Abstract
Knee osteoarthritis is a degenerative condition characterized by progressive cartilage degradation, subchondral damage, and bone remodelling. Among the approaches implemented to achieve symptomatic and functional improvements, injection treatments have gained increasing attention due to the possibility of intra-articular delivery with reduced side effects compared to systemic therapies.
In addition to well-established treatment options such as hyaluronic acid (HA), cortico-steroids (CS) and oxygen-ozone therapy, many other promising products have been employed in the last decades such as polydeoxyribonucleotide (PDRN) and biologic agents such as platelet-rich plasma (PRP) and mesenchymal stem cells (MSCs). Moreover, ultrasound-guided intra-meniscal injection and X-ray-guided subchondral injection techniques have been introduced into clinical practice.
Even when not supported by high evidence consensus, intra-articular CS and HA injections have gained precise indications for symptomatic relief and clinical improvement in OA. Biological products are strongly supported by in vitro evidence but there is still a lack of robust clinical evidence. PRP and MSCs seem to relieve OA symptoms through a regulation of the joint homeostasis, even if their capability to restore articular cartilage is still to be proved in vivo.
Due to increasing interest in the subchondral bone pathology, subchondral injections have been developed with promising results in delaying joint replacement. Nevertheless, due to their recent development and the heterogeneity of the injected products (biologic agents or calcium phosphate), this approach still lacks strong enough evidence to be fully endorsed.
Combined biological treatments, nano-molecular approaches, monoclonal antibodies and ‘personalized’ target therapies are currently under development or under investigation with the aim of expanding our armamentarium against knee OA.
Cite this article: EFORT Open Rev 2021;6:501-509. DOI: 10.1302/2058-5241.6.210026
Keywords: injection, osteoarthritis, stem cells
Introduction
Osteoarthritis (OA) is a degenerative disease with a tendency to worsen over time, characterized by articular cartilage degradation, subchondral damage, and bone remodelling, most commonly affecting weight-bearing joints such as the knee and hip. The aim of OA treatment is to control symptoms until the severity of the condition mandates surgical intervention; an early therapy may be a vital step for delaying the progression to end-stage disease. 1 Symptomatic control can be achieved through different therapeutic strategies such as lifestyle modifications, exercise therapy, pharmacological therapies and injections of various substances.2 Pharmacological approaches based for example on non-steroidal anti-inflammatory drugs (NSAIDs) and paracetamol have been shown to be effective in controlling pain in OA,3 but due to their systemic administration, they have also been associated with gastrointestinal, cardiovascular and renal adverse events, especially in patients already presenting with comorbidities.4 Injections have therefore gained increasing attention thanks to their more direct effect on the target tissues and reduced side effects due to intra-articular delivery. Among the most frequently used products we include corticosteroids (CS), hyaluronic acid (HA), polynucleotides, oxygen-ozone therapy, platelet-rich plasma (PRP) and mesenchymal stem cells (MSCs). CS and HA are ranked first as the most frequently injected substances and their use is supported by a large amount of literature, although some controversial findings have emerged.5 Conversely, there exists a more limited amount of evidence in support of the use of PRP and MSCs, due to their more recent discovery and introduction as OA treatments. Autologous biologic products, according to their capability of modulating the joint environment by releasing a series of growth factors and immune-modulatory molecules, could play a beneficial role in reducing the local inflammation and promoting cartilage and synovial anabolism.6 In addition, advances in research have not only brought about new products to be injected but also novel techniques of injection such as subchondroplasty and intra-meniscal application. The aim of the present narrative review is to summarize the different mechanisms of action of the various injectables for knee OA, presenting also their clinical results and potential areas of future development.
Off-the-shelf products
Corticosteroids (CS)
Intra-articular injection of CS is perhaps the most common conservative approach in the treatment of knee OA. The rationale behind its use relies on its immunosuppressive activity in the knee joint acting at different levels of the inflammatory cascade. In particular, it acts by blocking the synthesis of pro-inflammatory signalling molecules, such as interleukin 1 (IL-1), leukotrienes, prostaglandins and catabolic proteins such as metalloproteinases.2 These combined actions may be accountable for the pain relief observed in patients treated with CS.
