Abstract
The coronavirus disease 2019 (COVID-19) pandemic has emerged as a major threat to all healthcare systems across the globe, and it was declared a public health emergency of international concern by the World Health Organization (WHO). The novel coronavirus affects the respiratory system, producing symptoms such as fever, cough, dyspnea, and pneumonia. The association between COVID-19 and coagulation has been previously reported. Due to several inflammatory changes that occur in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections such as alterations in the levels of clotting factors, platelet activation leads to thrombus formation in coronary and cerebral vessels, leading to myocardial infarction and cerebrovascular accidents, respectively. Unfortunately, the progression of hypercoagulability in COVID-19 is rapid in patients with and without comorbidities. Hence, the proper monitoring of thrombotic complications in patients with COVID-19 is essential to avoid further complications. The implementation of guidelines for antithrombotic treatments based on the presentation of the disease is recommended. This review discusses the symptoms and mechanisms of upregulated coagulation in patients with COVID-19.
Keywords: COVID-19, SARS-CoV-2, Hypercoagulation, Thrombosis, Stroke, D-dimer
INTRODUCTION
At the end of December 2019, there was an unusual emergence of pneumonia cases of unknown origin in Wuhan, Hubei province, China [1]. All the patients had a history of exposure to the Huanan seafood market. Throat swabs were collected from all the suspected patients, and the causative agent was found to be a coronavirus by the Chinese Centre for Disease Control and Prevention; subsequently, the disease was named as coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) [2, 3]. COVID-19 is known to be caused by SARS-CoV-2, a member of the human-infecting betacoronavirus. Previous outbreaks such as severe acute respiratory syndrome (SARS) in China in 2002–2003 and Middle East respiratory syndrome in Saudi Arabia in 2011 were also attributed to the zoonotic origin of betacoronavirus [4]. These coronaviruses can also cause respiratory, hepatic, and central nervous system-related diseases [5]. Further epidemiological investigation showed that the zoonotic origins were rhinolophid bats (natural hosts) and pangolin mammals (intermediate hosts) [6]. An increasing number of COVID-19 cases were reported from other countries, and the WHO has raised a public health emergency of international concern [3].
The virus infects humans through various modes of transmission such as direct contact with an infected person via coughing, sneezing, or through other body fluids; inhalation of infected droplets; and, indirect contact with the surface used by infected persons (according to the WHO) [7]. When the SARS-CoV-2 enters the lower respiratory tract, it binds to the angiotensin-converting enzyme 2 (ACE) receptors, causing the downregulation of the renin-angiotensin system and increased vascular permeability, resulting in pulmonary edema and acute respiratory distress syndrome (ARDS) [1, 5, 8]. An attack of SARS-CoV-2 on ACE2 receptors triggers the activation of T cells and increases the release of pro-inflammatory mediators, causing various other outcomes [9]. A recent study in China reported that the most common symptoms of the SARS-CoV-2 infection were fever (98%), cough (76%), and fatigue (44%), and the least-common symptoms were headache (8%), hemoptysis (5%), and diarrhea (3%) [10].
COVID-19 mainly affects the respiratory, cardiac, and gastrointestinal systems; however, a state of severe infection can lead to several complications. The current study emphasizes the importance of analyzing the possibilities of thrombosis in patients with COVID-19. Thromboembolic events result in ischemic limbs, myocardial infarction, stroke, and so on. These are life-threatening conditions that require immediate hospitalization and rapid recovery to prevent disability. It is well known that patients with COVID-19 with histories of stroke and cardiovascular diseases have poor prognoses [11, 12]. Several pathological changes such as increased pro-inflammatory mediators, hypoxia, and other co-morbid conditions contribute to hypercoagulation and thrombus formation in the bloodstream, leading to thromboembolic events. Furthermore, a link between COVID-19 and coagulation has been reported [13]. However, the exact pathophysiology of hypercoagulability in COVID-19 remains unclear. Additionally, proper guidelines and diagnostic procedures for thrombotic complications in patients with severe COVID-19 should be strengthened with prophylactics; for example, anticoagulation therapy is advised to reduce disability and mortality rates.
