 2017;45:3–9. doi: 10.1007/s00240-016-0943-0. [DOI] [PubMed] [Google Scholar]
- 14.Norman RW, Bath SS, Robertson WG, Peacock M. When should patients with symptomatic urinary stone disease be evaluated metabolically? J Urol. 1984;132:1137–1139. doi: 10.1016/s0022-5347(17)50064-6. [DOI] [PubMed] [Google Scholar]
- 15.Pearle MS, Asplin JR, Coe FL, Rodgers A, Worcester EM. Medical management of urolithiasis [abstract]; 2nd International Consultation on Stone Disease; 2007 Sep 5; Paris, France. Paris: Health Publications; 2008. pp. 57–84. [Google Scholar]
- 16.Scales CD, Jr, Curtis LH, Norris RD, Springhart WP, Sur RL, Schulman KA, et al. Changing gender prevalence of stone disease. J Urol. 2007;177:979–982. doi: 10.1016/j.juro.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 17.Nam GE, Kim YH, Han K, Jung JH, Park YG, Lee KW, et al. Obesity Fact Sheet in Korea, 2018: data focusing on waist circumference and obesity-related comorbidities. J Obes Metab Syndr. 2019;28:236–245. doi: 10.7570/jomes.2019.28.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 19.Parks JH, Coward M, Coe FL. Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int. 1997;51:894–900. doi: 10.1038/ki.1997.126. [DOI] [PubMed] [Google Scholar]
- 20.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 21.Chen HW, Chen YC, Yang FM, Wu WJ, Li CC, Chang YY, et al. Mediators of the effects of gender on uric acid nephrolithiasis: a novel application of structural equation modeling. Sci Rep. 2018;8:6077. doi: 10.1038/s41598-018-24485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parks JH, Coe FL, Strauss AL. Calcium nephrolithiasis and medullary sponge kidney in women. N Engl J Med. 1982;306:1088–1091. doi: 10.1056/NEJM198205063061805. [DOI] [PubMed] [Google Scholar]
- 23.Kramer G, Klingler HC, Steiner GE. Role of bacteria in the development of kidney stones. Curr Opin Urol. 2000;10:35–38. doi: 10.1097/00042307-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 25.Viaene L, Meijers BK, Vanrenterghem Y, Evenepoel P. Evidence in favor of a severely impaired net intestinal calcium absorption in patients with (early-stage) chronic kidney disease. Am J Nephrol. 2012;35:434–441. doi: 10.1159/000338299. [DOI] [PubMed] [Google Scholar]
- 26.Kim JK, Cho YS, Park SY, Joo KJ, Min SK, Lee YG, et al. Recent surgical treatments for urinary stone disease in a Korean population: national population-based study. Int J Urol. 2019;26:558–564. doi: 10.1111/iju.13928. [DOI] [PubMed] [Google Scholar]
- 27.Brikowski TH, Lotan Y, Pearle MS. Climate-related increase in the prevalence of urolithiasis in the United States. Proc Natl Acad Sci U S A. 2008;105:9841–9846. doi: 10.1073/pnas.0709652105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min SK, Son SW, Seo KH, Kug JS, An SI, Choi YS, et al. Changes in weather and climate extremes over Korea and possible causes: a review. Asia Pac J Atmos Sci. 2015;51:103–121. [Google Scholar]