Abstract
Purpose
To assess the correlation between post-void residual urine ratio (PVR-R) and pathological bladder emptying diagnosed by pressure-flow studies (PFS) in males with lower urinary tract symptoms (LUTS).
Materials and Methods
PVR-R and PVR urine were evaluated in 410 males underwent PFS for LUTS. PVR-R was the percentage of PVR to bladder volume (voided volume+PVR). Schafer and International Continence Society (ICS) nomograms, Bladder Contractility Index (BCI) were used to diagnose bladder outlet obstruction (BOO) and detrusor underactivity (DUA). We subdivided the cohort in 4 groups: Group I, BOO+/DUA+; Group II, BOO-/DUA+; Group III, BOO+/DUA−; Group IV, BOO−/DUA− (control group). We subdivided the 4 groups according to PVR-R strata: (1) 0%–20%; (2) 21%–40%; (3) 41%–60%; (4) 61%–80%; (5) 81%–100%.
Results
Group I had a greater median PVR-R (50%) with a >40% in 61.4% of the cohort. Median PVR-R was 16.6% in Group II, 24% in Group III, and 0% in the control Group. According to ICS nomograms and BCI, median PVR-R and PVR were significantly higher (p<0.001) in obstructed and underactive males. PVR-R threshold of 20% allowed to recognize males with voiding disorders with high sensibility, specificity, PPV, and NPV. A PVR-R cut-off of 40% identified males with associated BOO and DUA and more severe voiding dysfunction.
Conclusions
A higher PVR-R is related to a more severe pathological bladder emptying, and to the association of BOO and DUA. PVR-R may have a clinical role in first assessment of males with LUTS and severe voiding dysfunction.
Keywords: Bladder outlet obstruction, Detrusor underactivity, Lower urinary tract symptoms, Males, Urodynamics
Graphical Abstract
INTRODUCTION
Post-void residual (PVR) urine is a controversial part of routine clinical assessment in males with lower urinary tract symptoms (LUTS) [1]. PVR is measured by transabdominal ultrasound, bladder scan or catheterization [2,3,4,5,6]. A missing established PVR threshold for treatment decision is the main limit of this parameter, due to large test-retest variability and lack of outcome studies [7]. Moreover, the diagnostic accuracy of PVR is low. The use of a PVR threshold of 50 mL has a positive predictive value (PPV) of 63% and a negative predictive value (NPV) of 52% as a predictor of bladder outlet obstruction (BOO) [8]. The identification of patients at risk for acute urinary retention may be achieved monitoring the changes over time of PVR measurements [9]. Furthermore, in patients with LUTS, high baseline PVR is associated with an increased risk of symptom progression [10,11].
Bladder voiding efficiency (BVE; [voided volume/total bladder capacity]×100) has been introduced to overcome some limits of PVR [12,13]. This parameter has been found to be more reliable than PVR also in the evaluation of patients with detrusor underactivity (DUA) [14]. In DUA subjects PVR variations were related to bladder volume (BV), while BVE had no significant relationship with BV and did not change in repeated measurements in the same patient.
Since the role of PVR in males with LUTS has given only poor and inconclusive data, we investigated a new and more functional parameter, post-void residual urine ratio (PVR-R). PVR-R is a percentage representing the ratio of PVR to BV. This parameter indicates the non-functional bladder storage of urine after micturition and is better related to the voiding emptying than the PVR per se. The same PVR value can have different meanings and relevance in patients with different bladder capacities, and also in the same male based on the bladder filling at the uroflowmetry (UF). Consequently, PVR is not comparable between individuals and not even in the same person. This may be a possible explanation of the poor and controversial predictive value of PVR. Contrary, PVR-R is a ratio linked to bladder capacity and voided volume and, hence, more reliable in representing the degree of failure of the bladder emptying.
The aim of this study was to assess, in males with LUTS, the relationship between PVR-R and pathological bladder emptying diagnosed by Pressure-flow studies (PFS).
