Abstract
Urothelial carcinoma of the upper urinary tract is uncommon and presents unique challenges for diagnosis and management. Nephroureterectomy has been the preferred management option, but it is associated with significant morbidity. Nephron-sparing treatments are a valuable alternative and provide similar efficacy in select cases. A PubMed literature review was performed in English language publications using the following search terms: urothelial carcinoma, upper tract, nephron-sparing, intraluminal and systemic therapy. Contemporary papers published within the last 10 years were primarily included. Where encountered, systematic reviews and meta-analyses were given priority, as were randomized controlled trials for newer treatments. Core guidelines were referenced and citations reviewed for inclusion. A summary of epidemiological data, clinical diagnosis, staging, and treatments focusing on nephron-sparing approaches to upper tract urothelial carcinoma (UTUC) are outlined. Nephron-sparing management strategies are viable options to consider in patients with favorable features of UTUC. Adjunctive therapies are being investigated but the data remains mixed. Protocol variability and dosage differences limit statistical interpretation. New mechanisms to improve treatment dwell times in the upper tracts are being designed with promising preliminary results. Studies investigating systemic therapies are ongoing but implications for nephron-sparing management are uncertain. Nephron-sparing management is an acceptable treatment modality best suited for favorable disease. More work is needed to determine if intraluminal and/or systemic therapies can further optimize treatment outcomes beyond resection alone.
Keywords: Carcinoma, transitional cell; Drug therapy; Organ sparing treatments; Urinary tract
Graphical Abstract

INTRODUCTION
Urothelial carcinoma (UC) is the 5th most common malignancy overall in the United States [1]. In 2019 in the United States, over 80,000 patients were projected to be newly diagnosed with UC of the bladder, with 17,670 dying from the disease [2,3]. In most situations, UC is identified in the lower urinary tracts, while upper tract urothelial carcinoma (UTUC) accounts for anywhere from 5% to 10% of new cases [4]. Of the patients found to have bladder cancer, 0.8% of them developed upper tract disease with 71% of cases developing within 5 years of bladder cancer diagnosis [5]. Wright et al. [5] also found on a review of almost 100,000 patients in the Surveillance, Epidemiology, and End Results (SEER) cancer registry that patients with high grade bladder cancer, non-muscle invasive disease (pTa, pT1), and primary tumors location at the trigone/ureteral orifice were significantly more likely to have upper tract rumor recurrence. To date, the management of UTUC has largely been derivative-informed by what is known about bladder cancer and the treatments thereof. However, there are notable genetic differences between lower and upper-tract disease despite the histologic similarities. This is mirrored by the fact that 60% of UTUC are invasive at the time of diagnosis relative to 15% to 25% of bladder tumors [6].
The relative rarity of the disease, the diagnostic challenges inherent to accessing the upper urinary tract, the clinical confounding which occurs when separating lower from upper tract symptomology, and an incomplete understanding of the underlying genetics has resulted in stunted therapeutic development in this space. The established Gold Standard for those with high-grade UTUC is nephroureterectomy with bladder cuff excision and this is well supported by both the American Urological Association (AUA) and European Association of Urology (EAU) Guidelines. However, this population is typically elderly with significant competing medical comorbidity, and the loss of a renal unit is potentially devastating in terms of quality of life. A large study by Zabor et al. [7] found that only 45% of those undergoing radical nephrectomy return to their preoperative glomerular filtration rate by 2 years. For those that develop end-stage renal disease, most of these patients will not be candidates for transplantation. Also, renal insufficiency prohibits systemic therapies that can offered in either the adjuvant or salvage settings.
Fortunately, endoscopic approaches are being developed to reduce both diagnostic and therapeutic morbidity. Minimally invasive, nephron-sparing techniques are supported for low-grade/low-volume disease in most candidates with comparable oncologic outcomes in well-selected patients [8]. Risk-adapted strategies for UTUC are emerging and can now be found in guideline statements. Here we review the contemporary data in support of nephron-sparing management of UTUC-focusing on advances in enhanced imaging, optical diagnostics, intraluminal therapies to include novel delivery mechanisms, and the role of systemic therapy for those undergoing segmental resection due to imperative indications.
DIAGNOSTICS AND STAGING
Patients with UTUC present with microscopic or gross hematuria in 70% to 80% of cases [9]. Flank pain is more uncommon and occurs in approximately 20% of patients [9]. Not surprisingly, the addition of constitutional symptoms (anorexia, unexplained weight loss, and night sweats) portends advanced disease and a more unfavorable prognosis [9]. AUA Guidelines recommend a formal hematuria evaluation for those patients presenting with the aforementioned symptoms, and this routinely means contrasted imaging of the upper urinary tracts and cystoscopic evaluation of the bladder. In terms of diagnostic performance, the sensitivity of CT urography is 0.67 to 1.00 and the specificity is 0.93 to 0.99 [10]. But high-grade flat lesions like carcinoma in situ (CIS) are typically missed. Magnetic resonance imaging urography adds very little in terms of sensitivity but can be considered in those unable to tolerate contrast due to pre-existing chronic kidney disease (CKD) [9]. In a comprehensive review issued by the Canadian Association of Radiologists, patients with mild-to-moderate CKD (glomerular filtration rate [GFR] between 30 and 60 mL/min/1.73 m2) could be offered standard doses of gadolinium-based contrast agents. In those with severe CKD (GFR <30 mL/min/1.73 m2) or on dialysis, newer gadolinium-based agents (gadobenate dimeglumine, gadobutrol, gadoterate, gadoteridol) can still be offered with an exceeding low risk for developing nephrogenic systemic fibrosis [11,12].
