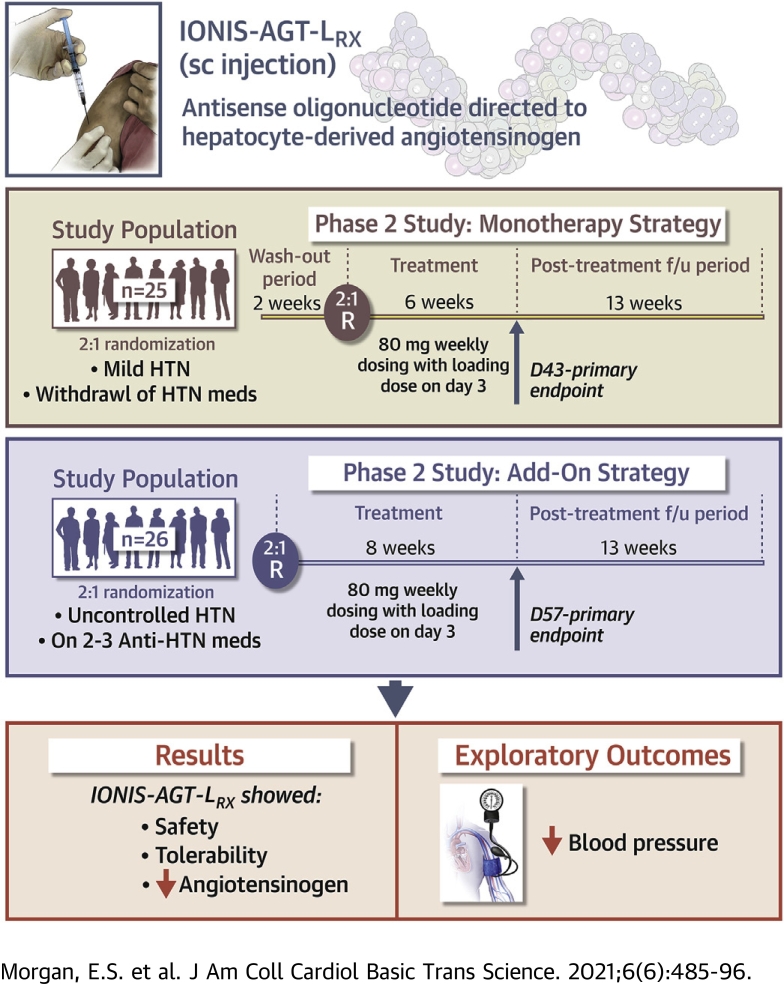
Visual Abstract
Key Words: angiotensinogen, antisense, hepatocyte, hypertension, oligonucleotide, RAAS
Abbreviations and Acronyms: ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; AGT, angiotensinogen; ASO, antisense oligonucleotide; CI, confidence interval; DBP, diastolic blood pressure; EDTA, ethylenediaminetetraacetic acid; GalNAc3, triantennary N-acetyl galactosamine; K+, potassium; PS, phosphorothioate; RAAS, renin-angiotensin-aldosterone system; SBP, systolic blood pressure
Highlights
-
•
Targeting AGT is a novel approach to inhibit the RAAS pathway.
-
•
AGT is primarily synthesized in the liver.
-
•
IONIS-AGT-LRx is an ASO directed to hepatocyte-derived AGT.
-
•
In 2 phase 2 trials as monotherapy and as an add-on to 2 to 3 medications for hypertension, IONIS-AGT-LRx was well tolerated with a significant reduction in plasma AGT levels.
-
•
IONIS-AGT-LRx is being developed for hypertension and heart failure indications.
Summary
Targeting angiotensinogen (AGT) may provide a novel approach to more optimally inhibit the renin-angiotensin-aldosterone system pathway. Double-blind, placebo-controlled clinical trials were performed in subjects with hypertension as monotherapy or as an add-on to angiotensin-converting enzyme inhibitors/angiotensin receptor blockers with IONIS-AGT-LRx versus placebo up to 2 months. IONIS-AGT-LRx was well tolerated with no significant changes in platelet count, potassium levels, or liver and renal function. IONIS-AGT-LRx significantly reduced AGT levels compared with placebo in all 3 studies. Although not powered for this endpoint, trends were noted in blood pressure reduction. In conclusion, IONIS-AGT-LRx significantly reduces AGT with a favorable safety, tolerability, and on-target profile. (A Study to Assess the Safety, Tolerability and Efficacy of IONIS-AGT-LRx; NCT04083222; A Study to Assess the Safety, Tolerability and Efficacy of IONIS-AGT-LRx, an Antisense Inhibitor Administered Subcutaneously to Hypertensive Subjects With Controlled Blood Pressure; NCT03714776; Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Ionis AGT-LRx in Healthy Volunteers; NCT03101878)
Chronic overactivity of the renin-angiotensin-aldosterone system (RAAS) pathway is considered a major contributor to the pathogenesis of cardiovascular disorders, including hypertension and heart failure (1,2). Although inhibition of the RAAS pathway by angiotensin-converting enzyme inhibitors (ACEis) or type I angiotensin receptor blockers (ARBs) represents one of the most effective treatments for hypertension and heart failure with reduced ejection fraction (3, 4, 5, 6, 7), a limitation of this approach is the higher risk of on-target toxicity manifested primarily by hyperkalemia and renal dysfunction, which necessitates lower clinical doses and limits more optimal inhibition of these pathways and clinical benefits (8). The downstream inhibition of the RAAS pathway can also result in upstream compensatory pathways that further limit their therapeutic efficacy (9, 10, 11).
Targeting the top of the RAAS pathway by silencing liver-derived angiotensinogen (AGT) (12) is a novel mechanism for RAAS inhibition. This approach has 2 potential advantages compared with current RAAS inhibitors. First, by inhibiting AGT production in the liver and minimizing inhibition of RAAS in the kidney, it may exhibit a better safety profile by allowing renal homeostasis and tubuloglomerular feedback to remain intact, thereby mitigating increases in potassium (K+) and renal dysfunction. Second, it may minimize escape mechanisms that act to restore angiotensin II levels or maintain angiotensin II signaling. More complete inhibition of locally generated angiotensin II in the vasculature or cardiac tissues may be advantageous in patients with resistant hypertension or heart failure (13,14).
IONIS-AGT-LRx (Ionis Pharmaceuticals, Carlsbad, California) is a hepatocyte-directed antisense oligonucleotide (ASO) drug designed target to AGT mRNA and reduce the synthesis of AGT protein in the liver, and consequently, reduce AGT levels in plasma. In this paper, we describe the safety profile, tolerability, and AGT reduction of IONIS-AGT-LRx in a phase 1 trial in healthy volunteers (Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Ionis AGT-LRx in Healthy Volunteers; NCT03101878) and 2 phase 2 trials: 1) in patients with mild hypertension after washout of antihypertensive medications (A Study to Assess the Safety, Tolerability and Efficacy of IONIS-AGT-LRx, an Antisense Inhibitor Administered Subcutaneously to Hypertensive Subjects With Controlled Blood Pressure; NCT03714776); and 2) in patients with uncontrolled blood pressure who received antihypertensive background therapy with 2 to 3 antihypertensive medications (A Study to Assess the Safety, Tolerability and Efficacy of IONIS-AGT-LRx; NCT04083222).