MATERIALS AND METHODS
Between January 2013 and June 2019, PVR-R and PVR were evaluated in a cohort of males underwent PFS for LUTS in all the urodynamics were performed in the Department of Urology, AOUI Verona. Ethical standards were performed according to the 1964 Declaration of Helsinki and its later amendments. Informed consent was acquired from all patients enrolled before inclusion in the study. Ethics Committee for Clinical Trials (CESC) of the provinces of Verona and Rovigo determined that the approval for this investigation was unnecessary since it only involved standard clinical practice. This research was registered in the audit was performed in the hospital where the urodynamics were performd, AOUI Verona. Demographic data of the patients were recorded. PVR-R was calculated as the percentage of PVR to total BV. Total BV was considered as a sum of voided volume (VV) and PVR. Hence, PVR-R was defined as follows: PVR-R=(PVR/total BV)×100.
Data was prospectively collected and retrospectively analyzed. Urodynamic diagnosis of males with BOO, DUA, BOO associated to DUA, or no pathological voiding were achieved. Hence, data of all the types of pathological bladder emptying, and also normal voiding, were correlated with PVR-R. Due to the lack of standardized urodynamics criteria of BOO and DUA in females, we decided to exclude females from our study. Free UF was the first examination, and was performed with patient arriving with full bladder greater than 150 mL. After performing free UF and evaluating the PVR by catheterization, the patients were prepared for invasive urodynamics and two PFS were performed in the same urodynamic section in the lapse-time of approximately two hours, according to The Good Urodynamic Practices [11,15]. To achieve the most accurate diagnosis of BOO, DUA, or mixed conditions, and to reduce any bias related to a sole examination, we decided to perform two PFS. PVR urine was measured by catheterization. To reduce PVR variability, in each patient this parameter was measured three times in the same day: after the two PFS and after a free UF. The lowest PVR was considered the most accurate to represent the better capability of the patients to empty the bladder. DUA was defined by Schafer nomograms classes as “very weak” and “weak” and by Bladder Contractility Index (BCI) as <100. Males with Schaefer nomograms obstruction classes II–VI and with International Continence Society (ICS) nomograms obstructed class (score >40) were considered as BOO [13]. According to urodynamics, we subdivided the cohort in four groups: Group I, BOO+/DUA+; Grouop II, BOO−/DUA+; Group III, BOO+/DUA−; Group IV, BOO−/DUA−. Group IV included males with non-pathological bladder emptying, and was considered as the control group. The first analysis graded patients according to detrusor contractility. A second evaluation was performed comparing patients by obstructive urodynamic diagnosis stratification. Comparison of both PVR-R and PVR in obstructed patients according to ICS nomograms and in underactive males according to BCI was performed. Lastly, we subdivided the four groups according to PVR-ratio strata as follows: (1) 0%–20%; (2) 21%–40%; (3) 41%–60%; (4) 61%–80%; (5) 81%–100%. The sensibility, specificity, PPV and NPV of PVR-R were also evaluated. In the pathological groups (Groups I–III) we evaluated sensibility, specificity, PPV and NPV of both the 40% and 20% PVR-R thresholds, while in the control group (Group IV) we evaluated these parameters using a cutoff of PVR-R <20%.
Statistical analysis
For the statistical analysis SPSS ver. 19.0 for Windows (IBM Corp., Armonk, NY, USA) was used. Continuous variables were reported as median, while the ratio between voided volume and BV was presented as percentage. Statistical test used were Mann–Whitney test, considering significant p<0.05. Sensibility, specificity, PPV, and NPV were evaluated.
