Abstract
Background
The target of our study was to investigate if the size (greater than and less than 1 cm) of ground-glass opacities (GGOs) of adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) of the lung influences the rate of their evolution.
Methods
We retrospectively analyzed patients with AIS and MIA who underwent surgery at Shanghai Chest Hospital, Shanghai Jiao Tong University between January 2018 and July 2019, focusing on histopathology, surgical procedure, epidermal growth factor receptor (EGFR) mutations, and computed tomography (CT) images.
Results
A total of 224 AIS (n=117) and MIA (n=107) tumors were analyzed. The patients with a tumor diameter <1 cm were distinctly younger than those with tumors >1 cm in size (P<0.001). Pure ground-glass opacities (pGGO) occurred significantly more in patients with nodules <1 cm, while part-solid/mixed ground-glass opacities (mGGO) predominated in patients with nodules >1 cm (P=0.047). There was no significant difference in GGO evolution for GGOs of different sizes. Mutations of EGFR were more common in patients with MIA than in those with AIS (P<0.001).
Conclusions
We found that GGO size and variation (pGGO or mGGO) did not correlate to tumor stability, therefore larger GGOs can undergo standard follow-up protocols to evaluate their evolution over time.
Keywords: Ground-glass opacities (GGOs), size, adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), progression, follow-up
Introduction
Adenocarcinoma is the most common histological type of non-small cell lung cancer (NSCLC), and accounts for 40% of all cases (1,2). On computed tomography (CT) scans, preinvasive lesions such as atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), and minimally invasive adenocarcinoma (MIA) present as ground-glass opacities (GGO), which are considered inert nodules because they are less aggressive and evolve slowly (3,4). Follow up CT scan is the preferred strategy for patients with GGO’s, and surgical resection is indicated an increase in the dimensions of the nodule is detected (5). In 2015 the World Health Organization defined 2 new types of adenocarcinoma, AIS and MIA. The AIS type was defined as a small (≤3 cm), localized adenocarcinoma consisting of a pure lepidic component without an invasive component. The MIA type was defined as a small (≤3 cm), solitary adenocarcinoma with a predominantly lepidic growth, and an invasive component of ≤5 mm that lacked vascular, lymph gland, alveolar, or pleural invasion (6-10). It has been hypothesized that morphologically, these two subtypes show a gradual malignant progression from AIS to MIA (11,12).
In clinical practice, GGO’s larger than 1 cm are considered to have a higher risk of evolving into a malignancy than those smaller than 1 cm (13). The management of these nodules is unclear, as some surgeons opt for immediate surgical resection while others recommend a strict follow-up and surgery when the nodule begins to evolve. Due to insufficient evidence, neither of these strategies has emerged as a gold standard approach. Therefore, it is necessary to compare the clinical, biological, and genetic characteristics of AIS and MIA that are greater than and less than 1 cm in size.
The aim of this study was to compare the radiological evolution of GGOs (AIS and MIA) greater than and less than 1 cm in size during regular follow-up and to investigate the genetic characteristics of each. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-21-1994).
Methods
Patient selection
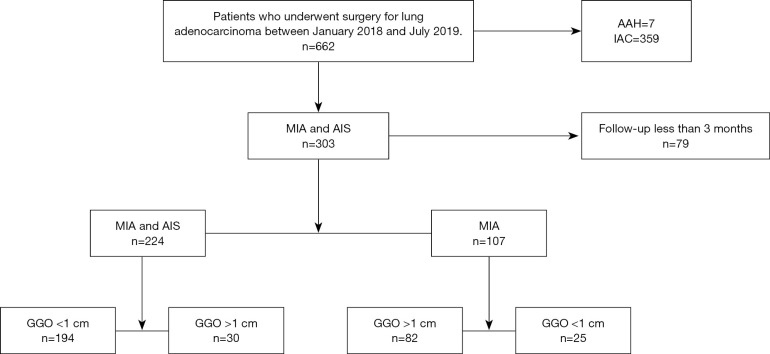
This retrospective analysis was approved by the Institutional Review Board of the Shanghai Chest Hospital, Shanghai Jiao Tong University (No: IS2155) and written informed consent was provided by all participants. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). We selected patients with lung adenocarcinoma who underwent surgery at our hospital between January 2018 and July 2019 for retrospective analysis. The inclusion criteria were patients that had been diagnosed with AIS or MIA who underwent follow-up for at least 3 months. Exclusion criteria were patients who had AAH and invasive adenocarcinoma or patients with AIS or MIA who had a follow-up time of less than 3 months. We retrospectively enrolled 224 participants in this study (Figure 1).