Methods
Design of the ASO IONIS-AGT-LRx
IONIS-AGT-LRx is a second-generation, ligand-conjugated ASO. The ASO is covalently linked to triantennary N-acetyl galactosamine (GalNAc3), a high-affinity ligand for the hepatocyte-specific asialoglycoprotein receptor, to form an GalNAc3-ASO conjugate (15).The GalNAc3 ligand enhances ASO delivery to hepatocytes, resulting in 10- to 30-fold potency increases in pre-clinical and clinical studies (16,17). The oligonucleotide portion of the drug consists of 20 nucleotides that are connected sequentially by phosphorothioate (PS) linkages. The nucleotide sequence of IONIS-AGT-LRx is complementary to a 20-nucleotide stretch within the 3ʹ UTR of human AGT and binds to the mRNA by Watson-Crick base pairing. IONIS-AGT-LRx is designed to serve as a substrate for RNase H1, which is achieved by a chimeric design, so that a 10 PS deoxynucleotide center is flanked by 5 PS nucleotides modified with 2′-O-(2-methoxyethyl). Thus, the resulting “2′-O-(2-methoxyethyl) gapmer” has a 5-10-5 design, giving it enhanced affinity for its cognate RNA sequence and increased nuclease resistance relative to a non-chimeric PS deoxynucleotide. The hybridization of IONIS-AGT-LRx to AGT mRNA results in RNase H1-dependent cleavage of the mRNA, thus preventing production of the AGT protein. Reduction in AGT mRNA correlates directly with a subsequent reduction in AGT protein levels in blood.
Pre-clinical assessment of IONIS-AGT-LRx in human AGT-transgenic mice
The results of the pre-clinical studies and phase 1 study are shown in the Supplemental Appendix and in Supplemental Figures 1 and 2.
Phase 1: IONIS-AGT-LRx in healthy volunteers
The methods for this trial are presented in the Supplemental Appendix.
Phase 2: IONIS-AGT-LRx monotherapy study
The phase 2 monotherapy study was a randomized, double-blind, placebo-controlled trial that evaluated IONIS-AGT-LRx at 6 sites in the United States. Patients aged 18 to 72 years, inclusive, with controlled hypertension on 2 antihypertensive medications (confirmed during a 1-week run-in period), 1 of which was an ACEi or an ARB and the other was a beta-blocker, calcium channel blocker, or diuretic, were enrolled (Supplemental Figure 3). Patients with a screening plasma AGT level <20 μg/ml or with K+ >4.85 and urine protein/creatinine ratio ≥0.3 mg/mg were excluded. All antihypertensive medications were stopped for 14 days (washout). Patients who met the inclusion criteria of a systolic blood pressure (SBP) >140 to ≤165 mm Hg after washout were randomized 2:1 to 80 mg IONIS-AGT-LRx or placebo. Patients were also stratified by screening plasma AGT level (≤30 μg/ml vs. >30 μg/ml). All patients received weekly, in-clinic, subcutaneous injections for 6 weeks with a loading dose administered on day 3, then were followed for 12 weeks in the post-treatment period. The primary efficacy endpoint was the comparison of percent change in plasma AGT from baseline to study week 7 (day 43) between 80 mg IONIS-AGT-LRx and placebo. Exploratory endpoints included post-baseline changes in SBP, diastolic blood pressure (DBP), percentage of patients who reached the goals of in-clinic SBP ≤140 mm Hg, DBP ≤90 mm Hg, and both over time. Blood pressure was measured by study personnel at every in-clinic visit in a quiet room after 5 min of resting in a chair with feet on the floor. Three consecutive blood pressure measurements were averaged to obtain an average blood pressure. When the dosing period was completed, the study investigators were allowed to re-initiate antihypertensive medications per clinical judgment. The CONSORT Diagram is shown in Supplemental Figure 3.
Phase 2: IONIS-AGT-LRx Add-on Study to standard of care in patients with uncontrolled hypertension on 2 or 3 antihypertensive medications
This phase 2 randomized, double-blind, placebo-controlled add-on trial evaluating IONIS-AGT-LRx was conducted at 9 sites in the United States. Patients aged 18 to 75 years, inclusive, on a stable regimen of 2 to 3 antihypertensive medications, including an ACEi/ARB and 1 or 2 additional antihypertensives in the beta-blocker, calcium channel blocker, or non-potassium sparing diuretic classes were eligible (Supplemental Figure 4). The inclusion criteria also required that patients have an average SBP within >140 and ≤170 mm Hg and DBP >80 mm Hg at screening and pre-dose day 1. Subjects with a screening AGT level <20 μg/ml or with K+ >4.9 and urine protein/creatinine ratio ≥0.3 mg/mg were excluded. Patients were stratified by screening ACEi/ARB dose then randomized 2:1 to 80 mg IONIS-AGT-LRx or placebo, respectively. All patients received weekly in-clinic subcutaneous injections for 8 weeks with a loading dose administered on day 3, and then were followed for 12 weeks in the post-treatment period. The primary efficacy endpoint was the comparison of percent change from baseline to study week 9 (day 57) in plasma AGT between 80 mg IONIS-AGT-LRx and placebo. Exploratory endpoints included post-baseline changes in SBP, DBP, and the percentage of patients who reached goals of SPB ≤140 mm Hg, DBP ≤80 mm Hg, and both during the study. Blood pressure was measured as in the monotherapy study. The CONSORT diagram is shown in Supplemental Figure 3.
Advarra (Columbia, Maryland), a central institutional review board, approved all 3 clinical trials.
Laboratory parameters
Chemistry and hematology parameters were measured by an automatic analyzer. AGT levels were measured in ethylenediaminetetraacetic acid (EDTA) plasma by an enzyme-linked immunoassay developed by Medpace Reference Labs (Cincinnati, Ohio). Plates were pre-coated with rabbit anti-human immunoglobulin monoclonal antibody (IBL-America, Minneapolis, Minnesota); EDTA-plasma in 1;10,000 dilution was added and AGT was detected with horse radish peroxidase mouse anti-human AGT monoclonal Fab’ fragment (IBL-America). Purified human AGT was used to generate the standard curve, and linearity was confirmed in the working range. Hemolysis (up to 1,600 mg/dl hemoglobin), lipemia (up to triglycerides of 2,265 mg/dl), and icterus (bilirubin up to 35.7 mg/dl) did not interfere with assay performance. Spiking and recovery bias was acceptable at −14.8% to 16.6%. The precision measured as the coefficient of variability was 9%. The analytical measuring range was 4.7 to 300 μg/ml. Studies in healthy human adults showed the 95% reference interval for AGT levels was 13.9 to 75.9 μg/ml. AGT levels were also measured in spot urine using the same method. Angiotensin II levels were measured by radioimmunoassay in EDTA plasma (Quest Diagnostics Nichols Institute, Cincinnati, Ohio). Plasma renin activity and renin mass were measured on EDTA plasma by liquid chromatography tandem mass spectrometry (Quest Diagnostics Nichols Institute) and an immunoradiometric assay (ARUP Laboratories, Salt Lake City, Utah), respectively. Aldosterone was measured by chemiluminescent immunoassay on EDTA plasma (Medpace Reference Labs).