Flexible ureteroscopy is essential and serves to directly visualize tumors and provide a mechanism for specimen retrieval. Ureteroscopic biopsy is notoriously challenging, but approaches 90% accuracy regardless of the total volume of tissue sample obtained [13]. Nevertheless, tumor depth and stage can be difficult to assess. Prior studies have shown significant discordance between ureteroscopy and final pathology in terms of stage [14]. For this reason, tumor grade is routinely used to approximate staging given the association between high-grade pathology and invasive disease [15]. A complete reference for the clinical staging of UTUC can be found in Table 1.
Table 1. Clinical staging for UTUC.
| Clinical staging of upper tract urothelial carcinoma | |
|---|---|
| Tx | Tumor invasion cannot be assessed |
| Tis | Carcinoma in situ (may coexist with papillary and sessile tumors) |
| Ta | Non-invasive on biopsy |
| T1 | Invasion of lamina propria on biopsy |
| T2 | Invasion of muscularis (rarely identified with biopsy alone) |
| T3 | Invasion of peri-ureteral fat, renal parenchyma, or sinus fat (suggested by imaging) |
| T4 | Invasion of adjacent organs |
| N0 | No involvement of lymph nodes on standard imaging |
| N1 | Lymphadenopathy on standard imaging |
UTUC, upper tract urothelial carcinoma.
Fortunately, enhanced imaging and photodynamic diagnostic systems are in development [16,17,18,19]. Optical coherence tomography and confocal laser endomicroscopy are particularly interesting-these technologies potentially offer a visual means for tissue diagnosis. A study by Bus et al. [20] demonstrated an 83% staging concordance with final histopathology using optical coherence tomography; sensitivity and specificity for tumor invasion was 100% and 92%, respectively. It stands to reason that improved diagnostics will result in more appropriate endoscopic treatment allocation.
The utility of cytology has also been investigated. Of note, abnormal cytology may suggest high-grade UTUC if the lower urinary tract has been completely evaluated and determined to be negative (this would typically include both random bladder and prostatic biopsies). In a contemporary study by Malm et al. [21], barbotage cytology identified 91% of all cancers; interestingly barbotage cytology and biopsy histology were equally efficient in detecting cancer. In contrast, the performance of fluorescence in situ hybridization (FISH) has not been as impressive, with a reported sensitivity of approximately 50% [22,23,24]. Thus, if performed, cytology should be obtained selectively and efforts should be undertaken to reduce cross contamination with other areas within the urinary tract; the utility of FISH alone is unproven, but it may yet serve a role to adjudicate equivocal cytology.
TREATMENT OPTIONS
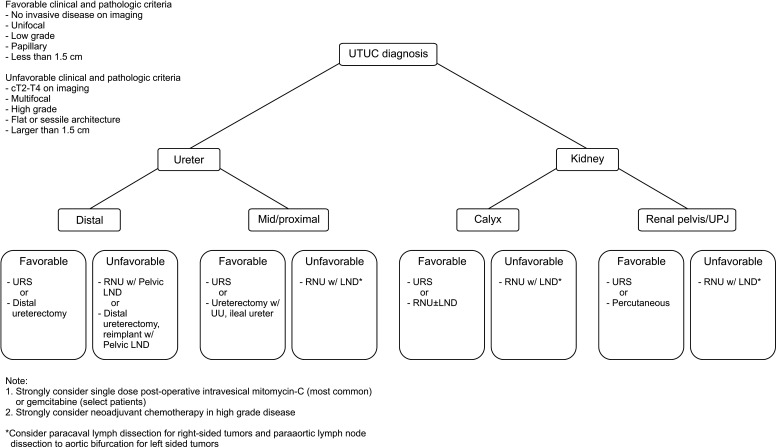
The treatment of UTUC requires assessment of location, volume, and grade. Obtaining adequate stage can be challenging as highlighted previously. The National Cancer Center Network (NCCN) and EAU Guidelines offer site and grade specific recommendations that are fairly well delineated. In Fig. 1, these recommendations have been used to formulate a treatment algorithm where disease characteristics help guide appropriate utilization of endoscopic approaches. In terms of nephron-sparing options, endoscopic resection vs. excision, uretero-ureterostomy, ileal ureter substitution, and distal ureterectomy are the most common options; the extent of both resection and reconstruction will necessarily be informed by the patient's underlying comorbidity.
Fig. 1. UTUC treatment algorithm. UTUC treatment options by site and risk strata. Risk categories defined in the upper left corner. UTUC, upper tract urothelial carcinoma; UPJ, ureteropelvic junction; URS, ureteroscopy; RNU, radical nephroureterectomy; LND, lymph node dissection; UU, ureteroureterostomy.
In terms of endoscopic management, both antegrade and retrograde approaches have been well described. Decisions pertaining to approach are guided by tumor location and size, with larger tumors in the renal pelvis (>1.5–2.0 cm by NCCN and EAU Guidelines) being best managed percutaneously; small tumors in the distal ureter can be safely managed ureteroscopically [4]. Laser energy, frequently with holmium yttrium aluminum garnet (Ho:YAG), is used to ablate tumors with a safe depth of penetration (<0.4 mm).
