Abstract
Myeloid sarcoma of the breast is a rare malignancy, can be seen after bone marrow transplantation. Although there are no specific features for this malignancy which is difficult to diagnose, some common features draw attention in the published case reports. Since there is no consensus on the treatment of myeloid sarcoma of the breast, we aimed to explain our own diagnosis and treatment methods in this case report.
Keywords: Breast, myeloid sarcoma, breast sarcoma, bone marrow transplantation
Key Points
• Myeloid sarcoma(MS) is an aggressive tumor.
• Breast tissue is a rare region for MS development.
• The development of MS of the breast tissue after bone marrow transplantation (BMT) in patients with acute myeloid leukemia (AML) is remarkable.
• If a breast mass is detected, the diagnosis should be supported by immunhistochemistry and biopsy.
• Systemic treatment should be started as soon as possible.
Introduction
Myeloid sarcoma (MS) is an aggressive tumor characterized by leukemic proliferation with or without mature myeloid cells, which can be seen anywhere in the body except bone marrow (1). MS is a hematological malignancy, rarely encountered as a soft tissue mass in the extramedullary system called granulocytic sarcoma or chloroma (2, 3, 4). Although MS can develop in any part of the body, it is frequently seen in bone, lymph nodes, soft tissue, and skin (1, 5). Breast tissue is a rare region for MS development, and only 3% of cases with,MS in breast tissue were reported in the Mayo Clinic series (6, 7). The development of MS of the breast tissue after bone marrow transplantation (BMT) in patients with acute myeloid leukemia (AML) is remarkable (8).
Case Presentation
A 31-year-old female presented to the General Surgery Department Breast and Endocrine Unit with the complaint of a palpable mass in both breasts. Seventeen years ago, the patient underwent surgery and chemotherapy due to osteosarcoma in the left ankle. Six years ago, she underwent a bilateral breast fibroadenoma excision, and four years ago, she underwent an excision due to phyllodes tumor in the right breast. Four years ago, bone marrow biopsy, peripheral smears, and hematological tests during postoperative controls were performed and they showed abnormal findings. After she was diagnosed with AML, BMT was performed. One month ago, she had a total thyroidectomy due to a malignant tumor in the left thyroid lobe. She experienced a postoperative pathology of papillary thyroid carcinoma with AML infiltration and follicular variant. During that period, she had masses in both breasts. On physical examination, 4–5 cm masses were palpated on the bilateral breasts. On the recent breast ultrasonography (USG), it was observed that the patient had masses in the lower outer quadrant of the right breast, whose borders were ambiguous. A 2.5 cm residual phyllodes tumor with a heterogeneous structure and an approximately 1 cm fibroadenoma with a slightly faint border at 3 o’clock were noted in the left breast. In the same period, fluorodeoxyglucose-18 (FDG-18) [FDG- positron emission tomography/computed tomography (PET)/CT] imaging showed that the mass in the right breast maximum standardized uptake values [(SUVmax) = 3.9] had activity involvement. There were no other positive findings in the PET/CT scan of the patient. Since the patient had a systemic disease, PET/CT imaging was performed after breast USG. This does not normally fit the breast imaging sequence and it is an exception for this patient. Due to the coronavirus disease 2019 (COVID-19) pandemic, there was a disruption in the follow-up. The control PET/CT imaging was performed after six months showing massive lesions of 4 cm in the right breast and 5 cm and 1.5 cm in the left breast (SUVmax = 9) and a 1 cm (SUVmax = 3.7) increase in the metabolism areas in both axilla (Figure 1). It was decided to perform a breast and axilla Tru-Cut biopsy. The results of the biopsies were interpreted MS containing diffuse blastic cell infiltration. Microscopic images of diffuse blastic cell infiltration in breast tissue with haematoxylin and eosin stain (H&E) are shown in Figure 2. When the blastic cells were examined immunohistochemically, they showed diffuse strong cytoplasmic staining with cluster of differentiation-45 (CD45), CD34, CD117, and human leukocyte antigen-DR (HLA-DR) and focal strong cytoplasmic staining with myeloperoxidase (MPO). Immunohistochemical staining images of breast tissue showing blast cells positive for CD45, CD34, CD117, HLA-DR, and MPO are shown in Figure 3.
Figure 1.
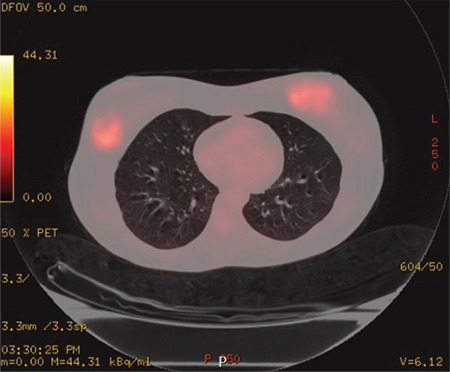
Axial paranchimal fused PET/CT image showing massive lesions of 4 cm in the right breast and 5 cm and 1.5 cm in the left breast (SUVmax = 9) and 1 cm (SUVmax = 3.7) increase in metabolism areas in both axilla
PET/CT: Positron emission tomography/computed tomography
Figure 2.