Statistical analysis
Descriptive summary statistics including number of subjects, mean ± SD, or 95% confidence interval (CI) for continuous variables, and counts and percentages for categorical variables are presented. All statistical tests were conducted using 2-sided tests with a 5% type I error rate. There was no statistical rationale for the selected sample size, and the outcomes were considered as descriptive. In the phase 1 study, the placebo subjects were pooled and analyzed as separate single- and multiple-dose placebo groups. One-way analysis of variance was applied to compare between individual IONIS-AGT-LRx treatment and pooled placebo by least-square mean (Wilcoxon rank sum test if data were substantial from normality). In the phase 2 monotherapy study, the primary efficacy endpoint was the comparison of percent change from baseline to study week 7 in plasma AGT between the IONIS-AGT-LRx 80 mg group and the placebo group, and analyses were based on a per protocol set (at least 5 doses and no protocol deviations that affected efficacy). Analysis of covariance was used with treatment and a randomization stratification factor (screening AGT concentration level) as independent variables. In the phase 2 add-on study, the primary efficacy endpoint was the comparison of percent change from baseline to study week 9 in plasma AGT between the IONIS-AGT-LRx 80 mg group and the placebo group, and the analyses were based on the per protocol set (at least 7 doses and no protocol deviations that affected efficacy). Analysis of covariance was used with treatment and a randomization stratification factor (screening ACE/ARB dose level) as independent variables. In the cases in which data were substantial different from normality, the nonparametric Van Elteren test was applied instead. The Cochran-Mantel-Haenszel test was used to compare the proportional difference between treatment groups while stratifying by randomization factor. All safety analyses were conducted in the safety population, defined as all subjects who were randomized and received at least 1 dose of IONIS-AGT-LRx or placebo. For threshold effects, the achieved blood pressure cutoff of <140/90 mm Hg was a pre-specified analysis. The achieved SBP and DBP cutoffs of >5, >10, and >15 mm Hg reduction were not pre-specified. All statistics were performed with SAS Enterprise Guide 6.1 software (SAS Institute, Cary, North Carolina).
Results
Pre-clinical assessment of IONIS-AGT-LRx in human AGT-transgenic mice and phase 1 healthy volunteer study
The results of the pre-clinical studies and phase 1 study are shown in the Supplemental Appendix and in Supplemental Tables 1 to 3.
Phase 2 monotherapy study
The baseline characteristics of the monotherapy study are shown in Table 1. In the monotherapy trial, 21 of 77 (27%) screened subjects did not meet AGT criteria. At screening, there was no difference in mean AGT levels in the placebo group versus the IONIS-AGT-LRx group (27.4 ± 13.1 μg/ml vs. 23.5 ± 3.7 μg/ml; p = 0.80). All patients had confirmed controlled SBP/DBP on hypertension therapy at screening. The AGT levels tended to decline following washout of anti-hypertensive medications. An expected, a rise in blood pressure occurred after stopping the anti-hypertensive medications for 14 days, reaching a mean SBP of 149 ± 15 and 146 ± 9 mm Hg and a mean DBP of 88 ± 10 and 86 ± 7 mm Hg for the placebo and IONIS-AGT-LRx groups, respectively. Renal function and K+ levels were normal at baseline.
Table 1.
Baseline Characteristics of the Monotherapy Study
| Placebo (n = 8) | IONIS-AGT-LRx (n = 17) | |
|---|---|---|
| Age (yrs) | 57 ± 4 | 60 ± 7 |
| Male | 2 (25) | 10 (59) |
| Female | 6 (75) | 7 (41) |
| White | 5 (63) | 10 (59) |
| BMI (kg/m2) | 29.5 ± 3.8 | 28.6 ± 2.8 |
| Hyperlipidemia | 5 (63) | 12 (71) |
| Type 2 diabetes mellitus | 1 (13) | 7 (41) |
| AGT (μg/ml) screening | 27.4 ± 13.1 | 23.5 ± 3.7 |
| >30 μg/ml | 1 (13) | 1 (6) |
| AGT (μg/ml) baseline | 26.9 ± 19.1 | 20.7 ± 4.7 |
| SBP screening (mm Hg) | 129 ± 13 | 126 ± 10 |
| DBP screening (mm Hg) | 82 ± 9 | 78 ± 6 |
| SBP post-washout (mm Hg) | 149 ± 15 | 146 ± 9 |
| DBP post-washout (mm Hg) | 88 ± 10 | 86 ± 7 |
| High-dose ACEi/ARB∗ | 3 (38) | 10 (59) |
| Creatinine (mg/dl) | 0.77 ± 0.19 | 0.88 (0.24) |
| eGFR (ml/min/1.73 m2) | 93 ± 13 | 90 ± 15 |
| Potassium (mmol/l) | 4.3 ± 0.3 | 4.3 ± 0.4 |
| Angiotensin II (ng/l) | 24 ± 23 | 18 ± 6 |
| Aldosterone (ng/dl) | 11.9 ± 9.5 | 11.9 ± 7.5 |
| Renin mass (pg/ml) | 13.7 ± 20.0 | 15.0 ± 22.5 |
| Plasma renin activity (ng/ml/h) | 1.4 ± 2.1 | 1.5 ± 1.9 |
Values are mean ± SD or n (%).
The angiotensinogen (AGT) baseline values were defined as the averaged values collected between day −7 and before the first dose of study drug.
BMI = body mass index; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; SBP = systolic blood pressure.
High-dose angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEi/ARB) was defined based on the average approved doses per package insert.