Indeed, the latest 2019 Osteoarthritis Research Society International (OARSI) guidelines have assigned to intra-articular CS injections a recommendation level of 1B (‘high consensus’), the same level as for HA. In particular, their use is suggested for short-term pain relief compared to hyaluronic acid, which instead requires a longer time to provide its more beneficial effects in terms of pain control (2–4 weeks).7
Concerning the indications for CS injections, synovitis has been seen as a single predictor of treatment response: therefore, patients with an inflammatory phenotype of OA, characterized by stiffness, joint swelling and effusion, are more likely to respond to CS compared to HA.8
The latest Cochrane meta-analysis of 27 randomized controlled trials (RCTs) found CS to be more beneficial in pain reduction (lower visual analogue scale (VAS)) and function improvement (measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score) than control interventions, but this difference was gradually lost after up to 13 weeks of follow-up, at which point no statistical difference was detected. Nevertheless, the reliability of these results is poor due to the low methodological quality of the included RCTs, hence the quality of evidence in support of CS injections for knee OA was graded as ‘low’.9 Most recent studies found CS injections to be superior to oral NSAIDs, even if the author suggests a possible bias represented by the intra-articular (IA) placebo effect.10
Despite its beneficial effects, CS knee injections are associated with some known side effects. The latest meta-analysis on the safety of this procedure reported rare and mild side effects such as temporary joint pain, erythema and itching, but no cases of joint infection.11
Hyaluronic acid (HA)
Hyaluronic acid (HA) is a glycosaminoglycan that provides joint lubrication and shock absorbency and acts as the backbone for the proteoglycans of the extracellular matrix. In normal adult knees, HA concentration ranges from 2.5 to 4.0 mg/ml, whereas in OA it decreases by 33–50%.12 Besides their different origins and biological characteristics, viscosupplements currently available are classified based on the molecular weight (MW), method of preparation, and dosage. Currently, no clinical trials indicate a clear advantage of one product over another, even though a higher MW allows optimal binding on cell surfaces. In addition, there is no evidence to support that HA viscosupplementation affects OA progression. HA effects may last up to 26 weeks.12 The benefits in terms of pain relief and function improvement associated with HA have been recently rediscussed.13 Osteoarthritis Research Society International (OARSI) guidelines assigned a level of recommendation 1B/2 to the use of HA in the treatment of knee OA.
Intra-articular HA is particularly recommended for long-term treatment, as it is associated with symptom improvement beyond 12 weeks (i.e. more durable effects compared to CS), and has demonstrated a more favourable safety profile than repeated corticosteroids injections.7 On the other hand, the American Academy of Orthopaedic Surgeons (AAOS), in light of inconclusive evidence, has neither endorsed nor discouraged HA use. However, the real benefits of HA in the early stages of joint degeneration need to be confirmed by specifically designed studies.
Polynucleotides
Polydeoxyribonucleotide (PDRN) is composed by polymers of various chain lengths capable of binding large amounts of water molecules, hence capable of reorganizing the cartilage surface.14 Moreover, PDRN in vitro experiments have shown enhancement of chondrocyte survivorship if compared to HA exposure, with a reduced proteoglycans degradation.15
PDRN effects seem to be related to viscoelastic properties, cell grown induction, collagen and cell migration and anti-inflammatory capabilities. Moreover, in animal models, not only symptomatic improvement has been reported, but also a decrease of proinflammatory factors, such as IL-6, tumor necrosis factor-alpha (TNF-α) and high mobility group box 1 (HMGB-1). Despite the need for further studies, PDRN has been shown to reduce cartilage oligomeric matrix protein (COMP) levels, which themselves have recently been investigated as a biomarker of arthritis.16
PDRN offers mechanical protection towards the damaged cartilage, replacing the synovial fluid and restoring an ideal microenvironment for matrix production. The PDRN preparation appears colourless, transparent, viscoelastic and it is provided in pre-filled glass sterile disposable syringes containing a solution of 2 ml (the concentration of polynucleotides is 20 mg/ml). PDRN intra-articular treatment has been shown to produce faster improvement of activities of daily living compared to HA.17 According to a meta-analysis, intra-articular PDRN injections provided more significant pain reduction for up to two months after the procedure and equal functional improvements if compared to HA.18 Therefore, the clinical advantages of PDRN, unlike HA, could be better exploited by extending the interval between the different injections, thus reducing the frequency of injections. However, there are still no large-scale RCTs determining the effect of PDRN injections on knee OA.
Oxygen-ozone therapy
The rationale behind the use of intra-articular ozone (O3) therapy arose from the assumption that chronic oxidative stress plays an important role in OA. Ozone is a molecule discovered in the mid-19th century consisting of three atoms of oxygen in a dynamically unstable structure. Clinical experiences and research have considered O3 as a powerful anti-inflammatory, immune-modulatory substance. Due to its high reactivity, it may be able to reduce oxidative stress, stimulate fibroblastic joint repair and may promote new cartilage growth. It can be safely administered intra-articularly as an O3-O2 gas mixture (Fig. 1). When dissolved into the synovial fluid, it can generate reactive oxygen species (ROS) and lipid oxidation products that may inhibit proteolytic enzymes. Indeed, O3 therapy leads to a localized increase in oxygen delivery by promoting vasodilation and angiogenesis.19
Fig. 1.

Oxygen-ozone preparation before intra-articular injection.
