Abstract
Purpose
P21-activated kinase 4 (PAK4), a serine/threonine kinase that regulates a number of fundamental cellular processes, has been suggested as a prognostic factor for various human tumors. The aim of the present study was to evaluate the clinical implications of phospho-Ser474 PAK4 (pPAK4S474), an activated form of PAK4, in surgically treated renal cell carcinoma (RCC).
Materials and Methods
Samples from 131 patients with surgically treated RCC were immunostained to detect PAK4 and pPAK4S474. Expression of PAK4 and pPAK4S474 was compared with clinicopathological characteristics and survival after nephrectomy.
Results
PAK4 and pPAK4S474 were expressed predominantly in the nucleus. Overall, 57.3% (75/131) and 24.4% (29/119) of specimens exhibited high expression of pPAK4S474 and PAK4, respectively. High expression of pPAK4S474 was associated with adverse pathologic characteristics, including advanced tumor stage and grade (p=0.036 and p=0.002, respectively), whereas this association was not significant for PAK4 expression (each p>0.05). Kaplan-Meier estimates showed that high expression of pPAK4S474 was associated with shorter recurrence-free survival in a subgroup with localized RCC and with cancer-specific survival in the total RCC cohort (log-rank test: p=0.001 and p=0.005, respectively), whereas PAK4 expression was not. Multivariate Cox regression analysis identified that high pPAK4S474 expression was an independent predictor of recurrence in the subgroup with localized RCC.
Conclusions
pPAK4S474 may be a more accurate prognostic factor than total PAK4 in RCC patients. This marker would be useful for identifying patients with pathologically localized disease who may require further interventions.
Keywords: P21-activated kinase 4, Recurrence, Renal cell carcinoma, Survival
Graphical Abstract
INTRODUCTION
Renal cell carcinoma (RCC) accounts for 2% to 3% of all adult malignancies and for 85% of malignant neoplasms arising from the kidney [1,2]. Similar to other malignancies, RCC presents with significant clinical heterogeneity, ranging from indolent to highly aggressive [3]. Several histological factors, including the tumor, nodes, metastasis (TNM) staging, nuclear grade, and histological type have been the most reliable RCC prognostic factors [4]. However, none of the predictive tools based on conventional clinicopathological parameters can determine the prognosis of RCC sufficiently [5]. To overcome these limitations, intensive efforts to identify molecular markers that identify patients actually at risk of death and recurrence following surgery are needed. These markers might not only be useful prognostic markers but also potential therapeutic targets in the adjuvant setting [6].
The p21-activated kinases (PAKs) belonging to the serine/threonine protein kinases are well recognized as downstream effectors of the Rho family of GTPases [7,8,9]. The recent discovery of several novel isoforms means that PAKs are now categorized into two subgroups based on architectural similarities [7,9]: Group I PAKs (PAK1-3) and the newly recognized Group II PAKs (PAK4-6). They regulate a wide range of cellular processes, including cell proliferation, angiogenesis, tumorigenesis, and metastasis [7,10]. PAKs are overexpressed and/or amplified in a variety of human cancers, and their role in cell transformation makes them attractive therapeutic targets [11,12,13,14]. In RCC, PAK1 regulates cell proliferation, invasion, anchorage-independent growth, and apoptosis, as well as resistance to 5-fluorouracil [15]. Recently, PAK4 was identified as an independent marker of poor prognosis in patients with surgically treated non-metastatic RCC [16]. Evidence suggests that PAKs play a crucial role in the biology of RCC, studies indicate an association between total PAKs expression and aggressiveness and poor survival; however, no published study to date has examined the effect of active phosphorylated PAK4 on RCC prognosis. We hypothesized that PAK4 activity rather than expression of total PAK4 reflects the real role of PAK4 in RCC. PAK4 activation involves autophosphorylation at serine 474 (S474) [17,18]. Therefore, in the present study, we used immunohistochemical (IHC) staining to detect expression of phospho-Ser474 PAK4 (pPAK4S474) in tissues from patients with surgically-treated RCC to elucidate its prognostic implications.
MATERIALS AND METHODS
1. Patients and tissue samples
The study was carried out in agreement with all applicable laws and regulations, good clinical practices, and the ethical principles described in the Declaration of Helsinki. The Ethics Committee of Chungbuk National University approved the protocol (IRB approval number: GR2010-12-010), and all patients provided written informed consent. Sample collection and analysis were approved by the Institutional Review Board of Chungbuk National University.