Figure 1.
Distribution of lung adenocarcinoma surgeries in our center. AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; GGO, ground glass opacity; AAH, atypical adenomatous hyperplasia; IAC, invasive adenocarcinoma.
Definition of change in GGOs on thin section CT
We retrospectively analyzed the high-resolution CT scans of all 224 participants. All nodules on CT images were divided into two groups as follows: homogenous ground glass nodule without solid component (pure GGOs, pGGO), and ground-glass nodule with solid components [part-solid/mixed GGOs (mGGO)] (14).
The scanning parameters of routine CT were as follows: detector collimation, 64 mm × 0.625 mm; pitch, 1.08–1.375; section thickness and interval, 5.0 and 5.0 mm; 2–7 s scan time; matrix, 512×512; field of view (FOV), 400 mm; 120 kVp and 230–280 mA. When a GGO was found, a high-resolution (HR)CT target scan followed for the suspected area with following, parameters: collimation, 64 mm × 0.625 mm; pitch, 0.64; section thickness and interval, 1.0–1.5 mm, no overlap reconstruction; 1–3 s scan time; matrix, 1,024×1,024; FOV, 120–180 mm.
All scans were performed without any contrast material. All images were evaluated by two radiological specialists with more than 5 years of experience, a final consensus regarding diagnosis of GGO progression was archived by plenary reading.
The size of each lesion was recorded by evaluating the largest diameter by a caliper tool in the software. The “CT density” was defined as the mean density [Hounsfield units (HU)] measured at 3 spots within the GGO part of each lesion with the software tool.
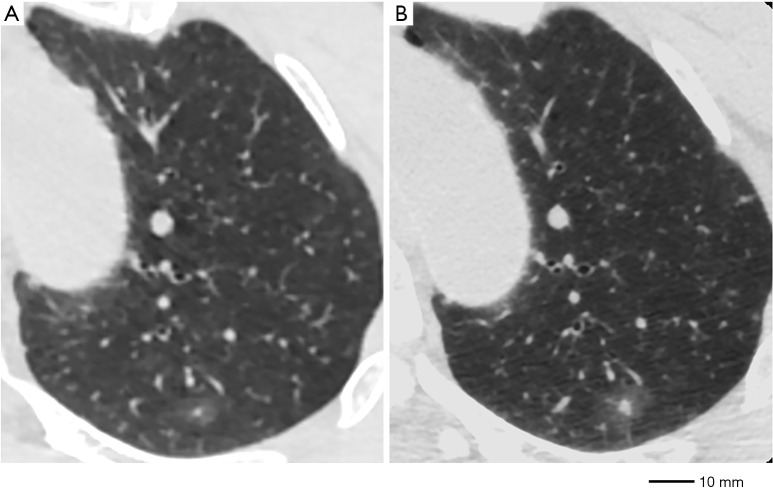
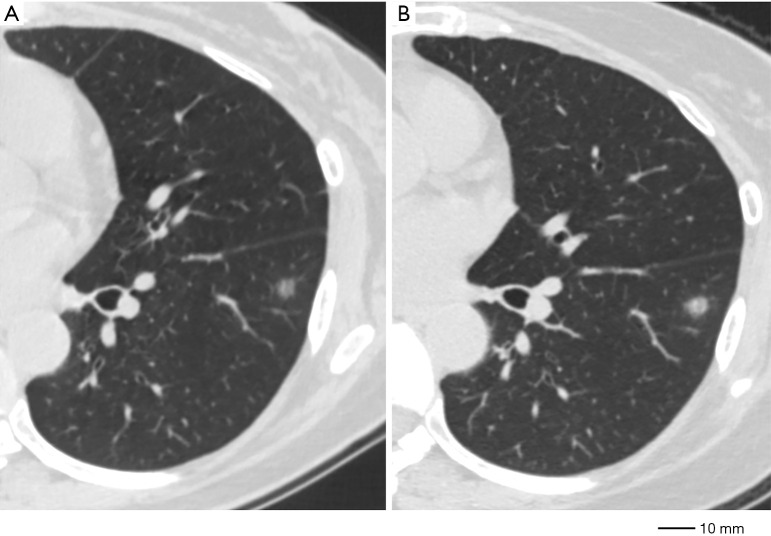
A GGO was deemed “changed” when any one of the following two situations were recognized: gross increase in the greatest dimension by at least 2 mm from the initial triple-source (TS)CT (Figures 2,3), gross increase in the CT density by at least 50 HU (Figures 2,3).
