Abstract
Functional nanoporous materials are widely explored for CO2 separation, in particular, small-pore aluminosilicate zeolites having a “trapdoor” effect. Such an effect allows the specific adsorbate to push away the sited cations inside the window followed by exclusive admission to the zeolite pores, which is more advantageous for highly selective CO2 separation. Herein, we demonstrated that the protonated organic structure-directing agent in the small-pore silicoaluminophosphate (SAPO) RHO zeolite can be directly exchanged with Na+, K+, or Cs+ and that the Na+ form of SAPO-RHO exhibited unprecedented separation for CO2/CH4, superior to all of the nanoporous materials reported to date. Rietveld refinement revealed that Na+ is sited in the center of the single eight-membered ring (s8r), while K+ and Cs+ are sited in the center of the double 8-rings (d8rs). Theoretical calculations showed that the interaction between Na+ and the s8r in SAPO-RHO was stronger than that in aluminosilicate RHO, giving an enhanced “trapdoor” effect and record high selectivity for CO2 with the separation factor of 2196 for CO2/CH4 (0.02/0.98 bar). The separation factor of Na-SAPO-RHO for CO2/N2 was 196, which was the top level among zeolitic materials. This work opens a new avenue for gas separation by using diverse silicoaluminophosphate zeolites in terms of the cation-tailored “trapdoor” effect.
The sodium form of silicoaluminophosphate RHO zeolite exhibits a pronounced cation-tailored “trapdoor” effect, showing an unprecedented selectivity adsorption separation performance for CO2/CH4 and CO2/N2.
Introduction
The CO2 concentration in the atmosphere has increased rapidly in recent decades due to the dramatic increase of emissions from industries and power plants, which is believed to have significant influence on global warming.1 In CO2 capture and sequestration (CCS), two important issues are the separation of CO2 from post-combustion (CO2/N2) and natural gas mixtures (CO2/CH4).2 Industrially, the separation of CO2/CH4 and CO2/N2 is mainly based on the strong chemical adsorption of amine solutions towards CO2, which has the drawbacks of complicated operation, strong corrosiveness to equipment, high energy consumption for regeneration, and easy deactivation. Hence, the low-cost and high-efficiency capture and separation of CO2 has always been highly desired.2a,3
Compared to chemical adsorption, weak physical adsorption based processes for the separation of CO2/CH4 and CO2/N2 have attracted much attention due to the characteristics of clean, simple operation, and low-energy consumption.4 Over recent years, a variety of solid porous materials have been investigated for the separation of CO2/CH4 and CO2/N2,2a,c,3b,5 including carbon-based materials,6 zeolites,7 metal–organic frameworks (MOFs),8 N or amine-functionalized solid porous materials,9 porous organic solids, etc.10 Taking into account the key factors governing the separation efficiency of CO2 such as adsorption capacity, selectivity, adsorption/desorption kinetics, and cost, zeolites, in particular, the small-pore zeolites with the “trapdoor” effect, have more advantages over other materials for industrial utilization.4a,b
The “trapdoor” effect of zeolites was first observed in small-pore chabazite (CHA) zeolites that can even perform “size-inverse” separation.11 For chabazite structures with a low Si/Al ratio (<3), K+, Rb+, or Cs+ ions fully occupy 8-ring windows that connect the cha cages. Larger CO molecules have a stronger interaction with the cations than smaller N2 molecules, which induces temporary and reversible cation deviation from the window sites and allows for exclusive admission of CO (0.376 nm) instead of N2 (0.364 nm). Such separation also gives a high selectivity of 93 for CO2/CH4 separation over a large pressure range.11
Among the small-pore zeolites, aluminosilicate Rho (RHO) with remarkable structural flexibility was found to have high selectivity for CO2 in the separation of CO2 and CH4.12 The idealized RHO framework (space group: Im3̄m) is constructed by double 8-rings (d8rs) and lta cages as the composite building units (CBUs). Each lta cage connects with six d8rs in six directions in space, while each d8r links two lta cages, generating a three-dimensional (3D) channel system with 8-ring pore openings (0.36 nm × 0.36 nm).13
Previous studies show that the hydrated cation form and the dehydrated proton form of zeolite Rho have the highest symmetry of Im3̄m. The framework of the dehydrated cation form of zeolite Rho undergoes distortions to the non-centrosymmetric I4̄3m.14 During the distortion, the 8-ring geometry is twisted from a circle to an elliptical shape, which reduces the pore aperture and thus adjusts the separation selectivity. Alteration in the type of cations can not only distort the 8-ring geometry but also tune the interaction between the cations and the adsorbates. Notably, the “trapdoor” effect in the dehydrated cation form of zeolite Rho was observed in the selective separation of CO2/CH4.14c,15 Zeolite Rho showed exceptionally high selectivity for CO2 in the separation of CO2/CH4 and the separation factor was as high as 960, which becomes a benchmark set by zeolites in the separation of CO2 from CH4.14c,15a
The SAPO RHO-type, denoted as DNL-6 (hereafter denoted as SAPO-RHO), was first synthesized by Su et al. with diethylamine (DEA) as an organic structure-directing agent (OSDA) in the presence of cetyltrimethylammonium bromide (CTAB) in 2011.16 Recently, a series of commercialized OSDAs were identified with a novel approach called RSS (Refining, Summarizing, and Searching) for the successful synthesis of SAPO-RHO.17 Considering the remarkable CO2 selectivity of the cationic forms of aluminosilicate RHO attributed to its “trapdoor” effect, we suppose that the cationic forms of SAPO-RHO might have a better CO2 selectivity because the framework of silicoaluminophosphate is more flexible than that of aluminosilicate and the “trapdoor” effect in SAPO could be well tailored. However, in general, the introduction of inorganic cations to the SAPO-RHOvia conventional ion-exchange of cations with the protonated SAPO-RHO zeolite will inevitably result in a serious crystallinity loss or even collapse of the framework.7c,18
Herein, we prepared the inorganic cationic form of SAPO-RHO zeolites via direct ion-exchange of protonated OSDA-containing SAPO-RHO with Na+, K+, or Cs+. The Na+ form of SAPO-RHO (denoted as Na-SAPO-RHO) with an optimized cation content showed unprecedented selective separation performance for CO2 from CH4 and N2. The Rietveld refinement and theoretical calculations provided an insight into the intriguing CO2 separation performance arising from the pronounced “trapdoor” effect. Breakthrough experiments suggested that Na-SAPO-RHO is a promising candidate for CO2 capture in biogas purification and flue gas separation via adsorption-based separation processes.