Tissue samples from 131 patients with primary RCC who underwent radical or partial nephrectomy were collected between May 2000 and December 2015. The pathology samples were re-examined by a pathologist to confirm the presence of tumor. All tumors were macrodissected within 15 minutes of surgical resection, fresh-frozen in liquid nitrogen, and stored at -80℃ until use. Regarding the fresh-frozen sections from nephrectomy specimens, each cancer specimen was confirmed as representative tumor tissue by analysis of adjacent tissue. The 7th edition of the American Joint Committee on Cancer classification system was used for evaluating pathological staging, and nuclear differentiation was graded according to the Fuhrman nuclear grading system [19,20]. All patients were evaluated postoperatively every 3 months for the first 2 years, every 6 months for the next 2 years, and yearly thereafter.
These evaluations included physical examinations, and radiologic investigations. Recurrence was defined as locoregional recurrence or newly identified distant metastasis based on clinical and radiographic findings. Deaths were determined by reviewing medical records and/or confirmed by interview with the family of the patient.
2. Tissue microarray and immunohistochemistry
Paraffin blocks from 131 patients with primary RCC who underwent radical or partial nephrectomy were used for IHC analysis. Tissue microarray and IHC were performed in accordance with the previous studies [18,21]. The anti-pPAK4S474 and total PAK4 antibodies were purchased from Cell Signaling Technology (Boston, MA, USA). Staining intensity and the proportion of positively stained epithelial cells were evaluated. Staining intensity was classified as follows: low expression (none or weak intensity) vs. high expression (moderate or strong intensity). Each specimen was examined and scored separately by three investigators, and discrepancies were discussed until agreement was reached.
3. Statistical analysis
Patients were categorized into two groups according to staining intensity: low pPAK4S474 and none/weak total PAK4 expression, and high pPAK4S474 and moderate/strong total PAK4 expression. Clinical and pathological characteristics were compared between the two groups using the chi square or Fisher's exact test (categorical variables) or the Student's t-test (numerical variables). The survival distributions, including tumor recurrence and cancer-specific death (CSD) were estimated by the Kaplan-Meier method and compared by a log-rank test. Univariate and multivariate survival analyses were performed using the Cox proportional hazard regression model. Recurrence was assessed in 97 patients with pathologically localized disease (T1-2N0M0), and cancerspecific survival (CSS) was evaluated using the total study cohort. Differences were considered significant at p<0.05, and all reported p-values were two-sided. All analyses were performed using IBM SPSS ver. 24.0 software (IBM Corp., Armonk, NY, USA).
RESULTS
The clinicopathologic data of 131 subjects are summarized in Table 1. The mean age of the patients was 59.5 years and 74.8% were male. A radical and partial nephrectomy was performed in 107 (81.7%) and 24 patients (18.3%), respectively (Table 1).
Table 1. Baseline characteristics of 131 patients with surgically-treated renal cell carcinoma.
| Variable | Value | |
|---|---|---|
| Mean age (y) | 59.5±12.8 | |
| Median follow-up (mo) | 55.9 (19.1–97.2) | |
| BMI (kg/m2) | 24.5±3.5 | |
| Sex | ||
| Male | 98 (74.8) | |
| Female | 33 (25.2) | |
| Smoking | ||
| Never | 71 (54.2) | |
| Ex-smoker | 35 (26.7) | |
| Current | 25 (19.1) | |
| DM | 23 (17.6) | |
| HTN | 52 (39.7) | |
| Operative methods | ||
| Radical nephrectomy | 107 (81.7) | |
| Partial nephrectomy | 24 (18.3) | |
| Operative technique | ||
| Laparoscopic approach | 81 (61.8) | |
| Open approach | 50 (38.2) | |
| Tumor laterality | ||
| Right | 67 (51.1) | |
| Left | 64 (48.9) | |
| Histology | ||
| Clear cell | 114 (87.0) | |
| Papillary | 7 (5.3) | |
| Chromophobe | 10 (7.6) | |
| Tumor size (mm) | 54.9±37.8 | |
| TNM stage | ||
| pT1 | 90 (68.7) | |
| pT2 | 7 (5.3) | |
| pT3–4 | 18 (13.7) | |
| Any pT, ≥pN1, or cM1 | 16 (12.2) | |
| Fuhrman grade | ||
| 1−2 | 80 (61.1) | |
| 3−4 | 51 (38.9) | |
Values are presented as mean±standard deviation, median (interquartile range), or number (%).
BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; TNM, tumor, nodes, metastasis.
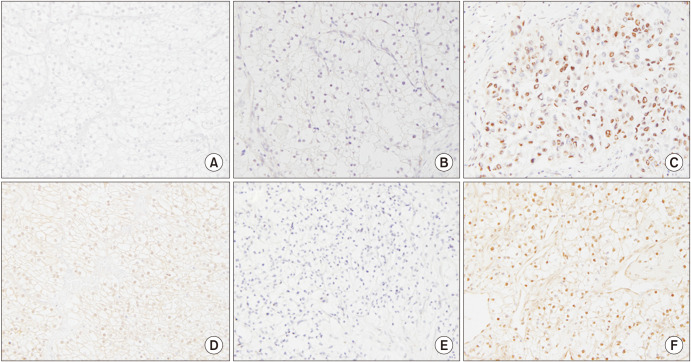
Expression of PAK4 and pPAK4S474 was predominant in cell nuclei in RCC specimens (Fig. 1). Overall, 57.3% (75/131) and 24.4% (29/119) of specimens exhibited high expression of pPAK4S474 and PAK4, respectively. Cytoplasmic expression of pPAK4S474 and PAK4 was weak in 18.3% (24/131) and 28.6% (34/119) of specimens, respectively. Table 2 summarizes the demographic and histologic features of PAK4 and pPAK4S474 expression in cell nuclei in 131 primary RCC samples. There were no significant differences in demographic characteristics (mean age, body mass index, sex distribution, smoking history, or presence of diabetes or hypertension) between the high and low PAK4 and pPAK4S474 expression groups (all p>0.05) (Table 2). With respect to pathologic features, high expression of pPAK4S474 was associated with both advanced tumor stage and grade (p=0.036 and p=0.002, respectively). High pPAK4S474 expression was also significantly associated with tumor size (p=0.038). By contrast, PAK4 expression was not associated with tumor size, tumor stage, or grade (Table 2).
Fig. 1. Representative images showing immunohistochemical staining for pPAK4S474 and PAK4 (magnification, ×400). (A) Control image of pPAK4S474 in tonsil tissue; (B) absent nuclear staining of pPAK4S474 in RCC tissue; (C) strong nuclear staining of pPAK4S474 in RCC tissue; (D) control image of PAK4 in tonsil tissue; (E) absent nuclear staining of PAK4 in RCC tissue; and (F) strong nuclear staining of PAK4 in RCC tissue. PAK4, p21-activated kinase 4; pPAK4S474, phospho-Ser474 PAK4; RCC, renal cell carcinoma.
Table 2. Comparison of clinical and pathological variables according to expression of phosphorylated PAK4 (pPAK4) and PAK4 in patients with surgically-treated renal cell carcinoma.
