Highlights
-
•
B cells regulate atherosclerotic plaque formation through production of antibodies and cytokines, and effects are subset specific (B1 and B2).
-
•
Putative human atheroprotective B1 cells function similarly to murine B1 in their spontaneous IgM antibody production. However, marker strategies in identifying human and murine B1 are different.
-
•
IgM antibody to oxidation specific epitopes produced by B1 cells associate with human coronary artery disease.
-
•
Neoantigen immunization may be a promising strategy for atherosclerosis vaccine development, but further study to determine relevant antigens still need to be done.
-
•
B-cell–targeted therapies, used in treating autoimmune diseases as well as lymphoid cancers, might have potential applications in treating cardiovascular diseases. Short- and long-term cardiovascular effects of these agents need to be assessed.
Key Words: B-cell, atherosclerosis, immunoglobulins
Abbreviations and Acronyms: ApoE, apolipoprotein E; APRIL, A proliferation−inducing ligand; BAFF, B-cell–activating factor; BAFFR, B-cell–activating factor receptor; BCMA, B-cell maturation antigen; BCR, B-cell receptor; Breg, regulatory B cell; CAD, coronary artery disease; CTLA4, cytotoxic T-lymphocyte–associated protein 4; CVD, cardiovascular disease; CXCR4, C-X-C motif chemokine receptor 4; GC, germinal center; GITR, glucocorticoid-induced tumor necrosis factor receptor–related protein; GITRL, glucocorticoid-induced tumor necrosis factor receptor–related protein ligand; GM-CSF, granulocyte-macrophage colony–stimulating factor; ICI, immune checkpoint inhibitor; IFN, interferon; IL, interleukin; IVUS, intravascular ultrasound; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; mAb, monoclonal antibody; MDA-LDL, malondialdehyde-modified low-density lipoprotein; MI, myocardial infarction; OSE, oxidation-specific epitope; OxLDL, oxidized low-density lipoprotein; PC, phosphorylcholine; PD-1, programmed cell death protein 1; PDL1, programmed death ligand 1; PD-L2, programmed death ligand 2; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TACI, transmembrane activator and CAML interactor; TNF, tumor necrosis factor; Treg, regulatory T cell
Central Illustration
Summary
Because atherosclerotic cardiovascular disease is a leading cause of death worldwide, understanding inflammatory processes underpinning its pathology is critical. B cells have been implicated as a key immune cell type in regulating atherosclerosis. B-cell effects, mediated by antibodies and cytokines, are subset specific. In this review, we focus on elaborating mechanisms underlying subtype-specific roles of B cells in atherosclerosis and discuss available human data implicating B cells in atherosclerosis. We further discuss potential B cell–linked therapeutic approaches, including immunization and B cell–targeted biologics. Given recent evidence strongly supporting a role for B cells in human atherosclerosis and the expansion of immunomodulatory agents that affect B-cell biology in clinical use and clinical trials for other disorders, it is important that the cardiovascular field be cognizant of potential beneficial or untoward effects of modulating B-cell activity on atherosclerosis.
A wealth of data have implicated smoking, hypertension, diabetes, hyperlipidemia, and lifestyle in the risk of developing atherosclerotic cardiovascular disease (CVD), and interventions to modify these factors are effective and the mainstay of current CVD prevention approaches (1). Yet despite these important interventions, CVD remains the leading cause of death worldwide (2), underscoring the need for a deeper understanding of the underpinnings of atherosclerotic lesion formation. Nearly 50 years ago, Ross and Glomset published a seminal paper in the New England Journal of Medicine on the pathogenesis of atherosclerosis, defining it as a “response to injury” (3), with the injurious stimuli including the risk factors mentioned above. Gerlis (4) and Schwartz and Mitchell (5) reported the presence of immune cells within atherosclerotic plaques and the adventitia of arteries in 1956 and 1962, respectively. These important observations accompanied by the biomedical research advances by many others over the ensuing decades led to our current understanding of atherosclerosis as a chronic inflammatory disease (6, 7, 8, 9, 10).
A major initiating event in atherosclerosis is deposition of lipids in the arterial wall. These lipids, acted on by oxidative enzymes produced by vessel wall cells, subsequently harbor oxidation-specific epitopes (OSEs). These OSEs in turn stimulate vascular cells, such as smooth muscle cells and endothelial cells, to produce adhesion molecules, cytokines, and chemokines that attract additional leukocytes, such as circulating monocytes and T cells, to the vessel wall (10). Monocytes that enter the arterial wall differentiate into macrophages. The mechanisms whereby T cells and macrophages contribute to atherosclerosis has been extensively reviewed elsewhere (11, 12, 13). In brief, macrophages can take up oxidized lipids to become foam cells. In addition, OSEs and other lesion-derived neoantigens function as danger-associated molecular patterns, which can engage Toll-like receptors and other pattern-recognition receptors (14,15), further stimulating inflammatory pathways in macrophages and T cells. T cells can also encounter local antigen and cytokines which also contribute to lesion progression through amplification of the inflammatory response (11,16).
Recent use of cutting-edge high-dimensional analysis of murine and human atherosclerotic lesions (17,18) confirms and extends earlier studies that T cells and macrophages are predominant cell types in the atherosclerotic lesion itself. B Cells, in contrast, are not a predominant immune cell type identified within the atherosclerotic lesions (17,18). However, they are in abundance in perivascular adipose tissue, which, in addition to the spleen and bone marrow, serves as a niche for immunoglobulin (Ig) production (14). Like other tissues with chronic inflammation, atherosclerotic vessels also harbor tertiary lymphoid organs, which contain Ig- and cytokine-producing B cells (14,19,20). Cytokines and Igs produced by B cells at these sites are thought to be important regulators of inflammation in atherosclerosis lesion formation. Moreover, circulating Igs produced in secondary lymphoid organs are found in atherosclerotic lesions, providing support that B cells outside of lesions may have important lesion-modifying effects (19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31). The present review predominantly highlights key mechanisms whereby B cell–derived Igs modulate atherosclerosis, with a focus on the implications for potential B-cell–targeted therapies for atherosclerotic CVD and potential CVD complications of B-cell–targeted therapies in clinical trials or clinical use for other diseases.
Brief General Overview of B-Cell Biology
B cells are lymphocytes that play major roles in both innate and adaptive immunity mainly through production of antibodies and cytokines. Murine B cells are broadly divided into B1 and B2 cells, that have distinct characteristics and cell surface markers reviewed in detail elsewhere (32). In brief, B1 cells develop mostly in the fetal liver and undergo self-renewal in the periphery (33). They reside predominantly in serosal cavities, but can travel to spleen and bone marrow where they produce predominantly IgM antibodies in a T-cell–independent manner (27,34,35). Murine B1 cells can be subdivided into B1a and B1b cells, which are distinguished by CD5 expression: B1a cells are CD5+ and B1b cells are CD5− (36,37). B2 cells are known as conventional B cells and participate in adaptive immunity. They arise from lymphoid progenitors in bone marrow, and differentiate into immature B cells through Ig heavy and light chain rearrangement (38). These immature B cells leave bone marrow to travel to secondary lymphoid organs, undergoing transitional stages to become mature B cells, which further differentiate into marginal zone B cells and follicular B cells (38,39). Follicular B cells represent a majority of B2 cells and participate in adaptive immune responses as they become activated by antigens stimulation via T-cell help in the germinal center (GC) (38). These activated B cells in the GC then undergo affinity maturation, class switching, and somatic hypermutation to produce highly antigen-specific predominantly IgG antibodies. These GC B cells then further differentiate into plasma cells or memory B cells (38). In addition, certain B-cell subtypes regulate inflammatory reactions through cytokine production. Regulatory B cells (Breg) are known to produce anti-inflammatory cytokine interleukin (IL) 10, which suppresses T-helper cells and inhibits macrophage Ag presentation and proinflammatory cytokine production (40).
B Cells and Atherosclerosis in Mice
An important role for B cells in murine atherosclerosis has been clearly demonstrated (20, 21, 22, 23, 24,30,31,41,42), and this topic has been recently reviewed in detail elsewhere (26,38,43,44). In brief, as depicted in Figure 1, B1 cells protect against atherosclerosis development through secreting IgM, which binds OSEs on low-density lipoprotein (LDL), preventing lipid uptake and inflammatory cytokine production by macrophages, thus reducing formation of foam cells and limiting inflammation. In addition, B1-derived IgM binds epitopes on apoptotic cells and enhances clearance of these cells, further contributing to limiting inflammation (14). Innate response activator is a B1a-derived B-cell subset induced by lipopolysaccharides to produce granulocyte-macrophage colony–stimulating factor (GM-CSF), promoting extramedullary hematopoiesis in the spleen and atherosclerosis. On the other hand, B2 cells are largely considered to be atherogenic via production of pathogenic IgG, activation of T cells, and induction of proinflammatory cytokines such as interferon (IFN) γ (20, 21, 22,24). The evidence supporting these B-cell subset–specific effects goes back nearly 20 years. In 2002, Caligiuri et al. showed that removal of the spleen in apolipoprotein E gene knockout (ApoE−/−) mice exacerbated atherosclerosis, whereas adoptive transfer of B cells to these splenectomized mice (41) attenuated disease development. In the same year, Major et al. demonstrated that LDL receptor–deficient (LDLR−/−) mice with <1.0% of their normal B-cell population in the bone marrow had a 30% to 40% increase in atherosclerotic lesion area (42) These two studies provided novel evidence that B cells protect against atherosclerosis development. Follow-on studies supported data implicating loss of anti–oxidized LDL (OxLDL) antibodies (42) as mice unable to secrete IgM had increased atherosclerotic disease (25,27,45). Interestingly, studies using a CD20 monoclonal antibody (mAb) or B-cell–activating factor receptor deletion (BAFFR−/−) to deplete B cells in hyperlipemic mice resulted in decreased atherosclerosis compared with control mice despite similar serum cholesterol levels (20, 21, 22,24). B2 depletion increased IL-17 production, suppressed T-cell production of IFN-γ, decreased proliferation of CD4 T cells in the spleen, lessened production of other proinflammatory cytokines, such as tumor necrosis factor (TNF) α and IL-1, and chemokines, such as monocyte chemoattractant protein 1, and reduced atherosclerosis (21,24). Furthermore, murine B2 cells produce IgG antibodies that bind OSEs and stimulate inflammation, at least in part through the Fc- receptor (46, 47, 48). These data countered the notion that B cells were atheroprotective. Notably, in these studies, the predominant B-cell subtype depleted with anti-CD20 or BAFFR deficiency was the B2 cell. Follow-on studies, using adoptive transfer of specific B-cell subsets confirmed that B-cell effects on atherosclerosis were subset specific, with B1 cells inhibiting (23) and B2 cells promoting (24) atherosclerosis.
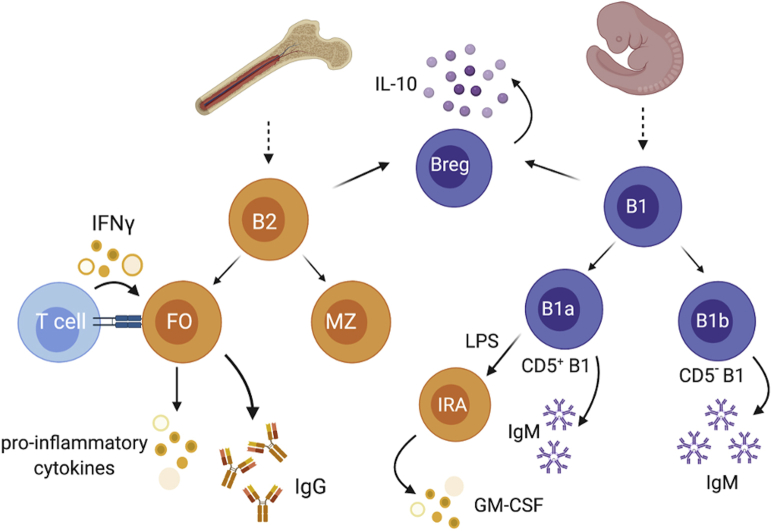
Figure 1.
