Abstract
Pes cavus in its different forms is not a pathological entity, but rather the manifestation of multiple diseases.
Cavovarus, a form of cavus foot, should never be considered a physiological deformity. A neurological condition should always be excluded.
The evolution of pes cavovarus is unpredictable because of the large number of conditions involved in its aetiology, as well as their variable degree of expression. About 66% of cavovarus feet are the result of subtle neurological diseases, which only become evident later in life.
Although surgery may not change quality of life, recent studies suggest that it may improve foot posture and reduce walking instability.
The aim of treatment is to preserve a painless, plantigrade, mobile foot. Management consists of correcting bone deformity while preserving movement, and the wise use of rebalancing techniques. Arthrodesis should only be a salvage procedure.
Cite this article: EFORT Open Rev 2021;6:510-517. DOI: 10.1302/2058-5241.6.210021
Keywords: cavovarus, cavus, children
Introduction
The foot resembles a tripod where the three legs consist of the tip of the heel, the first metatarsal (1MTT) and the fifth metatarsal (5MTT). The concept is useful to explain the pes cavus. When the tips of the tripod move closer the arch becomes higher. The leg of the tripod that moves the most will determine the type of pes cavus.
Pes cavus defined as a foot highly arched, is not infrequent in childhood, and is an asymptomatic normal foot variant. However, certain forms of pes cavus, such as pes cavovarus (PCV) or pes calcaneocavus, may be related to a neurological lesion and its secondary muscle imbalance and be symptomatic.
The differentiation between the various forms of pes cavus is paramount to understand its pathophysiology and apply the appropriate treatment. Some forms involve only the longitudinal arch, while others like PCV are complex deformities and require a different management. We should be cautious when using the term pes cavus in an interchangeably manner with pes cavovarus, as is commonly done, because the diagnostic and therapeutic implications are completely different.
Pes cavovarus denotes the presence of a three-dimensional deformity of the foot, but it is a descriptive feature not a diagnosis. Although PCV may be idiopathic in origin, in most cases, there is an underlying neurological condition. Therefore, one of the most important challenges is to elucidate the responsible cause. Finding the correct diagnosis can be difficult, often beyond the scope of an orthopaedic surgeon, and referral to a neuropediatric colleague may be necessary. But the search for the cause should never be ignored. Firstly, because the nature of the condition may determine the quality and life expectancy of the patient, and secondly, because our management plan will largely depend on the subsequent diagnosis.
A comprehensive review of this condition is extremely difficult. The literature is full of confounding factors: lack of uniformity on the background diagnosis, the report of undifferentiated results during skeletal growth and adulthood, the heterogenicity of treatments applied, together with the lack of long-term series. As a result, we have focused our review on the management principles, leaving recommendations over management and results for secondary consideration.
Epidemiology
The true incidence of pes cavus is unknown. There are reports suggesting that its presence increases with age, ranging from a 2% at three years of age to up to a 7% at the age of 16.1 Although the incidence could be much higher in the adult population, ranging from 10.5–25%.2,3
Aetiology
Some feet in absence of disease may be a variant of normality.4 A congenital variant of congenital cavus feet has also been described.4 In general, any cause that produces muscle imbalance of the foot may result in a pes cavovarus. Briefly, we can categorize pes cavus according to the origin of the causative insult (see Table 1).
Table 1.
Summary of the causes related to the aetiology of a pes cavus deformity
|
1. Peripheral nerve
a. Charcot–Marie–Tooth b. Peripheral nerve injuries c. Polineuritis |
|
2. Central nervous system
a. Cerebral palsy b. Friederich’s ataxia c. Poliomielitis d. Lysosomal storage diseases10 e. Familial paraplegia |
|
3. Spinal abnormalities
a. Spina bifida11 b. Dyastomyelia and syrongomyielia c. Spinal cord tumours, lipomas and tethered cord |
|
4. Other causes
a. Muscular dystrophies12 b. Post-traumatic: peroneus brevis injury13 c. Secondary to vascular ischemia and compartmental syndrome d. Secondary to clubfoot e. Associated to syndromes14 |
| 5. Idiopathic |
Charcot–Marie–Tooth (CMT) disease is a hereditary sensory and motor neuropathy (HSMN), with an estimated incidence of between 3–82/100,000.5 It constitutes the most commonly inherited peripheral neuropathy.6 More than 90 genes are involved in its cause.7 It is a heterogenous group of disorders but with quite a homogenous clinical phenotype. Predominantly transmitted in an autosomal dominant fashion, with variable penetrance, transmission linked to X-chromosome is also well described. Negative family history does not preclude CMT, as about 10% arise by de novo mutations.8 The condition produces a loss of myelin and axonal degeneration of peripheral nerves. Initial classification was based on motor nerve conduction velocity studies, but its complexity has increased with the adding of phenotype and genetic cause. Types 1, 2, and 3 are seen in children. The most common type, 1A, exhibits spotty nerve myelodegeneration, the second commonest form is the X-linked form. The condition is often progressive9 and the distal muscle wasting will keep advancing with age.