RESULTS
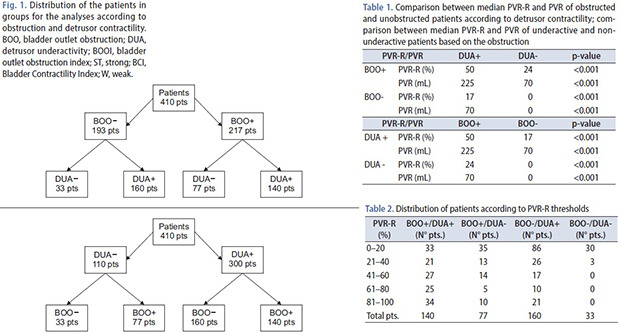
We analyzed 410 males PFS. Fig. 1 represents cohorts' subdivision and analysis. The mean patients age was 61.6+14.6 years, in Group I was 66.6+11.9 years, in Group II 58.7+18.8 years, in Group III 61.8+12.6 years, in Group IV 56.3+16.4 years. The patients who underwent urological therapy (alpha-adrenergic agents, 5-alpha reductase) were 36.3% (149/410), while males with indwelling catheter or in clean intermittent catheterization regimen were 10.2% (42/410). Table 1 reports the comparison between median PVR-R and PVR of obstructed and unobstructed patients, according to detrusor contractility. In the latter table, median PVR-R and PVR of underactive and non-underactive patients, according to obstruction, are also compared. The distribution of patients in each group based on PVR-R is reported in Table 2. Group I had a greater median PVR-R (50%) with a PVR-R >40% in 61.4% of the cohort. PVR-R was three times lower in Group II (16.6%) and only 30.0% of patients showed a PVR-R >40%. Group III found a median PVR-R of 24% and 37.7% of males had a PVR-R >40%. The control Group showed a median PVR-R of 0% and no subject had a PVR-R >40%.
Fig. 1. Distribution of the patients in groups for the analyses according to obstruction and detrusor contractility. BOO, bladder outlet obstruction; DUA, detrusor underactivity; BOOI, bladder outlet obstruction index; ST, strong; BCI, Bladder Contractility Index; W, weak.
Table 1. Comparison between median PVR-R and PVR of obstructed and unobstructed patients according to detrusor contractility; comparison between median PVR-R and PVR of underactive and non-underactive patients based on the obstruction.
| PVR-R/PVR | DUA+ | DUA− | p-value | |
|---|---|---|---|---|
| BOO+ | PVR-R (%) | 50 | 24 | <0.001 |
| PVR (mL) | 225 | 70 | <0.001 | |
| BOO− | PVR-R (%) | 17 | 0 | <0.001 |
| PVR (mL) | 70 | 0 | <0.001 | |
PVR-R, post-void residual urine ratio; DUA, detrusor underactivity; BOO, bladder outlet obstruction.
Table 2. Distribution of patients according to PVR-R thresholds.
| PVR-R (%) | BOO+/DUA+ (N° pts.) | BOO+/DUA− (N° pts.) | BOO−/DUA+ (N° pts.) | BOO−/DUA− (N° pts.) |
|---|---|---|---|---|
| 0–20 | 33 | 35 | 86 | 30 |
| 21–40 | 21 | 13 | 26 | 3 |
| 41–60 | 27 | 14 | 17 | 0 |
| 61–80 | 25 | 5 | 10 | 0 |
| 81–100 | 34 | 10 | 21 | 0 |
| Total pts. | 140 | 77 | 160 | 33 |
PVR-R, post-void residual urine ratio; BOO, bladder outlet obstruction; DUA, detrusor underactivity.
According to ICS nomograms, median PVR-R and PVR were significantly higher (p<0.001) in obstructed patients (Table 3). According to BCI nomograms, males with DUA showed a statistically greater median PVR-R and PVR (p<0.001) (Table 3).
Table 3. Comparison between median PVR-R and PVR of unobstructed and obstructed patients according to ICS nomograms; comparison between median PVR-R and PVR of underactive and non-underactive males according to BCI.
| Population | Unobstructeda | Obstructeda | p-value |
|---|---|---|---|
| N° pts. (n=410) | 193 | 217 | |
| PVR-R (%) | 6 | 45 | <0.001 |
| PVR (mL) | 20 | 190 | <0.001 |
PVR-R, post-void residual urine ratio; ICS, International Continence Society; BCI, Bladder Contractility Index.
a:Unobstructed and obstructed according to ICS nomograms.
Sensibility, specificity, PPV, and NPV of PVR-R in each group are reported in Table 4. Using a PVR-R threshold of 40%, we found in males with concomitant obstruction and underactivity (Group I) a high specificity and PPV, and a moderate sensibility and NPV. In patients of Group II and III, according to PVR-R cut-off of 40%, specificity and PPV were high, while sensibility and NPV were low. Considering a PVR-R threshold of 20%, in males of Group I (DAU and BOO patients), sensibility, specificity, PPV, and NPV were high. In unobstructed and underactive males (Group II), using a PVR-R cut-off of 20%, specificity and PPV were high, while sensibility and NPV were low. In obstructed and non-underactive patients (Group III), according to a PVR-R threshold of 20%, specificity and PPV were high, while sensibility and NPV were moderate. In the control group of non-pathological patients (unobstructed and non-underactive), considering a PVR-R cut-off <20%, sensibility, specificity, PPV, and NPV were high.