Despite controversial results, O3 therapy has shown better results in terms of pain relief, joint function and quality of life compared to placebo or corticosteroids injections. The kinematics of O3 effects seem to be slow but lasting. In some trials HA treatment was clinically superior to O3 therapy20 and therefore oxygen-ozone therapy still lacks a large consensus. In terms of safety profile, O3 proved to be a safe procedure with almost zero adverse events: O3 is bacteriostatic, fungicidal, and viricidal, therefore the infection risk is minimal.21
Biologic products
Platelet-rich plasma (PRP)
PRP is an autologous blood derivate characterized by a higher platelet concentration than peripheral blood (Fig. 2). The rationale behind the employment of platelets relies on the ability of the latter to release biologically active proteins that are able to promote tissue healing; a property that becomes even more relevant when the target tissue has low healing potential as cartilage.22 In particular, this beneficial effect of PRP on knee homeostasis is exerted by means of a number of growth factors released by platelets as insulin-like growth factor (IGF), tissue growth factor (TGF), epidermal growth factor (EGF), platelet derived growth factor (PDGF), vascular endothelium growth factor (VEGF) and fibroblast growth factor (FGF).23 In particular, PRP effects can be ascribed to its effect on the Wnt/β-catenin pathway, which is implicated in OA development.24 The Wnt family of proteins plays a central role in inflammation signalling cascades stimulating the release of catabolic molecules such as metalloproteinases, which are responsible for cartilage degradation and progressive degeneration of all the articular tissues. Moreover, the Wnt pathway is significantly involved in Type II collagen degradation and chondrocyte apoptosis.25 The clinical application of PRP injections for knee OA has been investigated by an increasing number of clinical studies, including many RCTs, that have demonstrated its safety and overall clinical benefits. Looking at high-quality studies, the majority have shown that PRP is superior to hyaluronic acid (HA), especially in the case of low-grade articular degeneration,26,27 whereas in severe OA less satisfactory outcomes have been documented, with results quite similar in comparison to viscosupplementation.26,28 A potential explanation for these contradictory results might be the high variability of PRP products. Indeed, PRP formulations may be prepared by different methods resulting in different compositions, thus acting as a source of bias in the analysis of clinical outcomes. This limitation in the ability of clinical studies to investigate the real efficacy of PRP may be overcome by future studies employing a novel coding system as proposed by Kon et al.29 An example of PRP’s formulation variability is the amount of leukocytes present.29 In fact, both leukocyte-rich PRP and leukocyte-poor PRP products are available. Nevertheless, comparison between these two formulations has been performed showing no inter-product differences; similarly, no clear evidence exists on the ideal number of injections and their timing to maximize the clinical results.30
Fig. 2.
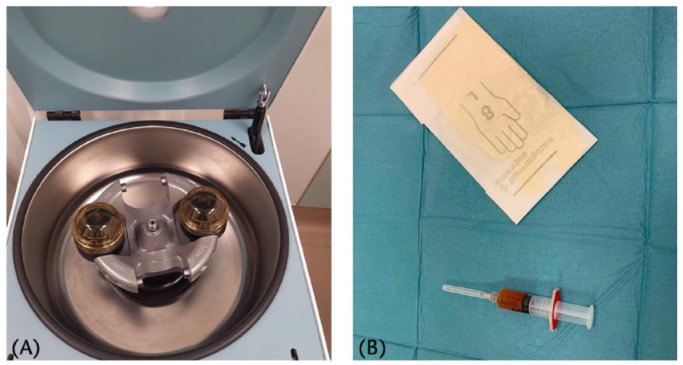
PRP preparation process. (A) Centrifugation after blood sample collection; (B) final output consisting in this case of leukocyte-poor PRP.
Note. PRP, platelet-rich plasma.
Mesenchymal stem cells (MSCs)
MSC treatment consists of intra-articular injections of stem cells associated to a pool of immune-modulatory and anti-inflammatory stromal molecules. Many adult tissues are populated by MSCs (adipose tissue, muscles, dermis, periosteum, synovial membrane, synovial fluid, etc.),31 but, in clinical practice, they are usually harvested from either bone marrow or adipose tissue. Even if many issues are still to be verified, MSCs, given their capacity to differentiate in mesenchymal derived tissue such as osteoblasts, chondrocytes and adipocytes, may have not only an anti-inflammatory, pro-angiogenetic and anti-apoptotic function, but also a reparative or regenerative role.32,33 Their effects are consequences of direct cell–cell interaction and secretion of factors.34 Similar to PRP, MSCs affect Wnt/β-catenin expression, thus controlling OA progression.25,35 However, differentiation potential is dependent on several factors such as architectural extracellular and inter-cellular segmental characterization, environmental factors, growth factors, and adequate pool of MSCs.36 In addition, having low expression of antigen-presenting molecules, MSCs are non-immunogenic;37 moreover, cartilage tissue, due to lack of vascular and lymphatic system, is particularly immune-privileged. That is why the use of allogenic MSCs may be feasible, and preliminary phase I and II studies are demonstrating their long-term efficacy and safety.
In clinical practice, MSCs can be used as a cell suspension, after expansion in culture or enzymatic digestion, or they can be concentrated directly in the operating room (OR), which is currently the favoured approach due to the stringent regulations and higher costs affecting the procedures undergoing extensive manipulation in the lab. MSC products differ markedly in composition, and the most suitable strategy is far from clear, with biological and practical considerations currently guiding the development of treatment strategies. Expanded MSCs allow a more reproducible treatment but a two-step procedure is needed with an increase in costs, manipulation-related infection risks and invasiveness. Cell expansion transforms MSCs in advanced-therapy medicinal products (ATMPs), subjected to more rigorous regulatory requirements, similar to those of conventional drugs. This is why in both the USA and Europe ‘minimally manipulated’ MSCs, such as bone marrow aspirate concentrate (BMAC) and adipose-derived stromal vascular fraction (SVF) are the most exploited strategies for clinical application.