Results and discussion
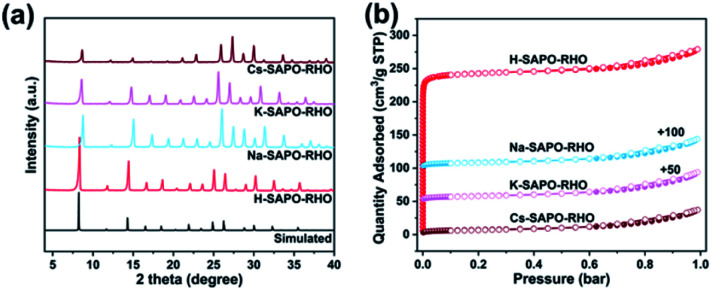
The SAPO-RHO with the Si/(Si + Al + P) mole ratio of 0.18 was hydrothermally synthesized in the presence of DEA, CTAB, and seeds at 473 K for 48 h. The direct ion-exchange (three cycles) of the Na+, K+, or Cs+ salt solution with the as-synthesized SAPO-RHO was performed and the resultant product is denoted as M-SAPO-RHO (M = Na, K, and Cs). The exchange degree for Na+, K+, and Cs+ is 87.13, 65.75, and 72.87%, respectively. The detailed information of the synthesis and ion exchange is provided in the ESI.† The powder X-ray diffraction (PXRD) (Fig. S1†) and the scanning electron microscopy (SEM) analyses (Fig. S2†) of the as-synthesized SAPO-RHO and the ion-exchanged M-SAPO-RHOs show that all the SAPO-RHOs are well-defined crystals with high crystallinity. Fig. 1a shows the PXRD patterns of the H-SAPO-RHO and the calcined M-SAPO-RHOs (873 K in air for 4 h). It is worth noting that the diffraction peaks of the calcined M-SAPO-RHO samples obviously shift to a high angle compared with those of the H-SAPO-RHO and simulated XRD of the idealized RHO framework, indicating the constriction of the unit cell and distortion of the framework. The texture properties of the SAPO-RHOs were characterized by N2 adsorption/desorption at 77 K (Table S1†) and the corresponding isotherms are provided in Fig. 1b. Compared with H-SAPO-RHO, the N2 adsorption of M-SAPO-RHOs was greatly restricted, as observed in zeolite Rho.15a
Fig. 1. (a) Simulated XRD pattern of SAPO-RHOs and experimental ones of the calcined M-SAPO-RHOs; (b) N2 adsorption/desorption isotherms of the calcined SAPO-RHO (H-SAPO-RHO) and M-SAPO-RHOs (M = Na, K, and Cs) at 77 K.
The unit cell compositions of the calcined SAPO-RHOs given in Table S1† were determined based on energy-dispersive spectroscopy (EDS). The EDS mapping (Fig. S3†) analysis clearly shows that Na+, K+, or Cs+ ions are uniformly distributed in the cavities of the corresponding SAPO-RHO.
Considering the fact of that the separation investigations were performed under the dry conditions, we analyzed the structures of dehydrated M-SAPO-RHOs via the Rietveld refinement against PXRD data. Here, we take Na-SAPO-RHO as an example to illustrate the Rietveld refinement process. The initial SAPO-RHO structural model was deduced from the idealized RHO framework. Because of the alternating distribution of Al and P in the SAPO-RHO framework, its space group was reduced to I432 (No. 211) from Im3̄m (No. 229). However, it was very challenging to achieve reasonable refinement results for dehydrated Na-SAPO-RHO (denoted as de-Na-SAPO-RHO) based on the I432 space group, since its bond lengths and bond angles deviate heavily from the ideal ones. After scrutinizing the cubic unit cell parameter of de-Na-SAPO-RHO (a = 14.47 Å), it is quite close to the one of dehydrated distorted zeolite Na-Rho (a = 14.38 Å),15a indicating that the crystallographic structure of de-Na-SAPO-RHO becomes distorted from the ideal framework as well. A distorted SAPO-RHO structural model (space group I23) was constructed based on the dehydrated distorted zeolite Na-Rho (I4̄3m), including the initial atomic coordinates for Al, P, and four O atoms in the asymmetric unit. Table S2† shows the space group changes of the H-type and dehydrated cation exchanged zeolite Rho and SAPO-RHO.
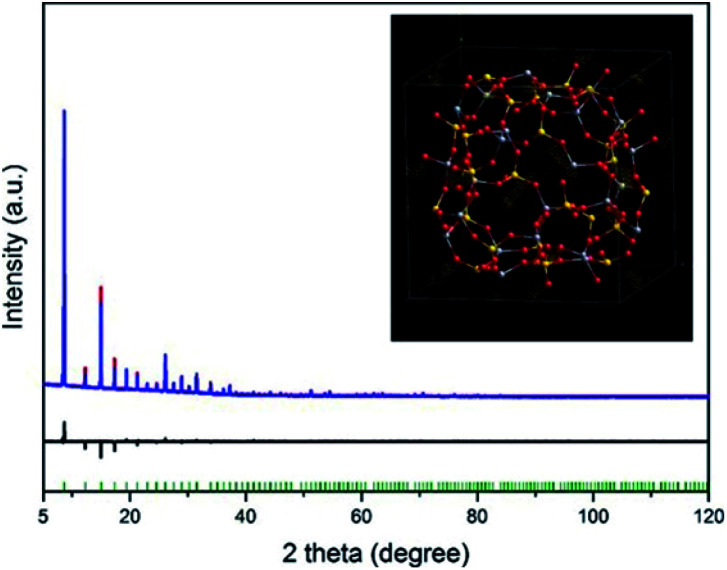
After profile fitting and optimizing the framework, the scale factor between the simulated PXRD data of the optimized framework and the experimental PXRD data was identified against high angle PXRD data (2θ: 60–120°), where the influence of the extra-framework species (Na+) in the cavities was negligible. Subsequently, the electron difference density map was calculated by applying the scale factor to the entire range (Fig. 2). Since the guest water molecules were already excluded, the isolated electron density within the cavity indicated the initial positions of the Na+ ions (inset of Fig. 2). Finally, the Rietveld refinement converged at Rp = 0.0147, Rwp = 0.0213, and GOF = 1.526, which revealed that most of Na+ ions were located in the elliptical single 8-rings (s8rs) and coordinated with framework O atoms (closest Na–O distance: 2.540 Å) and the other fraction (1.92 Na+ ions per unit cell in average) located close to the single 6-rings (s6rs) of lta cage as shown in Fig. 3a.