Table 4. Sensibility, specificity, PPV, and NPV of the four groups according to PVR-R thresholds of 40% and 20%.
| Group | PVR-R (%) | Sensibility | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Group I | 40 | 0.67 | 0.85 | 0.89 | 0.58 |
| 20 | 0.78 | 0.83 | 0.89 | 0.85 | |
| Group II | 40 | 0.30 | 0.84 | 0.86 | 0.26 |
| 20 | 0.44 | 0.83 | 0.89 | 0.32 | |
| Group III | 40 | 0.44 | 0.84 | 0.82 | 0.48 |
| 20 | 0.64 | 0.83 | 0.85 | 0.60 | |
| Group IV | <20 | 0.78 | 0.83 | 0.89 | 0.85 |
PPV, positive predictive value; NPV, negative predictive value; PVR-R, post-void residual ratio; BCI, Bladder Contractility Index; BOOI, bladder outlet obstruction index.
Group I: BCI<100+BOOI>40. Group II: BCI<100+BOOI<20. Group III: BCI≥100+BOOI>40. Group IV: BCI≥100+BOOI<20.
DISCUSSION
The amount of urine volume that may define a clinically relevant PVR has no consensus in literature [2]. Poorly associated with pathological voiding emptying, standard PVR urine fails as a predictive factor [7,8]. We investigated a novel parameter, PVR-R, which is related to both the total bladder capacity and total voided volume. For this reason, PVR-R might identify more clearly and properly the bladder emptying impairment, and it is comparable among patients. On the contrary, the standard PVR is a simple volume without relationship to the bladder filling and voided volume, and this is one of the main limits of this parameter. Abrams [12] described the BVE as a product of bladder contractility against urethral resistance, measured according to the degree of bladder emptying, and defined as: (voided volume/total bladder capacity)×100. BVE is a factor focused on VV, indicating the bladder contractility function of the patients, and representing the rate of successful emptying. Conversely, PVR-R is a parameter which quantify the degree of failure of bladder emptying, centered on the PVR. BVE measures the strength of the bladder during the voiding phase, while PVR-R measures bladder deficiency. Consequently, BVE and PVR-R are opposite parameters and cannot be considered as the same expression of bladder emptying measure. BVE and PVR-ratio are useful to better define the bladder voiding function under all possible aspects. However, both these two parameters may be measured to achieve a proper and correct evaluation of males with LUTS.
We found a correlation between PVR-R and pathological bladder emptying. Males with BOO and concomitant DUA had the most critical clinical condition. Median bladder emptying in this group was half of the bladder capacity, representing a high value of PVR-R of 50%. The counter-proof of the relationship between the pathological voiding and PVR-R was the significant lowering of PVR-R when BOO and DUA were not associated. Finally, in males with pathological emptying (BOO or DUA) the median PVR-R did not reach 0% as in the control group. Most of the patients with BOO and concurrent DUA showed PVR-R >40%. This PVR-R threshold has proven a high specificity and PPV, with a moderate sensibility and NPV. Therefore, using 40% as cut-off, PVR-R had a high reliability in identifying males with the potential association of obstruction and detrusor contractility disorders, and with a more severe voiding dysfunction. Therefore, males under investigation for LUTS, with high values of PVR-R (>40%) should be considered as “red flag patients” due to their very great risk to have the most impaired bladder emptying condition. Patients with PVR >40% might also represent the males whom a further urodynamic investigation may be required. These males might be the main candidates for pharmacological or surgical treatments, and might also have the potential worse results after BOO treatments, due to the mixed bladder emptying disorder.