| Variable | pPAK4 expression | p-value | PAK4 expression | p-value | |||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
| Patients | 56 (42.7) | 75 (57.3) | 90 (75.6) | 29 (24.4) | |||
| Mean age (y) | 59.5±12.4 | 59.5±13.1 | 0.994 | 60.3±13.0 | 59.9±12.6 | 0.218 | |
| BMI (kg/m2) | 24 ±3.7 | 24.4±3.4 | 0.713 | 24.6±3.5 | 24.6±3.6 | 0.955 | |
| Sex | 0.839 | 1.000 | |||||
| Male | 41 (73.2) | 57 (76.0) | 66 (73.3) | 21 (72.4) | |||
| Female | 15 (26.8) | 18 (24.0) | 24 (26.7) | 8 (27.6) | |||
| Smoking | 0.950 | 0.374 | |||||
| Never | 31 (55.4) | 40 (53.3) | 47 (52.2) | 16 (55.2) | |||
| Ex-smoker | 15 (26.8) | 20 (26.7) | 26 (28.9) | 5 (17.2) | |||
| Current | 10 (17.9) | 15 (20.0) | 17 (18.9) | 8 (27.6) | |||
| DM | 12 (21.4) | 11 (14.7) | 0.358 | 18 (20.0) | 4 (13.8) | 0.587 | |
| HTN | 23 (41.1) | 29 (38.7) | 0.857 | 38 (42.2) | 8 (27.6) | 0.192 | |
| Histology | 0.274 | 0.206 | |||||
| Clear cell | 50 (89.3) | 64 (85.3) | 76 (84.4) | 27 (93.1) | |||
| Papillary | 1 (1.8) | 6 (8.0) | 5 (5.6) | 2 (6.9) | |||
| Chromophobe | 5 (8.9) | 5 (6.7) | 9 (10.0) | 0 (0.0) | |||
| Tumor size (mm) | 47.3±31.4 | 60.6±41.2 | 0.038 | 54.2±35.8 | 52.3±36.1 | 0.803 | |
| TNM stage | 0.036 | 0.964 | |||||
| pT1 | 46 (82.1) | 44 (58.7) | 64 (71.1) | 20 (69.0) | |||
| pT2 | 2 (3.6) | 5 (6.7) | 5 (5.6) | 2 (6.9) | |||
| pT3–4 | 5 (8.9) | 13 (17.3) | 10 (11.1) | 4 (13.8) | |||
| Any pT, ≥pN1, or cM1 | 3 (5.4) | 13 (17.3) | 11 (12.2) | 3 (10.3) | |||
| Fuhrman grade | 0.002 | 0.271 | |||||
| 1−2 | 43 (76.8) | 37 (49.3) | 53 (58.9) | 21 (72.4) | |||
| 3−4 | 13 (23.2) | 38 (50.7) | 37 (41.1) | 8 (27.6) | |||
Values are presented as number (%) or mean±standard deviation.
p-values were obtained using the Student's t-test and chi-square tests.
PAK4, p21-activated kinases; BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; TNM, tumor, nodes, metastasis.