BMAC
BMAC is commonly obtained from the iliac crest using needle aspiration and concentration through centrifugation (one or multiple) occurring directly in the OR to obtain a product for immediate use (Fig. 3). Furthermore, recent technological developments have provided a concentration system without the need for centrifugation. With regard to clinical outcomes, even if BMAC has been shown to have a positive effect on function and pain, its regenerative effects and superiority compared to viscosupplementation and corticosteroids are still to be proved.38,39
Fig. 3.
BMAC preparation in the OR. (A) Harvesting of bone marrow aspirate; (B) centrifugation; (C) final output rich in MSCs.
Note. BMAC, bone marrow aspirate concentrate; OR, operating room; MSCs, mesenchymal stem cells.
SVF
SVF contains 300-fold more MSCs when compared to BMAC, and is composed of a heterogenous cell population: preadipocytes, vascular endothelial cells, smooth muscle cells and pericytes (ASCs), leucocytes, and erythrocytes.40 Biologically, the maintenance of the stromal cell niche architecture seems to be a great advantage.41 Indeed, this beneficial aspect is thought to arise from the cross talk between progenitors, resident cells and immune cells, possibly displaying a wound-healing phenotype (i.e. M2 alternatively activated macrophages). SVF has proved to be a safe treatment with positive clinical consequences and a radiological and histological improvement compared to BMAC.42,43,44
SVF products differ in terms of preparation methods: originally SVF was obtained by enzymatic digestion with collagenase and trypsin, but, following safety and regulatory concerns, minimally manipulative methods were developed,45 exploiting mechanical forces such as centrifugation or microfragmentation in order to obtain a readily injectable product. Although we still lack clearly defined protocols to optimize the outcomes, such an approach may pave the way for a simpler application of adipose-derived MSCs in clinical practice by reducing operative times and costs.46
Potential contraindications
The aforementioned biologic products are reaching a wider number of clinics, who must be aware of some potential contraindications for their use. Although no absolute contraindication exists, there are some conditions warranting caution and that should be considered for correct patient counselling and management. In the case of PRP, low basal platelet count (< 150,000/mm3), the presence of haematological diseases, coagulopathies (both congenital or acquired) or chronic anti-aggregant or steroidal therapy may impair or reduce the biologic efficacy of PRP.47 PRP and MSCs should be also avoided in cases of recent diagnosis of malignancies, in particular hematologic ones: in fact, stem cells have theoretically an increased transformation and tumourigenic potential that suggests avoiding their use in oncological patients. More debated is the administration of biologic products in patients affected by concurrent rheumatic diseases: although in these cases a systemic therapy may be necessary to control the progression of the disease, intra-articular injections of PRP or MSCs may still contribute to reduce local symptoms and improve the joint functional status.
Combined therapies
Another strategy that has been attempted in the conservative treatment of knee OA is a combination therapy based on a single intra-articular administration of multiple substances. Different combinations have been investigated, among PRP, MSCs, HA and corticosteroids. A recent meta-analysis showed that combined IA injections of CS and HA were superior to HA alone in the reduction of pain both in the short and long-term outcomes.48 Similar results were found in a study comparing PRP and HA against HA and PRP alone, where the combined therapy achieved better clinical outcomes.49,50 Preliminary results on a PRP + MSCs combination showed significant reduction of symptoms up to 12 months after the procedure.51 However, besides the preliminary encouraging results, combination therapy should be proposed with caution, especially when adopting biologic products, and further data should be collected before endorsing its routine application.
Subchondral injections
Subchondral injections refer to minimally invasive intra-osseous delivery under fluoroscopic guidance of products such as PRP, MSCs or as calcium phosphate (CaP) (Fig. 4); when the latter is employed the procedure takes the name of subchondroplasty.52 The rationale behind this procedure stems from an increasing interest in the role of the subchondral bone in OA. The latter is defined as the bony component lying distal to the calcified cartilage and it is directly linked to it by means of channels, which transport a high number of tiny branches of vessels and nerves.53
Fig. 4.
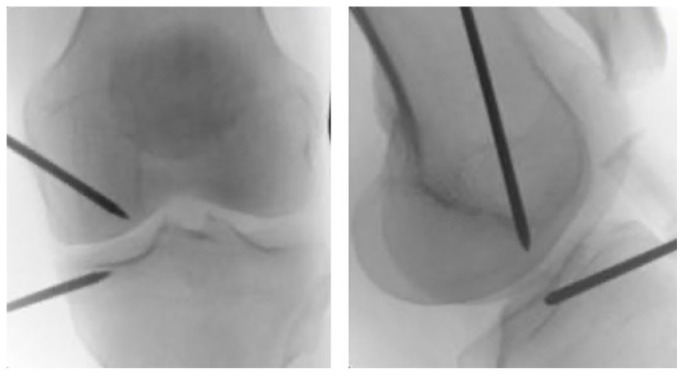
Intra-osseous placement of the trocars, under fluoroscopic guidance. BMAC was injected into the bone marrow lesions of the medial femoral condyle and tibial plateau.