Fig. 2. Plots for locating the Na+ ions in the de-Na-SAPO-RHO by applying the appropriate scale factor to the whole pattern. The inset is the difference electron density map to locate initial positions of Na+ through Rietveld refinement. The observed, calculated, and difference curves are in blue, red, and black, respectively. The vertical bars indicate the positions of the Bragg peaks (λ = 1.5406 Å).
Fig. 3. Crystallographic structures of (a) de-Na-SAPO-RHO, (b) de-K-SAPO-RHO, and (c) de-Cs-SAPO-RHO and their corresponding final Rietveld refinement plots. The observed, calculated, and difference curves are in blue, red, and black, respectively. The vertical bars indicate the positions of the Bragg peaks (λ = 1.5406 Å).
Locations and occupancies of K+ and Cs+ ions in the de-K-SAPO-RHO and de-Cs-SAPO-RHO were determined by utilizing the same method. Unlike Na+ ions sitting in the center of s8rs, K+ ions in the de-K-SAPO-RHO were located at the d8rs (center) with the closest K–O distance of 2.713 Å (Fig. 3b). In addition, a small portion of K+ ions (0.88 per unit cell) settled at the side of s6rs of the lta cage. For de-Cs-SAPO-RHO, Cs+ ions resided in the center of d8rs (closest Cs–O distance: 3.185 Å), while a few Cs+ ions (0.6 per unit cell) were close to the center of s6r (Fig. 3c). It is worth noting that cations transfer from s8r to d8r with the increase of the atomic number of the alkaline metal (Na < K < Cs), resulting from a gradually prolonged cation–O bond distance. The detailed structural data for all the SAPO-RHOs are given in Tables S3 and S4.†
To further investigate the structural distortion in cation exchanged SAPO-RHO, a control sample of hydrated Cs-SAPO-RHO was measured. It is worth noting that the unit cell dimensions of the hydrated Cs-SAPO-RHO are the same as the dehydrated one. Hydrated Cs-SAPO-RHO still possesses the space group of I23 instead of I432. It is distinct from the metal exchanged zeolite Rho whose structural distortion only occurred in the process of dehydration. Therefore, it can be clearly concluded that the distortion of the framework of SAPO-RHO is caused solely by the cations of Na+, K+, or Cs+.
The separation performance of the dehydrated H-SAPO-RHOs and M-SAPO-RHOs (M = Na, K, and Cs) were first evaluated by the pure-component equilibrium adsorption isotherms for CO2, N2, and CH4 at 273 K (Fig. S4†), 298 K (Fig. 4a–c), and 313 K (Fig. S4†) between 0 and 1 bar. The results in Fig. 4 and S4† show that all M-SAPO-RHOs have a higher uptake for CO2 than for CH4 and N2, and the uptake of all gases decreases with the increase of temperature. The CO2 uptake at 298 K and 1 bar is in the order of H-SAPO-RHO (4.41 mmol g−1) > Na-SAPO-RHO (3.53 mmol g−1) > K-SAPO-RHO (0.87 mmol g−1) > Cs-SAPO-RHO (0.45 mmol g−1) (Fig. 4a, Table 1). A higher CO2 (dynamic diameter of CO2: 0.33 nm) uptake in the H-SAPO-RHO at 1 bar is attributed to the unblocked 8-ring pore openings and the small size of protons, leaving more room for CO2 in the lta cage, while it is of interest to note that an appreciable CO2 uptake (1.21 mmol g−1 at 0.02 bar) is observed in the Na-SAPO-RHO although six Na+ ions per unit cell occupy the elliptical s8r pore openings. It results from the fact that CO2 with strong quadrupole moment can interact strongly with Na+ ions and push them away instantaneously from the center of the s8rs to allow the CO2 molecules to pass through, as observed in the “trapdoor” effect.11,14c,15a,19 In addition, an abrupt increase of CO2 uptake on K-SAPO-RHO above 0.2 bar is observed. Such a shape of the isotherm of CO2 has been also observed in Na form of MER zeolite with 8MR window, which is attributed to the structural flexibility of the elliptical 8MR window in MER zeolite.20
Fig. 4. Comparison of the (a) CO2, (b) CH4, and (c) N2 adsorption isotherms of all SAPO-RHOs at 298 K between 0 and 1 bar; (d) comparison of the CO2 uptake for all the SAPO-RHOs under 0.02 and 0.15 bar at 298 K respectively; (e) CO2/CH4 separation factors at 0.02/0.98 bar and (f) CO2/N2 separation factors at 0.15/0.85 bar at 298 K for all the SAPO-RHOs.