Broad Overview of Subsets of Murine B Cells That Are Involved in Atherosclerosis
Murine B cells can be divided into B cell subsets based on established cell surface markers. The B1 B cells are atheroprotective and can be divided into B1a and B1b based on CD5 expression with B1a being CD5+ and B1b being CD5-. B1a and B1b have unique capability to produce atheroprotective IgM in a T cell independent manner. In response to LPS, B1a cells can migrate to spleen and produce GM-CSF, promoting extramedullary hematopoiesis and atherogenesis (IRA B cells). Both B1 and B2 cells can give rise to IL-10 producing regulatory B cells (Breg). Breg is defined by its capability to produce anti-inflammatory cytokines like IL-10. B2, on the other hand, promotes atherosclerosis through production of atherogenic IgG, activation of T cells, and induced production of inflammatory cytokines (eg, IFN). GM-CSF = granulocyte-macrophage colony-stimulating factor; IgG = immunoglobulin G; IgM = immunoglobulin M; IL = interleukin; IFN = interferon; LPS = lipopolysaccharide.
Extrapolation of murine B-cell subtypes to humans has been challenging owing to differences in surface markers defining subtypes (19,44,49,50). However, emerging evidence has demonstrated common surface markers on human and murine B1 cells (e.g., C-X-C motif chemokine receptor 4 [CXCR4]) that associate with plasma levels of atheroprotective IgM to OSEs. Mechanistic gain- and loss-of-function studies in mice confirmed a causal relationship for CXCR4 on B1a cells and bone marrow production of IgM to OSEs (27). That CXCR4 expression on B1 cells results in increased IgM to OSEs in mice and humans and is inversely associated with coronary artery plaque volume as measured with the use of intravascular ultrasound (IVUS) in humans (27) underscores that parallels between mice and human can exist and that combining both preclinical mechanistic studies and studies in humans may be the best approach to understanding B-cell–mediated regulation of atherosclerosis. The identification of the putative human equivalents of the murine B1 cells that produce IgM to OSEs (27,51) and the abundance of evidence, albeit largely associative, that B cells are important in human disease (52) and that IgM to OSEs is inversely associated with coronary artery disease (CAD) (39,40,51) is discussed in detail below.
B Cells and Atherosclerosis in Humans
A historical perspective
More than 100 years ago, Sir Clifford Allbutt in his book Diseases of the Artery (53) noted that “round cell growth in the adventitia in arteriosclerosis is correlated with absorption of depraved matter from the diseased intima.” With the advent of specific immunohistologic reagents in the ensuring decades, these round cells were found to be lymphocytes with a predominance of B cells (4,5,54,55). Key data implicating B cells in human atherosclerosis come from important studies in patient cohorts that reported on the relationship between CAD and antibodies to OSEs (47,56, 57, 58, 59, 60). Consistent with the data in mice demonstrating B1-derived IgM–attenuated atherosclerosis and B2-derived IgG–promoted atherosclerosis, in a cohort of 504 patients undergoing medically indicated coronary angiography, univariate analysis revealed that IgM to OSEs was inversely and IgG to OSEs positively associated with coronary stenoses >50% (56). Analysis of 748 cases and 1,723 controls in the European Prospective Investigation into Cancer and Nutrition—Norfolk study suggested that IgM and IgG autoantibodies and immune complexes could modify risk prediction for CVD (61). Indeed, a subsequent report of incident CVD (ischemic stroke, myocardial infarction (MI), new-onset unstable angina, acute coronary interventions, and vascular death) over 15 years of follow-up in the Bruneck Study revealed that subjects with high IgG to OSEs had higher risk of CVD, whereas those with high IgM to OSEs had lower risk. Using these biomarkers as variables improved CVD risk prediction, enabling reclassification of subjects into more correct risk categories (59). In the Dallas Heart Study, autoantibodies to malondialdehyde-modified LDL (MDA-LDL) were measured in 3,509 subjects that were followed for 10.5 years. Multivariable-adjusted Cox regression analysis demonstrated that IgG to MDA-LDL was independently associated with time-to-incident major adverse cardiovascular events (60). Analysis of subjects with CAD development and respective control subjects in NORDIL (the Nordic Diltiazem Study) supported the hypothesis that lower IgM to MDA-LDL was associated with CAD development and further showed that there was an inverse relationship with IgM to MDA-LDL and necrotic core volume as measured with the use of IVUS (59).
A few studies have implicated low levels of IgG to a specific ApoB100 peptide (p210) and CAD events (60,62) along with the association of low IgM to native and MDA-modified versions of p210 and p45 (60). Other studies demonstrated that low levels of IgG to native p210 but not IgM to MDA-modified p210 were inversely associated with severity of CAD and MI risk (52), raising the interesting possibility that isotype responses may be idiotype specific. In addition, these studies highlight the complexity and controversy related to IgG autoantibodies and CVD. As in murine models, evidence for a protective role for IgM to OSEs in human atherosclerosis is more consistent than the evidence for IgG.
In addition to this wealth of evidence that antibodies produced exclusively by B cells are associated with CVD, additional data support an important role for B cells in human atherosclerosis. Huan et al. performed a network-driven analysis incorporating whole-blood gene expression profiles and CAD single-nucleotide polymorphism analysis constructed from 188 subjects with CAD and 188 age- and sex-matched control subjects from the Framingham Heart Study with Bayesian networks. Results clearly identified B-cell–centered immune function to be related to CAD pathogenesis. Gene ontology enrichment analysis identified B-cell activation, B-cell differentiation, and B-cell receptor (BCR) signaling pathways as significantly enriched in CAD. Of the top 20 CAD key driver genes, B-cell genes predominated, supporting a critical role for B cells in human atherosclerosis and suggesting that B-cell–targeted therapies may be useful to prevent or treat atherosclerosis (63). Yet while much is known about B-cell subtypes in murine atherosclerosis, much less is known about B-cell subtypes in human disease.
Human B-cell subsets
It is not possible to directly translate murine B-cell subtypes to humans. Issues that contribute to this limitation include differences in surface markers and in responses in immune cells between mice and human (64). Multiple studies completed by the Inflammation and Host Response to Injury Large-Scale Collaborative Research Program demonstrate that immune responses in mice often do not predict immune responses in humans (39,40). Until recently, there have been limited robust assays that allow comprehensive immune profiling in humans. Prior standards set by the Human Immunophenotyping Consortium relied on the use of 5 markers to define human B-cell subtypes (65). However, these still lack the capacity to identify the human equivalent of murine IgM-producing B1 cells, which seems particularly relevant in CVD as IgM to MDA-LDL (produced by B1 cells) is implicated in reducing inflammation and atherosclerosis. The recent publication of an integrated multiomic single-cell atlas of human B cells provides unprecedented high-dimensional data and identified 12 unique human B-cell clusters (51). It is likely that the human B1 cells are part of the CD45RB+CD27+CD73− memory cluster, but this needs further exploration.
B1 cells
Because of the difficulties with using murine marker strategies to identify the human equivalent of IgM-producing B1 cells, Griffin et al. identified putative human B1 cells by sort-purifying B-cell fractions and testing for 3 fundamental murine B1 cell functions: spontaneous IgM secretion, efficient T-cell stimulations, and tonic intracellular signaling (66). They found that CD20+CD27+CD43+ identified the human B-cell subset that fulfilled these criteria. Although some controversies have surrounded the identification of surface markers that define these cells (67,68), it is clear that cells within this subset produce IgM to modified phospholipids linked to atherosclerosis (66,69) and are inversely associated with CAD (27). Similarly to those in mice, these B1 cells comprise <5% to 10% of circulating B cells. Griffin went on to show that these B1 cells can be further subdivided into orchestrator B1 cells (CD11b+) and secretor B1 cells (CD11b−). Secretor B1 cells secrete large amounts of IgM and orchestrator B1 cells produce both IgM and anti-inflammatory cytokine IL-10 to suppress T-cell activation (70).
In support of a role for human B1 cells in protecting from atherosclerosis, Meeuwsen et al. (71) demonstrated higher numbers of unswitched memory B cells (CD27+CD43+ B1-like) associated with fewer secondary cardiovascular events, defined as any or combination of cardiovascular death, stroke, MI, coronary intervention, and peripheral intervention following carotid endarterectomy. Apart from an association between B1 cell frequency and cardiovascular incidence, recent studies demonstrated that the amount of the chemokine receptor CXCR4 on human CD20+CD27+CD43+ B1 cells was significantly associated with circulating levels of IgM antibodies specific for MDA-LDL (27), suggesting that CXCR4 is a crucial marker for identifying IgM to MDA-LDL–producing B1 cells in humans. In support of this associative finding, gain- and loss-of-function studies in mice demonstrated that CXCR4 expression on B1a cells induced migration to bone marrow and enhanced production of IgM to MDA-LDL (27). Moreover, CXCR4 expression on circulating human CD20+CD27+CD43+ B1 cells inversely correlated with coronary artery plaque burden and necrosis as measured by means of IVUS with virtual histology (23), and female mice lacking B-cell CXCR4 had increased atherosclerosis (72). Confirming the causal link between CXCR4 expression on B1a cells and production of IgM to MDA-LDL via regulation of trafficking to the bone marrow is not possible in humans. However, the common associative data in mice and humans strengthens the likelihood for a common or related mechanism and provides rationale for pursuing strategies that may augment CXCR4 on human B1 cells primed to produce IgM to OSEs.
B2 cells
B2 Cells develop in the bone marrow and travel to secondary lymphoid organs where they can transform into mature naïve B cells in follicular regions or differentiate to become memory B cells or antibody-producing plasma cells. In the early antibody response, plasmablasts are rapidly produced but are short lived (71). In humans, increased plasmablasts are associated with atherosclerosis (73). The plasmablasts then further develop into plasma cells, which secrete much higher levels of antibodies, including high-affinity IgG (74). This IgG, as mentioned above, has been shown to correlate with coronary artery stenosis in some human studies (58,75,76).
Regulatory B cells
Regulatory B cells (Bregs) are a group of B cells that suppress the immune system and control inflammation, often through the secretion of IL-10. Thus far, cell surface markers for murine Bregs have been identified, but markers to identify human Bregs remain unclear (77, 78, 79) Because of their anti-inflammatory nature, it has been hypothesized that IL-10–producing Bregs suppress plaque development (80). Decreased serum levels of IL-10 have long been associated with human CVD (81,82). IL-10 is produced by many cells, and it is unclear whether IL-10 produced specifically by B cells is sufficient to attenuate atherosclerosis, although patients with a history of atherosclerotic events had lower levels of IL-10+ B cells (72).