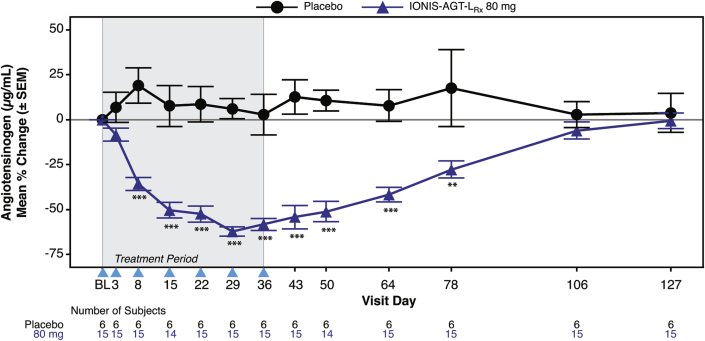
IONIS-AGT-LRx was well tolerated with no hypotensive events, hyperkalemia, or renal abnormalities (Supplemental Table 4). One patient in the placebo group experienced acute pancreatitis. After 6 weeks of dosing at day 43, a significant mean absolute reduction in AGT levels was noted in the IONIS-AGT-LRx group compared with the placebo group (−11.2 ± 6.0 μg/ml vs. 2.0 ± 4.6; p < 0.001). Similarly, the mean percent reduction in AGT levels was significantly lower with IONIS-AGT-LRx compared with the placebo group (−54 ± 24.8% vs. 12.6 ± 23.3%; p < 0.001) (Figure 1). Statistically significant differences were noted by day 8 and persisted to day 78. In the AGT-LRx group, 6 treated patients had AGT levels below the lower level of detection of the assay and were assigned the lower limit of detection of the AGT assays value of 4.7 μg/ml. The reductions were sustained and demonstrated reversibility in the post-treatment period.
Figure 1.
Mean Percent Changes in AGT Over Time in IONIS-AGT-LRx Versus Placebo in the Monotherapy Study
The shaded area represents the dosing window, the arrowheads show the timepoint when the dose was given, and the unshaded area shows the follow-up period. The primary endpoint was at day 43. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 IONIS-AGT-LRx versus placebo at the specified timepoint. AGT = angiotensinogen.
Exploratory analyses are shown in Table 2. There was a nonsignificant larger reduction in SBP (−8 mm Hg; 95% CI: −17 to 2 mm Hg) or DBP (−1 mm Hg; 95% CI: −8 to 5 mm Hg) observed with IONIS-AGT-LRx compared with placebo but these did not reach statistical significance. A higher percentage of subjects treated with IONIS-AGT-LRx compared with placebo achieved ≥5, ≥10, and ≥15 mm Hg reductions in SBP and DBP. Similarly, a higher percentage of patients treated with IONIS-AGT-LRx compared with placebo reached SBP ≤140 mm Hg or DBP ≤90 mm Hg. There were no significant changes in angiotensin II, aldosterone, or renin mass or activity.
Table 2.
Exploratory Analyses in the Monotherapy Study
| Results at Day 43 | Placebo (n = 6) | IONIS-AGT-LRx (n = 15) |
|---|---|---|
| Mean absolute change in SBP (mm Hg) | −2 (−19 to 16) | −8 (−17 to 2) |
| Median (quartile 1 to quartile 3) | 0 (−4 to 3) | −12 (−23 to 2) |
| Mean absolute change in DBP (mm Hg) | 4 (−4 to 12) | −1 (−8 to 5) |
| Median (quartile 1 to quartile 3) | 4 (−3 to 6) | −2 (−6 to 4) |
| Patients with ≥5 mm Hg reduction in SBP | 1 (17) | 8 (53) |
| Patients with ≥10 mm Hg reduction in SBP | 1 (17) | 8 (53) |
| Patients with ≥15 mm Hg reduction in SBP | 1 (17) | 5 (33) |
| Patients with ≥5 mm Hg reduction in DBP | 0 (0) | 4 (27) |
| Patients with ≥10 mm Hg reduction in DBP | 0 (0) | 3 (20) |
| Patients with ≥15 mm Hg reduction in DBP | 0 (0) | 3 (20) |
| Patients reaching SBP ≤140 mm Hg | 3 (50) | 10 (67) |
| Patients reaching DBP ≤90 mm Hg | 3 (50) | 11 (73) |
| Mean change in angiotensin II (ng/dl) | 1 ± 5 | 1 ± 8 |
| Mean change in aldosterone (ng/dl) | 0.1 ± 4.2 | 0.0 ± 3.0 |
| Mean change in renin mass (pg/ml) | −1.9 ± 4.2 | 15.7 ± 33.8 |
| Mean change in plasma renin activity (ng/ml/h) | 0.66 ± 2.01 | −0.11 ± 1.74 |
Values are mean (95% confidence interval), n (%), or n (%), or mean ± SD unless otherwise indicated.
Blood pressure results occurred after antihypertensive medications were reinitiated and were removed from this analysis.
Abbreviations as in Table 1.
Phase 2 add-on study
The baseline characteristics are shown in Table 3. In the add-on trial, 11 of 64 (17.2%) subjects did not meet AGT criteria. Most patients were women. Baseline mean AGT levels were 25.5 ± 4.4 μg/ml and 25.1 ± 3.3 μg/ml in the placebo and IONIS-AGT-LRx groups, respectively. Approximately two-thirds of patients were taking 2 antihypertensive medications and one-third were on 3 antihypertensive medications. Baseline mean SBP was 152 ± 8 mm Hg and DBP was 87 ± 8 mm Hg in the placebo group, and SBP was 154 ± 11 mm Hg and DBP was 89 ± 9 mm Hg in the IONIS-AGT-LRx group.
Table 3.
Baseline Characteristics of the Add-On Study
| Placebo (n = 8) | IONIS-AGT-LRx (n = 18) | |
|---|---|---|
| Age (yrs) | 61 ± 10 | 60 ± 8 |
| Male | 2 (25) | 4 (22) |
| Female | 6 (75) | 14 (78) |
| White | 4 (50) | 15 (83) |
| BMI (kg/m2) | 29.4 ± 4.2 | 28.1 ± 4.6 |
| Hyperlipidemia | 4 (50) | 13 (72) |
| Type 2 diabetes mellitus | 3 (38) | 5 (28) |
| AGT (μg/ml) baseline | 25.5 ± 4.4 | 25.1 ± 3.3 |
| SBP (mm Hg) | 152 ± 8 | 154 ± 11 |
| DBP (mm Hg) | 87 ± 8 | 89 ± 9 |
| Baseline diuretic used | 6 (75) | 11 (61) |
| Number of anti hypertensive meds | ||
| 2 | 6 (75) | 11 (61) |
| 3 | 2 (25) | 7 (39) |
| High-dose ACEi/ARB∗ | 3 (38) | 7 (39) |
| Creatinine (mg/dl) | 0.87 ± 0.11 | 0.77 ± 0.19 |
| eGFR (ml/min/1.73 m2) | 84 ± 15 | 89 ± 13 |
| Potassium (mmol/l) | 4.3 ± 0.5 | 4.2 ± 0.4 |
| Angiotensin II (ng/l) | 19 ± 6 | 32 ± 35 |
| Aldosterone (ng/dl) | 13.6 ± 4.5 | 9.7 ± 5.1 |
| Renin mass (pg/ml) | 8.2 ± 6.1 | 36.2 ± 72.7 |
| Plasma renin activity (ng/ml/h) | 1.0 ± 1.0 | 3.9 ± 5.8 |
Values are mean ± SD or n (%).
Abbreviations as in Table 1.
High-dose ACEi/ARB was defined based on the average approved doses per package insert.