The 40% PVR-R threshold had low sensibility and NPV in unobstructed and underactive patients (Group II) and in obstructed and non-underactive males (Group III). Therefore, we evaluated a lower PVR-R cut off (20%) in all pathological groups (Groups I–III). By lowering the PVR-R threshold, specificity and PPV remained high, and both sensibility and NPV were improved in all these groups. In the control group (Group IV), the PVR-R threshold <20% showed high sensibility, specificity, PPV, and NPV. Therefore, patients with PVR-R values below this cut-off are very likely not associated with a pathological condition of bladder emptying. Finally, according to our data, the PVR-R threshold of 20% can be considered a suitable limit to differentiate males who are at low risk (<20%) or at high risk (>20%) of emptying disorders such as BOO/DUA, although it is not able to differentiate the type of voiding dysfunction. This risk increases as the PVR-R rises.
PVR-R might have relevant implications in the clinical assessment and follow-up of males with LUTS. The PVR-R threshold of 20% can be a reliable and effective tool in the initial assessment of males with LUTS, with a high sensibility and specificity in identifying patients with pathological bladder emptying conditions. On the contrary, standard PVR is poor reliable. Indeed, a low PVR (i.e., ≤100 mL) could be misleadingly considered as normal, while it could represent a high and pathological percentage of residual urine compared to the bladder filling. Therefore, the PVR can be a confounding parameter in the evaluation of males with LUTS, while the PVR-R allows to identify the effective failure of the bladder emptying. In addition, the PVR-R is truly comparable during the follow-up of males and can be used to recognize a worsening condition more clearly than the standard PVR.
PVR-R may also play a role in the preoperative assessment of candidates for surgery for LUTS and presumed benign prostatic hyperplasia (BPH), helping clinicians to better recognize males who may need further investigation and invasive urodynamics. Data of a recent Cochrane and meta-analysis and of the RCT UPSTREAM study did not demonstrate the usefulness of preoperative urodynamics (UD) in these candidates to surgery [16,17]. However, several studies have shown that males with DUA, associated or not to BOO, had satisfactory results at short-time follow-up after surgery, but not long lasting-time [18,19,20,21,22,23]. Moreover, poorer surgical outcomes have been reported in underactive males than in non-underactive obstructed patients. Unfortunately, without preoperative invasive UD, it is not possible to identify the patients with DUA who may have a higher risk of a recurrence of LUTS during the follow-up. Although PVR-R cannot be used to distinguish the type of voiding dysfunction, the rate of this parameter relevantly increased in case of association of BOO and DUA. Hence, candidates to surgery for LUTS suggestive of BPH, with PRV-R higher than 40%, are likelihood to have associated voiding disorders, or a more relevant bladder emptying impairment. For this reason, PVR-R could be a useful tool to select males who may require a preoperative UD investigation, allowing to achieve a better preoperative counseling and management. Conversely, the standard PVR is not reliable in the identification of these males.
In our study, correlation between PVR-R and pathological bladder emptying was urodynamically proven in a very large cohort. Since invasive urodynamics is still the only accepted tool to diagnose BOO and DUA, we considered it as a strength of the study. The assessment of PVR was obtained by several measurements in each patient in the same day, lowering the variability of this parameter and possible bias. Thus, this is another strength of our study. Furthermore, the control group demonstrated no PVR-R differences between the two studies, showing that the number of PVR measurements in our investigations were sufficient. The large group of the patients evaluated in the study was also an important feature. Finally, we found PVR-R thresholds significantly associated to the bladder emptying disorders, although the lower cut-off (20%) was not able to differentiate between a BOO or DUA disorder. This is a limit of our study. However, the identification of the underlying pathophysiology of LUTS is not the aim of clinical use of PVR-R. Only invasive urodynamics can diagnose the specific pathophysiology of LUTS. PVR-R may be useful in the initial assessment of males with LUTS when urologists need to recognize which patients may have more severe voiding disorders and may require further investigation. Thus, although PVR-R pathological thresholds cannot identify the pathophysiology of voiding disorders, its use can aid in the classification of males at high or low risk of voiding. However, improved selection of patients that may benefit from further investigations with urodynamics may be guided by the data we collected. Another limit of the study may be the inclusion of the patients on clean intermittent catheterization or indwelling catheter. However, this category of males represents a part of the subjects with voiding dysfunctions referring to clinicians and aim of our real-life study was to collect the most complete data in all the types of patients with voiding dysfunctions. Nevertheless, these males were only a minor part of the cohort. Our Department is in a Tertiary Hospital, and our urodynamic clinic is a referral center. For this reason, most of the patients, after urodynamics, underwent the following clinical management in other urological Departments, and consequently we could not perform their follow-up. This may be another limit of our study.