Comparisons of equilibrium CO2 uptake and selectivity on various zeolites and SAPOs.
| Material | CO2 uptake [mmol g−1] | CH4 uptake [mmol g−1] | N2 uptake [mmol g−1] | α(CO2/CH4) (0.02/0.98 bar) | α(CO2/N2) (0.15/0.85 bar) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| 0.02 bar | 0.15 bar | 1.0 bar | 0.98 bar | 0.85 bar | ||||
| H-SAPO-RHOa | 0.44 | 1.84 | 4.41 | 0.83 | 0.210 | 26 | 50 | This work |
| Na-SAPO- RHO a | 1.21 | 2.42 | 3.53 | 0.027 | 0.070 | 2196 | 196 | This work |
| K-SAPO-RHOa | 0.01 | 0.046 | 0.87 | 0.024 | 0.010 | 20 | 29 | This work |
| Cs-SAPO-RHOa | 0.01 | 0.054 | 0.45 | 0.017 | 0.014 | 30 | 22 | This work |
| Na-1-SAPO-RHOa | 0.81 | 2.04 | 3.43 | 0.090 | 0.116 | 441 | 105 | This work |
| Na-2-SAPO-RHOa | 0.71 | 2.28 | 3.38 | 0.042 | 0.089 | 828 | 145 | This work |
| Na-4-SAPO-RHOa | 0.60 | 1.82 | 3.08 | 0 | 0 | ∞ | ∞ | This work |
| H-SAPO-RHOa | 0.49 | 1.95 | 4.60 | 0.38 | 0.23 | 63 | 48 | 24 |
| SAPO-34b | 0.24 | 1.20 | 3.26 | 0.65 | — | 20 | — | 25 |
| Na-SAPO-34b | 0.89 | 2.10 | 3.40 | 0.62 | 0.26 | 75 | 44 | 25 |
| SAPO-17c | 0.29 | 1.29 | 3.27 | — | 0.31 | — | 23 | 26 |
| SAPO-35c | 0.29 | 1.87 | 3.68 | — | 0.32 | — | 33 | 26 |
| SAPO-56c | 0.76 | 2.87 | 5.44 | — | 0.39 | — | 42 | 26 |
| Na-RHOa | 2.06 | 3.30 | 4.23 | 0.11 | — | 960 | — | 15a |
| Na-CHAa | 2.64 | 3.95 | 4.70 | 1.60 | 0.60 | 81 | 37 | 27 |
| Na-KFId | — | 3.40 | — | — | 0.27 | — | 71 | 28 |
| Na-Xa | 2.00 | 3.17 | 5.00 | 0.67 | 0.23 | 147 | 78 | 29 |
| Na-MERa | 1.50 | 2.50 | 3.80 | 0.32 | — | 229 | — | 30 |
| K-MERa | 1.93 | 2.93 | 3.57 | 0.052 | — | 1818 | — | 30 |
| NaKA (K = 17%)a | 1.00 | 2.30 | 3.43 | — | 0.02 | — | 660 | 22 |
| Na-Aa | 3.20 | 3.90 | 4.80 | 1.34 | 0.65 | 117 | 34 | 31 |
| Mg-MOF-74a | 2.30 | 5.65 | 8.00 | 1.11 | — | 102 | — | 32 |
| SIFSIX-3-Zna | 1.95 | 2.30 | 2.55 | 0.79 | 0.46 | 121 | 28 | 32a |
| UTSA-280a | 0.48 | 1.58 | 2.78 | 0.10 | 0.15 | 235 | 60 | 33 |
The adsorption data were measured at 298 K.
The adsorption data were measured at 293 K.
The adsorption data were measured at 273 K.
The adsorption data were measured at 303 K.
For CH4 and N2, however, the adsorption on Na-SAPO-RHO is very limited (0.027 mmol g−1 at 0.98 bar for CH4 and 0.070 mmol g−1 at 0.85 bar for N2), which might result from the fact that (1) their dynamic diameters (CH4: 0.38 nm, N2: 0.36 nm) are larger than effective pore openings; (2) their weaker interactions with Na+ ions are insufficient to push the Na+ ions away from the center of the s8rs to allow the CH4 and N2 molecules to pass, i.e., the “trapdoor” remains shut. Notice that the gradual increase of the N2 uptake on Na-SAPO-RHO with increase of pressure is observed as with H-SAPO-RHO (Fig. 4c) and the H form RHO zeolite.21 Elemental analysis shows that the exchange degree of Na+ in Na-SAPO-RHO is ca. 87.13%, leaving 12.87% of H+ balancing the negative charge of the framework of SAPO-RHO. Thus, the gradual increase of the N2 uptake in Na-SAPO-RHO can be attributed to the existence of H+.
Different from the case in Na-SAPO-RHO, when CO2, CH4, and N2 pass through K-SAPO-RHO or Cs-SAPO-RHO, the K+ or Cs+ ions located in the center of d8rs must first move from d8r to s8r. Since K+ or Cs+ ions are located at the center of the d8rs and coordinate with more framework oxygen atoms compared with Na+ ions in the s8r, pushing K+ or Cs+ ions is energetically much more difficult than moving Na+ ions. Thus, there is a lower uptake of CO2 (0.01 mmol g−1 at 0.02 bar for both K-SAPO-RHO and Cs-SAPO-RHO), N2 (0.010 mmol g−1 for K-SAPO-RHO and 0.014 mmol g−1 for Cs-SAPO-RHO at 0.85 bar), and CH4 (0.024 mmol g−1 for K-SAPO-RHO and 0.017 mmol g−1 for Cs-SAPO-RHO at 0.98 bar) in the K-SAPO-RHO and Cs-SAPO-RHO than that in the Na-SAPO-RHO over the entire pressure range as expected (Table 1).
To further evaluate the selectivity of the M-SAPO-RHOs in the separation of CO2/CH4 and CO2/N2, the separation factor α of CO2/CH4 (0.02/0.98 bar) and CO2/N2 (0.15/0.85 bar) were calculated on the basis of single-component isotherms (Table 1). The separation factor is highly associated with the CO2/CH4/N2 uptake at the operated pressure. As shown in Fig. 4b and c and Table 1, the CH4 and N2 uptakes at the pressure range for Na/K/Cs-SAPO-RHO are comparable and extremely low (0.017–0.027 mmol g−1 for CH4 and 0.010–0.070 mmol g−1 for N2). The separation factor thus mainly depends on the uptake of CO2. Fig. 4d shows the CO2 uptake of Na/K/Cs-SAPO-RHO at low pressure area and the separation factors for CO2/CH4 and CO2/N2 are summarized in Fig. 4e and f, respectively. The detailed uptakes for CO2/CH4/N2 in SAPO-RHOs at various pressures and the corresponding values reported in the literature are provided in Table 1.