Comparative studies between radical nephrourectomy and endoscopic resection have shown equivalent disease-specific and overall survival in those being treated for low-grade disease [25,26,27]. Table 2 [27,28,29,30,31,32,33,34] presents larger contemporary studies where both overall and cancer specific survival were assessed in those undergoing nephron-sparing approaches. Grasso et al. [28] reported their 15-year experience with 160 consecutive patients and found 2-, 5-, and 10-year cancer specific survival (CSS) rates of 98%, 87%, and 81%. Those offered endoscopic management for high-grade disease secondary to imperative indication (i.e., solitary renal unit, baseline renal insufficiency, inability to tolerate surgery) did worse, with a median survival of 29.2 months; the overall survival at two years was only 54% [28]. Another study by Motamedinia et al. [29] reported their 30-year experience with 141 patients. Here, those with low-grade disease that were managed endoscopically had a radiographic free survival of 71.5 months; the radiographic free survival was 36.4 months in the setting of high-grade disease. Interestingly, this study did not find that multi-focality predicted radiographic recurrence, progression, or death. Also, tumor grade had less significance when controlling for age, imperative indication, and history of concomitant bladder cancer [29]. While endoscopic treatment is associated with a greater frequency of recurrence in some studies, these cases are routinely amendable to repeat endoscopic management while preserving oncologic benefit [25].
Table 2. Contemporary studies demonstrating comparative outcomes for nephron-sparing approaches.
| Author | Year | Study design | Approach (n) | FU (mo) | High risk pathology (percent ≥pT2/3, HG) | OS | CSS | RF outcomes | |
|---|---|---|---|---|---|---|---|---|---|
| Segmental ureterectomy | |||||||||
| Kim et al. [30] | 2021 | Retrospective | SU (40) | 23.2 | SU 56.8% (≥pT2) | SU 71.5% (3 y) | SU 82.6% (3 y) | SU 35.3% (CKD≥III) | |
| RNU (40) | RNU 57.9% (≥pT2) | RNU 87.5% (3 y)* | RNU 93% (3 y) | RNU 85% (CKD≥III) | |||||
| Li et al. [31] | 2019 | Retrospective | SU (73) | 35.8 | SU 47.8% (≥pT2) | NR | SU 31.3% (3 y)a | NR | |
| RNU (182) | RNU 52.9% (≥pT2) | RNU 38% (3 y)a | |||||||
| Fang et al. [32] | 2016 | Meta-analysis | SU (983) | 25.6–58 | SU 9.1%–31.4% (≥pT3) | SU 40%–72% (5 y) | SU 54%–90% (5 y) | RNU 9.3 mL/m2 lower* | |
| RNU (2,980) | RNU 19.5%–44.4% (≥pT3) | RNU 43%–67% (5 y) | RNU 64%–86% (5 y) | ||||||
| Percutaneous & endoscopic resection/ablation | |||||||||
| Scotland et al. [33] | 2018 | Retrospective | ES (80) | 44.3 | 51.2% (HG) | 75% (5 y); 39% (10 y)b | 84% (5y); 65% (10 y)b | Post ES GFR 9.3 mL/m2 lower | |
| Motamedinia et al. [29] | 2016 | Retrospective | PC (141) | 66 | 45% (HG) | LG 126 months | NR | NR | |
| HG 59.6 months | |||||||||
| Yakoubi et al. [27] | 2014 | Meta-analysis | ES (322) | 18–58 | 10%–25% (≥pT2) | ES 62%–75% (5 y)c | ES 67%-87% (5 y)c | NR | |
| RNU (680) | 26%–67% (≥pT2) | RNU 58%–76% (5 y)c | RNU 64%–92% (5 y)c | ||||||
| Grasso et al. [28] | 2012 | Prospective | ES (82) | 38.2 | 55.6% (HG) | NR | ES/LG 87% (5y); 81% (10 y) | NR | |
| RNU (80) | RNU/HG 53% (5y); 53% (10 y) | ||||||||
| Cutress et al. [34] | 2012 | Meta-analysis | ES (149) | 20–51 | ES 13.4% (HG) | ES 72% (3 y) | ES 91% (3 y) | NR | |
| PC (47) | PC 46.8% (HG) | PC 79% (3 y) | PC 89% (3 y) | ||||||
FU, follow-up; HG, high-grade; OS, overall survival; CSS, cancer specific survival; RF, renal function; SU, segmental ureterectomy; RNU, radical nephroureterectomy; CKD, chronic kidney disease; NR, not reported; ES, endoscopic resection and/or ablation; GFR, glomerular filtration rate; PC, percutaneous resection and/or ablation; LG, low-grade.
a:CSS calculated as subset of patients with ≥pT2 disease. b:17/80 (21.3%) receiving palliative resection. c:No statistical difference but authors warn of significant study heterogeneity.
*If statistical significance (p-value <0.05) reported.