Clinical Implications
Understanding the roles of B cells in human atherosclerosis is still in its infancy, and the majority of supporting evidence is associative. Nonetheless, these preliminary associations allow us to generate hypotheses to test in mechanistic studies in preclinical models that address key findings in humans. In addition, the advent of high-dimensional immune-subtyping technologies like CyTOF and RNAseq have strengthened our armamentarium of tools to identify human B-cell subtypes. Moreover, the expansion of immunotherapies that target specific B-cell surface proteins for cancer, autoimmune, and other disease (83, 84, 85, 86, 87, 88, 89, 90) provides important clinical opportunities to assess the effect of these agents on specific B-cell subtypes, production of Igs and cytokines linked to CVD, and even CVD end points themselves. The following sections review potential B-cell–based therapeutic possibilities for human CVD and potential opportunities to learn more about mechanisms whereby B cells may regulate atherosclerosis in humans through exploring CVD end points in individuals getting B-cell–targeted therapies for other disorders. We discuss potential CVD therapies with evidence in murine models, such as use of immunization, biologics that lead to B-cell depletion or reduced survival, and targeted T-cell costimulatory and immune checkpoint therapies, and highlight the need for evaluation of CVD end points in clinical trials and clinical care using these agents.
Immunization
In addition to murine models of atherosclerosis, OxLDL has been shown to be present in the arterial wall in larger animal models of hypercholesteremia and in humans (91, 92, 93). Given the protective role of IgM to OSEs in blocking OxLDL uptake by macrophages to produce foam cells or stimulating downstream inflammatory pathways, it is appealing to hypothesize that immunization to induce antibodies to OxLDL would inhibit atherosclerosis formation. With immunization as a preventive approach for atherosclerosis, patients could receive an injection of immunogen that could potentially lead to an expansion of B cells and production of IgM specific to the injected immunogen (Figure 2). The binding of IgM on the OxLDL adduct then further blocks OxLDL from being taken up by macrophages, resulting in reduction of plaque formation. Although immunization of various forms of OxLDL (e.g., MDA-LDL, malondialdehyde-acetaldehyde adduct) were shown to be atheroprotective in murine and rabbit models (75), specific OxLDL forms to be used as immunogens in humans remain to be determined. This highlights the need to better develop approaches to characterize modified LDL moieties and other antigens in human plaques to determine the relevant antigens/immunogens to advance immunization as a potential therapy.
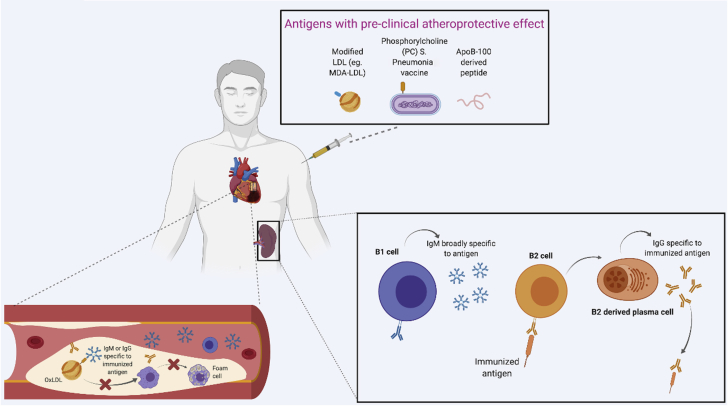
Figure 2.
Immunization
Various types of antigens (eg, MDA-LDL, PC, ApoB-100 peptide) were shown to have an atheroprotective effect in pre-clinical models. Whether any of these antigens work and how they work in human are still unanswered questions. A potential mechanism underlining atheroprotective effect of the immunization includes stimulation of antigen specific immunoglobulin (IgM or IgG) production. These immunoglobulins can potentially bind to oxidized LDL to prevent formation of foam cells leading to a plaque formation. However, detailed characterization of B cells responsible for immunoglobulin production to limit atherosclerosis is still needed. ApoB = apoliprotein B; MDA-LDL = malondialdehyde modified low density lipoprotein; OxLDL = oxidized low density lipoprotein; other abbreviations as in Figure 1.
Streptococcus pneumoniae immunization
B cells in ApoE−/− murine models have been shown to generate IgM that binds the phosphorylcholine (PC) moiety found on OxLDL but not native LDL (94,95). These anti-PC antibodies were identified to be identical to the T15 idiotype natural IgM that is produced by B1 cells (96,97) and known to bind PC moieties of polysaccharides on the cell wall of Streptococcus pneumoniae and confer protection against pneumococcal infection (98). Notably, patients with a recent diagnosis of pneumococcal pneumonia were found to have increased IgM specific to OxLDL, suggesting that immunization for S. pneumoniae may attenuate atherosclerosis. Indeed, immunization of LDLR–/– mice with S. pneumoniae resulted in expansion of splenic B cells that produced anti-PC IgM that recognized the PC moiety found on both the bacterial cell wall and OxLDL and reduced atherosclerosis in the mice (96,99). There is evidence in humans that immunization of toddlers with a 9-valent pneumococcal conjugate vaccine resulted in higher IgM titers than IgG for some serotypes and persistence of functionally active IgM for a least a year (99). These data and a wealth of other work raise the interesting question of whether human pneumococcal vaccination might prevent cardiovascular events. Many observational studies of S. pneumoniae vaccine in human subjects have analyzed cardiovascular end points with conflicting and confounded findings (100, 101, 102, 103), underscoring the need for a randomized controlled clinical trial. The AUSPICE (Australian Study for the Prevention Through Immunization of Cardiovascular Events) randomized 4,725 individuals age 55 to 60 years with no self-reported prior MI or stroke to receive either pneumococcal vaccine or placebo (104). Results are pending, so whether the PC epitope present on the cell wall of S. pneumoniae and on OxLDL is the right immunogen for immunization in humans have yet to be clearly shown.
Immunization with other modified lipids or lipid components
In addition to animal studies supporting pneumococcal immunization to attenuate atherosclerosis, other immunogens have been considered (60,63,69,104, 105, 106, 107, 108, 109). Early studies showed that immunization with homologous MDA-LDL inhibited the progression of atherogenesis in mice and Watanabe heritable hyperlipidemic rabbits (108,109). Notably, IgM to MDA-LDL has been inversely associated with atherosclerosis as measured by coronary angiography, coronary artery calcium, and events in humans (58, 59, 60,110). Yet, although immunization with MDA-LDL led to atheroprotection in experimental models, use of MDA-LDL as an immunogen may be impractical because when MDA is used to modify LDL, a wide variety of related MDA adducts are formed, not all of which may be atheroprotective. Malondialdehyde-acetaldehyde–type adducts have emerged as important immunodominant epitopes that lead to atheroprotective responses (111) and thus may hold promise as a potential immunogen to develop a vaccine approach to retard or prevent atherosclerosis. In addition, there are data to support specific apoB100 peptides as effective immunogens (60,62,107). Clearly, more work needs to be done in both humans and other animals to define the relevant antigens in atherosclerotic plaques to develop potential strategies to induce a humoral response to limit the impact of these neoantigens on atherosclerosis formation.
B-Cell Therapies
B-cell–targeted therapies are available and in clinical use for a variety of disease states, including B-cell leukemias, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple sclerosis, and multiple myeloma. Although B-cell–targeted therapies are not currently used for atherosclerosis, given the compelling data for subset-specific effects of B cells on atherosclerosis in preclinical models described above and the consistent association with B-cell and B-cell–derived products in human atherosclerosis, it is interesting to hypothesize that they may affect human atherosclerosis. Not only may they have potential therapeutic impact on atherosclerosis but it is also important to consider potential effects of these B-cell–targeted agents on atherosclerosis in patients treated with these agents for other disease conditions. It would clearly be prudent for patients receiving B-cell–targeted therapies to be followed for both short- and long-term cardiovascular effects. We discuss the potential atherosclerotic effects of B-cell therapies that are currently available, undergoing clinical trials, or currently in preclinical studies.
B-cell receptor modulation and activation
Antigen engagement with the BCR directs the cell to activate and differentiate into an antibody-generating plasma cell. Binding of antigen to the BCR can also induce a complex signaling cascade that increases intracellular Ca2+ and results in transcription of genes that promote release of chemokines such as CCL3 and CCL4. CCL3 and CCL4 then enhance infiltration of the local tissue by monocytes and T cells (112, 113, 114). Several therapies have been developed to target or modulate BCR activation (Figure 3). Inhibition of the BCR signaling pathway has been effective in treating cancers such as chronic lymphocytic leukemia (114, 115, 116). Ibrutinib and acalabrutinib inhibit Bruton tyrosine kinase, a key step in the cascade, thus decreasing release of chemokines (117,118). Acalabrutinib has been used for the treatment of relapsed or refractory mantle cell lymphoma (83) Similarly, epratuzumab is an agonist of CD22, which when activated results in inhibition of the downstream Ca2+ influx in the BCR activation cascade (119). It is interesting to hypothesize that use of BCR signaling inhibitors may be atheroprotective due to diminished proinflammatory chemokine release, but to date reported cardiovascular consequences of these agents have been largely an increase in atrial fibrillation (120).
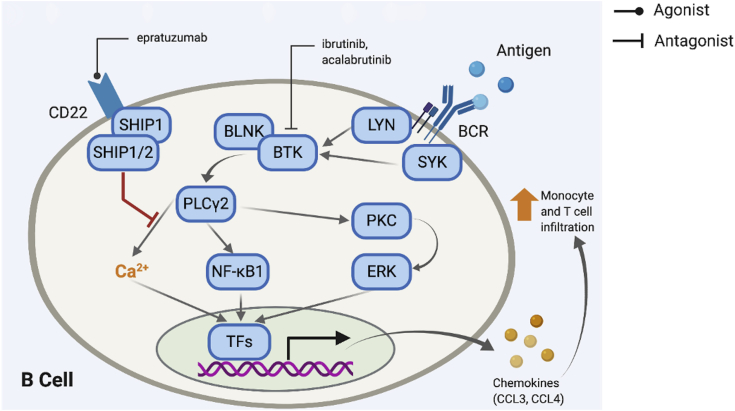
Figure 3.
B-Cell Therapies That Target BCR Modulation and Activation
Binding of Ag to the B cell receptor (BCR) induces a signaling cascade that increases intracellular Ca2+ and also results in transcription of genes that increase chemokine release. Ibrutinib and acalabrutinib inhibit BTK, a key step in the cascade, thus possibly decreasing release of chemokines such as CCL3 and CCL4, which enhances tissue infiltration by monocytes and T cells. Similarly, epratuzumab is an agonist of CD22, which, when activated, results in inhibition of the BCR activation cascade. BLNK = B cell linker protein; BTK - Bruton tyrosine kinase; ERK = extracellular signal-regulated kinase; NF-kB1 = nuclear factor kappa Beta subunit 1; PKC = protein kinase C.
The role of BCR signaling in determining B-cell fate and the relative proportion of different B-cell subsets adds an additional layer of complexity to development of therapies targeting the BCR pathways (121). In general, loss of inhibition of the BCR pathway skews B-cell development toward the B1a or B1 cell fate in murine and human cells, respectively (122,123). Even binding self-antigen by IgM, which prevents binding of these antigens to BCR, can influence B-cell development (124). Careful assessment not only for atrial fibrillation and any attendant increase in bleeding risk with needed anticoagulation therapy to reduce stroke risk, in addition to longer-term impacts related to atherosclerosis, need to be considered.