IONIS-AGT-LRx was well tolerated with no serious adverse events, hypotensive events, or renal abnormalities (Supplemental Table 5). One patient in the IONIS-AGT-LRx group with no history of diabetes mellitus, screening K+ of 4.8 mmol/l, and an estimated glomerular filtration rate of 84 ml/min/1.73 m2 at day 1 developed asymptomatic hyperkalemia with no electrocardiographic changes at day 8 after only 2 doses of study drug that peaked to 5.9 mmol/l at day 22. Study drug was discontinued, benazepril (20 mg/day) held, and hydrochlorothiazide increased from 12.5 to 25 mg/day. The K+ returned to 4.1 mmol/l within 5 days. At approximately 42 days after the last dose, the patient was re-challenged with benazepril 20 mg/day and K+ increased to 5.7 mmol/l. Benazepril was permanently withdrawn, and the hydrochlorothiazide was reduced to 12.5 and the K+ increased back to 5.8 mmol/l. The estimated glomerular filtration rate was not significantly changed throughout this period. The investigator deemed this event unrelated to the study drug.
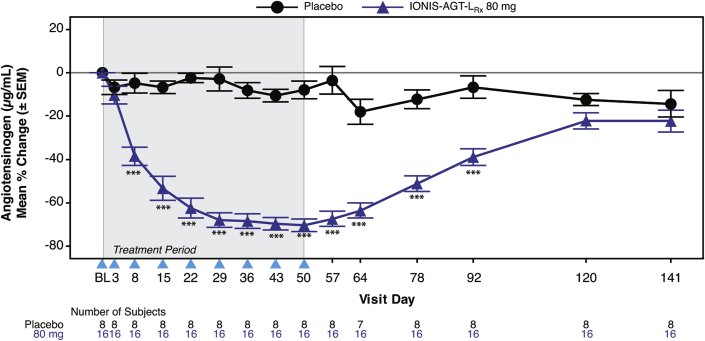
After 8 weeks of dosing, at day 57, a significant absolute reduction in mean AGT levels was noted in the IONIS-AGT-LRx group compared with the placebo group (−17.0 ± 4.1 μg/ml vs. −1.1 ± 4.5 μg/ml; p < 0.001). Similarly, the mean percent reduction in AGT levels was significantly lower in the IONIS-AGT-LRx group compared with the placebo group (−67 ± 14.1% vs. 3.4 ± 17.8%; p < 0.001 (Figure 2). Statistically significant differences were noted by day 8 and persisted to day 92. In the IONIS-AGT-LRx group, 2 treated patients had AGT levels below the lower level of detection and were assigned values of 4.7 μg/ml.
Figure 2.
Mean Percent Changes in AGT Over Time in IONIS-AGT-LRx Versus Placebo in the Add-On Study
The shaded area represents the dosing window, the arrowheads show the timepoint when the dose was given, and the unshaded area shows the follow-up period. The primary endpoint was at day 57. ∗p < 0.05; ∗∗p < 0.01, ∗∗∗p < 0.001 IONIS-AGT-LRx versus placebo at the specified timepoint. Abbreviation as in Figure 1.
Exploratory analyses are shown in Table 4. There was a numerically larger reduction in SBP (−12 mm Hg; 95% CI: −21 to −4 mm Hg) and DBP (−6 mm Hg; 95% CI: −11 to −1 mm Hg) observed with IONIS-AGT-LRx compared with placebo but this did not reach statistical significance. A higher percentage of subjects treated with IONIS-AGT-LRx compared with placebo achieved ≥5, ≥10, and ≥15 mm Hg reductions in SBP and DBP. Similarly, a higher percentage of patients treated with IONIS-AGT-LRx compared with placebo reached SBP ≤140 mm Hg or DBP ≤90 mm Hg. There was no significant difference based on whether patients were on 2 or 3 antihypertensive medications. There were no significant changes in angiotensin II, aldosterone or renin mass or activity.
Table 4.
Exploratory Analyses in the Add-On Study
| Results at Day 57 | Placebo (n = 8) | IONIS-AGT-LRx (n = 16) |
|---|---|---|
| Mean absolute change in SBP (mm Hg) | −5 (−13 to 4) | −12 (−21 to −4) |
| Median (quartile 1 to quartile 3) | −5 (−8 to 0) | −10 (−19 to −2) |
| Mean absolute change in DBP (mm Hg) | 1 (−7 to 9) | −6 (−11 to −1) |
| Median (quartile 1 to quartile 3) | −1 (−8 to 10) | −7 (−9 to 2) |
| Patients with ≥5 mm Hg reduction in SBP | 5 (63) | 11 (69) |
| Patients with ≥10 mm Hg reduction in SBP | 1 (13) | 8 (50) |
| Patients with ≥15 mm Hg reduction in SBP | 1 (13) | 7 (44) |
| Patients with ≥5 mm Hg reduction in DBP | 3 (38) | 10 (63) |
| Mean change in angiotensin II (ng/dl) | 2 ± 10 | −4 ± 19) |
| Mean change in aldosterone (ng/dl) | 0.2 ± 2.9 | −0.8 ± 4.7 |
| Mean change in renin mass (pg/ml) | 16.9 ± 41.7 | 71.9 ± 248.3 |
| Mean change in plasma renin activity (ng/ml/h) | −0.2 ± 0.86 | −1.53 ± 3.76 |
| Patients with ≥10 mm Hg reduction in DBP | 1 (13) | 3 (19) |
| Patients with ≥15 mm Hg reduction in DBP | 0 (0) | 2 (13) |
| Patients reaching SBP ≤140 mm Hg | 2 (25) | 8 (50) |
| Patients reaching DBP ≤80 mm Hg | 1 (13) | 9 (56) |
Values are mean (95% confidence interval), n (%), or mean ± SD unless otherwise indicated.
Abbreviations as in Table 1.
Waterfall plots of individual blood pressure changes in both trials
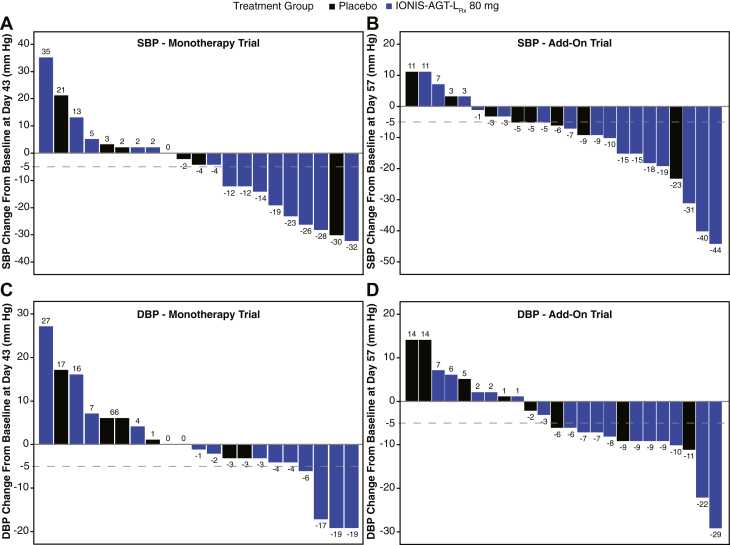
Waterfall plots are shown of the systolic and diastolic blood pressure changes in individual patients in the monotherapy (Figures 3A and 3B) and the add-on trial (Figures 3C and 3D). More patients in the IONIS-AGT-LRx arm had a decline in SBP and DBP, with maximum excursions of −32 in SBP and −19 mm Hg in the monotherapy trial and −44 mm Hg in SBP and −29 mm Hg DBP in the add-on trial. There was no significant correlation of the percent change of AGT from baseline to the primary endpoint and either change in SBP or DBP in the treatment arms of the 2 trials individually or combined (monotherapy: r = 0.20; 95% CI: −0.17 to 0.52; p = 0.28; add-on trial: r = 0.23; 95% CI: −0.14 to 0.54; p = 0.22).