Note. BMAC, bone marrow aspirate concentrate.
Indeed, bone marrow lesions (BML), an alteration of the bone marrow visible on T2-weighted magnetic resonance imaging (MRI), is commonly identified in osteoarthritic knees.54 In addition, subchondral sclerosis is generally recognized as a hallmark of OA.
There is an etiopathogenetic hypothesis suggesting that OA may start as subchondral bone pathology, leading to destruction of the overlying articular cartilage. Hence, subchondral injections exploit the possibility to fill the trabecular space within the BML with substances capable of ameliorating the bone integrity. Two recent systematic reviews on the topic55,56 showed that, independently from the employed substance, the subchondral injection is a safe procedure able to relieve OA-related symptoms. In particular, subchondroplasty was able to reduce the rate of conversion to arthroplasty therefore delaying this procedure in patients with BML. This is particularly relevant if we consider that patients with BMLs are nine times more likely to progress to TKA compared to patients without BML within three years.57
Nevertheless, the quality of evidence in support of the efficacy of this procedure is poor. In addition, in spite of CaP being the most employed substance in subchondral injections, there is still no comparative analysis demonstrating its superiority over PRP and MSCs. Moreover, there is still no evidence regarding the quantity of substance that should be injected into the subchondral bone. One limitation of this technique may be represented by the fluoroscopic guidance for the intraosseous insertion of the cannula: in fact, this imaging technique, as opposed to MRI, is not able to perfectly localize the exact position of the bone marrow lesions. On the other hand, a potential strength of subchondroplasty is represented by the ability of this procedure to address osteoarthritis pathophysiology at its core: the affected area of the subchondral bone.
Intra-meniscal injections
Meniscal degenerative tear and/or meniscal degeneration are often an early sign of knee OA and could elicit significant pain even in the absence of relevant chondral damage.58 Arthroscopic meniscectomy has shown conflicting results related to both the augmented risk of OA progression and reduced efficacy when compared to placebo surgery.59 The first-line management of degenerative meniscal lesions without ‘mechanical’ symptoms is conservative.60 The conservative approach appears challenging and consists of physiotherapy, NSAIDs, HA or corticosteroid intra-articular injections, and also the aforementioned biological products. In recent years there have been attempts to target selectively the meniscal tissue with intra-meniscal injections. Among them, PRP, due to its anti-inflammatory and regenerative effects, has recently been spreading in clinical practice. Guenoun et al performed an ultrasound-guided intra-meniscal, meniscal wall and intra-articular injection procedure, demonstrating that intra-meniscal PRP treatment is feasible, safe and efficient. A prolonged clinical and functional improvement was observed. Despite promising in vitro results, no MRI healing signs were noted.61 Randomized controlled studies with a higher number of patients are needed to confirm these encouraging results.
Micro-fragmented adipose tissue (MFAT) rich in SVF, once processed, may also be used for intra-meniscal injections; it acts as trophic mediator by secreting a variety of cytokines and growth factors, inhibiting fibrosis and apoptosis, enhancing angiogenesis and stimulating the differentiation of tissue-intrinsic reparative or stem cells. Mild mechanical forces may preserve the microarchitecture of the stromal vascular niche, where pericytes are located. MFAT may not only work as biological vehicle but also as an adipose tissue filler for meniscus regeneration. Malanga et al demonstrated that MFAT is a safe and potentially efficacious treatment option. A clinical improvement in pain, function, and quality of life measures, with no side effects, was achieved.62
Future perspectives
A strong limitation of the injective techniques is still the limited longevity of a sufficient drug dose inside the joint in order to exert a durable therapeutic effect. To overcome this limit, numerous new nano-technological approaches have been developed and are currently under testing.63 Indeed, the possibility of employing nano-carriers to keep the injected substances inside the knee environment and slowly release their contents over time, may achieve what most injective strategies have failed so far to obtain: long-lasting symptomatic relief. Another new experimental approach is represented by gene delivery. The concept is to deliver intra-articularly viral vectors with cDNAs coding for therapeutic proteins such as TGF-β1, IL-1Ra, interferon-beta (IFN-β), aiming at a sustained, endogenous drug delivery system.64
In addition, new injectable substances have been developed in order to slow down, halt or even to reverse the destruction of the joint tissues either by promoting cartilage anabolism or inhibiting its catabolism. In the first category we include the recombinant human fibroblast growth factor 18 (rhFGF-18, Sprifermin): it has been investigated both in vitro and in rat models showing its ability to expand hyaline cartilage-producing chondrocytes leading to an overall increase in cartilage volume.65 However, despite these encouraging pre-clinical findings, the first RCT assessing its efficacy in humans found no difference between rhFGF-18 and a placebo, thus again revealing the challenges of addressing the complex in vivo human joint system.66
Among the cartilage catabolism inhibitors, we should mention IL-1 receptor antagonist (IL-1Ra): its use as an intra-articular injectable was found to be safe in humans and effective in reducing cartilage degeneration and synovial inflammation in animal models.67 Moreover, monoclonal antibodies directed against nerve growth factor (NGF) (tanezumab, fulranumab, and fasinumab) have also been suggested as potentially therapeutic in OA. Indeed, anti-NGF injections for knee OA were found to be superior in pain reduction compared to a placebo 68. There are also molecules able to simultaneously promote cartilage synthesis and taper inflammatory processes responsible for cartilage degradation, such as kartogenin. This small molecule has been reported to promote cartilage regeneration and inhibit joint inflammation in small animal models, but its efficacy in humans still needs to be investigated in clinical trials.69
Last but not least, all the therapeutic strategies mentioned in the present review should be viewed in the perspective of the ‘personalized medicine’. Indeed, not only the development of new drugs is relevant, but also the correct indication to the right patient plays a key role. Radiologic and biochemical strategies for early diagnosis, a better understanding of the pathogenesis and prognostic factors of OA, which is a multi-faceted disease with different genotypes and phenotypes, and the patient’s individual features should drive the choice of the ‘right’ injectables, modelling a more personalized therapeutic plan for each patient.70
Footnotes
ICMJE Conflict of interest statement: BDM reports consultancy for Cartiheal Ltd, outside the submitted work.