B-cell and plasma cell depletion
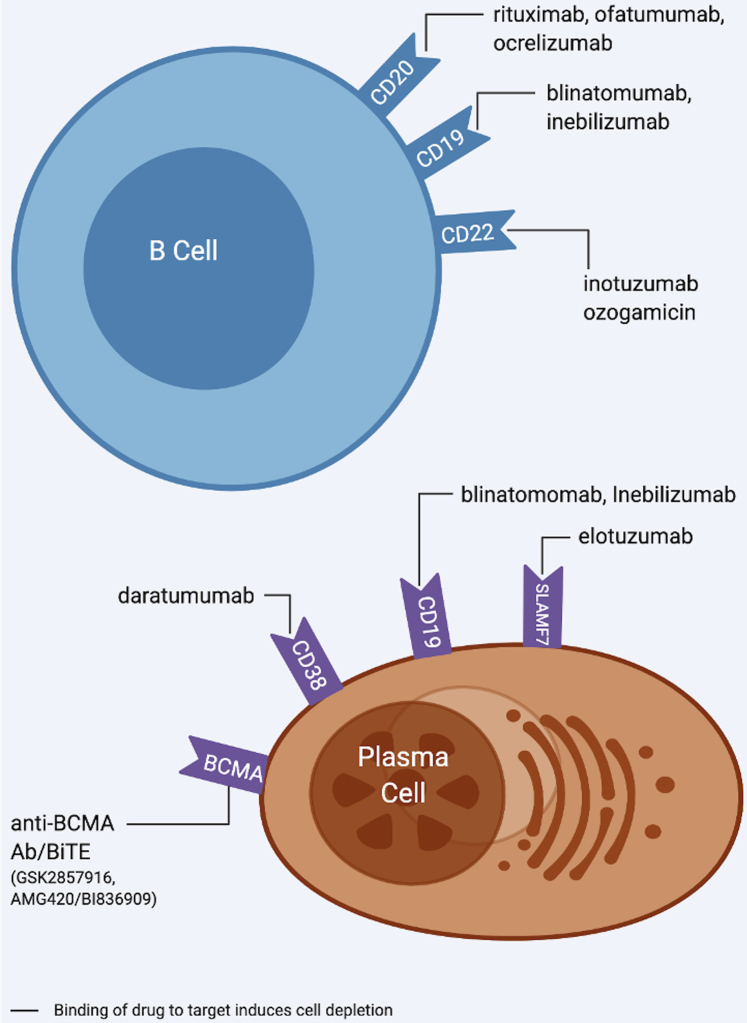
Therapies that deplete B cells and plasma cells target various surface receptors, triggering cell death via apoptosis and/or lysis. The following are B and plasma cell depletion drugs and their targets: rituximab-CD20, ofatumumab-CD20, ocrelizumab-CD20, blinatomumab-CD19, inebilizumab-CD19, inotuzumab-CD22, ozogamicin-CD22, elotuzumab-SLAMF7, daratumumab-CD38, GSK2857916-BCMA, and AMG420/BI836909-BCMA (125,126) (Figure 4). Likely, the impact of these drugs on atherosclerosis would be influenced by the B-cell subset that is most affected.
Figure 4.
B-Cell and Plasma Cell Depletion Therapies
These therapies target surface receptors that are pan markers of B cells (CD20, CD19, CD22) and plasma cells (CD38, CD19, SLAMF7, BCMA). The impact of these therapies on atherosclerosis is unknown but maybe governed by the B cell subtypes/plasma cells that are depleted.
Murine studies using a CD20-specific monoclonal antibody resulted in a preferential depletion of B2 cells with loss of IgG and less diet-induced atherosclerosis (21,24). Although both B1 and B2 cells express CD20, murine studies demonstrated that CD20 mAb treatment depleted 95% to 98% of B cells in the bone marrow, blood, spleen, lymph nodes, and gut-associated lymphoid tissues, but only 30% to 43% of B1 cells and 43% to 78% of B2 cells in the peritoneal cavity. The peritoneal cavity is the major reservoir of B1 cells and appears to protect from anti-CD20 depletion, which could account for the preferential depletion of B2 cells (126). Given that B2 cells are involved in proinflammatory processes that promote atherosclerosis, it would be reasonable to hypothesize that anti-CD20 mAb therapy would be atheroprotective. Indeed, RA patients taking rituximab demonstrate decreased inflammation, improved endothelial function, and decreased carotid intima-media thickness (cIMT) (86,127). Data from clinical trials of rituximab for autoimmune diseases, however, do not support differences in major adverse cardiovascular events (128).
Although data suggest that drugs that target CD20 largely affect B2 cells and not B1 cells, therapies targeting CD19 and CD22 can affect both B-cell subsets. These drugs are used for treatment of acute B-cell lymphoblastic leukemia (129,130). While these drugs may affect processes that both aggravate and attenuate atherosclerosis, to our knowledge there are no data as to whether they affect atherosclerosis in humans. CD19 is also found on the cell surface of plasma cells. In addition, other therapies that target plasma cells (elotuzumab-SLAMF7, daratumumab-CD38, GSK2857916-BCMA, AMG420/BI836909-BCMA) have been used to reduce clonal plasma cells in the bone marrow due to multiple myeloma (85,86). These therapies also have potential to affect plasma cells involved in producing antibodies that could modulate atherosclerosis and have been associated with vascular thrombosis in humans (88). Yet, the role of plasma cells in murine atherosclerosis is controversial. Depletion of plasma cells in mice has been shown to attenuate atherosclerotic lesion size (89) and at the same time increasing plaque instability (89). In contrast, in a murine model of deficient antibody production, there were increased atherosclerotic lesions and necrotic cores (131). As such, it will be important to study the effects of these agents on antibody isotypes that target OSEs and monitor both short- and long-term follow-up for coronary events such as MI.
B-cell survival
B-cell survival is dependent on several factors, including BAFF and A proliferation-inducing ligand (APRIL) binding to cell surface receptors. BAFF binds BAFFR, transmembrane activator and CAML interactor (TACI), and B-cell maturation antigen (BCMA), while APRIL binds TACI and BCMA. Binding of these ligands in conjunction with antigen to the BCR induces an intracellular signaling cascade resulting in activation of nuclear factor κB and increased B-cell survival (132) (Figure 5).
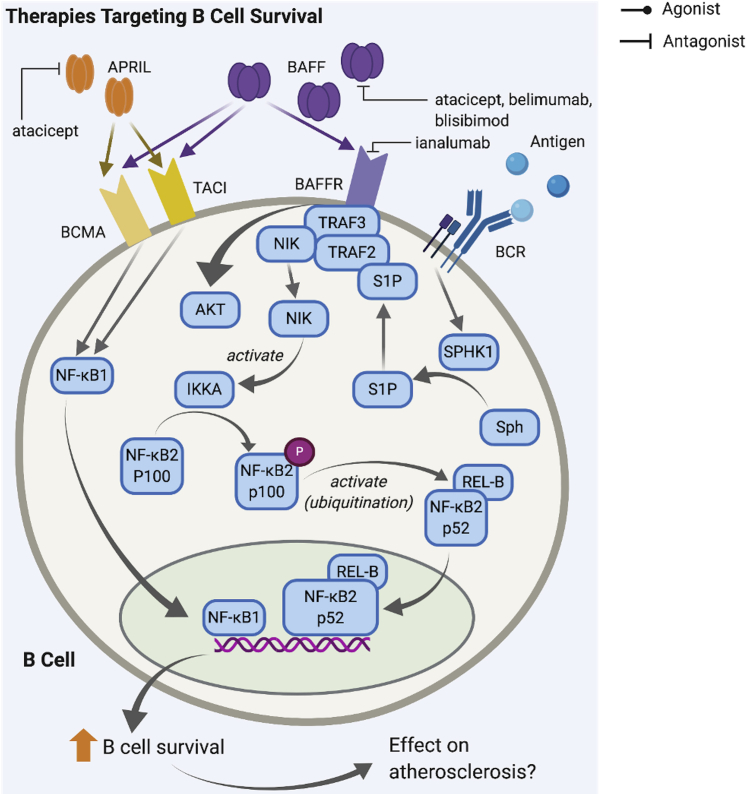
Figure 5.
B-Cell Therapies That Target B-Cell Survival Inhibit Interaction of APRIL and BAFF With TACI, BCMA, and BAFFR on B Cells
Binding of APRIL and BAFF ligands activates an intracellular signaling cascade via NF-κB that results in transcription of genes that enhance B cell survival. Atacicept inhibits binding of APRIL on BCMA and TACI and binding of BAFF on BAFFR, while belimumab, blisibimod and ianalumab target only binding between BAFF and BAFFR. APRIL = A proliferation-inducing ligand; BAFF = B-cell activating factor; BAFFR = B-cell activating factor receptor; BCMA = B-cell maturation antigen; BCR = B cell receptor; TACI = transmembrane activator and CAML interactor; other abbreviations as in Figure 2.
The impact of APRIL- and BAFF-targeting therapies on development of atherosclerosis continues to be a subject of debate. A major effect of blocking BAFF-BAFFR interaction in mice is reduced B2-cell survival. BAFFR-deficient LDLR−/− mice were found to have decreased B2 cells with the levels of B1a cells and IgM remaining unaffected (20). Similar findings were reported in BAFFR-deficient ApoE−/− mice and ApoE−/−mice treated with anti-BAFFR mAb (22). Those studies showed reduced atherosclerosis lesion formation and thus therapeutic value in targeting BAFF-BAFFR. However, other studies have indicated a possible atheroprotective role of BAFF as well. B-cell cultures treated with BAFF had greater transcription factor activator protein 1 activation and more IL-10–producing B cells (133). A possible mechanism underlying the anti-inflammatory effect is the expansion of regulatory T cells (Tregs) in response to BAFFR activation (134). Overexpression of BAFF also increased anti-OxLDL IgM production and significantly attenuated atherosclerosis in hyperlipidemic mice (135). Thus, further elucidation of the B-cell subsets and processes that are affected by drugs targeting APRIL and BAFF is needed.
Therapies targeting BAFF/BAFFR and APRIL are being used in the clinical setting mainly as therapy for treating graft-versus-host disease and autoimmune conditions, including RA, SLE, and myasthenia gravis (136, 137, 138). Belimumab (anti-BAFF mAb) was approved for treatment of SLE (139). Other drugs, including blisibimod (anti-BAFF mAb) (140), ianalumab (anti-BAFFR mAb) (141), and atacicept (anti-BAFF and anti-APRIL mAb) (142), also targeting the same pathways, are in clinical trials and no impact on cardiovascular events have been reported to date. These drugs could potentially be repurposed as atherosclerosis therapy once the roles of BAFF/APRIL in atherosclerosis are better clarified.
Immune checkpoint inhibitors
Although immune checkpoint inhibitors (ICIs) have drastically altered the landscape of oncology therapy by interfering with cancer cell–T-cell interactions and overcoming tumor tolerance, recent evidence suggests that B cells have a potential role in response to these therapies (143). In addition, use of ICIs is associated with cardiotoxicity (e.g., myocarditis, acute MI, and vasculitis) (144,145) as well as increasing evidence pointing to a link between ICI use and atherosclerosis-driven cardiovascular events (146). The mechanism underlying these cardiotoxic/atherosclerotic effects may be an inflammatory process resulting from use of ICIs, although much more work remains to be done to fully understand their impact on atherosclerosis (147,148). The cardiovascular effects of ICI therapies and drugs targeting T-cell costimulation have recently been reviewed in detail by Simons et al. (149), but highlights as they related to B cells will be briefly described below.