Other causes include peripheral nerve injuries: the peroneal nerve injury being the most likely to result in a pes cavus. Among the causes related to the central nervous system, cerebral palsy is the most common, especially in the hemiplegic form. Friederich’s ataxia, a familiar progressive ataxia secondary to a continuous degeneration of the posterior spinal cord columns, should always be taken in account, as cavovarus feet may be the presenting symptom.
Among the spinal causes, spina bifida, with unilateral or bilateral involvement, is the more common cause; cavus feet being more frequent in sacral lesions.11 Always consider the spine when facing a unilateral case, remembering syringomyelia or a tethered cord.
Despite an extensive search sometimes the cause cannot be found, and we label them as idiopathic . This group still represents a significant proportion of the total.15
Classification
Accepting pes cavus as an increase of height of the longitudinal arch of the foot, we can identify different types of pes cavus:
- a. Those forms where only the sagittal plane of the foot is involved:4
- Anterior form: when there is a flexion deformity of the forefoot relative to the hindfoot.
- Posterior pes cavus: where an isolated verticalization of the calcaneus with compensatory plantar flexion of the ankle is present.
- Mixed forms: a combination of the previous types.
- b. Forms with involvement of the sagittal and coronal plane: pes cavovarus (PCV) – here the primary deformity is the pronation of the forefoot. Cavovarus deformity can also be classified (Cavovarus flexibility classification system (forefoot-hindfoot) according to Mosca:16
- Flexible-Flexible
- Stiff-Flexible
- Rigid-Flexible
- Rigid-Stiff
- Rigid-Rigid
- Late Rigid-Rigid classified by the following
• Flexible
- Dynamic deformity of the forefoot or hindfoot that corrects with tendon transfers.
- Dynamic and flexible deformity of the hindfoot that corrects after correction of the forefoot and with tendon transfers.
• Stiff
- Structural deformity of the forefoot or hindfoot that corrects with soft tissue release.
• Rigid
- Structural deformity of the forefoot or hindfoot that requires osteotomies or arthrodesis for correction.
Pathophysiology
The pathophysiology in pes calcaneocavus is related to weakness of the triceps surae, resulting in an excessive dorsiflexion of the os calcis, and the consequent shortening of the foot length with the appearance of a pes cavus.
The process in pes cavovarus is a bit more complex. It is the result of unbalanced forces applied on a growing foot, leading to a progressive deformity. Controversy still exists about the initial events, although the most accepted sequence, at least in CMT, appears to be the denervation of the foot’s intrinsic muscles. This results in clawing toes and an increase in the height of the foot’s arch as well as an equinus deformity of the forefoot over the hindfoot. Due to the relative strength of the hallux muscle, the flexion is more accentuated on the first ray, resulting in a pronation deformity in the transverse plane. Once the deformity is established it tends to progress.
Following intrinsic involvement, the denervation has been suggested to continue towards the lateral compartment, with peroneus brevis more involved than longus, and then the anterior tibialis muscle becomes affected.17 However, other authors suggest that the first involved muscle is the peroneus brevis, followed by the tibialis anterior, and then the intrinsic muscle.18 Nevertheless, computerized tomography (CT) scan studies19 suggest that intrinsic involvement always comes first, which can be followed by either the peroneal nerve innervated muscles, or by the tibial nerve innervated muscles. It appears clear that the pattern of muscle involvement is at least highly variable20 and is not only dependant on the aetiology, and the type of mutation but also on its penetrance.
Additionally, this deformity may occur in children during periods of rapid growth. When this happens, a lack of growth of the muscle on the short side of the deformity can result in shortening, as muscle growth requires stretching to reach its appropriate size. Conversely, its antagonist muscle for identical reasons will end up with a functional overlength. The severity and the rate of progression will ultimately depend on the form of CMT, its causative gene and the type of mutation.21 Typically, the deformity tends to appear at the beginning of the second decade of life, when the heel varus is initially flexible, but in most patients will progress and become stiff by the end of the same decade.22
Clinical presentation
A familiar history of feet deformity is important. It has been suggested23 that the simple presence of bilateral PCV denotes a 76% chance of having CMT disease. However, this finding has not been confirmed in other studies.15,24 Therefore, the aetiology of a significant proportion of cavovarus feet remains unknown.