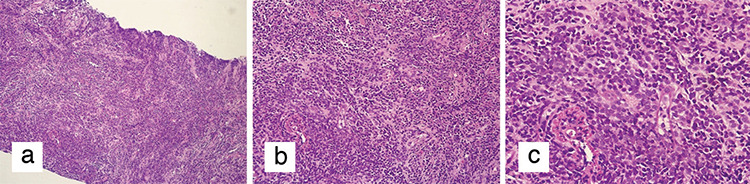
Microscopic images of diffuse blastic cell infiltration in breast tissue with haematoxylin and eosin stain (H&E). a) Diffuse blastic cell infiltration in breast tissue (H&E x100). b) Diffuse blastic cell infiltration in breast tissue (H&E x200). c) Diffuse blastic cell infiltration in breast tissue (H&E x400)
Figure 3.
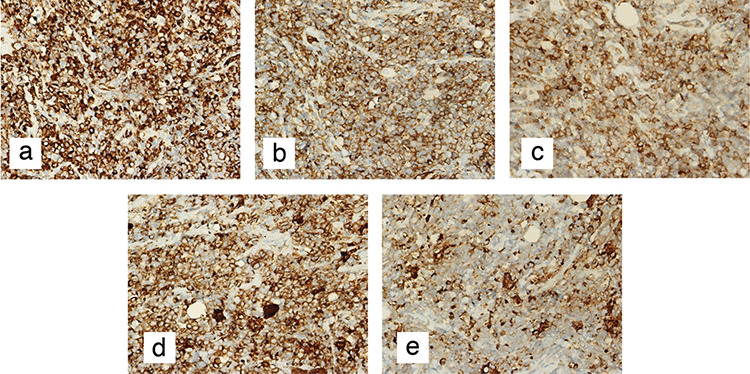
Immunohistochemical staining images of breast tissue. a) Immunohistochemical staining of blast cells showing diffuse strong cytoplasmic positivity for CD45 (CD45 x400). b) Immunohistochemical staining of blast cells showing diffuse strong cytoplasmic positivity for CD34 (CD34 x400). c) Immunohistochemical staining of blast cells showing diffuse strong cytoplasmic positivity for CD117 (CD117 x400). d) Immunohistochemical staining of blast cells showing diffuse strong cytoplasmic positivity for HLA-DR (HLA-DR x400). e) Immunohistochemical staining of blast cells showing focal strong cytoplasmic positivity for MPO (MPO x400)
CD: Cluster of differentiation; HLA-DR: Human leukocyte antigen-DR isotype; MPO: Myeloperoxidase
The patient was evaluated in the oncology council. Due to the aggressive spread, a BMT and systemic chemotherapy were planned. The mass in the patient’s breast did not need a palliative resection. The patient was followed up after the planned therapies.
Discusson and Conclusion
Although MS is an extramedullary hematological malignancy, in which myeloid cells show various degrees of maturation, it is frequently seen in patients with previously diagnosed myeloid leukemia (9, 10). According to the World Health Organization, MS is diagnosed based on three classes: blastic (consisting of myeloblasts), immature (consisting of myeloblasts and promyelocytes), and differentiated (consisting of promyelocytes and more mature neutrophils) (8). Although MS can develop in any part of the body, the development of MS of the breast is extremely rare. For this reason, patients with MS in the breast are often misdiagnosed as having lobular breast carcinoma, non-Hodgkin lymphoma, or small-round-blue-cell tumor (11). The first area where extramedullary relapse occurs after stem cell transplantation is the breast, and relapse can occur 2–73 months after transplantation (average 17 months) (8). MS may occur as a unilateral or bilateral breast mass, but it usually does not cause nipple retraction (2, 12, 13, 14). Although there was no specific finding of MS in the breast when examining the studies, case reports emphasize the findings of irregular, spiculated, angular or unclear margins, and posterior shadowing (7, 15). MS diagnosis can be supported by MPO, CD34, CD 43, CD 117, and CD 68 (12). Specific CD markers can be very useful in diagnosis. CD 117, CD 68, and CD 43 are positive in most cases, and CD 45 is reactive in 75% of the cases (12).
There is no consensus on the treatment of MS of the breast, but surgical resection (lumpectomy or mastectomy) with systemic chemotherapy is generally recommended (7, 8). Simultaneous radiotherapy is controversial (8). Disease-free survival is predicted between three and twelve years with systemic chemotherapy (8). In light of the literature, the diagnosis was supported by MPO, CD 34, and CD 117 staining in our patient, who developed bilateral breast MS four years after BMT for AML treatment. Considering the patient’s diagnosis of MS in both breasts and the inclusion of AML infiltration of the cancerous tissue in the thyroidectomy material, it was decided that the patient undergoes BMT and receive systemic treatment again. Moreover, systemic chemotherapy was planned after the transplantation.