ICIs target the interaction of immune checkpoint receptors on T cells with their ligands on antigen-presenting cells such as B cells (Figure 6). programmed cell death protein 1 (PD-1) on T cells binds PD ligand (PDL) 1 or 2 on B cells. Similarly, cytotoxic T-lymphocyte–associated protein 4 (CTLA4) on T cells binds CD80 and CD86 on B cells. In most cases, PD1-PDL1/L2 and CTLA4-CD80/86 interactions inhibit T-cell receptor–major histocompatibility complex class II molecules and CD28-CD80/86 activation of T cells, resulting in increased T-cell activity and proliferation. CD80/CD86 on B cells likely has a predominantly atherogenic effect, as CD86 promotes the induction of TH1 immunity (150). In addition, numbers of CD86+ B cells also correlate with increased stenosis and incidence of stroke in humans (151).
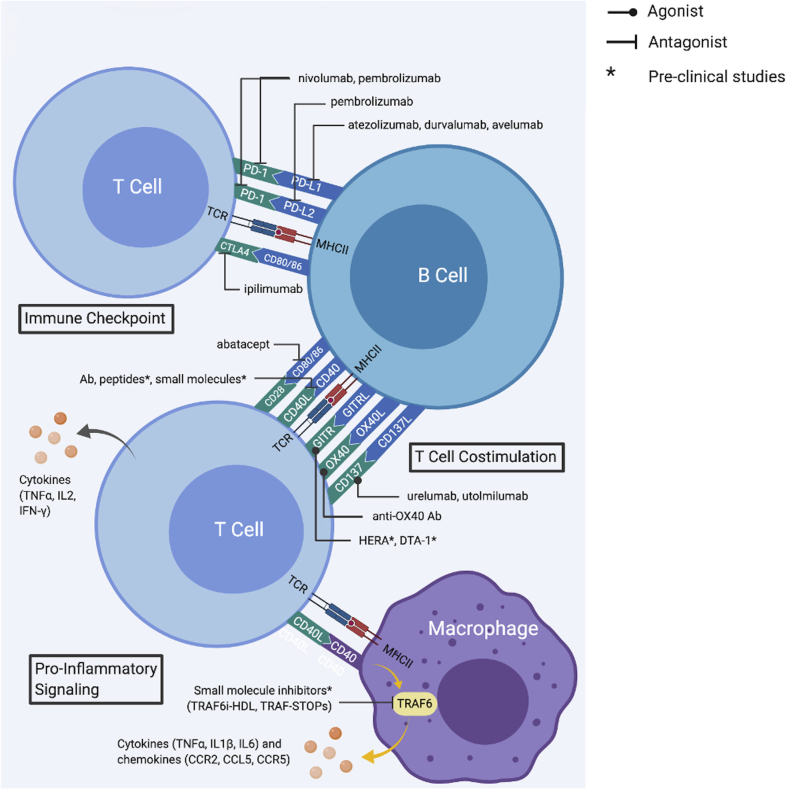
Figure 6.
Therapies Targeting Interactions of Immune Checkpoint Receptors to Disrupt T-Cell Activation Through T- and B-Cell Communication
This family of therapies can be divided into immune checkpoint inhibitors (ICIs) and T cell co-stimulation classes. ICIs mainly target interactions between PD-1 and PD-L1/L2 or CTLA4 and CD80/86, which generally inhibits T cell activation and proliferation caused by TCR and MHCII interaction. Therefore, ICIs increase T cell activation which could result in atherogenic effects. T cell costimulation serves to promote T cell response to foreign antigens and to limit undesired responses to self-antigens. This class of drugs mainly targets CD28-CD80/86, CD40L/CD40, GITR/GITRL, OX40/OX40L, and CD137/CD137L interactions. IL2 = interleukin-2; MHCII =major histocompatibility complex class II molecules; TNFα = tumor necrosis factor- alpha; other abbreviation as in Figure 2.
PDL1/2 on B cells as well as other antigen-presenting cells mainly have atheroprotective effects. Deletion of PDL1/2 in LDLR deficient (PD-L1/2−/−LDLR−/−) revealed larger lesions with increased numbers of lesional CD4 and CD8 T cells and elevated serum levels of TNFα (152). While the role of the PD-1/PDL1 interaction has been well-studied, the PD-1/PDL2 interaction is less understood but may play a vital role in atherosclerosis. In murine studies, 50% to 70% of peritoneal atheroprotective B1a cells expressed PDL2. This subpopulation of B1a cells are highly enriched for VH11/VH12 repertoire, which has capability in binding phosphatidylcholine moiety on OxLDL (153). Perhaps these cells represent a subpopulation of B1a cells that play a greater role in atheroprotection. Notably, advanced coronary artery imaging with fluorodeoxyglucose positron-emission tomography revealed significant inflammatory activity in subjects receiving ICI [CTLA-4 and/or PD-1 inhibitors (154)], underscoring the important need for vigilant consideration of the risk for vascular inflammation.
Examples of drugs and their targets include nivolumab (inhibits PD-1), pembrolizumab (inhibits PD-1 and PDL2), atezolizumab (inhibits PD-L1), durvalumab (inhibits PDL1), avelumab (inhibits PDL1), and ipilimumab (inhibits CTLA4) (155, 156, 157, 158).
T-cell costimulation
T-cell costimulation serves to promote T-cell response to foreign antigens and to limit undesired responses to self-antigens through binding of costimulatory molecules expressed on antigen-presenting cells. Two major families of these costimulatory molecules include the B7 and TNF families (159). B7-family molecules CD80 and CD86 are expressed on B cells to activate CD28 receptors on T cells. CD80/86−/−LDLR−/− mice have reduced diet-induced atherosclerosis lesion formation and less proinflammatory IFN-γ cytokine production by T cells (160). TNF-family molecules CD40, CD137L (41BBL), OX40L, and glucocorticoid-induced TNF receptor–related protein ligand (GITRL) are also found on B cells. Apart from performing as a costimulatory molecule, CD40 can also up-regulate CD80/CD86 expression (156,161). Using an anti-CD40L antibody or knocking out CD40L resulted in decreased lesion size in some mouse studies and unchanged lesion size in other studies; in addition, some studies report slower atherosclerotic progression with blocking CD40L (162, 163, 164). Clinical trials of ruplizumab, an antibody targeting the CD40-CD40L interaction, showed promising results but was halted because of complications related to thrombosis (165). Currently, in addition to antibodies, inhibitory peptides and small molecules are undergoing preclinical study (157,165).
CD137L binds to CD137 on T cells, especially CD8 T cells, to control T-cell activation. ApoE−/− mice treated with anti-CD137 agonist have increased levels of aortic plaque development, inflammatory cytokines in the lesions, and CD8 infiltration (158). Urelumab and utolmilumab, anti-CD137 agonist antibodies, are currently in clinical trials. The agonistic activity of these drugs likely causes higher CD8 infiltration and potentially increased atherosclerosis (166). Ox40L on B cells is not constitutively expressed, but can be induced through CD28/CD80–86, CD40-CD40L interactions, or cytokine IL-18 (157).
OX40L binds to OX40 expressed on activated CD4 and CD8 T cells and Tregs. Interaction between OX40/OX40L leads to prolongation of T-cell response and increased T-cell proliferation and survival, thus promoting atherosclerosis. OX40L−/− mice have reduced atherosclerosis plaque, whereas mice with overexpression of OX40L have increased plaque volume (167,168). Agonistic anti-OX40 antibodies were previously found to have antitumor effects and are currently undergoing clinical trials; these antibodies have the potential to have atherogenic effects due to increased immune response activation (169).
GITRL on B cells mostly binds to GITR on CD4 effector memory T cells and Tregs to promote effector function and proliferation/survival of these T cells (170). Transplantation of bone marrow from B-cell–restricted overexpression of GITRL transgenic mice to LDLR−/− mice shows reduced diet-induced aortic atherosclerosis plaque, potentially through balancing regulation CD4 effector memory T cells and Tregs (171). Several agonists of GITR are being studied in the context of cancer therapy but may also have an effect on atherosclerosis (170, 171, 172, 173). Because of its effect on both CD4 T cells and Tregs, the overall impact of GITR activation on the level of inflammation is unknown. However, constitutive activation of B-cell GITR in mice showed an overall atheroprotective effect and decreased atherosclerosis severity (171).
Conclusions
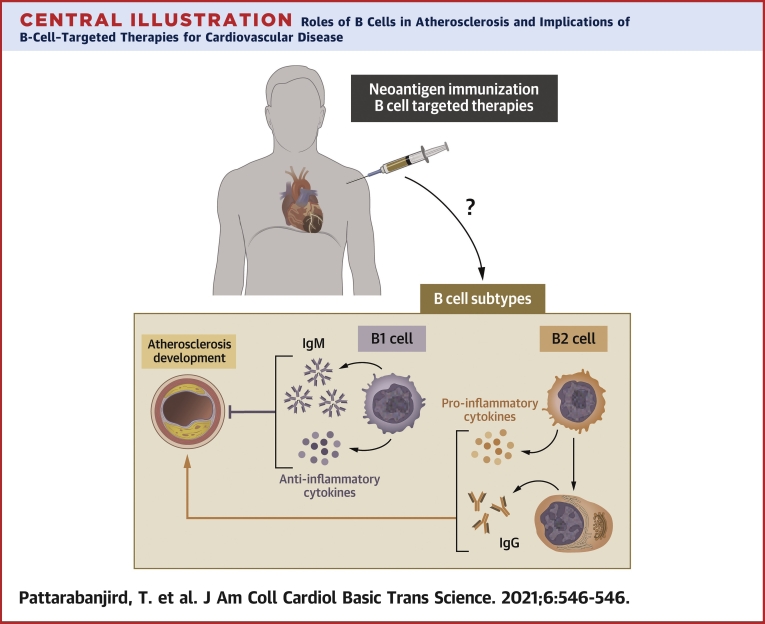
In summary, B cells have been clearly shown to be important regulators of diet-induced atherosclerosis in mice. Accumulating evidence in humans suggests a key role for B-cell–derived Igs and cytokines in human atherosclerotic disease(Central Illustration). Despite a plethora of new biological therapeutic agents that target B cells, however, clinical trials in cardiovascular medicine are limited. There is opportunity to learn about cardiovascular impact of some of these agents through careful assessment of short- and long-term indexes of subclinical and clinical cardiovascular events in trials of these agents for other indications or even in post-approval assessment. This is not only important for advancing our knowledge about potential use of B-cell–targeted agents and ICIs for cardiovascular disease, but also for a better understanding of the cardiovascular benefits or adverse consequences of using these agents for cancer or autoimmunity.
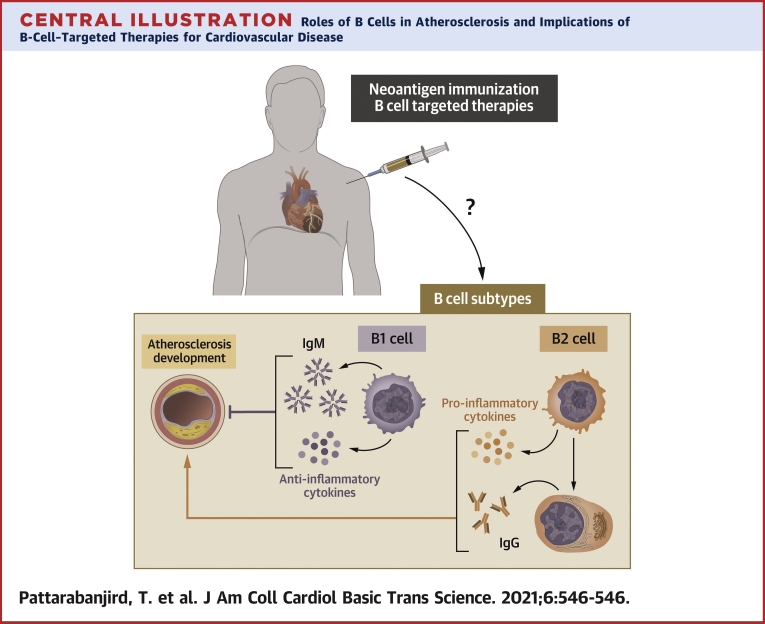
Central Illustration.