The most common reported symptoms independent of aetiology include plantar callosities, foot pain, and unsteady gait. However, footwear difficulty was the simple most common complaint among these children.25
CMT is a debilitating condition, loss of force may start as soon as four years of age, but remain clinically undetectable until later age. Foot deformity is frequently the presenting feature; however, its incidence ranges from 27% in children to 70% in adolescents. The most common complaints in this population are ankle instability during walking (63%), and frequent falls (47%). Pain has been reported in up to 60% of patients.26
Be aware that hip dysplasia may be a feature of CMT and could be the presenting symptom.27 Intrinsic hand atrophy is well reported but is less common than foot involvement. Bowel and bladder symptoms should be searched for. In patients with anterior cavus foot the resultant deformity leads to an increase in pressure under the metatarsal heads, leading to metatarsalgia. Conversely, in pes calcaneocavus the excessive pressure over the calcaneal tuberosity linked with the sensory impairment frequently associated in these patients when associated with spina bifida, may result in plantar ulcers.
Physical examination
Clinical examination should include a shoeless assessment of the patient’s gait. The presence of a foot drop or an extensor recruitment to compensate for weak dorsiflexion should be noted. Abnormal heel and tandem walking may be an early sign of alert. When a calcaneocavus foot is present a peg-leg gait may appear as a result of the poor push-off. The Trendelenburg test should be included in the dynamic assessment.
Foot examination should include:
Checking side involvement: uni or bilateral.
Assessing the hindfoot position and differentiate between the type of cavus deformity.
Assessing the flexibility of the hindfoot, using the Coleman block test, or equivalents.28,29 In the Coleman test the patient is asked to stand with the heel and lateral border of the foot over a 1-inch-high block while the medial metatarsals contact the floor (Fig. 1). When the hindfoot is flexible the heel will return to a neutral or valgus position.
Identification of the apex of the deformity.
Other foot anomalies: assessing associated toes deformities. Assessing where calosities are present, mostly at head of the first and fifth and base of the fifth metatarsals.
Assessing the mobility of the foot joints (flexibility) and whether manual correction of the deformity is possible. Assessing ankle mobility and Achilles length.
Fig. 1.
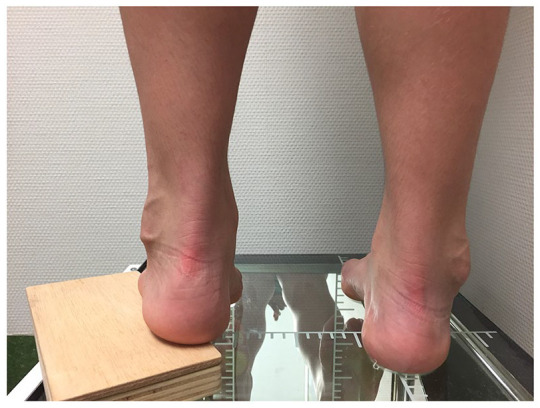
Coleman test: block placed under the lateral border of the foot and heel, while the medial side of the floor is lying on the floor. Observe the correction of the heel varus.
The examination should also include: a detailed spine examination looking for hairy patches, dimples or structural deformities. Hip assessment including motion and a Trendelenburg test to rule out hip dysplasia. Finally, the hands should be evaluated for wasting of the intrinsic muscles.
A basic neurological examination should include muscle power, tendon reflexes, and a sensory examination. Knee and ankle reflexes in the hypertrophic form (CMT1A) are typically absent. Sensory changes include loss of vibration and position sense and vasomotor signs.
Radiological assessment
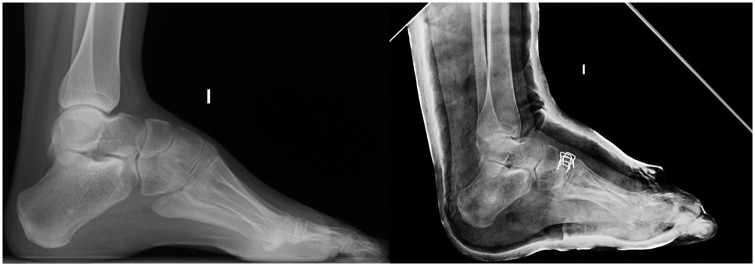
The initial radiologic assessment of the cavus foot should include a standing anteroposterior (AP) and lateral films of both feet. Look for the location of the lateral malleolus on the lateral film. A more posterior location than usual is common in severe forms and indicates the presence of an external rotation deformity at the ankle.30,31 Modified views with the ankle in internal rotation should be requested in order to get a proper lateral view of the foot (Fig. 2).
Fig. 2.
Standing lateral film of the foot. Note the posterior location of the lateral malleolus and the distorted image of the talar dome, indicating external rotation deformity at the level of the ankle.