INTRALUMINAL THERAPIES
Endoscopic treatments offer acceptable outcomes for patients with low-grade/low-volume disease. However, rates of recurrence requiring salvage nephroureterectomy remains too high, and this is especially true in those with high-grade disease. Rates of extirpative salvage for low-grade and high-grade UC are estimated at 16.7% and 28.6%, respectively [8]. To reduce rates of recurrence while attempting to preserve renal function, several intraluminal therapies have been deployed into the upper urinary tracts. Bacillus Calmette-Guerin (BCG) was one of the first and is perhaps the best studied adjuvant therapy. The use of BCG for resected papillary tumors and the primary treatment CIS is largely regarded as standard of care in those meeting criteria for intermediate-or high-risk non-muscle invasive bladder cancer. Its use is supported by both the AUA and EAU Guidelines [35]. But efficacy in the upper-urinary tracts remains uncertain, the results confounded by variable dosages, unique mechanisms of delivery, and institutional variation in terms of indications. Indeed, there is no level 1 evidence supporting BCG for UTUC and retrospective data is largely negative [36]. Rastinehad et al. [37] found that BCG post-resection or ablation did not result in significant differences in recurrence rates regardless of grade. Low-grade recurrence was 26% for endoscopic management alone vs. 33% in those receiving adjuvant BCG; high-grade recurrence 38% was for endoscopic management alone vs. 39% in those receiving adjuvant BCG. The use of BCG may be more ideally suited for CIS, as Carmignani et al. [38] have shown that an induction course of BCG could convert positive cytology to negative with a mean recurrence rate of 32% at 19 to 57 months follow-up. While encouraging, negative cytology alone is an insufficient benchmark for remission and other more definitive diagnostic measures, like ureteroscopy with repeat biopsy, should be employed for surveillance.
Mitomycin C (MMC) has also been studied. Metcalfe et al. [39] reported data in 28 renal units receiving intraluminal therapy after complete endoscopic resection for Ta/T1 tumors, 3-year recurrence-free, progression-free, and importantly nephrouretectomy-free survival was 60%, 80%, and 76%, respectively; 3-year overall survival was 92.9%. Another series by Aboumarzouk et al. [40] evaluating MMC instillation into 20 renal units with biopsy proven low-grade UTUC showed a recurrence-free survival of 65% at a mean follow-up of 24 months. Only 1 (5%) patient was noted to have a long-term complication. Fortunately no patients went on to develop post-operative renal impairment or systemic side-effects [40]. MMC has also been constituted into a gelatinous matrix called Mitogel (UGN-101) in an effort to achieve more sustained contact along the upper tract urothelium. In an open-label, single-arm, phase 3 trial, Kleinmann et al. [41], treated patients with biopsy-proven primary or recurrent low-grade UTUC with 6 instillations of weekly UGN-101 up to a maximum dose of 60 mg per instillation. Of note, only tumors in either the renal pelvis or the calyces were eligible for treatment. The primary outcome was complete response defined as a negative 3-month ureteroscopic evaluation to include negative urine cytology. Seventy-four patients were able to receive at least one dose. Forty-two patients (59%) had a complete response with a median follow-up of 11 months. Of those patients with a complete response, only 6 recurred. In terms of adverse events, the most frequently reported events were ureteric stenosis in 31 (44% in total, the majority being Grades 1–2), urinary tract infection in 23 (32%), hematuria in 22 (31%), and flank pain in 21 (30%). No deaths were reported [41]. Another intriguing intraluminal therapy with a novel delivery mechanism pairing is gemcitabine impregnated ureteral stents. Preliminary in vitro studies have shown stable diffusion kinetics up to 72 hours with complete stent dissolution after about 9 days. There was a reduction of 75% of viable tumor burden with minimal toxicity to normal cell lines [42].
Last, Balasubramanian et al. [43] reported their findings in a cohort of 51 patients (58 renal units) which received salvage topical therapy (either BCG, MMC, or gemcitabine) for recurrent UTUC (Ta, T1, and Tis). Of note, 18 renal units required additional topical therapy—44% (8/18) for refractory disease and 56% (10/18) as a reinduction course following a period of remission. Five renal units had CIS unresponsive to initial therapy and only 20% (1/5) of these responded to additional intraluminal therapy. Globally these data support that there are some cases of UTUC that can be salvaged with additional courses of intraluminal therapy, but those presenting with CIS may not do as well [43].
It is also worth noting that intraluminal therapies are frequently deployed into the lower urinary tracts prophylactically around the time of surgical resection to decrease the risk of bladder seeding. The estimated rate of bladder recurrence following radical nephrouretectomy is anywhere from 22% to 47% [44,45]. A meta-analysis by Wu et al. [46] demonstrated a bladder tumor incidence of 24% in cases where intravesical chemotherapy (predominantly MMC) was used versus 36.9% in cases where it was not-the pooled odds ratio for bladder tumor recurrence being 0.45 (p-value<0.05). Gemcitabine has also been used with similar efficacy and may offer a better side-effect profile. Interestingly, studies have also shown benefit with intravesical irrigations using either physiologic saline or distilled water. In a retrospective series by Yamamoto et al. [47], patients receiving these bladder irrigations had a 25.0% recurrence rate versus 52.5% in those that did not (p-value<0.05). Unfortunately, retrospective study design and small sample size clouds interpretation of these findings. With that said, the concept does warrant further evaluation given the impressive risk reduction at low treatment related toxicity. Concurrently, comparative studies evaluating the relative efficacy of these agents are not available. Nevertheless, given the robust data supporting intra-vesical chemotherapy, the EAU Guidelines do recommend a single post-operative dose of intravesical mitomycin after nephrouretectomy, typically within 24 hours of resection [48].