Roles of B Cells in Atherosclerosis and Implications of B-Cell–Targeted Therapies for Cardiovascular Disease
IgG = immunoglobulin G; IgM = immunoglobulin M.
In addition to directly targeting B cells, vaccination to stimulate B cells to produce atheroprotective antibodies is a very appealing approach owing to the low cost of vaccines. However, much more work in humans needs to be done to understand the relevant antigens and ways to promote production of specific isotypes that would have protective rather than proatherogenic activity. Several key questions remain unanswered. What human B cell produces IgM to MDA-LDL or other relevant antigens? Can that cell be modulated to enhance production? Would there by cross-reactivity with other self-antigens? Given the promise of potential benefit in humans based on preclinical mechanistic studies and the wealth of compelling human associative studies, we hope this review serves to accelerate research in this important yet understudied area.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Sumanth Prabhu, MD, served as Guest Editor for this paper. Michael R. Bristow, MD, PhD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Arnett D.K., Blumenthal R.S., Albert M.A. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani S.S., Alonso A., Benjamin E.J. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 3.Ross R., Glomset J.A. The pathogenesis of atherosclerosis. N Engl J Med. 1976;295:369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 4.Gerlis L.M. The significance of adventitial infiltrations in coronary atherosclerosis. Br Heart J. 1956;18:166–172. doi: 10.1136/hrt.18.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz C.J., Mitchell J.R. Cellular infiltration of the human arterial adventitia associated with atheromatous plaques. Circulation. 1962;26:73–78. doi: 10.1161/01.cir.26.1.73. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 7.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby P., Loscalzo J., Ridker P.M. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. 2018;72:2071–2081. doi: 10.1016/j.jacc.2018.08.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 10.Libby P., Lichtman A.H., Hansson G.K. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabas I., Lichtman A.H. Monocyte-macrophages and T cells in atherosclerosis. Immunity. 2017;47:621–634. doi: 10.1016/j.immuni.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saigusa R., Winkels H., Ley K. T Cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17:387–401. doi: 10.1038/s41569-020-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore K.J., Koplev S., Fisher E.A. Macrophage trafficking, inflammatory resolution, and genomics in atherosclerosis: JACC macrophage in CVD series (part 2) J Am Coll Cardiol. 2018;72:2181–2197. doi: 10.1016/j.jacc.2018.08.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srikakulapu P., Hu D., Yin C. Artery tertiary lymphoid organs control multilayered territorialized atherosclerosis B-cell responses in aged mice. Arterioscler Thromb Vasc Biol. 2016;36:1174–1185. doi: 10.1161/ATVBAHA.115.306983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg D., Witztum J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2020;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 16.Doran A.C., Yurdagul A., Jr., Tabas I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20:254–267. doi: 10.1038/s41577-019-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zernecke A., Winkels H., Cochain C. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circ Res. 2020;127:402–426. doi: 10.1161/CIRCRESAHA.120.316903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez D.M., Rahman A.H., Fernandez N. Immune profiling of atherosclerotic plaques identifies innate and adaptive dysregulations associated with ischemic cerebrovascular events. bioRxiv. 2019:721688. [Google Scholar]
- 19.Sage A.P., Nus M., Baker L.L., Finigan A.J., Masters L.M., Mallat Z. Regulatory B cell–specific interleukin-10 is dispensable for atherosclerosis development in mice. Arterioscler Thromb Vasc Biol. 2015;35:1770–1773. doi: 10.1161/ATVBAHA.115.305568. [DOI] [PubMed] [Google Scholar]
- 20.Kyaw T., Tay C., Krishnamurthi S. B1a B Lymphocytes are atheroprotective by secreting natural IgM that increases IgM Deposits and reduces necrotic cores in atherosclerotic lesions—novelty and significance. Circ Res. 2011;109:830–840. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 21.Ait-Oufella H., Herbin O., Bouaziz J.-D. B Cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyaw T., Tay C., Hosseini H. Depletion of B2 but not B1a B cells in BAFF receptor–deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sage A.P., Tsiantoulas D., Baker L. BAFF receptor deficiency reduces the development of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:1573–1576. doi: 10.1161/ATVBAHA.111.244731. [DOI] [PubMed] [Google Scholar]
- 24.Kyaw T., Tay C., Khan A. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185:4410–4419. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 25.Cherepanova O.A., Srikakulapu P., Greene E.S. Novel autoimmune IgM antibody attenuates atherosclerosis in IgM deficient low-fat diet-fed, but not western diet-fed ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2020;40:206–219. doi: 10.1161/ATVBAHA.119.312771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Upadhye A., Sturek J.M., McNamara C.A. B Lymphocyte–mediated protective immunity in atherosclerosis. Arterioscler Thromb Vasc Biol. 2020;40:309–322. doi: 10.1161/ATVBAHA.119.313064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upadhye A., Srikakulapu P., Gonen A. Diversification and CXCR4-dependent establishment of the bone marrow B-1a cell pool governs atheroprotective IgM production linked to human coronary atherosclerosis. Circ Res. 2019;125:e55–e70. doi: 10.1161/CIRCRESAHA.119.315786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upadhye A., Srikakulapu P., McNamara C.A. Cell- and sex-specific role of FcR (FcReceptor) IIb in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:1269–1271. doi: 10.1161/ATVBAHA.119.312916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipinski M.J., Campbell K.A., Duong S.Q. Loss of Id3 Increases VCAM-1 expression, macrophage accumulation and atherogenesis in LDLR−/− mice. Arterioscler Thromb Vasc Biol. 2012;32:2855–2861. doi: 10.1161/ATVBAHA.112.300352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenfeld S.M., Perry H.M., Gonen A. B-1b Cells secrete atheroprotective IgM and attenuate atherosclerosis. Circ Res. 2015;117:e28–e39. doi: 10.1161/CIRCRESAHA.117.306044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doran A.C., Lipinski M.J., Oldham S.N. B-Cell aortic homing and atheroprotection depend on Id3. Circ Res. 2012;110:e1–e12. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper M.D. The early history of B cells. Nat Rev Immunol. 2015;15:191–197. doi: 10.1038/nri3801. [DOI] [PubMed] [Google Scholar]
- 33.Kantor a A.B., Herzenberg L.A. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 34.Alugupalli K.R., Gerstein R.M. Divide and conquer: division of labor by B-1 B cells. Immunity. 2005;23:1–2. doi: 10.1016/j.immuni.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Baumgarth N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Frontiers in immunology. 2016;7:324. doi: 10.3389/fimmu.2016.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy R.R. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Berland R., Wortis H.H. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 38.Ghosn E.E.B., Sadate-Ngatchou P., Yang Y., Herzenberg L.A., Herzenberg L.A. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natil Acad Sci U S A. 2011;108:2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosn E.E.B., Yamamoto R., Hamanaka S. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc Natl Acad Sci. 2012;109:5394–5398. doi: 10.1073/pnas.1121632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanaba K., Bouaziz J.D., Haas K.M., Poe J.C., Fujimoto M., Tedder T.F. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Caligiuri G., Nicoletti A., Poirier B., Hansson G.K. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Major A.S., Fazio S., Linton M.F. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 43.Perry H.M., Bender T.P., McNamara C.A. B Cell subsets in atherosclerosis. Front Immunol. 2012;3:373. doi: 10.3389/fimmu.2012.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srikakulapu P., McNamara C.A. B cells and atherosclerosis. Am J Physiol Heart Circ Physiol. 2017;312:H1060–H1067. doi: 10.1152/ajpheart.00859.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis M.J., Malik T.H., Ehrenstein M.R., Boyle J.J., Botto M., Haskard D.O. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor–deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saad A.F., Virella G., Chassereau C., Boackle R.J., Lopes-Virella M.F. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res. 2006;47:1975–1983. doi: 10.1194/jlr.M600064-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Oksjoki R., Kovanen P.T., Lindstedt K.A., Jansson B., Pentikainen M.O. OxLDL-IgG immune complexes induce survival of human monocytes. Arterioscler Thromb Vasc Biol. 2006;26:576–583. doi: 10.1161/01.ATV.0000201041.14438.8d. [DOI] [PubMed] [Google Scholar]
- 48.Nagarajan S. Anti-OxLDL IgG blocks OxLDL interaction with CD36, but promotes FcγR, CD32A-dependent inflammatory cell adhesion. Immunol Lett. 2007;108:52–61. doi: 10.1016/j.imlet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Lebien T.W., Tedder T.F. B Lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosser E.C., Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Glass D.R., Tsai A.G., Oliveria J.P. An integrated multi-omic single-cell atlas of human B cell identity. Immunity. 2020;53:217–232.e5. doi: 10.1016/j.immuni.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sjögren P., Fredrikson G.N., Samnegard A. High plasma concentrations of autoantibodies against native peptide 210 of apoB-100 are related to less coronary atherosclerosis and lower risk of myocardial infarction. Eur Heart J. 2008;29:2218–2226. doi: 10.1093/eurheartj/ehn336. [DOI] [PubMed] [Google Scholar]
- 53.Albutt T.C. Macmillan; London: 1915. Diseases of the arteries including angina pectoris. [Google Scholar]
- 54.Moos M.P., John N., Grabner R. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E–deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 55.Houtkamp M.A., de Boer O.J., van der Loos C.M., van der Wal A.C., Becker A.E. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol. 2001;193:263–269. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH774>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 56.Tsimikas S., Brilakis E.S., Lennon R.J. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Tsimikas S., Willeit P., Willeit J. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–2229. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 58.Prasad A., Clopton P., Ayers C. Relationship of autoantibodies to MDA-LDL and ApoB-immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler Thromb Vasc Biol. 2017;37:1213–1221. doi: 10.1161/ATVBAHA.117.309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van den Berg V.J., Haskard D.O., Fedorowski A. IgM antimalondialdehyde low density lipoprotein antibody levels indicate coronary heart disease and necrotic core characteristics in the Nordic Diltiazem (NORDIL) study and the Integrated Imaging and Biomarker Study 3 (IBIS-3) EBioMedicine. 2018;36:63–72. doi: 10.1016/j.ebiom.2018.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Björkbacka H., Alm R., Persson M., Hedblad B., Nilsson J., Fredrikson G.N. Low levels of apolipoprotein B-100 autoantibodies are associated with increased risk of coronary events. Arterioscler Thromb Vasc Biol. 2016;36:765–771. doi: 10.1161/ATVBAHA.115.306938. [DOI] [PubMed] [Google Scholar]