Significantly, the separation factor of 2196 of Na-SAPO-RHO for CO2/CH4 is more than twice that of 960, superior to all of the nanoporous materials reported to date (Table 1). The separation factor of Na-SAPO-RHO for CO2/N2 is also as high as 196, which is the top level among zeolitic materials. The unprecedented high separation factor of Na-SAPO-RHO for CO2/CH4 and CO2/N2 is in fact due to the much lower uptake of CH4 (0.027 mmol g−1 at 0.98 bar) and N2 (0.070 mmol g−1 at 0.85 bar) than that in all other nanoporous materials. We also evaluated the uptake rate (i.e., rate of adsorption) of M-SAPO-RHOs. Considering that the adsorption capacity for CH4 and N2 was very low, we measured only the rate of adsorption for CO2. The rate of adsorption curves for H-SAPO-RHO, Na-SAPO-RHO, K-SAPO-RHO, and Cs-SAPO-RHO at 298 K and 1.0 bar are provided in Fig. S5.† As shown in Fig. S5,† the rate of adsorption of H-SAPO-RHO and Na-SAPO-RHO is much higher than that of K-SAPO-RHO and Cs-SAPO-RHO, which is attributed to the trapdoor effect caused by different cations.
To estimate the error in selectivity of the Na-SAPO-RHO, we prepared two more batches of the Na-SAPO-RHO (denoted as batches 2 and 3, the original Na-SAPO-RHO is denoted as batch 1) and conducted N2 adsorption/desorption (isotherms in Fig. S6 and textural properties in Table S5†).
Moreover, the pure component adsorption isotherms of CO2, CH4, and N2 for the three batches of Na-SAPO-RHO were also measured (Fig. S7a–c†) and the average uptake of CO2, CH4, and N2 with error bars plotted is shown in Fig. S7d–f.† As shown in Fig. S7,† the adsorption capacities of the three batches of Na-SAPO-RHO for CO2, CH4, and N2 are very similar to each other. The separation factors of the three batches of Na-SAPO-RHO for CO2/CH4 and CO2/N2 were calculated from their adsorption isotherms and plotted as shown in Fig. S8a and b,† respectively, which show that error in selectivity from batch to batch is very little.
To elucidate how the locations of inorganic cations affect the gas separation performance, we carried out the periodic DFT calculations using the Vienna ab initio simulation package (VASP 5.4.4). The details of calculations are presented in the ESI.† The results illustrate that N2 and CH4 have weaker interactions with inorganic cations in the Na/K/Cs-SAPO-RHO framework than CO2 as shown in Table S6† and they only interact with inorganic cations locating in the s6rs. It is of significance to note that the CO2 molecules in the Na-SAPO-RHO sample are captured by two Na+ ions that are distributed in the s6rs and s8rs, respectively (Fig. S9†). However, it is distinct from the situations observed in K/Cs-SAPO-RHO in which CO2 interacts with K+/Cs+ ions in the s6rs solely (Fig. S11†). These results indicate that CO2 molecules have a stronger interaction with Na-SAPO-RHO than with K/Cs-SAPO-RHO, which well explains a higher uptake of CO2 in Na-SAPO-RHO than in K/Cs-SAPO-RHO at the low pressure.
To gain a deep understanding on the different performance between Na-SAPO-RHO and zeolite Na-Rho, density functional theory (DFT) calculations were conducted based on the cluster modes. As shown in Fig. 5, two neutral Na5Al5Si43O72(OH)48 and Na4Si4Al24P20O72(OH)48 clusters cut from zeolite Na-Rho and Na-SAPO-RHO were utilized for the theoretical calculations. The details of calculations are presented in the ESI.† The calculated results show that the energy required to push the Na+ away from the center of the elliptical s8rs of the Na5Al5Si43O72(OH)48 and Na4Si4Al24P20O72(OH)48 clusters is 5.30 eV and 6.48 eV, respectively. The results show that Na+ ions have a stronger interaction with the SAPO framework than those with the aluminosilicate framework, indicating the enhanced “trapdoor effect” in SAPO-RHO. This means that more energy would be needed for CO2, CH4, and N2 to push the Na+ away from the center of the s8rs of Na-SAPO-RHO than from that of zeolite Na-Rho, leading to a lower uptake of CO2/CH4/N2 in Na-SAPO-RHO than that in Na-Rho. The strong “trapdoor” effect is especially disadvantageous for CH4 and N2 to push Na+ away because the interaction between Na+ and CH4 or N2 is much weaker than that between Na+ and CO2.11,14c,15a,22 This explains why the decrease in the uptake of CH4 and N2 in Na-SAPO-RHO is much more pronounced as compared to that of CO2, resulting in a higher selectivity for CO2.
Fig. 5. Molecular structures of two neutral Na5Al5Si43O72(OH)48 and Na4Si4Al24P20O72(OH)48 clusters and the binding energy of Na+ in the center of elliptical s8rs in two clusters.