EK reports consultancy for Cartiheal Ltd, Fidia Farmaceutici Spa and Greenbone Ortho Srl, payment for lectures including service on speakers’ bureaus from Zimmer Biomet, and stock/stock options in Cartiheal Ltd, all outside the submitted work.
The other authors declare no conflict of interest relevant to this work.
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. de Girolamo L, Kon E, Filardo G, et al. Regenerative approaches for the treatment of early OA. Knee Surg Sports Traumatol Arthrosc 2016;24:1826–1835. [DOI] [PubMed] [Google Scholar]
- 2. Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res 2018;11:2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the US bone and joint initiative. Semin Arthritis Rheum 2014;43:701–712. [DOI] [PubMed] [Google Scholar]
- 4. Cooper C, Chapurlat R, Al-Daghri N, et al. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: what does the literature say? Drugs Aging 2019;36:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen C, Lefèvre-Colau M-M, Poiraudeau S, Rannou F. Evidence and recommendations for use of intra-articular injections for knee osteoarthritis. Ann Phys Rehabil Med 2016;59:184–189. [DOI] [PubMed] [Google Scholar]
- 6. Lim W, Park SH, Kim B, Kang SW, Lee JW, Moon YL. Relationship of cytokine levels and clinical effect on platelet-rich plasma-treated lateral epicondylitis. J Orthop Res 2018;36:913–920. [DOI] [PubMed] [Google Scholar]
- 7. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578–1589. [DOI] [PubMed] [Google Scholar]
- 8. Heidari P, Heidari B, Babaei M. Efficacy and predictive factors of response to intra-articular corticosteroids in knee osteoarthritis. Reumatologia 2020;58:424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jüni P, Hari R, Rutjes AWS, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev 2015;CD005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015;162:46–54. [DOI] [PubMed] [Google Scholar]
- 11. Ayub S, Kaur J, Hui M, et al. Efficacy and safety of multiple intra-articular corticosteroid injections for osteoarthritis: a systematic review and meta-analysis of randomized controlled trials and observational studies. Rheumatology (Oxford) 2021; doi: 10.1093/rheumatology/keaa808. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 12. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy 2015;31:2213–2221. [DOI] [PubMed] [Google Scholar]
- 13. Rutjes AWS, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 2012;157:180–191. [DOI] [PubMed] [Google Scholar]
- 14. Mun JU, Cho HR, Choi YS, Kim YU. Effect of multiple intra-articular injections of polynucleotides on treatment of intractable knee osteoarthritis: a case report. Medicine (Baltimore) 2017;96:e9127–e9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gennero L, Denysenko T, Calisti GF, et al. Protective effects of polydeoxyribonucleotides on cartilage degradation in experimental cultures. Cell Biochem Funct 2013;31:214–227. [DOI] [PubMed] [Google Scholar]
- 16. Tseng S, Reddi AH, Di Cesare PE. Cartilage oligomeric matrix protein (COMP): a biomarker of arthritis. Biomark Insights 2009;4:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giarratana LS, Marelli BM, Crapanzano C, et al. A randomized double-blind clinical trial on the treatment of knee osteoarthritis: the efficacy of polynucleotides compared to standard hyaluronian viscosupplementation. Knee 2014;21:661–668. [DOI] [PubMed] [Google Scholar]
- 18. Kim MS, Cho RK, In Y. The efficacy and safety of polydeoxyribonucleotide for the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2019;98:e17386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manoto SL, Maepa MJ, Motaung SK. Medical ozone therapy as a potential treatment modality for regeneration of damaged articular cartilage in osteoarthritis. Saudi J Biol Sci 2018;25:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc 2017;25:485–492. [DOI] [PubMed] [Google Scholar]
- 21. Sconza C, Respizzi S, Virelli L, et al. Oxygen-ozone therapy for the treatment of knee osteoarthritis: a systematic review of randomized controlled trials. Arthroscopy 2020;36:277–286. [DOI] [PubMed] [Google Scholar]
- 22. Anitua E, Sánchez M, Orive G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv Drug Deliv Rev 2010;62:741–752. [DOI] [PubMed] [Google Scholar]
- 23. Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop 2010;34:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu J, Huang J-F, Qin X-X, et al. Platelet-rich plasma inhibits Wnt/β-catenin signaling in rabbit cartilage cells activated by IL-1β. Int Immunopharmacol 2018;55:282–289. [DOI] [PubMed] [Google Scholar]