The standard measurements for foot deformity should include:
Meary angle (normal value: 0°): The longitudinal axis of the first metatarsal (1MTT) and the talus normally forms a straight line. When a foot cavus is present, the lines intersect at the apex of the deformity (normally at the dorsal aspect of the first cuneiform body). Increase is indicative of 1MTT plantar flexion.
Calcaneal pitch is the angle formed by a line along the plantar surface of the os calcis and a line that goes through the floor. The normal value is < 25°. A calcaneal pitch > 30° is indicative of posterior cavus (calcaneocavus).
Hibbs angle: formed by a line through the calcaneus and the axis of the 1MTT. Normal value < 45°.
AP–talus–1MTT angle: (12 abduction, –10 adduction) in a normal foot the line formed by the longitudinal axis of the talus and the 1MTT are parallel or intersect at the body or neck of the talus.16 In malalignment the axes intersect at the level of talonavicular or the head of the talus. The 1MTT is always adducted in relation to the talus (Fig. 3, Fig. 4).
Fig. 3.
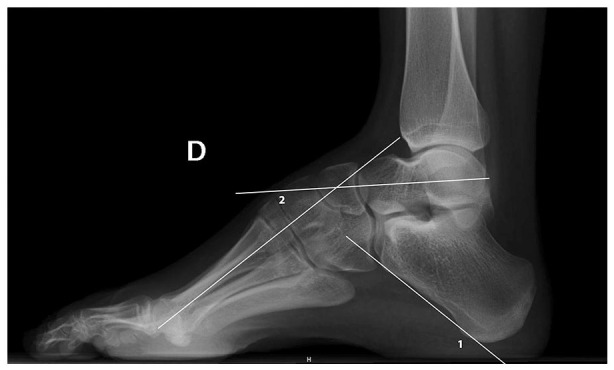
Lateral standing film of a cavovarus foot. (1) indicating the calcaneal pitch angle, (2) indicating the abnormal Meary angle.
Fig. 4.
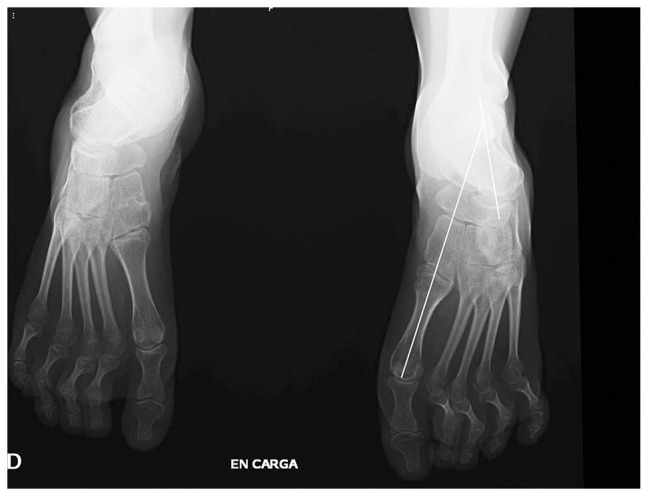
Standing AP film, in a severe pes cavovarus. Note the abnormal relationship of the talus and 1MTT.
Notes. AP, anteroposterior; 1MTT, first metatarsal.
Other radiographic views
The radiological evaluation of a cavovarus foot should also include the use of standing films with the Coleman block test in order to assess the flexibility of the foot.16 The Saltzman el-Khouty projection may be used to assess the position of the heel in relation to the tibia. However, any existing rotation may accentuate the apparent varus.
Other diagnostic evaluation
Brain and spine magnetic resonance imaging (MRI) are compulsory in cases of unilateral cavus foot. Nerve velocity studies should be included if there is a suspicion of a HMSN. Although normally reduced in CMT, in CMT type 2 they may be near normal. Genetic testing is part of the routine workup in a study of PCV, although the increasing number of mutations described makes genetic diagnosis sometimes difficult.
Management
There is a lack of consensus about what constitutes the ideal treatment for pes cavovarus,32 which is reflected by the wide variation in treatment applied in different centres.33 There are numerous factors that may explain this disparity. Firstly, the wide aetiological spectrum responsible for this deformity; secondly the variable severity of involvement even in patients sharing a common mutation and finally, and even more importantly, the paucity of long-term reports about these techniques.34,35
Another factor rarely discussed in the literature is that of the differences in treatment between children and adults. Many studies report adults and children together, making no stratification of the results according to age. The importance of segregating these populations lies in the fact that children represent the most severe spectrum of the disease, as early manifestation is synonymous with major severity and greater tendency to progression. On the other hand, muscle imbalance in children occurs in a growing skeleton, therefore the uneven forces will favour a skewed bone growth and an abnormal development of the bony structures. Not surprisingly, it has been suggested that surgical treatment should be delayed until the end of growth, as results then are much more predictable, and the chances of relapses reduce.4,36
Conservative treatment has been recommended in the non-progressive flexible cavus foot, as insoles supporting the lateral side of the foot or with metatarsal bars, unloading the areas of excessive pressure. Other therapies such as stretching, activity modification, and modified footwear have also been described.13,17,27,28 Trials with the use of Botulin toxin have proven ineffective.37 A French group proposed the use of casting and night splinting until achieving skeletal maturity, which showed a modest beneficial result.38
The objectives of a successful treatment are to achieve a painless, plantigrade, mobile foot. In our view, conservative treatment plays no role. Furthermore, delaying surgery until an older age only favours an increase of the severity and rigidity of the deformity, precluding the possibility of achieving a close to normal foot.