NEPHRON-SPARING SURGICAL RESECTION
Beyond endoscopic therapies with or without intraluminal instillations to manage low-grade/low-volume disease, patients can elect for surgical resection with ureteral reconstruction vs replacement if underlying disease factors warrant a more aggressive approach. The 2 most commonly cited options highlighted by the NCCN and the EAU UTUC Guidelines are distal ureterectomy with reimplantation or segmental ureteral resection with uretero-ureterostomy. For carefully selected patients meeting the low-risk criteria as outlined in the EAU guidelines (unifocal disease, tumor size <2 cm, low-grade cytology, low-grade biopsy, and no invasive aspect on CT urography), the oncologic outcomes between radical nephrourectomy and distal ureterectomy with reimplantation appear similar. A contemporary study by Seisen et al. [49] compared the oncologic outcomes of radical nephrourectomy, distal ureterectomy, and endoscopic treatment for clinically organ confined UTUC of the distal ureter. Overall, 128 (42.1%), 134 (44.1%), and 42 (13.8%) were treated with radical nephrourectomy, distal ureterectomy, and endoscopic surgery, respectively. Here the authors found equivalent rates of overall, cancer-specific, and intravesical recurrence-free survival across the 3 surgical procedures. Interestingly, when adjusting for comorbidity using the American Society of Anesthesiologists score, both distal ureterectomy (hazard ratio [HR] 0.80, p=0.01) and endoscopic surgery (HR 0.84, p=0.02) were independent predictors of overall survival [49]. As stated previously, this may support conservative treatments in select patients with significant comorbidity. Similarly, in a large French multi-institutional study, the 5-year probability of CSS, recurrence-free survival, and metastasis-free survival for segmental ureterectomy and radical nephrourectomy were 87.9% and 86.3% (p=0.99); 37% and 47.9% (p=0.48); and 81.9% and 85.4% (p=0.51), respectively [50]. Using SEER data, Lughezzani et al. [51] showed an overall cancer specific mortality (CSM) of 77.6% at 5-years after either nephrourectomy or segmental resection. However, when stratifying by disease stage, those with non-organ confined disease (either pT1-2N1-3 or pT3-4N1-3 for the purposes of this study) had a 5-year CSM as low as 28.7% [51]. Last, ileal substitution is an option for those with more extensive ureteral involvement. A more recent single center retrospective review from Ou et al. [52] identified 80 patients that underwent ileal ureter creation for UTUC and 2- and 5-year CSS were 87.55% and 75.0%, respectively.
SYSTEMIC THERAPY
Multi-modal therapy including systemic cisplatin-based chemotherapy is largely considered standard of care for lowerurinary tract UC. It should come as no surprise that systemic therapy is also being studied in the upper urinary tracts. Sequencing of therapy for UTUC can be quite challenging in lite of the fact that higher-grade disease frequently results in nephrectomy, and the potential nephrotoxicity as a result of surgical resection or cisplatin exposure or both is a significant concern.
The landmark POUT (peri-operative chemotherapy vs. surveillance in upper tract urothelial cancer) trial was a phase 3 randomized controlled clinical trial that recruited patients with invasive and/or node-positive UTUC to either surveillance or adjuvant cisplatin-based chemotherapy. Adjuvant chemotherapy was found to significantly improve disease-free survival (HR 0.45, p<0.01) at a median follow-up of 30.3 months. The 3-year event free estimates were 71% and 46% for chemotherapy and surveillance, respectively [53]. Data in the neoadjuvant space is largely retrospective. A meta-analysis by Kim et al. [54] including 4 large studies found that neoadjuvant chemotherapy had a 4.76-fold higher probability of having pathologic N0 status relative to the control group. Data from a small phase II trial of NAC followed by extirpative surgery by Margulis et al. [55] was not as reassuring. In this study on 3 patients (10.3%) achieved ypT0N0 status; the complete response rate was only 13.8%. In terms of immunotherapy (IO), the data remains immature. There are several ongoing clinical trials evaluating IOs in the adjuvant setting, several of which that are no longer accruing, but study results are not estimated to be available until 2022 at the earliest [56].
There is precious little data in terms of nephron-sparing surgery and systemic therapy. This is likely due to a multitude of factors to include the relative rarity of UTUC, the challenges inherent to adequate staging in the upper urinary tracts, the reluctance to offer systemic therapy without clear evidence of invasive disease, and the dilemma in pursuing nephron-sparing surgical options but also treating systemically with agents known to cause nephrotoxicity.
CONCLUSIONS
UTUC is fairly uncommon and presents unique diagnostic and therapeutic challenges. As highlighted before, the management of UTUC has largely been derivative of lower urinary tract UC despite divergent genetics and clinical behavior in the upper urinary tracts. Also, despite incredible advances made in endoscopic instrument design to include the availability of enhanced imaging (notably optical coherence tomography and confocal laser endomicroscopy), our ability to stage UTUC remains inferior to what can be accomplished in the bladder. Accurate assessment of disease burden and tumor aggressiveness are key to better treatment allocation, and this is especially important when treatment choices potentially result in renal extirpation and the possibility of CKD.
Both AUA and EAU Guidelines now support nephron-sparing approaches in well-selected patients, but the argument has been made that rates of salvage nephrouretectomy remain too high. Intraluminal immunotherapy (BCG) and chemotherapy (MMC and gemcitabine) have been studied with mixed results and are not yet supported by guidelines. Limitations in this treatment space reside in the difficulty of achieving adequate dwell times in the upper urinary tracts, and the sporadic indications, treatment regimens, and dosages which cloud the efficacy landscape. Novel delivery mechanisms are being engineered with some encouraging results in terms of response rates and tolerability – long-term outcomes such as progression-free and overall survival remain unknown. The benefits of systemic therapy for those requiring nephron-sparing approaches (endoscopic ablation, distal ureterectomy, and ileal ureter substitution) for imperative indications has not been adequately studied.