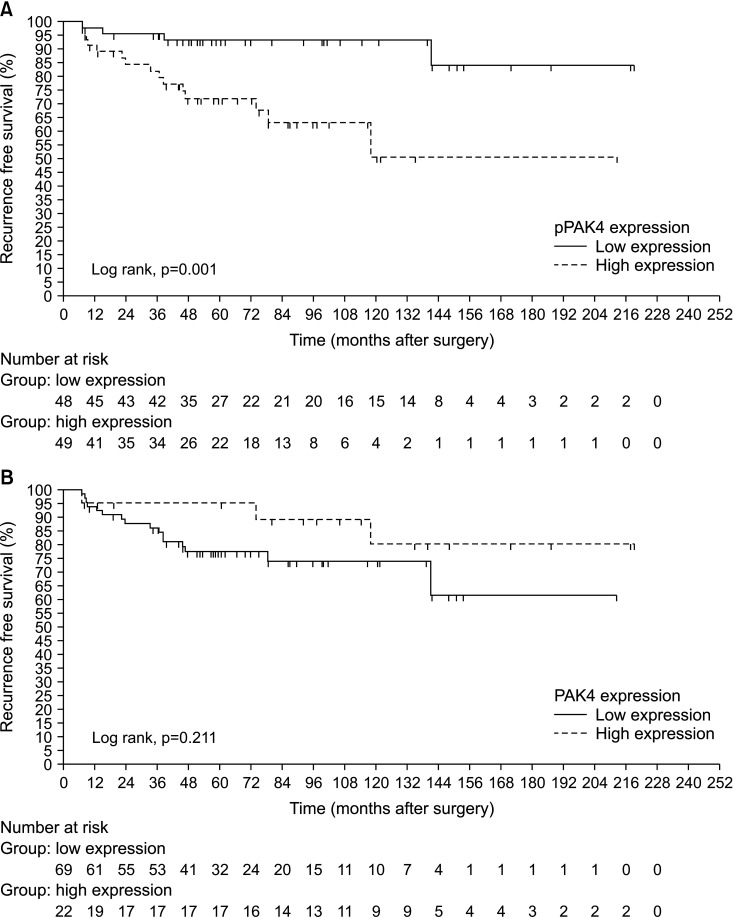
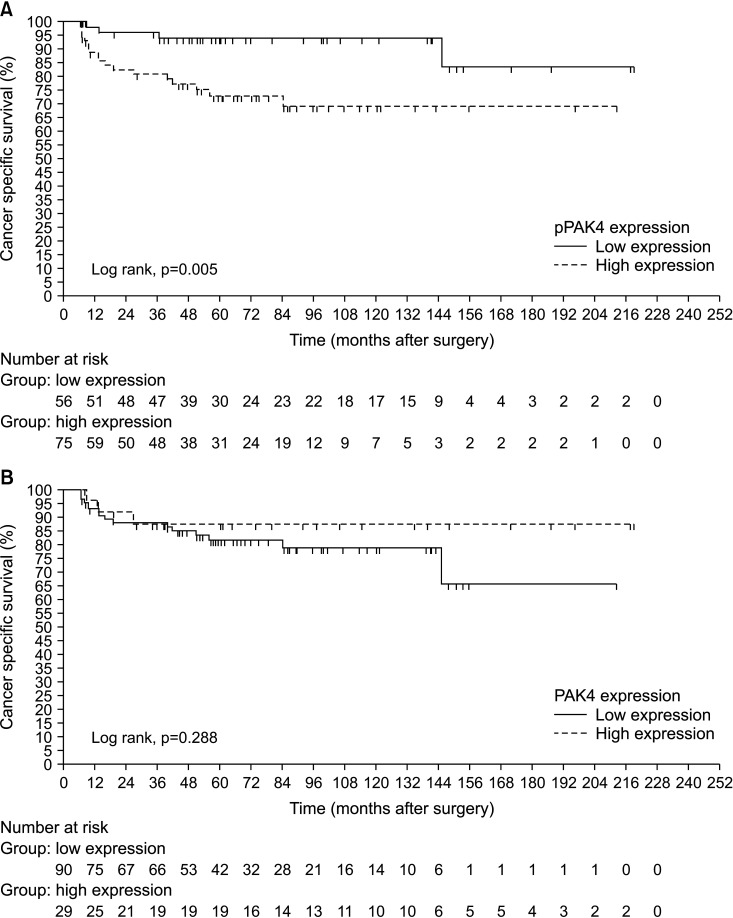
During a median 55.9 months (interquartile range, 19.1–97.2 months), 19 of the 97 patients (19.6%) with localized RCC (T1-2N0M0) experienced recurrence and 22 of the total cohort (n=131) (16.8%) died of RCC. Patients with localized RCC who experienced shorter RFS exhibited significantly higher expression of pPAK4S474 than patients who did not (log-rank test, p=0.001; Fig. 2A). High expression of pPAK4S474 was also significantly associated with shorter CSS in the total RCC cohort (log rank test, p=0.005; Fig. 3A). By contrast, PAK4 expression showed no significant association with time to recurrence or CSD (log-rank test: p=0.211 and p=0.288, respectively; Figs. 2B, 3B). Multivariate Cox regression analysis revealed that high pPAK4S474 expression was an independent predictor of recurrence in the subgroup with localized RCC (T1-2N0M0) (hazard ratio: 5.729; 95% confidence interval, 1.780–18.436; p=0.003) (Table 3). Univariate analysis identified high pPAK4S474 expression as a significant predictor of CSD, but this was not significant in multivariate analysis (Table 4).
Fig. 2. Kaplan-Meier survival curves predicting recurrence according to expression of (A) pPAK4S474 and (B) PAK4 in localized RCC (T1-2N0M0) patients. PAK4, p21-activated kinase 4; pPAK4S474, phospho-Ser474 PAK4; RCC, renal cell carcinoma.
Fig. 3. Kaplan-Meier survival curves predicting cancer-specific death according to expression of (A) pPAK4S474 and (B) PAK4 in the total cohort of RCC patients. PAK4, p21-activated kinase 4; pPAK4S474, phospho-Ser474 PAK4; RCC, renal cell carcinoma.
Table 3. Univariate and multivariate Cox regression models predicting tumor recurrence in a subgroup with localized RCC (T1-2N0M0) after surgery.