- 61.Ravandi A., Boekholdt S.M., Mallat Z. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized low density lipoprotein with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk study. J Lipid Res. 2011;52:1829–1836. doi: 10.1194/jlr.M015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asciutto G., Dias N.V., Edsfeldt A. Low levels of IgG autoantibodies against the apolipoprotein B antigen p210 increases the risk of cardiovascular death after carotid endarterectomy. Atherosclerosis. 2015;239:289–294. doi: 10.1016/j.atherosclerosis.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 63.Huan T., Zhang B., Wang Z. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33:1427–1434. doi: 10.1161/ATVBAHA.112.300112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seok J., Warren H.S., Cuenca A.G. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maecker H.T., McCoy J.P., Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griffin D.O., Holodick N.E., Rothstein T.L. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70−. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Descatoire M., Weill J.-C., Reynaud C.-A., Weller S. A human equivalent of mouse B-1 cells? J Exp Med. 2011;208:2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reynaud C.-A., Weill J.-C. Gene profiling of CD11b+ and CD11b− B1 cell subsets reveals potential cell sorting artifacts. J Exp Med. 2012;209:433–434. doi: 10.1084/jem.20120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engelbertsen D., Vallejo J., Quach T.D. Low levels of IgM antibodies against an advanced glycation endproduct–modified apolipoprotein B100 peptide predict cardiovascular events in nondiabetic subjects. J Immunol. 2015;195:3020–3025. doi: 10.4049/jimmunol.1402869. [DOI] [PubMed] [Google Scholar]
- 70.Griffin D.O., Rothstein T.L. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011;208:2591–2598. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meeuwsen J.A.L., van Duijvenvoorde A., Gohar A. High levels of (un)switched memory B cells are associated with better outcome in patients with advanced atherosclerotic disease. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Döring Y., Jansen Y., Cimen I. B-Cell–specific CXCR4 protects against atherosclerosis development and increases plasma IgM levels. Circ Res. 2020;126:787–788. doi: 10.1161/CIRCRESAHA.119.316142. [DOI] [PubMed] [Google Scholar]
- 73.Nutt S.L., Hodgkin P.D., Tarlinton D.M., Corcoran L.M. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 74.Rincón-Arévalo H., Quintero J.C., Fortich F. Low frequency of IL-10+ B cells in patients with atherosclerosis is related with inflammatory condition. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ye J., Bromage E.S., Kaattari S.L. The strength of B cell interaction with antigen determines the degree of IgM polymerization. J Immunol. 2010;184:844–850. doi: 10.4049/jimmunol.0902364. [DOI] [PubMed] [Google Scholar]
- 76.Yla-Herttuala S., Palinski W., Butler S.W., Picard S., Steinberg D., Witztum J.L. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 77.Tsimikas S., Palinski W., Witztum J.L. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor–deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:95–100. doi: 10.1161/01.atv.21.1.95. [DOI] [PubMed] [Google Scholar]
- 78.Mauri C., Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 79.Mauri C., Menon M. Human regulatory B cells in health and disease: therapeutic potential. J Clin Invest. 2017;127:772–779. doi: 10.1172/JCI85113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peng B., Ming Y., Yang C. Regulatory B cells: the cutting edge of immune tolerance in kidney transplantation. Cell Death Dis. 2018;9:109. doi: 10.1038/s41419-017-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ait-Oufella H., Taleb S., Mallat Z., Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 82.Smith D.A., Irving S.D., Sheldon J., Cole D., Kaski J.C. Serum levels of the antiinflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation. 2001;104:746–749. doi: 10.1161/hc3201.094973. [DOI] [PubMed] [Google Scholar]
- 83.Herman S.E.M., Mustafa R.Z., Gyamfi J.A. Ibrutinib inhibits BCR and NF-κB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123:3286–3295. doi: 10.1182/blood-2014-02-548610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamaguchi Y., Uchida J., Cain D.W. The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. J Immunol. 2005;174:4389–4399. doi: 10.4049/jimmunol.174.7.4389. [DOI] [PubMed] [Google Scholar]
- 85.Kantarjian H., Stein A., Gökbuget N. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kantarjian H.M., DeAngelo D.J., Stelljes M. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375:740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y., Sanchez L., Siegel D.S., Wang M.L. Elotuzumab for the treatment of multiple myeloma. J Hematol Oncol. 2016;9:55. doi: 10.1186/s13045-016-0284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abdallah N., Kumar S.K. Daratumumab in untreated newly diagnosed multiple myeloma. Ther Adv Hematol. 2019;10 doi: 10.1177/2040620719894871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trudel S., Lendvai N., Popat R. Targeting B-cell maturation antigen with GSK2857916 antibody–drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19:1641–1653. doi: 10.1016/S1470-2045(18)30576-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Plummer C., Driessen C., Szabo Z., Mateos M.-V. Management of cardiovascular risk in patients with multiple myeloma. Blood Cancer J. 2019;9:26. doi: 10.1038/s41408-019-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fichtlscherer S., Breuer S., Heeschen C., Dimmeler S., Zeiher A.M. Interleukin-10 serum levels and systemic endothelial vasoreactivity in patients with coronary artery disease. J Am Coll Cardiol. 2004;44:44–49. doi: 10.1016/j.jacc.2004.02.054. [DOI] [PubMed] [Google Scholar]
- 92.Rosenfeld M.E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J.L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arterioscler Thromb Vasc Biol. 1990;10:336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- 93.Senders M.L., Que X., Cho Y.S. PET/MR imaging of malondialdehyde-acetaldehyde epitopes with a human antibody detects clinically relevant atherothrombosis. J Am Coll Cardiol. 2018;71:321–335. doi: 10.1016/j.jacc.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Briley-Saebo K.C., Nguyen T.H., Saeboe A.M. In vivo detection of oxidation-specific epitopes in atherosclerotic lesions using biocompatible manganese molecular magnetic imaging probes. J Am Coll Cardiol. 2011;59:616–626. doi: 10.1016/j.jacc.2011.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palinski W., Horkko S., Miller E. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E–deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horkko S., Binder C.J., Shaw P.X. Immunological responses to oxidized LDL. Free Radic Biol Med. 2000;28:1771–1779. doi: 10.1016/s0891-5849(00)00333-6. [DOI] [PubMed] [Google Scholar]
- 97.Shaw P.X., Horkko S., Chang M.K. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kearney J.F. Immune recognition of OxLDL in atherosclerosis. J Clin Invest. 2000;105:1683–1685. doi: 10.1172/JCI10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Briles D.E., Forman C., Hudak S., Claflin J.L. Antiphosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simell B., Nurkka A., Ekström N., Givon-Lavi N., Käyhty H., Dagan R. Serum IgM antibodies contribute to high levels of opsonophagocytic activities in toddlers immunized with a single dose of the 9-valent pneumococcal conjugate vaccine. Clin Vaccine Immunol. 2012;19:1618–1623. doi: 10.1128/CVI.00248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lamontagne F., Garant M.-P., Carvalho J.-C., Lanthier L., Smieja M., Pilon D. Pneumococcal vaccination and risk of myocardial infarction. CMAJ. 2008;179:773–777. doi: 10.1503/cmaj.070221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tseng H.F., Slezak J.M., Quinn V.P., Sy L.S., van den Eeden S.K., Jacobsen S.J. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. JAMA. 2010;303:1699–1706. doi: 10.1001/jama.2010.529. [DOI] [PubMed] [Google Scholar]
- 103.Siriwardena A.N., Gwini S.M., Coupland C.A.C. Influenza vaccination, pneumococcal vaccination and risk of acute myocardial infarction: matched case–control study. CMAJ. 2010;182:1617–1623. doi: 10.1503/cmaj.091891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eurich D.T., Johnstone J.J., Minhas-Sandhu J.K., Marrie T.J., Majumdar S.R. Pneumococcal vaccination and risk of acute coronary syndromes in patients with pneumonia: population-based cohort study. Heart. 2012;98:1072–1077. doi: 10.1136/heartjnl-2012-301743. [DOI] [PubMed] [Google Scholar]
- 105.Ren S., Hure A., Peel R. Rationale and design of a randomized controlled trial of pneumococcal polysaccharide vaccine for prevention of cardiovascular events: the Australian Study for the Prevention Through Immunization of Cardiovascular Events (AUSPICE) Am Heart J. 2016;177:58–65. doi: 10.1016/j.ahj.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 106.Shah P.K., Chyu K.-Y., Dimayuga P.C., Nilsson J. Vaccine for Atherosclerosis. J Am Coll Cardiol. 2014;64:2779–2791. doi: 10.1016/j.jacc.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 107.Hansson Gr, Nilsson J. Vaccination against atherosclerosis? Induction of atheroprotective immunity. Semin Immunopathol. 2009;31:95–101. doi: 10.1007/s00281-009-0151-x. [DOI] [PubMed] [Google Scholar]
- 108.Fredrikson G.N., Soderberg I., Lindholm M. Inhibition of atherosclerosis in ApoE-null mice by immunization with ApoB-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23:879–884. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- 109.Freigang S., Miller E., Witztum J.L., Palinski W. Immunization of LDL receptor deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol. 1998;18:1972–1982. doi: 10.1161/01.atv.18.12.1972. [DOI] [PubMed] [Google Scholar]
- 110.Palinski W., Miller E., Witztum J.L. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci U S A. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Budoff M.J., Ahmadi N., Gul K.M. Aged garlic extract supplemented with B vitamins, folic acid and l-arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev Med. 2009;49:101–107. doi: 10.1016/j.ypmed.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 112.Gonen A., Hansen L.F., Turner W.W. Atheroprotective immunization with malondialdehyde-modified LDL is hapten specific and dependent on advanced MDA adducts: implications for development of an atheroprotective vaccine. J Lipid Res. 2014;55:2137–2155. doi: 10.1194/jlr.M053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takahashi K., Sivina M., Hoellenriegel J. CCL3 and CCL4 are biomarkers for B cell receptor pathway activation and prognostic serum markers in diffuse large B cell lymphoma. Br J Haemotol. 2015;171:726–735. doi: 10.1111/bjh.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quiroga M.P., Balakrishnan K., Kurtova A.V. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009;114:1029–1037. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ten Hacken E., Burger J.A. Microenvironment interactions and B-cell receptor signaling in Chronic lymphocytic leukemia: implications for disease pathogenesis and treatment. Biochim Biophys Acta. 2016;1863:401–413. doi: 10.1016/j.bbamcr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rai K.R., Jain P. Chronic lymphocytic leukemia (CLL)—then and now. Am J Hematol. 2016;91:330–340. doi: 10.1002/ajh.24282. [DOI] [PubMed] [Google Scholar]