The above results show that the Na-SAPO-RHO has a better performance than the K- and Cs-SAPO-RHO in the separation of CO2/CH4 and CO2/N2. Notice that the exchange degree in Na-SAPO-RHO is 87.13% upon 3 cycles of ion-exchange. To investigate the influence of ion-exchange degree on the separation performance, we further investigated the CO2 separation of the Na-SAPO-RHOs with the exchange degree of 42.41, 72.64, and 100% upon 1, 2, and 4 cycles of the ion-exchange process. The corresponding products are denoted as Na-1-SAPO-RHO, Na-2-SAPO-RHO, and Na-4-SAPO-RHO, respectively. The compositions and characterization results of the Na-SAPO-RHOs are provided in Table S1, Fig. S12 and S13 in the ESI.†
The single component equilibrium adsorption isotherms of the Na-(1-4)-SAPO-RHOs at 298 K with the pressure up to 1 bar for CO2, CH4, and N2 are displayed in Fig. S14a–c,† respectively and the detailed uptakes of all components at various pressures are summarized in Table 1. The CO2 uptakes of all Na-SAPO-RHOs at 0.02 and 0.15 bar are shown in Fig. S14d.† It is found that the uptake of CH4 and N2 gradually decreases with the increase of the ion-exchange degree (CH4: 0.090, 0.042, and 0.027 mmol g−1 at 0.98 bar for 1, 2, and 3 cycles of exchanged samples, respectively; N2: 0.116, 0.089, and 0.070 mmol g−1 at 0.85 bar for 1, 2, and 3 cycles of exchanged samples, respectively) and reaches 0 at the 4th cycle of ion-exchange (Table 1 and Fig. S14b and c†). Such results might be attributed to the strong “trapdoor” effect of Na+ on CH4 and N2 and the increased Na+ blocking of the s8r with the increase of exchanged Na+. Notice that, the Na-SAPO-RHO (3 cycles of ion-exchange) has the best CO2 uptake capacity (1.21 mmol g−1 at 0.02 bar) and selectivity performance (α(CO2/CH4): 2196; α(CO2/N2): 196) among the Na-SAPO-RHOs (Table 1 and Fig. S14e and f†). This suggests that the amount and distribution of both Na+ and H+ in Na-SAPO-RHO play an important role in determining gas adsorption. In this respect, further understanding is needed on the basis of future detailed structural characterization and theoretical calculations.
The breakthrough experiments of the Na-SAPO-RHO were conducted using binary CO2/CH4 (50/50, v/v) and CO2/N2 (15/85, v/v) gas mixtures at 298 K and atmospheric pressure (Schematic S1†), mimicking the industrial process conditions of biogas2a,23 and flue gas2a,34 respectively and the corresponding breakthrough curves are given in Fig. S15.† According to Fig. S15,† CO2 can be completely separated from CH4 and N2. On the basis of the breakthrough results, we also calculated the dynamic separation selectivity of the batch 1 of Na-SAPO-RHO for CO2/CH4 and CO2/N2 (Fig. S16 and S17†). The detailed information for the calculation is included in Section 8 of the ESI.† As shown in Fig. S17,† although the selectivity predicted by the breakthrough curves is slightly lower than that estimated from the multiple pure component adsorption measurements, Na-SAPO-RHO is also highly selective under dynamic conditions, rendering this zeolite potentially useful for selective CO2 adsorption in practical application.
Conclusions
Silicoaluminophosphate RHO zeolite was hydrothermally synthesized in the presence of diethylamine as an OSDA with the assistance of seeds. Na+, K+, or Cs+ was introduced into the as-prepared SAPO-RHOvia direct ion-exchange and complete replacement of protonated diethylamine by Na+ , while maintaining the zeolite crystallinity, has been successfully achieved upon four cycles of ion-exchange. The structure of ion-exchanged SAPO-RHOs after three cycles is determined by the Rietveld refinement. Structural analyses show that Na+ ions are mainly sited in the center of s8rs, while the K+ and Cs+ ions are mainly distributed in the center of d8rs. Na+ ion-exchanged SAPO-RHO upon three cycles of ion-exchange (Na-SAPO-RHO) exhibits an unprecedented separation factor of 2196 for CO2/CH4 and 196 for CO2/N2, which is much superior to K+ and Cs+ exchanged SAPO-RHOs with the same cycles of ion-exchange. Significantly, the Na+ form of SAPO-RHO exhibited unprecedented separation for CO2/CH4, superior to all the nanoporous materials reported to date. Theoretical calculations show that the interaction between Na+ and 8-rings in SAPO-RHO is much stronger than that in aluminosilicate Rho, which leads to a superior separation performance of Na-SAPO-RHO. Complete ion-exchange of Na+ in SAPO-RHO further increases the separation factor for CO2/CH4 and CO2/N2 by further enhancing the “trapdoor” effect. Breakthrough experiments demonstrate that Na-SAPO-RHO can completely separate CO2 from CH4 or N2. These superior features make Na-SAPO-RHO a promising candidate for CO2 capture in biogas purification and flue gas separation via adsorption-based separation processes. The present work introduces a new promising system based on silicoaluminophosphate zeolites for highly selective gas separation in terms of the cation-tailored “trapdoor” effect.
Author contributions
W. Y. and J. Y. designed and supervised the project; P. B. and Y. W. were involved in the design of the experiments; X. W., H. S., and B. W. performed the experiments; N. Y. and P. G. performed the structural analyses; M. X. and T. C. contributed to the calculations; P. L. and L. L. conducted the adsorption analyses; X. W. wrote the first draft; and W. Y., P. G. and J. Y. deeply revised the manuscript. X. W., N. Y., and M. X. contributed equally to this work.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China (U1967215, 21835002, and 21621001) and the 111 Project of China (B17020). Dr. Peng Guo acknowledges financial support from the National Natural Science Foundation of China (No. 21972136), CAS Pioneer Hundred Talents Program (Y706071202), Dalian National Laboratory for Clean Energy, (DNL) Cooperation Fund, and Chinese Academy of Sciences (DNL201908). Dr. Nana Yan acknowledges financial support from the CAS Special Research Assistant Program and the scholarship from STOE.