Figure 2.
A case showing GGO dynamic CT change in size increase and solid part growth. (A) GGO in the left upper lobe, measuring 8 mm in diameter, −544 HU in CT density. (B) Diameter grew to 14 mm and CT density increased to −365 after 3 months (with solid component growth from 1 to 3 mm in diameter). GGO, ground glass opacity; CT, computed tomography; HU, Hounsfield units.
Figure 3.
A case showing GGO dynamic CT change in size and CT density. (A) GGO in the left lower lobe, measuring 6.6 mm in diameter, −398 HU in CT density. (B) Diameter grew to 8.4 mm and CT density increased to −220 HU after 72 months. GGO, ground glass opacity; CT, computed tomography; HU, Hounsfield units.
All CT images and all CT densities were compared for MIA and AIS. Lesions with CT scan changes during the follow-up period were defined as unstable nodules. Lesions that did not evolve during the follow-up period were defined as stable nodules.
Clinical evaluation
All tumor sections were classified according to the 2015 WHO classification for lung adenocarcinoma (15). The AIS type was defined as a small (≤3 cm), localized adenocarcinoma consisting of a lepidic component without an invasive component (16). The MIA type was defined as a small (≤3 cm), solitary adenocarcinoma with a predominantly lepidic growth, showing ≤5 mm invasive component and that lacked vascular, lymph gland, alveolar, or pleural invasion (8). All hematoxylin-and-eosin (H&E) stained slides of the surgically resected specimens were independently reviewed by two pathologists to evaluate the histological classification. In addition, we analyzed the EGFR mutation status based on the peptide nucleic acid-locked nucleic acid polymerase chain reaction (PCR) clamp method, a method that can detect known mutations by PCR primers (17).
Surgical strategy
All participants underwent a lung resection (wedge resection, segmentectomy, or lobectomy) and lymphadenectomy (sampling or radical). GGOs near lung hilum were removed by up-front anatomical resection (segmentectomy based on CT 3D reconstruction or lobectomy). All surgical operations were performed by thoracic surgeons at the Shanghai Chest Hospital, Shanghai Jiao Tong University, wherein all surgical procedures are standardized.
Statistical analysis
Clinicopathological data of the AIS/MIA groups were compared in all participants by SPSS 26.0 software (IBM Corp., Armonk, NY, USA). The chi-square test or Fisher’s exact test was used for categorical variables, and the independent-samples t-test was used for continuous variables. A P value <0.05 was considered statistically significant.
Results
Participant demographic characteristics
The demographic characteristics of the 224 participants are summarized in Table 1. Of the 224 participants, 169 were female and 55 were male. The mean age was 50.71 years (±12.06; range, 24–79). A total of participants (11.1%) had a history of smoking and 85 (37.9%) had a positive family history for tumors. The participants were divided into two groups according to the diameter of the tumor: group 1, tumor size <1 cm (n=194) and group 2, tumors size ≥1 cm (n=30). Among the participants in group 1, 112 of the tumors were AIS and 82 were MIA. Among those in group 2, 5 were AIS and 25 were MIA. Group 1 had an average age of 48.92 years (±11.17, range, 24–76 years), while group 2 had an average age of 62.27 years (±11.3, range, 39–79 years). Individual participant history revealed that 74 (38.1%) participants in group 1 had a family history positive for tumors in, while 11 (36.6%) in group 2 had a positive family history. A total of 19 participants (9.8%) in group 1 and 6 (20%) in group 2 had a positive history of smoking. The average follow-up time was 16.56 months (±18.057, range, 3–132) in group 1, and 18.37 months (±17.709, range, 3–60) in group 2 (P=0.610).