- 117.Woyach J.A., Johnson A.J., Byrd J.C. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120:1175–1184. doi: 10.1182/blood-2012-02-362624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu H., Hu C., Wang A. Ibrutinib selectively targets FLT3-ITD in mutant FLT3-positive AML. Leukemia. 2016;30:754–757. doi: 10.1038/leu.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang M., Rule S., Zinzani P.L. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–667. doi: 10.1016/S0140-6736(17)33108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sieger N., Fleischer S.J., Mei H.E. CD22 ligation inhibits downstream B cell receptor signaling and Ca2+ flux upon activation. Arthrit Rheum. 2013;65:770–779. doi: 10.1002/art.37818. [DOI] [PubMed] [Google Scholar]
- 121.Tang C.P.S., McMullen J., Tam C. Cardiac side effects of bruton tyrosine kinase (BTK) inhibitors. Leuk Lymphoma. 2018;59:1554–1564. doi: 10.1080/10428194.2017.1375110. [DOI] [PubMed] [Google Scholar]
- 122.Graf R., Seagal J., Otipoby K.L. BCR-dependent lineage plasticity in mature B cells. Science. 2019;363:748–753. doi: 10.1126/science.aau8475. [DOI] [PubMed] [Google Scholar]
- 123.Pan C., Baumgarth N., Parnes J.R. CD72-deficient mice reveal nonredundant roles of CD72 in B cell development and activation. Immunity. 1999;11:495–506. doi: 10.1016/s1074-7613(00)80124-7. [DOI] [PubMed] [Google Scholar]
- 124.Pao L.I., Lam K.P., Henderson J.M. B Cell–specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 125.Tsiantoulas D., Kiss M., Bartolini-Gritti B. Secreted IgM deficiency leads to increased BCR signaling that results in abnormal splenic B cell development. Sci Rep. 2017;7:3540. doi: 10.1038/s41598-017-03688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hofmann K., Clauder A.-K., Manz R.A. Targeting B cells and plasma cells in autoimmune diseases. Front Immunol. 2018;9:835. doi: 10.3389/fimmu.2018.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ramos-Casals M., Sanz I., Bosch X., Stone J.H., Khamashta M.A. B-Cell–depleting therapy in systemic lupus erythematosus. Am J Med. 2012;125:327–336. doi: 10.1016/j.amjmed.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Novikova D.S., Popkova T.V., Lukina G.V. The effects of rituximab on lipids, arterial stiffness and carotid intima-media thickness in rheumatoid arthritis. J Korean Med Sci. 2016;31:202–207. doi: 10.3346/jkms.2016.31.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kerekes G., Soltész P., Dér H. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol. 2009;28:705–710. doi: 10.1007/s10067-009-1095-1. [DOI] [PubMed] [Google Scholar]
- 130.Morris-Rosenfeld S., Lipinski M.J., McNamara C.A. Understanding the role of B cells in atherosclerosis: potential clinical implications. Expert Rev Clin Immunol. 2014;10:77–89. doi: 10.1586/1744666X.2014.857602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tay C., Liu Y.H., Kanellakis P. Follicular B cells promote atherosclerosis via T cell–mediated differentiation into plasma cells and secreting pathogenic immunoglobulin G. Arterioscler Thromb Vasc Biol. 2018;38:e71–e84. doi: 10.1161/ATVBAHA.117.310678. [DOI] [PubMed] [Google Scholar]
- 132.Centa M., Jin H., Hofste L. Germinal center–derived antibodies promote atherosclerosis plaque size and stability. Circulation. 2019;139:2466–2482. doi: 10.1161/CIRCULATIONAHA.118.038534. [DOI] [PubMed] [Google Scholar]
- 133.Sage A.P., Nus M., Chakraborty J.B. X-Box binding protein-1 dependent plasma cell responses limit the development of atherosclerosis. Circ Res. 2017;121:270–281. doi: 10.1161/CIRCRESAHA.117.310884. [DOI] [PubMed] [Google Scholar]
- 134.Pieper K., Grimbacher B., Eibel H. B-Cell biology and development. J Allergy Clin Immunol. 2013;131:959–971. doi: 10.1016/j.jaci.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 135.Yang M. Novel function of B cell-activating factor in the induction of IL-10–producing regulatory B cells. J Immunol. 2010;184:3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 136.Walters S., Webster K.E., Sutherland A. Increased CD4 T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol. 2009;182:793–801. doi: 10.4049/jimmunol.182.2.793. [DOI] [PubMed] [Google Scholar]
- 137.Jackson S.W., Scharping N.E., Jacobs H.M., Wang S., Chait A., Rawlings D.J. Cutting edge: BAFF Overexpression reduces atherosclerosis via TACI-dependent B cell activation. J Immunol. 2016;197:4529–4534. doi: 10.4049/jimmunol.1601198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huda R. New approaches to targeting B Cells for myasthenia gravis therapy. Front Immunol. 2020;11:240. doi: 10.3389/fimmu.2020.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee Y.H., Song G.G. Comparative efficacy and safety of intravenous or subcutaneous belimumab in combination with standard therapy in patients with active systemic lupus erythematosus: a bayesian network meta-analysis of randomized controlled trials. Lupus. 2018;27:112–119. doi: 10.1177/0961203317713143. [DOI] [PubMed] [Google Scholar]
- 140.Merrill J.T., Shanahan W.R., Scheinberg M., Kalunian K.C., Wofsy D., Martin R.S. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77:883–889. doi: 10.1136/annrheumdis-2018-213032. [DOI] [PubMed] [Google Scholar]
- 141.Dörner T., Posch M.G., Li Y. Treatment of primary Sjögren’s syndrome with ianalumab (VAY736) targeting B cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann Rheum Dis. 2019;78:641–647. doi: 10.1136/annrheumdis-2018-214720. [DOI] [PubMed] [Google Scholar]
- 142.Merrill J.T., Wallace D.J., Wax S. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol. 2018;70:266–276. doi: 10.1002/art.40360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Helmink B.A., Reddy S.M., Gao J. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johnson D.B., Chandra S., Sosman J.A. Immune Checkpoint Inhibitor Toxicity in 2018. Jama. 2018;320:1702–1703. doi: 10.1001/jama.2018.13995. [DOI] [PubMed] [Google Scholar]
- 145.Michel L., Rassaf T., Totzeck M. Cardiotoxicity from immune checkpoint inhibitors. Int J Cardiol Heart Vasc. 2019;25:100420–100520. doi: 10.1016/j.ijcha.2019.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lutgens E., Seijkens T.T.P. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. Journal for immunotherapy of cancer. 2020;8 doi: 10.1136/jitc-2019-000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rouwet E., Lutgens E. 2016 Jeffrey M. Immune Checkpoints in atherosclerosis: toward immunotherapy for atheroprotection. Arterioscler Thromb Vasc Biol. 2018;38:1678–1688. doi: 10.1161/ATVBAHA.118.307742. [DOI] [PubMed] [Google Scholar]
- 148.Kusters P.J.H., Lutgens E., Seijkens T.T.P. Exploring immune checkpoints as potential therapeutic targets in atherosclerosis. Cardiovasc Res. 2018;114:368–377. doi: 10.1093/cvr/cvx248. [DOI] [PubMed] [Google Scholar]
- 149.Simons K.H., de Jong A., Jukema J.W., de Vries M.R., Arens R., Quax P.H.A. T Cell co-stimulation and co-inhibition in cardiovascular disease: a double-edged sword. Nat Rev Cardiol. 2019;16:325–343. doi: 10.1038/s41569-019-0164-7. [DOI] [PubMed] [Google Scholar]
- 150.Ewing M.M., Karper J.C., Abdul S. T-Cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int J Cardiol. 2013;168:1965–1974. doi: 10.1016/j.ijcard.2012.12.085. [DOI] [PubMed] [Google Scholar]
- 151.Mantani P.T., Ljungcrantz I., Andersson L. Circulating CD40+ and CD86+ B cell subsets demonstrate opposing associations with risk of stroke. Arterioscler Thromb Vasc Biol. 2014;34:211–218. doi: 10.1161/ATVBAHA.113.302667. [DOI] [PubMed] [Google Scholar]
- 152.Gotsman I., Grabie N., Dacosta R., Sukhova G., Sharpe A., Lichtman A.H. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhong X., Tumang J.R., Gao W., Bai C., Rothstein T.L. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for VH11/VH12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 154.Calabretta R., Hoeller C., Pichler V. Immune checkpoint inhibitor therapy induces inflammatory activity in large arteries. Circulation. 2020;142:2396–2398. doi: 10.1161/CIRCULATIONAHA.120.048708. [DOI] [PubMed] [Google Scholar]
- 155.Dine J., Gordon R., Shames Y., Kasler M., Barton-Burke M. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurs. 2017;4:127–135. doi: 10.4103/apjon.apjon_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yun T.J., Chaudhary P.M., Shu G.L. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol. 1998;161:6113–6121. [PubMed] [Google Scholar]
- 157.Croft M., So T., Duan W., Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nozawa K., Ohata J., Sakurai J. Preferential blockade of CD8+ T cell responses by administration of anti–CD137 ligand monoclonal antibody results in differential effect on development of murine acute and chronic graft-versus-host diseases. J Immunol. 2001;167:4981–4986. doi: 10.4049/jimmunol.167.9.4981. [DOI] [PubMed] [Google Scholar]
- 159.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Buono C., Pang H., Uchida Y., Libby P., Sharpe A.H., Lichtman A.H. B7-1/B7-2 costimulation regulates plaque antigen–specific T-cell responses and atherogenesis in low-density lipoprotein receptor–deficient mice. Circulation. 2004;109:2009–2015. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- 161.Sahoo N.C., Rao K.V., Natarajan K. CD80 expression is induced on activated B cells following stimulation by CD86. Scand J Immunol. 2002;55:577–584. doi: 10.1046/j.1365-3083.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 162.Bosmans L.A., Bosch L., Kusters P.J.H., Lutgens E., Seijkens T.T.P. The CD40-CD40L dyad as immunotherapeutic target in cardiovascular disease. J Cardiovasc Transl Res. 2021;14(1):13–22. doi: 10.1007/s12265-020-09994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schönbeck U., Sukhova G.K., Shimizu K., Mach F., Libby P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci U S A. 2000;97:7458–7463. doi: 10.1073/pnas.97.13.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mach F., Schönbeck U., Sukhova G.K. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen J., Song Y., Bojadzic D. Small-molecule inhibitors of the CD40–CD40L costimulatory protein-protein interaction. J Med Chem. 2017;60:8906–8922. doi: 10.1021/acs.jmedchem.7b01154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chu D.-T., Bac N.D., Nguyen K.-H. An update on anti-CD137 antibodies in immunotherapies for cancer. Int J Mol Sci. 2019;20:1822. doi: 10.3390/ijms20081822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Foks A.C., van Puijvelde G.H.M., Bot I. Interruption of the OX40-OX40 ligand pathway in LDL receptor–deficient mice causes regression of atherosclerosis. J Immunol. 2013;191:4573–4580. doi: 10.4049/jimmunol.1200708. [DOI] [PubMed] [Google Scholar]
- 168.Wang X., Ria M., Kelmenson P.M. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat Genet. 2005;37:365–372. doi: 10.1038/ng1524. [DOI] [PubMed] [Google Scholar]
- 169.Deng J., Zhao S., Zhang X. OX40 (CD134) and OX40 ligand, important immune checkpoints in cancer. Onco Targets Ther. 2019:7347–7353. doi: 10.2147/OTT.S214211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Azuma M. Role of the glucocorticoid-induced TNFR-related protein (GITR)–GITR ligand pathway in innate and adaptive immunity. Crit Rev Immunol. 2010;30:547–557. doi: 10.1615/critrevimmunol.v30.i6.40. [DOI] [PubMed] [Google Scholar]
- 171.Meiler S., Smeets E., Winkels H. Constitutive GITR activation reduces atherosclerosis by promoting regulatory CD4+ T-cell responses—brief report. Arterioscler Thromb Vasc Biol. 2016;36:1748–1752. doi: 10.1161/ATVBAHA.116.307354. [DOI] [PubMed] [Google Scholar]
- 172.Richards D.M., Marschall V., Billian-Frey K. HERA-GITRL activates T cells and promotes antitumor efficacy independent of FcγR-binding functionality. J Immunother Cancer. 2019;7:191. doi: 10.1186/s40425-019-0671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nocentini G., Ronchetti S., Petrillo M.G., Riccardi C. Pharmacological modulation of GITRL/GITR system: therapeutic perspectives. Br J Pharmacol. 2012;165:2089–2099. doi: 10.1111/j.1476-5381.2011.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]