The principles of treatment of pes cavovarus laid down by Mosca in 200139 are still applicable: first of all, correct all of the segmental deformities while preserving motion, secondly balance the remaining forces and lastly, leave reasonable treatment options available for possible recurrence of deformity and pain. This third principle stresses the importance that patients and families understand that in most cases we are confronted with a progressive condition, which has no treatment, and where pes cavovarus is only one of its consequences. Therefore, despite obtaining an excellent correction of the deformity, the progressive nature of the condition favours the chances of recurrence and the need for further surgery.
A careful clinical examination, together with the information given by the Coleman test and the radiological studies, may help us identify the deformities and their apex. In our experience the hardest challenge lies in detecting the muscular imbalance and deciding on the ideal rebalancing procedure. The heterogenous and inconsistent pattern of muscle involvement in patients with common pathology,19,20,28 that can even be asymmetrical in the same patient,40 makes this task extremely difficult.
The first step in treatment consists of assessing the flexibility of the foot and locating the apex of the deformities. At early stages pes cavovarus deformity could be considered a primary forefoot deformity with compensatory changes at the level of the midfoot and hindfoot.28,29,34 A positive Coleman test or equivalent indicates that the varus component of the hindfoot is compensatory, and that the real culprit is the equinus and pronation of the forefoot. The way to approach this situation varies greatly between authors. While some attempt correction by osteotomies of the 1MTT,7,34,35,41 others prefer to do the correction closer to the apex,16,29 and others use a combination of both techniques.28
The criticism of the first metatarsal osteotomy is that it produces the correction away from the deformity apex, which is normally located at cuneiforms level. Besides, an osteotomy in a growing child should be performed at diaphyseal level, due to the risk of growth plate injury, which moves correction even further away from the apex. Correcting the deformity at the cuneiforms has the inherent mechanical advantage of achieving correction at the apex of the deformity, despite the fact that some studies have suggested a lesser effect over the hindfoot correction.42
When correction of the anterior deformity does not suffice to address the hindfoot varus, the addition of a calcaneal osteotomy may be necessary to achieve a valgus hindfoot. The type of osteotomy ranges from simple sliding osteotomies to the classical Dwyer osteotomy. Dwyer osteotomy combined with internal rotation may be the most efficient combination to achieve correction.43
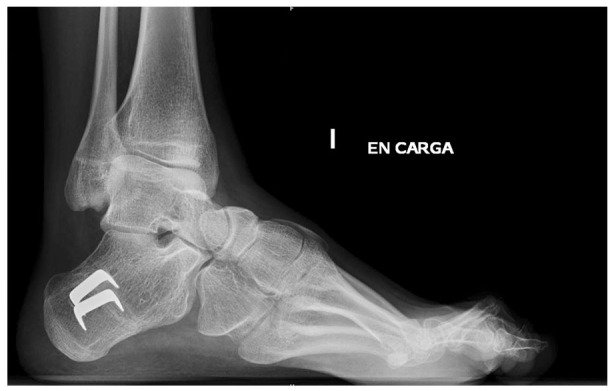
Once the deformity has become more rigid, midfoot tarsectomy may be necessary to achieve a plantigrade foot. There are a number of midfoot osteotomies, such as the Jappas, or the Akron osteotomy, that allow a tri-dimensional correction, preserving some mobility at the subtalar and Chopart joints. The outcomes of these osteotomies have been related to the age at the time of procedure, with higher tendency to relapse in younger patients (Fig. 5).32
Fig. 5.
Severe cavovarus deformity treated by Tarsal osteotomy.