In this case report, we described the management of a patient with MS of the breast, which is a rare tumor. It should be kept in mind that MS may develop in the breast after BMT in patients with AML and those patients should be followed up using breast ultrasound. If a mass is detected, the diagnosis should be supported by immunhistochemistry and biopsy, and systemic treatment should be started as soon as possible.
Footnotes
Informed Consent: Written informed consent was obtained from the patient before participation in this study.
Peer-review: Externally peer-reviewed.
Author Contributions
Conception: E.V.; Design: E.V.; Supervision: S.A.G., N.Z.U.; Materials: U.K., Ç.V., Z.G., N.Z.U.; Data Collection and/or Processing: U.K., Ç.V., Z.G.; Analysis and/or Interpretation: E.V., S.A.G, N.Z.U.; Literature Search: E.V.; Writing: E.V.; Critical Review: S.A.G.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declare that this study received no financial support.
References
- 1.Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinicopathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340–350. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]
- 2.Valbuena JR, Admirand JH, Gualco G, Medeiros LJ. Myeloid Sarcoma Involving the Breast. Arch Pathol Lab Med. 2005;129:32–38. doi: 10.5858/2005-129-32-MSITB. [DOI] [PubMed] [Google Scholar]
- 3.Roth MJ, Medeiros LJ, Elenitoba-Johnson K, Kuchnio M, Jaffe ES, Stetler-Stevenson M. Extramedullary myeloid cell tumors. An immunohistochemical study of 29 cases using routinely fixed and processed paraffin-embedded tissue sections. Arch Pathol Lab Med. 1995;119:790–798. [PubMed] [Google Scholar]
- 4.Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309–316. doi: 10.1177/2040620711410774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vardiman JW. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: an overview with emphasis on the myeloid neoplasms. Chem Biol Interact. 2010;184:16–20. doi: 10.1016/j.cbi.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Bubulac L, Bardaş A, Popa DC, Vasilache ED, Ionescu BO, Coriu D, et al. Breast myeloid sarcoma after allogeneic stem cell transplantation for acute myelomonocytic leukemia - case report. Rom J Morphol Embryol. 2019;60:707–711. [PubMed] [Google Scholar]
- 7.Zhai J, Kong X, Yang X, Gao J, Xuan L, Wang X, et al. An uncommon granulocytic sarcoma of the breast: a case report and literature review. Onco Targets Ther. 2018;11:3685–3690. doi: 10.2147/OTT.S149149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toumeh A, Phinney R, Kobalka P, Mohamed I. Bilateral myeloid sarcoma of the breast and cerebrospinal fluid as a relapse of acute myeloid leukemia after stem-cell transplantation: A case report. J Clin Oncol. 2012;30:e199–e201. doi: 10.1200/JCO.2011.40.2255. [DOI] [PubMed] [Google Scholar]
- 9.Stewart RL, Dell CM, Samayoa L. Myeloid sarcoma of the breast misdiagnosed as poorly differentiated mammary carcinoma with lobular features. Breast J. 2015;21:192–193. doi: 10.1111/tbj.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozsoy A, Akdal Dolek B, Barca N, Aktas H, Araz L, Kulacoglu S. Ultrasound findings in a case of myeloid sarcoma of the breast. J Belg Soc Radiol. 2016;100:15. doi: 10.5334/jbr-btr.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomaa W, Ghanim A, Emam E, Bayoumi K, Ghanim A. Primary myeloid sarcoma of the breast: a case report and review of literature. J Microsc Ultrastruct. 2018;6:212–214. doi: 10.4103/JMAU.JMAU_15_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu HY, Liu L, Gu L, Luo YH. Clinical characteristics and management of primary granulocytic sarcoma of the breast: A case report. Medicine (Baltimore) 2019;98:e16648. doi: 10.1097/MD.0000000000016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham I. A clinical review of breast involvement in acute leukemia. Leuk Lymphoma. 2006;47:2517–2526. doi: 10.1080/10428190600967022. [DOI] [PubMed] [Google Scholar]
- 14.Thachil J, Richards RM, Copeland G. Granulocytic sarcoma—a rare presentation of a breast lump. Ann R Coll Surg Engl. 2007;89:W7–W9. doi: 10.1308/147870807X227827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinoshita T, Yokokawa M, Yashiro N. Multicentric granulocytic sarcoma of the breast: mammographic, sonographic, and MR findings of granulocytic sarcoma of the breasts. Clin Imaging. 2006;30:271–274. doi: 10.1016/j.clinimag.2005.11.004. [DOI] [PubMed] [Google Scholar]