COAGULATION IN COVID-19
COVID-19 leads to complications in the respiratory system, cardiovascular diseases, and so on. However, the occurrence of thromboembolic events in critically ill patients with COVID-19 may cause severe outcomes such as cerebrovascular accidents. Elderly patients with comorbid conditions such as respiratory disease, hypertension, diabetes, obesity that favors ARDS, acute cerebrovascular disease (stroke), and various complications in COVID-19 patients [14]. Hematological manifestations such as thrombocytopenia and lymphopenia have been observed in patients with COVID-19 [1, 15, 16]. Thrombocytopenia, commonly seen in critically ill patients with COVID-19, progresses to several malfunctions in the coagulation system, leading to the development of disseminated intravascular coagulation (DIC) [17]. The spiked outer layer of SARS-CoV-2 binds to ACE2 receptors, stimulating nuclear factor kappa B-driven inflammatory factors involved in thrombogenesis, including increased production of pro-inflammatory cytokines, such as monocyte chemoattractant protein, transforming growth factor beta 1, interleukin (IL) 6, and tumor necrosis factor (TNF) a [16]. Several conditions, such as the increased release of pro-inflammatory mediators, hypoxia, and sepsis-induced coagulation might be potential risk factors for hypercoagulability in patients with COVID-19 [14]. A recent study in the USA highlighted the role of coagulopathy in patients with severe COVID-19, and approximately 71.4% of them met the criteria for DIC of the International Society for Thrombosis and Hemostasis [18].
Sepsis is a life-threatening condition that accounts for approximately 40% of the mortality rate. Vascular complications are seen in several other viral infections such as influenza, avian influenza (H5N1), swine flu (H1N1), SARS, parvovirus infection, herpes simplex virus infection, cytomegalovirus infection, and infections of other respiratory viruses [19]. On the other hand, the inflammatory phase is critical for host defense mechanisms because it causes tissue damage, endothelial cell disruption, and uncontrolled coagulation activation. Inflammation is not only limited by the initiation of the coagulation activity but coagulation also stimulates the inflammation process. Coagulation is a defense reaction of innate immunity that confines the infection to the thrombi and limits the proliferation and dissemination of pathogens [20]. Platelets play a vital role in the coagulation mechanisms. Pathogens and pro-inflammatory mediators influence tissue factor expression, which in turn activates the coagulation process [19]. Platelets are activated by pro-inflammatory mediators such as the platelet-activating factor, and this is followed by thrombin generation and fibrin formation. This process is facilitated by inhibiting the physiological mechanisms of anticoagulation systems such as the anti-thrombin system, activated protein C system, and tissue factor pathway inhibitor, thereby producing microvascular clots and leading to ischemic changes in the vital organs [21].
A study in Singapore conducted during the SARS epidemic reported a few cases of large artery ischemic stroke with viral respiratory complaints. Thrombotic complications involving large arteries, despite the presence of mild vascular risk factors and administration of therapeutic doses of heparin, indicated the pro-coagulant state in patients with SARS infections [22]. Embolism from the proximal sites and significant hypotension due to sepsis resulted in stroke. Unfortunately, the use of intravenous immunoglobulin (IVIg) has been linked to an increased risk of thrombosis [22, 23]. IVIg is widely used to treat autoimmune neuromuscular disorders, and it is found to produce some rare but severe outcomes [23]. After IVIg infusion, following changes including high levels of immunoglobulin, immune complex formation, and platelet aggregation, the blood viscosity tended to increase, thereby reducing arterial blood flow and leading to thrombosis [24]. It can be resolved by providing thrombolytic therapy, limiting maximum infusion, etc.