- 25. De Santis M, Di Matteo B, Chisari E, et al. The role of Wnt pathway in the pathogenesis of OA and its potential therapeutic implications in the field of regenerative medicine. BioMed Res Int 2018;2018:7402947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord 2012;13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cerza F, Carnì S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med 2012;40:2822–2827. [DOI] [PubMed] [Google Scholar]
- 28. Sánchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy 2012;28:1070–1078. [DOI] [PubMed] [Google Scholar]
- 29. Kon E, Di Matteo B, Delgado D, et al. Platelet-rich plasma for the treatment of knee osteoarthritis: an expert opinion and proposal for a novel classification and coding system. Expert Opin Biol Ther 2020;20:1447–1460. [DOI] [PubMed] [Google Scholar]
- 30. Kon E, Filardo G, Condello V, et al. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am J Sports Med 2011;39:1668–1675. [DOI] [PubMed] [Google Scholar]
- 31. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007;213:341–347. [DOI] [PubMed] [Google Scholar]
- 32. Di Matteo B, Vandenbulcke F, Vitale ND, et al. Minimally manipulated mesenchymal stem cells for the treatment of knee osteoarthritis: a systematic review of clinical evidence. Stem Cells Int 2019;2019:1735242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol 2013;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc 2013;21:1717–1729. [DOI] [PubMed] [Google Scholar]
- 35. Tornero-Esteban P, Peralta-Sastre A, Herranz E, et al. Altered expression of Wnt signaling pathway components in osteogenesis of mesenchymal stem cells in osteoarthritis patients. PLoS One 2015;10:e0137170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marmotti A, de Girolamo L, Bonasia DE, et al. Bone marrow derived stem cells in joint and bone diseases: a concise review. Int Orthop 2014;38:1787–1801. [DOI] [PubMed] [Google Scholar]
- 37. Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 2009;17:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shapiro SA, Arthurs JR, Heckman MG, et al. Quantitative T2 MRI mapping and 12-month follow-up in a randomized, blinded, placebo controlled trial of bone marrow aspiration and concentration for osteoarthritis of the knees. Cartilage 2019;10:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vad V, Barve R, Linnell E, Harrison J. Knee osteoarthritis treated with percutaneous chondral-bone interface optimization: a pilot trial. Surg Sci 2016;7:1–12. [Google Scholar]
- 40. Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 2006;8:166–177. [DOI] [PubMed] [Google Scholar]
- 41. Ghaemi SR, Harding FJ, Delalat B, Gronthos S, Voelcker NH. Exploring the mesenchymal stem cell niche using high throughput screening. Biomaterials 2013;34:7601–7615. [DOI] [PubMed] [Google Scholar]
- 42. Hudetz D, Borić I, Rod E, et al. The effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes (Basel) 2017;8:E270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bansal H, Comella K, Leon J, et al. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med 2017;15:141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44. Roato I, Belisario DC, Compagno M, et al. Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: clinical and histological observations. Int Orthop 2019;43:15–23. [DOI] [PubMed] [Google Scholar]
- 45. Desando G, Bartolotti I, Martini L, et al. Regenerative features of adipose tissue for osteoarthritis treatment in a rabbit model: enzymatic digestion versus mechanical disruption. Int J Mol Sci 2019;20:E2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Condé-Green A, Kotamarti VS, Sherman LS, et al. Shift toward mechanical isolation of adipose-derived stromal vascular fraction: review of upcoming techniques. Plast Reconstr Surg Glob Open 2016;4:e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med 2015;43:1575–1582. [DOI] [PubMed] [Google Scholar]
- 48. Smith C, Patel R, Vannabouathong C, et al. Combined intra-articular injection of corticosteroid and hyaluronic acid reduces pain compared to hyaluronic acid alone in the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2019;27:1974–1983. [DOI] [PubMed] [Google Scholar]
- 49. Karasavvidis T, Totlis T, Gilat R, Cole BJ. Platelet-rich plasma combined with hyaluronic acid improves pain and function compared with hyaluronic acid alone in knee osteoarthritis: a systematic review and meta-analysis. Arthroscopy 2020;37(4):1277-1287. [DOI] [PubMed] [Google Scholar]