CONCLUSIONS
Currently, PVR has shown several limitations in the evaluation of males with LUTS [7,8,13]. PVR-R is a novel and accurate measurement of bladder emptying. A higher PVR-R is related to a more severe pathological bladder emptying, especially when BOO and DUA are associated. A PVR-R threshold of 20% may be useful for differentiate between males at low or high risk of voiding disorders. A greater PVR-R cut-off of 40% may be used to identify males that are likelihood to suffer of a mixed BOO/DUA condition. Therefore, PVR-R may have a relevant clinical role in first assessment of males with LUTS, and might also improve the selection of patients whom require preoperative UD investigations. PVR-R can overcome the limits of PVR recognizing which males may be at higher risk of voiding dysfunctions. We consider that this this finding adds new data our current knowledge.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Emanuele Rubilotta.
- Data acquisition: Antonio D'Amico.
- Statistical analysis: Nicolò Trabacchin.
- Data analysis and interpretation: Emanuele Rubilotta, Matteo Balzarro, and Rita Righetti.
- Drafting of the manuscript: Jerry G. Blaivas.
- Critical revision of the manuscript: Emanuele Rubilotta, Jerry G. Blaivas, and Alessandro Antonelli.
- Supervision: Alessandro Antonelli.
- Approval of the final manuscript: Emanuele Rubilotta and Jerry G. Blaivas.
References
- 1.Abrams P. New words for old: lower urinary tract symptoms for “prostatism”. BMJ. 1994;308:929–930. doi: 10.1136/bmj.308.6934.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gravas S, Cornu JN, Drake MJ, Gacci M, Gratzke C, Herrmann TRW, et al. Diagnostic evaluation. In: Gravas S, Cornu JN, Drake MJ, Gacci M, Gratzke C, Herrmann TRW, et al., editors. EAU guidelines on management of non-neurogenic male lower urinary tract symptoms (LUTS), incl. benign prostatic obstruction (BPO) Arnhem, The Netherlands: European Association of Urology; 2018. pp. 6–14. [Google Scholar]
- 3.Asimakopoulos AD, De Nunzio C, Kocjancic E, Tubaro A, Rosier PF, Finazzi-Agrò E. Measurement of post-void residual urine. Neurourol Urodyn. 2016;35:55–57. doi: 10.1002/nau.22671. [DOI] [PubMed] [Google Scholar]
- 4.Goode PS, Locher JL, Bryant RL, Roth DL, Burgio KL. Measurement of postvoid residual urine with portable transabdominal bladder ultrasound scanner and urethral catheterization. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:296–300. doi: 10.1007/s001920070020. [DOI] [PubMed] [Google Scholar]
- 5.Ouslander JG, Simmons S, Tuico E, Nigam JG, Fingold S, Bates-Jensen B, et al. Use of a portable ultrasound device to measure post-void residual volume among incontinent nursing home residents. J Am Geriatr Soc. 1994;42:1189–1192. doi: 10.1111/j.1532-5415.1994.tb06987.x. [DOI] [PubMed] [Google Scholar]
- 6.Nygaard IE. Postvoid residual volume cannot be accurately estimated by bimanual examination. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:74–76. doi: 10.1007/BF01902376. [DOI] [PubMed] [Google Scholar]
- 7.Fantl JA, Newman DK, Colling J, DeLancey JOL, Keeys C, Loughery R, et al. Urinary incontinence in adults: acute and chronic management. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research (AHCPR); 1996. [Google Scholar]