Table 1. General characteristics.
| Characteristic | Total (n=224) | Group 1 (AIS + MIA <1 cm) (n=194) | Group 2 (AIS + MIA ≥1 cm) (n=30) | P value |
|---|---|---|---|---|
| Age (years) | 50.71 (±12.057; 24–79) | 48.92 (±11.172; 24–76) | 62.27 (±11.298; 39–79) | <0.001* |
| Gender | 0.230 | |||
| Male | 55 (24.6%) | 45 (23.2%) | 10 (33.3%) | |
| Female | 169 (75.4%) | 149 (76.8%) | 20 (66.7%) | |
| Smoking history | 25 (11.2%) | 19 (9.8%) | 6 (20%) | 0.117 |
| Family tumor history | 85 (37.9%) | 74 (38.1%) | 11 (36.6%) | 0.877 |
| Follow-up (months) | 16.80 (±17.982) | 16.56 (±18.057) | 18.37 (±17.709) | 0.610 |
Data are presented as mean (SD), range (–) or n (%). *P<0.05. AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; SD, standard deviation.
Clinicopathological features and CT examination
Participant clinicopathological features are summarized in Table 2, which reveals statistical differences between the two groups in terms of clinical tumor size (P<0.001), pathological tumor diameter (P<0.001), surgical procedure (P<0.001), and histological type (P=0.047). The CT image analysis showed that in group 1 had more participants with pGGO’s than group 2, with 115 (59.3%) and 12 (40%) participants, respectively (P=0.047). In group 1, 85 participants (43.8%) presented with multiple primary lung cancer with an average tumor diameter of 6.95 mm (±1.74, range, 3–10), while the number of participants with the same presentation in group 2 was 16 (53.3%), with an average tumor size of 13.88 mm (±2.78, range, 11–23). In group 1 nodule dimensions increased in 26 participants (13.4%), while 5 patients (2.5%) developed CT density increases. A total of 7 participants (23.3%) in group 2 experienced an increase in nodule size, while 2 (6.7%) developed CT density increases. We did not find a significant relationship between the pathological tumor diameter and nodule dimension changes (P=0.168) or CT density increases (P=0.238). With regards to surgery in group 1, 112 participants (57.7%) received a wedge resection with an average nodule diameter of 6.46 mm (±1.58; range, 3–10), 39 (20.1%) underwent segmentectomy with an average nodule diameter of 7.08 mm (±1.69; range, 4–10), and 43 (22.2%) received lobectomy with an average nodule diameter of 8.26 mm (±1.38, range, 5–10). In group 2, 6 participants (20%) received a wedge resection with an average nodule diameter of 12 mm (±1.10, range, 11–14), 8 (26.7%) underwent a segmentectomy with an average nodule diameter of 13.75 mm (±1.58; range, 11–15), and 16 (53.3%) received a lobectomy with an average nodule diameter of 15 mm (±3.7, range, 12–25). Tumors larger than 1 cm in diameter were more likely to be treated with lobectomy rather than with wedge resection.
Table 2. Clinicopathological features.
| Variable | Total (n=224) | Group 1 (AIS + MIA <1 cm) (n=194) | Group 2 (AIS + MIA ≥1 cm) (n=30) | P value |
|---|---|---|---|---|
| Multiple primary lung cancer | 101 (45.1%) | 85 (43.8%) | 16 (53.3%) | 0.329 |
| Clinical tumor size (cm) | 8.41 (±3.011; 3–26) | 7.65 (±1.979; 3–14) | 13.30 (±3.879; 8–26) | <0.001* |
| Pathological tumor size (cm) | 7.93 (±3.098; 3–25) | 6.98 (±1.712; 3–10) | 14.07 (±3.05; 11–25) | <0.001* |
| Size change (n) | 33 (14.7%) | 26 (13.4%) | 7 (23.3%) | 0.168 |
| CT density change (n) | 7 (3.1%) | 5 (2.5%) | 2 (6.7%) | 0.238 |
| CT image (n) | 0.047* | |||
| pGGO | 127 (56.7%) | 115 (59.3%) | 12 (40%) | |
| mGGO | 97 (43.3%) | 79 (40.7%) | 18 (60%) | |
| CT density (average) | −484.31 (172.895) | −484.51 (179.554) | −483.00 (123.705) | 0.965 |
| Surgical procedure (n) | <0.001* | |||
| Wedge resection | 118 (52.7%) | 112 (57.7%) | 6 (20%) | |
| Segmentectomy | 47 (21%) | 39 (20.1%) | 8 (26.7%) | |
| Lobectomy | 59 (26.3%) | 43 (22.2%) | 16 (53.3%) |
Data are presented as mean (SD), range (–) or n (%). *P<0.05. AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; CT, computed tomography; pGGO, pure ground-glass opacity; mGGO, mixed solidity ground glass opacity; SD, standard deviation.