Electronic supplementary information (ESI) available: Details for synthesis, ion-exchange, characterization, and simulation. CCDC 2056929–2056932. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc00619c
Notes and references
- Boot-Handford M. E. Abanades J. C. Anthony E. J. Blunt M. J. Brandani S. Mac Dowell N. Fernández J. R. Ferrari M.-C. Gross R. Hallett J. P. Haszeldine R. S. Heptonstall P. Lyngfelt A. Makuch Z. Mangano E. Porter R. T. J. Pourkashanian M. Rochelle G. T. Shah N. Yao J. G. Fennell P. S. Energy Environ. Sci. 2014;7:130–189. [Google Scholar]
- (a) Bae Y. S. Snurr R. Q. Angew. Chem., Int. Ed. 2011;50:11586–11596. doi: 10.1002/anie.201101891. [DOI] [PubMed] [Google Scholar]; (b) Patel H. A. Byun J. Yavuz C. T. ChemSusChem. 2017;10:1303–1317. doi: 10.1002/cssc.201601545. [DOI] [PubMed] [Google Scholar]; (c) Pardakhti M. Jafari T. Tobin Z. Dutta B. Moharreri E. Shemshaki N. S. Suib S. Srivastava R. ACS Appl. Mater. Interfaces. 2019;11:34533–34559. doi: 10.1021/acsami.9b08487. [DOI] [PubMed] [Google Scholar]
- (a) Yang H. Xu Z. Fan M. Gupta R. Slimane R. B. Bland A. E. Wright I. J. Environ. Sci. 2008;20:14–27. doi: 10.1016/s1001-0742(08)60002-9. [DOI] [PubMed] [Google Scholar]; (b) Kenarsari S. D. Yang D. Jiang G. Zhang S. Wang J. Russell A. G. Wei Q. Fan M. RSC Adv. 2013;3:22739–22773. [Google Scholar]
- (a) Yang R. T., Gas Separation by Adsorption Processes, Butterworth, Boston, 1987 [Google Scholar]; (b) Yang R. T., Adsorbents: Fundamentals and Applications, Wiley-Interscience, New York, 2003 [Google Scholar]; (c) Ben-Mansour R. Habib M. A. Bamidele O. E. Basha M. Qasem N. A. A. Peedikakkal A. Laoui T. Ali M. Appl. Energy. 2016;161:225–255. [Google Scholar]; (d) Khraisheh M. Mukherjee S. Kumar A. Al Momani F. Walker G. Zaworotko M. J. J. Environ. Manage. 2020;255:109874. doi: 10.1016/j.jenvman.2019.109874. [DOI] [PubMed] [Google Scholar]
- (a) Lu A. H. Hao G. P. Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem. 2013;109:484–503. [Google Scholar]; (b) Wang J. Huang L. Yang R. Zhang Z. Wu J. Gao Y. Wang Q. O'Hare D. Zhong Z. Energy Environ. Sci. 2014;7:3478–3518. [Google Scholar]; (c) Lee S. Y. Park S. J. J. Ind. Eng. Chem. 2015;23:1–11. [Google Scholar]; (d) Zhou D. D. Zhang X. W. Mo Z. W. Xu Y. Z. Tian X. Y. Li Y. Chen X. M. Zhang J. P. EnergyChem. 2019;1:100016. [Google Scholar]
- (a) Siriwardane R. V. Shen M. S. Fisher E. P. Poston J. A. Energy Fuels. 2001;15:279–284. [Google Scholar]; (b) Creamer A. E. Gao B. Environ. Sci. Technol. 2016;50:7276–7289. doi: 10.1021/acs.est.6b00627. [DOI] [PubMed] [Google Scholar]; (c) Zhang Z. Cano Z. P. Luo D. Dou H. Yu A. Chen Z. J. Mater. Chem. A. 2019;7:20985–21003. [Google Scholar]; (d) Wang S. Li Y. Dai S. Jiang D.-E. Angew. Chem., Int. Ed. 2020;59:19645–19648. doi: 10.1002/anie.202005931. [DOI] [PubMed] [Google Scholar]
- (a) Chue K. T. Kim J. N. Yoo Y. J. Cho S. H. Yang R. T. Ind. Eng. Chem. Res. 1995;34:591–598. [Google Scholar]; (b) Kim J. Lin L. C. Swisher J. A. Haranczyk M. Smit B. J. Am. Chem. Soc. 2012;134:18940–18943. doi: 10.1021/ja309818u. [DOI] [PubMed] [Google Scholar]; (c) Cheung O. Hedin N. RSC Adv. 2014;4:14480–14494. [Google Scholar]; (d) Wang Y.-Y. Zhang Q. Yu J.-H. Chem. J. Chin. Univ. 2020;41:616–622. [Google Scholar]; (e) Liu S.-S. Chai Y.-C. Guan N.-J. Li L.-D. Chem. J. Chin. Univ. 2021;42:268–288. [Google Scholar]
- (a) Sumida K. Rogow D. L. Mason J. A. McDonald T. M. Bloch E. D. Herm Z. R. Bae T.-H. Long J. R. Chem. Rev. 2012;112:724–781. doi: 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]; (b) Furukawa H. Cordova K. E. O'Keeffe M. Yaghi O. M. Science. 2013;341:1230444. doi: 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]; (c) Trickett C. A. Helal A. Al-Maythalony B. A. Yamani Z. H. Cordova K. E. Yaghi O. M. Nat. Rev. Mater. 2017;2:17045. [Google Scholar]; (d) Hu Z. Wang Y. Shah B. B. Zhao D. Adv. Sustainable Syst. 2019:3. [Google Scholar]; (e) Jiang Y. Tan P. Qi S. Gu C. Peng S. Wu F. Liu X. Sun L. CCS Chem. 2020;2:1659–1668. [Google Scholar]; (f) Mao V. Y. Milner P. J. Lee J.-H. Forse A. C. Kim E. J. Siegelman R. L. McGuirk C. M. Porter-Zasada L. B. Neaton J. B. Reimer J. A. Long J. R. Angew. Chem., Int. Ed. 2020;59:19468–19477. doi: 10.1002/anie.201915561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Hu X. Liu L. Luo X. Xiao G. Shiko E. Zhang R. Fan X. Zhou Y. Liu Y. Zeng Z. Li C. Appl. Energy. 2020;260:114244. [Google Scholar]; (b) Varghese A. M. Karanikolos G. N. Int. J. Greenhouse Gas Control. 2020;96:103005. [Google Scholar]