Figure 3.
Waterfall Plots of the SBP and DBP in the Monotherapy and Add-On Trials
(A) Systolic blood pressure (SBP) and (B) diastolic blood pressure (DBP) in the monotherapy trial. (C) SBP and (D) DBP in the add-on trial.
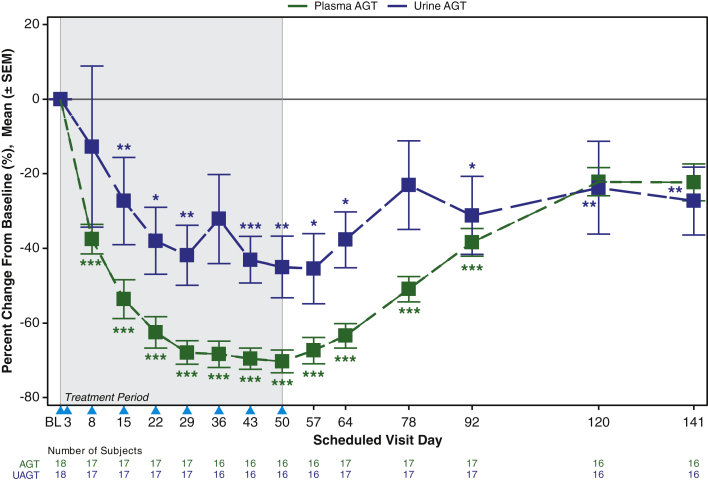
Comparison of urinary versus plasma AGT knockdown in the add-on study
The impact of IONIS-AGT-LRx on renal AGT versus liver AGT may be potentially evaluated by assessing AGT levels in plasma, which are liver-derived, and AGT levels in urine, which are kidney-derived. Figure 4 demonstrates a urine/plasma gradient in AGT reduction starting at day 8 after only 2 doses with a maintained separation until day 50. Once IONIS-AGT-LRx has gone through 2 to 3 half-lives, both urine and plasma AGT levels return toward baseline and then merge.
Figure 4.
Impact of IONIS-AGT-LRx on UAGT Versus Plasma AGT Levels
AGT was measured in plasma and in spot urine and plotted over time during the treatment phase and in recovery off-drug. A gradient was noted in urine and plasma that resolved in recovery when IONIS-AGT-LRx was beginning to wear off. The p values represent comparisons of either urine AGT (UAGT) or plasma AGT versus placebo based on the analysis of covariance or Van Elteren test. The shaded area represents the dosing window, the arrowheads show the timepoint when the dose was given, and the unshaded area shows the follow-up period. ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗p < 0.001. AGT = angiotensinogen. Abbreviations as in Figure 1.
Changes in renal function in both studies
Supplemental Figure 5 demonstrates the temporal trends in K+ and renal function in both phase 2 studies. No significant temporal trends were noted in both parameters.
Discussion
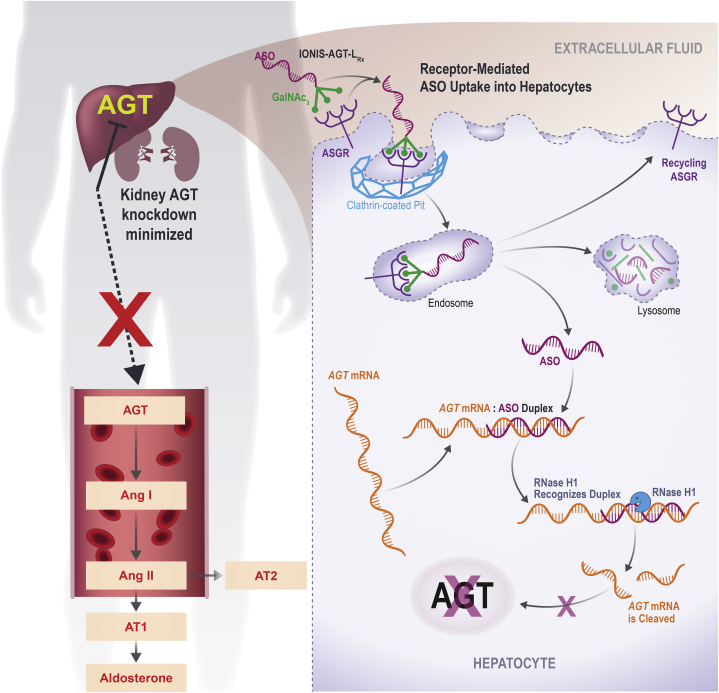
This study described the generation and initial clinical experience of a liver-targeted ASO directed to AGT. Figure 5 demonstrates the mechanism of action for IONIS-AGT-LRx using a receptor-mediated ASO uptake into hepatocytes to reduce the production of hepatic AGT and minimize the knockdown of renal AGT. The pre-clinical and 3 short-term clinical trials in relatively low-risk subjects demonstrated that IONIS-AGT-LRx resulted in significant AGT reductions and was well tolerated with no significant adverse off-target effects. At these levels of AGT reduction and in subjects with mostly preserved renal function, no on-target side effects such as hypotension, hyperkalemia, and renal dysfunction were noted. These data provided a rationale to study AGT reduction in patients with resistant hypertension and heart failure.
Figure 5.
Ligand-Conjugated Antisense Technology
GalNAc-conjugate moiety delivers the ASO to the hepatocytes where hepatic AGT is made. Targeting the top of RAAS pathway by reducing liver-derived AGT is a novel mechanism for RAAS inhibition. This GalNAc conjugation will minimize renal AGT reduction and thereby potentially provide a better safety profile than other RAAS inhibitors. AGT = angiotensinogen; ASGR = asialoglycoprotein receptor; ASO = antisense oligonucleotide; GaINAc3 = triantennary N-acetyl galactosamine; mRNA = messenger RNA; RAAS = renin-angiotensin-aldosterone system.