We further divided all MIA participants into two groups according to the diameter of the tumor (Table 3): group 3, tumor size <1 cm (n=82) vs. group 4, tumor size ≥1 cm (n=25). Those in group 3 had an average diameter of 7.29 mm (±1.718), while those in group 4 had an average diameter of 14.36 mm (±3.817). In group 3, 32 participants (39%) had a pGGO vs. 9 participants (36%) in group 4 (P=0.785). In group 3, 10 participants (12.2%) had an increase in nodule size, while 5 (6.1%) developed CT density increases. In group 4, 7 participants (28%) had an increase in nodule size, while 2 (8%) developed CT density increases. We did not detect a significant relationship between the pathological tumor diameter and nodule dimension changes (P=0.069), CT density increases (P=0.664), type of GGO (P=0.785), or CT density (P=0.134). With regards to surgery, in group 3, 38 participants (46.3%) received wedge resection (average diameter, 6.61±1.57 mm; range, 3–10 mm), 21 (25.6%) underwent segmentectomy (average diameter, 7.29±1.74 mm; range, 4–10 mm), and 23 (28.1%) received lobectomy (average diameter, 8.43±1.34 mm; range, 6–10 mm). In group 4, 5 participants (20%) received wedge resection (average diameter, 12.2±1.10 mm; range, 11–14 mm), 5 (20%) underwent segmentectomy (average diameter, 14.6±0.89 mm; range, 13–15 mm), and 15 (60%) received lobectomy (average diameter, 15±3.84 mm; range, 12–25 mm) in group 4. Tumors larger than 1 cm in diameter were more likely to undergo a lobectomy rather than a wedge resection (P=0.018).
Table 3. MIA clinicopathological features.
| Variable | Total (n=107) | Group 1 (MIA <1 cm) (n=82) | Group 2 (MIA ≥1 cm) (n=25) | P value |
|---|---|---|---|---|
| Multiple primary lung cancer (n) | 50 (46.7%) | 36 (43.9%) | 14 (56%) | 0.289 |
| Clinical tumor size (cm) | 9.36±3.508 [3–26] | 8.05±1.962 [3–14] | 13.64±4.051 [8–26] | <0.001* |
| Pathological tumor size (cm) | 8.94±3.685 [3–25] | 7.29±1.718 [3–10] | 14.36±3.817 [11–25] | <0.001* |
| Size change (n) | 17 (15.9) | 10 (12.2%) | 7 (28%) | 0.069 |
| CT density change (n) | 7 (6.5%) | 5 (6.1%) | 2 (8%) | 0.664 |
| CT image (n) | 0.785 | |||
| pGGO | 41 (38.3%) | 32 (39%) | 9 (36%) | |
| mGGO | 66 (61.7%) | 50 (61%) | 16 (64%) | |
| CT density (average) | −433.50±173.876 | −419.56±182.795 | −479.24±133.845 | 0.134 |
| Surgical procedure (n) | 0.011* | |||
| Wedge resection | 43 (40.2%) | 38 (46.3%) | 5 (20%) | |
| Segmentectomy | 26 (24.3%) | 21 (25.6%) | 5 (20%) | |
| Lobectomy | 38 (35.5%) | 23 (28.1%) | 15 (60%) |
Data are presented as mean (SD), range [–] or n (%). *P<0.05. MIA, minimally invasive adenocarcinoma; CT, computed tomography; pGGO, pure ground-glass opacity; mGGO, mixed solidity ground glass opacity; SD, standard deviation.
Genetic information
We tested 63 of 117 AIS participants (53.9%) and 70 of 107 MIA participants (65.4%) for EGFR mutations (18) (Table 4). Among those tested, 17 participants (27%) in the AIS group and 37 (52.9%) in the MIA group had EGFR mutations. The results showed that EGFR mutations were more frequent in MIA than in AIS (P=0.002). Among the 17 AIS participants, 3 had deletion mutations in exon 18, 5 in exon 19, 2 in exon 20, and 7 in exon 21. Among the 37 MIA participants, 3 had deletion mutations in exon 18, 20 in exon 19, 2 in exon 20, and 12 in exon 21 (19). We did not detect an obvious difference in exon ratio between AIS and MIA (P=0.303). In group 1, the EGFR mutation was found in 45 of the 115 participants (39.1%) who underwent genetic testing. In group 2, the EGFR mutation was found in 9 of the 18 participants (50%) who underwent genetic testing. No statistically significant difference was found between pathological tumor diameter and EGFR mutations (P=0.383).