- 8.Oelke M, Höfner K, Jonas U, de la Rosette JJ, Ubbink DT, Wijkstra H. Diagnostic accuracy of noninvasive tests to evaluate bladder outlet obstruction in men: detrusor wall thickness, uroflowmetry, postvoid residual urine, and prostate volume. Eur Urol. 2007;52:827–834. doi: 10.1016/j.eururo.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Roehrborn CG. Alfuzosin 10 mg once daily prevents overall clinical progression of benign prostatic hyperplasia but not acute urinary retention: results of a 2-year placebo-controlled study. BJU Int. 2006;97:734–741. doi: 10.1111/j.1464-410X.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 10.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr, Dixon CM, Kusek JW, et al. Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 11.Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–274. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 12.Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: three simple indices to define bladder voiding function. BJU Int. 1999;84:14–15. doi: 10.1046/j.1464-410x.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- 13.Yono M, Ito K, Oyama M, Tanaka T, Irie S, Matsukawa Y, et al. Variability of post-void residual urine volume and bladder voiding efficiency in patients with underactive bladder. Low Urin Tract Symptoms. 2021;13:51–55. doi: 10.1111/luts.12325. [DOI] [PubMed] [Google Scholar]
- 14.Nitti VW. Pressure flow urodynamic studies: the gold standard for diagnosing bladder outlet obstruction. Rev Urol. 2005;7(Suppl 6):S14–S21. [PMC free article] [PubMed] [Google Scholar]
- 15.Rosier PFWM, Schaefer W, Lose G, Goldman HB, Guralnick M, Eustice S, et al. International Continence Society Good Urodynamic Practices and Terms 2016: urodynamics, uroflowmetry, cystometry, and pressure-flow study. Neurourol Urodyn. 2017;36:1243–1260. doi: 10.1002/nau.23124. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Jeong CW, Oh SJ. Effect of preoperative urodynamic detrusor underactivity on transurethral surgery for benign prostatic hyperplasia: a systematic review and meta-analysis. J Urol. 2018;199:237–244. doi: 10.1016/j.juro.2017.07.079. [DOI] [PubMed] [Google Scholar]
- 17.Drake MJ, Lewis AL, Young GJ, Abrams P, Blair PS, Chapple C, et al. Diagnostic assessment of lower urinary tract symptoms in men considering prostate surgery: a noninferiority randomised controlled trial of urodynamics in 26 hospitals. Eur Urol. 2020;78:701–710. doi: 10.1016/j.eururo.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Rubilotta E, Balzarro M, Gubbiotti M, Antonelli A. Outcomes of transurethral resection of the prostate in unobstructed patients with concomitant detrusor underactivity. Neurourol Urodyn. 2020;39:2179–2185. doi: 10.1002/nau.24470. [DOI] [PubMed] [Google Scholar]
- 19.Blaivas JG, Forde JC, Davila JL, Policastro L, Tyler M, Aizen J, et al. Surgical treatment of detrusor underactivity: a short term proof of concept study. Int Braz J Urol. 2017;43:540–548. doi: 10.1590/S1677-5538.IBJU.2016.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seki N, Kai N, Seguchi H, Takei M, Yamaguchi A, Naito S. Predictives regarding outcome after transurethral resection for prostatic adenoma associated with detrusor underactivity. Urology. 2006;67:306–310. doi: 10.1016/j.urology.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Thomas AW, Cannon A, Bartlett E, Ellis-Jones J, Abrams P. The natural history of lower urinary tract dysfunction in men: the influence of detrusor underactivity on the outcome after transurethral resection of the prostate with a minimum 10-year urodynamic follow-up. BJU Int. 2004;93:745–750. doi: 10.1111/j.1464-410X.2003.04719.x. [DOI] [PubMed] [Google Scholar]
- 22.Masumori N, Furuya R, Tanaka Y, Furuya S, Ogura H, Tsukamoto T. The 12-year symptomatic outcome of transurethral resection of the prostate for patients with lower urinary tract symptoms suggestive of benign prostatic obstruction compared to the urodynamic findings before surgery. BJU Int. 2010;105:1429–1433. doi: 10.1111/j.1464-410X.2009.08978.x. [DOI] [PubMed] [Google Scholar]
- 23.Wada N, Kikuchi D, Tateoka J, Abe N, Banjo H, Tsuchida M, et al. Long-term symptomatic outcome after transurethral resection of the prostate: a urodynamics-based assessment. Int J Urol. 2019;26:1071–1075. doi: 10.1111/iju.14104. [DOI] [PubMed] [Google Scholar]