The development of specific liver-targeted ASOs has significantly improved the clinical efficacy of target knockdown, as well as decreasing the doses needed by 10- to 30-fold (18,19), as well as the safety and tolerability (20). With human-specific IONIS-AGT-LRx, as predicted from the use of the GalNAc moiety for hepatocyte targeting, potency for reducing both circulating AGT levels and liver mRNA knockdown were significantly improved compared with the non-GalNAc version. Furthermore, in pre-clinical studies, kidney AGT knockdown was minimally affected by IONIS-AGT-LRx, whereas the non-GalNac ASO led to significant kidney AGT knockdown, consistent with previous studies that used rodent-specific AGT ASOs (21). Thus, the hepatocyte-targeted approach allowed similar liver activity compared with the nontargeted approach but at lower doses that reduced drug exposure and activity in the kidney. This was suggested by the temporal gradient noted in AGT levels in the urine versus the plasma. Additional studies in a larger number of subjects will be needed to confirm this observation.
The primary objectives of all 3 clinical studies was to demonstrate the safety and tolerability, as well as on-target effects, at the levels of AGT knockdown achieved. With single doses as high as 80 mg and weekly doses at 80 mg, along with 1 loading dose that reached a total of 400 mg/month, there was no evidence of liver test elevation, renal dysfunction, or decreases in platelet count. In particular, the lower acute and cumulative doses of the GalNAc ASOs compared with non-GalNAc ASOs did not result in any cases of drug-related thrombocytopenia to date in patients who received up to 1 year of dosing (19,20,22,23). In this cohort of patients whose lowest entry estimated glomerular filtration rate was >60 ml/min/1.73 m2, 1 case of hyperkalemia without change in estimated glomerular filtration rate was present in the IONIS-AGT-LRx group in the add-on trial. Larger studies in subjects with lower renal function will be needed to ascertain whether on-target effects will be improved compared with ACEis/ARBs (24), concomitant aldosterone (25), and with effects seen in previous studies of renin inhibitors (26, 27, 28).
IONIS-AGT-LRx resulted in robust plasma AGT lowering in both trials in patients with hypertension and was more pronounced in the add-on study in patients already on at least 2 medications, 1 of which was an ACE or an ARB. The effect occurred fairly rapidly with one-half the reduction noted in the first 8 days. The difference in AGT reduction in the studies might be due to differences in clinical characteristics or baseline AGT levels. In the monotherapy group, the AGT levels tended to decline when the ACEis/ARBs were washed out, whereas in the add-on study they were higher. This suggests the ACEs/ARBs and other antihypertensive medications might upregulate AGT synthesis in the liver. AGT is known to be under control of estrogen that upregulates its synthesis, and this might explain why more women were enrolled in the trial than men, because the studies had an entry criterion of AGT >20 μg/dl (29,30).
In exploratory analysis, there was a numerically higher reduction in SBP and DBP in both trials, as well as more patients reaching specific thresholds of reduction (<5, <10, and <15 mm Hg) and reaching SBP ≤140 mm Hg and DBP ≤90 mm Hg. The waterfall plots showed most subjects responded, even those already on ACEis/ARBs. These numerical changes were clinically significant but were not statistically significant, and the trial size was not powered for these endpoints. Importantly, after the study drug was withdrawn, there was no rebound hypertension.
Based on the preceding data, 3 clinical trials are currently underway with IONIS-AGT-LRx; 1) A Study to Assess the Safety, Tolerability and Efficacy of IONIS-AGT-LRx in ASTRAAS (ASO Targeting the RAAS System; NCT04714320) will recruit 150 participants with uncontrolled blood pressure who are on ≥3 antihypertensive medications and evaluate the effect of IONIS-AGT-LRx on in-office and 24-h ambulatory SBP, DBP, and plasma AGT; 2) ASTRAAS-HF (A Study to Assess the Safety, Tolerability and Efficacy of IONIS-AGT-LRx in Participants With Chronic Heart Failure With Reduced Ejection Fraction; NCT04836182) will assess IONIS-AGT-LRx as an add-on to standard of care in participants with heart failure with reduced ejection fraction; and 3) (A Study to Assess the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of ION904 (NCT04731623) is currently recruiting subjects with a more potent ASO to AGT using recently described novel chemistry (31).
Additional approaches that target AGT include siRNA and immunization approaches (32, 33, 34). The most critical clinical needs are in subjects with resistant hypertension and heart failure, especially with preserved ejection fraction (35). Additional indications may also include chronic kidney disease, hepatic steatosis, and atherosclerosis (36,37). The hypothesis that liver-targeted AGT inhibition will provide more efficacious RAAS blockade at a similar or improved on-target safety profile remains to be tested as clinical development of these agents advances.
Study Limitations
First, the monotherapy and add-on studies were small in sample size and were not powered for blood pressure endpoints. The safety and blood pressure trends indicate larger studies with a longer treatment duration should be conducted to corroborate these results. Second, the analytical measuring range of the plasma AGT assay was 4.7 to 300 µg/ml, as such several patients had AGT levels below the lower limit of detection and were assigned a value of 4.7 µg/ml. Thus, it is possible the mean percent reductions in AGT were underestimated. Future studies will require more sensitive AGT assays with lower limits of detection to more accurately ascertain the mean percent reduction in AGT levels.
Conclusions
IONIS-AGT-LRx showed a favorable safety, tolerability, and on-target profile, significantly reduced AGT, and provided numerically favorable reduction in both SBP and DBP. Ongoing trials are assessing its effect in studies in hypertension and heart failure.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The RAAS pathway is a well-accepted target for therapies for that treat hypertension and heart failure. Targeting AGT, which is at the top of this pathway, is a novel approach in improving efficacy of RAAS inhibition. One potential advantage of targeting AGT is that is it primarily synthesized in the liver, thus potentially allowing kidney homeostasis to remain intact and improving the therapeutic index.
TRANSLATIONAL OUTLOOK: Inhibiting hepatocyte-derived AGT may allow more potent and safer inhibition of the RAAS pathway. Clinical indications to test this hypothesis may include patients with hypertension, particularly resistant hypertension, heart failure with preserved or reduced ejection fraction, Marfan syndrome, and kidney diseases.
Funding Support and Author Disclosures
This work was supported by Ionis Pharmaceuticals. Dr. Tsimikas is a co-inventor of and receives royalties from patents owned by University of California, San Diego, on oxidation-specific antibodies and of biomarkers related to oxidized lipoproteins; and is a co-founder and has an equity interest in Oxitope, Inc and its affiliates, Kleanthi Diagnostics, LLC, and Covicept Therapeutics, Inc. Dr. Bakris has been a consultant to Ionis Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Tracy Reigle for generating the artwork and Julia Trunfio for administrative assistance.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For expanded Methods and Results sections as well as supplemental tables, and figures, please see the online version of this paper.