In conclusion, nephron-sparing approaches should be strongly considered for those with low-volume/low-grade disease as there appears to be equivalent oncological outcomes with reduced treatment-related morbidity. The added utility of intraluminal therapy is in question, as study results are mixed; improved diagnostics and treatment regimens may yet shift this paradigm and additional clinical trial results are eagerly anticipated.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Jason M. Farrow and Chandru P. Sundaram.
- Data acquisition: Jason M. Farrow, Gustavo M. Gryzinski, and Sean Q. Kern.
- Statistical analysis: Jason M. Farrow and Gustavo M. Gryzinski.
- Data analysis and interpretation: Jason M. Farrow, Chandru P. Sundaram, Sean Q. Kern, and Gustavo M. Gryzinski.
- Drafting of the manuscript: Jason M. Farrow, Sean Q. Kern, and Gustavo M. Gryzinski.
- Critical revision of the manuscript: Jason M. Farrow.
- Obtaining funding: Chandru P. Sundaram.
- Administrative, technical, or material support: Jason M. Farrow and Chandru P. Sundaram.
- Supervision: Chandru P. Sundaram.
- Approval of the final manuscript: Chandru P. Sundaram.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts & figures 2019. Atlanta: American Cancer Society; 2019. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Fiuk JV, Schwartz BF. Upper tract urothelial carcinoma: paradigm shift towards nephron sparing management. World J Nephrol. 2016;5:158–165. doi: 10.5527/wjn.v5.i2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright JL, Hotaling J, Porter MP. Predictors of upper tract urothelial cell carcinoma after primary bladder cancer: a population based analysis. J Urol. 2009;181:1035–1039. doi: 10.1016/j.juro.2008.10.168. discussion 1039. [DOI] [PubMed] [Google Scholar]
- 6.Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224–1233. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 7.Zabor EC, Furberg H, Lee B, Campbell S, Lane BR, Thompson RH, et al. Long-term renal function recovery following radical nephrectomy for kidney cancer: results from a multicenter confirmatory study. J Urol. 2018;199:921–926. doi: 10.1016/j.juro.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metcalf M, Pierorazio PM. Future strategies to enhance kidney preservation in upper urinary tract urothelial carcinoma. Transl Androl Urol. 2020;9:1831–1840. doi: 10.21037/tau.2019.11.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 2015;68:868–879. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Cowan NC, Turney BW, Taylor NJ, McCarthy CL, Crew JP. Multidetector computed tomography urography for diagnosing upper urinary tract urothelial tumour. BJU Int. 2007;99:1363–1370. doi: 10.1111/j.1464-410X.2007.06766.x. [DOI] [PubMed] [Google Scholar]
- 11.Schieda N, Blaichman JI, Costa AF, Glikstein R, Hurrell C, James M, et al. Gadolinium-based contrast agents in kidney disease: comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can Assoc Radiol J. 2018;69:136–150. doi: 10.1016/j.carj.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Woolen SA, Shankar PR, Gagnier JJ, MacEachern MP, Singer L, Davenport MS. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: a systematic review and meta-analysis. JAMA Intern Med. 2020;180:223–230. doi: 10.1001/jamainternmed.2019.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojas CP, Castle SM, Llanos CA, Santos Cortes JA, Bird V, Rodriguez S, et al. Low biopsy volume in ureteroscopy does not affect tumor biopsy grading in upper tract urothelial carcinoma. Urol Oncol. 2013;31:1696–1700. doi: 10.1016/j.urolonc.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Smith AK, Stephenson AJ, Lane BR, Larson BT, Thomas AA, Gong MC, et al. Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: implications for conservative management. Urology. 2011;78:82–86. doi: 10.1016/j.urology.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Clements T, Messer JC, Terrell JD, Herman MP, Ng CK, Scherr DS, et al. High-grade ureteroscopic biopsy is associated with advanced pathology of upper-tract urothelial carcinoma tumors at definitive surgical resection. J Endourol. 2012;26:398–402. doi: 10.1089/end.2011.0426. [DOI] [PubMed] [Google Scholar]
- 16.Hao YC, Xiao CL, Liu K, Liu YQ, Ma LL. [Application of narrow-band imaging flexible ureteroscopy in the diagnosis, treatment and follow-up of upper tract urothelial carcinomas] Zhonghua Wai Ke Za Zhi. 2018;56:222–226. doi: 10.3760/cma.j.issn.0529-5815.2018.03.011. Chinese. [DOI] [PubMed] [Google Scholar]
- 17.Kata SG, Aboumarzouk OM, Zreik A, Somani B, Ahmad S, Nabi G, et al. Photodynamic diagnostic ureterorenoscopy: a valuable tool in the detection of upper urinary tract tumour. Photodiagnosis Photodyn Ther. 2016;13:255–260. doi: 10.1016/j.pdpdt.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Somani BK, Moseley H, Eljamel MS, Nabi G, Kata SG. Photodynamic diagnosis (PDD) for upper urinary tract transitional cell carcinoma (UT-TCC): evolution of a new technique. Photodiagnosis Photodyn Ther. 2010;7:39–43. doi: 10.1016/j.pdpdt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Traxer O, Geavlete B, de Medina SG, Sibony M, Al-Qahtani SM. Narrow-band imaging digital flexible ureteroscopy in detection of upper urinary tract transitional-cell carcinoma: initial experience. J Endourol. 2011;25:19–23. doi: 10.1089/end.2009.0593. [DOI] [PubMed] [Google Scholar]