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (continuous) | 1.001 (0.964−1.039) | 0.969 | - | ||
| Sex (female vs. male) | 0.721 (0.238−2.189) | 0.564 | - | ||
| HTN (yes vs. no) | 1.365 (0.545−3.418) | 0.506 | - | ||
| DM (yes vs. no) | 2.097 (0.796−5.525) | 0.134 | - | ||
| BMI (continuous) | 1.030 (0.872−1.217) | 0.726 | - | ||
| Smoking history (yes vs. no) | 1.579 (0.619−4.029) | 0.339 | - | ||
| OP methods (PN vs. RN) | 0.041 (0.000−32.744) | 0.349 | - | ||
| TNM stage | |||||
| pT1a | 1 | ||||
| pT1b | 5.246 (1.660−16.580) | 0.005 | 4.118 (1.217−13.938) | 0.023 | |
| pT2 | 7.464 (1.836−30.351) | 0.005 | 6.475 (1.568−26.745) | 0.010 | |
| Fuhrman grade (G3–4 vs. G1–2) | 2.037 (0.813−5.105) | 0.129 | - | ||
| Tumor histology (non-ccRCC vs. ccRCC) | 4.300 (1.608−11.496) | 0.004 | 4.117 (1.373−12.349) | 0.012 | |
| PAK4 expression (high vs. low) | 0.458 (0.131–1.600) | 0.221 | - | ||
| pPAK4S474 expression (high vs. low) | 5.443 (1.729−17.129) | 0.004 | 5.729 (1.780−18.436) | 0.003 | |
RCC, renal cell carcinoma; HR, hazard ratio; CI, confidence interval; HTN, hypertension; DM, diabetes mellitus; BMI, body mass index; OP, operation; PN, partial nephrectomy; RN, radical nephrectomy; TNM, tumor, nodes, metastasis; ccRCC, clear cell RCC; PAK4, p21-activated kinase 4; pPAK4S474, phospho-Ser474 PAK4.
Table 4. Univariate and multivariate Cox regression models predicting cancer-specific death in the total cohort after surgery.
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (continuous) | 1.007 (0.973−1.041) | 0.700 | - | ||
| Sex (female vs. male) | 0.762 (0.279−2.080) | 0.596 | - | ||
| HTN (yes vs. no) | 2.132 (0.917−4.957) | 0.079 | - | ||
| DM (yes vs. no) | 1.417 (0.522−3.850) | 0.494 | - | ||
| BMI (continuous) | 0.786 (0.668−0.925) | 0.004 | 0.817 (0.645−1.035) | 0.094 | |
| Smoking history (yes vs. no) | 0.759 (0.324−1.778) | 0.525 | - | ||
| OP methods (PN vs. RN) | 0.042 (0.000−23.542) | 0.327 | - | ||
| TNM stage | |||||
| pT1–2 | 1 | ||||
| pT3–4 | 11.146 (3.628−34.239) | <0.001 | 11.566 (2.277−58.746) | 0.003 | |
| Any pT, ≥pN1, or cM1 | 24.767 (8.001−76.670) | <0.001 | 5.900 (0.789−44.151) | 0.084 | |
| Fuhrman grade (G3–4 vs. G1–2) | 19.889 (4.623−85.570) | <0.001 | 9.011 (0.982−82.666) | 0.052 | |
| Tumor histology (non-ccRCC vs. ccRCC) | 2.121 (0.781−5.761) | 0.140 | - | ||
| PAK4 expression (high vs. low) | 0.512 (0.147–1.783) | 0.293 | - | ||
| pPAK4S474 expression (high vs. low) | 4.282 (1.434−12.783) | 0.009 | 1.307 (0.222−7.704) | 0.767 | |
HR, hazard ratio; CI, confidence interval; HTN, hypertension; DM, diabetes mellitus; BMI, body mass index; OP, operation; PN, partial nephrectomy; RN, radical nephrectomy; TNM, tumor, nodes, metastasis; ccRCC, clear cell renal cell carcinoma; PAK4, p21-activated kinase 4; pPAK4S474, phospho-Ser474 PAK4.
DISCUSSION
The present study evaluated the clinical implications of pPAK4S474 expression (representing PAK4 activity) in surgically-treated RCC. There was a significant association between pPAK4S474 expression and RCC aggressiveness and adverse survival of RCC patients. Notably, total PAK4 expression was not associated with survival and pathological characteristics. Therefore, the data suggest that pPAK4 levels are a more accurate prognostic factor than total PAK4 levels in RCC patients. Moreover, we found that high pPAK4S474 expression was an independent predictor of recurrence in a subgroup of patients with localized RCC. Thus, pPAK4S474 may help clinically to identify patients diagnosed at T1-2N0M0 whose surgery only may not be curative.