Numerous soft tissue procedures have been reported to balance the foot. Plantar fascial release may have some effect on releasing the retracted foot intrinsic muscle, helping to decrease the longitudinal arch of the foot, but when a claw toe deformity is present, fascial release may be contraindicated.7 The next most widely used transfer is the peroneus longus to peroneus brevis, in an attempt to weaken the plantarflexion of the first metatarsal while reinforcing the weaker eversion of the hindfoot. The Jones procedure is used when a first toe claw deformity is present, but in children the arthrodesis of the interphalangeal joint is replaced by a tenodesis with the Extensor Hallucis Brevis.16 Other tendon transfers used are the tibialis posterior to the dorsum of the foot, to debilitate heel inversion while enhancing weak ankle dorsiflexion. More controversial is the transfer of the tibialis anterior, as this muscle is normally involved in early phases of the disease.34 However, the classical dogma that a transferred muscle always loses power has been recently questioned.7,44
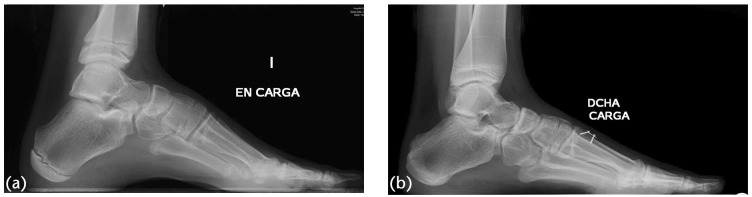
There is another possible approach in young children (up to 10–12 years of age) presenting with a rigid deformity of the forefoot and a flexible hindfoot (as demonstrated by the Coleman block test).25 This consists of performing a dorsal hemiepiphysiodesis of the first metatarsal, associated with a plantar fascial release and in selected cases adding a peroneus longus to brevis transfer. The reasons to select this approach are multiple. Firstly, the guided growth procedure allows a correction closer to the apex of the deformity. Secondly, the correction happens in a progressive way, allowing for the soft tissue to adapt to the changes in a more gradual fashion. Finally, and most importantly, is the dynamic nature of the correction produced. Opposite to other procedures where a limited amount of correction is made (directly related to the size of the osteotomy wedges used), growth inhibition allows for the amount of correction to be modulated in time and intensity. So far, this procedure, in selected cases, has shown good correction and maintenance of the results at skeletal maturity (Fig. 6).45
Fig. 6.
(a) Girl aged 10 years with severe pes cavus, standing lateral X-ray before surgery. (b) The same girl treated by dorsal hemiepiphysiodesis of the 1MTT and fascial plantar release four years after the initial procedure.
Note. 1MTT, first metatarsal.
Although multiple algorithms have been designed for the management of these patients,16,46,47 we believe that the most important step in treatment is an accurate assessment and an individualized plan. Long-term results have shown varied results. While some series have reported some encouraging results,34 in others the results have been more disappointing.29 A recent review form Australia showed that although foot posture and self-reported daily falls improved in CMT patients going through surgery, the natural course of the disease remained unchanged, and so did their quality of life. The gait parameters including strength, balance and long jump did not differ significantly from those of the natural history.7
Conclusions
Pes cavus and especially cavovarus, is a deformity, representing in many cases the first manifestation of a neurological disease. A detailed general and foot examination is necessary to determine the aetiology and design a treatment plan. The aim of treatment is to preserve a painless, plantigrade foot and treatment should be tailored for each individual. On treating these patients always leave rescue options, as these may be needed later. Do not forget to council the patients and families about the implications of the diagnosis and the long-term expectations.
Footnotes
ICMJE Conflict of interest statement: The author declares no conflict of interest relevant to this work.
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Reimers J, Pedersen B, Brodersen A. Foot deformity and the length of the triceps surae in Danish children between 3 and 17 years old. J Pediatr Orthop B 1995;4:71–73. [DOI] [PubMed] [Google Scholar]
- 2. Sachithanandam V, Joseph B. The influence of footwear on the prevalence of flat foot: a survey of 1846 skeletally mature persons. J Bone Joint Surg Br 1995;77:254–257. [PubMed] [Google Scholar]