Table 4. EGFR mutation characteristics.
| Characteristics | Total | AIS, n=63 | MIA, n=70 | P value | Group 1, n=115 | Group 2, n=18 | P value | Unstable group, n=27 | Stable group, n=106 | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| EGFR mutation | 54 (40.6) | 17 (27.0) | 37 (42.9) | 0.002* | 45 (39.1) | 9 (50.0) | 0.383 | 15 (55.6) | 39 (36.8) | 0.076 |
| Exon 18 mutation | 3 (4.8) | 3 (4.3) | 0.303 | 5 (4.3) | 1 (5.6) | 0.790 | 1 (3.7) | 5 (4.7) | 0.828 | |
| Exon 19 mutation | 5 (7.9) | 20 (28.6) | 22 (19.1) | 3 (16.7) | 6 (22.2) | 19 (18.0) | ||||
| Exon 20 mutation | 2 (3.2) | 2 (2.9) | 3 (2.6) | 1 (5.6) | 1 (3.7) | 3 (2.8) | ||||
| Exon 21 mutation | 7 (11.1) | 12 (17.1) | 15 (13.0) | 4 (22.2) | 7 (26.0) | 12 (11.3) |
Data are presented as n (%). *P<0.05. EGFR, epidermal growth factor receptor; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma.
Difference between unstable group and stable group
We divided all participants into two groups according to CT image changes: an “unstable group” who experienced CT image changes during the follow-up period (n=40) and a “stable group” (n=184) who had no obvious CT image changes during the follow-up period. In the stable group 106 out of 184 (57.6%) participants were tested for EGFR mutations, while 27 out of 40 (67.5%) in the unstable group were tested. With regards to the result, 39 participants (36.8%) in the stable group and 15 (55.6%) in the unstable group had EGFR mutations. There was no statistically significant difference between the two groups in terms of the incidence of EGFR mutations (P=0.076).
Prognosis of unstable group and stable group
All 224 participants were participating in routine post-surgical follow-up every 6 months, and at the moment they are all alive and 1 of them has experienced recurrence. Performance status (PS) scores of all participants were all between 1 and 2 without any significant difference between the unstable group and stable group.
Discussion
There is a lot we do not know about the newly defined adenocarcinoma subtypes of AIS and MIA, as demonstrated by Inamura in a recent publication (15). Our study demonstrated several important differences between different sizes of AIS and MIAs in terms of age, CT findings, and surgical procedure.
Morphologically, it has been hypothesized that AIS gradually progresses to become the more malignant MIA (11,12). The proportion of the solid component, namely pGGO (non-solid component) and mGGO (part-solid component), is generally correlated with tumor progression (20). In addition, several studies have found that the solid component of a GGO in adenocarcinoma enhances the biological invasion of the tumor (21). Previous studies have reported that the imaging pattern of GGO’s was associated with the IASLC/ATS/ERS histological subtypes of adenocarcinoma (19). The proportion of the solid component in advanced-stage lesions was significantly higher than in earlier stage lesions (22). According to CT findings, we found that the proportion of pGGO was higher in smaller lesions. Lesions smaller than 1 cm were more likely to be a pGGO while those larger than 1 cm were most often mGGO.
We found that participants with a tumor diameter of less than 1 cm were considerably younger than those with a tumor diameter greater than 1 cm (48.92±11.17 vs. 62.27±11.3 years). The subtype and diameter of a tumor are important factors in the choice of surgical procedure (23). Tumors larger than 1 cm in diameter were more likely to be treated with a lobectomy than with a wedge resection or a segmentectomy. Since it is difficult to differentiate an MIA from an invasive carcinoma in small nodules, MIA patients were also more likely to undergo a lobectomy rather than a wedge resection.
Mutations of EGFR are one of the most common oncogenic driver mutations of lung adenocarcinoma, especially in the East Asian population (24,25). It has been hypothesized that EGFR-mutated adenocarcinoma transforms from an AIS to a MIA and eventually an IAC (26). In our research, EGFR mutation rates were 52.9% in MIA and 27% in AIS. Our study showed that EGFR mutations were more frequent in MIA than in AIS (P=0.002). In addition, we did not identify any obvious differences in exon ratio among AIS when compared to MIAs (P=0.303). We also did not find a relationship between pathological tumor diameter and EGFR mutation. The ratio of EGFR mutations was significantly correlated with tumor subtypes but not with tumor diameter.