- 20.Bus MT, de Bruin DM, Faber DJ, Kamphuis GM, Zondervan PJ, Laguna Pes MP, et al. Optical diagnostics for upper urinary tract urothelial cancer: technology, thresholds, and clinical applications. J Endourol. 2015;29:113–123. doi: 10.1089/end.2014.0551. [DOI] [PubMed] [Google Scholar]
- 21.Malm C, Grahn A, Jaremko G, Tribukait B, Brehmer M. Diagnostic accuracy of upper tract urothelial carcinoma: how samples are collected matters. Scand J Urol. 2017;51:137–145. doi: 10.1080/21681805.2017.1295102. [DOI] [PubMed] [Google Scholar]
- 22.Chen AA, Grasso M. Is there a role for FISH in the management and surveillance of patients with upper tract transitional-cell carcinoma? J Endourol. 2008;22:1371–1374. doi: 10.1089/end.2008.0096. [DOI] [PubMed] [Google Scholar]
- 23.Johannes JR, Nelson E, Bibbo M, Bagley DH. Voided urine fluorescence in situ hybridization testing for upper tract urothelial carcinoma surveillance. J Urol. 2010;184:879–882. doi: 10.1016/j.juro.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 24.McHale T, Ohori NP, Cieply KM, Sherer C, Bastacky SI. Comparison of urinary cytology and fluorescence in situ hybridization in the detection of urothelial neoplasia: an analysis of discordant results. Diagn Cytopathol. 2019;47:282–288. doi: 10.1002/dc.24108. [DOI] [PubMed] [Google Scholar]
- 25.Cutress ML, Stewart GD, Tudor EC, Egong EA, Wells-Cole S, Phipps S, et al. Endoscopic versus laparoscopic management of noninvasive upper tract urothelial carcinoma: 20-year single center experience. J Urol. 2013;189:2054–2060. doi: 10.1016/j.juro.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Pan S, Smith AD, Motamedinia P. Minimally invasive therapy for upper tract urothelial cell cancer. J Endourol. 2017;31:238–245. doi: 10.1089/end.2016.0475. [DOI] [PubMed] [Google Scholar]
- 27.Yakoubi R, Colin P, Seisen T, Léon P, Nison L, Bozzini G, et al. Radical nephroureterectomy versus endoscopic procedures for the treatment of localised upper tract urothelial carcinoma: a meta-analysis and a systematic review of current evidence from comparative studies. Eur J Surg Oncol. 2014;40:1629–1634. doi: 10.1016/j.ejso.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Grasso M, Fishman AI, Cohen J, Alexander B. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: a 15-year comprehensive review of 160 consecutive patients. BJU Int. 2012;110:1618–1626. doi: 10.1111/j.1464-410X.2012.11066.x. [DOI] [PubMed] [Google Scholar]
- 29.Motamedinia P, Keheila M, Leavitt DA, Rastinehad AR, Okeke Z, Smith AD. The expanded use of percutaneous resection for upper tract urothelial carcinoma: a 30-year comprehensive experience. J Endourol. 2016;30:262–267. doi: 10.1089/end.2015.0248. [DOI] [PubMed] [Google Scholar]
- 30.Kim TH, Lee CU, Kang M, Jeon HG, Jeong BC, Seo SI, et al. Comparison of oncologic and functional outcomes between radical nephroureterectomy and segmental ureterectomy for upper urinary tract urothelial carcinoma. Sci Rep. 2021;11:7828. doi: 10.1038/s41598-021-87573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Pan Y, Hu J. Oncologic outcomes comparison of partial ureterectomy and radical nephroureterectomy for urothelial carcinoma. BMC Urol. 2019;19:120. doi: 10.1186/s12894-019-0557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang D, Seisen T, Yang K, Liu P, Fan X, Singla N, et al. A systematic review and meta-analysis of oncological and renal function outcomes obtained after segmental ureterectomy versus radical nephroureterectomy for upper tract urothelial carcinoma. Eur J Surg Oncol. 2016;42:1625–1635. doi: 10.1016/j.ejso.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scotland KB, Kleinmann N, Cason D, Hubbard L, Tanimoto R, Healy KA, et al. Ureteroscopic management of large ≥2 cm upper tract urothelial carcinoma: a comprehensive 23-year experience. Urology. 2018;121:66–73. doi: 10.1016/j.urology.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 34.Cutress ML, Stewart GD, Wells-Cole S, Phipps S, Thomas BG, Tolley DA. Long-term endoscopic management of upper tract urothelial carcinoma: 20-year single-centre experience. BJU Int. 2012;110:1608–1617. doi: 10.1111/j.1464-410X.2012.11169.x. [DOI] [PubMed] [Google Scholar]
- 35.Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196:1021–1029. doi: 10.1016/j.juro.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 36.Verges DP, Lallas CD, Hubosky SG, Bagley DH., Jr Endoscopic treatment of upper tract urothelial carcinoma. Curr Urol Rep. 2017;18:31. doi: 10.1007/s11934-017-0675-x. [DOI] [PubMed] [Google Scholar]
- 37.Rastinehad AR, Ost MC, Vanderbrink BA, Greenberg KL, El-Hakim A, Marcovich R, et al. A 20-year experience with percutaneous resection of upper tract transitional carcinoma: is there an oncologic benefit with adjuvant bacillus Calmette Guérin therapy. Urology. 2009;73:27–31. doi: 10.1016/j.urology.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Carmignani L, Bianchi R, Cozzi G, Grasso A, Macchione N, Marenghi C, et al. Intracavitary immunotherapy and chemotherapy for upper urinary tract cancer: current evidence. Rev Urol. 2013;15:145–153. [PMC free article] [PubMed] [Google Scholar]