- 3. Aminian A, Sangeorzan BJ. The anatomy of cavus foot deformity. Foot Ankle Clin 2008;13:191–198, v. [DOI] [PubMed] [Google Scholar]
- 4. Wicart P. Cavus foot, from neonates to adolescents. Orthop Traumatol Surg Res 2012;98:813–828. [DOI] [PubMed] [Google Scholar]
- 5. Deenen JC, Horlings CG, Verschuuren JJ, Verbeek AL, van Engelen BG. The epidemiology of neuromuscular disorders: a comprehensive overview of the literature. J Neuromuscul Dis 2015;2:73–85. [PubMed] [Google Scholar]
- 6. Reilly MM, Murphy SM, Laurá M. Charcot–Marie–Tooth disease. J Peripher Nerv Syst 2011;16:1–14. [DOI] [PubMed] [Google Scholar]
- 7. Lin T, Gibbons P, Mudge AJ, Cornett KMD, Menezes MP, Burns J. Surgical outcomes of cavovarus foot deformity in children with Charcot–Marie–Tooth disease. Neuromuscul Disord 2019;29:427–436. [DOI] [PubMed] [Google Scholar]
- 8. Mathis S, Goizet C, Tazir M, et al. Charcot–Marie–Tooth diseases: an update and some new proposals for the classification. J Med Genet 2015;52:681–690. [DOI] [PubMed] [Google Scholar]
- 9. Neumann JA, Nickisch F. Neurologic disorders and cavovarus deformity. Foot Ankle Clin N Am. 2019; 24:195–203. [DOI] [PubMed] [Google Scholar]
- 10. Alderson J, Ghosh PS. Clinical reasoning: pes cavus and neuropathy: think beyond Charcot–Marie–Tooth disease. Neurology 2019;93:e823–e826. [DOI] [PubMed] [Google Scholar]
- 11. Gunay H, Sozbilen MC, Gurbuz Y, Altinisik M, Buyukata B. Incidence and type of foot deformities in patients with spina bifida according to level of lesion. Childs Nerv Syst 2016;32:315–319. [DOI] [PubMed] [Google Scholar]
- 12. Schilling L, Forst R, Forst J, Fujak A. Orthopaedic disorders in myotonic dystrophy Type 1: descriptive clinical study of 21 patients. BMC Musculoskelet Disord 2013;14:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ziebarth K, Krause F. Updates in pediatric cavovarus deformity. Foot Ankle Clin 2019;24:205–217. [DOI] [PubMed] [Google Scholar]
- 14. Reinker KA, Stevenson DA, Tsung A. Orthopaedic conditions in Ras/MAPK related disorders. J Pediatr Orthop 2011;31:599–605. [DOI] [PubMed] [Google Scholar]
- 15. Karakis I, Gregas M, Darras BT, Kang PB, Jones HR. Clinical correlates of Charcot–Marie–Tooth disease in patients with pes cavus deformities. Muscle Nerve 2013;47:488–492. [DOI] [PubMed] [Google Scholar]
- 16. Mosca VS. Foot and ankle deformities. In: Mosca VS, ed. Priciples and management of pediatric foot and ankle deformities and malformations. Philadelphia, PA: Wolters Kluwer Health, 2014:61–118. [Google Scholar]
- 17. VanderHave KL, Hensinger RN, King BW. Flexible cavovarus foot in children and adolescents. Foot Ankle Clin 2013;18:715–726. [DOI] [PubMed] [Google Scholar]
- 18. Huber M. What is the role of tendon transfer in the cavus foot? Foot Ankle Clin 2013;18:689–695. [DOI] [PubMed] [Google Scholar]
- 19. Price AE, Maisel R, Drennan JC. Computed tomographic analysis of pes cavus. J Pediatr Orthop 1993;13:646–653. [PubMed] [Google Scholar]
- 20. Stilwell G, Kilcoyne RF, Sherman JL. Patterns of muscle atrophy in the lower limbs in patients with Charcot–Marie–Tooth disease as measured by magnetic resonance imaging. J Foot Ankle Surg 1995;34:583–586. [DOI] [PubMed] [Google Scholar]
- 21. Pareyson D, Marchesi C. Diagnosis, natural history, and management of Charcot–Marie–Tooth disease. Lancet Neurol 2009;8:654–667. [DOI] [PubMed] [Google Scholar]
- 22. Aktas S, Sussman MD. The radiological analysis of pes cavus deformity in Charcot Marie Tooth disease. J Pediatr Orthop B 2000;9:137–140. [DOI] [PubMed] [Google Scholar]
- 23. Nagai MK, Chan G, Guille JT, Kumar SJ, Scavina M, Mackenzie WG. Prevalence of Charcot–Marie–Tooth disease in patients who have bilateral cavovarus feet. J Pediatr Orthop 2006;26:438–443. [DOI] [PubMed] [Google Scholar]
- 24. Mohamed AR, Rodriguez-Casero MV, Kornberg AJ, Ryan MM. Neurophysiologic findings in children presenting with pes cavus. J Peripher Nerv Syst 2010;15:238–240. [DOI] [PubMed] [Google Scholar]
- 25. Sanpera I, Jr, Frontera-Juan G, Sanpera-Iglesias J, Corominas-Frances L. Innovative treatment for pes cavovarus: a pilot study of 13 children. Acta Orthop 2018;89:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burns J, Crosbie J. Weight bearing ankle dorsiflexion range of motion in pes cavus compared to normal and pes planus feet. Foot 2004;15:91–94. [Google Scholar]