Reduced hemostasis that favors the coagulation process is another prominent change in patients with COVID-19. A recent study showed significant increases in the D-dimer levels, fibrin/fibrinogen (FIB) degradation products (FDP), and lower levels of antithrombin (AT) and prothrombin time activity in patients with COVID-19 compared to the levels in healthy control groups [25]. Together, these factors stimulate the development of consumption coagulopathy (DIC) and worsen existing clinical conditions. DIC is a condition characterized by increased systemic coagulation and decreased hemostasis due to fibrin deposition and thrombus formation, thereby provoking pulmonary embolism and multi-organ dysfunction [20]. Hence, the routine monitoring of D-dimer levels and FDP can help in the early diagnosis and management.
HYPOXIA-INDUCED COAGULATION
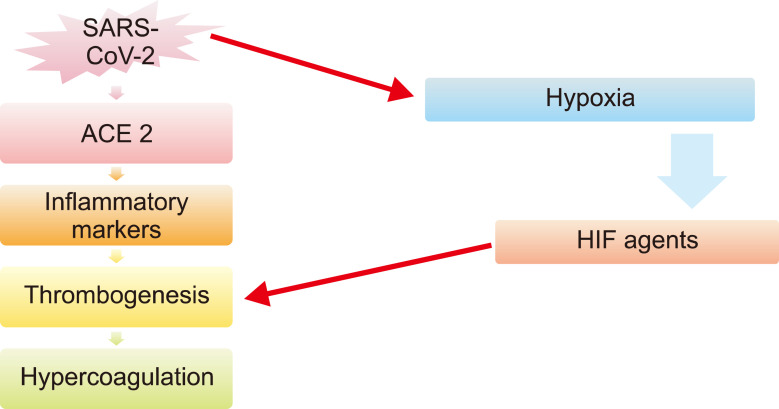
SARS-CoV-2 causes hypoxia due to various reasons including serious lung injury, myocardial infarction, other heart complications, and malignancies [26, 27]. Hypoxia occurs when oxygen demand is higher than oxygen supply. Hypoxia in patients with COVID-19 causes vascular occlusion, increases blood viscosity, stimulates thrombogenesis through hypoxia-inducible factors (HIF agents), and indirectly induces coagulation by inducing pro-inflammatory cytokines such as TNFa and IL-1 (Fig. 1) [28].
Fig. 1.
Suggested pathways of hypercoagulation by SARS-CoV-2.
Abbreviations: ACE2, angiotensin converting enzyme 2; HIF agents, hypoxia-inducible factors.
D-DIMER LEVELS
The D-dimer level is a reliable and sensitive index for assessing fibrin deposition, and it helps to suspect thrombus formation. Elevated D-dimer levels indicated hypercoagulation in patients with COVID-19. Moreover, increased coagulation abnormality was prevalent among patients with severe pneumonia having elevated D-dimer levels [29]. D-dimer levels higher than 1 mg/mL were found to worsen the severity of COVID-19 along with increasing the possibility of mortality [30]. Elevated D-dimer levels are commonly observed in patients under critical care [31, 32]. Furthermore, D-dimer levels are also elevated in stroke, DIC, and venous thromboembolism (VTE) [33].
The D-dimer test may give false-negative results even in thrombosis and in cases of aged thrombi in VTE (14 days, which becomes less amenable for plasmin digestion), hypofibrinolysis, and VTE receiving a therapeutic dose of heparin or on oral anticoagulation [33]. Patients with COVID-19 with elevated D-dimer levels treated with heparin showed lower mortalities than those who did not receive heparin treatment [14]. Patients with D-dimer levels higher than 1,000 ng/mL are suspected to be 20 times more likely to die from infection than those with lower levels. A recent study reported that nearly 5.7% of patients with severe infection having elevated D-dimer and CRP levels developed cardiovascular diseases. On an average, the onset of stroke in COVID-19 takes approximately 12 days [31].