Recent large scale whole genome sequencing studies of clear cell RCC by the Cancer Genome Atlas Research Network discovered novel and prevalent genomic alterations, including frequent inactivation of chromatin remodeling genes PBRM1, SETD2, and BAP1 [22]. However, PAK4 was not identified as a top ranked gene in clear cell RCC, suggesting that changes in its expression or activity may be more relevant to tumorigenesis and progression of RCC [22]. This notion complements and is consistent with findings by Park et al. [18], who demonstrated that the PAK4-Slug axis promotes epithelial-mesenchymal transition and worsens prognosis of prostate cancer. They found that pPAK4 expression was significantly higher in prostate cancer tissues with a high Gleason score (≥8) than in those with a low Gleason score (≤7), although staining for cytoplasmic PAK4 did not correlate with Gleason scores [18]. In prostate cancer tissues, total PAK4 was detected in both the cell cytoplasm and nucleus, whereas pPAK4 signals were predominant in the nucleus [18]. In the present study, IHC of RCC tissues showed a similar result; pPAK4 was distributed mainly in the cell nucleus (57.3% nuclear vs. 18.3% cytoplasmic), whereas PAK4 expression was detected evenly in the nucleus and the cytoplasm (24.4% nuclear vs. 28.6% cytoplasmic). Considering that pPAK4 is mainly nuclear, identification of its nuclear target(s) would be helpful to better understand the underlying molecular mechanism. With this in mind, it is notable that the Slug transcription factor was identified as a nuclear target of PAK4 in prostate cancer. Park et al. [18] identified Slug as a PAK4-specific EMT-inducing factor. PAK4 regulated Slug in a phosphorylation (at S158 and S254)-dependent manner; this phosphorylation increased the transcriptional activity and stability of Slug. It is tempting to speculate that Slug also acts downstream of PAK4 in RCC.
Liu et al. [16] investigated the role of PAK4 expression in recurrence and survival of patients with non-metastatic clear cell RCC following surgery, and demonstrated that high PAK4 expression is associated with early recurrence and poor survival. However, our data did not show any association between total PAK4 expression and pathological characteristics and survival. At present, the reason for this disparity is not clear. One possible explanation would be the different study designs; we included diverse subtypes of histology, clear cell RCC, papillary RCC, and the chromophobe RCC. To accurately determine the prognostic role of PAK4 and pPAK4S474 in RCC, additional large cohort studies would be helpful. Liu et al. [16] also showed that high PAK4 expression is an adverse prognostic marker in a subgroup of patients with low Fuhrman grade (grade 1–2) and in a subgroup with early T stage (T1–2) disease. In a similar context, we found that pPAK4S474 expression functions as an independent predictor of recurrence in a subgroup of patients with localized RCC. Taken together, these findings suggest that expression of PAK4 and pPAK4S474 are prognostic markers for early phase RCC progression.
A possible limitation of the present study is the relatively small sample size examined, which may reduce the statistical power. Considering that diverse types of RCC histology are included for evaluation, expanding the sample size is recommended. To better understand the prognostic value of pPAK4, other complementary omics approaches would be worthwhile. Identification of a nuclear target(s) of PAK4 as a potential prognostic marker(s) is also warranted.
CONCLUSIONS
High expression of pPAK4S474 is an adverse prognostic factor in patients with surgically resected RCC. This marker appears to be especially useful for identifying patients for whom surgery is believed to be potentially curative, i.e., patients with pathological T1-2N0M0 disease. Compared with total PAK4 expression, pPAK4S474 (which is representative of PAK4 activity) may be a more accurate prognostic factor in patients with surgically treated RCC.
ACKNOWLEDGMENTS
This work was supported by the research grant of the Chungbuk National University in 2020.
The specimens used in this study were provided by Chungbuk National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs. All samples from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols. The authors wish to thank Ms. Eun-Ju Shim from the National Biobank of Korea at Chungbuk National University Hospital for sample preparation and technical assistance.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Ho Won Kang, Eung-Gook Kim, and Seok Joong Yun.
- Data acquisition: Hee Youn Lee, Kyeong Kim, and Sung Pil Seo.
- Statistical analysis: Ho Won Kang, Xuan-Mei Piao, and Won Tae Kim.
- Data analysis and interpretation: Yun-Sok Ha, Yeong Uk Kim, and Won Tae Kim.
- Drafting of the manuscript: Ho Won Kang and Xuan-Mei Piao.
- Critical revision of the manuscript: Yun-Sok Ha, Yeong Uk Kim, Yong-June Kim, Sang-Cheol Lee, Eun-Young Shin, Eung-Gook Kim, and Wun-Jae Kim.
- Administrative, technical, or material support: Seok Joong Yun and Wun-Jae Kim.