Tumors with altered CT images during follow-up were defined as unstable tumors. Some reports suggest that larger GGOs are more likely to have invasive characteristics, and therefore it is reasonable to expect a higher incidence of CT image changes in larger GGOs (27). According to our study, there was no significant difference between stable and unstable GGOs between the two size groups. We did not find a link between EGFR mutations and the stability of tumors. Our findings are important as it is often believed that in larger GGOs a conservative approach should be bypassed, however in our study size did not correlate to tumor stability, which indicated that there is no need to circumvent follow up for surgery in larger GGOs, rather follow-up should be integral to evaluate the stability of the tumor over time. Another important finding was that of the stability of group 2 (tumors >1 cm), which also had a statistically significantly larger proportion of mGGO, yet these tumors were just as stable as those in group 1. Our results showed that participants in group 1 received more wedge resections and segmentectomies, while group 2 received more lobectomies, which demonstrated that surgeons are more willing to perform a lobectomy in the case of a GGO greater than 1 cm in size; despite that, according to our results, size did not influence the stability of the GGO.
There were several limitations to this study. This was a retrospective study in which selection biases were inevitable. Our research was carried out by a single institution located in East Asia. East Asian patients have different genetic characteristics in lung adenocarcinoma to those of Western patient populations, such as more frequent EGFR mutations (28); therefore, our findings may not necessarily be applied to the broader international population.
Conclusions
Despite beliefs that larger GGO’s are more likely to be invasive in nature, our study population showed no correlation between larger GGOs (>1 cm) and tumor stability. We therefore encourage a follow-up first approach in larger GGOs, as our study also revealed these lesions tend to undergo lobectomies instead of sublobectomies more readily.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This article was supported by the National Natural Science Foundation of China (Grant No.81972176), Shanghai Science and Technology Committee (18411966100), Shanghai Talent Development Fund (2019073), Shanghai Natural Science Foundation (Grant No. 18ZR1435100). Shanghai “Rising Stars of Medical Talent” Youth Development Program (Specialist Program), “Chenxing” award program for outstanding young scholars of Shanghai Jiaotong University.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board of Shanghai Chest Hospital (No: IS2155) and written informed consent was provided by all patients. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-21-1994
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-21-1994
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-1994). The authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Zhang P, Li S, Lv C, et al. BPI-9016M, a c-Met inhibitor, suppresses tumor cell growth, migration and invasion of lung adenocarcinoma via miR203-DKK1. Theranostics 2018;8:5890-902. 10.7150/thno.27667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T, Luo J, Gu H, et al. Impact of Solid Minor Histologic Subtype in Postsurgical Prognosis of Stage I Lung Adenocarcinoma. Ann Thorac Surg 2018;105:302-8. 10.1016/j.athoracsur.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 3.Inamura K. Clinicopathological Characteristics and Mutations Driving Development of Early Lung Adenocarcinoma: Tumor Initiation and Progression. Int J Mol Sci 2018;19:1259. 10.3390/ijms19041259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen T, Luo J, Gu H, et al. Should minimally invasive lung adenocarcinoma be transferred from stage IA1 to stage 0 in future updates of the TNM staging system? J Thorac Dis 2018;10:6247-53. 10.21037/jtd.2018.10.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding S, Long F, Jiang S. Acute myocardial infarction following erlotinib treatment for NSCLC: A case report. Oncol Lett 2016;11:4240-4. 10.3892/ol.2016.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T, Luo J, Wang R, et al. Visceral pleural invasion predict a poor survival among lung adenocarcinoma patients with tumor size ≤ 3cm. Oncotarget 2017;8:66576-83. 10.18632/oncotarget.16476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida H, Shimizu Y, Sakaguchi H, et al. Distinctive clinicopathological features of adenocarcinoma in situ and minimally invasive adenocarcinoma of the lung: A retrospective study. Lung Cancer 2019;129:16-21. 10.1016/j.lungcan.2018.12.020 [DOI] [PubMed] [Google Scholar]