The early diagnosis of thrombotic changes in severe COVID-19 cases using laboratory findings such as D-dimer levels, FDP, and prothrombin time may be helpful in managing further complications. The International Society of Thrombosis and Hemostasis recommends the use of prophylactic doses of low-molecular-weight heparin or unfractionated heparin for pharmacological prophylaxis or, alternatively, mechanical prophylaxis [16]. Heparin, which acts as an anticoagulant and an anti-inflammatory agent, was used in patients with COVID-19 with a low incidence of bleeding that was found in a few cases [34]. Patients with COVID-19 with D-dimer levels >3.0 m/mL as well as those with sepsis-induced coagulation score ≥4 showed significant reductions in mortality in heparin users compared to non-users [34]. Hence, guidelines and the proper management of thrombosis in patients with COVID-19 may help in reducing the mortality rate.
CONCLUSION
Blood hypercoagulability in patients with COVID-19 has been a major cause of morbidity and mortality. The present review describes the symptoms and mechanisms of upregulated coagulation in patients with COVID-19. Further studies are required to elucidate the pathophysiological mechanisms that may be helpful for exploring early biomarkers and drug targets so that this fatal event can be avoided at an early stage in the patient population with COVID-19.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills S. COVID-19, public health measures, legal considerations: a medical perspective. Judic Rev. 2020;25:71–9. doi: 10.1080/10854681.2020.1760575. [DOI] [Google Scholar]
- 3.Sohrabi C, Alsafi Z, O'Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–6. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 5.Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam TT, Jia N, Zhang YW, et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–5. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 7.Karia R, Gupta I, Khandait H, Yadav A, Yadav A. COVID-19 and its modes of transmission. SN Compr Clin Med. 2020:1–4. doi: 10.1007/s42399-020-00498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abassi ZA, Skorecki K, Heyman SN, Kinaneh S, Armaly Z. Covid-19 infection and mortality: a physiologist's perspective enlightening clinical features and plausible interventional strategies. Am J Physiol Lung Cell Mol Physiol. 2020;318:L1020–2. doi: 10.1152/ajplung.00097.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential thera-peutic tools. Virol Sin. 2020;35:266–71. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin C, Zhou L, Hu Z, et al. Clinical characteristics and outcomes of COVID-19 patients with a history of stroke in Wuhan, China. Stroke. 2020;51:2219–23. doi: 10.1161/STROKEAHA.120.030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu H, Rhee JW, Cheng P, et al. Cardiovascular complications in patients with COVID-19: consequences of viral toxicities and host immune response. Curr Cardiol Rep. 2020;22:32. doi: 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020;11:322–5. doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99:1205–8. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colling ME, Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc Med. 2020;25:471–8. doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–5. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goeijenbier M, van Wissen M, van de Weg C, et al. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84:1680–96. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minasyan H, Flachsbart F. Blood coagulation: a powerful bactericidal mechanism of human innate immunity. Int Rev Immunol. 2019;38:3–17. doi: 10.1080/08830185.2018.1533009. [DOI] [PubMed] [Google Scholar]
- 21.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Umapathi T, Kor AC, Venketasubramanian N, et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004;251:1227–31. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalakas MC, Clark WM. Strokes, thromboembolic events, and IVIg: rare incidents blemish an excellent safety record. Neurology. 2003;60:1736–7. doi: 10.1212/01.WNL.0000074394.15882.83. [DOI] [PubMed] [Google Scholar]
- 24.Okuda D, Flaster M, Frey J, Sivakumar K. Arterial thrombosis induced by IVIg and its treatment with tPA. Neurology. 2003;60:1825–6. doi: 10.1212/01.WNL.0000068334.04500.08. [DOI] [PubMed] [Google Scholar]
- 25.Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–20. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 26.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Milbrandt EB, Reade MC, Lee M, et al. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol Med. 2009;15:438–45. doi: 10.2119/molmed.2009.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avula A, Nalleballe K, Narula N, et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–9. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–90. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripodi A. D-dimer testing in laboratory practice. Clin Chem. 2011;57:1256–62. doi: 10.1373/clinchem.2011.166249. [DOI] [PubMed] [Google Scholar]
- 34.Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–60. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]