- (a) Wu J. Xu F. Li S. Ma P. Zhang X. Liu Q. Fu R. Wu D. Adv. Mater. 2019;31:1802922. doi: 10.1002/adma.201802922. [DOI] [PubMed] [Google Scholar]; (b) Duan F. Liu X. Qu D. Li B. Wu L. CCS Chem. 2020;2:2676–2687. [Google Scholar]
- Shang J. Li G. Singh R. Gu Q. Nairn K. M. Bastow T. J. Medhekar N. Doherty C. M. Hill A. J. Liu J. Z. Webley P. A. J. Am. Chem. Soc. 2012;134:19246–19253. doi: 10.1021/ja309274y. [DOI] [PubMed] [Google Scholar]
- Palomino M. Corma A. Jordá J. L. Rey F. Valencia S. Chem. Commun. 2012;48:215–217. doi: 10.1039/c1cc16320e. [DOI] [PubMed] [Google Scholar]
- Robson H. E., Shoemaker D. P., Ogilvie R. A. and Manor P. C., in Molecular Sieves, American Chemical Society, 1973, ch. 9, vol. 121, pp. 106–115 [Google Scholar]
- (a) Parise J. B. Cox D. E. J. Phys. Chem. 1984;88:1635–1640. [Google Scholar]; (b) Parise J. B. Gier T. E. Corbin D. R. Abrams L. Jorgensen J. D. Prince E. J. Phys. Chem. 1984;88:2303–2307. [Google Scholar]; (c) Lozinska M. M. Mowat J. P. S. Wright P. A. Thompson S. P. Jorda J. L. Palomino M. Valencia S. Rey F. Chem. Mater. 2014;26:2052–2061. [Google Scholar]; (d) Balestra S. R. G. Hamad S. Ruiz-Salvador A. R. Domínguez−García V. Merkling P. J. Dubbeldam D. Calero S. Chem. Mater. 2015;27:5657–5667. [Google Scholar]
- (a) Lozinska M. M. Mangano E. Mowat J. P. S. Shepherd A. M. Howe R. F. Thompson S. P. Parker J. E. Brandani S. Wright P. A. J. Am. Chem. Soc. 2012;134:17628–17642. doi: 10.1021/ja3070864. [DOI] [PubMed] [Google Scholar]; (b) Lozinska M. M. Mangano E. Greenaway A. G. Fletcher R. Thompson S. P. Murray C. A. Brandani S. Wright P. A. J. Phys. Chem. C. 2016;120:19652–19662. [Google Scholar]
- Su X. Tian P. Li J. Zhang Y. Meng S. He Y. Fan D. Liu Z. Microporous Mesoporous Mater. 2011;144:113–119. [Google Scholar]
- Yan N. Wang L. Liu X. Wu P. Sun T. Xu S. Han J. Guo P. Tian P. Liu Z. J. Mater. Chem. A. 2018;6:24186–24193. [Google Scholar]
- Xiang X. Yang M. Gao B. Qiao Y. Tian P. Xu S. Liu Z. RSC Adv. 2016;6:12544–12552. [Google Scholar]
- De Baerdemaeker T. De Vos D. Nat. Chem. 2013;5:89. doi: 10.1038/nchem.1560. [DOI] [PubMed] [Google Scholar]
- Choi H. J. Jo D. Min J. G. Hong S. B. Angew. Chem., Int. Ed. 2021;60:4307–4314. doi: 10.1002/anie.202012953. [DOI] [PubMed] [Google Scholar]
- Ke Q. Sun T. Wei X. Guo Y. Xu S. Wang S. Chem. Eng. J. 2019;359:344–353. [Google Scholar]
- Liu Q. Mace A. Bacsik Z. Sun J. Laaksonen A. Hedin N. Chem. Commun. 2010;46:4502–4504. doi: 10.1039/c000900h. [DOI] [PubMed] [Google Scholar]
- (a) Zhou K. Chaemchuen S. Verpoort F. Renewable Sustainable Energy Rev. 2017;79:1414–1441. [Google Scholar]; (b) Wang S. Bai P. Sun M. Liu W. Li D. Wu W. Yan W. Shang J. Yu J. Adv. Sci. 2019;6:1901317. doi: 10.1002/advs.201901317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X. Tian P. Fan D. Xia Q. Yang Y. Xu S. Zhang L. Zhang Y. Wang D. Liu Z. ChemSusChem. 2013;6:911–918. doi: 10.1002/cssc.201200907. [DOI] [PubMed] [Google Scholar]
- Luo Y. Funke H. H. Falconer J. L. Noble R. D. Ind. Eng. Chem. Res. 2016;55:9749–9757. [Google Scholar]
- Cheung O. Liu Q. Bacsik Z. Hedin N. Microporous Mesoporous Mater. 2012;156:90–96. [Google Scholar]
- Guo Y. Sun T. Gu Y. Liu X. Ke Q. Wei X. Wang S. Chem.–Asian J. 2018;13:3222–3230. doi: 10.1002/asia.201800930. [DOI] [PubMed] [Google Scholar]
- Liu Q. Pham T. Porosoff M. D. Lobo R. F. ChemSusChem. 2012;5:2237–2242. doi: 10.1002/cssc.201200339. [DOI] [PubMed] [Google Scholar]
- Walton K. S. Abney M. B. Douglas LeVan M. Microporous Mesoporous Mater. 2006;91:78–84. [Google Scholar]
- Georgieva V. M. Bruce E. L. Verbraeken M. C. Scott A. R. Casteel W. J. Brandani S. Wright P. A. J. Am. Chem. Soc. 2019;141:12744–12759. doi: 10.1021/jacs.9b05539. [DOI] [PubMed] [Google Scholar]
- Rzepka P. Wardecki D. Smeets S. Müller M. Gies H. Zou X. Hedin N. J. Phys. Chem. C. 2018;122:17211–17220. [Google Scholar]
- (a) Nugent P. Belmabkhout Y. Burd S. D. Cairns A. J. Luebke R. Forrest K. Pham T. Ma S. Space B. Wojtas L. Eddaoudi M. Zaworotko M. J. Nature. 2013;495:80–84. doi: 10.1038/nature11893. [DOI] [PubMed] [Google Scholar]; (b) Herm Z. R. Swisher J. A. Smit B. Krishna R. Long J. R. J. Am. Chem. Soc. 2011;133:5664–5667. doi: 10.1021/ja111411q. [DOI] [PubMed] [Google Scholar]
- Lin R. Li L. Alsalme A. Chen B. Small Structures. 2020;1:2000022. [Google Scholar]
- Bower J. K. Barpaga D. Prodinger S. Krishna R. Schaef H. T. McGrail B. P. Derewinski M. A. Motkuri R. K. ACS Appl. Mater. Interfaces. 2018;10:14287–14291. doi: 10.1021/acsami.8b03848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.