- 27. Schwend RM, Drennan JC. Cavus foot deformity in children. J Am Acad Orthop Surg 2003;11:201–211. [DOI] [PubMed] [Google Scholar]
- 28. Mubarak SJ, Van Valin SE. Osteotomies of the foot for cavus deformities in children. J Pediatr Orthop 2009;29:294–299. [DOI] [PubMed] [Google Scholar]
- 29. Wicart P, Seringe R. Plantar opening-wedge osteotomy of cuneiform bones combined with selective plantar release and dwyer osteotomy for pes cavovarus in children. J Pediatr Orthop 2006;26:100–108. [DOI] [PubMed] [Google Scholar]
- 30. Perera A, Guha A. Clinical and radiographic evaluation of the cavus foot: surgical implications. Foot Ankle Clin 2013;18:619–628. [DOI] [PubMed] [Google Scholar]
- 31. Akoh CC, Phisitkul P. Clinical examination and radiographic assessment of the cavus foot. Foot Ankle Clin N Am 2019; 24:183–193. [DOI] [PubMed] [Google Scholar]
- 32. Weiner DS, Jones K, Jonah D, Dicintio MS. Management of the rigid cavus foot in children and adolescents. Foot Ankle Clin 2013;18:727–741. [DOI] [PubMed] [Google Scholar]
- 33. Laurá M, Singh D, Ramdharry G, et al. ; Inherited Neuropathies Consortium. Prevalence and orthopedic management of foot and ankle deformities in Charcot–Marie–Tooth disease. Muscle Nerve 2018;57:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ward CM, Dolan LA, Bennett DL, Morcuende JA, Cooper RR. Long-term results of reconstruction for treatment of a flexible cavovarus foot in Charcot–Marie–Tooth disease. J Bone Jt Surg - Ser A 2008;90:2631–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leeuwesteijn AEEPM, de Visser E, Louwerens JWK. Flexible cavovarus feet in Charcot–Marie–Tooth disease treated with first ray proximal dorsiflexion osteotomy combined with soft tissue surgery: a short-term to mid-term outcome study. Foot Ankle Surg 2010;16:142–147. [DOI] [PubMed] [Google Scholar]
- 36. Simon AL, Seringe R, Badina A, Khouri N, Glorion C, Wicart P. Long term results of the revisited Meary closing wedge tarsectomy for the treatment of the fixed cavo-varus foot in adolescent with Charcot–Marie–Tooth disease. Foot Ankle Surg 2019;25:834–841. [DOI] [PubMed] [Google Scholar]
- 37. Burns J, Scheinberg A, Ryan MM, Rose KJ, Ouvrier RA. Randomized trial of botulinum toxin to prevent pes cavus progression in pediatric Charcot–Marie–Tooth disease type 1A. Muscle Nerve 2010;42:262–267. [DOI] [PubMed] [Google Scholar]
- 38. d’Astorg H, Rampal V, Seringe R, Glorion C, Wicart P. Is non-operative management of childhood neurologic cavovarus foot effective? Orthop Traumatol Surg Res 2016;102:1087–1091. [DOI] [PubMed] [Google Scholar]
- 39. Mosca VS. The cavus foot. J Pediatr Orthop 2001;21:423–424. [PubMed] [Google Scholar]
- 40. Burns J, Ouvrier R, Estilow T, et al. Symmetry of foot alignment and ankle flexibility in paediatric Charcot–Marie–Tooth disease. Clin Biomech (Bristol, Avon) 2012;27:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim BS. Reconstruction of cavus foot: a review. Open Orthop J 2017;11:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Viehweger E, Jacquemier M, Launay F, Giusiano B, Bollini G. First cuneiform osteotomy alters hindfoot architecture. Clin Orthop Relat Res 2005;441:356–365. [DOI] [PubMed] [Google Scholar]
- 43. An TW, Michalski M, Jansson K, Pfeffer G. Comparison of lateralizing calcaneal osteotomies for varus hindfoot correction. Foot Ankle Int 2018;39:1229–1236. [DOI] [PubMed] [Google Scholar]
- 44. Gray K, Burns J, Little D, Bellemore M, Gibbons P. Is tibialis anterior tendon transfer effective for recurrent clubfoot? Clin Orthop Relat Res 2014;472:750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sanpera I, Corominas L, Frontera-Juan G. Rsults at skeletal maturity of a new technique for treatment of pes cavus. J Child Orthop 2019;13(suppl 1):s30. [Google Scholar]
- 46. Kaplan JRM, Aiyer A, Cerrato RA, Jeng CL, Campbell JT. Operative treatment of the cavovarus foot. Vol. 39, Foot and Ankle International. SAGE Publications Inc, 2018:1370–1382. [DOI] [PubMed] [Google Scholar]
- 47. Louwerens JWK. Operative treatment algorithm for foot deformities in Charcot–Marie–Tooth disease. Oper Orthop Traumatol 2018;30:130–146. [DOI] [PubMed] [Google Scholar]