- 9.Yang D, Li Y, Liu J, et al. Study on solitary pulmonary nodules: correlation between diameter and clinical manifestation and pathological features. Zhongguo Fei Ai Za Zhi 2010;13:607-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T, Luo J, Wang R, et al. Prognosis of limited resection versus lobectomy in elderly patients with invasive lung adenocarcinoma with tumor size less than or equal to 2 cm. J Thorac Dis 2018;10:2231-9. 10.21037/jtd.2018.04.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura H, Koizumi H, Kimura H, et al. Epidermal growth factor receptor mutations in adenocarcinoma in situ and minimally invasive adenocarcinoma detected using mutation-specific monoclonal antibodies. Lung Cancer 2016;99:143-7. 10.1016/j.lungcan.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 13.Boland JM, Froemming AT, Wampfler JA, et al. Adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive pulmonary adenocarcinoma--analysis of interobserver agreement, survival, radiographic characteristics, and gross pathology in 296 nodules. Hum Pathol 2016;51:41-50. 10.1016/j.humpath.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 14.Kakinuma R, Ashizawa K, Kuriyama K, et al. Measurement of focal ground-glass opacity diameters on CT images: interobserver agreement in regard to identifying increases in the size of ground-glass opacities. Acad Radiol 2012;19:389-94. 10.1016/j.acra.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 15.Inamura K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front Oncol 2017;7:193. 10.3389/fonc.2017.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borczuk AC. Assessment of invasion in lung adenocarcinoma classification, including adenocarcinoma in situ and minimally invasive adenocarcinoma. Mod Pathol 2012;25 Suppl 1:S1-10. 10.1038/modpathol.2011.151 [DOI] [PubMed] [Google Scholar]
- 17.Jiang L, Mino-Kenudson M, Roden AC, et al. Association between the novel classification of lung adenocarcinoma subtypes and EGFR/KRAS mutation status: A systematic literature review and pooled-data analysis. Eur J Surg Oncol 2019;45:870-6. 10.1016/j.ejso.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 18.Biton J, Mansuet-Lupo A, Pecuchet N, et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti-PD-1 in Lung Adenocarcinoma. Clin Cancer Res 2018;24:5710-23. 10.1158/1078-0432.CCR-18-0163 [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Zhang T, Han X, et al. Impact of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of stage IA adenocarcinoma of the lung: Correlation between computed tomography images and EGFR and KRAS gene mutations. Exp Ther Med 2015;9:2095-103. 10.3892/etm.2015.2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takashima S, Maruyama Y, Hasegawa M, et al. CT findings and progression of small peripheral lung neoplasms having a replacement growth pattern. AJR Am J Roentgenol 2003;180:817-26. 10.2214/ajr.180.3.1800817 [DOI] [PubMed] [Google Scholar]
- 21.Aoki T, Tomoda Y, Watanabe H, et al. Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology 2001;220:803-9. 10.1148/radiol.2203001701 [DOI] [PubMed] [Google Scholar]
- 22.Kuriyama K, Seto M, Kasugai T, et al. Ground-glass opacity on thin-section CT: value in differentiating subtypes of adenocarcinoma of the lung. AJR Am J Roentgenol 1999;173:465-9. 10.2214/ajr.173.2.10430155 [DOI] [PubMed] [Google Scholar]
- 23.Fang W, Xiang Y, Zhong C, et al. The IASLC/ATS/ERS classification of lung adenocarcinoma-a surgical point of view. J Thorac Dis 2014;6:S552-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosell R, Karachaliou N. Large-scale screening for somatic mutations in lung cancer. Lancet 2016;387:1354-6. 10.1016/S0140-6736(15)01125-3 [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Zhou M, Ma Y, et al. Hybridized Polyoxometalate-Based Metal-Organic Framework with Ketjenblack for the Nonenzymatic Detection of H2 O2. Chem Asian J 2018. [Epub ahead of print]. 10.1002/asia.201800758 [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto H, Shimizu J, Horio Y, et al. Disproportionate representation of KRAS gene mutation in atypical adenomatous hyperplasia, but even distribution of EGFR gene mutation from preinvasive to invasive adenocarcinomas. J Pathol 2007;212:287-94. 10.1002/path.2165 [DOI] [PubMed] [Google Scholar]
- 27.Hiramatsu M, Inagaki T, Inagaki T, et al. Pulmonary ground-glass opacity (GGO) lesions-large size and a history of lung cancer are risk factors for growth. J Thorac Oncol 2008;3:1245-50. 10.1097/JTO.0b013e318189f526 [DOI] [PubMed] [Google Scholar]
- 28.Chougule A, Prabhash K, Noronha V, et al. Frequency of EGFR mutations in 907 lung adenocarcioma patients of Indian ethnicity. PLoS One 2013;8:e76164. 10.1371/journal.pone.0076164 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as