- 39.Metcalfe M, Wagenheim G, Xiao L, Papadopoulos J, Navai N, Davis JW, et al. Induction and maintenance adjuvant Mitomycin C topical therapy for upper tract urothelial carcinoma: tolerability and intermediate term outcomes. J Endourol. 2017;31:946–953. doi: 10.1089/end.2016.0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aboumarzouk OM, Somani B, Ahmad S, Nabi G, Townell N, Kata SG. Mitomycin C instillation following ureterorenoscopic laser ablation of upper urinary tract carcinoma. Urol Ann. 2013;5:184–189. doi: 10.4103/0974-7796.115746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinmann N, Matin SF, Pierorazio PM, Gore JL, Shabsigh A, Hu B, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol. 2020;21:776–785. doi: 10.1016/S1470-2045(20)30147-9. [DOI] [PubMed] [Google Scholar]
- 42.Barros AA, Browne S, Oliveira C, Lima E, Duarte AR, Healy KE, et al. Drug-eluting biodegradable ureteral stent: new approach for urothelial tumors of upper urinary tract cancer. Int J Pharm. 2016;513:227–237. doi: 10.1016/j.ijpharm.2016.08.061. [DOI] [PubMed] [Google Scholar]
- 43.Balasubramanian A, Metcalfe MJ, Wagenheim G, Xiao L, Papadopoulos J, Navai N, et al. Salvage topical therapy for upper tract urothelial carcinoma. World J Urol. 2018;36:2027–2034. doi: 10.1007/s00345-018-2349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seisen T, Colin P, Rouprêt M. Risk-adapted strategy for the kidney-sparing management of upper tract tumours. Nat Rev Urol. 2015;12:155–166. doi: 10.1038/nrurol.2015.24. [DOI] [PubMed] [Google Scholar]
- 45.Xylinas E, Kluth L, Passoni N, Trinh QD, Rieken M, Lee RK, et al. Prediction of intravesical recurrence after radical nephroureterectomy: development of a clinical decision-making tool. Eur Urol. 2014;65:650–658. doi: 10.1016/j.eururo.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Wu P, Zhu G, Wei D, Liu S, Walsh K, Li D, et al. Prophylactic intravesical chemotherapy decreases bladder tumor recurrence after nephroureterectomy for primary upper tract urothelial carcinoma: a systematic review and meta-analysis. J BUON. 2015;20:1229–1238. [PubMed] [Google Scholar]
- 47.Yamamoto S, Sakamoto S, Imamura Y, Sazuka T, Nakamura K, Inoue T, et al. Intravesical irrigation might prevent bladder recurrence in patients undergoing radical nephroureterectomy for upper urinary tract urothelial carcinoma. Int J Urol. 2019;26:791–796. doi: 10.1111/iju.14014. [DOI] [PubMed] [Google Scholar]
- 48.Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. 2021;79:62–79. doi: 10.1016/j.eururo.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 49.Seisen T, Nison L, Remzi M, Klatte T, Mathieu R, Lucca I, et al. Oncologic outcomes of kidney sparing surgery versus radical nephroureterectomy for the elective treatment of clinically organ confined upper tract urothelial carcinoma of the distal ureter. J Urol. 2016;195:1354–1361. doi: 10.1016/j.juro.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 50.Colin P, Ouzzane A, Pignot G, Ravier E, Crouzet S, Ariane MM, et al. Comparison of oncological outcomes after segmental ureterectomy or radical nephroureterectomy in urothelial carcinomas of the upper urinary tract: results from a large French multicentre study. BJU Int. 2012;110:1134–1141. doi: 10.1111/j.1464-410X.2012.10960.x. [DOI] [PubMed] [Google Scholar]
- 51.Lughezzani G, Jeldres C, Isbarn H, Sun M, Shariat SF, Alasker A, et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of 2299 patients. Eur J Cancer. 2009;45:3291–3297. doi: 10.1016/j.ejca.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Ou YC, Hu CY, Cheng HL, Yang WH. Long-term outcomes of total ureterectomy with ileal-ureteral substitution treatment for ureteral cancer: a single-center experience. BMC Urol. 2018;18:73. doi: 10.1186/s12894-018-0389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birtle A, Johnson M, Chester J, Jones R, Dolling D, Bryan RT, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet. 2020;395:1268–1277. doi: 10.1016/S0140-6736(20)30415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim DK, Lee JY, Kim JW, Hah YS, Cho KS. Effect of neoadjuvant chemotherapy on locally advanced upper tract urothelial carcinoma: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2019;135:59–65. doi: 10.1016/j.critrevonc.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 55.Margulis V, Puligandla M, Trabulsi EJ, Plimack ER, Kessler ER, Matin SF, et al. Phase II trial of neoadjuvant systemic chemotherapy followed by extirpative surgery in patients with high grade upper tract urothelial carcinoma. J Urol. 2020;203:690–698. doi: 10.1097/JU.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teo MY, Rosenberg JE. Perioperative immunotherapy in muscle-invasive bladder cancer and upper tract urothelial carcinoma. Urol Clin North Am. 2018;45:287–295. doi: 10.1016/j.ucl.2017.12.011. [DOI] [PubMed] [Google Scholar]