Appendix
References
- 1.Ferrario C.M., Strawn W.B. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 2.Weber K.T. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–1697. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 3.Cohn J.N., Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 4.Swedberg K., Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) Am J Cardiol. 1988;62:60–66a. doi: 10.1016/s0002-9149(88)80087-0. [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer M.A., Swedberg K., Granger C.B. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S., Sleight P., Pogue J., Bosch J., Davies R., Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 7.van Vark L.C., Bertrand M., Akkerhuis K.M. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur Heart J. 2012;33:2088–2097. doi: 10.1093/eurheartj/ehs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makani H., Bangalore S., Desouza K.A., Shah A., Messerli F.H. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ. 2013;346:f360. doi: 10.1136/bmj.f360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrario C.M., Ahmad S., Varagic J. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. Am J Physiol Heart Circ Physiol. 2016;311:H404–H414. doi: 10.1152/ajpheart.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nobakht N., Kamgar M., Rastogi A., Schrier R.W. Limitations of angiotensin inhibition. Nat Rev Nephrol. 2011;7:356–359. doi: 10.1038/nrneph.2011.29. [DOI] [PubMed] [Google Scholar]
- 11.Bomback A.S., Klemmer P.J. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–492. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 12.Lu H., Cassis L.A., Kooi C.W., Daugherty A. Structure and functions of angiotensinogen. Hypertens Res. 2016;39:492–500. doi: 10.1038/hr.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roig E., Perez-Villa F., Morales M. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21:53–57. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- 14.Narayan H., Webb D.J. New evidence supporting the use of mineralocorticoid receptor blockers in drug-resistant hypertension. Curr Hypertens Rep. 2016;18:34. doi: 10.1007/s11906-016-0643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakash T.P., Yu J., Migawa M.T. Comprehensive structure-activity relationship of triantennary N-acetylgalactosamine conjugated antisense oligonucleotides for targeted delivery to hepatocytes. J Med Chem. 2016;59:2718–2733. doi: 10.1021/acs.jmedchem.5b01948. [DOI] [PubMed] [Google Scholar]
- 16.Prakash T.P., Graham M.J., Yu J. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucl Acids Res. 2014;42:8796–8807. doi: 10.1093/nar/gku531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viney N.J., van Capelleveen J.C., Geary R.S. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 18.Crooke S.T., Witztum J.L., Bennett C.F., Baker B.F. RNA-targeted therapeutics. Cell Metab. 2018;27:714–739. doi: 10.1016/j.cmet.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Tsimikas S., Karwatowska-Prokopczuk E., Gouni-Berthold I. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 20.Crooke S.T., Baker B.F., Xia S. Integrated assessment of the clinical performance of GalNAc3-conjugated 2’-O-methoxyethyl chimeric antisense oligonucleotides: I. Human volunteer experience. Nucl Acid Ther. 2019;29:16–32. doi: 10.1089/nat.2018.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullick A.E., Yeh S.T., Graham M.J., Engelhardt J.A., Prakash T.P., Crooke R.M. Blood pressure lowering and safety improvements with liver angiotensinogen inhibition in models of hypertension and kidney injury. Hypertension. 2017;70:566–576. doi: 10.1161/HYPERTENSIONAHA.117.09755. [DOI] [PubMed] [Google Scholar]
- 22.Gaudet D., Karwatowska-Prokopczuk E., Baum S.J. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J. 2020;41:3936–3945. doi: 10.1093/eurheartj/ehaa689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viney N.J., Guo S., Tai L.J. Ligand conjugated antisense oligonucleotide for the treatment of transthyretin amyloidosis: preclinical and phase 1 data. ESC Heart Fail. 2021;8:652–661. doi: 10.1002/ehf2.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamout H., Lazich I., Bakris G.L. Blood pressure, hypertension, RAAS blockade, and drug therapy in diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21:281–286. doi: 10.1053/j.ackd.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Sternlicht H., Bakris G.L. Spironolactone for resistant hypertension--hard to resist? Lancet. 2015;386:2032–2034. doi: 10.1016/S0140-6736(15)00264-0. [DOI] [PubMed] [Google Scholar]
- 26.Parving H.H., Persson F., Lewis J.B., Lewis E.J., Hollenberg N.K., AVOID Study Investigators Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer M.A., Brenner B.M., McMurray J.J. Aliskiren in type 2 diabetes and cardiorenal end points. N Engl J Med. 2013;368:1065–1066. doi: 10.1056/NEJMc1300257. [DOI] [PubMed] [Google Scholar]
- 28.Kristensen S.L., Mogensen U.M., Tarnesby G. Aliskiren alone or in combination with enalapril vs. enalapril among patients with chronic heart failure with and without diabetes: a subgroup analysis from the ATMOSPHERE trial. Eur J Heart Fail. 2018;20:136–147. doi: 10.1002/ejhf.896. [DOI] [PubMed] [Google Scholar]
- 29.Wu C., Lu H., Cassis L.A., Daugherty A. Molecular and pathophysiological features of angiotensinogen: a mini review. N Am J Med Sci (Boston) 2011;4:183–190. doi: 10.7156/v4i4p183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer M., Baessler A., Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:672–677. doi: 10.1016/s0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson C., Monia B.P., Ryden-Bergsten K. Single dose safety, pharmacokinetics, and pharmacodynamics of a potent PCSK9 synthesis inhibitor, AZD8233, in subjects with elevated LDL cholesterol (abstr.) Circulation. 2020;142:A13913. [Google Scholar]
- 32.Uijl E., Colafella K.M.M., Sun Y. Strong and sustained antihypertensive effect of small interfering RNA targeting liver angiotensinogen. Hypertension. 2019;73:1249–1257. doi: 10.1161/HYPERTENSIONAHA.119.12703. [DOI] [PubMed] [Google Scholar]
- 33.Nakagami H., Morishita R. Recent advances in therapeutic vaccines to treat hypertension. Hypertension. 2018;72:1031–1036. doi: 10.1161/HYPERTENSIONAHA.118.11084. [DOI] [PubMed] [Google Scholar]
- 34.Ren L., Colafella K.M.M., Bovee D.M., Uijl E., Danser A.H.J. Targeting angiotensinogen with RNA-based therapeutics. Curr Opin Nephrol Hypertens. 2020;29:180–189. doi: 10.1097/MNH.0000000000000586. [DOI] [PubMed] [Google Scholar]
- 35.Fiuzat M., Lowy N., Stockbridge N. Endpoints in heart failure drug development: history and future. J Am Coll Cardiol HF. 2020;8:429–440. doi: 10.1016/j.jchf.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Ye F., Wang Y., Wu C. Angiotensinogen and megalin interactions contribute to atherosclerosis-brief report. Arterioscler Thromb Vasc Biol. 2019;39:150–155. doi: 10.1161/ATVBAHA.118.311817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao X.R., Rong J.B., Lu H.S. Angiotensinogen in hepatocytes contributes to Western diet-induced liver steatosis. J Lipid Res. 2019;60:1983–1995. doi: 10.1194/jlr.M093252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.