- Supervision: Yong-June Kim, Sang-Cheol Lee, Seok Joong Yun, Eun-Young Shin, Eung-Gook Kim, and Wun-Jae Kim.
- Approval of the final manuscript: all authors.
References
- 1.Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:706–720. doi: 10.1093/annonc/mdz056. [DOI] [PubMed] [Google Scholar]
- 2.Kang HW, Seo SP, Kim WT, Yun SJ, Lee SC, Kim WJ, et al. Trends in clinical, operative, and pathologic characteristics of surgically treated renal mass in a Korean center: a surgical series from 1988 through 2015. Investig Clin Urol. 2019;60:184–194. doi: 10.4111/icu.2019.60.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbieri CE, Chinnaiyan AM, Lerner SP, Swanton C, Rubin MA. The emergence of precision urologic oncology: a collaborative review on biomarker-driven therapeutics. Eur Urol. 2017;71:237–246. doi: 10.1016/j.eururo.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. Oncologist. 2011;16 Suppl 2:4–13. doi: 10.1634/theoncologist.2011-S2-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haake SM, Weyandt JD, Rathmell WK. Insights into the genetic basis of the renal cell carcinomas from the Cancer Genome Atlas. Mol Cancer Res. 2016;14:589–598. doi: 10.1158/1541-7786.MCR-16-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol. 2002;34:713–717. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 8.Knaus UG, Bokoch GM. The p21Rac/Cdc42-activated kinases (PAKs) Int J Biochem Cell Biol. 1998;30:857–862. doi: 10.1016/s1357-2725(98)00059-4. [DOI] [PubMed] [Google Scholar]
- 9.Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63. doi: 10.1007/s10555-008-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar R, Sanawar R, Li X, Li F. Structure, biochemistry, and biology of PAK kinases. Gene. 2017;605:20–31. doi: 10.1016/j.gene.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kichina JV, Goc A, Al-Husein B, Somanath PR, Kandel ES. PAK1 as a therapeutic target. Expert Opin Ther Targets. 2010;14:703–725. doi: 10.1517/14728222.2010.492779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye DZ, Field J. PAK signaling in cancer. Cell Logist. 2012;2:105–116. doi: 10.4161/cl.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeo D, He H, Baldwin GS, Nikfarjam M. The role of p21-activated kinases in pancreatic cancer. Pancreas. 2015;44:363–369. doi: 10.1097/MPA.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 14.Bautista L, Knippler CM, Ringel MD. p21-activated kinases in thyroid cancer. Endocrinology. 2020;161:bqaa105. doi: 10.1210/endocr/bqaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Sullivan GC, Tangney M, Casey G, Ambrose M, Houston A, Barry OP. Modulation of p21-activated kinase 1 alters the behavior of renal cell carcinoma. Int J Cancer. 2007;121:1930–1940. doi: 10.1002/ijc.22893. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Yang Y, Liu Y, Liu H, Zhang W, Xu L, et al. p21-Activated kinase 4 predicts early recurrence and poor survival in patients with nonmetastatic clear cell renal cell carcinoma. Urol Oncol. 2015;33:205. doi: 10.1016/j.urolonc.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Yun CY, You ST, Kim JH, Chung JH, Han SB, Shin EY, et al. p21-activated kinase 4 critically regulates melanogenesis via activation of the CREB/MITF and β-catenin/MITF pathways. J Invest Dermatol. 2015;135:1385–1394. doi: 10.1038/jid.2014.548. [DOI] [PubMed] [Google Scholar]
- 18.Park JJ, Park MH, Oh EH, Soung NK, Lee SJ, Jung JK, et al. The p21-activated kinase 4-Slug transcription factor axis promotes epithelial-mesenchymal transition and worsens prognosis in prostate cancer. Oncogene. 2018;37:5147–5159. doi: 10.1038/s41388-018-0327-8. [DOI] [PubMed] [Google Scholar]
- 19.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 20.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Park MH, Lee HS, Lee CS, You ST, Kim DJ, Park BH, et al. p21-activated kinase 4 promotes prostate cancer progression through CREB. Oncogene. 2013;32:2475–2482. doi: 10.1038/onc.2012.255. [DOI] [PubMed] [Google Scholar]
- 22.Linehan WM, Ricketts CJ. The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications. Nat Rev Urol. 2019;16:539–552. doi: 10.1038/s41585-019-0211-5. [DOI] [PubMed] [Google Scholar]