- 50. Sun S-F, Lin G-C, Hsu C-W, Lin H-S, Liou IS, Wu SY. Comparing efficacy of intraarticular single crosslinked Hyaluronan (HYAJOINT Plus) and platelet-rich plasma (PRP) versus PRP alone for treating knee osteoarthritis. Sci Rep 2021;11:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pintat J, Silvestre A, Magalon G, Gadeau AP, Pesquer L, Perozziello A, Peuchant A, Mounayer C, Dallaudière B. Intra-articular Injection of Mesenchymal Stem Cells and Platelet-Rich Plasma to Treat Patellofemoral Osteoarthritis: Preliminary Results of a Long-Term Pilot Study. J Vasc Interv Radiol. 2017;28(12):1708-1713. [DOI] [PubMed] [Google Scholar]
- 52. Sundaram K, Vargas-Hernández JS, Sanchez TR, et al. Are subchondral intraosseous injections effective and safe for the treatment of knee osteoarthritis? A systematic review. J Knee Surg 2019;32:1046–1057. [DOI] [PubMed] [Google Scholar]
- 53. Li G, Yin J, Gao J, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther 2013;15:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kon E, Ronga M, Filardo G, et al. Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg Sports Traumatol Arthrosc 2016;24:1797–1814. [DOI] [PubMed] [Google Scholar]
- 55. Di Matteo B, Polignano A, Onorato F, et al. Knee intraosseous injections: a systematic review of clinical evidence of different treatment alternatives. Cartilage 2020; doi: 10.1177/1947603520959403. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nairn LN, Subramaniam M, Ekhtiari S, Axelrod DE, Grant JA, Khan M. Safety and early results of Subchondroplasty® for the treatment of bone marrow lesions in osteoarthritis: a systematic review. Knee Surg Sports Traumatol Arthrosc 2020; doi: 10.1007/s00167-020-06294-w. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 57. Scher C, Craig J, Nelson F. Bone marrow edema in the knee in osteoarthrosis and association with total knee arthroplasty within a three-year follow-up. Skeletal Radiol 2008;37:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Howell R, Kumar NS, Patel N, Tom J. Degenerative meniscus: pathogenesis, diagnosis, and treatment options. World J Orthop 2014;5:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sihvonen R, Paavola M, Malmivaara A, Järvinen TLN. Finnish Degenerative Meniscal Lesion Study (FIDELITY): a protocol for a randomised, placebo surgery controlled trial on the efficacy of arthroscopic partial meniscectomy for patients with degenerative meniscus injury with a novel ‘RCT within-a-cohort’ study design. BMJ Open 2013;3:e002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Beaufils P, Becker R, Kopf S, et al. Surgical management of degenerative meniscus lesions: the 2016 ESSKA meniscus consensus. Knee Surg Sports Traumatol Arthrosc 2017;25:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guenoun D, Magalon J, de Torquemada I, et al. Treatment of degenerative meniscal tear with intrameniscal injection of platelets rich plasma. Diagn Interv Imaging 2020;101:169–176. [DOI] [PubMed] [Google Scholar]
- 62. Malanga GA, Chirichella PS, Hogaboom NS, Capella T. Clinical evaluation of micro-fragmented adipose tissue as a treatment option for patients with meniscus tears with osteoarthritis: a prospective pilot study. Int Orthop 2021;45:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ummarino A, Gambaro FM, Kon E, Torres Andón F. Therapeutic manipulation of macrophages using nanotechnological approaches for the treatment of osteoarthritis. Nanomaterials (Basel) 2020;10:E1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Evans CH, Ghivizzani SC, Robbins PD. Gene delivery to joints by intra-articular injection. Hum Gene Ther 2018;29:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gigout A, Guehring H, Froemel D, et al. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthritis Cartilage 2017;25:1858–1867. [DOI] [PubMed] [Google Scholar]
- 66. Dahlberg LE, Aydemir A, Muurahainen N, et al. A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clin Exp Rheumatol 2016;34:445–450. [PubMed] [Google Scholar]
- 67. Dawson J, Engelhardt P, Kastelic T, Cheneval D, MacKenzie A, Ramage P. Effects of soluble interleukin-1 type II receptor on rabbit antigen-induced arthritis: clinical, biochemical and histological assessment. Rheumatology (Oxford) 1999;38:401–406. [DOI] [PubMed] [Google Scholar]
- 68. Krupka E, Jiang G-L, Jan C. Efficacy and safety of intra-articular injection of tropomyosin receptor kinase A inhibitor in painful knee osteoarthritis: a randomized, double-blind and placebo-controlled study. Osteoarthritis Cartilage 2019;27:1599–1607. [DOI] [PubMed] [Google Scholar]
- 69. Wang S-J, Qin J-Z, Zhang T-E, Xia C. Intra-articular injection of kartogenin-incorporated thermogel enhancing osteoarthritis treatment. Front Chem 2019;7:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Di Matteo B, Murrell WD, Görtz S, Kon E. Osteoarthritis: an ancient disease, an unsolved conundrum. Int Orthop 2021;45:313–317. [DOI] [PubMed] [Google Scholar]



